Abstract
Context
Sex-specific effects of pregnancy calcium carbonate supplementation have been reported in 8-12 year old Gambian children, indicating faster growth in boys but slower growth in girls born to calcium-supplemented mothers.
Objective
To determine whether the pregnancy supplement resulted in sex-specific effects on offspring IGF1 and other growth-related indices in mid-childhood.
Design
Analysis of archived data obtained in mid-childhood from the children of rural Gambian mothers who had been randomised to 1500 mgCa/d (Ca) or placebo (P) from 20 weeks pregnancy to delivery (ISRCTN96502494).
Participants and Methods
Of the 526 children born and followed in infancy, 290 had early-morning, fasting plasma assayed for IGF1, IGFBP3, leptin, insulin and calcium-related indices and had anthropometry performed at age 7.5(SD1.2) years (N/group: Males(M)-Ca=64, Females(F)-Ca=77; M-P=76, F-P=73). Sex-specific effects of maternal supplementation were considered using regression with sexes separated and together to test for sex*supplement interactions.
Results
Boys had lower IGF1, IGFBP3, leptin and insulin than girls (p≤0.004). IGF1 was higher in M-Ca than M-P (+14.2 (SE7.7)%, P=0.05) but lower in F-Ca than F-P (-17.8 (SE7.4)%, P=0.01); sex*supplement interaction P=0.001. IGF1 concentrations (ng/ml, geometric mean [-1SE,+1SE]) were M-Ca=78.1[4.3,4.5], M-P=67.8[3.4,3.6]; F-Ca=99.5[4.8,5.1], F-P=118.9[6.4,6.8]). Similar sex*supplement interactions were seen for IGFBP3 and IGF1-adjusted-for-IGFBP3 but group differences were smaller. There were no significant supplement effects on the other biochemical indices.
Conclusions
Calcium carbonate supplementation of pregnant Gambian mothers resulted in higher IGF1 in boys and lower IGF1 in girls during mid-childhood, consistent with the reported maternal supplement effects on growth of the offspring in later childhood.
Keywords: calcium, insulin-like growth factor-1, growth, pregnancy, programming, supplementation
Introduction
We have previously reported unexpected and sex-specific long-term effects of calcium carbonate supplementation on the growth and bone development of rural Gambian children [1, 2]. Firstly, 12 months of calcium supplementation in prepubertal 8-12 year olds (ISRCTN28836000) advanced the timing of the pubertal growth spurt in boys, such that peak height velocity was attained earlier than in the placebo group [1], with corresponding advancement of the peak velocities of bone expansion and mineral accrual [2]. No such effect was seen in girls [1, 2]. These findings paralleled those of our trials in British 16-18 year olds in which calcium carbonate supplementation for 12 months increased stature and bone growth in adolescent boys [3] but not in post-menarcheal girls [4].
Secondly, calcium carbonate supplementation of rural Gambian mothers from 20 weeks pregnancy to term (ISRCTN96502494) resulted in shorter stature and smaller bone size and mineral accrual of girls at age 8-12 years and a trend towards greater linear growth in boys [5, 6], when no detectable effects had been observed at birth, in infancy or at mean age 7.5 years [7–9]. This suggests, as in the first study, that the calcium carbonate supplement had resulted in sex-specific effects on growth, this time of the offspring, indicated by slower growth in girls and accelerated growth in boys by age 8-12 years.
A possible explanation for these findings is that calcium carbonate supplementation in childhood or in utero altered the activation of the metabolic events that trigger the initiation of puberty, advancing skeletal growth and development in boys but delaying in girls. This would imply an alteration, in a sex-specific manner, in the activation of the hypothalamic-pituitary-gonadal (HPA) axis, which occurs several years prior to the growth spurt and before the appearance of visible pubertal signs [10]. Insulin-like growth factor 1 (IGF1) is a major driver of this process [11, 12] and a principal anabolic factor mediating postnatal and pubertal bone growth [13]. Circulating IGF1 in children and adolescents has been shown to be raised in response to supplementation with calcium carbonate [14] and milk [15, 16] and, in mid-childhood, is predictive of age at menarche in girls [17]. This gives rise to the possibility that the findings of the Gambian trials were related to effects of the supplement, in childhood and in utero, on circulating IGF1 concentrations that differed between boys and girls.
To investigate this hypothesis, we have conducted an analysis of archived biochemical data obtained at mean age 7.5 years from the cohort of Gambian children whose mothers had participated in the pregnancy calcium supplementation trial. The aim was to test whether the maternal supplement had resulted in sex-specific effects on the circulating IGF1 and other growth-related indices of their offspring in mid-childhood, prior to the age when the differential effects of the supplement on their growth were detected.
Subjects and Methods
Participants and study design
Data used in this study were from the children of rural Gambian mothers who had participated in a randomised, placebo-controlled trial of calcium supplementation in pregnancy between 1995-2000 (ISRCTN96502494), who had delivered a healthy singleton baby. These children took part in an investigation of the effects of pregnancy calcium supplementation on childhood blood pressure and growth between November 2005 and August 2006 at age 5-10 years [9]. Scientific approval for the follow-up study was obtained from the Medical Research Council (MRC) Laboratories The Gambia Scientific Co-ordinating Committee. Ethical approval was granted by The Gambia Government/MRC Laboratories Ethics Committee and the London School of Hygiene and Tropical Medicine Ethics Committee. Full written informed consent was obtained from the parents or guardians of each child after the purpose of the study had been explained in the local language. All the families, investigators and field workers in The Gambia and the laboratory staff in Cambridge were blinded to the supplement group allocations.
The mothers in the pregnancy trial were residents of the rural province of West Kiang, The Gambia, West Africa, latitude 13°N. In this resource-poor region, undernutrition is common, calcium intakes are low, childhood growth is poor and puberty is delayed [7, 18, 19]. Full details of the pregnancy trial are elsewhere [8] but, in brief, the calcium supplement was 1500 mg per day elemental calcium given as calcium carbonate (3 tablets of orange-flavoured Calcichew, Nycomed Pharma AS distributed in the UK by Shire Pharmaceutical Development Ltd); the matching placebo supplement was of cellulose-lactose (Nycomed Pharma AS). The mean (SD) calcium intake of mothers, measured in a sub-set of these women at 20 weeks of pregnancy, was 355 (190) mg/day) [7]. Randomisation was in weekly blocks of 4 subjects to minimise confounding by season. The supplements were consumed daily in the late afternoon from 20 weeks of pregnancy to term. The supplements were well accepted and compliance was high (averaging 97% in both groups).
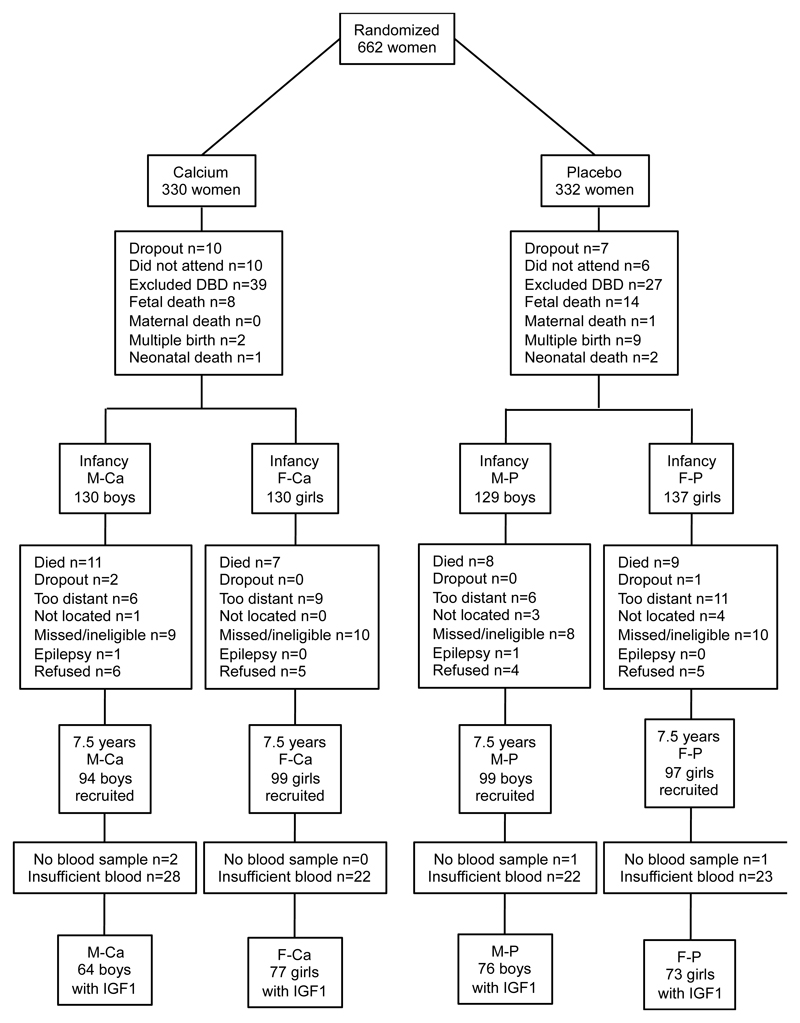
The children in the follow-up study had been traced and enrolled following village sensitisation meetings. Of the 526 children born and followed into infancy, 389 children took part; 193 whose mothers had been in the calcium-supplemented group, 196 in the placebo group. IGF1 had been determined in blood samples where there was sufficient volume (n=290). These were from boys and girls born to mothers who had received calcium supplementation (Males (M)-Ca, n=64; Females (F)-Ca, n=77) or placebo (M-P, n=76; F-P, n=73). The flow diagram of the children, by sex and trial group allocation of their mother, and the characteristics of those lost to follow up, is in Figure 1. The children with an IGF1 value were older by 0.5 (0.1) years (P ≤0.001), and were consequently taller and heavier, than the other children who participated in the follow-up study but their weights, heights and head circumferences during infancy were not significantly different (all P>0.3).
Figure 1.
Flow diagram of the children in the study by sex and by trial group allocation of their mother. M-Ca = male children of mothers in the calcium supplemented group; F-Ca = female children of mothers in the calcium supplemented group; M-P = male children of mothers in the placebo group; F-P = female children of mothers in the placebo group; DBD = mothers who delivered a term baby before their due date and who therefore had started supplementation at a later stage of gestation than specified for the trial.
Blood collection and anthropometry
A venous blood sample (10ml) had been obtained from each child between 08.00h and 10.00h after an overnight fast. Blood was collected into EDTA for the assay of parathyroid hormone (PTH) and into lithium heparin for the other analytes reported here. Each sample was placed in an insulated box containing pre-cooled freezer packs and transported to the MRC Laboratory in Keneba, West Kiang, for immediate processing and storage at -80°C. Samples were subsequently transported on dry ice to MRC Human Nutrition Research, Cambridge, UK (now the MRC Elsie Widdowson Laboratory) and stored at -80°C prior to analysis between July and October 2006.
On the day of blood collection, the height, weight, mid-upper arm circumference (MUAC) and triceps skinfold thickness (TST) of each child were measured [9]. Date of birth and infant growth data were available from the original trial [8].
Biochemical assays
The plasma samples were analysed for IGF1, IGF-binding protein 3 (IGFBP3), PTH, 1,25-dihydroxyvitamin D (1,25(OH)2D), 25-hydroxyvitamin D (25(OH)D), leptin, insulin, calcium, phosphate and albumin. IGF1, IGFBP3 and intact PTH were assayed by Immulite 1000 (Diagnostic Products Corporation, Los Angeles, USA), 1,25(OH)2D by radioimmunassay (IDS), 25(OH)D by chemiluminescent immunoassay (Liaison, DiaSorin Inc., Stillwater, USA), the other analytes were measured on a Konelab analyser (Thermo Fischer Scientific, Vantaa, Finland) using the following Thermo Scientific kits: calcium, arsenazo III method; phosphorus, ammonium molybdate method; albumin, bromocresol purple method. PTH values <7.0 pg/ml were excluded because, as advised by the manufacturers, these were likely due to haemolysis or to interference by excess EDTA when sample volume per tube was insufficient, resulting in implausibly low values. Leptin was assayed by Quantikine Solid-Phase ELISA (R&D Systems, Minneapolis, USA). Insulin was measured on a 1235 AutoDELFIA automatic immunoassay system using a 2-step time–resolved fluorometric assay (Dako Ltd, Turku, Finland) [20]
Quality assurance was achieved using control materials supplied by the kit manufacturers and commercial materials as follows: minerals and albumin, Roche serum control (Roche Diagnostic Corporation, Indianapolis, USA), Lyphochek (Bio-Rad Laboratories, Herts, UK), NEQAS Clin Chem (Birmingham, UK) and an internal plasma drift control; PTH, NEQAS (Edinburgh, UK); for 25(OH)D; and 1,25(OH)2D, DEQAS (www.deqas.org).
Statistical analysis
Data were analysed using DataDesk 6.3.1 (Data Description Inc, Ithaca, NY). Summary statistics by group are presented as mean (SE) for normally distributed values, and as geometric mean (-1SE, +1SE) for positively skewed data. The latter were derived by calculating the mean (SE) in data transformed to natural logarithms followed by back transformation. Conventionally, IGF1 divided by IGFBP3 is reported as an index of available IGF1. In this data set, IGF1 was more closely related to the square root of IGFBP3 (loge[IGF1] = k + 0.54loge[IGFBP3], P≤0.0001). For comparison, both the index IGF1:√IGFBP3 and the conventional IGF1:IGFBP3 are reported.
A square root power transformation provided the best normalisation for the IGF1 distribution, as has been reported by others [21], and was used in all models. This transformation was also used to model the two indices relating IGF1 to IGFBP3. All other data were modelled after transformation to natural logarithms. Mean (SE) differences between groups are presented as sympercents (difference/mean) derived by multiplying the mean (SE) difference in natural logarithms multiplied by 100 [22, 23].
ANOVA and ANCOVA models were established using the Linear Model software within DataDesk, firstly with supplement groups separated to test for differences between boys and girls in each group, then with boys and girls separated to test for differences between the supplement groups in each sex, and finally with all data combined to test for a sex*supplement group interaction. Following this, potential covariates were added into full models with stepwise back elimination of non-significant variables (those with P>0.05).
The possibility that any sex and supplement effect might differ across the age range of the cohort was tested for in these models by including current age and appropriate age interaction terms. Infant length at 52 weeks postpartum was included to adjust for the potential influence of size in early life [5], there having been no significant effect of the maternal supplement in either boys or girls in infancy [8]. Length at 52 weeks was selected to adjust for inter-individual variation in infant size because it had been shown previously in growth models of this cohort to produce the greatest reductions in the residual variance compared to other anthropometric measures between 2 weeks and 52 weeks postpartum [5]. Current height, weight and BMI were also considered as potential predictors of the biochemical factors. In addition, possible effects of season of birth and maternal calcium intake on IGF1 and the response to the supplementation were investigated but no significant effects were observed and are not discussed further.
Height, weight and BMI standard deviation scores (SD(Z)-scores: HAZ, WAZ, BMIAZ) relative to British children of the same age were calculated at the time of the original study [9]. These measures are presented as summary statistics but were not included as potential covariates in statistical models to avoid artificially inflating or diminishing any sex differences because of the known differences in maturational delay between Gambian boys and girls when expressed relative to the growth trajectories of Western children [18].
Results
The mean (SE) age of the children was 7.5 (0.1) years (SD 1.2, range 5.4-10.5 years) and was not significantly different between the four sex-supplement groups (P = 0.9). The children had lower attained height, weight and BMI relative to British reference children of the same age, as shown by mean SD-scores below zero (mean (SE) boys: HAZ = -1.03 (0.07), WAZ = -1.48 (0.08), BMIAZ = -1.04 (0.07); girls: HAZ = -0.82 (0.07), WAZ -1.35 (0.08), BMIAZ = -1.27 (0.08)).
Table 1 presents the IGF1, IGFBP3 and anthropometric data by sex. Overall, boys had significantly lower IGF1, IGFBP3, the two IGF1/IGFBP3 indices, MUAC and TST than girls and greater BMI throughout the age range (age*sex interaction: all P>0.4). There was no significant difference between boys and girls in height or weight. Age was a significant positive predictor of all variables in Table 1 except BMI (P = 0.1) but infant size (length at 52 weeks) was only a significant predictor of the anthropometric variables. Age adjustment made little difference to the size or significance of the sex differences observed in IGF1 and IGFBP3. When adjusted for current age and infant size, boys had attained significantly less height, weight, MUAC and TST than girls relative to their size in infancy (all P ≤0.001).
Table 1. IGF1, IGFBP3 and anthropometry of the children by sex.
| Boys | Girls | P | |
|---|---|---|---|
|
|
|
||
| n=140 | n=150 | ||
| Age years | 7.6 (0.1) | 7.5 (0.1) | 0.6 |
| IGF1 ng/ml a,b | 72.3 (2.7,2.8) | 108.5 (4.0,4.2) | ≤0.001 |
| IGFBP3 µg/ml | 2.57 (0.08) | 2.90 (0.08) | 0.03 |
| IGF1:√IGFBP3 a,b | 46.7 (1.6,1.6) | 66.1 (2.2,2.3) | ≤0.001 |
| IGF1: IGFBP3 a,b | 29.9 (1.1,1.1) | 40.3 (1.6,1.7) | ≤0.001 |
| Height cm | 119.2 (0.6) | 119.1 (0.7) | 0.9 |
| Weight kg | 20.2 (0.3) | 19.8 (0.3) | 0.3 |
| BMI kg/m2 | 14.1 (0.1) | 13.9 (0.1) | 0.05 |
| MUAC cm | 16.1 (0.1) | 16.5 (0.1) | 0.005 |
| TST mm | 5.8 (0.1) | 7.1 (0.1) | ≤0.001 |
Data are mean (SE), or for positively skewed distributions, geometric mean (-1SE,+1SE) calculated with data transformed to logarithms followed by back transformation. BMI, body mass index; MUAC, mid-upper arm circumference; TST, triceps skinfold thickness.
Positively skewed distribution.
The P values are from models with the dependent variable power-transformed to square root, all others from models with data transformed to natural logarithms.
To convert mass units to molar units the following can be used: IGF1 ng/ml = 0.133 nM, IGFBP3 µg/ml = 33 nM
There were no significant differences in IGF1, IGFBP3 or anthropometric variables in the children between the maternal supplement groups with sexes combined (all P >0.5). However, a pattern of sex-specific differences between the supplement groups emerged when boys and girls were considered separately (Table 2 and illustrated for IGF1 in Figure 2).
Table 2. IGF1, IGFBP3 and anthropometry of Gambian boys and girls by maternal supplement group.
| Boys | Girls | Sex*S/P | |||||||
|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|||||||
| Calcium (M-Ca, n=64) |
Placebo (M-P, n=76) |
%Δ (SE) | P | Calcium (F-Ca, n=77) |
Placebo (F-P, n=73) |
%Δ (SE) | P | P | |
| IGF1 ng/mla,b | 78.1 (4.3,4.5) | 67.8 (3.4,3.6) | +14.2 (7.7) | 0.05 | 99.5 (4.8,5.1)c | 118.9 (6.4,6.8)c | -17.8 (7.4) | 0.01 | 0.001 |
| IGFBP3 µg/ml | 2.72 (0.13) | 2.44 (0.09) | +11.3 (6.2) | 0.07 | 2.81 (0.11)d | 3.00 (0.12)c | -7.9 (7.3) | 0.3 | 0.05 |
| IGF1:√IGFBP3a,b | 48.7 (2.6,2.8) | 45.0 (1.8,1.9) | +8.0 (6.8) | 0.2 | 61.5 (2.7,2.8)e | 71.3 (3.6,3.8)c | -14.7 (6.8) | 0.02 | 0.008 |
| IGF1:IGFBP3a,b | 30.5 (1.8,2.0) | 29.5 (1.2,1.3) | +3.4 (7.3) | 0.4 | 38.2 (2.0,2.1)c | 42.7 (2.5,2.7)c | -11.3 (8.0) | 0.2 | 0.1 |
| Height cm | 119.6 (0.9) | 118.8 (0.9) | +0.7 (1.0) | 0.5 | 118.5 (0.9) | 119.7(1.0) | -1.0 (1.1) | 0.4 | 0.3 |
| Weight kg | 20.6 (0.4) | 19.8 (0.3) | +3.9 (2.6) | 0.1 | 19.5 (0.4)d | 20.2 (0.5) | -2.8 (2.9) | 0.3 | 0.08 |
| BMI kg/m2 | 14.3 (0.1) | 13.9 (0.1) | +2.6 (1.2) | 0.04 | 13.8 (0.1)e | 13.9 (0.1) | -1.0 (1.4) | 0.5 | 0.06 |
| MUAC cm | 16.3 (0.2) | 15.9 (0.1) | +2.1 (1.3) | 0.1 | 16.4 (0.2) | 16.7 (0.2)e | -1.3 (1.4) | 0.4 | 0.08 |
| TST mm | 5.9 (0.2) | 5.7 (0.2) | +4.4 (3.8) | 0.2 | 7.0 (0.2)c | 7.2 (0.2)c | -4.8 (3.5) | 0.2 | 0.08 |
Data are mean (SE), or, for positively skewed distributions, geometric mean (-1SE,+1SE) calculated with data transformed to logarithms followed by back transformation. BMI, body mass index; MUAC, mid-upper arm circumference; TST, triceps skinfold thickness.
%Δ (SE) = mean (SE) percentage difference, calculated as a sympercent using data transformed to natural logarithms; sex*S/P = sex*supplement group interaction term in joint models.
P values are the significance of differences between the supplement groups with sexes separated and for the sex*supplement group interactions in joint models with data transformed to natural logarithms. There were no significant age*supplement group interactions in any of these models
Positively skewed distribution.
The P values are from models with the dependent variable power-transformed to square root.
Significance of difference between the sexes within each supplement group: c P≤0.001, d P≤0.05, e P≤0.01
To convert mass units to molar units the following can be used: IGF1 ng/ml = 0.133 nM, IGFBP3 µg/ml = 33 nM
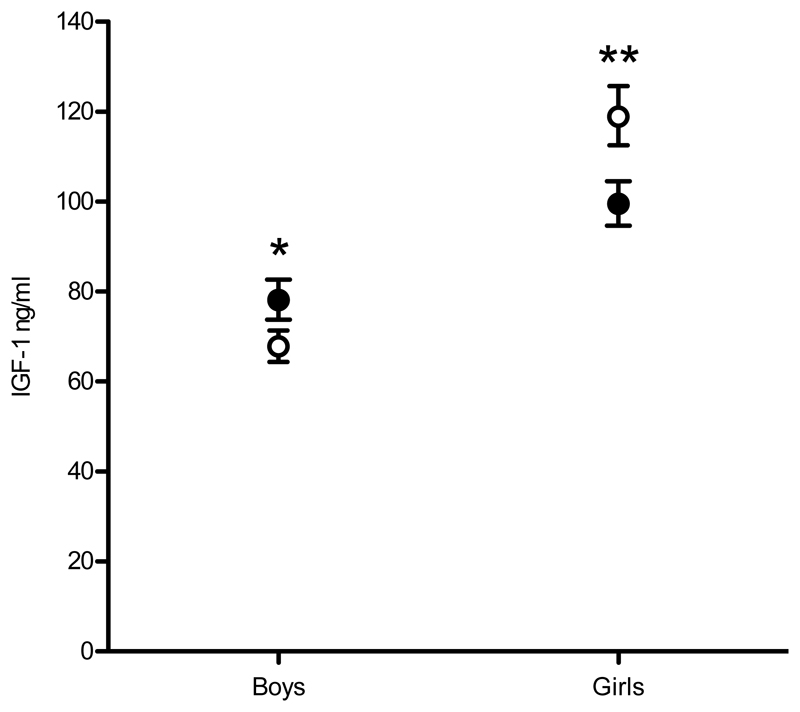
Figure 2.
Effect of the maternal calcium supplement on IGF1 in Gambian boys and girls at mean age 7.5 years. Data are geometric mean (-1SE, +1SE). Closed circles = children of mothers in the calcium supplemented group; open circles = children of mothers in the placebo group; ** P = 0.01; * P = 0.05; sex*supplement group interaction P = 0.001.
In boys, IGF1 was greater in M-Ca than M-P (+14.2 (7.7)%, P = 0.05) whereas, in girls, it was lower in F-Ca than F-P (-17.8 (7.4)%, P = 0.01), resulting in a significant sex*supplement group interaction term in the joint model (P = 0.001). Trends consistent with this pattern were also observed for IGFBP3, IGF1:√IGFBP3 and IGF1:IGFBP3, with significant interaction terms in joint models for IGFBP3 and IGF1:√IGFBP3 (Table 2). Adjustment for age had little or no effect on the size and significance of these differences, with the exception of IGF1 in boys (M-Ca v M-P = +12.3 (7.2)%, P = 0.07), but the sex*supplement group interaction in the joint model remained significant at P = 0.001. There were no significant age*supplement group interactions in any of these models, indicating that the sex-specific differences in IGF1 variables between the supplement groups were observed across the age range of the children.
For the anthropometry, M-Ca tended to have greater values for all variables than M-P whereas F-Ca tended to have lower values than F-P (Table 2). This difference was only statistically significant for BMI in boys (M-Ca v M-P = +2.6 (1.2)%, p=0.04), equating to a BMIAZ difference of +0.25 (0.1) of a standard deviation. The tendency of boys in the calcium group towards greater weight was significant after adjustment for age and infant size (M-Ca v M-P: weight = +3.3 (1.7)%, P = 0.05). The sex*supplement interaction in joint models was not significant for any anthropometric variable before adjustment but was for weight (P = 0.02) and BMI (P = 0.05) after adjustment for age and infant size. There was no indication of a significant interaction of age with any of these effects of supplement group, except for MUAC in boys where the difference between M-Ca and M-P was greater in older children (age*supplement group P = 0.05).
There were highly significant correlations between IGF1, IGFBP3, the IGF/BP3 indices and all the anthropometric variables in both boys and girls. Adding current height and/or weight into models diminished the size and significance of the sex-specific differences in IGF1 by supplement group, but the pattern of differences was unchanged (e.g. IGF1 adjusted for age, height and weight: in boys, M-Ca v M-P = +9.4 (6.8)% P = 0.1; in girls, F-Ca v F-P = -12.8 (6.2)%, P = 0.02; sex*supplement group interaction: P = 0.007). Adjusting for BMI instead of weight gave similar results, with height remaining as a significant co-variable. In boys, but not girls, there was a significant height*supplement group interaction for IGF1 and its indices (IGF1, P = 0.02; IGF1:√IGFBP3, P = 0.002; IGF1:BP3 P = 0.002), but not for IGFBP3 (P = 0.1), indicating that the difference in IGF1 between M-Ca and M-P in boys was more pronounced in taller children. Age was not a significant predictor, suggesting that the height interaction spanned the age range of the boys. There were no significant interactions with weight or BMI for any of these variables in either sex.
Table 3 provides the summary statistics for the other growth and calcium-related analytes by sex-supplement group. There were no significant differences for any variable between the maternal supplement groups with sexes separated or combined, and there were no significant sex*supplement group interactions. Boys had lower leptin and insulin concentrations than girls (M v F in unadjusted models: leptin = -72.5 (8.7)%, P <0.001; insulin = -22.3 (6.2)%, P <0.001; these differences increased slightly after adjusting for age, height and weight (or BMI)), but there were no significant sex differences in the other factors. For both these growth factors, the pattern of differences in mean values between the four sex-supplement groups was similar to that observed for IGF1 and the anthropometry, i.e. higher values for boys but lower for girls in the calcium group compared with placebo, but these differences were not significant.
Table 3. Growth- and calcium-related factors of Gambian boys and girls by maternal supplement group.
| Boys | Girls | Sex*S/P | |||||||
|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|||||||
| Calcium (M-Ca, n=64) | Placebo (M-P, n=76) | %Δ (SE) | P | Calcium (F-Ca, n=77) | Placebo (F-P, n=73) | %Δ (SE) | P | P | |
| Leptin ng/mla | 0.80 (0.07,0.07) | 0.70 (0.05,0.06) | +13.6 (11.9) | 0.3 | 1.49 (0.13,0.14) c | 1.57 (0.13,0.14)c | -5.4 (12.8) | 0.7 | 0.3 |
| Insulin pmol/la | 21.0 (1.2,1.3) | 18.1 (1.0,1.1) | +14.7 (8.6) | 0.2 | 23.4 (1.5,1.6) | 25.2 (1.5,1.6)c | -7.4 (8.9) | 0.4 | 0.08 |
| PTH pg/mla,b | 31.1 (2.5,2.7) | 34.5 (2.3,2.5) | -10.1 (10.7) | 0.3 | 31.6 (2.5,2.7) | 36.0 (2.5,2.7) | -12.8 (11.0) | 0.2 | 0.9 |
| 1,25(OH)2D pmol/la | 231 (10,10) | 236 (8.6,9.0) | -1.8 (5.7) | 0.7 | 244 (10,10) | 251 (11,11) | -3.0 (6.0) | 0.6 | 0.9 |
| 25OHD nmol/l | 57.2 (1.5) | 58.2 (1.4) | -1.5 (3.9) | 0.7 | 59.7 (1.7) | 57.9 (1.5) | +2.3 (3.9) | 0.6 | 0.5 |
| Calcium mmol/l | 2.37 (0.01) | 2.38 (0.01) | -0.7 (0.8) | 0.4 | 2.38 (0.02) | 2.39 (0.02) | -0.8 (1.1) | 0.5 | 0.9 |
| Phosphate mmol/l | 1.62 (0.02) | 1.60 (0.02) | +1.2 (1.7) | 0.5 | 1.56 (0.02) | 1.60 (0.02) | -3.0 (2.0) | 0.1 | 0.1 |
| Albumin g/l | 38.7 (0.4) | 38.7 (0.3) | -0.1 (1.3) | 0.9 | 38.9 (0.3) | 39.6 (0.4) | -1.9 (1.4) | 0.2 | 0.4 |
Data are mean (SE), or a geometric mean (-1SE,+1SE) calculated with data transformed to logarithms followed by back transformation. PTH, parathyroid hormone; 1,25(OH)2D, 1,25-dihydroxyvitamin D; 25OHD, 25-hydroxyvitamin D; %Δ (SE) = mean (SE) percentage difference, calculated as a sympercent using data transformed to natural logarithms; sex*S/P = sex*supplement group interaction term in joint models. P values are the significance of differences between the supplement groups with sexes separated and for the sex*supplement group interactions in joint models with data transformed to natural logarithms.
n=254 (M-Ca = 59, M-P = 65, F-Ca = 66, F-P = 64).
Significance of difference between the sexes within each supplement group: P ≤0.001. There were no other significant differences in any measure between calcium and placebo in either sex or between boys and girls in each supplement group or age*supplement group interactions in any of these models
Age was a significant predictor of plasma insulin (P <0.001, positive), calcium (P = 0.02, negative) and albumin (P <0.001, negative), and infant size was a predictor of 1,25(OH)2D (P = 0.01, negative) but there were no significant sex or supplement group interactions with age or infant size. There were highly significant positive inter-relationships between leptin, insulin and the anthropometric variables in both boys and girls, which were not age dependent. For insulin, but not leptin, there was an interaction between supplement group and BMI such that the tendency in boys for insulin to be greater in M-Ca than M-P increased with greater BMI (P = 0.04) whereas the tendency in girls for insulin to be lower in F-Ca than F-P increased with greater BMI (P = 0.06); sex*supplement*BMI interaction in the joint model P = 0.006. There were no relationships of the calcium-related variables with height, weight or BMI after accounting for age.
Discussion
The purpose of this analysis of archived biochemical data from the children of Gambian mothers who had participated in a pregnancy calcium supplementation trial was to consider whether there was evidence of a sex-specific effect on IGF-1 and other growth- and calcium-related factors at mean age 7.5 years that presaged the differential growth effects observed in the same children later in childhood [5]. This proved to be the case for IGF1, with greater values in boys (+14%) and lower values in girls (-18%) whose mothers had received the calcium supplement compared to their counterparts whose mothers had received placebo. Consistent trends similar to the pattern for IGF1 were seen for IGFBP3 and for IGF1 adjusted for IGFBP3. No significant sex-specific effects on the other growth- and calcium-related factors were seen, although the sex-supplement group differences in leptin and insulin were in the same direction as for IGF1. Subtle anthropometric differences were apparent that mirrored those seen at the later age [5], especially the tendency to greater weight and BMI in the boys whose mothers had received the calcium supplement, but there was no evidence of the differential effects of the maternal supplement on height growth seen later in childhood.
The diet in rural Gambia is low in calcium, with intakes averaging 300-400 mg/d in women and around 200 mg/d in infants and children [7, 19], considerably less than international recommendations. The results of this study suggest that the increase in calcium intake of the supplemented mothers in the second half of pregnancy programmed the growth trajectories of their offspring through a mechanism involving alterations in the growth hormone (GH)-IGF1 axis, directly or indirectly. This aligns with concepts developed by others (eg reviews by [24–27]) whereby the nutritional status of the mother in late pregnancy provides a “forecast” of the nutritional environment into which the child will be born and programmes its subsequent growth and metabolic pathways [24]. The potential for nutritional programming of offspring IGF1 in utero is recognised from trials of milk supplementation in pregnancy and early life [28], from observational studies of maternal milk consumption [29], and from extensive studies in animals [30], although possible differences in the effects on males and females were not explored in many of these studies.
The mechanism by which the maternal calcium carbonate supplement may have altered the growth trajectories of the offspring in a sex-specific manner is unclear. However, sexual dimorphism in fetal growth is well recognised and girls have higher circulating IGF1 than boys at birth and throughout childhood [31, 32]. There are known sex-differences in the response to environmental factors during fetal life such as maternal diet [33], famine [34] and micronutrient supplementation [35], and in pregnancies affected by conditions such as asthma and cigarette use [36]. Many of these studies report sex-specific effects on insulin-like growth factors, their binding proteins and other related growth factors.
Several possible mechanisms have been proposed to explain these phenomena, including sex-dependent effects on placental size and function [33, 36] and on epigenetic modifications through DNA-methylation of growth-related genes [33, 34]. The former possibility appears a less likely explanation for the long-term effects of the maternal calcium carbonate supplement because no discernible effects were observed on offspring growth at birth or during the subsequent 12 months [8]. Because responsiveness to GH largely occurs post-natally [31] and there is marked sexual dimorphism in the pattern of GH secretion [24, 31], it is possible that the Gambian study indicates sex-specific effects on intrauterine programming of GH, on expression of GH receptors at the liver and other tissues, or on other factors in the hypothalamic-pituitary-gonadal axis upstream of IGF1 and IGFBP3 production and bioactivity [24].
Higher plasma IGF1 in mid-childhood is associated with an earlier pubertal growth spurt [21], earlier menarche in girls [17] and faster linear growth in later childhood [37]. It is possible, therefore, that the sex-specific effects of the maternal supplement on IGF1 at age 7.5y predict faster growth and an earlier puberty in the boys and slower growth and later puberty in the girls for those children whose mothers received the calcium carbonate supplement. This study is limited by the post-hoc nature of the hypothesis under test and by the relatively limited number of children from the original trial who provided sufficient blood sample for IGF1 analysis, although the final numbers and the reasons for loss to follow-up were evenly balanced across the four groups. To confirm the apparent sex-specific and long-term effects of the maternal supplement on IGF1 and childhood growth, longitudinal studies are now in progress involving the entire cohort of children from the maternal pregnancy trial as they enter and pass through adolescence.
Highlights.
Pregnancy calcium supplementation (ISRCTN96502494) of Gambian mothers resulted in faster childhood growth in boys, slower growth in girls.
At mean age 7.5 years, plasma IGF1 of offspring was altered in a sex-specific manner before growth effects were evident.
Plasma IGF1 was higher in girls than boys.
Plasma IGF1 was higher in boys of calcium-supplemented mothers than those of mothers who consumed placebo.
Plasma IGF1 was lower in girls of calcium-supplemented mothers than those of mothers who consumed placebo.
Acknowledgements
This study and the original data collection on which it was based were funded by European Union Sixth Framework [FOOD-CT-2005-007036] and by the Medical Research Council (MRC) [Programmes U105960371, U123261351, MC-A760-5QX00] and the Department for International Development (DfID) under the MRC/DfID Concordat. We wish to acknowledge the contributions to this paper of all those involved in the original pregnancy supplementation trial and the follow-up data collection at age 7.5 years, in particular Ann Laidlaw, Janet Bennett, Gail Goldberg of MRC Elsie Widdowson Laboratory, Cambridge, UK (formerly MRC Human Nutrition Research); Yankuba Sawo, Kabiru Ceesay, Landing MA Jarjou, and other staff members of MRC Unit The Gambia, Keneba, The Gambia
Footnotes
Declarations of Interest: None
Author contributions
AP conceived the hypothesis relating IGF1 to maternal calcium supplementation, conducted the statistical analyses and drafted the manuscript; KW provided scientific advice on the analysis and interpretation of the growth and biochemical data; SN was responsible for the biochemical analyses at MRC Human Nutrition Research; SH, SEM, AP designed and conducted the follow-up study of the Gambian children at age 7.5 years and are responsible for the data archive. All authors critically reviewed and approved the final article.
References
- [1].Prentice A, Dibba B, Sawo Y, Cole TJ. The effect of prepubertal calcium carbonate supplementation on the age of peak height volocity in Gambian adolescents. Am J Clin Nutr. 2012;96:1042–1050. doi: 10.3945/ajcn.112.037481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Ward KA, Cole TJ, Laskey MA, Ceesay M, Mendy MB, Sawo Y, Prentice A. The effect of prepubertal calcium carbonate supplementation on skeletal development in Gambian boys - a 12-year follow-up study. J Clin Endocrinol Metab. 2014;99:3169–3176. doi: 10.1210/jc.2014-1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Prentice A, Ginty F, Stear SJ, Jones SC, Laskey MA, Cole TJ. Calcium supplementation increases stature and bone mineral mass of 16-18 year old boys. J Clin Endocrinol Metab. 2005;90(6):3153–3161. doi: 10.1210/jc.2004-2114. [DOI] [PubMed] [Google Scholar]
- [4].Stear SJ, Prentice A, Jones SC, Cole TJ. Effect of a calcium and exercise intervention on the bone mineral status of 16-18-y-old adolescent girls. Am J Clin Nutr. 2003;77:985–992. doi: 10.1093/ajcn/77.4.985. [DOI] [PubMed] [Google Scholar]
- [5].Ward KA, Jarjou L, Prentice A. Long-term effects of maternal calcium supplementation on childhood growth differ between males and females in a population accustomed to a low calcium intake. Bone. 2017;103:31–38. doi: 10.1016/j.bone.2017.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Schoenbuchner S, Moore S, Jarjou L, Prentice A, Ward K. Maternal calcium supplementation in a rural Gambian population associated with reduced height and weight among adolescent female, but not male, offspring. Bone Abstr. 2017;6:160. [Google Scholar]
- [7].Jarjou L, Prentice A, Sawo Y, Laskey MA, Bennett J, Goldberg GR, Cole TJ. Randomized, placebo-controlled calcium supplementation study of pregnant Gambian women: effects on breast-milk calcium concentration and infant birth weight, growth and bone mineral accretion in the first year of life. Am J Clin Nutr. 2006;83:657–666. doi: 10.1093/ajcn.83.3.657. [DOI] [PubMed] [Google Scholar]
- [8].Goldberg GR, Jarjou LMA, Cole TJ, Prentice A. Randomized, placebo-controlled, calcium supplementation trial in pregnant Gambian women accustomed to a low calcium intake: effects on maternal blood pressure and infant growth. Am J Clin Nutr. 2013;98:972–982. doi: 10.3945/ajcn.113.059923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Hawkesworth S, Sawo Y, Fulford AJ, Goldberg GR, Jarjou LMA, Prentice A, Moore SE. Effect of maternal calcium supplementation on offspring blood pressure in 5-10 year old rural Gambian children. Am J Clin Nutr. 2010;92:741–747. doi: 10.3945/ajcn.2010.29475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Palmert MR, Dunkel L. Delayed puberty. New Engl J Med. 2012;366:443–453. doi: 10.1056/NEJMcp1109290. [DOI] [PubMed] [Google Scholar]
- [11].Cannata D, Vijayakumar A, Fierz Y, LeRoith D. The GH/IGF-1 axis in growth and development: new insights derived from animal models. Adv Pediatr. 2010;57:331–351. doi: 10.1016/j.yapd.2010.09.003. [DOI] [PubMed] [Google Scholar]
- [12].Wolfe A, Divall S, Wu S. The regulation of reproductive neuroendocrine function by insulin and insulin-like growth factor-1 (IGF-1) Front Neuroendocrinol. 2014;35:558–572. doi: 10.1016/j.yfrne.2014.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Maes C, Kronenberg HM. In: Pediatric Bone. Glorieux FH, Pettifor JM, Jüppner H, editors. Academic Press; Amsterdam: 2012. Postnatal growth: growth plate biology, bone formation and remodeling; pp. 55–82. [Google Scholar]
- [14].Ginty F, Prentice A, Laidlaw A, McKenna L, Jones SC, Stear SJ, Cole TJ. In: Nutritional Aspects of Osteoporosis. Burckhardt P, Dawson-Hughes B, Heaney R, editors. Elsevier Science; USA: 2004. Calcium carbonate supplementation is associated with higher plasma IGF-1 in 16-18 year old boys and girls; pp. 45–57. [Google Scholar]
- [15].Hoppe C, Mølgaard C, Juul A, Michaelsen KF. High intakes of skimmed milk, but not meat, increase IGF-1 and IGFBP-3 in eight-year-old boys. Eur J Clin Nutr. 2004;58:1211–1216. doi: 10.1038/sj.ejcn.1601948. [DOI] [PubMed] [Google Scholar]
- [16].Cadogan J, Eastell R, Jones N, Barker ME. Milk intake and bone mineral acquisition in adolescent girls: randomised, controlled intervention trial. BMJ. 1997;315:1255–1260. doi: 10.1136/bmj.315.7118.1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Thankamony A, Ong KK, Ahmed ML, Ness AR, Holly JMP, Duger DB. Higher levels of IGF-1 and adrenal androgens at age 8 years are associated with earlier age at menarche in girls. J Clin Endocrinol Metab. 2012;97:E786–E790. doi: 10.1210/jc.2011-3261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Prentice AM, Ward KA, Goldberg GR, Jarjou LMA, M SE, Fulford AJ, Prentice A. Critical windows for nutritional interventions against stunting. Am J Clin Nutr. 2013;97:911–8. doi: 10.3945/ajcn.112.052332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Dibba B, Prentice A, Ceesay M, Stirling DM, Cole TJ, Poskitt EME. Effect of calcium supplementation on bone mineral accretion in Gambian children accustomed to a low calcium diet. Am J Clin Nutr. 2000;71:544–549. doi: 10.1093/ajcn/71.2.544. [DOI] [PubMed] [Google Scholar]
- [20].Hawkesworth S, Walker CG, Sawo Y, Fulford AJC, Jarjou LMA, Goldberg GR, Prentice A, Prentice AM, Moore SE. Nutritional supplementation during pregnancy and offspring cardiovascular disease risk in The Gambia. Am J Clin Nutr. 2011;94(suppl):1853S–1860S. doi: 10.3945/ajcn.110.000877. [DOI] [PubMed] [Google Scholar]
- [21].Cole TJ, Ahmed ML, Preece MA, Hindmarsh P, Dunger DB. The relationship between Insulin-like growth Factor 1, sex steroids and timing of the pubertal growth spurt. Clin Endocrinol. 2015;82:862–869. doi: 10.1111/cen.12682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Cole TJ. Sympercents: symmetric differences on the 100 log(e) scale simplify the presentation of log transformed data. Stat Med. 2000;19:3109–3125. doi: 10.1002/1097-0258(20001130)19:22<3109::aid-sim558>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- [23].Cole TJ, Altman DG. Statistics Notes: Percentage differences, symmetry, and natural logarithms. BMJ. 2017;358 doi: 10.1136/bmj.j3683. j3683. [DOI] [PubMed] [Google Scholar]
- [24].Holt RIG. Fetal programming of the growth hormone-insulin-like growth factor axis. Trends Endocrinol Metab. 2002;13:392–397. doi: 10.1016/s1043-2760(02)00697-5. [DOI] [PubMed] [Google Scholar]
- [25].Fowden AL, Forhead AJ. Endocrine mechanisms of intrauterine programme. Reproduction. 2004;127:515–526. doi: 10.1530/rep.1.00033. [DOI] [PubMed] [Google Scholar]
- [26].Roth CL, Divall S. Consequences of early life programing by genetic and environmental influences: a synthesis regarding pubertal timing. Endocr Develop. 2016;29:134–152. doi: 10.1159/000438883. [DOI] [PubMed] [Google Scholar]
- [27].Gluckman PD, Pinal CS. Regulation of fetal growth by the somatotrophic axis. J Nutr. 2003;133:1741–1746S. doi: 10.1093/jn/133.5.1741S. [DOI] [PubMed] [Google Scholar]
- [28].Ben-Shlomo Y, Holly J, McCarthy A, Savage P, Davies D, Davey Smith G. Prenatal and postnatal milk supplementation and adult Insulin-like Growth Factor 1: Long-term follow-up of a randomized controlled trial. Cancer Epidemiol Biomarkers Prev. 2005;14:1336–1339. doi: 10.1158/1055-9965.EPI-04-0908. [DOI] [PubMed] [Google Scholar]
- [29].Hrolfsdottir L, Rytter D, Hammer Bech B, Brink Henriksen T, Danielsen I, Steingrimsdottir L, Olsen SF, Halldorsson TI. Maternal milk consumption, birth size and adult height of offspring: a prospective cohort study with 20 years of follow-up. Eur J Clin Nutr. 2013;67:1036–1041. doi: 10.1038/ejcn.2013.151. [DOI] [PubMed] [Google Scholar]
- [30].Fowden AL. The insulin-like growth factors and feto-placental growth. Placenta. 2003;24:803–812. doi: 10.1016/s0143-4004(03)00080-8. [DOI] [PubMed] [Google Scholar]
- [31].Geary MPP, Pringle PJ, Rodeck CH, Kingdom JC, Hindmarsh PC. Sexual dimorphism in the growth hormone and insulin-like growth factor axis at birth. J Clin Endocrinol Metab. 2003;88:3708–3714. doi: 10.1210/jc.2002-022006. [DOI] [PubMed] [Google Scholar]
- [32].Soldin OP, Dahlin JRB, Gresham EG, King J, Soldin SJ. IMMULITE® 2000 age and sex-specific reference intervals for alpha fetoprotein, homocysteine, insulin, insulin-like growth factor-1, insulin-like growth factor binding protein-3, C-peptide, immunoglobulin E and intact parathyroid hormone. Clin Biochem. 2008;41:937–942. doi: 10.1016/j.clinbiochem.2008.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Tarrade A, Panchecko P, Junien C, Gabory A. Placental contribution to nutritional programming of health and diseases: epigenetics and sexual dimorphism. J Exp Biol. 2015;218:50–58. doi: 10.1242/jeb.110320. [DOI] [PubMed] [Google Scholar]
- [34].Tobi EW, Lumey LH, Talens RP, Kremer D, Putter H, Stein AD, Slagboom PE, Heijmans BT. DNA methylation differences after exposure to prenatal famine are common and timing- and sex-specific. Hum Mol Gen. 2009;18:4046–4053. doi: 10.1093/hmg/ddp353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Roberfroid D, Huybregts L, Lanou H, Henry M-C, Meda N, Kolsteren P, for the Micronutriments et Santé de la Mère et de l'Enfant Study (MISAME) Group Effect of maternal multiple micronutrient supplements on cord blood hormones: a randomized controlled trial. Am J Clin Nutr. 2010;91:1649–1658. doi: 10.3945/ajcn.2009.28855. [DOI] [PubMed] [Google Scholar]
- [36].Clifton VL, Hodyl NA, Murphy VE, Giles WB, Baxter RC, Smith R. Effect of maternal asthma, inhaled glucocorticoids and cigarette use during pregnancy on the newborn insulin-loke growth factor axis. Growth Horm IGF Res. 2009;20:39–48. doi: 10.1016/j.ghir.2009.07.004. [DOI] [PubMed] [Google Scholar]
- [37].Dalskov S, Ritz C, Larnkiaer A, Damsgaard CT, Petersen RA, Sørensen LB, Ong KK, Astrup A, Michaelsen KF, Mølgaard C. Associations between adiposity, hormones, and gains in height, whole-body height-adjusted bone size, and size-adjusted bone mineral content in 8- to 11-year old children. Osteoporos Int. 2016;27:1619–1629. doi: 10.1007/s00198-015-3428-z. [DOI] [PubMed] [Google Scholar]