Abstract
Rationale
Emerging evidence supports a crucial role for tertiary lymphoid organs (TLOs) in chronic obstructive pulmonary disease (COPD) progression. However, mechanisms of immune cell activation leading to TLO in COPD remain to be defined.
Objectives
To examine the role of lung dendritic cells (DC) in T follicular helper (Tfh)-cell induction, a T-cell subset critically implicated in lymphoid organ formation, in COPD.
Methods
Myeloid cell heterogeneity and phenotype was studied in an unbiased manner via single-cell RNA sequencing on HLA-DR+ cells sorted from human lungs. The in vitro capability of FACS-sorted DC-subsets of control and COPD lungs to polarize IL-21+CXCL13+ Tfh-like cells was measured. In situ imaging analysis was performed on COPD stage IV GOLD lungs with TLO.
Measurements and Main Results
ScRNAseq analysis revealed a high level of heterogeneity among human lung myeloid cells. Among these, cDC2 showed increased induction of IL-21+CXCL13+ Tfh-like cells. Importantly, the capacity to induce IL-21+ Tfh-like cells was higher in cDC2s from COPD patients compared with control patients. Increased Tfh-induction by COPD cDC2 correlated with increased presence of Tfh-like cells in COPD lungs as compared to controls, and cDC2 co-localized with Tfh-like cells in TLOs of COPD. Mechanistically, cDC2 exhibited a unique migratory signature and (transcriptional) expression of several pathways and genes related to DC-induced Tfh-priming. Importantly, blocking the co-stimulatory OX40L-OX40 axis reduced Tfh-induction by control lung cDC2.
Conclusions
In COPD lung, we found lung EBI2+ OX-40L-expressing cDC2 that induces IL-21+ Tfh-like cells, suggesting an involvement of these cells in TLO formation.
Introduction
Chronic obstructive pulmonary disease (COPD) is currently the third leading cause of death worldwide (1) and is characterized by progressive airway inflammation, emphysema and impaired lung function resulting from inhaled oxidants such as cigarette smoke (CS). COPD severity and tissue destruction correlate with development of tertiary lymphoid organs (TLO) (2, 3). While rarely developing in healthy individuals, lung TLO formation is significantly increased in COPD (GOLDI/II) patients (4). Finally, in severe/end-stage COPD patients (GOLD III/IV), TLO numbers and size further increase and can be found in nearly 50% of the small airways (2, 4). TLOs consist of well-defined B-cell follicles surrounded by T-cells interspersed with dendritic cells (DC), reminiscent of the structural organization also seen in secondary lymphoid organs (5). Several studies have shown that absence of TLOs via either use of B-cell deficient mice (6) or antibodies blocking B-cell recruitment (7) or survival (4, 8), prevented CS-induced emphysema in a murine COPD model. However, mechanisms governing TLO formation during COPD remain to be elucidated.
Peripheral T follicular helper (Tfh-)like cells exhibit phenotypic overlap with ‘bona fida’ Tfh-cells and regulate local B-cell isotype switching in peripheral diseased organs, including skin (9), synovial tissue (10) and lung (11) via IL-21 secretion. Importantly, we and others have also described the presence of IL-21+ Tfh-like cells in TLO of idiopathic pulmonary arterial hypertension (IPAH) (12) and COPD (3) lungs, suggesting these cells are involved in TLO formation and maintenance.
Human DC are heterogeneous and consists of different subsets, including two conventional (c)DC populations (CD141+ cDC1 and the CD1c+ cDC2) and plasmacytoid (p)DC (13, 14). It has been shown that mice, in which DC were depleted, failed to develop and maintain lung TLO in response to allergens or virus infection (15–17). In contrast, repeated pulmonary delivery of activated DC was sufficient to induce TLO formation (15, 18). These studies highlight a crucial role for DC in TLO formation and maintenance. However, how DC induce TLO, especially during COPD, is unknown.
We hypothesized that human lung DC induce Tfh-like cell polarization and hence contribute to subsequent lymphoid neogenesis during COPD. We first used an unbiased approach to address the complexity of Lineage(Lin)−HLA-DR+ lung cells and found a high level of heterogeneity. We then demonstrated that cDC2 are the most efficient subset in inducing IL-21+ Tfh-like cells. Importantly, cDC2 isolated from COPD lungs showed increased potential to polarize Tfh-like cells. Mechanistically, we found that cDC2 expressed a unique migratory signature, suggesting these cells are highly capable to migrate to the site of TLO formation and subsequently interact with CD4+ T-cells. Furthermore, blocking the co-stimulatory OX40L-OX40 axis reduced Tfh-induction by cDC2 derived from non-obstructed control lungs. Some of the results have been previously reported in the form of an abstract (19).
Methods
Detailed description of materials and methods can be found in the online methods supplement.
Human lung samples
Lung samples were obtained from non-obstructed control or COPD subjects. Study and consent procedures were reviewed and approved by the Swedish Research Ethical Committee in Gothenburg, Sweden (FEK 675-12/2012 and 1026-15, March 2016) in accordance with the principles of the Declaration of Helsinki. Written informed consent was obtained preoperatively. Table 1 shows demographics and lung function. Additional information about the source and processing of the human lung tissue samples is described in the online methods supplement.
Table 1. Summary of subject demographics, smoking history, and spirometry.
| Group | Control | COPD GOLD II | COPD GOLD IV |
|---|---|---|---|
| Subjects, n | 35 | 7 | 5 |
| Sex: M,F | 14, 21 | 3, 4 | 0, 5 |
| Age, yr | 65(10) | 70 (6) | 61 (8) |
| Smoking, pack-years (active and former) | 28(12) | 41 (10) | 45 (9) |
| Smoking status: never, active, former | 8, 1, 26 | 0, 0, 7 | 0, 0, 5 |
| FEV1, % pred | 94(17) | 57 (7) | 27 (17) |
| DLco, % pred | 74 (14) | 63(10) | 31 (4) |
Definition of abbreviations: % pred = percentage of the predicted value; COPD = Chronic Obstructive Pulmonary Disease
Data are represented as mean (SD)
In vitro DC/T-cell co-cultures
Mixed Leukocyte Reactions (MLR) were set up between FACS-sorted lung DC populations and allogeneic naïve blood CD4+ T-cells as described in the online supplement. T-cell proliferation and polarization, including cytokine and transcription factor profiling, were subsequently analyzed via flow cytometry as described in the online supplement.
Ex vivo phenotyping of lung leukocyte populations
Flow cytometry was used to assess expression of extracellular and intracellular phenotypic protein markers by lung DC and T-cell subsets as described in the online supplement. Single-cell RNA transcriptomes of the lung HLA-DR+ fraction were generated and analyzed as described in the online supplement.
In situ imaging of GOLD IV COPD lung TLO
To image CH25H and CD19 mRNA topography in the lung TLO, RNAScope was performed as described in the online supplement. Fluorescence microscopy was used to determine the presence and anatomical localization of cDC2 in lung TLO as described in the online supplement.
Results
Unbiased single cell RNA sequencing analysis of human lung Lin−HLA-DR+ cells.
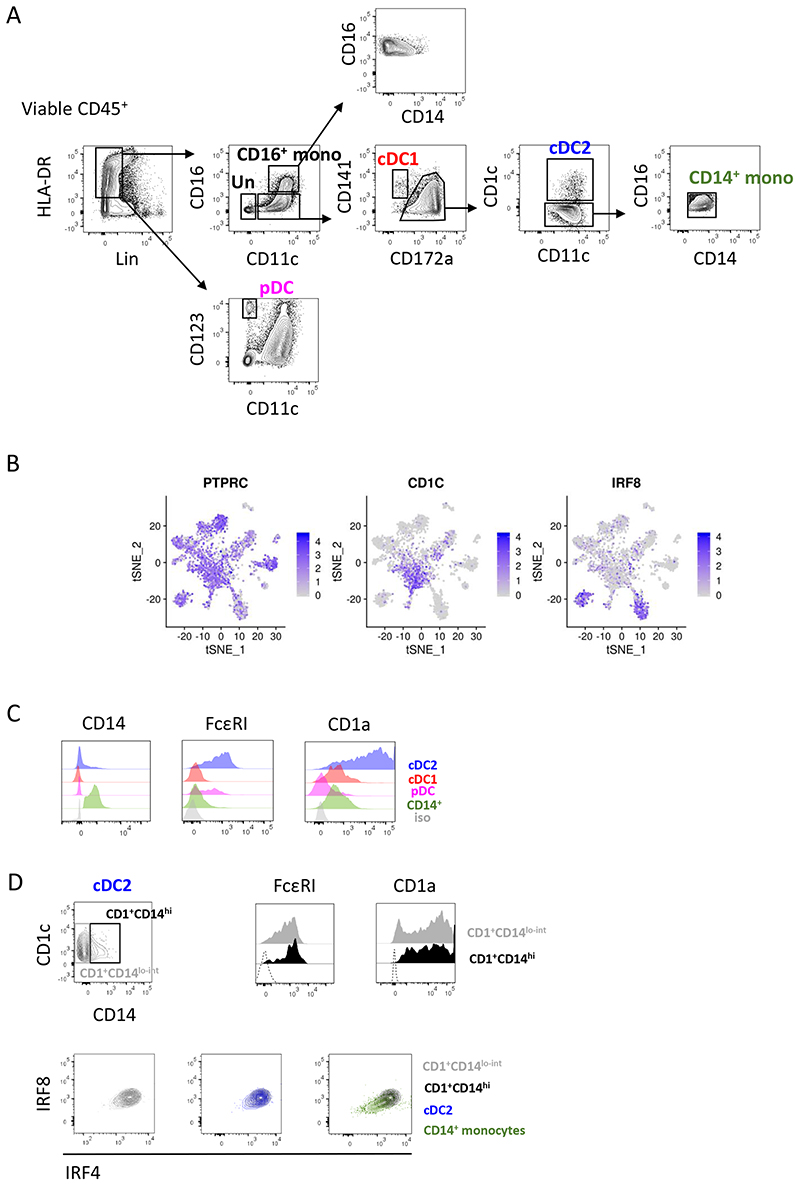
Myeloid cells represent a heterogeneous population and comprise several subtypes (14). To date, definition of human lung myeloid cells was biased by the limited markers available to identify and isolate the cells. To identify the different myeloid cell populations in the human lung in an unbiased way, we performed single-cell RNA sequencing on Lin−HLA-DR+ cells from non-obstructed lungs. Different DC (including cDC1, cDC2 and pDC) and monocyte subsets (including CD14+, CD16+ and CD14+CD16+ monocytes) were FACS-sorted based on the expression of DC and monocyte subset defining surface markers (Figure E1A) (14) and pooled afterwards in enriched proportions before sequencing.
Unsupervised clustering identified 14 clusters (Figure 1A). Differential gene expression between clusters was analyzed (Figure 1B). Cluster 1 highly expressed cDC2-associated genes such as CD1C, CLEC10A, FCER1A and CD1A. Cluster 2 highly expressed monocyte/macrophage-related genes such as MRC1, CTSD, MARCO and VSIG4 while cluster 3 is characterized by cell cycle gene expression, including TOP2A, CENPF1 and STMN1. Cluster 4 expressed high levels of pDC-associated genes, including TCF4, GZMB, CLEC4C and BCL11A while cluster 5 exhibited high expression levels of monocyte/macrophage-related genes, including S100A8, S100A9, FCN1 and VCAN. Cluster 6 expressed natural killer (NK) cell-associated genes, like GZMA, CD96 and GNLY while cluster 7 displayed expression of monocyte/macrophage-related genes, including FCGR3A, CTSS, PECAM1 and MAFB. Cluster 8 expressed high levels of cDC1 genes, including CLEC9A, IRF8, ID2 and XCR1. Cluster 9 exhibited expression of type II alveolar epithelial cells (AEC), including SFTPC, SFTPB, SFTPD and EPCAM. Cluster 10 expressed endothelial cell-related genes including VWF, CAV1 and GIMAP7. Furthermore, both cluster 9 and cluster 10 lack expression of the pan-leukocyte marker gene PTPRC (coding for CD45), further confirming their non-immune cell nature (Figure E1B). Cluster 11 highly expressed genes associated with DC activation, including CCR7, CCL22, LAMP3 and BIRC3. Both cluster 12 and cluster 14 expressed monocyte/macrophage-related genes, like CXCL10, CXCL11, CCL8 and GBP1 for cluster 12 and LYVE1, C1QA, CD163 and CD14 for cluster 14. Finally, cluster 13 exhibited expression of mast cell genes, including KIT, CPA3 and MS4A2. The complete list of top 20 differentially expressed genes is available in Table S1.
Figure 1. Human non-obstructed lungs contain a highly heterogeneous myeloid cell compartment.
Myeloid cells, purified from non-obstructed human peritumoral lung tissues (n=3), were analyzed by single-cell RNA sequencing using the Seurat package. Combined single-cell transcriptomes were analyzed. (A) t-SNE representation of cell clusters identified using unsupervised clustering. Each dot represents an individual cell. Colors represent identified clusters. (B) Heatmap of scaled expression of (log values of Unique Molecular Identifiers (UMI)) for the top 20 differentially expressed genes of each cluster (based on log fold change). (C) Signature scores (arbitrary units) of individual cells for indicated gene signatures.
To confirm the identity of the different clusters, we calculated signature scores for each single cell using published transcriptome signatures for human blood (20) and lung (21) leukocyte subsets (Figure 1C). Cluster 1 had the highest score for the cDC2 signature while cluster 2 exhibited score increase for CD14++CD16+ monocyte and CD14+ and CD16+ monocyte cell signatures. Cluster 3 showed an overlap with a signature defining a proliferating monocyte/macrophage (Mac/mono cycl.) subset as observed in (21). Cluster 4 displayed a clear overlap with the pDC signature while cluster 5 exhibited a high score for the CD14+ monocyte and CD14+CD16+ monocyte signature. Cluster 6 overlapped with the NK cell signature and cluster 7 with the CD16+ monocyte and CD14++CD16+ monocyte cell signature. Cluster 8 showed a clear overlap with the cDC1 signature while cluster 9 and cluster 10 overlapped with signatures of type II AEC and endothelial cells respectively. For cluster 11, we observed a high overlap with an ‘activated DC’ signature. As expected, both cluster 12 and cluster 14 had high signature scores for macrophage phenotypes (mac1 and mac2 respectively) observed earlier in the lung (21). Finally, cluster 13 had a high score for a mast cell signature.
Based on the relevance in terms of their potency to prime T-cell activation and polarization we opted to primarily focus on cDC2, cDC1 and pDC, rather than on macrophage subsets, NK-cells, mast cells and structural cells for the rest of the study. All monocyte subsets are weak stimulators of naïve T-cells (22). Therefore, we finally opted to include only CD14+ monocytes as a reference monocyte population as these cells embody the ‘classical’ monocyte subset (22). Single-cell transcriptome data confirmed that cDC1, cDC2, pDC and CD14+ monocytes each represent homogeneous cell populations. Furthermore, cells in the ‘activated DC’ cluster expressed cDC2 and cDC1 hallmark genes, including CD1c and IRF8 respectively (Figure E1B). Therefore, this cluster represented a mixture of cDC1 and cDC2 with a distinct activation status rather than a separate DC subset with a distinct ontogeny.
However, additional flow cytometry analysis revealed heterogeneous expression of several myeloid cell markers by cDC2, including FcεRI, CD1a and the monocyte marker CD14 (Figure E1C). This could imply potential presence of a CD14hiFcεRIhiCD1ahi monocyte-derived (mo)DC population within the cDC2 gate. However, CD14lo and CD14hi cDC2 fractions displayed a similar heterogeneous expression pattern of both FcεRI and CD1a, hence we couldn’t identify a clear CD14hiFcεRIhiCD1ahi subset (Figure E1D). Moreover, CD14lG and CD14hi fractions exhibited a similar (cDC2) expression profile of the lineage-defining transcription factors IRF4 and IRF8 (14), clearly distinct from that exhibited by CD14+ monocytes, considered as the precursors of moDC (14) (Figure E1D). Thus, there were no immediate indications that the CD14+ cDC2 represent an ontogenetically different subset. Moreover, full comprehension of the ontogenetic relationship between CD14lo and CD14hi cDC2 fractions requires further investigation and is beyond the scope of this study. Therefore, we decided to isolate the different lung DC subsets as outlined in Figure E1A.
Lung cDC2 are the most potent subset to polarize naïve CD4+ T-cells into Tfh-like cells
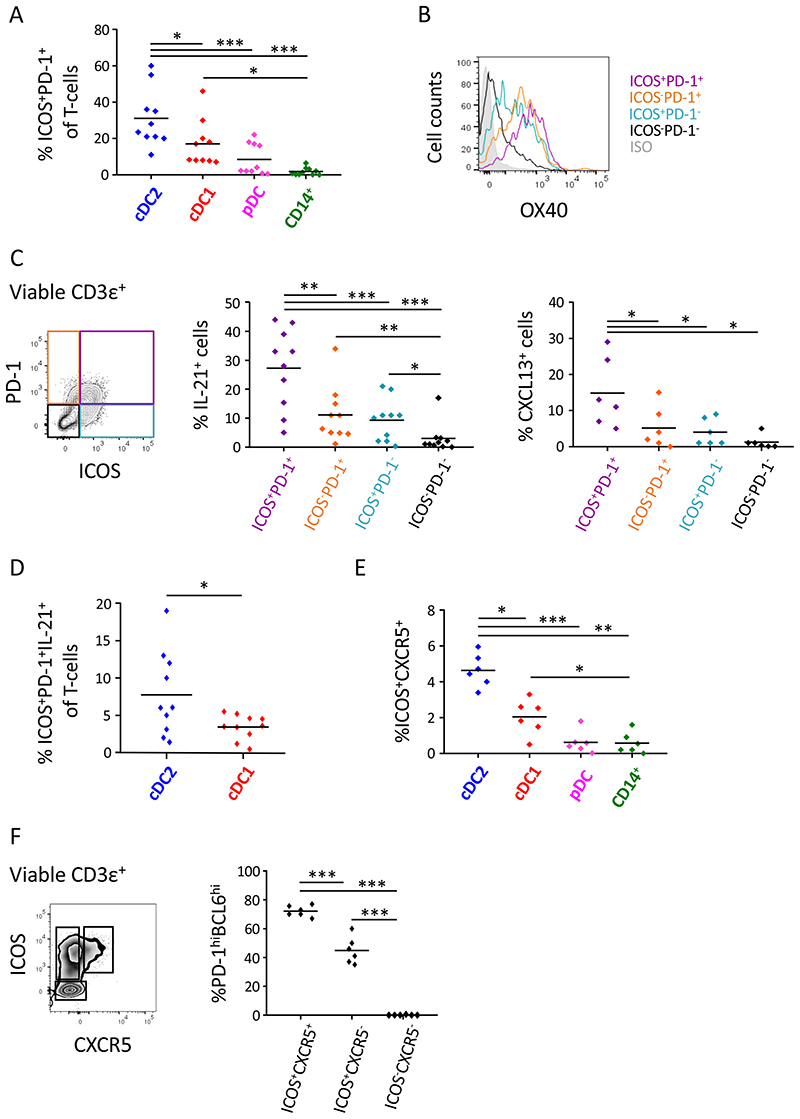
To assess the capacity of the different human lung DC subsets to polarize naïve CD4+ T-cells into Tfh- or Tfh-like cells, FACS-sorted lung DC subsets were co-cultured with allogeneic naïve blood CD4+ T-cell (MLR). The degree of Tfh-polarization was assessed at d7 of the culture. Compared to other DC subsets, cDC2 induced the highest proportion of CD4+ T-cells expressing high levels of ICOS and PD-1, two hallmark Tfh-markers (Figure 2A and E2A). Of note, lung pDCs and CD14+ monocytes are poor stimulators of naïve CD4+ T-cell proliferation (Figure E3A), underlying the lower proportions of ICOShiPD-1hi T-cells in these co-cultures. However, cDC2 and cDC1 induced similar levels of T-cell proliferation (Figure E3A) implying that there was an intrinsic qualitative difference between both cDC subsets to promote Tfh-like cell skewing. Compared to ICOS−PD-1+, ICOS+PD-1− and ICOS−PD1− T-cell subsets in the co-culture, ICOShiPD-1hi T-cells were characterized by the highest surface levels of OX40, another critical Tfh-cell marker (Figure 2B). Moreover, compared to the three other T-cell populations, ICOShiPD-1hi T-cells were the dominant producers of IL-21 and CXCL13, the hallmark Tfh cytokine and chemokine respectively, confirming the Tfh-like cell nature of this T-cell population (Figure 2C and E2B). In contrast, there was no significant difference in secretion of the Th1 cytokine IFN-γ by ICOShiPD-1hi T-cells as compared to ICOS−PD-1+ T-cells (Figure E3B), demonstrating that the ICOShiPD-1hi T-cells do not simply represent a generally increased activation state. Importantly, compared to cDC1, lung cDC2 induced increased proportions of ICOShiPD-1hi IL-21 secreting T-cells (Figure 2D). Of note, there was no significant difference in the induction of IFN-γ secretion by ICOS+PD-1+ T-cells induced by cDC1 and cDC2, implying a degree of selectivity in the T-cell cytokine responses elicited by cDC2 versus cDC1 (Figure E3C).
Figure 2. Lung cDC2 are the most potent inducers of Tfh-like cell polarization.
DC subsets were purified from non-obstructed peritumoral lung tissue and co-cultured with allogeneic naive blood CD4+ T-cells. (A) Percentages of ICOS+PD-1+ T-cells in the different DC/T-cell co-cultures were determined at d7 of the co-culture via flow cytometry. Summary data graph with each symbol representing an individual donor (n=10). (B) Flow cytometry histogram of OX40 staining on ICOS+PD-1+ (purple), ICOS−PD-1+ (orange), ICOS+PD-1− (blue) and ICOS− PD-1− (black) T-cell subsets in cDC2/T-cell co-cultures. Representative data from 3 donors is shown. (C) Intracellular IL-21 (n=10) and CXCL13 (n=6) staining of ICOS+PD-1+ (purple), ICOS−PD-1+ (orange), ICOS+PD-1− (blue) and ICOS−PD-1− (black) T-cell subsets in cDC2/T-cell co-cultures after restimulation with PMA and ionomycin in the presence of Golgi-plug and Golgi-stop. Summary data graph in which each symbol represents an individual donor. (D) Percentages of ICOS+PD-1+IL-21+ T-cells in cDC2/T-cell and cDC1/T-cell co-cultures were determined. Summary data graph in which each symbol represents an individual donor (n=10). (E) Proportions of ICOS+CXCR5+ T-cells in the different DC/T-cell co-cultures were determined at day 4. Summary data graph in which each symbol represents an individual donor (n=6). (F) Percentages of PD-1hiBCL6hi cells in ICOS+CXCR5+, ICOS+CXCR5− and ICOS− CXCR5− T-cell subsets in the cDC2/T-cell co-cultures were determined via flow cytometry. Summary data graph in which each symbol represents an individual donor (n=6). *p<0.05, **p<0.01, ***p<0.001, Tukey’s multiple comparison test (A, C, E and F) and paired student’s t-test (D).
To further confirm the induction of Tfh-cells in the co-cultures, we analyzed expression of CXCR5, a classical Tfh-cell surface marker, and Bcl6, key transcription factor driving Tfh-cell development (23). Expression of CXCR5 was transient and not detectable at day 7 (data not shown and (24)). Therefore, we determined the proportion of CXCR5hiICOShiPD-1hiBcl6hi Tfh-like cells in the different co-cultures at day 4, a time point that corresponds with peak CXCR5 expression (24). In line with our previous results from day 7, cDC2 were the most efficient inducers of ICOShiCXCR5hi T-cells as compared to cDC1, pDC and CD14+ monocytes (Figure 2E and E2C). Importantly, in contrast to ICOS+CXCR5− and ICOS−CXCR5−, ICOShiCXCR5hi T-cells were almost exclusively PD-1hiBcl6hi, further supporting their Tfh-like nature (Figure 2F and E2D).
Collectively, these results demonstrate that human resident lung cDC2 are the most potent DC subset to polarize Tfh-like cells from naïve CD4+ T-cells.
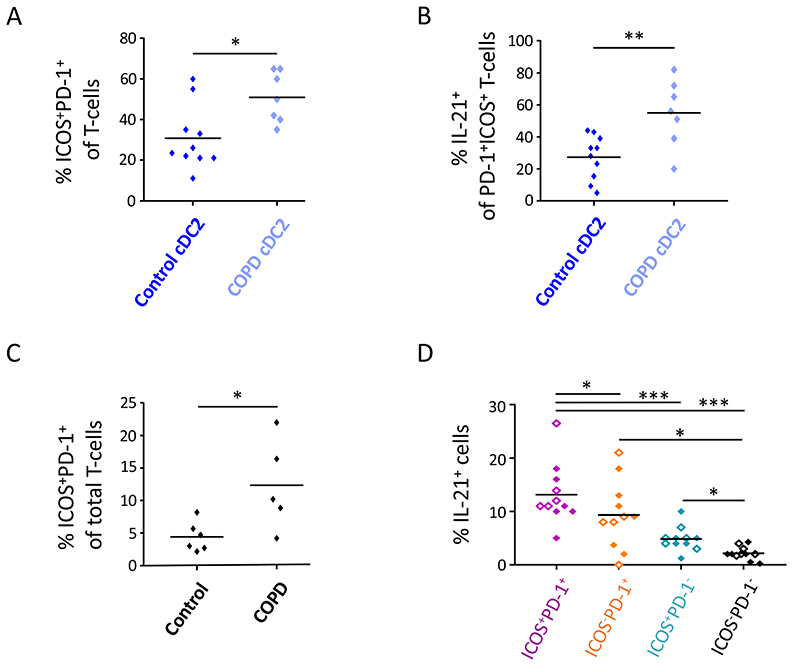
cDC2 from GOLD II COPD lungs show increased potential to induce Tfh-like cells, which correlated with increased presence of Tfh-like cells in the lung tissue
We next assessed whether lung cDC2 from COPD patients exhibited an increased capacity to induce Tfh-like cells as compared to cDC2 from non-obstructed lungs. To this end, we isolated cDC2 from GOLD II COPD peripheral lung tissue and co-cultured these cells with allogeneic naïve blood CD4+ T-cells. COPD lung cDC2 induced increased proportions of Tfh-like cells as compared to control cDC2 from non-obstructed lungs (Figure 3A and E4A). Importantly, Tfh-like cells induced by COPD cDC2 contained an increase in the frequency of IL-21+ cells as compared to Tfh-like cells induced by cDC2 from non-obstructed lungs (Figure 3B and E4B). Of note, the difference in Tfh-like cell induction between COPD and control cDC2 could not be attributed to a difference in their potential to stimulate T-cell proliferation (Figure E4C). Furthermore, control and COPD cDC2 contained a similar proportion of CD14hi cells, indicating that a difference in CD14hi cDC2 fraction did not underlie the difference in Tfh-like cell induction by control and COPD cDC2 (Figure E4D).
Figure 3. cDC2 from COPD GOLD II lungs display increased potential to promote Tfh-like cell skewing which is associated with the increased presence of Tfh-like cells in the COPD lung.
(A) and (B) cDC2 were isolated from COPD GOLD II peritumoral lung tissues (n=7) and co-cultured with allogeneic naïve CD4+ T-cells. Proportions of ICOS+PD-1+ T-cells (A) and ICOS+PD-1+IL-21+ T-cells (B) were determined at day 7 and compared to the respective T-cell proportions induced by cDC2 from non-obstructed peritumoral lung tissues as previously shown in Figure 2 (n=10). Shown is summary data graphs in which each symbol represents an individual donor. (C) Percentages of ICOS+PD-1+ Tfh-like cells were determined in peritumoral lung tissue resections of COPD and non-obstructed control subjects via flow cytometry. Shown is summary data graph in which each symbol represents an individual donor (n=6 for controls and n=5 for COPD subjects). (D) Intracellular IL-21 staining of lung tissue ICOS+PD-1+ (purple), ICOS−PD-1+ (orange), ICOS+PD-1− (blue) and ICOS−PD-1− (black) T-cell subsets after PMA/ionomycin restimulation (+ Golgi-plug/Golgi-stop) in DC-free in vitro cultures. Shown are pooled data from control (full diamonds) and COPD (open diamonds) lung resections (n=11). Each symbol represents an individual donor. *p<0.05, **p<0.01, ***p<0.001, Student’s t-test (A-C) and Tukey’s multiple comparison test (D).
We next investigated whether there is a corresponding increased presence of Tfh-like cells in peripheral lung tissue of GOLD II COPD subjects. Flow cytometry analysis revealed the presence of ICOShiPD-1hi T-cells in both control and COPD lung tissue. Importantly, compared to control lungs, the frequency of ICOShiPD-1hi T-cells was increased GOLD II COPD lungs (Figure 3C and E4D). In line with our in vitro findings, lung ICOShiPD-1hi T-cells were the dominant IL-21 producers as compared to ICOS−PD-1hi, ICOShiPD-1− and ICOS−PD-1− T-cell fractions, confirming the Tfh-like nature of these cells (Figure 3D).
Lung cDC2 express a unique migratory signature distinct from cDC1
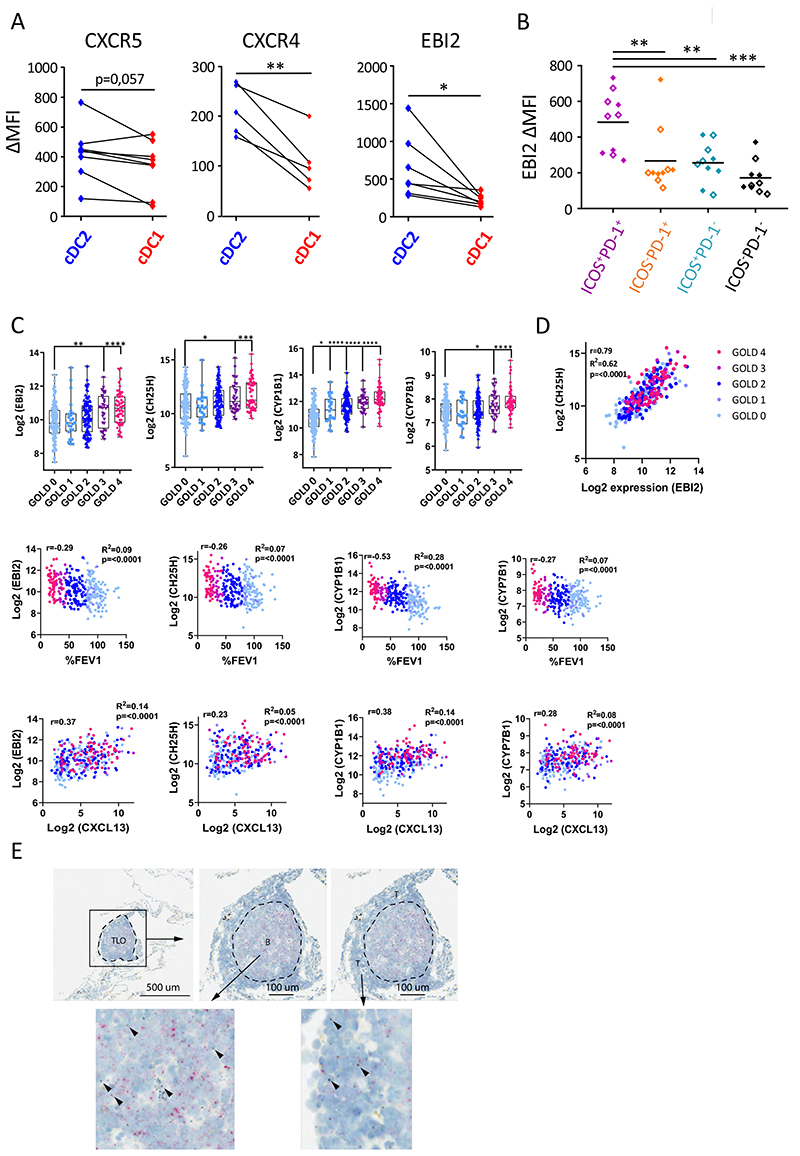
The chemoattractants CXCL12 and CXCL13 are instrumental for TLO-formation during COPD by mediating the recruitment and localization of the critical cell types, including T cells, B cells and DCs (7, 25, 26). Therefore, we analyzed expression of the corresponding receptors CXCR4 and CXCR5 on cDC2 and cDC1 via flow cytometry. In control lungs, cDC2 tended to exhibit increased expression of CXCR5 while CXCR4 levels were significantly increased as compared to cDC1 (Figure 4A and E5A). However, there was no difference in CXCR5 and CXCR4 expression between control and COPD cDC2 (Figure E5B).
Figure 4. cDC2 exhibit a unique migratory pattern.
(A) Surface levels of CXCR5, CXCR4 and EBI2 were measured on cDC2 and cDC1 from non-obstructed peritumoral lung resections via flow cytometry (n=7 for CXCR5, n=5 for CXCR4 and n=7 for EBI2). Summary data graphs (mean MFI corrected for background intensity) for the indicated markers are shown. Each symbol represents an individual donor. (B) Surface EBI2 levels on ICOS+PD-1+ (purple), ICOS− PD-1+ (orange), ICOS+PD-1− (blue) and ICOS−PD-1− (black) T-cell subsets in the lung measured via flow cytometry. Summary data graph (mean MFI corrected for background intensity) of pooled control (full diamonds) and COPD (open diamonds) lung samples (n=10). Each symbol represents an individual donor. (C) Correlations of whole lung EBI2, CH25H, CYP1B1 and CYP7B1 mRNA expression with COPD disease severity (GOLD stage and %FEV1) and whole lung CXCL13 mRNA as a marker for TLO formation. (D) Correlation of whole lung CH25H mRNA expression with whole lung EBI2 mRNA expression. Data in C and D are derived from a publicly available GSE-set (GSE47460). Healthy control subjects n=116; GOLD I n=24; GOLD II n=97; GOLD III n=32 and GOLD IV n=54. (E) In situ visualization of CH25HmRNA (brown) in COPD GOLD IV explanted lung tissue TLOs via RNAscope duplex technology (n=5). CD19 mRNA (red) is used to delineate the B-cell follicle of the TLO. *p<0.05, **p<0.01, ***p<0.001, (A) Student’s t-test, (B) Tukey’s multiple comparison test (C) and Holm-Sidak’s multiple test correction was used. To test for correlation of expression for the indicated genes within all study subjects, linear regression analysis and Pearson’s correlation test were used to calculate the correlation coefficient r, R2 and p-value of correlation.
In addition, 7α,25-dihydroxycholesterol, a cholesterol derivative, is a key chemoattractant in organizing the lymphoid microenvironment (27). The receptor for 7a,25-dihydroxycholesterol, EBI2 (GPR183) is expressed on a variety of leukocytes, including T-cells, B-cells, ILC3 and DC (27). Importantly, the oxysterol-EBI2 axis was recently shown to be a key regulator of lung TLO formation in a mouse model of COPD (28). Moreover, EBI2 controls cDC2 positioning at the B-T zone border of mouse lymphoid organs (29). In non-obstructed lungs, cDC2 expressed increased EBI2 surface levels as compared to cDC1 (Figure 4A). Of note, control and COPD cDC2 displayed similar expression levels of EBI2 (Figure E5B). Additionally, we investigated the expression of EBI2 on T cell subsets in the peripheral lung tissue in control and COPD subjects. Compared to ICOS−PD-1hi, ICOShiPD-1− and ICOS− PD-1− lung T-cells, the dominant IL-21 producing ICOShiPD-1hi T-cell fraction displayed the highest levels of surface EBI2 as well (Figure 4B and E5C).
To further study the association between the oxysterole-EBI2 axis and TLO-formation in COPD, we analyzed a publicly available dataset containing total lung transcriptome data from a cohort of COPD patients (GOLD I – IV) and healthy control subjects (Figure 4C) (GSE47460 derived from lung samples obtained through the NHLBI-funded Lung Tissue Research Consortium (LTRC) as part of the Lung Genomic Research Consortium (LGRC)). mRNA transcripts encoding EBI2 and mRNA encoding enzymes in the cholesterol metabolic pathway (i.e. CH25H, CYP1B1 and CYP7B1) positively correlated with COPD disease stage and hence inversely correlated with %FEV1 (Figure 4C). Importantly, lung EBI2, CH25H, CYP1B1 and CYP7B1 mRNA expression all correlated with CXCL13 mRNA expression, a marker for TLO formation during COPD disease (Figure 4C). Finally, lung CH25H mRNA strongly correlated with EBI2 mRNA levels (Figure 4D).
The correlation between the oxysterole-EBI2 axis and TLO-formation encouraged us to investigate the presence of the cholesterol metabolic pathway in TLOs of end-stage (GOLD IV) COPD patients. Indeed, RNAscope analysis of lung TLOs confirmed the expression of CH25H mRNA, encoding for one of the upstream enzyme involved in cholesterol degradation (27) in both the B cell follicular area and the T cell zone of the TLO (Figure 4E).
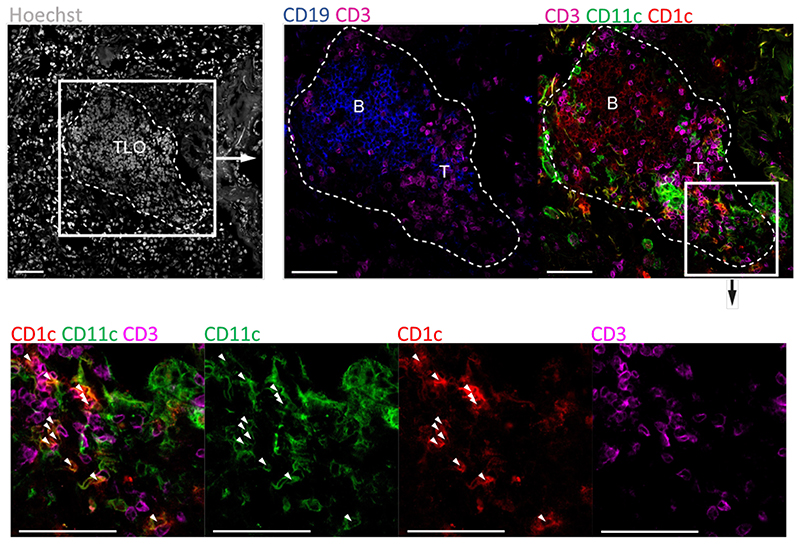
Finally, we performed confocal imaging of GOLD IV COPD lung tissue to determine the anatomical localization of cDC2 in the TLOs. This immunofluorescence analysis showed that cDC2 were indeed abundantly present in the follicular T cell zone of the TLOs, linking the unique migratory signature of cDC2 to the actual presence of these cells to TLOs during COPD (Figure 5).
Figure 5. cDC2 reside in the follicular T-cell zone of established COPD GOLD IV TLO.
Representative confocal fluorescence images of TLOs located in COPD GOLD IV explanted lungs (n=5). CD19 (blue) (AF594) and CD3ε (purple) (AF647) was used to define the B- and T-cell zone of the TLO respectively. CD11c (green) (FITC) and CD1c (red) (AF542) were used to identify cDC2 (white arrows). Hoechst was used as nuclear counter staining (grey). Scale bars 100μm.
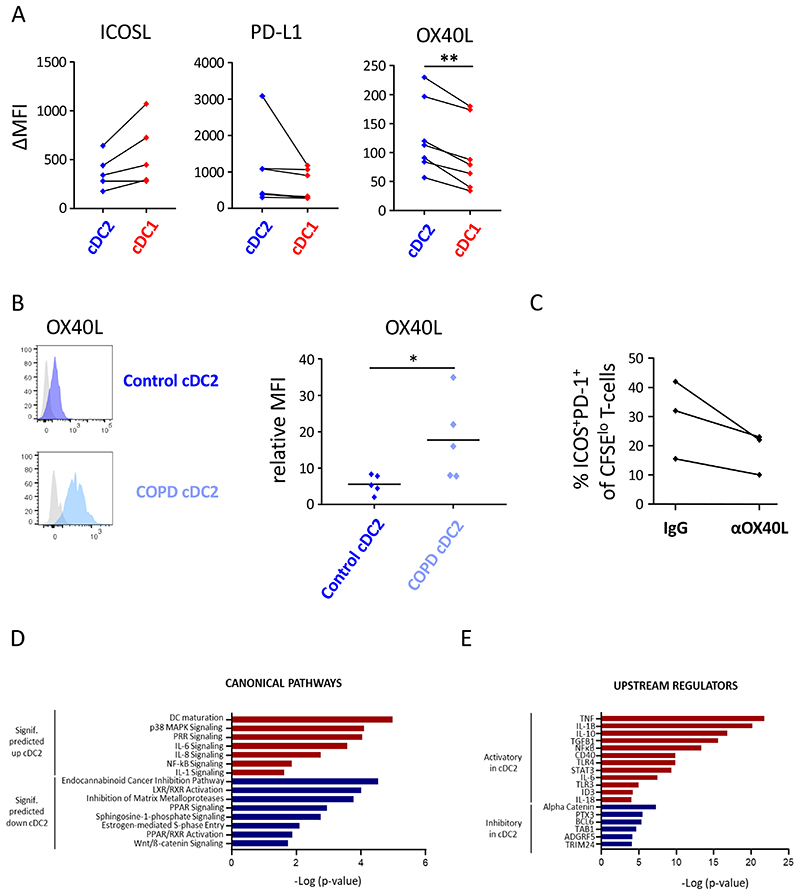
Lung cDC2 express increased levels of OX40L and transcriptional signatures related to Tfh-cell priming
To gain insights into the mechanism used by cDC2 to induce Tfh-like cells, we performed flow cytometric analysis of the co-stimulatory repertoire expressed on the different lung DC subsets. ICOSL, PDL1 and OX40L are known to deliver critical co-stimulatory signals to skew naïve T cells into Tfh-cells (23, 24). In non-obstructed control lungs, cDC2 and cDC1 subsets expressed similar levels of ICOSL and PDL1. However, we observed a significant increase in OX40L expression in cDC2 cells as compared to cDC1 (Figure 6A). Importantly, compared to control cDC2s, OX40L levels were even further increased on COPD cDC2 (Figure 6B). We next investigated whether OX40L was involved in Tfh-like cell polarization by lung cDC2. cDC2 isolated from non-obstructed control lung tissue were co-cultured with allogeneic naïve CD4+ T-cells in the presence of a blocking antibody for OX40L (oxelumab) or IgG isotype control. ICOS+PD-1+ Tfh-like cell priming was analyzed at d7 of the co-culture. Compared to the IgG control, blocking OX40L reduced ICOS+PD-1+ Tfh-like cell induction in each experiment (Figure 6C), confirming the importance of the OX40L-OX40 axis.
Figure 6. cDC2 express increased levels of OX40L and transcriptional signatures related to Tfh-cell priming.
Surface levels of ICOSL, PD-L1 and OX40L were measured on cDC2 and cDC1 from (A) non-obstructed and (B) COPD GOLDII (OX40L) peritumoral lung tissue via flow cytometry. Summary data graphs of the indicated co-stimulatory markers are depicted in A and representative flow cytometry histograms and summary data graph for OX40L is shown in B. Each symbol represents an individual donor (n=5 for ICOSL, n=6 for PD-L1, n=7 for control OX40L control and n=5 for COPD OX40L). *p<0.05, **p<0.01, Student’s t-test. (C) cDC2 were isolated from non-obstructed peritumoral lung resections (n=3) and co-cultured with allogeneic naïve CD4+ T-cells in the presence of an OX40L blocking antibody (oxelumab) or an IgG isotype control. Proportions of ICOS+PD-1+ T-cells were determined at day 7. Shown is the combined data graph in which each symbol represents an individual donor. (D) and (E) cDC2 and cDC1 were FACS-sorted from non-obstructed peritumoral lung resections (n=5) and the RNA transcriptomic profile of these subsets was generated via NGS. (D) Canonical pathways significantly (signif) upregulated (red) and downregulated (blue) (Fisher’s exact test, -log10 P values for each represented pathway are shown) in transcriptional signatures in cDC2 vs cDC1 as predicted by Ingenuity Pathway Analysis (IPA). (E) Significant putative regulators with predicted activating (red) or inhibitory (blue) influence on transcriptional signatures in cDC2 vs cDC1 from non-obstructed lungs, as determined by IPA.
To further understand the mechanisms underlying the enhanced ability of cDC2 to polarize Tfh-like cells, we compared the transcriptional profile of cDC2 with cDC1. cDC2 and cDC1 were FACS-sorted and their transcriptomic profile was generated via Next Generation Sequencing (NGS). Interestingly, this analysis revealed that genes encoding for signaling components of the IL-6 (p = 0,0002) and IL-1 (p = 0,02) pathways, critical for Tfh-cell development (30, 31), were significantly upregulated in cDC2 (Figure 6D). Furthermore, transcripts involved in general DC maturation and activation, features of DC contributing to Tfh generation (32) were also upregulated in cDC2 compared to cDC1 (Figure 6D). Consistent with these observations, biocomputational analysis identified signaling through CD40 (p = 1,15 × 10−10); secretion of multiple effector cytokines such as TNF (p = 1,58 × 10−22), IL-1β (p = 6,01 × 10−21), IL-6 (p = 2,86 × 10−8) and IL-18 (p = 9,43 × 10−5) and transcription factors and signaling mediators like NF-κB (p = 4,36 × 10−14), STAT3 (p = 4,06 × 10−10) and ID3 (p = 5,96 × 10−5) as putative upstream regulators of transcriptional signatures in lung cDC2 (Figure 6E). In contrast, transcriptional pathways and genes downregulated in cDC2 had no immediate connection to Tfh-priming by DC or were negatively associated with Tfh polarization, eg. LXR (p = 9,54 × 10−5) (33) (Figure 6E). Collectively, transcriptomic analysis indicates that lung cDC2 are characterized by signatures related to key pathways involved in Tfh- or Tfh-like cell polarization.
Discussion
The current study reveals a potent ability for cDC2 to induce IL-21 and CXCL13 secreting Tfh-like cells, suggesting a crucial role for cDC2 in TLO formation during COPD.
ScRNA-seq revealed a high level of heterogeneity among human lung Lin−HLA-DR+ cells. In agreement with previous studies investigating human blood and lung tumor myeloid cell heterogeneity (20, 21), we identified clusters of cDC1, cDC2, pDC, a cluster containing ‘activated DC’, the three monocyte subsets, being CD14+ (‘classical’) monocytes, CD16+ (‘non-classical’) monocytes and CD14++CD16+ (‘intermediate’) monocytes and several additional monocyte/macrophage populations. Furthermore, we found a population of NK-cells, mast cells, type II AEC and endothelial cells. HLA-DR expression by NK-cells and mast cells was reported previously (34, 35). Although type II AEC and endothelial cells can express HLA-DR (36, 37) we believe that these rather represented a minor contamination. Compared to the Zilionis et al. study, we found less monocyte/macrophage populations/phenotypes in the lung tissue. This discrepancy could be explained by the fact that alveolar cells, containing multiple alveolar macrophage phenotypes, were not part of our analysis. Furthermore, we sampled lung tissue distant from the tumor bed, likely lacking several tumor-infiltrating myeloid cell populations (TIMs) (21). Finally, this dataset has some limitations that warrant future investigations, notably the lack of a larger validation cohort, the relatively low numbers of cells analyzed and the read-depth which might have limited the power to define lung cell subsets in this experiment.
The role of DC during COPD remains controversial. Several studies demonstrated that DC exhibit an increased co-stimulatory repertoire (38) and that, especially CD1c+ DC (cDC2), drive Th17-responses during cigarette smoke induced lung emphysema in both humans and mice (39–42). In contrast, a recent report stated that human lung CD1c+ DC displayed a regulatory function during COPD, suppressing pathogenic T-cell responses and inducing regulatory T-cells (43). In our study, compared to other DC subsets, cDC2 were the most potent in skewing naïve CD4+ T-cells into IL-21 and CXCL13 secreting Tfh-like cells. These findings are in line with a recent murine study, demonstrating that lung cDC2, but not cDC1, were driving antigen-specific Tfh-induction (44). Furthermore, recent research showed that human tonsil cDC2 were the most efficient in Tfh-cell polarization as well (45). Strikingly, by using lung resident cDC2 in our study, we also demonstrated that human cDC2 residing in non-lymphoid peripheral organs can induce Tfh-like cells without requirement of prior migration to draining lymph nodes.
The increased capability of COPD cDC2 to induce Tfh-like cells was associated with increased presence of Tfh-like cells in the COPD parenchyma. To our knowledge, we are the first to demonstrate the presence of a Tfh-like cell during early (GOLDI/II) stages of COPD. We and others showed already the presence of peripheral, extrafollicular IL-21 secreting PD-1+ICOS+ Tfh-like cells, that lack CXCR5 and/or Bcl6 expression, in rheumatoid arthritis (10), skin fibrosis (9) and an HDM-driven asthma model (11). The effect of Tfh-like cells on COPD development and progression remains to be elucidated. However, Ladjemi et al recently confirmed the presence of IL-21+ T-cells that did not co-express CXCR5 in the TLOs of late-stage COPD lungs, further supporting a role for an extrafollicular Tfh-like cell type and IL-21 (3).
cDC2 exhibited a unique migratory signature, including increased expression of CXCR4, CXCR5 and EBI2, suggesting that these cells efficiently migrate to the site of TLO formation during COPD. Levels of CXCL12 and CXCL13, ligands for CXCR4 and CXCR5 respectively, are increased in human COPD lungs (7, 25, 26). A recent study also highlighted the crucial role of the oxysterol-EBI2 axis in COPD TLO formation (28). We now demonstrated the presence of CH25H mRNA in both T- and B-cell zone of TLOs during late-stage COPD. These results imply that cholesterol metabolism is important for maintaining the structure of established TLO as well by continuously recruiting T-, B-cells and cDC2. In line with this premise, we were able to detect cDC2 in the T-cell zone of GOLD IV COPD lung TLO. This observation indicates that cDC2 are also important for maintaining established TLOs most likely via sustained antigen-presentation and induction of T(fh-like) cell polarization and proliferation.
Furthermore, we found that, compared to cDC1, cDC2 exhibited increased (transcriptional) expression of pathways and genes related to DC-induced Tfh-priming, including OX40L. In agreement with previous reports (24, 46), we confirmed that the OX40L-OX40 axis promoted human Tfh-polarization. Mediators that stimulate OX40L expression, including TSLP, IL-1, IL-33 and GM-CSF, are abundantly present in the lung (47, 48) and elevated levels are observed in COPD subjects (47, 49, 50), likely underlying the increased OX40L levels expressed by COPD cDC2. Additionally, several genes encoding for cytokine mediators that deliver Tfh-skewing signals, including IL-6, IL-1β and TGFβ have already been shown to be increased during COPD (51). This implies that these signals might further expand the cDC2-induced Tfh-like cell polarization during COPD.
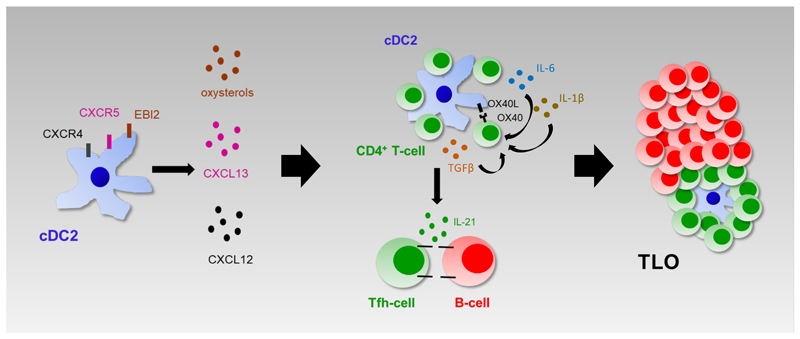
Collectively, we propose a model (Figure 7) in which, during COPD, locally produced chemokines, like CXCL12 and CXCL13, and cholesterol metabolites attract cDC2 and CD4+ T-cells to the site of TLO formation. Upon encounter, cDC2 skew IL-21+ Tfh-like cell polarization via OX40L and cytokine signals. Finally, the chronicity of this self-amplifying loop results in the formation of well-organized TLOs in which Tfh-like cell clonality and proliferation is further sustained by cDC2. Thus, our study reveals a new (immune) mechanism underlying TLO formation during COPD. However, additional studies will be required to fully comprehend the role of this pathway, and of TLO formation in general, in COPD pathogenesis and progression.
Figure 7. cDC2 drive lymphoid neogenesis during COPD; a working model.
Elevated pulmonary levels of CXCL12, CXCL13 and cholesterol metabolites, produced during COPD, attract cDC2 and CD4+ T-cells to the site of TLO formation. Upon encounter, cDC2 drive IL-21+ Tfh-like cell polarization via the OX40L-OX40 axis and the secretion of cytokines like IL-6, IL-1β and TGF-β. The chronicity of this self-amplifying loop results in the formation of well-established TLOs during late-stage COPD in which Tfh-like cell clonality and proliferation is further sustained by cDC2.
Supplementary Material
At a Glance Commentary.
Scientific Knowledge of on the Subject
Chronic obstructive pulmonary disease (COPD) severity and tissue destruction correlate with the development of tertiary lymphoid organs (TLO). T follicular helper (Tfh)-cells represent a specialized CD4+ T-cell subset, key for lymphoid organ formation. Dendritic cells (DC) are potent inducers of CD4+ T-cell responses, including Tfh-cell responses. However, how human lung DC polarize Tfh-cells during COPD and hence contribute to the generation of TLOs remains to be elucidated.
What this Study Adds to the Field
Single cell RNA sequencing showed that the myeloid cell compartment in the human non-obstructed lung is highly heterogeneous, containing multiple DC and monocyte/macrophage subsets. Among these, CD1c+ conventional (c)DC (cDC2) were the most potent inducers of Tfh-cell polarization. Importantly, compared to cDC2 from non-obstructed control lungs, cDC2 derived from COPD lungs showed increased potential to polarize Tfh-cells. Mechanistically, cDC2 exhibited a unique migratory signature, including expression of the oxysterol receptor EBI2, known to control spatial organization of immune cells in TLO. Furthermore, we demonstrated the crucial contribution of the OX40-OX40L co-stimulatory axis to cDC2 mediated Tfh-cell induction. Additionally, cDC2 exhibited (transcriptional) expression of several other pathways and genes related to DC-induced Tfh-priming. Together, our study revealed a novel immune mechanism underlying TLO formation during COPD pathogenesis.
Impact: Our study reveals a new (immune) mechanism underlying TLO formation during COPD and argues for increased investigation of the role of this pathway, and TLO formation in general, in COPD pathogenesis and progression. In addition, the data provide conceptual advances regarding the formation of TLO during other respiratory and non-respiratory diseases and contribute to the field of human lung dendritic cells and T follicular helper cells.
Acknowledgments
We are grateful to all the lung tissue donors. We thank the surgeons and nurses from the Thoraxkliniken, Sahlgrenska University Hospital and Gothenburg University, Gothenburg, Sweden for sample collection. We also wish to thank Mr Aiman Alzetani, Professor Christian Ottensmeier and the rest of the Target Lung team for sample collection in Southampton.
References
- 1.Collaborators GBDCoD. Global, regional, and national age-sex specific mortality for 264 causes of death, 1980-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet. 2017;390:1151–1210. doi: 10.1016/S0140-6736(17)32152-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hogg JC, Chu F, Utokaparch S, Woods R, Elliott WM, Buzatu L, Cherniack RM, Rogers RM, Sciurba FC, Coxson HO, Pare PD. The nature of small-airway obstruction in chronic obstructive pulmonary disease. N Engl J Med. 2004;350:2645–2653. doi: 10.1056/NEJMoa032158. [DOI] [PubMed] [Google Scholar]
- 3.Ladjemi MZ, Martin C, Lecocq M, Detry B, Aboubakar Nana F, Moulin C, Weynand B, Fregimilicka C, Bouzin C, Thurion P, Carlier F, et al. Increased IgA Expression in Lung Lymphoid Follicles in Severe COPD. Am J Respir Crit Care Med. 2018 doi: 10.1164/rccm.201802-0352OC. [DOI] [PubMed] [Google Scholar]
- 4.Polverino F, Cosio BG, Pons J, Laucho-Contreras M, Tejera P, Iglesias A, Rios A, Jahn A, Sauleda J, Divo M, Pinto-Plata V, et al. B Cell-Activating Factor. An Orchestrator of Lymphoid Follicles in Severe Chronic Obstructive Pulmonary Disease. Am J Respir Crit Care Med. 2015;192:695–705. doi: 10.1164/rccm.201501-0107OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Randall TD. Bronchus-associated lymphoid tissue (BALT) structure and function. Adv Immunol. 2010;107:187–241. doi: 10.1016/B978-0-12-381300-8.00007-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.John-Schuster G, Hager K, Conlon TM, Irmler M, Beckers J, Eickelberg O, Yildirim AO. Cigarette smoke-induced iBALT mediates macrophage activation in a B cell-dependent manner in COPD. Am J Physiol Lung Cell Mol Physiol. 2014;307:L692–706. doi: 10.1152/ajplung.00092.2014. [DOI] [PubMed] [Google Scholar]
- 7.Bracke KR, Verhamme FM, Seys LJ, Bantsimba-Malanda C, Cunoosamy DM, Herbst R, Hammad H, Lambrecht BN, Joos GF, Brusselle GG. Role of CXCL13 in cigarette smoke-induced lymphoid follicle formation and chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2013;188:343–355. doi: 10.1164/rccm.201211-2055OC. [DOI] [PubMed] [Google Scholar]
- 8.Seys LJ, Verhamme FM, Schinwald A, Hammad H, Cunoosamy DM, Bantsimba-Malanda C, Sabirsh A, McCall E, Flavell L, Herbst R, Provoost S, et al. Role of B Cell-Activating Factor in Chronic Obstructive Pulmonary Disease. Am J Respir Crit Care Med. 2015;192:706–718. doi: 10.1164/rccm.201501-0103OC. [DOI] [PubMed] [Google Scholar]
- 9.Taylor DK, Mittereder N, Kuta E, Delaney T, Burwell T, Dacosta K, Zhao W, Cheng LI, Brown C, Boutrin A, Guo X, et al. T follicular helper-like cells contribute to skin fibrosis. Sci Transl Med. 2018;10 doi: 10.1126/scitranslmed.aaf5307. [DOI] [PubMed] [Google Scholar]
- 10.Rao DA, Gurish MF, Marshall JL, Slowikowski K, Fonseka CY, Liu Y, Donlin LT, Henderson LA, Wei K, Mizoguchi F, Teslovich NC, et al. Pathologically expanded peripheral T helper cell subset drives B cells in rheumatoid arthritis. Nature. 2017;542:110–114. doi: 10.1038/nature20810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Coquet JM, Schuijs MJ, Smyth MJ, Deswarte K, Beyaert R, Braun H, Boon L, Karlsson Hedestam GB, Nutt SL, Hammad H, Lambrecht BN. Interleukin-21-Producing CD4(+) T Cells Promote Type 2 Immunity to House Dust Mites. Immunity. 2015;43:318–330. doi: 10.1016/j.immuni.2015.07.015. [DOI] [PubMed] [Google Scholar]
- 12.Perros F, Dorfmuller P, Montani D, Hammad H, Waelput W, Girerd B, Raymond N, Mercier O, Mussot S, Cohen-Kaminsky S, Humbert M, et al. Pulmonary lymphoid neogenesis in idiopathic pulmonary arterial hypertension. Am J Respir Crit Care Med. 2012;185:311–321. doi: 10.1164/rccm.201105-0927OC. [DOI] [PubMed] [Google Scholar]
- 13.Guilliams M, Ginhoux F, Jakubzick C, Naik SH, Onai N, Schraml BU, Segura E, Tussiwand R, Yona S. Dendritic cells, monocytes and macrophages: a unified nomenclature based on ontogeny. Nat Rev Immunol. 2014;14:571–578. doi: 10.1038/nri3712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guilliams M, Dutertre CA, Scott CL, McGovern N, Sichien D, Chakarov S, Van Gassen S, Chen J, Poidinger M, De Prijck S, Tavernier SJ, et al. Unsupervised High-Dimensional Analysis Aligns Dendritic Cells across Tissues and Species. Immunity. 2016;45:669–684. doi: 10.1016/j.immuni.2016.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.GeurtsvanKessel CH, Willart MA, Bergen IM, van Rijt LS, Muskens F, Elewaut D, Osterhaus AD, Hendriks R, Rimmelzwaan GF, Lambrecht BN. Dendritic cells are crucial for maintenance of tertiary lymphoid structures in the lung of influenza virus-infected mice. J Exp Med. 2009;206:2339–2349. doi: 10.1084/jem.20090410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Muniz LR, Pacer ME, Lira SA, Furtado GC. A critical role for dendritic cells in the formation of lymphatic vessels within tertiary lymphoid structures. J Immunol. 2011;187:828–834. doi: 10.4049/jimmunol.1004233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Halle S, Dujardin HC, Bakocevic N, Fleige H, Danzer H, Willenzon S, Suezer Y, Hammerling G, Garbi N, Sutter G, Worbs T, et al. Induced bronchus-associated lymphoid tissue serves as a general priming site for T cells and is maintained by dendritic cells. J Exp Med. 2009;206:2593–2601. doi: 10.1084/jem.20091472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.van Rijt LS, Vos N, Willart M, Muskens F, Tak PP, van der Horst C, Hoogsteden HC, Lambrecht BN. Persistent activation of dendritic cells after resolution of allergic airway inflammation breaks tolerance to inhaled allergens in mice. Am J Respir Crit Care Med. 2011;184:303–311. doi: 10.1164/rccm.201101-0019OC. [DOI] [PubMed] [Google Scholar]
- 19.Naessens T, M Y, Hamrud E, Gehrmann U, Mattsson J, Skogberg G, Israelsson E, Thörn K, Melville F, Deys L, Bracke K, et al. Human lung CD1c dendritic cells orchestrate lymphoid neogenesis during COPD. ERJ. 2019 doi: 10.1164/rccm.201906-1123OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Villani AC, Satija R, Reynolds G, Sarkizova S, Shekhar K, Fletcher J, Griesbeck M, Butler A, Zheng S, Lazo S, Jardine L, et al. Single-cell RNA-seq reveals new types of human blood dendritic cells, monocytes, and progenitors. Science. 2017;356 doi: 10.1126/science.aah4573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zilionis R, Engblom C, Pfirschke C, Savova V, Zemmour D, Saatcioglu HD, Krishnan I, Maroni G, Meyerovitz CV, Kerwin CM, Choi S, et al. Single-Cell Transcriptomics of Human and Mouse Lung Cancers Reveals Conserved Myeloid Populations across Individuals and Species. Immunity. 2019;50:1317–1334.:e1310. doi: 10.1016/j.immuni.2019.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Boyette LB, Macedo C, Hadi K, Elinoff BD, Walters JT, Ramaswami B, Chalasani G, Taboas JM, Lakkis FG, Metes DM. Phenotype, function, and differentiation potential of human monocyte subsets. PLoS One. 2017;12:e0176460. doi: 10.1371/journal.pone.0176460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Crotty S. T follicular helper cell differentiation, function, and roles in disease. Immunity. 2014;41:529–542. doi: 10.1016/j.immuni.2014.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pattarini L, Trichot C, Bogiatzi S, Grandclaudon M, Meller S, Keuylian Z, Durand M, Volpe E, Madonna S, Cavani A, Chiricozzi A, et al. TSLP-activated dendritic cells induce human T follicular helper cell differentiation through OX40-ligand. J Exp Med. 2017;214:1529–1546. doi: 10.1084/jem.20150402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Litsiou E, Semitekolou M, Galani IE, Morianos I, Tsoutsa A, Kara P, Rontogianni D, Bellenis I, Konstantinou M, Potaris K, Andreakos E, et al. CXCL13 production in B cells via Toll-like receptor/lymphotoxin receptor signaling is involved in lymphoid neogenesis in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2013;187:1194–1202. doi: 10.1164/rccm.201208-1543OC. [DOI] [PubMed] [Google Scholar]
- 26.Roos AB, Sanden C, Mori M, Bjermer L, Stampfli MR, Erjefalt JS. IL-17A Is Elevated in End-Stage Chronic Obstructive Pulmonary Disease and Contributes to Cigarette Smoke-induced Lymphoid Neogenesis. Am J Respir Crit Care Med. 2015;191:1232–1241. doi: 10.1164/rccm.201410-1861OC. [DOI] [PubMed] [Google Scholar]
- 27.Cyster JG, Dang EV, Reboldi A, Yi T. 25-Hydroxycholesterols in innate and adaptive immunity. Nat Rev Immunol. 2014;14:731–743. doi: 10.1038/nri3755. [DOI] [PubMed] [Google Scholar]
- 28.Jia J, Conlon TM, Sarker RS, Tasdemir D, Smirnova NF, Srivastava B, Verleden SE, Gunes G, Wu X, Prehn C, Gao J, et al. Cholesterol metabolism promotes B-cell positioning during immune pathogenesis of chronic obstructive pulmonary disease. EMBO Mol Med. 2018;10 doi: 10.15252/emmm.201708349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lu E, Dang EV, McDonald JG, Cyster JG. Distinct oxysterol requirements for positioning naive and activated dendritic cells in the spleen. Sci Immunol. 2017;2 doi: 10.1126/sciimmunol.aal5237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nish SA, Schenten D, Wunderlich FT, Pope SD, Gao Y, Hoshi N, Yu S, Yan X, Lee HK, Pasman L, Brodsky I, et al. T cell-intrinsic role of IL-6 signaling in primary and memory responses. Elife. 2014;3:e01949. doi: 10.7554/eLife.01949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chakarov S, Fazilleau N. Monocyte-derived dendritic cells promote T follicular helper cell differentiation. EMBO Mol Med. 2014;6:590–603. doi: 10.1002/emmm.201403841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yamasaki S, Shimizu K, Kometani K, Sakurai M, Kawamura M, Fujii SI. In vivo dendritic cell targeting cellular vaccine induces CD4(+) Tfh cell-dependent antibody against influenza virus. Sci Rep. 2016;6:35173. doi: 10.1038/srep35173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ryu H, Chung Y. Dyslipidemia promotes germinal center reactions via IL-27. BMB Rep. 2018;51:371–372. doi: 10.5483/BMBRep.2018.51.8.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Erokhina SA, Streltsova MA, Kanevskiy LM, Telford WG, Sapozhnikov AM, Kovalenko EI. HLA-DR(+) NK cells are mostly characterized by less mature phenotype and high functional activity. Immunol Cell Biol. 2018;96:212–228. doi: 10.1111/imcb.1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lotfi-Emran S, Ward BR, Le QT, Pozez AL, Manjili MH, Woodfolk JA, Schwartz LB. Human mast cells present antigen to autologous CD4(+) T cells. J Allergy Clin Immunol. 2018;141:311–321.:e310. doi: 10.1016/j.jaci.2017.02.048. [DOI] [PubMed] [Google Scholar]
- 36.Taflin C, Favier B, Baudhuin J, Savenay A, Hemon P, Bensussan A, Charron D, Glotz D, Mooney N. Human endothelial cells generate Th17 and regulatory T cells under inflammatory conditions. Proc Natl Acad Sci U S A. 2011;108:2891–2896. doi: 10.1073/pnas.1011811108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zissel G, Ernst M, Rabe K, Papadopoulos T, Magnussen H, Schlaak M, Muller-Quernheim J. Human alveolar epithelial cells type II are capable of regulating T-cell activity. J Investig Med. 2000;48:66–75. [PubMed] [Google Scholar]
- 38.Freeman CM, Martinez FJ, Han MK, Ames TM, Chensue SW, Todt JC, Arenberg DA, Meldrum CA, Getty C, McCloskey L, Curtis JL. Lung dendritic cell expression of maturation molecules increases with worsening chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2009;180:1179–1188. doi: 10.1164/rccm.200904-0552OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shan M, Cheng HF, Song LZ, Roberts L, Green L, Hacken-Bitar J, Huh J, Bakaeen F, Coxson HO, Storness-Bliss C, Ramchandani M, et al. Lung myeloid dendritic cells coordinately induce TH1 and TH17 responses in human emphysema. Sci Transl Med. 2009;1:4ra10. doi: 10.1126/scitranlsmed.3000154. [DOI] [PubMed] [Google Scholar]
- 40.Shan M, You R, Yuan X, Frazier MV, Porter P, Seryshev A, Hong JS, Song LZ, Zhang Y, Hilsenbeck S, Whitehead L, et al. Agonistic induction of PPARgamma reverses cigarette smoke-induced emphysema. J Clin Invest. 2014;124:1371–1381. doi: 10.1172/JCI70587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lu W, You R, Yuan X, Yang T, Samuel EL, Marcano DC, Sikkema WK, Tour JM, Rodriguez A, Kheradmand F, Corry DB. The microRNA miR-22 inhibits the histone deacetylase HDAC4 to promote T(H)17 cell-dependent emphysema. Nat Immunol. 2015;16:1185–1194. doi: 10.1038/ni.3292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.You R, Lu W, Shan M, Berlin JM, Samuel EL, Marcano DC, Sun Z, Sikkema WK, Yuan X, Song L, Hendrix AY, et al. Nanoparticulate carbon black in cigarette smoke induces DNA cleavage and Th17-mediated emphysema. Elife. 2015;4:e09623. doi: 10.7554/eLife.09623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tsoumakidou M, Tousa S, Semitekolou M, Panagiotou P, Panagiotou A, Morianos I, Litsiou E, Trochoutsou AI, Konstantinou M, Potaris K, Footitt J, et al. Tolerogenic signaling by pulmonary CD1c+ dendritic cells induces regulatory T cells in patients with chronic obstructive pulmonary disease by IL-27/IL-10/inducible costimulator ligand. J Allergy Clin Immunol. 2014;134:944–954.:e948. doi: 10.1016/j.jaci.2014.05.045. [DOI] [PubMed] [Google Scholar]
- 44.Krishnaswamy JK, Gowthaman U, Zhang B, Mattsson J, Szeponik L, Liu D, Wu R, White T, Calabro S, Xu L, Collet MA, et al. Migratory CD11b(+) conventional dendritic cells induce T follicular helper cell-dependent antibody responses. Sci Immunol. 2017;2 doi: 10.1126/sciimmunol.aam9169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Durand M, Walter T, Pirnay T, Naessens T, Gueguen P, Goudot C, Lameiras S, Chang Q, Talaei N, Ornatsky O, Vassilevskaia T, et al. Human lymphoid organ cDC2 and macrophages play complementary roles in T follicular helper responses. J Exp Med. 2019 doi: 10.1084/jem.20181994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jacquemin C, Schmitt N, Contin-Bordes C, Liu Y, Narayanan P, Seneschal J, Maurouard T, Dougall D, Davizon ES, Dumortier H, Douchet I, et al. OX40 Ligand Contributes to Human Lupus Pathogenesis by Promoting T Follicular Helper Response. Immunity. 2015;42:1159–1170. doi: 10.1016/j.immuni.2015.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ying S, O’Connor B, Ratoff J, Meng Q, Fang C, Cousins D, Zhang G, Gu S, Gao Z, Shamji B, Edwards MJ, et al. Expression and cellular provenance of thymic stromal lymphopoietin and chemokines in patients with severe asthma and chronic obstructive pulmonary disease. J Immunol. 2008;181:2790–2798. doi: 10.4049/jimmunol.181.4.2790. [DOI] [PubMed] [Google Scholar]
- 48.Naessens T, Schepens B, Smet M, Pollard C, Van Hoecke L, De Beuckelaer A, Willart M, Lambrecht B, De Koker S, Saelens X, Grooten J. GM-CSF treatment prevents respiratory syncytial virus-induced pulmonary exacerbation responses in postallergic mice by stimulating alveolar macrophage maturation. J Allergy Clin Immunol. 2016;137:700–709.:e709. doi: 10.1016/j.jaci.2015.09.031. [DOI] [PubMed] [Google Scholar]
- 49.Morissette MC, Shen P, Thayaparan D, Stampfli MR. Disruption of pulmonary lipid homeostasis drives cigarette smoke-induced lung inflammation in mice. Eur Respir J. 2015;46:1451–1460. doi: 10.1183/09031936.00216914. [DOI] [PubMed] [Google Scholar]
- 50.Kearley J, Silver JS, Sanden C, Liu Z, Berlin AA, White N, Mori M, Pham TH, Ward CK, Criner GJ, Marchetti N, et al. Cigarette smoke silences innate lymphoid cell function and facilitates an exacerbated type I interleukin-33-dependent response to infection. Immunity. 2015;42:566–579. doi: 10.1016/j.immuni.2015.02.011. [DOI] [PubMed] [Google Scholar]
- 51.Barnes PJ. Inflammatory mechanisms in patients with chronic obstructive pulmonary disease. J Allergy Clin Immunol. 2016;138:16–27. doi: 10.1016/j.jaci.2016.05.011. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.