Abstract
Background
Attention deficit/hyperactivity disorder (ADHD), autism spectrum disorder (autism) and schizophrenia are highly heritable neurodevelopmental disorders, affecting the lives of many individuals. It is important to increase our understanding of how the polygenic risk for neurodevelopmental disorders manifests during childhood in boys and girls.
Methods
Polygenic risk scores (PRS) for ADHD, autism and schizophrenia were calculated in a subsample of 15 205 children from the Norwegian Mother, Father and Child Cohort Study (MoBa). Mother-reported traits of repetitive behavior, social communication, language and motor difficulties, hyperactivity and inattention were measured in children at 6 and 18 months, 3, 5 and 8 years. Linear regression models in a multigroup framework were used to investigate associations between the three PRS and dimensional trait measures in MoBa, using sex as a grouping variable.
Results
Before the age of 2, the ADHD PRS was robustly associated with hyperactivity and inattention, with increasing strength up to 8 years, and with language difficulties at age 5 and 8. The autism PRS was robustly associated with language difficulties at 18 months, motor difficulties at 36 months, and hyperactivity and inattention at 8 years. We did not identify robust associations for the schizophrenia PRS. In general, the PRS associations were similar in boys and girls. The association between ADHD PRS and hyperactivity at 18 months was, however, stronger in boys.
Conclusions
Polygenic risk for autism and ADHD in the general population manifests early in childhood and broadly across behavioral measures of neurodevelopmental traits.
Keywords: Polygenic risk score, neurodevelopmental disorders, ADHD, autism, schizophrenia, repetitive behavior, social communication, language and motor difficulties, hyperactivity, inattention, MoBa
Introduction
Neurodevelopmental disorders (NDDs) are characterized by childhood-onset impairments in developmental domains such as language, motor skills, communication and social interaction, behavioral flexibility, and regulation of attention, activity and impulses (APA, 2013; Thapar, Cooper, & Rutter, 2017). Common neurodevelopmental disorders include attention deficit/hyperactivity disorder (ADHD) and autism spectrum disorder (autism) (Thapar et al., 2017). Schizophrenia, although typically diagnosed in adolescence or early adulthood, is commonly associated with developmental difficulties in childhood and therefore by some also considered to be a neurodevelopmental disorder (Riglin et al., 2017).
ADHD, characterized by inattention and/or hyperactivity-impulsivity, is the most common neurodevelopmental disorder in childhood, affecting approximately 4-5% of children worldwide and usually diagnosed around age 6 years (Kessler et al., 2007; Polanczyk, Willcutt, Salum, Kieling, & Rohde, 2014; Visser et al., 2014). Autism, characterized by communication and social interaction impairments along with restricted and repetitive behavior (Grove et al., 2019; Sullivan, Daly, & O’Donovan, 2012), affects about 1.5% of children in high-income countries (Lyall et al., 2017) and is diagnosed at a median age of 4 years (Baio et al., 2018). Schizophrenia, characterized by long-standing delusions and hallucinations, disorganized speech or behavior (Sullivan et al., 2012), affects about 1% of the population in adulthood (Segal, 2010). ADHD and autism are more prevalent in males compared to females (Surén et al., 2012; Willcutt, 2012). For schizophrenia, although the prevalence is similar, males have an earlier onset than females (Li, Ma, Wang, Yang, & Wang, 2016). Mechanisms underlying sex differences are largely unknown, and recognition and diagnosis might be biased toward male manifestations (May, Adesina, McGillivray, & Rinehart, 2019). Although ADHD, autism and schizophrenia are categorically defined for clinical purposes,they have also been viewed as the extremes of continuous trait dimensions found in the general population (Demontis et al., 2019; Robinson et al., 2016; Rössler, 2013). This is supported by analyses finding high genetic correlations of diagnosed ADHD and autism with ADHD and autism symptoms in the general population (Demontis et al., 2019; Robinson et al., 2016; Rössler, 2013).
Neurodevelopmental disorders like ADHD, autism and schizophrenia are all highly heritable (70%−90%) and etiologically complex, involving a large number of potentially overlapping genetic and environmental risk factors (Burmeister, McInnis, & Zöllner, 2008; Faraone & Larsson, 2019; Homberg et al., 2016; Mulligan et al., 2009; Sullivan et al., 2012, 2018). The genetic influence on neurodevelopmental disorders includes both common (present in >1% of the population) and rare variants. Genome-wide association studies (GWAS) indicate that a substantial part of the genetic liability to neurodevelopmental disorders are conferred by common single nucleotide polymorphisms (SNPs) distributed across the genome (Grove et al., 2019; Sullivan et al., 2012). SNP heritability has been estimated to 22% for ADHD, 12% for autism and 45% for schizophrenia (Sullivan et al., 2018). Common SNPs have individually low impact, but together explain a substantial proportion of the risk. Genetic risk for neurodevelopmental disorders might manifest in subclinical symptoms in the general population. Prospective and longitudinal general population cohorts, like the Norwegian Mother, Father and Child Cohort Study (MoBa), represent a unique framework for studying manifestations of genetic risk (Robinson, Shaver, & Wrightsman, 2013).
Only a few studies have used polygenic risk scores (PRS) to examine manifestations of genetic risk for neurodevelopmental disorders in early childhood (Jansen et al., 2018; Martin, Hamshere, Stergiakouli, O’Donovan, & Thapar, 2014; Nivard et al., 2017; Riglin et al., 2017). In the Avon Longitudinal Study of Parents and Children cohort (ALSPAC), PRS for schizophrenia predicted impairments of language and social communication at age 7−9 years (Riglin et al., 2017), and PRS for ADHD were associated with hyperactivity and inattention at 7−9 years (Martin et al., 2014). A lack of measures of neurodevelopmental traits prior to the age of 4 years has been a limitation for multiple studies. Inclusion of earlier measures is needed to increase our understanding of when, during development, genetic risk for various neurodevelopmental disorders is expressed in observable traits. To improve early identification and support, the knowledge gap on developmental influences on the phenotypic manifestations of genetic risk for psychiatric disorders is important to address (Sullivan et al., 2018).
Previous studies have focused on the genetic risk of specific disorders (Burmeister et al., 2008; Faraone & Larsson, 2019; Homberg et al., 2016; Sullivan et al., 2012, 2018) often using diagnostic measures in older children as outcomes. Where trait measures of neurodevelopment have been studied, they have rarely been measured in children younger than 4 years old.
The aim of the current study was to investigate when and how polygenic risk for ADHD, autism and schizophrenia was associated with neurodevelopmental traits (repetitive behavior, social communication difficulties, language and motor difficulties, hyperactivity and inattention) from 6 months up to 8 years, and whether they were associated differently in boys and girls.
Methods
Sample
We used children from MoBa as our sample. MoBa is a longitudinal pregnancy cohort including approximately 114,500 children, their mothers and fathers (Magnus et al., 2006, 2016). Blood samples were collected from the children’s umbilical cord at birth (Paltiel et al., 2014). The genotyping, imputation and quality control are described in Appendix S1, found in the Supporting Information. Genotype data was available for a subsample of 15,205 of the children (48.9% girls), which, is a genetic homogenous sample of unrelated individuals. In MoBa, pregnant women were recruited from 1999 to 2008. The women consented to participation in 40.6% of the pregnancies. Written informed consent was obtained from all participants upon recruitment. The initial data collection was based on a license from the Norwegian Data Protection Agency and approval from The Regional Committee for Medical Research Ethics. The MoBa cohort is currently regulated by the Norwegian Health Registry Act. The current study was approved by the Regional Committees for Medical and Health Research Ethics (2016/ 1702) and has undergone a Data Protection Impact Assessment.
Measures
Mother-reported questionnaire data were available from when children were aged 6 and 18 months, 3, 5 and 8 years. The questionnaires included several dimensional measures of traits related to neurodevelopment. Version 12 of the quality assured MoBa data files was used to conduct the analyses. For each measurement instrument, a mean score of the items was computed, with at least half the items required to be nonmissing. Items were reverse-coded where necessary so that high scores reflected greater symptom load. For more information about the scales used in this study, see Appendix S2.
Social communication, difficulties and repetitive behavior were measured using items from the Ages and Stages Communication scale at 6 months (only social communication) (Richter & Janson, 2007), the Modified Checklist for Autism in Toddlers (M-CHAT) (Baron-Cohen, Allen, & Gillberg, 1992; Robins, Fein, Barton, & Green, 2001) at 18 months and the Social Communication Questionnaire (SCQ) (Rutter, Bailey, & Lord, 2003) at 3 and 8 years. When the children were 5 years old, the mothers reported on a short version (Ronald, Happé, & Plomin, 2008) of the Childhood Autism Spectrum Test (CAST; formerly named Childhood Asperger Syndrome Test) (Scott, Baron-Cohen, Bolton, & Brayne, 2002), which includes items similar to the SCQ and M-CHAT.
Inattention and hyperactivity/impulsivity were assessed using the Diagnostic and Statistical Manual of Mental Disorders (DSM)-oriented ADHD problems scale of the Child Behavior Checklist (CBCL) (Achenbach, Dumenci, & Rescorla, 2001) at 18 months and 3 years, the revised Conners’ Parent Rating Scale (CPRS-R) (Kumar & Steer, 2003) at 5 years, and the full Parent/Teacher Rating Scale for Disruptive Behavior Disorders (RS-DBD) (Silva et al., 2005) at 8 years.
Language difficulties were measured at 18 months, 3 and 5 years using the Ages and Stages Questionnaire (ASQ). The ASQ has been found to be an effective screening tool for developmental difficulties (Richter & Janson, 2007). At 8 years, the Children’s Communication Checklist-2 (CCC-2) (Bishop, 2003) short version was used to identify language difficulties. Motor difficulties were measured at 6 and 18 months, and 3 years using ASQ, and at 5 years using Children’s Development Inventory (CDI) (Ireton, 1992).
Statistical analyses
Polygenic risk scores (PRS) were generated, using PRSice2 (Choi & O’Reilly, 2019), for each child in our analytic sample, based on summary statistics from European samples from the most recent Psychiatric Genomic Consortium (PGC) GWAS for each disorder; ADHD (20,183 cases and 35,191 controls) (Demontis et al., 2019); autism (18,381 cases and 27,969 controls) (Grove et al., 2019); and schizophrenia (36,989 cases and 113,075 controls) (Schizophrenia, 2014). PRS combines the effects of common SNPs observed in large-scale GWAS to capture the cumulative effect of risk alleles in an individual (Wray et al., 2014). All summary statistics from PGC were subject to quality control including filtering for minor allele frequencies (MAF>1%) and INFO threshold >.8. We used PRS built on a range of p value thresholds (<5e−8, < 1e−6, <1e−4, <.001, <.01, <.05, <.1., <.2, <.5. and 1) for inclusion of SNPs with progressively weaker associations with the disorders in the original GWAS, and assessed the consistency of results across thresholds as part of sensitivity checking.
In order to guard against inflated Type I error from overfitting, we performed a principal component (PC) analysis on this set of 10 PRS and used the first PRS-PC as exposure variable in the linear regression models. This was done using the prcomp function in R. The first PC reweighted the variants included to achieve maximum variation over all the 10 PRS thresholds used (Coombes, Ploner, Bergen, & Biernacka, 2020). The lavaan-package (Rosseel, 2012) was used to run linear regression models in R version 3.4.4, testing associations between the PRS-PC for the three neurodevelopmental disorders and dimensional measures of development at all ages. The correlations between PRS-PCs were 0.20 between ADHD and autism (95% CIs: 0.18−0.22); 0.09 between ADHD and schizophrenia (95% CIs: 0.08−0.11); and 0.01 between autism and schizophrenia (95% CIs: 0.00−0.03). Sex differences were investigated by including sex as a grouping variable in a multigroup framework. Each PRS was adjusted for the covariates genotyping batch and 10 top genetic principal components. PRS-PCs and outcome measures were standardized to zero mean and unit variance prior to analyses. All results are on associations between PRS-PC, but the abbreviation PRS will be used in the text.
In order to account for multiple testing, we corrected the critical p value threshold corresponding to an alpha level of 5% for the number of effective tests run (Bonferroni correction). To determine the number of effective tests, we ran a principal component analysis on all 25 outcome measures (Leppert et al., 2019). The number of tests was defined as the number of principal components explaining 80% variance, which was 16. An alpha level of 5% is therefore reflected in a corrected p value of.0031 (.05/16).
Results
Descriptive statistics for the neurodevelopmental trait scales are presented in Table 1. For information not divided by sex, including Cronbach’s alpha for each scale, see Table S1. In our main figures and tables, we present results based on the three PRS-PCs.
Table 1. Descriptive statistics for measures of neurodevelopmental traits at all ages for children with genotype data.
| Age | Variable | N Boys | N Girls | Mean (SD) Boys | Mean (SD) Girls | No of items in scale |
|---|---|---|---|---|---|---|
| 6 months | Social communication | 6,913 | 6,638 | 5.41 (0.77) | 5.38 (0.73) | 5 |
| Motor difficulties | 6,915 | 6,640 | 6.73 (1.23) | 6.73 (1.20) | 6 | |
| 18 months | Repetitive behavior | 5,971 | 5,644 | 6.36 (0.63) | 6.34 (0.60) | 6 |
| Social communication | 5,971 | 5,643 | 15.44 (0.87) | 15.36 (0.77) | 15 | |
| Language difficulties | 5,962 | 5,645 | 4.46 (1.59) | 3.91 (1.32) | 3 | |
| Motor difficulties | 5,971 | 5,646 | 6.64 (1.27) | 6.75 (1.33) | 6 | |
| Hyperactivity | 5,965 | 5,634 | 3.50 (0.94) | 3.42 (0.90) | 2 | |
| Inattention | 5,966 | 5,636 | 3.15 (0.95) | 3.06 (0.92) | 2 | |
| 36 months | Repetitive behavior | 4,818 | 4,622 | 15.96 (2.53) | 15.57 (2.37) | 12 |
| Social communication | 4,829 | 4,627 | 28.35 (1.79) | 28.03 (1.55) | 26 | |
| Language difficulties | 4,839 | 4,632 | 6.69 (1.15) | 6.49 (0.86) | 6 | |
| Motor difficulties | 4,821 | 4,622 | 5.48 (1.42) | 4.80 (1.08) | 4 | |
| Hyperactivity | 4,826 | 4,616 | 6.23 (1.59) | 6.23 (1.62) | 4 | |
| Inattention | 4,829 | 4,617 | 3.20 (0.97) | 3.15 (0.94) | 2 | |
| 5 years | Repetitive behavior | 1,603 | 1,508 | 5.47 (0.72) | 5.36 (0.61) | 5 |
| Social communication | 1,604 | 1,510 | 11.71 (0.97) | 11.57 (0.81) | 11 | |
| Language difficulties | 3,735 | 3,579 | 6.73 (1.20) | 6.61 (1.02) | 6 | |
| Motor difficulties | 3,743 | 3,585 | 13.36 (1.74) | 12.55 (1.10) | 12 | |
| Hyperactivity | 3,741 | 3,578 | 4.24 (1.39) | 3.99 (1.23) | 3 | |
| Inattention | 3,744 | 3,585 | 12.60 (3.67) | 11.80 (3.10) | 9 | |
| 8 years | Repetitive behavior | 3,789 | 3,575 | 12.70 (1.24) | 12.52 (0.95) | 12 |
| Social communication | 3,773 | 3,560 | 28.82 (2.51) | 28.27 (2.24) | 26 | |
| Language difficulties | 3,776 | 3,567 | 21.45 (4.87) | 20.66 (4.29) | 16 | |
| Hyperactivity | 3,783 | 3,572 | 12.93 (4.17) | 11.97 (3.45) | 9 | |
| Inattention | 3,786 | 3,571 | 14.45 (4.32) | 13.15 (3.51) | 9 |
The difference in data availability for the repetitive behavior and social communication variable at 5 years compared to other measures in Table 1 is due to the version of the 5-year questionnaire containing those measures being sent only to a subset of MoBa participants.
Polygenic risk score for ADHD
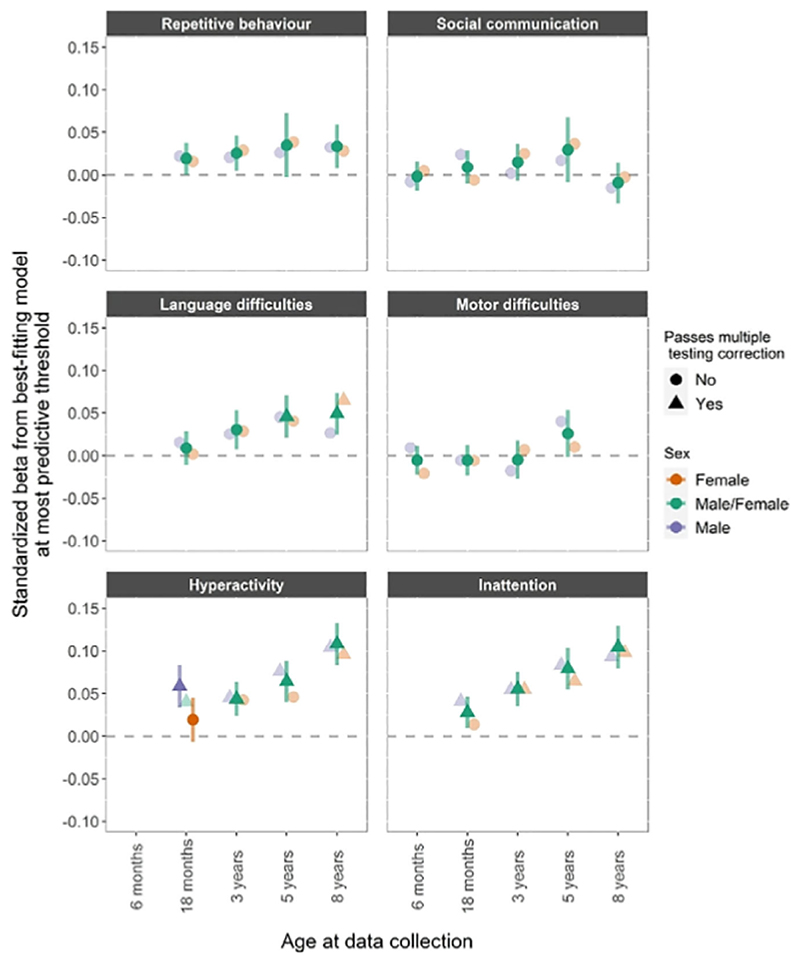
The associations between ADHD PRS and neurodevelopmental traits at different time points and in boys and girls are shown in Figure 1. There was a pattern of increasing associations with age, and most of the associations did not differ across the sexes. There was strong evidence of a positive association with language difficulties at 5 years (β =.046, CI = 0.021−0.070) and at 8 years (β.049, CI = 0.025−0.073) for both sexes. As expected, polygenic risk for ADHD was also robustly associated with increased hyperactivity and inattention across development, except in 18-month-old girls.
Figure 1.
ADHD PRS. PRS for ADHD and phenotypic measures of repetitive behavior, social communication difficulties, language and motor difficulties, hyperactivity and inattention. Estimates from linear regression models with sex as a grouping variable in a multi group framework. The darker fill intensity indicate which model (sex difference or no sex difference) provided a better fit to the PCA-PRS. Estimates from the better-fitting (sex difference or no sex difference) model also have 95% confidence interval bars, whilst those from the poorer-fitting model are presented only as point estimates for reference. Results presented in a triangle means they passed multiple testing correction with a p value <.0031, corresponding to an alpha of 5%
Polygenic risk score for autism
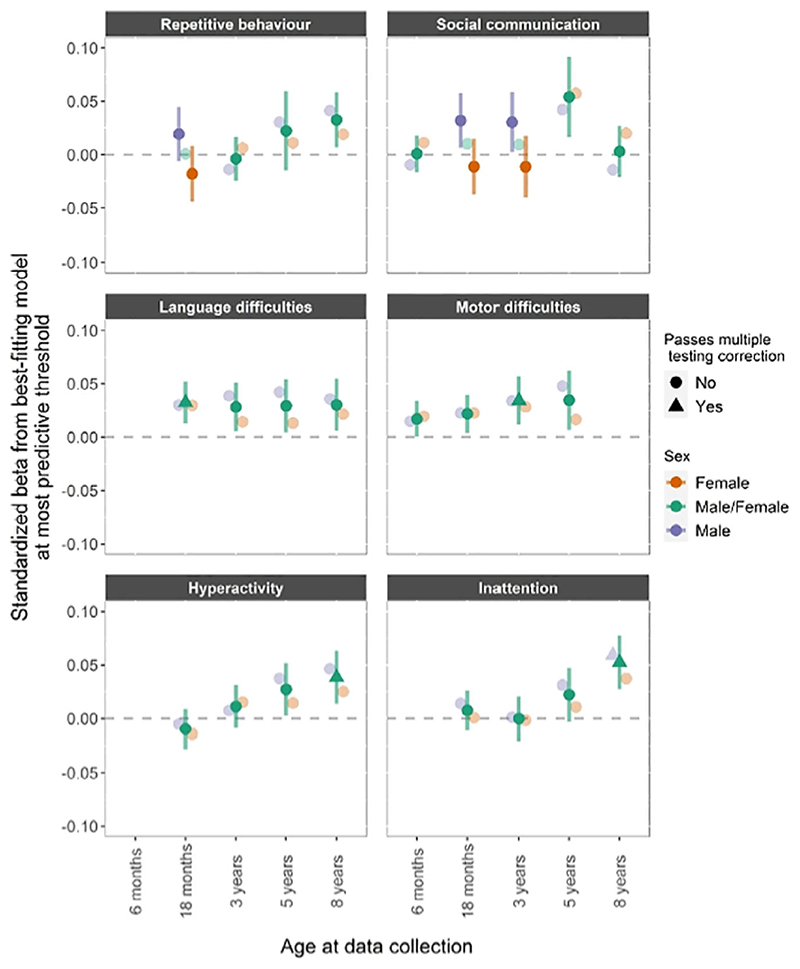
The associations between autism PRS and neurodevelopmental traits at different time points and in boys and girls are shown in Figure 2.
Figure 2.
Autism PRS. PRS for autism and phenotypic measures of repetitive behavior, social communication difficulties, language and motor difficulties, hyperactivity and inattention. Estimates from linear regression models with sex as a grouping variable in a multi group framework. The darker fill intensity indicate which model (sex difference or no sex difference) provided a better fit to the PCA-PRS. Estimates from the better-fitting (sex difference or no sex difference) model also have 95% confidence interval bars, whilst those from the poorer-fitting model are presented only as point estimates for reference. Results presented in a triangle means they passed multiple testing correction with a p value <.0031, corresponding to an alpha of 5%
PRS for autism showed a consistent association with language and motor difficulties, although strong evidence of associations with language was only shown at 18 months (β =.032, CI = 0.012−0.052) and with motor difficulties at 3 years (β =.034, CI = 0.012−0.057). We found strong evidence for an association between PRS for autism and hyperactivity at 8 years (β =.038, CI = 0.013−0.063), and inattention at 8 years (β =.052, CI = 0.027−0.077). PRS for autism showed some evidence of association with social communication difficulties at age 5 and repetitive behavior at age 8, but these associations were not robust to multiple testing correction.
Polygenic risk score for schizophrenia
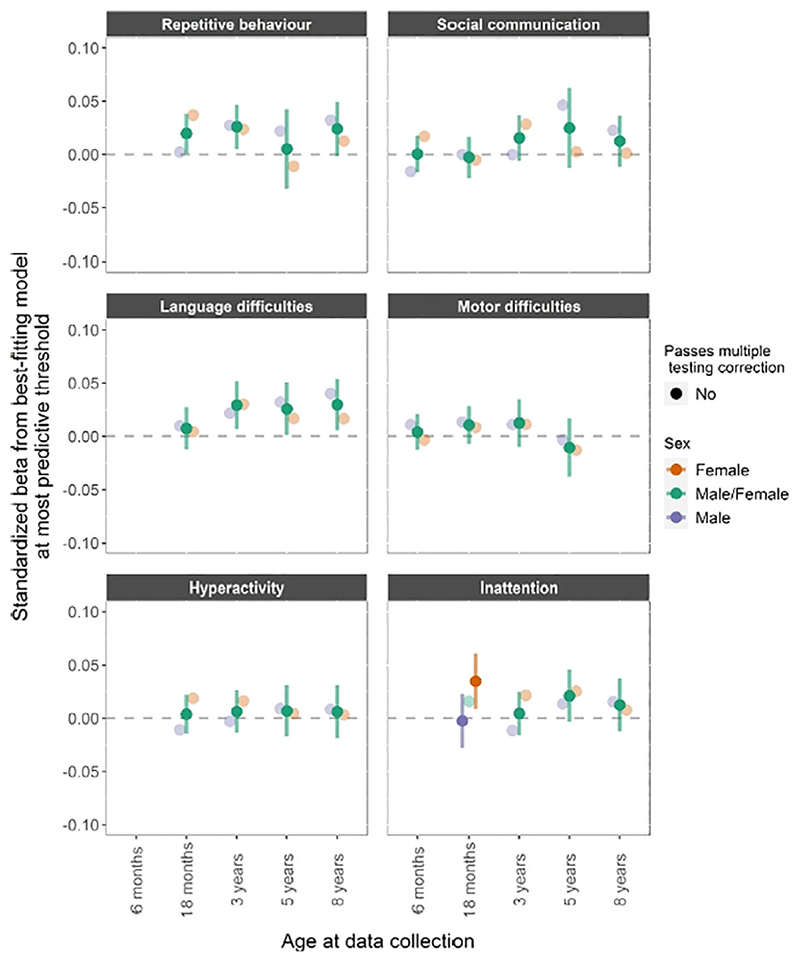
The associations between schizophrenia PRS and neurodevelopmental traits across age and sex are shown in Figure 3. There was only weak evidence (i.e., not passing multiple correction) for association with language difficulties at 3 years (β =.029, CI = 0.007−0.052), 5 years (β =.026, CI = 0.001−0.050) and 8 years (β.030, CI 0.006−0.054).
Figure 3.
Schizophrenia PRS. PRS for schizophrenia and phenotypic measures of repetitive behavior, social communication difficulties, language and motor difficulties, hyperactivity and inattention. Estimates from linear regression models with sex as a grouping variable in a multi group framework. The darker fill intensity indicate which model (sex difference or no sex difference) provided a better fit to the PCA-PRS. Estimates from the better-fitting (sex difference or no sex difference) model also have 95% confidence interval bars, whilst those from the poorer-fitting model are presented only as point estimates for reference. Results presented in a triangle means they passed multiple testing correction with a p value <.0031, corresponding to an alpha of 5%
To aid interpretation of a complex set of results, we provide several additional contextual items in the Supporting Information. Figure S1 shows the correlation between all neurodevelopmental traits presented in this paper. Figures S2−S10 display the associations of the six neurodevelopmental traits of repetitive behavior, social communication difficulties, language and motor difficulties, hyperactivity and inattention at 10 different p value thresholds for sensitivity analysis and easier comparison with previous studies using PRS. In order to visualize how PRSs associate with the trait as burden of risk variants increases, decile plots are presented in Supporting Information for the PRS-trait association of the largest effect size (Figures S11 and S12).
Discussion
The aim was to identify early manifestations of polygenic risk for ADHD, autism and schizophrenia in children between 6 months and 8 years in the general population. This is important so potential mediators and moderators of risk can be examined, and to determine the usefulness of polygenic risk scores for prediction of neurodevelopmental difficulties. We found that polygenic risk was robustly associated with neurodevelopmental measures as early as 18 months. Previous studies have not been able to investigate such associations at this young age. Before the age of 2, polygenic risk for ADHD and autism showed an association with at 1east one of the neurodevelopmental trait measures.
The main pattern seems to be that the polygenic risk manifested similarly in early childhood neurodevelopmental traits for boys and girls, although we did observe a stronger association between hyperactivity and ADHD PRS in boys at 18 months. The sex differential PRS associations showed limited consistency across age and sensitivity analyses. Replication in well-powered samples is needed, and the use of sex-stratified GWAS of neurodevelopmental disorders could be informative. In line with our finding that most PRS associations were similar across the sexes, other studies have found little consistent sex differences in SNP heritabilities or genetic correlations among neurodevelopmental and mental health traits (Martin et al., 2018, 2021). This suggests that polygenic factors are unlikely to fully explain the sex bias in symptoms and diagnoses of neurodevelopmental disorders. Further investigations are needed of the role of sex in the likely complex interactions between genomics, environment and hormonal factors in influencing neurodevelopmental outcomes (Khramtsova, Davis, & Stranger, 2019; May et al., 2019).
Early manifestations of ADHD genetic risk
In line with studies showing phenotypic overlap between ADHD and language difficulties (Korrel, Mueller, Silk, Anderson, & Sciberras, 2017), and previously observed associations between ADHD PRS and pragmatic language abilities (Martin et al., 2014), we found robust evidence of an association between polygenic risk for ADHD and language difficulties at ages 5 and 8.
Our results suggest that associations between ADHD polygenic risk and hyperactivity and inattention in the general population are robust as early as age 18 months and persist and strengthen with age to midchildhood. This supports and extends previous findings from other cohorts (Martin et al., 2014). The only identified sex difference was at 18 months, with boys showing a positive association with both hyperactivity and inattention.
Early manifestations of autism genetic risk
We identified an association between autism PRS and language difficulties which was robust only at age 18 months but consistent across all ages and for different SNP inclusion thresholds, indicating that polygenic risk for autism may contribute to language development across early and midchildhood. We found a robust association between autism PRS and motor difficulties at 3 years, which was relatively consistent across sensitivity analyses (i.e., at different p value thresholds) and age. These findings indicate that polygenic risk for autism may manifest in motor difficulties at an early age and throughout early childhood. Future studies should examine whether autism polygenic risk is associated with motor difficulties measured at older ages. Autism PRS was also robustly associated with hyperactivity and inattention at age 8, with consistent associations in sensitivity analyses except for the more stringent thresholds for SNP inclusion (see Figure S7).
Early manifestations of schizophrenia genetic risk
For schizophrenia PRS, we observed only weak evidence of association with repetitive behavior at 3 years, language difficulties at age 3, 5 and 8 years, and attention problems at age 18 months for girls. A number of possible explanations exist for why we did not identify any robust evidence of associations between the schizophrenia PRS and childhood neurodevelopmental measures. First, we examined trait associations at younger ages than most previous studies. Since schizophrenia has a late onset, signs of polygenc risk for schizophrenia may manifest at a later stage. Associations found in other cohorts from midchildhood (e.g., Nivard et al., 2017; Riglin et al., 2017; St Pourcain et al., 2018) have been of small magnitude, although increasing somewhat in strength with age. Thus, our power to detect earliest signs of the schizphrenia PRS manifestations might be low. Second, genetic risk for schizophrenia may be expressed in other phenotypes not captured by current measures. Genetic variants showing an association with schizophrenia may only weakly index the risk for some phenotypic measures and may more strongly reflect genetic risk for other characteristics of the disorder, such as negative symptoms.
Limitations
The study has some limitations. Some are limitations inherent to analyses using PRS based on current GWAS including small effect sizes, GWAS sample sizes and ascertainment methods (Demontis et al., 2019; Grove et al., 2019; St Pourcain et al., 2018). Rare genetic variants are not captured by GWAS, so we are only able to examine associations with common variants. Some limitations were specific to our study. First, it is important to note that, due to limited number of available measures, these analyses are specific for given time points, and not analyzed as developmental trajectories. Second, there are demographic differences between the discovery GWAS samples and our sample. The discovery sample consists of both children and adults with a diagnosis. There is an ongoing discussion about whether childhood and adult onset ADHD is actually the same disorder (Moffitt et al., 2015). If not, the PRS for ADHD would yield different results than if the discovery sample was based purely on children. Third, despite the use of one of the largest population-birth cohorts worldwide, the current subsample may not be adequately powered to identify small effects in some domains. Fourth, as with all longitudinal studies, MoBa is subject to attrition (Nilsen et al., 2013). Previous studies have shown that predictors of attrition include presence of behavioral difficulties, such as ADHD, in the study child (Wolke et al., 2009). Selective attrition could lead to bias in our estimates, likely in the form of an underestimation of associations between the PRS and the neurodevelopmental traits. Lastly, given the extent of genetic correlations between neurodevelopmental disorders and an array of other conditions and behaviors (Demontis et al., 2019; Grove et al., 2019; Schizophrenia, 2014), it is likely that genetic risk for these disorders manifests more broadly in childhood than is captured in the current study. Future work should investigate this, though if it is assumed that effect sizes in the current study represent an upper bound for effects in other domains, power will be a limitation until sample sizes increase or the predictive power of PRS is substantially enhanced.
Conclusions
To our knowledge, our study is the first to investigate manifestations of polygenic risk for multiple neurodevelopmental disorders in a general population sample of children as young as six months of age. The results show that polygenic risk for autism and ADHD is detectable in neurodevelopmental traits in the general population at an early age. These polygenic risks are relatively broadly expressed and appears predominantly consistent across sex, with some exceptions that should be replicated and further investigated. Even larger population-based longitudinal studies with both genetic data and robust and consistent measures of neurodevelopmental traits are required to reliably detect how genetic risk for neurodevelopmental disorders are phenotypically expressed from infancy through childhood and adulthood.
Supplementary Material
Key points.
Liability to neurodevelopmental disorders is partially underpinned by incremental effects of many common genetic variants.
These variants are also associated with a range of neurodevelopmental traits in the general population across childhood and sex.
Our results narrow the knowledge gap on how polygenic risk for neurodevelopmental disorders manifests both early in development and broadly across a range of domains, with some specificity for boys and girls. Findings from genetic analyses such as these can in the future help pinpoint which early behavioral profiles clinicians should attend to when assessing risk for neurodevelopmental problems.
Acknowledgements
R.B.A. was supported by the Research Council of Norway (274611). L.J.H. and A.H. were supported by the South-Eastern Norway Regional Health Authority (grants 2018058, 2018059, 2020022). G.D.S. works in the Medical Research Council Integrative Epidemiology Unit at the University of Bristol, which is supported by the Medical Research Council (MC_UU_00011/1). O.A.A. was supported by the Research Council of Norway (229129; 213837; 248778; 223273; 249711); the South-East Norway Regional Health Authority (2017−112); KG Jebsen Stiftelsen (SKGJ) and H2020 grant # 847776 CoMorMent. The Norwegian Mother, Father and Child Cohort Study is supported by the Norwegian Ministry of Health and Care Services and the Ministry of Education and Research. We are grateful to all the participating families in Norway who take part in this on-going cohort study. We thank the Norwegian Institute of Public Health (NIPH) for generating high-quality genomic data. This research is part of the HARVEST collaboration, supported by the Research Council of Norway (#229624). We also thank the NORMENT Centre for providing genotype data, funded by the Research Council of Norway (#223273), South East Norway Health Authority and KG Jebsen Stiftelsen. We further thank the Center for Diabetes Research, the University of Bergen for providing genotype data and performing quality control and imputation of the data funded by the ERC AdG project SELECTionPREDISPOSED, Stiftelsen Kristian Gerhard Jebsen, Trond Mohn Foundation, the Research Council of Norway, the Novo Nordisk Foundation, the University of Bergen, and the Western Norway health Authorities (Helse Vest). The authors declare that they have no competing or potential conflicts of interest.
Footnotes
Conflict of interest statement: No conflicts declared.
References
- Achenbach TM, Dumenci L, Rescorla LA. Ratings of relations between DSM IV diagnostic categories and items of the CBCL/6-18, TRF, and YSR. University of Vermont; Burlington, VT: 2001. pp. 1–9. [Google Scholar]
- APA. Diagnostic and statistical manual of mental disorders (DSM-5®) 5th. American Psychiatric Association; Washington, DC: 2013. [Google Scholar]
- Baio J, Wiggins L, Christensen DL, Maenner MJ, Daniels J, Warren Z, et al. Dowling NF. Prevalence of autism spectrum disorder among children aged 8 years autism and developmental disabilities monitoring network, 11 sites, United States, 2014. MMWR Surveillance Summaries. 2018;67:1. doi: 10.15585/mmwr.ss6706a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baron-Cohen S, Allen J, Gillberg C. Can autism be detected at 18 months? The needle, the haystack and the CHAT. British Journal of Psychiatry. 1992;161(839) doi: 10.1192/bjp.161.6.839. [DOI] [PubMed] [Google Scholar]
- Bishop DV. The children’s communication checklist: CCC-2. Harcourt Assessment; 2003. [Google Scholar]
- Burmeister M, McInnis MG, Zöllner S. Psychiatric genetics: Progress amid controversy. Nature Reviews Genetics. 2008;9:527–540. doi: 10.1038/nrg2381. [DOI] [PubMed] [Google Scholar]
- Choi SW, O’Reilly PF. PRSice-2: Polygenic Risk Score software for biobank-scale data. Gigascience. 2019;8:giz082. doi: 10.1093/gigascience/giz082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coombes BJ, Ploner A, Bergen SE, Biernacka JM. A principal component approach to improve association testing with polygenic risk scores. Genetic Epidemiology. 2020;44:676–686. doi: 10.1002/gepi.22339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demontis D, Walters RK, Martin J, Mattheisen M, Als TD, Agerbo E, et al. Neale BM. Discovery of the first genome-wide significant risk loci for attention deficit/hyperactivity disorder. Nature Genetics. 2019;51:63–75. doi: 10.1038/s41588-018-0269-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faraone SV, Larsson H. Genetics of attention deficit hyperactivity disorder. Molecular Psychiatry. 2019;24:562–575. doi: 10.1038/s41380-018-0070-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grove J, Ripke S, Als TD, Mattheisen M, Walters RK, Won H, et al. Børglum AD. Identification of common genetic risk variants for autism spectrum disorder. Nature Genetics. 2019;51:431–444. doi: 10.1038/s41588-019-0344-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Homberg JR, Kyzar EJ, Scattoni ML, Norton WH, Pittman J, Gaikwad S, et al. Kalueff AV. Genetic and environmental modulation of neurodevelopmental disorders: translational insights from labs to beds. Brain Research Bulletin. 2016;125:79–91. doi: 10.1016/j.brainresbull.2016.04.015. [DOI] [PubMed] [Google Scholar]
- Ireton H. Child development inventory. Behavior Science Systems; Minneapolis, MN: 1992. [Google Scholar]
- Jansen PR, Polderman TJC, Bolhuis K, Ende J, Jaddoe VWV, Verhulst FC, et al. Tiemeier H. Polygenic scores for schizophrenia and educational attainment are associated with behavioural problems in early childhood in the general population. Journal of Child Psychology and Psychiatry. 2018;59:39–47. doi: 10.1111/jcpp.12759. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Amminger GP, Aguilar-Gaxiola S, Alonso J, Lee S, Ustun TB. Age of onset of mental disorders: a review of recent literature. Current Opinion in Psychiatry. 2007;20:359. doi: 10.1097/YCO.0b013e32816ebc8c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khramtsova EA, Davis LK, Stranger BE. The role of sex in the genomics of human complex traits. Nature Reviews Genetics. 2019;20:173–190. doi: 10.1038/s41576-018-0083-1. [DOI] [PubMed] [Google Scholar]
- Korrel H, Mueller KL, Silk T, Anderson V, Sciberras E. Research Review: Language problems in children with Attention-Deficit Hyperactivity Disorder a systematic mcta-analytic review. Journal of Child Psychology and Psychiatry. 2017;58:640–654. doi: 10.1111/jcpp.12688. [DOI] [PubMed] [Google Scholar]
- Kumar G, Steer RA. Factorial validity of the Conners’ Parent Rating Scale-revised: Short form with psychiatric outpatients. Journal of Personality Assessment. 2003;80:252–259. doi: 10.1207/S15327752JPA8003_04. [DOI] [PubMed] [Google Scholar]
- Leppert B, Havdahl A, Riglin L, Jones HJ, Zheng J, Davey Smith G, et al. Stergiakouli E. Association of maternal neurodevelopmental risk alleles with early-life exposures. JAMA Psychiatry. 2019;76:834–842. doi: 10.1001/jamapsychiatry.2019.0774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li R, Ma X, Wang G, Yang J, Wang C. Why sex differences in schizophrenia? Journal of Translational Neuroscience. 2016;1:37. [PMC free article] [PubMed] [Google Scholar]
- Lyall K, Croen L, Daniels J, Fallin MD, Ladd-Acosta C, Lee BK, et al. Newschaffer C. The changing epidemiology of autism spectrum disorders. Annual Review of Public Health. 2017;38:81–102. doi: 10.1146/annurev-publhealth-031816-044318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magnus P, Birke C, Vejrup K, Haugan A, Alsaker E, Daltveit AK, et al. Stoltenberg C. Cohort profile update: the Norwegian mother and child cohort study (MoBa) International Journal of Epidemiology. 2016;45:382–388. doi: 10.1093/ije/dyw029. [DOI] [PubMed] [Google Scholar]
- Magnus P, Irgens LM, Haug K, Nystad W, Skjærven R, Stoltenberg C. Cohort profile: the Norwegian mother and child cohort study (MoBa) International Journal of Epidemiology. 2006;35:1146–1150. doi: 10.1093/ije/dyl170. [DOI] [PubMed] [Google Scholar]
- Martin J, Hamshere ML, Stergiakouli E, O’Donovan MC, Thapar A. Genetic risk for attention-deficit/hyperactivity disorder contributes to neurodevelopmental traits in the general population. Biological Psychiatry. 2014;76:664–671. doi: 10.1016/j.biopsych.2014.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin J, Khramtsova EA, Goleva SB, Blokland GAM, Traglia M, Walters RK, et al. Stahl E. Examining sex-differentiated genetic effects across ncuropsychiatric and behavioral traits. Biological Psychiatry. 2021;89:1127–1137. doi: 10.1016/j.biopsych.2020.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin J, Walters RK, Demontis D, Mattheisen M, Lee SH, Robinson E, et al. Werge T. A genetic investigation of sex bias in the prevalence of attentiondeficit/hyperactivity disorder. Biological Psychiatry. 2018;83:1044–1053. doi: 10.1016/j.biopsych.2017.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May T, Adesina I, McGillivray J, Rinehart NJ. Sex differences in neurodevelopmental disorders. Current Opinion in Neurology. 2019;32:622–626. doi: 10.1097/WCO.0000000000000714. [DOI] [PubMed] [Google Scholar]
- Moffitt TE, Houts R, Asherson P, Belsky DW, Corcoran DL, Hammerle M, et al. Caspi A. Is adult ADHD a childhood-onset neurodevelopmental disorder? Evidence from a four-decade longitudinal cohort study. The American Journal of Psychiatry. 2015;172:967–977. doi: 10.1176/appi.ajp.2015.14101266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulligan A, Anney RJL, O’Regan M, Chen W, Butler L, Fitzgerald M, et al. Gill M. Autism symptoms in attention-deficit/hyperactivity disorder: A familial trait which correlates with conduct, oppositional defiant, language and motor disorders. Journal of Autism Developmental Disorders. 2009;39:197–209. doi: 10.1007/s10803-008-0621-3. [DOI] [PubMed] [Google Scholar]
- Nilsen RM, Suren P, Gunnes N, Alsaker ER, Bresnahan M, Hirtz D, et al. Stoltenberg C. Analysis of selfselection bias in a population-based cohort study of autism spectrum disorders. Paediatric Perinatal Epidemiology. 2013;27:553–563. doi: 10.1111/ppe.12077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nivard MG, Gage SH, Hottenga JJ, van Beijsterveldt CEM, Abdcllaoui A, Bartels M, et al. Middeldorp CM. Genetic overlap between schizophrenia and developmental psychopathology: longitudinal and multivariate polygenic risk prediction of common psychiatric traits during development. Schizophrenia Bulletin. 2017;43:1197–1207. doi: 10.1093/schbul/sbx031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paltiel L, Anita H, Skjerden T, Harbak K, Bækkcn S, Nina Kristin S, et al. Magnus P. The biobank of the Norwegian Mother and Child Cohort Study present status. Norsk Epidemiologi. 2014;24:1–2. [Google Scholar]
- Polanezyk GV, Willcutt EG, Salum GA, Kieling C, Rohde LA. ADHD prevalence estimates across three decades: An updated systematic review and meta-regression analysis. International Journal of Epidemiology. 2014;43:434–442. doi: 10.1093/ije/dyt261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter J, Janson H. A validation study of the Norwegian version of the ages and stages Questionnaires. Acta Paediatrica. 2007;96:748–752. doi: 10.1111/j.1651-2227.2007.00246.x. [DOI] [PubMed] [Google Scholar]
- Riglin L, Collishaw S, Richards A, Thapar AK, Maughan B, O’Donovan MC, Thapar A. Schizophrenia risk alleles and neurodevelopmental outcomes in childhood: A population-based cohort study. The Lancet Psychiatry. 2017;4:57–62. doi: 10.1016/S2215-0366(16)30406-0. [DOI] [PubMed] [Google Scholar]
- Robins DL, Fein D, Barton ML, Green JA. The Modified Checklist for Autism in Toddlers: An initial study investigating the early detection of autism and pervasive developmental disorders. Journal of Autism Deνelopmental Disorders. 2001;31:131–144. doi: 10.1023/a:1010738829569. [DOI] [PubMed] [Google Scholar]
- Robinson EB, St Pourcain B, Anttila V, Kosmicki JA, Bulik-Sullivan B, Grove J, Daly MJ. Genetic risk for autism spectrum disorders and neuropsychiatric variation in the general population. Nature Genetics. 2016;48:552–555. doi: 10.1038/ng.3529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson JP, Shaver PR, Wrightsman LS. Measures of personality and social psychological attitudes: Measures of social psychological attitudes. Vol. 1 Academic Press; San Diego, CA: 2013. [Google Scholar]
- Ronald A, Happé F, Plomin R. A twin study investigating the genetic and environmental aetiologies of parent, teacher and child ratings of autistic-like traits and their overlap. European Child Adolescent Psychiatry. 2008;17:473–483. doi: 10.1007/s00787-008-0689-5. [DOI] [PubMed] [Google Scholar]
- Rosseel Y. Lavaan: An R package for structural equation modeling and more. Version 0.5 12 (BETA) Journal of Statistical Software. 2012;48:1–36. [Google Scholar]
- Rössler W. What is normal? The impact of psychiatric classification on mental health practice and research. Frontiers in Public Health. 2013;1:68. doi: 10.3389/fpubh.2013.00068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutter M, Bailey A, Lord C. The social communication questionnaire. Western Psychological Services; Torrance, CA: 2003. [Google Scholar]
- Schizophrenia, W. G. o. t. P. G. C. Biological insights from 108 schizophrenia-associated genetic loci. Nature. 2014;511:421–427. doi: 10.1038/nature13595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott FJ, Baron-Cohen S, Bolton P, Brayne C. The CAST (Childhood Asperger Syndrome Test) Preliminary development of a UK screen for mainstream primary-school-age children. Autism. 2002;6:9–31. doi: 10.1177/1362361302006001003. [DOI] [PubMed] [Google Scholar]
- Segal DL. In: The Corsini Encyclopedia of psychology. Weiner lB, Craighead WE., editors. John Wiley & Sons, Inc; 2010. Diagnostic and statistical manual of mental disorders (DSM-IV-TR) pp. 1–3. [DOI] [Google Scholar]
- Silva RR, Alpert M, Pouget E, Silva V, Trosper S, Reyes K, Dummit S. A rating scale for disruptive behavior disorders, based on the DSM-IV item pool. Psychiatric Quarterly. 2005;76:327–339. doi: 10.1007/s11126-005-4966-x. [DOI] [PubMed] [Google Scholar]
- St Pourcain B, Robinson EB, Anttila V, Sullivan BB, Mailer J, Golding J, et al. Davey Smith G. ASD and schizophrenia show distinct developmental profiles in common genetic overlap with population-based social communication difficulties. Molecular Psychiatry. 2018;23:263–270. doi: 10.1038/mp.2016.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan PF, Agrawal A, Bulik CM, Andreassen OA, Børglum AD, Breen G, O’Donovan MC. Psychiatric genomics: An update and an agenda. American Journal of Psychiatry. 2018;175:15–27. doi: 10.1176/appi.ajp.2017.17030283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan PF, Daly MJ, O’Donovan M. Genetic architectures of psychiatric disorders: The emerging picture and its implications. Nature Reviews Genetics. 2012;13:537–551. doi: 10.1038/nrg3240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suren P, Bakken IJ, Aase H, Chin R, Gunnes N, Lie KK, et al. Stoltenberg C. Autism spectrum disorder, ADHD, epilepsy, and cerebral palsy in Norwegian children. Pediatrics. 2012;130:e152–e158. doi: 10.1542/peds.2011-3217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thapar A, Cooper M, Rutter M. Neurodevclop-mcntal disorders. The Lancet Psychiatry. 2017;4:339–346. doi: 10.1016/S2215-0366(16)30376-5. [DOI] [PubMed] [Google Scholar]
- Visser SN, Danielson ML, Bitsko RH, Holbrook JR, Kogan MD, Ghandour RM, et al. Blumberg SJ. Trends in the parent-report of health care provider- diagnosed and medicated attention-deficit/hyperactivity disorder: United States, 2003 2011. Journal of the American Academy of Child Adolescent Psychiatry. 2014;53:34–46.:e32. doi: 10.1016/j.jaac.2013.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willcutt EG. The prevalence of DSM IV attention-deficit/hyperactivity disorder: a meta-analytic review. Neurotherapeutics: the Journal of the American Society for Experimental NeuroTherapeutics. 2012;9:490–499. doi: 10.1007/s13311-012-0135-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolke D, Waylen A, Samara M, Steer C, Goodman R, Ford T, Lamberts K. Selective drop-out in longitudinal studies and non-biased prediction of behaviour disorders. The British Journal of Psychiatry. 2009;195:249–256. doi: 10.1192/bjp.bp.108.053751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wray NR, Lee SH, Mehta D, Vinkhuyzen AA, Dudbridge F, Middeldorp CM. Research review: Polygenic methods and their application to psychiatric traits. Journal of Child Psychology and Psychiatry. 2014;55:1068–1087. doi: 10.1111/jcpp.12295. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.