Abstract
Background
Bone loss is accelerated after the menopause in women, as is the risk of hip fracture, but little is known about the importance of age at menopause, time since menopause and total reproductive years for risk of hip fracture.
Methods
Between 2004 and 2008, the China Kadoorie Biobank recruited 125,336 postmenopausal women who had a natural menopause and recorded 1,327 incident cases of hip fracture during the first 10 years of follow-up. Multivariable Cox regression was used to estimate hazard ratios and 95% confidence intervals (CI) for incident hip fracture for age at menopause, time since menopause and total reproductive years.
Results
The mean (SD) age at menopause was 48.8 (4.0) years. Compared with women who reached menopause before age 53 years, women with a later age at menopause had a 22% (95%CI: 11-35%) lower risk of hip fracture. Compared to women who were <5 years since menopause, those who were 5-9, 10-14, 15-19, and ≥20 years since menopause had hazard ratios of hip fracture of 1.43 (95%CI: 1.01-2.04), 2.10 (95% CI: 1.71-2.57), 2.50 (95%CI: 2.21-2.83) and 2.33 (95%CI: 1.97-2.75), respectively. Women with a longer (≥36 years) versus shorter (<30 years) duration of total reproductive years had a 19% (95%CI: 9-28) lower risk of hip fracture.
Conclusions
Women with younger age at menopause, longer interval since menopause or shorter duration of total reproductive years had the highest risks of hip fracture.
Introduction
Hip fracture is one of the most serious complications of falls in older people worldwide, and is associated with a high mortality, disability and economic burden.1–4 Despite reports of an increased incidence of hip fracture over the last two decades in China, 5 little is known about the determinants of hip fracture in China.6 Previous studies in China have reported a 23% higher mortality rate at one year following a hip fracture,6 and reliable data on modifiable causes of hip fracture are needed to guide the design of programs for prevention of hip fracture in China.7
Older women are more likely to suffer from hip fracture compared with older men,8 9 due to higher risks of osteoporosis and falls.10 11 Peak bone mass and rates of bone loss are highly correlated with age as are the risks of osteoporosis and hip fracture12. It is well established that estrogen levels are positively associated with bone mineral density.13–15 Women typically achieve peak bone mass in their 30s, and subsequently suffer from progressive bone loss with increasing age.16–19 Indeed, both blood levels of estrogen and bone mineral density decline rapidly after the onset of menopause.20–24
Given that current age and age at menopause are both associated with risks of hip fracture, it is likely that lifetime duration of exposure to endogenous estrogens may also be correlated with risks of hip fracture in older women.25–27 A longer duration of exposure to endogenous estrogen is likely to be associated with lower risks of hip fracture. Although extreme age at menarche or menopause are associated with risks of hip fracture, little is known about the effects of total reproductive years on hip fracture.28
The aims of the present study were to examine the associations of age at menopause, time since menopause, and duration of total reproductive years with risks of incident hip fracture in post-menopausal women recruited into the China Kadoorie Biobank (CKB) study.
Methods
Baseline Survey
Details of the design and study procedures used in CKB have been previously published.29 Briefly, 512,891 adults (210,222 men, 302,669 women) aged 30-79 years were recruited from 10 regions (5 urban, 5 rural) in China from 2004 to 2008. Data on demographic, socioeconomic, dietary and other lifestyle factors (e.g. smoking, tea, alcohol drinking, and physical activity), indoor air pollution, personal and family medical history, sleeping and psychological wellbeing and women’s reproductive factors (e.g. menstrual history, history of childbearing, breastfeeding, hysterectomy, ovariectomy, or use of oral contraceptives) were collected by trained health workers using a laptop-based questionnaire. Physical measurements were recorded and a blood sample was collected from each participant for a long-term storage. Ethics approval was obtained from the relevant international, national and local authorities, and all participants provided written informed consent.
Follow-up for morbidity and mortality
Study participants were followed-up for cause-specific morbidity and mortality by linkage with death and disease registers and for hospitalized events by linkage with the national health insurance system. Active follow-up was performed on an annual basis to minimize any additional loss to follow up. All deaths and diseases were coded using the International Classification of Diseases 10th edition (ICD-10) and were blinded to baseline exposures. The primary endpoints were incident hip fractures, defined by codes S72.0-S72.9 in ICD-10.
Statistical methods
A total of 145,172 postmenopausal women aged 50 years or greater were recruited at baseline after excluding individuals who had missing or inconsistent values for reproductive factors. Participants who had a history of cancer, fracture, hysterectomy or oophorectomy were excluded (n=19,836), leaving 125,336 women for the present analyses.
The baseline characteristics are presented as means (SD) for continuous variables and as percentages for categorical variables. Cox proportional hazards models were used to estimate hazard ratios (HRs) and 95% CI for the associations between age at menopause, time since menopause, total reproductive years and risk of fatal or nonfatal hip fracture. Time since menopause was defined as the time (in years) between age at menopause and age at risk of hip fracture event. Analyses were conducted using time since entry into the study as the time scale and stratified by (time-varying) age-at-risk (except for time since menopause) and region to allow each group and region to have different baseline hazard rates for the disease. Analyses were stratified by area of residence and age at risk (Model 1), and were additionally adjusted for the highest level of attained education (≤primary, ≥secondary), household income (<5000, 5000-19999, and ≥20000 yuan) and marital status (Model 2). Further adjustments included disease history of diabetes, chronic obstructive pulmonary disease (COPD), stroke, coronary health disease (CHD), body mass index (BMI), self-rated health, smoking status (current smokers, ex-smokers, never smokers), alcohol consumption (weekly drinker, ex or occasional drinker, never regular drinkers), dairy intake (weekly, less than once a week, never), fruit intake (weekly, less than once a week, never), soybean products intake (weekly, less than once a week, never), calcium or zinc or iron intake (intake, never intake), physical activity (MET-hr/day) and reproductive factors including age at menarche (except for total reproductive years analysis), parity, number of abortions, age at first birth, breast feeding duration per child, and use of oral contraceptives (OC) (Model 3). For analyses of time since menopause, age was used as the underlying time scale with time since menopause (in 5-year groups) as a time-varying variable. Associations were compared between subgroups of women by region, age, BMI, parity and duration of breast feeding per child. Additional sensitivity analyses excluded: (i) those who had parity >10, (ii) who had age at menopause <43 years; and (iii) those who never used OC. Group-specific variances were used for variables with 3 or more categories to facilitate comparisons between any two categories.30
Results
Baseline characteristics
Among 125,336 women included, the mean (SD) age at baseline was 60.4 (6.7) years and 54.4% were resident in rural areas. Few women were current smokers (6.0%), regular alcohol drinkers (2.3%), regular fruit consumers (6.9%), or ever used OC (9.3%). Around 80% of women were married, 98.9% of women had given birth and 98.1% of those had reported breastfeeding their children. Compared with women who had a later age at menopause or longer duration of reproductive years, women with an earlier age at menopause or fewer reproductive years were, on average, more likely to live in rural areas, and be slightly leaner, less educated, have lower household income, smoke more, consume more fruit and dairy products but consume less soy products (Table 1).
Table 1. Baseline characteristics of postmenopausal women by age at menopause.
| Characteristics | Total | Age at menopause | ||||
|---|---|---|---|---|---|---|
| Mean (SD) or median (ranges) or percentage (%) | <43 | 43-47 | 48-50 | 51-52 | ≥53 | |
| N (%) | 125,336 | 9,658 (7.7) | 27,999 (22.3) | 47,887 (38.2) | 21,552 (17.2) | 18,240 (14.6) |
| Mean (SD) age at base line, y | 60.4 (6.7) | 62.6 (7.1) | 60.8(7.0) | 59.8 (6.9) | 59.5 (6.2) | 61.5 (5.6) |
| Lifestyle factors and physical measurement | ||||||
| Rural, % | 54.4 | 65.4 | 57.5 | 54.1 | 52.0 | 47.6 |
| Education primary or below, % | 74.8 | 84.8 | 77.3 | 73.8 | 71.6 | 72.4 |
| Married, % | 81.9 | 75.4 | 80.5 | 82.8 | 84.5 | 81.7 |
| Low household income, % | 15.1 | 23.4 | 16.7 | 14.1 | 12.7 | 14.1 |
| Current regular smoking, % | 6.0 | 9.0 | 6.9 | 5.7 | 5.1 | 5.2 |
| Weekly regular drinker, % | 2.3 | 2.9 | 2.4 | 2.3 | 2.4 | 2.1 |
| Physical activity, MET h/day | 12.9(8.4, 20.8) | 12.2 (8.4, 20.6) | 12.8(8.4, 21.0) | 13.0(8.4, 21.0) | 13.5(8.9, 21.4) | 12.2(8.4, 19.3) |
| BMI (SD), kg/m2 | 23.9 (3.7) | 23.3 (3.7) | 23.7 (3.7) | 23.8 (3.6) | 24.2 (3.6) | 24.5 (3.7) |
| Dietary factors, % | ||||||
| Weekly dairy consumption | 67.8 | 75.2 | 69.2 | 67.3 | 67.1 | 63.7 |
| Weekly fruit consumption | 6.9 | 9.8 | 7.4 | 6.7 | 5.8 | 6.5 |
| Weekly soy consumption | 57.7 | 49.3 | 56.2 | 57.6 | 60.7 | 61.3 |
| Regular calcium consumption | 10.5 | 8.9 | 10.2 | 10.4 | 11.0 | 11.7 |
| Medical History | ||||||
| Diabetes | 9.6 | 8.9 | 9.2 | 9.1 | 10.0 | 11.9 |
| COPD | 8.8 | 12.2 | 9.6 | 8.4 | 7.8 | 8.1 |
| CHD | 5.6 | 5.3 | 5.7 | 5.1 | 5.6 | 6.8 |
| Stroke | 2.3 | 2.3 | 2.6 | 2.1 | 2.2 | 2.7 |
| Poor self-rated health | 62.1 | 65.3 | 62.1 | 62.8 | 59.6 | 61.2 |
| Reproductive factors, | ||||||
| Age at Menarche, y | 16.0 (2.0) | 16.0 (2.1) | 15.9 (1.9) | 16.0 (2.0) | 16.0 (1.9) | 16.2 (2.0) |
| Nulliparous, % | 1.1 | 2.1 | 1.2 | 1.1 | 0.8 | 0.8 |
| Oral contraceptive pill used, % | 9.3 | 5.9 | 8.8 | 9.4 | 10.7 | 10.2 |
| Number of live births | 2.9 (1.5) | 3.4 (1.6) | 3.0 (1.5) | 2.9 (1.5) | 2.7 (1.4) | 3.0 (1.4) |
| Parity≥3 children, % | 55.4 | 67.6 | 58.2 | 53.2 | 49.0 | 58.0 |
| Age at first birth, ya | 22.9 (3.5) | 22.2 (3.3) | 22.7 (3.4) | 23.1 (3.5) | 23.1 (3.5) | 22.9 (3.5) |
| Never breastfed child, %a | 1.9 | 1.9 | 2.0 | 2.0 | 1.9 | 1.6 |
| Breastfeeding per child, monthsa | 15.7 (7.8) | 16.6 (8.2) | 16.1 (8.1) | 15.5 (7.7) | 15.5 (7.8) | 15.6 (7.6) |
Values are percentages for categorical variables, and means (SD) or median (25th and 75th percentiles) for continuous variables. MET:, metabolic equivalent of task.
Among parous women only
All participants had reached menopause prior to recruitment at baseline in the present report.
Age at menopause
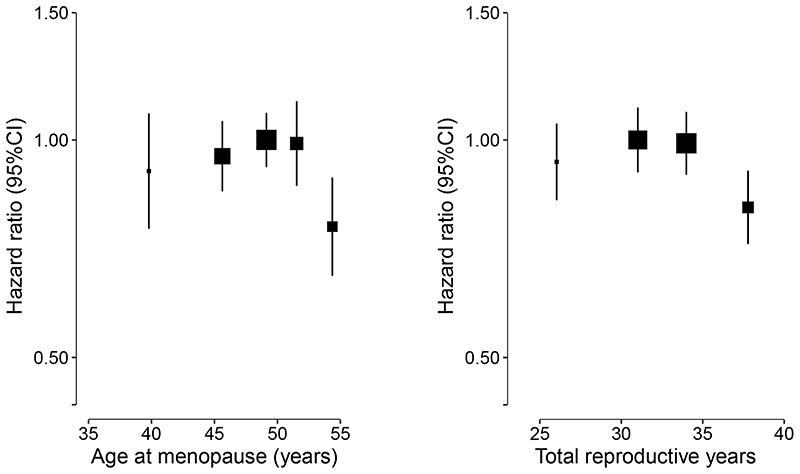
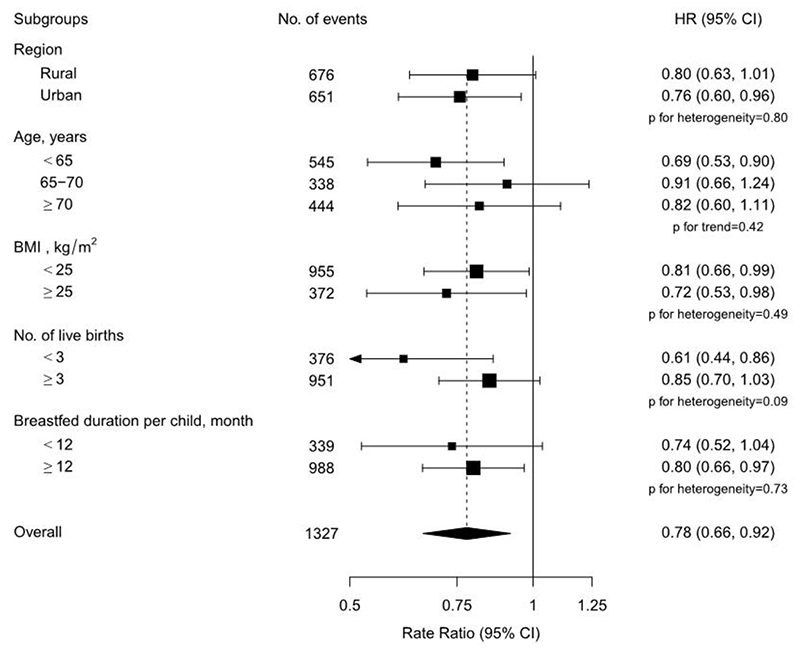
Overall, among postmenopausal women, there were no statistical differences in the association between age at menopause and risk of hip fracture among women who reached menopause before the age of 53 years, but a lower risk of hip fracture was observed among women who reached menopause at age of 53 years or greater (Figure 1, p for heterogeneity= 0.04). Compared to women who reached menopause before age 53 years, women who reached menopause at age of 53 years or greater had a 22% lower risk of hip fracture. Further, women who had onset of menopause at ≥53 years had a 24% (95%CI: 11-35) lower risk of hip fracture compared with those who had onset of menopause at age 48-50 years (Table 2).The associations of age at menopause with hip fracture were broadly consistent across different population subgroups including region, age, BMI, parity and mean duration of breastfeeding (Figure 3), and in sensitivity analyses among a subset of women who never used OCs or who had a later age at menopause (⩾43 years).
Figure 1. Adjusted hazard ratios (HRs) and 95% CIs for incident hip fracture by age at menopause and total reproductive years.
Models were stratified by age and study area, and adjusted for level of attained education, household income, marital status, diabetes, COPD, stroke, CHD, self-rated health, smoking status, alcohol use, dairy intake, fruit intake, soy intake, calcium intake, physical activity, body mass index (BMI) and reproductive factors including age at menarche, parity, abortion, age at first birth, breastfeeding duration per child, and oral contraceptive use. Squares represent the HR with area inversely proportional to the variance of the log HR. Vertical lines indicate the corresponding 95% CI.
Table 2. HR (95% CI) for incident hip fracture associated with age at menopause.
| Age at menopause≥53 vs not |
Age at menopause | |||||
|---|---|---|---|---|---|---|
| <43 | 43-47 | 48-50 | 51-52 | ≥53 | ||
| No. of events | 118 | 311 | 521 | 217 | 160 | |
| Model 1 | 0.75 (0.64,0.89) | 0.94 (0.78,1.12) |
0.95 (0.85, 1.06) |
1.00 (0.92,1.09) |
0.96 (0.84,1.10) |
0.73 (0.62,0.85) |
| Model 2 | 0.75 (0.64,0.89) | 0.93 (0.78,1.12) |
0.95 (0.85, 1.06) |
1.00 (0.92,1.09) |
0.96 (0.84,1.10) |
0.73 (0.62,0.85) |
| Model 3 | 0.78 (0.66,0.92) | 0.91 (0.75,1.09) |
0.95 (0.85, 1.06) |
1.00 (0.92,1.09) |
0.99 (0.86,1.13) |
0.76 (0.65,0.89) |
Model 1: Stratified by age at risk and study area.
Model 2: In addition to Model 1, adjusted to education, household income and marital status.
Model 3: In addition to Model 2, adjusted to diabetes, COPD, stroke, CHD, self-rated health, smoking status, alcohol use, dairy intake, fruit intake, soy intake, calcium intake, physical activity, body mass index (BMI) and reproductive factors including age at menarche, parity, abortion, age at first birth, breastfeeding duration per child, and oral contraceptive use.
Figure 3. Adjusted hazard ratios (HR) and 95% CI for incident hip fracture comparing postmenopausal women who had an age at menopause ≥53 with who had an age at menopause <53, by baseline characteristics.
Models were stratified by age and study area, and adjusted for level of attained education, household income, marital status, diabetes, COPD, stroke, CHD, self-rated health, smoking status, alcohol use, dairy intake, fruit intake, soy intake, calcium intake, physical activity, body mass index (BMI) and reproductive factors including age at menarche, parity, abortion, age at first birth, breastfeeding duration per child, and oral contraceptive use. Each square represents the HR. Horizontal lines indicate the corresponding 95% CI. The diamond indicates the overall estimate and its 95% CI.
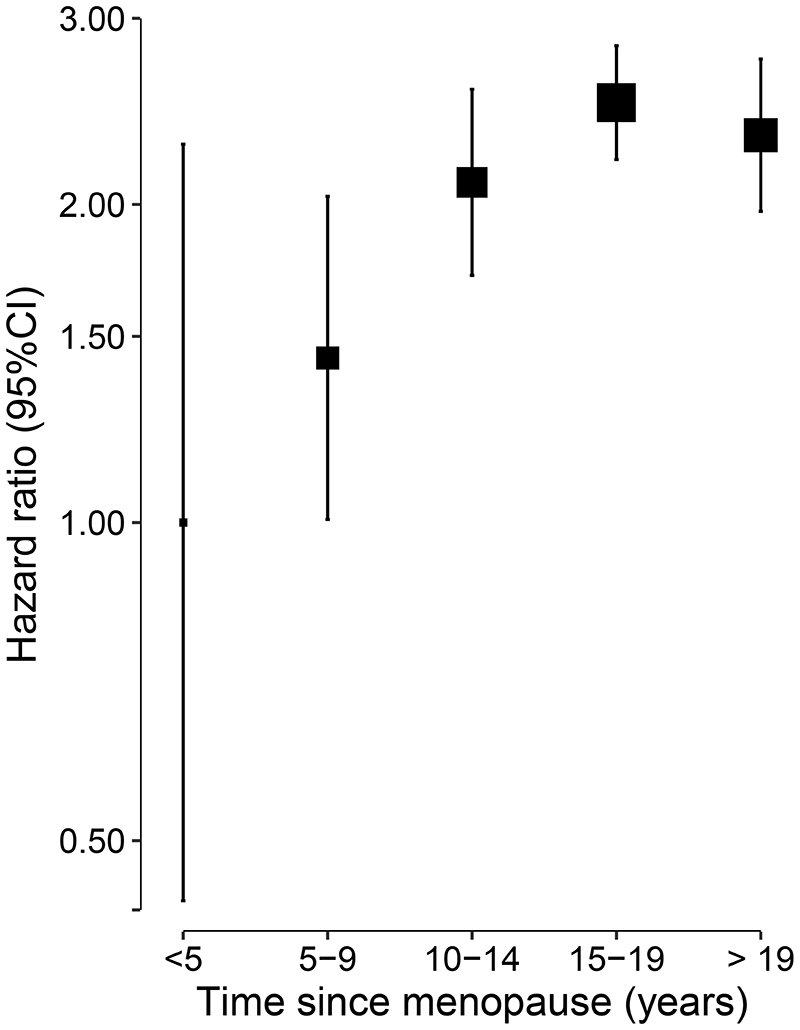
Time since menopause
Compared with women who were <5 years postmenopausal, higher risks of fatal or nonfatal hip fracture were observed among women who were 5-9, 10-14, 15-19, and ≥20 years post-menopause (Figure 2). Compared to women who were <5 years postmenopausal, women who were 5-9, 10-14, 15-19, and ≥20 years post-menopause had hazard ratios as 1.43 (95%CI: 1.01-2.04), 2.10 (95%CI: 1.71-2.57), 2.50 (95%CI: 2.21-2.83) and 2.33 (95%CI: 1.97-2.75), respectively (p trend=0.008). The risks of hip fracture increased with longer duration since menopause, but were attenuated by 20 years after menopause.
Figure 2. Adjusted hazard ratio (HR) and 95% CI for incident hip fracture by, time since menopause.
Models were stratified by age and study area, and adjusted for level of attained education, household income, marital status, diabetes, COPD, stroke, CHD, self-rated health, smoking status, alcohol use, dairy intake, fruit intake, soy intake, calcium intake, physical activity, body mass index (BMI) and reproductive factors including age at menarche, parity, abortion, age at first birth, breastfeeding duration per child, and oral contraceptive use. Squares represent the HR with area inversely proportional to the variance of the log HR. Vertical lines indicate the corresponding 95% CI.
Total reproductive years
The association between total reproductive years and risk of incident hip fracture were consistent with the associations between age at menopause and risk of incident hip fracture. Among those women who had a shorter duration of total reproductive years (<36 years), no statistically significant differences were identified between reproductive years and risk of hip fracture, but the risk of hip fracture was significantly lower in women who had the longest duration of reproductive years (⩾36 years) (Table 3, Figure 1, p for heterogeneity=0.03). Women who had the longest duration of reproductive years, compared to those who had total reproductive years between 30 to 32 years had a 19% (95%CI: 9-28) lower risk of hip fracture. Moreover, the association did not differ substantially when analyses were performed among subgroups of women defined by region, age, BMI, number of live births, mean breastfeeding duration, nor in subsequent sensitivity analyses. However, the on the risk of incident hip fractures was significantly lower among younger women and decreased with increasing age.
Table 3. HR (95% CI) for incident hip fracture associated with total reproductive years.
| Total reproductive years≥36 vs not |
Total reproductive years | ||||
|---|---|---|---|---|---|
| <30 | 30-32 | 33-35 | ≥36 | ||
| No. of events | 272 | 368 | 390 | 297 | |
| Model 1 | 0.80(0.70,0.91) | 0.95 (0.85,1.08) |
1.00 (0.90, 1.11) |
0.98 (0.88,1.08) |
0.78 (0.70,0.87) |
| Model 2 | 0.80(0.70,0.91) | 0.95 (0.85,1.08) |
1.00 (0.90, 1.11) |
0.98 (0.88,1.08) |
0.78 (0.69,0.87) |
| Model 3 | 0.83(0.72,0.94) | 0.93 (0.83,1.05) |
1.00 (0.90, 1.11) |
0.99 (0.90,1.09) |
0.81 (0.72,0.91) |
Model 1: Stratified by age at risk and study area.
Model 2: In addition to Model 1, adjusted to education, household income and marital status.
Model 3: In addition to Model 2, adjusted to diabetes, COPD, stroke, CHD, self-rated health, smoking status, alcohol use, dairy intake, fruit intake, soy intake, calcium intake, physical activity, body mass index (BMI) and reproductive factors including, parity, abortion, age at first birth, breastfeeding duration per child, and oral contraceptive use.
Discussion
In the present study of 125,336 postmenopausal Chinese women, a later age at menopause and a longer total duration of reproductive years were each associated with a lower risk of hip fracture. The risk of hip fracture increased steadily by time since menopause but attenuated at about 20 years after menopause. To our knowledge, this is the first large prospective study to investigate the associations of time since menopause and total reproductive years with risk of hip fracture in Chinese women.
The findings of the present study are consistent with those of previous studies, indicated that a later age at menopause was associated with a lower risk of hip fracture.31–33 Compared to women who reached menopause before age 53 years, women who reached menopause at age of 53 years or greater had a 22% lower risk of hip fracture. In addition, the significantly lower risk of hip fracture was observed in women who reached menopause at 53 years or older. No differences were observed in risk of hip fracture between the other groups. Additional adjustment for dietary and reproductive factors, and BMI substantially reduced the relative risks for hip fracture associated with estimates for menopause at age 48-50 years versus age 53 or older compared to models that were adjusted for age and region only. This suggests that these factors may confound the associations between age at menopause and risk of hip fracture.
Previous studies of bone mineral density (BMD), indicated that once women reach menopause, age at menopause has a very limited effect on the long-term bone mineral density.20 21 31 It has been suggested that women with an early age of menopause have a higher rate of bone loss compared to those having a later age at menopause. The differences in the rate of bone loss between women having early and later age at menopause decline after reaching the age of 65 years.20 21 In a prospective study 561,609 women living in the UK with a mean duration of follow-up of 6.2 years, women with age at menopause <45 years had a 22% higher risk of hip fracture compared to those who had menopause at 50 years.31 No differences in risk of hip fracture were observed between women had menopause at age 50 years and over and those aged 45-49 years. Similarly, a 17% (OR=1.17 95% CI: 1.03-1.33) higher risk of hip fracture was reported among women having an average 2.6 years younger age at menopause from a case-control study with 100 postmenopausal women conducted in Taiwan.32 In contrast, two case-control studies, one in Beijing, China (involving 118 cases and 226 controls), and one in Italy (involving 206 cases and 590 controls) reported no differences in the risk of hip fracture and age at menopause.33 34
A linear trend was observed in the association between increasing time since menopause and risk of hip fracture in the first 20 years after menopause, but the observed trend appears to attenuate thereafter. Likewise, BMD is positively correlated with current estrogen levels. Both BMD and estrogen levels are likely to reach their lowest levels at about 20 years after the menopause. However, previous studies had reported that BMD has a relatively higher initial speed of decrease in the first 5-10 years after menopause, but the rate of bone loss slows down thereafter.35 36 The present study demonstrated that women who were reached menopause greater than 15 years previously had the highest risk of hip fracture.
Similar associations between total reproductive years and risk of hip fracture were observed as those between age at menopause and hip fracture. A longer total duration of reproductive years was associated with a lower risk of hip fracture. A longer duration of reproductive years was associated with a higher BMD in a Chinese study, which is consistent with the findings of the present study37. Moreover, a prospective study of 63,000 Norwegian women also reported an inverse association between total reproductive years and risk of hip fractures in those aged 50-79 years38. In a subgroup analysis, however, a decreasing trend of association was observed between total reproductive years and increasing age group. This may reflect the cumulative lifetime exposure to endogenous estrogens, which attenuates when the BMD reaches a stable level after a larger initial reduction. The association between estrogen level and risk of hip fracture attenuated after reaching a lower level of bone mass.
This is the first large prospective study to examine the associations between menopausal characteristics and risk of hip fracture in Chinese, and also the first study to explore the effect of time since menopause on long-term risks of hip fracture. The analyses were robust to adjustment for a wide range of potential confounders including socio-demographic, physical and lifestyle characteristics, medical history and relevant reproductive factors. The study had several limitations, including that hip fractures included both fragility and non-fragility hip fractures which could underestimate the associations of menopause with hip fracture. Women with hip fractures occurring between their menopause and recruitment were excluded. No assessments of bone mineral density or hormone levels were recorded in the present study, and no validation of reported fracture diagnosis was performed. Likewise, no data were collected on the use of hormone replacement therapy in the present study. Potential confounders in the present study were collected at baseline which could have changed during the follow-up. Age at menarche and age at menopause were also self-reported retrospectively by the participants. Thus, recall bias could have been more significant in those who were older at baseline due to a longer recall period.
Conclusions
The present report involving 125,336 older women, demonstrated that a later age at menopause and longer duration of reproductive years were associated with a lower risk of incident hip fractures in postmenopausal women aged 50 years or greater. Longer intervals since menopause were associated higher risks of hip fracture, but the excess risks of hip fracture were attenuated by 20 years after reaching menopause. Recognition of the importance of the relationships of reproductive years with hip fracture may guide more intensive treatment for affected individuals to reduce their risks of hip fracture. Further studies investigating the associations between menopause characteristics and BMD or bone remodeling are needed to confirm the findings of the present study.
Two sentence summary.
In a study of 125,336 postmenopausal women who had a natural menopause, 1,327 incident cases of hip fracture were recorded during the first 10 years of follow-up. The study demonstrated that women with younger age at menopause, longer interval since menopause or shorter duration of total reproductive years had the highest risks of hip fracture.
Acknowledgments
The chief acknowledgment is to the participants, the project staff, staff of the China CDC and its regional offices for access to death and disease registries. The Chinese National Health Insurance scheme provided electronic linkage to all hospitalization data. The China Kadoorie Biobank study is jointly coordinated by the University of Oxford and the Chinese Academy of Medical Sciences. The funding body for the baseline survey was the Kadoorie Charitable Foundation, Hong Kong, China and the funding sources for the long-term continuation of the study include UK Wellcome Trust (202922/Z/16/Z, 104085/Z/14/Z, 088158/Z/09/Z), Chinese National Natural Science Foundation (81390540, 81390541, 81390544), and the National Key Research and Development Program of China (2016YFC0900500, 2016YFC0900501, 2016YFC0900504, 2016YFC1303904). Core funding was provided to the CTSU, University of Oxford, by the British Heart Foundation, the UK Medical Research Council, and Cancer Research UK. The funders played no role in the design or conduct of the study, including data collection, management, analysis, or interpretation of the results; preparation, review, or approval of the manuscript; or the decision to submit the manuscript for publication.
Footnotes
Competing interests:
We declare that we have no conflicts of interest.
Author contributions
KP, MW, RI, and RC designed and planned the paper. KP performed data analyses and wrote the first draft of the manuscript. PY generated the figures. RC, LY, DB, CK, MT and YC provided advice on the scientific interpretation of the results and revisions to the manuscript. RC and ZC, as the members of CKB steering committee, designed and supervised the overall conduct of the study and obtained funding. YG, YC, ZB, and ZC coordinated the data acquisition (both for baseline and resurveys, and the long-term follow-up. All authors provided critical comments on the manuscript.
Access to data and data analysis:
RCl, DB, KP and ZC had full access to all the data in the study and take responsibility for the integrity of all data and accuracy of the data analysis. Data from the baseline survey, first resurvey, and cause-specific mortality are available to all bona fide researchers (www.ckbiobank.org). Additional data are also made available on a collaborative basis by contacting the study investigators. All data requests are reviewed monthly by the CKB Data Access Committee, which is composed of senior scientists from Beijing and Oxford.
Contributor Information
Members of the China Kadoorie Biobank collaborative group:
Junshi Chen, Zhengming Chen (PI), Robert Clarke, Rory Collins, Yu Guo, Liming Li (PI), Jun Lv, Richard Peto, Robin Walters, Daniel Avery, Derrick Bennett, Ruth Boxall, Fiona Bragg, Yumei Chang, Yiping Chen, Zhengming Chen, Robert Clarke, Huaidong Du, Simon Gilbert, Alex Hacker, Michael Holmes, Christiana Kartsonaki, Rene Kerosi, Garry Lancaster, Kuang Lin, John McDonnell, Iona Millwood, Qunhua Nie, Jayakrishnan Radhakrishnan, Paul Ryder, Sam Sansome, Dan Schmidt, Rajani Sohoni, Iain Turnbull, Robin Walters, Jenny Wang, Lin Wang, Neil Wright, Ling Yang, Xiaoming Yang, Zheng Bian, Yu Guo, Xiao Han, Can Hou, Biao Jing, Chao Liu, Jun Lv, Pei Pei, Yunlong Tan, Canqing Yu, Qingdao CDC, Licang CDC, Provincial CDC, Nangang CDC, Provincial CDC, Meilan CDC, Provincial CDC, Suzhou CDC, Provincial CDC, Liuzhou CDC, Provincial CDC, Pengzhou CDC, Provincial CDC, Maiji CDC, Provincial CDC, Huixian CDC, Provincial CDC, Tongxiang CDC, Provincial CDC, and Liuyang CDC
References
- 1.Johnell O, Kanis J. An estimate of the worldwide prevalence, mortality and disability associated with hip fracture. Osteoporosis International. 2004;15(11):897–902. doi: 10.1007/s00198-004-1627-0. [DOI] [PubMed] [Google Scholar]
- 2.Braithwaite RS, Col NF, Wong JB. Estimating hip fracture morbidity, mortality and costs. Journal of the American Geriatrics Society. 2003;51(3):364–70. doi: 10.1046/j.1532-5415.2003.51110.x. [DOI] [PubMed] [Google Scholar]
- 3.Brainsky A, Glick H, Lydick E, et al. The economic cost of hip fractures in community - dwelling older adults: a prospective study. Journal of the American Geriatrics Society. 1997;45(3):281–87. doi: 10.1111/j.1532-5415.1997.tb00941.x. [DOI] [PubMed] [Google Scholar]
- 4.Farahmand BY, Michaelsson K, Ahlbom A, et al. Survival after hip fracture. Osteoporosis International. 2005;16(12):1583–90. doi: 10.1007/s00198-005-2024-z. [DOI] [PubMed] [Google Scholar]
- 5.Xia WB, He SL, Xu L, et al. Rapidly increasing rates of hip fracture in Beijing, China. Journal of Bone and Mineral Research. 2012;27(1):125–29. doi: 10.1002/jbmr.519. [DOI] [PubMed] [Google Scholar]
- 6.Li S, Sun T, Liu Z. Excess mortality of 1 year in elderly hip fracture patients compared with the general population in Beijing, China. Archives of Osteoporosis. 2016;11(1):35. doi: 10.1007/s11657-016-0289-9. [DOI] [PubMed] [Google Scholar]
- 7.Tian M, Gong X, Rath S, et al. Management of hip fractures in older people in Beijing: a retrospective audit and comparison with evidence-based guidelines and practice in the UK. Osteoporosis International. 2016;27(2):677–81. doi: 10.1007/s00198-015-3261-4. [DOI] [PubMed] [Google Scholar]
- 8.Hopkins R, Pullenayegum E, Goeree R, et al. Estimation of the lifetime risk of hip fracture for women and men in Canada. Osteoporosis International. 2012;23(3):921–27. doi: 10.1007/s00198-011-1652-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cummings SR, Black DM, Rubin SM. Lifetime risks of hip, Colles’, or vertebral fracture and coronary heart disease among white postmenopausal women. Archives of Internal Medicine. 1989;149(11):2445–48. [PubMed] [Google Scholar]
- 10.Tinetti ME, Doucette J, Claus E, et al. Risk factors for serious injury during falls by older persons in the community. Journal of the American Geriatrics Society. 1995;43(11):1214–21. doi: 10.1111/j.1532-5415.1995.tb07396.x. [DOI] [PubMed] [Google Scholar]
- 11.Ambrose AF, Paul G, Hausdorff JM. Risk factors for falls among older adults: a review of the literature. Maturitas. 2013;75(1):51–61. doi: 10.1016/j.maturitas.2013.02.009. [DOI] [PubMed] [Google Scholar]
- 12.Samelson EJ, Hannan MT. Epidemiology of osteoporosis. Current Rheumatology Reports. 2006;8(1):76–83. doi: 10.1007/s11926-006-0030-6. [DOI] [PubMed] [Google Scholar]
- 13.Morcov C, Vulpoi C, Brănişteanu D. Relationship between bone mineral density, weight, and estrogen levels in pre and postmenopausal women. The Medical-Surgical Journal. 2012;116(4):946–50. [PubMed] [Google Scholar]
- 14.Ho-Pham LT, Nguyen ND, Nguyen TV. Quantification of the relative contribution of estrogen to bone mineral density in men and women. BMC Musculoskeletal Disorders. 2013;14(1):366. doi: 10.1186/1471-2474-14-366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Riggs BL, Khosla S, Melton LJ., III A unitary model for involutional osteoporosis: estrogen deficiency causes both type I and type II osteoporosis in postmenopausal women and contributes to bone loss in aging men. Journal of Bone and Mineral Research. 1998;13(5):763–73. doi: 10.1359/jbmr.1998.13.5.763. [DOI] [PubMed] [Google Scholar]
- 16.Chew CK, Clarke BL. Causes of low peak bone mass in women. Maturitas. 2018;111:61–68. doi: 10.1016/j.maturitas.2017.12.010. [DOI] [PubMed] [Google Scholar]
- 17.Sasson H, Carpenter C. Achievement of peak bone mass in women is critically dependent on adolescent calcium intake. OA Sports Medicine. 2013;1:16. [Google Scholar]
- 18.Dasarathy J, Labrador H. Bone Health in Women. Primary Care: Clinics in Office Practice. 2018;45(4):643–57. doi: 10.1016/j.pop.2018.07.011. [DOI] [PubMed] [Google Scholar]
- 19.Riggs BL, Melton LJ., III Involutional osteoporosis. New England journal of medicine. 1986;314(26):1676–86. doi: 10.1056/NEJM198606263142605. [DOI] [PubMed] [Google Scholar]
- 20.Sirola J, Kröger H, Honkanen R, et al. Factors affecting bone loss around menopause in women without HRT: a prospective study. Maturitas. 2003;45(3):159–67. doi: 10.1016/s0378-5122(03)00150-6. [DOI] [PubMed] [Google Scholar]
- 21.Ahlborg H, Johnell O, Nilsson B, et al. Bone loss in relation to menopause: a prospective study during 16 years. Bone. 2001;28(3):327–31. doi: 10.1016/s8756-3282(00)00451-8. [DOI] [PubMed] [Google Scholar]
- 22.Recker R, Lappe J, Davies K, et al. Characterization of perimenopausal bone loss: a prospective study. Journal of Bone and Mineral Research. 2000;15(10):1965–73. doi: 10.1359/jbmr.2000.15.10.1965. [DOI] [PubMed] [Google Scholar]
- 23.Sowers MR, Zheng H, Jannausch ML, et al. Amount of bone loss in relation to time around the final menstrual period and follicle-stimulating hormone staging of the transmenopause. The Journal of Clinical Endocrinology & Metabolism. 2010;95(5):2155–62. doi: 10.1210/jc.2009-0659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Finkelstein JS, Brockwell SE, Mehta V, et al. Bone mineral density changes during the menopause transition in a multiethnic cohort of women. The Journal of Clinical Endocrinology & Metabolism. 2008;93(3):861–68. doi: 10.1210/jc.2007-1876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Compston JE. HRT and osteoporosis. British medical bulletin. 1992;48(2):309–44. doi: 10.1093/oxfordjournals.bmb.a072549. [DOI] [PubMed] [Google Scholar]
- 26.Cummings SR, Kelsey JL, Nevitt MC, et al. Epidemiology of osteoporosis and osteoporotic fractures. Epidemiologic reviews. 1985;7(1):178–208. doi: 10.1093/oxfordjournals.epirev.a036281. [DOI] [PubMed] [Google Scholar]
- 27.Cummings SR, Browner WS, Bauer D, et al. Endogenous hormones and the risk of hip and vertebral fractures among older women. New England Journal of Medicine. 1998;339(11):733–38. doi: 10.1056/NEJM199809103391104. [DOI] [PubMed] [Google Scholar]
- 28.Cumming RG, Klineberg RJ. Breastfeeding and other reproductive factors and the risk of hip fractures in elderly women. International Journal of Epidemiology. 1993;22(4):684–91. doi: 10.1093/ije/22.4.684. [DOI] [PubMed] [Google Scholar]
- 29.Chen Z, Chen J, Collins R, et al. China Kadoorie Biobank of 0.5 million people: survey methods, baseline characteristics and long-term follow-up. International Journal of Epidemiology. 2011;40(6):1652–66. doi: 10.1093/ije/dyr120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Easton DF, Peto J, Babiker AG. Floating absolute risk: an alternative to relative risk in survival and case-control analysis avoiding an arbitrary reference group. Statistics in Medicine. 1991;10(7):1025–35. doi: 10.1002/sim.4780100703. [DOI] [PubMed] [Google Scholar]
- 31.Banks E, Reeves GK, Beral V, et al. Hip fracture incidence in relation to age, menopausal status, and age at menopause: prospective analysis. PLoS Medicine. 2009;6(11):e1000181. doi: 10.1371/journal.pmed.1000181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen F-P, Hsu K-H, Fu T-S, et al. Risk factor for first-incident hip fracture in Taiwanese postmenopausal women. Taiwanese Journal of Obstetrics and Gynecology. 2016;55(2):258–62. doi: 10.1016/j.tjog.2015.12.017. [DOI] [PubMed] [Google Scholar]
- 33.Parazzini F, Tavani A, Ricci E, et al. Menstrual and reproductive factors and hip fractures in post menopausal women. Maturitas. 1996;24(6):191–96. doi: 10.1016/s0378-5122(96)82009-3. [DOI] [PubMed] [Google Scholar]
- 34.Huo D, Lauderdale DS, Li L. Influence of reproductive factors on hip fracture risk in Chinese women. Osteoporosis International. 2003;14(8):694–700. doi: 10.1007/s00198-003-1429-9. [DOI] [PubMed] [Google Scholar]
- 35.Wark JD. Osteoporotic fractures: background and prevention strategies. Maturitas. 1996;23(2):193–207. doi: 10.1016/0378-5122(95)00974-4. [DOI] [PubMed] [Google Scholar]
- 36.Bernstein CN, Leslie WD, Leboff MS. AGA technical review on osteoporosis in gastrointestinal diseases. Gastroenterology. 2003;124(3):795–841. doi: 10.1053/gast.2003.50106. [DOI] [PubMed] [Google Scholar]
- 37.Kang H, Chen YM, Han G, et al. Associations of Age, BMI, and Years of Menstruation with Proximal Femur Strength in Chinese Postmenopausal Women: A Cross-Sectional Study. International Journal of Environmental Research & Public Health. 2016;13(2):157. doi: 10.3390/ijerph13020157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jacobsen BK, Nilssen S, Heuch I, et al. Reproductive factors and fatal hip fractures. A Norwegian prospective study of 63,000 women. Journal of Epidemiology & Community Health. 1998;52(10):645–50. doi: 10.1136/jech.52.10.645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bush T, Wells H, James M, et al. Effects of hormone therapy on bone mineral density-results from the postmenopausal estrogen/progestin interventions (PEPI) trial. Journal of the American Medical Association. 1996;276(17):1389–96. [PubMed] [Google Scholar]
- 40.Lufkin EG, Wahner HW, O’fallon WM, et al. Treatment of postmenopausal osteoporosis with transdermal estrogen. Annals of internal Medicine. 1992;117(1):1–9i. doi: 10.7326/0003-4819-117-1-1. [DOI] [PubMed] [Google Scholar]
- 41.De Villiers T, Gass M, Haines C, et al. Global consensus statement on menopausal hormone therapy. Climacteric. 2013;16(2):203–04. doi: 10.3109/13697137.2013.771520. [DOI] [PubMed] [Google Scholar]
- 42.Cauley JA, Seeley DG, Ensrud K, et al. Estrogen replacement therapy and fractures in older women. Annals of Internal Medicine. 1995;122(1):9–16. doi: 10.7326/0003-4819-122-1-199501010-00002. [DOI] [PubMed] [Google Scholar]
- 43.Session DR, Kelly AC, Jewelewicz R. Current concepts in estrogen replacement therapy in the menopause. Fertility and Sterility. 1993;59(2):277–84. doi: 10.1016/s0015-0282(16)55667-2. [DOI] [PubMed] [Google Scholar]
- 44.Jin F, Tao M, Teng Y, et al. Knowledge and attitude towards menopause and hormone replacement therapy in Chinese women. Gynecologic and obstetric investigation. 2015;79(1):40–45. doi: 10.1159/000365172. [DOI] [PubMed] [Google Scholar]
- 45.Sherrington C, Fairhall NJ, Wallbank GK, et al. Exercise for preventing falls in older people living in the community. Cochrane database of systematic reviews. 2019;(1) doi: 10.1002/14651858.CD012424.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kannus P, Parkkari J, Niemi S, et al. Prevention of hip fracture in elderly people with use of a hip protector. New England Journal of Medicine. 2000;343(21):1506–13. doi: 10.1056/NEJM200011233432101. [DOI] [PubMed] [Google Scholar]