Abstract
Background and aims
People with IBD often experience pain, fatigue, and bowel incontinence and are at an increased risk of anxiety and depression. Our aim was to assess the impact of these symptoms on health-related quality of life (QoL) in IBD.
Methods
In the IBD-BOOST survey, over 26,000 people with IBD across the UK were approached; 8486 participant-completed surveys were returned. Participants’ QoL was measured using the EQ-5D-5L questionnaire and their QoL calculated on a scale ranging from 1 (perfect health) to -0.594 (worst health). Item nonresponse was imputed. Stages of linear regression models assessed the associations of symptoms with QoL controlling for IBD type, socio-demographic characteristics, co-morbidities and, in further analysis, for IBD activity and IBD control.
Results
The EQ-5D-5L questionnaire was fully completed by 8093 (95.4%) participants (mean age 50 years (SD 15); 49% with Crohn’s disease). The mean QoL was 0.76 (SD 0.23). From the three IBD-related symptoms, pain was associated with the largest QoL decrement (-0.159) followed by fatigue (-0.140) and incontinence (-0.048). Co-occurrence of pain and fatigue further reduced QoL. Clear graded associations were observed between symptom severity and QoL decrements (p<0.001). Depression and anxiety were also associated with significant QoL decrements (-0.102 and -0.110 for moderate-to-severe anxiety and moderately severe depression, respectively). Worse IBD control and higher IBD activity were associated with lower QoL.
Conclusions
We report strong associations between symptoms of pain, fatigue, incontinence, anxiety, and depression and their severity on QoL in IBD. These estimates could inform further IBD management interventions.
Introduction
Inflammatory Bowel Disease (IBD), predominantly comprising Crohn’s Disease (CD) and ulcerative colitis (UC), is a chronic condition causing inflammation in the gastrointestinal tract and is characterised by episodes of remission and relapse. It has been reported that in 2017 there were 6.8 million people diagnosed with IBD globally, 3·9 million women and nearly 3·0 million men.(1)
Disease onset typically occurs during young adulthood and treatment can be complex including medical therapy with corticosteroids, 5-aminosalicylic drugs, immunosuppressants, and biologics as well as surgery in some patients. People with IBD often experience urgency, an imperative and urgent need to defecate which when severe or when toilet facilities are not available, can result in faecal incontinence, an involuntary loss of faeces. Many people living with IBD report bowel incontinence (75%), abdominal pain (62%), and fatigue (41%).(2) They are also at increased risk of anxiety and depression compared to the general population.(3) These symptoms can occur even when IBD is in remission and are associated with significantly reduced quality of life (QoL) as people living with IBD pursue employment, family planning, and personal achievements.(4)
Health-related quality of life (HRQoL), a self-reported measure of the functional impact of illness on individuals’ daily life that is assessed using either a generic or disease-specific questionnaire, is an important outcome in clinical trials.(5) Generic HRQoL instruments have the advantage of enabling comparisons of effects across different diseases and are widely used in health economic and policy analyses. The five-level version of the EuroQoL five-dimensional (EQ-5D-5L) questionnaire(6) is a frequently used generic HRQoL measure that asks respondents to indicate their health status on five dimensions: mobility, self-care, usual activities, pain and discomfort, and anxiety and depression. EQ-5D-5L has been demonstrated to be feasible, consistent, and valid in patients with IBD.(7)
Previous studies have assessed the individual impact of symptoms of pain,(8) fatigue,(9) incontinence(10), and stress and depression(11) on HRQoL in IBD using generic or disease-specific measures. However, no study has yet assessed the relationship between pain, fatigue, incontinence, anxiety, and depression collectively on QoL in IBD. To address this gap, data from a large UK cross-sectional survey, the IBD-BOOST survey, were used to quantify associations between symptoms of fatigue, pain, incontinence, and the additional contribution of anxiety and depression, on QoL in adults with IBD.
Materials and Methods
IBD-BOOST survey
The IBD-BOOST survey was a multi-site, cross-sectional, participant-completed survey in the UK that recruited adult participants with IBD from (i) hospital outpatient clinics, (ii) the UK IBD BioResource (www.ibdbioresource.nihr.ac.uk), and (iii) the Crohn’s and Colitis UK (www.crohnsandcolitis.org.uk) charity between February 2019 and July 2022. Outpatient clinic patients were recruited via letter, email, or mobile phone text sent by clinical teams containing a link to the online survey. This link directed patients to an online participant information sheet, screening form, consent form, and the survey. Alternatively, patients received paper copies of these materials via post. The IBD BioResource had ethics committee permission to approach people who had indicated a willingness to participate in research. Therefore, email invitations were sent to patients previously recruited to the BioResource. CCUK approached their members by post or email. Participants with incomplete surveys received up to two reminders by text message, post or email. Adults with IBD were also able to participate by following an online survey link posted on social media and selected IBD-related websites.
The survey included fourteen sections that collected information on participants’ sociodemographic characteristics, disease history and health, current treatment for IBD, IBD control and disease activity levels, symptoms of fatigue, pain, and bowel incontinence, anxiety and depression, and HRQoL.
Measurement of health-related QoL in the IBD-BOOST survey
The HRQoL of IBD-BOOST survey participants was measured using the EQ-5D-5L questionnaire. Participants were asked to rate their health across five domains - mobility, self-care, usual activities, pain/discomfort, and anxiety/depression – by selecting one of five possible levels: “no problems”, “slight problems”, “moderate problems”, “severe problems” or “extreme problems”. Using the UK National Institute for Health and Care Excellence (NICE) recommended scoring algorithm, these problems were mapped onto EQ-5D utility values with 1 representing a perfect health state, 0 representing a health state equivalent to death, and values less than 0 representing health states worse than death.(12)
Fatigue, pain, and bowel incontinence measures
The Patient-Reported Outcomes Measurement Information System (PROMIS) (www.promishealth.org) questionnaires were used to measure patient-reported symptoms of fatigue, pain, and bowel incontinence in the past seven days. In this study, we used the seven-item PROMIS Fatigue Short Form v1.0, the three-item PROMIS Pain Intensity v1.0, and the four-item PROMIS Gastrointestinal Bowel Incontinence v1.0. Raw scores for each of the symptoms’ scale range from 7 to 35, 3 to 15, and 4 to 20, respectively, with higher scores representing worse symptoms. PROMIS measures have demonstrated reliability, precision, and construct validity.(13)
Prior to analysis, PROMIS fatigue and PROMIS pain scores were converted to standardised T-scores and then dichotomised, with a score of 60 or above indicating the presence of the symptom. PROMIS incontinence scores of 5 or above indicated bowel incontinence.
Anxiety and depression measures
To measure anxiety and depression, the Generalised Anxiety Disorder (GAD)-7(14) and the Patient Health Questionnaire (PHQ)-9(15) were administered. GAD-7 scores range from 0-21 with scores of 0-4, 5-9, 10-14, and 15-21 indicating no anxiety, mild anxiety, moderate anxiety, and severe anxiety, respectively. Prior to analysis, moderate and severe scores (10–21) were combined into a moderate-to-severe anxiety category. PHQ-9 scores range from 0 to 27 (with a score of 0-4 indicating no depression and higher scores corresponding to more severe depression). For analysis, scores of 0-4, 5-9, and greater than 10 were categorised as no depression, mild-to-moderate, and moderately severe depression, respectively. Both the GAD-7 and PHQ-9 have demonstrated excellent reliability, construct and criterion validity, and sensitivity to change (16, 17).
IBD activity and control
Disease activity of participants diagnosed with Crohn’s disease or Ulcerative Colitis was measured using a practical and non-invasive tool, the two-item Patient Reported Outcome PRO-2.(18, 19) The PRO-2 for CD is comprised of 2 items: abdominal pain and stool frequency whilst the PRO-2 for UC is comprised of rectal bleeding and stool frequency. A higher score indicates worse levels of IBD activity. Similarly, IBD control was measured using the validated IBD-Control questionnaire,(20) comprising 13 items in addition to a 0-100 visual analogue scale (VAS). IBD control scores range from 0-16 (with lower scores representing worse disease control) and tertiles (1: 15-16 [best control], 2: 9-14 and 3: 0-8 [worst control]) were used in the analysis.
Statistical methods
Statistical modelling of QoL
We used regression modelling to estimate the strength of association between key exposures and EQ-5D-5L QoL utility. The EQ-5D utility data have an upper limit of 1 and are typically negatively skewed, with a high proportion of participants reporting full health (utility value of 1). Although different types of regression models have been proposed to accommodate these distributional features, ordinary least squares (OLS) regression has remained the preferred model, both in terms of parsimony and because it minimises bias.(21)
Our approach to modelling QoL involved three pre-specified stages of model development. All stages included the following core patient characteristics – IBD type (Crohn’s disease, ulcerative colitis, or other type of IBD), previous IBD surgery, and use of biologic medications as binary variables, sociodemographic (age, gender, smoking status, body mass index [BMI]) and clinical (physical and mental health comorbidities) characteristics, and nationwide quintiles of index of multiple deprivation (IMD), a measure of socioeconomic deprivation in the UK(22) (from quintile 1, the most deprived, to quintile 5, the least deprived) (Table S1).
Initially, a model was fitted including only IBD disease characteristics (IBD type, prior IBD surgery, use of biologic medication and time since diagnosis) and confounders (age, gender, smoking status, BMI, IMD, and physical/mental comorbidities). Additional variables (time since diagnosis, pregnancy, education, employment, and relationship status) were retained only if statistically significantly associated with QoL (p<0.05). At stage 1, IBD-related symptoms (fatigue, pain, and bowel incontinence) were included (dichotomised or categorised by severity) as key exposures alongside the covariates and confounders retained in the initial model. At stage 2, interactions between the dichotomised IBD-related symptoms with more than 20% overlap were included and retained only if statistically significant. At stage 3, anxiety and depression symptoms were included as main effects to investigate their independent association with QoL, and with further interaction terms to examine whether they modify the relationship between IBD-related symptoms and QoL. This model development strategy is summarised in Table S2.
Sensitivity analyses
We assessed the effect of the addition of adjustments for IBD activity and IBD control in the models on the estimated associations between symptoms and QoL. In a further analyses, the associations were estimated (a) separately among participants with CD and among participants with UC or other IBD, and (b) separately among women and men.
All analyses were performed using Stata v.17.0 (StataCorp).
Missing data
Multivariable multivariate imputation by chained equations (MICE) was performed to replace missing responses in returned questionnaires assuming that participants’ missing responses for the EQ-5D-5L questionnaire, symptoms, and other measures were missing at random.(23) Missing EQ-5D-5L questionnaire responses were imputed by predictive mean matching after all covariates, including the visual analogue scale score, were included in the imputation model. Missing values of other measures included in the model were imputed using the same approach. We imputed 10 datasets replacing missing data, performed statistical analyses (described below) on each dataset, and combined their estimates using Rubin’s rules.(24)
Ethical statement
This study involves human participants and was approved by North West – Greater Manchester West Research Ethics Committee (REC reference: 18/NW/0613). Participants gave informed consent to participate in the study before taking part.
Results
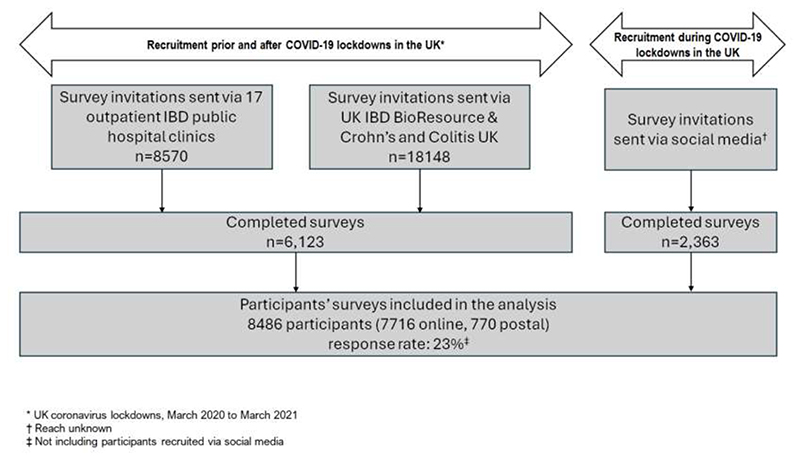
In the IBD-BOOST survey, over 26,000 people with IBD across the UK were approached; 8486 of them (7716 online, 770 postal) completed the IBD-BOOST survey (response rate of 23% - Figure 1). Missing data were generally low (<5%), except for socioeconomic deprivation (11%), incontinence (10%), and disease activity scores (11%-15%). Key demographic and clinical characteristics are presented in Table 1 (with further details in Tables S3 and S4). The vast majority of participants (91%) were white Caucasians and forty-nine percent of participants had Crohn’s disease. Participants’ mean age was 50 years and their mean duration of IBD was 14 years. The EQ-5D-5L questionnaire was completed by 8153 participants, with 8121 (99.6%) of them responding to all five domains. Many participants reported problems across QoL domains (62% with pain/discomfort, 52% with anxiety/depression, 45% with usual activities, 33% with mobility, and 17% with self-care). The mean QoL utility across the study participants was 0.76 (SD 0.23). As expected, participants with pain reported the highest percentage of problems with pain/discomfort domain (97%) compared with those with fatigue (87%) and incontinence (63%). This was also the case for problems with mobility (63% for participants with pain compared to 64% and 40% for participants with fatigue and incontinence, respectively). Participants with fatigue reported the highest percentage of problems with self-care (40%). The proportion of participants who reported problems with usual activities and anxiety/depression was similar amongst participants with fatigue or pain (79% & 76%, respectively) (Figure S1). For each IBD-related symptom, the highest percentage of reported problems for any EQ-5D domain was for pain/discomfort.
Figure 1. Recruitment of IBD-BOOST Survey participants.
Table 1. Selected demographic and clinical characteristics of the 8486 IBD-Boost Survey participants.
| N | (%) or Mean (SD) | |
|---|---|---|
| IBD Type | ||
| Crohn’s disease | 4168 | (49.1%) |
| Other IBD (including missing1) | 4318 | (50.1 %) |
| Gender | ||
| Male | 3285 | (38.7%) |
| Female | 4888 | (57.6%) |
| Missing | 313 | (3.7%) |
| Age (years) | 49.8 | (15.4) |
| Missing | 304 | (3.6%) |
| Ethnicity | ||
| White | 7751 | (91.3%) |
| Mixed | 118 | (1.4%) |
| Asian | 209 | (2.5%) |
| Black | 35 | (0.4%) |
| Any other | 71 | (0.8%) |
| Missing | 302 | (3.6%) |
| Educational level | ||
| No formal education | 219 | (2.6%) |
| School (GCSE or AS/A-levels) | 2277 | (26.8%) |
| Further and Higher education | 5661 | (66.7%) |
| Missing | 329 | (3.9%) |
| Employment status | ||
| Employed | 5071 | (59.8%) |
| Unemployed due to illness | 499 | (5.9%) |
| Unemployed | 218 | (2.6%) |
| Student or Homemaker | 467 | (5.5%) |
| Retired | 1921 | (22.6%) |
| Missing | 310 | (3.7%) |
| Relationship status | ||
| Living alone | 2378 | (28.0%) |
| Living with someone | 5779 | (68.0%) |
| Missing | 329 | (3.9%) |
| IMD quintile | ||
| 1 (most deprived) | 1124 | (13.2%) |
| 2 | 1357 | (16.0%) |
| 3 | 1611 | (19.0%) |
| 4 | 1709 | (20.1%) |
| 5 (least deprived) | 1756 | (20.7%) |
| Missing | 929 | (10.9%) |
| Fatigue (PROMIS score ≥ 60) | 2036 | (24.0%) |
| Missing | 42 | (5.0%) |
| Pain (PROMIS score ≥ 60) | 1769 | (20.9%) |
| Missing | 262 | (3.1%) |
| Incontinence (PROMIS score ≥ 5) | 4562 | (53.8%) |
| Missing | 839 | (9.9%) |
| GAD-7 anxiety level | ||
| No anxiety (GAD-7 score 0-4) | 4353 | (51.3%) |
| Mild anxiety (GAD-7 score 5-9) | 2097 | (24.7%) |
| Moderate to severe anxiety (GAD-7 score 10-21) | 1733 | (20.4%) |
| Missing | 303 | (3.6%) |
| PHQ-9 depression level | ||
| No depression (PHQ-9 score 0-4) | 3741 | (44.1%) |
| Mild to moderate depression (PHQ-9 score 5-9) | 2132 | (25.1%) |
| Moderately severe depression (PHQ-9 score 10- 27) |
2306 | (27.2%) |
| Missing | 307 | (3.6%) |
| IBD activity score (PRO-2) for Crohn's Disease | 9.6 | (10.4) |
| Missing | 615 | (14.8%) |
| IBD activity score (PRO-2) for Ulcerative Colitis | 1.2 | (1.5) |
| Missing | 456 | (10.6%) |
| IBD control tertile | ||
| 1 (best control) | 1903 | (22.4%) |
| 2 | 3291 | (38.8%) |
| 3 (worst control) | 2862 | (33.7%) |
| Missing | 430 | (5.0%) |
| EQ-5D-5L utility | 0.76 | (0.23) |
| Missing | 385 | (4.5%) |
66 participants did not report their IBD diagnosis. Mean (SD) presented across participants with non-missing data. IBD control tertiles (1: 15-16 [best control], 2: 9-14 and 3: 0-8 [worst control]). IBD: Inflammatory bowel disease; GCSE: General Certificate of Secondary Education IMD: Indices of Multiple Deprivation; BMI: Body Mass Index; PROMIS: Patient-Reported Outcomes Measurement Information System; GAD-7: Generalised Anxiety Disorder Assessment; PHQ-9: Patient Health Questionnaire-9; PRO-2: Patient-Reported Outcome; EQ-5D-5L: 5-level EQ-5D version; SD: standard deviation.
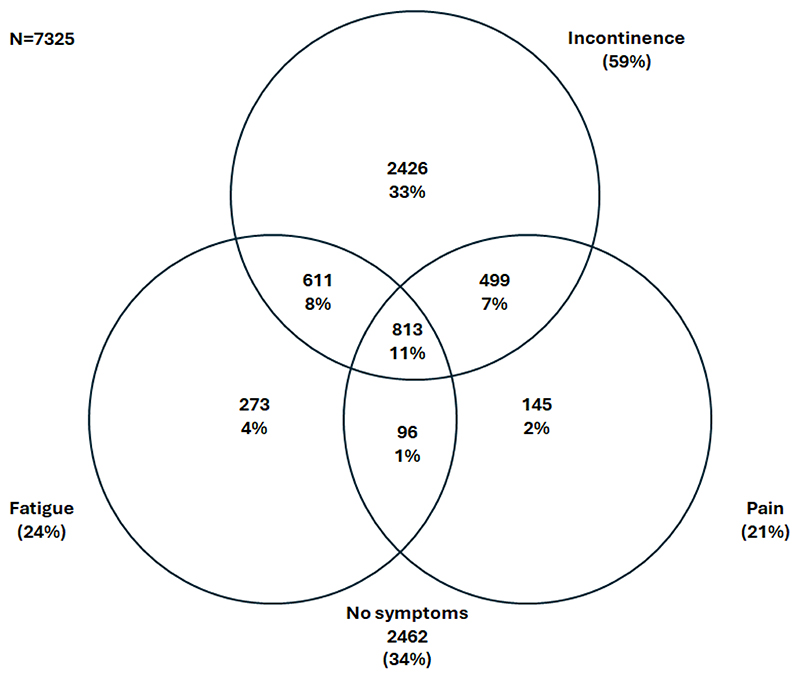
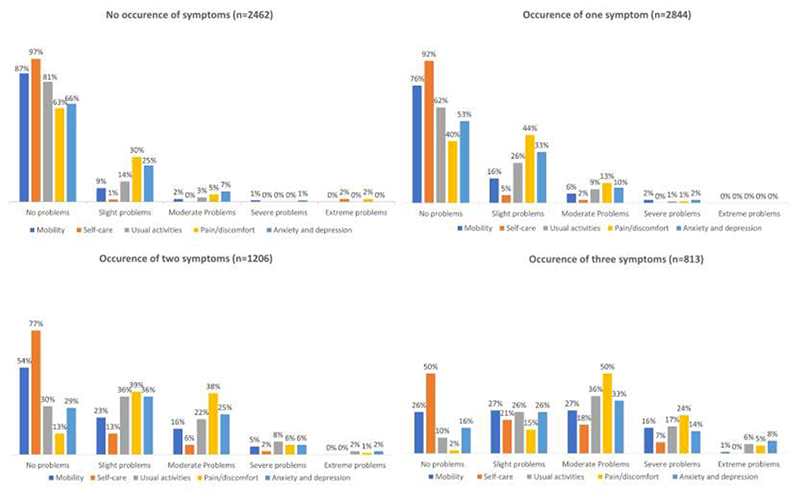
Amongst participants with available symptom data, 59% experienced bowel incontinence, 24% fatigue, and 21% pain in the past week. While 33% of participants experienced incontinence only, 4% fatigue only, 2% pain only, a substantial number of participants experienced multiple symptoms – 8% experienced fatigue and incontinence, 7% pain and incontinence, 1% fatigue and pain, and 11% of participants reported all three symptoms in the past 7 days (Figure 2). Participants experiencing fatigue, pain, or incontinence reported issues across QoL domains with participants who experienced co-occurring symptoms reporting lower QoL. Specifically, of the 813 participants (11%) who experienced all three symptoms of fatigue, pain, and incontinence, severe or extreme problems were reported for the pain/discomfort domain by 29%, for usual activity by 23%, for anxiety/depression by 22%, for mobility by 17%, and for self-care by 7% (Figure 3).
Figure 2. Co-occurrence of fatigue, pain, and bowel incontinence among IBD-BOOST Survey participants with complete data.
Participants with any missing data about fatigue, pain, and bowel incontinence symptoms (n=1161) excluded. Number (%) presented.
Figure 3. Distribution of responses to EQ-5D-5L questionnaire by (co-)occurrence of fatigue, pain, and bowel incontinence symptoms.
Participants with any missing data about fatigue, pain and bowel incontinence symptoms (n=1161) excluded.
QoL of people living with IBD
In the initial QoL model of people living with IBD, the QoL of the reference individual – a 50-year old man with Crohn’s disease who is a non-smoker, has a normal weight, lives alone in an area with socio-economic deprivation in quintile 3, educated to GCSE or AS/A-levels, is employed, has no history of IBD surgery and possesses no physical and mental health comorbidities - was estimated at 0.862 (standard error (SE) 0.011) (Table S5). Being female, unemployed, a smoker, being outside the normal BMI weight range, living alone, use of biological treatment and having physical or mental comorbidities were all independently associated with lower QoL. In particular, being unemployed due to illness was associated with a large QoL decrement [-0.321 (SE 0.010)] relative to being employed and living with someone was associated with higher QoL [0.026 (0.005)] relative to living alone. All else equal, QoL was similar between people living with Crohn’s disease or other IBD (Table S5).
Decrements in QoL associated with symptoms of fatigue, pain, and bowel incontinence (Stage 1)
Adding dichotomised fatigue, pain, and bowel incontinence symptoms in the model were all associated with significant QoL decrements. Pain was associated with the largest QoL utility decrement [-0.159 (SE 0.005)], followed by fatigue [-0.140 (SE 0.005)] and incontinence [-0.048 (SE 0.004)] (Table 2 – Stage 1). Clear associations between level of symptom severity and QoL utility were also observed. Compared with a PROMIS symptom score <50 for pain and fatigue, a PROMIS score ≥60 was associated with a QoL decrement of -0.184 (SE 0.006) for pain and -0.161 (SE 0.006) for fatigue (both ptrend<0.001). A PROMIS incontinence score ≥10 was associated with a QoL decrement of -0.063 (SE 0.006) compared with a PROMIS incontinence score <5 (ptrend<0.001) (Table S6).
Table 2. Quality of life (EQ-5D utility) associated with patient characteristics and key symptoms of inflammatory bowel disease (N=8486 participants).
| Characteristic | Stage 1 Mean (SE) |
Stage 2 Mean (SE) |
Stage 3 Mean (SE) |
|---|---|---|---|
| QoL of reference individual1 | 0.928 (0.009)* | 0.927 (0.009)* | 0.960 (0.009)* |
| Female sex | -0.007 (0.004) | -0.008 (0.004)* | -0.004 (0.004) |
| Age at time of EQ-5D questionnaire (per 10 years, centred at 50) | 0.006 (0.002)* | 0.006 (0.002)* | -0.002 (0.002) |
| Time since diagnosis (ref: 0-3 years) | |||
| 4 years and over | 0.004 (0.005) | 0.004 (0.005) | -0.003 (0.005) |
| Pregnant | 0.012 (0.023) | 0.013 (0.023) | -0.002 (0.022) |
| Smoking status (ref: never smoker) | |||
| Ex-smoker | -0.015 (0.004)* | -0.016 (0.004)* | -0.013 (0.004)* |
| Current smoker | -0.028 (0.007)* | -0.028 (0.007)* | -0.018 (0.007)* |
| BMI (ref: normal weight) | |||
| Underweight | -0.036 (0.012)* | -0.035 (0.012)* | -0.029 (0.011)* |
| Overweight | -0.020 (0.004)* | -0.020 (0.004)* | -0.016 (0.004)* |
| IMD quintile (ref: 3) | |||
| 1 | -0.011 (0.007) | -0.011 (0.007) | -0.008 (0.006) |
| 2 | 0.003 (0.006) | 0.003 (0.006) | 0.001 (0.006) |
| 4 | -0.002 (0.006) | -0.002 (0.006) | -0.001 (0.006) |
| 5 | 0.001 (0.006) | 0.000 (0.006) | 0.002 (0.005) |
| Education level (ref: GCSE or AS/A-levels) | |||
| No education | -0.016 (0.012) | -0.016 (0.012) | -0.009 (0.011) |
| Further and Higher education | 0.003 (0.004) | 0.003 (0.004) | 0.002 (0.004) |
| Employment status (ref: employed) | |||
| Unemployed due to illness | -0.216 (0.008)* | -0.215 (0.008)* | -0.201 (0.008)* |
| Unemployed | -0.066 (0.012)* | -0.065 (0.012)* | -0.055 (0.011)* |
| Student or Homemaker | -0.015 (0.008) | -0.015 (0.008) | -0.016 (0.007)* |
| Retired | -0.033 (0.006)* | -0.033 (0.006)* | -0.038 (0.005)* |
| Living circumstances (ref: living alone) | |||
| Living with someone | 0.018 (0.004)* | 0.018 (0.004)* | 0.012 (0.004)* |
| IBD type (ref: Crohn's disease) | |||
| OtherIBD | -0.001 (0.004) | -0.001 (0.004) | -0.001 (0.004) |
| IBD operation | -0.003 (0.005) | -0.002 (0.005) | -0.005 (0.004) |
| Use of biologic medication | -0.006 (0.004) | -0.006 (0.004) | -0.003 (0.004) |
| Physical comorbidity | -0.031 (0.004)* | -0.031 (0.004) | -0.028 (0.004)* |
| Mental comorbidity | -0.096 (0.005)* | -0.096 (0.005) | -0.055 (0.004)* |
| PROMIS pain | -0.159 (0.005)* | -0.145 (0.007)* | -0.106 (0.006)* |
| PROMIS fatigue | -0.140 (0.005)* | -0.130 (0.006)* | -0.064 (0.006)* |
| PROMIS incontinence | -0.048 (0.004)* | -0.049 (0.004)* | -0.028 (0.004)* |
| PROMIS pain AND PROMIS fatigue | -0.032 (0.010)* | -0.049 (0.009)* | |
| GAD-7 scale for anxiety (ref: no anxiety)** | |||
| Mild anxiety | -0.038 (0.005)* | ||
| Moderate to severe anxiety | -0.102 (0.006)* | ||
| PHQ-9 scale for depression (ref: no depression)** | |||
| Mild to moderate depression | -0.050 (0.005)* | ||
| Moderately severe depression | -0.110 (0.007)* |
PROMIS pain (≥ 60); PROMIS fatigue (≥ 60); PROMIS bowel incontinence (≥ 5);
p-value<0.05;
ptrend<0.05.
Interactions between fatigue and incontinence and pain and incontinence (Stage 2) excluded as not statistically significant
50 year old man with Crohn’s disease, diagnosed in last 3 years, not pregnant, without operation and not receiving biologic medication, never smoker, normal weight, living alone and in an area of average socioeconomic deprivation, educated at GCSE or AS/A levels, employed, without physical or mental health comorbidities, and, in models Stage 2 and 3, without respective symptoms.
IBD: Inflammatory bowel disease; IMD: Indices of Multiple Deprivation; SD: Standard Deviation; BMI: Body Mass Index; EQ-5D-5L: 5-level EQ-5D version; PROMIS: Patient-Reported Outcomes Measurement Information System; GAD-7: Generalised Anxiety Disorder Assessment; PHQ-9: Patient Health Questionnaire-9
Decrements in QoL accounting for interactions between fatigue, pain, and bowel incontinence (Stage 2)
At stage 2, the interactions between fatigue, pain, and incontinence were added to the QoL model (Table 2 – Stage 2). The three-way interaction between the symptoms and the interactions between fatigue and incontinence and pain and incontinence were not statistically significant and thus excluded. Strong interaction was observed only between pain and fatigue [-0.032 (SE 0.010)] indicating even larger reductions in QoL amongst patients experiencing these co-occurring symptoms.
Further contribution of anxiety and depression to reduced QoL (Stage 3)
At stage 3, adjusting for anxiety and depression only marginally weakened the associations of other patient characteristics and symptoms, with QoL. Adding anxiety and depression into the model indicated that they were both associated with lower QoL (Table 2 – Stage 3) with graded associations across levels of symptom severity (both ptrend<0.05). Severe levels of depression [-0.110 (SE 0.007)] and anxiety [-0.102 (SE 0.006)] were associated with the largest reductions in QoL utility. In a further analysis exploring interactions between fatigue, pain, incontinence, anxiety, and depression, significant interaction between anxiety and fatigue was noted (Table S7).
Sensitivity analyses
Higher levels of IBD activity and lower levels of IBD control were both associated with significantly lower QoL and their inclusion in QoL models marginally weakened the associations between symptoms and QoL (Table S8).
Similar decrements in QoL associated with pain, fatigue, incontinence, anxiety and depression were observed in separate analyses among participants with CD and other types of IBD, (Table S9) and separately among women and men (Table S10).
Discussion
In this study, we estimated decrements in QoL associated with IBD-related symptoms of pain, fatigue, and bowel incontinence, and the further contributions of anxiety and depression. We found that pain and fatigue were associated with larger QoL decrements compared to bowel incontinence. We also identified clear graded associations between symptom severity and QoL, suggesting that people with IBD who experience more severe symptoms have lower QoL. We note significant negative interactions between pain and fatigue, suggesting that the co-occurrence of these symptoms was associated with even larger decrements in QoL. Anxiety and depression are shown to further impact QoL of people living with IBD.
To our knowledge, there are no studies assessing decrements in QoL associated with fatigue, pain, or incontinence in IBD using the EuroQoL EQ-5D generic health-related QoL instrument and therefore no direct comparisons can be made with our study. Our study is the first to provide estimates of QoL associated with the common symptoms of fatigue, pain, and incontinence, and interactions between them in patients with IBD. While the associations of fatigue and pain with QoL are somewhat larger than the association between incontinence and QoL, given the high frequency of incontinence reported in this study, the overall impact of this symptom on QoL is substantial. Our statistical model also highlighted the relationship between anxiety and depression and QoL in people with IBD.
In line with other studies,(25) we found no significant difference between the QoL of participants diagnosed with CD compared to participants with other types of IBD. Similar to other studies,(26) we also reported that being unemployed and living alone were associated with lower QoL. Other sociodemographic factors identified in our study as associated with lower QoL included having BMI outside the healthy weight range and being a smoker.
Two previous studies using the SF-36(27) and an IBD-specific QoL instruments (IBDQ-9),(28) respectively, reported that fatigue is associated with impaired QoL. Similarly, a study using the short inflammatory bowel disease questionnaire (SIBDQ),(29) reported a significant correlation between pain intensity and reduced QoL in IBD. An association between incontinence and lower QoL, measured using IBD-Q, has also been reported.(30) The interaction between pain and fatigue in IBD has also been previously highlighted.(31) Although individual associations between symptoms of fatigue, pain and incontinence and QoL have been noted, our study is the first to quantify the joint impact of these symptoms and their co-occurrence on QoL and to do so using generic HRQoL measure.
A considerably larger proportion of IBD patients report anxiety and/or depression compared to the general population.(32) In our study, 49% of respondents reported suffering from anxiety and 56% from depression while for the general population the levels are much lower at 6% and 3%, respectively.(32) The current study found that severe levels of depression and anxiety were associated with substantial QoL decrements [-0.110 (SE 0.007) and -0.102 (SE 0.006), respectively].
Our study aligns with previous reports in IBD(33, 34) indicating that IBD activity and poor IBD control are significant contributors to lower QoL. IBD activity and control are also associated with the symptoms studied and therefore, we initially excluded them to allow more comprehensive assessment of the associations between symptoms with QoL. However, although the associations between symptoms and QoL were somewhat reduced following adjustment for disease activity and control, they remained substantial indicating need for improved symptom management in addition to managing disease activity and control.
We also compared the study estimates of symptoms’ impact on QoL in IBD with other chronic conditions that used EQ-5D-5L to measure QoL utility. Specifically, a study examining the associations between symptoms and QoL in people with Multiple Sclerosis (MS), indicated that fatigue, pain, anxiety, and depression all had substantial negative impacts on QoL.(35) Comparing the associations of anxiety and depression with QoL with other chronic conditions, a study investigating the QoL of adult patients with rheumatoid arthritis,(36) found negative correlation between anxiety and depression and HRQoL. These results concur with the findings from our study, showing that pain and fatigue are common symptoms and significant predictors of HRQoL in chronic diseases, and that anxiety and depression emerge as important contributors to HRQoL in these populations.
Several limitations of the present study need to be acknowledged. First, in view of the cross-sectional study design, we cannot be confident that the estimated associations between symptom and QoL are causal. Further prospective longitudinal studies are needed to strengthen the evidence. Second, the present study did not include a control group and therefore we are unable to investigate in detail whether associations between symptoms such as depression and anxiety and QoL in IBD are similar to those reported in other populations. Third, the study was carried out during the COVID-19 pandemic in the UK and required a change in recruitment method from hospital outpatient databases to self-selection via social media. Therefore, there is some risk of participants with a non-verified IBD diagnosis being included in the study. Fourth, the study did not aim to recruit a representative sample of the UK IBD patient population. The study sample, for example, is likely to include a higher proportion of hospital-managed IBD patients (as IBD Bioresource also recruited from hospitals). However, the IBD-BOOST’s large and well-characterised survey population allowed adjustments for a wide range of individual patient characteristics and the study results are expected to generalise to a wider IBD patient population. Lastly, in a large-scale online survey such as this it was impossible to have an objective marker of IBD activity such as faecal calprotectin, and we recognise that self-reported disease activity has a low correlation with endoscopic activity, making our PRO definition of disease activity liable to confounding by symptoms such as those attributable IBS.
In conclusion, we report, for the first time, detailed estimates of decrements in QoL associated with the symptoms of fatigue, pain, and bowel incontinence in IBD. We also highlight the associations of anxiety and depression with QoL in the IBD population. These findings add to the existing literature(37) for the need for change in IBD management practice, with increased focus on directly managing these symptoms given their negative impacts on QoL goes beyond the impact mediated through IBD disease activity and control. New interventions to better manage these symptoms in people with IBD are likely to improve their QoL.
Supplementary Material
Key messages.
People with IBD experience fatigue, pain, incontinence and are at increased risk of anxiety and depression. These symptoms limit peoples’ quality of life, ability to work and socialise.
No study has assessed the relationship between these symptoms collectively and QoL in IBD. To address this gap, data from a large survey were used to quantify associations between fatigue, pain, incontinence, and the contribution of anxiety and depression, on QoL in IBD.
Our findings underline the need for interventions to improve the management of these common symptoms in people with IBD.
Short summary.
The study reports strong associations between symptoms of pain, fatigue and incontinence their severity on quality of life (QoL) in IBD. Pain is associated with the largest QoL reduction (-0.159), followed by fatigue (-0.140) and incontinence (-0.048). Anxiety and depression further reduced QoL.
Acknowledgements
We thank the participants who contributed their time to the study and the hospital personnel, Crohn’s and Colitis UK charity and NIHR IBD-Bioresource who facilitated recruitment of study participants and Bowel Research UK who helped us advertise the study.
Funding
This work was supported by the UK NIHR Programme Grants for Applied Research (RP-PG-0216-20001) and Bowel Research UK studentship (CR). Further support from the National Institute for Health Research Barts Biomedical Research Centre (NIHR203330) is acknowledged. The study was designed and analysed independently of all funders and the views expressed are those of the author(s) and not necessarily those of the NIHR, the Department of Health and Social Care or any other funder.
Footnotes
Author Contributions
BM, CR and CN conceived and designed the study. CR, LM, TH, IS, AH, CN, and BM contributed to acquisition of data, CR, FCB, TH, and BM analysed the data. All authors contributed to the interpretation of the data, the development of the manuscript, and critically reviewed/revised the manuscript for important intellectual content. All authors approved the final version of the manuscript before submission.
Conflict of Interest
CN declares the following conflicts of interest: Speaker fees from: Janssen, WebMD, Medscape, Merck Pharmaceutical; Tillotts Pharma UK. Pfizer advisory board.
AH reports consulting fees from AbbVie, BMS, Celltrion, Dr Falk Pharma, Galapagos, Lilly, Janssen, Pfizer and Takeda; has received speaker fees and sponsorship for academic meetings from Abbvie, BMS, Celltrion, Galapagos, Lilly, Jannsen, Pfizer and Takeda.
JOL served as a consultant and an advisory board participant for AbbVie, Bristol Myers Squibb, Celgene, Celltrion, Eli Lilly, Engytix, Ferring Pharmaceuticals, Galapagos, Gilead, GSK, Janssen, MSD, Napp, Pfizer, Shire, Takeda, and Vifor Pharma; has received speaker fees and sponsorship for academic meetings from AbbVie, BMS, Celltrion, Ferring Pharmaceuticals, Janssen, MSD, Napp, Norgine, Pfizer, Shire, Tillotts Pharma, and Takeda; and has received investigator-led research grants from AbbVie, Gilead, Pfizer, Shire, and Takeda.
Other Authors declare no conflicts of interest related to this work.
Data availability statement
Data are available from CN on reasonable request.
References
- 1.Alatab S, Sepanlou SG, Ikuta K, Vahedi H, Bisignano C, Safiri S, et al. The global, regional, and national burden of inflammatory bowel disease in 195 countries and territories, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. The Lancet gastroenterology & hepatology. 2020;5(1):17–30. doi: 10.1016/S2468-1253(19)30333-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Perler BK, Ungaro R, Baird G, Mallette M, Bright R, Shah S, et al. Presenting symptoms in inflammatory bowel disease: descriptive analysis of a community-based inception cohort. BMC gastroenterology. 2019;19:1–8. doi: 10.1186/s12876-019-0963-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Choi K, Chun J, Han K, Park S, Soh H, Kim J, et al. Risk of anxiety and depression in patients with inflammatory bowel disease: a nationwide, population-based study. Journal of clinical medicine. 2019;8(5):654. doi: 10.3390/jcm8050654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Irvine JE. Quality of life of patients with ulcerative colitis: past, present, and future. Inflammatory Bowel Diseases. 2008;14(4):554–65. doi: 10.1002/ibd.20301. [DOI] [PubMed] [Google Scholar]
- 5.Haapamäki J, Turunen U, Roine RP, Färkkilä MA, Arkkila PET. Impact of demographic factors, medication and symptoms on disease-specific quality of life in inflammatory bowel disease. Quality of Life Research. 2009;18:961–9. doi: 10.1007/s11136-009-9514-y. [DOI] [PubMed] [Google Scholar]
- 6.Herdman M, Gudex C, Lloyd A, Janssen MF, Kind P, Parkin D, et al. Development and preliminary testing of the new five-level version of EQ-5D (EQ-5D-5L) Quality of life research. 2011;20:1727–36. doi: 10.1007/s11136-011-9903-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rencz F, Lakatos PL, Gulácsi L, Brodszky V, Kürti Z, Lovas S, et al. Validity of the EQ-5D-5L and EQ-5D-3L in patients with Crohn’s disease. Quality of Life Research. 2019;28:141–52. doi: 10.1007/s11136-018-2003-4. [DOI] [PubMed] [Google Scholar]
- 8.Vartiainen P, Mäntyselkä P, Heiskanen T, Hagelberg N, Mustola S, Forssell H, et al. Validation of EQ-5D and 15D in the assessment of health-related quality of life in chronic pain. Pain. 2017;158(8):1577–85. doi: 10.1097/j.pain.0000000000000954. [DOI] [PubMed] [Google Scholar]
- 9.Cohen BL, Zoëga H, Shah SA, Leleiko N, Lidofsky S, Bright R, et al. Fatigue is highly associated with poor health-related quality of life, disability and depression in newly-diagnosed patients with inflammatory bowel disease, independent of disease activity. Alimentary Pharmacology & Therapeutics. 2014;39(8):811–22. doi: 10.1111/apt.12659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wilson M. The impact of faecal incontinence on the quality of life. British Journal of Nursing. 2007;16(4):204–7. doi: 10.12968/bjon.2007.16.4.22978. [DOI] [PubMed] [Google Scholar]
- 11.Iglesias-Rey M, Barreiro-de Acosta M, Caamaño-Isorna F, Rodríguez IV, Ferreiro R, Lindkvist B, et al. Psychological factors are associated with changes in the health-related quality of life in inflammatory bowel disease. Inflammatory bowel diseases. 2014;20(1):92–102. doi: 10.1097/01.MIB.0000436955.78220.bc. [DOI] [PubMed] [Google Scholar]
- 12.Hernandez Alava M, Pudney S, Wailoo A. Estimating the Relationship Between EQ-5D-5L and EQ-5D-3L: Results from a UK Population Study. Pharmacoeconomics. 2023;41(2):199–207. doi: 10.1007/s40273-022-01218-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Spiegel BMR, Hays RD, Bolus R, Melmed GY, Chang L, Whitman C, et al. Development of the NIH patient-reported outcomes measurement information system (PROMIS) gastrointestinal symptom scales. The American journal of gastroenterology. 2014;109(11):1804. doi: 10.1038/ajg.2014.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Spitzer RL, Kroenke K, Williams JBW, Löwe B. A brief measure for assessing generalized anxiety disorder: the GAD-7. Archives of internal medicine. 2006;166(10):1092–7. doi: 10.1001/archinte.166.10.1092. [DOI] [PubMed] [Google Scholar]
- 15.Kroenke K, Spitzer RL, Williams JBW, Löwe B. The patient health questionnaire somatic, anxiety, and depressive symptom scales: a systematic review. General hospital psychiatry. 2010;32(4):345–59. doi: 10.1016/j.genhosppsych.2010.03.006. [DOI] [PubMed] [Google Scholar]
- 16.Toussaint A, Hüsing P, Gumz A, Wingenfeld K, Härter M, Schramm E, et al. Sensitivity to change and minimal clinically important difference of the 7-item Generalized Anxiety Disorder Questionnaire (GAD-7) Journal of affective disorders. 2020;265:395–401. doi: 10.1016/j.jad.2020.01.032. [DOI] [PubMed] [Google Scholar]
- 17.Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. Journal of general internal medicine. 2001;16(9):606–13. doi: 10.1046/j.1525-1497.2001.016009606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jairath V, Khanna R, Zou GY, Stitt L, Mosli M, Vandervoort MK, et al. Development of interim patient-reported outcome measures for the assessment of ulcerative colitis disease activity in clinical trials. Alimentary pharmacology & therapeutics. 2015;42(10):1200–10. doi: 10.1111/apt.13408. [DOI] [PubMed] [Google Scholar]
- 19.Khanna R, Zou G, D'Haens G, Feagan BG, Sandborn WJ, Vandervoort MK, et al. A retrospective analysis: the development of patient reported outcome measures for the assessment of Crohn's disease activity. Alimentary pharmacology & therapeutics. 2015;41(1):77–86. doi: 10.1111/apt.13001. [DOI] [PubMed] [Google Scholar]
- 20.Bodger K, Ormerod C, Shackcloth D, Harrison M. Development and validation of a rapid, generic measure of disease control from the patient's perspective: the IBD-control questionnaire. Gut. 2014;63(7):1092–102. doi: 10.1136/gutjnl-2013-305600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pullenayegum EM, Tarride JE, Xie F, Goeree R, Gerstein HC, O'Reilly D. Analysis of health utility data when some subjects attain the upper bound of 1: are Tobit and CLAD models appropriate? Value in Health. 2010;13(4):487–94. doi: 10.1111/j.1524-4733.2010.00695.x. [DOI] [PubMed] [Google Scholar]
- 22.Noble S, McLennan D, Noble M, Plunkett E, Gutacker N, Silk M, et al. The English indices of deprivation 2019. 2019.
- 23.White IR, Royston P, Wood AM. Multiple imputation using chained equations: issues and guidance for practice. Statistics in medicine. 2011;30(4):377–99. doi: 10.1002/sim.4067. [DOI] [PubMed] [Google Scholar]
- 24.Rubin DB. Multiple imputation for survey nonresponse. Wiley; New York: 1987. [Google Scholar]
- 25.Zhou Y, Ren W, Irvine EJ, Yang D. Assessing health-related quality of life in patients with inflammatory bowel disease in Zhejiang, China. Journal of clinical nursing. 2010;19(1-2):79–88. doi: 10.1111/j.1365-2702.2009.03020.x. [DOI] [PubMed] [Google Scholar]
- 26.Bernklev T, Jahnsen J, Henriksen M, Lygren I, Aadland E, Sauar J, et al. Relationship between sick leave, unemployment, disability, and health-related quality of life in patients with inflammatory bowel disease. Inflammatory bowel diseases. 2006;12(5):402–12. doi: 10.1097/01.MIB.0000218762.61217.4a. [DOI] [PubMed] [Google Scholar]
- 27.Jelsness-Jørgensen LP, Bernklev T, Henriksen M, Torp R, Moum BA. Chronic fatigue is associated with impaired health-related quality of life in inflammatory bowel disease. Alimentary pharmacology & therapeutics. 2011;33(1):106–14. doi: 10.1111/j.1365-2036.2010.04498.x. [DOI] [PubMed] [Google Scholar]
- 28.Villoria A, García V, Dosal A, Moreno L, Montserrat A, Figuerola A, et al. Fatigue in out-patients with inflammatory bowel disease: prevalence and predictive factors. PLoS One. 2017;12(7):e0181435. doi: 10.1371/journal.pone.0181435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schirbel A, Reichert A, Roll S, Baumgart DC, Büning C, Wittig B, et al. Impact of pain on health-related quality of life in patients with inflammatory bowel disease. World Journal of Gastroenterology. 2010;16(25):3168. doi: 10.3748/wjg.v16.i25.3168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Falling CL, Siegel CA, Salwen-Deremer JK. Inflammatory Bowel Disease and Pain Interference: A Conceptual Model for the Role of Insomnia, Fatigue, and Pain Catastrophizing. Crohn's & Colitis 360. 2022;4(3):otac028. doi: 10.1093/crocol/otac028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Graff LA, Walker JR, Bernstein CN. Depression and anxiety in inflammatory bowel disease: a review of comorbidity and management. Inflammatory Bowel Diseases. 2009;15(7):1105–18. doi: 10.1002/ibd.20873. [DOI] [PubMed] [Google Scholar]
- 32.MIND.ORG.UK. Mental health facts and statistics. [Accessed 11 July 2023]. https://www.mind.org.uk/information-support/types-of-mental-health-problems/statistics-and-facts-about-mental-health/how-common-are-mental-health-problems .
- 33.Hagelund LM, Elkjær Stallknecht S, Jensen HH. Quality of life and patient preferences among Danish patients with ulcerative colitis–results from a survey study. Current Medical Research and Opinion. 2020;36(5):771–9. doi: 10.1080/03007995.2020.1716704. [DOI] [PubMed] [Google Scholar]
- 34.Huppertz-Hauss G, Lie Høivik M, Jelsness-Jørgensen L-P, Henriksen M, Høie O, Jahnsen J, et al. Health-related quality of life in patients with inflammatory bowel disease 20 years after diagnosis: results from the IBSEN study. Inflammatory bowel diseases. 2016;22(7):1679–87. doi: 10.1097/MIB.0000000000000806. [DOI] [PubMed] [Google Scholar]
- 35.Zhang Y, Taylor BV, Simpson S, Jr, Blizzard L, Campbell JA, Palmer AJ, et al. Feelings of depression, pain and walking difficulties have the largest impact on the quality of life of people with multiple sclerosis, irrespective of clinical phenotype. Multiple Sclerosis Journal. 2021;27(8):1262–75. doi: 10.1177/1352458520958369. [DOI] [PubMed] [Google Scholar]
- 36.Wan SW, He H-G, Mak A, Lahiri M, Luo N, Cheung PP, et al. Health-related quality of life and its predictors among patients with rheumatoid arthritis. Applied Nursing Research. 2016;30:176–83. doi: 10.1016/j.apnr.2015.07.004. [DOI] [PubMed] [Google Scholar]
- 37.Keefer L, Bedell A, Norton C, Hart AL. How should pain, fatigue, and emotional wellness be incorporated into treatment goals for optimal management of inflammatory bowel disease? Gastroenterology. 2022;162(5):1439–51. doi: 10.1053/j.gastro.2021.08.060. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are available from CN on reasonable request.