Abstract
Most of the variation in outcome following severe traumatic brain injury (TBI) remains unexplained by currently recognised prognostic factors. Neuroinflammation may account for some of this difference. We hypothesised that TBI generated variable autoantibody responses between individuals that would contribute to outcome.
We developed a custom protein microarray to detect autoantibodies to both central nervous system and systemic antigens in serum from the acute-phase (the first seven days), late (6-12 months) and long-term (6-13 years) intervals after TBI in human patients.
We identified two distinct patterns of immune response to TBI. The first was a broad response to the majority of antigens tested, predominantly IgM-mediated in the acute-phase, then IgG-dominant at late and long-term time-points. The second was responses to specific antigens, most frequently myelin-associated glycopeptide (MAG), which persisted for several months post-TBI but then subsequently resolved.
Exploratory analyses suggested that patients with a greater acute IgM response experienced worse outcomes than predicted from current known risk factors, suggesting a direct or indirect role in worsening outcome. Furthermore, late persistence of anti-MAG IgM autoantibodies correlated with raised serum neurofilament light concentrations at these time points, suggesting an association with ongoing neurodegeneration over the first year post-injury.
Our results show that autoantibody production occurs in some individuals following TBI, can persist for many years, and is associated with worse patient outcome. The complexity of responses mean that conventional approaches based on measuring responses to single antigenic targets may be misleading.
Keywords: Traumatic brain injury, autoantibodies, secondary injury, neurodegeneration, neuroinflammation
Abbreviations
- BBB
Blood-brain barrier
- CLDN5
Claudin 5
- CNS
Central nervous system
- COL5a2
Collagen type 5 alpha 2 chain
- DAMP
Danger-associated molecular pattern
- GFAP
Glial fibrillary acidic protein
- GOS
Glasgow Outcome Score
- GOSE
Glasgow Outcome Score Extended
- Ig
Immunoglobulin
- MAC
Membrane attack complex
- MAG
Myelin-associated glycopeptide
- MBP
Myelin basic protein
- MFI
Median fluorescence intensity
- NfL
Neurofilament light
- SCI
Spinal cord injury
- SELE
E-selectin
- TBI
Traumatic brain injury
- TJP-1
Tight junction protein 1
Introduction
Traumatic brain injury (TBI) is the leading cause of death and disability in young adults in the developed world (1). Despite significant advances in knowledge there are few reliable early prognostic markers, and there is marked heterogeneity in outcome between individuals with seemingly similar initial primary injuries. Indeed, the best established prognostic models in TBI (such as the CRASH and IMPACT models) explain less than 40% of outcome variance (2). Secondary injury, the process where (amongst others) metabolic and inflammatory consequences of TBI cause additional neurological injury, is likely to contribute significantly to this heterogeneity, and is potentially therapeutically modifiable (3). In addition, TBI is now increasingly believed to trigger a chronic neurodegenerative process in a subset of patients (4–13).
The mechanisms underlying early secondary injury and late neurodegeneration are not well understood, but neuroinflammation has been implicated in both processes, and represents a potential therapeutic target. Most work to date has focussed on innate immunity and microglial activation, which may persist decades after injury. The intensity of the late microglial response, in particular, appears to correlate with late functional outcome and white matter damage (14–16). However to-date, therapeutic modulation of these systems has not had any clinical impact (17, 18).
In addition to local innate immune activation, TBI disrupts both brain tissue and the blood-brain barrier (BBB), releasing brain antigens into the systemic circulation, and into the cervical lymph nodes via glymphatic and meningeal lymphatic systems (19, 20), generating both humoral and cellular adaptive autoimmune responses, which may be detrimental (21–23). The phenomenon of destructive autoimmunity triggered by central nervous system (CNS) injury is well established, with notable examples including sympathetic ophthalmia, and NMDAr encephalitis following herpes simplex encephalitis (24, 25). As far back as the 1960s, studies of small patient cohorts have described autoantibodies to individual brain antigens following TBI (21, 23, 26–29). Given the plethora of different brain antigens released after TBI, it is unlikely that measuring a single autoantibody captures the true extent of autoimmune response.
Indeed, although the cognate antigens have been poorly characterised, past studies show that autoantibodies to multiple CNS targets are likely to be produced following TBI (21).
The function and clinical relevance of these autoantibodies is uncertain. They may represent an important mechanism for clearing cellular debris (30), however there are also suggestions from experimental spinal cord injury (SCI) that they may be pathogenic: Injection of sera from mice with SCI into the hippocampi of uninjured mice induced glial activation with prominent neuron loss, whereas sera from B-cell knockout mice that had also undergone SCI had no such effect. Furthermore, B-cell competent mice with SCI developed ectopic meningeal lymphoid follicles, resembling those seen in patients with multiple sclerosis (MS), providing mechanistic insights into how traumatic adaptive immune activation could cause ongoing CNS injury, even after the BBB had been reconstituted after injury (31).
We hypothesised that the release of brain antigens into the systemic circulation and cervical lymph nodes following injury, in the context of a heightened danger-associated molecular pattern (DAMP) milieu, generates autoantibodies, against a variety of brain proteins, which may be pathogenic. To screen for such autoantibodies, we developed a CNS human protein microarray, with brain and non-brain antigens, and analysed samples from the acute phase and at two late time-points after moderate to severe TBI. We hypothesised that autoantibodies present in the acute phase would associate with worse clinical outcome at 6-12 months post-TBI, and their persistence in the longer term would associate with biomarkers of ongoing neurodegeneration.
Materials and methods
Study populations
Written consent for TBI patients was obtained from legal representatives prior to enrolment in the acute phase, and further written consent for ongoing use of data and study participation were obtained from the patients at follow up. The studies described were approved either by the Cambridgeshire local research ethics committee (REC 97/290 and 13/EE/0119), or regional ethical board in Stockholm (#2005/1526/31/2).
Healthy controls were recruited through the University of Cambridge (REC 97/290) and Cambridge Biomedical Research Centre (REC 11/33/0007), and all provided written consent. A further bank of 28 “positive control” samples from patients with autoimmune thyroid disease, type 1 diabetes, multiple sclerosis or autoimmune encephalitis provided by Sanja Ugrinovic (Department of Immunology, Addenbrookes Hospital, UK) and Patrick Waters (Nuffield Department of Clinical Neurosciences, University of Oxford, UK) which were used in the early development of the protein microarray contributed normative data.
Procedures
Sample collection and storage
Serum samples were collected at up to four different time-points: 1) “acute” – within 72 hours of injury (before an adaptive immune response should have occurred), 2) “subacute – 7 days post-injury, 3) “late” – 6-12 months post-injury, and 4) “long-term” – 6-13 years post-injury. A number of individuals donated samples at multiple time-points; a schematic diagram of the samples utilised is shown in Supplementary Fig. 1A. The samples were aliquoted, labelled with pseudoanonymised identifiers, and frozen immediately at -70°C. Samples from the validation cohort were shipped on dry ice with temperature monitoring to the University of Cambridge.
Demographic, clinical, and outcome information
Demographic information and IMPACT score variables (age, post-resuscitation GCS motor score, pupil reactivity, occurrence of hypoxia or hypotension, Marshall CT classification (32), presence of subarachnoid or epidural haemorrhage on CT, blood glucose and haemoglobin concentration (33)) were recorded by the research team at the time of admission. Injuries were characterised using the Injury Severity Scale (34). Glasgow Outcome Score Extended (35) was recorded for the discovery cohort at between 6 and 9 months post-TBI, and Glasgow Outcome Score (36) was recorded for the validation cohort at 9-13 months post-TBI, as per the respective original protocols for both of these studies.
Autoantibody screening
Autoantibody screening was undertaken using a custom protein microarray based on the HuProt™ (version 2.0) platform (37). The microarray was devised in collaboration with Cambridge Protein Arrays Ltd. (Cambridge, UK) and CDI laboratories (Puerto Rico) to detect autoantibodies to a broad selection of CNS, BBB and systemic antigens. The microarrays consisted of a glass microscope slide with a thin SuperEpoxy coating, printed with quadruplicate spots of recombinant yeast-expressed whole proteins, each fused with GST (glutathione-S-transferase). The array included 79 targets: 52 brain related, 5 blood-brain-barrier related, and 22 non-brain related (full antigen list detailed in Supplementary Table 1). Each slide accommodated up to 12 individual serum samples. Samples from the same patient at different time-points (acute, subacute and late) were assayed on the same slide. The long-term (6-13 years post-injury) cohort were run in a separate batch alongside healthy controls; the 28 age and sex-matched healthy controls used as their comparators were recruited contemporaneously.
In brief, the slides were blocked in 2% BSA/ 0.1% PBS-Tween overnight at 4°C, washed, and then incubated with 200μl of 1:1000 diluted serum at room temperature for 2 hours. The slides were washed again, incubated at room temperature for 2 hours with fluorophore-conjugated goat anti-human IgM-μ chain-Alexa488 (Invitrogen, Carslbad, CA, USA, Cat. No. A21215) and goat anti-human IgG-Fc-DyLight550 (Invitrogen Cat. No. SA5-10135) secondary antibodies, washed, and then scanned using a Tecan LS400 scanner and GenePix Pro v4 software, with the output being median fluorescence value of the quadruplicate spots for each protein. Supplementary Fig. 2A-C demonstrate the reproducibility of microarray results. Antigen-specificity of the antibody binding is shown in Supplementary Fig. 2D.
Anti-GFAP autoantibody ELISA
To assess the impact of the yeast expression system used for the protein microarray, verification of the anti-GFAP autoantibody signal seen on the protein microarray was performed using a custom ELISA with E. coli expressed protein. 96-well ELISA plates were coated with 100μl 100ng/ml recombinant E. coli expressed GFAP (Dx-SyS, Mountain View, CA, USA, Cat No. DXAG-001) and incubated overnight at 4°C. The plates were blocked with 200μl blocking buffer (Thermofisher Cat. No. 37542) for 2 hours at 37°C with constant rocking, washed three times with 200μl TBS-T, and then incubated with 100μl dilute serum (1:100 in blocking buffer) for 2 hours at room temperature. After a further three washes, the plates were incubated with 100μl dilute (1:10000) Horseradish Peroxidase (HRP) conjugated anti-human IgM (Jackson Immunoresearch Cat. No. 109-036-129) or IgG (Jackson Immunoresearch Cat. No. 109-036-098) antibody for 1 hour, washed three times, and 100μl 3,3’,5,5’-Tetramethylbenzidine (TMB) solution added (Thermofisher Cat. No. 34028). After 5 minutes, 100μl TMB stop solution (Thermofisher Cat. No. N600) was added, and the plates read with a TECAN infinite M200 Pro ELISA reader set to 450nm (correction wavelength 540 nm), taking a blank well as zero.
Immunoglobulin fraction isolation
To determine whether the microarray results represented binding of antibody or some other serum component (38), IgM and IgG were isolated from samples using Kappa and Lambda magnetic beads (PureProteome™). Serum was diluted with PBS (25 μl to a volume of 100 μl) and incubated with 300 μl bead slurry (prepared according to the manufacturer’s instruction) for 60 minutes at room temperature with continuous end-over-end rotation. The beads were separated using a magnetic rack, and the immunodepleted serum removed. The immunoglobulin fraction was eluted from the beads using three washes with 150 μl 0.1M Glycine HCl, and the resultant eluent was neutralised with 45 μl 1M Tris HCl. Estimation of IgG recovery in the eluent was estimated by NanoDrop™ to be approximately 50%, and thus the eluent was diluted to the equivalent of 1 in 500 before being run as standard on the protein microarray, so as to ensure that the immunoglobulin concentration closely approximated that of 1 in 1000 serum. The immunodepleted serum was run as standard on the protein microarray having compensated for the additional dilution.
Total immunoglobulin quantification
Total IgG and IgM were measured using the standard clinical assay at Addenbrookes Hospital, UK.
Neurofilament light and glial fibrillary acidic protein quantification
Acute GFAP concentrations in the discovery cohort were measured by Randox Laboratories Ltd (Crumlin, County Antrim, BT29 4QY, United Kingdom), using a sandwich chemiluminescent immunoassay (Evidence Investigator™ Cerebral Custom Array IV). Serum samples were transported on dry ice.
For quantification of NfL and GFAP concentrations in the validation, late and long-term cohorts, serum samples were shipped on dry ice to the Clinical Neurochemistry Laboratory, Sahlgrenska University Hospital where the analyses were performed using commercially available kits (NF-Light Advantage and GFAP discovery kit, respectively) on the Single molecule array (Simoa) platform, according to instructions from the manufacturer (Quanterix, Billerica, MA, USA)
Statistical analysis
Descriptive data are presented using median and interquartile range (for continuous variables) or number and percentage (for categorical variables). Between group differences were calculated using Mann-Whitney U tests for unpaired continuous variables and Wilcoxon Matched-Pairs Signed Rank tests for paired continuous variables; complete statistics for all comparisons are tabulated in Supplementary Table 2. The association between two continuous variables was assessed using linear regression. Categorical data were compared using chi-squared analysis. Variance of groups was compared using an F-test. Temporal profiles of paired samples were assessed using Friedman with post-hoc Dunn tests. All p-values stated are unadjusted and two-tailed. The Benjamini-Hochberg procedure, with a false discovery rate set to 10%, was used to account for multiple comparisons where appropriate, and p-values which remained <0.05% after correction are highlighted.
Outcome prediction
The risk of poor outcome was calculated for the acute cohorts using the IMPACT prognostic calculator Core+CT+Lab model (http://www.tbi-impact.org/?p=impact/calc). Where individual covariate data were not available (n = 2% of values; all relating to laboratory results), a value was imputed by using the median value of all patients in that cohort. To assess whether autoantibody responses were associated with outcome, we used a sliding dichotomy approach (39) to group patients according to whether their outcome was “worse than expected” or “better than / as expected” compared with the predictions made by the IMPACT Calculator (Supplementary Fig. 1B&C).
Protein microarray
The degree of antibody binding was quantified by the median fluorescence of the four quadruplicate spots for each antigen. Fluorescence values for each antigen were then normalised by dividing by the median value of all antigens for that patient. Both raw (non-normalised) and normalised values were then converted into Z-scores based on respective normal distributions generated from data for each antigen from 269 samples (all samples from the discovery and validation cohorts, healthy controls and “positive” controls); the top and bottom 2% of values for each antigen were excluded to remove artefactual outliers or strong positive results so that the interpretation of Z-scores approximates those of a Gaussian distribution. This broad selection of subjects included both “well” and critically ill participants to ensure it would include variation attributable to non-specific binding resulting from acute phase reactants (38). While this approach may have reduced sensitivity to detection of antibody responses, we accepted this as a price for the increased specificity that resulted. A representative distribution of Z-scores within all samples assayed is displayed in Supplementary Fig. 1D. For the initial screen, a positive autoantibody result was defined by a threshold of Z≥3. An increase of Z≥1 between paired samples, providing the second sample showed a Z >3, was used to define the development of a new autoantibody.
Analyses were performed using Graphpad Prism (version 8.1.0; Graphpad Software) and SPSS (version 25; IBM SPSS).
Results
Study Populations and measurements
Banked samples of serum collected at up to two time-points during the first week of admission (“acute”: 0-3 days post-TBI, and “subacute”: 7-10 days post-TBI) from two retrospective cohorts of patients with moderate to severe TBI, recruited from two separate centres, were studied sequentially as discovery and validation cohorts. The discovery cohort (n=25) was recruited from patients admitted to the Neurosciences Critical Care Unit, Addenbrookes Hospital, UK between January 2012 and August 2013, and the validation cohort (n=66) was recruited from patients admitted to the Neurointensive Care Unit, Karolinska University Hospital, Sweden between January 2007 and October 2012. The demographic details of both acute cohorts are summarised in Table 1. The validation cohort were older (p=0.001), more likely to have a mass lesion (p=0.001) and traumatic subarachnoid haemorrhage (p=0.001), but had lower overall trauma severity (based on the injury severity scale (ISS); p=0.0003). They had a poorer predicted outcome based on the IMPACT variables (p<0.0001), but Glasgow Outcome Score (GOS) at follow up was not different (p=0.125). The validation cohort was followed up at a median of 12 months post-injury compared with 7 months for the discovery group.
A subset of the discovery cohort (n=20) provided serum samples 6-12 months post-injury, forming a third “late” cohort. A fourth “long-term” cohort (n=34, 13 of whom had contributed to the discovery cohort, and six of whom had samples at all four time-points) provided new serum samples 6 to 13 years post-TBI (see Supplementary Fig. 1A for details of sample time-points for individual patients).
Healthy controls (n=45) were recruited through the University of Cambridge and Cambridge Biomedical Research Centre (demographic comparisons with relevant cohorts shown in Table 1B).
Acute upregulation of IgM and IgG responses
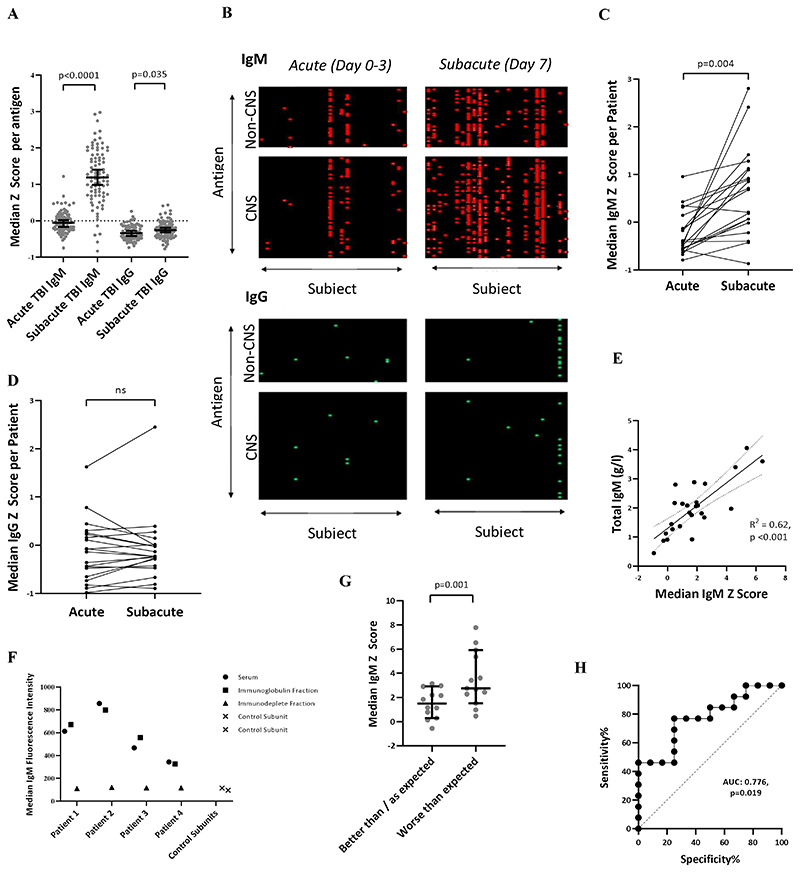
IgM antibodies to most antigens were considerably upregulated between the acute (day 0-3 post-TBI) and subacute (day 7) time-points (n=20, p<0.0001), with a smaller increase in IgG (p=0.035) (Fig. 1A&B; Supplementary Table 2). These findings from the discovery cohort were replicated in the validation cohort (p<0.001 and p=0.003, for IgM and IgG; Supplementary Table 2). At day 7, there was a greater variation in IgM response between subjects compared to acute samples (F=0.409, p=0.004; Fig. 1C). There was no similar effect in the IgG response (F=1.16, p=0.75; Fig. 1D). Total serum IgM was increased in half of patients (Supplementary Fig. 1E), and correlated with the median IgM Z-score derived from the protein microarray (R2=0.62, p<0.001) (Fig. 1E). Following immunodepletion, the median IgM signal reduced to the level of the control subunits on the microarray (i.e. subunits processed in the absence of serum) (Fig. 1F). In comparison, total serum IgG was low, with 13/25 (52%) of patients’ values below normal reference levels (Supplementary Fig. 1F) and there was no relationship with mean IgG Z-score on the protein microarray (Supplementary Fig. 1G).
Figure 1.
(A) Marked increase in polyantigenic IgM and smaller increase in polyantigenic IgG is seen between the acute and subacute time points at a group-level in the 20 patients with paired serum from the discovery cohort. Each datapoint refers to the median Z-score of an antigen across the cohort. (B) Heatmaps, where antigens Z>3 are highlighted, display the polyantigenic nature of IgM, and to a lesser degree, IgG, responses. (C&D) The median Z score of all antigens per patient captures the degree of inter-individual variation of IgM, and comparatively homogenous IgG, polyantigenic responses. (E) Total serum IgM concentration correlated with an individual’s median IgM Z-score derived from the protein microarray. (F) Following immunodepletion, the median IgM signal was reduced the level of control units processed in the absence of serum. (G) Comparison of polyantigenic IgM response between the dichotomised prognostic groups in the Discovery cohort suggests that more marked responses are associated with an outcome worse than predicted by the IMPACT variables. (H) ROC curve analysis displaying the ability of polyantigenic IgM response to differentiate between “Better than / as expected” vs. “Worse than expected” prognosis groups. Statistical tests for A: Wilcoxon Matched-Pairs Signed Rank test; C&D: F-test from one-way ANOVA E: Linear regression G: Mann-Whitney U test H: Receiver operating characteristic curve.
New autoantibodies develop in the acute phase of TBI
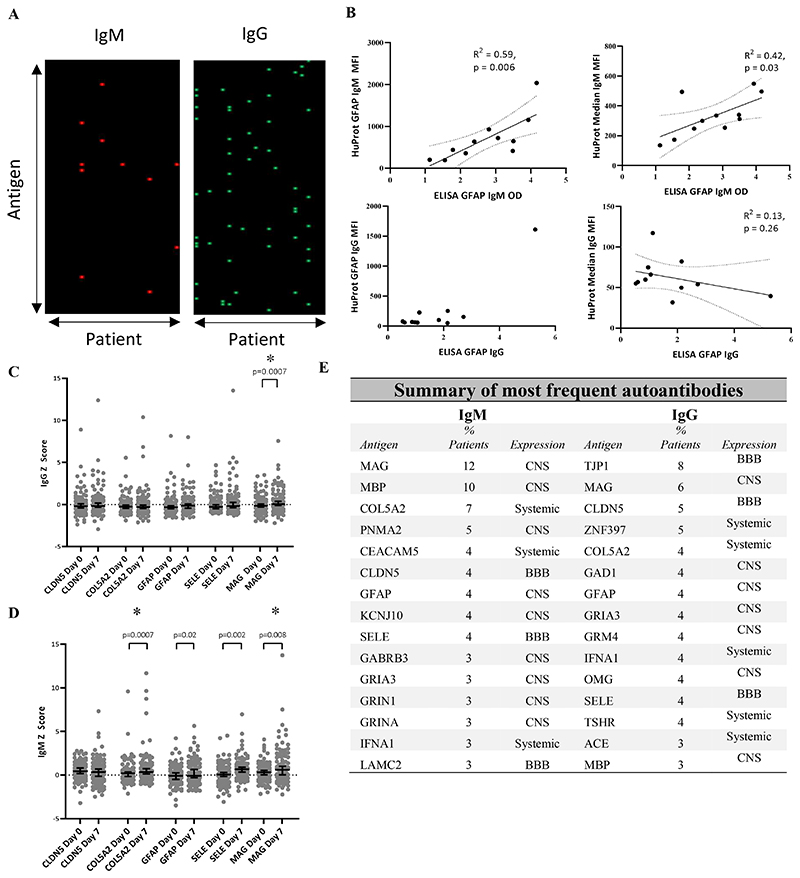
To identify antigens driving high autoantibody responses, the microarray fluorescence data were normalised (see methods) and new autoantibody responses defined as an increase of Z score ≥1 between paired samples, providing the second sample showed a Z ≥3. Five of 20 patients in the discovery cohort (25%) developed new IgM autoantibodies and 13/20 (65%) patients developed new IgG autoantibodies between the acute and subacute time-points (Fig. 2A). We confirmed these responses to be specific to immunoglobulin binding to its cognate antigen (Supplementary Fig. 2D). To exclude that these findings were related to the yeast-expression of proteins, we performed ELISA using E. coli-expressed GFAP to confirm the presence of anti-GFAP autoantibodies, to which both IgM and IgG were seen on the protein microarray. The ELISA IgM signal was largely dominated by the broad upregulation of IgM (as measured by the median IgM Z score across all antigens on the protein microarray); the strong anti-GFAP IgG signal seen on the protein microarray was replicated in the IgG ELISA (Fig. 2B).
Figure 2.
(A) Heatmaps displaying dominant IgM and IgG autoantibodies to specific antigens newly detected at 7-10 days post-injury (defined as Z>3 with a Z increase of >1 from the paired acute sample). Each row corresponds to an antigen, and each column to an individual patient. (B) Comparison between anti-GFAP IgM and IgG autoantibodies as measured by ELISA using E. coli-expressed GFAP and the corresponding data generated by the protein microarray. The IgM results from the ELISA showed concordance with the normalised anti-GFAP IgM signal from the protein microarray, but also showed a relationship with the median IgM fluorescence value across all antigens, suggesting that the IgM ELISA signal was strongly influenced by the degree of global IgM upregulation. The strong positive anti-GFAP IgG signal was seen in both assays, and the IgG ELISA did not appear to be affected by the global IgG binding tendency captured on the protein microarray. (C&D) Comparison of whole group IgM and IgG Z score change between acute and subacute samples for the five most frequently seen autoantibodies.. (E) Top 15 most frequent autoantibodies for both IgM and IgG isotypes seen following TBI at the Subacute time point across both discovery and validation cohorts. “Expression” refers to the predominant site of expression. CNS = Central nervous system; BBB = Blood brain barrier Statistical tests: (B) linear regression, (C&D) Wilcoxon Matched-Pairs Signed Rank test; * denotes comparisons which remain significant even after removal of all values where Z≥3.
The presence of particularly strong responses to individual antigens was replicated in the validation cohort, where new IgM and IgG autoantibodies were seen in 42/66 (64%) and 37/66 (56%) patients respectively.
At the subacute time-point, antigens with high IgG responses were almost ten times more likely to also have an IgM response compared to antigens with a low IgG response (5/16 [31%] vs. 25/568 [4.4%], OR 9.87, p=0.0001). In contrast, at the acute time-point, there was no such relationship (1/11) [9%] vs 20/538 [2.6%], OR=2.52, p = 0.37).
After combining the discovery and validation cohorts, autoantibodies were seen to 51 different antigens (44 IgM, 31 IgG), of which, 24/51 (47%) antigens induced both IgM and IgG responses. The most frequently seen overall targets were MAG, COL5a2, CLDN5, GFAP and SELE (Fig 2E). While CNS targets comprised most of the 15 most commonly seen autoantibodies (9/15 IgM, 7/15 IgG), autoantibodies to BBB antigens (TJP1, CLDN5 and SELE) were also common (3/15 for both IgM and IgG). There were also autoantibodies to systemic antigens (3/15 IgM, 5/15 IgG); we commonly observed responses against COL5a2, a ubiquitous collagen, autoantibodies against which are recognised in respiratory disease and implicated in rejection of lung transplants (40, 41). In the discovery group (where trauma computerised tomography series reports were available), the subacute IgG response to COL5a2 was higher in patients with lung contusions than those without (p=0.04, Supplementary Table 2).
Unexpectedly, autoantibodies appeared to be more likely to develop against extracellular or plasma membrane expressed proteins than those restricted to the intracellular compartment, both for CNS (IgM OR 2.04, p = 0.02; IgG OR 1.3 p = 0.32) and non-CNS (IgM OR 3.95, p = 0.01; IgG OR 3.87, p = 0.006), once the relative number of antigens in each compartment was accounted for.
Having identified these antigens through a stringent threshold of Z>3 autoantibody response, we compared Z-scores for the five most frequently seen autoantibodies for every patient. 4/5 IgM responses (against COL5a2, GFAP, SELE and MAG) and 1/5 IgG responses (against MAG) were increased in the subacute group compared to the acute samples (Fig. 2C&D).
To assess the relationship between acute protein release and subsequent autoantibody response, the acute serum GFAP concentration was compared with subacute GFAP autoantibody responses. A significant relationship was seen between in the discovery cohort (Spearman rho 0.58; p=0.008). However, in the validation cohort – with an alternative GFAP assay - this finding was not replicated (Spearman rho 0.19; p=0.12).
Subacute IgM autoantibody responses may be associated with worse late clinical outcome
The discovery and validation cohorts were pooled to investigate the association of autoantibody responses with clinical parameters. Younger age was associated with a higher IgM response (R2=0.19, p<0.0001), but none of the following parameters correlated with either IgM or IgG responses: Sex, Glasgow Coma Scale Motor Score, Injury Severity Score and IMPACT score.
In the 25 patients from the discovery cohort, those with an outcome worse than predicted had a higher median IgM Z-score at Day 7 than those whose outcome was as good as, or better than, expected (p=0.01) (Fig. 1G; Supplementary Table 2). The median IgM Z-score at day 7 could differentiate these two groups (AUC 0.776, p=0.019; Fig. 1H). There was no difference between groups in the number of new IgM or IgG autoantibodies (p=0.70).
In the validation cohort, however, there was no relationship between any autoantibody profile and outcome (median IgM Z p=0.15; number of normalised IgM antibodies Z>3 p=0.67; number of normalised IgG antibodies Z>3 p=0.33) (Supplementary Table 2). However, the older age of this cohort meant that the IgM response was less marked, the clinical outcome was less granular, as only GOS had been collected (Supplementary Fig. 1C), and the GOS was recorded at a median of twelve months rather than seven.
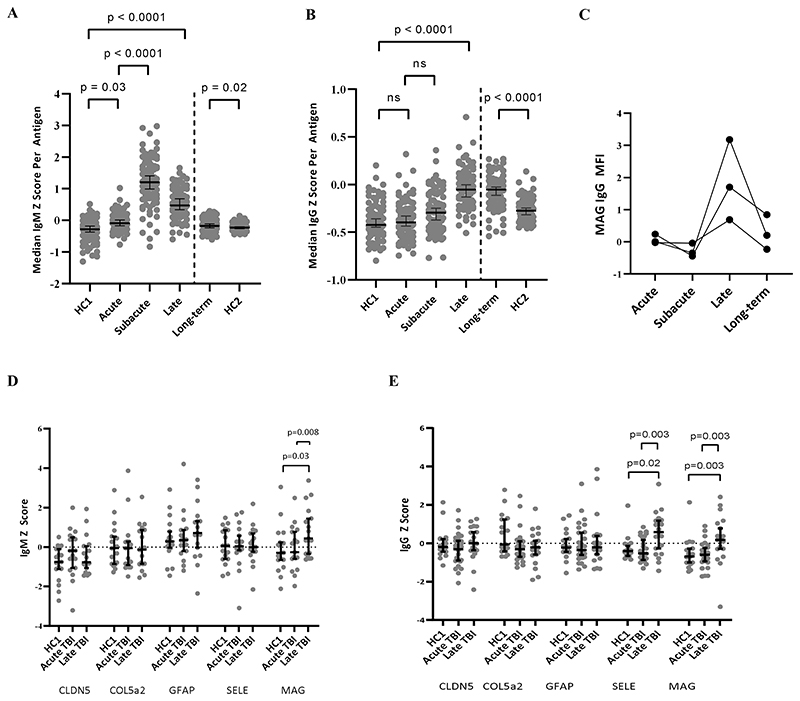
IgM and IgG autoreactivity persists for several years post-injury
A subset of 20 patients from the discovery cohort provided samples at a late time-point (6-12 months post-TBI, Supplementary Table 1B) which had significantly higher median non-normalized Z-scores for both IgM and IgG than both controls (p <0.0001 for both; Supplementary Table 2) and their acute samples (p<0.0002 for IgM and p<0.0001 for IgG, respectively; Fig. 3A&B, Supplementary Table 2). The IgM response peaked at the subacute time-point before falling towards (but not back to) baseline, whereas IgG reactivity was maximal at the late time-point (p<0.0001 for all comparisons except acute vs. subacute IgG where p=0.058).
Figure 3. Temporal profiles of autoantibody responses.
(A) Broad IgM upregulation peaking at the subacute time-point (Day 7 post-TBI), and waning over the ensuing months to years, but never returning to baseline. (B) Broad IgG upregulation peaking at the late time-point (6-12 months post-TBI) and remaining at this level long-term (6-13 years post-TBI). (C) The temporal profiles of dominant anti-MAG IgG autoantibodies from three patients with serum taken at 4 time-points at late time-points (6-9 months) showing a return towards baseline long-term (7-13 years). (D&E) Screening for the five most frequently seen autoantibodies in the acute phase reveals late persistent IgM to MAG and IgG to MAG and SELE. HC = healthy controls. HC group 1, acute, subacute and late samples were assayed contemporaneously in one experimental batch; HC group 2 and long-term samples were assayed contemporaneously as a separate experimental batch. Statistical tests for paired TBI samples: Wilcoxon Matched-Pairs Signed Rank test; for controls vs. TBI: Mann-Whitney U test.
A further long-term cohort who had sustained a moderate to severe TBI between 6 and 13 years previously (n=34, of whom 13 had contributed to the discovery cohort) were screened for autoantibodies, and showed persistent upregulation of IgM and (particularly) IgG compared to healthy controls (p=0.016 and p<0.0001 for IgM and IgG, respectively; Fig. 3A&B, Supplementary Table 2).
The five most frequently detected autoantibodies seen during the acute-phase were assessed in the late (6-12 months post-TBI) samples after normalisation. There were persistent IgG autoantibodies against MAG and SELE, but also IgM autoantibodies to MAG (Fig. 3D&E). However, these autoantibodies was not seen in the long-term samples (p>0.05 for all comparisons with controls at this time-point; Supplementary Table 2), suggesting a waning of response over years (Fig. 3C).
To assess whether late IgG autoantibodies were the result of subacute class-switching, the data from the 11 patients with samples taken at acute, subacute and late time-points were analysed for temporal relationships. Those antigens where a late IgG Z≥3 was seen were more likely to have a corresponding subacute IgM of Z≥2 (this lower threshold again used to capture more subtle responses) than those antigens where late IgG Z<3 (7/27 [26%] vs. 58/919 [6%], OR 5.2, p<0.0001). There was no such relationship seen between late IgG and acute IgM (1/27 [4%] vs. 38/919 [4%], OR= 0.89, p=0.91, suggesting that the late IgG are indeed related to subacute IgM production.
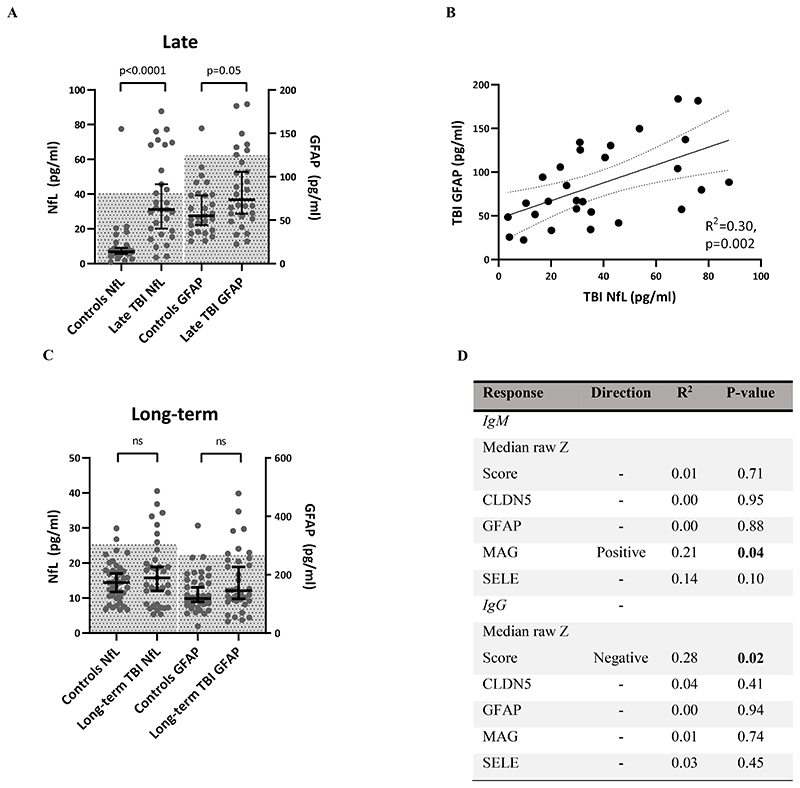
Markers of neurodegeneration are seen in a subset of patients years after TBI, and differ depending on autoantibody profile
Both NfL and GFAP concentrations were significantly higher at a group-level in the late TBI (6-12 months post-injury) cohort than healthy controls (NfL p<0.0001; GFAP p=0.05; Fig. 4A; Supplementary Table 2), and there was an association between the concentrations of the two proteins (R2=0.3, p=0.002); Fig. 4B). Although the effective half-life of NfL in the serum has not been fully delineated (but believed to be in the region of a few weeks), the effective half-life of GFAP is between 24-48 hours (42), and thus the presence of raised GFAP levels, whilst less marked, is highly suggestive of an active injurious process, rather than slow clearing of protein released at the time of injury. Whilst at the long-term (6-13 year) time-point there was no significant group difference in serum GFAP or NfL concentration between TBI patients and healthy controls (GFAP p=0.11, NfL p=0.4; Fig. 4C, Supplementary Table 2), a larger proportion of TBI patients had neural injury biomarkers above the control normal range (defined as values within 2 standard deviations of the control population mean) (GFAP 7 [20%] vs. 1 [0.25%], p=0.01; NfL 7 [20%] vs. 2 [0.5%], p=0.046), suggesting that at least a subset of patients experience ongoing neurodegeneration, in keeping with previous studies (4, 7–9, 11, 13).
Figure 4.
(A) late TBI patients have higher serum NfL and GFAP concentrations than healthy controls at a group level (hatched area = mean +/- 2 standard deviations). (B) Late serum NfL and GFAP concentrations covary. (C) There is no difference at a group level between long-term TBI patients and healthy controls, but more TBI patients lie outside the normal range (hatched area = mean +/- 2 standard deviations).. (D) Anti-MAG IgM weakly correlates with high serum NfL concentrations, whereas polyantigenic IgG antibodies correlate with low serum NfL concentrations. Statistical tests for A&C:Mann-Whitney U test; for B&D: Linear regression.
As NfL appeared to be the more sensitive of the two biomarkers to discriminate from healthy controls, the late autoantibody profiles were regressed against serum NfL concentrations. Although no association survived adjustment for multiple comparisons, two hypotheses were generated by this analysis: 1) high anti-MAG IgM reactivity associates with high NfL, and 2) median IgG Z scores associate with low NfL (Fig. 4D). High anti-MAG IgM weakly correlated with high NfL (R2=0.15, p=0.029) in the late-term cohort, but this relationship was largely driven by a single individual.
Discussion
In this study, we identified two discrete autoantibody responses following TBI.
The first is an upregulation of (initially) predominantly IgM autoantibodies against many antigens, commencing within the first week of TBI but persisting for several years. This picture is reminiscent of an alteration in the “natural autoantibody” repertoire, which are low-affinity polyreactive species produced by B1 cells, and thought to have, amongst other functions, a role in the clearance of cellular debris (43). That such a response might occur acutely in response to massive cell injury is easy to appreciate, and is in keeping with murine experiments which suggest that circulating autoantibodies following TBI bind to dying neurons (30). However, the ongoing alteration of this system years after the injury is surprising, and its implications as yet unknown. This polyantigenic IgM upregulation was so marked in some patients that it could be detected by measuring total serum IgM concentration. In young patients, a large IgM response appeared to be an independent predictor of worse outcome; whilst the intention of this response may be to clear the debris of cell death, it is conceivable that in severe trauma the subsequent immune-complex load could be detrimental. If the association with worse outcome is borne out in larger studies, measuring total serum IgM concentration could represent a useful prognostic biomarker, and perhaps a marker for stratifying patients for immunomodulatory studies. This response diminished with increasing age, in keeping with the known effect of ageing on both B-1 (polyreactive IgM) and B-2 (antigen-specific) responses (44, 45), and so may be of less importance in older patients.
The second autoantibody response comprises a disproportionate rise in antibody concentration against a small number of neural antigens (of which MAG was the most common), but also to blood-brain-barrier antigens (TJP1, CLDN5) and systemic antigens (such as COL5a2). In keeping with our results, Western blot experiments (21) have also identified GFAP as a frequent autoantibody target after TBI. The temporal profile of these responses to specific antigens largely recapitulated a typical primary adaptive immune response, with early IgM and later IgG production. The primacy of MAG as an autoantigen at late time-points is particularly interesting given that white matter tract degeneration is a key phenomenon driving late post-traumatic neurodegeneration (11, 16, 46), and the association with serum NfL concentrations in our cohort suggests that these autoantibodies may be playing a role in this process, although we cannot judge whether they are contributing to ongoing damage, or part of the repair effort.
Previous studies addressing the production of autoantibodies following TBI have largely looked for responses to a single antigen, such as S100b (23), myelin basic protein (26) and beta tubulin (28), and have found a response to the target in question. Our data, in keeping with data from Western blot experiments (21) suggests that autoantibodies are produced to a multitude of specific targets following TBI, and so measuring responses to a single antigen gives an inadequate representation of the repertoire. Furthermore, it appears that there is a marked, separate response of antibodies which bind to the majority of proteins on the microarray, reminiscent of polyreactive natural antibodies; this response dominated the IgM signal on ELISA, and therefore would compromise the validity of results generated by single-antigen assays.
There are two main limitations of this study. Firstly, the protein array platform utilises proteins expressed by yeast, and therefore may not present the target proteins in an authentic conformation. It may therefore have a lower sensitivity for certain proteins than other antibody detection techniques, and conversely may detect antibodies which would not bind to authentic human proteins. Despite this caveat, as demonstrated by (i) use of purified immunoglobulin fractionated from sera and (ii) through pre-incubation with cognate antigens, results obtained from the protein microarray were shown to be specific and reproducible; furthermore results were replicated by ELISA using protein from a different expression system, and one of the major antibody targets detected was GFAP, replicating Western Blot experiment results using human brain lysate.(21) The corresponding rise in total serum IgM concentration additionally validates the broad upregulation of IgM detected on the protein microarray, and highlights the strength of the protein microarray technology to investigate the complexity of autoantibody responses, even if it does not have the accuracy of assays designed to detect autoantibodies to a specific single antigen using authentic human protein.
The second limitation is the baseline differences between the clinical cohorts, especially the fewer young patients in the validation cohort which reduced the power to investigate the association between the upregulated IgM response and clinical outcome.
In conclusion, we have used protein microarray technology to screen for novel autoantibody production following moderate to severe TBI. This approach has elucidated two distinct patterns of response: 1) a diffuse upregulation of mainly IgM responses, which peaks at day 7, and which persist for years post-injury, and 2) a much greater autoantibody response to a few specific antigens which follow a vaccination-like temporal profile, and persist for months but return to baseline years post-injury. Our data would suggest that the polyantigenic IgM response in the acute phase may be detrimental to clinical outcome, and that persistent anti-MAG IgM autoantibodies associate with surrogate markers of late neurodegeneration.
Supplementary Material
Key points.
Traumatic brain injury triggers autoantibody production
Alterations in autoantibody repertoire persist for years post-injury
Acknowledgements
We thank Paddy Waters (Neuroimmunology Group, Nuffield Department of Clinical Neurosciences, John Radcliffe Hospital, Oxford, UK), and Sanja Ugrinovic (Department of Infectious Diseases, Cambridge University Hospitals, Cambridge, UK) for sharing samples which contributed to the development of the protein microarray and normative data. We gratefully acknowledge the participation of all NIHR Cambridge BioResource volunteers, and thank the NIHR Cambridge BioResource centre and staff for their contribution. We thank the National Institute for Health Research and NHS Blood and Transplant.
Funding
EJN, AH, AJC, PJH and DKM are supported by the Medical Research Council (UK) within the framework of ERA-NET NEURON. ERZ is supported by the Ministero della Salute (Italy) within the framework of ERA-NET NEURON. DKM is supported by an NIHR Senior Investigator Award and European Union 7th Framework program (EC grant 602150). EJN is supported by the Intensive Care Society Young Investigator Award, Addenbrookes Charitable Trust Cambridge Clinical Research Fellowship and NIHR Brain Injury Healthcare Technology Co-operative Seedcorn funding. EPT acknowledges funding from Region Stockholm (ALF), the Swedish Brain Foundation (Hjärnfonden) and the Swedish Society for Medical Research (Svenska Sállskapet for Medicinsk Forskning). HZ is a Wallenberg Scholar supported by grants from the Swedish Research Council (#2018-02532), the European Research Council (#681712), Swedish State Support for Clinical Research (#ALFGBG-720931), Centrum för Idrottsforskning (#P2019-0198), and the UK Dementia Research Institute at UCL. PJH is funded by the NIHR (Research Professorship and Cambridge BRC) and Royal College of Surgeons of England. JJ is funded by the Wellcome Trust (RG79413). VFJN is funded by an Academy of Medical Sciences / The Health Foundation Clinician Scientist Fellowship. HSM is supported by the Cambridge Trust and the Rosetrees Trust
Data availability
Data from this study is available to academic collaborators on application to the corresponding author.
References
- 1.Jennett B. Epidemiology of head injury. J Neurol Neurosurg Psychiatry. 1996;60:362–369. doi: 10.1136/jnnp.60.4.362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lingsma HF, Roozenbeek B, Steyerberg EW, Murray GD, Maas AI. Early prognosis in traumatic brain injury: from prophecies to predictions. Lancet Neurol. 2010;9:543–554. doi: 10.1016/S1474-4422(10)70065-X. [DOI] [PubMed] [Google Scholar]
- 3.Werner C, Engelhard K. Pathophysiology of traumatic brain injury. BJA Br J Anaesth. 2007;99:4–9. doi: 10.1093/bja/aem131. [DOI] [PubMed] [Google Scholar]
- 4.Whitnall L. Disability in young people and adults after head injury: 5-7 year follow up of a prospective cohort study. J Neurol Neurosurg Psychiatry. 2006;77:640–645. doi: 10.1136/jnnp.2005.078246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hammond FM, Grattan KD, Sasser H, Corrigan JD, Rosenthal M, Bushnik T, Shull W. Five years after traumatic brain injury: A study of individual outcomes and predictors of change in function. NeuroRehabilitation. 2004;19:25–35. [PubMed] [Google Scholar]
- 6.Himanen L, Portin R, Isoniemi H, Helenius H, Kurki T, Tenovuo O. Longitudinal cognitive changes in traumatic brain injury: a 30-year follow-up study. Neurology. 2006;66:187–192. doi: 10.1212/01.wnl.0000194264.60150.d3. [DOI] [PubMed] [Google Scholar]
- 7.Millis SR, Rosenthal M, Novack TA, Sherer M, Nick TG, Kreutzer JS, High WM, Jr, Ricker JH. Long-term neuropsychological outcome after traumatic brain injury. J Head Trauma Rehabil. 2001;16:343–355. doi: 10.1097/00001199-200108000-00005. [DOI] [PubMed] [Google Scholar]
- 8.Till C, Colella B, Verwegen J, Green RE. Postrecovery Cognitive Decline in Adults With Traumatic Brain Injury. Arch Phys Med Rehabil. 2008;89:S25–S34. doi: 10.1016/j.apmr.2008.07.004. [DOI] [PubMed] [Google Scholar]
- 9.Ruff RM, Young D, Gautille T, Marshall LF, Barth J, Jane JA, Kreutzer J, Marmarou A, Levin HS, Eisenberg HM, Foulkes MA. Verbal learning deficits following severe head injury: heterogeneity in recovery over 1 year. Spec Suppl. 1991;75:S50–S58. [Google Scholar]
- 10.Kumar R, Husain M, Gupta RK, Hasan KM, Haris M, Agarwal AK, Pandey Cm, Narayana PA. Serial Changes in the White Matter Diffusion Tensor Imaging Metrics in Moderate Traumatic Brain Injury and Correlation with Neuro-Cognitive Function. J Neurotrauma. 2009;26:481–495. doi: 10.1089/neu.2008.0461. [DOI] [PubMed] [Google Scholar]
- 11.Newcombe VFJ, Correia MM, Ledig C, Abate MG, Outtrim JG, Chatfield D, Geeraerts T, Manktelow AE, Garyfallidis E, Pickard JD, Sahakian BJ, et al. Dynamic Changes in White Matter Abnormalities Correlate With Late Improvement and Deterioration Following TBI: A Diffusion Tensor Imaging Study. Neurorehabil Neural Repair. 2016;30:49–62. doi: 10.1177/1545968315584004. [DOI] [PubMed] [Google Scholar]
- 12.Greenberg G, Mikulis DJ, Ng K, DeSouza D, Green RE. Use of Diffusion Tensor Imaging to Examine Subacute White Matter Injury Progression in Moderate to Severe Traumatic Brain Injury. Arch Phys Med Rehabil. 2008;89:S45–S50. doi: 10.1016/j.apmr.2008.08.211. [DOI] [PubMed] [Google Scholar]
- 13.Sander AM, Roebuck TM, Struchen MA, Sherer M, High WM., Jr Long-Term Maintenance of Gains Obtained in Postacute Rehabilitation by Persons with Traumatic Brain Injury. J Head Trauma Rehabil. 2001;16:356–373. doi: 10.1097/00001199-200108000-00006. [DOI] [PubMed] [Google Scholar]
- 14.Ramlackhansingh AF, Brooks DJ, Greenwood RJ, Bose SK, Turkheimer FE, Kinnunen KM, Gentleman S, Heckemann RA, Gunanayagam K, Gelosa G, Sharp DJ. Inflammation after trauma: Microglial activation and traumatic brain injury. Ann Neurol. 2011;70:374–383. doi: 10.1002/ana.22455. [DOI] [PubMed] [Google Scholar]
- 15.Johnson VE, Stewart JE, Begbie FD, Trojanowski JQ, Smith DH, Stewart W. Inflammation and white matter degeneration persist for years after a single traumatic brain injury. Brain. 2013;136:28–42. doi: 10.1093/brain/aws322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Scott G, Hellyer PJ, Ramlackhansingh AF, Brooks DJ, Matthews PM, Sharp DJ. Thalamic inflammation after brain trauma is associated with thalamo-cortical white matter damage. J Neuroinflammation. 2015;12 doi: 10.1186/s12974-015-0445-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Scott G, Zetterberg H, Jolly A, Cole JH, De Simoni S, Jenkins PO, Feeney C, Owen DR, Lingford-Hughes A, Howes O, Patel MC, et al. Minocycline reduces chronic microglial activation after brain trauma but increases neurodegeneration. Brain. 2018;141:459–471. doi: 10.1093/brain/awx339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Maas AI, Murray G, Henney H, Kassem N, Legrand V, Mangelus M, Muizelaar J-P, Stocchetti N, Knoller N. Efficacy and safety of dexanabinol in severe traumatic brain injury: results of a phase III randomised, placebo-controlled, clinical trial. Lancet Neurol. 2006;5:38–45. doi: 10.1016/S1474-4422(05)70253-2. [DOI] [PubMed] [Google Scholar]
- 19.Plog BA, Dashnaw ML, Hitomi E, Peng W, Liao Y, Lou N, Deane R, Nedergaard M. Biomarkers of Traumatic Injury Are Transported from Brain to Blood via the Glymphatic System. J Neurosci. 2015;35:518–526. doi: 10.1523/JNEUROSCI.3742-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Absinta M, Ha S-K, Nair G, Sati P, Luciano NJ, Palisoc M, Louveau A, Zaghloul KA, Pittaluga S, Kipnis J, Reich DS. Human and nonhuman primate meninges harbor lymphatic vessels that can be visualized noninvasively by MRI. eLife. 2017;6 doi: 10.7554/eLife.29738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang Z, Zoltewicz JS, Mondello S, Newsom KJ, Yang Z, Yang B, Kobeissy F, Guingab J, Glushakova O, Robicsek S, Heaton S, et al. Human Traumatic Brain Injury Induces Autoantibody Response against Glial Fibrillary Acidic Protein and Its Breakdown Products. PLoS ONE. 2014;9:e92698. doi: 10.1371/journal.pone.0092698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cox AL, Coles AJ, Nortje J, Bradley PG, Chatfield DA, Thompson SJ, Menon DK. An investigation of auto-reactivity after head injury. J Neuroimmunol. 2006;174:180–186. doi: 10.1016/j.jneuroim.2006.01.007. [DOI] [PubMed] [Google Scholar]
- 23.Marchi N, Bazarian JJ, Puvenna V, Janigro M, Ghosh C, Zhong J, Zhu T, Blackman E, Stewart D, Ellis J, Butler R, et al. Consequences of Repeated Blood-Brain Barrier Disruption in Football Players. PLoS ONE. 2013;8:e56805. doi: 10.1371/journal.pone.0056805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.C ET, Jr, Kilmartin D, Agarwal M, Zierhut M. Sympathetic Ophthalmia. Ocul Immunol Inflamm. 2017;25:149–151. doi: 10.1080/09273948.2017.1305727. [DOI] [PubMed] [Google Scholar]
- 25.Gelfand JM. Autoimmune encephalitis after herpes simplex encephalitis: insights into pathogenesis. Lancet Neurol. 2018;17:733–735. doi: 10.1016/S1474-4422(18)30279-5. [DOI] [PubMed] [Google Scholar]
- 26.Ngankam L, Kazantseva NV, Gerasimova MM. [Immunological markers of severity and outcome of traumatic brain injury] Zhurnal Nevrol Psikhiatrii Im SS Korsakova Minist Zdr Meditsinskoĭ Promyshlennosti Ross Fed Vserossiiskoe Obshchestvo Nevrol Vserossiiskoe Obshchestvo Psikhiatrov. 2011;111:61–65. [PubMed] [Google Scholar]
- 27.Sorokina EGZ, Semenova hB, Bazarnaya NA, Meshcheryakov SV, Reutov VP, Goryunova AV, Pinelis VG, Granstrem OK, Roshal LM. Autoantibodies to Glutamate Receptors and Products of Nitric Oxide Metabolism in Serum in Children in the Acute Phase of Craniocerebral Trauma. Neurosci Behav Physiol. 2009;39:329–334. doi: 10.1007/s11055-009-9147-1. [DOI] [PubMed] [Google Scholar]
- 28.Škoda D, Kranda K, Bojar M, Glosová L, Bäurle J, Kenney J, Romportl D, Pelichovská M, Cvachovec K. Antibody formation against β-tubulin class III in response to brain trauma. Brain Res Bull. 2006;68:213–216. doi: 10.1016/j.brainresbull.2005.05.032. [DOI] [PubMed] [Google Scholar]
- 29.Shamrei RK. [The value of determining autoantibodies in the diagnosis and expertise of closed brain injury] Voen-Meditsinskiĭ Zhurnal. 1969;4:39–43. [PubMed] [Google Scholar]
- 30.Stein TD, Fedynyshyn JP, Kalil RE. Circulating Autoantibodies Recognize and Bind Dying Neurons Following Injury to the Brain. J Neuropathol Exp Neurol. 2002;61:1100–1108. doi: 10.1093/jnen/61.12.1100. [DOI] [PubMed] [Google Scholar]
- 31.Ankeny DP, Lucin KM, Sanders VM, McGaughy VM, Popovich PG. Spinal cord injury triggers systemic autoimmunity: evidence for chronic B lymphocyte activation and lupus-like autoantibody synthesis. J Neurochem. 2006;99:1073–1087. doi: 10.1111/j.1471-4159.2006.04147.x. [DOI] [PubMed] [Google Scholar]
- 32.Marshall LF, Marshall SB, Klauber MR, Van Berkum Clark M, Eisenberg H, Jane JA, Luerssen TG, Marmarou A, Foulkes MA. The diagnosis of head injury requires a classification based on computed axial tomography. J Neurotrauma. 1992;9(Suppl 1):S287-292. [PubMed] [Google Scholar]
- 33.Steyerberg EW, Mushkudiani N, Perel P, Butcher I, Lu J, McHugh GS, Murray GD, Marmarou A, Roberts I, Habbema JDF, Maas AIR. Predicting Outcome after Traumatic Brain Injury: Development and International Validation of Prognostic Scores Based on Admission Characteristics. PLoS Med. 2008;5 doi: 10.1371/journal.pmed.0050165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Baker SP, O’Neill B, Haddon W, Long WB. The injury severity score: a method for describing patients with multiple injuries and evaluating emergency care. J Trauma. 1974;14:187–196. [PubMed] [Google Scholar]
- 35.Jennett B, Bond M. ASSESSMENT OF OUTCOME AFTER SEVERE BRAIN DAMAGE: A Practical Scale. The Lancet. 1975;305:480–484. doi: 10.1016/s0140-6736(75)92830-5. [DOI] [PubMed] [Google Scholar]
- 36.Teasdale GM, Pettigrew LEl, Wilson JtL, Murray G, Jennett B. Analyzing Outcome of Treatment of Severe Head Injury: A Review and Update on Advancing the Use of the Glasgow Outcome Scale. J Neurotrauma. 1998;15:587–597. doi: 10.1089/neu.1998.15.587. [DOI] [PubMed] [Google Scholar]
- 37.Jeong JS, Jiang L, Albino E, Marrero J, Rho HS, Hu J, Hu S, Vera C, Bayron-Poueymiroy D, Rivera-Pacheco ZA, Ramos L, et al. Rapid Identification of Monospecific Monoclonal Antibodies Using a Human Proteome Microarray. Mol Cell Proteomics MCP. 2012;11 doi: 10.1074/mcp.O111.016253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Guven E, Duus K, Lydolph MC, Jørgensen CS, Laursen I, Houen G. Non-specific binding in solid phase immunoassays for autoantibodies correlates with inflammation markers. J Immunol Methods. 2014;403:26–36. doi: 10.1016/j.jim.2013.11.014. [DOI] [PubMed] [Google Scholar]
- 39.Murray GD, Barer D, Choi S, Fernandes H, Gregson B, Lees KR, Maas AIR, Marmarou A, Mendelow AD, Steyerberg EW, Taylor GS, et al. Design and Analysis of Phase III Trials with Ordered Outcome Scales: The Concept of the Sliding Dichotomy. J Neurotrauma. 2005;22:511–517. doi: 10.1089/neu.2005.22.511. [DOI] [PubMed] [Google Scholar]
- 40.Iwata T, Philipovskiy A, Fisher AJ, Presson RG, Chiyo M, Lee J, Mickler E, Smith GN, Petrache I, Brand DB, Burlingham WJ, et al. Anti-Type V Collagen Humoral Immunity in Lung Transplant Primary Graft Dysfunction. J Immunol Baltim Md 1950. 2008;181:5738–5747. doi: 10.4049/jimmunol.181.8.5738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tiriveedhi V, Gautam B, Sarma NJ, Askar M, Budev M, Aloush A, Hachem R, Trulock E, Myers B, Patterson AG, Mohanakumar T. Pre-transplant antibodies to Ka1 tubulin and Collagen-V in lung transplantation: Clinical Correlations. J Heart Lung Transplant Off Publ Int Soc Heart Transplant. 2013;32:807–814. doi: 10.1016/j.healun.2013.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Thelin EP, Zeiler FA, Ercole A, Mondello S, Büki A, Bellander B-M, Helmy A, Menon DK, Nelson DW. Serial Sampling of Serum Protein Biomarkers for Monitoring Human Traumatic Brain Injury Dynamics: A Systematic Review. Front Neurol. 2017;8 doi: 10.3389/fneur.2017.00300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Palma J, Tokarz-Deptula B, Deptuła J, Deptuła W. Natural antibodies – facts known and unknown. Cent-Eur J Immunol. 2018;43:466–475. doi: 10.5114/ceji.2018.81354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rodriguez-Zhurbenko N, Quach TD, Hopkins TJ, Rothstein TL, Hernandez AM. Human B-1 Cells and B-1 Cell Antibodies Change With Advancing Age. Front Immunol. 2019;10 doi: 10.3389/fimmu.2019.00483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sasaki S, Sullivan M, Narvaez CF, Holmes TH, Furman D, Zheng N-Y, Nishtala M, Wrammert J, Smith K, James JA, Dekker CL, et al. Limited efficacy of inactivated influenza vaccine in elderly individuals is associated with decreased production of vaccine-specific antibodies. J Clin Invest. 2011;121:3109–3119. doi: 10.1172/JCI57834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sullivan GM, Mierzwa AJ, Kijpaisalratana N, *Haiying Tang, Wang Y, Song S-K, Selwyn R, Armstrong RC. Oligodendrocyte Lineage and Subventricular Zone Response to Traumatic Axonal Injury in the Corpus Callosum. J Neuropathol Exp Neurol. 2013;72:1106–1125. doi: 10.1097/NEN.0000000000000009. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data from this study is available to academic collaborators on application to the corresponding author.