Summary
Background
B cells produce alloantibodies and activate alloreactive T cells, negatively affecting kidney transplant survival. In contrast, regulatory B cells are associated with transplant tolerance. There is an unmet need for immunotherapies that inhibit B cell effector function, including antibody secretion, whilst sparing regulators and minimising infection risk. B lymphocyte stimulator (BLyS) is a cytokine that promotes B cell activation, and has not previously been targeted in kidney transplant recipients.
Aim
We sought to examine the safety and activity (the latter measured by a reduction in naïve B cells from baseline to week 24) of an anti-BLyS antibody, belimumab, in addition to standard of care immunosuppression in adult kidney transplant recipients. We utilised an experimental medicine study design with multiple secondary and exploratory endpoints to gain further insight into the effect of belimumab on the generation of de novo IgG and on the regulatory B cell compartment.
Methods
We conducted a phase 2 double-blind randomised placebo-controlled trial of belimumab, in addition to standard of care immunosuppression (basiliximab, mycophenolate mofetil, tacrolimus and prednisolone), in adults aged 18-75 receiving a kidney transplant. Subjects were randomised at a single centre, Addenbrooke’s Hospital (UK), stratified according to receipt of a living or deceased donor organ. Within each stratum subjects were randomised by telephone in a 1:1 ratio to receive intravenous belimumab 10mg/kg or placebo (day 0, 14, 28 and every four weeks thereafter for a total of seven infusions). Coprimary endpoints were safety and change in naïve B cells from baseline to Week 24. This trial has completed and is registered as ClinicalTrials.gov number NCT01536379 and EudraCT number 2011-006215-56.
Findings
28 kidney transplant recipients were randomised to belimumab (n=14) or placebo (n=14) treatment between 13th September 2013 and 8th February 2015. Twelve belimumab and 13 placebo patients were transplanted and went on to receive at least one dose of belimumab/placebo (modified intention to treat (MITT) population). We observed similar rates of adverse events in belimumab and placebo groups, including serious infection (1/12 (8%) and 5/13 (38%) respectively during the six month on-treatment phase; 0/13 (0%) and 2/13 (15%) during the six month post-treatment follow-up phase). There was one death in the on-treatment phase in a patient in the placebo group. The co-primary endpoint of a reduction in naïve B cells from baseline to week 24 was not met; treatment with belimumab resulted in a trend towards, but not a statistically significant reduction in naïve B cells from baseline to week 24 (adjusted mean difference -34·4 cells/mm3 (95% confidence interval (CI) -109·5 to 40·7)).
Interpretation
Treatment with belimumab in addition to standard of care immunosuppression in low immunological risk renal transplant recipients was not associated with an adverse safety signal nor an increased risk of infection.
Funding
GlaxoSmithKline
Introduction
Transplantation is the optimal renal replacement therapy for most patients with end-stage renal failure (ESRF). Current immunosuppressive regimens carry an increased risk of infection but limit T cell activation such that T cell mediated rejection (TCMR) occurs in less than 20% of kidney transplant recipients and is largely treatable. In contrast, there remain significant challenges in the field of humoral alloimmunity. De-novo donor-specific human leucocyte antigen (HLA)–specific immunoglobulin G (IgG) antibodies (DSA) and some non-HLA IgG antibodies are associated with acute antibody-mediated rejection (ABMR) and allograft loss1–3. Furthermore, sensitised patients with pre-formed DSA have an elevated risk of acute and chronic ABMR, reducing graft survival4. B cells are not only the precursors of antibody-producing plasma cells, but act as antigen presenting cells and secrete proinflammatory cytokines, including interleukin (IL)-6, that can activate T cells driving TCMR. Indeed, the presence of B cells in TCMR biopsies is a negative prognostic factor associated with steroid resistance5. Hence, there is an urgent unmet need in solid organ transplantation for immunotherapeutic strategies that target B cells and plasma cells6, ideally without increasing infectious complications.
Efforts to inhibit pathological humoral immunity are complicated by the fact that B cells may also regulate immune responses via IL-10 production7,8. These regulatory B cells are enriched in transitional (CD24/CD38high)7 and memory (CD24high/CD27+) B cell compartments in humans8. Several lines of evidence suggest that regulatory B cells may be important in transplantation. Firstly, although a single dose of the anti-CD20 antibody rituximab at induction had no impact on transplant outcomes9,10, the use of a second dose within one to two weeks of transplantation was associated with TCMR in kidney transplant recipients11 and cardiac allograft vasculopathy12. Secondly, B cell transcripts and IL-10 producing B cells are increased in the peripheral blood of tolerant kidney transplant recipients13–15. Finally, kidney transplant recipients with a higher number of transitional B cells have reduced rejection16. Similarly, patients with a higher B cell IL-10:TNFα ratio have a slower decline in transplant function and reduced allograft loss17,18. In contrast, patients with rejection following ABO incompatible transplantation had fewer CD24high/CD27+ memory B cells19. Together, these data show that B cells can play both a positive and negative role in transplantation and highlight the need to identify immunosuppressants that preserve the immunoregulatory aspect of B cell function.
B lymphocyte stimulator (BLyS; also known as BAFF) is a cytokine that enhances B cell survival and proliferation20 and contributes to the plasma cell niche21. The humanised anti-BLyS IgG1 antibody, belimumab, is licenced for use in patients with SLE22, a disease characterised by high circulating BLyS23. In renal transplant recipients, elevated serum BLyS is associated with the development of de novo DSA24, high-titre HLA antibodies25, and an increased frequency of ABMR26. Experimental models suggest that BLyS neutralisation may be effective in preventing rejection27,28. BLyS inhibition, used as monotherapy, had little impact on HLA antibody titres in sensitised subjects on the transplant waiting list29,30, but to date, this axis has not been targeted in human transplant recipients.
Currently, only 10% of compounds entering clinical trials reach patients as medicines. Experimental medicine seeks to improve this by ‘identify(ing) mechanisms of pathophysiology or disease, or … demonstrat(ing) proof-of-concept evidence of the validity and importance of new discoveries or treatments’ (UK Medical Research Council), thus providing detailed, mechanistic phenotypic data that precedes and informs late phase clinical development.
We undertook a phase 2 randomised, double-blind, placebo-controlled experimental medicine trial of belimumab in addition to standard of care immunosuppression in renal transplant recipients to assess its safety and activity, as measured by a reduction in naïve B cells from baseline to week 24, and its effect on a number of exploratory endpoints. The use of belimumab was not associated with increased infection but led to a reduction in naïve B cells and activated memory B cells. The residual B cell compartment in belimumab-treated subjects was skewed towards cells with a regulatory phenotype, with an increased capacity to produce IL-10 relative to IL-6. Following belimumab treatment, whole blood gene expression and protein microarray analysis indicated fewer circulating plasmablasts, reduced de novo IgG antibody formation and a reduction in kidney and endothelial cell-specific antibodies known to negatively impact graft outcome. In addition, belimumab treatment had a significant effect on the CD4 T cell transcriptome, with a marked reduction in cell cycle gene expression. Our data suggest that belimumab may be a useful therapeutic strategy to explore further in standard immunological risk kidney transplant recipients.
Methods
Study Design
In this phase IIA, randomised, double-blind, sponsor unblind study, subjects were recruited at a single UK transplant centre (Addenbrooke’s Hospital, Cambridge). The study was approved by the local Research Ethics Committee (East of England - Cambridge East). The study protocol is available at www.gsk-clinicalstudyregister.com
Study Patients
Patients aged 18 to 75 years receiving a kidney transplant were eligible for inclusion. Exclusion criteria included donor age <5 or >70 years, ABO blood type incompatibility, 0-0-0 HLA mismatch, a positive T and/or B cell cross-match, and previous recipient exposure to B cell targeted therapy (full eligibility criteria are in study protocol, supplementary material). All patients provided written informed consent.
Randomisation and Masking
Subjects were stratified according to whether the recipient received a living or deceased donor organ. Within each stratum, subjects were randomised in a 1:1 ratio to either belimumab or placebo. Randomisation was performed by the investigators using the GlaxoSmithKline telephone based Registration and Medication Ordering System (RAMOS). This was a double-blind study in which the investigator, patient and sponsor (with the exception of statistics personnel unblinded for interim/sample size re-estimation) were blinded to the treatment assignment. An unblinded pharmacist prepared the infusion according to the subject’s randomised treatment.
Procedures
Subjects received seven doses of belimumab 10mg/kg (GlaxoSmithKline, Rockville, MD, USA) or placebo (0.9% sodium chloride solution) by intravenous infusion over one hour at days 0, 14, 28 and then every four weeks to week 20, in addition to basiliximab (20mg IV days 0 and 3), tacrolimus (0.15mg/kg daily: target trough level 6-10μg/L first six months post transplantation, 5-8μg/L thereafter), mycophenolate mofetil (500mg twice daily) and prednisolone (20mg daily initially, weaning to 5mg daily in month 1-2). Patients received infection prophylaxis with nystatin (1st month), co-trimoxazole (months 1-6) and valganciclovir (months 1-6), and were followed for 12 months (see study protocol in supplementary material).
For adverse event reporting the on-treatment phase commenced from the start of the first infusion of belimumab/placebo and ended 28 days after the last dose. The post-treatment phase began the following day. For other primary and secondary endpoints the on-treatment phase ended 35 days after the last dose to allow inclusion of the On-Trt Week 24 visit on day 168 +/- 7 days. Details of visit windows can be found in the supplementary appendix.
Outcomes
The first co-primary endpoint was safety assessed by: review of AEs and SAEs, classified using the Medical Dictionary for Regulatory Activities (MedDRA) and including AEs of special interest; and change from baseline and number of subjects outside the normal range for vital signs and safety laboratory assessments, including immunoglobulin levels and white cell count.
The second co-primary endpoint was change in naïve B cells from baseline to Week 24, a measure of functional BLyS neutralisation validated in patients with SLE31.
Secondary and exploratory endpoints included pharmacodynamics, immunological biomarkers (e.g. regulatory B cells, assessment of peripheral blood transcriptional profiles, and non-HLA antibodies) and clinical endpoints. Full details of the secondary and exploratory endpoints can be found in the supplementary appendix and study protocol.
B Cell Stimulation Assays
Frozen peripheral blood mononuclear cells (PBMC) were thawed and cultured in vitro with CpG DNA Oligodeoxynucleotides (100nM; Hycult Biotech) and CD40L (1μg/ml; R&D Systems). Phorbol 12-myristate 13-acetate (PMA) (50ng/ml; Sigma-Aldrich), ionomycin (500ng/ml; Sigma-Aldrich) and brefeldin A (5μg/ml; BioLegend) were added for the last five hours of culture and IL-6 and IL-10 quantified by flow cytometry.
Microarray Analyses
RNA was extracted from whole blood or from purified leukocyte subsets as described previously32, and hybridised to Human Gene ST 2.1 microarrays (Affymetrix). Microarray data are available in the ArrayExpress database (http://www.ebi.ac.uk/arrayexpress) under accession number E-MTAB-5906.
Patient serum was hybridised to ProtoArray (Invitrogen) protein microarrays according to manufacturer’s instructions.
Details of gene expression and protein array analyses are in the supplementary appendix.
Statistical Analysis
Primary analyses were undertaken using a pre-specified modified intention to treat population (MITT) including all patients randomised who received at least one infusion of belimumab/placebo. Secondary and exploratory biomarker outputs were undertaken using a pre-specified per protocol population (PP) that included all MITT subjects who received at least five infusions of belimumab/placebo, were followed up beyond week 24 and received no prohibited medications or plasma exchange (referred to as PP1 population in the study protocol).
No formal statistical hypotheses were defined in the reporting and analysis plan (RAP). For the primary and secondary biomarker endpoints, a mixed model for repeated measures (MMRM) approach was used to produce adjusted mean differences between belimumab and placebo with corresponding 95% confidence intervals (details in supplementary appendix). For each of the primary and secondary endpoints, error diagnostics from the residuals were examined to ensure that the model did not depart from the assumptions underlying analysis of variance. If the assumptions were seriously violated, then non-parametric alternatives were performed as defined in the RAP. Non-parametric statistical analyses were performed on exploratory endpoints post hoc and these are marked as such.
The target sample size of 20 evaluable subjects was informed by feasibility assessment but calculated with the aim of achieving a reduction in naïve B cell count from baseline to 24 weeks. Assumptions for the sample size calculation are provided in the supplemental methods.
Interim re-estimations of sample size were performed in November 2014 and February 2015. An extended safety review team, consisting at a minimum of a safety physician, a clinical development physician and a statistician, oversaw the study.
Analyses were performed using SAS version 9.3 except for transcriptomic and protein microarray analyses, which were performed using R version 3.3.1.
ClinicalTrials.gov number NCT01536379; EudraCT number 2011-006215-56
Role of the Funding Source
The study was designed by the sponsor, GlaxoSmithKline, in close collaboration with principal investigators at Addenbrooke’s Hospital. Authors affiliated to GlaxoSmithKline, Addenbrooke’s Hospital and the University of Cambridge were jointly responsible for trial design, data collection, analysis and interpretation, report writing and the decision to submit the paper for publication.
Results
Twenty-eight subjects were randomised to belimumab (n=14) or placebo (n=14) between 13th September 2013 and 8th February 2015. Twelve belimumab and 13 placebo patients went on to receive at least one dose of belimumab/placebo (Fig. 1) in addition to standard immunosuppression (Table S3). Baseline patient characteristics were comparable between groups (Table 1). Belimumab pharmacokinetic (PK) parameters were similar to those observed in SLE22 and were not affected by ESRF (Fig. S1A; Table S12). Belimumab effectively removed circulating BLyS, and levels remained suppressed up to week 12 post-treatment, rebounding thereafter (Fig. S1B).
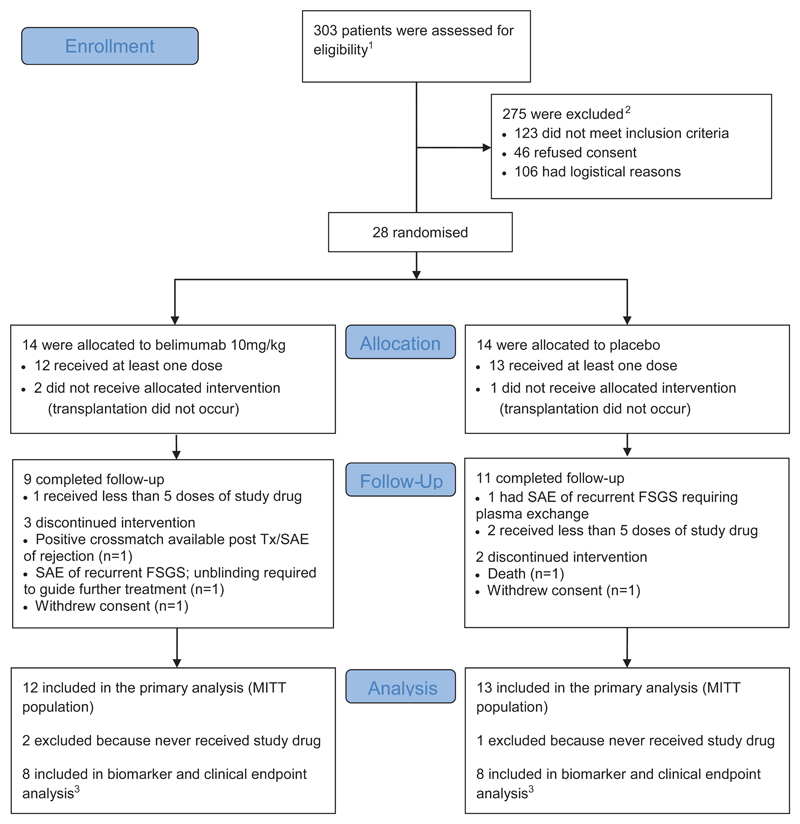
Figure 1. Enrolment, Randomisation and Follow-up.
The randomised treatment was given in addition to standard immunosuppression consisting of basiliximab, mycophenolate mofetil, tacrolimus and corticosteroids (see methods for full details).
1260 at Addenbrooke’s Hospital, Cambridge (28 randomised); 43 at Guy's and St Thomas’ NHS Foundation Trust, London (0 randomised).
2Reasons for not meeting inclusion criteria shown in Supplementary Table 1. Logistical reasons included unavailability of study staff or randomised study drug, insufficient time for consent and recipient resident out of region.
3The pre-specified per protocol (PP) population used for biomarker and clinical endpoint analysis consisted of all subjects in the modified intention to treat (MITT) population who received at least five infusions of belimumab/placebo, no prohibited medications or plasma exchange and at least 24 weeks follow-up.
FSGS Focal Segmental Glomerulosclerosis, SAE Serious Adverse Event
Table 1. Baseline Demographics and Clinical Outcomes.
| Placebo (n=13) | Belimumab 10mg/kg (n=12) |
|||
|---|---|---|---|---|
| Baseline demographic data, n | 13 | 12 | ||
|
| ||||
| Age (years), Mean (SD) | 51.0 (14.0) | 54.3 (11.0) | ||
| Range | 24-73 | 32-72 | ||
|
| ||||
| Sex, n (%) | ||||
| - Female | 4 (31) | 7 (58) | ||
| - Male | 9 (69) | 5 (42) | ||
|
| ||||
| Race, n (%) | ||||
| - White/Caucasian | 12 (92) | 11 (92) | ||
| - Asian | 1 (8) | 1 (8) | ||
|
| ||||
| Mean number (SD) of HLA-A/B/DR mismatches | ||||
| - HLA-A | 1.7 (0.5) | 1.8 (0.5) | ||
| - HLA-B | 0.9 (0.6) | 1.1 (0.3) | ||
| - HLA-DR | 0.6 (0.5) | 0.8 (0.6) | ||
|
| ||||
| Number of previous kidney transplants, n (%) | ||||
| - 0 | 11 (85) | 11 (92) | ||
| - 1 | 2 (15) | 1 (8) | ||
|
| ||||
| Pre-transplant renal replacement modality, n (%) | ||||
| - None | 3 (23) | 2 (17) | ||
| - Haemodialysis | 7 (54) | 7 (58) | ||
| - Peritoneal dialysis | 3 (23) | 3 (25) | ||
|
| ||||
| HLA antibody at baseline (MFI>2000)3, n (%) | ||||
| - Non-DSA | 5 (38) | 3 (25) | ||
| - DSA 2 | 0 | 1 (8) | ||
|
| ||||
| Donor type, n (%) | ||||
| - Living | 3 (23) | 4 (33) | ||
| - Donation after circulatory death | 7 (54) | 5 (42) | ||
| - Donation after brain death | 3 (23) | 3 (25) | ||
|
| ||||
| Donor age (years) | ||||
| Mean (SD) | 52.9 (8.8) | 58.3 (8.1) | ||
| Range | 37-68 | 47-69 | ||
|
| ||||
| Donor creatinine-last value prior to transplant (μmol/L) | ||||
| Mean (SD) | 64.8 (17.5) | 84.3 (30.7) | ||
| Median (Min, Max) | 66.0 (37-92) | 78.0 (51-167) | ||
|
| ||||
| Clinical outcome data1, n | 8 | 8 | ||
|
| ||||
| Acute transplant rejection by week 522, n (%) | ||||
| - Borderline, not treated | 2 (25) | 0 | ||
| - Borderline, treated | 1 (12.5) | 0 | ||
| - Type IIa, treated | 0 | 1 (12.5) | ||
|
| ||||
| Cumulative HLA class I (MFI >500)3 | Sub 22 | Sub 28 | Sub 8 | Sub 27 |
| Before treatment | 39612 | 20046 | 127392 | 4894 |
| On treatment Wk 2 | 24658 | 14731 | 56453 | 4493 |
| On treatment Wk 4 | 26129 | 21466 | 33810 | 3438 |
| On treatment Wk 8 | 28930 | 19528 | 34420 | 4632 |
| On treatment Wk 12 | 26709 | 26500 | 25985 | 6320 |
| On treatment Wk 24 | 23152 | 18376 | 26491 | 8103 |
| Post treatment Wk 28 | 19204 | 18892 | 24607 | 4349 |
|
| ||||
| Cumulative HLA class II (MFI >500)3 | Sub 4 | Sub 1 | ||
| Before treatment | 2382 | 16009 | ||
| On treatment Wk 2 | 916 | 9305 | ||
| On treatment Wk 4 | 1211 | 12346 | ||
| On treatment Wk 8 | 768 | 12553 | ||
| On treatment Wk 12 | 606 | 10540 | ||
| On treatment Wk 24 | 577 | 13408 | ||
| Post treatment Wk 28 | 785 | 9075 | ||
|
| ||||
| Graft survival, n (%) | 8 (100) | 8 (100) | ||
|
| ||||
| Creatinine at 52 weeks (μmol/L) | ||||
| Mean (SD) | 142.5 (65.7) | 110.6 (43.6) | ||
| Median (Min, Max) | 114 (86-282) | 94 (68-201) | ||
|
| ||||
| eGFR at 52 weeks (ml/min/1.73m2), Mean (SD) | 53.00 (22.10) | 57.42 (15.08) | ||
| Median (Min, Max) | 54.03 (20.41-86.63) | 56.77 (30.25-81.93) | ||
Clinical outcome data described for the per protocol population.
Acute rejection was defined on biopsy according to Banff 2009 criteria: Acute antibody mediated rejection (Grade I, II or III) or acute T cell mediated rejection (Borderline, Type IA, Type IB, Type IIA, Type IIB, Type III). An additional subject randomised to belimumab had a positive pre-transplant MHC class II flow crossmatch (fulfilling an exclusion criterion but unknown at the time of enrolment and transplant). They experienced Type IIA acute cellular rejection (day 6) and grade II antibody mediated rejection (day 34). They were withdrawn once the crossmatch was recognised following a single dose of belimumab.
A cut-off of MFI>2000 was used to define preformed HLA antibody at baseline, since this is the threshold for clinical significance determined by the tissue-typing laboratory. Cumulative HLA class I and class II MFI calculated by summing the normalised MFI values for any single antigen bead with a value above the level of detection (500). An additional subject in the belimumab group underwent adoptive transfer of donor HLA-specific allosensitization following kidney transplantation from a highly sensitized donor.
SD standard deviation, n number of subjects, HLA human leukocyte antigen, MFI mean fluorescence intensity measured by Luminex assay, DSA Donor specific antibody, Min minimum, Max maximum, and eGFR estimated glomerular filtration rate.
The first co-primary endpoint was safety. We observed AE in 11/12 (92%) belimumab-treated subjects and 10/13 (77%) placebo-treated subjects during the 6 month on-treatment phase and 10/12 (83%) and 9/13 (69%) respectively during the 6 month post-treatment follow-up phase. AE occurring in more than one subject with a higher incidence in the belimumab group were diarrhoea, urinary tract infection, dizziness and vomiting (on treatment) and nasopharyngitis, urinary tract infection and type two diabetes (post treatment). For each event, the difference in frequency compared to placebo was no more than two subjects and not considered to represent a new safety signal. The number of patients experiencing serious AE were similar (5/12 (42%) of belimumab-treated and 7/13 (54%) of placebo-treated subjects during the 6 month on-treatment phase and 2/12 (17%) and 2/13 (15%) respectively during the 6 month post-treatment follow-up phase). A key question we wished to address in this study was whether the use of belimumab in renal transplantation might be associated with an excess risk of infection. Belimumab has previously been used in patients with autoimmune diseases, in combination with steroids, and in some cases, an anti-proliferative agent (e.g., azathioprine, MMF or methotrexate)22. This is the first study to use belimumab with basiliximab induction and with triple maintenance immunosuppression (tacrolimus, MMF and prednisolone) in patients with ESRF at baseline, a condition known to be associated with a heightened susceptibility to infection33. Therefore, post-transplant infection was an important end-point, particularly given its association with an increased risk of rejection and allograft loss34. At 6 and 12 month follow-up, we observed no excess serious infection events in the belimumab group (1/12 (8%) and 5/13 (38%) in belimumab and placebo-treated patients respectively during the 6 month on-treatment phase and 0/13 (0%) and 2/13 (15%) respectively in the 6 month post-treatment follow-up phase). We also found no excess frequency of BK virus and cytomegalovirus (CMV) infection in belimumab-treated subjects (Table 2). There were no new diagnoses of malignancy in either group during the follow up period and one death, from a cardiovascular cause, in the placebo group (Table 2). In summary, in this preliminary study, belimumab was not associated with an excess risk of serious infection or malignancy when used with standard transplant immunosuppression.
Table 2. Safety Findings During On-treatment and Post-treatment Phases, by Treatment Group.
| On treatment 1 | Post-treatment | |||
|---|---|---|---|---|
| Number (%) Subjects | Number (%) Subjects | |||
| Placebo | Belimumab | Placebo | Belimumab | |
| (n=13) | (n=12) | (n=13) | (n=12) | |
| Any adverse event (AE) | 10 (77) | 11 (92) | 9 (69) | 10 (83) |
|
| ||||
| Any serious AE (SAE) | 7 (54) | 5 (42) | 2 (15) | 2 (17) |
|
| ||||
| Any severe AE | 6 (46) | 8 (67) | 3 (23) | 3 (25) |
|
| ||||
| Deaths 2 | 1 (8) | 0 | 0 | 0 |
|
| ||||
| Most common AE 3 | ||||
| - Leukopenia | 4 (31) | 4 (33) | 3 (23) | 3 (25) |
| - Diarrhoea | 3 (23) | 5 (42) | 1 (8) | 1 (8) |
| - Urinary tract infection 4 | 3 (23) | 4 (33) | 1 (8) | 2 (17) |
| - Anaemia 5 | 4 (31) | 3 (25) | 0 | 1 (8) |
| - Lower respiratory tract infection 6 | 2 (15) | 2 (17) | 2 (15) | 0 |
| - Dyspepsia7 | 2 (15) | 2 (17) | 2 (15) | 0 |
| - Nasopharyngitis | 2 (15) | 1 (8) | 1 (8) | 2 (17) |
| - Alanine aminotransferase increased | 2 (15) | 2 (17) | 0 | 0 |
| - Diabetes Mellitus8 | 1 (8) | 1 (8) | 0 | 2 (17) |
| - Endoscopic evidence upper GI inflammation9 | 2 (15) | 1 (8) | 0 | 0 |
| - Dizziness | 0 | 2 (17) | 0 | 1 (8) |
| - Vomiting | 0 | 2 (17) | 0 | 0 |
|
| ||||
| Infections | ||||
| - All | 7 (54) | 5 (42) | 8 (62) | 6 (50) |
| - Serious infections (n≥1) | 5 (38) | 1 (8) | 2 (15) | 0 |
| - Severe infections (n≥1) | 5 (38) | 2 (17) | 3 (23) | 0 |
| - Opportunistic infections | 3 (23) | 2 (17) | 3 (23) | 3 (25) |
| - BK viraemia | 2 (15) | 1 (8) | 1 (8) | 1 (8) |
| - BK viraemia-associated nephropathy | 0 | 1 (8) | 1 (8) | 0 |
| - Cytomegalovirus (CMV) viraemia10 | 1 (8) | 0 | 1 (8) | 2 (17) |
|
| ||||
| Malignant neoplasm | 0 | 0 | 0 | 0 |
|
| ||||
| Depression/Suicide/Self injury | 0 | 0 | 0 | 0 |
|
| ||||
| Post infusion systemic reaction11 | 0 | 1 (8) | NA | NA |
|
| ||||
| Recurrence of focal segmental glomerulonephritis12 | 1 (8) | 1 (8) | 0 | 0 |
|
| ||||
| Hypogammaglobulinemia of grade ≥3 (<4.0g/L) | 2 (15) | 3 (25) | 0 | 0 |
The on-treatment phase commences from the start of the first infusion of randomised study drug and ends 28 days after the last dose. The post-treatment phase begins the following day.
Death due to fatal myocardial infarction and acute cardiac failure.
Includes all AEs (by grouped MedDRA preferred terms) occurring in more than one subject during the study not including opportunistic infections (listed separately), ordered by overall frequency.
Grouped preferred terms include urinary tract infection, Escherichia urinary tract infection, urinary tract infection enterococcal and urosepsis.
Grouped preferred terms include anaemia, anaemia vitamin B12 deficiency and iron deficiency anaemia.
Grouped preferred terms include pneumonia and lower respiratory tract infection.
Grouped preferred terms include dyspepsia and gastrooesophageal reflux disease.
Grouped preferred terms include diabetes mellitus and type two diabetes mellitus; one post treatment AE was worsening of diabetes, onset on treatment, in context of steroids for SAE (colitis)
Grouped preferred terms include oesophagitis, duodenal ulcer, duodenitis and ulcerative gastritis.
Grouped preferred terms include cytomegalovirus test positive, cytomegalovirus viraemia, culture positive (verbatim term CMV).
The infusion reaction reported was mild, settled spontaneously and did not recur on subsequent infusions.
Grouped preferred terms include focal segmental glomerulosclerosis (FSGS) and glomerulonephritis (recurrence of FSGS verbatim term). NA denotes not applicable.
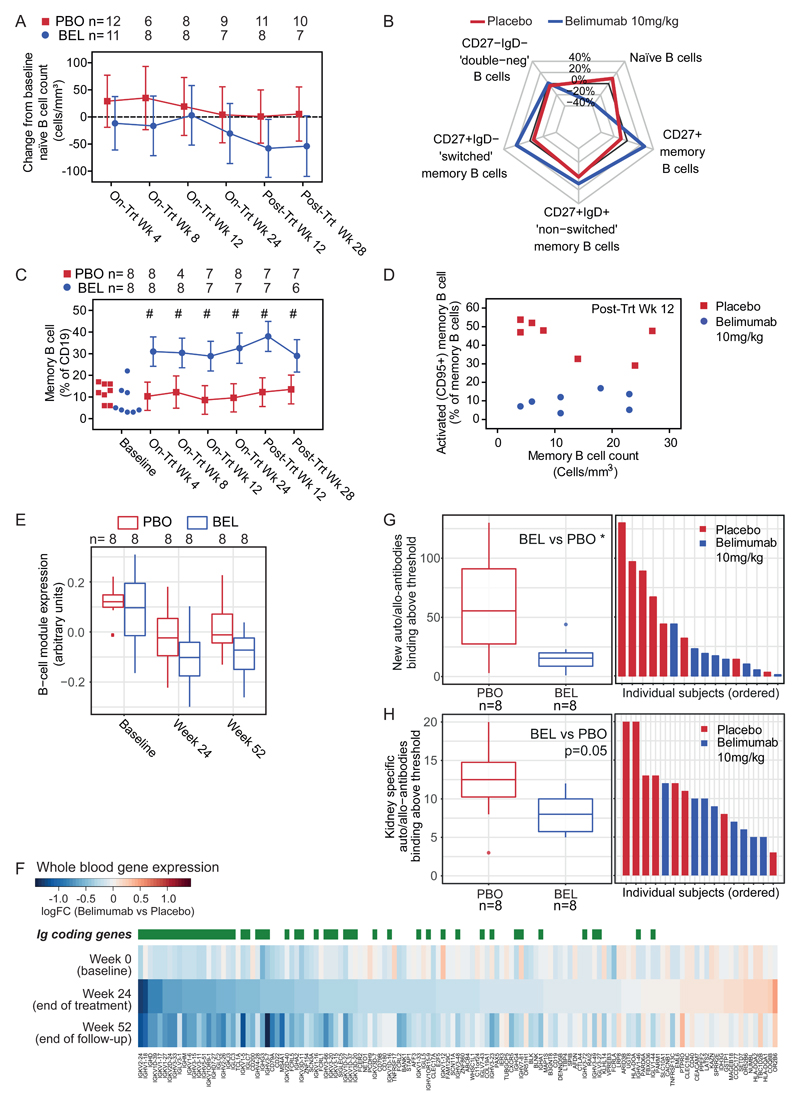
BLyS is known to support the survival of transitional and naïve B cells20. Therefore, we assessed naïve B cell count as a co-primary endpoint. This co-primary endpoint of a reduction in naïve B cells from baseline to Week 24 was not met. When considering the MITT population, there was a trend towards a reduction, but no statistically significant difference in naïve B cells (adjusted mean difference naïve B cells from baseline to week 24 - 34·4 cells/mm3 (95% CI -109·5 to 40·7)) (Fig. 2A; Table S4). At later time points in the PP population that included all MITT subjects who received at least five infusions of belimumab/placebo, significant differences in naïve B cell count were observed; adjusted mean difference naïve B cells from baseline to post treatment week 28 was -61.6 cells/mm3 (95% CI -122.6 to -0·7) (Fig S1D; Table S4). A pre-specified non-parametric sensitivity analysis on the MITT population showed a greater reduction from baseline naïve B cell count with belimumab at post treatment week 12 and 28 (median difference at post treatment week 28 -48.3 cells/mm3 (95% CI -144.7 to -13.0)) (Fig. S1E; Table S5).
Figure 2. Belimumab Affects Naïve B Cells, Activated Memory B Cells and Circulating Plasmablasts, and Reduces Allo/Auto-antibody Generation.
Panel A shows the adjusted mean (95% CI) change from baseline in naïve (CD20+CD27-) B cell count (cells/mm3), by visit and treatment.
Panel B is a radar plot summarising the median difference from baseline to week 24 compared to baseline (i.e. percentage at week 24 – percentage at week 0) for the labelled B cell populations by belimumab or placebo, where each B-cell population is expressed as a percentage of total B cells.
Panel C shows memory (CD20+CD27+) B cells expressed as a percentage of B cells by visit and treatment group.
Panel D shows the relationship between memory B cell count (cells/mm3) and activated memory B cell (CD95+CD27+) percentage at the post treatment week 12 timepoint for individual subjects labelled by treatment group.
Panel E shows expression (arbitrary units) of a B cell whole blood gene expression module by treatment group at baseline, end of on-treatment phase (Week 24) and end of follow-up (Week 52). Horizontal lines correspond to median and interquartile ranges.
Panel F shows a heat map of differential gene expression (belimumab versus placebo groups) for genes in the B cell module at baseline, week 24 and week 52. Genes are ordered by fold-change at week 24 and immunoglobulin-coding transcripts are highlighted. Colour corresponds to log2-fold change, with blue indicating higher expression in the placebo group (Placebo) relative to the belimumab group (Belimumab).
Panel G shows the number of unique ProtoArray antigen specificities with significant antibody binding at Week 24 but not at baseline. Horizontal lines on boxplot (left) correspond to median and interquartile ranges. Ordered individual participant data are also shown (right), with participants coloured by treatment group. The Wilcoxon rank-sum test was used to compare the belimumab treatment group (BEL) and placebo treatment group (PBO); * denotes p<0.05.
Panel H shows the number of kidney-specific unique ProtoArray antigen specificities (as defined in 37) with significant antibody binding at Week 24. Horizontal lines on boxplot (left) correspond to median and interquartile ranges. Ordered individual participant data are also shown (right), with participants coloured by treatment group. The Wilcoxon rank-sum test was used to compare the belimumab treatment group (BEL) and placebo treatment group (PBO); exact p value displayed.
Panel A uses the MITT population. Panels B, C, D, G and H use the PP population. Panels E and F use the MITT population for baseline and the PP population thereafter. C shows raw values at baseline for comparison and adjusted mean estimate with 95% confidence intervals at subsequent timepoints. Adjusted mean estimates and 95% confidence intervals are obtained from MMRM model, with fixed categorical effects of treatment, visit, donor type and treatment-by-visit interaction and fixed continuous covariates of baseline and baseline-by-visit interaction. A compound symmetry variance structure was used to model the within-patient errors, shared across treatments. # indicates that the 95% confidence interval of the treatment difference does not include zero. D shows data for individual PP subjects labelled by treatment group. Panels B, C, D, E, F, G and H represent analyses performed post hoc.
Biopsy proven TCMR (Banff grade I-III) occurs with a frequency of 15.3% in the 6 months post-transplant, in those receiving basiliximab-based induction therapy35. B cell depletion with the anti-CD20 antibody rituximab has been associated with increased TCMR11 and cardiac allograft vasculopathy12. Since belimumab also depletes some B cell subsets, we sought to confirm that its use was not associated with an increase in alloimmune responses. The overall frequency of TCMR (including borderline episodes, Banff classification) was similar in belimumab (n=1/12 (16.7%)) and placebo-treated subjects (n=3/13 (23%), Table 1). One additional subject was randomised to the belimumab group and received a single dose at day 0 but was withdrawn from the study due to a positive B lymphocyte flow cytometry cross-match (an exclusion criterion), the results of which became available post-transplant. This subject subsequently developed Banff IIA TCMR rejection with features suspicious of early acute humoral rejection (day 6), with a further biopsy during treatment demonstrating grade II ABMR (day 34), both of which resolved following treatment with methylprednisolone (day 3-5), plasma exchange (day 8 & 38-52) and anti-thymocyte globulin (day 8-21) (details in supplementary appendix). In the context of pre-formed HLA antibodies and suboptimal induction immunosuppression their rejection episode was not unexpected; however given there was also one further episode of Banff grade IIA cellular rejection in the belimumab PP treatment group and only three Banff ‘borderline’ rejections in the placebo treated group (one was treated with corticosteroids), close monitoring for rejection episodes in larger studies will be required to better understand this observation. Overall, transplant function at one year, graft and patient survival were not negatively affected by the addition of belimumab to standard of care immunosuppression (Table 1 & 2).
Total B cell numbers were comparable throughout the study (Fig. S1C; Table S13). We observed fewer transitional (CD24brCD38brIgD+) B cells at post-treatment week 12 (adjusted mean difference -9156 cells/ml (95% CI -18307 to -6) (Fig. S1F; Table S14) with relative sparing of memory (CD20+CD27+) B cells (Fig. S1G; Table S15) such that the proportion of memory B cells was increased throughout the treatment and follow up period (maximal adjusted mean difference 25.7% (95% CI 16.0 to 35.5) at post-treatment week 12; post hoc analysis) (Fig. 2B, C; Table S7). In contrast to the effect of belimumab on the total memory B cell pool, activated memory (CD95+CD27+) B cells were decreased from post-treatment week 12 to 28 (Fig. 2D, S1H; Table S16) (adjusted mean difference -14538.52 cells/ml (95% CI - 24875.4 to -4201.6) at post-treatment week 12). There was also a reduction in circulating CD19+CD27+CD38hi plasmablasts measured by flow cytometry at post-treatment week 12 (adjusted mean difference -1055.5 count/ml (95% CI -1889.7 to -221.2; post hoc analysis) (Fig. S1I; Table S17). In order to further understand the impact of belimumab on B cell activation and differentiation into antibody-secreting cells, we assessed the whole blood transcriptome. Weighted gene co-expression network analysis identified a B cell gene expression module, identified as such based on the overrepresentation of genes annotated as part of the BCR signaling pathway in the Gene Ontology (Fig. S1L), with attenuated expression in belimumab-treated subjects (post hoc analysis; Fig. 2E). The most attenuated genes in this module coded for IgG, suggesting a strong effect on antibody-secreting cells and complementing our observation of fewer circulating plasmablasts and reduced de novo non-HLA antibody formation (described below).
Eight study participants had anti-HLA antibodies (non donor-specific) at transplantation (3/12 in the belimumab group and 5/13 in the placebo group, Table 1). We observed a variable reduction in pre-existing HLA class I and II antibody levels, but the small sample size precludes any definitive conclusions on the effect of belimumab (Fig. S2). Two patients recruited to the study developed de novo DSA during follow-up; both had been withdrawn following a single dose of belimumab for reasons unrelated to antibody development (Fig S2C; see appendix for details).
De novo HLA and non-HLA IgG autoantibodies can have a deleterious effect on allograft function2,3,36. In order to sensitively measure the ability of belimumab to inhibit the development of de novo IgG antibodies, we compared antibody binding to a human protein array in serum samples taken at time zero and week 24. This demonstrated that the addition of belimumab to standard of care immunosuppression significantly reduced de novo IgG antibody formation (median number new antibody specificities binding above threshold 55.5 placebo vs 15.5 belimumab; post hoc analysis p=0.0474) (Fig. 2G; Table S8). In addition to effects on de novo antibodies, we also observed an effect on pre-formed non-HLA antibodies that have been implicated in allograft damage; at week 24 we saw a trend towards reduced IgG specific for kidney antigens37 following belimumab treatment (median number antibodies binding kidney specific antigen above threshold 12.5 placebo vs 8.0 belimumab; post hoc analysis p=0.0524 (Fig. 2H; Table S8); median mean protoarray signal 1307.3 placebo vs 983.5 belimumab; post hoc analysis p=0.065 (Fig. S2D; Table S8), and a significant reduction in anti-EDIL3 (EGF-like repeats and discoidin I-like domains 3) antibodies at week 24 (median of mean protoarray signal 353.6 placebo vs 261.9 belimumab; post hoc analysis p=0.0379) (Fig. S2E; Table S8), an endothelial cell-specific antibody associated with posttransplant glomerulopathy36. The presence of antibodies to glial cell-derived neurotrophic factor (GDNF) at the time of transplantation has been associated with more severe chronic allograft injury (interstitial fibrosis and tubular atrophy) on 24 month biopsies3; we observed a reduction in the number of subjects with detectable anti-GDNF antibodies at week 24 compared with time 0 in the belimumab group (6/8 and 2/8 at time 0 and 24 weeks respectively), in contrast to the placebo group (6/8 and 6/8 at time 0 and week 24 respectively; post hoc analysis) (Fig. S2F). Together, these data emphasise that belimumab modulates a clinically important aspect of B cell function post-transplant.
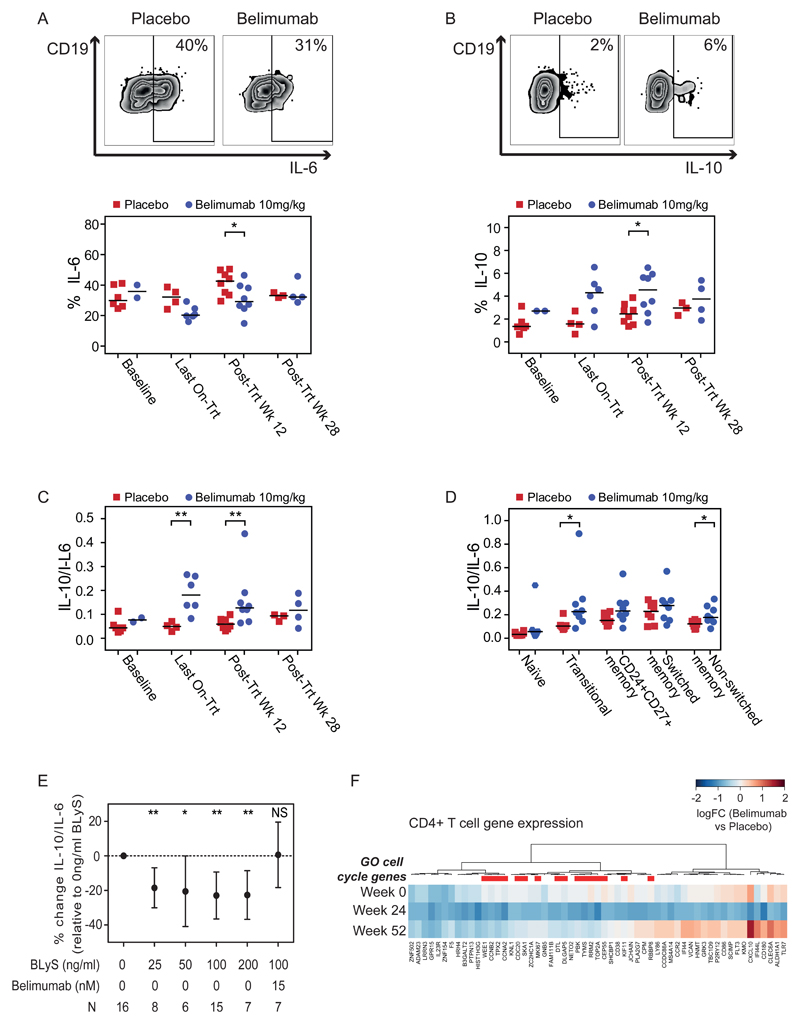
Transitional B cells have been associated with good outcomes in renal transplant, and this has been proposed to be due to their enrichment with IL-10 producing regulatory B cells15–18. IL-10 producing B cells have also been identified within memory subsets8. We sought to address how belimumab treatment affects B cell cytokine production post-transplant. PBMC were stimulated ex vivo with CpG and CD40L, and B cell IL-10 and IL-6 production assessed (Fig. 3A, B; Table S9). Despite reductions in transitional B cells (Fig. S1F; Table S14), at week 12 post-treatment subjects receiving belimumab had a reduction in IL-6-producing B cells (median 42.62% placebo vs. 29.18% belimumab; post hoc analysis p=0.0379) and an increase in IL-10+ B cells (median 2.46% placebo vs. 4.56% belimumab; post hoc analysis p=0.0499) (Fig. 3A, B; Table S9), skewing the cytokine ratio toward a more regulatory profile compared with controls (median 0.059 placebo vs. 0.127 belimumab; post hoc analysis p=0.0070) (Fig. 3C). This skewing of cytokine production towards IL-10 was observed in both transitional and memory B-cell subsets (Fig. 3D; Table S10). To validate our findings and confirm that the effect of belimumab on B cell cytokine production was B cell intrinsic we cultured PBMC enriched for memory B cells from healthy controls with BLyS in the presence or absence of belimumab. This demonstrated that BLyS stimulation significantly decreased B cell IL-10 relative to IL-6 (mean % change from baseline with 100ng/ml BLyS -22.90% (95%CI -36.52, -9.28); post hoc analysis p=0.0029) and was abrogated by the addition of belimumab (mean % change from baseline with 100ng/ml BLyS + 15nM belimumab 0.63% (95%CI -18.28, 19.54); post hoc analysis p=0.9377) (Fig. 3E; Table S11) providing new insights for Breg biology.
Figure 3. Memory B Cell IL-10/IL-6 Ratio is BLyS Dependent. Belimumab Treatment Increases Regulatory B Cells and is Associated with Reduced T cell Proliferation.
Peripheral blood mononuclear cells (PBMC) were stimulated ex vivo for 5 hours and intracellular cytokine production quantified by flow cytometry (Panel A-D). Individual data points represent individual subjects with horizontal lines signifying the median for each treatment group. Wilcoxon rank-sum tests were performed to compare samples by treatment and visit.
Panel A shows representative flow cytometry plots for IL-6 (upper) and the percentage of CD19+ B cells expressing IL-6 (lower).
Panel B shows representative flow cytometry plots for IL-10 (upper) and the percentage of CD19+ B cells expressing IL-10 by treatment group and timepoint (lower).
Panel C shows the calculated ratio of IL-10/IL-6 with higher values indicating a more anti-inflammatory cytokine milieu.
Panel D shows the IL-10/IL-6 ratio for individual subsets of naïve (CD27-), transitional (CD24hiCD38hiIgD+), CD24+CD27+ memory, switched memory (CD27+IgD-), and non switched memory (CD27+IgD+) B cells at post treatment Wk 12.
Panel E shows the mean IL-10/IL-6 ratio (relative to 0ng/mL BLyS) with 95% confidence intervals for PBMC from healthy volunteers enriched for CD27+ memory B cells and stimulated for 48 hours with increasing quantities of BLyS. In the presence of increasing quantities of BLyS a more inflammatory cytokine milieu was observed. This change was blocked by the addition of belimumab. N, number of healthy volunteers tested for each experimental condition. T-tests were performed to determine whether the mean percentage changes from baseline differed significantly from 0 for each experimental condition.
Panel F shows a heatmap of differential gene expression in circulating CD4+ T-cells for the most downregulated genes at week 24 in the belimumab group relative to the placebo group, with genes ordered by unsupervised hierarchical clustering. Genes annotated to the cell-cycle (GO:0007049) are highlighted. Colour corresponds to log2-fold change, with blue indicating higher expression in the placebo group (Placebo) relative to the belimumab group (Belimumab).
Panels A-D use the PP population and Panel F uses the MITT population for baseline and PP population thereafter. P-values in Panels A-D were calculated using Wilcoxon rank-sum tests and indicated where significant; in all other cases the values are not statistically significantly different. P-values in Panel E were calculated using Student’s t-test. * p<0.05, ** p<0.01, *** p<0.001, NS not significant (p≥0.05). All analyses were performed post hoc.
We also investigated whether T cell activation was altered by belimumab treatment, since B cells are potent antigen presenting cells and IL-6 may augment T cell activation38 whilst IL-10 can regulate T cell responses39. There was no difference in the number of circulating T cells by treatment (Fig. S1J-K); Table S18-19) but transcriptomic analysis of purified circulating CD4 T cells revealed reduced expression of cell-cycle genes in belimumab-treated subjects (post hoc analysis; Fig. 3F, Table S2) suggesting that BLyS neutralisation may also inhibit T cell proliferation.
Discussion
A key goal of this study was to determine the safety profile of belimumab in combination with standard transplant immunosuppression. Although belimumab has previously been used in patients with SLE with renal involvement, it has not been used in those with established ESRF, which in itself confers an increased risk of infection33. Despite this, and a significantly greater burden of concomitant immunosuppression than used previously, we observed no excess infections with the addition of belimumab. Observed AEs were consistent with that expected for the underlying population, concurrent medications and known safety profile of belimumab.
The co-primary endpoint of a reduction in naïve B cells from baseline to week 24 was not met in the MITT population, due to the impact of subjects that received only one dose of belimumab on a group with limited sample size. However, sensitivity and pre-specified analyses performed on the PP population that included subjects who received at least five doses of belimumab/placebo, confirmed that belimumab did have a significant biological effect, greatest at post treatment week 12. Sample size calculation relied on data from studies of belimumab in SLE; it is likely that the more intense immunosuppression given in the immediate post-transplant period masked earlier differences.
Although the co-primary endpoint of a reduction in naïve B cells from baseline to week 24 was not met, data from secondary and mechanistic end-points suggest but do not definitely prove, potential beneficial effects of belimumab post-transplant. Despite depletion by belimumab of the transitional B cell subset known to contain regulatory B cells, remaining B cells after belimumab treatment demonstrated an increased capacity to produce the immunoregulatory cytokine IL-10 relative to IL-6. Since B cells are the major source of IL-6 in secondary lymphoid organs40, this observation is significant and of potential clinical benefit in transplantation: IL-6 promotes B cell differentiation into antibody-forming plasma cells, contributes to the plasma cell niche41, enhances T follicular helper cell differentiation (critical for the germinal centre response), and inhibits the generation of regulatory T cells38. Indeed, an IL-6R antagonist has been used to treat patients with chronic ABMR42.
We present flow cytometry, transcriptomic and protein microarray data suggesting that BLyS neutralisation reduced antibody-forming cells and resulted in a lower incidence of de novo non-HLA autoantibody formation post-transplant. In particular, observed trends in kidney and endothelial specific IgG raise the possibility of improved longer-term transplant outcomes2,3,36. Our study also highlights the role of BLyS in memory B cell activation. Belimumab-treated subjects had fewer circulating activated (CD95+) memory B cells, suggesting a rationale for using belimumab as a longer-term adjuvant in sensitised transplant subjects with pre-existing donor-specific memory B cells, in addition to lymphocyte depletion and antibody removal. This strategy is currently being studied in a recently established clinical trial using belimumab in combination with bortezomib, plasma exchange and rituximab as a desensitisation therapy (ClinicalTrials.gov Identifier: NCT02500251). Finally, a decrease in T cell proliferation markers demonstrates the potential for belimumab to modulate both cellular and humoral alloimmunity.
Our study has several limitations; the sample size was not powered for clinical endpoints, limiting the broader interpretation of our findings. Early phase studies with modest numbers cannot provide definitive data on safety; with this caveat we report no major adverse safety signal. Despite modest numbers, we were able to implement a range of assays measuring both clinical and immunological parameters so that our major findings are backed by multiple independent readouts, for example flow cytometry, transcriptional differences and antibody array, which together support robust and clinically useful effects on the B cell compartment post-transplant.
The study population (standard immunological risk transplant recipients without DSA) was selected in light of previous studies in SLE showing a significant effect of belimumab on naïve B cells; we therefore hypothesised that the depletion and inhibition of naïve B cells may prevent the development of de novo HLA antibodies in low risk patients. However, these patients have a low risk of developing DSA (around 10% per annum) and a trial powered to detect a difference would need several thousand patients, unfeasible for a Phase 2 experimental medicine study. The transplant community has acknowledged that the unmet need for novel effective therapies for ABMR and to improve long-term graft outcomes may be best addressed through non-traditional trial designs that include surrogate endpoints43.
Although our study did not include sensitised subjects at greatest risk of ABMR and graft loss, the occurrence of ABMR in the sensitised subject that was inadvertently included in the study provides valuable information for the design of future trials in this area, suggesting that such patients may well need antibody removal and pan-lymphocyte depletion in addition to belimumab. Further studies will be required to evaluate the safety and efficacy of belimumab alongside lymphocyte depleting treatments including thymoglobulin or alemtuzumab, agents that previously associated with an increase in serum BLyS44. Whether belimumab would have the same pro-regulatory effect on the reconstituted B cell pool when used in combination with lymphocyte-depletion remains to be determined. An observed rebound in serum BLyS following cessation of belimumab raises the question of whether belimumab should be administered for a more extended period post-transplant, with graduated discontinuation alongside monitoring of serum BLyS.
This study exemplifies an experimental medicine approach and suggests potential efficacy of belimumab as an immunomodulatory agent in transplantation that can limit new antibody formation, providing a useful platform to support design of future clinical studies to explore the further use of belimumab in renal transplantation. In addition, our study provides new insights into BLyS biology and the mechanism of action of belimumab, including novel preliminary data on its effect on the regulatory B cell compartment and autoantibody production, which are also of relevance in autoimmunity.
Supplementary Material
Panel: Research in context.
Evidence before this study
Therapeutic agents that limit humoral alloimmunity in kidney transplant recipients are currently lacking. B lymphocyte stimulator (BLyS; also known as BAFF) is a cytokine that promotes B cell survival, and we hypothesised that this pathway may represent a useful therapeutic target in transplantation. We searched PubMed using the terms ‘BAFF’ and ‘kidney transplantation’ and ‘Clinical trial’ and Clinicaltrials.gov using the terms ‘kidney transplantation and ‘BAFF’ to establish the current clinical evidence-base in this area. Of the six studies identified in this search, only two included agents that targeted the BAFF/BLyS pathway, using monoclonal antibodies that bind BAFF/BLyS (tabalumab and belimumab), and only one of these was completed and published (Mujtaba et al. 2016). In fact, neither of these studies attempted BAFF/BLyS blockade in kidney transplant recipients. Both used an anti-BAFF/BLyS antibody as monotherapy in patients with end-stage kidney failure, with the aim of reducing pre-formed HLA antibodies to a level that permitted safe transplantation, but observed little effect. No trial to date has addressed the question of whether blockade of BAFF/BLyS could be a useful addition to current maintenance immunosuppressive agents following kidney transplantation.
Added value of this study
This study adds value to the existing evidence base by delivering the first data on the use of belimumab in kidney transplant recipients, showing no excess risk of infection and a reduction in naïve B cells, activated memory B cells, circulating plasmablasts, and kidney-specific IgG. We also provide the first data on the impact of belimumab to reduce de novo IgG formation and increase regulatory B cell numbers in the peripheral blood. Our study informs clinical trials modulating the BLyS/BAFF axis in transplant recipients, and those using belimumab outside of the field of transplantation in systemic lupus erythematosus (SLE).
Implications of all the available evidence
Our data suggest that belimumab may be a useful therapeutic strategy in standard immunological risk kidney transplant recipients to prevent de novo donor-specific antibody formation. The observed lowering of activated memory B cells and preformed IgG suggest an additional potential role in sensitised kidney transplant recipients or in those with antibody-mediated rejection. Therefore, the use of belimumab warrants further study in these patient groups, in the context of larger randomised controlled trials.
Acknowledgments
Study funded by GlaxoSmithKline; ClinicalTrials.gov number NCT01536379; EudraCT number 2011-006215-56.
CJEW and MRC are supported by the National Institute of Health Research (NIHR) Cambridge Biomedical Research Centre and the NIHR Blood and Transplant Research Unit. We thank all the patients who participated in the study, without whom this work would not have been possible. We also thank the Wellcome trust for their role in funding GDB, A J Want, S S Franco, P A Wilson, the GSK Clinical Unit Cambridge for technical support, Addenbrooke's Hospital Histocompatibility and Immunogenetics laboratory and pathology services for their assistance with sample processing, ThermoFisher for their advice on ProtoArray analysis, and D Game and colleagues at Guy's and St Thomas' National Health Service (NHS) Foundation Trust for their contribution to patient screening.
Footnotes
NOTICE: following is the author's peer reviewed version of a work that was accepted for publication in The Lancet. Changes resulting from the publishing process, such as editing, corrections, structural formatting, and other quality control mechanisms are not reflected in this document. Changes have been made to this work since it was submitted for publication. A definitive version was subsequently published in The Lancet, Volume 391, Issue 10140, Page 2619-2630 on June 30th 2018 (epub June 14th).
Contributors:
GDB, SMF, NT, PAL, DNS, AG, CJEW, RBJ, LRD, COS, KGCS, RBH and MRC were involved in study design.
GDB, NT, AOS and MRC recruited participants.
GDB, SMF, NT, PAL, AG, CJEW, AOS, JAC, KEF, AR, LPE, RBH and MRC collected data.
GDB, SMF, PAL, DNS, AG, JAC, KEF and AR were involved in data analysis.
DNS performed statistical analysis.
GDB, SMF, NT, PAL, DNS, AG, AR, LPE, COS, KGCS, RBH and MRC interpreted the data GDB, SMF and DNS produced figures.
MRC and GDB wrote the first draft of the manuscript; all authors contributed to its revision and approved the final version.
Disclosures
GDB was funded by a Wellcome Trust Translational Medicine and Therapeutics (TMAT) PhD grant (102728/z/13/z). SMF received funding from GlaxoSmithKline (GSK) for a GSK-Wellcome Trust TMAT PhD. SMF, DNS, AG, KEF, AR, L-PE, COS, and RBH are employees of and hold stock in GSK. JAC is employed by a recruitment agency, working on contract at GSK. RBJ did a secondment to GSK, funded by the company. LRD is a previous employee and a stockholder of GSK, and employee and stockholder of Celgene Corporation. GDB, PAL, and MRC have received grants from GSK outside the submitted work. NT has received support to attend clinical meetings from Astellas Pharma and Alexion Pharmaceuticals.
CJEW has received support to attend clinical meetings from Organ Assist and reports consultancy fees from GSK. KGCS reports consultancy fees from MedImmune, UCB, and Kymab. MRC is funded by a Medical Research Council New Investigator Research Grant (MR/N024907/1) and an Arthritis Research UK Cure Challenge Research Grant (21777), and also receives support from the National Institute of Health Research Cambridge Biomedical Research Centre. RBH has a patent PB65956 pending. AR has a patent issued for recombinant factor H and variants and conjugates thereof (US20150139975 A1), and an EU application pending (WO2011077102 A1). AR's spouse David Kavanagh is head of the National Renal Complement Therapeutics Centre, UK; Chief Investigator for NCT02949128, Alexion Pharmaceuticals; founding board member and scientific adviser to Gyroscope Therapeutics (stock options; consultancy fees paid to Newcastle University, Newcastle, UK); and consults for Alexion Pharmaceuticals and Akari Therapeutics (Newcastle University, Newcastle, UK, receives consulting fees). A-MO declares no competing interests.
References
- 1.Lefaucheur C, Loupy A, Vernerey D, et al. Antibody-mediated vascular rejection of kidney allografts: a population-based study. Lancet. 2013;381(9863):313–9. doi: 10.1016/S0140-6736(12)61265-3. [DOI] [PubMed] [Google Scholar]
- 2.Dragun D, Muller DN, Brasen JH, et al. Angiotensin II type 1-receptor activating antibodies in renal-allograft rejection. N Engl J Med. 2005;352(6):558–69. doi: 10.1056/NEJMoa035717. [DOI] [PubMed] [Google Scholar]
- 3.Sigdel TK, Li L, Tran TQ, et al. Non-HLA antibodies to immunogenic epitopes predict the evolution of chronic renal allograft injury. J Am Soc Nephrol. 2012;23(4):750–63. doi: 10.1681/ASN.2011060596. [DOI] [PubMed] [Google Scholar]
- 4.Bentall A, Cornell LD, Gloor JM, et al. Five-year outcomes in living donor kidney transplants with a positive crossmatch. Am J Transplant. 2013;13(1):76–85. doi: 10.1111/j.1600-6143.2012.04291.x. [DOI] [PubMed] [Google Scholar]
- 5.Sarwal M, Chua MS, Kambham N, et al. Molecular heterogeneity in acute renal allograft rejection identified by DNA microarray profiling. N Engl J Med. 2003;349(2):125–38. doi: 10.1056/NEJMoa035588. [DOI] [PubMed] [Google Scholar]
- 6.Inaba A, Clatworthy MR. Novel immunotherapeutic strategies to target alloantibodyproducing B and plasma cells in transplantation. Curr Opin Organ Transplant. 2016;21(4):419–26. doi: 10.1097/MOT.0000000000000338. [DOI] [PubMed] [Google Scholar]
- 7.Blair PA, Norena LY, Flores-Borja F, et al. CD19(+)CD24(hi)CD38(hi) B cells exhibit regulatory capacity in healthy individuals but are functionally impaired in systemic Lupus Erythematosus patients. Immunity. 2010;32(1):129–40. doi: 10.1016/j.immuni.2009.11.009. [DOI] [PubMed] [Google Scholar]
- 8.Iwata Y, Matsushita T, Horikawa M, et al. Characterization of a rare IL-10-competent B-cell subset in humans that parallels mouse regulatory B10 cells. Blood. 2011;117(2):530–41. doi: 10.1182/blood-2010-07-294249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tyden G, Ekberg H, Tufveson G, Mjornstedt L. A randomized, double-blind, placebocontrolled study of single dose rituximab as induction in renal transplantation: a 3-year follow-up. Transplantation. 2012;94(3):e21–2. doi: 10.1097/01.tp.0000418580.88642.e1. [DOI] [PubMed] [Google Scholar]
- 10.van den Hoogen M, Kamburova EG, Baas MC, et al. Rituximab as induction therapy after renal transplantation: a randomized, double-blind, placebo-controlled study of efficacy and safety. Am J Transplant. 2015;15(2):407–16. doi: 10.1111/ajt.13052. [DOI] [PubMed] [Google Scholar]
- 11.Clatworthy MR, Watson CJ, Plotnek G, et al. B-cell-depleting induction therapy and acute cellular rejection. N Engl J Med. 2009;360(25):2683–5. doi: 10.1056/NEJMc0808481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chandraker A, Kobashigawa J, Stehlik J, et al. Rituximab Induction in Cardiac Transplantation Is Associated with Accelerated Coronary Artery Vasculopathy: CTOT11. American Journal of Transplantation. 2016;16(Supplement S3):403. [Google Scholar]
- 13.Pallier A, Hillion S, Danger R, et al. Patients with drug-free long-term graft function display increased numbers of peripheral B cells with a memory and inhibitory phenotype. Kidney Int. 2010;78(5):503–13. doi: 10.1038/ki.2010.162. [DOI] [PubMed] [Google Scholar]
- 14.Newell KA, Asare A, Kirk AD, et al. Identification of a B cell signature associated with renal transplant tolerance in humans. J Clin Invest. 2010;120(6):1836–47. doi: 10.1172/JCI39933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chesneau M, Pallier A, Braza F, et al. Unique B cell differentiation profile in tolerant kidney transplant patients. Am J Transplant. 2014;14(1):144–55. doi: 10.1111/ajt.12508. [DOI] [PubMed] [Google Scholar]
- 16.Shabir S, Girdlestone J, Briggs D, et al. Transitional B lymphocytes are associated with protection from kidney allograft rejection: a prospective study. Am J Transplant. 2015;15(5):1384–91. doi: 10.1111/ajt.13122. [DOI] [PubMed] [Google Scholar]
- 17.Cherukuri A, Salama A, Carter C, Clark B, Rothstein D, Baker R. Human Regulatory B Cells (BRegs) Are Characterised by Both IL-10 and TNF-α Expression and Are Reduced in Numbers with Altered Function in Renal Transplant Recipients with Immunological Graft Injury. American Journal of Transplantation. 2013;13(s5, A645):229. [Google Scholar]
- 18.Cherukuri A, Rothstein DM, Clark B, et al. Immunologic human renal allograft injury associates with an altered IL-10/TNF-alpha expression ratio in regulatory B cells. J Am Soc Nephrol. 2014;25(7):1575–85. doi: 10.1681/ASN.2013080837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schlosser HA, Thelen M, Dieplinger G, et al. Prospective analyses of circulating B-cell subsets in AB0-compatible and AB0-incompatible kidney transplant recipients. Am J Transplant. 2016 doi: 10.1111/ajt.14013. [DOI] [PubMed] [Google Scholar]
- 20.Mackay F, Schneider P, Rennert P, Browning J. BAFF AND APRIL: a tutorial on B cell survival. Annu Rev Immunol. 2003;21:231–64. doi: 10.1146/annurev.immunol.21.120601.141152. [DOI] [PubMed] [Google Scholar]
- 21.Avery DT, Kalled SL, Ellyard JI, et al. BAFF selectively enhances the survival of plasmablasts generated from human memory B cells. J Clin Invest. 2003;112(2):286–97. doi: 10.1172/JCI18025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Navarra SV, Guzman RM, Gallacher AE, et al. Efficacy and safety of belimumab in patients with active systemic lupus erythematosus: a randomised, placebo-controlled, phase 3 trial. Lancet. 2011;377(9767):721–31. doi: 10.1016/S0140-6736(10)61354-2. [DOI] [PubMed] [Google Scholar]
- 23.Steri M, Orru V, Idda ML, et al. Overexpression of the Cytokine BAFF and Autoimmunity Risk. N Engl J Med. 2017;376(17):1615–26. doi: 10.1056/NEJMoa1610528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Thibault-Espitia A, Foucher Y, Danger R, et al. BAFF and BAFF-R levels are associated with risk of long-term kidney graft dysfunction and development of donor-specific antibodies. Am J Transplant. 2012;12(10):2754–62. doi: 10.1111/j.1600-6143.2012.04194.x. [DOI] [PubMed] [Google Scholar]
- 25.Snanoudj R, Candon S, Roelen DL, et al. Peripheral B-cell phenotype and BAFF levels are associated with HLA immunization in patients awaiting kidney transplantation. Transplantation. 2014;97(9):917–24. doi: 10.1097/01.TP.0000438211.34842.5e. [DOI] [PubMed] [Google Scholar]
- 26.Banham G, Prezzi D, Harford S, et al. Elevated pretransplantation soluble BAFF is associated with an increased risk of acute antibody-mediated rejection. Transplantation. 2013;96(4):413–20. doi: 10.1097/TP.0b013e318298dd65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Parsons RF, Yu M, Vivek K, et al. Murine islet allograft tolerance upon blockade of the Blymphocyte stimulator, BLyS/BAFF. Transplantation. 2012;93(7):676–85. doi: 10.1097/TP.0b013e318246621d. [DOI] [PubMed] [Google Scholar]
- 28.Kwun J, Page E, Hong JJ, et al. Neutralizing BAFF/APRIL with atacicept prevents early DSA formation and AMR development in T cell depletion induced nonhuman primate AMR model. Am J Transplant. 2015;15(3):815–22. doi: 10.1111/ajt.13045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mujtaba MA, Komocsar WJ, Nantz E, et al. Effect of Treatment With Tabalumab, a B Cell-Activating Factor Inhibitor, on Highly Sensitized Patients With End-Stage Renal Disease Awaiting Transplantation. Am J Transplant. 2016 doi: 10.1111/ajt.13557. [DOI] [PubMed] [Google Scholar]
- 30.Naji A. One Year Exploratory Study to Evaluate the Efficacy and Safety of Belimumab for Normalization of Alloantibody Levels in Sensitized Patients Awaiting Kidney Transplantation. Clinicaltrialsgov. NCT01025193 . [Google Scholar]
- 31.Stohl W, Hiepe F, Latinis KM, et al. Belimumab reduces autoantibodies, normalizes low complement levels, and reduces select B cell populations in patients with systemic lupus erythematosus. Arthritis Rheum. 2012;64(7):2328–37. doi: 10.1002/art.34400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lyons PA, Koukoulaki M, Hatton A, et al. Microarray analysis of human leucocyte subsets: the advantages of positive selection and rapid purification. BMC Genomics. 2007;8:64. doi: 10.1186/1471-2164-8-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vanholder R, Ringoir S. Infectious morbidity and defects of phagocytic function in end-stage renal disease: a review. J Am Soc Nephrol. 1993;3(9):1541–54. doi: 10.1681/ASN.V391541. [DOI] [PubMed] [Google Scholar]
- 34.Almond PS, Matas A, Gillingham K, et al. Risk factors for chronic rejection in renal allograft recipients. Transplantation. 1993;55(4):752–6. doi: 10.1097/00007890-199304000-00013. discussion 6–7. [DOI] [PubMed] [Google Scholar]
- 35.Group. Haynes R, Harden P, et al. Alemtuzumab-based induction treatment versus basiliximab-based induction treatment in kidney transplantation (the 3C Study): a randomised trial. Lancet. 2014;384(9955):1684–90. doi: 10.1016/S0140-6736(14)61095-3. [DOI] [PubMed] [Google Scholar]
- 36.Jackson AM, Sigdel TK, Delville M, et al. Endothelial cell antibodies associated with novel targets and increased rejection. J Am Soc Nephrol. 2015;26(5):1161–71. doi: 10.1681/ASN.2013121277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li L, Wadia P, Chen R, et al. Identifying compartment-specific non-HLA targets after renal transplantation by integrating transcriptome and "antibodyome" measures. Proc Natl Acad Sci U S A. 2009;106(11):4148–53. doi: 10.1073/pnas.0900563106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jordan SC, Choi J, Kim I, et al. Interleukin-6, A Cytokine Critical to Mediation of Inflammation, Autoimmunity and Allograft Rejection: Therapeutic Implications of IL-6 Receptor Blockade. Transplantation. 2017;101(1):32–44. doi: 10.1097/TP.0000000000001452. [DOI] [PubMed] [Google Scholar]
- 39.Maynard CL, Weaver CT. Diversity in the contribution of interleukin-10 to T-cell-mediated immune regulation. Immunol Rev. 2008;226:219–33. doi: 10.1111/j.1600-065X.2008.00711.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Barr TA, Shen P, Brown S, et al. B cell depletion therapy ameliorates autoimmune disease through ablation of IL-6-producing B cells. J Exp Med. 2012;209(5):1001–10. doi: 10.1084/jem.20111675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kometani K, Kurosaki T. Differentiation and maintenance of long-lived plasma cells. Curr Opin Immunol. 2015;33:64–9. doi: 10.1016/j.coi.2015.01.017. [DOI] [PubMed] [Google Scholar]
- 42.Choi J, Aubert O, Vo A, et al. Assessment of Tocilizumab (Anti-Interleukin-6 Receptor Monoclonal) as a Potential Treatment for Chronic Antibody-Mediated Rejection and Transplant Glomerulopathy in HLA-Sensitized Renal Allograft Recipients. Am J Transplant. 2017 doi: 10.1111/ajt.14228. [DOI] [PubMed] [Google Scholar]
- 43.O'Connell PJ, Kuypers DR, Mannon RB, et al. Clinical Trials for Immunosuppression in Transplantation: The Case for Reform and Change in Direction. Transplantation. 2017;101(7):1527–34. doi: 10.1097/TP.0000000000001648. [DOI] [PubMed] [Google Scholar]
- 44.Bloom D, Chang Z, Pauly K, et al. BAFF is increased in renal transplant patients following treatment with alemtuzumab. Am J Transplant. 2009;9(8):1835–45. doi: 10.1111/j.1600-6143.2009.02710.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.