Abstract
Our objective was to investigate associations of body size (birth weight and body mass index) and growth in height, body fat (adiposity) and lean mass during childhood and adolescence, with risk markers for diabetes in young South Asian adults.
We studied 357 men and women aged 21 years from the Pune Children’s Study birth cohort. Exposures were 1) birth weight, 21-year BMI, both of these mutually adjusted, and their interaction, and 2) uncorrelated conditional measures of growth in height and proxies for gain in adiposity and lean mass from birth-8 years (childhood) and 8-21 years (adolescence) constructed from birth weight, and weight, height, and skinfolds at 8 and 21 years. Outcomes were plasma glucose and insulin concentrations during an oral glucose tolerance test and derived indices of insulin resistance and secretion.
Higher 21-year BMI was associated with higher glucose and insulin concentrations and insulin resistance, and lower disposition index. After adjusting for 21-year BMI, higher birth weight was associated with lower 120-minute glucose and insulin resistance, and higher disposition index. In the growth analysis, greater adiposity gain during childhood and adolescence was associated with higher glucose, insulin and insulin resistance, and lower disposition index, with stronger effects from adolescent gain. Greater childhood lean gain and adolescent height gain were associated with lower 120-minute glucose and insulin.
Consistent with other studies, lower birth weight and higher childhood weight gain increases diabetes risk. Disaggregation of weight gain showed that greater child/adolescent adiposity gain, and lower lean and height gain may increase risk.
Keywords: birth weight, child/adolescent body composition, glucose-insulin metabolism, diabetes risk, India
Introduction
Low- and middle-income countries (LMICs) are experiencing a rapidly escalating epidemic of non-communicable diseases, especially type 2 diabetes and cardiovascular disease (CVD)1-3. India is predicted to have over 100 million people with diabetes by 20304. This is generally ascribed to genetic predisposition accompanied by recent rapid socioeconomic transition leading to physical inactivity and obesity. However, there is evidence to suggest that susceptibility to diabetes is influenced by factors earlier in life, including intra-uterine undernutrition (leading to low birth weight) and rapid childhood weight gain5,6. A number of studies have shown, in children and adults, that lower birth weight in combination with higher current weight or body mass index (BMI) is associated with higher insulin resistance and a greater risk of type 2 diabetes5,7-12.
The Pune Children’s Study was established in 1991 to prospectively study the relationship of birth weight and childhood growth to diabetes and CVD risk. At 8 years of age, lower birth weight and higher current weight were associated with higher central adiposity, lipid concentrations, blood pressure and insulin resistance7. We now report our findings at 21years of age. We investigated the associations of birthweight, 21-year body mass index, and growth in height, and proxies for adiposity and lean gain from birth to 8 years and 8 to 21 years with 21-year risk markers for diabetes. We hypothesised that lower birth weight, greater adiposity gain in childhood and/or adolescence, and less height and lean mass gain in childhood and/or adolescence would be associated with higher diabetes risk factors at 21 years.
Participants and Methods
The Pune Children’s Study has been described previously7. Children born at full term (>37 weeks’ gestation) in the KEM Hospital, Pune between 1987 and 1989 were studied (n=477). The children were selected to obtain, as near as possible, equal numbers in eight birth weight bands representing 250g increments7. At 8 years, we recorded anthropometry (weight, height, triceps, biceps, subscapular and suprailiac skinfolds). An oral glucose tolerance test (OGTT; WHO protocol, using a dose of 1.75 g/kg body weight) was carried out and glucose, insulin and lipids were measured. In 2009 we were able to trace 383 of these children (now aged 21 years): 6 had died (1kidney failure, 2 severe infective illness, 2 accidents, 1 unknown) and 17 declined to participate. Of the 360 who were studied, 3 did not have relevant data at 8 years of age and we therefore analysed 357 who attended both the 8 and 21 years follow-up. Ethics permission for the study was obtained from the KEM Hospital Ethics Committee and informed written consent was obtained from all participants.
The participants were admitted to the Diabetes Unit, KEM Hospital, the evening before the investigations and fasted overnight after a standard dinner. Weight was measured to the nearest 5 g using an electronic weighing scale (Conweigh Electronic Instruments, Mumbai). Standing height was measured to the nearest 0.1 cm using a wall-mounted stadiometer. Waist and hip circumferences were measured to the nearest 0.1 cm using a non-stretchable measuring tape. Skinfolds were measured to the nearest millimeter using Harpenden skinfold calipers. Participants’ total and regional body fat and lean mass were assessed using dual energy X-ray absorptiometry (DXA; Lunar Prodigy, GE, Madison, WI, USA). At 8 and 21 years, anthropometry was measured by a small number of research staff, who were trained to use standardised methods according to written protocols. Regular follow-up training, and inter-observer and intra-observer variation studies were carried out regularly.
The following morning, fasting venous blood samples were taken for measurement of plasma glucose and insulin. An oral glucose tolerance test was carried out, giving 75 g anhydrous glucose in water, followed by further blood samples for measurement of plasma glucose and insulin at 30 and 120 minutes13.
The Standard of Living Index (SLI)14 was used to assess the family’s socio-economic status; higher scores indicate higher social class. This is a standardised questionnaire-based index and is based on information about housing, amenities and possessions.
Laboratory analyses
Plasma glucose was measured using standard enzymatic methods (Hitachi 902, Roche Diagnostics, Mannheim, Germany). Between-batch coefficients of variation were <3% in the normal range. Plasma insulin was measured using a Delfia technique (Victor 2, Wallac, Turku, Finland); between-batch coefficients of variation for insulin measurements were <6%. HOMA-IR (index of insulin resistance) and HOMA-β (index of β-cell function) were calculated using the online Oxford HOMA calculator15. Insulin secretion was measured as Insulinogenic Index (increment in the plasma insulin divided by that in the plasma glucose at 30 min)16. The Matsuda index of insulin sensitivity was computed by the formula k/sqrt(fasting glucose*120 min glucose*fasting insulin*120 min insulin) where k (constant)=10,00017. Disposition Index was calculated as insulinogenic index * Matsuda index. This represents β-cell function for the prevailing insulin resistance, as originally described by Bergman18.
Definitions
Body mass index (BMI) was used to define underweight (<18.5 kg/m2), overweight (≥25.0 and <30.0 kg/m2) and obesity (≥30.0 kg/m2)19,20. Body fat percent was used to define adiposity (≥25% for males and ≥35% for females). Hyperglycemia was classified according to the American Diabetes Association (75 g OGTT) criteria: (impaired fasting glucose {IFG} as fasting plasma glucose 5.6-6.9 mmol/L and 120 min <7.8 mmol/L; impaired glucose tolerance {IGT} as fasting plasma glucose <5.6 mmol/L and 120 min 7.8-11.0 mmol/L and diabetes mellitus as fasting plasma glucose ≥7.0 mmol/L or 120 min ≥11.1 mmol/L)21. We defined prediabetes as IFG or IGT.
Statistical methods
Associations of birth weight and 21-year BMI with diabetes risk markers were initially examined by multiple linear regression analysis, adjusting for age and sex, according to the Fewtrell-Lucas-Cole 4-model approach (using birth weight alone, current BMI alone, both of these combined, and the interaction between the two as predictors)22. Outcomes were body composition (waist and hip circumferences, waist/hip ratio (WHR), and fat and lean mass, and fat percentage by DXA), plasma glucose and insulin concentrations, and the indices derived from these.
To examine associations between outcomes at 21years and growth (changes in size) during childhood and adolescence, we generated conditional growth variables at 8 and 21 years. Our aim was to understand the relationship of growth from birth to 8 years (‘childhood’) independent of size at birth, and growth from 8 to 21years (‘adolescence’) independent of size at birth and at 8 years. Within an individual, different body measurements at a given age, and the same body measurements at different ages, are strongly correlated, and special methods are required to study the relationship of the growth of specific components during specific periods with outcomes. Conditional variables adjust for concurrent and prior body measurements, in order to create variables that represent change in particular measurements during particular time periods, independent of other measurements and other time periods23,24. We constructed 3-compartment conditional variables23, using height as a marker for linear skeletal size, sum of skinfolds (independent of height) as a proxy for adiposity, and body weight (independent of height and skinfolds) as a proxy for lean mass, and used these to predict risk markers for diabetes assessed at 21 years.
The sequence of constructing the conditional variables is shown below. Birth weight is considered on its own, as the only available measure of size at birth and is a composite measure for all components. We then regressed height at 8 years on birth weight and expressed the residual as a z-score (‘height gain 0-8y’). We then regressed sum of skinfolds at 8 years on birth weight and height at 8 years and expressed the residual as a z score (‘adiposity gain 0-8y’). We regressed weight at 8 years on birth weight, height at 8 years and sum of skinfolds at 8 years and expressed the residual as a z score (‘lean gain 0-8y’). We then repeated this sequence at 21 years as shown below. These conditional growth variables can be interpreted as height gain, adiposity gain and lean mass gain from birth to 8 years, and from 8 to 21 years, above or below that expected, in this population, given the earlier size measurements. A positive value indicates greater than expected growth, and a negative value indicates lower than expected growth. By construction, the seven conditional variables are uncorrelated with each other, and can be included together in regression models.
| BW | Birth weight |
| Ht8 | BW | Conditional height growth from birth to 8 years, independent of birth weight (‘height gain 0-8y’) |
| SF8 | BW Ht8 | Conditional skinfold gain from birth to 8 years, independent of height gain (‘adiposity gain 0-8y’) |
| Wt8 | BW Ht8 SF8 | Conditional weight gain from birth to 8 years, independent of height and adiposity gain (‘lean mass gain 0-8y’) |
| Ht21 | BW Ht8 SF8 Wt8 | Conditional height growth from 8 to 21 years, independent of BW and all 8 year measurements (‘height gain 8-21y’) |
| SF21 | BW Ht8 SF8 Wt8 Ht21 | Conditional skinfold gain from 8 to 21 years, independent of birth weight, all 8 year measurements and 21-year height gain (‘adiposity gain 8-21y’) |
| Wt21 | BW Ht8 SF8 Wt8 Ht21 SF21 | Conditional weight gain from 8 to 21 years, independent of birth weight, all 8 year measurements and 21-year height and adiposity gain (‘lean mass gain 8-21y’) |
We present sex adjusted results as there was no effect modification by gender. Given the limitations of social class categories as defined by SLI, we used SLI as a continuous variable in our analyses. All exposure and outcome variables were expressed as age and sex standardised z-scores. To account for testing of multiple outcomes, we set the significance level at p<0.01 and present regression coefficients with 99% confidence intervals.
To test the representativeness (selection bias) of our study sample, we compared body size and glucose-insulin variables between participants and non-participants using regression imputation. We did not impute data to analyse outcomes. We developed an imputation model using the variables significantly associated with each other at 8 years in a multiple regression model. We applied this regression imputation for participants to calculate 21-year values for variables which were significantly different between participants and non-participants at 8 years of age. We then compared the observed values of the participants with the imputed values of the non-participants.
Statistical analysis was performed using SPSS 16 (SPSS Inc., Chicago, USA).
Results
General characteristics (Table 1)
Table 1. Characteristics of the participants at 8 and 21years.
| Men | Women | ||
|---|---|---|---|
| N=191 | N=166 | ||
| Birth weight kg | 2.82 (0.4) | 2.72 (0.3) | 0.03 |
| 8 years | |||
| Age years | 8.4 (0.1) | 8.4 (0.1) | 0.83 |
| Height cm | 124.7 (10.6) | 124.3 (6.1) | 0.75 |
| Weight kg | 21.6 (3.6) | 21.1 (3.9) | 0.20 |
| BMI kg/m2 | 13.7 (1.5) | 13.5 (1.7) | 0.41 |
| Sum of skinfolds mm | 21.3 (18.5, 25.4) | 28.1 (23.5, 33.9) | <0.001 |
| 21 years | |||
| Age years | 21.4 (0.4) | 21.4 (0.4) | 0.93 |
| Height cm | 172.0 (6.6) | 156.9 (6.4) | <0.001 |
| Weight kg | 65.3 (13.1) | 52.3 (10.6) | <0.001 |
| BMI kg/m2 | 22.0 (4.0) | 21.2 (4.1) | 0.08 |
| Sum of skinfolds mm | 61.0 (37.3, 89.2) | 71.4 (47.8, 96.2) | 0.002 |
| Waist circumference cm | 81.17 (10.92) | 73.88 (9.51) | <0.001 |
| Hip circumference cm | 93.61 (8.71) | 93.25 (8.32) | 0.69 |
| Waist-hip ratio | 0.8 (0.05) | 0.7 (0.05) | <0.001 |
| Lean mass kg (DXA) | 46.46 (5.83) | 30.21 (3.79) | <0.001 |
| Fat mass kg (DXA) | 15.66 (9.12) | 19.13 (8.03) | <0.001 |
| % body fat (DXA) | 22.36 (9.64) | 35.18 (8.44) | <0.001 |
| Glucose-insulin measures and indices | |||
| Fasting glucose mmol/L | 5.1 (0.6) | 5.0 (0.4) | 0.01 |
| 30 min glucose mmol/L | 8.3 (1.3) | 7.7 (1.4) | <0.001 |
| 120 min glucose mmol/L | 5.5 (4.8, 6.5) | 5.7 (4.8, 6.5) | 0.48 |
| Fasting insulin pmol/L † | 41.4 (26.1, 64.2) | 48.0 (31.8, 68.4) | 0.06 |
| 30 min insulin pmol/L † | 495.6 (362.5, 705.6) | 505.0 (327.4, 727.9) | 0.94 |
| 120 min insulin pmol/L † | 297.0 (172.6, 488.2) | 302.0 (199.5, 491.4) | 0.34 |
| HOMA-IR† | 0.9 (0.6, 1.4) | 1.0 (0.7, 1.5) | 0.08 |
| HOMA-β† | 85.1 (60.2, 106.1) | 94.5 (72.4, 122.5) | 0.004 |
| Matsuda Index† | 6.0 (3.8, 8.9) | 5.2 (3.5, 8.0) | 0.18 |
| Insulinogenic Index† | 52.2 (37.8, 78.2) | 58.1 (39.1, 83.1) | 0.18 |
| Disposition Index† | 330.41 (191.55, 512.50) | 290.96 (193.02, 520.39) | 0.63 |
Values are mean (SD), or † median (25th-75th centiles) for skewed variables. P values (last column) tested using ANOVA or Mann-Whitney test as appropriate.
Of 477 children who were studied at 8 years of age, 357 (75%) participated in the 21-year study (191 males). Non-participants had higher 8-year BMI (14.0 v 13.6 kg/m2; p=0.05), but similar 8-year height, and glucose and insulin concentrations compared to participants. Participants’ 21-year BMI was similar to the imputed values for non-participants (21.6 vs 21.9 kg/m2; p>0.05). At 21 years, 188 (52.6%) were of normal weight, 94 (26.4%) were underweight, 66 (18.5%) were overweight, and 9 (2.5%) were obese; 169 (48.3%) were adipose (using body fat percentage assessed by DXA). Three were already known to have diabetes (all on insulin treatment); an additional 5 were diagnosed with diabetes in this study, and 61 with prediabetes (40 IFG and 21 IGT). Five percent of participants regularly smoked, 4% chewed tobacco in some form, while less than 1% regularly consumed alcohol. The mean total SLI score was 41 (SD 7); about 97% were classified as belonging to higher social class according to the National Family Health Survey (NFHS) classification14. There were no differences in characteristics between boys and girls at 8 years for anthropometric and biochemical measurements. At 21 years, men were taller, heavier, and had higher glucose concentrations but lower percentage body fat than women.
Fewtrell-Lucas-Cole analysis (Table 2)
Table 2. Associations of birthweight and 21 year BMI with diabetes risk markers (Fewtrell-Lucas-Cole model).
| Adult outcomes | Model 1 Birth weight | Model 2 21year BMI | Model 4 Interaction model Birth weight (BW), 21year BMI and the interaction (BW X 21year BMI) – data shown only for the interaction term | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 21 year BMI | ||||||||||
| β (99% CI) | P | β (99% CI) | P | β (99% CI) | P | β (99% CI) | P | β (99% CI) | P | |
| Waist circumference | 0.15 (0.01, 0.29) | 0.01 | 0.92 (0.88, 0.97) | <0.001 | 0.05 (0.01, 0.09) | 0.02 | 0.92 (0.88, 0.99) | <0.001 | -0.006 (-0.06, 0.04) | 0.86 |
| Hip circumference | 0.22 (0.08, 0.35) | <0.001 | 0.92 (0.85, 0.97) | <0.001 | 0.12 (0.08, 0.17) | <0.001 | 0.90 (0.86, 0.94) | <0.001 | 0.01 (-0.04, 0.06) | 0.17 |
| Waist-hip ratio | -0.01 (-0.12, 0.11) | 0.91 | 0.48 (0.38, 0.57) | <0.001 | -0.06 (-0.15, 0.03) | 0.15 | 0.49 (0.39, 0.58) | <0.001 | -0.03 (-0.12, 0.06) | 0.55 |
| Fat mass(DXA) | 0.13 (-0.01, 0.27) | 0.03 | 0.94 (0.88, 0.98) | <0.001 | 0.03 (-0.02, 0.08) | 0.09 | 0.93 (0.88, 0.98) | <0.001 | 0.02 (-0.03, 0.07) | 0.07 |
| Lean Mass(DXA) | 0.35 (0.22, 0.48) | <0.001 | 0.54 (0.42, 0.65) | <0.001 | 0.30 (0.18, 0.41) | <0.001 | 0.51 (0.40, 0.62) | <0.001 | 0.006 (-0.10, 0.11) | 0.073 |
| % body fat(DXA) | 0.04 (-0.07, 0.15) | 0.41 | 0.71 (0.65, 0.77) | <0.001 | -0.03 (-0.09, 0.02) | 0.21 | 0.71 (0.65, 0.77) | <0.001 | -0.02 (-0.07, 0.04) | 0.28 |
| Fasting glucose | -0.02 (-0.12, 0.08) | 0.53 | 0.15 (0.05, 0.25) | 0.002 | -0.04 (-0.13, 0.06) | 0.35 | 0.15 (0.05, 0.25) | 0.002 | -0.05 (-0.14, 0.04) | 0.97 |
| 30 min glucose | -0.08 (-0.22, 0.05) | 0.14 | 0.14 (0.00, 0.27) | 0.01 | -0.10 (-0.23, 0.04) | 0.08 | 0.15 (0.01, 0.28) | 0.006 | -0.03 (-0.16, 0.10) | 0.74 |
| 120 min glucose | -0.12 (-0.26, 0.01) | 0.02 | 0.31 (0.17, 0.44) | <0.001 | -0.16 (-0.29, -0.02) | 0.002 | 0.33 (0.19, 0.45) | <0.001 | -0.02 (-0.15, 0.10) | 0.51 |
| Fasting insulin | -0.02 (-0.16, 0.11) | 0.43 | 0.37 (0.24, 0.50) | <0.001 | -0.06 (-0.19, 0.06) | 0.18 | 0.38 (0.24, 0.50) | <0.001 | 0.07 (-0.05, 0.20) | 0.96 |
| 30 min insulin | -0.08 (-0.22, 0.05) | 0.41 | 0.20 (0.06, 0.33) | 0.01 | -0.11 (-0.24, 0.03) | 0.28 | 0.21 (0.07, 0.34) | 0.008 | 0.04 (-0.09, 0.18) | 0.32 |
| 120 min insulin | -0.18 (-0.31, -0.04) | 0.03 | 0.30 (0.16, 0.42) | <0.001 | -0.21 (-0.34, -0.08) | 0.006 | 0.32 (0.18, 0.44) | <0.001 | 0.07 (-0.06, 0.19) | 0.88 |
| HOMA-IR | -0.02 (-0.15, 0.12) | 0.54 | 0.37 (0.23, 0.49) | <0.001 | -0.06 (-0.18, 0.07) | 0.20 | 0.37 (0.24, 0.50) | <0.001 | 0.06 (-0.03, 0.16) | 0.27 |
| HOMA-β | -0.01 (-0.15, 0.12) | 0.54 | 0.32 (0.19, 0.45) | <0.001 | -0.05 (-0.18, 0.08) | 0.20 | 0.33 (0.19, 0.46) | <0.001 | 0.10 (-0.02, 0.23) | 0.27 |
| Matsuda index | 0.13 (-0.01, 0.27) | 0.03 | -0.40 (-0.53, -0.28) | <0.001 | 0.18 (0.05, 0.30) | <0.001 | -0.42 (-0.55, -0.30) | <0.001 | -0.06 (-0.18, 0.06) | 0.04 |
| Insulinogenic index | -0.05 (-0.18, 0.09) | 0.47 | 0.08 (-0.05, 0.22) | 0.15 | -0.06 (-.019, 0.08) | 0.38 | 0.09 (-0.05, 0.22) | 0.13 | 0.05 (-0.09, 0.18) | 0.36 |
| Disposition index | 0.10 (-0.03, 0.24) | 0.05 | -0.23 (-0.36, -0.09) | <0.001 | 0.13 (0.00, 0.27) | 0.01 | -0.24 (-0.37, -0.10) | <0.001 | -0.05 (-0.19, 0.07) | 0.37 |
| IFG | 1.12 (0.80, 1.57) | 0.41 | 1.64 (1.18, 2.28) | 0.01 | 1.05 (0.74, 1.49) | 0.56 | 1.63 (1.17, 2.27) | 0.01 | 0.88 (0.61, 1.26) | 0.88 |
| IGT | 0.96 (0.61, 1.51) | 0.81 | 1.85 (1.18, 2.92) | 0.02 | 0.91 (0.57, 1.43) | 0.67 | 1.87 (1.18, 2.95) | 0.09 | 0.97 (0.61, 1.53) | 0.92 |
| IFG+IGT+DM | 1.01 (0.77, 1.33) | 0.95 | 1.79 (1.36, 2.35) | <0.001 | 0.95 (0.72, 1.27) | 0.74 | 1.80 (1.37, 2.36) | <0.001 | 0.84 (0.63, 1.13) | 0.88 |
All exposure and outcome variables are Z standardized and adjusted for gender and current age; Values are β regression coefficients (99% CI)
Linear regression used for continuous outcomes; Logistic regression used for dichotomous outcomes and represent odds ratios
IFG: Impaired Fasting Glucose; IGT: Impaired Glucose Tolerance; DM: Diabetes Mellitus; IFG, IGT and DM compared against NGT (Normal Glucose Tolerance)
Body size and composition
In the analysis using birth weight alone as a predictor, birth weight was directly related to 21-year waist and hip circumferences, and lean mass. Birth weight was unrelated to WHR or percent body fat. Using current BMI alone, BMI was positively related to waist and hip circumferences, WHR, fat mass, lean mass and percentage body fat. The effect size was greater for fat mass than for lean mass. In the combined model using both birthweight and 21-year BMI as predictors, the positive associations of birthweight with 21-year waist and hip circumferences and lean mass remained significant. Analyses with and without SLI showed similar results.
Glucose, insulin and indices (Table 2)
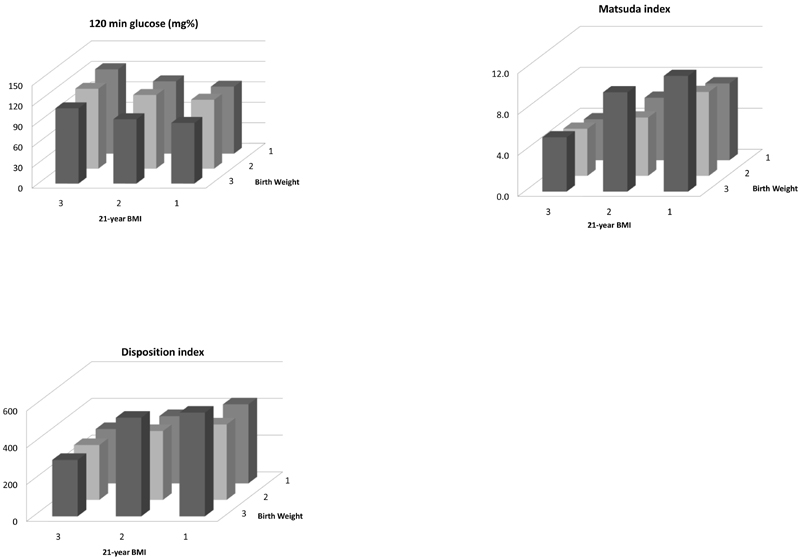
In the analysis using birth weight alone as a predictor, birth weight was inversely related to 120-min insulin; there were no associations with IFG, IGT or diabetes mellitus. Current BMI was strongly positively related to glucose and insulin concentrations at all time points during OGTT, and to IFG, IGT and diabetes mellitus. BMI was also positively related to HOMA-β and HOMA-IR, and inversely to Matsuda and Disposition Indices. In the combined model using both birthweight and 21-year BMI as predictors, the negative association of birth weight with 120-min insulin remained significant. In addition, a negative association of birth weight with 120-min glucose, a positive association with Matsuda Index and a positive association of borderline significance with Disposition Index (p=0.014) were now apparent. The associations of BMI with glucose-insulin variables including IFG, IGT and diabetes mellitus remained of similar strength in the combined model. There were no interactions between birth weight and 21 year BMI for any of the outcomes. The highest 120-min glucose concentrations, lowest Matsuda Index and lowest Disposition Index were in participants who were in the lowest tertile of birth weight and highest tertile of 21-year BMI (Figure 1).
Figure 1. 120 min glucose concentrations, Matsuda index and Disposition index according to tertiles of birth weight and 21-year BMI; statistics shown in Table 2.
Conditional growth analysis (Table 3a and 3b)
Table 3a. Associations of conditional growth variables (height gain, adiposity gain (gain in sum of skinfolds independent of height) and lean gain (gain in weight independent of height and sum of skinfolds) with diabetes risk factors (body size and composition) at 21 years.
| Variables | Birth weight | Height gain 0-8y | Adiposity gain 0-8y | Lean gain 0-8y | Height gain 8-21y | Adiposity gain 8-21y | Lean gain 8-21y | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| β (99% CI) | P | β (99% CI) | P | β (99% CI) | P | β (99% CI) | P | β (99% CI) | P | β (99% CI) | P | β (99% CI) | P | |
| BMI | 0.09 (0.08, 0.11) | <0.001 | 0.14 (0.13, 0.15) | <0.001 | 0.35 (0.33, 0.36) | <0.001 | 0.38 (0.36, 0.41) | <0.001 | -0.04 (-0.06, -0.01) | <0.001 | 0.73 (0.72, 0.75) | <0.001 | 0.39 (0.38, 0.40) | <0.001 |
| Waist circumference | 0.13 (0.09, 0.17) | <0.001 | 0.23 (0.19, 0.28) | <0.001 | 0.27 (0.22, 0.32) | <0.001 | 0.36 (0.29, 0.42) | <0.001 | 0.16 (0.09, 0.23) | <0.001 | 0.76 (0.72, 0.80) | 0.03 | 0.29 (0.26, 0.44) | <0.001 |
| Hip circumference | 0.19 (0.16, 0.23) | <0.001 | 0.31 (0.27, 0.34) | <0.001 | 0.16 (0.11, 0.20) | <0.001 | 0.54 (0.48, 0.60) | <0.001 | 0.29 (0.22, 0.36) | <0.001 | 0.69 (0.65, 0.73) | 0.05 | 0.35 (0.31, 0.39) | <0.001 |
| WHR | -0.003 (-0.09, 0.08) | 0.72 | 0.03 (-0.05, 0.12) | 0.24 | 0.25 (0.14, 0.36) | <0.001 | -0.005 (-0.14, 0.13) | 0.19 | -0.07 (-0.22, 0.08) | 0.23 | 0.46 (0.37, 0.54) | <0.001 | 0.09 (0.003, 0.17) | <0.001 |
| Fat mass | 0.11 (0.07, 0.15) | <0.001 | 0.29 (0.26, 0.33) | <0.001 | 0.32 (0.28, 0.36) | <0.001 | 0.29 (0.23, 0.35) | <0.001 | 0.10 (0.03, 0.16) | 0.001 | 0.78 (0.74, 0.81) | <0.001 | 0.22 (0.19, 0.26) | <0.001 |
| Lean Mass | 0.32 (0.26, 0.38) | <0.001 | 0.36 (0.30, 0.42) | <0.001 | -0.32 (-0.39, -0.24) | <0.001 | 0.85 (0.75, 0.95) | <0.001 | 0.68 (0.57, 0.78) | <0.001 | 0.26 (0.20, 0.32) | <0.001 | 0.49 (0.43, 0.55) | <0.001 |
| % body fat | 0.02 (-0.01, 0.07) | 0.10 | 0.18 (0.14, 0.23) | <0.001 | 0.31 (0.26, 0.37) | <0.001 | 0.11 (0.04, 0.19) | <0.001 | -0.06 (-0.13, 0.02) | 0.18 | 0.63 (0.59, 0.68) | <0.001 | 0.12 (0.08, 0.17) | <0.001 |
All dependent and independent variables are Z standardized and adjusted for gender and current age; Values are β regression coefficients (99% CI)
Linear regression used for continuous outcomes
Table 3b. Associations of conditional growth variables (height gain, adiposity gain (gain in sum of skinfolds independent of height) and lean gain (gain in weight independent of height and sum of skinfolds) with diabetes risk factors (glucose-insulin metabolism) at 21 years.
| Variables | Birth weight | Height gain 0-8y | Adiposity gain 0-8y | Lean gain 0-8y | Height gain 8-21y | Adiposity gain 8-21y | Lean gain 8-21y | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| β (99% CI) | P | β (99% CI) | P | β (99% CI) | P | β (99% CI) | P | β (99% CI) | P | β (99% CI) | P | β (99% CI) | P | |
| Fasting glucose | -0.03 (-0.17, 0.10) | 0.45 | 0.004 (-0.13, 0.14) | 0.18 | 0.15 (-0.01, 0.32) | 0.28 | -0.004 (-0.22, 0.21) | 0.93 | -0.14 (-0.39, 0.09) | 0.35 | 0.17 (0.03, 0.30) | <0.001 | -0.09 (-0.22, 0.04) | 0.02 |
| 30 min glucose | -0.08 (-0.21, 0.06) | 0.12 | -0.07 (-0.21, 0.06) | 0.51 | 0.08 (-0.09, 0.25) | 0.68 | -0.08 (-0.30, 0.14) | 0.008 | -0.10 (-0.34, 0.14) | 0.77 | 0.21 (0.07, 0.35) | <0.001 | -0.04 (-0.18, 0.09) | 0.51 |
| 120 min glucose | -0.12 (-0.25, 0.00) | 0.007 | 0.01 (-0.11, 0.13) | 0.41 | 0.17 (0.01, 0.32) | 0.002 | -0.23 (-0.44, -0.03) | 0.002 | -0.23 (-0.46, -0.01) | 0.004 | 0.40 (0.27, 0.53) | <0.001 | 0.01 (-0.10, 0.14) | 0.42 |
| Fasting insulin | -0.03 (-0.16, 0.08) | 0.37 | 0.04 (-0.08, 0.17) | 0.36 | 0.04 (-0.10, 0.19) | 0.08 | -0.003 (-0.20, 0.19) | 0.30 | 0.04 (-0.17, 0.26) | 0.95 | 0.46 (0.34, 0.58) | <0.001 | 0.03 (-0.09, 0.15) | 0.50 |
| 30 min insulin | -0.09 (-0.22, 0.04) | 0.41 | 0.01 (-0.11, 0.15) | 0.60 | 0.03 (-0.12, 0.20) | 0.42 | -0.07 (-0.29, 0.14) | 0.23 | -0.007 (-0.24, 0.23) | 0.22 | 0.32 (0.18, 0.45) | <0.001 | -0.04 (-0.17, 0.09) | 0.38 |
| 120 min insulin | -0.19 (-0.31, -0.07) | 0.01 | 0.03 (-0.08, 0.15) | 0.43 | 0.15 (0.01, 0.30) | 0.008 | -0.29 (-0.48, -0.10) | 0.002 | -0.15 (-0.36, -0.04) | 0.001 | 0.47 (0.35, 0.58) | <0.001 | -0.01 (-0.12, 0.10) | 0.69 |
| HOMA-IR | -0.03 (-0.15, 0.09) | 0.35 | 0.03 (-0.09, 0.16) | 0.26 | 0.05 (-0.10, 0.20) | 0.56 | -0.006 (-0.21, 0.19) | 0.29 | 0.03 (-0.18, 0.25) | 0.95 | 0.46 (0.33, 0.58) | <0.001 | 0.01 (-0.10, 0.14) | 0.54 |
| HOMA-β | -0.02 (-0.15, 0.10) | 0.45 | 0.03 (-0.09, 0.15) | 0.30 | -0.001 (-0.15, 0.15) | 0.35 | -0.01 (-0.22, 0.18) | 0.18 | 0.09 (-0.13, 0.32) | 0.40 | 0.41 (0.28, 0.54) | <0.001 | 0.07 (-0.05, 0.19) | 0.05 |
| Matsuda index | 0.14 (0.03, 0.25) | <0.001 | -0.03 (-0.15, 0.07) | 0.40 | -0.13 (-0.27, -0.001) | 0.01 | 0.19 (0.007, 0.37) | 0.003 | 0.09 (-0.10, 0.29) | 0.21 | -0.56 (-0.67, -0.45) | <0.001 | -0.002 (-0.11, 0.11) | 0.69 |
| Insulinogenic index | -0.05 (-0.19, 0.08) | 0.51 | 0.04 (-0.10, 0.18) | 0.75 | 0.006 (-0.16, 0.18) | 0.38 | -0.02 (-0.26, 0.20) | 0.38 | 0.01 (-0.24, 0.26) | 0.63 | 0.14 (0.001, 0.28) | 0.004 | -0.02 (-0.16, 0.28) | 0.46 |
| Disposition index | 0.10 (-0.02, 0.24) | 0.04 | 0.04 (-0.08, 0.17) | 0.95 | -0.10 (-0.26, 0.06) | 0.34 | 0.09 (-0.12, 0.30) | 0.12 | 0.05 (-0.18, 0.29) | 0.97 | -0.30 (-0.44, -0.17) | <0.001 | 0.001 (-0.13, 0.13) | 0.90 |
All dependent and independent variables are Z standardized and adjusted for gender and current age; Values are β regression coefficients (99% CI)
Linear regression used for continuous outcomes
Body size and composition
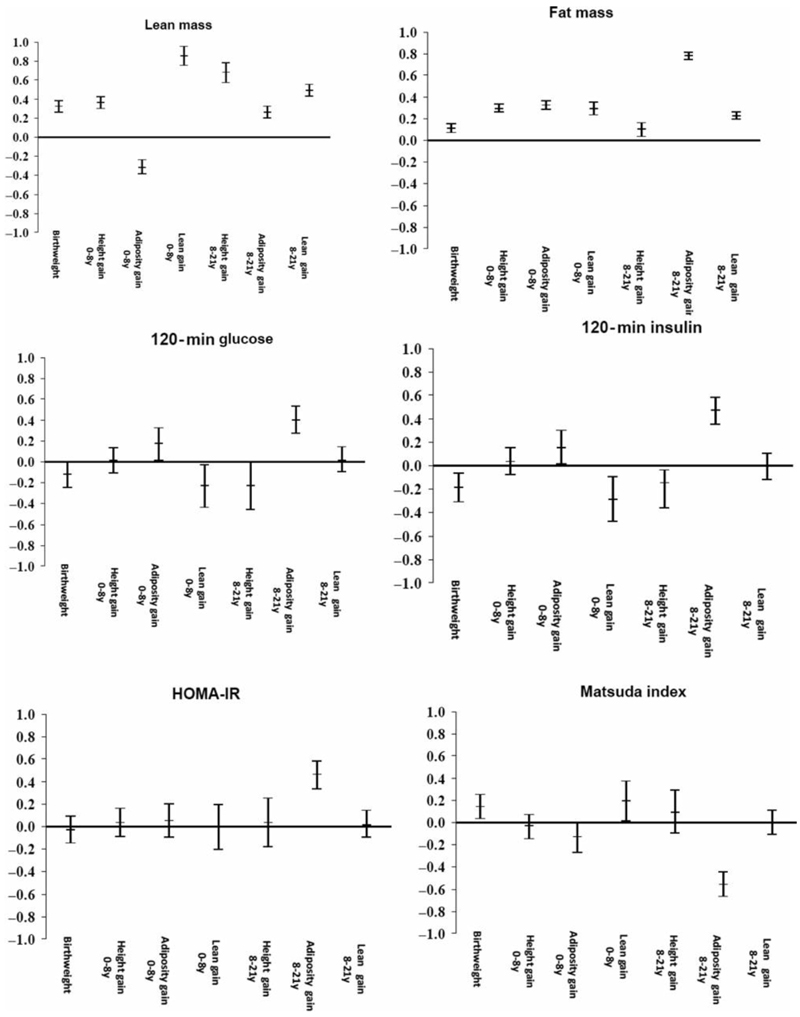
Greater adiposity gain 0-8y and 8-21y was associated with higher levels of all 21-year adiposity measures (BMI, waist circumference and WHR, fat mass and fat percent). Adiposity gain 0-8y was negatively associated with 21-year lean mass. Greater height gain 0-8y and 8-21y was strongly positively associated with 21-year lean mass and less strongly with measures of adiposity.
Glucose, insulin and indices
Greater adiposity gain 0-8y was positively associated with 120-minute glucose and insulin and negatively with Matsuda index. Adiposity gain 8-21y was associated with almost all the glucose and insulin variables (positively with glucose and insulin concentrations at all time points, HOMA-IR, and HOMA-β, and negatively with Matsuda and Disposition indices). Greater lean mass gain 0-8y and greater height gain 8-21y was associated with lower glucose and insulin concentrations at 120 minutes. Greater lean mass gain 0-8y was associated with higher Matsuda index.
Figure 2 depicts these relationships for selected variables.
Figure 2. Associations of conditional growth variables (height gain, adiposity gain (gain in sum of skinfolds independent of height) and lean gain (gain in weight independent of height and sum of skinfolds) with selected diabetes risk factors; statistics shown in Table 3a and 3b.
Discussion
We related prospective measurements of size and proxies of change in body composition in childhood and adolescence to 21-year glucose-insulin metabolism. Those who were born light and developed a ‘high’ adult BMI (‘small becoming big’) had the highest 120-min glucose concentration, the lowest insulin sensitivity and the poorest β-cell function in relation to prevailing insulin resistance. These results are consistent with our findings in this cohort at 8 years. There were no interactions between birth weight and current BMI in these analyses, indicating that the associations of birth weight and adult BMI with outcomes were additive rather than multiplicative. We have previously shown an interaction between birth weight and current size at 8 years on insulin resistance at 8 years7. Using the longitudinal data and 3-component conditional growth analysis, we were able to create independent proxies for linear, adipose and lean mass post-natal growth. We found that greater adiposity gain during childhood and adolescence was associated with higher adult adiposity, lower lean mass, higher glucose concentrations, lower insulin sensitivity and poorer β-cell function. These are known risk factors for future diabetes. In contrast, greater lean mass gain during childhood and greater height gain during adolescence were associated with lower glucose concentrations and higher insulin sensitivity, factors protective against future diabetes. Another longitudinal cohort study in India has recently shown that a greater gain in adiposity, distinct from linear growth and lean mass growth, was associated with higher insulin resistance (HOMA-IR) in adolescence25. Our cohort is the first report of a follow-up into adult life using this approach. There are several birth cohorts with data collected more continuously throughout childhood but with more limited measures of growth (height and weight, but no skinfolds). These have shown that lower weight at birth and greater weight gain relative to height during childhood and adolescence are associated with an increased risk of adult diabetes11,23,26,27. Our study had the advantage of skinfold measurements during childhood, in addition to weight and height, which allowed us to estimate for the first time the effects of linear growth, and proxy measures of adiposity gain (skinfold gain independent of height) and lean mass gain (weight gain independent of height and skinfolds) on the evolving risk of diabetes.
The contributions of size at birth and post-natal growth to later diabetes risk are debated. The association of lower birth weight with higher risk of diabetes appears to be due to an impaired β-cell response to glucose load on the background of higher insulin resistance. The latter could be contributed by low lean body mass which is the major tissue disposing off the load and excess adiposity which secretes many adipocytokines which interfere with insulin action. In animal models of maternal undernutrition resulting in small size at birth, there is impairment of pancreatic β-cell and skeletal muscle development with relative preservation of adipose tissue28. This body composition is reminiscent of the thin-fat body composition of Indian newborns29. The thrifty phenotype hypothesis proposed that impaired fetal development led principally to a reduced ‘capacity’ of the β-cells to produce insulin which led to diabetes in later life on exposure to the ‘load’ of insulin resistance from post-natal obesity30. Our data suggest that small size at birth leads to both reduced capacity (impaired insulin secretion) and increased load (increased insulin resistance) from early life.
Patterns of postnatal growth associated with higher risk of diabetes in our study included greater adiposity gain both during childhood as well as adolescence. Adiposity gain between birth and 8 years was associated with higher adult fat mass and lower adult lean mass - an accentuation of the ‘thin-fat’ phenotype. These body composition changes could account for lower insulin sensitivity independent of that seen with lower birth weight. This is corroborated by the greater insulin sensitivity associated with greater lean gain between birth and 8 years. Adiposity gain between 8 years and 21 years was similarly associated with higher adult fat mass and lower insulin sensitivity but also with lower β-cell function. This could indicate failure of an already compromised β-cell reserve (capacity) after a prolonged period of higher insulin resistance (load)31.
Strengths and limitations
The Pune Children’s Study is one of few studies with prospective follow up of a birth cohort with measurements of height, weight and skinfolds, and glucose-insulin across childhood and young adulthood. We are able to relate serial measurements of not only weight and height, but also measures of body fat (skinfolds) to adult outcomes. It also goes beyond measurements of fasting glucose and insulin concentrations in childhood to dynamic measures of glucose and insulin metabolism from oral glucose tolerance tests. Our loss to follow-up of ~25% is one of the lowest for a long–term longitudinal study and adds to its internal validity. Limitations of our study were that there were no pregnancy data, and newborn weight was the only measurement recorded at birth. Skinfolds were the only childhood measure of body fat available; there are better measures of total body fat now, such as DXA. However, we know of no cohort studies with adult follow-up that have used these measurements in childhood. The participants were all born in one hospital in Pune and who were available for follow-up from childhood to young adulthood; this limits the representativeness given the population size and socio-economic diversity of India. However, the KEM is the second largest hospital in Pune with a general wing (where charges are cheaper and discounts are offered to those on low income) and a private wing (where charges are higher). Due to its reputation for providing quality health care, it attracts people from a wide range of socioeconomic classes. We do not have data on pubertal staging and are therefore not able to assess its effects on growth and metabolism. Most of our participants were classified as belonging to upper social class; this factor may also limit the generalisability. However, the classification of social class based on SLI cut-offs has limitations; the majority of participants in our rural cohort were also classified as upper social class based on this score. The study was conducted during 2009-11; over the past decade, India has undergone significant socio-economic transition, mostly in urban areas, characterised by increased disposable income, adoption of energy-dense diets, lowered levels of physical activity and higher stress levels. Pune has transformed into a cosmopolitan city with a population of over 5 million accompanied by a growth in the Information Technology and automobile sector industries. Although our study may not reflect the current socio-economic conditions in Pune, we will attempt to capture these changes in our next follow-up of the cohort when they are 35-40 years old. Given the limitations of the observational design, we are unable to prove any causal associations. Because our ideas of adiposity gain and lean gain are based on derived measures, we consider our observations hypothesis generating needing further confirmation. Such a study is in progress.
Summary
In a 21-year old birth cohort previously studied at 8 years of age, we found that diabetes risk factors continued to be associated with lower birth weight in addition to the expected positive associations with current BMI. We have also been able to highlight windows of growth and evolving body composition changes across childhood and adolescence which are associated with diabetes risk. Our findings suggest that greater adiposity gain at all ages is associated with higher diabetes risk while a greater lean mass gain, especially in childhood, and height gain in adolescence is associated with lower diabetes risk. Given the importance of nutrition for growth, our findings highlight the importance of optimal nutrition throughout the lifecycle including the intrauterine period. The ‘small becoming big’ paradigm highlights the dilemma associated with advising on weight gain in children who were born low birth weight. More research is needed to investigate interventions which will promote lean rather than adipose growth in childhood and adolescence.
Acknowledgements
We are grateful to the study participants for taking part in this study. We thank Dr KJ Coyaji, Medical Director of the KEM Hospital, Dr VS Padbidri, former Director Research and Dr L Garda, Director Research for providing research facilities. We thank Ms LV Ramdas, Ms S Hardikar, Ms S Wagle, Ms V Deshpande, Ms D Raut, Ms P Hardikar, Ms S Kasture, Ms V Kantikar, Ms K Advani, Ms N Gurav, Ms R Saswade and Mr A Patil-Kendre for their contribution in collection of the data. We also thank Mr TM Deokar, Mr SD Chougule, Mr AB Gaikwad, Mr ML Hoge, Mr V Wagh, Mr SN Khemkar, Mr SB Wagh, Mr BS Jadhav and Dr A Bavdekar for their invaluable contribution to the study. Dr Sharad Gore helped with statistical queries. We also acknowledge the support of Sneha-India.
Financial support
The study was funded by The Wellcome Trust, UK (Grant Number: 083460/Z/07/Z), the Medical Research Council, UK and the Department for International Development, UK.
Footnotes
Conflict of interest
None of the authors had any financial or personal conflicts of interest associated with this manuscript.
Ethical standards
The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national guidelines on human experimentation (Indian Council of Medical Research) and with the Helsinki Declaration of 1975, as revised in 2008, and has been approved by the institutional committee (KEM Hospital Research Centre Ethics Committee).
Author contributor statement
CSY and CHDF designed the research, analysed and interpreted the data and wrote the manuscript. KK, HL, and SMJ analysed and interpreted the data, and wrote the manuscript. DSB, CJ and PCY carried out data collection and critically reviewed the manuscript. SAB and ANP designed the research and critically reviewed the manuscript. All authors approved the final version.CSY is the guarantor of this work and as such, had full access to all the data in the study and takes responsibility of the integrity of the data and the accuracy of the data analysis.
References
- 1.Gaziano TA, Bitton A, Anand S, Abrahams-Gessel S, Murphy A. Growing epidemic of coronary heart disease in low- and middle-income countries. Curr Probl Cardiol. 2010;35:72–115. doi: 10.1016/j.cpcardiol.2009.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.GBD 2015 Mortality and Causes of Death Collaborators. Global, regional, and national life expectancy, all-cause mortality, and cause-specific mortality for 249 causes of death, 1980-2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet. 2016;388:1459–1544. doi: 10.1016/S0140-6736(16)31012-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.International Diabetes Federation. IDF Diabetes Atlas. 7th edn. Brussels, Belgium: International Diabetes Federation; 2015. http://www.idf.org/diabetesatlas . [Google Scholar]
- 4.Wild S, Roglic G, Green A, Sicre R, King H. Global prevalence of diabetes. Estimates for the year 2000 and projections for 2030. Diabetes Care. 2004;27:1047–53. doi: 10.2337/diacare.27.5.1047. [DOI] [PubMed] [Google Scholar]
- 5.Hales CN, Barker DJP. Type 2 (non-insulin-dpendant) diabetes mellitus : the thrifty phenotype hypothesis. Diabetologia. 1992;35:595–601. doi: 10.1007/BF00400248. [DOI] [PubMed] [Google Scholar]
- 6.Yajnik CS. Early life origins of insulin resistance and type 2 diabetes in India and other Asian countries. J Nutr. 2004;134:205–10. doi: 10.1093/jn/134.1.205. [DOI] [PubMed] [Google Scholar]
- 7.Bavdekar A, Yajnik CS, Fall CHD, Bapat S, Pandit AN, Deshpande V, Bhave S, Kellingray SD, Joglekar C. Insulin resistance syndrome in 8-year-old Indian children: small at birth, big at 8 years, or both? Diabetes. 1999;48:2422–9. doi: 10.2337/diabetes.48.12.2422. [DOI] [PubMed] [Google Scholar]
- 8.Phillips DIW, Barker DJP, Hales CN, Hirst S, Osmond C. Thinness at birth and insulin resistance in adult life. Diabetologia. 1994;37:150–4. doi: 10.1007/s001250050086. [DOI] [PubMed] [Google Scholar]
- 9.Whincup PF, Cook DG, Adshead F, Taylor SJC, Walker M, Papacosta O, Alberti KGMM. Childhood size is more strongly related than size at birth to glucose and insulin levels in 10–11 year-old children. Diabetologia. 1997;40:319–26. doi: 10.1007/s001250050681. [DOI] [PubMed] [Google Scholar]
- 10.Ong KK, Petry CJ, Emmett PM, Sandhu MS, Kiess W, Hales CN, Ness AR, Dunger DB, ALSPAC study team Insulin sensitivity and secretion in normal children related to size at birth, postnatal growth, and plasma insulin-like growth factor-I levels. Diabetologia. 2004;47:1064–70. doi: 10.1007/s00125-004-1405-8. [DOI] [PubMed] [Google Scholar]
- 11.Bhargava SK, Sachdev HPS, Fall CHD, Osmond C, Ramakrishnan L, Barker DJP, Biswas SKD, Ramji S, Prabhakaran D, Reddy KS. Relation of serial changes in childhood body mass index to impaired glucose tolerance in young adulthood. New England J Med. 2004;350:865–75. doi: 10.1056/NEJMoa035698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Eriksson JG, Forsen T, Tuomilheto J, Jaddoe VWV, Osmond C, Barker DJP. Effect of size at birth and childhood growth on the insulin resistance syndrome in elderly individuals. Diabetologia. 2002;45:342–8. doi: 10.1007/s00125-001-0757-6. [DOI] [PubMed] [Google Scholar]
- 13.Part 1: Diagnosis and Classification of Diabetes Mellitus. Geneva: World Health Organization; 1999. Definition, Diagnosis and Classification of Diabetes Mellitus and its Complications Report of a WHO Consultation. [Google Scholar]
- 14.International Institute for Population Sciences and Macro International. National Family Health Survey (NHFS-3), India 2005-06. Mumbai: IIPS; 2007. [Google Scholar]
- 15.The Oxford Centre for Diabetes, Endocrinology and Metabolism, Diabetes Trials Unit. HOMA calculator. [accessed 15 July 2015]. Available from http://www.dtu.ox.ac.uk .
- 16.Wareham NJ, Phillips DI, Byrne CD, Hales CN. The 30 minute insulin incremental response in an oral glucose tolerance test as a measure of insulin secretion. Diabet Med. 1995;12:931. doi: 10.1111/j.1464-5491.1995.tb00399.x. [DOI] [PubMed] [Google Scholar]
- 17.DeFronzo RA, Matsuda M. Reduced time points to calculate the composite index. Diabetes Care. 2010;33:e93. doi: 10.2337/dc10-0646. [DOI] [PubMed] [Google Scholar]
- 18.Bergman RN, Phillips LS, Cobelli C. Physiologic evaluation of factors controlling glucose tolerance in man. Measurement of insulin sensitivity and β-cell glucose sensitivity from the response to intravenous glucose. J Clin Invest. 1981;68:1456–67. doi: 10.1172/JCI110398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.BMI classification. World Health Organization. Global database on body mass index. [accessed 01 August 2015]. Available from http://www.who.int/bmi .
- 20.World Health Organization. Physical status: the use and interpretation of anthropometry. Report of a WHO Expert Committee. World Health Organ Tech Rep Ser. 1995;854:1–452. [PubMed] [Google Scholar]
- 21.American Diabetes Association. Standards of medical care in diabetes—2014. Diabetes Care. 2014;37:S14–80. doi: 10.2337/dc14-S014. [DOI] [PubMed] [Google Scholar]
- 22.Lucas A, Fewtrell MS, Cole TJ. Fetal origins of adult disease-the hypothesis revisited. BMJ. 1999;24(319):245–9. doi: 10.1136/bmj.319.7204.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Adair LS, Fall CHD, Osmond C, Stein AD, Martorell R, Ramirez-Zea M, Sachdev HPS, Dahly DL, Bas I, Norris SA, Micklesfield L, et al. Associations of linear growth and relative weight gain during early life with adult health and human capital in countries of low and middle income: findings from five birth cohort studies. Lancet. 2013;382:525–34. doi: 10.1016/S0140-6736(13)60103-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Osmond C, Fall CHD. In: Chapter 11 p275-300 in Handbook of Statistics 37; Disease modelling and public health, Part B. Srinivasa Rao ASR, Pyne S, Rao CR, editors. North Holland/Elsevier; Amsterdam, Netherlands: 2017. Conditional Growth Models: An Exposition and Some Extensions. [Google Scholar]
- 25.Krishnaveni GV, Veena SR, Srinivasan K, Osmond C, Fall CHD. Linear growth and fat and lean tissue gain in childhood: associations with cardiometabolic and cognitive outcomes in adolescent Indian children. PLoS One. 2015;10:e0143231. doi: 10.1371/journal.pone.0143231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Eriksson JG. Early growth, and coronary heart disease and type 2 diabetes: experiences from the Helsinki Birth Cohort Studies. Int J Obes. 2006;30:S18–22. doi: 10.1038/sj.ijo.0803515. [DOI] [PubMed] [Google Scholar]
- 27.Norris SA, Osmond C, Gigante D, Kuzawa CW, Ramakrishnan L, Lee NR, Ramirez-Zea M, Richter LM, Stein AD, Tandon N, Fall CHD and the COHORTS Group Size at birth, weight gain in infancy and childhood, and adult diabetes risk in five low- or middle-income country birth cohorts. Diabetes Care. 2012;35:72–9. doi: 10.2337/dc11-0456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Warner MJ, Ozanne SE. Mechanisms involved in the developmental programming of adult diseae. Biochemistry J. 2010;427:333–47. doi: 10.1042/BJ20091861. [DOI] [PubMed] [Google Scholar]
- 29.Yajnik CS, Fall CHD, Coyaji KJ, Hirve SS, Rao S, Barker DJP, Joglekar C, Kellingray S. Neonatal anthropometry: the thin-fat Indian baby; the Pune Maternal Nutrition Study. Int J Obesity. 2003;27:173–80. doi: 10.1038/sj.ijo.802219. [DOI] [PubMed] [Google Scholar]
- 30.Wells JC. The thrifty phenotype: an adaptation in growth or metabolism? Am J Hum Biol. 2011;23:65–75. doi: 10.1002/ajhb.21100. [DOI] [PubMed] [Google Scholar]
- 31.Wells JC, Pomeroy E, Walimbe SR, Popkin BM, Yajnik CS. The elevated susceptibility to diabetes in India: an evolutionary perspective. Front Public Health. 2016;4:145. doi: 10.3389/fpubh.2016.00145. [DOI] [PMC free article] [PubMed] [Google Scholar]