Abstract
Gait is emerging as a potential diagnostic tool for cognitive decline. . The ‘Deep and Frequent Phenotyping for Experimental Medicine in Dementia Study’ (D&FP) is a multicentre feasibility study embedded in the United Kingdom Dementia Platform designed to determine participant acceptability and feasibility of extensive and repeated phenotyping to determine the optimal combination of biomarkers to detect disease progression and identify early risk of Alzheimer’s Disease. Gait is included as a clinical biomarker. The tools to quantify gait in the clinic and home, and suitability for multi-centre application have not been examined. Six centres from the National Institute for Health Research Translational Research Collaboration in Dementia initiative recruited 20 individuals with early onset Alzheimer’s disease. Participants wore a single wearable (tri-axial accelerometer) and completed both clinic-based and free-living gait assessment. A series of macro (behavioural) and micro (spatiotemporal) characteristics were derived from the resultant data using previously validated algorithms. Results indicate good participant acceptability, and potential for use of body-worn sensors in both the clinic and the home. Recommendations for future studies have been provided. Gait has been demonstrated to be a feasible and suitable measure, and future research should examine its suitability as a biomarker in Alzheimer’s Disease.
Keywords: Gait, wearables, phenotyping, Alzheimer’s disease, cognition, free-living
Introduction
Dementia prevalence is increasing worldwide with consequential societal and economic costs [1]. As curative therapies have yet to be developed, research has broadened to facilitate preventative and interventional measures. Identification of biomarkers aims to aid early diagnosis [2]. This will allow people with cognitive impairment and dementia, along with their relatives, to optimise treatment, maintain independence and quality of life, improve current understanding of preclinical pathology and improve diagnostic accuracy. Gait has been proposed as a clinical biomarker for dementia [3].
A relationship between gait and cognition has been established [4]. Safe gait requires complex cognitive processes. Reduced gait speed is linked to falls, mortality and cognitive decline [5]. More specific spatiotemporal measures of gait have been linked to particular cognitive domains; for example impaired attention and executive function are associated with slower pace and increased variability during walking [6]. Changes in gait can occur up to 12 years prior to diagnosis of cognitive impairment [7]. Gait impairments in spatiotemporal characteristics have also been found in APOE-4 carriers, a genetic risk for Alzheimer’s disease [8, 9]. Therefore, gait could be a useful clinical biomarker even before the development of clinical dementia.
Quantitative gait analysis provides evidence that people with dementia walk more slowly with increased variability and impaired temporal gait control compared to cognitively-intact older adults ([3, 4, 10]). Motion capture analysis systems, pressurised sensor walkways and accelerometers have all been useful in identifying gait impairments [11, 12]. However, the majority of these analytical tools require specialised laboratories, which are not widely available, costly and only allow infrequent assessment [13–15]. Use of wearable technology, such as small body-worn sensors, allows gait to be assessed in both clinic and the home (termed free-living gait) [14]. Free-living gait analysis is particularly useful, as not only does it allow objective observation of an individual’s day-to-day gait, it also provides measurements of both microstructural (micro) and macrostructural (macro) characteristics of gait. Micro characteristics are discrete spatiotemporal gait characteristics contained in each walking bout (e.g. step length, step time) [16] and can be collected both in the clinic and at home [17]. Macro characteristics refer to the volume (e.g. total walking time, total steps) and pattern (e.g. variability of bout length) of walking and can only be collected over prolonged periods of time i.e. free-living gait assessment [16]. While micro gait characteristics are sensitive to changes in cognition and the brain [4], macro characteristics can provide an easily accessible and detailed picture of changes to an individual’s overall gait function. Free-living gait assessment may provide a cost-effective means to assess specific gait characteristics and functional abilities in people with cognitive impairment. This proposal is supported by the successful use of wearable technology in cognitively impaired populations in both the clinic [18–21] and home [22]. However, to this author’s knowledge, there is no studies using a combined approach of clinic and free-living gait data, assessing both clinically relevant micro and macro outcomes.
Gait is one of the potential clinical biomarkers investigated in the ‘Deep and Frequent Phenotyping for Experimental Medicine in Dementia Study’ (DFP), a multicentre feasibility study embedded in the United Kingdom Dementia Platform. It is designed to determine participant acceptability of extensive and repeated phenotyping, and to establish the operational practicability to inform the conduct of a full trial utilising the National Institute for Health Research Translational Research Collaboration in Dementia (NIHR TRC-D) infrastructure. The aim of this study was to determine the feasibility of conducting clinical and free-living gait assessments in a dementia population as part of the D&FP protocol. This includes establishing practical considerations for use of wearable sensors, including acceptability by participants, staff training, technical considerations and limitations and providing recommendations for future research.
Methods
Participants
Six specialist centres of excellence in the UK that constitute the NIHR Translational Research Collaboration in Dementia (NIHR Biomedical Research Centers or Dementia Units associated with the following NHS Trusts: Oxford University Hospitals (OUH), South London and Maudsley (SLaM), Cambridge University Hospitals (CUHT), University College Hospital London (UCLH), West London Mental Health Care (WLMHC) and Newcastle Hospitals) took part in this non-interventional multi-site study. Each aimed to recruit four patients with probable Alzheimer’s disease (AD), diagnosed according to National Institute of Aging-Alzheimer’s Association (NIA-AA) criteria [23].
Participants inclusion criteria were: between 55 and 80; a Rosen modified Hanchinski ischemic score ≤4; Mini-Mental State Examination (MMSE) score above 20; healthy as determined by a physician; being on stable medication dose for any non-significant medical conditions for at least one month and stable dose for at least 3 months if treated with cholinesterase inhibitors and/or memantine; and able to walk independently for at least 15 metres (with a single walking aid if required).
Control subjects were not recruited for this pilot study. However, previous data from similar aged controls using the same body-worn sensors and derived gait outcomes (as seen in Table 3 [17]) was used to make observational comparisons between controls and individuals with AD, as detailed in the discussion.
Table 3.
Micro gait characteristics (reported as either (mean ± SD) or (median (interquartile range)) as per control reference data [17]) for clinic gait assessment (Time 1 and 2) and free-living gait assessment, as derived from a theoretical model of gait [15]. Control data has previously been published for both clinical and free-living protocols and is used here as a reference to show expected findings in a healthy ageing cohort.
| Clinical Gait Assessment | Free-living Gait | ||||
|---|---|---|---|---|---|
|
| |||||
| Time 1 | Time 2 | Control Reference | One time only | Control Reference | |
|
|
|||||
| Domain/Gait Characteristics | (n=17) | (n=17) | (n=50) | (n=16) | (n=50) |
| Pace | |||||
| Step Velocity (m/s) | 0.976 ± 0.186 | 0.995 ± 0.181 | 1.393 ± 0.207 | 1.044 (0.146) | 1.097 (0.48) |
| Step Length (m) | 0.559 ± 0.083 | 0.569 ± 0.075 | 0.726 ±0.095 | 0.61 (0.054) | 0.601 (0.183) |
| Step Time Variability (s) | 0.049 (0.065) | 0.032 (0.016) | 0.073 (0.301) | 0.169 (0.034) | 0.175 (0.156) |
| Swing Time Variability (s) | 0.028 (0.046) | 0.027 (0.019) | 0.018 (0.133) | 0.145 (0.017) | 0.147 (0.125) |
| Stance Time Variability (s) | 0.055 (0.065) | 0.033 (0.018) | 0.022 (0.109) | 0.179 (0.035) | 0.188 (0.161) |
| Variability (SD) | |||||
| Step Velocity Variability (m/s) | 0.087 (0.07) | 0.083 (0.051) | 0.073 (0.301) | 0.357 (0.048) | 0.383 (0.494) |
| Step Length Variability (m) | 0.048 (0.043) | 0.052 (0.045) | 0.033 (0.096) | 0.147 (0.019) | 0.151 (0.079) |
| Rhythm | |||||
| Step Time (s) | 0.583 ± 0.061 | 0.579 ± 0.056 | 0.525 ± 0.047 | 0.61 (0.029) | 0.593 (0.144) |
| Swing Time (s) | 0.420 ± 0.058 | 0.424 ± 0.060 | 0.371 ± 0.040 | 0.461 (0.041) | 0.449 (0.113) |
| Stance Time (s) | 0.739 ± 0.067 | 0.730 ± 0.058 | 0.679 ± 0.061 | 0.762 (0.031) | 0.741 (0.166) |
| Asymmetry | |||||
| Step Time Asymmetry (s) | 0.024 (0.035) | 0.019 (0.024) | 0.007 (0.140) | 0.095 (0.006) | 0.093 (0.086) |
| Swing Time Asymmetry (s) | 0.017 (0.021) | 0.021 (0.016) | 0.010 (0.126) | 0.086 (0.01) | 0.084 (0.064) |
| Stance Time Asymmetry (s) | 0.02 (0.025) | 0.027 (0.018) | 0.007 (0.140) | 0.095 (0.009) | 0.094 (0.086) |
| Postural Control | |||||
| Step Length Asymmetry (m) | 0.033 (0.026) | 0.031 (0.031) | 0.007 (0.060) | 0.083 (0.015) | 0.081 (0.043) |
Ethics
The study was approved by a National Research Ethics Committee London on the 19th of Sep 2014 – IRAS reference 14/LO/1467, IRAS project ID: 156309. All participants had mental capacity for informed consent.
Protocol
Research staff training
All research staff attended a training day with a one-hour introductory session inclusive of both general and study specific gait analysis theory. This also included a brief demonstration of the testing protocol and data sharing platform, as well as a question and answer based session. All participating staff were new to the emerging field of wearable technology and had no previous experience of administering gait assessments. A resource pack containing additional training resources and materials (written and video-based standardised operating procedures, wearable sensors and adhesives) was provided to respective centre staff at this initial training day.
Wearable technology
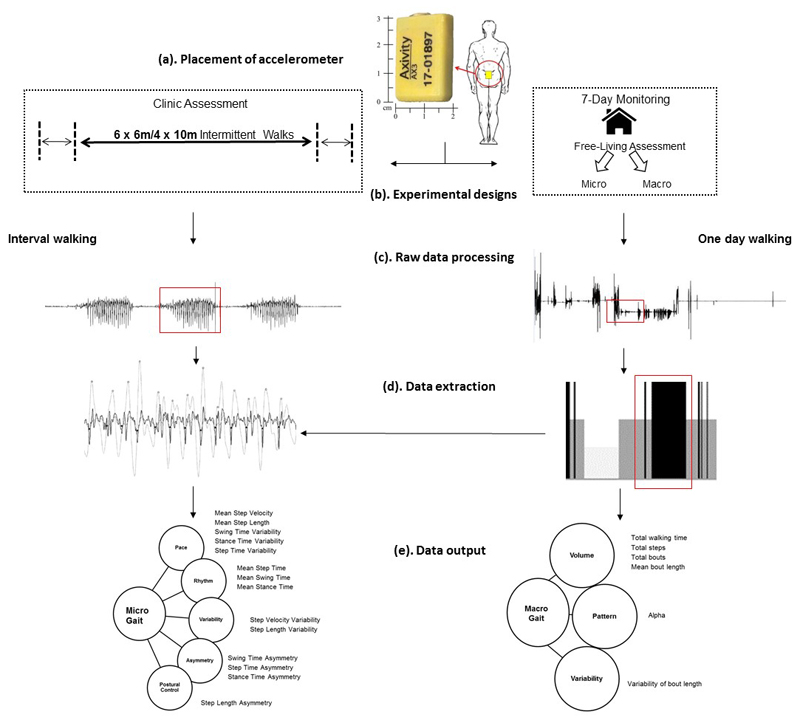
Participants were asked to wear a low-cost tri-axial accelerometer-based wearable (Axivity AX3; Axivity, York, UK; Dimensions: 23.0mm x32.5mm x7.6mm, weight 9g) located on the fifth lumbar vertebra (L5; see Figure 1). The wearable was attached using double sided tape and Hypafix (BSN Medical Limited, Hull, UK) and was programmed to capture with a sampling frequency of 100Hz (16 bit resolution, range ±8g). Recorded signals were stored locally on the sensor’s internal memory (512MB) as a raw binary file and then downloaded upon the completion of each testing session. Participants were provided with additional adhesives and attachment instructions for the duration of the 7-day free-living assessment, and received a prepaid envelope and instructions for the return of the wearable to their host centre. Participants were informed that the wearable was shower-resistant but could not be submerged in water (i.e. in a bath/swimming), and that it should remain in place throughout the duration of the week. They were also provided instructions on how to reattach the wearable in the event it was removed or came off by itself.
Figure 1.
(a) Example of body worn monitor placement for both the clinic based and free-living data collection on L5 centrally located on the lower back; (b) Gait protocols for clinic and home based assessments for the D&FP feasibility study; (c) The raw vertical acceleration signal segmented into walking bouts (d) Left; Example of gait characteristic extraction from walking bouts: detecting initial contacts (black stars) and final contacts (white circles). Right: Identification of walking bouts (black bars) from free-living data from which gait characteristics are extracted; (e) Left: Conceptual model of gait representing domains and 14 gait micro characteristics. Right: Macro characteristics of gait described by domains of volume, pattern and variability.
Gait assessment
Participants were required to complete three gait assessments (Figure 1); two clinic based assessments (Day 1 and Day 169) and one free-living assessment as described above (beginning on day 85). Clinic assessments of gait included: a straight line walk over a distance of 6 or 10m – depending upon availability of clinic space. This was repeated 4 times in centres employing 10m walks, and 6 times in centres employing 6m walks under single-task conditions. Assessment of free-living gait involved participants wearing the wearable on their lower back for 7-days. During this time, participants were instructed to go about normal activities as usual. Gait outcomes were derived from a theoretical model [15] and included a range of characteristics sensitive to cognition [4].
Clinical and Neuropsychological Assessment
Participants were also required to undergo a cognitive testing battery, here we report a limited set of tests with which we compare to gait measures. Participants were scored on the Mini Mental State Exam (MMSE) to provide a score of global cognition [24] and the Clinical Dementia Rating Scale (CDR) to rate the severity of dementia [25]. Participants also completed the Clock Drawing Executive Test (CLOX), a measure used to identify executive impairment [26]. Additionally, participants were scored on the Bristol Activities of Daily Living Scale (BADLS) to identify impairments in activities of daily living [27] and the Geriatric Depression Scale (GDS), to identify depressive symptoms [28].
Data transfer from individual sites
Raw acceleration data for both clinic-based and free-living assessments, along with pseudonymised patient information were transferred from external sites to Newcastle University using a commercially available cloud storage-system (Dropbox, Dropbox Inc.). This system is the most popular cloud based data transfer program [29], and has critical appraisal for robust in-built security measures [30]. All site-specific research staff were required to create a user account and to join a secure ‘shared’ folder with the gait correspondent at Newcastle University. Data were uploaded and shared immediately after completing a testing session and a confirmation email was provided to external sites after the data was downloaded to the secure databases at Newcastle University.
Outcomes
Gait outcomes chosen were based on a theoretical model of gait derived from principal component analysis [15] to allow exploration of a wide yet comprehensive range of variables (see Figure 1). It has previously been demonstrated in older adults and validated in Parkinson’s disease. Clinic based assessments of micro gait characteristics were derived as follows. Temporal algorithms identify initial contact (IC) and final contact (FC) events within the gait cycle. These are estimated from the filtered vertical accelerations by a Gaussian continuous wavelet transform (CWT) [31] which allows for timing estimations. Micro (spatiotemporal) outcomes are estimated using IC/FC events along with an inverted pendulum model [32]. Using the relationship between time and length (Eqn 1.), accurate estimations of gait velocity can then be provided (taken from Del Din and colleagues [33]). Variations of characteristics such as variability and asymmetry calculations facilitate a detailed investigation of the step-to-step fluctuations and inter-limb coordination, respectively. Variability was estimated from the standard deviation between all steps. Asymmetry was determined as the absolute difference between left and right steps (Eqn 2) [31].
| (1) |
| (2) |
For 7-day free-living gait assessments a logical heuristics paradigm was embedded into walking bout identification and quantification algorithms. Macro (behavioural) gait characteristics such as volume of time spent walking and number of bouts performed were generated based on the walking bouts detected across all days. In addition a set of non-linear descriptors were also derived, including: (i) shape and power-law distribution (alpha, α) based on a logarithmic scale from their density and length and (ii) the within bout variability (S2) estimated using a maximum likelihood technique [34]. Alpha refers to the distribution of total walking time, describing a ratio between long and short walking bouts. Higher alpha values means walking time is made up of proportionally more short bouts, while low alpha values means it is made up of proportionally more long bouts.
Data Analysis
Descriptive analysis was taken of both clinic and free-living data. Mann Whitney U tests were employed to assess differences between gait at the first and second visit. The relationship between cognition (as measured by the MMSE and CLOX 1 and 2) and gait (as measured by micro gait characteristics in Time 1, micro and macro gait characteristics in free-living data) was explored using Spearman’s Rho. Preliminary analyses were performed to identify correlations between age and sex and the variables of interest – no correlations were found.
Results
Twenty participants were successfully recruited (see Table 1). Participants were removed from analysis at Time 1 due to problems with adherence to data collection protocol (n=2) and noisy signal due to incorrect sensor placement (n=1); and at Time 2 due to problems with adherence to data collection protocol (n=2) and noisy signal (n=1). Participants’ free-living gait data was removed from analysis due to failure to complete full seven day gait assessment (n=2), problems with adherence to data collection protocol (n=1), and a monitor was lost in the post (n=1). Table 1 provides the success rates for participant recruitment, and Table 2 provides the number of participants for each time point within the study. Overall, from our 20 participants, we had 83% completion of all data.
Table 1.
Success rates of data collection and analysis across the six participating centres.
| Centre | Participants Recruited | Gait Assessments Collected (Analysed) | Success Rate | |||
|---|---|---|---|---|---|---|
| (n) | Day 1 | Day 85 | Day 169 | Collected | Analysed | |
| 1 | 4 | 4 (3) | 3 (3) | 3 (3) | 10/12 (83%) | 9/10 (90%) |
| 2 | 3 | 3 (2) | 3 (3) | 3 (3) | 9/9 (100%) | 8/9 (89%) |
| 3 | 4 | 4 (4) | 4 (4) | 4 (4) | 12/12 (100%) | 12/12 (100%) |
| 4 | 4 | 4 (4) | 4 (4) | 4 (4) | 12/12 (100%) | 12/12 (100%) |
| 5 | 2 | 2 (2) | 2 (2) | 2 (2) | 6/6 (100%) | 6/6 (100%) |
| 6 | 3 | 3 (2) | 3 (0) | 2 (1) | 9/9 (89%) | 3/9 (33%) |
Table 2.
Demographic and baseline assessment information for study participants included in data analysis.
| Time 1 (n=17) | Time 2 (n=17) | 7-day Free-living (n=16) | |
|---|---|---|---|
| Age (years) | 67.41 (7.8) | 68.5 (7.200) | 70.9 (8.3) |
| Sex (m/f) | 9/8 | 7/10 | 8/8 |
| Height (m) | 1.68 (0.09) | 1.685 (0.089) | 1.69 (0.09) |
| MMSE (0-30) | 25 (3) | n/a | n/a |
| CDR (0 -3) | 0.9(.3) | n/a | n/a |
| BADLS (0-60) | 3 (3) | n/a | n/a |
| GDS (0-15) | 2 (1) | n/a | n/a |
| CLOX1 (0-15) | 11 (4) | n/a | n/a |
| CLOX2 (0-15) | 13 (2) | n/a | n/a |
Demographics and cognitive measures for Time 1, Time 2 and free-living assessment for all subjects are provided in Table 2. Participants were older adults (mean age at baseline: 67 years) with very mild Alzheimer’s disease impairment (mean MMSE score at baseline: 25; CDR: 0.9). Participants were not depressed (mean GDS score at baseline: 2/15) with minor impairments in activities of daily living (mean BADLS at baseline: 3/60) and executive function (mean CLOX1 and CLOX2 at baseline: 11/15 and 13/15 respectively).
Clinic-based Data
As previously detailed current algorithmic methods facilitate the generation of three of these characteristics in combination with 11 other discrete outcomes (see Table 3). No significant differences in gait were found between the first and second visit.
Free-living Data
The logical heuristics algorithms for detection and quantification of walking bouts, and the linear and non-linear macro analysis were able to extract all three of these parameters with the addition of several potentially more sensitive measures (see Table 4). Data in Table 3 also provides micro characteristic gait outcomes derived from data collected in both clinic and free-living conditions throughout the feasibility study. We have also included a similarly aged control group from previous works following the same protocol – this is to provide a descriptive comparison regarding expected outcomes from a cognitively intact older population.
Table 4.
Macro gait characteristics (median (interquartile range)) for free-living gait assessment
| Macro gait characteristics | 7-day Free-living Activity (n=16) |
|---|---|
| Total Daily Walk Time (mins) | 214 (66) |
| Total Daily Steps | 13268 (2791) |
| Total Daily Bouts | 707 (233) |
| Mean Bout Length (secs) | 17 (4) |
| Variability | 0.829 (0.059) |
| Alpha | 1.627 (0.065) |
Relationship of clinical and free-living gait outcomes and cognitive function
Clinical gait outcomes and cognition
There was a strong negative correlation between executive function (CLOX 1) and stance time, as measured in the clinic, RHO= - .539, p = .026, with higher levels of executive function associated with shorter stance time (strength of correlation interpreted according to Cohen [35]’s guidelines).
Free-living gait outcomes and cognition
In free-living gait, there was a strong negative correlations between global cognition (MMSE) and step velocity, RHO = - .513, p = .042, and step length, RHO = - .519, p = .039, with lower MMSE scores associated with slower velocity and longer step length. There was a strong positive correlation between global cognition and step velocity variability, RHO = - .522, p = .038, with lower MMSE scores associated with higher variability. There was also a strong negative correlation between global cognition and number of daily bouts, RHO = - .595, p = .015, with lower MMSE scores associated with more daily bouts. Table 5 outlines all associations between cognitive and gait variables.
Table 5.
Relationships between cognition and micro characteristics of gait (Spearman’s coefficient (p value)). Significant correlations are highlighted in bold.
| Clinical Gait Assessment (n=17) | Free-living Gait Assessment (n=16) | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| MMSE | CLOX1 | CLOX2 | MMSE | CLOX1 | CLOX2 | |
| Pace | ||||||
| Step Velocity (m/s) | -.094 (.719) | .195(.453) | .141 (.589) | -.513* (.042) | .161 (.552) | .199 (.459) |
| Step Length (m) | -.219 (.398) | -.017 (.947) | .001 (.996) | -.519* (.039) | -.073 (.788) | .033 (.903) |
| Step Time Variability (s) | -.355 (.161) | -.283 (.271) | -.137 (.599) | -.043 (.874) | .063 (.818) | .130 (.632) |
| Swing Time Variability (s) | -.303 (.236) | -.104 (.692) | -.065 (.804) | .012 (.965) | -.048 (.861) | -.023 (.934) |
| Stance Time Variability (S) | -.368 (.146) | -.271 (.294) | -.127 (.626) | -.004 (.987) | .089 (.742) | .153 (.573) |
| Variability (SD) | ||||||
| Step Velocity Variability (m/s) | -.421 (.092) | -.073 (.781) | -.009 (.973) | -.522* (.038) | .313 (.239) | .275 (.303) |
| Step Length Variability (m) | -.253 (.328) | 0.000 (1.000) | .140 (.592) | -.116 (.670) | .269 (.313) | .014 (.960) |
| Rhythm | ||||||
| Step Time (s) | -.168 (.518) | -.472 (.056) | -.387 (.125) | .187 (.488) | -.289 (.278) | -.234 (.383) |
| Swing Time (s) | -.102 (.698) | -.290 (.258) | -.251 (.331) | .096 (.723) | -.274 (.305) | -.198 (.463) |
| Stance Time (s) | -.287 (.263) | -.539* (.026) | -.384 (.129) | .212 (.431) | -.244 (.362) | -.131 (.628) |
| Asymmetry | ||||||
| Step Time Asymmetry (s) | -.033 (.900) | -.238 (.358) | .009 (.973) | -.251 (.349) | -.009 (.974) | .374 (.153) |
| Swing Time Asymmetry (s) | -.217 (.403) | -.362 (.153) | -.216 (.405) | -.074 (.785) | -.065 (.810) | .275 (.303) |
| Stance Time Asymmetry (s) | .069 (.791) | -.183 (.482) | .041 (.875) | -.196 (.468) | -.006 (.983) | .355 (.177) |
| Postural Control | ||||||
| Step Length Asymmetry (m) | -.211 (.417) | -.151 (.563) | .024 (.926) | -.393 (.132) | .076 (.780) | .035 (.898) |
Discussion
The aim of this study was to determine the acceptability and feasibility of gait assessment in the clinic and the home using body-worn sensors in a dementia population. This study is novel in reporting both micro and macro gait outcomes in both the clinic and the home in a mild AD cohort across multiple sites nationally. Outcomes reported were comprehensive and clinically appropriate measures for both micro, looking at aspects of pace, variability, rhythm, asymmetry and postural control, and macro gait characteristics, looking at aspects of volume, pattern and variability of walking behaviours. This allows a widely representative picture of gait, which is necessary when considering gait’s potential clinical utility.
Data were successfully collected from the majority of participants; demonstrating people with mild AD can complete clinical gait assessments and the utility of body-worn sensors as a non-invasive clinical tool that can quantitatively assess gait over prolonged periods. This study two lab gait assessments and one free-living assessment; this has not previously been done in the literature [18–20, 22]. No significant differences were found between the first and second visits; this may be due to the short time period between visits and the increased familiarity with testing conditions. However, the majority of participants attended the follow-up visit, showing willingness to undergo clinical gait assessment multiple times. Previous research has also found that body-worn sensors generally elicit positive feedback from participants, noting that they are comfortable and easy to wear.
Although this study did not collect data from controls, inferences can be made from a previous data set of similar aged controls using the same body-worn sensors and derived gait outcomes as seen in Table 3 [17]. Regarding micro gait characteristics, within the clinic people with mild AD dementia demonstrated impairment across all domains; they walked more slowly and were more asymmetrical with impaired variability and postural control of gait compared to this reference control group. This indicates that gait is a potentially useful discriminatory tool between dementia and cognitively intact older adults. These findings were not as prominent in free-living gait assessment, possibly due to familiarity of home environment or due to increased complexity of free-living gait (i.e. environmental demands, obstacles, turning, walking while engaging in multiple activities [36]). For example, controls may engage in more complex walking activities such as large grocery trips or longer recreational walks, while people with dementia may stay closer to home and only engage in familiar activities they feel confident engaging in. The sample size is however very small and larger groups are needed to explore differences in free-living gait across group. Further research is necessary to assess the potential of free-living gait as a useful complementary diagnostic marker for dementia.
Another interesting finding was the reported average daily step count of 14,983 and average walking time of 220 minutes – almost 5000 steps and 70 minutes over the recommended daily step goal and time engaging in physical activity for adults respectively [37]. This goes against the norm, with the literature generally reporting approximately 6000 steps a day in studies using a pedometer [38]. Although this could be due to a small group of research-enthused participants, different sensors, such as pedometers, use different bout lengths to identify walking (i.e. some might identify over 60 seconds of activity as walking [39], others may identify over 10 steps as walking). This is considered problematic, as 90% of walking takes place in under 60 second bouts [14]; a higher cut-off threshold can therefore miss data and report lower step counts. Del Din, et al. [14] report a difference of approximately 7,500 steps when using a > 60 second cut-off threshold compared to including all walking bouts. Gietzelt, et al. [22] found controls had a higher number of walking bouts compared to people with dementia; however, the study only included walking bouts > 20 seconds. For patient populations, it could be argued that including all walking bout lengths is beneficial in order to provide a truer picture – people with dementia may spend more time in short bouts of walking compared to long bouts. Adopting a previously validated algorithm and data analysis process, this study identified walking as three or more steps with no minimum resting period [16, 17]. We propose the differences in the average daily activity demonstrated in this study and previous studies is due to a more sensitive measure of walking and a high proportion of steps taking place in short bouts of walking.
As previously stated, strong associations between gait and cognition have been established, along with suggestions that gait impairment predicts cognitive decline [3, 4, 6, 40, 41]. This study similarly found correlations between global cognition and characteristics of pace and variability in free-living gait and a relationship between executive function and temporal characteristics of gait in the clinic. Lower cognitive scores were associated with slower pace and impaired timing and variability. Although these findings reflect previous literature, it was expected that a stronger relationship would be seen between gait and cognition in the clinic. This may become more obvious as cognitive impairment progresses; this particular cohort was still very early stage AD. Additionally, these cognitive measures were employed as they are commonly used in clinics – more sensitive and specific measures used during in-depth neuropsychological testing may have produced stronger results. It was also found that lower cognitive scores were associated with more daily bouts of walking – our participants may have been taking an increased number of short bouts. Future research should look at this in relationship to variability of bout length and alpha, the ratio of long to short bouts, scores to better understand its meaning.
Limitations and recommendations for the future
Several key considerations for future research should be discussed. This study was a multi-centre study, which led to between-site differences in gait protocol and data collection. Body-worn sensors have the ability to assess sensitive and specific measures of gait, negating the need for a more technical gait laboratory and team of experts on-site. However, it is imperative that protocols are performed uniformly across sites in order to reduce heterogeneity. Therefore, when adopting this approach, fidelity to protocol should be regularly ascertained. Data was also lost during the study. This was not due to participants, but largely due to problems with the data collection protocol – this is an important outcome from this feasibility study, highlighting the need for efficient and regular training and refresher courses for research staff regarding use of technology and implementation of protocols. Clinic-based protocols differed in length of intermittent walks (either 6m or 10m). This was influenced by availability of space. Although it is recommended that longer walks provide more sensitive measures of steady state gait, only one centre could provide such a space. Therefore, it may be more feasible for future research to use shorter walks (6m or less) standardised across centres to ensure more accurate data pooling. It has also been shown that dual-task gait is a sensitive predictor of dementia [42, 43]; future research should encapsulate a dual-task protocol to improve gait’s utility as a clinical biomarker. Although the core aim of this study was to assess feasibility of such studies, the findings were limited by the small sample size and lack of a control group for comparative purposes. Therefore, these findings are only preliminary. A larger sample and comparison with a healthy older adult control cohort will facilitate and strengthen data interpretation to determine utility of gait as a clinical biomarker in early stages of cognitive impairment and prodromal dementia. This will form part of the main DFP study.
Clinical Implications
Body-worn sensors can provide an enriched picture of an individual’s gait function and habitual walking activities that could act as a complementary diagnostic tool for clinicians. Clinical use of body-worn sensors in annual health assessments could track gait changes over time and act as a red flag for cognitive impairment. The ability to use these sensors in a home environment has added benefit because it provides continuous data over prolonged time periods which is more representative of gait rather than a one-off assessment, and reduced need to attend for assessment [14]. Free-living gait also captures environmental and cognitive challenges not seen in controlled clinical settings, and may provide a more realistic picture of gait without confounding factors such as observer bias and unfamiliar testing conditions [14]. Assessment of macro gait characteristics can also provide an easily understandable insight into changes in behaviour related to cognitive decline. Physical inactivity has been associated with dementia but reports are limited by use of self-report measures, which may be biased and unreliable [44]. Body-worn sensors could provide objective insights into volume and patterns of walking activity across healthy older adults to dementia populations [14]. This could also improve current physical rehabilitation strategies as interventions can be personalised to habitual activity [45].
Conclusion
In conclusion, it is feasible to assess quantitative gait characteristics in both the clinic and the home in a cognitively impaired cohort. Going forward, care to standardise training and assess fidelity are important. Body-worn sensors hold great potential as a clinical tool that can provide a personalised picture of individual’s current gait function and could map changes across time. Future research aims to assess clinic and free-living gait in a cohort of prodromal Alzheimer’s disease as part of a larger study establishing a range of clinical biomarkers for dementia.
Table 6.
Relationships between cognition and macro characteristics of gait (Spearman’s coefficient (p value). Significant correlations are highlighted in bold.
| MMSE | CLOX1 | CLOX2 | |
|---|---|---|---|
| Total Daily Walk Time (mins) | -.436 (.091) | -.077 (.776) | -.015 (.956) |
| Total Daily Steps | -.350 (.184) | -.083 (759) | -.139 (.608) |
| Total Daily Bouts | -.595* (.015) | .055 (.839) | .047 (863) |
| Mean Bout Length (secs) | .087 (.747) | -.229 (.393) | .092 (.734) |
| Variability | .388 (.137) | -.159 (.556) | -.196 (.466) |
| Alpha | -.110 (.686) | .138 (.609) | .174 (.520) |
Acknowledgements
The Deep and Frequent Phenotyping study is funded by MRC and NIHR as part of Dementias Platform UK. The authors would like to thank the NIHR Translational Research Collaboration in Dementia (constituting of six specialist centres of excellence; NIHR Biomedical Research Centers or Dementia Units associated with the following NHS Trusts: Oxford University Hospitals (OUH), South London and Maudsley (SLaM), Cambridge University Hospitals (CUHT), University College Hospital London (UCLH), West London Mental Health Care (WLMHC) and Newcastle Hospitals), the participants, and the research staff at each of the six respective sites who completed data collection and transfer. The members of the Brain and Movement Research Group at Newcastle are funded by the National Institute for Health Research (NIHR) Newcastle Biomedical Research Unit and Newcastle Biomedical Research Centre based at Newcastle upon Tyne Hospitals NHS Foundation Trust and Newcastle University. The views expressed are those of the authors and not necessarily those of the NHS, the NIHR or the Department of Health.
Footnotes
Conflict of Interest/Disclosure Statement
The authors have no conflict of interest to report.
References
- [1].Prince M, Wimo A, Guerchet M, Ali G, Wu YT, Prina M. World Alzheimer Report 2015. The global impact of dementia. An analysis of prevalence, incidence, cost and trends. Alzheimer's Disease International; London: 2015. [Google Scholar]
- [2].Korolev IO, Symonds LL, Bozoki AC, Initi AsDN. Predicting Progression from Mild Cognitive Impairment to Alzheimer's Dementia Using Clinical, MRI, and Plasma Biomarkers via Probabilistic Pattern Classification. Plos One. 2016;11:e0138866. doi: 10.1371/journal.pone.0138866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Beauchet O, Annweiler C, Callisaya ML, De Cock AM, Helbostad JL, Kressig RW, Srikanth V, Steinmetz JP, Blumen HM, Verghese J, Allali G. Poor Gait Performance and Prediction of Dementia: Results From a Meta-Analysis. J Am Med Dir Assoc. 2016;17:482–490. doi: 10.1016/j.jamda.2015.12.092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Morris R, Lord S, Bunce J, Burn D, Rochester L. Gait and cognition: Mapping the global and discrete relationships in ageing and neurodegenerative disease. Neurosci Biobehav Rev. 2016;64:326–345. doi: 10.1016/j.neubiorev.2016.02.012. [DOI] [PubMed] [Google Scholar]
- [5].Studenski S, Perera S, Patel K, Rosano C, Faulkner K, Inzitari M, Brach J, Chandler J, Cawthon P, Connor EB, Nevitt M, et al. Gait speed and survival in older adults. JAMA. 2011;305:50–58. doi: 10.1001/jama.2010.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Verghese J, Wang C, Lipton RB, Holtzer R, Xue X. Quantitative gait dysfunction and risk of cognitive decline and dementia. J Neurol Neurosurg Psychiatry. 2007;78:929–935. doi: 10.1136/jnnp.2006.106914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Buracchio T, Dodge HH, Howieson D, Wasserman D, Kaye J. The trajectory of gait speed preceding mild cognitive impairment. Arch Neurol. 2010;67:980–986. doi: 10.1001/archneurol.2010.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].MacAulay RK, Allaire T, Brouillette R, Foil H, Bruce-Keller AJ, Keller JN. Apolipoprotein E genotype linked to spatial gait characteristics: predictors of cognitive dual task gait change. PloS one. 2016;11:e0156732. doi: 10.1371/journal.pone.0156732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Sakurai R, Montero-Odasso M. Apolipoprotein E4 Allele and Gait Performance in Mild Cognitive Impairment: Results From the Gait and Brain Study. Journals of Gerontology Series A: Biomedical Sciences and Medical Sciences. 2017 doi: 10.1093/gerona/glx075. glx075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Mc Ardle R, Morris R, Wilson J, Galna B, Thomas AJ, Rochester L. What can quantitative gait analysis tell us about dementia and its subtypes? A structured review. Journal of Alzheimers Disease. 2017;60 doi: 10.3233/JAD-170541. [DOI] [PubMed] [Google Scholar]
- [11].Tao WJ, Liu T, Zheng RC, Feng HT. Gait Analysis Using Wearable Sensors. Sensors. 2012;12:2255–2283. doi: 10.3390/s120202255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Lord S, Galna B, Rochester L. Moving forward on gait measurement: toward a more refined approach. Mov Disord. 2013;28:1534–1543. doi: 10.1002/mds.25545. [DOI] [PubMed] [Google Scholar]
- [13].Kaye J, Mattek N, Dodge H, Buracchio T, Austin D, Hagler S, Pavel M, Hayes T. One walk a year to 1000 within a year: Continuous in-home unobtrusive gait assessment of older adults. Gait & Posture. 2012;35:197–202. doi: 10.1016/j.gaitpost.2011.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Del Din S, Godfrey A, Mazza C, Lord S, Rochester L. Free-living monitoring of Parkinson's disease: Lessons from the field. Mov Disord. 2016;31:1293–1313. doi: 10.1002/mds.26718. [DOI] [PubMed] [Google Scholar]
- [15].Lord S, Galna B, Verghese J, Coleman S, Burn D, Rochester L. Independent domains of gait in older adults and associated motor and nonmotor attributes: validation of a factor analysis approach. J Gerontol A Biol Sci Med Sci. 2013;68:820–827. doi: 10.1093/gerona/gls255. [DOI] [PubMed] [Google Scholar]
- [16].Hickey A, Del Din S, Rochester L, Godfrey A. Detecting free-living steps and walking bouts: validating an algorithm for macro gait analysis. Physiol Meas. 2017;38:N1–N15. doi: 10.1088/1361-6579/38/1/N1. [DOI] [PubMed] [Google Scholar]
- [17].Del Din S, Godfrey A, Galna B, Lord S, Rochester L. Free-living gait characteristics in ageing and Parkinson's disease: impact of environment and ambulatory bout length. J Neuroeng Rehabil. 2016;13:46. doi: 10.1186/s12984-016-0154-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Gillain S, Warzee E, Lekeu F, Wojtasik V, Maquet D, Croisier J-L, Salmon E, Petermans J. The value of instrumental gait analysis in elderly healthy, MCI or Alzheimer's disease subjects and a comparison with other clinical tests used in single and dual-task conditions. Annals of physical and rehabilitation medicine. 2009;52:453–474. doi: 10.1016/j.rehab.2008.10.004. [DOI] [PubMed] [Google Scholar]
- [19].Chung P-C, Hsu Y-L, Wang C-Y, Lin C-W, Wang J-S, Pai M-C. Circuits and Systems (ISCAS), 2012 IEEE International Symposium; IEEE; 2012. pp. 1323–1326. [Google Scholar]
- [20].Greene BR, Kenny RA. Assessment of cognitive decline through quantitative analysis of the timed up and go test. IEEE Transactions on Biomedical Engineering. 2012;59:988–995. doi: 10.1109/TBME.2011.2181844. [DOI] [PubMed] [Google Scholar]
- [21].Mc Ardle R, Morris R, Wilson J, Galna B, Thomas AJ, Rochester L. What can quantitative gait analysis tell us about dementia and its subtypes? A structured review. J Alzheimers Dis. 2017;60 doi: 10.3233/JAD-170541. [DOI] [PubMed] [Google Scholar]
- [22].Gietzelt M, Wolf K, Kohlmann M, Marschollek M, Haux R. Measurement of Accelerometry-based Gait Parameters in People with and without Dementia in the Field. Methods Inf Med. 2013;52:319–325. doi: 10.3414/ME12-02-0009. [DOI] [PubMed] [Google Scholar]
- [23].McKhann GM, Knopman DS, Chertkow H, Hyman BT, Jack CR, Jr, Kawas CH, Klunk WE, Koroshetz WJ, Manly JJ, Mayeux R, Mohs RC, et al. The diagnosis of dementia due to Alzheimer's disease: recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement. 2011;7:263–269. doi: 10.1016/j.jalz.2011.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”: a practical method for grading the cognitive state of patients for the clinician. Journal of psychiatric research. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- [25].Hughes CP, Berg L, Danziger WL, Coben LA, Martin RL. A new clinical scale for the staging of dementia. Br J Psychiatry. 1982;140:566–572. doi: 10.1192/bjp.140.6.566. [DOI] [PubMed] [Google Scholar]
- [26].Royall DR, Cordes JA, Polk M. CLOX: an executive clock drawing task. J Neurol Neurosurg Psychiatry. 1998;64:588–594. doi: 10.1136/jnnp.64.5.588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Fish J. Encyclopedia of Clinical Neuropsychology. Springer; 2011. Bristol Activities of Daily Living Scale; pp. 452–453. [Google Scholar]
- [28].Brink TL, Yesavage JA, Lum O. Geriatric depression scale. 1983.
- [29].Drago I, Mellia MM, Munafo M, Sperotto A, Sadre R, Pras A. Proceedings of the 2012 ACM conference on Internet measurement conference - IMC '12; Boston, Massachusetts, USA: ACM; 2012. [Google Scholar]
- [30].Ruff N, Ledoux F, Eads IW. Application Security Forum. 2012.
- [31].McCamley J, Donati M, Grimpampi E, Mazza C. An enhanced estimate of initial contact and final contact instants of time using lower trunk inertial sensor data. Gait Posture. 2012;36:316–318. doi: 10.1016/j.gaitpost.2012.02.019. [DOI] [PubMed] [Google Scholar]
- [32].Zijlstra A, Zijlstra W. Trunk-acceleration based assessment of gait parameters in older persons: a comparison of reliability and validity of four inverted pendulum based estimations. Gait Posture. 2013;38:940–944. doi: 10.1016/j.gaitpost.2013.04.021. [DOI] [PubMed] [Google Scholar]
- [33].Del Din S, Hickey A, Woodman S, Hiden H, Morris R, Watson P, Nazarpour K, Catt M, Rochester L, Godfrey A. IEEE Workshop on Statistical Signal Processing; IEEE; 2016. [Google Scholar]
- [34].Chastin SF, Granat MH. Methods for objective measure, quantification and analysis of sedentary behaviour and inactivity. Gait Posture. 2010;31:82–86. doi: 10.1016/j.gaitpost.2009.09.002. [DOI] [PubMed] [Google Scholar]
- [35].Cohen J. Statistical power analysis for the behavioral sciences. Vol. 2 Hilsdale. NJ: Lawrence Earlbaum Associates; 1988. [Google Scholar]
- [36].Hickey A, Stuart S, O'donovan K, Godfrey A. Walk on the wild side: the complexity of free-living mobility assessment. J Epidemiol Community Health. 2017 doi: 10.1136/jech-2016-208752. jech-2016-208752. [DOI] [PubMed] [Google Scholar]
- [37].World Health Organization. Global recommendations on physical activity for health. 2010. [PubMed]
- [38].Choi BC, Pak AW, Choi JC, Choi EC. Daily step goal of 10,000 steps: a literature review. Clin Invest Med. 2007;30:E146–151. doi: 10.25011/cim.v30i3.1083. [DOI] [PubMed] [Google Scholar]
- [39].Lara J, O'Brien N, Godfrey A, Heaven B, Evans EH, Lloyd S, Moffatt S, Moynihan PJ, Meyer TD, Rochester L, Sniehotta FF, et al. Pilot Randomised Controlled Trial of a Web-Based Intervention to Promote Healthy Eating, Physical Activity and Meaningful Social Connections Compared with Usual Care Control in People of Retirement Age Recruited from Workplaces. PLoS One. 2016;11:e0159703. doi: 10.1371/journal.pone.0159703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Verghese J, Lipton RB, Hall CB, Kuslansky G, Katz MJ, Buschke H. Abnormality of gait as a predictor of non-Alzheimer's dementia. N Engl J Med. 2002;347:1761–1768. doi: 10.1056/NEJMoa020441. [DOI] [PubMed] [Google Scholar]
- [41].Verghese J, Lipton R, Ayers E. Spatial navigation and risk of cognitive impairment: A prospective cohort study. Alzheimer's & Dementia. 2017 doi: 10.1016/j.jalz.2017.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Montero-Odasso MM, Sarquis-Adamson Y, Speechley M, Borrie MJ, Hachinki VC, Wells J, Riccio PM, Schapira M, Sejdic E, Camicioli RM. Association of Dual-Task Gait With Incident Dementia in Mild Cognitive Impairment: Results From the Gait and Brain Study. JAMA neurology. 2017 doi: 10.1001/jamaneurol.2017.0643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].MacAulay RK, Wagner MT, Szeles D, Milano NJ. Improving Sensitivity to Detect Mild Cognitive Impairment: Cognitive Load Dual-Task Gait Speed Assessment. Journal of the International Neuropsychological Society. 2017:1–9. doi: 10.1017/S1355617717000261. [DOI] [PubMed] [Google Scholar]
- [44].van Alphen HJM, Volkers KM, Blankevoort CG, Scherder EJA, Hortobágyi T, van Heuvelen MJG. Older adults with dementia are sedentary for most of the day. PloS one. 2016;11:e0152457. doi: 10.1371/journal.pone.0152457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Pavel M, Jimison HB, Wactlar HD, Hayes TL, Barkis W, Skapik J, Kaye J. The role of technology and engineering models in transforming healthcare. IEEE Rev Biomed Eng. 2013;6:156–177. doi: 10.1109/RBME.2012.2222636. [DOI] [PubMed] [Google Scholar]