Abstract
Ovarian cancer is a fatal malignancy in women with a low survival rate that demands new therapeutic paradigms. Cancer cells acquire various exclusive alterations to proliferate, invade, metastasize, and escape cell death, acting independently of growth-inducing or growth-inhibiting signals. The nature of cellular signaling in tumorigenesis is interwoven. Wnt signaling is an evolutionarily conserved signaling cascade that has been shown to regulate ovarian cancer pathogenesis. The molecular mechanism of Wnt signaling underlying the development of ovarian cancer, drug resistance, and relapse is not completely understood. Extracellularly secreted Wnt signaling inhibitors are crucial regulators of ovarian cancer tumorigenesis and malignant properties of cancer stem cells. Wnt inhibitors arbitrated modifications affecting Wnt pathway proteins on the cell membranes, in the cytoplasm, and in the nucleus have been shown to span essential contributions in the initiation, progression, and chemoresistance of ovarian cancer. Although many extrinsic inhibitors developed targeting the downstream components of the Wnt signaling pathway, investigating the molecular mechanisms of endogenous secreted inhibitors might substantiate prognostic or therapeutic biomarkers development. Given the importance of Wnt signaling in ovarian cancer, more systematic studies combined with clinical studies are requisite to probe the precise mechanistic interactions of Wnt antagonists in ovarian cancer. This review outlines the latest progress on the Wnt antagonists and ovarian cancer-specific regulators such as micro-RNAs, small molecules, and drugs regulating these Wnt antagonists in ovarian tumourigenesis.
Keywords: Ovarian cancer, Wnt signaling, Wnt antagonists, Cancer stem cell, Biomarker, Novel targets
1. Introduction
Ovarian cancer (OC) with the 5-year survival rate of 20–30% is fatal disease worldwide (Wu et al., 2018). The most common type of ovarian cancer develops from the surface epithelial cells trapped as inclusion cysts in the ovary. A growing number of studies say the genesis of ovarian cancer can be from different ovarian origins, such as the fallopian tube. However, the proper mechanism behind ovarian localization, ovulation, and migration of tumor-forming cells into the ovary remains unclear. Within the ovary, repair molecules and post-ovulatory environment including secretion of chemokines and cytokines can provide tumorigenic factors that support tumor formation, metastasis, and cell survival. Ovarian follicular fluid can also act as a mutational initiation by releasing ROS that drives the transformation of fimbrial epithelial cells (Drake et al., 2003). 70% of the cases are asymptomatic in the early stage and usually detected only in the later metastatic stages, which makes treatment difficult, and hence the fatality rate is also reasonably high (Arend et al., 2013). The standard treatment of epithelial ovarian cancer is a combination of platinum compounds with taxane compounds, yet, many patients become resistant to these chemotherapies and relapse. Epidemiological studies have shown that anovulation, the use of oral contraception, or tubal ligation reduces the risk of ovarian cancer (Luvero et al., 2014). The epithelial ovarian cancers are divided based on their histologic classification as high grade serous (50–75%) (Lisio et al., 2019), low grade serous (2–5%), mucinous (2–5%), endometroid (5–25%), and clear-cell carcinoma (10–25%) (Chen et al., 2003). A plenty of interactive studies have shown the Wnt/β-catenin pathway activation is fundamental in chemoresistance and target specific inhibition of ovarian cancer stem cells (CSC) (Nagaraj et al., 2015). It is firmly accepted that Wnt signaling pathway influence tumourigenesis in ovarian cancer (Arend et al., 2013); (Nguyen et al., 2019). The fallopian tube of women who had risk-reducing salpingo-oophorectomy found high involvement of Wnt/β-catenin signaling in the serous ovarian carcinoma precursor lesions (Novak et al., 2015). Increasing evidence from literature show that several natural antagonist of Wnt signaling are expressed physiologically in the ovary. There are reports that show epigenetic silencing of these antagonist during ovarian cancer progression. Understanding the interaction of these antagonists with Wnt signaling in the ovarian cancer and their expression will aid in development of specific personalized therapeutic strategies for Wnt dependent ovarian cancer. In this review, we will summarize the different Wnt antagonist characterized particularly in the ovary and their interaction with Wnt signaling. In addition, highlight the different strategies currently available to alter the expression of these Wnt antagonists.
1.1. Process of Wnt signaling
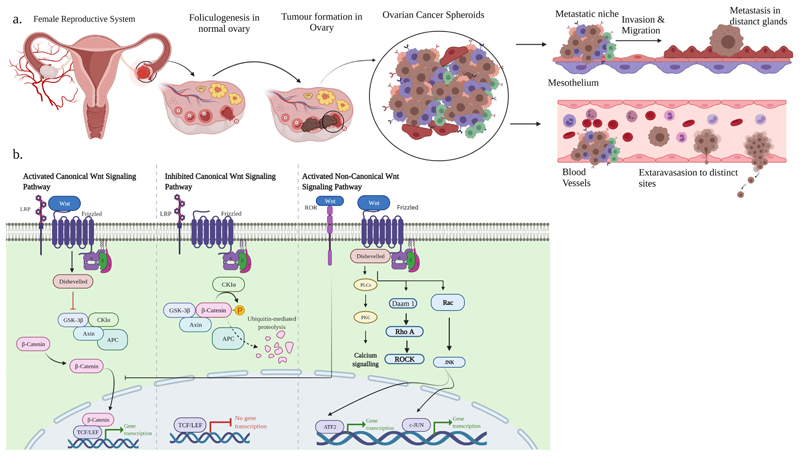
The Wnt signaling pathway controls a diverse set of cell fate decisions in embryonic development and adult tissue maintenance (Ricken* et al., 2002; Yao et al., 2004). There are two primary Wnt signaling pathways: the canonical Wnt/β-catenin pathway and the non-canonical Wnt pathway. The non-canonical pathway operates independently of the protein β-catenin and is again classified as planar cell polarity pathway and Wnt-Ca2+ pathway. In the canonical Wnt/β-catenin dependent pathway, Wnt proteins bind to the cell surface frizzled (Fzd) receptors, initiating a signaling cascade that facilitates stabilization and nuclear translocation of β-catenin. β-catenin then binds to TCF/LEF transcription factors in the nucleus, leading to transcription and expression of Wnt-responsive genes. The canonical Wnt signaling results in free β-catenin accumulation. In the presence of Wnt ligands, upon binding to the Fzd receptor, LRP5, and LRP6 receptor, the Wnt pathway is turned ‘on’ and, Casein kinase (CK1) and glycogen synthase kinase 3β (GSK3β) phosphorylate LRP5/6. This recruits Dsh molecules to interact with Fzd receptors. This complex inactivates GSK3β and blocks the phosphorylation of β-catenin by causing its dissociation from Axin. The non-phosphorylated β-catenin in the cytoplasm translocates to the nucleus where it associates with the transcription factor TCF/LEF family by displacing Groucho and interacting with co-activator such as B-cell lymphoma 9/legless and Pygopus (pygo) to promote transcriptional induction of target genes such as c-myc, Cyclin D1, Vascular endothelial growth factor (VEGF), Survivin (BIRC5), and matrix metalloproteinases (MMPs). Therefore, the presence of β-catenin stimulates transcription of genes about survival and proliferation (Teeuwssen and Fodde, 2019; Gatcliffe et al., 2008). When there is no receptor, or no Wnt ligand, or the receptor is blocked; the canonical pathway is ‘off’. Some Wnt signaling inhibitors are competing with Wnt ligands to bind the Fzd receptors and hinder the activation of Wnt signaling. In the absence of Wnt ligands, the destruction complex of Axin, APC, and GSK3β degrades β-catenin. β-catenin is phosphorylated by CK1 and GSK3β, followed by ubiquitination and proteasomal degradation. Absence or low cytoplasmic β-catenin levels allow Groucho to be recruited to transcription factor TCF/LEF, which blocks the activation of the β-catenin target genes and ensures transcriptional repression (Fig. 1b).
Fig. 1.
a. Illustration of tumor formation in the ovary gives rise to all types of cells with aberrant mutations and stemness characteristics. The tumor-initiating cells detach from the primary tumor cells and undergo EMT transition. These cancer stem cells (CSC) spread through invasion, migration, and metastasize to different organs. b.Overview of Wnt/β-catenin dependent and Wnt/β-catenin independent pathway. Canonical Wnt pathway is activated by binding Wnt ligand to Frizzled (Fzd) receptor, and LRP5/6 co-receptor leads to the phosphorylation of CK1α and GSK3β, which recruits Disheveled (Dvl) protein. The activated Dvl protein inactivates the β-catenin destruction complex. It stabilizes β-catenin accumulation in the cytoplasm, which then translocates into the nucleus leading to the transcription of various β-catenin target genes. In inhibited state (i.e., absence of Wnt ligands), GSK3β phosphorylates β-catenin in the cytoplasm and undergoes β-TrCp mediated ubiquitination and proteasomal degradation. In the absence of nuclear β-catenin, the transcription of various β-catenin target genes is repressed. In the non-canonical pathway (Wnt/PCP), Wnt proteins bind the ROR-Fzd receptor and recruit the Dvl protein. Dvl binds GTPase Rho and activates GTPase Rac1/Rho triggered Rho kinase (ROCK) and JNK proteins which control transcriptional reactions. Wnt/Ca2+ signaling is stimulated by G-protein-initiated phospholipase C (PLC) forwarding to intracellular calcium levels and upregulating Ca2+ dependent cytoskeletal reactions.
The non-canonical pathway mediates cell polarity or facilitates an increase in intracellular calcium. At the start, the Wnt ligand binds to Fzd receptors and stimulates disheveled (Dsh) protein recruitment. Some Wnt ligands like Wnt5a, activate the planar cell polarity (PCP) pathway and Wnt-Ca2+ pathway (Qi et al., 2014). In PCP signaling, the transduction of Wnt signaling is independent of the LRP5/6 and activates Rac and Rho GTPases to mediate cytoskeletal changes. Wnt/Fzd/Dsh can activate heterotrimeric G proteins in Wnt-Ca2+ signaling, which recruit protein kinase C (PKC), phospholipase C calcium-calmodulin kinase 2 (CamK2), and calcineurin phosphate (Teeuwssen and Fodde, 2019).
1.2. Wnt signaling in ovarian cancer
Several of the Wnt ligands and receptors have been implicated in ovarian cancer. The importance of the Wnt signaling pathway in the ovary was first studied when Wnt-7a protein revealed an effect on sex-specific differentiation of reproductive tract in mice model (Parr and McMahon, 1998). Wnt-4 protein verified a significant function in embryonic female germline development (Vainio et al., 1999). Aberrant expression of Wnt ligands (Wnt-2b, Wnt-5a, Wnt-11) validated in five ovarian surface epithelial cancer cell lines (SKOV-3, CaOV-3, OVCAR-3, SW626, Hey) was the first study to suggest Wnt signaling involvement in ovarian cancer (Ricken* et al., 2002). In addition, assessing 45 ovarian endometroid adenocarcinoma tissues and two cell lines revealed genetic alterations in the Wnt/β-catenin pathway such as the β-catenin gene (CTNNB1), APC, AXIN genes (Wu et al., 2001).
Silencing of Wnt2b downregulated the proliferation, invasion, and angiogenesis of ovarian cancer cells in vitro. In addition, expression of Wnt2b in OC tissues positively correlated with metastasis (Yu et al., 2022). Inhibition of Frizzled-7 expression is high in OC tissues compared to normal tissues and correlated with poor survival. A new antibody-drug conjugate, septuximab vedotin (F7-ADC), designed against Fzd-7, inhibits the OC cell growth in vitro and xenograft mice models (Do et al., 2022). Immunohistochemical expression of Wnt-1, Wnt-5a in normal ovary and ovarian cancer suggested wnt-5a expression activates non-canonical Wnt pathway that promotes malignant ovarian cancer (Filho et al., 2009). In ovarian cancer, 23 genes out of 100 target genes were over-expressed by Wnt signaling mediated transcription. The micro-RNAs have many regulatory functions that inhibit the translation of mRNAs which has become an eminent entity in biological research. Predominantly miR-21 is expressed in many epithelial carcinomas, displaying its role in ovarian cancer tumor growth, migration, invasion, and escape apoptosis through Wnt/β-catenin signaling and CD44 biomarker activation (Wang et al., 2019). Usage of β-catenin inhibitor iCG-001/PRI-724 in OC platinum resistant patient-derived xenografts (PDX) models, suggested the involvement of Wnt/β-catenin signaling in the platinum resistance of OC and stem cell like properties of circulating tumor cells (Nagaraj et al., 2015). Furthermore, cancer cells and tissues with TP52 mutation and corresponding increased expression of β-catenin show resistance to taxane drugs (Shu et al., 2022). Activation of focal adhesion kinase (FAK) results in increased active β-catenin protein in ovarian tumorospheres and increased chemoresistance. In accordance, a FAK inhibitor inhibited chemoresistance and apoptosis by decreasing the β-catenin expression in ovarian cancer tumorospheres and tumor-carrying mice models treated with platinum drugs (Diaz Osterman et al., 2019). The long intergenic non-protein coding RNA 1094 (LINC01094) is highly expressed in OC and up-regulated the tumor proliferation, invasion, and migration by activating the Wnt/β-catenin pathway (Chen et al., 2021). The Wnt/β-catenin pathway regulates many critical facets of ovarian cancer development, including cancer cell proliferation, survival (Arend et al., 2013; Koni et al., 2020), enhancing metastasis and tumor angiogenesis (Tang et al., 2018), immune suppression (Cannon et al., 2015), maintaining cancer stem cells (CSCs), and chemoresistance (Nagaraj et al., 2015).
1.3. Wnt signaling in ovarian cancer stem cells
Aberrant Wnt signaling, both canonical and non-canonical is critical for cancer development. In addition, it has a key role in cancer stem cells (CSCs) survival, invasion, metastasis, including chemoresistance and relapse reactivation and clonal expansion of quiescent CSCs. The malignant properties of CSCs necessitate the development of targeted therapies to inhibit their functionality or sensitize them and thus improve clinical outcomes. The CSCs are heterogeneous and vigorous and are regulated by interactions with the tumor microenvironment (Muñoz-Galván and Carnero, 2020). Ovarian CSCs (OCSCs) were recognized by Bapat et al. from the ascites of OC patients (Bapat et al., 2005). Stem cell-like cells have been spotted in the mice’s ovarian surface epithelium, tubal epithelium, and coelomic epithelium (Szotek et al., 2008). Reported OCSCs markers expressed in these cells are CD44, CD133, CD24, CD117, Nestin, Nanog, and Oct3/4 and functional markers like ALDH1A1 and ABC transporters are the indices of chemoresistance, tumor-causing potential, spheroid formation capability. Yet, these CSC markers are not indeed expressed conjointly and may not indicate the whole collection of CSC populations, even as there have also been anomalous data of stemness property with each of these markers (Bregenzer et al., 2019; Saha et al., 2021; Srivastava et al., 2021). Furthermore, dual expression of stem cells fate markers, such as CD133 and ALDH1A1, has been reported to indicate increased stem-like properties than cells expressing a single CSC marker (Lupia and Cavallaro, 2017). As discussed earlier, OCSCs undergoing an epithelial-mesenchymal transition in ascites might enhance ovarian cancer cells’ stemness, metastasis, and chemoresistance (Fig. 1a). OCSCs also play a unique role in metastasis, which allows them to survive in non-adherent conditions within the lymphatic and vascular systems and the ascites fluid within the peritoneal cavity (Lupia and Cavallaro, 2017). The niche of OCSCs is unknown but inferred that they could be exploited from the ovarian stem cell niche such as surface epithelium and tubal epithelium (Flesken-Nikitin et al., 2013). The Wnt/β-catenin pathway is intricate in cancer development and for maintenance and function of OCSCs (Nguyen et al., 2019). Side population cells isolated from HGSOC had high expression of ABCG2, CD133, CD117, and the expression β-catenin target genes AXIN2, DKK1, and anti-apoptotic factors confirmed by RT-PCR suggests the involvement of canonical Wnt signaling in the occurrence of OCSCs (Gui et al., 2021). In OCSCs, Wnt 5 protein interacts with tyrosine kinase-like receptor ROR1, which induces migration, invasion, spheroid formation, and tumor infusement in immuno-compromised mice. Inhibition of tyrosine kinase receptor with ROR1-specific monoclonal antibody suppresses these phenotypes, rejecting xenograft growth and depleting OCSCs (S. Zhang et al., 2019b). Moreover, the OCSC marker c-KIT is over-expressed in hypoxic tumor microenvironments, which activates β-catenin and ABC transporters that cause resistance to the chemo-drugs cisplatin/paclitaxel (Chau et al., 2013). Interestingly, inhibition of c-KIT with imatinib in combination with cisplatin/paclitaxel suppressed tumor growth in OC cells and in vivo models. Wnt pathway inhibitor that is currently being tested is ipafricept (OMP-54F28), a fusion protein comprising the extracellular part of frizzled receptor 8 (Fzd8) fused to an IgG1 Fc fragment that competes with frizzled receptors for binding Wnt ligands (Jimeno et al., 2017). The previously tested chimeric protein shows high efficacy against tumor growth and CSCs, alone or combined with other agents in preclinical models (Le et al., 2015). Mariya et al. studied the expression of matrix metalloproteinase-10 (MMP-10), a β-catenin target gene attributed to chemoresistance, and the maintenance of epithelial ovarian cancer stem cells by activating Wnt/β-catenin signaling (Mariya et al., 2016). Homolog 2-interacting protein (DAB2IP), a tumor suppressor inhibited the expression of Wnt 5b, a non-canonical Wnt pathway initiator, and other stemness-associated genes that might prevent ovarian cancer relapse (Zong et al., 2020). Theaflavin-3, 3′-digallate (TF3), a polyphenol, inhibited the ovarian cancer spheroid formation by upregulating the expression of caspase 3/7 and inhibiting the Wnt/β-catenin signaling pathway (Pan et al., 2018). Guo et al. demonstrated the ingestion of organic pollutants provoked the development of ovarian cancer. Bisphenol-A induced the formation of ovarian cancer spheroids developed from OVCAR-3 cells with cancer stem cell-like properties by upregulating the Wnt signaling pathway (Guo et al., 2020). Over-expression of STAT3 induced ovarian spheroid development, and DKK1, a Wnt antagonist, suppressed its activity. The study displayed STAT-miR-92a-DKK1 might regulate cancer stemness and ovarian cancer progression (Chen et al., 2017). The molecular mechanisms and pathology of OCSCs and Wnt signaling in the fatal process of metastasis are still unclear. Targeting CSCs and Wnt pathway is necessary to avoid recurrence and to develop a personalized cancer treatment.
1.4. Drug resistance in OC and OCSC
Epithelial ovarian cancer is related to high mortality because of the difficulty of early screening, advance stage present, and high recurrence rates of the disease (Freimund et al., 2018). Recurrence refers to the time interval between the last drug administered and the occurrence of relapse. Many theories tried to explain the development of resistance to chemotherapy, but cancer stem cells are significant contributing factors to resistance and recurrence (Soliman et al., 2021). Nearly 75% of patients, who are highly responsive to initial anti-cancer treatment, recur during two years and fail to respond to available chemotherapeutic compounds due to acquired resistance (Banno et al., 2014; Stronach et al., 2015). Surgery is the first line of treatment for subjects with relapse in OC—however, the development of chemoresistance leads to treatment failure with a fatality rate of 90%. Earlier, platinum drugs causing DNA damage were suitable for anti-tumor treatment for OC patients. However, recent research has found that it can inhibit cell growth and cytotoxicity via nuclear, mitochondrial, and cytoplasmic coordinated integration. Drug resistance and relapse are thus attributed to multiple factors such as metabolic reprogramming (Norouzi-Barough et al., 2018), achievement of metastatic ability, altered apoptotic & autophagy pathways, chemotherapy drug response (Shen et al., 2021), oxidative stress, dysregulation of glucose metabolism (Park et al., 2022), lipid metabolism (Lu et al., 2022), DNA repair (Royfman et al., 2021), epigenetics (Watson et al., 2019), micro-RNAs (Zhao et al., 2022), and non-coding RNAs (Liu et al., 2022). Hence, recognizing novel drug targets and designing potent chemotherapeutic agents to control drug resistance in OC is essential. Among the various altered metabolic and signaling pathways in cancer cells, dysregulation of several drug-metabolizing enzymes (DMEs) such as cytochrome P450 (CYP; critical players in the phase I-dependent metabolism) system, glutathione-S-transferase (GST), and uridine diphosphate-glucuronosyltransferase (UGT) superfamilies plays a critical role in acquiring drug resistance. Also, another challenge is reduced cellular accumulation, that is; decreased influx or increased efflux of anti-cancer drugs via different membrane transporters, to name a few ATP-binding cassette (ABC) transporters, solute carriers (SLC) transporters (Norouzi-Barough et al., 2018). Persistent up-regulation of the Nrf2 pathway causes oxidative stress in cancer cells over-express genes involved in drug metabolism and causes resistance to chemotherapeutic agents, including cisplatin, doxorubicin and etoposide (Norouzi-Barough et al., 2018). It is speculated that OCSCs contribute to primary tumor growth, metastasis, relapse, and acquired chemoresistance (Lupia and Cavallaro, 2017). CSCs maintain a state of quiescence, remaining in G0 for a prolonged period (Giornelli and Mandó, 2017). Recent most treatments administer therapeutics that target actively dividing cells into the S or M phases but not the cells in the G0 phase. Thus, CSC quiescence contributes to the acquisition of chemo-resistance in OC. As the proportion of OCSCs increases at relapse, a challenge for novel therapeutics is determining the efficacy of the drug in both adult stem cells and CSCs in the quiescent state (Giornelli and Mandó, 2017; Zhan et al., 2013). The association between CSCs and OC progression reveals the potential to improve targeted therapies for OC patients. However, further biological characterization of CSCs, particularly the underlying mechanisms regulating their function, is required. The development of novel CSC treatments requires a thorough understanding of the complex genomic profile of OCs since their heterogeneity dictates the differential responses to treatment, particularly the specificity and efficacy of drugs (Pieterse et al., 2019; Zhan et al., 2013).
Of interest, the Wnt signaling has been reported to mediate the cancer cells undergoing EMT to acquire stem-like properties that enable them to become drug-resistant, arbitrating dissemination and metastasis. Dysregulation of the Wnt pathway has been associated with increased drug resistance and poor survival of patients (Barghout et al., 2015). Wnt/β-catenin inhibitor CG-001/PRI-724 is in phase I of clinical trials shown to sensitize the platinum drugs in HGOC and OCSCs (Nagaraj et al., 2015). Wnt antagonists, namely, vantictumab (anti-FZD) and ipafricept (FZD8-Fc), when administered in combination with taxane drugs, exhibited anti-tumor efficiency as well as sensitizes OCSC by inducing mitotic cell death (Fischer et al., 2017). Several endogeneous antagonists have also been shown to chemosensitize the OCSCs. Endogenous Wnt antagonist secreted frizzled-related protein 4 (SFRP4) has pro-apoptotic factors that chemo-sensitize the OC cells (Deshmukh et al., 2017; Nagaraj et al., 2021; Saran et al., 2012). Epigenetic suppression of SFRP5 inhibited epithelial-mesenchymal transitions and exhibited resistance to taxane drugs in OC (Su et al., 2010). The role of other Wnt antagonist in OCSCs function is yet to be explored.
2. Wnt antagonists
Wnt signaling activity is controlled at different levels by a wide range of evolutionarily conserved modulators. These modulators can either act extracellularly by affecting receptor-ligand interactions or intracellular by interfering with the various components of Wnt signaling cascade. Alterations in these modulators may be responsible for the aberrent Wnt signaling seen in most cancer tissue.
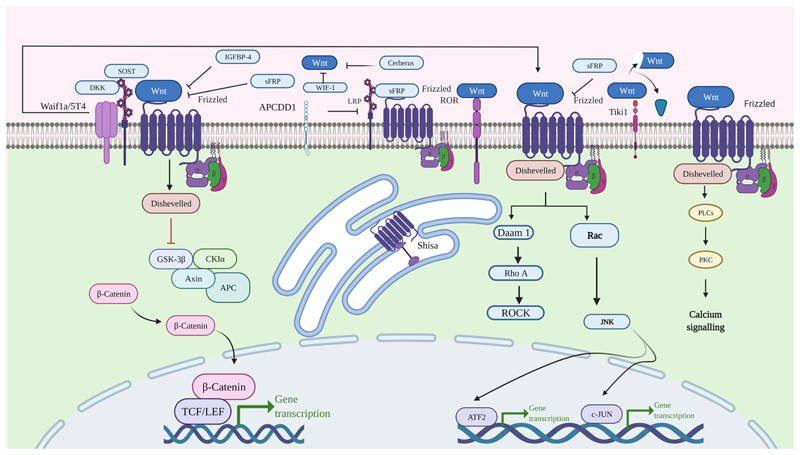
Wnt antagonists are groups of secreted and transmembrane proteins. They inhibit Wnt signaling in two ways: blocking ligand-receptor interactions or Wnt receptor maturation (Fig. 2). The discovered secreted Wnt antagonists are the Dickkopf proteins (DKKs), secreted Frizzled-related proteins (SFRPs), Wnt-inhibitory factor 1 (WIF-1), Wise/SOST, Cerberus, insulin-like growth factor binding protein 4 (IGFBP-4), Shisa, Wnt-activated inhibitory factor 1 (Waif1/5T4), adenomatosis polyposis coli down-regulated 1 (APCDD1), and Tiki1 (Belo et al., 2009; Bhuvanalakshmi et al., 2019; Bovolenta et al., 2008; Jones and Jomary, 2002; Kawano and Kypta, 2003; Niehrs, 2006).
Fig. 2. Schematic representation of endogenously expressed extracellular Wnt antagonist interfering Wnt signaling pathway.
2.1. Dickkopf proteins
Dickkopfs (DKKs) belong to an evolutionarily conserved secreted glycoproteins family (Glinka et al., 1998) consisting of four members, DKK1, DKK2, DKK3, and DKK4. These DKKs comprise 255–350 amino acids and contribute to two conserved cysteine-rich domains (CRDs). The carboxy-terminal CRD shares homology with the colipase fold, and DKK_N is unique to the DKKs. Apart from the two CRDs, a soggy (sgy) domain is present only in DKK3 (Niehrs, 2006). A far related DKK family member also called Dickkopf-like protein 1, shares sequence homology with the sgy domain of DKK3 (Krupnik et al., 1999). DKK1, -2, and -4 in humans located on the same chromosome 4/5/8/10 paralogy group. These genes are duplicated early in vertebrate evolution, whereas DKK3 is not a part of this group (Glinka et al., 1998; Luke et al., 2003; Pollard and Holland, 2000). DKKs proteins with conserved cysteine domains specifically inhibit the Wnt/β-catenin signaling cascade by blocking low-density lipoprotein receptor-related protein (LRP5/6) from complexing with Frizzled (Luke et al., 2003; Mao et al., 2001; Semënov et al., 2001) (Fig. 2). All DKKs have reported anti-tumor and/or chemosensitization function in ovarian cancer.
2.1.1. DKK1
DKK1 is a secreted Wnt inhibitor of LRP6. DKK1 inhibits the cell’s response to the Wnt ligands by binding LRP6 and Kremen (co-receptor) which removes LRP6 protein from the cell surface by endocytosis (Mao et al., 2002). Loss of DKK1 makes LRP6 free from inhibition, thereby enhancing the Wnt signaling. Evidence of Wnt signaling suppression by DKK1 is extensive. Yet, its molecular mechanism in ovarian physiology and tumorigenesis remains elusive. Studies have shown the DKK1 expression in both sexes during gonadal development in mice models (Combes et al., 2011; Manuylov et al., 2008) and have a dual functional role in gonadal development. Aberrant expression of DKK1 with higher progesterone levels might impair the implantation rates in the endometrium (Liu et al., 2010). While investigating the role of the Chromebox homolog 2 (CBX2) and CBX2 regulating genes in follicular development of ovary by genome-wide association study, DKK1 was one of the genes up-regulated in granulosa cells (Bouazzi et al., 2019). Genetic mutation in DKK1 is implicated in polycystic ovarian syndrome (PCOS) with metabolic dysfunction and hyperandrogenism (Jones et al., 2011). Elevated levels of DKK1 expression in 178 ovarian serous papillary adenocarcinoma tumor biopsies could initiate tumor cell invasion and cancer progression (Wang and Zhang, 2011). Also, DKK1 expression was high in ovarian carcinoma compared to ovarian cystadenoma and normal ovarian tissues (Shizhuo et al., 2009). The DKK1 was shown to be up-regulated and inhibits the process of epithelial-mesenchymal transition (EMT) by abnormal expression of TET1 proteins in epithelial ovarian carcinoma cell lines (SKOV-3 & OVCAR-3) (Duan et al., 2017). Many cancers reported the DKK1 dysregulation and epigenetic modification by promoter hypermethylation. Many studies have reported the abnormal expression of miRNAs in tumorigenesis and metastasis. In a recent study, STAT3 silences DKK1 by miRNA-92a in epithelial ovarian cancer. The miRNA-92a promoted Wnt/β-catenin signaling regulating stem cell properties in ovarian spheroids by interfering with Wnt antagonist DKK1. The miRNA-92a targets the 3′UTR sequence of DKK 1 and inhibits its expression (Chen et al., 2017) (Chen et al., 2017) (Table 1) (Fig. 3). In another study, the expression of miR-372 was linked to DKK1 expression. The over-expressed miR-372 suppressed the proliferation of ovarian cancer cells (OVCAR-3 & A2780) by down-regulating the DKK1 protein (Guan et al., 2017) (Table 1) (Fig. 3). DKK1 is one of the proteins (categorized as type 1 signatures as long-term relapse-free survivors) identified through SOMAscan affinity proteomics to be expressed in the peritoneal ascites of OC patients with no relapse at 24 months after the first line of surgical treatment (Finkernagel et al., 2019). In addition, the basal level expression of DKK1 was lower in A2780 ovarian cancer cells than in A2780-cis resistant cells. siRNA silenced DKK1 sensitized A2780 cells to cisplatin but sensitizing A2780-cis resistant cells was complex (Salim et al., 2015) implicating DKK1 role in drug sensitization. The above studies suggest that DKK1 to be a critical player in the development of ovarian tumor cells.
Table 1. miRNAs targeting Wnt antagonist in ovarian cancer.
| Non-coding RNAs | Cancer type | Function | Reference |
|---|---|---|---|
| miR-92a-1 | Epithelial Ovarian Cancer | Increased expression promotes oncogenic activation, & chemoresistance. Targets DKK1 | (Chen et al., 2017) |
| miR-372 | Increased expression downregulates DKK1 activation | (Guan et al., 2017) | |
| miR-126–5p | Increased expression represses DKK3, & promote platinum resistance | (Wu et al., 2019) | |
| miR-1180 | Loss of tumour suppressor function, & promotes chemoresistance. Targets SFRP1 | (Gu et al., 2019) | |
| miR-1207 | Promote cancer stem cell like fate & loss of tumour suppressor function. Targets SFRP1 | (Wu et al., 2015) | |
| miR-181a | Increased chemoresistance and cancer stemness. Targets SFRP4 | (Nagaraj et al., 2021) |
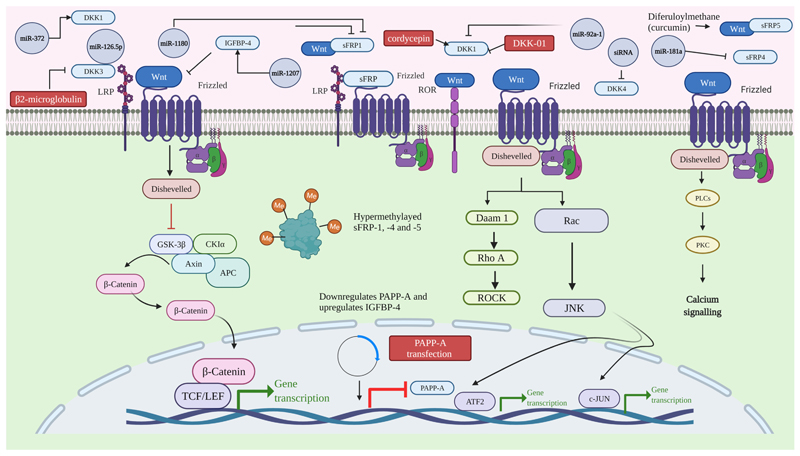
Fig. 3. The visual presentation gives an overview of miRNAs and small molecules which bind Wnt antagonist to promote/repress the Wnt signaling pathway.
2.1.2. DKK2
Like DKK1, DKK2 also suppresses the Wnt/β-catenin signaling by binding the LRP6 receptor (Mao et al., 2001; Mao and Niehrs, 2003). An in vitro study reported epigenetic suppression DKK2 in 78 ovarian carcinoma tissues with normal ovarian tissues and ovarian cancer (SKOV-3 & ES-2) cell lines. DKK2 is epigenetically silenced by methylation and downregulated as the ovarian cancer grade progresses compared with normal ovarian tissues. When DKK2 is over-expressed in ovarian cancer cell lines, it reduces the expression of Wnt/β-catenin target genes involved in cell growth, cell migration, and cell invasion (Zhu et al., 2012). Patients with higher expression of DKK2 and G protein-coupled receptor as showed better survival (Fraungruber et al., 2021).
2.1.3. DKK3
In-silico analysis revealed that higher DKK3 expression associated with the relapse-free survival rate of ovarian cancer patients (Chen and Zheng, 2018; Wang et al., 2016). Higher promoter hypermethylation of DKK3 in ovarian granulosa tumor tissues compared with normal control tissues was observed (Xu et al., 2016). An in-silico and in vitro analysis displayed low DKK3 protein and mRNA expression in ovarian epithelial tumors compared with normal ovarian tissues (You et al., 2011). DKK3 expression was high in ovarian cancer cells and had proapoptotic and tumor suppressor properties in mucinous and clear cell ovarian carcinoma (Takata et al., 2015). miR-126-5p competitively regulate DKK3 in epithelial ovarian cancer progression & promotes platinum resistance (Wu et al., 2019).
2.1.4. DKK4
DKK4 antagonizes the β-catenin pathway in some cancers (Cai et al., 2018). Like other homologs, DKK4 binds LRP5/6/kremen receptors to mediate LRP6 endocytosis, thereby interfering with β-catenin mediated signal transduction (Fatima et al., 2012). DKK4 mRNA and protein were elevated in 33 epithelial ovarian cancer (EOC) tissues compared with those in 33 benign ovarian tumors (Wang et al., 2017). The mutation-specific ectopic promoter methylation of DKK4 has been studied in colorectal cancer and thyroid carcinoma (Baehs et al., 2009; Mancikova et al., 2017). While DKK4 has been studied in pathological conditions, very little work has been carried out to understand its role in ovarian cancer.
2.2. Secreted Frizzled Related Proteins (SFRPs)
The SFRP1–5 is the most prominent family of extracellularly secreted Wnt inhibitors that competes for binding the CRD domain of the Frizzled receptors. Based on sequence homology and phylogenetic analysis, SFRP1, — 2, and -5 form a subgroup that diverges from the one developed by SFRP3 and - 4 (Jones and Jomary, 2002). The CRDs of SFRPs show 30–50% sequence similarity with those of Fz receptors and contain ten conserved cysteine residues, and the carboxy-terminal part of SFRPs contains a Netrin-related motif (NTR) and amino acids ranging between 295 and 346. The promotor regions of SFRPs are hypermethylated, inducing transcriptional silencing (Schiefer et al., 2014). SFRPs modulate the Wnt signaling pathway by binding directly with Wnt ligands or competing with Wnt ligands to bind Frizzled receptors to intercept the interaction between the Wnt proteins and Frizzled receptors (Bafico et al., 1999). The precise mechanism of how SFRPs regulate the Wnt pathway remains unclear. The promoters of different isoforms of SFRPs have also been found to undergo epigenetic alterations in various human cancers, including SFRP2 in gastric cancer, SFRP3 in hepatocellular carcinoma, SFRP4 in mesothelioma, and SFRP5 in breast cancer (Cheng et al., 2007; Deshmukh et al., 2019; Lin et al., 2014; Veeck et al., 2008). An alternative mechanism of SFRPs reports that they translocate into the nucleus and bind with β-catenin. Although SFRPs regulates Wnt signaling by binding to Wnt proteins or Frizzled receptors (Bafico et al., 1999), they may also undergo endocytosis by forming complexes with Wnt receptors to import themselves into the cytosol. Other observations suggest that SFRPs bind with β-catenin, which functions as a carrier for nuclear translocation. However, experimental evidence for this is currently unavailable. The study also shows that the exosomes take up secreted extracellular SFRPs and the immunofluorescence data demonstrated that SFRP1-5 co-localized with β-catenin in both the cytoplasm and nucleus. The silenced β-catenin can block the β-catenin dependent nuclear translocation of SFRPs and interrupt the β-catenin-TCF/LEF complex interaction. SFRPs suppress the interaction of TCF4 with β-catenin, which suggests that nuclear SFRPs act as β-catenin-interacting proteins that regulate the Wnt signaling pathway by modulating the association between TCF4 and β-catenin. Therefore, the regulation of the Wnt signaling pathway by SFRPs is achieved by the intracellular modulation of SFRPs and their extracellular functions (Liang et al., 2019). The past studies show SFRPs regulate the Wnt signaling pathway by associating with β-catenin in the nucleus where SFRP1, SFRP2, and SFRP5 suppressed the interaction of β-catenin and TCF4 complex, while SFRP3 and SFRP4 facilitated the association of β-catenin with TCF4. This intracellular interaction of SFRPs revises the Wnt signaling transcriptional activity and may alter the CSC properties of cancer cells.
2.2.1. SFRP1
Among the SFRPs family, there are extensive reports on SFRP1 which has a reported biphasic role. At decreased concentrations, SFRP1 increases Wnt activity; however, reduces Wnt activity under high concentrations of SFRP1 (Uren et al., 2000). Loss of CRD in SFRP1 resulted in reversal of Wnt3a inhibition and modifying the amino acids present in the NLD domain in SFRP1 upregulated canonical Wnt signaling (Bhat et al., 2007). In fish embryos, isolated NLD inhibited Wnt mirroring SFRP1 whole protein while CRD does not produce any inhibitory effect (Lopez-Rios et al., 2008). Ovarian stimulation protocols for in vitro fertilization reported altered gene expression of Wnt inhibitors with up-regulation of SFRP1 in endometrium (Wu et al., 2020). SFRP1 expression is upregulated by cir-ANKHD1, a circular RNA and SFRP1 repressed by miR-27a-3p which indicates that cir-ANKHD1/SFRP1/miR-27-3p association might have a role in granulosa cell proliferation and apoptosis (Li et al., 2021). Abnormal expression and hypermethylation status of SFRP1 was reported across 60 clinical epithelial ovarian cancer samples and cell lines (OVCAR-4, SKOV-3, COV-644, TOV-21-G). SFRP1 mutation has been shown to initiate the proliferation, migration, and invasion abilities of epithelial ovarian cancer cells by activating Wnt/β-catenin pathway (H. Zhang et al., 2019a) and is positively associated with progression free survial (Flanagan et al., 2013). The SFRP1 methylation status is high in high-grade serous ovarian carcinoma, and unmethylated in low-grade serous ovarian carcinoma, and normal ovarian tissues (Al-Shabanah, 2014; Kardum et al., 2017). The over-expressed miR-1207 target and suppress SFRP1 inhibitor to promote Wnt/β-catenin mediated ovarian tumorigenesis progression and increased the cancer stem cell-like property (Wu et al., 2015) (Table 1) (Fig. 3). The miR-1180 detected in conditioned media of bone marrow derived mesenchymal stem cells target SFRP1 to induce ovarian cancer cell (SKOV-3 & COC1) growth and cisplatin resistance by activating Wnt/β-catenin pathway (Gu et al., 2019) (Table 1) (Fig. 3). A high expression of SFRP1 promotes resistance to topotecan, a drug used as a second line treatment for OC induces disease progression and drug resistance by altering immune microenvironment (Menyhárt et al., 2019).
2.2.2. SFRP2
Various studies in animal models and human tissue samples reported the expression and methylation of SFRP2, a Wnt inhibitor in granulosa cells and ovarian tumors (Chermuła et al., 2019). In addition, increased methylation of SFRP2 in ovarian tumors is correlated with cancer recurrence (Su et al., 2009). In ovarian cancer cell lines (SKOV-3 & OVCAR-3), TET1 (Ten-eleven translocation methylcytosine dioxygenase 1) protein demethylated and upregulated Wnt antagonist SFRP2 along with DKK2 to inhibit Wnt/β-catenin signaling and prevent epithelial-mesenchymal transition (EMT) and cancer cell metastasis (Duan et al., 2017). SFRP2 inhibited Wnt7a-Fzd 5 mediated Wnt/β-catenin signaling in ovarian cancer (SKOV-3) cell line (Yoshioka et al., 2012). Using a protein-protein interaction network, SFRP2 was identified as part of the differentially expressed genes regulating ovarian cancer stem cells (Zhang et al., 2020). In aglandular caruncular areas of the endometrium, the expression of SFRP2 can inhibit the WNT signaling pathway and prevent endometrial adenogenesis in the inter-caruncular areas of the uterus. Additionally, Estrogen-induced adenogenesis inhibition can be conciliated by aberrant induction of SFRP2 and loss of several critical Wnts, leading to Wnt signaling (Hayashi and Spencer, 2006), and another study recorded SFRP2 methylation in the endometrium (Marichereda et al., 2018). RNA-sequencing results identified increased SFRP2 protein differentially expressed in high-grade serous ovarian tumor samples correlated with bowel metastasis (Mariani et al., 2019). As SFRP2 expression and methylation are associated with poor survival and recurrence in ovarian cancer patients, SFRP2 can be used as an early screening biomarker.
2.2.3. SFRP3
SFRP1–4 have been shown to bind to Wnt3a with affinities in the nanomolar range, SFRP1 and – 2, but not SFRP3 and – 4, attached to Wnt5a. Studies have shown Secreted frizzled-related protein Family (SFRP3) complexes with Wnt1 and XWnt8 to block canonical Wnt signaling in Xenopus embryos (Leyns et al., 1997; Lin et al., 1997; Wang et al., 1997). The crystal structures of the CRDs from mouse Fz8 and mouse SFRP3 have been determined and revealed the potential for the CRDs to dimerize (Dann et al., 2001). These results indicate that SFRPs form complexes with frizzled receptors through CRD domain binding, inhibiting the access to the Wnts and blocking the Wnt signaling (Lin et al., 1997). An immunohistochemistry analysis displays a high expression of SFRP3 in the intrauterine placenta of humans and minimal expression in trophoblastic tumors, which are expected to have associations with intrauterine placental dysfunction trophoblastic tumor development (Partl et al., 2014). Though the expression of SFRP3 and its promoter methylation have been studied in various disease models (Kristensen et al., 2014) and other cancers (Schlensog et al., 2018), it is yet to be explored in ovarian physiology and/ovarian cancer.
2.2.4. SFRP4
The Wnt antagonist, Secreted frizzled-related protein 4 (SFRP4), is involved in cell proliferation and differentiation and plays a crucial role in carcinogenesis. As the SFRP family’s most prominent member, SFRP4 has a length of 346 amino acids with a molecular weight of 39.9 kDa. This SFRP4 protein has a C-terminal and N-terminal domain. The C-terminal part has a conserved region of six cysteine residues followed by the Netrin-like domain (NLD). A past study analyzed the SFRP4 function and apoptosis in the ovaries of Wistar rats. There was an increase in the mRNA expression levels of SFRP4 in the thecal layers of follicles in treated rats. Though the precise mechanism of SFRP4 in ovulation is unfamiliar, its function as a modulator of apoptosis in the ovary proposed its role as a local initiator of apoptosis (Wu et al., 2020). Also, a synergistic effect of SFRP4 and Wnt/Ca2 + pathway activation intercepting Wnt/β-catenin signaling in cell proliferation and metastasis of cancer cells (Muley et al., 2010). The pro-apoptotic property of SFRP4 is studied in 153 serous carcinomas and found to decrease the levels of β-catenin (Drake et al., 2009). A study validated that SFRP4 expression negatively associated with β-catenin expression in 84% of serous ovarian carcinoma samples using immunohistochemistry on tissue microarrays. Another study expressed that reduced expression of SFRP4 in chemosensitive ovarian cancer cell line A2780 marked increased resistance to chemotherapeutics. Transfection of chemo-resistant cell lines with SFRP4 increased their sensitivity to chemotherapy. The role of SFRP4 as an anti-proliferative, pro-apoptotic factor in the chemo-response of ovarian tumors is established (Saran et al., 2012). Research on SFRP4 loss supported the role of SFRP4 as a potential tumor suppressor gene in ovarian cancer by intercepting the Wnt/β-catenin signaling pathway. SFRP4 was expressed on the protein level in 721 ovarian cancer histotypes using RT-qPCR, western blot, and immunohistochemistry. Studies have shown increased levels of SFRP4 expression in the tubular epithelium of the ovary but a significant decrease in the ovarian malignancies (Jacob et al., 2012). SFRP4 disrupted the cancer growth when administered in mouse xenograft model of ovarian cancer. This data indicates SFRP4 as a therapeutic agent in control of angiogenesis. In in vitro assays, SFRP4 inhibited endothelial cell migration, and the in vivo assays demonstrated decreased vascularity in mouse models. Also, the protein-induced the ROS levels in endothelial cells, thus facilitating endothelial cell apoptosis. In addition to many apoptosis-related genes identified in the ovary, SFRP4 appears to inhibit the cell proliferation process (Muley et al., 2010). The over-expression of miR-181a increased Wnt/β-catenin signaling by inhibiting the Wnt antagonist SFRP4 in ovarian cancer. The miR-181a/SFRP4 mediated activation of Wnt signaling promoted chemoresistance and stemness (Nagaraj et al., 2021) (Table 1) (Fig. 3). These outcomes illustrated that SFRP4 is a predictive marker of chemo-sensitivity and therapeutic agent in ovarian cancer.
2.2.5. SFRP5
SFRP5 is downregulated and hypermethylated in disease conditions. In advanced epithelial ovarian cancer, SFRP5 can be a potential prognostic biomarker. In ovarian clear cell adenocarcinoma (OCCA), SFRP5 gene promoter methylation plays a vital role in OCCA development, and it is a potential prognostic marker and acts as a therapeutic target (Ho et al., 2010). Epigenetic changes in SFRP5 accompany malignant changes and chemoresistance in ovarian cancer by upregulation of the Wnt signaling pathway and Twist gene-mediated epithelial-mesenchymal transition and Akt 2 signaling (Su et al., 2010). RT-PCR studies in human granulosa cells revealed the expression patterns of SFRP5 along with LH-dependent genes which play a pivotal role in follicular development (Maman et al., 2011). In ovarian cancer, methylation of SFRP5 may contribute to cell proliferation, and migration via the canonical Wnt signaling pathway and is also associated with tumour recurrence (Lin et al., 2018). Through many studies, it is known SFRP5 is methylated, and SFRP5 demethylation by compound curcumin with 5-aza-2′-deoxycytidine (DAC) activated SFRP5 which inhibited colony formation and epithelial-mesenchymal transition on ovarian cancer (Yen et al., 2019).
2.3. IGFBP-4 protein
IGF signaling is an essential pathway in follicular development and tissue repair after ovulation (Forbes et al., 2012). IGFBP-4 binds the Fzd-8 receptor and LRP5/6 co-receptor and inhibits canonical Wnt signaling in an IGF-independent fashion to promote cardiogenesis (Zhu et al., 2008). IGFBP-4 has an anti-estrogenic function that is activated and involved in the ovarian follicle selection in response to estrogen in the ovary (Durai et al., 2006). In human granulosa cells, IGFBP-4 has a potent IGF-independent antigonadotropic effect but more has to be explored to understand the mode of action of IGFBP-4 in granulosa cells (Wright et al., 2002). Up-regulation of IGFBP-4 in estrogen receptor-expressing ovarian cancer cells indicates that they are estrogen-regulated, and its expression with standard marker CA125 attributes to the development of ovarian cancer (Walker et al., 2007). RNA-sequence transcriptome analysis identified high expression of IGFBP4 in all stages of epithelial ovarian cancer. In the serum samples of ovarian cancer patients, IGFBP4 levels are increased and expressed much before cancer antigen 125 (Mosig et al., 2012). Ascites of ovarian cancer patients showed increased expression of IGFBP-4 which looks like a predictor of ovarian cancer progression (Yunusova et al., 2016). Cisplatin treated patient-derived ovarian cancer xenografts expressed IGFBP-4 at the low cis-responsive state and are associated with the epithelial-mesenchymal transition (Ricci et al., 2017). Increased mRNA expression of IGFBP-4 in serous ovarian cancer attributes to a low overall survival rate (Zheng et al., 2020) suggestive of its potential as a tumor progression biomarker.
3. Other Wnt antagonists unexplored in ovarian cancer
In addition to the Wnt antagonists discussed above, there are a few conserved small protein inhibitors that have not been characterized yet in ovarian physiology and cancer. These include WIF, Wise/SOST, Cerberus, Shisa, Waif1, APCDD1, and Tiki1. Their common feature is to antagonize Wnt signaling by preventing ligand-receptor interactions or Wnt receptor maturation. These Wnt antagonist functions have been studied to a little extent in other clinical conditions, but elaborate studies have not been done in any cancers to date.
3.1. Wnt inhibitory factor – 1 (WIF-1)
The expression of WIF-1 has been primarily observed in the human retina and is abundant in the brain, lung, and cartilage (Hsieh et al., 1999; Hu et al., 2008; Hunter et al., 2004; Surmann-Schmitt et al., 2009). In Drosophila, WIF-1 blocks the activity of Fz2 by blocking XWnt8 and Wingless (Hsieh et al., 1999). WIF-1 inhibits the functions of both canonical and non-canonical Wnts such as Wnt3a, Wnt5a, Wnt7a, and Wnt11a (Surmann-Schmitt et al., 2009), and these Wnts have a role in ovarian cancer pathogenesis. WIF-1 gene mutation or loss contributes towards carcinogenesis. Promoter hyper-methylation of WIF-1 was increased colorectal cancer tissues than adjacent normal tissues (Jalilvand and Soltanpour, 2020). There was a decrease in mRNA levels, and a protein expression level of WIF-1 with high expression levels of β-catenin indicates tumourigenesis activity of WIF-1 in non-small cell lung carcinoma (Tang et al., 2017).
3.2. Waif1/5T4
Waif 1a is a single-pass plasma membrane protein. It comprises various leucine-rich repeats in the extracellular part, and a short carboxyl terminus without conserved motifs embryos revealed Wnt-activated inhibitory factor 1a as a novel direct Wnt/β-catenin target expressed in zebrafish, Xenopus, mouse, and mammalian cells. Waif1 interacts with LRP6 and blocks both Wnt3a- and DKK1-induced LRP6 internalization without changing Wnt3a initiated phosphorylation of LRP6 (Kagermeier-Schenk et al., 2011). Overexpression of Waif1/5T4 in human carcinomas corresponds with a poor survival prognosis. This highlights the role of Waif1/5T4 in cancer biology.
3.3. APCDD1
In vitro studies revealed that APCDD1 can bind with Wnt3a and the extracellular domain of LRP5 and inhibits the canonical Wnt signaling pathway, and blocks Fz and Wnt interaction. Adenomatosis polyposis coli down-regulated 1 (APCDD1) is a membrane-bound glycoprotein, and it was first identified in SW480 colon cancer cells due to genes that were down-regulated post APC induction (Jukkola et al., 2004; Takahashi et al., 2002).
3.4. Wise/SOST
Wise, also known as SOSTDC1 (sclerostin domain-containing) Ectodin, and USAG-1 (uterine sensitization-associated gene-1), form a cystine knot and integrates with SOST to the CAN subfamily of cystine knot-containing BMP antagonists (Avsian-Kretchmer and Hsueh, 2004; Ellies et al., 2006; Itasaki et al., 2003; Lintern et al., 2009). In Xenopus, different assays revealed that Wise might either inhibit or activate Wnt signaling (Itasaki et al., 2003).
Wise inhibits Wnt1, Wnt3a, and Wnt8 activities in cell culture reporter assays (Blish et al., 2008; Itasaki et al., 2003; Yanagita et al., 2004). Sclerostin (SOST) inhibits Wnt signaling via LRP5 and LRP6 by interacting with the first two YWTD-EGF repeat domains (Li et al., 2005; Semënov et al., 2005). Similar to DKK1, SOST has the potential to inhibit the formation of the Wnt1-induced Fz8–LRP6 complex (Semènov et al., 2005). Wise and SOST block BMP signaling in cell culture models. However, their physiological role behind the blockade of Wnt signaling via Wise/SOST is still unclear (Avsian-Kretchmer and Hsueh, 2004; Laurikkala et al., 2003).
3.5. Shisa
The Shisa Protein Family comprises nine subfamilies (Furushima et al., 2007; Pei and Grishin, 2012). This protein has a CRD peptide in the amino-terminal, a transmembrane region, and a proline-rich car-boxy-terminal (Pei and Grishin, 2012). In Xenopus embryos, Shisa, along with BMP inhibitors, helps to expand anterior neural structures and initiates the secondary head. It acts as Wnt and fibroblast growth factor (FGF) signaling antagonist during head formation by trapping Fz and the FGF receptor in the endoplasmic reticulum and inhibiting their maturation (Yamamoto et al., 2005). In mice, mShias expressions have been observed in anterior visceral endoderm, anterior mesendoderm, anterior neuroectoderm, and somitic mesoderm. Upregulation of Shisa blocks Wnt signaling in mammalian cells. Knockdown of mShisa in mice has no phenotype in them. This suggests that other Wnt antagonists like DKK, SFRP may have compensated for these mShisa (Furushima et al., 2007).
3.6. Cerberus protein
Primarily Cerberus was identified and isolated from Xenopus. Upregulation of Cerberus in Xenopus induces ectopic head formation (Bouwmeester et al., 1996). Studies have shown that Cerberus obtained from Xenopus binds to Nodal, BMP, and Wnt proteins through independent sites and blocks all three signaling pathways, simultaneously leads to the formation of the head and inhibiting truck formation. (Piccolo et al., 1999).
3.7. Tiki1
In Xenopus, Tiki1 is identified as an organizer-specific gene via function cDNA screening technique, and it is necessary for neural development. Tiki1 is an evolutionary conserved transmembrane metalloprotease that removes eight amino-terminal residues from Wnt and forms oxidized Wnt oligomers. These oligomers compromised receptor-binding ability. Thus, it blocks Wnt signaling (Zhang et al., 2012). In humans, Tiki1 and Tiki2 genes are misregulated in human cancer and other pathological conditions.
4. Drugs & small compounds targeting Wnt antagonists
Many Wnt antagonists have been shown to play a crucial role in inhibiting ovarian cancer progression, proliferation, survival, chemoresistance, and migration. Targeting Wnt antagonist may potentially inhibit the Wnt signaling pathway, ovarian cell proliferation, and metastasis, ultimately leading to the apoptosis of cancer cells. Researchers have developed several small inhibitors and vaccines to target these Wnt antagonists to test whether they are suitable for treating ovarian cancer. We have tabulated (Table 2) (Fig. 3) various drugs that have been previously used or are currently under clinical trial-specific to the activation of wnt antagonists in ovarian cancer. DKN-01 is a humanized monoclonal antibody, which binds to Dickkopf-1 (Dkk1) and promotes a Wnt signaling cascade. Studies performed in EOC cell lines and the ID8 ovarian cancer model showed that DKN-01 does not affect tumor growth in OC (Betella et al., 2020). Apart from this in ovarian cancer cell lines (SKOV-3 & OVCAR-3), cordycepin increases DKK1 levels and inhibits canonical Wnt signaling (Jang et al., 2019). β2-microglobulin has been shown to activate cell growth by suppressing DKK-3-induced apoptosis (Kim et al., 2015). In both EOC tissues and benign ovarian tumors, siRNA-mediated knockdown of DKK4 has promoted the phosphorylation of c-JUN and JNK, inhibited invasive cell ability, and inhibited the formation of actin filaments (Wang et al., 2017). In patients with primary ovarian tumor, 17 beta-estradiol (E(2)), propyl pyrazole triol, and letrozole were shown to up-regulate the levels of IGFBP-4 (Walker et al., 2007). PAPP-A transfection in HRA ovarian cancer cell line has been shown to downregulate PAPP-A while increasing IGFBP-4 levels (Tanaka et al., 2004). A natural compound, diferuloylmethane (curcumin), derived from Curcuma longa, reduced the SKOV-3 ovarian cell line proliferation. And combined treatment by 5-aza-2′-deoxycytidine (DAC) and curcumin in SKOV3 cells have shown to reverse the methylation status of SFRP5 by hindering the expression of DNA methyltransferase DNMT3a protein, colony formation, and EMT. The inhibitory effect of combination treatment on SKOV3 cell growth was higher than the effects of the individual treatments (Yen et al., 2019).
Table 2. Drugs targeting Wnt antagonist in Ovarian cancer.
| Antagonist | Drug | Cell line / Model | Mechanism | Reference |
|---|---|---|---|---|
| DKK1 | DKN-01 | EOC cell lines and the ID8 ovarian cancer model | A monoclonal antibody inhibits DKK1 and promotes Wnt signaling (Betella et al., Inhibition does not affect tumor growth | (Betella et al., 2020) |
| Cordycepin | SKOV3 and OVCAR3 cell lines | Increased Dickkopf-related protein 1 (Dkk1) levels and inhibited β-catenin signaling | (Jang et al., 2019) | |
| DKK3 | β2-microglobulin | SKOV3 carcinoma cells DKK3 transfected OVCAR-3 carcinoma cells | Activates cell growth by suppressing DKK-3-induced apoptosis Controls cell cycle- and apoptosis-associated proteins | (Kim et al., 2015) |
| SKOV3 carcinoma cells | Obstructs VEGFR-2/Akt/mTOR signaling cascade | |||
| DKK4 | siRNA-mediated knockdown | EOC tissues and benign ovarian tumors | Inhibited cell invasive ability Promoted the phosphorylation of c-JUN and JNK Inhibited formation of actin filaments | (Wang et al., 2017) |
| IGFBP-4 | 17beta-estradiol (E2) & propyl pyrazole triol & letrozole | Sections from primary tumors of patients treated with letrozole | Upregulates IGFBP-4 | (Walker et al., 2007) |
| PAPP-A transfection | Human Rectal Adenocarcinoma (HRA) cell lines | Downregulation of PAPP-A increases IGFBP-4 | (Tanaka et al., 2004) | |
| SFRP5 | Diferuloylmethane (curcumin) | SKOV3 cell lines | Reduced cell proliferation | (Yen et al., 2019) |
| Diferuloylmethane (curcumin) + DAC | By impeding the expression of DNMT3a protein, reverses the methylation status of SFRP5, inhibits colony formation, and EMT |
To date, researchers have developed small inhibitors, antibodies, and siRNAs to target the DKK family of proteins, IGFBP-4, Waif1/5T4, and SFRP5, to inhibit Wnt signaling pathways in ovarian cancer. Apart from ovarian cancer, many studies have been explored with Wnt antagonists in various other pathophysiological conditions including bones (Kalam et al., 2017); colorectal and prostate cancer (Amato and Stepankiw, 2012); osteoporosis (Table 3). In addition, there are novel small molecule mimics and inhibitors listed in Pubchem and Drug Bank databases (Table 3). The role of these in ovarian cancer is yet to be explored. For example, MVA-5T4 is a cancer vaccine that targets an oncofetal antigen 5TA (Waif1/5T4, a cell surface Wnt antagonist) which is also highly expressed in breast and ovarian cancer. MVA-5TA is safe and highly immunogenic. A phase II clinical trial (NCT01556841) of MVA-5T4 in ovarian cancer, fallopian tube cancer, and peritoneal cancer was completed, but its results are yet to be posted. Taken together, the evidence currently available suggests that targeting other Wnt antagonists may enhance the overall survival rate of the patients. Recent developments in computational aided drug designing tools have facilitated the development of newer drugs and drug repurposing. Further research in the field may unravel the role of all the Wnt antagonists in ovarian cancer and help develop novel therapeutic approaches.
Table 3. Drugs targeting Wnt antagonist in other pathophysiological conditions.
| Antagonist | Model Used | Drug | Mechansim | Phase of Clinical trial Status | Reference |
|---|---|---|---|---|---|
| DKK1 | Ovotoxic mice | Letrozole | Increase DKK1 in estrogen deficient mice in both lumbar and femur bones | – | (Kalam et al., 2017) |
| SOST | Ovotoxic mice | Letrozole | increase SOST in estrogen deficienct mice in both lumbar and femur bones | – | (Kalam et al., 2017) |
| DrugBank | Romosozumab | Inhibitor | – | (Wishart et al., 2018) | |
| IGFBP-4 | DrugBank | Mecasermin | Carrier | – | (Wishart et al., 2018) |
| 5T4 (Waif1/ 5T4) | Colorectal, renal and prostate cancer patients | MVA-5T4 | Antibody; Vaccine | III; II (22409460; 01556841) | (Amato and Stepankiw, 2012) |
| SFRP1 | PubChem | 5-(benzenesulfonyl)-N-[3-(dimethylamino) propyl]–2-ethylbenzenesulfonamide | Inhibitor | (Kim et al., 2021) | |
| SFRP4 | DrugBank | Cyclothiazide | Inhibitor | – | (Wishart et al., 2018) |
5. Conclusions and future perspectives
Wnt/β-catenin signaling and its components are associated with cancer progression. Most of the current research targets β-catenin and its destruction complexes to prevent tumorigenesis. Wnt signaling inhibitors and negative regulators are also equally critical in OC. Fewer investigations delineate the roles of Wnt signaling antagonists in OC tumorigenesis, chemoresistance, and cancer relapse. The overactivation of the Wnt/β-catenin pathway in the tumorigenesis of EOC opens up the idea that targeted inhibition of this pathway with endogenous antagonists will have strong therapeutic potentials by probing strictly at what explicit mechanisms and proteins are involved in building OC cells drug-resistant. Recent studies have identified the expression of several Wnt signaling regulators in cancer cells. Challenging research anticipates the importance of antagonists and negative regulators of Wnt signaling in ovarian carcinogenesis. The expression of every inhibitor varies in different cancer and even stages. To identify the best possible markers and prove their prognostic and diagnostic value large cohort of patients has to be included and perform prospective studies. Tissue-based screening has made its way into the clinic; however, the conditions are diagnosed in the later stage only. Early detection demands tests on liquid biopsies such as blood, serum, and plasma. Genetic and epigenetic alteration of Wnt antagonists and negative regulators can be used as early detection biomarkers and other standard markers to allow rapid detection of OC progression in early stages. Epigenetic modifications of Wnt antagonists in particular DKK and SFRP lead to chemoresistance in ovarian cancer. The studies which reported the outcome of small-molecule inhibitors against these Wnt antagonists in ovarian cancer models strengthen their role in ovarian cancer development and progression. There is yet unexplored Wnt antagonist in ovarian physiology and pathophysiology.
More research into the mechanism of Wnt antagonists alone or in combination with other ovarian biomarkers, could prove clinically useful for a better understanding of the pathology of OC and improving early detection and treatment. OC displays intrinsic resistance to the platinum and taxane group of drugs, and the platinum/taxane refractory phenotype typically develops during the treatment course. Therefore, identification of molecular determinants which could drive platinum/ taxane refractory phenotype could be used as biomarkers or as sensitizing targets for platinum/taxane compounds in ovarian cancer. Added investigations demand characterization of Wnt antagonists and their interactome to reveal how they sensitize ovarian cancer to chemotherapy by using in vitro and in vivo patient-derived xenografts and organoid models. These might pave the way to individualized diagnosis or therapies in the future. Advanced imaging techniques and analytical methods must identify important structural information on atomic structures of protein complexes, discrete domains, and protein interaction interfaces. Additional small molecular inhibitors or activators with specific targets against Wnt antagonists and their mechanism of action would direct therapeutics. A stronger perception of these antagonists will be the up-shot of loss-of-function studies and the availability of purified Wnt proteins or receptor proteins to help determine each antagonist’s binding affinities and specificities. Few other studies reveal the opposite roles of every Wnt antagonist in different in vitro / in vivo models. Extra insights seek domain characterization and its interaction with signaling compounds to assess cells’ inhibitory or activating properties. Employing progressive analytical technologies to inspect the mechanistic signal transduction events in vivo would give insight into the biological aspects of Wnt signaling in a spatiotemporal fashion. Since some antagonist has biphasic functions, such analysis would be essential in the development of these as prognostic/diagnostic markers and/ therapeutic targets.
Mechanistic studies on drug resistance are limited to small ovarian cancer cell lines panels. Further studies are required on the full spectrum of primary cell lines, tissue samples, organoids (Nanki et al., 2020) as well as in vivo models (Alkema et al., 2016), considering the high degrees of molecular heterogeneity and distinct subtypes of ovarian cancer. Although a few synthetic antagonists to Wnt/β-catenin signaling is in clinical trial in OC, currently there is no clinical trial with endogenous Wnt antagonists. Due to ovarian cancer subtypes’ heterogeneity, in-depth learning of Wnt antagonists and their well-defined molecular functions with complete genotyping and phenotyping profiles in OC tissues is inadequate. Although different attributes of each component of Wnt antagonist signaling are beginning to be explored, their definite mechanism in ovarian cancer growth, drug resistance, cancer stem cells, and relapse is still not completely understood. Fortunately, the development of new technologies, including DNA microarray, proteomic analysis, molecular/pathways-targeted therapies, RNA interference (RNAi), and clustered regularly-interspaced short palindromic repeat-associated nuclease Cas (CRISPR/Cas) screens have provided new opportunities to assess expression-function relationship of Wnt antagonist particularly in context of drug response and resistance phenotype (Longley and Johnston, 2005).
There is a demand for high specificity and efficacy in drug resistant OC therapeutics and diagnostics. Applying the drug combination strategy, especially drugs with different mechanisms of action or inhibitor compounds/proteins aiding in sensitization of anti-cancer drugs, can improve diagnostic/treatment efficacy. Studies so far with the Wnt antagonists have clearly shown that these have capacity to improve drug sensitivity to both OC and OCSCs. In addition, these Wnt antagonist also show great promise as diagnostic markers for OC progression. Thus, combined with above mentioned in depth characterization studies, the exploitation of Wnt antagonists as such or their signaling in therapy and/diagnosis would be essential in OC. Small molecule or peptide mimics of these Wnt antagonists could be developed to target OC and OCSCs. Studies so far also suggest that combining the chemotherapy with these antagonists show better clearance of the disease. These Wnt antagonist mimics can pose less toxicity, good compatibility, and less immune response compared to synthetic compounds. Thus, theragnostics based on the Wnt antagonist may provide a novel approach to treat the aggressive Wnt-driven OC.
Acknowledgments
Krithicaa Narayanaa Y is supported by Senior Research Fellowship [RBMH/FW/2020/35] from the Indian Council of Medical Research, New Delhi, India. This work is also supported by the DBT/Wellcome Trust India Alliance Fellowship [IA/E/14/1/501793] awarded to Dr. Lakshmi R Perumalsamy. In addition, we gratefully acknowledge the support from SERB-POWER grant from Science and Engineering Research Board, Department of Science and Technology, India to Dr Lakshmi R Perumalsamy and Dr Arun Dharmarajan [SPG/2021/000995]. All the figures in this review were created with BioRender.com.
Abbreviations
- APC
Adenomatous polyposis coli
- APCDD1
Adenomatosis polyposis coli down-regulated 1
- BIRC5
Survivin, Baculoviral inhibitor of apoptosis repeat-containing 5
- BMP
Bone morphogentic protein
- Ca2+
Calcium
- CD
Cluster of Differentiation
- CK1
Casein kinase
- CRD
Cysteine rich domain
- CSC
Cancer stem cells
- CTNNB1
β-catenin
- DKK
Dickkopf
- Dsh
Disheveled
- EMT
Epithelial-mesenchymal transition
- EOC
Epithelial ovarian cancer
- FDA
Food and Drug Administeration
- FGF
Fibroblast growth factor
- Fzd
Frizzled
- GSK3β
Glycogen synthase kinase 3β
- IGFBP-4
Insulin-like growth factor binding protein 4
- JNK
c-Jun kinase enzyme
- LRP 5/6
Low-density lipoprotein receptor related protein
- MET
Mesenchymal-epithelial transition
- miRNA
micro Ribonucleic acid
- MMP
Matrix metalloproteinses
- mRNA
messenger RNA
- NLD
Netrin like domain
- OC
Ovarian cancer
- PCOS
Polycystic ovarian syndrome
- PDX
Patient-derived xenograft
- PCP
Planar cell polarity
- PKC
Protein kinase C
- ROS
Reactive oxygen species
- SFRP
Secreted frizzled related protein
- siRNA
small interfering RNA
- TCF/LEF
T – cell factor/lymphoid enhancer factor
- UTR
Untranslated region
- VEGF
Vascular endothelial growth factor
- Waif1
Wnt-activated inhibitory factor 1
- WIF
Wnt Inhibitory factor
- Wnt
Wingless-related integration site
Footnotes
CRediT authorship contribution statement
Krithicaa Narayanaa Y: Conceptualization, Investigation, Writing – original draft preparation, Visualization. Naveen Kumar P: Writing – original draft. Sudha Warrier: Writing – review & editing. Lakshmi R Perumalsamy: Conceptualization, Writing – review & editing, Supervision. Arun Dharmarajan: Conceptualization, Writing – review & editing, Supervision, Project administration.
Competing interests
The authors declare no potential competing interests.
References
- Alkema NG, Wisman GBA, Van Der Zee AGJ, Van Vugt MATM, De Jong S. Studying platinum sensitivity and resistance in high-grade serous ovarian cancer: different models for different questions. Drug Resist Updat. 2016;24:55–69. doi: 10.1016/j.drup.2015.11.005. [DOI] [PubMed] [Google Scholar]
- Al-Shabanah OA, Hafez MM, Hassan ZK, Sayed-Ahmed MM, Abozeed WN, Alsheikh A, Al-Rejaie SS. Methylation of SFRPs and APC genes in ovarian cancer infected with high risk human papillomavirus. Asian Pac J Cancer Prev. 2014;15:2719–2725. doi: 10.7314/apjcp.2014.15.6.2719. [DOI] [PubMed] [Google Scholar]
- Amato RJ, Stepankiw M. Evaluation of MVA-5T4 as a novel immunotherapeutic vaccine in colorectal, renal and prostate cancer. Future Oncol. 2012;8:231–237. doi: 10.2217/fon.12.7. [DOI] [PubMed] [Google Scholar]
- Arend RC, Londoño-Joshi AI, Straughn JM, Buchsbaum DJ. The Wnt/β-catenin pathway in ovarian cancer: a review. Gynecol Oncol. 2013 doi: 10.1016/j.ygyno.2013.09.034. [DOI] [PubMed] [Google Scholar]
- Avsian-Kretchmer O, Hsueh AJW. Comparative genomic analysis of the eight-membered ring cystine knot-containing bone morphogenetic protein antagonists. Mol Endocrinol. 2004;18:1–12. doi: 10.1210/me.2003-0227. [DOI] [PubMed] [Google Scholar]
- Baehs S, Herbst A, Thieme SE, Perschl C, Behrens A, Scheel S, Jung A, Brabletz T, Göke B, Blum H, Kolligs FT. Dickkopf-4 is frequently down-regulated and inhibits growth of colorectal cancer cells. Cancer Lett. 2009;276:152–159. doi: 10.1016/j.canlet.2008.11.003. [DOI] [PubMed] [Google Scholar]
- Bafico A, Gazit A, Pramila T, Finch PW, Yaniv A, Aaronson SA. Interaction of frizzled related protein (FRP) with Wnt ligands and the frizzled receptor suggests alternative mechanisms for FRP inhibition of Wnt signaling. J Biol Chem. 1999;274:16180–16187. doi: 10.1074/jbc.274.23.16180. [DOI] [PubMed] [Google Scholar]
- Banno K, Yanokura M, Iida M, Adachi M, Nakamura K, Nogami Y, Umene K, Masuda K, Kisu I, Nomura H, Kataoka F, et al. Application of microRNA in diagnosis and treatment of ovarian cancer. Biomed Res Int. 2014;2014 doi: 10.1155/2014/232817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bapat SA, Mali AM, Koppikar CB, Kurrey NK. Stem and progenitor-like cells contribute to the aggressive behavior of human epithelial ovarian cancer. Cancer Res. 2005;65:3025–3029. doi: 10.1158/0008-5472.CAN-04-3931. [DOI] [PubMed] [Google Scholar]
- Barghout SH, Zepeda N, Xu Z, Steed H, Lee CH, Fu Y. Elevated β-catenin activity contributes to carboplatin resistance in A2780cp ovarian cancer cells. Biochem Biophys Res Commun. 2015;468:173–178. doi: 10.1016/j.bbrc.2015.10.138. [DOI] [PubMed] [Google Scholar]
- Belo JA, Silva AC, Borges AC, Filipe M, Bento M, Gonalves L, Vitorino M, Salgueiro A, Texeira V, Tavares AT, Marques S. Generating asymmetries in the early vertebrate embryo: the role of the Cerberus-like family. Int J Dev Biol. 2009;53:1399–1407. doi: 10.1387/ijdb.072297jb. [DOI] [PubMed] [Google Scholar]
- Betella I, Turbitt WJ, Szul T, Wu B, Martinez A, Katre A, Wall JA, Norian L, Birrer MJ, Arend R. Wnt signaling modulator DKK1 as an immunotherapeutic target in ovarian cancer. Gynecol Oncol. 2020;157:765–774. doi: 10.1016/j.ygyno.2020.03.010. [DOI] [PubMed] [Google Scholar]
- Bhat RA, Stauffer B, Komm BS, Bodine PVN. Structure–Function analysis of secreted frizzled-related protein-1 for its Wnt antagonist function. J Cell Biochem. 2007;102:1519–1528. doi: 10.1002/jcb.21372. [DOI] [PubMed] [Google Scholar]
- Bhuvanalakshmi G, Gamit N, Patil M, Arfuso F, Sethi G, Dharmarajan A, Kumar AP, Warrier S. Stemness, pluripotentiality, and wnt antagonism: SFRP4, a wnt antagonist mediates pluripotency and stemness in glioblastoma. Cancers. 2019;11:25. doi: 10.3390/cancers11010025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blish KR, Wang W, Willingham MC, Du W, Birse CE, Krishnan SR, Brown JC, Hawkins GA, Garvin AJ, D’Agostino RB, Torti FM, et al. A human bone morphogenetic protein antagonist is down-regulated in renal cancer. Mol Biol Cell. 2008;19:457–464. doi: 10.1091/mbc.E07-05-0433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouazzi L, Sproll P, Eid W, Biason-Lauber A. The transcriptional regulator CBX2 and ovarian function: a whole genome and whole transcriptome approach. Sci Rep. 2019;9 doi: 10.1038/s41598-019-53370-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouwmeester T, Kim SH, Sasai Y, Lu B, De Robertis EM. Cerberus is a head-inducing secreted factor expressed in the anterior endoderm of Spemann’s organizer. Nature. 1996;382:595–601. doi: 10.1038/382595a0. [DOI] [PubMed] [Google Scholar]
- Bovolenta P, Esteve P, Ruiz JM, Cisneros E, Lopez-Rios J. Beyond Wnt inhibition: new functions of secreted Frizzled-related proteins in development and disease. J Cell Sci. 2008 doi: 10.1242/jcs.026096. [DOI] [PubMed] [Google Scholar]
- Bregenzer ME, Horst EN, Mehta P, Novak CM, Repetto T, Mehta G. The role of cancer stem cells and mechanical forces in ovarian cancer metastasis. Cancer. 2019 doi: 10.3390/cancers11071008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai X, Yao Z, Li L, Huang J. Role of DKK4 in tumorigenesis and tumor progression. Int J Biol Sci. 2018;14:616–621. doi: 10.7150/ijbs.24329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannon MJ, Ghosh D, Gujja S. Signaling circuits and regulation of immune suppression by ovarian tumor-associated macrophages. Vaccines. 2015;3:448–466. doi: 10.3390/vaccines3020448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chau WK, Ip CK, Mak ASC, Lai HC, Wong AST. c-Kit mediates chemoresistance and tumor-initiating capacity of ovarian cancer cells through activation of Wnt/β-catenin-ATP-binding cassette G2 signaling. Oncogene. 2013;32:2767–2781. doi: 10.1038/onc.2012.290. [DOI] [PubMed] [Google Scholar]
- Chen GY, Zheng HC. The clinicopathological and prognostic significances of Dkk3 expression in cancers: a bioinformatics analysis. Cancer Biomark. 2018;23:323–331. doi: 10.3233/CBM-181245. [DOI] [PubMed] [Google Scholar]
- Chen H, Liu Y, Liu P, Dai Q, Wang P. LINC01094 promotes the invasion of ovarian cancer cells and regulates the Wnt/β-catenin signaling pathway by targeting miR-532-3p. Exp Ther Med. 2021;22 doi: 10.3892/etm.2021.10662. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Chen MW, Yang ST, Chien MH, Hua KT, Wu CJ, Hsiao SM, Lin H, Hsiao M, Su JL, Wei LH. The STAT3-miRNA-92-Wnt Signaling Pathway Regulates Spheroid Formation and Malignant Progression in Ovarian Cancer. Cancer Res. 2017;77:1955–1967. doi: 10.1158/0008-5472.CAN-16-1115. [DOI] [PubMed] [Google Scholar]
- Chen VW, Ruiz B, Killeen JL, Coté TR, Wu XC, Correa CN, Howe HL. Pathology and classification of ovarian tumors. Cancer. 2003;97:2631–2642. doi: 10.1002/cncr.11345. [DOI] [PubMed] [Google Scholar]
- Cheng YY, Yu J, Wong YP, Man EPS, To KF, Jin VX, Li J, Tao Q, Sung JJY, Chan FKL, Leung WK. Frequent epigenetic inactivation of secreted frizzled-related protein 2 (SFRP2) by promoter methylation in human gastric cancer. Br J Cancer. 2007;97:895–901. doi: 10.1038/sj.bjc.6603968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chermuła B, Brazert M, Izycki D, CiesiÓłka S, Kranc W, Celichowski P, Ozegowska K, Nawrocki MJ, Jankowski M, Jeseta M, Antosik P, et al. New gene markers of angiogenesis and blood vessels development in porcine ovarian granulosa cells during short-term primary culture in vitro. Biomed Res Int. 2019;2019 doi: 10.1155/2019/6545210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Combes AN, Bowles J, Feng CW, Chiu HS, Khoo PL, Jackson A, Little MH, Tam PPL, Koopman P. Expression and functional analysis of Dkk1 during early gonadal development. Sex Dev. 2011;5:124. doi: 10.1159/000327709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dann CE, Hsieh JC, Rattner A, Sharma D, Nathans J, Leahy DJ. Insights into Wnt binding and signalling from the structures of two Frizzled cysteine-rich domains. Nature. 2001;412:86–90. doi: 10.1038/35083601. [DOI] [PubMed] [Google Scholar]
- Deshmukh A, Kumar S, Arfuso F, Newsholme P, Dharmarajan A. Secreted Frizzled-related protein 4 (sFRP4) chemo-sensitizes cancer stem cells derived from human breast, prostate, and ovary tumor cell lines. Sci Rep. 2017;7 doi: 10.1038/s41598-017-02256-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deshmukh A, Arfuso F, Newsholme P, Dharmarajan A. Epigenetic demthylation of sFRPs, with emphasis on sFRP4 activation leading to Wnt signalling suppression and histone modifications in breast, prostate, and ovary cancer stem cells. Int J Biochem Cell Biol. 2019;109:23–32. doi: 10.1016/j.biocel.2019.01.016. [DOI] [PubMed] [Google Scholar]
- Diaz Osterman CJ, Ozmadenci D, Kleinschmidt EG, Taylor KN, Barrie AM, Jiang S, Bean LM, Sulzmaier FJ, Jean C, Tancioni I, Anderson K, et al. FAK activity sustains intrinsic and acquired ovarian cancer resistance to platinum chemotherapy. Elife. 2019;8 doi: 10.7554/eLife.47327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Do M, Wu CCN, Sonavane PR, Juarez EF, Adams SR, Ross J, Baena ARy, Patel C, Mesirov JP, Carson DA, Advani SJ, et al. A FZD7-specific antibody-drug conjugate induces ovarian tumor regression in preclinical models. Mol Cancer Ther. 2022;21:113–124. doi: 10.1158/1535-7163.MCT-21-0548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drake J, Shearwood AM, White J, Friis R, Zeps N, Charles A, Dharmarajan A. Expression of secreted frizzled-related protein 4 (SFRP4) in primary serous ovarian tumours. Eur J Gynaecol Oncol. 2009;30:133–141. [PubMed] [Google Scholar]
- Drake JM, Friis RR, Dharmarajan AM. The role of sFRP4, a secreted frizzled- related protein in ovulation. Apoptosis. 2003;8:389–397. doi: 10.1023/a:1024181203729. [DOI] [PubMed] [Google Scholar]
- Duan H, Yan Z, Chen W, Wu Y, Han J, Guo H, Qiao J. TET1 inhibits EMT of ovarian cancer cells through activating Wnt/β-catenin signaling inhibitors DKK1 and SFRP2. Gynecol Oncol. 2017;147:408–417. doi: 10.1016/j.ygyno.2017.08.010. [DOI] [PubMed] [Google Scholar]
- Durai R, Davies M, Yang W, Yang SY, Seifalian A, Goldspink G, Winslet M. Biology of insulin-like growth factor binding protein-4 and its role in cancer (review) Int J Oncol. 2006 [PubMed] [Google Scholar]
- Ellies DL, Viviano B, McCarthy J, Rey J-P, Itasaki N, Saunders S, Krumlauf R. Bone density ligand, sclerostin, directly interacts with LRP5 but Not LRP5G171V to modulate wnt activity. J Bone Miner Res. 2006;21:1738–1749. doi: 10.1359/jbmr.060810. [DOI] [PubMed] [Google Scholar]
- Fatima S, Lee NP, Tsang FH, Kolligs FT, Ng IOL, Poon RTP, Fan ST, Luk JM. Dickkopf 4 (DKK4) acts on Wnt/β-catenin pathway by influencing β-catenin in hepatocellular carcinoma. Oncogene. 2012;3138(31):4233–4244. doi: 10.1038/onc.2011.580. [DOI] [PubMed] [Google Scholar]
- Filho LB, Oshima CTF, de O Lima F, de O Costa H, de S Damaão R, Gomes TS, Gonçalves WJ. Canonical and noncanonical Wnt pathway: A comparison among normal ovary, benign ovarian tumor and ovarian cancer. Oncol Rep. 2009;21:313–320. [PubMed] [Google Scholar]
- Finkernagel F, Reinartz S, Schuldner M, Malz A, Jansen JM, Wagner U, Worzfeld T, Graumann J, Pogge von Strandmann E, Müller R. Dual-platform affinity proteomics identifies links between the recurrence of ovarian carcinoma and proteins released into the tumor microenvironment. Theranostics. 2019;9:6601. doi: 10.7150/thno.37549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer MM, Cancilla B, Yeung VP, Cattaruzza F, Chartier C, Murriel CL, Cain J, Tam R, Cheng CY, Evans JW, O'Young G, et al. WNT antagonists exhibit unique combinatorial antitumor activity with taxanes by potentiating mitotic cell death. Sci Adv. 2017;3 doi: 10.1126/sciadv.1700090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flanagan JM, Wilhelm-Benartzi CS, Metcalf M, Kaye SB, Brown R. Association of somatic DNA methylation variability with progression-free survival and toxicity in ovarian cancer patients. Ann Oncol Soc J Eur Soc Med Oncol. 2013;24:2813–2818. doi: 10.1093/annonc/mdt370. [DOI] [PubMed] [Google Scholar]
- Flesken-Nikitin A, Hwang C, Il, Cheng CY, Michurina TV, Enikolopov G, Nikitin AY. Ovarian surface epithelium at the junction area contains a cancer-prone stem cell niche. Nature. 2013;495:241–245. doi: 10.1038/nature11979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forbes BE, McCarthy P, Norton RS. Insulin-like growth factor binding proteins: a structural perspective. Front Endocrinol (Lausanne) 2012;3 doi: 10.3389/fendo.2012.00038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraungruber P, Kaltofen T, Heublein S, Kuhn C, Mayr D, Burges A, Mahner S, Rathert P, Jeschke U, Trillsch F. G protein-coupled estrogen receptor correlates with Dkk2 expression and has prognostic impact in ovarian cancer patients. Front Endocrinol. 2021;12:564002. doi: 10.3389/fendo.2021.564002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freimund AE, Beach JA, Christie EL, Bowtell DDL. Mechanisms of drug resistance in high-grade serous ovarian cancer. Hematol Oncol Clin North Am. 2018;32:983–996. doi: 10.1016/j.hoc.2018.07.007. [DOI] [PubMed] [Google Scholar]
- Furushima K, Yamamoto A, Nagano T, Shibata M, Miyachi H, Abe T, Ohshima N, Kiyonari H, Aizawa S. Mouse homologues of Shisa antagonistic to Wnt and Fgf signalings. Dev Biol. 2007;306:480–492. doi: 10.1016/j.ydbio.2007.03.028. [DOI] [PubMed] [Google Scholar]
- Gatcliffe TA, Monk BJ, Planutisy K, Holcombey RF. Wnt signaling in ovarian tumorigenesis. doi: 10.1111/j.1525-1438.2007.01127.x. n.d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giornelli GH, Mandó P. A Theoretical View of Ovarian Cancer Relapse. Overian Cancer Rev. 2017 [Google Scholar]
- Glinka A, Wu W, Delius H, Monaghan AP, Blumenstock C, Niehrs C. Dickkopf-1 is a member of a new family of secreted proteins and functions in head induction. Nature. 1998;391:357–362. doi: 10.1038/34848. [DOI] [PubMed] [Google Scholar]
- Gu ZW, He YF, Wang WJ, Tian Q, Di W. MiR-1180 from bone marrow- derived mesenchymal stem cells induces glycolysis and chemoresistance in ovarian cancer cells by upregulating the Wnt signaling pathway. J Zhejiang Univ Sci B. 2019;20:219–237. doi: 10.1631/jzus.B1800190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan X, Zong ZH, Chen S, Sang XB, Wu Ddan, Wang L li, Liu Y, Zhao Y. The role of miR-372 in ovarian carcinoma cell proliferation. Gene. 2017;624:14–20. doi: 10.1016/j.gene.2017.04.043. [DOI] [PubMed] [Google Scholar]
- Gui Z, Yan H, Wu J, Zhang M, Wu A, He J. Aberrant activation of Wnt/catenin signaling and overexpression of ABCG2 contributes to apoptosis down regulation and tumor progression of high grade ovarian cancer. Acta Biochim Pol. 2021;68:127–133. doi: 10.18388/abp.2020_5488. [DOI] [PubMed] [Google Scholar]
- Guo Y, Li B, Yan X, Shen X, Ma J, Liu S, Zhang D. Bisphenol A and polychlorinated biphenyls enhance the cancer stem cell properties of human ovarian cancer cells by activating the WNT signaling pathway. Chemosphere. 2020;246 doi: 10.1016/j.chemosphere.2019.125775. [DOI] [PubMed] [Google Scholar]
- Ho CM, Lai HC, Huang SH, Chien TY, Lin MC, Chang SF. Promoter methylation of sFRP5 in patients with ovarian clear cell adenocarcinoma. EurJ Clin Invest. 2010;40:310–318. doi: 10.1111/j.1365-2362.2010.02266.x. [DOI] [PubMed] [Google Scholar]
- Hsieh JC, Kodjabachian L, Rebbert ML, Rattner A, Smallwood PM, Samos CH, Nusse R, Dawid IB, Nathans J. A new secreted protein that binds to Wnt proteins and inhibits their activites. Nature. 1999;398:431–436. doi: 10.1038/18899. [DOI] [PubMed] [Google Scholar]
- Hu YA, Gu X, Liu J, Yang Y, Yan Y, Zhao C. Expression pattern of Wnt inhibitor factor 1(Wif1) during the development in mouse CNS. Gene Expr Patterns. 2008;8:515–522. doi: 10.1016/j.gep.2008.06.001. [DOI] [PubMed] [Google Scholar]
- Hunter DD, Zhang M, Ferguson JW, Koch M, Brunken WJ. The extracellular matrix component WIF-1 is expressed during, and can modulate, retinal development. Mol Cell Neurosci. 2004;27:477–488. doi: 10.1016/j.mcn.2004.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itasaki N, Jones CM, Mercurio S, Rowe A, Domingos PM, Smith JC, Krumlauf R. Wise, a context-dependent activator and inhibitor of Wnt signalling. Development. 2003;130:4295–4305. doi: 10.1242/dev.00674. [DOI] [PubMed] [Google Scholar]
- Jacob F, Ukegjini K, Nixdorf S, Ford CE, Olivier J, Caduff R, Scurry JP, Guertler R, Hornung D, Mueller R, Fink DA, et al. Loss of Secreted Frizzled-Related Protein 4 Correlates with an Aggressive Phenotype and Predicts Poor Outcome in Ovarian Cancer Patients. PLoS One. 2012;7:e31885. doi: 10.1371/journal.pone.0031885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jalilvand A, Soltanpour MS. Promoter hypermethylation of wnt/p-catenin signaling pathway inhibitor WIF-1 gene and its association with MTHFR C677T polymorphism in patients with colorectal cancer. Oman Med J. 2020;35:1–6. doi: 10.5001/omj.2020.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang HJ, Yang KE, Hwang IH, Huh YH, Kim DJ, Yoo HS, Park SJ, Jang IS. Cordycepin inhibits human ovarian cancer by inducing autophagy and apoptosis through Dickkopf-related protein 1/p-catenin signaling. Am J Transl Res. 2019;11:6890. [PMC free article] [PubMed] [Google Scholar]
- Jimeno A, Gordon M, Chugh R, Messersmith W, Mendelson D, Dupont J, Stagg R, Kapoun AM, Xu L, Uttamsingh S, Brachmann RK, et al. A first-in-human phase I study of the anticancer stem cell agent ipafricept (OMP- 54F28), a decoy receptor for Wnt ligands, in patients with advanced solid tumors. 2017 doi: 10.1158/1078-0432.CCR-17-2157. [DOI] [PubMed] [Google Scholar]
- Jones MR, Chua A, Chen YDI, Li X, Krauss RM, Rotter JI, Legro RS, Azziz R, Goodarzi MO. Harnessing expression data to identify novel candidate genes in polycystic ovary syndrome. PLoS One. 2011;6 doi: 10.1371/journal.pone.0020120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones SE, Jomary C. Secreted Frizzled-related proteins: searching for relationships and patterns. BioEssays. 2002;24:811–820. doi: 10.1002/bies.10136. [DOI] [PubMed] [Google Scholar]
- Jukkola T, Sinjushina N, Partanen J. Drapc1 expression during mouse embryonic development. Gene Expr Patterns. 2004;4:755–762. doi: 10.1016/j.modgep.2004.03.006. [DOI] [PubMed] [Google Scholar]
- Kagermeier-Schenk B, Wehner D, Özhan-Kizil G, Yamamoto H, Li J, Kirchner K, Hoffmann C, Stern P, Kikuchi A, Schambony A, Weidinger G. Waif1/5T4 Inhibits Wnt/β-Catenin Signaling and Activates Noncanonical Wnt Pathways by Modifying LRP6 Subcellular Localization. Dev Cell. 2011;21:1129–1143. doi: 10.1016/j.devcel.2011.10.015. [DOI] [PubMed] [Google Scholar]
- Kalam A, Talegaonkar S, Vohora D. Effects of raloxifene against letrozole-induced bone loss in chemically-induced model of menopause in mice. Mol Cell Endocrinol. 2017;440:34–43. doi: 10.1016/j.mce.2016.11.005. [DOI] [PubMed] [Google Scholar]
- Kardum V, Karin V, Glibo M, Skrtic A, Martic TN, Ibisevic N, Skenderi F, Vranic S, Serman L. Methylation-associated silencing of SFRP1 gene in high-grade serous ovarian carcinomas. Ann Diagn Pathol. 2017;31:45–49. doi: 10.1016/j.anndiagpath.2017.07.002. [DOI] [PubMed] [Google Scholar]
- Kawano Y, Kypta R. Secreted antagonists of the Wnt signalling pathway. J Cell Sci. 2003 doi: 10.1242/jcs.00623. [DOI] [PubMed] [Google Scholar]
- Kim BR, Lee EJ, Seo SH, Lee SH, Rho SB. Dickkopf-3 (DKK-3) obstructs VEGFR-2/Akt/mTOR signaling cascade by interacting of β2-microglobulin (β2M) in ovarian tumorigenesis. Cell Signal. 2015;27:2150–2159. doi: 10.1016/j.cellsig.2015.08.008. [DOI] [PubMed] [Google Scholar]
- Koni M, Pinnarò V, Brizzi MF. The Wnt Signalling Pathway: A Tailored Target in Cancer. Int J Mol Sci. 2020;21:1–26. doi: 10.3390/ijms21207697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kristensen IB, Christensen JH, Lyng MB, Møller MB, Pedersen L, Rasmussen LM, Ditzel HJ, Abildgaard N. Expression of osteoblast and osteoclast regulatory genes in the bone marrow microenvironment in multiple myeloma: only up-regulation of Wnt inhibitors SFRP3 and DKK1 is associated with lytic bone disease. Leuk Lymphoma. 2014;55:911–919. doi: 10.3109/10428194.2013.820288. [DOI] [PubMed] [Google Scholar]
- Krupnik VE, Sharp JD, Jiang C, Robison K, Chickering TW, Amaravadi L, Brown DE, Guyot D, Mays G, Leiby K, Chang B, et al. Functional and structural diversity of the human Dickkopf gene family. Gene. 1999;238:301–313. doi: 10.1016/s0378-1119(99)00365-0. [DOI] [PubMed] [Google Scholar]
- Laurikkala J, Kassai Y, Pakkasjärvi L, Thesleff I, Itoh N. Identification of a secreted BMP antagonist, ectodin, integrating BMP, FGF, and SHH signals from the tooth enamel knot. Dev Biol. 2003;264:91–105. doi: 10.1016/j.ydbio.2003.08.011. [DOI] [PubMed] [Google Scholar]
- Le PN, McDermott JD, Jimeno A. Targeting the Wnt pathway in human cancers: therapeutic targeting with a focus on OMP-54F28. Pharmacol Ther. 2015;146:1–11. doi: 10.1016/j.pharmthera.2014.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leyns L, Bouwmeester T, Kim SH, Piccolo S, De Robertis EM. Frzb-1 is a secreted antagonist of Wnt signaling expressed in the Spemann organizer. Cell. 1997;88:747–756. doi: 10.1016/s0092-8674(00)81921-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Zhang Y, Kang H, Liu W, Liu P, Zhang J, Harris SE, Wu D. Sclerostin binds to LRP5/6 and antagonizes canonical Wnt signaling. J Biol Chem. 2005;280:19883–19887. doi: 10.1074/jbc.M413274200. [DOI] [PubMed] [Google Scholar]
- Li X, Gao F, Fan Y, Xie S, Li C, Meng L, Li L, Zhang S, Wei H. A novel identified circ-ANKHD1 targets the miR-27a-3p/SFRP1 signaling pathway and modulates the apoptosis of granulosa cells. Environ Sci Pollut Res Int. 2021;28:57459–57469. doi: 10.1007/s11356-021-14699-4. [DOI] [PubMed] [Google Scholar]
- Liang CJ, Wang ZW, Chang YW, Lee KC, Lin WH, Lee JL. SFRPs Are Biphasic Modulators of Wnt-Signaling-Elicited Cancer Stem Cell Properties beyond Extracellular Control. Cell Rep. 2019;28:1511–1525.:e5. doi: 10.1016/j.celrep.2019.07.023. [DOI] [PubMed] [Google Scholar]
- Lin HW, Fu CF, Chang MC, Lu TP, Lin HP, Chiang YC, Chen CA, Cheng WF. CDH1, DLEC1 and SFRP5 methylation panel as a prognostic marker for advanced epithelial ovarian cancer. Epigenomics. 2018;10:1397–1413. doi: 10.2217/epi-2018-0035. [DOI] [PubMed] [Google Scholar]
- Lin K, Wang S, Julius MA, Kitajewski J, Moos M, Luyten FP. The cysteine-rich frizzled domain of Frzb-1 is required and sufficient for modulation of Wnt signaling. Proc Natl Acad Sci USA. 1997;94:11196–11200. doi: 10.1073/pnas.94.21.11196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin YW, Shih YL, Lien GS, Suk FM, Hsieh CB, Yan MDeL. Promoter methylation of SFRP3 is frequent in hepatocellular carcinoma. Dis Markers. 2014 doi: 10.1155/2014/351863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lintern KB, Guidato S, Rowe A, Saldanha JW, Itasaki N. Characterization of wise protein and its molecular mechanism to interact with both Wnt and BMP signals. J Biol Chem. 2009;284:23159–23168. doi: 10.1074/jbc.M109.025478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisio MA, Fu L, Goyeneche A, Gao ZH, Telleria C. High-grade serous ovarian cancer: Basic sciences, clinical and therapeutic standpoints. Int J Mol Sci. 2019 doi: 10.3390/ijms20040952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu M, Zhang H, Li Y, Wang S. Noncoding RNAs interplay in ovarian cancer therapy and drug resistance. Cancer Biother Radiopharm. 2022 doi: 10.1089/cbr.2021.0339. [DOI] [PubMed] [Google Scholar]
- Liu Y, Kodithuwakku SP, Ng PY, Chai J, Ng EHY, Yeung WSB, Ho PC, Lee KF. Excessive ovarian stimulation up-regulates the Wnt-signaling molecule DKK1 in human endometrium and may affect implantation: an in vitro coculture study. Hum Reprod. 2010;25:479–490. doi: 10.1093/humrep/dep429. [DOI] [PubMed] [Google Scholar]
- Longley DB, Johnston PG. Molecular mechanisms of drug resistance. J Pathol. 2005;205:275–292. doi: 10.1002/path.1706. [DOI] [PubMed] [Google Scholar]
- Lopez-Rios J, Esteve P, Ruiz JM, Bovolenta P. The Netrin-related domain of Sfrp1 interacts with Wnt ligands and antagonizes their activity in the anterior neural plate. Neural Dev. 2008;3:19. doi: 10.1186/1749-8104-3-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu J, Li Y, Li YA, Wang L, Zeng AR, Ma XL, Qiang JW. In vivo detection of dysregulated choline metabolism in paclitaxel-resistant ovarian cancers with proton magnetic resonance spectroscopy. J Transl Med. 2022;20:92. doi: 10.1186/s12967-022-03292-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luke GN, Castro LFC, McLay K, Bird C, Coulson A, Holland PWH. Dispersal of NK homeobox gene clusters in amphioxus and humans. Proc Natl Acad Sci U S A. 2003;100:5292–5295. doi: 10.1073/pnas.0836141100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lupia M, Cavallaro U. Ovarian cancer stem cells: Still an elusive entity? ProcMol Cancer. 2017 doi: 10.1186/s12943-017-0638-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luvero D, Milani A, Ledermann JA. Treatment options in recurrent ovarian cancer: latest evidence and clinical potential. Ther Adv Med Oncol. 2014;6:229. doi: 10.1177/1758834014544121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maman E, Yung Y, Cohen B, Konopnicki S, Dal Canto M, Fadini R, Kanety H, Kedem A, Dor J, Hourvitz A. Expression and regulation of sFRP family members in human granulosa cells. Mol Hum Reprod. 2011;17:399–404. doi: 10.1093/molehr/gar010. [DOI] [PubMed] [Google Scholar]
- Mancikova V, Montero-Conde C, Perales-Paton J, Fernandez A, Santacana M, Jodkowska K, Inglada-Pérez L, Castelblanco E, Borrego S, Encinas, M, Matias-Guiu X, et al. Multilayer OMIC data in medullary thyroid carcinoma identifies the STAT3 pathway as a potential therapeutic target in RETM918T tumors. Clin Cancer Res. 2017;23:1334–1345. doi: 10.1158/1078-0432.CCR-16-0947. [DOI] [PubMed] [Google Scholar]
- Manuylov NL, Smagulova FO, Leach L, Tevosian SG. Ovarian development in mice requires the GATA4-FOG2 transcription complex. Development. 2008;135:3731–3743. doi: 10.1242/dev.024653. [DOI] [PubMed] [Google Scholar]
- Mao B, Niehrs C. Kremen2 modulates Dickkopf2 activity during Wnt/LRP6 signaling. Gene. 2003;302:179–183. doi: 10.1016/s0378-1119(02)01106-x. [DOI] [PubMed] [Google Scholar]
- Mao B, Wu W, Li Y, Hoppe D, Stannek P, Glinka A, Niehrs C. LDL-receptor-related protein 6 is a receptor for Dickkopf proteins. Nature. 2001;411:321–325. doi: 10.1038/35077108. [DOI] [PubMed] [Google Scholar]
- Mao B, Wu W, Davidson G, Marhold J, Li M, Mechler BM, Dellus H, Hoppe D, Stannek P, Walter C, Glinka A, et al. Kremen proteins are Dickkopf receptors that regulate Wnt/β-catenin signalling. Nature. 2002;417:664–667. doi: 10.1038/nature756. [DOI] [PubMed] [Google Scholar]
- Mariani A, Wang C, Oberg AL, Riska SM, Torres M, Kumka J, Multinu F, Sagar G, Roy D, Jung DB, Zhang Q, et al. Genes associated with bowel metastases in ovarian cancer. Gynecol Oncol. 2019;154:495–504. doi: 10.1016/j.ygyno.2019.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marichereda VG, Bykova NA, Bubnov VV, Manasova GS, Moskalenko TY, Volyanska AG, Shevchenko IM, Adamovska TM. The analysis of methylation of DNA promoter of SFRP2 gene in patients with hyperplastic processes of the endometrium . Exp Oncol. 2018 [PubMed] [Google Scholar]
- Mariya T, Hirohashi Y, Torigoe T, Tabuchi Y, Asano T, Saijo H, Kuroda T, Yasuda K, Mizuuchi M, Saito T, Sato N. Matrix metalloproteinase-10 regulates stemness of ovarian cancer stem-like cells by activation of canonical Wnt signaling and can be a target of chemotherapy-resistant ovarian cancer. Oncotarget. 2016;7:26806–26822. doi: 10.18632/oncotarget.8645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menyhvárt O, Fekete JT, Győrffy B. Gene Expression Indicates Altered Immune Modulation and Signaling Pathway Activation in Ovarian Cancer Patients Resistant to Topotecan. Int J Mol Sci. 2019;20 doi: 10.3390/ijms20112750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosig RA, Lobl M, Senturk E, Shah H, Cohen S, Chudin E, Fruscio R, Marchini S, D'Incalci M, Sachidanandam R, Dottino P, Martignetti JA. IGFBP-4 tumor and serum levels are increased across all stages of epithelial ovarian cancer. J Ovarian Res. 2012;5:3. doi: 10.1186/1757-2215-5-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muley A, Majumder S, Kolluru GK, Parkinson S, Viola H, Hool L, Arfuso F, Ganss R, Dharmarajan A, Chatterjee S. Secreted frizzled-related protein 4: An angiogenesis inhibitor. Am J Pathol. 2010;176:1505–1516. doi: 10.2353/ajpath.2010.090465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munoz-Galvan S, Carnero A. Targeting cancer stem cells to overcome therapy resistance in ovarian cancer. Cells. 2020;9 doi: 10.3390/cells9061402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagaraj AB, Joseph P, Kovalenko O, Singh S, Armstrong A, Redline R, Resnick K, Zanotti K, Waggoner S, Di Feo A. Critical role of Wnt/ß-catenin signaling in driving epithelial ovarian cancer platinum resistance. Oncotarget. 2015;6:23720–23734. doi: 10.18632/oncotarget.4690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagaraj AB, Knarr M, Sekhar S, Connor RS, Joseph P, Kovalenko O, Fleming A, Surti A, Nurmemmedov E, Beltrame L, Marchini S, et al. The miR-181a-SFRP4 axis regulates Wnt activation to drive stemness and platinum resistance in ovarian cancer. Cancer Res. 2021;81:2044–2055. doi: 10.1158/0008-5472.CAN-20-2041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nanki Y, Chiyoda T, Hirasawa A, Ookubo A, Itoh M, Ueno M, Akahane T, Kameyama K, Yamagami W, Kataoka F, Aoki D. Patient-derived ovarian cancer organoids capture the genomic profiles of primary tumours applicable for drug sensitivity and resistance testing. Sci Rep. 2020;101(10):1–11. doi: 10.1038/s41598-020-69488-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen VHL, Hough R, Bernaudo S, Peng C. Wnt/β-catenin signalling in ovarian cancer: Insights into its hyperactivation and function in tumorigenesis. J Ovarian Res. 2019;12 doi: 10.1186/s13048-019-0596-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niehrs C. Function and biological roles of the Dickkopf family of Wnt modulators. Oncogene. 2006 doi: 10.1038/sj.onc.1210054. [DOI] [PubMed] [Google Scholar]
- Norouzi-Barough L, Sarookhani MR, Sharifi M, Moghbelinejad S, Jangjoo S, Salehi R. Molecular mechanisms of drug resistance in ovarian cancer. J Cell Physiol. 2018;233:4546–4562. doi: 10.1002/jcp.26289. [DOI] [PubMed] [Google Scholar]
- Novak M, Lester J, Karst AM, Parkash V, Hirsch MS, Crum CP, Karlan BY, Drapkin R. Stathmin 1 and p16INK4A are sensitive adjunct biomarkers for serous tubal intraepithelial carcinoma. Gynecol Oncol. 2015;139:104–111. doi: 10.1016/j.ygyno.2015.07.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan H, Kim E, Rankin GO, Rojanasakul Y, Tu Y, Chen YC. Theaflavin-3, 3'-digallate inhibits ovarian cancer stem cells via suppressing Wnt/β-Catenin signaling pathway. J Funct Foods. 2018;50:1–7. doi: 10.1016/j.jff.2018.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park GBin, Jeong JY, Choi S, Yoon YS, Kim D. Glucose deprivation enhances resistance to paclitaxel via ELAVL2/4-mediated modification of glycolysis in ovarian cancer cells. Anticancer Drugs. 2022;33:e370–e380. doi: 10.1097/CAD.0000000000001215. [DOI] [PubMed] [Google Scholar]
- Parr BA, McMahon AP. Sexually dimorphic development of the mammalian reproductive tract requires Wnt-7a. Nat. 1998:707–710. doi: 10.1038/27221. [DOI] [PubMed] [Google Scholar]
- Partl JZ, Fabijanovic D, Skrtic A, Vranic S, Martic TN, Serman L. Immunohistochemical expression of SFRP1 and SFRP3 proteins in normal and malignant reproductive tissues of rats and humans. Appl Immunohistochem Mol Morphol AIMM. 2014;22:681–687. doi: 10.1097/PAI.0000000000000019. [DOI] [PubMed] [Google Scholar]
- Pei J, Grishin NV. Unexpected diversity in Shisa-like proteins suggests the importance of their roles as transmembrane adaptors. Cell Signal. 2012;24:758–769. doi: 10.1016/j.cellsig.2011.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piccolo S, Agius E, Leyns L, Bhattacharyya S, Grunz H, Bouwmeester T, De Robertis EM. The head inducer cerberus is a multifunctional antagonist of Nodal, BMP and Wnt signals. Nature. 1999;397:707–710. doi: 10.1038/17820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pieterse Z, Amaya-Padilla MA, Singomat T, Binju M, Madjid BD, Yu Y, Kaur P. Ovarian cancer stem cells and their role in drug resistance. Int J Biochem Cell Biol. 2019;106:117–126. doi: 10.1016/j.biocel.2018.11.012. [DOI] [PubMed] [Google Scholar]
- Pollard SL, Holland PWH. Evidence for 14 homeobox gene clusters in human genome ancestry. Curr Biol. 2000;10:1059–1062. doi: 10.1016/s0960-9822(00)00676-x. [DOI] [PubMed] [Google Scholar]
- Qi H, Sun B, Zhao X, Du J, Gu Q, Liu Y, Cheng R, Dong X. Wnt5a promotes vasculogenic mimicry and epithelial-mesenchymal transition via protein kinase Cα in epithelial ovarian cancer. Oncol Rep. 2014;32:771–779. doi: 10.3892/or.2014.3229. [DOI] [PubMed] [Google Scholar]
- Ricci F, Fratelli M, Guffanti F, Porcu L, Spriano F, Dell’Anna T, Fruscio R, Damia G. Patient-derived ovarian cancer xenografts re-growing after a cisplatinum treatment are less responsive to a second drug re-challenge: a new experimental setting to study response to therapy. Oncotarget. 2017;8:7441–7451. doi: 10.18632/oncotarget.7465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *Ricken* A, Lochhead P, Kontogiannea M, Farookhi R. Wnt Signaling in the Ovary: Identification and Compartmentalized Expression of wnt-2, wnt-2b, and Frizzled-4 mRNAs. Endocrinology. 2002;143:2741–2749. doi: 10.1210/endo.143.7.8908. [DOI] [PubMed] [Google Scholar]
- Royfman R, Whiteley E, Noe O, Morand S, Creeden J, Stanbery L, Hamouda D, Nemunaitis J. BRCA1/2 signaling and homologous recombination deficiency in breast and ovarian cancer. Future Oncol. 2021;17:2817–2830. doi: 10.2217/fon-2021-0072. [DOI] [PubMed] [Google Scholar]
- Saha S, Parte S, Roy P, Kakar SS. Ovarian Cancer Stem Cells: Characterization and Role in Tumorigenesis. Adv Exp Med Biol. 2021;1330:151–169. doi: 10.1007/978-3-030-73359-9_10. [DOI] [PubMed] [Google Scholar]
- Salim H, Zong D, Hååg P, Novak M, Mörk B, Lewensohn R, Lundholm L, Viktorsson K. DKK1 is a potential novel mediator of cisplatin-refractoriness in non-small cell lung cancer cell lines. BMC Cancer. 2015;15 doi: 10.1186/s12885-015-1635-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saran U, Arfuso F, Zeps N, Dharmarajan A. Secreted frizzled-related protein 4 expression is positively associated with responsiveness to Cisplatin of ovarian cancer cell lines in vitro and with lower tumour grade in mucinous ovarian cancers. BMC Cell Biol. 2012;13:25. doi: 10.1186/1471-2121-13-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiefer L, Visweswaran M, Perumal V, Arfuso F, Groth D, Newsholme P, Warrier S, Dharmarajan A. Epigenetic regulation of the secreted frizzled-related protein family in human glioblastoma multiforme. Cancer Gene Ther. 2014;21:297–303. doi: 10.1038/cgt.2014.30. [DOI] [PubMed] [Google Scholar]
- Schlensog M, Magnus L, Heide T, Eschenbruch J, Steib F, Tator M, Kloten V, Rose M, Noetzel E, Gaisa NT, Knüchel R, et al. Epigenetic loss of putative tumor suppressor SFRP3 correlates with poor prognosis of lung adenocarcinoma patients. Epigenetics. 2018;13:214–227. doi: 10.1080/15592294.2016.1229730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sëmenov M, Tamai K, He X. SOST is a ligand for LRP5/LRP6 and a Wnt signaling inhibitor. J Biol Chem. 2005;280:26770–26775. doi: 10.1074/jbc.M504308200. [DOI] [PubMed] [Google Scholar]
- Semënov MV, Tamai K, Brott BK, Kuhl M, Sokol S, He X. Head inducer dickkopf-1 is a ligand for Wnt coreceptor LRP6. Curr Biol. 2001;11:951–961. doi: 10.1016/s0960-9822(01)00290-1. [DOI] [PubMed] [Google Scholar]
- Shen L, Xia M, Zhang Y, Luo H, Dong D, Sun L. Mitochondrial integration and ovarian cancer chemotherapy resistance. Exp Cell Res. 2021;401:112549. doi: 10.1016/j.yexcr.2021.112549. [DOI] [PubMed] [Google Scholar]
- Shizhuo W, Tao J, Shulan Z, Bing Z. The expression and significance of Dickkopf-1 in epithelial ovarian carcinoma. Int J Biol Markers. 2009;24:165–170. doi: 10.1177/172460080902400306. [DOI] [PubMed] [Google Scholar]
- Shu C, Zheng X, Wuhafu A, Cicka D, Doyle S, Niu Q, Fan D, Qian K, Ivanov AA, Du Y, Mo X, et al. Acquisition of taxane resistance by p53 inactivation in ovarian cancer cells. Acta Pharmacol Sin. 2022 doi: 10.1038/s41401-021-00847-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soliman AA, Elzarkaa AA, Malik E. Epithelial ovarian cancer and cancer stem cells. Adv Exp Med Biol. 2021;1330:21–32. doi: 10.1007/978-3-030-73359-9_2. [DOI] [PubMed] [Google Scholar]
- Srivastava M, Ahlawat N, Srivastava A. Ovarian cancer stem cells: newer horizons. J Obstet Gynaecol India. 2021;71:115–117. doi: 10.1007/s13224-020-01412-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stronach EA, Cunnea P, Turner C, Guney T, Aiyappa R, Jeyapalan S, de Sousa CH, Browne A, Magdy N, Studd JB, Sriraksa R, et al. The role of interleukin-8 (IL-8) and IL-8 receptors in platinum response in high grade serous ovarian carcinoma. Oncotarget. 2015;6:31593–31603. doi: 10.18632/oncotarget.3415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su HY, Lai HC, Lin YW, Chou YC, Liu CY, Yu MH. An epigenetic marker panel for screening and prognostic prediction of ovarian cancer. Int J Cancer. 2009;124:387–393. doi: 10.1002/ijc.23957. [DOI] [PubMed] [Google Scholar]
- Su H-Y, Lai H-C, Lin Y-W, Liu C-Y, Chen C-K, Chou Y-C, Lin S-P, Lin W-C, Lee H-Y, Yu M-H. Epigenetic silencing of SFRP5 is related to malignant phenotype and chemoresistance of ovarian cancer through Wnt signaling pathway. Int J Cancer. 2010;127:555–567. doi: 10.1002/ijc.25083. [DOI] [PubMed] [Google Scholar]
- Surmann-Schmitt C, Widmann N, Dietz U, Saeger B, Eitzinger N, N akamura Y, Rattel M, Latham R, Hartmann C, von der Mark H, Schett G, et al. Wif-1 is expressed at cartilage-mesenchyme interfaces and impedes Wnt3a-mediated inhibition of chondrogenesis. J Cell Sci. 2009;122:3627–3637. doi: 10.1242/jcs.048926. [DOI] [PubMed] [Google Scholar]
- Szotek PP, Chang HL, Brennand K, Fujino A, Pieretti-Vanmarcke R, Celso C, Lo Dombkowski D, Preffer F, Cohen KS, Teixeira J, Donahoe PK. Normal ovarian surface epithelial label-retaining cells exhibit stem/progenitor cell characteristics. Proc Natl Acad Sci U S A. 2008;105:12469–12473. doi: 10.1073/pnas.0805012105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi M, Fujita M, Furukawa Y, Hamamoto R, Shimokawa T, Miwa N, Ogawa M, Nakamura Y. Isolation of a novel human gene, APCDD1, as a direct target of the-catenin/T-cell factor 4 complex with probable involvement in colorectal. Carcinogenesis. 2002;1 [PubMed] [Google Scholar]
- Takata A, Terauchi M, Hiramitsu S, Uno M, Wakana K, Kubota T. Dkk-3 induces apoptosis through mitochondrial and fas death receptor pathways in human mucinous ovarian cancer cells. Int J Gynecol Cancer. 2015;25:372–379. doi: 10.1097/IGC.0000000000000340. [DOI] [PubMed] [Google Scholar]
- Tanaka Y, Kobayashi H, Suzuki M, Hirashima Y, Kanayama N, Terao T. Genetic downregulation of pregnancy-associated plasma protein-A (PAPP-A) by bikunin reduces IGF-I-dependent Akt and ERK1/2 activation and subsequently reduces ovarian cancer cell growth, invasion and metastasis. Int J Cancer. 2004;109:336–347. doi: 10.1002/ijc.11700. [DOI] [PubMed] [Google Scholar]
- Tang MKS, Yue PYK, Ip PP, Huang RL, Lai HC, Cheung ANY, Tse KY, Ngan HYS, Wong AST. Soluble E-cadherin promotes tumor angiogenesis and localizes to exosome surface. Nat Commun. 2018;9 doi: 10.1038/s41467-018-04695-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang Q, Zhao H, Yang B, Li L, Shi Q, Jiang C, Liu H. WIF-1 gene inhibition and Wnt signal transduction pathway activation in NSCLC tumorigenesis. Oncol Lett. 2017;13:1183–1188. doi: 10.3892/ol.2017.5566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teeuwssen Fodde. Wnt signaling in ovarian cancer stemness, EMT, and therapy resistance. J Clin Med. 2019;8:1658. doi: 10.3390/jcm8101658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Üren A, Reichsman F, Anest V, Taylor WG, Muraiso K, Bottaro DP, Cumberledge S, Rubin JS. Secreted frizzled-related protein-1 binds directly to wingless and is a biphasic modulator of Wnt signaling. J Biol Chem. 2000;275:4374–4382. doi: 10.1074/jbc.275.6.4374. [DOI] [PubMed] [Google Scholar]
- Vainio S, Heikkilä M, Kispert A, Chin N, McMahon AP. Female development in mammals is regulated by Wnt-4 signalling. Nat. 1999:405–409. doi: 10.1038/17068. [DOI] [PubMed] [Google Scholar]
- Veeck J, Geisler C, Noetzel E, Alkaya S, Hartmann A, Knüchel R, Dahl E. Epigenetic inactivation of the secreted frizzled-related protein-5 (SFRP5) gene in human breast cancer is associated with unfavorable prognosis. Carcinogenesis. 2008;29:991–998. doi: 10.1093/carcin/bgn076. [DOI] [PubMed] [Google Scholar]
- Walker G, MacLeod K, Williams ARW, Cameron DA, Smyth JF, Langdon SP. Insulin-like growth factor binding proteins IGFBP3, IGFBP4, and IGFBP5 predict endocrine responsiveness in patients with ovarian cancer. Clin Cancer Res. 2007;13:1438–1444. doi: 10.1158/1078-0432.CCR-06-2245. [DOI] [PubMed] [Google Scholar]
- Wang S, Zhang S. Dickkopf-1 is frequently overexpressed in ovarian serous carcinoma and involved in tumor invasion. Clin Exp Metastas-.- 2011;286(28):581–591. doi: 10.1007/s10585-011-9393-9. [DOI] [PubMed] [Google Scholar]
- Wang S, Krinks M, Lin K, Luyten FP, Moos M. Frzb, a secreted protein expressed in the Spemann organizer, binds and inhibits Wnt-8. Cell. 1997;88:757–766. doi: 10.1016/s0092-8674(00)81922-4. [DOI] [PubMed] [Google Scholar]
- Wang S, Wei H, Zhang S. Dickkopf-4 is frequently overexpressed in epithelial ovarian carcinoma and promotes tumor invasion. BMC Cancer. 2017;171(17):1–9. doi: 10.1186/s12885-017-3407-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Wang S, Zhou S, Yu L, Zhang L, Mei L. A network-pathway based module identification for predicting the prognosis of ovarian cancer patients. J Ovarian Res. 2016;9:1–8. doi: 10.1186/s13048-016-0285-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Yang X, Yuan M, Xian S, Zhang L, Yang D, Cheng Y. Promotion of ovarian cancer cell invasion, migration and colony formation by the miR-21/Wnt/CD44v6 pathway. Oncol Rep. 2019;42:91. doi: 10.3892/or.2019.7153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson ZL, Yamamoto TM, McMellen A, Kim H, Hughes CJ, Wheeler LJ, Post MD, Behbakht K, Bitler BG. Histone methyltransferases EHMT1 and EHMT2 (GLP/G9A) maintain PARP inhibitor resistance in high-grade serous ovarian carcinoma. Clin Epigenetics. 2019;11 doi: 10.1186/s13148-019-0758-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright RJ, Holly JMP, Galea R, Brincat M, Mason HD. Insulin-Like Growth Factor (IGF)-Independent Effects of IGF Binding Protein-4 on Human Granulosa Cell Steroidogenesis. Biol Reprod. 2002;67:776–781. doi: 10.1095/biolreprod.101.001511. [DOI] [PubMed] [Google Scholar]
- Wu G, Liu A, Zhu J, Lei F, Wu S, Zhang X, Ye L, Cao L, He S. MiR-1207 overexpression promotes cancer stem cell-like traits in ovarian cancer by activating the Wnt/β-catenin signaling pathway. Oncotarget. 2015;6:28882–28894. doi: 10.18632/oncotarget.4921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu G, Cao L, Zhu J, Tan Z, Tang M, Li Z, Hu Y, Yu R, Zhang S, Song L, Li J. Loss of RBMS3 confers platinum resistance in epithelial ovarian cancer via activation of miR-126-5p/β-catenin/CBP signaling. Clin Cancer Res. 2019;25:1022–1035. doi: 10.1158/1078-0432.CCR-18-2554. [DOI] [PubMed] [Google Scholar]
- Wu J, Sun H, Yang L, Deng Y, Yan Y, Wang S, Yang G, Ma H. Improved survival in ovarian cancer, with widening survival gaps of races and socioeconomic status: a period analysis, 1983-2012. J Cancer. 2018;9:3548–3556. doi: 10.7150/jca.26300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu JL, Keller P, Kanchwala M, Xing C, Babayev SN, Carr BR, Bukulmez O, Word RA. Controlled ovarian stimulation protocols alter endometrial histomorphology and gene expression profiles. Reprod Sci. 2020;27:895–904. doi: 10.1007/s43032-019-00093-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu R, Zhai Y, Fearon ER, Cho KR. Diverse mechanisms of β-catenin deregulation in ovarian endometrioid adenocarcinomas. Cancer Res. 2001;61 [PubMed] [Google Scholar]
- Xu Y, Li X, Wang H, Xie P, Yan X, Bai Y, Zhang T. Hypermethylation of CDH13, DKK3 and FOXL2 promoters and the expression of EZH2 in ovary granulosa cell tumors. Mol Med Rep. 2016;14:2739–2745. doi: 10.3892/mmr.2016.5521. [DOI] [PubMed] [Google Scholar]
- Yamamoto A, Nagano T, Takehara S, Hibi M, Aizawa S. Shisa promotes head formation through the inhibition of receptor protein maturation for the caudalizing factors, Wnt and FGF. Cell. 2005;120:223–235. doi: 10.1016/j.cell.2004.11.051. [DOI] [PubMed] [Google Scholar]
- Yanagita M, Oka M, Watabe T, Iguchi H, Niida A, Takahashi S, Akiyama T, Miyazono K, Yanagisawa M, Sakurai T. USAG-1: a bone morphogenetic protein antagonist abundantly expressed in the kidney. Biochem Biophys Res Commun. 2004;316:490–500. doi: 10.1016/j.bbrc.2004.02.075. [DOI] [PubMed] [Google Scholar]
- Yao HHC, Matzuk MM, Jorgez CJ, Menke DB, Page DC, Swain A, Capel B. Follistatin operates downstream of Wnt4 in mammalian ovary organogenesis. Dev Dyn. 2004;230:210–215. doi: 10.1002/dvdy.20042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yen H-Y, Tsao C-W, Lin Y-W, Kuo C-C, Tsao C-H, Liu C-Y. Regulation of carcinogenesis and modulation through Wnt/β-catenin signaling by curcumin in an ovarian cancer cell line. Sci Rep. 2019;91(9):1–14. doi: 10.1038/s41598-019-53509-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshioka S, King ML, Ran S, Okuda H, MacLean JA, McAsey ME, Sugino N, Brard L, Watabe K, Hayashi K. WNT7A regulates tumor growth and progression in ovarian cancer through the WNT/β-catenin pathway. Mol Cancer Res. 2012;10:469–482. doi: 10.1158/1541-7786.MCR-11-0177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- You A, Fokas E, Wang LF, He H, Kleb B, Niederacher D, Engenhart-Cabillic R, An HX. Expression of the Wnt antagonist DKK3 is frequently suppressed in sporadic epithelial ovarian cancer. J Cancer Res Clin Oncol. 2011;137:621–627. doi: 10.1007/s00432-010-0916-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu S, Pen X, Zheng H, Gao Q, Wang H. Downregulated Wnt2B expression suppresses proliferation, invasion, and angiogenesis of ovarian cancer cells through inhibiting the Wnt/β-catenin signaling pathway. Cancer Biother Radiopharm. 2022 doi: 10.1089/cbr.2021.0004. [DOI] [PubMed] [Google Scholar]
- Yunusova NV, Villert AB, Spirina LV, Frolova AE, Kolomiets LA, Kondakova IV. Insulin-like growth factors and their binding proteins in tumors and ascites of ovarian cancer patients: association with response to neoadjuvant chemotherapy. Asian Pac J Cancer Prev. 2016;17:5315–5320. doi: 10.22034/APJCP.2016.17.12.5315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhan Q, Wang C, Ngai S. Ovarian cancer stem cells: a new target for cancer therapy. Biomed Res Int. 2013;2013 doi: 10.1155/2013/916819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Sun D, Qiu J, Yao L. SFRP1 inhibited the epithelial ovarian cancer through inhibiting wnt/β-catenin signaling. Acta Biochim Pol. 2019a;66:392–400. doi: 10.18388/abp.2019_2757. [DOI] [PubMed] [Google Scholar]
- Zhang S, Zhang H, Ghia EM, Huang J, Wu L, Zhang J, Lam S, Lei Y, He J, Cui B, Widhopf GF, et al. Inhibition of chemotherapy resistant breast cancer stem cells by a ROR1 specific antibody. Proc Natl Acad Sci U S A. 2019b;116:1370–1377. doi: 10.1073/pnas.1816262116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Abreu JG, Yokota C, MacDonald BT, Singh S, Coburn KLA, Cheong SM, Zhang MM, Ye QZ, Hang HC, Steen H, et al. Tiki1 is required for head formation via wnt cleavage-oxidation and inactivation. Cell. 2012;149:1565–1577. doi: 10.1016/j.cell.2012.04.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Su Y, Wu X, Xiao R, Wu Y, Yang B, Wang Z, Guo L, Kang X, Wang C. Integrative analysis of the common genetic characteristics in ovarian cancer stem cells sorted by multiple approaches. J Ovarian Res. 2020;13 doi: 10.1186/s13048-020-00715-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao L, Liang X, Wang L, Zhang X. The role of miRNA in ovarian cancer: an overview. Reprod Sci. 2022 doi: 10.1007/s43032-021-00717-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng R, Chen W, Xia W, Zheng J, Zhou Q. The prognostic values of the insulin-like growth factor binding protein family in ovarian cancer. Biomed Res Int. 2020;2020 doi: 10.1155/2020/7658782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu J, Zhang S, Gu L, Di W. Epigenetic silencing of DKK2 and Wnt signal pathway components in human ovarian carcinoma. Carcinogenesis. 2012;33:2334–2343. doi: 10.1093/carcin/bgs278. [DOI] [PubMed] [Google Scholar]
- Zhu W, Shiojima I, Ito Y, Li Z, Ikeda H, Yoshida M, Naito AT, Nishi JI, Ueno H, Umezawa A, Minamino T, et al. IGFBP-4 is an inhibitor of canonical Wnt signalling required for cardiogenesis. Nature. 2008;454:345–349. doi: 10.1038/nature07027. [DOI] [PubMed] [Google Scholar]
- Zong X, Wang W, Ozes A, Fang F, Sandusky GE, Nephew KP. EZH2-mediated downregulation of the tumor suppressor DAB2IP maintains ovarian cancer stem cells. Cancer Res. 2020;80:4371–4385. doi: 10.1158/0008-5472.CAN-20-0458. [DOI] [PMC free article] [PubMed] [Google Scholar]