Structured Summary
Background
The gastrointestinal microbiota has an important role in mucosal immune homeostasis and may contribute to maintaining mucosal healing in Crohn’s disease.
Aim
To identify changes in the microbiota, metabolome, and protease activity associated with mucosal healing in established paediatric Crohn’s disease.
Methods
Twenty-five participants aged 3-18 years with a diagnosis of Crohn’s disease, a disease duration over six months, and maintenance treatment with biological therapy were recruited. They were divided into a low calprotectin group (faecal calprotectin <100μg/g, “mucosal healing”, n=11), and a high calprotectin group (faecal calprotectin >100μg/g, “mucosal inflammation”, n=11). 16S gene-based metataxonomics, 1H NMR spectroscopy-based metabolic profiling, and protease activity assays were performed on stool samples.
Results
Relative abundance of Dialister species was six-times greater in the low calprotectin group (q = 0.00999). Alpha and beta diversity, total protease activity, and inferred metagenomic profiles did not differ between groups. Pentanoate (valerate) and lysine were principal discriminators in a machine-learning model which differentiated high and low calprotectin samples using NMR spectra (R2 0.87, Q2 0.41). Mean relative concentration of pentanoate was 1.35-times greater in the low calprotectin group (95% CI 1.03 – 1.68, p = 0.036) and was positively correlated with Dialister. Mean relative concentration of lysine was 1.54-times greater in the high calprotectin group (95% CI 1.05 – 2.03, p = 0.028).
Conclusions
This multiomic study identifies an increase in Dialister species and pentanoate, and a decrease in lysine, in patients with “mucosal healing”. It supports further investigation of these as potential novel therapeutic targets in Crohn’s disease.
Keywords: microbiome, metabolome, protease activity, pentanoate, valerate, Dialister
Introduction
Crohn’s disease is a chronic inflammatory disease caused by an inappropriate immunological response to the gastrointestinal microbiota in genetically susceptible individuals. Transmural inflammation throughout the gastrointestinal tract causes pain, bleeding, weight loss, and diarrhoea; ultimately resulting in long term complications such as stricture and fistula formation. Children with Crohn’s disease typically have more aggressive disease and worse long-term outcomes than those with adult onset disease.1
Treatment of paediatric Crohn’s disease (CD) aims to control symptoms and to suppress mucosal inflammation. Adequate suppression of inflammation results in mucosal healing, which is associated with improved long-term outcomes.1,2 Low faecal calprotectin levels are a well validated non-invasive marker of mucosal healing in Crohn’s disease.3–6 The faecal calprotectin threshold which defines mucosal healing has not been universally agreed, studies support values in the range of 100-300μg/g3–8, with a value of 100μg/g used most frequently9. In paediatric CD Weinstein-Nakar and colleagues found faecal calprotectin levels under 100μg/g were predictive of mucosal and transmural healing.7
Inducing and maintaining mucosal healing is a major challenge. Over 25% of all paediatric CD patients are now escalated to anti-TNF and other biological therapies,10 but approximately one in three patients will not have a sustained response to maximal medical therapy.11,12
The gastrointestinal microbiome has an important role in mucosal immune homeostasis and evidence from epidemiological studies and mouse models suggest it is involved in the pathogenesis of Crohn’s disease.13,14 There are several mechanisms by which the microbiota could promote mucosal healing or exacerbate mucosal inflammation in established disease.15
Changes to the microbiota’s metabolic capacity alters the mucosal concentration of active metabolites. The short chain fatty acids (SCFAs) acetate, propionate, butyrate, and pentanoate (also known as valerate) are produced by bacterial metabolism of dietary fibre and amino acids,16 and are present in reduced concentration in Crohn’s disease.17 SCFAs act as a primary energy source for enterocytes, promote tight junction formation, and induce antimicrobial protein formation.18 Butyrate18,19 and pentanoate20 induce anti-inflammatory cytokine production and modify immune cell differentiation via their activation of free fatty acid receptors and inhibition of histone deacetylase. Immunomodulatory properties of other metabolites, including bile acid metabolites21 and amino acids22, have also been described.
Protease activity in the gastrointestinal lumen is influenced by microbial metabolism. Bacteria produce proteases, and also degrade or inhibit host pancreatic and inflammatory proteases. Protease activity is increased in IBD23, and can influence disease activity by inducing invasive behaviour in commensal bacteria24 or by degrading tight junction proteins25. Pro-inflammatory signalling cascades are initiated by protease activated receptors on enterocytes, fibroblasts, and mucosal immune cells.24,25
Previous studies have characterised the microbiome of paediatric CD patients relative to controls and found reduced diversity, high instability, and altered relative abundance of multiple bacterial genera.26,27 Increased mucosal inflammation has been associated with a greater reduction in alpha diversity, as well as reduced abundance of SCFA producers such as Faecalibacterium prausnitzii26 and Roseburia hominis28,29. Conversely, inducing remission with anti-TNF induction makes the microbiota more similar to that of healthy children.30 It is not known whether inflammation associated microbial differences persist in established disease, or whether they contribute to ongoing mucosal healing or inflammation.
This study aims to identify changes in the microbiota which are associated with mucosal healing in established paediatric Crohn’s disease and to investigate the functional implications of these changes using inferred metagenomics, 1H NMR metabolomics, and protease activity assays.
Materials and Methods
Study design and participant recruitment
Participants were recruited to the ‘Metabolic and Microbiome Profiling in Paediatric IBD’ study, a multi-centre clinical cohort study in North-West London. The parents or carers of all study participants provided informed consent to join the study, and the study participants signed age appropriate assent forms. The study design and objectives were approved by London Bloomsbury research ethics committee (REC reference 17/LO/2049). All patients included in this analysis had a confirmed diagnosis of paediatric Crohn’s disease and a disease duration of greater than six months. All were receiving maintenance biological therapy, which was defined as regular treatment with infliximab, adalimumab, or vedolizumab beyond the induction phase.
Clinical data was obtained from review of electronic medical records and from a questionnaire completed at induction to the study. C-reactive protein, erythrocyte sedimentation rate, haemoglobin concentration, and stool calprotectin concentration were tested as part of routine clinical care.
All participants provided a sample of a whole stool for microbiome, metabolome and protease activity analysis. Stool samples were collected using a ‘fecotainer’ system (Excretas Medical, Netherlands). The sample was immediately refrigerated to 4°C and was homogenised, divided, and frozen at minus 80°C within four hours of production. Sample order was randomised prior to further analysis.
The low calprotectin group was defined by a calprotectin concentration of less than 100μg/g, which was used as a surrogate for mucosal healing, and the high calprotectin group by a concentration of 100μg/g or over. Calprotectin value was used to assign groups if it was measured in the same stool sample as that used for the study analyses, or if two stable calprotectin values were recorded within the two months before and after collection of the sample used for study analyses and there had been no change in clinical disease activity or treatment in this period. Samples without a corresponding calprotectin measurement meeting these requirements were not included in the high and low calprotectin groups, but were included for analyses which did not require calprotectin value i.e. correlation analyses between microbiome and metabolome.
Baseline characteristics were compared between groups using Welch t test for continuous variables, and the Chi squared test for categorical variables (where test validity requirements were met).
Metataxonomic analysis
Metataxonomic analysis (16S rRNA gene sequencing) was performed on DNA extracted from thawed stool samples. The V1-2 region of the 16S rRNA gene was used for taxonomic discrimination.31–33
DNA extraction and sequencing
DNA was extracted using the Powerlyzer PowerSoil DNA isolation kit (Qiagen, Hilden, Germany) according to the manufacturers protocol except samples were lysed by bead beating for 3 minutes at speed 8 using a Bullet Blender Storm instrument (Thistle scientific, Glasgow, UK). Sample libraries were prepared using a protocol adapted from Illumina’s 16S Metagenomic Sequencing Library Preparation Protocol,34 which has been previously described.35 The V1-V2 region of 16S rRNA gene were amplified using the primers listed in Supplementary Table 1. SequalPrep Normalization Plate Kit (Thermo Fischer Scientific, Illinois, USA) was used to normalise index PCR reactions, and NEBNext Library Quant Kit for Illumina (New England Biolabs, Hitchin, UK) was used to quantify sample libraries. Sequencing was performed on an Illumina MiSeq platform (Illumina Inc., San Diego, California, USA) using paired-end 300 bp chemistry and the MiSeq Reagent Kit v3 (Illumina).
Data analysis
16S rRNA gene sequence data was analysed using the DADA2 pipeline (version 1.12)36 in R (R foundation, Vienna). Outlier analysis (ROUT, Q=1) of the number of sequences from each sample was performed using GraphPad Prism (GraphPad Software, San Diego, California). The vegan package in R was used to calculate Shannon index, species richness, and Pielou evenness for all samples. A neighbour-joining phylogenetic tree was constructed using the R packages DECIPHER and phangorn, and the phyloseq package37 was used to calculate weighted UniFrac distances. Weighted UniFrac distance was compared between groups using non-metric multidimensional scaling (NMDS) and permutational analysis of variance (PERMANOVA), performed using the R packages vegan and ggplot 2.
The amplicon sequence variant (ASV) table produced by DADA2 was formatted by grouping rare variants followed by subsampling data to the level of the sample with the lowest summed read count which was 7114 reads. No samples were identified as an outlier for summed read count using GraphPad Prism (ROUT, Q = 1). STAMP38 was used to analyse difference in relative abundance between high and low calprotectin groups at all taxonomic levels from genus to phylum. Two group comparison was performed in STAMP using two-sided White’s non-parametric t test with Benjamini-Hochberg correction for multiple testing (q < 0.05 was considered significant). Results were filtered to select only those where the difference in the mean proportion of sequences was greater than 1%.
Inferred metagenomic analysis
Inferred metagenomic analysis was performed using Piphillin, which is the best performing tool for predicting metagenome composition in clinical biosamples.39 Three outputs were analysed: KEGG pathways, KEGG orthologs, and BioCyc features. All were formatted by subsampling to the level of the sample with the lowest summed counts (1.51x106, 7.98 x 105, and 2.84 x106 respectively). Two group comparison was performed in STAMP using the same parameters as above. An effect size filter of 0.1% difference between pathway, ortholog, or feature expression was used.
Protease activity assay
A mixture of one-part thawed stool sample and two-parts phosphate buffered saline (PBS) were vortexed with microbeads at 2850 revolutions per minute for 5 minutes using the Vortex Genie 2 with microcentrifuge tube adaptor (Scientific industries Inc, USA). The homogenised mixture was centrifuged at 20000 x g for 20 minutes. Faecal water was prepared by passing 500μl supernatant through 5.0μm centrifugal filter (12000 x g, 3 minute spin). PBS was added in appropriate volume to prepare 10 and 100 fold dilutions of faecal water.
Protein concentration of faecal water dilutions was measured and compared against bovine serum albumin standards using the colorimetric Pierce BCA Protein assay following the manufacturer’s protocol (Thermo Fischer Scientific, Illinois, USA). Protein concentration in the faecal water was normalised to 1mg/ml by addition of PBS. Dilutions of the 1mg/ml faecal water and trypsin standard were prepared. These were added to an FTC-Casein substrate and working reagent from the Pierce Fluorescent Protease Assay kit (Thermo Fischer Scientific) as per the manufacturers protocol. Proteolysis of the substrate was measured by fluorescent resonance energy transfer using a FLUOstar OPTIMA (BMG LABTECH, Germany). Total protease activity of faecal water was expressed relative to the activity of trypsin standards in ng/ml concentration (relative units). All assays performed in triplicate.
The activity of protease subtypes was assessed by performing the above protease activity assay, but with the co-incubation of a protease inhibitor (Roche diagnostics, Switzerland) with the faecal solution for five minutes, prior to addition of the working reagent. Optimal inhibitor concentrations were determined in preliminary work and inhibitor specificity confirmed by searching the MEROPS database40. The following inhibitors were used: Antipain-dihydrochloride (50 μg/ml), Leupeptin (5 μg/ml), or a combination of antipaindihydrochloride (50 μg/ml), aprotinin (2 μg/ml), and Pefabloc (1mg/ml). Assays were performed in duplicate. Degree of inhibition was calculated as ratio of protease activity in inhibited and uninhibited wells. Protease subtype activity (Δ protease activity) was calculated as total protease activity multiplied by degree of inhibition. Outliers for protease and protease subtype activity were identified using GraphPad Prism (ROUT, Q = 1) and excluded from further analysis. Welch’s T test was performed to compare total protease and protease subtype activity between groups. Bonferroni correction was used to correct p values from protease subtype activity analysis.
Metabolome analysis
Sample preparation for NMR analysis
Faecal water extraction was carried out by homogenising each sample with water (UHPLC grade, Fisher Chemical) at a ratio of 1:2 (mg of wet weight of faecal sample: μL of water). The mixture was vortexed for 5 min and centrifuged at 18,000 x g and 4°C for 20 minutes, 540 μL of the supernatant were mixed with 60 μL of 1.5 M KH2PO4 buffer (pH 7.4, 100% of deuterium oxide (D2O), 2 mM sodium azide and 1% of TSP (3-trimethylsilyl-[2,2,3,3,-2H4]-propionic acid sodium salt). The mixture was centrifuged at 18,000 x g at 4 °C for 1 min. An aliquot of 580 μL of the supernatant was transferred into a 5 mm outer diameter NMR tube as described previously41.
1H NMR spectroscopy
Water-suppressed 1H NMR spectroscopy was performed at 300 K on a Bruker 600 MHz Avance III HD spectrometer (Bruker Biospin, Karlsruhe, Germany). The 1D 1H NMR spectra were acquired using standard one-dimensional pulse sequence, with saturation of the water resonance (noesygppr1d pulse program) during both the relaxation delay (RD = 4s) and mixing time (tm = 10 ms). The two magnetic field z-gradients implemented by this pulse sequence are applied for 1 ms. The receiver gain was set to 90.5 and acquisition time (ACQ) to 2.73s for all experiments. Each 1-dimensional (1D) 1H NMR spectrum was acquired using 4 dummy scans, 32 scans, 64 K data points and with a spectral window of 20 ppm. Prior to Fourier Transformation, each free induction decay (FID) was multiplied by an exponential function corresponding to a line broadening of 0.3 Hz.
2D 1H-1H J-resolved experiment was also acquired for each sample to detect the J-couplings in the second dimension using the pulse program with suppression of the water resonance during the relaxation delay (jresgpprqf). The acquisition parameters used for this experiment were as follows: 16 dummy scans and 2 scans, 8K points with spectral window of 16.7 ppm for f2 and 40 increments with a spectral window of 78 Hz for f1, incremented delay of 3μs, RD of 2 s, and ACQ of 0.41 s. The receiver gain was set to 90.5. A sine-bell apodization function was applied on both dimensions, followed by Fourier transformation, tilting by 45°, and symmetrisation along f142.
2D-NMR experiments including 1H-1H Total Correlation Spectroscopy (TOCSY) and 1H-13C Heteronuclear Single Quantum Coherence (HSQC) were acquired for a representative sample for identification purposes.
Spectral data analysis
1D 1H NMR spectra were phased, baseline corrected and calibrated to TSP peak at δ1H 0 ppm prior to being digitized over the range δ1H -0.5 to 11 ppm, and imported into MATLAB (2014a, MathWorks, Natick, U.S.). Spectral regions containing residual water (δ1H 4.69 to 4.93), TSP (δ1H -0.50 to 0.62), and noise (δ1H 9.35 to 11.00) were removed prior to probabilistic quotient normalization (PQN)43.
The data set was auto-scaled and modelled using Partial Least Squares Discriminant Analysis (PLS-DA) in a Monte-Carlo Cross-Validation (MCCV) framework using Storey-Tibshirani method of correction for multiple testing44. Variables with q < 0.05 were considered to be significant. Goodness of fit (R2Y) was calculated using the training data, and the goodness of prediction (Q2Y) from test data. For discriminant analysis, calprotectin was used as dummy variable (Y = 0 and 1 for high and low calprotectin respectively).
Identification of metabolites
The structural identification of significant metabolites was achieved by 2D-NMR experiments and Subset Optimization by Reference Matching (STORM) on 1D 1H NMR data set45. Internal and external databases such as the Human Metabolome Data Base (HMDB; http://hmdb.ca/)46 and/or the Biological Magnetic Resonance Data Bank (BMRB; http://www.bmrb.wisc.edu) were used for confirmation of assignments.
Relative concentrations were calculated for metabolites that had significant correlation with Dialister and those that were observed as discriminators on the MCCV-PLS-DA model. This was performed by taking the integral from normalised spectra of a representative resonance peak of pentanoate at 1.30 (δ1H range from 1.27 to 1.33), lysine at 1.73 (δ1H range from 1.69 to 1.76), beta-alanine at 2.56 (δ1H range from 2.55 to 2.58) and phenylalanine at 7.43 ppm (δ1H range from 7.41 to 7.45). The integral of a peak represents a relative concentration of its corresponding metabolite. Mean metabolite relative concentration was compared between high and low calprotectin groups.
Metataxonomic-Metabolome correlation analysis
Full resolution NMR spectral features were correlated with relative abundance data at five taxonomic levels (genera – phyla) using Spearman’s rank correlation coefficient with Storey-Tibshirani method for multiple testing correction. A threshold of | ρ | ≥ 0.5 with q < 0.05 was used to indicate a significant correlation.
Metabolic network analysis
Metabolites that had significant correlation with Dialister and those that were observed as discriminators on the MCCV-PLS-DA model were used to construct a condensed multicompartmental metabolic reaction network using MetaboNetworks v.2.3.47 This software is an agnostic bioinformatic tool that can reconstruct the pathway relationship observed in a real system, which is visualised as classical representation (KEGG). It calculates the shortest paths (number of reactions) between metabolites, including only the reactions that can occur in humans considered as a supra-organism. A custom metabolic reaction network was constructed using enzymes linked to Homo sapiens genes and enzymes linked to bacteria present in the gastrointestinal tract. Species from genera with >1.5% relative abundance in either the high or low calprotectin group (Supplementary Figure 2), and that had genomic data available on the KEGG organism database were selected for inclusion in the analysis.
Metabolic potential of Dialister species
In order to identify the most appropriate reference genome to assess the metabolic potential of Dialister, species level analysis was performed for the Dialister genus. The 334 base pair ASVs from Dialister (Supplementary Table 2) were matched against 16S sequences in the rRNA/ITS database (National Centre for Biotechnology Information (NCBI), updated 4th July 2020) using the megaBLAST48 nucleotide search tool. Species identity was assigned if there was 98.7% sequence match to a reference strain49. This confidence threshold has high accuracy for species identification when using the V1-2 region of the 16S rRNA gene.33
The NCBI reference sequence linked to the representative strain was used to generate a metabolic pathway map using MetaCyc (https://metacyc.org).50
Sensitivity analyses
The metataxonomic, protease activity, and metabolome data analysis were repeated for two sensitivity analyses. The first was performed due to the uncertainty surrounding the appropriate threshold of faecal calprotectin which indicates mucosal healing. Values of 100 to 300 μg/g have been suggested as appropriate thresholds. Participants with faecal calprotectin concentrations between 100μg/g and 300μg/g were therefore excluded, leaving only those with faecal calprotectin levels which are widely accepted to predict mucosal healing or mucosal inflammation.
The second sensitivity analysis excluded participants who were receiving vedolizumab. Vedolizumab is an α4β7 integrin antagonist, it has a different mechanism of action compared to anti-TNF agents and could conceivably induce distinct changes in the microbiota. Participants receiving vedolizumab were excluded in order to remove this as a potential confounding factor.
Results
Participant characteristics
The ‘Metabolic and Microbiome Profiling in Paediatric IBD’ study recruited 53 children. Of these, 25 patients with established paediatric Crohn’s disease on maintenance biological therapy were eligible for inclusion in this analysis (Supplementary Figure 1). There were eleven patients in each of the low and high calprotectin groups. Three patients met inclusion criteria, but did not have a calprotectin value, these patients were included in analyses which did not require calprotectin value.
Height was significantly lower in the high calprotectin group (difference in mean z score -1.22, 95% CI -2.26 to -0.18, p = 0.025). There were no significant differences in other baseline characteristics. Disease location, and treatment with biological and immunomodulatory therapy were similar between groups (Table 1).
Table 1.
Baseline characteristics of the low and high calprotectin groups
| Low Calprotectin | High Calprotectin | p value | |
|---|---|---|---|
| Number | 11 | 11 | |
| Age Mean ± SD (years) |
13.4 ± 3.30 | 14.4 ± 2.20 | 0.414 |
| Disease Duration Mean ± SD (months) |
30.7 ± 13.5 | 29.8 ±13.8 | 0.878 |
| Time on biologics Mean ± SD (months) |
18.6 ± 11.4 | 19.1 ± 13.8 | 0.921 |
| Height (z score) | 0.534 ± 1.35 | -0.689 ± 0.689 | 0.025 |
| BMI (z score) | 0.174 ± 1.17 | 0.0648 ± 1.24 | 0.846 |
| Biological therapy | Infliximab 91% Vedolizumab 9% |
Infliximab 91% Vedolizumab 9% |
0.99 |
| Immunomodulator | Azathioprine 18% Methotrexate 18% Mercaptopurine 9% |
Azathioprine 45% Methotrexate 0% Mercaptopurine 0% |
- |
| Disease location | L1 0% L2 45% L3 45% L4a/b 54% Perianal 9% |
L1 9% L2 36% L3 45% L4a/b 63% Perianal 27% |
- |
P values calculated using unpaired unequal variance t test (Welch’s test) for continuous variables, and Chi squared test for categorical variables where validity requirements met
Clinical disease activity was not significantly different between groups. Mean paediatric Crohn’s disease activity index (PCDAI) ± standard deviation was 19 ± 22 in the high calprotectin group versus 13.8 ± 14 in the low calprotectin group (p= 0.50). Median calprotectin in the low calprotectin group was 38 μg/g (range 23 – 97) compared to 1338 μg/g (range 141-3933) in the high calprotectin group (p=0.0016).
Metataxonomic analysis
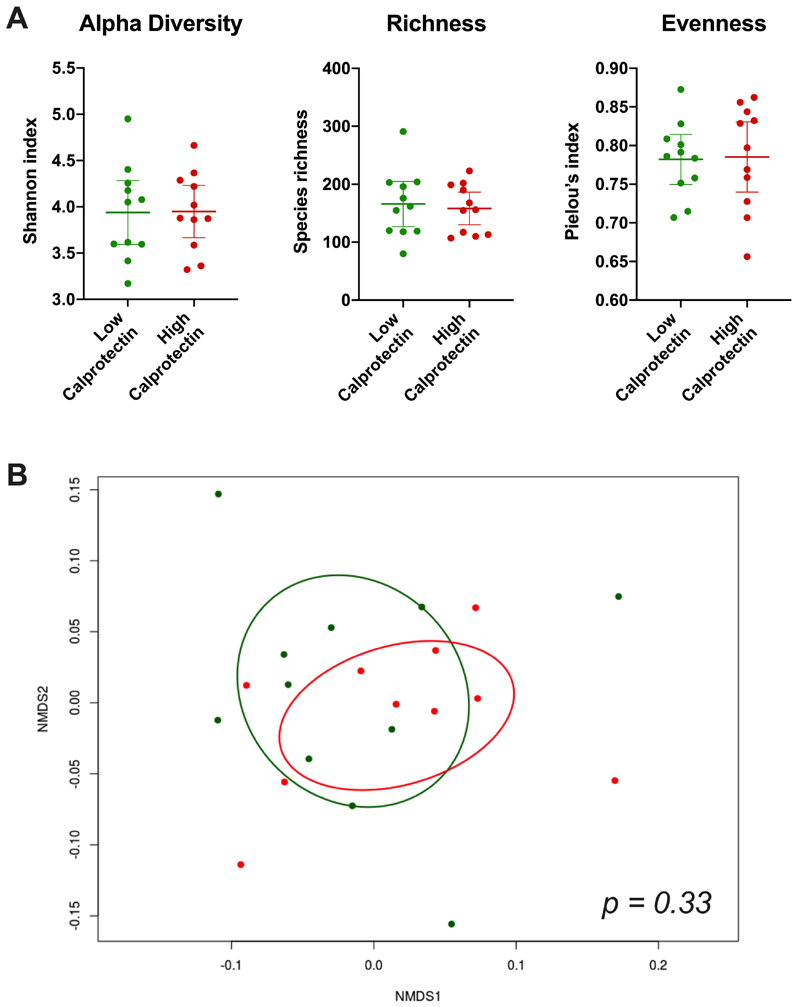
Bacterial diversity metrics and community composition were similar in the high and low calprotectin groups. There was no significant difference in Shannon diversity index, Pielou’s evenness index, or species richness between groups (Figure 1A). No samples were identified as outliers by these diversity metrics. An NMDS plot of weighted UniFrac distance showed no clear separation of the two groups by beta diversity (Figure 1B). This finding was consistent with non-significance on PERMANOVA of weighted UniFrac distance (p=0.33). The sample with the highest calprotectin concentration (3933 μg/g) was identified as an outlier on weighted UniFrac distance and was not included in the NMDS plot or PERMANOVA calculation.
Figure 1. Comparison of microbial diversity metrics and community composition between high and low calprotectin groups.
A) Scatter plots with mean and 95% confidence interval for alpha diversity (Shannon index), species richness, and evenness (Pielou’s index). B) Non-metric multidimensional scaling (NMDS) plot of weighted UniFrac distance with 95% confidence ellipses. Red dots and ellipse = high calprotectin, green dots and ellipse = low calprotectin. P value calculated using PERMANOVA.
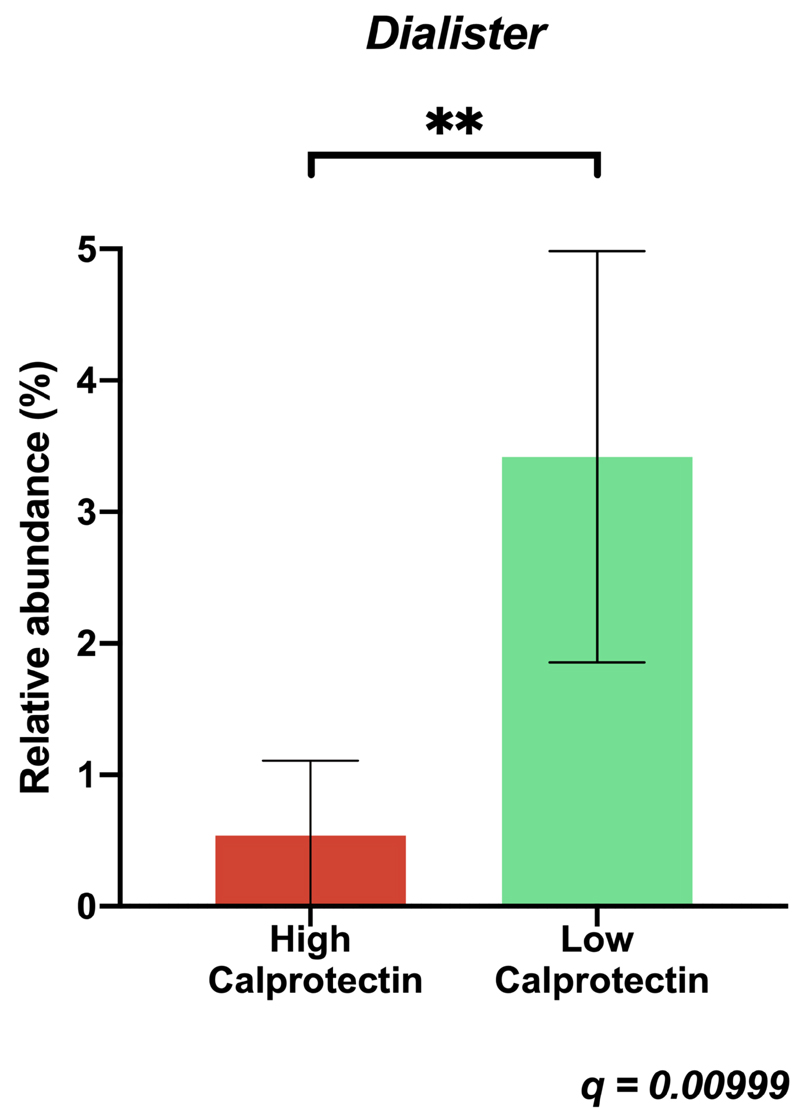
There was a six-fold increase in the relative abundance of the Dialister genus in the low calprotectin group compared to the high calprotectin group. Dialister species were the 7th most abundance genus in the low calprotectin group with a mean proportion of sequences of 3.4% (SD 2.22), compared to the 30th most abundant in the high calprotectin group with a mean proportion of sequences of 0.53% (SD 0.79) (difference of means -2.88%, 95% CI -4.31 to -1.53, q = 0.00999) (Figure 2). The relative abundance of Dialister was negatively correlated with calprotectin (Spearman’s ρ = - 0.73, p = 0.0001). There were no other significant differences in relative abundance with q <0.05 and difference in the mean proportion of sequences >1% at genus level (Supplementary Figure 2), or at higher taxonomic levels.
Figure 2. Mean relative abundance of Dialister in high and low calprotectin groups.
Bars indicate 95% confidence intervals. q value calculated using two-sided White’s non-parametric t test with Benjamini-Hochberg correction for multiple testing.
Inferred metagenomic analysis identified no significant differences in KEGG ortholog, KEGG pathway, or BioCyc feature expression between the two groups.
Protease activity
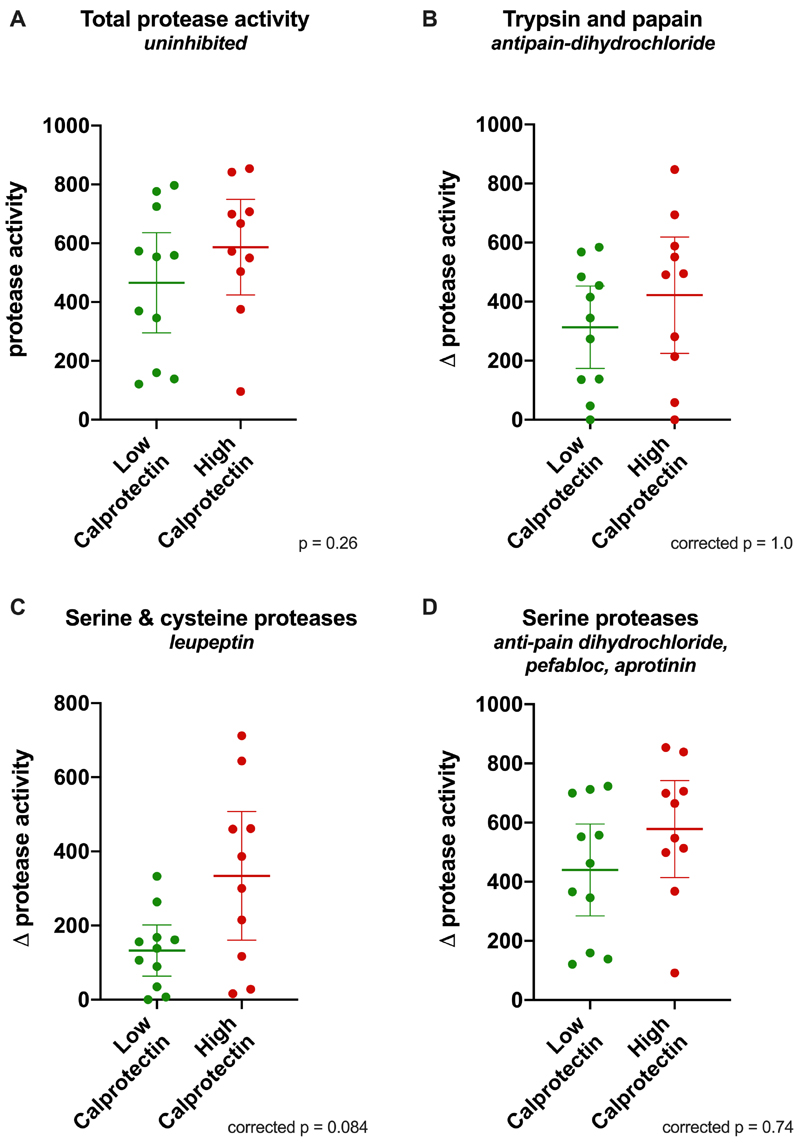
Protease activity was assayed in all thawed stool samples. Outlier analysis detected an anomalously high protease activity of 1809 relative units in the sample with the highest calprotectin concentration (3933 μg/g), this result was removed from further analysis of protease and protease subtype activity.
There was no significant difference in total protease activity between the groups. Mean protease activity was 587 relative units (SD 227) in the high calprotectin group versus 465 relative units (SD 254) in the low calprotectin group (difference of means 121, 95% CI -99 to 342, p = 0.26) (Figure 3A).
Figure 3. Scatter plots of protease activity (expressed as equivalent concentration of trypsin in μg/ml) in patients with high and low calprotectin.
Bars indicate mean and 95% confidence interval. Unpaired t test was performed for all samples, Bonferroni correction was applied for protease subtype activity. A: total protease activity (uninhibited). B-D: protease subtype activity, expressed as reduction in protease activity caused by addition of inhibitors (Δ protease activity); anti-pain dihydrochloride (B); leupeptin (C); anti-pain dihydrochloride, pepstatin, aprotinin (D).
The activity of protease subtypes was assayed by addition of different combinations of protease inhibitors to control the spectrum of inhibition. After Bonferroni correction there was no significant difference in the activity of any protease subtypes (Figure 3 B-D).
1H NMR based metabolic profiling
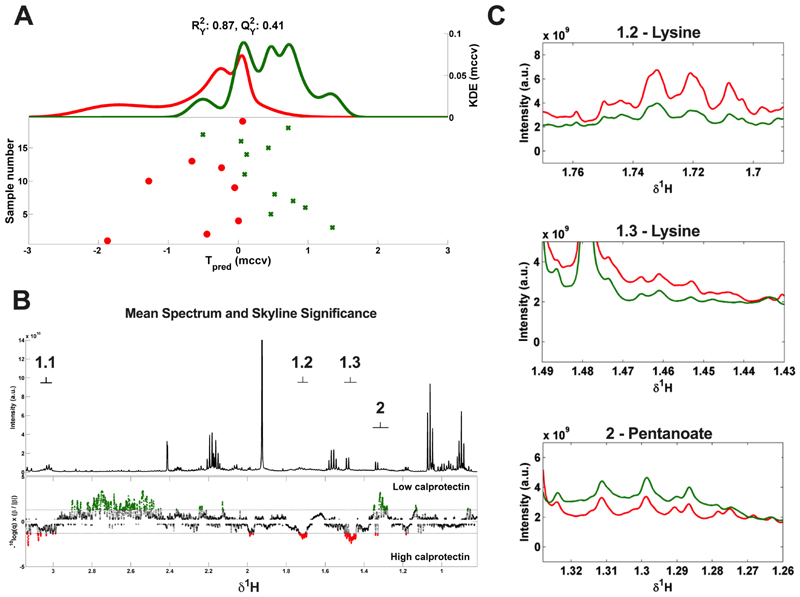
1H NMR spectroscopy was performed on faecal water prepared from thawed stool samples. Analysable spectra were not obtained for three samples from the high calprotectin group and one sample without a calprotectin value. The remaining data set was modelled using high and low calprotectin groups as a dichotomous classifier (n=19). The MCCV-PLS-DA model discriminated samples from high and low calprotectin groups with explained variance (R2Y) of 0.87 and capability of prediction (Q2Y) of 0.41 (Figure 4A).
Figure 4.
(A) MCCV-PLS-DA score plot derived from 1D 1H-NMR spectra of faecal water samples, indicating the differentiation between high calprotectin group (red) and low calprotectin group (green). The model is comprised of Kernel Density Estimate (KDE) of the predicted scores (Tpred) for both groups. Dots represent the metabolic profile of each patient from the study cohort when its corresponding NMR spectrum and calprotectin values were available (n=19). The fit and predictability of the model were obtained and expressed as R2Y (explained variance) and Q2Y (capability of prediction) values. (B) MCCV–PLS-DA loading plot. Top depicts the average 1D 1H-NMR spectrum of the 19 faecal water samples. The bottom depicts the Manhattan plot showing -log10(q) × sign of regression coefficient (β) of the MCCV–PLS-DA model that in conjunction represent the contribution of each variable on it. In green, metabolites are shown that are significantly higher in the low calprotectin group, and in red metabolites significantly higher in the high calprotectin group. Labels: 1.1-1.3 = lysine (1.73(m), 1.46(m), 3.04 (t)), 2 = pentanoate/valerate (1.30(m)). (C) Fragments from the average 1D 1H-NMR spectra from the high calprotectin (red, n = 8) and low calprotectin (green, n = 11) groups, showing the less overlapped resonances detected as significant in the MCCV-PLS-DA model corresponding to lysine (1.2, 1.73(m), 1.3, 1.46(m)) and pentanoate/valerate (2, 1.30(m)). MCCV = Monte Carlo Cross-Validation. PLS-DA = Partial Least Squares Discriminant Analysis.
The principal metabolic discriminators used by the model were pentanoate, which was increased in the low calprotectin group, and lysine which was increased in the high calprotectin group (Figure 4). Peak assignments of both metabolites were confirmed by multiple two-dimensional NMR experiments and STORM method (Supplementary Table 3 and Supplementary Figure 4). Other regions of the 1D spectra detected as significant by the model did not correspond to assignable metabolites (Figure 4B).
The relative concentrations of pentanoate and lysine in the high and low calprotectin groups were approximated using the integrals of their resonance peaks at 1.30 ppm (δ1H range from 1.27 to 1.33) and 1.73 (δ1H range from 1.69 to 1.76) respectively (Figure 4C). The mean concentration of pentanoate was 1.35 times greater in the low calprotectin group compared to the high calprotectin group (95% CI 1.03 – 1.68 times greater, p = 0.0361) and had strong negative correlation with calprotectin (ρ = -0.61, p = 0.006). The relative concentration of lysine was 1.54 times greater in the high calprotectin group (95% CI 1.05 – 2.03 times greater, p = 0.028) and had strong positive correlation with calprotectin (ρ = 0.58, p = 0.009).
Multiomic integration
Relative abundance data from metataxonomic analysis was correlated with 1D 1H NMR spectral features using Spearman’s rank correlation coefficient with Storey-Tibshirani correction for multiple testing (n=21). Dialister species abundance had significant positive correlation (ρ >0.5, p <0.05) with the concentration of pentanoate and ß-alanine, and significant negative correlation (ρ < -0.5, p <0.05) with phenylalanine (Supplementary Figure 3 and Supplementary Table 4). The presence of these metabolites was confirmed by multiple two-dimensional NMR approaches and STORM method (Supplementary Table 4 and Supplementary Figure 4). Unlike pentanoate, there was no significant difference in the relative concentration of beta-alanine or phenylalanine between the high and low calprotectin groups (p = 0.25 and p = 0.12 respectively). Four other bacterial genera (Escherichia/Shigella, Unclassified Lachnospiraceae, Roseburia, and Veillonella) had significant correlations with metabolites, as shown in Supplementary Table 4.
Metabolic potential of Dialister species
Metataxonomic and metabolite data were used to create a condensed reaction network in MetaboNetworks (Supplementary Figure 5).47 This illustrates metabolic pathways available in the microbiome of participants, and how they lead to biosynthesis or degradation of significant metabolites.
The potential contribution of Dialister to the production or degradation of significant metabolites was assessed by comparing its metabolic network map to the microbiome reaction network. Species level analysis of the Dialister genus was performed to identify the most appropriate reference genome to use to generate its metabolic network map. This revealed that 79% of the difference in the Dialister genus relative abundance between the groups was attributable to Dialister invisus, and that the relative abundance of Dialister invisus was significantly higher in the low calprotectin group (p=0.0069) (Supplementary Table 2). A metabolic network map was created from the genome of a representative strain from the Dialister invisus species (Dialister invisus JCM 17566). This network map acts as a partial approximation of the true metabolic potential of Dialister species, it is unable to account for variation amongst the different strains of the species, and does not contain all metabolic processes which occur within this strain.
The metabolic network of Dialister invisus JCM 17566 includes the succinate pathway, which is a major route for propionate formation. Propionate can be coupled to yield pentanoate (Supplementary Figure 5). The sequenced genome of Dialister invisus JCM 17566 does not feature any annotated genes for the biosynthesis or degradation of beta-alanine or phenylalanine.
Sensitivity analyses
Two sensitivity analyses were performed. The first excluded participants with faecal calprotectin values within the range of suggested thresholds for mucosal healing (100μg/g – 300μg/g), leaving only those with calprotectin values very highly predictive of mucosal healing or inflammation. One patient from the high calprotectin group with a faecal calprotectin value of 141μg/g was excluded. The second sensitivity analysis excluded patients receiving maintenance therapy with vedolizumab rather than an anti-TNF agent. This removed potential bias resulting from different therapy induced changes to the microbiota. Two patients were excluded, one from each group. The metataxonomic and metabolomic findings of the primary analysis were reproduced in both sensitivity analyses (Supplementary Table 5).
Discussion
This study investigates changes in the microbiota associated with mucosal healing, as indicated by calprotectin level <100μg/g, in a cohort of patients with established paediatric CD receiving maintenance biological therapy. There were no significant differences in alpha or beta diversity between the high and low calprotectin groups.
Interestingly, there was significantly greater relative abundance of bacteria from the Dialister genus in patients who had low calprotectin levels, and the relative abundance of Dialister had a strong negative correlation with calprotectin. The difference in Dialister relative abundance was largely attributable to the Dialister invisus species.
Dialister are obligately anaerobic or microaerophilic members of the firmicutes phylum. Amongst healthy adults they have been noted to have a bimodal abundance distribution, being either abundant or nearly absent, with decreased temporal stability in intermediate states.51 Dialister species are known to have reduced abundance in adult and paediatric Crohn’s disease patients relative to healthy controls.52,53 Interestingly Dialister also has higher relative abundance in Crohn’s disease patients who are in remission following ileocaecal resection than those who have post-operative recurrence.54 It is possible that Dialister, with its bimodal abundance distribution, acts as a biomarker of mucosal inflammation. Alternatively, Dialister may influence disease activity via its metabolic activity.
An estimate of Dialister invisus’s metabolic potential was made by producing a metabolic map from its protein coding genes which have been linked to metabolic pathways. This is not able to provide a complete account of its metabolic activity but did identify potential production of propionate. Production of propionate and acetate by Dialister invisus has been confirmed in culture experiments.55 Propionate is utilised in pentanoate formation. The positive correlation of Dialister with pentanoate observed in our study is consistent with Dialister promoting the formation of pentanoate, either through increasing the availability of its precursor or by a more direct mechanism.
Pentanoate, also known as valerate, was identified as a key indicator of mucosal healing by a machine learning model utilising spectral data from 1H NMR metabolomic analysis to distinguish high and low calprotectin groups. The relative concentration of pentanoate was higher in the low calprotectin group and was negatively correlated with calprotectin. Pentanoate has previously been found to be deficient in stool samples from patients with Crohn’s disease compared to controls,17 and an inverse relationship with clinical disease activity has been described in adult Crohn’s disease.56,57 In paediatric Crohn’s disease levels of pentanoate have been shown to increase with successful exclusive enteral nutrition treatment.58 An association with mucosal healing has not previously been described.
In vitro evidence shows that pentanoate has marked immunomodulatory effects on lymphocytes through promoting AKT-mTOR signalling and enhancing histone acetyltransferase activity, leading to increased IL-10 and suppressed IL-17 production.20 Pentanoate treatment decreased Th17 cell differentiation and reduced acute inflammation in a mouse model of experimental autoimmune encephalitis.20 In addition to its immunomodulatory effects pentanoate has also been found to impair vegetative growth of Clostridioides difficile in vitro, and supplementation has been proposed as a possible treatment strategy in C. difficile infection.59 The association of pentanoate with mucosal healing demonstrated in our study is consistent with its described effects on lymphocytes and pathogenic microbiota. It supports a model in which reduced concentration of pentanoate contributes to the pathogenesis of Crohn’s disease.
Lysine was also identified as a discriminator by the machine learning model; its relative concentration was higher in the high calprotectin group and was positively correlated with calprotectin. Faecal lysine has previously been found to be present in higher concentration in Crohn’s disease than in healthy controls,60 but it is not known whether it has any biological significance. Lysine is known to competitively inhibit uptake of arginine at the CAT1 transporter, and thus could reduce uptake of arginine which has immunomodulatory properties.61 Alternatively, the increase in lysine may be secondary to inflammation induced changes in microbial amino acid metabolism, and could be a useful biomarker of disease activity.
Beta-alanine and phenylalanine were correlated with Dialister, however their relative concentrations were not significantly different between the high and low calprotectin groups and the machine learning model did not identify either metabolite as a discriminator. It is not clear that beta-alanine or phenylalanine are associated with mucosal healing.
Given the significant metataxonomic and metabolomic differences between the high and low calprotectin groups it is surprising no differences were detected by inferred metagenomic analysis. However, apart from Dialister there were no large-scale changes in microbial community composition in our study, and the lack of difference likely reflects the limitations of inferred approaches in detecting smaller metagenomic differences.
No difference in total protease activity or protease subtype activity was found between high and low calprotectin groups. Protease subtype analysis was limited by non-specificity of protease inhibitors, therefore several significant protease classes such as matrixmetalloproteases could not be assessed.
This study has several limitations. It is a cross sectional analysis of 25 participants, longitudinal analysis of a larger data set would give further insights on how the microbiota and metabolome change with disease activity and would aid detection of smaller changes. Use of metagenomic sequencing would enhance understanding of how changes in the microbiota influence the metabolome, and targeted metabolomic approaches would allow quantification and improved detection of metabolites.
Use of a faecal calprotectin level under 100μg/g as a biomarker for mucosal healing is a potential limitation of this study, as there is uncertainty regarding the appropriate threshold to indicate mucosal healing, with values in the range of 100-300μg/g supported. A sensitivity analysis which excluded patients with ‘borderline’ faecal calprotectin values was performed to address this and confirmed all findings from the primary analysis. There is additional concern the accuracy of faecal calprotectin may be lower in ileal disease9, but in this cohort only one patient had isolated ileal disease and they had a very raised calprotectin level over 2000μg/g.
In this study clinical disease activity did not correlate closely with calprotectin. This observation is consistent with many other studies in adult and paediatric Crohn’s disease and highlights the need to consider both clinical remission and mucosal healing as distinct therapeutic targets62.
This study addresses an important and clinically relevant question – are there microbial or metabolic features in established paediatric CD which could contribute to sustained mucosal inflammation or promote mucosal healing? It reports a comprehensive multiomic analysis of a well phenotyped cohort, which is representative of many patients with established paediatric CD. It has several important findings. Lysine is increased in patients with ongoing mucosal inflammation, this association warrants further investigation to identify potentially relevant disease mechanisms and biomarkers. Most significantly, Dialister invisus is more abundant in patients with mucosal healing and has the potential to facilitate pentanoate formation. Pentanoate, which has known immunomodulatory properties highly relevant to Crohn’s disease, is also increased in those with mucosal healing and is correlated with Dialister relative abundance. The Dialister pentanoate relationship could therefore represent a novel and targetable therapeutic axis which promotes mucosal healing in Crohn’s disease.
Supplementary Material
Acknowledgements
We would like to thank Dr Matthew Hyde and Professor Modi of the Neonatal research unit at Chelsea and Westminster Hospital for providing clinical research facilities, and Dr Warren Hyer, Eunice Goto, and Kay Crook for facilitating recruitment and sample collection. We would also like to thank all of the patients and their families for their participation.
Funding
This work was funded by the National Institute of Health Research (NIHR) and the NIHR Imperial Biomedical Research Centre (BRC). The Division of Digestive Disease at Imperial College London receives financial support from the National Institute of Health Research (NIHR) Imperial Biomedical Research Centre (BRC) based at Imperial College Healthcare NHS Trust and Imperial College London. This article is independent research funded by the NIHR BRC, and the views expressed in this publication are those of the authors and not necessarily those of the NHS, NIHR, or the Department of Health.
References
- 1.Ruemmele FM, Veres G, Kolho KL, et al. Consensus guidelines of ECCO/ESPGHAN on the medical management of pediatric Crohn’s disease. J Crohns Colitis. 2014;8(10):1179–1207. doi: 10.1016/j.crohns.2014.04.005. [DOI] [PubMed] [Google Scholar]
- 2.Pineton de Chambrun G, Peyrin-Biroulet L, Lémann M, Colombel J-F. Clinical implications of mucosal healing for the management of IBD. Nat Rev Gastroenterol Hepatol. 2010;7(1):15–29. doi: 10.1038/nrgastro.2009.203. [DOI] [PubMed] [Google Scholar]
- 3.Vazquez Moron JM, Pallares Manrique H, Machancoses FH, Ramos Lora M, Ruiz Frutos C. Accurate cut-offs for predicting endoscopic activity and mucosal healing in Crohn’s disease with fecal calprotectin. Rev Esp Enferm Dig. 2017;109(2):130–136. doi: 10.17235/reed.2017.4542/2016. [DOI] [PubMed] [Google Scholar]
- 4.Mosli MH, Zou G, Garg SK, et al. C-Reactive Protein, Fecal Calprotectin, and Stool Lactoferrin for Detection of Endoscopic Activity in Symptomatic Inflammatory Bowel Disease Patients: A Systematic Review and Meta-Analysis. Am J Gastroenterol. 2015;110(6):802–819. doi: 10.1038/ajg.2015.120. quiz 820. [DOI] [PubMed] [Google Scholar]
- 5.Kennedy NA, Jones G-R, Plevris N, Patenden R, Arnott ID, Lees CW. Association Between Level of Fecal Calprotectin and Progression of Crohn’s Disease. Clin Gastroenterol Hepatol Off Clin Pract J Am Gastroenterol Assoc. 2019;17(11):2269–2276.e4. doi: 10.1016/j.cgh.2019.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kostas A, Siakavellas SI, Kosmidis C, et al. Fecal calprotectin measurement is a marker of short-term clinical outcome and presence of mucosal healing in patients with inflammatory bowel disease. World J Gastroenterol. 2017;23(41):7387–7396. doi: 10.3748/wjg.v23.i41.7387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Weinstein-Nakar I, Focht G, Church P, et al. Associations Among Mucosal and Transmural Healing and Fecal Level of Calprotectin in Children With Crohn’s Disease. Clin Gastroenterol Hepatol. 2018;16(7):1089–1097.e4. doi: 10.1016/j.cgh.2018.01.024. [DOI] [PubMed] [Google Scholar]
- 8.Reenaers C, Bossuyt P, Hindryckx P, Vanpoucke H, Cremer A, Baert F. Expert opinion for use of faecal calprotectin in diagnosis and monitoring of inflammatory bowel disease in daily clinical practice. United Eur Gastroenterol J. 2018;6(8):1117–1125. doi: 10.1177/2050640618784046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Simon EG, Wardle R, Thi AA, Eldridge J, Samuel S, Moran GW. Does fecal calprotectin equally and accurately measure disease activity in small bowel and large bowel Crohn’s disease?: a systematic review. Intest Res. 2019;17(2):160–170. doi: 10.5217/ir.2018.00114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ashton JJ, Borca F, Mossotto E, et al. Increased prevalence of anti-TNF therapy in paediatric inflammatory bowel disease is associated with a decline in surgical resections during childhood. Aliment Pharmacol Ther. 2019;49(4):398–407. doi: 10.1111/apt.15094. [DOI] [PubMed] [Google Scholar]
- 11.Ding NS, Hart A, De Cruz P. Systematic review: predicting and optimising response to anti-TNF therapy in Crohn’s disease - algorithm for practical management. Aliment Pharmacol Ther. 2016;43(1):30–51. doi: 10.1111/apt.13445. [DOI] [PubMed] [Google Scholar]
- 12.Grossi V, Lerer T, Griffiths A, et al. Concomitant Use of Immunomodulators Affects the Durability of Infliximab Therapy in Children With Crohn’s Disease. Clin Gastroenterol Hepatol. 2015;13(10):1748–1756. doi: 10.1016/j.cgh.2015.04.010. [DOI] [PubMed] [Google Scholar]
- 13.Shouval DS, Rufo PA. The Role of Environmental Factors in the Pathogenesis of Inflammatory Bowel Diseases: A Review. JAMA Pediatr. 2017;171(10):999–1005. doi: 10.1001/jamapediatrics.2017.2571. [DOI] [PubMed] [Google Scholar]
- 14.Gkouskou KK, Deligianni C, Tsatsanis C, Eliopoulos AG. The gut microbiota in mouse models of inflammatory bowel disease. Front Cell Infect Microbiol. 2014;4:28. doi: 10.3389/fcimb.2014.00028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lopetuso LR, Petito V, Zambrano D, et al. Gut Microbiota: A Key Modulator of Intestinal Healing in Inflammatory Bowel Disease. Dig Dis. 2016;34(3):202–209. doi: 10.1159/000444460. [DOI] [PubMed] [Google Scholar]
- 16.Neis EPJG, Dejong CHC, Rensen SS. The role of microbial amino acid metabolism in host metabolism. Nutrients. 2015;7(4):2930–2946. doi: 10.3390/nu7042930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhuang X, Li T, Li M, et al. Systematic Review and Meta-analysis: Short-Chain Fatty Acid Characterization in Patients With Inflammatory Bowel Disease. Inflamm Bowel Dis. 2019;25(11):1751–1763. doi: 10.1093/ibd/izz188. [DOI] [PubMed] [Google Scholar]
- 18.Parada Venegas D, De la Fuente MK, Landskron G, et al. Short Chain Fatty Acids (SCFAs)-Mediated Gut Epithelial and Immune Regulation and Its Relevance for Inflammatory Bowel Diseases. Front Immunol. 2019;10:277. doi: 10.3389/fimmu.2019.00277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schulthess J, Pandey S, Capitani M, et al. The Short Chain Fatty Acid Butyrate Imprints an Antimicrobial Program in Macrophages. Immunity. 2019;50(2):432–445.e7. doi: 10.1016/j.immuni.2018.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Luu M, Pautz S, Kohl V, et al. The short-chain fatty acid pentanoate suppresses autoimmunity by modulating the metabolic-epigenetic crosstalk in lymphocytes. Nat Commun. 2019;10(1):760. doi: 10.1038/s41467-019-08711-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Duboc H, Rajca S, Rainteau D, et al. Connecting dysbiosis, bile-acid dysmetabolism and gut inflammation in inflammatory bowel diseases. Gut. 2013;62(4):531–539. doi: 10.1136/gutjnl-2012-302578. [DOI] [PubMed] [Google Scholar]
- 22.Liu Y, Wang X, Hu C-AA. Therapeutic Potential of Amino Acids in Inflammatory Bowel Disease. Nutrients. 2017;9(9) doi: 10.3390/nu9090920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jablaoui A, Kriaa A, Mkaouar H, et al. Fecal Serine Protease Profiling in Inflammatory Bowel Diseases. Front Cell Infect Microbiol. 2020;10:21. doi: 10.3389/fcimb.2020.00021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Carroll IM, Maharshak N. Enteric bacterial proteases in inflammatory bowel disease-pathophysiology and clinical implications. World J Gastroenterol. 2013;19(43):7531–7543. doi: 10.3748/wjg.v19.i43.7531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vergnolle N. Protease inhibition as new therapeutic strategy for GI diseases. Gut. 2016;65(7):1215–1224. doi: 10.1136/gutjnl-2015-309147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gevers D, Kugathasan S, Denson LAA, et al. The treatment-naive microbiome in new-onset Crohn’s disease. Cell Host Microbe. 2014;15(3):382–392. doi: 10.1016/j.chom.2014.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Goyal A, Yeh A, Bush BR, et al. Safety, Clinical Response, and Microbiome Findings Following Fecal Microbiota Transplant in Children With Inflammatory Bowel Disease. Inflamm Bowel Dis. 2018;24(2):410–421. doi: 10.1093/ibd/izx035. [DOI] [PubMed] [Google Scholar]
- 28.Kolho K-L, Korpela K, Jaakkola T, et al. Fecal Microbiota in Pediatric Inflammatory Bowel Disease and Its Relation to Inflammation. Am J Gastroenterol. 2015;110(6):921–930. doi: 10.1038/ajg.2015.149. [DOI] [PubMed] [Google Scholar]
- 29.Lewis JD, Chen EZ, Baldassano RN, et al. Inflammation, Antibiotics, and Diet as Environmental Stressors of the Gut Microbiome in Pediatric Crohn’s Disease. Cell Host Microbe. 2015;18(4):489–500. doi: 10.1016/j.chom.2015.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kowalska-Duplaga K, Kapusta P, Gosiewski T, et al. Changes in the Intestinal Microbiota Are Seen Following Treatment with Infliximab in Children with Crohn’s Disease. J Clin Med. 2020;9(3) doi: 10.3390/jcm9030687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gołębiewski M, Tretyn A. Generating amplicon reads for microbial community assessment with next-generation sequencing. J Appl Microbiol. 2020;128(2):330–354. doi: 10.1111/jam.14380. [DOI] [PubMed] [Google Scholar]
- 32.Alcon-Giner C, Caim S, Mitra S, et al. Optimisation of 16S rRNA gut microbiota profiling of extremely low birth weight infants. BMC Genomics. 2017;18(1):841. doi: 10.1186/s12864-017-4229-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Johnson JS, Spakowicz DJ, Hong B-Y, et al. Evaluation of 16S rRNA gene sequencing for species and strain-level microbiome analysis. Nat Commun. 2019;10(1):5029. doi: 10.1038/s41467-019-13036-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Illumina 16S Metagenomic Sequencing Library Preparation [Google Scholar]
- 35.Mullish BH, Pechlivanis A, Barker GF, Thursz MR, Marchesi JR, McDonald JAK. Functional microbiomics: Evaluation of gut microbiota-bile acid metabolism interactions in health and disease. Methods. 2018;149:49–58. doi: 10.1016/j.ymeth.2018.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Callahan BJ, McMurdie PJ, Rosen MJ, Han AW, Johnson AJA, Holmes SP. DADA2: High-resolution sample inference from Illumina amplicon data. Nat Methods. 2016;13(7):581–583. doi: 10.1038/nmeth.3869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McMurdie PJ, Holmes S. phyloseq: an R package for reproducible interactive analysis and graphics of microbiome census data. PLoS One. 2013;8(4):e61217. doi: 10.1371/journal.pone.0061217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Parks DH, Tyson GW, Hugenholtz P, Beiko RG. STAMP: statistical analysis of taxonomic and functional profiles. Bioinformatics. 2014;30(21):3123–3124. doi: 10.1093/bioinformatics/btu494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Iwai S, Weinmaier T, Schmidt BL, et al. Piphillin: Improved Prediction of Metagenomic Content by Direct Inference from Human Microbiomes. PLoS One. 2016;11(11):e0166104. doi: 10.1371/journal.pone.0166104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rawlings ND, Waller M, Barrett AJ, Bateman A. MEROPS: the database of proteolytic enzymes, their substrates and inhibitors. Nucleic Acids Res. 2014;42(Database issue):D503–9. doi: 10.1093/nar/gkt953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gratton J, Phetcharaburanin J, Mullish BH, et al. Optimized Sample Handling Strategy for Metabolic Profiling of Human Feces. Anal Chem. 2016;88(9):4661–4668. doi: 10.1021/acs.analchem.5b04159. [DOI] [PubMed] [Google Scholar]
- 42.Dona AC, Jimenez B, Schafer H, et al. Precision high-throughput proton NMR spectroscopy of human urine, serum, and plasma for large-scale metabolic phenotyping. Anal Chem. 2014;86(19):9887–9894. doi: 10.1021/ac5025039. [DOI] [PubMed] [Google Scholar]
- 43.Dieterle F, Ross A, Schlotterbeck G, Senn H. Probabilistic quotient normalization as robust method to account for dilution of complex biological mixtures. Application in 1H NMR metabonomics. Anal Chem. 2006;78(13):4281–4290. doi: 10.1021/ac051632c. [DOI] [PubMed] [Google Scholar]
- 44.Posma JM, Garcia-Perez I, Ebbels TMD, et al. Optimized Phenotypic Biomarker Discovery and Confounder Elimination via Covariate-Adjusted Projection to Latent Structures from Metabolic Spectroscopy Data. J Proteome Res. 2018;17(4):1586–1595. doi: 10.1021/acs.jproteome.7b00879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Posma JM, Garcia-Perez I, De Iorio M, et al. Subset optimization by reference matching (STORM): an optimized statistical approach for recovery of metabolic biomarker structural information from 1H NMR spectra of biofluids. Anal Chem. 2012;84(24):10694–10701. doi: 10.1021/ac302360v. [DOI] [PubMed] [Google Scholar]
- 46.Wishart DS, Feunang YD, Marcu A, et al. HMDB 4.0: the human metabolome database for 2018. Nucleic Acids Res. 2018;46(D1):D608–D617. doi: 10.1093/nar/gkx1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Posma JM, Robinette SL, Holmes E, Nicholson JK. MetaboNetworks, an interactive Matlab-based toolbox for creating, customizing and exploring sub-networks from KEGG. Bioinformatics. 2013;30(6):893–895. doi: 10.1093/bioinformatics/btt612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Morgulis A, Coulouris G, Raytselis Y, Madden TL, Agarwala R, Schäffer AA. Database indexing for production MegaBLAST searches. Bioinformatics. 2008;24(16):1757–1764. doi: 10.1093/bioinformatics/btn322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yarza P, Yilmaz P, Pruesse E, et al. Uniting the classification of cultured and uncultured bacteria and archaea using 16S rRNA gene sequences. Nat Rev Microbiol. 2014;12(9):635–645. doi: 10.1038/nrmicro3330. [DOI] [PubMed] [Google Scholar]
- 50.Caspi R, Billington R, Ferrer L, et al. The MetaCyc database of metabolic pathways and enzymes and the BioCyc collection of pathway/genome databases. Nucleic Acids Res. 2016;44(D1):D471–80. doi: 10.1093/nar/gkv1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lahti L, Salojärvi J, Salonen A, Scheffer M, de Vos WM. Tipping elements in the human intestinal ecosystem. Nat Commun. 2014;5(1):4344. doi: 10.1038/ncomms5344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Joossens M, Huys G, Cnockaert M, et al. Dysbiosis of the faecal microbiota in patients with Crohn’s disease and their unaffected relatives. Gut. 2011;60(5):631–637. doi: 10.1136/gut.2010.223263. [DOI] [PubMed] [Google Scholar]
- 53.Kowalska-Duplaga K, Gosiewski T, Kapusta P, et al. Differences in the intestinal microbiome of healthy children and patients with newly diagnosed Crohn’s disease. Sci Rep. 2019;9(1):18880. doi: 10.1038/s41598-019-55290-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mondot S, Lepage P, Seksik P, et al. Structural robustness of the gut mucosal microbiota is associated with Crohn's disease remission after surgery. Gut. 2016;65(6):954 LP–962. doi: 10.1136/gutjnl-2015-309184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sakamoto M, Ikeyama N, Toyoda A, et al. Dialister hominis sp. nov., isolated from human faeces. Int J Syst Evol Microbiol. 2020;70(1):589–595. doi: 10.1099/ijsem.0.003797. [DOI] [PubMed] [Google Scholar]
- 56.De Preter V, Machiels K, Joossens M, et al. Faecal metabolite profiling identifies medium-chain fatty acids as discriminating compounds in IBD. Gut. 2015;64(3):447 LP–458. doi: 10.1136/gutjnl-2013-306423. [DOI] [PubMed] [Google Scholar]
- 57.De Preter V, Joossens M, Ballet V, et al. Metabolic profiling of the impact of oligofructose-enriched inulin in Crohn’s disease patients: a double-blinded randomized controlled trial. Clin Transl Gastroenterol. 2013;4(1):e30–e30. doi: 10.1038/ctg.2012.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tjellström B, Högberg L, Stenhammar L, et al. Effect of exclusive enteral nutrition on gut microflora function in children with Crohn’s disease. Scand J Gastroenterol. 2012;47(12):1454–1459. doi: 10.3109/00365521.2012.703234. [DOI] [PubMed] [Google Scholar]
- 59.McDonald JAK, Mullish BH, Pechlivanis A, et al. Inhibiting Growth of Clostridioides difficile by Restoring Valerate, Produced by the Intestinal Microbiota. Gastroenterology. 2018;155(5):1495–1507.e15. doi: 10.1053/j.gastro.2018.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Marchesi JR, Holmes E, Khan F, et al. Rapid and Noninvasive Metabonomic Characterization of Inflammatory Bowel Disease. J Proteome Res. 2007;6(2):546–551. doi: 10.1021/pr060470d. [DOI] [PubMed] [Google Scholar]
- 61.Sugihara K, Morhardt TL, Kamada N. The Role of Dietary Nutrients in Inflammatory Bowel Disease. Front Immunol. 2019;9:3183. doi: 10.3389/fimmu.2018.03183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Peyrin-Biroulet L, Sandborn W, Sands BE, et al. Selecting Therapeutic Targets in Inflammatory Bowel Disease (STRIDE): Determining Therapeutic Goals for Treat-to-Target. Am J Gastroenterol. 2015;110(9):1324–1338. doi: 10.1038/ajg.2015.233. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.