Abstract
We developed a functional lineage tracing tool termed CaTCH (CRISPRa Tracing of Clones in Heterogeneous cell populations). CaTCH combines precise clonal tracing of millions of cells with the ability to retrospectively isolate founding clones alive prior to and during selection, allowing functional experiments. Using CaTCH, we captured rare clones representing as little as 0.001% of a population and investigated the emergence of resistance to targeted melanoma therapy in vivo.
A single genome can encode hundreds of different cell types with diverse functions, providing the basis for all complex organisms. Recently, single cell studies have revealed a remarkable degree of cellular heterogeneity, even within clonal cell populations. The formation and evolutionary selection of sub-clonal populations shape important biological processes from organismal development, tissue differentiation1–3, and cellular reprogramming4–6 to the development of diseases7–9 and therapy resistance8–10. Traditional lineage tracing methods can be used to map the heterogeneity of cell populations before and after evolutionary selection11–13, but do not allow the functional characterization of founder clones at early stages and in comparison with their post-selection counterparts.
To fill this gap, we developed CaTCH (CRISPRa tracing of clones in heterogeneous cell populations), a novel technique combining precise clone tracing of millions of cells with the ability to isolate specific clones alive at any time point from complex cell populations (Fig. 1a). To perform CaTCH, cells are first transduced with a dCas9 fused to a transcriptional activator (dCas9-VPR construct) and are then uniquely labelled with a stably integrated DNA barcode (BC construct) fused to an inducible GFP-reporter. After experimental selection, the barcodes of enriched and depleted clones are identified by next generation sequencing (NGS) to design sgRNAs that are complementary to the barcode. The sgRNA constructs are transduced into the heterogeneous cell population and guide the dCas9-VPR to the barcode reporter specifically in the clone of interest, activating GFP expression (Fig. 1b). The clone of interest can then be isolated alive by FACS and functionally characterized. By preserving cells before or during experimental selection, the founders of selected clones can be identified at any stage of the selection process.
Fig. 1. CaTCH – A barcode-guided CRISPRa-inducible reporter for isolation of specific cell clones.
a, The CaTCH workflow. After transduction of a heterogeneous cell population with the dCas9VPR construct and the barcode (BC) reporter, a part of the cells is used for a selection experiment to identify clones with a desired phenotype. The clone’s specific barcode sequence is identified by NGS and used to design complementary sgRNA constructs. The sgRNAs are transduced into the retained, unselected population and specifically guide the dCas9-VPR to the barcode of interest to activate GFP expression. The GFP positive clones can be isolated alive by FACS and used to perform functional studies. b, Schematics and FACS plots of CaTCH reporter activation in bulk A375 cells harboring a single barcode (BC). GFP expression is only induced if a sgRNA complementary to the barcode is present. The experiment was repeated independently 3 times with similar results. c, Experimental layout to determine the sensitivity of CaTCH reporter activation in a spike-in experiment. d, FACS plots of the spike-in experiment shown in c. The top row shows the GFP signal, while the bottom row additionally visualizes the spiked-in iRFP+ cell populations. e, Quantification of Fig. 1d, including additional spike-in ratios (the corresponding FACS plots are shown in Extended Data Fig. 3a). n=3 biologically independent samples, n=6 for 0.001% spike-in. Data displayed as mean ± standard deviation (SD). f, Experimental outline to trace and CaTCH-isolate pre-existing, therapy-resistant cell clones: NrasG12D-iRFP expressing clones with a known BC were spiked into a complex barcoded background population and treated with vehicle or RAFi. g, Enrichment of iRFP-positive cells over 31 days of RAFi-treatment measured by FACS; Extended Data Fig. 4a displays the corresponding plots. n=3 biologically independent samples, except for vehicle day 7 n=1, data displayed as mean ± standard error of mean (SEM). h, FACS plots display the CaTCH reporter activation of spiked-in cells. GFP+ cells were sorted, expanded, and their BC sequenced. The bar graph shows the percentage of identified reads corresponding to the target BC after 31 days of vehicle or RAFi-treatment (n=3 biologically independent samples, data displayed as mean ± standard deviation) or after CaTCH isolation (n=1).
To establish CaTCH, we systematically tested and optimized each component. To select a suitable dCas9-transcriptional activator fusion, we transduced A375 melanoma cells with either dCas9-VP64 or dCas9-VPR14, as well as a GFP-reporter controlled by a doxycycline inducible promoter (TRE3G). After transduction with sgRNAs targeting binding sites within TRE3G, we found that cells expressing dCas9-VPR showed higher GFP activation rates and levels compared to dCas9-VP64, confirming the higher sensitivity and efficiency of dCas9-VPR in reporter settings14 (Extended Data Fig. 1a).
To optimize the barcode reporter, we first evaluated the barcode position for sgRNA-guided GFP-reporter activation. We designed two CaTCH reporter constructs with the same set of 8 distinct barcodes, but with different distances to the promoter. We targeted the barcodes sequentially and found that the optimal distance for CRISPRa-mediated reporter activation is 58-130 bp upstream of the transcription start site (TSS), similar to endogenous loci15 (Extended Data Fig. 1b). Targeting the reporter with multiple sgRNAs simultaneously and introducing a spacer between targeted barcodes further enhanced reporter activation (Extended Data Fig. 1c). We designed our final barcode cassette to harbor three independent barcodes, which serve as sgRNA binding sites. The center barcode site functions as a spacer and as an additional target that can be used if the standard sgRNA targets are inefficient. We utilized a semi-random barcode design with fixed nucleotide positions to assure optimal sgRNA binding and to achieve high complexity of our barcoding library (Extended Data Fig. 2a). As dual sgRNA targeting enhances the signal strength of the GFP-reporter (Extended Data Fig. 2b), we established a rapid, one-step cycle-ligation protocol to efficiently clone dual sgRNA expression constructs. Finally, we established a reporter barcode library comprising more than 130 million unique barcodes at an even distribution (Extended Data Fig. 2c).
To test the sensitivity of reporter activation, we engineered cells containing a single known target barcode and transduced them with a constitutive iRFP-expression construct to trace them independently of CaTCH components (Fig. 1c). After expansion, 97% of cells remained iRFP-positive, which we spiked at different ratios (10% to 0.001%) into an iRFP-negative, complexly barcoded background population. After transducing the mixed population with sgRNAs targeting the known barcode of the iRFP-positive cells, the GFP-reporter was activated in ~45% of cells (range: 33-56) over an extensive range of spike-ins, but not in the iRFP-negative cells (Fig. 1d,e, Extended Data Fig. 3a). CaTCH was able to detect as few as 0.001% target cells in a complex barcoded population with high specificity. Finally, we compared a different order and different types of construct delivery16 (lipofection vs. lentiviral transduction) and found that the highest efficiency is achieved when the BC reporter is stably integrated and the sgRNA is delivered last, using lentiviral transduction (Extended Data Fig. 3b-e).
To probe the utility of CaTCH, we performed a proof-of-concept experiment simulating preexisting therapeutic resistance. We used the mouse melanoma cell line YUMM1.7 (BrafV600E/wt, Pten-/-, Cdkn2a-/-)17 that is sensitive to RAF inhibitors (RAFi) and engineered resistant cells by expressing constitutively active NrasG12D, which is known to drive RAFi resistance in human melanoma18. We labeled NrasG12D-expressing cells with iRFP and a distinct barcode and mixed 0.035% of these resistant cells into a RAFi-sensitive, complexly barcoded population (Fig. 1f). When treated with RAFi, NrasG12D-clones enriched to 90-97% (Fig. 1g, Extended Data Fig. 4a). To retrieve the treatment-naïve NrasG12D founder clones from the untreated starting population, we transduced these cells with the sgRNA constructs targeting the barcode within the NrasG12D-clones (Extended Data Fig. 4b). We detected a GFP-high population (0.012% of parent) that consisted exclusively of iRFP-positive cells, indicating a specific activation of the barcode reporter (Fig. 1h). We FACS-sorted the GFP-high cells, expanded them, and found a ~1000-fold enrichment of the correct barcode with a purity of ~90% as determined by NGS and FACS (Fig. 1h, Extended Data Fig. 4c). Both therapy-selected and their CaTCH-isolated, treatment-naïve founder clones were resistant to RAFi-treatment and harbored the NrasG12D mutation in the majority of cells (Extended Data Fig. 4d,e). This proof-of-concept study establishes the feasibility of CaTCH to label and trace rare cell clones with a distinct phenotype and subsequently isolate founder clones in their unperturbed state for functional analyses.
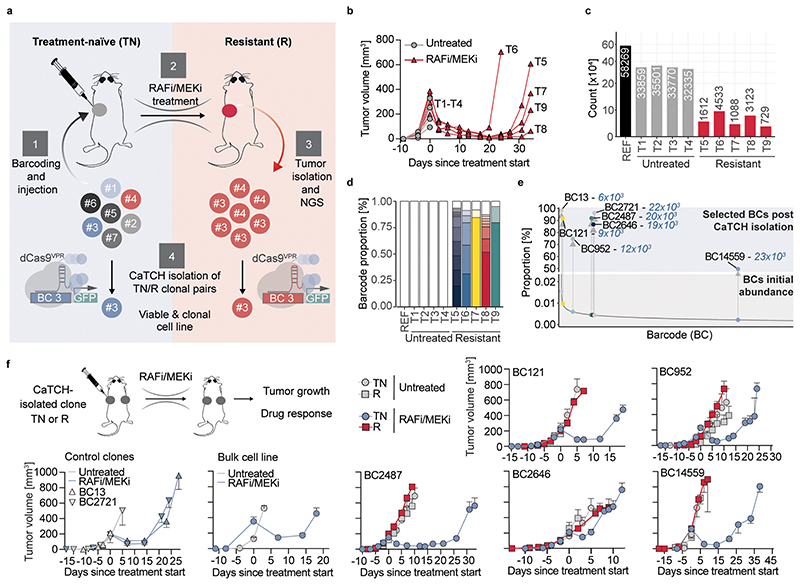
Melanoma patients frequently acquire resistance to RAFi/MEKi in the clinic8. To what extent resistance reflects selection of a pre-existing resistant clones, as modeled above, or the acquisition of resistance traits is still debated19–22. CaTCH offers a new approach to answering this question by enabling the isolation and characterization of therapy-resistant clones alongside with their treatment-naïve founders. We took advantage of the genetically engineered mouse melanoma model YUMM1.7 that recapitulates a biologically relevant immune-microenvironment17. We injected a population of barcoded YUMM1.7 cells into immunocompetent mice and treated one cohort with RAFi/MEKi until tumor relapse (Fig. 2a). In the RAFi/MEKi cohort, tumors regressed initially, but relapsed 24-34 days post-injection (Fig. 2b, Extended Data Fig. 5a). In untreated tumors, we found a high barcode complexity of ~34,000 clones, indicating a high tumor-initiating capacity, whereas in RAFi/MEKi resistant tumors, the barcode complexity was strongly reduced to ~2000 clones (Fig. 2c, Extended Data Fig. 5b). In each resistant tumor, 2-6 barcodes dominated, indicating a considerable clonal selection under therapy (Fig. 2d). The dominant barcodes were not shared between resistant tumors (Extended Data Fig. 5c,d). In a similar resistance-screen in vitro, clonal heterogeneity remained high in resistant samples with only a 5-6 fold reduction of barcodes and a maximal enrichment to ~0.05%, indicating different selection pressures in vivo and in vitro (Extended Data Fig. 6a-e).
Fig. 2. CaTCH isolated treatment-naïve clones initially respond to RAFi/MEKi treatment and acquire resistance in vivo.
a, Experimental outline: (1) A population of barcoded cells is injected subcutaneously into C57BL/6 mice. (2) After RAFi/MEKi treatment and development of resistance (3) tumors are excised, BC-sequenced and taken into culture. (4) Clones of interest are CaTCH-isolated from the tumor-derived, resistant (R) cells and from the treatment-naïve (TN) starting population. b, Individual tumor growth curves of RAFi/MEKi-treated or vehicle treated melanoma tumors. Corresponding survival curve is shown in Extended Data Fig. 5a. (Untreated=4 tumors isolated from 4 mice; RAFi/MEKi=5 tumors isolated from 5 mice). c, Total number of unique BCs identified by NGS (REF=starting cell line, T1-T9=individual tumors, one tumor per mouse). d, BC composition identified by NGS. BCs more abundant than 1% of the sample are highlighted individually in color, BCs comprising less than 1% barcode proportion were summed up (white bars). e, BC proportion before (bottom, grey) and after (top, blue) CaTCH-isolation from the TN starting population. BCs are sorted by their distribution in the starting population from high (left) to low (right). Isolated BCs are highlighted by dots, their fold-change enrichment after CaTCH-isolation is indicated in blue. BCs were named by their abundance-rank in the starting population (1=most abundant). f, Experimental outline and in vivo RAFi/MEKi treatment response of tumors established from single CaTCH-isolated clones. Data displayed as mean ± standard error of mean (SEM). The corresponding survival curves are shown in Extended Data Fig. 7d. n=tumors, two tumors per mouse, BC121: TNUT n=6, RUT n=6, TNRAFi/MEKi n=10, RRAFi/MEKi n=10; BC952: TNUT n=6, RUT n=6, TNRAFi/MEKi n=8, RRAFi/MEKi n=10; BC2487: TNUT n=6, RUT n=6, TNRAFi/MEKi n=8, RRAFi/MEKi n=10; BC2646: TNUT n=6, RUT n=4, TNRAFi/MEKi n=10, RRAFi/MEKi n=6, experiment performed twice; BC14559: TNUT n=6, RUT n=6, TNRAFi/MEKi n=6, RRAFi/MEKi n=10, experiment performed twice; BC13: TNUT n=6, TNRAFi/MEKi n=8; BC2721: TNUT n=6, TNRAFi/MEKi n=10; BULK: TNUT n=6, TNRAFi/MEKi n=10.
To discriminate pre-existing from acquired resistance mechanisms in our in vivo model, we used CaTCH to isolate 5 clonal pairs (BC121, BC952, BC2487, BC2646, BC14559) both from resistant (R) tumors and the treatment-naïve (TN) starting population. We also isolated two control clones (BC13, BC2721) that were only present in treatment-naïve but not in resistant tumors. After CaTCH isolation, single barcodes were enriched up to 23,000-fold from an initial abundance ≥ 0.002% in the treatment-naïve population to a purity of up to 98% in the CaTCH-isolated population (Fig. 2e, Extended Data Fig. 7a). BC14559 displayed an unusually low purity of ~50% and co-enriched with BC43158. Using CaTCH, we targeted both barcodes independently, isolated and expanded both clones, and confirmed a co-enrichment of both barcodes irrespective of which barcode was used for isolation, suggesting that both barcodes were integrated in the same clone (Extended Data Fig. 7b). Using conventional barcoding approaches with NGS readout, these barcodes would have been considered as two independent clones, unless they would be sub-cultivated in single cell cultures.
To compare the therapeutic response in resistant clones and their treatment-naïve founders, we injected our 5 clonal pairs and 5 treatment-naïve, control clones (the 2 CaTCH isolated and 3 randomly selected, FACS-sorted clones) into mice and treated them with RAFi/MEKi. None of the resistant clones, but all control clones and all treatment-naïve founder clones, except for BC2646TN, responded to therapy before relapsing (Fig. 2f, Extended Data Fig. 7c,d). To investigate the underlying cellular programs responsible for the reduced therapeutic response of BC2646TN, we established tumors of BC2646TN and tumors of 2 therapy-responsive and 2 resistant control clones in mice, and analysed their transcriptome after 3 days of treatment with RAFi/MEKi or vehicle (Extended Data Fig. 8a,b). Compared to the therapy-responsive controls, the resistant clones and BC2646TN showed a reduced transcriptional response to RAFi/MEKi, including only minor changes in cell division and proliferation programs, and a higher expression of key regulators of cellular fitness, survival, and known resistance factors such as c-MET or EGFR (Extended Data Fig. 8c-g, Supplementary Tables 1-5). These data provide a potential explanation for the reduced response of BC2646TN to targeted therapy.
We also investigated resistance mechanisms on a genomic level by whole exome sequencing and found that resistant clones showed a higher number of mutations than their treatment-naïve counterparts (Extended Data Fig. 9a-c). One functionally relevant candidate is a de novo KrasG12R mutation, which we identified in the resistant BC14559R clone, but not in its treatment-naïve counterpart, providing experimental evidence that it was acquired by this clone during drug treatment (Extended Data Fig. 9d). While KrasG12R is rarely found in melanoma, it is known to drive tumorigenesis through activating the MAPK pathway23,24, and thereby may promote RAFi/MEKi resistance in our model. These data demonstrate that CaTCH is well suited to mechanistically analyse founder clones and compare them with their post-selection counterparts to gain insights into the genomic and transcriptomic alterations induced by selective bottlenecks.
Here, we established CaTCH, a method to isolate and analyse specific cell clones from a heterogeneous population. CaTCH does not only allow for comparisons between the start- and endpoint of a selection but can also provide insights into intermediate stages during dynamic adaption processes. Although related methods for clone isolation have recently emerged16,25,26, their technical execution is largely different, and based on the published data, CaTCH has the highest efficiency and resolution in cell tracing and isolation capabilities. We utilized CaTCH to investigate subclone evolution during targeted therapy in vivo and found that RAFi/MEKi-resistance largely does not arise from selection of pre-existing resistant clones but is an acquired state achievable by most clones in our model27,28. Strategies that interfere with such adaptation processes could delay the onset of resistance and improve the durability of treatment responses8,29–31. Future applications of CaTCH range from basic biology to regenerative medicine, for example, to shed light on clonal selection and adaptation processes during metastasis, reprogramming, and differentiation.
Methods
One-step cloning of dual sgRNA-expression construct
SgRNAs were ordered as sense and antisense oligos with the following overhangs: sgRNA1 – CACC-SENSE, ANTISENSE-CAAA; sgRNA2 CTTGTTT-SENSE-GTTTA, AAA-ANTISENSE-CAAATTCTC; complementary sense:antisense pairs were annealed and phosphorylated using 1μl 100 μM sense oligo, 1μl 100 μM antisense oligo, 1 μl 10x T4-DNA-ligation buffer (Thermo Fisher Scientific), 0.5 μl T4 PNK (NEB), 6.5 μl water at 37 °C for 30 min, 95 °C for 5 min and then ramped down to 25 °C at 5 °C per minute. 1 μl of 1:200 diluted annealed sgRNA1 was the inserted into a cycle-ligation reaction with 100 ng empty backbone (pRRL_hU6_mU6_EF1as_Thy1.1), 2 μl 10x CutSmart buffer (NEB), 1 μl 20 mM ATP (Roche), 1 μl Esp3I (NEB), 0.5 μl 400 U/μl T4 DNA Ligase (NEB) to a final volume of 20 μl, cycling 24 times between 37 °C and 16 °C for 5 min each. 1 μl of 1:200 diluted annealed sgRNA2 and 1 μl BbsI-HF (NEB) were then added to the reaction followed by cycling 24 times between 37 °C and 16 °C for 5 min each with a final incubation of 2 h at 37 °C. It is important to add BbsI-HF only after the first round of cycle-ligation, as the unedited cloning site for sgRNA1 harbours additional BbsI cut sites to remove Esp3I-uncut plasmids. 10 μl of the reaction were then transformed into Stbl3 cells.
CaTCH reporter plasmid library cloning
pRRL_P7_SBS12Seq_BC_reporter_mCMV_eGFP_EF1as_eBFP2 empty backbone was digested with BsmBI (NEB) and dephosphorylated using Antarctic phosphatase (NEB) with a subsequent QIAquick Spin Column (Qiagen, manufacturers protocol) purification. The CaTCH reporter library was ordered as an Ultramer DNA oligo from IDT and 172.5 ng library was amplified in 23 separate reactions with 10 PCR cycles each, using Platinum Pfx DNA Polymerase (Thermo Fisher Scientific) according to the manufacturers protocol with the following primers: Fwd – TGACAGTGAGCGCGTCTCTG, Rev – TTGTCCGCGTCGATCCTAGG. The reactions were pooled over two QIAquick Spin Columns (Qiagen, manufacturer’s protocol) and agarose gel purified using three QIAquick Spin Columns. 100 ng amplified library were inserted into a cycle-ligation with 4 μg cut backbone in 20 separate reactions, using Esp3L (Thermo Fisher Scientific) and T7 DNA ligase (NEB) in FastDigest buffer (Thermo Fisher Scientific) with 1 mM DTT (Thermo Fisher Scientific) and 1 mM ATP (Roche), cycling 40 times between 37 °C and 23 °C for 5 min each, followed by a 2 hour digestion at 37 °C. Religated empty plasmids were digested with addition of BsmBI (NEB) for 1 h at 55 °C. Reactions were pooled and phenol extracted with addition of 1 volume cold phenol pH 8 (Sigma) and PhaseLock Gel Light tubes (QuantaBio) and precipitated from the aqueous phase using 1/10 volume 3 M NaAc pH 5.2, Pellet Paint Co-Precipitant (Novagen) and 3 volumes ice cold 100% ethanol, incubating at -20 °C overnight. The DNA pellet was spun down at maximum speed at 4 °C, washed twice with 70% ethanol and resuspended in 20 μl EB-buffer. The library was electroporated into MegaX DH10B T1R Electrocomp Cells (Invitrogen), using ice cold Gene Pulser/MicroPulser cuvettes according to the manufacturers recommendations on a GenePulser Xcell Electroporation System (Bio-Rad). The Bacteria were recovered in pre-warmed recovery medium shaking at 225 rpm at 37 °C for 1 h. A serial dilution of the recovered bacteria was plated onto LB-Amp plates to estimate electroporation efficiencies and the remainder of the bacteria was grown on LB agar plates containing 100 μg/ml ampicillin at 32 °C overnight. Colonies were then collected and transferred to 500 ml LB-Amp, shaking at 225 rpm at 32 °C for 6 h. After the incubation, the cells were pelleted at 4000 g, 4 °C for 30 min and pellets were frozen at -20 °C. Plasmids from each pellet were purified using NucleoBond Xtra Midi columns (Macherey-Nagel, follow manufacturers protocol) and eluted in 1x TE buffer. Individual plasmid library preparations were pooled to obtain the final CaTCH library.
NGS of CaTCH reporter plasmid library
80 ng library total was amplified and prepared for sequencing using the CaTCH barcode NGS strategy (see below). The library was sequenced with ~237 million 100 bp single-end reads reads on an Illumina HiSeqV4.
Genomic DNA isolation to retrieve barcodes
All genomic DNA isolation steps were performed in a plasmid and PCR-product free environment. Cell pellets were resuspended in 800 μl DNA extraction buffer (10 mM Tris-HCl pH 8, 150 mM NaCl, 10 mM EDTA pH 8) with 0.1% SDS and 0.2 mg/ml proteinase K (NEB) and incubated shaking at 1400 rpm at 56 °C for 24 - 48 h. Optional RNase (Roche) digestion was performed at 37 °C for 2 h. DNA was extracted by addition of 1 volume cold phenol pH 8 (Sigma) and the use of PhaseLock Gel Light tubes (QuantaBio) in two sequential rounds. The aqueous phase was transferred to a new tube and precipitation was initiated by addition of 1/10 volume 3 M NaAc pH 5.2, 1 μl Pellet Paint Co-Precipitant (Novagen) and 0.8 volumes isopropanol at -20 °C overnight. DNA pellets were spun down at maximum speed for 30 min at 4 °C, washed once with 70% ethanol and resuspended in EB-buffer. To facilitate DNA resuspension and later PCR efficiency the sample was fragmented in ten repeated freeze-thaw cycles.
CaTCH barcode NGS-library preparation
The first PCR was performed in a plasmid and PCR-product free environment. 12 μg genomic DNA was amplified in 12 individual 50 μl PCR reactions using Amplitaq Gold (Invitrogen) using the following protocol: 5 μl 10x Gold Buffer, 4 μl MgCl2 (25 mM), 0.4 dNTP-Mix (25 mM each), 0.2 μl AmpliTaq Gold DNA Polymerase (5 U/μl), 1 μg genomic DNA, 1.5 μl Primer (10 μM) Fwd - TGGAGTTCAGACGTGTGCTCTTC, 1.5 μl Primer (10 μM) Rev – TCTTTCCCTACACGACGCTCTTCCGATCTNNNNNN-Sample-tag(4 or 6 nt)GCCTAAAGCTTCTCCTGCCG, in final volume of 50 μl. Cycle conditions: 10 min at 95 °C; 29 cycles of 30 sec at 95 °C, 45 sec at 64 °C and 30 sec at 72 °C; final extension at 72 °C for 7 min. PCR products were pooled, concentrated over a spin column (in-house; alternatively QIAquick Spin columns can be used) and purified over a 2.5% agarose gel (correct band size at 391 bp). All spin column steps were performed following the manufacturer’s (Qiagen) instructions (including all optional wash steps and an extended incubation time with PE buffer of 30 min). DNA was eluted in 40 μl EB-buffer. 120 ng of purified PCR products were extended with NGS adapters in a second PCR using another 12 individual 50 μl PCR reactions (10 ng template each) with Amplitaq Gold (Invitrogen) (PCR reaction protocol identical to first PCR). Primers: Fwd – CAAGCAGAAGACGGCATACGAGATGTGACTGGAGTTCAGACGTGTGCT, Rev - AATGATACGGCGACCACCGAGATCTACACTCTTTCCCTACACGACGCT. Cycle conditions: 10 min at 95 °C; 5 cycles of 30 sec at 95 °C, 45 sec at 62 °C and 30 sec at 72 °C; final extension at 72 °C for 7 min. Products were again pooled, spin column concentrated and gel purified (3%, 449 bp product). Final DNA concentrations were assessed by Qubit dsDNA HS Assay (Invitrogen).
CaTCH barcode NGS strategy
For barcode identification of isolated clones, sequencing was performed with 8% PhiX either on an Illumina HiSeqV4 with 100 bp single-end reads or on an Illumina MiSeqNano with 150 bp single-end reads.
NGS of Nras
The Nras locus was amplified using the following primers: Fwd – TCTTTCCCTACACGACGCTCTTCCGATCT ACAAACTGGTGGTGGTTGGA Rev – TGGAGTTCAGACGTGTGCTCTTCCGATCT TGGTTCTGGATTAGCTGGATCG and P5 and P7 adapters were added in a second PCR using standard Illumina TruSeq primer. Amplicons were sequenced with 50 bp single-end reads on an Illumina HiSeqV4.
Bioinformatic analysis of CaTCH barcoding data
The reads were processed in Python (v3.6.7) using Biopython (v1.70)32. The reads were scanned for their barcode using the regular expression CGCCG[ATGC]{7}GCC[ATGC]{9}, and the sample and genotype tags were then identified at a fixed offset from the matched barcode, as per the design of the construct. A counter was kept for each distinct barcode sequence encountered. To prevent artificial inflation of the barcode counts due to random sequencing errors, barcodes with a Hamming distance of 2 or less (i.e. up to 2 substitutions) were subsequently assumed to represent the same barcode and their counts were combined. The barcode counts where then analysed in R (v3.5.1) with help of the data.table (v1.12.2) (https://cran.r-project.org/package=data.table) and tidyr packages. They were visualized using the R packages ggplot2 (v3.2.1)33 and ggrepel (v0.8.1) (https://cran.r-project.org/package=ggrepel). Numeric IDs were assigned to the barcodes in decreasing order of absolute abundance (count) in the reference sample. The proportional abundance of each barcode in each sample was calculated as the fraction of its count over the library size. Only barcodes with at least 50 reads were considered and barcodes representing more than 1% of the assigned reads were highlighted. These results were visualized using the R packages ggplot2 (v3.2.1)33 and ggrepel (v0.8.1) and pheatmap (v1.0.12).
Cell culture
YUMM 1.7 cells (BrafV600E/WTPTEN-/-CDKN2a-/-, actual corresponding Braf mutation in mouse is BRAFV584E/WT) were cultured in DMEM-F12 media; A375 cells, NIH/3T3 cells, HEK293 cells, Lenti-X 293T cells and PlatE cells were cultured in DMEM media; K562 cells, MOLM13 cells, U937 cells were culture in RPMI media; All media contained 10% FBS, 2 mM L-glutamine and 100 IU/ml penicillin/streptomycin. Cell stocks for liquid nitrogen storage were frozen in FBS containing 10% DMSO. All cells were grown at 37 °C with 5% CO2. All cell lines were tested negative for mycoplasma contamination on a regular basis. RAFi/MEKi-resistant cell lines isolated from mice were grown in 100 nM dabrafenib (RAFi) and 30 nM trametinib (MEKi). To generate dabrafenib-resistant melanoma lines, cells were exposed to 1 μM dabrafenib. After 4 - 6 weeks of continuous exposure, resistant cell lines were derived and cultured continuously on 1 μM dabrafenib. Doxycycline-inducible systems34 were activated by addition of 10 μg/ml doxycycline to the medium.
Lipofection
Transfections were performed on 6-well-plates with cells being 70-90% confluent in 2 ml complete growth medium without antibiotics. 4 μg of plasmid DNA was added to 250 μl Opti-MEM reduced serum media (Thermo Fisher Scientific) and mixed gently. 10 μl Lipofectamine 2000 transfection reagent (Thermo Fisher Scientific) was mixed with 250 μl Opti-MEM reduced serum media (Thermo Fisher Scientific) and incubated for 5 min at RT. Both mixes were then combined and left at RT for 20 min, before directly adding it to the cells.
In vitro resistance selection screen
In 6 replicates, 1x 106 YUMM 1.7 cells were seeded onto 15 cm tissue culture dishes and immediately treated with 100 nM dabrafenib and 30 nM trametinib or DMSO. Medium with fresh drug was exchanged every 3-4 days and after 6 days, drug-treated plates reached confluency. Cells were then trypsinized, and 1 x106 cells were reseeded for continuous drug treatment while the rest was taken for NGS. After 4 weeks all reseeded plates harbored slow growing colonies and were harvested for NGS. DMSO treated controls were split once, 1 x106 cells were reseeded and harvested for NGS once they reached confluency in order to have matched passage numbers and conditions to treated samples.
Plasmids and lentivirus generation
For lentivirus production, LentiX 293T cells were transfected on a 10 cm dish with 4 μg of the plasmid of interest, 2 μg Pax2 and 1 μg VSVG plasmid using 21 μg polyethylenimine (PEI), M.W. 25000, linear (Alfa Aesar). Virus was collected 48 h, 64 h and 72 h post transfection in DMEM containing 1% FBS, frozen and diluted for infection. The following lentiviral constructs were used: pRRL_hU6_mU6_EF1as_Thy1.1, pRRL_hU6_mU6_EF1as_Thy1.1_P2A_Neo, pRRL_hU6_EF1as_Thy1.1_P2A_Neo, pRRL_hU6_PGK_GFP_P2A_Neo, pRRL_PGK_dCas9_VP64_T2A_mCherry, pRRL_PGK_dCas9_VPR_P2A_mCherry, pRRL_EF1as_dCas9_VPR_P2A_mCherry, pRRL_P7_SBS12Seq_BC_reporter_mCMV_eGFP_EF1as_eBFP2, pRRL_P7_BC_reporter_mCMV_eGFP_EF1as_eBFP2, pRRL_SFFV_BLAS_IRES_iRFP720, pRRL_TRE3G_GFP_miRE_PGK_Neo, pGIPZ_SFFV_rtTA3_IRES_Ametrine Relevant plasmids have been deposited at Addgene. For overexpression of NrasG12D an eco-packaging system was used, and PlatE cells were infected with 20 μg plasmid DNA (pMSCV_iRFP720_IRES_NrasG12D) and 5 μg helper DNA (Gag-Pol helper plasmid) using CaCl2. Virus was harvested 48h, 64h and 72h post transfection. For infection of target cells with lentivirus and retrovirus, polybrene was added at a concentration of 8 μg/ml.
Mouse models
All experiments using animals were performed in accordance with our protocol approved by the Austrian Ministry (BMBWF-66.015/0011-V/3b/2018 or GZ: 340118/2017/25). For injections of the YUMM1.7 cells (BrafV600E/WTPTEN-/-CDKN2a-/- melanoma) 6-8-week-old male or female C57BL/6 mice were used as an immunocompetent model. All of these mice were received from the in-house breeding facility and housed under standard pathogen-free conditions.
Animal studies
For subcutaneous tumour cell injections, mice were anesthetized using ketaminhydrochlorid (100 mg/kg), xylazin (10 mg/kg) and acepromazin (3 mg/kg) or isoflurane and tumour cells were subcutaneously injected into the shaved flank in 50 μl growth factor reduced Matrigel/PBS (1:1) (Szabo Scandic). For in vivo resistance generation, mice were subcutaneously injected with 1 x106 melanoma cells and treatment was initiated at ~200 mm3. For experiments using clonal cell lines 1 x105 cells were injected. For RAFi/MEKi treatment, mice received a combination of 5 mg/kg dabrafenib and 0.15 mg/kg trametinib daily via oral gavage. Tumour size monitoring was performed using caliper measurement and calculated using the following formula volume = (D x d2)/2, in which D and d refer to the long and short tumour diameter, respectively.
Flow cytometry
For flow cytometry of whole tumours, tumours were dissected, cut into small pieces and dissociated for 1.5 hours using Collagenase A (1 mg/ml) in PBS. Single cell suspensions were strained through a 70 μm nylon mesh, washed in FACS buffer (0.5% BSA, 2 mM EDTA) and incubated for 10 min on 4 °C with anti-mouse Fc-Block CD16/CD32 antibody (clone 2.4G2, BD Pharmingen, 1:10). Cells were subsequently stained with a live-dead stain (Fixable viability dye eFluor 780, eBioscience, 1:1000) and a pan mouse immune-cell antibody CD45 PECy7 (clone 30-F11, BD Pharmingen, 1:500) in FACS buffer for 30 min at 4 °C to distinguish cancer cells from immune cells. The injected CaTCH-isolated TN/R cell pairs were further identified by their GFP expression signal. For flow cytometry of cultured cells, cells were detached using 0.05% Trypsin, inhibited with full medium and stained in FACS buffer (0.5% BSA, 2mM EDTA). Thy1.1/CD90.1 was detected using the following antibodies mouse CD90.1 AF700 (clone OX-7, 1:400 BioLegend) or mouse CD90.1 PE-Cy7 (clone OX-7, 1:400 BD Pharmingen). Cells were washed in PBS and resuspended in FACS buffer (0.5% BSA, 2mM EDTA). Data was acquired using a BD Fortessa and analysed using FlowJo 10.4.
Immunoblotting
RIPA buffer containing protease and phosphatase inhibitors (CST, Thermo Fisher) was used according to manufacturer’s instructions. Protein concentrations were determined using BCA Protein Assay Kit (Pierce). Proteins were separated using a 4 - 12% gradient Bis-Tris polyacrylamide gel in the MOPS buffer system (Invitrogen) and transferred onto nitrocellulose membranes (Bio-Rad) using standard protocols. Membranes were blocked with 5% milk in TBS-T and incubated with antibodies against pERK (clone D13.14.4E, CST, 1:2000), tERK (clone L34F12, CST, 1:2000), pAKT (clone D9E, CST, 1:2000), tAKT (clone 40D4, CST, 1:2000) and Histone H3 (polyclonal, abcam, 1:1000). After primary antibody incubation and washing, membranes were probed with HRP-coupled secondary antibodies and developed using the ECL system (GE Healthcare).
Gene expression analysis
Whole RNA was isolated from cells using a magnetic bead-based purification kit (in-house). Quantseq libraries were generated using the Lexogen Quantseq 3’ mRNA seq kit using 250 ng input RNA, according to manufacturer’s instructions. Sequencing was performed in-house on Illumina HiSeqV4 to obtain 50 bp single-end sequencing reads.
Whole exome sequencing
Genomic DNA was isolated from frozen cell pellets using the DNeasy Blood & Tissue kit (Qiagen). Library preparation and sequencing was performed by Macrogen, Inc. using the Agilent SureSelect Murine All Exon Library and NovaSeq 6000 with 2 x 150 bp and 100x coverage (= 50x on-target coverage).
Bioinformatic analysis of gene expression data
Analysis of QuantSeq data was performed with an in-house established pipeline: Adapter and polyA sequences were clipped with bbduk (v38.06) (https://sourceforge.net/projects/bbmap/). Abundant sequences (iGenomes GRCm38) are removed with bbmap (v38.06). Cleaned reads were aligned against the genome (GRCm38, primary_assembly) with STAR (v2.6.0c)35. Reads were counted towards their corresponding gene (Ensembl 94) with featureCounts (v1.6.2)36. Differentially expressed genes were detected with DESeq2 (v1.18.1)37. Coverage tracks are created with deepTools (normalized BPM; v3.0.2)38. GO-terms were extracted, and data was visualized using pcaExplorer39 and pheatmap (package version 1.0.12). Upstream mediator analysis was performed using the Ingenuity Pathway Analysis software (QIAGEN Inc., https://www.qiagenbioinformatics.com/products/ingenuitypathway-analysis)40. Additional heatmaps were generated using Heatmapper41. Venn diagrams were generated using Venny 2.1.
Bioinformatic analysis of exome sequencing data
Analysis of exome sequencing data was performed using the bcbio (https://github.com/bcbio/bcbio-nextgen) community resource using the following tools: alignment with bwa42, SNV variant calling with an ensemble of samtools43 and varscan44 retaining only variants passing the filtering in both tools. Common SNVs in all samples were determined using vcftools45 and excluded from all further analysis. Low-complexity regions (LCRs) were called analogous to Li H., Biofinformatics 201446 (10.1093/bioinformatics/btu356) on the mouse genome using SeqClean mdust (ftp://occams.dfci.harvard.edu/pub/bio/tgi/software/seqclean/) and all SNVs in LCRs were excluded from all further analysis. Variants were annotated using Annovar47 and the final annotated variant set was analysed and visualized using maftools48. The mouse oncoKB list was created based on the OncoKB49 human cancer gene list (downloaded September 26, 2019) and mapping mouse orthologs using the biomaRt R package50.
Alternative reporter activation approach16
sgRNAs were expressed from the pRRL_hU6_EF1as_Thy1.1_P2A_Neo construct. A375 cells were transduced with either single sgRNA or a sgRNA library, both at a low infection rate using lentivirus. SgRNA-positive cells were selected using G418 (Gibco) and the completed selection was confirmed by FACS staining for the Thy1.1 surface marker. To label cells with RFP pRRL_SFFV_BLAS_IRES_iRFP720 was stably transduced and cells were FACS sorted twice and cultured on blasticidin. The sgRNA-inducible reporter plasmid contained a mCMV-driven GFP downstream of 3 identical, equally spaced sgRNA binding sites within a pcDNA backbone. For reporter activation, this construct was then co-transfected with pRRL_PGK_dCas9_VPR_P2A_mCherry at a molar ratio of 3:1 using Lipofectamine 2000 (Thermo Fisher Scientific) and GFP signals were measured by FACS 48h after transfection.
Detailed CaTCH cell line generation
The cell line of interest was first infected large scale with pRRL_EF1as_dCas9_VPR_P2A_mCherry or pRRL_PGK_dCas9_VPR_P2A_mCherry and then FACS sorted up to two times for mCherry expression. Hereby, large cell numbers were sorted to maintain clonal representation and heterogeneity within the population. Then a defined number of these dCas9-VPR expressing cells was infected with the CaTCH barcoding library (pRRL_P7_SBS12Seq_BC_reporter_mCMV_eGFP_EF1as_eBFP2) at an infection rate below 20% to obtain mostly single integrations. This cell line was expanded and then FACS sorted for the library marker eBFP2 as well as a low eGFP signal (ca. 10 - 20% of parental) (Extended Data Fig. 10) to ensure integration into open chromatin, which will allow efficient reporter activation later on. Minimal CMV promoter function is well known to be influenced by positional effects51, including this sorting gate reduces the number of cells with no reporter activation. To further restrict the barcode complexity of the cell line to a defined maximum, we sorted a distinct number of events. This cell line was then expanded to just have enough cells for the downstream experiment, references for initial barcode complexities and to freeze cell stocks for liquid nitrogen storage.
Detailed CaTCH clonal cell line isolation
To isolate a defined cell clone from a heterogeneous CaTCH cell line, its CaTCH reporter has to be activated by transducing the whole population with a single, barcode-specific dual-sgRNA expression construct (i.e. pRRL_hU6_mU6_EF1as_Thy1.1). Therefore, the cell line was infected with the sgRNA on a 6-well format in several replicates to ensure a certain representation of the targeted clone. We aimed for high infection rates (50-70%) to increase the chance of activating the reporters of lowly abundant barcodes while still keeping toxicities low. Infection rates can be reduced if necessary, but cell numbers might need to be increased in return. These cells were expanded and FACS sorted for a rare GFP-high population, directly into a 96-well. The sorting gate was set by comparison with a non-activating sgRNA infected control cell line. The sorted cell line was checked for high GFP signal with a fluorescent microscope and expanded. When necessary, the cell line was sorted for GFP a 2nd time, to remove non-specifically sorted background cells. The success of the isolation procedure was assessed by extracting genomic DNA from the sorted cell lines and sequencing of the barcode cassette by NGS. In principle, other sgRNA delivery approaches are also compatible with CaTCH, such as non-integrating viral tools (e.g., integration-deficient lentiviruses and retroviruses, AAVs) or non-viral sgRNA delivery. For example, in vitro transcribed sgRNAs allow highly efficient delivery and could be directly delivered as a pre-assembled RNP complex with dCas9-VPR protein.
Extended Data
Extended Data Fig. 1. Development and optimization of the CaTCH reporter.
a, Functional comparison of dCas9-VPR to dCas9-VP64 using a doxycycline inducible reporter system with sgRNAs targeting either one (-53 bp or -203 distance to TSS), two, or seven target sites (tetO sites). Doxycycline induced activation via rtTA3 was used as a positive control. Reporter activation was measured by FACS. Left: Corresponding FACS histograms of GFP activation (normalized to mode); Right: Quantification of percent activated of parent (upper panel) and of signal strength in mean fluorescent intensity (MFI, lower panel), n=3 biologically independent samples, for CTRL n=2, bar graphs display the mean ± standard deviation (SD). b, Evaluating the optimal sgRNA positioning using two reporter constructs (D1 and D2). Left: FACS histograms of GFP activation with the individual sgRNAs in D1 or D2, ordered by sgRNA-distance to the TSS (-58 bp, -82 bp, -106 bp, -130 bp, -154 bp, -202 bp, -250 bp, -298 bp); Right: Quantification of percent activated and MFI; n=2 biologically independent samples, bar graphs display the mean. c, Determining the effect of multiple sgRNA target sites and their spacing on GFP activation by comparing a reporter construct with spaced BCs (R1) with a reporter construct with BCs side by side (R2). Left: FACS histograms of GFP activation; Right: Quantification of percent activated and MFI, n=2 biologically independent samples, bar graphs display the mean.
Extended Data Fig. 2. Design and Assessment of the CaTCH library.
a, Design of the CaTCH barcode cassette with 3 independent sgRNA target sites. Each barcode sequence is semi-random with defined base restrictions. b, CaTCH reporter activation by targeting sgRNA-sites individually or simultaneously. Left: FACS histograms of GFP activation; Right: Quantification of percent activated and MFI, n=3 biologically independent samples, n=1 for CTRL. All bar graphs display the mean ± standard deviation (SD). c, Complexity of the CaTCH library plasmid pool determined by NGS (~237 million reads). Left Y-axis: Barcode distribution in the library, shown in bars. Right Y-axis: Relative, cumulative barcode representation, shown as a red line. X-axis: Frequency of unique BC. The bar graph on the right shows the sum of all identified unique barcodes resulting in ~130 million unique barcodes detected in the library.
Extended Data Fig. 3. Order of construct delivery and delivery method influence the resolution of reporter activation.
a, Sensitivity of CaTCH reporter activation (full dataset of spike-in experiment from Fig. 1c-e). FACS plots on the top show the GFP signal only, while plots at the bottom additionally visualize the spiked in iRFP+ cell populations. The experiment was repeated independently 3 times with similar results and 6 times for the 0.001% spike in. b, Experimental outline of two different reporter activation strategies. CaTCH approach: After the (1) dCas9-VPR construct, the (2) BC-controlled GFP-reporter is stably introduced into the cells. Reporter activation is achieved by lentiviral (3) sgRNA transduction. Alternative approach: (1) First, a sgRNA is stably expressed and functions as an identifier in the cells. (2) Subsequently, an sgRNA-specific GFP-reporter plasmid and a separate dCas9-VPR construct are simultaneously, transiently co-transfected for reporter activation. c, FACS data of GFP-reporter activation of both approaches in a spike-in experiment similar to Fig. 1c. iRFP+ spiked-in cells are visualized in red. Gates were set to obtain 0 unspecific events in the 0% spike-in control after delivery of sgRNA/reporter plasmid and dCas9-VPR. Reporter activation was measured by GFP signal. Every experimental condition was performed in triplicates, except 0.001% spike-ins, which were performed in six replicates. d, Quantification of the GFP-reporter activation. Correctly activated = percent of iRFP+ (spike-in) of GFP+ (reporterpositive) events. Activation efficiency = percent of GFP+ within iRFP+ events. Data displayed as mean ± standard error of mean (SEM). n=3 biologically independent samples, n=6 for 0.001% spike-in. e, Delivery rates of a plasmid constitutively expressing GFP in several cell lines with lipofection or lentiviral delivery. Data measured by FACS, n=2 biologically independent samples. Bar graph displays the mean.
Extended Data Fig. 4. Tracing and isolation of pre-existing therapy resistant cell clones in vitro.
a, FACS plots corresponding to the quantification of NrasG12D spike in experiment of Fig. 1g. b, Experimental outline of CaTCH reporter activation and isolation displayed in Fig. 1h. c, FACS analysis of CaTCH isolated cells from Fig. 1h after expansion. Most GFP+ cells are also iRFP+, indicating isolation of the correct clone. The experiment was performed once. d, Immunoblot of MAPK-pathway (pERK) after short term RAFi-treatment in vitro of the bulk cell line, RAFi-selected NrasG12D cells, and treatment-naïve CaTCH-isolated NrasG12D cells. Quantification of pERK normalized to total protein levels is indicated by the numbers. The experiment was performed independently twice with similar results. e, NGS indicating the proportion of reads for NrasWT and NrasG12D, n=3 biologically independent samples for vehicle- and RAFi-treated samples, n=1 for others.
Extended Data Fig. 5. CaTCH reveals a strong clonal selection of RAFi/MEKi-treated melanoma cells in vivo.
a, Respective survival curves of resistance generation experiment in vivo from Fig. 2b. Statistical analysis was performed by two-sided Log-rank (Mantel-Cox) test, comparing RAFi-MEKi-treated to untreated. **P<0.01, ns=non-significant. (Untreated=4 tumors isolated from 4 mice; RAFi/MEKi=5 tumors isolated from 5 mice). b, Violin plot showing the distribution of identified BCs within samples. REF=starting cell line, T1-T9=individual tumors, one tumor per mouse; median: line, middle 50% of data: box, 1.5*IQR: whiskers; individually depicted points indicate outliers; the exact number of barcodes per individual tumor can be derived from Fig. 2c. c, Pearson correlation matrix of all identified BCs, n=58269 unique BCs. d, Heatmap showing normalized read-counts of selected BCs in the indicated samples.
Extended Data Fig. 6. CaTCH reveals a modest clonal selection of RAFi/MEKi-treated melanoma cells in vitro.
a, Experimental outline. The same population of CaTCH barcoded melanoma cells used in the in vivo experiment from Fig. 2 was seeded for RAFi/MEKi-treatment in vitro. 6 replicates were used per condition. RAFi/MEKi treated cells were sampled after 1 week and 5 weeks on treatment. DMSO treated samples were sampled after passaging them once. b, Total number of unique barcodes identified by NGS in the respective samples. REF1-2, two replicates of the starting cell line; DMSO R1-6, 6 DMSO treated replicates; RAFi/MEKi R1-6, 6 RAFi/MEKi treated replicates sampled after 1 or 5 weeks on treatment. c, Violin plot showing the distribution of identified BCs within samples from Extended Data Fig. 6b; median:line, middle 50% of data: box, 1.5*IQR: whiskers; the exact number of barcodes per individual sample can be derived from Extended Data Fig. 6b. d, Pearson correlation matrix of identified BCs, n=45928 unique BCs. e, BC composition identified by NGS. BCs comprising more than 1% of the sample are highlighted individually in color, BCs accounting for < 1% barcode proportion were summed up (white bars). Same colors indicate same BCs. Grey sections are different BCs.
Extended Data Fig. 7. CaTCH isolation of specific clones allows their phenotypic characterization.
a, NGS of CaTCH isolated clones identified in the in vivo experiment (see Fig. 2d). Shown is the BC composition after CaTCH isolation of the indicated BC from the starting population (treatment-naïve and depleted, Fig. 2e) or from tumor-derived RAFi/MEKi-resistant cell lines. BCs > 1% of the sample are highlighted individually in color, BCs < 1% were summed up in white bars. The correct, targeted BC within each sample is shown at the bottom section of every bar, in its respective color code from Fig. 2d, e. b, BC composition of different isolations of BC14559 or BC43158. Both BCs were always identified together, indicated in the respective BC color. The BC which was targeted for isolation is indicated below each bar. c, Tumor growth curves and survival curves of in vivo RAFi/MEKi treatment response of three randomly single cell sorted CaTCH-barcoded clones (C1, C2, C3). Sorted clones were transduced with a barcode-unspecific CaTCH reporter activating sgRNA and sorted for activated GFP signal before injection. Growth curves displayed as mean ± standard error of mean (SEM). Statistical analysis on survival curves was performed by two-sided Log-rank (Mantel-Cox) test, comparing individual clones on treatment to untreated. *P<0.05, ns=non-significant. n=tumors, two tumors per mouse, C1: UT n=6, RAFi/MEKi n=8; C2: UT n=6, RAFi/MEKi n=8; C3: UT n=4, RAFi/MEKi n=8). d, Survival curves corresponding to the tumor growth curves from Fig. 2f. Statistical analysis was performed by two-sided Log-rank (Mantel-Cox) test, comparing individual clones on treatment to untreated. *P<0.05, **P<0.01, ns=non-significant. n=mice. BULK: TNUT n=3, TNRAFi/MEKi n=5; BC121: TNUT n=3, RUT n=3, TNRAFi/MEKi n=5, RRAFi/MEKi n=5; BC952: TNUT n=3, RUT n=3, TNRAFi/MEKi n=4, RRAFi/MEKi n=5; BC2487: TNUT n=3, RUT n=3, TNRAFi/MEKi n=4, RRAFi/MEKi n=5; BC13: TNUT n=3, TNRAFi/MEKi n=4; BC2721: TNUT n=3, TNRAFi/MEKi n=5; BC2646: TNUT n=3, RUT n=2, TNRAFi/MEKi n=5, RRAFi/MEKi n=3; BC14559: TNUT n=3, RUT n=3, TNRAFi/MEKi n=3, RRAFi/MEKi n=5.
Extended Data Fig. 8. CaTCH-isolated clone with reduced response shows pre-existing transcriptional features of RAFi/MEKi-resistance in vivo.
a, Experimental outline and individual tumor growth curves of injected cell lines in vivo, aligned to treatment start. Tumors were harvested at day 0 (untreated) or at day 3 of RAFi/MEKi treatment (on RAFi/MEKi therapy). b, PCA plots of RNAseq analysis. Three tumors were sequenced per cell line and condition. This experiment was performed once. c, Heatmap of top 500 high-variance genes with three main gene clusters indicated on the right: Top GO term per cluster: Cluster 1 – Cellular response to interferon beta, Cluster 2 – negative regulation of cell proliferation, Cluster 3 – Cell division; see Supplementary Table 1 for extended lists. n=3 biologically independent samples (tumors) per cell line and condition; GO-terms and statistics (Fisher’s exact test) were derived in R using topGOtable (pcaExplorer). d, Heatmap showing differentially expressed genes (deviation from the mean > 1 or < -1) within GO term ‘cell division’ (GO:0035458); see Supplementary Table 2 for gene list. e, Venn diagram showing overlap of differentially regulated genes (on RAFi/MEKi therapy vs. untreated) within treatment-naïve clones, DESeq2, threshold of Log2FC ± 1; see Supplementary Table 3 for full gene lists. f, Top 50 mediators identified in Ingenuity upstream mediator analysis of RAFi/MEKi-treated tumors, compared to BC13, only selected mediators are displayed; see Supplementary Table 4 for full list. g, Heatmap showing normalized read counts of indicated genes across samples on RAFi/MEKi therapy; values for each tumor shown individually; see Supplementary Table 5 for full list.
Extended Data Fig. 9. Whole exome sequencing identifies de novo mutations in RAFi/MEKi-resistant clones.
a, Total number of detected variants per sample. b, Summary of variant classification visualized as box plots; n=5 (5 individual cell clones); box displays median with 25th and 75th quartile, whiskers correspond to minimum and maximum values; values correspond to the data in Supplementary Fig 9a. c, Overview of SNV classes. d, Read tracks of the KRAS locus at codon 12 for clones BC14559TN and BC14559R. The KRASG12R mutation was exclusively identified in BC14559R.
Extended Data Fig. 10. Gating strategy for FACS sorting of CaTCH library transduced cells.
Representative gating strategy for sorting of CaTCH library infected cells. The cells were previously infected with and sorted for dCas9-VPR (mCherry) and then infected with the CaTCH library (BFP). BFP-positive cells were sorted for low GFP (CaTCH reporter) expression, which enhances reporter functionality. Each plot shows the subpopulation gated for in the preceding plot to the left.
Supplementary Material
Reporting Summary.
Further information on research design is available in the Nature Research Reporting Summary linked to this article.
Acknowledgements
We thank all members of the Obenauf and Zuber lab for experimental support and discussions, in particular M. Roth, M. Muhar, I. Barbosa, V. Pinamonti, and I. Krecioch. We thank the Stark lab, especially C. Neumayr and M. Pagani for sharing of reagents and cell lines. We further thank M. Bosenberg for providing YUMM 1.7 mouse melanoma cells. This work was funded by the Starting Grants of the European Research Council (ERC-StG-759590 to A.C.O. and ERC-StG-336860 to J.Z.), the Vienna Science and Technology fund (#LS16-063 to A.C.O. and T.W.), and the Austrian Science Fund (SFB-F4710; to J.Z.). Research at the IMP is generously supported by Boehringer Ingelheim.
Footnotes
Author Contributions
C.U. and A.C.O. conceived the study, designed the experiments, interpreted the results and wrote the manuscript. A.C.O. supervised the study. C.U. developed experimental tools and the CaTCH library; performed most in vitro experiments and parts of the in vivo treatment studies; all flow cytometry analysis; data analysis and parts of the computational analysis. F.H. performed in vivo work and experimental work for QuantSeq library preparation as well as computational analysis of the data (Extended Data Fig. 8b, c, f), statistical analysis (Extended Data Fig. 5a; Extended Data Fig. 7c,d) and provided conceptual and experimental input. L.F. performed all in vivo treatment studies of isolated CaTCH clones (Fig. 2f; Extended Data Fig. 7c; Extended Data Fig. 8a) and the in vitro experiment and western blot in Extended Data Fig. 4d. J.J. provided conceptual input on library design, library cloning strategy, plasmids and technical input. K.F. provided computational analysis of CaTCH barcoding data (Fig. 2c, e; Extended Data Fig. 5b-d; Extended Data Fig. 6b-e). T.N. provided computational analysis of CaTCH barcoding and WES data (Extended Data Fig. 9) and Nras mutation analysis (Extended Data Fig. 4e). S.C. performed in vivo work and library preparations. L.H. established the in vivo model systems and provided conceptual and experimental input. J.J.L. analysed the CaTCH plasmid library NGS data and generated Extended Data Fig. 2b. T.R.B. provided the mRNA sequencing analysis pipeline and mRNA sequencing analysis as well as help with data analysis. M.F. provided the cloning-protocol, expertise and helped with CaTCH library cloning. T.W. and J.Z. provided experimental and technical input and co-wrote the manuscript. All authors read and approved the manuscript.
Ethical Regulation
We have complied with all relevant ethical regulations. All experiments using animals were performed in accordance with our protocol approved by the Austrian Ministry (BMBWF-66.015/0009-V/3b/2019 or GZ: 340118/2017/25).
Conflict of Interests
The authors declare no conflict of interest.
Data availability
All datasets generated during the current study are available from the corresponding authors upon reasonable request. RNAseq data have been deposited at the Gene Expression Omnibus (GEO) under accession number GSE139236.
References
- 1.Reya T, Morrison SJ, Clarke MF, Weissman IL. Stem cells, cancer, and cancer stem cells. Nature. 2001;414:105–111. doi: 10.1038/35102167. [DOI] [PubMed] [Google Scholar]
- 2.Mojtahedi M, et al. Cell Fate Decision as High-Dimensional Critical State Transition. PLOS Biol. 2016;14:e2000640. doi: 10.1371/journal.pbio.2000640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Simons BD, Clevers H. Strategies for Homeostatic Stem Cell Self-Renewal in Adult Tissues. Cell. 2011;145:851–862. doi: 10.1016/j.cell.2011.05.033. [DOI] [PubMed] [Google Scholar]
- 4.Shakiba N, et al. Cell competition during reprogramming gives rise to dominant clones. Science (80-) 2019;364:eaan0925. doi: 10.1126/science.aan0925. [DOI] [PubMed] [Google Scholar]
- 5.Biddy BA, et al. Single-cell mapping of lineage and identity in direct reprogramming. Nature. 2018;564:219–224. doi: 10.1038/s41586-018-0744-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yamanaka S. Elite and stochastic models for induced pluripotent stem cell generation. Nature. 2009;460:49–52. doi: 10.1038/nature08180. [DOI] [PubMed] [Google Scholar]
- 7.Tabassum DP, Polyak K. Tumorigenesis: it takes a village. Nat Rev Cancer. 2015;15:473–483. doi: 10.1038/nrc3971. [DOI] [PubMed] [Google Scholar]
- 8.Ramos P, Bentires-Alj M. Mechanism-based cancer therapy: resistance to therapy, therapy for resistance. Oncogene. 2015;34:3617–3626. doi: 10.1038/onc.2014.314. [DOI] [PubMed] [Google Scholar]
- 9.McGranahan N, Swanton C. Biological and Therapeutic Impact of Intratumor Heterogeneity in Cancer Evolution. Cancer Cell. 2015;27:15–26. doi: 10.1016/j.ccell.2014.12.001. [DOI] [PubMed] [Google Scholar]
- 10.Tirosh I, et al. Dissecting the multicellular ecosystem of metastatic melanoma by single-cell RNA-seq. Science (80-) 2016;352:189–196. doi: 10.1126/science.aad0501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kebschull JM, Zador AM. Cellular barcoding: lineage tracing, screening and beyond. Nat Methods. 2018;15:871–879. doi: 10.1038/s41592-018-0185-x. [DOI] [PubMed] [Google Scholar]
- 12.Bhang HC, et al. Studying clonal dynamics in response to cancer therapy using high-complexity barcoding. Nat Med. 2015;21:440–448. doi: 10.1038/nm.3841. [DOI] [PubMed] [Google Scholar]
- 13.Hata AN, et al. Tumor cells can follow distinct evolutionary paths to become resistant to epidermal growth factor receptor inhibition. Nat Med. 2016;22:262–269. doi: 10.1038/nm.4040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chavez A, et al. Comparison of Cas9 activators in multiple species. Nat Methods. 2016;13:563–567. doi: 10.1038/nmeth.3871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gilbert LA, et al. Genome-Scale CRISPR-Mediated Control of Gene Repression and Activation. Cell. 2014;159:647–661. doi: 10.1016/j.cell.2014.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Al’Khafaji AM, Deatherage D, Brock A. Control of Lineage-Specific Gene Expression by Functionalized gRNA Barcodes. ACS Synth Biol. 2018;7:2468–2474. doi: 10.1021/acssynbio.8b00105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Meeth K, Wang JX, Micevic G, Damsky W, Bosenberg MW. The YUMM lines: a series of congenic mouse melanoma cell lines with defined genetic alterations. Pigment Cell Melanoma Res. 2016;29:590–597. doi: 10.1111/pcmr.12498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shi H, et al. Acquired Resistance and Clonal Evolution in Melanoma during BRAF Inhibitor Therapy. Cancer Discov. 2014;4:80–93. doi: 10.1158/2159-8290.CD-13-0642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sharma SV, et al. A Chromatin-Mediated Reversible Drug-Tolerant State in Cancer Cell Subpopulations. Cell. 2010;141:69–80. doi: 10.1016/j.cell.2010.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ravindran Menon D, et al. A stress-induced early innate response causes multidrug tolerance in melanoma. Oncogene. 2015;34:4448–4459. doi: 10.1038/onc.2014.372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Trumpp A, Wiestler OD. Mechanisms of Disease: cancer stem cells—targeting the evil twin. Nat Clin Pract Oncol. 2008;5:337–347. doi: 10.1038/ncponc1110. [DOI] [PubMed] [Google Scholar]
- 22.Friedman R. Drug resistance in cancer: molecular evolution and compensatory proliferation. Oncotarget. 2016;7:11746–11755. doi: 10.18632/oncotarget.7459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hobbs GA, et al. Atypical KRAS G12R Mutant Is Impaired in PI3K Signaling and Macropinocytosis in Pancreatic Cancer. Cancer Discov. 2020;10:104–123. doi: 10.1158/2159-8290.CD-19-1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zafra MP, et al. An in vivo KRAS allelic series reveals distinct phenotypes of common oncogenic variants. bioRxiv. 2019;14:847509. doi: 10.1158/2159-8290.CD-20-0442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rebbeck C, et al. SmartCodes: functionalized barcodes that enable targeted retrieval of clonal lineages from a heterogeneous population. bioRxiv. 2018;376:2109–2117. [Google Scholar]
- 26.Akimov Y, Bulanova D, Abyzova M, Wennerberg K, Aittokallio T. DNA barcode-guided lentiviral CRISPRa tool to trace and isolate individual clonal lineages in heterogeneous cancer cell populations. bioRxiv. 2019;2:218–263. [Google Scholar]
- 27.Rambow F, et al. Toward Minimal Residual Disease-Directed Therapy in Melanoma. Cell. 2018;174:843–855.:e19. doi: 10.1016/j.cell.2018.06.025. [DOI] [PubMed] [Google Scholar]
- 28.Shaffer SM, et al. Rare cell variability and drug-induced reprogramming as a mode of cancer drug resistance. Nature. 2017;546:431–435. doi: 10.1038/nature22794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Greaves M, Maley CC. Clonal evolution in cancer. Nature. 2012;481:306–313. doi: 10.1038/nature10762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Calderwood SK. Tumor heterogeneity, clonal evolution, and therapy resistance: an opportunity for multitargeting therapy. Discov Med. 2013;15:188–94. [PMC free article] [PubMed] [Google Scholar]
- 31.Smith MP, et al. Inhibiting Drivers of Non-mutational Drug Tolerance Is a Salvage Strategy for Targeted Melanoma Therapy. Cancer Cell. 2016;29:270–284. doi: 10.1016/j.ccell.2016.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cock PJA, et al. Biopython: freely available Python tools for computational molecular biology and bioinformatics. Bioinformatics. 2009;25:1422–1423. doi: 10.1093/bioinformatics/btp163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bodenhofer U, Kothmeier A, Hochreiter S. APCluster: an R package for affinity propagation clustering. Bioinformatics. 2011;27:2463–2464. doi: 10.1093/bioinformatics/btr406. [DOI] [PubMed] [Google Scholar]
- 34.Loew R, Heinz N, Hampf M, Bujard H, Gossen M. Improved Tet-responsive promoters with minimized background expression. BMC Biotechnol. 2010;10:81. doi: 10.1186/1472-6750-10-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dobin A, et al. STAR: ultrafast universal RNA-seq aligner. Bioinformatics. 2013;29:15–21. doi: 10.1093/bioinformatics/bts635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liao Y, Smyth GK, Shi W. featureCounts: an efficient general purpose program for assigning sequence reads to genomic features. Bioinformatics. 2014;30:923–930. doi: 10.1093/bioinformatics/btt656. [DOI] [PubMed] [Google Scholar]
- 37.Love MI, Huber W, Anders S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014;15:550. doi: 10.1186/s13059-014-0550-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ramírez F, et al. deepTools2: a next generation web server for deep-sequencing data analysis. Nucleic Acids Res. 2016;44:W160–W165. doi: 10.1093/nar/gkw257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Marini F, Binder H. pcaExplorer: an R/Bioconductor package for interacting with RNA-seq principal components. BMC Bioinformatics. 2019;20:331. doi: 10.1186/s12859-019-2879-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Krämer A, Green J, Pollard J, Tugendreich S. Causal analysis approaches in Ingenuity Pathway Analysis. Bioinformatics. 2014;30:523–530. doi: 10.1093/bioinformatics/btt703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Babicki S, et al. Heatmapper: web-enabled heat mapping for all. Nucleic Acids Res. 2016;44:W147–W153. doi: 10.1093/nar/gkw419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Li H, Durbin R. Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics. 2009;25:1754–1760. doi: 10.1093/bioinformatics/btp324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Li H. A statistical framework for SNP calling, mutation discovery, association mapping and population genetical parameter estimation from sequencing data. Bioinformatics. 2011;27:2987–2993. doi: 10.1093/bioinformatics/btr509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Koboldt DC, et al. VarScan 2: Somatic mutation and copy number alteration discovery in cancer by exome sequencing. Genome Res. 2012;22:568–576. doi: 10.1101/gr.129684.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Danecek P, et al. The variant call format and VCFtools. Bioinformatics. 2011;27:2156–2158. doi: 10.1093/bioinformatics/btr330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Li H. Toward better understanding of artifacts in variant calling from high-coverage samples. Bioinformatics. 2014;30:2843–2851. doi: 10.1093/bioinformatics/btu356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wang K, Li M, Hakonarson H. ANNOVAR: functional annotation of genetic variants from high-throughput sequencing data. Nucleic Acids Res. 2010;38:e164. doi: 10.1093/nar/gkq603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mayakonda A, Lin D-C, Assenov Y, Plass C, Koeffler HP. Maftools: efficient and comprehensive analysis of somatic variants in cancer. Genome Res. 2018;28:1747–1756. doi: 10.1101/gr.239244.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chakravarty D, et al. OncoKB: A Precision Oncology Knowledge Base. JCO Precis Oncol. 2017:1–16. doi: 10.1200/PO.17.00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Durinck S, Spellman PT, Birney E, Huber W. Mapping identifiers for the integration of genomic datasets with the R/Bioconductor package biomaRt. Nat Protoc. 2009;4:1184–1191. doi: 10.1038/nprot.2009.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zuber J, et al. Toolkit for evaluating genes required for proliferation and survival using tetracycline-regulated RNAi. Nat Biotechnol. 2011;29:79–83. doi: 10.1038/nbt.1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All datasets generated during the current study are available from the corresponding authors upon reasonable request. RNAseq data have been deposited at the Gene Expression Omnibus (GEO) under accession number GSE139236.