Abstract
Several studies have used neuroimaging techniques in an attempt to characterize brain correlates of the attentional modulation of pain. Although these studies have advanced the knowledge in the field, important confounding factors such as imprecise theoretical definitions of attention, incomplete operationalization of the construct under exam, and limitations of techniques relying on measuring regional changes in cerebral blood flow have hampered the potential relevance of the conclusions. Here, we first provide an overview of the major theories of attention and of attention in the study of pain to bridge theory and experimental results. We conclude that load and motivational/affective theories are particularly relevant to study the attentional modulation of pain and should be carefully integrated in functional neuroimaging studies. Then, we summarize previous findings and discuss the possible neural correlates of the attentional modulation of pain. We discuss whether classical functional neuroimaging techniques are suitable to measure the effect of a fluctuating process like attention, and in which circumstances functional neuroimaging can be reliably used to measure the attentional modulation of pain. Finally, we argue that the analysis of brain networks and spontaneous oscillations may be a crucial future development in the study of attentional modulation of pain, and why the interplay between attention and pain, as examined so far, may rely on neural mechanisms shared with other sensory modalities.
Keywords: pain, attention, neuroimaging, bottom-up attention, top-down attention, brain networks
1. Introduction
Pain and nociception are not the same phenomena. Nociception refers to the peripheral and central nervous system processes triggered by the activation of nociceptors (Sherrington, 1906). Pain is a subjective experience, one of the possible outcomes of nociceptors activation. Several behavioral studies have shown that pain can induce attentional biases (but see (Crombez, Van Ryckeghem, Eccleston, & Van Damme, 2013) for an important meta-analysis on the topic), and may interrupt behavior (Eccleston & Crombez, 1999; Moore, Keogh, & Eccleston, 2012). However, attentional manipulations can also modulate the perception of pain and reaction times to nociceptive stimuli, especially when the concurrent pain-unrelated task requires effort and demands cognitive resources (Buhle & Wager, 2010; Legrain, Crombez, & Mouraux, 2011; Romero, Straube, Nitsch, Miltner, & Weiss, 2013; Verhoeven, Van Damme, Eccleston, Van Ryckeghem, Legrain, & Crombez, 2011).
In a recent review we offered a critical perspective on the influence of cognition/attention on the electrophysiological responses to nociceptive and painful stimuli, particularly on the functional relationship between attention and the magnitude of event related potentials (ERPs) (Legrain, Mancini, Sambo, Torta, Ronga, & Valentini, 2012). The aim of the present review is to discuss the contribution of neuroimaging studies to the study of attentional modulation of pain and nociceptive inputs with a special emphasis on theoretical and methodological perspectives 1,2.
The first functional neuroimaging studies on the attentional modulation of pain often referred to ‘attention’ as a monolithic construct. This was likely motivated by practical operational reasons and by the fact that the concept of attention is difficult to disentangle from the concept of consciousness or executive control. However, attention is not a unitary process. Therefore, it should be considered that different attentional processes can modulate pain and cortical responses to nociceptive stimuli via different mechanisms mediated by different neural substrates (Raz & Buhle, 2006). Here, we will attempt to highlight how interpreting attention as a unitary construct might have led to partially contradictive findings and, occasionally, over-generalized conclusions. We will first outline some key concepts of attention, in particular those relevant for a critical review of neuroimaging studies on the attentional modulation of pain.
Selective attention. Selective attention is one of the most used notions when referring to attention. The concept of selectivity was introduced more than a century ago by William James, (James, 1890) who defined attention as a restricted focus of consciousness on one out of several objects physically present in the environment. In this view, selective attention would constitute a means to filter the flow of incoming information in order to prioritize the processing of information according to its relevance. Why should it be important to select relevant information? According to the limited-capacity bottleneck theory (Broadbent, 1958), we are unable to process all the available information simultaneously; therefore, a selection is required. Importantly, this limited capacity could be related more to the limited number of actions that an individual can perform rather than the limited amount of sensory information that is processed. In this vein, selective attention would serve to prioritize the processing of information that enables us to select the most relevant among several possible actions (Allport, 1987; Hommel, 2010). This interpretation implies that selective attention to painful stimuli would therefore prioritize escape or defensive actions to maintain the integrity of the body.
‘Executive attention’ is a concept strictly linked to that of executive functions, proposed as part of attentional processes in the influential theory of attention by Posner and Petersen (Petersen & Posner, 2012; Posner & Petersen, 1990). Executive attention would refer to the ability to keep the effective processing of a target stimulus regardless of concomitant distraction by irrelevant elements. The concept of executive attention clearly overlaps with that of ‘selective attention’ (or according to the authors’ terminology focal attention). However, the definition of ‘executive attention’ by Petersen and Posner (2012) does not place much emphasis on spatial or motor aspects. Rather, it conceives executive attention as the process that enables us to maintain cognitive control and, for instance, to stay on task while filtering irrelevant distractive information. Moreover, in the Petersen and Posner model, each component of attention is wired in specific brain regions and networks. Executive attention is associated with the activity of the anterior cingulate cortex and networks comprising it (Dosenbach, Fair, Miezin, Cohen, Wenger, Dosenbach, Fox, Snyder, Vincent, Raichle, Schlaggar, & Petersen, 2007; Dosenbach, Visscher, Palmer, Miezin, Wenger, Kang, Burgund, Grimes, Schlaggar, & Petersen, 2006).
The concept of ‘executive attention’ is relevant for the study of pain in that it explains why the concomitant execution of pain-unrelated cognitive tasks can prevent the attentional capture by nociceptive∕ painful inputs (Buhle & Wager, 2010; Legrain, Crombez, & Mouraux, 2011; Legrain, Crombez, Plaghki, & Mouraux, 2013; Seminowicz & Davis, 2007a; Van Damme, Gallace, Spence, Crombez, & Moseley, 2009; Van Damme, Legrain, Vogt, & Crombez, 2009; Verhoeven, Van Damme, Eccleston, Van Ryckeghem, Legrain, & Crombez, 2011).
Posner and Petersen’s theory also describes other types of attention such us alerting attention, i.e. the ability to increase and maintain response readiness to an impending stimulus, and orienting attention, i.e. the ability to select specific stimuli among multiple sensory stimuli. For this latter concept, the authors refer to the influential work by Corbetta and Schulman (e.g. (Corbetta & Shulman, 2002)) on the dorsal attentional network, which we will explain in the next paragraph.
Bottom-up vs top-down processes. Some stimuli are particularly difficult to ignore and capture attention automatically even when they are far away from the focus of attention (Theeuwes, 1991). This involuntary capture of attention is defined as “bottom-up” or “stimulus driven”. Bottom-up attention is an exogenous attention, meaning that it is triggered by external cues or events and is opposed to the top-down, endogenous, and often voluntary deployment of attention (Egeth & Yantis, 1997; Knudsen, 2007). While top-down attention allows an individual to focus on what is relevant in terms of goals and motivations, bottom-up capture of attention constitutes a mechanism serving to re-orient attention towards salient stimuli whose physical features make them stand out from concurrent or preceding stimuli. Bottom-up capture of attention is also involved in the detection of changes in the incoming stream of sensory input. The bottom-up capture of attention can rely, for instance, on the detection of a mismatch between internal representations of environmental regularities (built on recent past experiences) and new sensory inputs disrupting such regularity (Escera & Malmierca, 2014; Näätänen & Kreegipuu, 2011; Polich, 2007; Sokolov, 1963).
At the cortical level these two systems are subserved by two distinct networks. The first, called ‘dorsal attentional network’ is involved in the top-down selection of stimuli and responses and encompasses the intraparietal cortex and the superior frontal cortex. The second (ventral fronto-parietal network) comprises the temporo-parietal cortex and the inferior frontal cortex and is engaged by salient or deviant stimuli (Corbetta & Shulman, 2002). These two systems work in synergy with activity of the ventral parietal network being suppressed during task execution, but activity in the dorsal parietal network being modulated by incoming relevant and salient stimuli (Corbetta, Patel, & Shulman, 2008). See figure 1 for an illustration of the different attentional networks as identified with fMRI. See figure 2 for an illustration of the dorsal and ventral attentional networks.
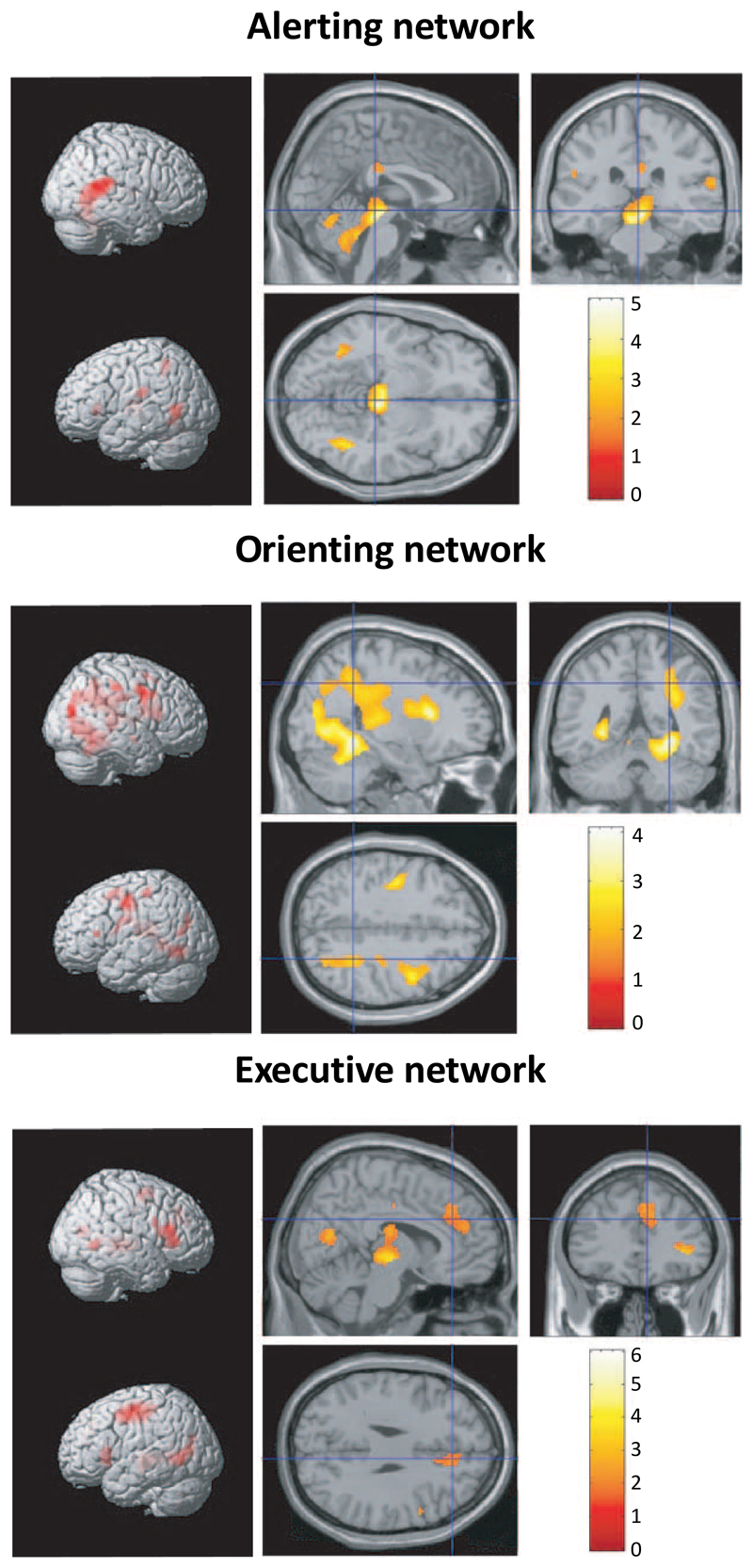
Figure 1.
Reproduced with permission and modified from (Raz, 2004; Raz & Buhle, 2006). Attentional networks as identified by fMRI (and in line with (Petersen & Posner, 2012). This figure is meant to illustrate that different attentional processes can be subserved by different attentional networks. The authors propose the existence of three attentional networks, the alerting, the orienting and the executive (see also note 5 for further description of brain networks). The alerting network shows thalamic activation, the orienting network shows parietal activation, and the executive network anterior cingulate cortex activations.
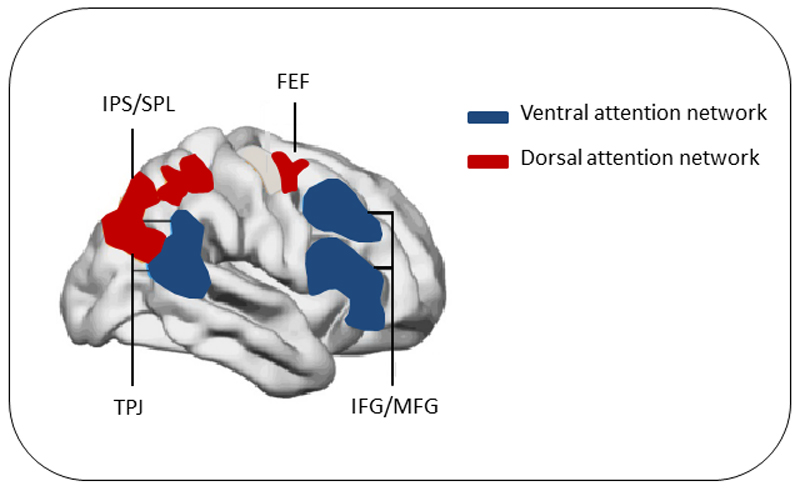
Figure 2.
Reproduced with permission and modified from Aboitiz et al., 2014 (Aboitiz, Ossandon, Zamorano, Palma, & Carrasco, 2014), Chica and Bartolomeo 2012 (Chica & Bartolomeo, 2012). Anatomy of the ventral and dorsal attention networks. FEF Frontal eye field, IPS/SPL Intra parietal sulcus/Superior parietal lobe, TPJ Temporo-parietal junction, IFG/MFG inferior frontal gyrus, medial frontal gyrus.
Despite the frequent usage of these terms in the literature on the study of pain and nociception, all these operational definitions of attention have significant conceptual overlaps, and basic research on attention has not opted for a unitary perspective. Moreover, the above-mentioned descriptions are not meant to be exhaustive or to be considered ‘specific’ for pain. Nevertheless, we believe that this conceptual organization is useful to offer insights on the neural mechanisms of the attentional modulation of nociception and pain. In the next paragraph, we will briefly discuss some of the major theoretical frameworks in the pain field, in which some of these operational definitions are embedded.
2. Theoretical models and conceptual frameworks used to study the effects of attention on pain
2.1. Major theories of attention to pain: gate, load, motivational and affective theories
We have all had the every-day life experience that the perception of pain varies considerably depending on the context. Therefore, it is not surprising that the ability of attention to modulate nociceptive processing and pain perception has always captured the interest of pain researchers. Several theories have tried to explain and conceptualize these interactions in a coherent framework.
Gate theories of pain. Melzack and Wall (Melzack & Wall, 1965) were the first to propose that the spinal transmission of nociceptive inputs can also be under the descending influence of supra-spinal mechanisms, including attention. Convincing experimental evidence for this notion comes from studies showing how cognitive tasks and distraction can modulate the nociceptive flexion reflex, a spinal reflex (Ruscheweyh, Kreusch, Albers, Sommer, & Marziniak, 2011; Willer, Roby, & Le Bars, 1984). Leventhal & Everhart (Leventhal & Everhart, 1979) and McCaul & Malott (McCaul & Malott, 1984) proposed that nociceptive processing involves several operations, which transform an input signal (i.e. nociceptive input) into output signals, one of them being the sensation of pain. In this regard, the processing of nociceptive information would be reduced by limited-resources constraints. As such, the processing of nociceptive and non-nociceptive information, would lead to a competition. Considering that, in most circumstances, nociceptive inputs may represent a higher threat to the organism as compared to non-nociceptive inputs; attention to nociceptive input would be prioritized with respect to other contextually relevant events. The important implication of this theory for the attentional modulation of pain, is that top-down processes can influence the spinal transmission of nociceptive inputs, possibly gating incoming afferent inputs.
Load theories of pain. Perceptual load theories (Lavie, 2005) propose that attentional effects on sensory processing depend on the interaction between task difficulty and stimuli features. In this vein, high load tasks requiring to process non-nociceptive information (e.g. performance of a difficult task involving the discrimination of non-nociceptive sensory stimuli) would consume shared cognitive resources that, in turn, would become less readily available for the processing of nociceptive input (Legrain, Crombez, Plaghki, & Mouraux, 2013). This would explain why nociceptive stimuli can be perceived as less painful when they occur in the context of a high cognitive load task not involving the processing of these nociceptive stimuli (Romero, Straube, Nitsch, Miltner, & Weiss, 2013).
Motivational theories of pain. Motivational theories of pain importantly address the discrepancy between experimental and clinical findings. When pain is investigated in an experimental context and consists of brief painful stimuli, the engagement in other cognitive tasks can reduce the experience of pain. However, when the experience of pain is intense, persistent and invalidating, it can take over and become the constant focus of attention. As such, managing pain can become a goal for chronic pain patients. This aspect of the interaction between attention and pain has been made explicit in the ‘motivational account for pain’ to explain the maladaptive effects in chronic pain states. Van Damme et al. (Van Damme, Legrain, Vogt, & Crombez, 2009) emphasized that pain must be conceived in the frame of goal pursuit. According to these authors, the ability of pain to distract individuals from current goals depends both on the characteristics of the goal and on those of the pain experience. For instance, the authors suggested that if pain occurs during the pursuit of a goal, it is likely to capture attention although it is not goal-relevant. However, chronic pain patients constantly deal with ongoing pain and, therefore, pain management itself becomes the goal, triggering an enhanced processing of pain-related information.
Affective theories. Affective theories highlight the role that affective aspects of the stimulus (e.g. affective signals of threat) have in modulating attention to it. For instance, it has been proposed that emotional signals can shape perception by partially operating with different processes and brain structures than those related to endogenous attentional processes (Pourtois, Schettino, & Vuilleumier, 2013). Studies in the pain domain have shown that affective factors such as catastrophizing and negative priming can enhance responses to pain (Dillmann, Miltner, & Weiss, 2000; Godinho, Magnin, Frot, Perchet, & Garcia-Larrea, 2006; Keogh, Ellery, Hunt, & Hannent, 2001; Vlaeyen, Timmermans, Rodriguez, Crombez, van Horne, Ayers, Albert, & Wellens, 2004; Wunsch, Philippot, & Plaghki, 2003). In addition to this, recent influential theories underline how emotional factors might play a role in the transition from acute to chronic pain (Baliki & Apkarian, 2015). These theories might also explain individual susceptibility and behavioural patterns, as individuals might put subjective affective weights on painful stimuli also when these are applied in experimental contexts.
2.2. Salience and relevance of pain: on the concept of bottom-up and top-down attention in pain research
The concept of ‘bottom-up’ and ‘top-down’ is shared by several approaches to study attention. How does pain research integrate these concepts? Experimental evidence shows that, during the execution of cognitive tasks not involving pain (e.g., the performance of a task involving the processing of auditory stimuli) the occurrence of a painful stimulus can strongly capture attention and impair performance, even when the painful stimulus is completely irrelevant for the task. This would suggest that nociceptive inputs can cause an involuntary shift of attention from its current focus towards the nociceptive stimulus (Crombez, Baeyens, & Eelen, 1994; Crombez, Eccleston, Baeyens, & Eelen, 1996, 1998; Crombez, Vervaet, Lysens, Baeyens, & Eelen, 1998; Eccleston & Crombez, 1999).
However, it would be simplistic to consider that painful stimuli always capture attention. It is true that nociceptive stimuli are often able to trigger attentional capture by making the subject disengage attention from the achievement of planned goals and interrupt ongoing activities in order to prioritize escape or survival (Loeser & Melzack, 1999; Melzack & Casey, 1968). It is also clear that orienting and maintaining attention towards or away from a nociceptive stimulus (or pain) depends on the balance between its salience and relevance3 to current behavioral goals, and most probably, also by the emotional relevance that the stimulus has for the person (Keogh, Ellery, Hunt, & Hannent, 2001). Legrain et al. (Legrain, Van Damme, Eccleston, Davis, Seminowicz, & Crombez, 2009) suggested that the capture of attention by brief nociceptive stimuli can be modulated by three factors. First, unattended nociceptive stimuli are more likely to capture attention if the attentional focus is on features that are shared with the nociceptive distracter (attentional set hypothesis; (Van Ryckeghem, Crombez, Eccleston, Legrain, & Van Damme, 2012). Second, nociceptive stimuli are less likely to capture attention if the goal is effortful and, thereby, recruits all available attentional resources (attentional load hypothesis; (Legrain, Bruyer, Guerit, & Plaghki, 2005). Third, nociceptive stimuli are less likely to capture attention if other cognitive processes, not necessarily an effortful task, are concomitantly engaged to maintain goal priorities and actively shield the processing of the attended stimulus from distraction (Legrain, Crombez, Plaghki, & Mouraux, 2013). For a representation of this model please refer to the figures in (Legrain, Perchet, & Garcia-Larrea, 2009; Legrain & Torta, 2015).
3. Functional imaging of the attentional modulation of pain
The first neuroimaging studies of pain (e.g. (Bushnell, Duncan, Hofbauer, Ha, Chen, & Carrier, 1999; Coghill, Talbot, Evans, Meyer, Gjedde, Bushnell, & Duncan, 1994; Jones, Brown, Friston, Qi, & Frackowiak, 1991; Peyron, Garcia-Larrea, Gregoire, Costes, Convers, Lavenne, Mauguiere, Michel, & Laurent, 1999; Rainville, Duncan, Price, Carrier, & Bushnell, 1997; Talbot, Marrett, Evans, Meyer, Bushnell, & Duncan, 1991; Tolle, Kaufmann, Siessmeier, Lautenbacher, Berthele, Munz, Zieglgansberger, Willoch, Schwaiger, Conrad, & Bartenstein, 1999) aimed to identify which areas of the brain respond to painful stimuli. Regional cerebral blood flow changes (rCBF) were interpreted as reflecting both pain- and attention-related activity (Derbyshire, Jones, Devani, Friston, Feinmann, Harris, Pearce, Watson, & Frackowiak, 1994; Hsieh, Stahle-Backdahl, Hagermark, Stone-Elander, Rosenquist, & Ingvar, 1996; Jones, Brown, Friston, Qi, & Frackowiak, 1991; Svensson, Minoshima, Beydoun, Morrow, & Casey, 1997). Subsequent reports tried to characterize differential activations to painful stimuli and cognitive tasks demanding attention (e.g. (Davis, Taylor, Crawley, Wood, & Mikulis, 1997; Derbyshire & Jones, 1998), by exploring activity in the cingulate cortex, a region thought to contain manifold subregions devoted to cognitive, sensory, and affective processing (Devinsky, Morrell, & Vogt, 1995; B. Vogt, 2009; B. A. Vogt, 2005; B. A. Vogt, Nimchinsky, Vogt, & Hof, 1995). In these studies pain stimuli and attentional tasks were presented in separate blocks. Altogether, these results suggested that painful stimuli and attentional tasks activate adjacent, but not overlapping, segments of the cingulate cortex. However, these studies could not provide any insight on the interactions between attention and pain, which were addressed in later studies (Bantick, Wise, Ploghaus, Clare, Smith, & Tracey, 2002; Brooks, Nurmikko, Bimson, Singh, & Roberts, 2002; Frankenstein, Richter, McIntyre, & Remy, 2001; Petrovic, Petersson, Ghatan, Stone-Elander, & Ingvar, 2000; Peyron, Garcia-Larrea, Gregoire, Costes, Convers, Lavenne, Mauguiere, Michel, & Laurent, 1999), wherein painful stimuli were presented during the execution of an attentional task. Later studies set out to study the effects of attention on pain and, occasionally, the effects of pain on attentional tasks. We review these studies in the next paragraphs, with a particular focus on the attentional component that they investigated.
3.1. The effect of selective attention on pain
Selective attention to pain has been tested in different ways. Some studies asked participants to attend to specific features of a painful stimulus. Other studies have instead used stimuli of another sensory modality as distracters, adding a component of intermodal attention. In the next two paragraphs, we will present and discuss the results and conclusion of those studies.
3.1.1. Attention to specific features of the painful stimulus
Kulkarni et al. (Kulkarni, Bentley, Elliott, Youell, Watson, Derbyshire, Frackowiak, Friston, & Jones, 2005) used Positron Emission Tomography (PET) to investigate whether attending to the location or unpleasantness of a painful stimulus applied on the left side of the body would result in a differential pattern of regional brain activity. The basic assumption was that attending to the location of the stimulus would increase activity in ‘sensory’ areas, whereas attending to its unpleasantness would increase activity in ‘emotional’ areas. The results supported their hypothesis by showing increased activations in the perigenual cingulate cortex, amygdala, orbitofrontal cortex, primary motor cortex, hypothalamus, and posterior insula when participants were attending to unpleasantness as compared to location. Conversely, increased activations were observed in the right primary sensory cortex (S1) and in the inferior parietal cortices when participants were attending to location as compared to unpleasantness. These results were taken to support the distinction between selective modulation of lateral (sensory) and medial (affective) pain systems. Although such a sharp division of these two systems, especially concerning the insula and the cingulate cortex, can be questioned (Buchel, Bornhovd, Quante, Glauche, Bromm, & Weiller, 2002; Coghill, Sang, Maisog, & Iadarola, 1999; Valentini, Betti, Hu, & Aglioti, 2013), these results suggested that different patterns of brain activity could reflect the processing of different features of the stimulus. These results also suggested that the amygdala could be involved in some aspects of the processing of experimental nociceptive stimuli, in line with affective attentional theories.
Other studies also supported the possibility that attentional selection of different features of a nociceptive stimulus may unveil partially distinguishable patterns of brain responses to painful stimuli. Oshiro and colleagues used functional magnetic resonance imaging (fMRI) to investigate spatial (e.g. stimulus location (Oshiro, Quevedo, McHaffie, Kraft, & Coghill, 2007)) and non-spatial (e.g. stimulus intensity (Oshiro, Quevedo, McHaffie, Kraft, & Coghill, 2009)) features of nociceptive stimuli. The authors used a delayed match-to-sample task in which participants had to compare the characteristics of a first stimulus to those of a stimulus presented after a delay, thus implying a more direct involvement of top-down attention driven by working memory processes. The findings of their two studies showed that matching the stimuli according to the spatial location or intensity yielded both different and common activations. In both tasks, the stimuli activated the cingulate cortex. However, matching the stimulus location increased activity in prefrontal and posterior parietal regions, whereas matching the stimulus on the basis of intensity increased activity in the anterior insula, in line with the notion that the insula is involved in pain intensity coding or serves as a ‘general magnitude estimator’ (zu Eulenburg, Baumgartner, Treede, & Dieterich, 2013).
In a subsequent study, Lobanov et al. (Lobanov, Quevedo, Hadsel, Kraft, & Coghill, 2013) aimed to integrate the two approaches by using a delayed match-to-sample task, but asking participants to either selectively attend to the spatial location or to the intensity of pairs of painful heat stimuli and to detect whether the second stimulus had the same or different cued feature of the first one. They observed that changes in the spatial location of the stimulus were easier to detect, but nonetheless yielded greater posterior parietal activations (intraparietal sulcus and superior parietal lobule), in all periods of the task, from the cue to the discrimination phase. They concluded that the posterior parietal cortex plays a role in the processing of spatial aspects of the noxious stimuli. However, it should be emphasized that the posterior parietal cortex is involved in the processing of spatial aspects of a variety of sensory stimuli, among which nociceptive ones.
Taken together, these studies indicate that attention to different features of nociceptive stimuli can recruit different areas (or networks as we will argue later). However, they also highlight the need to take into consideration factors other than selective attention before drawing conclusions about the effects of selective attention on nociceptive processing, in particular, the cognitive load required to perform the different tasks.
Overall, protocols contrasting brain responses to different features of the same painful stimuli can bear several advantages over other experimental approaches. Indeed, one problem of using intermodal attention is that it might become difficult to distinguish between brain activations that reflect differences in the characteristic of the incoming stimulus (for instance a tactile stimulus is usually less intense that a nociceptive one, therefore some activations may be related to the unmatched intensity of the stimulus) and brain activations that reflect attentional modulation of the incoming stimulus. This issue is resolved when the same incoming sensory stimulus is used.
3.1.2. Selective attention to pain, to another modality or ‘distraction’ from pain
Using PET, Peyron et al. (Peyron, Garcia-Larrea, Gregoire, Costes, Convers, Lavenne, Mauguiere, Michel, & Laurent, 1999) asked participants either to attend to thermal painful stimuli, to attend to auditory stimuli, or to perform no task at all, trying in this way to characterize possibly selective features of attention to pain per se. They found that selective attention to pain triggered stronger responses in prefrontal, posterior parietal and cingulate cortices, as compared to selective attention to auditory stimuli, which was associated with increased responses in temporal regions.
Brooks et al. (Brooks, Nurmikko, Bimson, Singh, & Roberts, 2002) used a similar approach to compare the effects of selectively attending to 15-second duration heat stimuli that were either painful or non-painful, or to visual stimuli presented concomitantly. They observed stronger responses in the anterior insula when participants focused on pain. In contrast, when attention was focused on the visual stimuli and directed away from the painful stimuli, they observed stronger responses in the mid-insula.
Although tempting, it is difficult to conclude that these results reflect the effects of selective attention to painful stimuli. Indeed, by definition, intermodal modulation of attention implies attentional fluctuations from pain to the other modality and vice versa. In addition, this approach cannot easily take into account the distracting effect of the unattended stimulus per se. Finally, stimuli belonging to different modalities were delivered at different spatial locations, therefore introducing a possible confound related to the deployment of spatial attention to the source of the sensory event. This is important as studies have shown that the spatial location of distracters modulates their effectiveness in capturing attention (e.g.(Van Ryckeghem, Crombez, Eccleston, Legrain, & Van Damme, 2012)). Therefore, although these studies have provided important information on the relationship between attention and pain, conclusions should be cautious due to the plurality of possible interpretations.
In another study, Tracey and colleagues (Tracey, Ploghaus, Gati, Clare, Smith, Menon, & Matthews, 2002) tested the effect of ‘focusing’ on pain vs. being distracted from pain, i.e. think of something else. As a control, they also asked participants to perform the same task when a warm, instead of a painful stimulus, was applied. Their behavioral results suggested that participants rated stimuli in the ‘non-attend’ session as less painful, but this was not the case for warm stimuli. However, it is important to mention that the same trend of reduction in the non-attend condition was observed for both modalities, and considering that only nine participants were included in the study, definite conclusions about the pain specificity of the results could be prone to false positives. Their fMRI results suggested that activity in the periaqueductal gray (PAG) increased for the non-attended condition, when BOLD signal in response to painful stimuli was subtracted from the BOLD signal in response to warm stimuli. The authors concluded that increase in PAG activity reflects top-down influences on descending inhibitory pathways.
3.2. Competition for attentional resources: the effects of working memory and executive control on pain processing
According to the limited attentional resources model, when participants perform a highly demanding cognitive task, the attentional load will reduce available resources needed to process task-irrelevant stimuli (Legrain, Van Damme, Eccleston, Davis, Seminowicz, & Crombez, 2009). This would result in a reduction of the pain triggered by irrelevant nociceptive stimuli (Bantick, Wise, Ploghaus, Clare, Smith, & Tracey, 2002). In this context, a highly effective task is the Stroop task, in which participants have to inhibit an automatic process and, instead, perform a non-automatic process (e.g. the word ‘red’ is displayed in blue, and participants have to name the color blue, instead of reading the word red).
Several studies have compared the brain responses elicited by painful stimuli while subjects perform a Stroop task vs. a low attentional demands task (Bantick, Wise, Ploghaus, Clare, Smith, & Tracey, 2002; Seminowicz & Davis, 2007a, 2007c; Valet, Sprenger, Boecker, Willoch, Rummeny, Conrad, Erhard, & Tolle, 2004). Some of these studies (e.g. (Bantick, Wise, Ploghaus, Clare, Smith, & Tracey, 2002)) have observed reduced activity to painful stimuli during the Stroop test in the mid-cingulate cortex. Others (e.g. (Seminowicz & Davis, 2007a, 2007b, 2007c; Seminowicz, Mikulis, & Davis, 2004)) found that performing the Stroop test can attenuate responses to moderately painful stimuli in primary and secondary somatosensory cortices (S1, S2) and in the anterior insula (Seminowicz, Mikulis, & Davis, 2004).
These differences could be related to crucial differences in the performed tasks. Bantick et al. asked participants to rate the intensity of the painful stimuli whereas Seminowicz et al. did not. This additional ‘pain rating’ task actually rendered the painful stimuli relevant for the participant’s behavioural goals. For this reason, in the study of Bantick et al., the nociceptive stimuli cannot truly be considered as task irrelevant. In addition to this, it has been shown that the effects of attentional manipulation on painful stimuli can vary depending on the interactions between the cognitive load and the perceived intensity of the stimuli (see also (Romero, Straube, Nitsch, Miltner, & Weiss, 2013)). While in the study of Valet et al., (2004), the Stroop test had a strong impact on stimulus-related activations, Seminowicz and Davis (2007) did not observe a complete disruption of pain related activity by the cognitive load and vice versa.
Using a different approach, Bingel et al. (Bingel, Rose, Glascher, & Buchel, 2007) explored the ‘distractive’ effects of pain and of the execution of a visual working memory task on visual processing. Participants performed an N-back task, which required comparing a letter appearing on an irrelevant background image (of different levels of visibility), to a letter appeared one or two images before. Laser stimuli of different intensity were presented concomitantly with the visual stimulus. BOLD signal in visual areas was modulated by the visibility of the background image but, importantly, the administration of painful stimuli reduced this BOLD increase. The authors proposed that the distractive effects of pain and of working memory on visual processing were driven by different brain regions: the rostral anterior cingulate cortex could explain the BOLD modulation of visual areas by pain, and the inferior parietal cortex by working memory.
Although these findings offer a new and interesting perspective, it should also be noted that no control condition with equally salient non-nociceptive stimuli was used, to investigate whether these effects were specific for pain. Moreover, specific regions of interest (ROIs) were chosen to test the effects of pain and working memory over visual activation, leaving open the possibility that areas other than the selected ones were also modulated. Finally, participants were asked to provide pain ratings, which made the painful stimulus behaviorally relevant. Therefore, it remains unclear whether BOLD changes in visual areas were dependent on distraction from pain or on the involvement in two tasks at the same time (working memory task and intensity rating task).
A study by Sprenger and colleagues supported the possibility that working memory modulates the processing of nociceptive inputs at the level of the spinal cord (Sprenger, Eippert, Finsterbusch, Bingel, Rose, & Buchel, 2012). These authors recorded spinal cord fMRI data while participants received painful stimuli during the execution of an N-back working memory task. Their results suggested that the distractive effect of the N-back task reduced the spinal activity related to the incoming nociceptive input. Importantly the authors also showed that the administration of naloxone, an opioid receptor antagonist, diminished - but did not abolish - the effect of working memory on the stimuli, suggesting that the effects of working memory are partially dependent on opioid-sensitive descending inhibitory circuits.
The fact that naloxone did not abolish completely the effects of working memory on painful stimuli may be important as it suggests that more than one mechanism operating both at spinal and at supraspinal levels, can participate in modulating the responses (Torta, Churyukanov, Plaghki, & Mouraux, 2015).
To summarize, the use of tasks involving working memory and executive functions can provide important insights especially in terms of attentional modulation of pain in the context of ‘load theories’. However, critical review of these studies also indicates that it is important to consider carefully that even apparently negligible tasks like providing a sensory rating can have an important impact on BOLD changes, as they constitute a task per se (Seminowicz & Davis, 2007c). Moreover, although tempting, conclusions about ‘specific’ effects on pain should be avoided, if other stimuli of comparable salience are not tested.
4. Advantages and disadvantages of using fMRI to study the interactions between pain and attention
As highlighted in previous paragraphs, different cognitive functions or different attentional processes may exert their effect on the processing of nociceptive input through different mechanisms, and these mechanisms could operate at supraspinal and/or spinal levels. One open issue regards how to disentangle, using fMRI, effects related to bottom-up and top-down mechanisms. Indeed, although some tasks can be expected to tap bottom-up mechanisms more than top-down mechanisms, it is unlikely that responses to a stimulus presented in a given context reflect completely one or the other process. For example, when a deviant stimulus is presented in a stream of standard stimuli, a bottom-up capture of attention occurs, that is possibly followed by top-down maintenance of the attentional focus on the deviant stimulus, if this acquired relevance for the ongoing behavioral goals. A major issue is that these processes are likely to occur within a time interval that cannot be resolved using standard fMRI, which is dependent on slow changes in the BOLD signal. Furthermore, some effects of attention on the BOLD responses sampled in a given brain region could depend on a ‘reactivation’ of that brain region, due to feedback projections (see (Pessoa, Kastner, & Ungerleider, 2003) for a review). In this respect, studies relying on the recording of ERPs are able to pinpoint more precisely the time intervals at which attention may exert its effects on the processing of nociceptive stimuli. However, ERP studies cannot provide any clear evidence about the engagement of subcortical structures that are instead crucial in attentional processing.
5. Do the ‘insula’ and the ‘cingulate cortex’ have a pivotal role in the attentional modulation of pain?
From a simplistic summary of the findings reported so far, we could conclude that the insula and the cingulate cortex are two key players in pain-attention interactions.
What is the possible functional role of these brain regions? Several lines of evidence suggest that it is difficult to attribute to these regions a selective role in the attentional modulation of pain, or even a unique function (Cauda, D'Agata, Sacco, Duca, Geminiani, & Vercelli, 2011; Chang, Yarkoni, Khaw, & Sanfey, 2013; Craig, 2009; Palomero-Gallagher, Mohlberg, Zilles, & Vogt, 2008; Palomero-Gallagher, Vogt, Schleicher, Mayberg, & Zilles, 2009; Shackman, Salomons, Slagter, Fox, Winter, & Davidson, 2011; Torta & Cauda, 2011; B. A. Vogt, 2016). In order to advance our understanding of their role during the experience of pain we should first consider their anatomo-functional complexity.
The insula is not a unitary structure, neither structurally nor functionally. In fact, studies have reported at least two (Cauda, Costa, Torta, Sacco, D'Agata, Duca, Geminiani, Fox, & Vercelli, 2012; Cauda, D'Agata, Sacco, Duca, Geminiani, & Vercelli, 2011; Cerliani, Thomas, Jbabdi, Siero, Nanetti, Crippa, Gazzola, D'Arceuil, & Keysers, 2011; Treister, Eisenberg, Gershon, Haddad, & Pud, 2010), three (Chang, Yarkoni, Khaw, & Sanfey, 2013; Deen, Pitskel, & Pelphrey, 2011) or even nine (Kelly, Toro, Di Martino, Cox, Bellec, Castellanos, & Milham, 2012) functionally distinct clusters. The anterior insula has been proposed to play a central role in the integration of exteroceptive and interoceptive sensory inputs, whereas the posterior insula has been proposed to be more specifically related to specific somatosensory functions (Cauda, Costa, Torta, Sacco, D'Agata, Duca, Geminiani, Fox, & Vercelli, 2012; Cauda, D'Agata, Sacco, Duca, Geminiani, & Vercelli, 2011; Cerliani, Thomas, Jbabdi, Siero, Nanetti, Crippa, Gazzola, D'Arceuil, & Keysers, 2011; Treister, Eisenberg, Gershon, Haddad, & Pud, 2010). In other words, the anterior insula could constitute an integration hub mediating attention and internally oriented self-cognition (Craig, 2002, 2009; zu Eulenburg, Baumgartner, Treede, & Dieterich, 2013). Furthermore, the anterior and posterior insula could work in synergy to modulate autonomic reactivity to salient stimuli (V. Menon & Uddin, 2010), painful stimuli being just one of the possible stimuli triggering these reactions.
Some authors have proposed that the insula is activated by stimuli belonging to several sensory modalities (zu Eulenburg, Baumgartner, Treede, & Dieterich, 2013), and serves the role of a ‘general magnitude estimator’ (Baliki, Geha, & Apkarian, 2009). This would explain why ‘attention to pain’ tends to increase both the intensity of pain perception and the magnitude of pain-related insular responses. At the same time, this notion would entail that attentional effects of pain-related insular responses are not specific for pain (Baliki et al., 2009). For all these reasons, stating that the insula is the site of interaction between pain and attention is probably reductive (Davis, Bushnell, Iannetti, St Lawrence, & Coghill, 2015; Feinstein, Khalsa, Salomons, Prkachin, Frey-Law, Lee, Tranel, & Rudrauf, 2015), and a supramodal perspective should be instead adopted. Furthermore, future studies should consider the most recent findings about the complexity of insular anatomy, structural connectivity and functional connectivity to avoid overly simplistic views.
The activation of the cingulate cortex is also observed consistently in studies of attentional modulation of pain. A potential role of the anterior, mid-cingulate cortex and dorsal anterior cingulate cortex was proposed already in the very first fMRI studies. These studies often concluded that attention and pain elicited responses in distinct subregions of the cingulate cortex (Moont, Crispel, Lev, Pud, & Yarnitsky, 2012; Nir, Sinai, Moont, Harari, & Yarnitsky, 2012; Peyron, Garcia-Larrea, Gregoire, Costes, Convers, Lavenne, Mauguiere, Michel, & Laurent, 1999).
Again, the complexity of this brain region should be discussed. On the one hand, studies have allowed important advances on the relationship between cytoarchitectonic and functional overlaps in the cingulate cortex, providing support at the receptor level of four distinct areas (Palomero-Gallagher, Vogt, Schleicher, Mayberg, & Zilles, 2009; B. A. Vogt, 2005; B. A. Vogt, Berger, & Derbyshire, 2003; B. A. Vogt & Laureys, 2005; B. A. Vogt & Vogt, 2003; B. A. Vogt, Vogt, Perl, & Hof, 2001). On the other hand, as already proposed in (B. A. Vogt, 2005), studies using a meta-analytic approach provide evidence of large functional overlaps. Both Shackman et al., (Shackman, Salomons, Slagter, Fox, Winter, & Davidson, 2011) and Torta & Cauda (Torta & Cauda, 2011) observed substantial activation overlap between responses to ‘pain’, and ‘attention’ (not necessarily pain-related). This overlap was especially evident in the mid-cingulate and dorsal anterior cingulate cortex. Such evidence is in line with the view that the response triggered by nociceptive stimuli in the cingulate cortex could largely reflect orienting of attention to sensory stimuli independently of the modality of the stimuli and regardless of whether these stimuli elicit a perception of pain (see (Kucyi, Hodaie, & Davis, 2012; Legrain, Van Damme, Eccleston, Davis, Seminowicz, & Crombez, 2009; Seeley, Menon, Schatzberg, Keller, Glover, Kenna, Reiss, & Greicius, 2007; Shackman, Salomons, Slagter, Fox, Winter, & Davidson, 2011; Torta, Costa, Duca, Fox, & Cauda, 2013). Further evidence supports this interpretation. First, the mid-cingulate cortex is anatomically and functionally connected to motor, premotor and parietal areas (Torta & Cauda, 2011; Yu, Zhou, Liu, Jiang, Dong, Zhang, & Walter, 2011) and could thus easily prompt flight or fight responses to potentially harmful stimuli. Second, salient sensory events such as novel stimuli trigger strong responses in the mid-cingulate cortex independently of sensory modality (Downar, Crawley, Mikulis, & Davis, 2002). Third, nociceptive evoked EEG responses possibly originating from the mid-cingulate cortex (Garcia-Larrea, Frot, & Valeriani, 2003) are enhanced when attention is more strongly captured by those nociceptive stimuli (Legrain, Guerit, Bruyer, & Plaghki, 2002, 2003). Conversely, the same EEG responses were reduced in amplitude when participants succeeded to not be distracted by the nociceptive stimuli (Legrain et al., 2013).
Importantly, although we have treated in this paragraph the insula and the cingulate cortex as two ‘stand-alone’ regions, significant evidence suggest that they operate in close synergy, although possibly keeping separate functional roles (V Menon, 2015), as discussed in the following paragraph about brain networks.
4. Moving from brain regions to brain networks
In the last years, neuroimaging research has suggested that brain functions emerge from the activity of large-scale networks, both at rest (Fox, Snyder, Vincent, Corbetta, Van Essen, & Raichle, 2005) and during task-execution (Laird, Fox, Eickhoff, Turner, Ray, McKay, Glahn, Beckmann, Smith, & Fox, 2012). Another approach to study the attentional effects over pain is thus to conceive these effects in terms of brain networks. A network approach would foster the understanding of the recurrent functional relationship between brain regions such as the insula and the cingulate cortex. Indeed, these two regions (more specifically the anterior insula and the dorsal anterior cingulate cortex) would form the so-called ‘salience network’ (Kucyi, Hodaie, & Davis, 2012; V Menon, 2015; Seeley, Menon, Schatzberg, Keller, Glover, Kenna, Reiss, & Greicius, 2007), and be part, as hubs, to more than a brain network (Cauda, Costa, Torta, Sacco, D'Agata, Duca, Geminiani, Fox, & Vercelli, 2012; Cauda, Torta, Sacco, Geda, D'Agata, Costa, Duca, Geminiani, & Amanzio, 2012; Chang, Yarkoni, Khaw, & Sanfey, 2013; V. Menon & Uddin, 2010; Torta, Costa, Duca, Fox, & Cauda, 2013; Yu, Zhou, Liu, Jiang, Dong, Zhang, & Walter, 2011). One of the major advantages of the network approach is that the functional relationship between two or more brain regions can be tested under the assumption that co-variations of the BOLD signal imply functional links. This approach has offered the possibility to move from ‘localizationist’ approaches to functional hypotheses.
Valet et al. (Valet, Sprenger, Boecker, Willoch, Rummeny, Conrad, Erhard, & Tolle, 2004) observed that during distraction from painful stimuli using a Stroop Task, the activity in the perigenual anterior cingulate cortex and the orbitofrontal cortex co-varied with activity in the PAG and posterior thalamus. This covariation was present when the painful stimuli were applied concomitantly with the Stroop Task, but was not observed when the Stroop Task was executed during the administration of non-painful stimuli. Seminowicz and Davis (Seminowicz & Davis, 2007b) investigated the interaction between cognitive load and pain intensity, using a network perspective. They first isolated a brain activity pattern associated with the cognitive load that could be identified as ‘attentional’. This network was composed of task-positive parts (e.g. areas synergistically active that increased their activity with increasing difficulty of the task, including the frontal and parietal regions and the anterior insula) and task-negative parts (e.g. areas that showed the opposite pattern, including the precuneus and the posterior cingulate cortex). When all conditions were included (pain and cognitive load)4, and an analysis run using the mask previously identified, the activation of the task-positive network was increased by pain. As suggested by the authors, these findings could reflect a substrate for the effects of pain on attention, or more simply general arousal measure capturing the activity of a network modulated by exteroception.
The network perspective opens a more ecologically grounded approach: that spontaneous brain oscillations and variations in attention can themselves influence pain-attention interactions (see (Kucyi & Davis, 2015). In this vein, Kucyi and colleagues, using a more advanced dynamic functional connectivity analysis, showed that the resting state network activity can shape the experience of pain. Furthermore, focusing on pain changes networks dynamics, likely promoting a decreased engagement of the default mode network (DMN, a network most active when there are no significant events in the surrounding environment and when participants are not involved in a specific task) and an increased engagement of two other networks, referred to as the ‘salience network’ and the ‘antinociceptive network’ (Kucyi, Salomons, & Davis, 2013)5. Similarly, Ter-Minassian et al. (Ter Minassian, Ricalens, Humbert, Duc, Aube, & Beydon, 2013) found reduced DMN activity during anticipation of pain while the so-called ‘dorsal attention network’ was, instead, in a more activated state.
Future studies may exploit more advanced network metrics provided by graph theory analysis (see Bullmore and Sporns, 2009) to elucidate the relationship between these different networks during different experimental manipulations of attention and pain.
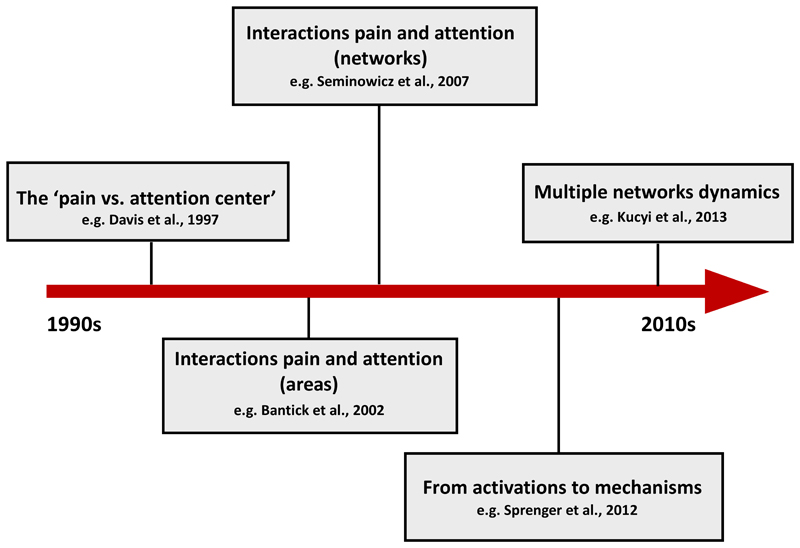
Figure 3 shows a summary of the evolution in the study of the attentional modulation of pain across the years.
Figure 3.
The evolution of the theoretical perspective in the study of the attentional modulations of pain. The ‘pain vs. attention center’. The first studies aimed at identifying which brain activations were related to pain and which ones to attention. Attention tasks and painful stimuli were often presented in separate blocks and differential activations taken to indicate that different brain regions elaborate pain perception (or nociceptive elaboration) and attentional tasks (e.g. Davis et al., 1997). ‘Interactions pain and attention (areas)’. Subsequent studies sought to determine which areas underpin the attentional modulation of pain. For this aim, noxious stimuli were applied during the execution of an attentional task and brain activations obtained in these conditions compared to brain activations elicited by noxious stimuli without a concomitant task (e.g. Bantick et al., 2002). ‘Interactions pain attention (networks)’. A further step moved the interest from single (or multiple areas) studied as working separately to brain networks. Attentional modulation of noxious stimuli were investigated by studying activation and deactivation of networks of areas (e.g. Seminowicz et al., 2007). ‘From activation to mechanisms’. The suggestion that cognitive factors, including attention, could modulate activity at the level of the spinal cord was proposed back in the 1960s. However, imaging evidence was available later on, showing reduced activations to noxious stimuli at the level of the spinal cord activations during the execution of a working memory task. These (de)activations were also used to predict pain reductions (Sprenger et al., 2012). ‘Multiple network dynamics’. More recently, it has been suggested that spontaneous mind wandering from pain is correlated with network dynamics, with strengthened or weakened function connectivity between the PAG and the DMN.
5. Concluding remarks
Pain has often been regarded as a unique sensory and emotional experience that would deeply differ from the experience emerging from the processing of stimuli in other sensory modalities. However, although descending mechanisms are thought increasingly to play an important role in pain modulation, as well as in the susceptibility to develop chronic pain, it is important to emphasize that the top-down modulation of sensory input is not a unique feature of the nociceptive system. In this vein, the top-down projections that are often assumed to be specifically involved in pain modulation may actually play a more general role, facilitating/inhibiting physiological reactions, for defensive actions/escape or survival (Mason, 2005). This possibility would further support the interpretation of pain as resulting from the activity of a general cortical system prioritizing and prompting action in response to potentially dangerous stimuli that are meaningful for body homeostasis (Legrain et al., 2011). Some authors have shown that spinal responses to innocuous and nociceptive cold can be differentially modulated by PAG activity, which would affect responses to nociceptive, but not to innocuous stimuli (Leith, Koutsikou, Lumb, & Apps, 2010). In addition, similar corticofugal mechanisms modulate the transmission of non-nociceptive somatosensory input at the level of dorsal column nuclei. Indeed, it has been shown that corticofugal projections can modulate the responses of dorsal column nuclei to tactile stimuli (Malmierca, Chaves-Coira, Rodrigo-Angulo, & Nunez, 2014; Nunez & Malmierca, 2007). This effect could contribute to the mechanisms of selective attention. Therefore, we conclude that at least a part of the attentional modulation of nociception is similar to the modulation observed in other sensory modalities. Yet, painful stimuli may gain a different affective weight compared to other modalities, as they have the potential to damage the body. In line with this view, we believe that motivational, load and affective theories may be particularly suitable to capture the complex interplay between attention and pain (Lavie, Beck, & Konstantinou, 2014; Pourtois, Schettino, & Vuilleumier, 2013; Van Damme, Legrain, Vogt, & Crombez, 2009). Indeed, they offer a clear framework to show how affective factors, contextual factors and cognitive load may shape the interruptive nature of pain.
Our review of the literature has also highlighted the importance of the choice of theoretical framework in the experimental design and interpretation of the results, considering that the concepts of attention and pain, as well as their neural substrates can be largely overlapping. In this sense, paradigms in which brain responses to different features of a painful stimulus are compared and paradigms using working memory tasks might be less prone to potentially confounding effects, and can lead to more solid conclusions. What is more, methodological aspects regarding the time of the BOLD signal response should also be considered more carefully when addressing a fluctuating phenomenon like attention.
Future directions in the study of attentional modulation of pain should conceive complex models that consider the individual valence and relevance of nociceptive stimuli. Moreover, future lines of research should take into account individual variability in the ability to engage in alternative tasks or disengaging from salient stimuli, how much individual differences are modifiable by experience or represent stable personality traits. Finally, research may benefit from the use of more ecological conditions to investigate spontaneous oscillations of attention at rest and during tasks. Figure 4 shows current cognitive models of attentional modulation of pain and nociceptive inputs and suggests possible future directions of study.
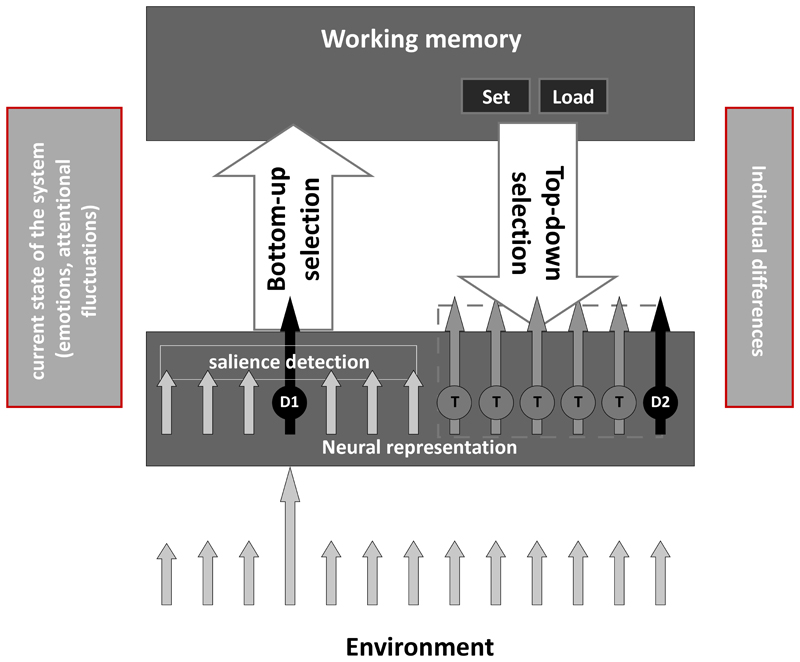
Figure 4.
Schematic illustration of the attentional processes that contribute to the elaboration of nociceptive stimuli. Priority access to working memory is gained depending on top-down selection (dependent on intentional control) and bottom-up selection (triggered by involuntary capture of attention). The intentional selection of the stimuli is made based on the relevance of cognitive goals (i.e. it depends on the possibility that stimuli are targets [T] of the task). Stimuli that are not goal-relevant (i.e. that are distractors [D]) can enter into the spot of attention, if they are salient enough to capture attention involuntarily (D1) or if they share some features with the targets (D2). For a thorough description of the model please refer to (Legrain & Torta, 2015; Legrain, Van Damme, Eccleston, Davis, Seminowicz, & Crombez, 2009). In an update of this model, we suggest that other factors can influence the balance between top-down and bottom-up processes (see left and right boxes). For instance the balance between top-down and bottom-up selection can be shaped by the emotional status of the person (e.g. (Sussman, Szekely, Hajcak, & Mohanty, 2016; Vanlessen, Rossi, De Raedt, & Pourtois, 2014), and by spontaneous fluctuations of attention (Kucyi, Salomons, & Davis, 2013). Anxious individuals for instance might find it problematic to focus on an alternative goal and be more susceptible to being attracted by bottom-up distracters. In contrast, spontaneous ‘mind wandering’ away from the stimuli might contribute to reduce the bottom-up distraction of the stimuli even independently from a concomitant non-pain related goal. Individual differences in terms of personality traits and cognitive abilities may also contribute to a flexible (or non-flexible) balancing between the two systems. It remains to be elucidated how much these characteristics are unchangeable by the experience or can be trained.
Acknowledgements
VL is supported by the Fund for Scientific Research of the French-speaking Community of Belgium (F.R.S.-FNRS). AM received support from an ERC starting grant “PROBING-PAIN” (336130).
Footnotes
Throughout the review, we will refer sometimes to ‘nociceptive’ and sometimes to ‘pain’ modulation. The rationale of using either term was based on the terminology used in the reviewed literature. We used the term ‘pain’ if the original article reported the term ‘pain’, nociception if it was unclear whether the stimuli could be qualified as painful. Furthermore, the use of the concept ‘pain’ can be misleading in imaging studies. Indeed, the activation of brain regions in response to nociceptive inputs is not sufficient to be referred to as ‘pain’ when no subjective report on the perceived quality of the stimulus is available.
In this review, we will elaborate on why attention cannot be considered as a unitary concept. However, we will also use the notion of ‘attentional modulation of pain’ as a general term to refer to all possible effects of attention on pain and nociception.
We use the term salience to refer to the physical properties of the stimulus that captures attention (bottom-up). We use relevance to refer to the characteristics of the stimulus that make it pertinent for cognitive goals (top-down).
The areas of the task-negative network were remarkably similar to those of the ‘default mode network’, see next note on the topic.
Several networks can be identified in the “resting brain”. Besides the aforementioned attentional networks, researchers have consistently identified the default mode network (DMN), which is thought to be composed by areas including the posterior cingulate cortex, the precuneus, and the medial prefrontal cortex; the salience network which includes as prominent areas the anterior insula and the dorsal anterior cingulate cortex; the ‘antinociceptive network’ would include the medial prefrontal cortex, the periaqueductal gray, and the rostroventral medulla. In other words, it would include descending inhibitory systems.
The authors declare the absence of any conflict of interest.
References
- Aboitiz F, Ossandon T, Zamorano F, Palma B, Carrasco X. Irrelevant stimulus processing in ADHD: catecholamine dynamics and attentional networks. Front Psychol. 2014;5:183. doi: 10.3389/fpsyg.2014.00183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allport DA. Selection for action: Some behavioral and neurophysiological considerations of attention and action. 1987. [Google Scholar]
- Baliki MN, Apkarian AV. Nociception, Pain, Negative Moods, and Behavior Selection. Neuron. 2015;87(3):474–491. doi: 10.1016/j.neuron.2015.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baliki MN, Geha PY, Apkarian AV. Parsing pain perception between nociceptive representation and magnitude estimation. Journal of Neurophysiology. 2009;101(2):875–887. doi: 10.1152/jn.91100.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bantick SJ, Wise RG, Ploghaus A, Clare S, Smith SM, Tracey I. Imaging how attention modulates pain in humans using functional MRI. Brain. 2002;125(Pt 2):310–319. doi: 10.1093/brain/awf022. [DOI] [PubMed] [Google Scholar]
- Bingel U, Rose M, Glascher J, Buchel C. fMRI reveals how pain modulates visual object processing in the ventral visual stream. Neuron. 2007;55(1):157–167. doi: 10.1016/j.neuron.2007.05.032. [DOI] [PubMed] [Google Scholar]
- Broadbent D. Perception and Communication. 1958. [Google Scholar]
- Brooks JC, Nurmikko TJ, Bimson WE, Singh KD, Roberts N. fMRI of thermal pain: effects of stimulus laterality and attention. Neuroimage. 2002;15(2):293–301. doi: 10.1006/nimg.2001.0974. [DOI] [PubMed] [Google Scholar]
- Buchel C, Bornhovd K, Quante M, Glauche V, Bromm B, Weiller C. Dissociable neural responses related to pain intensity, stimulus intensity, and stimulus awareness within the anterior cingulate cortex: a parametric single-trial laser functional magnetic resonance imaging study. J Neurosci. 2002;22(3):970–976. doi: 10.1523/JNEUROSCI.22-03-00970.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buhle J, Wager TD. Performance-dependent inhibition of pain by an executive working memory task. Pain. 2010;149(1):19–26. doi: 10.1016/j.pain.2009.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bushnell MC, Duncan GH, Hofbauer RK, Ha B, Chen JI, Carrier B. Pain perception: is there a role for primary somatosensory cortex? Proceedings of the National Academy of Sciences USA. 1999;96(14):7705–7709. doi: 10.1073/pnas.96.14.7705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cauda F, Costa T, Torta DM, Sacco K, D'Agata F, Duca S, Geminiani G, Fox PT, Vercelli A. Meta-analytic clustering of the insular cortex: Characterizing the meta-analytic connectivity of the insula when involved in active tasks. Neuroimage. 2012 doi: 10.1016/j.neuroimage.2012.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cauda F, D'Agata F, Sacco K, Duca S, Geminiani G, Vercelli A. Functional connectivity of the insula in the resting brain. Neuroimage. 2011;55(1):8–23. doi: 10.1016/j.neuroimage.2010.11.049. [DOI] [PubMed] [Google Scholar]
- Cauda F, Torta DM, Sacco K, Geda E, D'Agata F, Costa T, Duca S, Geminiani G, Amanzio M. Shared "core" areas between the pain and other task-related networks. PLoS One. 2012;7(8):e41929. doi: 10.1371/journal.pone.0041929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerliani L, Thomas RM, Jbabdi S, Siero JC, Nanetti L, Crippa A, Gazzola V, D'Arceuil H, Keysers C. Probabilistic tractography recovers a rostrocaudal trajectory of connectivity variability in the human insular cortex. Hum Brain Mapp. 2011 doi: 10.1002/hbm.21338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang LJ, Yarkoni T, Khaw MW, Sanfey AG. Decoding the role of the insula in human cognition: functional parcellation and large-scale reverse inference. Cereb Cortex. 2013;23(3):739–749. doi: 10.1093/cercor/bhs065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chica AB, Bartolomeo P. Attentional routes to conscious perception. Front Psychol. 2012;3:1. doi: 10.3389/fpsyg.2012.00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coghill RC, Sang CN, Maisog JM, Iadarola MJ. Pain intensity processing within the human brain: a bilateral, distributed mechanism. Journal of Neurophysiology. 1999;82(4):1934–1943. doi: 10.1152/jn.1999.82.4.1934. [DOI] [PubMed] [Google Scholar]
- Coghill RC, Talbot JD, Evans AC, Meyer E, Gjedde A, Bushnell MC, Duncan GH. Distributed processing of pain and vibration by the human brain. Journal of Neuroscience. 1994;14(7):4095–4108. doi: 10.1523/JNEUROSCI.14-07-04095.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbetta M, Patel G, Shulman GL. The reorienting system of the human brain: from environment to theory of mind. Neuron. 2008;58(3):306–324. doi: 10.1016/j.neuron.2008.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbetta M, Shulman GL. Control of goal-directed and stimulus-driven attention in the brain. Nature Review Neuroscience. 2002;3(3):201–215. doi: 10.1038/nrn755. [DOI] [PubMed] [Google Scholar]
- Craig AD. How do you feel? Interoception: the sense of the physiological condition of the body. Nat Rev Neurosci. 2002;3(8):655–666. doi: 10.1038/nrn894. [DOI] [PubMed] [Google Scholar]
- Craig AD. How do you feel--now? The anterior insula and human awareness. Nature Review Neuroscience. 2009;10(1):59–70. doi: 10.1038/nrn2555. [DOI] [PubMed] [Google Scholar]
- Crombez G, Baeyens F, Eelen P. Sensory and temporal information about impending pain: the influence of predictability on pain. Behav Res Ther. 1994;32(6):611–622. doi: 10.1016/0005-7967(94)90015-9. [DOI] [PubMed] [Google Scholar]
- Crombez G, Eccleston C, Baeyens F, Eelen P. The disruptive nature of pain: an experimental investigation. Behav Res Ther. 1996;34(11–12):911–918. doi: 10.1016/s0005-7967(96)00058-7. [DOI] [PubMed] [Google Scholar]
- Crombez G, Eccleston C, Baeyens F, Eelen P. Attentional disruption is enhanced by the threat of pain. Behav Res Ther. 1998;36(2):195–204. doi: 10.1016/s0005-7967(97)10008-0. [DOI] [PubMed] [Google Scholar]
- Crombez G, Van Ryckeghem DM, Eccleston C, Van Damme S. Attentional bias to pain-related information: a meta-analysis. Pain. 2013;154(4):497–510. doi: 10.1016/j.pain.2012.11.013. [DOI] [PubMed] [Google Scholar]
- Crombez G, Vervaet L, Lysens R, Baeyens F, Eelen P. Avoidance and confrontation of painful, back-straining movements in chronic back pain patients. Behav Modif. 1998;22(1):62–77. doi: 10.1177/01454455980221004. [DOI] [PubMed] [Google Scholar]
- Davis KD, Bushnell MC, Iannetti GD, Lawrence K, St, Coghill R. Evidence against pain specificity in the dorsal posterior insula. F1000Res. 2015;4:362. doi: 10.12688/f1000research.6833.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis KD, Taylor SJ, Crawley AP, Wood ML, Mikulis DJ. Functional MRI of pain- and attention-related activations in the human cingulate cortex. J Neurophysiol. 1997;77(6):3370–3380. doi: 10.1152/jn.1997.77.6.3370. [DOI] [PubMed] [Google Scholar]
- Deen B, Pitskel NB, Pelphrey KA. Three systems of insular functional connectivity identified with cluster analysis. Cereb Cortex. 2011;21(7):1498–1506. doi: 10.1093/cercor/bhq186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derbyshire SW, Jones AK. Cerebral responses to a continual tonic pain stimulus measured using positron emission tomography. Pain. 1998;76(1–2):127–135. doi: 10.1016/s0304-3959(98)00034-7. [DOI] [PubMed] [Google Scholar]
- Derbyshire SW, Jones AK, Devani P, Friston KJ, Feinmann C, Harris M, Pearce S, Watson JD, Frackowiak RS. Cerebral responses to pain in patients with atypical facial pain measured by positron emission tomography. J Neurol Neurosurg Psychiatry. 1994;57(10):1166–1172. doi: 10.1136/jnnp.57.10.1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devinsky O, Morrell MJ, Vogt BA. Contributions of Anterior Cingulate Cortex to Behaviour. Brain. 1995;118:279–306. doi: 10.1093/brain/118.1.279. [DOI] [PubMed] [Google Scholar]
- Dillmann J, Miltner WH, Weiss T. The influence of semantic priming on event-related potentials to painful laser-heat stimuli in humans. Neurosci Lett. 2000;284(1–2):53–56. doi: 10.1016/s0304-3940(00)00957-5. [DOI] [PubMed] [Google Scholar]
- Dosenbach NU, Fair DA, Miezin FM, Cohen AL, Wenger KK, Dosenbach RA, Fox MD, Snyder AZ, Vincent JL, Raichle ME, Schlaggar BL, et al. Distinct brain networks for adaptive and stable task control in humans. Proc Natl Acad Sci U S A. 2007;104(26):11073–11078. doi: 10.1073/pnas.0704320104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dosenbach NU, Visscher KM, Palmer ED, Miezin FM, Wenger KK, Kang HC, Burgund ED, Grimes AL, Schlaggar BL, Petersen SE. A core system for the implementation of task sets. Neuron. 2006;50(5):799–812. doi: 10.1016/j.neuron.2006.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downar J, Crawley AP, Mikulis DJ, Davis KD. A cortical network sensitive to stimulus salience in a neutral behavioral context across multiple sensory modalities. Journal of Neurophysiology. 2002;87(1):615–620. doi: 10.1152/jn.00636.2001. [DOI] [PubMed] [Google Scholar]
- Eccleston C, Crombez G. Pain demands attention: a cognitive-affective model of the interruptive function of pain. Psychol Bull. 1999;125(3):356–366. doi: 10.1037/0033-2909.125.3.356. [DOI] [PubMed] [Google Scholar]
- Egeth HE, Yantis S. Visual attention: control, representation, and time course. Annu Rev Psychol. 1997;48:269–297. doi: 10.1146/annurev.psych.48.1.269. [DOI] [PubMed] [Google Scholar]
- Escera C, Malmierca MS. The auditory novelty system: an attempt to integrate human and animal research. Psychophysiology. 2014;51(2):111–123. doi: 10.1111/psyp.12156. [DOI] [PubMed] [Google Scholar]
- Feinstein JS, Khalsa SS, Salomons TV, Prkachin KM, Frey-Law LA, Lee JE, Tranel D, Rudrauf D. Preserved emotional awareness of pain in a patient with extensive bilateral damage to the insula, anterior cingulate, and amygdala. Brain Struct Funct. 2015 doi: 10.1007/s00429-014-0986-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox MD, Snyder AZ, Vincent JL, Corbetta M, Van Essen DC, Raichle ME. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc Natl Acad Sci U S A. 2005;102(27):9673–9678. doi: 10.1073/pnas.0504136102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frankenstein UN, Richter W, McIntyre MC, Remy F. Distraction modulates anterior cingulate gyrus activations during the cold pressor test. Neuroimage. 2001;14(4):827–836. doi: 10.1006/nimg.2001.0883. [DOI] [PubMed] [Google Scholar]
- Garcia-Larrea L, Frot M, Valeriani M. Brain generators of laser-evoked potentials: from dipoles to functional significance. Neurophysiologie Clinique. 2003;33(6):279–292. doi: 10.1016/j.neucli.2003.10.008. [DOI] [PubMed] [Google Scholar]
- Godinho F, Magnin M, Frot M, Perchet C, Garcia-Larrea L. Emotional modulation of pain: is it the sensation or what we recall? J Neurosci. 2006;26(44):11454–11461. doi: 10.1523/JNEUROSCI.2260-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hommel B. Grounding attention in action control: The intentional control of selection. Cambridge, MA: MIT Press; 2010. [Google Scholar]
- Hsieh JC, Stahle-Backdahl M, Hagermark O, Stone-Elander S, Rosenquist G, Ingvar M. Traumatic nociceptive pain activates the hypothalamus and the periaqueductal gray: a positron emission tomography study. Pain. 1996;64(2):303–314. doi: 10.1016/0304-3959(95)00129-8. [DOI] [PubMed] [Google Scholar]
- James W. The principles of psychology. New York: 1890. [Google Scholar]
- Jones AK, Brown WD, Friston KJ, Qi LY, Frackowiak RS. Cortical and subcortical localization of response to pain in man using positron emission tomography. Proc Biol Sci. 1991;244(1309):39–44. doi: 10.1098/rspb.1991.0048. [DOI] [PubMed] [Google Scholar]
- Kelly C, Toro R, Di Martino A, Cox CL, Bellec P, Castellanos FX, Milham MP. A convergent functional architecture of the insula emerges across imaging modalities. Neuroimage. 2012;61(4):1129–1142. doi: 10.1016/j.neuroimage.2012.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keogh E, Ellery D, Hunt C, Hannent I. Selective attentional bias for pain-related stimuli amongst pain fearful individuals. Pain. 2001;91(1–2):91–100. doi: 10.1016/s0304-3959(00)00422-x. [DOI] [PubMed] [Google Scholar]
- Knudsen EI. Fundamental components of attention. Annu Rev Neurosci. 2007;30:57–78. doi: 10.1146/annurev.neuro.30.051606.094256. [DOI] [PubMed] [Google Scholar]
- Kucyi A, Davis KD. The dynamic pain connectome. Trends Neurosci. 2015;38(2):86–95. doi: 10.1016/j.tins.2014.11.006. [DOI] [PubMed] [Google Scholar]
- Kucyi A, Hodaie M, Davis KD. Lateralization in intrinsic functional connectivity of the temporoparietal junction with salience- and attention-related brain networks. J Neurophysiol. 2012;108(12):3382–3392. doi: 10.1152/jn.00674.2012. [DOI] [PubMed] [Google Scholar]
- Kucyi A, Salomons TV, Davis KD. Mind wandering away from pain dynamically engages antinociceptive and default mode brain networks. Proc Natl Acad Sci U S A. 2013;110(46):18692–18697. doi: 10.1073/pnas.1312902110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulkarni B, Bentley DE, Elliott R, Youell P, Watson A, Derbyshire SW, Frackowiak RS, Friston KJ, Jones AK. Attention to pain localization and unpleasantness discriminates the functions of the medial and lateral pain systems. Eur J Neurosci. 2005;21(11):3133–3142. doi: 10.1111/j.1460-9568.2005.04098.x. [DOI] [PubMed] [Google Scholar]
- Laird AR, Fox PM, Eickhoff SB, Turner JA, Ray KL, McKay DR, Glahn DC, Beckmann CF, Smith SM, Fox PT. Behavioral interpretations of intrinsic connectivity networks. J Cogn Neurosci. 2012;23(12):4022–4037. doi: 10.1162/jocn_a_00077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavie N. Distracted and confused?: selective attention under load. Trends Cogn Sci. 2005;9(2):75–82. doi: 10.1016/j.tics.2004.12.004. [DOI] [PubMed] [Google Scholar]
- Lavie N, Beck DM, Konstantinou N. Blinded by the load: attention, awareness and the role of perceptual load. Philos Trans R Soc Lond B Biol Sci. 2014;369(1641) doi: 10.1098/rstb.2013.0205. 20130205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legrain V, Bruyer R, Guerit JM, Plaghki L. Involuntary orientation of attention to unattended deviant nociceptive stimuli is modulated by concomitant visual task difficulty. Evidence from laser evoked potentials. Clinical Neurophysiology. 2005;116(9):2165–2174. doi: 10.1016/j.clinph.2005.05.019. [DOI] [PubMed] [Google Scholar]
- Legrain V, Crombez G, Mouraux A. Controlling attention to nociceptive stimuli with working memory. PLoS One. 2011;6(6):e20926. doi: 10.1371/journal.pone.0020926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legrain V, Crombez G, Plaghki L, Mouraux A. Shielding cognition from nociception with working memory. Cortex. 2013;49(7):1922–1934. doi: 10.1016/j.cortex.2012.08.014. [DOI] [PubMed] [Google Scholar]
- Legrain V, Guerit JM, Bruyer R, Plaghki L. Attentional modulation of the nociceptive processing into the human brain: selective spatial attention, probability of stimulus occurrence, and target detection effects on laser evoked potentials. Pain. 2002;99(1–2):21–39. doi: 10.1016/s0304-3959(02)00051-9. [DOI] [PubMed] [Google Scholar]
- Legrain V, Guerit JM, Bruyer R, Plaghki L. Electrophysiological correlates of attentional orientation in humans to strong intensity deviant nociceptive stimuli, inside and outside the focus of spatial attention. Neuroscience Letters. 2003;339(2):107–110. doi: 10.1016/s0304-3940(02)01485-4. [DOI] [PubMed] [Google Scholar]
- Legrain V, Mancini F, Sambo CF, Torta DM, Ronga I, Valentini E. Cognitive aspects of nociception and pain: bridging neurophysiology with cognitive psychology. Neurophysiol Clin. 2012;42(5):325–336. doi: 10.1016/j.neucli.2012.06.003. [DOI] [PubMed] [Google Scholar]
- Legrain V, Perchet C, Garcia-Larrea L. Involuntary orienting of attention to nociceptive events: neural and behavioral signatures. Journal of Neurophysiology. 2009;102(4):2423–2434. doi: 10.1152/jn.00372.2009. [DOI] [PubMed] [Google Scholar]
- Legrain V, Torta DM. In: Emotion and Cognition: a Complex Nexus. Pickering G, editor. Springer (Ed.); 2015. Cognitive psychology and neuropsychology of nociception and pain. In G. S. E. P. [Google Scholar]
- Legrain V, Van Damme S, Eccleston C, Davis KD, Seminowicz DA, Crombez G. A neurocognitive model of attention to pain: behavioral and neuroimaging evidence. Pain. 2009;144(3):230–232. doi: 10.1016/j.pain.2009.03.020. [DOI] [PubMed] [Google Scholar]
- Leith JL, Koutsikou S, Lumb BM, Apps R. Spinal processing of noxious and innocuous cold information: differential modulation by the periaqueductal gray. J Neurosci. 2010;30(14):4933–4942. doi: 10.1523/JNEUROSCI.0122-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leventhal H, Everhart D. Emotions in personality and psychopathology. Springer; 1979. Emotion, pain, and physical illness; pp. 261–299. [Google Scholar]
- Lobanov OV, Quevedo AS, Hadsel MS, Kraft RA, Coghill RC. Frontoparietal mechanisms supporting attention to location and intensity of painful stimuli. Pain. 2013;154(9):1758–1768. doi: 10.1016/j.pain.2013.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loeser JD, Melzack R. Pain: an overview. Lancet. 1999;353(9164):1607–1609. doi: 10.1016/S0140-6736(99)01311-2. [DOI] [PubMed] [Google Scholar]
- Malmierca E, Chaves-Coira I, Rodrigo-Angulo M, Nunez A. Corticofugal projections induce long-lasting effects on somatosensory responses in the trigeminal complex of the rat. Front Syst Neurosci. 2014;8:100. doi: 10.3389/fnsys.2014.00100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason P. Deconstructing endogenous pain modulations. J Neurophysiol. 2005;94(3):1659–1663. doi: 10.1152/jn.00249.2005. [DOI] [PubMed] [Google Scholar]
- McCaul KD, Malott JM. Distraction and coping with pain. Psychol Bull. 1984;95(3):516–533. [PubMed] [Google Scholar]
- Melzack R, Casey K. In: The skin senses. Kenshalo R, editor. Springfield; 1968. Sensory, motivational, and central control determinants of pain: a new conceptual model; pp. 423–443. [Google Scholar]
- Melzack R, Wall PD. Pain mechanisms: a new theory. Science. 1965;150(3699):971–979. doi: 10.1126/science.150.3699.971. [DOI] [PubMed] [Google Scholar]
- Menon V. Brain Mapping: An Encyclopedic Reference. Academic Press: Elsevier; 2015. Salience Network. [Google Scholar]
- Menon V, Uddin LQ. Saliency, switching, attention and control: a network model of insula function. Brain Struct Funct. 2010;214(5–6):655–667. doi: 10.1007/s00429-010-0262-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moont R, Crispel Y, Lev R, Pud D, Yarnitsky D. Temporal changes in cortical activation during distraction from pain: a comparative LORETA study with conditioned pain modulation. Brain Res. 2012;1435:105–117. doi: 10.1016/j.brainres.2011.11.056. [DOI] [PubMed] [Google Scholar]
- Moore DJ, Keogh E, Eccleston C. The interruptive effect of pain on attention. Q J Exp Psychol (Hove) 2012;65(3):565–586. doi: 10.1080/17470218.2011.626865. [DOI] [PubMed] [Google Scholar]
- Näätänen R, Kreegipuu K. In: The Oxford Book of Event-Related Potential Components. O. H. online, editor. Oxford University Press; 2011. The Mismatch Negativity (MMN) [Google Scholar]
- Nir RR, Sinai A, Moont R, Harari E, Yarnitsky D. Tonic pain and continuous EEG: prediction of subjective pain perception by alpha-1 power during stimulation and at rest. Clin Neurophysiol. 2012;123(3):605–612. doi: 10.1016/j.clinph.2011.08.006. [DOI] [PubMed] [Google Scholar]
- Nunez A, Malmierca E. Corticofugal modulation of sensory information. Adv Anat Embryol Cell Biol. 2007;187 1 p following table of contents, 1–74. [PubMed] [Google Scholar]
- Oshiro Y, Quevedo AS, McHaffie JG, Kraft RA, Coghill RC. Brain mechanisms supporting spatial discrimination of pain. J Neurosci. 2007;27(13):3388–3394. doi: 10.1523/JNEUROSCI.5128-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oshiro Y, Quevedo AS, McHaffie JG, Kraft RA, Coghill RC. Brain mechanisms supporting discrimination of sensory features of pain: a new model. J Neurosci. 2009;29(47):14924–14931. doi: 10.1523/JNEUROSCI.5538-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palomero-Gallagher N, Mohlberg H, Zilles K, Vogt B. Cytology and receptor architecture of human anterior cingulate cortex. J Comp Neurol. 2008;508(6):906–926. doi: 10.1002/cne.21684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palomero-Gallagher N, Vogt BA, Schleicher A, Mayberg HS, Zilles K. Receptor architecture of human cingulate cortex: evaluation of the four-region neurobiological model. Hum Brain Mapp. 2009;30(8):2336–2355. doi: 10.1002/hbm.20667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pessoa L, Kastner S, Ungerleider LG. Neuroimaging studies of attention: from modulation of sensory processing to top-down control. J Neurosci. 2003;23(10):3990–3998. doi: 10.1523/JNEUROSCI.23-10-03990.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen SE, Posner MI. The attention system of the human brain: 20 years after. Annu Rev Neurosci. 2012;35:73–89. doi: 10.1146/annurev-neuro-062111-150525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrovic P, Petersson KM, Ghatan PH, Stone-Elander S, Ingvar M. Pain-related cerebral activation is altered by a distracting cognitive task. Pain. 2000;85(1–2):19–30. doi: 10.1016/s0304-3959(99)00232-8. [DOI] [PubMed] [Google Scholar]
- Peyron R, Garcia-Larrea L, Gregoire MC, Costes N, Convers P, Lavenne F, Mauguiere F, Michel D, Laurent B. Haemodynamic brain responses to acute pain in humans: sensory and attentional networks. Brain. 1999;122(Pt 9):1765–1780. doi: 10.1093/brain/122.9.1765. [DOI] [PubMed] [Google Scholar]
- Polich J. Updating P300: an integrative theory of P3a and P3b. Clinical Neurophysiology. 2007;118(10):2128–2148. doi: 10.1016/j.clinph.2007.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posner MI, Petersen SE. The attention system of the human brain. Annu Rev Neurosci. 1990;13:25–42. doi: 10.1146/annurev.ne.13.030190.000325. [DOI] [PubMed] [Google Scholar]
- Pourtois G, Schettino A, Vuilleumier P. Brain mechanisms for emotional influences on perception and attention: what is magic and what is not. Biol Psychol. 2013;92(3):492–512. doi: 10.1016/j.biopsycho.2012.02.007. [DOI] [PubMed] [Google Scholar]
- Rainville P, Duncan GH, Price DD, Carrier B, Bushnell MC. Pain affect encoded in human anterior cingulate but not somatosensory cortex. Science. 1997;277(5328):968–971. doi: 10.1126/science.277.5328.968. [DOI] [PubMed] [Google Scholar]
- Raz A. Anatomy of attentional networks. Anat Rec B New Anat. 2004;281(1):21–36. doi: 10.1002/ar.b.20035. [DOI] [PubMed] [Google Scholar]
- Raz A, Buhle J. Typologies of attentional networks. Nat Rev Neurosci. 2006;7(5):367–379. doi: 10.1038/nrn1903. [DOI] [PubMed] [Google Scholar]
- Romero YR, Straube T, Nitsch A, Miltner WH, Weiss T. Interaction between stimulus intensity and perceptual load in the attentional control of pain. Pain. 2013;154(1):135–140. doi: 10.1016/j.pain.2012.10.003. [DOI] [PubMed] [Google Scholar]
- Ruscheweyh R, Kreusch A, Albers C, Sommer J, Marziniak M. The effect of distraction strategies on pain perception and the nociceptive flexor reflex (RIII reflex) Pain. 2011;152(11):2662–2671. doi: 10.1016/j.pain.2011.08.016. [DOI] [PubMed] [Google Scholar]
- Seeley WW, Menon V, Schatzberg AF, Keller J, Glover GH, Kenna H, Reiss AL, Greicius MD. Dissociable intrinsic connectivity networks for salience processing and executive control. Journal of Neuroscience. 2007;27(9):2349–2356. doi: 10.1523/JNEUROSCI.5587-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seminowicz DA, Davis KD. Interactions of pain intensity and cognitive load: the brain stays on task. Cereb Cortex. 2007a;17(6):1412–1422. doi: 10.1093/cercor/bhl052. [DOI] [PubMed] [Google Scholar]
- Seminowicz DA, Davis KD. Pain enhances functional connectivity of a brain network evoked by performance of a cognitive task. J Neurophysiol. 2007b;97(5):3651–3659. doi: 10.1152/jn.01210.2006. [DOI] [PubMed] [Google Scholar]
- Seminowicz DA, Davis KD. A re-examination of pain-cognition interactions: implications for neuroimaging. Pain. 2007c;130(1–2):8–13. doi: 10.1016/j.pain.2007.03.036. [DOI] [PubMed] [Google Scholar]
- Seminowicz DA, Mikulis DJ, Davis KD. Cognitive modulation of pain-related brain responses depends on behavioral strategy. Pain. 2004;112(1–2):48–58. doi: 10.1016/j.pain.2004.07.027. [DOI] [PubMed] [Google Scholar]
- Shackman AJ, Salomons TV, Slagter HA, Fox AS, Winter JJ, Davidson RJ. The integration of negative affect, pain and cognitive control in the cingulate cortex. Nature Review Neuroscience. 2011;12(3):154–167. doi: 10.1038/nrn2994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherrington CS. Observations on the scratch-reflex in the spinal dog. J Physiol. 1906;34(1–2):1–50. doi: 10.1113/jphysiol.1906.sp001139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokolov EN. Higher nervous functions; the orienting reflex. Annu Rev Physiol. 1963;25:545–580. doi: 10.1146/annurev.ph.25.030163.002553. [DOI] [PubMed] [Google Scholar]
- Sprenger C, Eippert F, Finsterbusch J, Bingel U, Rose M, Buchel C. Attention modulates spinal cord responses to pain. Curr Biol. 2012;22(11):1019–1022. doi: 10.1016/j.cub.2012.04.006. [DOI] [PubMed] [Google Scholar]
- Sussman TJ, Szekely A, Hajcak G, Mohanty A. It's all in the anticipation: How perception of threat is enhanced in anxiety. Emotion. 2016;16(3):320–327. doi: 10.1037/emo0000098. [DOI] [PubMed] [Google Scholar]
- Svensson P, Minoshima S, Beydoun A, Morrow TJ, Casey KL. Cerebral processing of acute skin and muscle pain in humans. J Neurophysiol. 1997;78(1):450–460. doi: 10.1152/jn.1997.78.1.450. [DOI] [PubMed] [Google Scholar]
- Talbot JD, Marrett S, Evans AC, Meyer E, Bushnell MC, Duncan GH. Multiple representations of pain in human cerebral cortex. Science. 1991;251(4999):1355–1358. doi: 10.1126/science.2003220. [DOI] [PubMed] [Google Scholar]
- Ter Minassian A, Ricalens E, Humbert S, Duc F, Aube C, Beydon L. Dissociating anticipation from perception: Acute pain activates default mode network. Hum Brain Mapp. 2013;34(9):2228–2243. doi: 10.1002/hbm.22062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theeuwes J. Cross-dimensional perceptual selectivity. Percept Psychophys. 1991;50(2):184–193. doi: 10.3758/bf03212219. [DOI] [PubMed] [Google Scholar]
- Tolle TR, Kaufmann T, Siessmeier T, Lautenbacher S, Berthele A, Munz F, Zieglgansberger W, Willoch F, Schwaiger M, Conrad B, Bartenstein P. Region-specific encoding of sensory and affective components of pain in the human brain: a positron emission tomography correlation analysis. Annals of Neurology. 1999;45(1):40–47. doi: 10.1002/1531-8249(199901)45:1<40::aid-art8>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- Torta DM, Cauda F. Different functions in the cingulate cortex, a meta-analytic connectivity modeling study. Neuroimage. 2011;56(4):2157–2172. doi: 10.1016/j.neuroimage.2011.03.066. [DOI] [PubMed] [Google Scholar]
- Torta DM, Churyukanov MV, Plaghki L, Mouraux A. The effect of heterotopic noxious conditioning stimulation on Adelta-, C- and Abeta-fibre brain responses in humans. Eur J Neurosci. 2015;42(9):2707–2715. doi: 10.1111/ejn.13071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torta DM, Costa T, Duca S, Fox PT, Cauda F. Parcellation of the cingulate cortex at rest and during tasks: a meta-analytic clustering and experimental study. Front Hum Neurosci. 2013;7:275. doi: 10.3389/fnhum.2013.00275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tracey I, Ploghaus A, Gati JS, Clare S, Smith S, Menon RS, Matthews PM. Imaging attentional modulation of pain in the periaqueductal gray in humans. J Neurosci. 2002;22(7):2748–2752. doi: 10.1523/JNEUROSCI.22-07-02748.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treister R, Eisenberg E, Gershon E, Haddad M, Pud D. Factors affecting - and relationships between-different modes of endogenous pain modulation in healthy volunteers. Eur J Pain. 2010;14(6):608–614. doi: 10.1016/j.ejpain.2009.10.005. [DOI] [PubMed] [Google Scholar]
- Valentini E, Betti V, Hu L, Aglioti SM. Hypnotic modulation of pain perception and of brain activity triggered by nociceptive laser stimuli. Cortex. 2013;49(2):446–462. doi: 10.1016/j.cortex.2012.02.005. [DOI] [PubMed] [Google Scholar]
- Valet M, Sprenger T, Boecker H, Willoch F, Rummeny E, Conrad B, Erhard P, Tolle TR. Distraction modulates connectivity of the cingulo-frontal cortex and the midbrain during pain--an fMRI analysis. Pain. 2004;109(3):399–408. doi: 10.1016/j.pain.2004.02.033. [DOI] [PubMed] [Google Scholar]
- Van Damme S, Gallace A, Spence C, Crombez G, Moseley GL. Does the sight of physical threat induce a tactile processing bias? Modality-specific attentional facilitation induced by viewing threatening pictures. Brain Res. 2009;1253:100–106. doi: 10.1016/j.brainres.2008.11.072. [DOI] [PubMed] [Google Scholar]
- Van Damme S, Legrain V, Vogt J, Crombez G. Keeping pain in mind: a motivational account of attention to pain. Neuroscience Biobehavioral Reviews. 2009;34(2):204–213. doi: 10.1016/j.neubiorev.2009.01.005. [DOI] [PubMed] [Google Scholar]
- Van Ryckeghem DM, Crombez G, Eccleston C, Legrain V, Van Damme S. Keeping pain out of your mind: The role of attentional set in pain. Eur J Pain. 2012 doi: 10.1002/j.1532-2149.2012.00195.x. [DOI] [PubMed] [Google Scholar]
- Vanlessen N, Rossi V, De Raedt R, Pourtois G. Feeling happy enhances early spatial encoding of peripheral information automatically: electrophysiological time-course and neural sources. Cogn Affect Behav Neurosci. 2014;14(3):951–969. doi: 10.3758/s13415-014-0262-2. [DOI] [PubMed] [Google Scholar]
- Verhoeven K, Van Damme S, Eccleston C, Van Ryckeghem DM, Legrain V, Crombez G. Distraction from pain and executive functioning: an experimental investigation of the role of inhibition, task switching and working memory. European Journal of Pain. 2011;15(8):866–873. doi: 10.1016/j.ejpain.2011.01.009. [DOI] [PubMed] [Google Scholar]
- Vlaeyen JW, Timmermans C, Rodriguez LM, Crombez G, van Horne W, Ayers GM, Albert A, Wellens HJ. Catastrophic thinking about pain increases discomfort during internal atrial cardioversion. J Psychosom Res. 2004;56(1):139–144. doi: 10.1016/S0022-3999(03)00081-3. [DOI] [PubMed] [Google Scholar]
- Vogt B. Cingulate neurobiology and disease. Oxford University Press; New York: 2009. [Google Scholar]
- Vogt BA. Pain and emotion interactions in subregions of the cingulate gyrus. Nature Reviews Neuroscience. 2005;6(7):533–544. doi: 10.1038/nrn1704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogt BA. Midcingulate cortex: Structure, connections, homologies, functions and diseases. J Chem Neuroanat. 2016;74:28–46. doi: 10.1016/j.jchemneu.2016.01.010. [DOI] [PubMed] [Google Scholar]
- Vogt BA, Berger GR, Derbyshire SW. Structural and functional dichotomy of human midcingulate cortex. European Journal of Neuroscience. 2003;18(11):3134–3144. doi: 10.1111/j.1460-9568.2003.03034.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogt BA, Laureys S. Posterior cingulate, precuneal and retrosplenial cortices: cytology and components of the neural network correlates of consciousness. Cognition, Emotion and Autonomic Responses: The Integrative Role of the Prefrontal Cortex and Limbic Structures. 2005;150:205–217. doi: 10.1016/S0079-6123(05)50015-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogt BA, Nimchinsky EA, Vogt LJ, Hof PR. Human Cingulate Cortex - Surface-Features, Flat Maps, and Cytoarchitecture. Journal of Comparative Neurology. 1995;359(3):490–506. doi: 10.1002/cne.903590310. [DOI] [PubMed] [Google Scholar]
- Vogt BA, Vogt L. Cytology of human dorsal midcingulate and supplementary motor cortices. J Chem Neuroanat. 2003;26(4):301–309. doi: 10.1016/j.jchemneu.2003.09.004. [DOI] [PubMed] [Google Scholar]
- Vogt BA, Vogt LJ, Perl DP, Hof PR. Cytology of human caudomedial cingulate, retrosplenial, and caudal parahippocampal cortices. J Comp Neurol. 2001;438(3):353–376. doi: 10.1002/cne.1320. [DOI] [PubMed] [Google Scholar]
- Willer JC, Roby A, Le Bars D. Psychophysical and electrophysiological approaches to the pain-relieving effects of heterotopic nociceptive stimuli. Brain. 1984;107(Pt 4):1095–1112. doi: 10.1093/brain/107.4.1095. [DOI] [PubMed] [Google Scholar]
- Wunsch A, Philippot P, Plaghki L. Affective associative learning modifies the sensory perception of nociceptive stimuli without participant's awareness. Pain. 2003;102(1–2):27–38. doi: 10.1016/s0304-3959(02)00331-7. [DOI] [PubMed] [Google Scholar]
- Yu C, Zhou Y, Liu Y, Jiang T, Dong H, Zhang Y, Walter M. Functional segregation of the human cingulate cortex is confirmed by functional connectivity based neuroanatomical parcellation. Neuroimage. 2011;54(4):2571–2581. doi: 10.1016/j.neuroimage.2010.11.018. [DOI] [PubMed] [Google Scholar]
- zu Eulenburg P, Baumgartner U, Treede RD, Dieterich M. Interoceptive and multimodal functions of the operculo-insular cortex: tactile, nociceptive and vestibular representations. Neuroimage. 2013;83:75–86. doi: 10.1016/j.neuroimage.2013.06.057. [DOI] [PubMed] [Google Scholar]