Abstract
Hypoxia plays an important role in pancreatic cancer progression. It drives various metabolic reprogramming in cells including that of lipids, which in turn, can modify the structure and function of cell membranes. Homeostatic adaptation of membranes is well-recognized, but how and if it is regulated in hypoxic pancreatic cancer and its relation to aggressive phenotype and metastasis remains elusive. Here we show hypoxia-induced extensive global lipid remodelling spanning changes in lipid classes, unsaturation levels, glyceryl backbone and acyl chain lengths. No major modulation of plasma membrane biophysical properties revealed a decoupling of lipidome modulation from membrane properties under hypoxia. This was supported by observing minor changes in the lipidome of plasma membranes under hypoxia. Further, hypoxia increased migration and invasion underpinned by reduced actin volume, cell cortical stiffness and facile tether dynamics. In conclusion, we demonstrate buffering of the lipidome alterations leading to a homeostatic membrane response. These findings will help to understand the hypoxic regulation of pancreatic membrane homeostasis and identify tangible theranostic avenues.
Keywords: Pancreatic cancer, Hypoxia, Cell membranes, Lipidomics, Membrane biophysics
1. Introduction
Pancreatic cancer (PAC) is a highly aggressive malignant cancer with <9 % five-year survival rate and poor prognosis [1]. The dismal prognosis is due to its rapid progression and highly metastatic potential [2,3]. PAC involves significant alterations of cellular metabolism including glucose, amino acids, fatty acids (FA), and lipids, indispensable for their growth and survival [4,5]. Further, multi-omic profiling of pancreatic cancer stem cells has revealed extensive reliance on the fatty acid pathway highlighting lipidome rewiring [6]. Another major hallmark of PAC is the presence of oxygen deprivation, i.e., hypoxia, which is correlated strongly to aggressiveness [7–9]. Hypoxia-inducible factor-1α(HIF-1α) mediated hypoxic gene regulation, in general, is linked with the perturbation of many metabolic pathways. Specifically, rewiring lipid metabolism has revealed a tight regulation of lipid synthesis, lipolysis, and lipid droplets [10]. Extracellular FA flux, TG synthesis, and synthesis of derivative phospholipids increase under hypoxia, while lipid catabolism is suppressed. Notably, the accumulation of cholesterol has also been reported [11]. To obviate lipotoxicity due to an imbalance between synthesis and catabolism, lipid storage in lipid droplets is aptly enhanced [12]. The latter serves as the major source for the production of essential sterol esters and importantly phospholipids for the biogenesis of new membranes.
Modulation of the lipidome foremost affects lipid membranes which are essential for the physiological functioning of mammalian cells. This is rendered by either maintaining the structural integrity or via non-structural functional roles in signalling and trafficking [13,14]. Membranes orchestrate various membrane-associated processes by leveraging biophysical properties such as fluidity, packing, stiffness, curvature, and lateral organization [15]. Changes in these properties alter lipid-protein diffusion, localization, lipid-protein interactions, and finally their activity [16,17]. These impose an exquisite regulatory control and hence are critically modulated in cancer for survival and proliferation [18]. For instance, membrane fluidity in cancer is altered multiple ways. In highly metastatic cancer it can increase by reduction of cholesterol and saturated lipids for facile movement, while in drug-resistant cancer, it can decrease to restrict drug permeability [18]. These suggest non-trivial and complex regulation of membrane properties and function under stress. Cancer cell membrane mechanics and lateral organization are also linked with their metastatic dissemination.
Because of their central role, effective maintenance of membrane properties is essential in both normal and disease states. However, the correlation between hypoxic stress, lipidome remodelling, associated membrane properties, and metastatic potential in pancreatic cancer is not known. Here we evaluate the pancreatic cell membrane response to hypoxia using lipidomics, membrane biophysics, and cell biology. Upon hypoxia induction, levels of glycerophospholipids and sphingolipids decreased accompanied by a disproportioned increase of cholesterol esters and triacylglycerides leading to increased lipid storage. Distinct changes in the saturation levels and acyl chain lengths in a lipid class-specific fashion were revealed. In addition, hypoxia favoured the accumulation of acyl lipids compared to their ether-linked counterparts. Next, decoupling of plasma membrane biophysical properties from the global lipidome alterations indicated a stable plasma membrane homeostatic response. This was confirmed by having minor changes in the plasma membrane lipid pool itself under hypoxia. Our data reveals that conservation of plasma membrane biophysical attributes may be an essential factor for cellular fitness under hypoxia to maintain high metastatic potential. These findings will drive gaining a deepened understanding of the underlying mechanisms involved in the hypoxic regulation of pancreatic membranes. This is a fundamental step toward the development of reliable lipid and membrane biophysical biomarkers as well as membrane-centric therapeutic interventions.
2. Results and discussions
2.1. Global lipidomics under hypoxia reveals rewiring of lipid classes, glycerol-backbone, and chain lengths
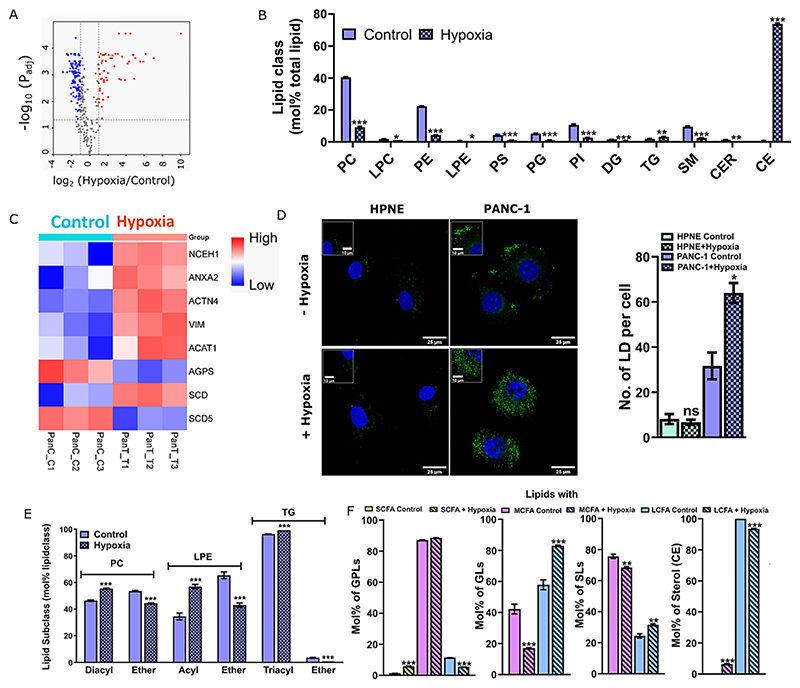
To investigate hypoxia-induced alterations in the global lipidome of pancreatic cancer, an in-depth quantitative mass spectrometric analysis was performed in the PANC-1 and HPNE cell lines. Hypoxia was induced using 200 μM of CoCl2 in the absence of serum and confirmed via HIF-1α expressiosn (Fig. S1A−B). Previous studies have shown that induction of hypoxia using CoCl2 is equivalent to ~1 % oxygen level [19,20]. As a control a non-cancerous pancreatic cell line, HPNE was used. Since both the cell lines are cultured in different media, we ruled out the effect of media composition on hypoxia induction by observing a similar level of HIF-1α expression in PANC-1 when cultured in HPNE media (Fig. S1B). In total, 290 and 259 lipids were identified and quantified in PANC-1 and HPNE cells, respectively (Supplementary file 1). From this pool, around 170 (PANC-1) and 20 (HPNE) lipid molecules were significantly altered under hypoxia compared to normoxic conditions (Figs. 1A, S1C). These lipids belonged to four main categories: glycerophospholipids (GPLs), sphingolipids (SPs), glycerolipids (GLs), and sterol lipids (STs) and covered 12 classes such as phosphatidylcholines (PC), lysophosphatidylcholine (LPC), phosphatidylinositol (PI), phosphatidylethanol-amine (PE), lyso-phosphatidylethanolamine (LPE), phosphatidylserine (PS), phosphatidylglycerol (PG), sphingomyelin (SM), triacylglyceride (TG), cholesteryl esters (CE), ceramides (CER) and diacylglycerol (DG). Mol% of TG and CE (belonging to GLs and STs categories, respectively) in the global lipidome were significantly upregulated in tumour cell line under hypoxia (Fig. 1B). TG and CE are predominant residents of lipid droplets and ER, but can also accumulate in plasma membranes (2−8 mol%), however Fig. 1B details the global changes in all TG/CE lipid pools (associated with both plasma and internal membranes). Label free proteomics revealed a higher expression of acyl-coenzyme A (CoA): cholesterol acyltransferase1 (ACAT1, enzyme required for the conversion of cholesterol to cholesterol ester) in PANC-1 under hypoxia, (Fig. 1C, Supplementary file 2) validating the increased abundance of CE in tumorigenic lipidome.
Fig. 1.
(A) Volcano plot depicting the significant fold change of different lipid species in PANC-1 cells cultured in the absence and presence of hypoxia in three independent experiments. Lipids with a min. fold change of 2 and adjusted P-value <0.05 are displayed in a colour-coordinated manner. (B) Mol% total lipid abundance at the lipid class level shows differential distributions. Data represent the average mol% within total lipid abundance for each lipid class ± standard error of the mean of three independent experiments. PC, LPE and TG in panel B represents both Ester- and Ether-linked lipids. (C) Heatmap showing a distinct pattern in LC-MS-based protein label free quantification (LFQ) intensity of selected targets in PANC-1 control and hypoxia induced cells for three independent experiments. Proteins with a min. log2 fold change of 1.5 and P-value <0.05 are displayed. (D) Lipid droplet staining with Bodipy showed a higher number of LDs in the cancer cells in the presence of hypoxia compared to normoxia and normal cell line HPNE. Data for LDs are presented as mean ± SEM of two independent experiments. Total 20 fields of view were taken per replicate and 60 cells were considered (Inset showing zoomed in lipid droplets; Scale bar: 25 μm). PANC-1 and HPNE are represented by blue and light green colour codes, respectively. Bar plots with patterns represent hypoxic conditions. (E) The mol% of each glycerol backbone linkage within lipid class. Alkyl ether (O) and plasmalogen (P) were combined as ether (E) lipids. (F) The mol% of lipids with varying lengths in their FA chain components (SCFA: small chain length fatty acid; <20, MCFA: medium chain length fatty acid; C20-C39, LFCA: long chain length fatty acid; C40-C60) within lipid category under control and hypoxia conditions. In all the cases, bar plots with patterns represent hypoxic conditions. Statistical analysis was determined using a student’s t-test (* P value <0.05, ** P value <0.01, and ***P value <0.001). To control false discovery two-stage linear step-up method of Benjamini, Krieger, and Yekutieli were performed, with Q = 1 %.
Increased abundance of CE and TG also implicate modulation of cellular lipid droplets (LDs) under hypoxia in pancreatic cancer cells as these lipids are major components of LDs [21]. Indeed, we observed a significant accumulation of LDs under HIF-1α upregulation (Fig. 1D). Consistently, differentially expressed proteins involved in LD biogenesis (NCEH1; ANXA2; ACTN4 and VIM) were upregulated in PANC-1 under hypoxic condition (Fig. 1C). These proteins were not modulated in HPNE under hypoxia thus accounting for low LD abundance (Fig. 1D). However, despite having reasonable amount of global cellular TG and CE levels in this cell line even under normal conditions, a low LD abundance is an intriguing finding and needs further investigation. GPLs, SPs, and DG were downregulated under hypoxia (Fig. 1B) in PANC-1 but not in HPNE (Fig. S1D).
Lipid metabolism has a high phenotypic plasticity allowing cells to adapt their membrane composition to different stress conditions. Along these lines, analysing co-regulated lipids can furnish critical hints about the physiological response of the cell to a certain stimulus and the molecular mechanisms underlying it. One such pair is the acyl/ether lipid ratio. Replacing the ester-carbonyl group in the sn-1 position of glyco- or glycerophospho-lipids generates ether lipids. We observed modulation of acyl vs. ether lipids in the most abundant lipid classes (Fig. 1E). For the GPLs, these included PC and LPE subclasses, wherein significant increase of diacyl-PC and acyl-LPE were observed under hypoxia with a concomitant reduction of their ether counterparts; PC-E and LPE-E. Likewise, a significant increase of triacylglycerol (TG-acyl) and a corresponding decrease of TG-E was observed in PANC-1. Decreased abundance of ether lipids under hypoxia in PANC-1 was validated by observing a reduced expression of alkylglyceronephosphate synthase (AGPS), a crucial enzyme involved in the synthesis of ether lipids, including ether glycerophospholipids [22] (Fig. 1C). Previously it has been shown that ether-containing ethanolamine phospholipids are significantly reduced in the serum of pancreatic cancer patients which also aligns with our data [23]. Also, the downregulation of ether lipid has been associated with the upregulation of the equivalent ester phospholipids [24], however, the molecular mechanism underlying this co-relation is not fully understood. In relevance to this work, whether this has an impact on membrane structure and function needs further clarification but factors such as the cross-sectional area of lipids, their melting temperatures, the differences in packing, and the interaction with other lipids such as cholesterol are likely to have a contribution.
Next, lipidomic data also revealed that hypoxia-induced an accumulation of long chain length lipid species of GLs and SLs in PANC-1. In contrast, medium-chained lipid species were upregulated within sterols (Fig. 1F). In case of GPLs, long chained lipids were downregulated, whereas small-chained lipids were upregulated with modulation of medium chain length species. Of note, more than one FA chain may contribute to the number of C-atoms in the overall chain length description for long chain fatty acid lipids. This data collectively, suggest remodelling of lipid chain lengths in response to hypoxia in a lipid-dependent manner. This could possibly drive lipid phase separation and/or lipid sorting to minimize hydrophobic mismatch and subsequently induce membrane re-organization. Further investigations to elucidate the functional aspect of this remodelling in organelle-centric fashion may provide additional cues. The upregulation of long-chained lipids is linked with increased expression of elongation of very long-chain fatty acids protein 1 (EVOLV1) in PANC-1 under hypoxia (Fig. S2C).
2.2. Increased unsaturation index at lipid category and class level in response to hypoxia
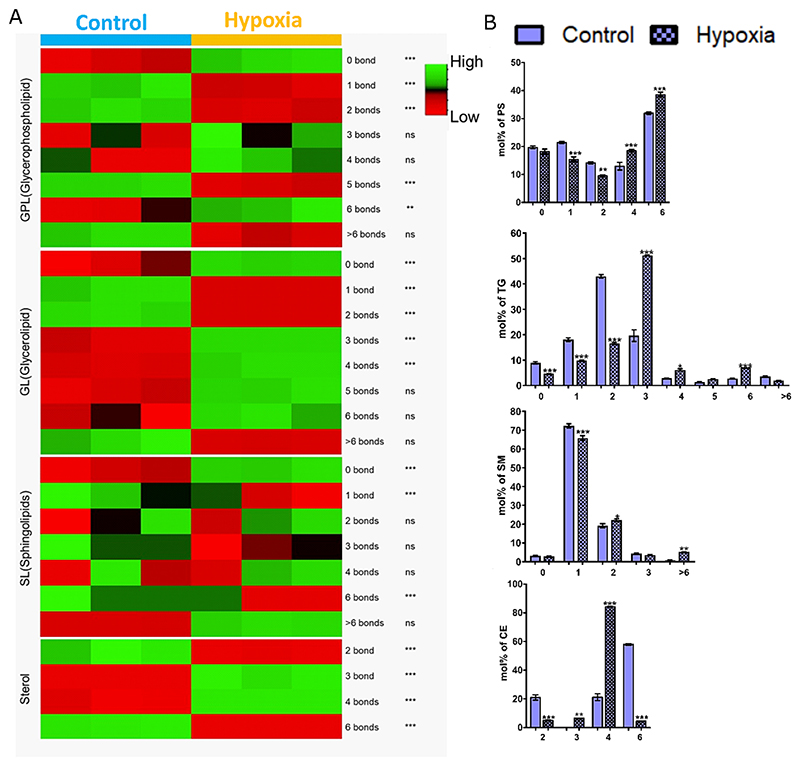
To better understand the effect of hypoxia on the unsaturation index of lipids, which are known to impact membrane biophysical properties, we investigated the poly-unsaturation levels in all lipid classes in both the cell lines (Figs. 2A, and S2Ai−iv). Hypoxia modulated multilevel poly-unsaturation within different lipid categories. Specifically, it increased GPLs species with 4 & 6 double bonds; SPs species with >6 double bonds; GLs with 3−6 double bonds, and STs with 3−4 double bonds in the cancer cell (Fig. 2A). Analysis of changes at the lipid class level revealed that the major contributor to the observed changes in GPLs was from PS (Fig. 2B). Similarly, the major contributors for the increase of SPs species with >6 double bonds were mainly from SM; with a concomitant increase in its chain lengths as well. For GLs and STs, the changes were found to be restricted to TG (along with longer chain lengths) and CE, respectively (Fig. 2B). No such changes were observed in HPNE (Fig. S2A) except for STs with 5 double bonds. Interestingly, the protein expression of many of the desaturases was modulated in PANC-1 cells suggesting a tight balance between saturation vs unsaturation under hypoxia. For example, the expression of stearoyl-CoA Desaturase (SCD) increased, but that of stearoyl-CoA Desaturase 5 (SCD5) decreased (Fig. 1C). At present, it is difficult to predict which enzymes are responsible for the observed changes in the polyunsaturation levels of fatty acids within different lipid classes under hypoxia. In HPNE, following proteomic profiling, no major changes were observed with these proteins.
Fig. 2.
(A) Heatmap depicting the Mol% total lipid abundance distribution at the lipid category level as per the double bond index (0,1,2,3,4,5 or >6 bonds), colour coded by row with green indicating high mol% abundance and red indicating low mol% abundance. (B) Mol% total lipid abundance distribution at the lipid class level of PANC-1 cells were summed up as per double bond index (0,1,2,3,4,5,6 & >6 bonds) and plotted using graphpad prism 8. In all the cases, bar plots with patterns represent hypoxic conditions. Statistical significance was determined by ANOVA followed by Bonferroni’s multiple comparison test, with multiplicity adjusted P value <0.01. * Adjusted P value <0.01, ** adjusted P value <0.001, and *** adjusted P value <0.0001.
2.3. Homeostatic maintenance of plasma membrane fluidity, order, and dynamics under hypoxic stress
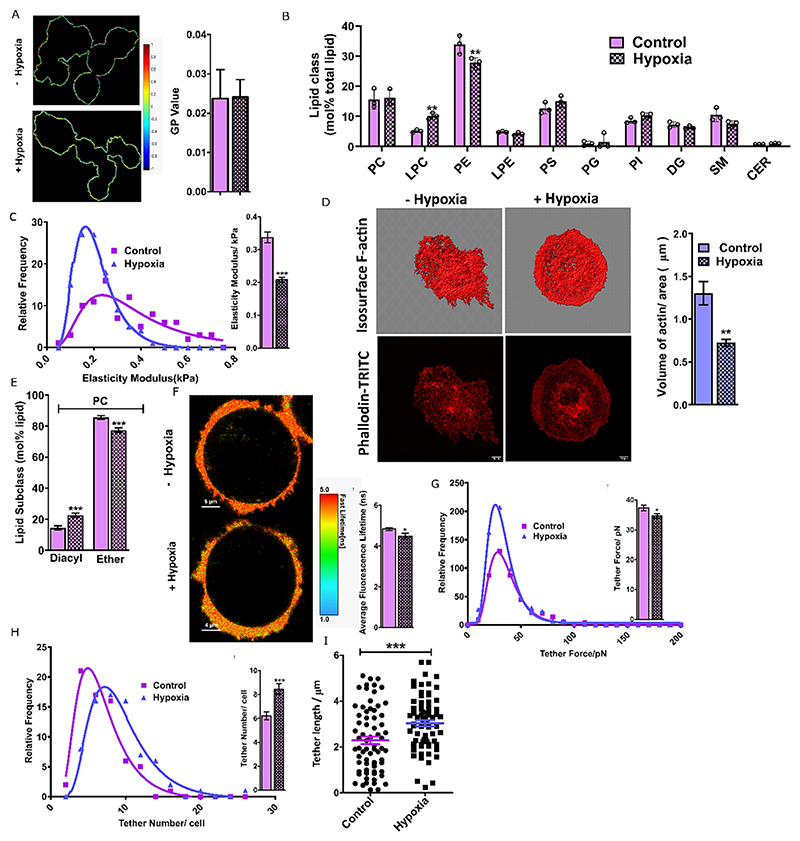
An important objective of this study was to investigate the influence of hypoxia on the membrane’s biophysical properties. To accomplish that, we analyzed membrane packing in cancerous and non-cancerous live cells using solvatochromic dyes (Laurdan, Di4-ANEPPDHQ) whose spectral characteristics are dependent on membrane properties such as order and hydration [25,26]. Deconvoluting the spectra to states with high (longer wavelength) and low hydration (short wavelength) within membrane bilayers provides a ratiometric index called generalized polarization (GP). GP can range from +1.0 (high order/low hydration) to -1.0 (low order/high hydration) in lipid membranes. Confocal spectral imaging of Di4-ANEPPDHQ in the live cell membrane was used to construct 2D GP maps of treated and untreated cells (Fig. 3A) and displayed a broad distribution of GP pixels (Fig. S3A) indicative of a heterogeneous plasma membrane organization. No effect on the plasma membrane order/hydration under hypoxia was observed in both cell types (Figs. 3A and S3B). Further, using the Laurdan probe and spectroscopy, a similar observation was confirmed (Fig. S3C). Next, we evaluated lipid bilayer depth-dependent plasma membrane fluidity in live cells using fluorescence anisotropy and specific lipid probes. While DPH (diphenylhexatriene) positions itself at the deep hydrophobic acyl chain regions (hydrophobic), its charged counterpart, TMA-DPH (trimethylamino-diphenylhexatriene) is located at the interfacial polar head group region of lipid bilayers [27]. Hypoxia induction did not change any significant level of fluidity in both cells (Fig. S3D). Membrane dynamics were also unaffected, confirmed by measuring no substantial changes in the lateral lipid diffusion in the plasma membranes of cancer and normal live cells under hypoxic and normoxic conditions (Fig. S3F−H). Taken together, the pancreatic cancer plasma cell membranes maintained their hydration, order, and dynamics under hypoxic stress with only minor perturbations.
Fig. 3.
(A) GP imaging (N = 3, n = 70) of di-4-ANEPPDHQ-labeled cell membranes of PANC-1 cell line and the corresponding GP values. (B) Mol% total lipid abundance (N = 3) at the lipid class level of the plasma membrane (PM), after removing TAGs and CEs, of PANC-1 cells cultured in the absence and presence of hypoxia. (C) Elastic moduli distribution of PANC-1 cells in the presence and absence of hypoxia, calculated using in a total of 200 force curves in two independent experiments. (D) Representative isosurface and Phalloidin-TRITC images (N = 3, n = 50) were used to quantify actin abundance. The bar graph represents the volume of actin per unit area in control and hypoxia-induced cells. Scale bar, 10 μm; 63× oil objective. (E) Mol% PC lipid subclass abundance distribution at the glycerol backbone linkage level of PM extracted lipid. Alkyl ether (O) and plasmalogen (P) were together abbreviated as ether lipids. (F) FLIM imaging (N = 2, n = 30) of PANC-1 cells with FliptR dye showed a decrease in average fluorescence lifetime after 24 h of hypoxia induction. (G) Relative frequency distributions of membrane tether forces, (H) tether number, and (I) tether length in the presence and absence of hypoxia. The mean values are provided in the bar graph. In all the cases, bar plots with patterns represent hypoxic conditions, and plasma membrane lipidomics data of PANC-1 is given in Magenta colour. The significance test was performed using a student’s t-test (*P < 0.01, two-tailed Student’s t-test). To control false discovery two-stage linear step-up method of Benjamini, Krieger, and Yekutieli were performed, with Q = 1 %.
The decoupling of hypoxia-induced global lipidome changes from plasma membrane biophysical properties prompted us to explore the lipidome changes specific to the plasma membrane itself. Mass analysis of lipids extracted from blebbed giant plasma membrane vesicles in hypoxic and normoxic conditions demonstrated minimal changes in the plasma membrane lipidome (Figs. 3B, S1G and Supplementary file 1) compared to global lipidomics. This hints that HIF-1α-induced lipid remodelling is organelle-specific in hypoxic-aggressive cancers and could preserve plasma cell membrane homeostasis for optimal membrane-associated cellular functions and survival.
2.4. Nanomechanical characterization of membranes under hypoxia
Malignancy is associated with changes in cell mechanics and hence we evaluated pancreatic cancer membrane mechanics under oxygen deprivation using atomic force microscopy (AFM). Hypoxia induced cell membrane softening in PANC-1 with no change in control HPNE cells (Figs. 3C and S3I). PC ether-linked lipids, which are known to modulate lipid raft domains in the plasma membrane were downregulated in PANC-1 and hence may account for disrupted raft membrane regions and hence softness (Fig. 3E). Second, a higher level of poly-unsaturation with various lipid classes was observed in PANC-1, making the membrane softer (Fig. 2A and B). Third, TG species have three lipid acyl chains attached to the head group, and quantification based on the number of saturated/unsaturated chains revealed that the abundance of TGs having three unsaturated chains (i.e., 0 chains are saturated; 0 SFA) increased in PANC-1 (Fig. S2B). Though TG is a predominant resident of lipid droplets and ER, they can also accumulate in plasma membranes (2−8 mol%) and could support the nanomechanical investigation of cell membrane stiffness. As we did not observe any major changes in the order, fluidity and packing of the plasma membrane, a softer cell membrane implies contribution from other factors. One such factor is the modulation of the actin cytoskeleton which is underneath the plasma membrane and impacts measurements of cell elasticity.
We observed decreased actin volume per area per cell in PANC-1 induced by HIF-1α underlying the decreased elastic modulus (Fig. 3D). Next, as membrane elasticity is also linked with membrane tension, we analyzed membrane tension in live cell membrane under normoxic and hypoxic induction using fluorescence lifetime imaging of a mechano-sensitive FliptR probe [28]. Upon hypoxia induction, the average life-time of the probe slightly decreased in PANC-1 cells with no change in HPNE cells (Figs. 3F and S3K). Decreased lifetime indicates either a reduction in lipid phase separation, local plasma membrane composition becoming more homogenous, or changes in membrane curvature; the latter being independently probed through tether analysis [29]. A minor reduction in plasma membrane cholesterol (Fig. S1G) could also result in increased softness and decreased tension, as cholesterol is known membrane rigidifier. This would render a homogenized plasma membrane composition.
We then investigated the impact of hypoxia on tether dynamics in pancreatic cancer. Membrane tethers are membrane nanotubes regulating cellular communication, adhesion, and immune responses [30]. Tether force, total extension of the tethers per cell, and tether numbers indicate alterations in the membrane reservoir and curvature. Tether force is associated with membrane stiffness and bending rigidity and tether length and number indicate membrane resistance to bending or curving. The accumulation of specific lipids can affect membrane tethers by inducing curvature and signals the plausible role of lipid sorting (induced by hypoxia during lipid remodelling) toward the formation of such curved regions at the site of insertion [31].
Hypoxia significantly reduced the median tether force in PANC-1 compared to normoxic conditions, whereas no change in HPNE cells was observed (Figs. 3G and S3L). These findings support the elastic modulus’s analysis and suggest that hypoxia induction reduces the force required for the tethers to be extended, reducing surface tension and bending rigidity/elastic modulus in a cell-line-dependent fashion. (39). The tether number/cell increased in hypoxic conditions along with an increase in the total extension of the last tether (i.e. tether length) in PANC-1 compared to HPNE (Figs. 3H−I and S3M−N). These collectively support facile tether dynamics, which could be crucial for cancer progression under hypoxic states. Finally, the formation of the membrane tethers is associated with the positively and negatively curved membrane at the tether circumference and base, respectively. Thus a tempting hypothesis emerges that hypoxia-induced lipidome rewiring might affect lipid sorting leading to selective enrichment of inverted cone-shape lipids, that may foster the formation of highly curved regions at the site of cell membrane insertion, eventually leading to more frequent membrane nanotube generation.
2.5. Hypoxia aggravates the migratory and invasive potential in pancreatic cancer
The aggressiveness of cancer cells is determined by their capacity to invade through the epithelial basement membrane. Hypoxia has been shown to promote cell migration and invasion in many cancers [32]. In the purview of membrane biophysics, the understanding of key factors underlying tumour dissemination is limited, but changes in cell mechanics which are associated with lipidome/membrane remodelling are integral to metastatic dissemination. This in turn governs cellular extensive deformation to migrate through tissues and enter blood vessels [33,34]. In accordance, recent studies have shown a strong correlation between decreasing cell stiffness and increasing invasive and metastatic efficiency [35–37]; the former was seen in our hypoxic perturbation of cortical stiffness in PANC-1.
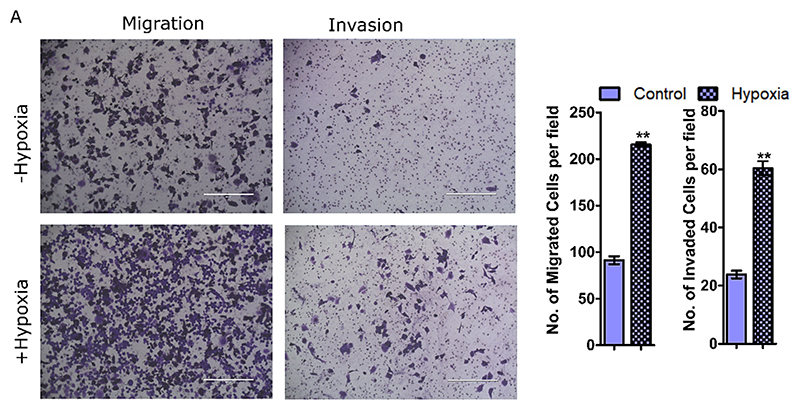
Using transwell migration and invasion assay, we observed that hypoxic pancreatic cancer cell exhibited enhanced migration and invasion compared to normoxic conditions (Fig. 4A). No change in the migration and a very slight change in invasion was observed in healthy pancreatic cells (Fig. S4A). Accumulation of unsaturated fatty acids including PUFAs has been positively correlated with higher migration and invasion [38]; a higher increase of these species was seen in PANC-1 (Fig. 2A, B). Similarly, an increased abundance of cholesterol esters (CE) aggravates the metastatic potential in many cancers such as renal cell carcinoma, prostate, breast, and pancreatic cancer [39–42]. The accumulation of CE also corroborates a higher migratory and invasion potential in PANC-1 cells induced under hypoxia. Finally, decreased cell stiffness in PANC-1 also correlates well with these observations. Surprisingly, the enhanced metastasis index in endometrial cancer progresses through the coordinated action of the EMT pathway and higher cell membrane fluidity [38]. Our data suggests a interplay of additional factors in hypoxic pancreatic cancer cells without the involvement of cell membrane fluidity. Furthermore, the plasma membrane tension in PANC-1 was attenuated under hypoxia and supports a higher migratory potential. This is due to the fact that an increased tension is associated with inhibited cancer cell migration and invasion by modulation of membrane curvature mediated by both lipids and membrane proteins [29].
Fig. 4.
(A) Representative images of transwell migration based on the visualization and quantification of the number of migratory cells. (B) Transwell matrigel invasion assay to show the invasive potential of hypoxia-treated PANC-1 cells. The significance test of two independent experiments of 4 randomly selected field per replicates were performed using a student’s t-test (*P < 0.01, two-tailed Student’s t-test). In all the cases, bar plots with patterns represent hypoxic conditions.
3. Conclusion
Hypoxia plays an active role in promoting tumour survival, progression, and invasion and is a common phenomenon in most malignant tumours, especially pancreatic cancer. Studying the effect and response of the hypoxic microenvironment in pancreatic cancer is thus gaining attention as an important therapeutic target. To adapt to the severe hypoxic environment, cancer cells change their metabolic phenotypes. Among them, aberrant metabolism of fatty acids, and lipids are some of the well-decorated hallmarks. The foremost impact of altered lipid metabolism is on the biophysical properties of cellular membranes, including plasma membranes. Cell membranes orchestrate various physiological functions where the membrane biophysical properties such as membrane order, hydration, fluidity, curvature, tension, etc. all have specific roles. By regulating the sorting, structure, diffusion, and interactions with other partners, these properties modulate the functions of membrane-embedded or associated biomolecules. Thus, the optimal response of cell membranes is crucial in both normal and disease states and led us to explore the same in pancreatic cancer cells under hypoxic stress.
Lipidomic analysis revealed enhanced accumulation of TG and CE under hypoxia with downregulation of GPLs and SPs. Increased abundance of CE and TG underlined a significant induction of lipid droplets. Lipid species with longer acyl chain lengths and increased desaturation levels also showed a significant increase. Furthermore, acyl-containing lipid species were upregulated and alkyl- and alkenyl-containing ether-linked PC, LPE, and TG lipid species were downregulated. This correlated well with the cellular abundance of specific enzymes responsible for lipid elongation or unsaturation. Biophysical membrane assays reported strong maintenance of plasma membrane properties such as fluidity, lipid diffusion, and order which remained largely unaltered under hypoxic stress. This was supported by observing no major variations in the plasma membrane lipidome under hypoxia unlike the global lipidome (inclusive of internal membranes). This implies a regulatory maintenance of plasma membrane biophysical attributes important for cellular process by mitigating substantial changes in the plasma membrane lipid composition. Enhanced migration and invasion under hypoxic states likely stems from a decreased cell cortical stiffness along with a reduction of actin volume. Finally, facile tether dynamics could be the critical factor responsible for higher migratory potential in hypoxic pancreatic cancer. In summary, we show distinctive lipid remodelling in both global and plasma membrane under hypoxia in pancreatic cancer and how the same is buffered driving a homeostatic plasma membrane biophysical response. The latter could account for a robust migration and invasion phenotype in hypoxic pancreatic cancers. A deeper understanding of the molecular mechanisms may furnish tangible avenues for targeting of pancreatic cancer using membrane-centric interventions.
Supplementary Material
Acknowledgments and funding
This work was supported by the Wellcome Trust/DBT India Alliance Fellowship (IA/I/21/1/505624) awarded to SK. This work is also supported by grants from DST-SERB (EMR/2016/005414, and WEA/2020/ 000032). Central Confocal and AFM Facilities at IIT Bombay are gratefully acknowledged. We are thankful to Prof. Ritu Aneja for the PANC-1 cell line.
Footnotes
CRediT authorship contribution statement
SK; Conceptualization, Supervision, Writing-Reviewing and Editing. PKA; Investigation, Data Curation, Methodology, Writing-Reviewing. SN; Data curation, Supervision, Editing, Software. GR; Supervision, Writing-Reviewing and Editing.
Preprint
The previous version of the current manuscript was submitted to bioXRiv pre-print server. Lipid remodelling by hypoxia aggravates migratory potential in pancreatic cancer while maintaining membrane homeostasis. Prema Kumari Agarwala, Shuai Nie, Gavin E. Reid, Shobhna Kapoor. bioRxiv 2022.12.08.519694; doi: https://doi.org/10.1101/2022.12.08.519694
Declaration of competing interest
The authors have no conflict of interest to declare.
Data availability
The data described in this article will be shared upon request. Inquiries should be directed to and will be fulfilled by the lead contact Shobhna Kapoor (shobhnakapoor@chem.iitb.ac.in), Department of Chemistry, Indian Institute of Technology Bombay, India.
References
- [1].Siegel RL, Miller KD, Wagle NS, Jemal A. Cancer statistics, 2023. CA Cancer J Clin. 2023 doi: 10.3322/caac.21763. [DOI] [PubMed] [Google Scholar]
- [2].Uccello M, Moschetta M, Mak G, et al. Towards an optimal treatment algorithm for metastatic pancreatic ductal adenocarcinoma (PDA) Curr Oncol. 2018;25:e90–e94. doi: 10.3747/co.25.3708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Waddell N, Pajic M, Patch AM, et al. Whole genomes redefine the mutational landscape of pancreatic cancer. Nature. 2015 doi: 10.1038/nature14169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Olivares O, Mayers JR, Gouirand V, et al. Collagen-derived proline promotes pancreatic ductal adenocarcinoma cell survival under nutrient limited conditions. Nat Commun. 2017 doi: 10.1038/ncomms16031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].McDonald OG, Li X, Saunders T, et al. Epigenomic reprogramming during pancreatic cancer progression links anabolic glucose metabolism to distant metastasis. Nat Genet. 2017 doi: 10.1038/ng.3753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Brandi J, Dando I, Pozza ED, et al. Proteomic analysis of pancreatic cancer stem cells: functional role of fatty acid synthesis and mevalonate pathways. J Proteome. 2017 doi: 10.1016/j.jprot.2016.10.002. [DOI] [PubMed] [Google Scholar]
- [7].Koong AC, Mehta VK, Le QT, et al. Pancreatic tumors show high levels of hypoxia. Int J Radiat Oncol Biol Phys. 2000;48:919–922. doi: 10.1016/s0360-3016(00)00803-8. [DOI] [PubMed] [Google Scholar]
- [8].Guillaumond F, Leca J, Olivares O, et al. Strengthened glycolysis under hypoxia supports tumor symbiosis and hexosamine biosynthesis in pancreatic adenocarcinoma. Proc Natl Acad Sci U S A. 2013 doi: 10.1073/pnas.1219555110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Chang Q, Jurisica I, Do T, Hedley DW. Hypoxia predicts aggressive growth and spontaneous metastasis formation from orthotopically grown primary xenografts of human pancreatic cancer. Cancer Res. 2011 doi: 10.1158/0008-5472.CAN-10-4049. [DOI] [PubMed] [Google Scholar]
- [10].Lee P, Chandel NS, Simon MC. Cellular adaptation to hypoxia through hypoxia inducible factors and beyond. Nat Rev Mol Cell Biol. 2020;21:268–283. doi: 10.1038/s41580-020-0227-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Mylonis I, Simos G, Paraskeva E. Hypoxia-inducible factors and the regulation of lipid metabolism. Cells. 2019;8:214. doi: 10.3390/cells8030214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Bensaad K, Favaro E, Lewis CA, et al. Fatty acid uptake and lipid storage induced by HIF-1α contribute to cell growth and survival after hypoxia-reoxygenation. Cell Rep. 2014;9:349–365. doi: 10.1016/j.celrep.2014.08.056. [DOI] [PubMed] [Google Scholar]
- [13].Garcia-Martinez V, Gimenez-Molina Y, Villanueva J, et al. Emerging evidence for the modulation of exocytosis by signalling lipids. FEBS Lett. 2018;592:3493–3503. doi: 10.1002/1873-3468.13178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Sheng R, Jung DJ, Silkov A, et al. Lipids regulate Lck protein activity through their interactions with the Lck Src homology 2 domain. J Biol Chem. 2016;291:17639–17650. doi: 10.1074/jbc.M116.720284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Sezgin E, Levental I, Mayor S, Eggeling C. The mystery of membrane organization: composition, regulation and roles of lipid rafts. Nat Rev Mol Cell Biol. 2017;18:361–374. doi: 10.1038/nrm.2017.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Kreutzberger AJB, Ji M, Aaron J, et al. Rhomboid distorts lipids to break the viscosity-imposed speed limit of membrane diffusion. Science (80-) 2019;363 doi: 10.1126/science.aao0076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Owen DM, Williamson DJ, Magenau A, Gaus K. Sub-resolution lipid domains exist in the plasma membrane and regulate protein diffusion and distribution. Nat Commun. 2012 doi: 10.1038/ncomms2273. [DOI] [PubMed] [Google Scholar]
- [18].Agarwala PK, Aneja R, Kapoor S. Lipidomic landscape in cancer: actionable insights for membrane-based therapy and diagnoses. Med Res Rev. 2022;42:983–1018. doi: 10.1002/med.21868. [DOI] [PubMed] [Google Scholar]
- [19].Marti HH, Jung HH, Pfeilschifter J, Bauer C. Hypoxia and cobalt stimulate lactate dehydrogenase (LDH) activity in vascular smooth muscle cells. Pflugers Arch - Eur J Physiol. 1994 doi: 10.1007/BF00374315. [DOI] [PubMed] [Google Scholar]
- [20].Semenza GL, Roth PH, Fang HM, Wang GL. Transcriptional regulation of genes encoding glycolytic enzymes by hypoxia-inducible factor 1. J Biol Chem. 1994 [PubMed] [Google Scholar]
- [21].Castoldi A, Monteiro LB, van Teijlingen Bakker N, et al. Triacylglycerol synthesis enhances macrophage inflammatory function. Nat Commun. 2020 doi: 10.1038/s41467-020-17881-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Chen L, Zhang W, He L, et al. Effect of alkylglycerone phosphate synthase on the expression levels of lncRNAs in glioma cells and its functional prediction. Oncol Lett. 2020 doi: 10.3892/ol.2020.11927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Ritchie SA, Akita H, Takemasa I, et al. Metabolic system alterations in pancreatic cancer patient serum: potential for early detection. BMC Cancer. 2013 doi: 10.1186/1471-2407-13-416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Benjamin DI, Cozzo A, Ji X, et al. Ether lipid generating enzyme AGPS alters the balance of structural and signaling lipids to fuel cancer pathogenicity. Proc Natl Acad Sci U S A. 2013;110:14912–14917. doi: 10.1073/pnas.1310894110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Lorent JH, Levental KR, Ganesan L, et al. Plasma membranes are asymmetric in lipid unsaturation, packing and protein shape. Nat Chem Biol. 2020;16:644–652. doi: 10.1038/s41589-020-0529-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Gunther G, Malacrida L, Jameson DM, et al. LAURDAN since Weber: the quest for visualizing membrane heterogeneity. Acc Chem Res. 2021 doi: 10.1021/acs.accounts.0c00687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Kaiser RD, London E. Location of diphenylhexatriene (DPH) and its derivatives within membranes: comparison of different fluorescence quenching analyses of membrane depth. Biochemistry. 1998 doi: 10.1021/bi980064a. [DOI] [PubMed] [Google Scholar]
- [28].Colom A, Derivery E, Soleimanpour S, et al. A fluorescent membrane tension probe. Nat Chem. 2018 doi: 10.1038/s41557-018-0127-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Tsujita K, Satow R, Asada S, et al. Homeostatic membrane tension constrains cancer cell dissemination by counteracting BAR protein assembly. Nat Commun. 2021 doi: 10.1038/s41467-021-26156-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Sun M, Graham JS, Hegedüs B, et al. Multiple membrane tethers probed by atomic force microscopy. Biophys J. 2005 doi: 10.1529/biophysj.104.058180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Batta G, Soltész L, Kovács T, et al. Alterations in the properties of the cell membrane due to glycosphingolipid accumulation in a model of Gaucher disease. Sci Rep. 2018 doi: 10.1038/s41598-017-18405-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Azam F, Mehta S, Harris AL. Mechanisms of resistance to antiangiogenesis therapy. Eur J Cancer. 2010 doi: 10.1016/j.ejca.2010.02.020. [DOI] [PubMed] [Google Scholar]
- [33].Wirtz D, Konstantopoulos K, Searson PC. The physics of cancer: the role of physical interactions and mechanical forces in metastasis. Nat Rev Cancer. 2011;11:512–522. doi: 10.1038/nrc3080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Gensbittel V, Kräter M, Harlepp S, et al. Mechanical adaptability of tumor cells in metastasis. Dev Cell. 2021;56:164–179. doi: 10.1016/j.devcel.2020.10.011. [DOI] [PubMed] [Google Scholar]
- [35].Cross SE, Jin YS, Rao J, Gimzewski JK. Nanomechanical analysis of cells from cancer patients. Nat Nanotechnol. 2007 doi: 10.1038/nnano.2007.388. [DOI] [PubMed] [Google Scholar]
- [36].Gossett DR, Tse HTK, Lee SA, et al. Hydrodynamic stretching of single cells for large population mechanical phenotyping. Proc Natl Acad Sci U S A. 2012 doi: 10.1073/pnas.1200107109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Han YL, Pegoraro AF, Li H, et al. Cell swelling, softening and invasion in a three-dimensional breast cancer model. Nat Phys. 2020 doi: 10.1038/s41567-019-0680-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Liu S, Dong P, Wang X, Gong W. Errα promotes pancreatic cancer progression by enhancing the transcription of PAI1 and activating the MEK/ERK pathway. Hpb. 2022;24:S259–S260. [PMC free article] [PubMed] [Google Scholar]
- [39].Liu Z, Liu X, Liu S, Cao Q. Cholesterol promotes the migration and invasion of renal carcinoma cells by regulating the KLF5/miR-27a/FBXW7 pathway. Biochem Biophys Res Commun. 2018 doi: 10.1016/j.bbrc.2018.05.122. [DOI] [PubMed] [Google Scholar]
- [40].Yue S, Li J, Lee S-Y, et al. Cholesteryl ester accumulation induced by PTEN loss and PI3K/AKT activation underlies human prostate cancer aggressiveness. Cell Metab. 2014;19:393–406. doi: 10.1016/j.cmet.2014.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Li J, Gu D, Lee SSY, et al. Abrogating cholesterol esterification suppresses growth and metastasis of pancreatic cancer. Oncogene. 2016 doi: 10.1038/onc.2016.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].de Gonzalo-Calvo D, López-Vilaró L, Nasarre L, et al. Intratumor cholesteryl ester accumulation is associated with human breast cancer proliferation and aggressive potential: a molecular and clinicopathological study. BMC Cancer. 2015:15. doi: 10.1186/s12885-015-1469-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data described in this article will be shared upon request. Inquiries should be directed to and will be fulfilled by the lead contact Shobhna Kapoor (shobhnakapoor@chem.iitb.ac.in), Department of Chemistry, Indian Institute of Technology Bombay, India.