Abstract
In the March 1st issue of Cancer Research, we identified the Wnt receptor Fzd7 as an attractive therapeutic target for the treatment of gastric cancer. In summary, we showed that pharmacological inhibition of Wnt receptors, or genetic deletion of Fzd7, blocks the initiation and growth of gastric tumours. Inhibiting Fzd receptors, specifically Fzd7, inhibits the growth of gastric cancer cells even in the presence of Adenomatous polyposis coli (Apc) mutation. Apc is located in the cytoplasm downstream of Fzd7 in the Wnt signaling cascade and APC mutations activate Wnt/β-catenin signaling, therefore this result seems counterintuitive. Here, we analyze this result in greater detail in the context of current knowledge of Wnt signaling, and discuss the wider implications of this aspect of Wnt signaling in other cancers.
Regulation of Wnt signaling intracellularly and at the plasma membrane
The Wnt signaling pathway regulates many cell functions, including proliferation, migration, apoptosis and differentiation [1]. It is essential during embryonic development and also in homeostasis of several adult tissues including the GI tract [2, 3], liver, breast and skin [1], and is deregulated in many cancers including colon, gastric, breast and liver [4]. Wnt ligands are secreted glycoproteins which bind to a ‘U’ shaped pocket in the dimer of Fzd receptors via a lipid modification of palmitoleic acid to form a receptor complex with co-receptors, including Lrp5/6 and Lgr4/5 [1]. This complex interacts with components of a cytoplasmic ‘destruction complex’ which contains Gsk3, Axin, Ck1 and Apc, to form a large signalosome [1]. In the absence of a Wnt ligand, newly translated β-catenin proteins are bound and phosphorylated by the destruction complex. However, in the presence of Wnt/Fzd binding, signalosome assembly disrupts this process and β-catenin is free to accumulate, translocate into the nucleus and associate with transcription factors Tcf/Lef to regulate target gene transcription [1] (Fig. 1A).
Figure 1. Wnt signalling with mutant Apc.
A. The levels of cytoplasmic β-catenin are regulated by the degradation complex which is inhibited when Wnt signalling is active (left hand panel). In Apc mutant cells it is often misconceived that Apc is completely deleted and therefore the degradation complex is non-functional and Wnt signalling cannot be regulated upstream of the degradation complex at the level of the receptor/ligand (illustration faded out, central panel). However mutant Apc is transcribed and translated resulting in a compromised, yet functional, β-catenin degradation complex which explains how upstream factors including sFRP, Dkk and Fzd inhibitors can still modulate Wnt signal activity (right hand panel). B. Immunohistochemistry for β-catenin in a human colorectal carcinoma showing increased nuclear localisation, as a surrogate marker of active Wnt, in the invasive front compared to the tumour center (from Brabletz et al. Pathol Res Pract. 1998;194:701-4).
Nuclear β-catenin, a hallmark of active Wnt signaling [1], is observed in ~30% of human gastric tumours [5]. Next generation sequencing has revealed that Wnt signaling is deregulated in gastric tumours at several points in the pathway including the ligand, receptors and intracellular transduction components [6, 7]. In addition to mutations, epigenetic changes are also observed in gastric tumours to Wnt inhibitors such as sFRP (binds directly to Wnt ligands) and Dkk1 (binds to and inhibits Lrp5/6 receptors), resulting in activation of Wnt signaling at the level of the receptor/ligand [1]. Indeed, RNF43, an E3 ubiquitin ligase that turns over Fzd on the cell surface [8] is mutated in ~18% of human gastric tumours, whilst many Wnt ligands are overexpressed [7]. This is around the same frequency (~18%) observed for APC mutations in GC, ~37% of which also contain an RNF43 mutation, indicating compound activation of Wnt signaling in the same tumour [7]. This observation that Wnt signaling is regulated intracellularly and at the plasma membrane is consistent with our experimental data in which we could inhibit the growth of Apc mutant gastric tumours by deleting Fzd7 and prompts a frequently asked question when presenting this work, ‘how can a cell with mutant Apc respond to inhibition of a Fzd receptor upstream?’.
Heterogenous Wnt signaling regulates distinct cell functions in a tumour
To answer this question, we first need to highlight that Wnt signaling is not a binary system whereby the pathway is on or off, but rather is highly regulated in a compound fashion to provide spatial and temporal variation depending on multiple environmental factors [9]. This was first demonstrated by Thomas Brabletz in 1998, who showed heterogenous expression of nuclear β-catenin (a surrogate marker of active Wnt signaling) within human CRCs, despite all tumour cells harboring APC mutation [10] (Fig. 1B). Furthermore, Wnt signaling is promoted by macrophage-derived TNF-α [11] and H. pylori infection can activate the Wnt pathway via Fzd7 [12], highlighting a role for the microenvironment for variable Wnt activity in tumours. This indicates that additional factors can regulate Wnt signal strength even in APC mutant cells. This can occur because the APC gene is not deleted but truncated and mutant APC is still transcribed and translated into a protein which is able to partially function in the destruction complex and respond to WNT ligands, albeit at a reduced level [13, 14]. Indeed, Wnt3 secretion maintains high Wnt signaling in APC mutant colon cancer cells [14], whilst APC is methylated, and subsequently inhibited further during tumour progression in colon tumours, highlighting variable Wnt activity as a feature of colon tumours [15]. This explains why APC mutant cells are sensitive to Fzd inhibition in our work, but what is the function of this variable Wnt signal?
Approximately 37% of APC mutant gastric tumours also contain RNF43 mutations, indicating compound activation of Wnt signaling in the same tumour [3]. However, only 5.5% of colon tumours have APC and RNF43 mutations, suggesting that GC and CRC preferentially select different mechanisms of optimal, ‘just right’, levels of Wnt signaling required for tumour growth and progression. The ‘just right’ model of Wnt signaling proposes APC mutations are selected for sub-maximal levels of Wnt signaling to provide a Wnt signal that is sufficient to transform cells, but not excessive and cytotoxic [16]. Experimental evidence from in vivo data helped confirm these models. For instance, the Apc1322T/+ mouse model retains a single β-catenin binding domain in the mutant Apc1322T allele, which develops more severe intestinal tumours, but with lower levels of Wnt signaling compared to other Apc mutant models that lack β-catenin binding capacity [17]. Additionally, we have previously shown that intestinal tumourigenesis is reduced in Cited1+/-; ApcMin/+ mice due to very high, cytotoxic Wnt signaling [18]. These data correlate with observations of improved clinical outcomes after relapse for molecular subtypes of colon cancer which display high Wnt signaling [19].
Notably, a common theme is emerging for epithelial tumours which indicates that cancer cells harbor mutations of intracellular components of the Wnt pathway with additional mutations or epigenetic modifications at the level of the receptor/ligand to permit further regulation of Wnt signaling (ligand and receptor overexpression) (Fig. 2). The net result, at least in colon cancer, appears to be to constrain Wnt signaling within an optimal ‘just right’ spectrum in the cancer cells, with bursts of intense signaling in a context dependent manner, for example at the invasive front of colon tumours (Fig 1). Given the context-dependent manner of Wnt signaling during development [9] this is perhaps not surprising. Importantly, despite multiple mutations within a cancer cell, correcting or inhibiting one gene in the Wnt pathway that is altered in cancer can potently inhibit tumour growth. This has important implications for cancer therapy. For example, restoring the function of APC in APC-mutant colon cancer cells inhibits tumour growth [20, 21], implying that mutation of APC is not just an initiating event in the genesis of colon cancer but provides the genetic platform that sustains the tumour. Restoring wild-type APC removes that platform. Furthermore, restoring sFRP for example [22], or blocking Fzd7 signalling in APC mutant colon cancer cells [23] or gastric cancer cells [7], inhibits Wnt signaling and tumour growth, again, despite the tumour cells harboring many other mutations, implicating the importance of “just right” Wnt signaling. Notably, Wnt signaling can be further stimulated by inhibiting GSK3 or addition of WNT3A in APC mutant colon cancer cells [14, 24], which suggests potential regulation also at the destruction complex even if APC is mutated [24]. Furthermore, other members of the Fzd family of Wnt receptors have been implicated in many cancer types, including GC in which we observed that Fzd2 and Fzd6 were also upregulated in human GC cells [7], and therefore future research will help determine their functional roles.
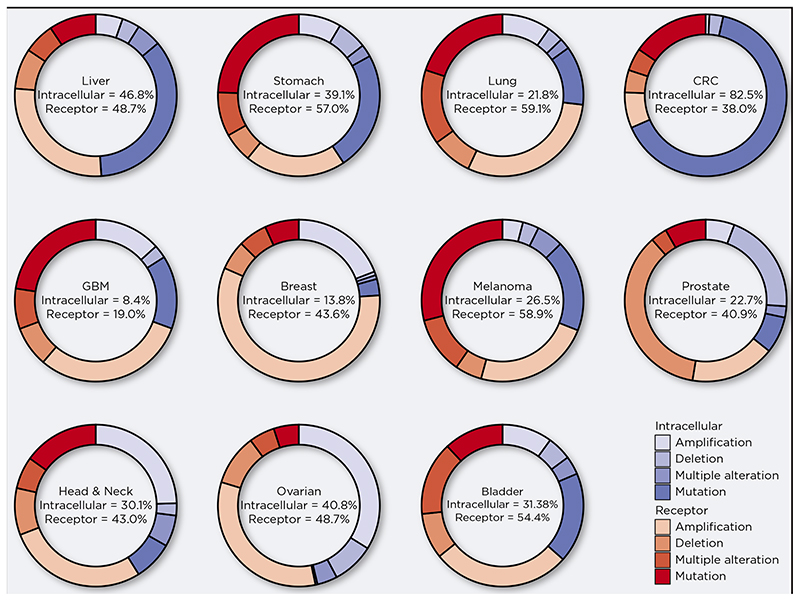
Figure 2. Alteration of Wnt pathway components in epithelial cancers.
Analysis of selected datasets from The Cancer Genome Atlas (TCGA) for mutations, amplifications and deletions of either intracellular (APC, β-catenin, Axin2 and Dvl) or receptor (e.g. WNTs, FZDs, RNF43, sFRP) pathway components. Relative frequency of genetic alterations shown as percentages. NSCLC = Non small cell lung cancer; CRC = Colorectal cancer; GBM = Glioblastoma. This figure was generated using data available at www.cbioportal.org.
Another important function of variable levels of Wnt signaling in tumours is to regulate cancer stem cell (CSC) activity. Stromal myofibroblast-derived hepatocyte growth factor (HGF) can increase Wnt signaling in APC mutant colon cancer cells and endow them with CSC features of self-renewal [25]. CSCs also display invasive and metastatic features, with CD44+ CSCs frequently observed at the invasive front of gastric tumours [26]. There are considerable similarities between migrating cells in the developing embryo and metastasizing tumour cells. During gastrulation populations of cells undergo an epithelial to mesenchymal transition (EMT) and become motile to set up the complex tissue patterning of the developing embryo [27]. Nuclear β-catenin is observed in migrating cells during early gastrulation, and β-catenin deficient mouse embryos fail to develop mesoderm [28]. Areas of increased Wnt signaling activity are often observed at the leading edge of tumours as can be seen in Figure 1 which shows increased expression of β-catenin at the invasive front of a human colon tumour. These data suggest an environmental regulation of Wnt signaling in APC mutant colon cells is high-jacking a developmental process to promote tumour invasion/metastasis.
Non-canonical PCP Wnt signaling is also critical for correct polarization of tissues and cell migration during gastrulation and is implicated in metastasis of several cancer types [29]. During neural crest migration, the small GTPase, and PCP signaling component, RhoA, acts to promote the retraction of cell protrusions [30]. In colon tumours, RhoA levels are reduced in metastatic sites compared to the primary tumour, and inactivation of RhoA increases canonical Wnt signaling to promote metastasis [31]. Furthermore, RhoA mutations are recurrent in genomically stable gastric tumours, suggesting they contribute to the invasive phenotype of diffuse gastric tumours [32].
The processes of EMT and MET are also regulated by Wnt signaling, with several Wnts and Fzds now implicated in both EMT and MET including FZD7 [24, 33, 34]. Wnt signaling can promote invasion of cancer cells by regulating genes involved in cell adhesion, including Eph/Ephrins and E-cadherin [35]. Several matrix metalloproteinase (MMP) genes are also regulated by Wnt signaling in numerous cancers [36, 37], including gastric cancer [38], which degrade proteins in the surrounding stroma including collagen, gelatin, fibronectin and laminin, to provide a permissive microenvironment to allow invading cells to migrate. These data illustrate that the heterogenous activation of Wnt signaling in tumours is regulated by deregulation of the pathway, at the level of the ligand/receptor, which is supported by RNASeq data (Fig. 2), and that different levels of Wnt activity regulate different cellular functions including stemness, EMT/MET and invasion during cancer progression.
It is worth mentioning that many right-sided CRCs, which are associated with poor prognosis, are not mutant for APC and instead have mutations to RNF43 (cbioportal website) or methylation of Dkk1 [39] or sFRP [22], which facilitate elevated Wnt signaling (Fig. 2). In the absence of an APC mutation, CRC cancers can acquire gain-of-function CTNNB1 mutations that render β-catenin resistant to proteasomal degradation [40]. However, the selection bias towards APC in preference to CTNNB1 mutations has been shown to reflect the abundance of colonic E-cadherin that can sequester mutant β-catenin, acting as a sink to prevent transformation [41]. Hepatocellular carcinoma (HCC) is the most common form of liver cancer, around 50% of which harbor similar mutations in the CTNNB1 gene [42]. Similar to CRC and GC, HCC also exhibit deregulation of Wnt components at the receptor/ligand level, including overexpression of Wnt ligands and FZD receptors, and down-regulation of sFRPs [4]. Here too CTNNB1 mutation alone does not cause liver cancer in mouse models but instead provides a platform in which tumour progression is accelerated in association with other factors including Kras [43] and Lkb1 [44].
Context specificity of Apc mutant cells to modulation of Wnt signaling
Despite compelling evidence outlined above that demonstrates Wnt signaling can be further regulated at the level of the receptor in Apc mutant cells, recent work indicates this phenomenon is context dependent. Intestinal stem cells (ISCs) are continuously engaged in a stochastic competitive process termed neutral-drift [45]. This process is neutral as all ISCs have equal probability of replacing their neighbour or being replaced. However, oncogenic mutations, in genes such as Apc, bias this competition in favour of Apc mutant cells such that they displace all wild-type ISCs cells from the niche and the crypt will become clonal with Apc-mutant progeny [46]. Indeed, lowering the overall level of Wnt ligands in an Apc mutant background further exacerbates the decreased fitness of non-mutant ISCs, allowing for the rapid clonality of Apc mutant ISCs, which accelerates intestinal tumour formation [47]. Similar size changes to the ISC pool and rate of clonality were observed following selective inhibition of a single binding site for R-Spondin, which is required to potentiate Wnt signaling [48], indicating that fluctuations in Wnt signaling can impact the dynamics of ISCs and in turn influence tumour initiation.
Some important insight has been gained recently into how APC mutant cells activate Wnt signaling. Wnt pathway activation was observed in APC mutant CRC cells which were unable to secrete Wnt ligands due to a mutation in PORCN (or treatment with PORCN inhibitor), and this activation was partially inhibited (~50%) in LRP6 mutant cells [49]. This response was not observed in CTNNB1 mutant CRC cells suggesting a specific role for mutant APC. Further analysis revealed that APC knockdown, but not APC2 knockdown, triggered the assembly of the Wnt signalasome and clathrin mediated endocytosis to activate Wnt signaling in CRC cells. However, as we observed that APC mutant GC cells can respond to PORCN inhibitors [7], further research will be needed to determine if similar mechanisms exist in other cancer types.
The recent molecular subtyping of multiple cancers has greatly enhanced our understanding of cancer genetics and disease stratification. Using a network-based approach, four consensus molecular subtypes (CMS) of CRC were recently identified, of which CMS2 and CMS3 display the highest levels of Wnt signaling and superior patient outcome. Interestingly, approximately 66% of CMS4 CRCs have mutations to APC and also display high stromal infiltrate, TGFβ signaling and have the poorest survival [19]. However, poor clinical prognosis in CMS4 CRC subtypes is associated with receptor-mediated Wnt activation, whereas oncogenic Wnt activation (mutant APC) is associated with improved patient outcome and CMS2 [50]. Collectively, this indicates disease/tumour progression needs to be considered when selecting the type (agonist vs antagonist) of WNT therapeutic in CRC, as increasing Wnt activity could be beneficial to patients with CMS4 tumours.
Summary
There is now substantial evidence that targeting the Wnt pathway at the level of the receptor/ligand is an attractive therapeutic strategy for inhibiting tumour growth, and potentially metastasis. Indeed, several drugs targeting the Wnt pathway at the level of the ligand/receptor are currently in clinical trials, some of which focus on metastatic disease. The monoclonal antibody Vantictumab (OMP-18R5 Oncomed/Bayer) binds to Fzd1, 2, 5, 7 and 8, is in Phase Ib clinical trials for pancreatic cancer, metastatic breast cancer and non-small cell lung cancer [51]. Porcupine inhibitors, which prevent the secretion of all Wnt ligands, are also in phase I clinical trials for advanced solid tumours (NCT01351103). We demonstrated Vantictumab was able to inhibit the growth of the growth of gastric tumours, with and without Apc mutations [7], suggesting this approach could also benefit gastric cancer patients. Further research in pre-clinical models is required to determine the therapeutic potential of targeting the Wnt pathway at the level of the ligand/receptor for metastatic disease, but the growing molecular evidence suggests this is an attractive strategy, and current clinical trials will help resolve this in the future, along with continued drug discovery programmes to target Wnt signaling, and combination therapies to optimize treatment.
Acknowledgments
Funding is gratefully acknowledged from the following: National Health and Medical Research Council of Australia (NHMRC, 566679, and APP1099302 awarded to E. Vincan, T.J. Phesse), Melbourne Health project grants (605030 and PG-002 awarded to E. Vincan and T.J. Phesse), Medical Research Council (MR/R026424/1 to T.J. Phesse), and Early career researcher grant (GIA-033 awarded to D.J. Flanagan), Cancer Council of Victoria project grants (CCV, APP1020716 awarded to E. Vincan and T.J. Phesse), CCV Fellowship awarded to D.J. Flanagan and Cardiff University/CMU Research Fellowship awarded to T.J. Phesse.
Footnotes
The authors declare no potential conflicts of interest.
References
- 1.Nusse R, Clevers H. Wnt/beta-Catenin Signaling, Disease, and Emerging Therapeutic Modalities. Cell. 2017;169(6):985–999. doi: 10.1016/j.cell.2017.05.016. [DOI] [PubMed] [Google Scholar]
- 2.Flanagan DJ, et al. Frizzled7 Functions as a Wnt Receptor in Intestinal Epithelial Lgr5(+) Stem Cells. Stem Cell Reports. 2015;4(5):759–767. doi: 10.1016/j.stemcr.2015.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Flanagan DJ, et al. Loss of the Wnt receptor frizzled 7 in the mouse gastric epithelium is deleterious and triggers rapid repopulation in vivo. Dis Model Mech. 2017;10(8):971–980. doi: 10.1242/dmm.029876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Phesse T, Flanagan D, Vincan E. Frizzled7: A Promising Achilles’ Heel for Targeting the Wnt Receptor Complex to Treat Cancer. Cancers (Basel) 2016;8(5) doi: 10.3390/cancers8050050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Clements WM, et al. beta-Catenin mutation is a frequent cause of Wnt pathway activation in gastric cancer. Cancer Res. 2002;62(12):3503–6. [PubMed] [Google Scholar]
- 6.Flanagan DJ, Vincan E, Phesse TJ. Winding back Wnt signalling: potential therapeutic targets for treating gastric cancers. Br J Pharmacol. 2017;174(24):4666–4683. doi: 10.1111/bph.13890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Flanagan DJ, et al. Frizzled-7 Is Required for Wnt Signaling in Gastric Tumors with and Without Apc Mutations. Cancer Res. 2019;79(5):970–981. doi: 10.1158/0008-5472.CAN-18-2095. [DOI] [PubMed] [Google Scholar]
- 8.Hao HX, et al. ZNRF3 promotes Wnt receptor turnover in an R-spondin-sensitive manner. Nature. 2012;485(7397):195–200. doi: 10.1038/nature11019. [DOI] [PubMed] [Google Scholar]
- 9.Wiese KE, Nusse R, van Amerongen R. Wnt signalling: conquering complexity. Development. 2018;145(12) doi: 10.1242/dev.165902. [DOI] [PubMed] [Google Scholar]
- 10.Brabletz T, et al. Nuclear overexpression of the oncoprotein beta-catenin in colorectal cancer is localized predominantly at the invasion front. Pathol Res Pract. 1998;194(10):701–4. doi: 10.1016/s0344-0338(98)80129-5. [DOI] [PubMed] [Google Scholar]
- 11.Oguma K, et al. Activated macrophages promote Wnt signalling through tumour necrosis factor-alpha in gastric tumour cells. EMBO J. 2008;27(12):1671–81. doi: 10.1038/emboj.2008.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Geng Y, et al. MicroRNA-27b suppresses Helicobacter pylori-induced gastric tumorigenesis through negatively regulating Frizzled7. Oncol Rep. 2016;35(4):2441–50. doi: 10.3892/or.2016.4572. [DOI] [PubMed] [Google Scholar]
- 13.Leedham SJ, et al. A basal gradient of Wnt and stem-cell number influences regional tumour distribution in human and mouse intestinal tracts. Gut. 2013;62(1):83–93. doi: 10.1136/gutjnl-2011-301601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Voloshanenko O, et al. Wnt secretion is required to maintain high levels of Wnt activity in colon cancer cells. Nat Commun. 2013;4:2610. doi: 10.1038/ncomms3610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Silva AL, et al. Boosting Wnt activity during colorectal cancer progression through selective hypermethylation of Wnt signaling antagonists. BMC Cancer. 2014;14:891. doi: 10.1186/1471-2407-14-891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Albuquerque C, et al. The ‘just-right’ signaling model: APC somatic mutations are selected based on a specific level of activation of the beta-catenin signaling cascade. Hum Mol Genet. 2002;11(13):1549–60. doi: 10.1093/hmg/11.13.1549. [DOI] [PubMed] [Google Scholar]
- 17.Pollard P, et al. The Apc 1322T mouse develops severe polyposis associated with submaximal nuclear beta-catenin expression. Gastroenterology. 2009;136(7):2204–2213.:e1-13. doi: 10.1053/j.gastro.2009.02.058. [DOI] [PubMed] [Google Scholar]
- 18.Meniel V, et al. Cited1 Deficiency Suppresses Intestinal Tumorigenesis. PLoS genetics. 2013;9(8) doi: 10.1371/journal.pgen.1003638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Guinney J, et al. The consensus molecular subtypes of colorectal cancer. Nat Med. 2015;21(11):1350–6. doi: 10.1038/nm.3967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Faux MC, et al. Restoration of full-length adenomatous polyposis coli (APC) protein in a colon cancer cell line enhances cell adhesion. Journal of cell science. 2004;117(Pt 3):427–39. doi: 10.1242/jcs.00862. [DOI] [PubMed] [Google Scholar]
- 21.Dow LE, et al. Apc Restoration Promotes Cellular Differentiation and Reestablishes Crypt Homeostasis in Colorectal Cancer. Cell. 2015;161(7):1539–52. doi: 10.1016/j.cell.2015.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Suzuki H, et al. Epigenetic inactivation of SFRP genes allows constitutive WNT signaling in colorectal cancer. Nat Genet. 2004;36(4):417–22. doi: 10.1038/ng1330. [DOI] [PubMed] [Google Scholar]
- 23.Vincan E, et al. Frizzled-7 receptor ectodomain expression in a colon cancer cell line induces morphological change and attenuates tumor growth. Differentiation. 2005;73(4):142–53. doi: 10.1111/j.1432-0436.2005.00015.x. [DOI] [PubMed] [Google Scholar]
- 24.Vincan E, et al. Frizzled-7 dictates three-dimensional organization of colorectal cancer cell carcinoids. Oncogene. 2007;26(16):2340–52. doi: 10.1038/sj.onc.1210026. [DOI] [PubMed] [Google Scholar]
- 25.Vermeulen L, et al. Wnt activity defines colon cancer stem cells and is regulated by the microenvironment. Nat Cell Biol. 2010;12(5):468–76. doi: 10.1038/ncb2048. [DOI] [PubMed] [Google Scholar]
- 26.Kodama H, et al. Prognostic impact of CD44-positive cancer stem-like cells at the invasive front of gastric cancer. Br J Cancer. 2017;116(2):186–194. doi: 10.1038/bjc.2016.401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Clevers H, Loh KM, Nusse R. Stem cell signaling. An integral program for tissue renewal and regeneration: Wnt signaling and stem cell control. Science. 2014;346(6205):1248012. doi: 10.1126/science.1248012. [DOI] [PubMed] [Google Scholar]
- 28.Haegel H, et al. Lack of beta-catenin affects mouse development at gastrulation. Development. 1995;121(11):3529–37. doi: 10.1242/dev.121.11.3529. [DOI] [PubMed] [Google Scholar]
- 29.Simons M, Mlodzik M. Planar cell polarity signaling: from fly development to human disease. Annu Rev Genet. 2008;42:517–40. doi: 10.1146/annurev.genet.42.110807.091432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mayor R, Theveneau E. The role of the non-canonical Wnt-planar cell polarity pathway in neural crest migration. Biochem J. 2014;457(1):19–26. doi: 10.1042/BJ20131182. [DOI] [PubMed] [Google Scholar]
- 31.Rodrigues P, et al. RHOA inactivation enhances Wnt signalling and promotes colorectal cancer. Nat Commun. 2014;5:5458. doi: 10.1038/ncomms6458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.TCGA. Comprehensive molecular characterization of gastric adenocarcinoma. Nature. 2014;513(7517):202–9. doi: 10.1038/nature13480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cao TT, et al. FZD7 is a novel prognostic marker and promotes tumor metastasis via WNT and EMT signaling pathways in esophageal squamous cell carcinoma. Oncotarget. 2017;8(39):65957–65968. doi: 10.18632/oncotarget.19586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schwab RHM, et al. Wnt is necessary for mesenchymal to epithelial transition in colorectal cancer cells. Dev Dyn. 2018;247(3):521–530. doi: 10.1002/dvdy.24527. [DOI] [PubMed] [Google Scholar]
- 35.Heuberger J, Birchmeier W. Interplay of cadherin-mediated cell adhesion and canonical Wnt signaling. Cold Spring Harb Perspect Biol. 2010;2(2):a002915. doi: 10.1101/cshperspect.a002915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liang J, et al. CDK8 Selectively Promotes the Growth of Colon Cancer Metastases in the Liver by Regulating Gene Expression of TIMP3 and Matrix Metalloproteinases. Cancer Res. 2018;78(23):6594–6606. doi: 10.1158/0008-5472.CAN-18-1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Deng B, et al. Down-regulation of Frizzled-7 expression inhibits migration, invasion, and epithelial-mesenchymal transition of cervical cancer cell lines. Med Oncol. 2015;32(4):102. doi: 10.1007/s12032-015-0552-8. [DOI] [PubMed] [Google Scholar]
- 38.Lowy AM, et al. beta-Catenin/Wnt signaling regulates expression of the membrane type 3 matrix metalloproteinase in gastric cancer. Cancer Res. 2006;66(9):4734–41. doi: 10.1158/0008-5472.CAN-05-4268. [DOI] [PubMed] [Google Scholar]
- 39.Bond CE, et al. Oncogenic BRAF mutation induces DNA methylation changes in a murine model for human serrated colorectal neoplasia. Epigenetics. 2018;13(1):40–48. doi: 10.1080/15592294.2017.1411446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Morin PJ, et al. Activation of beta-catenin-Tcf signaling in colon cancer by mutations in beta-catenin or APC. Science. 1997;275(5307):1787–90. doi: 10.1126/science.275.5307.1787. [DOI] [PubMed] [Google Scholar]
- 41.Huels DJ, et al. E-cadherin can limit the transforming properties of activating beta-catenin mutations. EMBO J. 2015;34(18):2321–33. doi: 10.15252/embj.201591739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vilchez V, et al. Targeting Wnt/beta-catenin pathway in hepatocellular carcinoma treatment. World J Gastroenterol. 2016;22(2):823–32. doi: 10.3748/wjg.v22.i2.823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Harada N, et al. Hepatocarcinogenesis in mice with beta-catenin and Ha-ras gene mutations. Cancer Res. 2004;64(1):48–54. doi: 10.1158/0008-5472.can-03-2123. [DOI] [PubMed] [Google Scholar]
- 44.Miyoshi H, et al. Hepatocellular carcinoma development induced by conditional beta-catenin activation in Lkb1+/- mice. Cancer Sci. 2009;100(11):2046–53. doi: 10.1111/j.1349-7006.2009.01284.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Snippert HJ, et al. Intestinal crypt homeostasis results from neutral competition between symmetrically dividing Lgr5 stem cells. Cell. 2010;143(1):134–44. doi: 10.1016/j.cell.2010.09.016. [DOI] [PubMed] [Google Scholar]
- 46.Vermeulen L, et al. Defining stem cell dynamics in models of intestinal tumor initiation. Science. 2013;342(6161):995–8. doi: 10.1126/science.1243148. [DOI] [PubMed] [Google Scholar]
- 47.Huels DJ, et al. Wnt ligands influence tumour initiation by controlling the number of intestinal stem cells. Nat Commun. 2018;9(1):1132. doi: 10.1038/s41467-018-03426-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yan KS, et al. Non-equivalence of Wnt and R-spondin ligands during Lgr5(+) intestinal stem-cell self-renewal. Nature. 2017;545(7653):238–242. doi: 10.1038/nature22313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Saito-Diaz K, et al. APC Inhibits Ligand-Independent Wnt Signaling by the Clathrin Endocytic Pathway. Dev Cell. 2018;44(5):566–581.:e8. doi: 10.1016/j.devcel.2018.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Michels BE, et al. Human colon organoids reveal distinct physiologic and oncogenic Wnt responses. J Exp Med. 2019;216(3):704–720. doi: 10.1084/jem.20180823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Smith DC, et al. First-in-human evaluation of the human monoclonal antibody vantictumab (OMP-18R5; anti-Frizzled) targeting the WNT pathway in a phase I study for patients with advanced solid tumors. Journal of Clinical Oncology. 2017;31(15_suppl):2540–2540. [Google Scholar]