Abstract
Aims
The forthcoming STAMPEDE2 trial has three comparisons in metastatic hormone-sensitive prostate cancer. We aim to determine clinical practices among STAMPEDE trial investigators for access to imaging and therapeutic choices and explore their interest in participation in STAMPEDE2.
Materials and methods
The survey was developed and distributed online to 120 UK STAMPEDE trial sites. Recipients were invited to complete the survey between 16 and 30 May 2022. The survey consisted of 30 questions in five sections on access to stereotactic ablative body radiotherapy (SABR), 177lutetium-prostate-specific membrane antigen-617 (177Lu-PSMA-617), choice of systemic therapies and use of positron emission tomography/computerised tomography and whole-body magnetic resonance imaging.
Results
From 58/120 (48%) sites, 64 respondents completed the survey: 55/64 (86%) respondents were interested to participate in SABR, 44/64 (69%) in 177Lu-PSMA-617 and 56/64 (87.5%) in niraparib with abiraterone comparisons; 45/64 (70%) respondents had access to bone, spine and lymph node metastases SABR delivery and 7/64 (11%) to 177Lu-PSMA-617. In addition to androgen deprivation therapy, 60/64 (94%) respondents used androgen receptor signalling inhibitors and 46/64 (72%) used docetaxel; 29/64 (45%) respondents would consider triplet therapy with androgen deprivation therapy, androgen receptor signalling inhibitors and docetaxel. Positron emission tomography/computerised tomography was available to 62/64 (97%) respondents and requested by 45/64 (70%) respondents for disease uncertainty on conventional imaging and 39/64 (61%) at disease relapse. Whole-body magnetic resonance imaging was available to 24/64 (38%) respondents and requested by 13/64 (20%) respondents in highly selected patients. In low-volume disease, 38/64 (59%) respondents requested scans at baseline and disease relapse. In high-volume disease, 29/64 (45%) respondents requested scans at baseline, best response (at prostate-specific antigen nadir) and disease relapse; 54/64 (84%) respondents requested computerised tomography and bone scan for best response assessment.
Conclusion
There is noteworthy disparity in clinical practice across current study sites, however most have expressed an interest in participation in the forthcoming STAMPEDE2 trial.
Keywords: 177lutetium-PSMA-617, metastatic hormone-sensitive prostate cancer, niraparib, positron emission tomography/computerised tomography, prostate-specific membrane antigen, stereotactic ablative body radiotherapy
Introduction
Prostate cancer causes around 12 000 deaths per year in the UK [1]. The STAMPEDE platform trial (ISRCTN78818544) is an innovative multi-arm multi-stage platform trial that has tested 10 different treatments in advanced prostate cancer, hypothesising improved outcomes with upstream treatment intensification. To date, three treatments added to androgen deprivation therapy (ADT) have improved outcomes: docetaxel, abiraterone acetate and prostate radiotherapy in low-burden metastatic disease [2–6] and have become standard of care in international guidelines [7,8]. STAMPEDE2 is a new platform trial continuing on from STAMPEDE and testing three new treatments with the ability to add further treatments in the future.
Radiation-based targeted therapies for metastatic prostate cancer have been of increasing interest. Metastasis-directed therapy with stereotactic ablative body radiotherapy (SABR) in metachronous oligometastatic disease has been shown to delay recurrence in prospective trials [9–13]. No randomised data exist in synchronous metastatic disease.
Comparably, in heavily pre-treated castrate-resistant prostate cancer (CRPC), two randomised trials showed that 177lutetium-prostate-specific membrane antigen-617 (177Lu-PSMA-617) improved progression-free survival [14,15] with results from the VISION trial [14] leading to the US Food and Drug Administration approval of 177Lu-PSMA-617 and subsequently its wider availability [16].
In addition to radiation-based therapies, molecular targeted therapies with poly (ADP-ribose) polymerase inhibitors (PARPi) in combination with androgen-receptor signalling inhibitors (ARSI) have been investigated in first-line CRPC. Phase III randomised trials recently reported on preferential improved survival in men with homologous recombination repair deficiency and breast cancer gene (BRCA) mutation subgroups [17–19].
The STAMPEDE2 trial aims to investigate these treatments in three new comparisons in men with metastatic hormone-sensitive prostate cancer (mHSPC). Here, we report on results from the STAMPEDE2 trial site survey conducted to explore the interest and technical capacities of STAMPEDE investigators to participate in the STAMPEDE2 trial and determine the patterns of current clinical practice for imaging and therapeutic choices.
Materials and methods
The site survey was designed by the STAMPEDE2 trial team in April 2022. The aims of the survey were to inform the design of the forthcoming STAMPEDE2 trial design and determine consensus on current practices in the UK reflected by the multiplicity of the STAMPEDE trial participating sites. The survey included a summary and rationale of the STAMPEDE2 trial design with three new comparisons in men with mHSPC, investigating SABR (comparison S), 177Lu-PSMA-617 (comparison P) and niraparib (PARPi) with abiraterone acetate plus prednisolone (abiraterone; comparison N). The survey constituted 30 questions in five sections: general questions, questions on access to novel imaging facilities, questions on use of systemic therapies at the treating site, questions on access to SABR delivery and questions on access to 177Lu-PSMA-617 (see Supplementary Appendix A). Multiple responses were permitted for selected questions. The survey was conducted using the online platform survey monkey (http://www.surveymonkey.co.uk) and was distributed via an e-mail link from the Medical Research Council Clinical Trials Unit (MRC CTU) to the 120 UK-based sites participating in the STAMPEDE trial (ISRCTN78818544). Principal investigators and/or first recipients of the survey were invited to complete the survey. The survey was active online between 16 and 30 May 2022. Descriptive analysis was utilised using Stata statistical software version 17.0 (Stata Corporation, College Station, TX, USA).
Results
During the 2-week period of the survey being active, 64 respondents completed the survey from 58 of the 120 (48%) STAMPEDE trial participating sites: 55/64 (86%) respondents were interested to participate in comparison S, 44/64 (69%) respondents were interested in comparison P and 56/64 (87.5%) respondents were interested in comparison N; 62/64 (97%) respondents had access to positron emission tomography/computerised tomography (PET/CT) scans. Of those, 35/62 (56%) had access to PET/CT scans at their treating centre, 23/62 (36%) had access at a neighbouring treating centre and 4/62 (6%) had access at a distance centre with a long referral pathway. Eleven of 64 (17%) respondents did not have direct access to PET/CT scans. Of those, 2/64 (3%) foresaw direct access at their treating centre in less than 12 months, 2/64 (3%) foresaw direct access in 1e3 years 1/64 (1.5%) foresaw direct access in more than 3 years and 6/64 (9%) were unsure or had no planned direct access to PET/CT scans. The types of PET/CT scans to which respondents had access to are summarised in Table 1.
Table 1. Type of available positron emission tomography/computerised tomography (PET/CT) imaging.
| Access to PET/CT imaging | n/N | % (95% confidence interval) |
|---|---|---|
| PET/CT | 62/64 | 97 (89–99) |
| 18F-choline PET/CT | 36/62 | 58 (45–70) |
| 18F-PSMA PET/CT | 25/62 | 40 (28–54) |
| 68Ga-PSMA PET/CT | 23/62 | 37 (25–50) |
F, fluorinated; Ga, gallium; PSMA, prostate-specific membrane antigen.
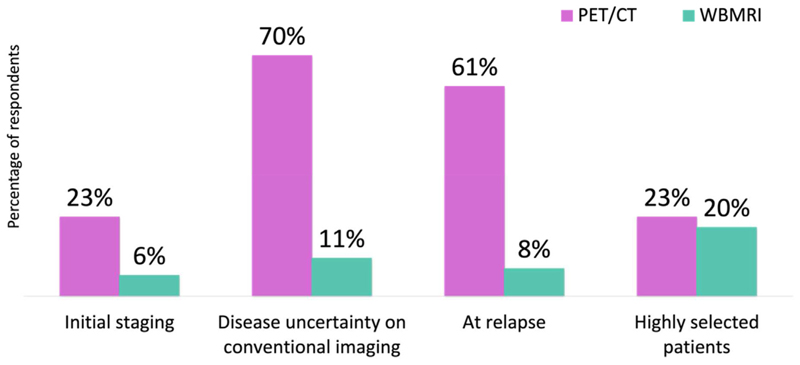
In total, 24/64 (37.5%) respondents had access to whole-body magnetic resonance imaging (WBMRI) and 38/64 (59%) did not have access to WBMRI. The timepoints for when clinicians requested novel imaging with PET/CT or WBMRI scans are summarised in Figure 1.
Fig 1. Timepoints for when clinicians request positron emission tomography/computerised tomography and whole-body magnetic resonance imaging.
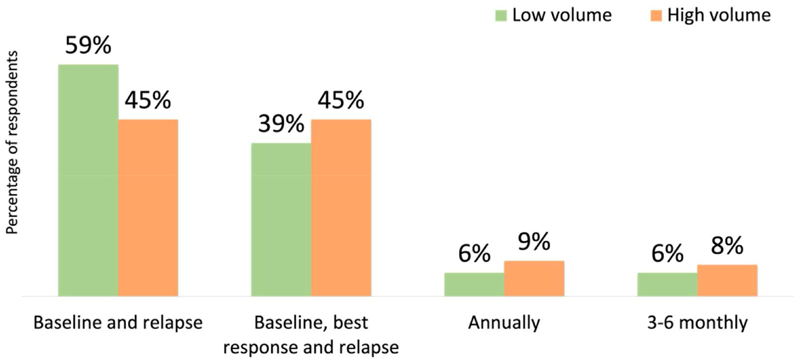
Questions on the frequency of imaging in mHSPC were divided based on disease volume (low volume versus high volume). For these questions, multiple responses were permitted. Figure 2 summarises the frequency of imaging in low- and high-volume mHSPC.
Fig 2. Frequency of imaging in low- and high-volume metastatic disease.
For best response assessment scans, 54/64 (84%) respondents selected CT and bone scans as the preferred imaging modality used, 3/64 (5%) respondents selected WBMRI and 2/64 (3%) respondents selected PET/CT.
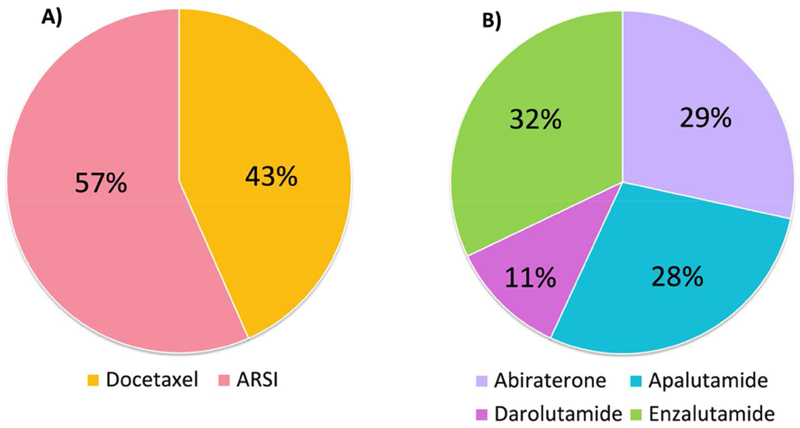
The choice of systemic doublet therapy in addition to ADT is summarised in Figure 3A. The choice of ARSI for systemic doublet therapy, providing all were approved and available on the National Health Service, is summarised in Figure 3B.
Fig 3. Systemic treatment.
(A) Choice of doublet therapy with androgen deprivation therapy. (B) Choice of androgen receptor signalling inhibitors.
Forty-seven of 64 respondents (73%) were likely to start ARSI therapy together with ADT and 14/64 (22%) respondents were likely to start ARSI at any another time after starting ADT. Of those, 7/14 (50%) started ARSI within 3 months of ADT, 3/14 (21%) started within 6e8 weeks and 4/14 (29%) started within 1 month of ADT.
Twenty-nine of 64 respondents (45%) were likely to use docetaxel chemotherapy as part of triplet therapy, if funding was available, 10/64 (16%) were not likely to use triplet therapy and 22/64 (34%) were unsure.
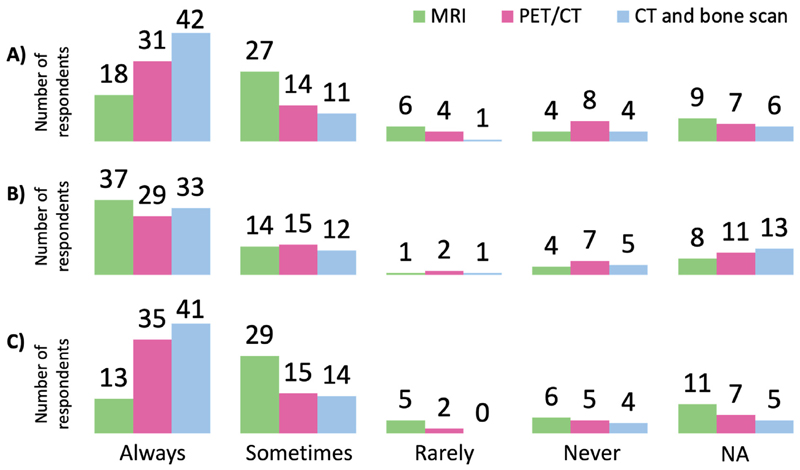
Forty-five of 64 respondents (70%) had access to SABR to treat spinal, non-spinal bone and nodal metastases at their treating centre and 22/64 (34%) had access through a neighbouring centre. For those who did not have direct access, 8/64 (12.5%) foresaw direct access at their treating centre in less than 1 year and 5/64 (8%) in 1–3 years. Figure 4 summarises the frequency of each imaging modality used for SABR planning for bone (non-spinal, Figure 4A), spine (Figure 4B) and lymph node metastases (Figure 4C).
Fig 4.
Frequency of each imaging modality requested for stereotactic ablative body radiotherapy planning to (A) bone (non-spinal), (B) spine and (C) lymph node metastases.
For the delivery of prostate radiotherapy and SABR in comparison S, most respondents were participating sites in other National Institute for Health and Care Research portfolio prostate trials, this included 39/64 (61%) respondents who participated in the PACE umbrella trial (ISRCTN17627211), 37/64 (58%) in the PIVOTALboost trial (ISRCTN80146950) and 17/64 (26.5%) in the CORE trial (ISRCTN45961438). In oligometastatic disease, 48/64 (75%) respondents would treat lymph nodes if found to be involved on conventional imaging and 47/64 (73%) if involved on PET/CT.
Seven of 64 respondents (11%) had direct access to 177Lu-PSMA-617; 18/64 (28%) respondents had access through a neighbouring centre. For respondents with no direct access to 177Lu-PSMA-617, 16/64 (25%) foresaw access in less than 1 year, 18/64 (28%) foresaw access in 1–3 years, 1/64 (2%) foresaw access in more than 3 years, 8/64 (12.5%) had no access planned and 21/64 (33%) were unsure.
Discussion
The STAMPEDE platform trial is a multi-arm multi-stage trial that has recruited 11 992 patients across 120 sites in the UK and Switzerland since its ethical approval in 2005. Responses to our survey were from UK-based participating sites only and have shown great interest for participation in the three new comparisons of the forthcoming STAMPEDE2 trial. Results from the survey have informed the final design of the STAMPEDE2 trial by concluding current practices in the UK related to access to novel imaging and choice of treatment in advanced prostate cancer.
We acknowledge that our survey received only a 48% response rate, which is moderate but not high and thus our findings cannot be regarded as fully representative of all STAMPEDE sites. The survey was conducted at a time of particular pressures within the National Health Service due to COVID-19, when there were limited staff for completion of the survey. One year on from this, the STAMPEDE2 trial is in set-up and enthusiasm from sites to take part in STAM-PEDE2 is evident, with a more responsive recent request eliciting strong interest to take part.
Our results showed the wide accessibility for PET/CT imaging. Most respondents requested PET/CT imaging at the time of disease relapse (61%) or disease uncertainty on conventional imaging (70%) given the greater accuracy of PET/CT for the detection of metastases when compared with suboptimal conventional imaging [20–22]. This practice reflects the established role of PET/CT imaging, in particular PSMA PET/CT in biochemically recurrent disease [23,24] and the initial staging of prostate cancer [22]. Results from these prospective trials have subsequently led to the endorsement of PSMA PET/CT imaging in updated international guidelines [7,8].
The improved sensitivity and specificity of PSMA PET/CT imaging for staging prostate cancer may redefine disease extent with potential stage migration and subsequent change in the patient’s treatment plan. Significant implications may arise from treatment alteration, leading to the omission of evidence-based treatment or overtreatment of what would otherwise be considered ‘microscopic’ disease. Evidence on clinical outcomes following PSMA PET/CT-directed treatment in mHSPC remains limited. In addition, current evidence from clinical trials for the management of prostate cancer is based on conventional imaging.
In non-metastatic prostate cancer, the survival benefit from combination treatment with ADT and radical doses of prostate radiotherapy is known [25,26]. Staging men in this group with PSMA PET/CT scans may detect occult metastatic disease resulting in the delivery of palliative doses of prostate radiotherapy or its omission. Similarly, the detection of low-volume metastatic disease may persuade the treating clinician to deliver SABR to metastatic sites with no real added benefit to men who will inevitably have excellent outcomes.
In low-burden metastatic disease detected on conventional imaging, the STAMPEDE M1:RT comparison demonstrated improved failure-free and overall survival with prostate radiotherapy [2,3]. Exploratory analysis showed that there was a continuum of benefit from prostate radiotherapy beyond three bone metastases seen on bone scans [27]. Additionally, bone scans were predictive of a response to prostate radiotherapy [28]. Oligometastatic disease has been defined as an intermediary metastatic state [29]. Its current definition is largely driven by the imaging modality used to describe the presence of a limited number of macroscopically visualised lesions [30–33]. In metachronous oligometastatic disease, SABR combined with standard of care improved progression-free survival [9–13]. A post-hoc analysis from the ORIOLE trial reported improved outcomes when all lesions visualised on PSMA PET/CT were treated with SABR [9].
WBMRI is a novel imaging modality with improved sensitivity than conventional imaging for bone metastases detection [34,35]. Standardised reporting guidelines have been published [36]. WBMRI can assess the cellularity of bone lesions and measure changes in apparent diffusion coefficient values, which has been correlated with treatment response [37–39]. The STAMPEDE2 trial comparison S eligibility will be determined by conventional imaging as per current clinical evidence. Considering the current status quo with access to novel imaging, an imaging sub-study will be integrated in STAMPEDE2 comparison S and treatment decisions using novel imaging will be stratified (see Supplementary Appendix B). The sub-study aims to explore patterns of treatment decisions and clinical outcomes for each imaging modality in the context of a large prospective clinical trial.
The survey concluded that almost half of clinicians (43%) used docetaxel as doublet therapy, despite National Institute for Clinical Excellence approval for enzalutamide and apalutamide in mHSPC following the COVID-19 pandemic [40,41]. Results from the STAMPEDE, CHAARTED and LATI-TUDE trials have shown that the addition of abiraterone or docetaxel to long-term ADT improves survival [4–6,42,43]. However, no trials have directly compared the two treatments to determine superiority of one over the other. A post-hoc analysis from the STAMPEDE trial compared outcomes from the abiraterone and docetaxel contemporaneous comparisons where recruitment overlapped, the results of which favoured abiraterone for improved failure-free survival and progression-free survival, with no significant difference with regards to other outcomes [44]. Subsequent exploratory analysis from the STAMPEDE trial reported on quality of life differences between the two treatments. The results after 2 years of treatment showed an improved global quality of life score with abiraterone [45]. In STAMPEDE2, based on our own patient-reported outcome data [46], we have adopted ARSI as the doublet treatment of choice. The choice of ARSI doublet treatment aligns with the investigational treatment in the trial. Additionally, by offering biomarker testing prior to starting ARSI, there is the opportunity for a second randomisation in comparison N for patients with a positive biomarker status (see Supplementary Appendix C).
Triplet therapy was likely to be used by 45% of respondents. At the time of the survey, the PEACE-1 and ARASENS trials had reported on the improved overall and progression-free survival with triplet therapy [47,48]. Subgroup analysis from the ARASENS trial confirmed that the survival benefit was consistent among all comers regardless of the disease volume and risk, with no increased toxicity from the addition of ARSI [49]. Since the survey, darolutamide has recently become available on the National Health Service for men with mHSPC through the ‘fast-track access for life-extending drugs’ scheme [50]. In the STAM-PEDE2 trial design, provision has been made for triplet therapy use across all comparisons. The decision to treat with triplet therapy will be stratified and will be at the treating clinician’s discretion.
Best response assessment scans may be useful when the clinical trial primary endpoint is radiographic progression as per the RECISTv1.1 criteria [51]. In the STAMPEDE2 trial, we strongly recommend undertaking best response assessment scans at 24 weeks from randomisation to correspond with the prostate-specific antigen nadir. The preferred choice of scans would be CT and bone scans to facilitate a validated like-for-like comparison with baseline and progression scans.
Radiotherapy quality assurance for the STAMPEDE2 trial comparison S will be led by the national Radiotherapy Clinical Trials Quality Assurance group and will be streamlined through the SABR expansion programme and other National Institute for Health and Care Research portfolio prostate cancer trials (PACE: ISRCTN17627211, PIVOTALboost: ISRCTN80146950 and PEARLS: ISRCTN36344989 trials). The survey results have shown that most centres had access to SABR. We, therefore, anticipate a smooth set-up and start to recruitment in comparison S.
Following the Food and Drug Administration approval for 177Lu-PSMA-617 in CRPC [16], 177Lu-PSMA-617 became available in the UK through the Early Access to Medicines Scheme [52], potentially expanding access across the UK. At the time of writing, the therapy is no longer available pending a National Institute for Clinical Excellence review. Treating centres with an infrastructure to support radioactive ligand therapy delivery will be prioritised to open for recruitment in the STAMPEDE2 trial comparison P. Additionally, this comparison is part sponsored by Advanced Accelerator Applications USA, Inc (AAA, a Novartis company; Millburn, NJ, USA) who will supply 177Lu-PSMA-617 and support additional trial costs.
Conclusion
The STAMPEDE2 trial will open three new investigative treatment comparisons for men diagnosed with mHSPC. There is significant variation in clinical practice across current study sites regarding access and application of novel imaging and the choice of therapy combinations at treatment initiation. Despite this, most existing trial centres have expressed great interest in participation in the STAMPEDE2 trial.
Supplementary Material
Funding
The authors acknowledge that this study represents independent research and did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors. H. Abdel-Aty is undertaking an MD (Res) at The Institute of Cancer Research and The Royal Marsden Hospital. She is also the STAMPEDE and STAMPEDE2 trial Clinical Research fellow, supported by The Medical Research Council Clinical Trials Unit at University College London. The STAM-PEDE2 trial is jointly funded by Cancer Research UK, Janssen Pharmaceuticals and Advanced Accelerator Applications USA, Inc. (AAA, a Novartis company; Millburn, NJ, USA). The Clinical Trials Unit receives core funding from UK Research and Innovation Medical Research Council (award MC_UU_00004/01).
Footnotes
Author contributions
HA and NJ are guarantors of integrity of the entire study. HA, NJ, GA, LB, CP, WC and NC devised the study concept and methodical approach for analysis. LO uploaded the survey online and collated the results for analysis. HA analysed the data. HA and NJ wrote the first draft of the manuscript. All authors were involved in reviewing the manuscript.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- [1].Prostate cancer survival statistics. Cancer Research UK; [Accessed 27 March 2023]. Available at: https://www.cancerresearchuk.org/health-professional/cancer-statistics/statistics-by-cancer-type/prostate-cancer/survival. [Google Scholar]
- [2].Parker CC, James ND, Brawley CD, Clarke NW, Ali A, Amos CL, et al. Radiotherapy to the prostate for men with metastatic prostate cancer in the UK and Switzerland: long-term results from the STAMPEDE randomised controlled trial. PLoS Med. 2022;19:e1003998. doi: 10.1371/journal.pmed.1003998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Parker CC, James ND, Brawley CD, Clarke NW, Hoyle AP, Ali A, et al. Radiotherapy to the primary tumour for newly diagnosed, metastatic prostate cancer (STAMPEDE): a randomised controlled phase 3 trial. Lancet. 2018;392:2353–2366. doi: 10.1016/S0140-6736(18)32486-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].James ND, Sydes MR, Clarke NW, Mason MD, Dearnaley DP, Spears MR, et al. Addition of docetaxel, zoledronic acid, or both to first-line long-term hormone therapy in prostate cancer (STAMPEDE): survival results from an adaptive, multiarm, multistage, platform randomised controlled trial. Lancet. 2016;387:1163–1177. doi: 10.1016/S0140-6736(15)01037-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].James ND, de Bono JS, Spears MR, Clarke NW, Mason MD, Dearnaley DP, et al. Abiraterone for prostate cancer not previously treated with hormone therapy. N Engl J Med. 2017;377:338–351. doi: 10.1056/NEJMoa1702900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].James ND, Clarke NW, Cook A, Ali A, Hoyle AP, Attard G, et al. Abiraterone acetate plus prednisolone for metastatic patients starting hormone therapy: 5-year follow-up results from the STAMPEDE randomised trial ( NCT00268476) Int J Cancer. 2022;151:422–434. doi: 10.1002/ijc.34018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Mottet N, Cornford P, van den Bergh RCN, Briers E, De Santis M, Gillessen S, et al. EAU-EANM-Arnhem, ESTRO-ESUR-ISUP-SIOG guidelines on prostate cancer. European Association of Urology; The Netherlands: 2022. [Accessed 27 March 2023]. Available at: https://d56bochluxqnz.cloudfront.net/documents/full-guideline/EAU-EANM-ESTRO-ESUR-ISUP_SIOG-Guidelines-on-Prostate-Cancer-2022.pdf. [Google Scholar]
- [8].Schaeffer EM, Srinivas S, Adra N, An Y, Barocas D, Bitting R, et al. NCCN Guidelines® Insights: Prostate Cancer, Version 1. 2023. Featured updates to the NCCN guidelines. J NCCN. 2022;20:1288–1298. doi: 10.6004/jnccn.2022.0063. [DOI] [PubMed] [Google Scholar]
- [9].Phillips R, Shi WY, Deek M, Radwan N, Lim SJ, Antonarakis ES, et al. Outcomes of observation vs stereotactic ablative radiation for oligometastatic prostate cancer: the ORIOLE phase 2 randomized clinical trial. JAMA Oncol. 2020;6:650–659. doi: 10.1001/jamaoncol.2020.0147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Ost P, Reynders D, Decaestecker K, Fonteyne V, Lumen N, De Bruycker A, et al. Surveillance or metastasis-directed therapy for oligometastatic prostate cancer recurrence: a prospective, randomized, multicenter phase II trial. J Clin Oncol. 2018;36(5):446–453. doi: 10.1200/JCO.2017.75.4853. [DOI] [PubMed] [Google Scholar]
- [11].Ost P, Reynders D, Decaestecker K, Fonteyne V, Lumen N, De Bruycker A, et al. Surveillance or metastasis-directed therapy for oligometastatic prostate cancer recurrence (STOMP): five-year results of a randomized phase II trial. J Clin Oncol. 2020;38:10. doi: 10.1200/JCO.2020.38.6_SUPPL.10. [DOI] [Google Scholar]
- [12].Palma DA, Olson R, Harrow S, Gaede S, Louie AV, Haasbeek C, et al. Stereotactic ablative radiotherapy for the comprehensive treatment of oligometastatic cancers: long-term results of the SABR-COMET phase II randomized trial. J Clin Oncol. 2020;38:2830–2838. doi: 10.1200/JCO.20.00818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Harrow S, Palma DA, Olson R, Gaede S, Louie AV, Haasbeek C, et al. Stereotactic radiation for the comprehensive treatment of oligometastases (SABR-COMET): extended long-term outcomes. Int J Radiat Oncol Biol Phys. 2022;114:611–616. doi: 10.1016/j.ijrobp.2022.05.004. [DOI] [PubMed] [Google Scholar]
- [14].Sartor O, de Bono J, Chi KN, Fizazi K, Herrmann K, Rahbar K, et al. Lutetium-177-PSMA-617 for metastatic castration-resistant prostate cancer. N Engl J Med. 2021;385:1091–1103. doi: 10.1056/NEJMoa2107322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Hofman MS, Emmett L, Sandhu S, Iravani A, Joshua AM, Goh JC, et al. TheraP: 177Lu-PSMA-617 (LuPSMA) versus cabazitaxel in metastatic castration-resistant prostate cancer (mCRPC) progressing after docetaxel — overall survival after median follow-up of 3 years (ANZUP 1603) 2022:5000. doi: 10.1200/JCO.2022.40.16_SUPPL.5000.40. [DOI] [Google Scholar]
- [16].FDA DISCO. Burst Edition: FDA approval of Pluvicto (lutetium Lu 177 vipivotide tetraxetan) for the treatment of adult patients with prostate-specific membrane antigen-positive metastatic castration-resistant prostate cancer who have been treated with androgen receptor pathway inhibition and taxane-based chemotherapy. FDA; [Accessed 27 March 2023]. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-disco-burst-edition-fda-approval-pluvicto-lutetium-lu-177-vipivotide-tetraxetan-treatment-adult . [Google Scholar]
- [17].Agarwal N, Azad A, Carles J, Fay AP, Matsubara N, Heinrich D, et al. TALAPRO-2: phase 3 study of talazoparib (TALA) + enzalutamide (ENZA) versus placebo (PBO) + ENZA as first-line (1L) treatment in patients (pts) with metastatic castration-resistant prostate cancer (mCRPC) J Clin Oncol. 2023;41:LBA17. doi: 10.1200/JCO.2023.41.6_SUPPL.LBA17. [DOI] [Google Scholar]
- [18].Chi KN, Rathkopf DE, Smith MR, Efstathiou E, Attard G, Olmos D, et al. Phase 3 MAGNITUDE study: first results of niraparib (NIRA) with abiraterone acetate and prednisone (AAP) as first-line therapy in patients (pts) with metastatic castration-resistant prostate cancer (mCRPC) with and without homologous recombination repair (HRR) gene alterations. J Clin Oncol. 2022;40:12. doi: 10.1200/JCO.2022.40.6_SUPPL.012. [DOI] [Google Scholar]
- [19].Clarke NW, Armstrong AJ, Thiery-Vuillemin A, Oya M, Shore N, Loredo E, et al. Abiraterone and olaparib for metastatic castration-resistant prostate cancer. NEJM Evid. 2022;1(9) doi: 10.1056/EVIDoa2200043. [DOI] [PubMed] [Google Scholar]
- [20].Hövels AM, Heesakkers RAM, Adang EM, Jager GJ, Strum S, Hoogeveen YL, et al. The diagnostic accuracy of CT and MRI in the staging of pelvic lymph nodes in patients with prostate cancer: a meta-analysis. Clin Radiol. 2008;63:387–395. doi: 10.1016/j.crad.2007.05.022. [DOI] [PubMed] [Google Scholar]
- [21].Heesakkers RA, Hövels AM, Jager GJ, van den Bosch HC, Witjes JA, Raat HP, et al. MRI with a lymph-node-specific contrast agent as an alternative to CT scan and lymph-node dissection in patients with prostate cancer: a prospective multicohort study. Lancet Oncol. 2008;9:850–856. doi: 10.1016/S1470-2045(08)70203-1. [DOI] [PubMed] [Google Scholar]
- [22].Hofman MS, Lawrentschuk N, Francis RJ, Tang C, Vela I, Thomas P, et al. Prostate-specific membrane antigen PET-CT in patients with high-risk prostate cancer before curative-intent surgery or radiotherapy (proPSMA): a prospective, randomised, multicentre study. Lancet. 2020;395:1208–1216. doi: 10.1016/S0140-6736(20)30314-7. [DOI] [PubMed] [Google Scholar]
- [23].Morris MJ, Rowe SP, Gorin MA, Saperstein L, Pouliot F, Josephson D, et al. Diagnostic performance of 18 F-DCFPyL-PET/CT in men with biochemically recurrent prostate cancer: results from the CONDOR phase III, multicenter study. Clin Cancer Res. 2021;27:3674–3682. doi: 10.1158/1078-0432.CCR-20-4573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Pienta KJ, Gorin MA, Rowe SP, Carroll PR, Pouliot F, Probst S, et al. A phase 2/3 prospective multicenter study of the diagnostic accuracy of prostate specific membrane antigen PET/CT with 18F-DCFPyL in prostate cancer patients (OSPREY) J Urol. 2021;206(1):52–61. doi: 10.1097/JU.0000000000001698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Widmark A, Klepp O, Solberg A, Damber JE, Angelsen A, Fransson P, et al. Endocrine treatment, with or without radiotherapy, in locally advanced prostate cancer (SPCG-7/SFUO-3): an open randomised phase III trial. Lancet. 2009;373:301–308. doi: 10.1016/S0140-6736(08)61815-2. [DOI] [PubMed] [Google Scholar]
- [26].Warde P, Mason M, Ding K, Kirkbride P, Brundage M, Cowan R, et al. Combined androgen deprivation therapy and radiation therapy for locally advanced prostate cancer: a randomised, phase 3 trial. Lancet. 2011;378:2104–2111. doi: 10.1016/S0140-6736(11)61095-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Ali A, Hoyle A, Haran A, Brawley CD, Cook A, Amos C, et al. Association of bone metastatic burden with survival benefit from prostate radiotherapy in patients with newly diagnosed metastatic prostate cancer: a secondary analysis of a randomized clinical trial. JAMA Oncol. 2021;7:555–563. doi: 10.1001/jamaoncol.2020.7857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Ali A, Hoyle AP, Parker CC, Brawley CD, Cook A, Amos C, et al. The automated bone scan index as a predictor of response to prostate radiotherapy in men with newly diagnosed metastatic prostate cancer: an exploratory analysis of STAMPEDE’s “M1|RT comparison”. Eur Urol Oncol. 2020;3:412–419. doi: 10.1016/j.euo.2020.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Hellman S, Weichselbaum RR. Oligometastases J Clin Oncol. 1995;13:8–10. doi: 10.1200/JCO.1995.13.1.8. [DOI] [PubMed] [Google Scholar]
- [30].Lecouvet FE, Oprea-Lager DE, Liu Y, Ost P, Bidaut L, Collette L, et al. Use of modern imaging methods to facilitate trials of metastasis-directed therapy for oligometastatic disease in prostate cancer: a consensus recommendation from the EORTC Imaging Group. Lancet Oncol. 2018;19:e534–e545. doi: 10.1016/S1470-2045(18)30571-0. [DOI] [PubMed] [Google Scholar]
- [31].Lievens Y, Guckenberger M, Gomez D, Hoyer M, Iyengar P, Kindts I, et al. Defining oligometastatic disease from a radiation oncology perspective: an ESTRO-ASTRO consensus document. Radiother Oncol. 2020;148:157–166. doi: 10.1016/j.radonc.2020.04.003. [DOI] [PubMed] [Google Scholar]
- [32].Guckenberger M, Lievens Y, Bouma AB, Collette L, Dekker A, deSouza NM, et al. Characterisation and classification of oligometastatic disease: a European Society for Radiotherapy and Oncology and European Organisation for Research and Treatment of Cancer consensus recommendation. Lancet Oncol. 2020;21:e18–e28. doi: 10.1016/S1470-2045(19)30718-1. [DOI] [PubMed] [Google Scholar]
- [33].Zilli T, Achard V, Pra AD, Hegemann NS, Jereczek-Fossa BA, Lancia A, et al. Recommendations for radiation therapy in oligometastatic prostate cancer: an ESTRO-ACROP Delphi consensus. Radiother Oncol. 2022;176:199–207. doi: 10.1016/j.radonc.2022.10.005. [DOI] [PubMed] [Google Scholar]
- [34].Jambor I, Kuisma A, Ramadan S, Huovinen R, Sandell M, Kajander S, et al. Prospective evaluation of planar bone scintigraphy, SPECT, SPECT/CT, 18F-NaF PET/CT and whole body 1. 5T MRI, including DWI, for the detection of bone metastases in high risk breast and prostate cancer patients: SKELETA clinical trial. Acta Oncol. 2016;55:59–67. doi: 10.3109/0284186X.2015.1027411. [DOI] [PubMed] [Google Scholar]
- [35].Van Nieuwenhove S, Van Damme J, Padhani AR, Vandecaveye V, Tombal B, Wuts J, et al. Whole-body magnetic resonance imaging for prostate cancer assessment: current status and future directions. J Magn Reson Imaging. 2022;55:653–680. doi: 10.1002/jmri.27485. [DOI] [PubMed] [Google Scholar]
- [36].Padhani AR, Lecouvet FE, Tunariu N, Koh DM, De Keyzer F, Collins DJ, et al. Metastasis reporting and data system for prostate cancer: practical guidelines for acquisition, interpretation, and reporting of whole-body magnetic resonance imaging-based evaluations of multiorgan involvement in advanced prostate cancer. Eur Urol. 2017;71:81. doi: 10.1016/j.eururo.2016.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Fujiwara M, Yoshida S, Takahara T, Soma T, Nakamura Y, Ishikawa Y, et al. Prostate and metastasis diffusion volume based on apparent diffusion coefficient as a prognostic factor in hormone-naïve prostate cancer. Clin Exp Metastasis. 2023;40:187–195. doi: 10.1007/s10585-023-10200-2. [DOI] [PubMed] [Google Scholar]
- [38].Perez-Lopez R, Mateo J, Mossop H, Blackledge MD, Collins DJ, Rata M, et al. Diffusion-weighted imaging as a treatment response biomarker for evaluating bone metastases in prostate cancer: a pilot study. Radiology. 2017;283:168–177. doi: 10.1148/radiol.2016160646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Perez-Lopez R, Lorente D, Blackledge MD, Collins DJ, Mateo J, Bianchini D, et al. Volume of bone metastasis assessed with whole-body diffusion-weighted imaging is associated with overall survival in metastatic castration-resistant prostate cancer. Radiology. 2016;280:151–160. doi: 10.1148/radiol.2015150799. [DOI] [PubMed] [Google Scholar]
- [40].Overview. Enzalutamide for treating hormone-sensitive metastatic prostate cancer. Guidance. NICE; [Accessed 27 March 2023]. https://www.nice.org.uk/guidance/ta712 . [Google Scholar]
- [41].Overview. Apalutamide with androgen deprivation therapy for treating high-risk hormone-relapsed non-metastatic prostate cancer. Guidance. NICE; [Accessed 27 March 2023]. https://www.nice.org.uk/guidance/ta740 . [Google Scholar]
- [42].Fizazi K, Tran N, Fein L, Matsubara N, Rodriguez-Antolin A, Alekseev BY, et al. Abiraterone plus prednisone in metastatic, castration-sensitive prostate cancer. New Engl J Med. 2017;377:352–360. doi: 10.1056/NEJMoa1704174. [DOI] [PubMed] [Google Scholar]
- [43].Sweeney CJ, Chen Y-H, Carducci M, Liu G, Jarrard DF, Eisenberger M, et al. Chemohormonal therapy in metastatic hormone-sensitive prostate cancer. N Engl J Med. 2015;373:737–746. doi: 10.1056/NEJMoa1503747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Sydes MR, Spears MR, Mason MD, Clarke NW, Dearnaley DP, de Bono JS, et al. Adding abiraterone or docetaxel to long-term hormone therapy for prostate cancer: directly randomised data from the STAMPEDE multi-arm, multi-stage platform protocol. Ann Oncol. 2018;29:1235–1248. doi: 10.1093/annonc/mdy072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Rush HL, Murphy L, Morgans AK, Clarke NW, Cook AD, Attard G, et al. Quality of life in men with prostate cancer randomly allocated to receive docetaxel or abiraterone in the STAMPEDE trial. J Clin Oncol. 2022;40:825–836. doi: 10.1200/JCO.21.00728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Attard G, Murphy L, Clarke NW, Sachdeva A, Jones C, Hoyle A, et al. Abiraterone acetate plus prednisolone with or without enzalutamide for patients with metastatic prostate cancer starting androgen deprivation therapy: final results from two randomised phase 3 trials of the STAMPEDE platform protocol. Lancet Oncol. 2023;24:443–456. doi: 10.1016/S1470-2045(23)00148-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Smith MR, Hussain M, Saad F, Fizazi K, Sternberg CN, Crawford ED, et al. Darolutamide and survival in metastatic, hormone-sensitive prostate cancer. N Engl J Med. 2022;386:1132–1142. doi: 10.1056/NEJMoa2119115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Fizazi K, Foulon S, Carles J, Roubaud G, McDermott R, chon A, et al. Abiraterone plus prednisone added to androgen deprivation therapy and docetaxel in de novo metastatic castration-sensitive prostate cancer (PEACE-1): a multicentre, open-label, randomised, phase 3 study with a 2 × 2 factorial design. Lancet. 2022;399:1695–1707. doi: 10.1016/S0140-6736(22)00367-1. [DOI] [PubMed] [Google Scholar]
- [49].Hussain M, Tombal B, Saad F, Fizazi K, Sternberg CN, Crawford ED, et al. Darolutamide Plus androgen-deprivation therapy and docetaxel in metastatic hormone-sensitive prostate cancer by disease volume and risk subgroups in the phase III ARASENS trial. J Clin Oncol. 2023;41:3595–3607. doi: 10.1200/JCO.23.00041. [DOI] [PubMed] [Google Scholar]
- [50].NHS England. NHS fast tracks life-extending prostate cancer drug to patients. [Accessed 27 March 2023]. https://www.england.nhs.uk/2022/11/nhs-fast-tracks-life-extending-prostate-cancer-drug/
- [51].Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45:228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- [52].Lutetium (177Lu) vipivotide tetraxetan: treatment protocol: information on the Pharmacovigilance system. [Accessed 27 March 2023]. Available at: https://www.gov.uk/government/publications/lutetium-177lu-vipivotide-tetraxetan-in-the-treatment-of-prostate-specific-membrane-antigen-psma-positive-metastatic-castration-resistant-prostate/lutetium-177lu-vipivotide-tetraxetanin-treatment-protocol-information-on-the-pharmacovigilance-system.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.