Summary
Background
The microcephaly epidemic, which started in Brazil in 2015, was declared a Public Health Emergency of International Concern in 2016. The hypothesis that the epidemic is caused by Zika virus (ZIKV) infection during pregnancy has not been investigated in a case-control study.
Methods
Preliminary report of a case-control study investigating the association between microcephaly and ZIKV infection during pregnancy. Cases and controls born in eight public hospitals in Recife, Brazil. Cases were neonates with microcephaly; controls were neonates matched by expected date of delivery and area of residence. Serum of cases and controls and Cerebrospinal Fluid (CSF) of cases were tested for Zika-specific IgM and quantitative reverse-transcriptase-polymerase-chain-reaction (RT-PCR). The main exposure, indicating laboratory confirmed ZIKV during pregnancy was positivity to Zika-specific IgM or RT-PCR in neonates. Mother´s serum was tested with plaque-reduction-neutralization-assays (PRNT50) for ZIKV and dengue virus. We estimated crude odds ratios (OR) and 95% confidence interval (95%CI) using a median unbiased estimator for binary data in an unconditional logistic regression model. We estimated ORs separately for cases with and without radiological brain abnormalities.
Findings
Thirty-two cases and 62 controls were recruited prospectively between January-May/2016. 64% of the mothers of controls had ZIKV infection. Thirteen of the 32 cases (40·6%) and none of the 62 controls had laboratory confirmed Zika with a crude overall OR=55·5 (95%CI=8·6 to ∞); OR=113·3 (95%CI=14·5 to ∞) for those with abnormal brain imaging and OR=24·7 (95%CI=2·9 to ∞) with normal brain imaging.
Interpretation
The microcephaly epidemic is a result of Congenital ZIKV Infection.
Funders
The study was funded by Brazilian Minister of Health (MoH), Pan American Health Organization (PAHO), Enhancing Research Activity in Epidemic Situations (ERAES).
Background
An increase in numbers of neonates with microcephaly was detected in Brazil in August 2015, which was to become a major epidemic by late 2015. Up until June 25, 2016, 8,165 cases have been notified, of which 1,638 were confirmed, 3,466 excluded and 3,061 remain under investigation.1 Soon the hypothesis was formulated that the microcephaly epidemic was caused by ZIKV infection during pregnancy. In November 2015, the Brazilian MoH declared a Public Health Emergency of National Importance.2 Microcephaly results from any insult that disturbs early brain growth, and can be caused by genetic variations, teratogenic agents, or other congenital infections.3
ZIKV infection in humans was initially limited to sporadic cases in a small number of countries and perceived not to cause outbreaks nor severe disease. Outbreaks were first detected in the Pacific Islands, in 2007 and 2013. Since 2007 transmission has been detected in 61countries and territories, most of them located in the Americas.4
In February 2016 the World Health Organization (WHO) declared in relation to the epidemic that started in Brazil that ´the cluster of microcephaly cases and other neurological disorders [....] constitutes a Public Health Emergency of International Concern (PHEIC)’.5
Since the hypothesis of the microcephaly epidemic being caused by Congenital Zika Infection was first proposed in Brazil6 there has been an accumulation of evidence supporting the hypothesis.7–17 The causal relationship between Zika virus and birth defects was strong enough to be considered causal, but that the argument would be stronger if confirmed by at least one case control study and a cohort study.18 Indeed, the evidence so far comes from case reports,16 case series,19,20 modeling studies,17 and the preliminary reporting of a cohort study.7 None included appropriate population control groups. This is a report of the preliminary analysis of a case-control study conducted, at request of the Brazilian MoH, to investigate the causes of the microcephaly epidemic in Brazil; the main hypothesis was that it is caused by congenital ZIKV infection.
Methods
Study design
A case-control study, with prospective recruitment of newly born cases and concurrent controls.
Study period
Neonates were born between January 15, 2016 and May 2, 2016.
Setting
The study site was the metropolitan region of Recife, Pernambuco State, Brazil.
Study population
Neonates born to women resident in Pernambuco and delivered in eight public maternity units. Neonates with anencephaly or encephalocele were excluded.
Case definition
Microcephaly defined as head circumference (HC) of two standard deviations below the mean for sex and gestational age in the Fenton Growth Chart.21
Control definition
Live neonates without microcephaly and with normal brain imaging by transfontanellar ultrasonography (USG) and no major birth defects detected by physical examination by a neonatologist.
Matching criteria
Two controls were selected for each case, matched by Health Region of residence and expected date of delivery (to ensure cases and controls were conceived at the same stage of the epidemic). The criteria for matching for expected date of delivery was specific for gestational age of cases. For cases born at term and post-term (37 weeks or more), controls were the next eligible neonates born of gestational aged 37 weeks or more. For early preterm cases (born at <34 weeks) controls were the next eligible neonates who were of <34 weeks gestational age at the date of birth of the case. For preterm cases (born between 34 and 36 weeks gestational age), controls were the next two eligible neonates born between 34 and 36 weeks of gestational age at the time of birth of the case.
Gestational age was estimated from fetal USG performed in the first trimester. If USG was not available, gestational age was estimated from the date of the last menstrual period recorded in the antenatal care card or informed by the woman.
Head circumference was measured in the delivery room using a non-stretch Teflon tape.
Cases
If microcephaly was detected, cord blood was collected. If microcephaly was confirmed in a second HC measurement 12-24 hours after birth, the neonate was considered eligible for the study.
Controls
Two controls were selected (2:1), amongst the first neonates born from the following morning in one of the study hospitals with normal HC, meeting the matching criteria. Cord blood was collected from the neonate eligible as a control.
Data collection
After the mothers signed the informed consent form, samples were collected, mothers interviewed and children referred for brain imaging. Interviews were conducted in the hospital by a trained female nurse, using a structured standardized questionnaire.
Imaging
Radiological brain imaging was performed for cases using CT-scan without contrast and for controls by transfontanellar USG by radiologist.
Sample collection and laboratory testing
CSF of cases was collected by the study neonatologists. Umbilical cord blood was collected in the delivery room from cases and controls; when necessary, peripheral blood was collected before the neonate left hospital. Blood samples were sent to the Virology and Experimental Therapy Department (LAVITE-FIOCRUZ-PE) where they were aliquoted and stored. Macerated tissue material from neonatal deaths and stillbirths underwent testing by RT-PCR.
Serum and CSF samples were analyzed by RT-PCR and for the detection of ZIKV-specific IgM antibodies. For the RT-PCR, RNA was extracted from specimens and subjected to reverse transcription and amplification; for serology, a capture ELISA based on the US Centers for Disease Control and Prevention (CDC) Emergency Use Authorization protocol was used, with reagents from CDC Fort Collins, USA.22 For the ELISA assay both ZIKV and DENV were tested in parallel to check for cross-reactivity. For mothers and neonates the presence of neutralizing antibodies to Zika virus and dengue virus (DENV1-4) was assessed by plaque reduction neutralization assay (PRNT50) in Vero cells, following a protocol described in details elsewhere.23 PRNT50 assay was carried out using the virus strain ZIKV PE/243 isolated in Pernambuco, Brazil. Serum of mothers and neonates were tested for specific IgM and IgG antibodies for toxoplasmosis, rubella and cytomegalovirus (the main infectious causes congenital microcephaly).24
Variable definition
Main outcome
Microcephaly, defined as head circumference at least 2 standard deviations (SD) below the mean for gestational age and sex.
Main exposure
For investigating the association, a positive reverse transcription-polymerase chain reaction (RT-PCR) and/or a positive IgM antibody against ZIKV were considered as laboratory confirmation.
Brain imaging (computed tomography scan (CT-scan) in cases and USG in controls) was classified for the purpose of this analysis, into normal or abnormal (including, calcification, ventriculomegaly, lissencephaly).
A neonate was considered to be small for gestation age (SGA) when the birth weight was below the 10th percentile for gestational age and sex in the Fenton Growth Chart.
Sample size calculation
The original study aimed to include 200 cases and 400 controls, to have 90% power, 95% precision to detect an association of a magnitude of two (OR=2) or larger, assuming that 67% of cases were exposed.
Statistical analysis
We described features of cases and controls and their mothers. We estimated the crude OR and 95%CI for the association between microcephaly and laboratory confirmation of ZIKV infection, considering the results in serum or CSF (cases) and results in serum alone (controls), overall and separately according to presence of abnormalities in brain imaging of the cases. To deal with the fact that all controls were ZIKV negative, the OR was calculated using the median unbiased estimator for binary data in an unconditional logistic regression model.25,26 This statistical approach is appropriate for zero cells, and applied to our situation in which the sample size for this preliminary analysis is comparatively small and data structure sparse. Another consequence of all controls being Zika negative was that although the design was matched, a conditional, matched analysis was not needed as matched and unmatched analysis will give the same result. We calculated OR adjusted for mother´s age and schooling of mothers (as a proxy of socioeconomic status) for the overall association. Since serum is easier to collect than CSF, we investigated the agreement between RT-PCR and Zika-specific IgM both for serum and CSF and between levels of Zika-specific IgM serum and CSF.
The protocol was approved by the Research Ethics Committee of PAHO (2015-12-0075) and Fiocruz-PE/CONEPE/CAAE (1.380.943).
Role of the funding sources
Interests have been declared in-line with WHO policy and no conflicts of interest identified from any of the contributors. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Results
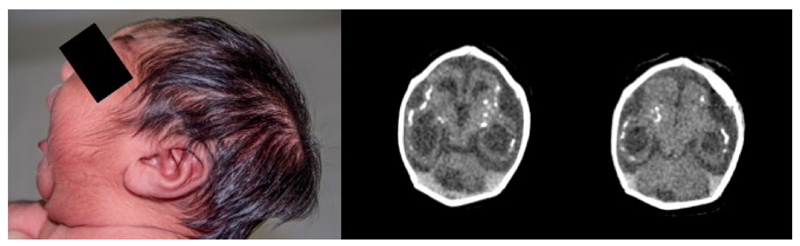
This preliminary analysis included 32 neonates with microcephaly (cases) and 62 neonates without microcephaly (controls). A photograph and cerebral tomography image of one of the cases showed features previously described during the current microcephaly epidemic27 (annex figure 1).
Figure 1. Newborn with microcephaly with confirmed zika laboratory exams and abnormal CT scan.
The neonate shows phenotypical features previously described during the microcephaly epidemic, including craniofacial disproportion, prominent externa occipital protuberance and excessive scalp skin (photo, left). Radiological features found on brain imaging (CT image, right) include reduced volume of cortical brain parenchyma, cortical and subcortical calcifications, simplified gyral pattern and ventriculomegaly.
Two cases had only one control. The participation rate was 100% for cases and 76% for controls. No control was excluded due to birth defects. For five cases, brain imaging was not undertaken: three died in intensive care before CT-scan was conducted, one was a stillborn, and another was in intensive care at the time of this analysis. Those five were included in the analysis for all cases and excluded from the analysis stratified by radiological brain imaging.
Gestational age (GA) was estimated based on antenatal care fetal USG. For cases, in 14 (43·7%) GA was calculated based on USG in the first trimester of pregnancy; 11 (34·4%) in the second, three (9·4%) in third trimester. Only one GA for cases (3·1%) was based on reported last menstrual period (LMP), and three (9·4%) by Capurro method (carried out by study neonatologists). For controls, in 27 (43·5%) GA was calculated based on antenatal care fetal USG in the first trimester, 22 (35·5%) at the second, five (8·1%) at third trimester, five (8·1%) by maternal report of LMP, and three (4·8%) by Capurro method.28
Approximately of 59% of the mothers of cases did not reported rash, versus 74% of controls, but the difference did not reach statistical significance at 5% level. Mothers of cases had less schooling than of controls, but this difference was not statistically significant. Mothers of cases had slightly higher frequency of ZIKV positivity by PRNT50 (80%) than the mothers of controls (64%), but this was not statistically significantly different (table 1). Around 10% of the mothers had ZIKV as a primary Flavivirus infection including the mothers of five cases. Overall 55% of the cases and controls had more than one Flavivirus infection, with DENV-3 and DENV-4 the predominant dengue serotypes (annex table 1). Results of PRNT50 for ZIKV and DENV were similar in mother - neonate pairs (data not shown).
A proportion of 34·4% of the cases had severe microcephaly (head circumference ≤3SD below the mean for sex and gestational age at birth). A higher proportion of cases were born with low birth weight and small for gestational age; these differences were statistically significant. Neonates small for gestational age was very frequent: 84·4% of the cases compared to 6·4% of controls. Of the 27 cases investigated for brain imaging, 11 had imaging with one or more abnormalities: seven had calcifications, five ventriculomegaly, one lissencephaly and six other abnormalities (table 1).
None of the 62 controls tested for RT-PCR in serum were positive; none of the 59 controls tested for Zika-specific IgM in serum were positive; 40·6% of cases tested positive for Zika (by RT-PCR or Zika-specific IgM) in CSF or serum, and 28·1% in serum only (table 2). No cross-reactivity with IgM dengue-specific IgM was observed among ZIKV IgM positive samples.
The overall crude OR for microcephaly and laboratory confirmed Zika infection was 55·5 (95%CI=8·6 to ∞); and very similar when adjusted by mother´s education and by mothers age. The magnitude of the association in cases with radiological brain abnormality at CT-scan was very strong: OR=113·3 (95%CI=14·5 to ∞); in cases with normal brain imaging was still strong and statistically significant (OR=24·7; 95%CI=2·9 to ∞). When only serum results were considered, the magnitude of the crude association was strong OR=31·7 (95%CI=4·7 to ∞), even after adjustment. The magnitude of the association among the cases with radiological brain abnormality at CT scan was very strong, OR=80·9 (95%CI=10·2 to ∞) and in cases with normal brain imaging, still strong but not statistically significant (OR=3·9; 95%CI=0·1 to ∞) (table 3).
Out of 13 ZIKV positive neonates four had normal imaging. Of the six cases whose mothers were seronegative to ZIKV (PRNT50) five neonates were also negative (to Zika specific IgM or RT-PCR in serum or CSF), and one was RT-PCR positive. Annex table 2 presents the characteristics of cases and controls.
There was good agreement between positivity at Zika-specific IgM in serum and CSF (Kappa 0·91, 95%CI=0·74 to 1·00). The proportion of cases that had Zika-specific IgM in CSF was 28% and in serum 25%, and the geometric mean in CSF was 23·62 (95%CI=18·8 to 29·6) and in serum 16·8 (95%CI=12·3 to 23·0). No neonate nor their mothers had toxoplasmosis, rubella and cytomegalovirus specific IgM in serum. A high proportion of mothers of cases and controls had IgG in serum: for toxoplasmosis this was 53% for cases and 44% for controls; for CMV, 87·5% for cases 76% for controls; and for rubella, 62·5% for cases and 74% for controls. These were not statistically significant (data not shown).
Discussion
We found a remarkably strong association between microcephaly and laboratory confirmation of ZIKV infection by RT-PCR or Zika-specific IgM in CSF or serum. The risk was over 50 times higher overall, and over 100 times in cases with abnormal brain imaging but was also present in cases with normal imaging. RT-PCR or Zika-specific IgM were only positive in neonates with microcephaly and negative in serum of all neonates in the control group. More than half of the neonates with microcephaly had normal radiological imaging. There was very good agreement of Zika-specific IgM positivity in serum and in CSF of neonates.
Our study is the first to estimate seroprevalence of ZIKV infection in pregnant women in an epidemic area in Brazil. The high ZIKV PRNT50 seropositivity (63%) among mothers of controls might suggest the high frequency of ZIKV infection in this group in Recife. Similar frequencies of Zika infection in the general population were found in Yap Island29 and in French Polynesia after their outbreaks17. We can not determine with any degree of certainty the timing of the Zika infection, before or during pregnancy in a case-control study. A cohort of pregnant women will be able to assess the timing of the onset of ZIKV infection and relate it to the full spectrum of the adverse outcome of pregnancy.
We found a higher frequency of multitypic Flavivirus infections including ZIKV and DENV-3/DENV-4 serotypes, the predominant DENV profile in the study area.23 We used the best available test authorized by Food and Drug Administration (CDC Zika IgM antibody capture enzyme-linked immunosorbent assay) for antibody testing.30 All IgM ZIKV positive samples were confirmed by PRNT50. This approach is recommended by CDC guidelines31 to rule out false-positive results.
Our study has limitations inherent to a preliminary analysis. The management team decided to perform the analysis mainly because the microcephaly epidemic is considered a public health emergency of international concern. There was a sense of urgency in finding the answer to the main study question, i.e. the association between the ZIKV infection and the microcephaly. Although this sample size had 82% power to demonstrate the association, we are aware that interim analysis can overestimate the strength of an association so the magnitude needs to be treated with some caution. The case-control study will continue to investigate the current and alternative hypotheses as well as the role of cofactors, and provide final estimates.
There was a clinical indication to collect CSF from cases, but for ethical reasons – lack of a clinical indication - no CSF was collected from controls; therefore the association in which laboratory confirmation includes CSF test results if not strictly a fair comparison. However, presence of Zika-specific IgM in CSF indicates an infection in the neural system of the neonate (as IgM does not cross the placenta or the blood-brain barrier and therefore it is produced by the neonate and not by the mother); and given the good concordance between serum and CSF, we consider it highly unlikely that neonates of the control group had Zika-specific IgM in CSF. The prospective recruitment of neonates with samples collected at birth ensures that the presence of Zika specific IgM or RT-PCR results originating from intrauterine, rather than post-natal ZIKV infection.
The striking association between microcephaly and laboratory confirmed ZIKV infection found in our study adds the necessary epidemiological – counterfactual - evidence (given the presence of a rigorously selected control group) - to the process of confirming causality.12,32
We expect one component of the congenital Zika syndrome to be intrauterine growth restriction; we found a very high proportion of neonates who were small for gestational age among cases of microcephaly. Brasil et al reported fetal growth restriction in fetuses of mothers who had Zika during pregnancy.7 In June 2016 WHO recommended the use of Intergrowth-21 Size at Birth Standards for identifying neonates with microcephaly.33 In our study if we had classified the cases using Intergrowth-21 instead of Fenton curve, seven cases would be misclassified, six of them with borderline measurements (two had abnormal brain image, another one was ZIKV IgM positive in the CSF). Therefore, the use of Fenton parameter in our study does not seem to introduce bias. Even if seven neonates were misclassified it would underestimate the strength of the association.
Surprisingly, in our study only seven of 27 cases with CT scan investigation had abnormal brain imaging. This is in contrast with the few series of children with microcephaly published, in which neonates with microcephaly investigated had abnormal brain imaging.19,20,32,34 It is not clear whether neonates with microcephaly and normal brain imaging were excluded.20,32 The association with ZIKV laboratory confirmation was also present in neonates with microcephaly with normal brain imaging, indicating that congenital ZIKV syndrome can be present in neonates with microcephaly and normal brain imaging.
In our results, the detection of Zika-specific IgM (CDC protocol) in neonates with microcephaly appears to be an adequate for the diagnosis of congenital ZIKV (although not for its exclusion). The question of Flavivirus cross-reactivity, particularly for dengue,23,32 may not be relevant in neonates, as intrauterine infection with dengue is unlikely, and maternal IgM do not cross the placenta barrier; this is consistent with a recent study of ZIKV and dengue specific IgM in maternal-neonate with microcephaly pairs where cross-reaction in neonates was not relevant for Zika-specific IgM.35 We suggest that Zika-specific IgM in serum is a useful alternative to in CSF when CSF collection is a challenge.
In view of the association between ZIKV infection and microcephaly with normal radiological brain imaging, it may be worth reconsidering guidance on excluding cases of microcephaly based on normal brain imaging in surveillance and diagnostics of congenital ZIKV.
If the causal link between ZIKV infection during pregnancy and microcephaly is true, ZIKV is the cause of the PHEIC, and we should prepare for the epidemic of microcephaly to expand to all countries with current autochthonous ZIKV transmission and to those where ZIKV transmission is likely to expand to.36,37
We conclude that the microcephaly epidemic is a result of congenital ZIKV infection. We recommend that the list of congenital infections normally referred to as TORCH, to be renamed as TORCHZ, and that we prepare for a global epidemic of microcephaly and other manifestations of Congenital Zika Syndrome.
Acknowledgements
Some of the authors received partial support from the National Advisory Board of Scientific and Technological Development (Conselho Nacional de Desenvolvimento Científico e Tecnológico) - CNPq (scholarship 306708/2014-0 to CMTM, 308818/2013-0 to RAAX, 308590/2013-9 to DBMF, 308491/2013-0 to MFPMA, 304174/2014-9 to CB and 306222/2013-2 to WVS); We are grateful to UPE, UFPE, LSHTM, and FIOCRUZ-PE for freeing the time of Investigators to work in the study (RAAX,TVBA,DBMF, LCR, WVS,MFPMA, CB,CMTM, SV, APLM, ETAM. RD, MTC, and UM). We would like to thank the director and staff of participating hospitals. We thank the mothers for their collaboration and generosity.
Footnotes
Contributors
TVBA, CMTM, LCR, RAAX and DBMR participated in all phases of the study.
All other authors participated in the interpretation of data for the work, revising the work critically, approved the final version, and agree to be accountable for all aspects of the work.
Declaration of interests
We declare no competing interests.
Contributor Information
Thalia Velho Barreto de Araújo, Federal University of Pernambuco, Av. Professor Moraes Rego, 1235 - Cidade Universitária, Recife - PE, 50670-901.
Laura Cunha Rodrigues, Email: Laura.Rodrigues@lshtm.ac.uk, London School of Hygiene & Tropical Medicine Keppel St, London WC1E 7HT, UK.
Ricardo Arraes de Alencar Ximenes, Email: raaximenes@uol.com.br, Federal University of Pernambuco and University of Pernambuco. Av. Professor Moraes Rego, 1235 - Cidade Universitária, Recife - PE, 50670-901.
Demócrito de Barros Miranda-Filho, Email: demofilho@gmail.com, University of Pernambuco, Av. Gov. Agamenon Magalhães, S/N - Santo Amaro, Recife - PE, 50100-010.
Ulisses Ramos Montarroyos, Email: ulisses_montarroyos@yahoo.com.br, University of Pernambuco, Av. Gov. Agamenon Magalhães, S/N - Santo Amaro, Recife - PE, 50100-010.
Ana Paula Lopes de Melo, Email: anap0001@gmail.com, Federal University of Pernambuco, Av. Professor Moraes Rego, 1235 - Cidade Universitária, Recife - PE, 50670-901.
Sandra Valongueiro, Email: svalong@gmail.com, Federal University of Pernambuco, Av. Professor Moraes Rego, 1235 - Cidade Universitária, Recife - PE, 50670-901.
Maria de Fátima Pessoa Militão de Albuquerque, Email: militaofatima@gmail.com, The Research Center Aggeu Magalhães (CPqAM) / Oswaldo Cruz Foundation (Fiocruz), Av. Professor Moraes Rego s/n - Cidade Universitária, Recife - PE, 50740-465.
Wayner Vieira Souza, Email: wayner@cpqam.fiocruz.br, The Research Center Aggeu Magalhães (CPqAM) / Oswaldo Cruz Foundation (Fiocruz). Av. Professor Moraes Rego s/n - Cidade Universitária, Recife - PE, 50740-465.
Cynthia Braga, Email: braga@cpqam.fiocruz.br, The Research Center Aggeu Magalhães (CPqAM) / Oswaldo Cruz Foundation (Fiocruz). Av. Professor Moraes Rego s/n - Cidade Universitária, Recife - PE, 50740-465.
Sinval Pinto Brandão Filho, Email: sinval@cpqam.fiocruz.br, The Research Center Aggeu Magalhães (CPqAM) / Oswaldo Cruz Foundation (Fiocruz). Av. Professor Moraes Rego s/n - Cidade Universitária, Recife - PE, 50740-465.
Marli Tenório Cordeiro, Email: marli.tenorio@gmail.com, The Research Center Aggeu Magalhães (CPqAM) / Oswaldo Cruz Foundation (Fiocruz). Av. Professor Moraes Rego s/n - Cidade Universitária, Recife - PE, 50740-465.
Enrique Vazquez, Email: evazquez@paho.org, Pan American Health Organization (PAHO-WHO) Lote 19 - Avenida das Nações - SEN - Asa Norte, Brasília - DF, 70312-970.
Danielle Di Cavalcanti Souza Cruz, Email: danidicavalcanti@hotmail.com, Instituto Materno Infantil Fernando Figueira R. dos Coelhos, 300 - Boa Vista, Recife - PE, 50070-550.
Cláudio Maierovitch Pessanha Henriques, Email: claudio.henriques@saude.gov.br, Fiocruz Brasília Avenida L3 Norte, S/N - Campus Universitário Darcy Ribeiro, Brasília - DF, 70910-900.
Luciana Caroline Albuquerque Bezerra, Email: lua_cad@yahoo.com.br, Pernambuco State Health Department (SES-PE). Av. Afonso Olindense, 1513 - Várzea, Recife - PE - CEP: 50.810-000.
Priscila Mayrelle da Silva Castanha, Email: castanha.priscila@gmail.com, The Research Center Aggeu Magalhães (CPqAM) / Oswaldo Cruz Foundation (Fiocruz). Av. Professor Moraes Rego s/n - Cidade Universitária, Recife - PE, 50740-465.
Rafael Dhalia, Email: rdhalia@gmail.com, The Research Center Aggeu Magalhães (CPqAM) / Oswaldo Cruz Foundation (Fiocruz). Av. Professor Moraes Rego s/n - Cidade Universitária, Recife - PE, 50740-465.
Ernesto Torres Azevedo Marques-Júnior, Email: marques@pitt.edu, The Research Center Aggeu Magalhães (CPqAM) / Oswaldo Cruz Foundation (Fiocruz); University of Pittsburgh. Pittsburgh, USA. Av. Professor Moraes Rego s/n - Cidade Universitária, Recife - PE, 50740-465.
Celina Maria Turchi Martelli, Email: turchicm@gmail.com, The Research Center Aggeu Magalhães (CPqAM) / Oswaldo Cruz Foundation (Fiocruz). Av. Professor Moraes Rego s/n - Cidade Universitária, Recife - PE, 50740-465.
References
- 1.Brasil Ministério da Saúde. Informe epidemiológico nº32 - Semana Epidemiologica 25/2016 - Monitoramento dos casos de microcefalia no Brasil. 2016. [accessed July 4, 2016]. http://combateaedes.saude.gov.br/images/pdf/informe_microcefalia_epidemiologico_32.pdf .
- 2.Brasil Ministério da Saúde. Informe epidemiológico nº1/2015 - Semana epidemiologica 46 - Monitoramento dos casos de microcefalias no Brasil. 2015. [accessed April 4, 2016]. http://portalsaude.saude.gov.br/images/pdf/2015/novembro/24/COES-Microcefalias---Informe-Epidemiol--gico---SE-46---24nov2015.pdf .
- 3.Ashwal S, Michelson D, Plawner L, Dobyns W. Practice parameter: Evaluation of the child with microcephaly. Neurology. 2009;73:887–97. doi: 10.1212/WNL.0b013e3181b783f7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.WHO. Situation Report : Zika virus, microcephaly, Guillain-Barré syndrome. 2016. Jun 23, [accessed July 5, 2016]. http://apps.who.int/iris/bitstream/10665/246112/1/zikasitrep-23Jun2016-eng.pdf?ua=1 .
- 5.WHO. WHO Director-General summarizes the outcome of the Emergency Committee regarding clusters of microcephaly and Guillain-Barré syndrome. [accessed March 3, 2016]. http://www.who.int/mediacentre/news/statements/2016/emergency-committee-zika-microcephaly/en/# .
- 6.Brito C. Zika Virus: A New Chapter in the History of Medicine. Acta Med Port. 2015;28:679–80. doi: 10.20344/amp.7341. [DOI] [PubMed] [Google Scholar]
- 7.Brasil P, Pereira Jr J, Raja Gabaglia C, et al. Zika Virus Infection in Pregnant Women in Rio de Janeiro — Preliminary Report. N Engl J Med. 2016 doi: 10.1056/NEJMoa1602412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.European Center for Disease Prevention and Control. Rapid Risk Assessment: Zika virus disease epidemic: potential association with microcephaly and Guillain-Barré syndrome (first update) [accessed March 3, 2016]. http://ecdc.europa.eu/en/publications/Publications/rapid-risk-assessment-zika-virus-first-update-jan-2016.pdf .
- 9.PAHO. Epidemiological Alert: Neurological syndrome, congenital malformations, and Zika virus infection. Implications for public health in the Americas. 2015. Dec 1, [accessed March 3, 2016]. http://www.paho.org/hq/index.php?option=com_docman&task=doc_view&Itemid=270&gid=32405&lang=en .
- 10.Calvet G, Aguiar RS, Melo AS, et al. Detection and sequencing of Zika virus from amniotic fluid of fetuses with microcephaly in Brazil: a case study. Lancet Infect Dis. 2016;16:653–60. doi: 10.1016/S1473-3099(16)00095-5. [DOI] [PubMed] [Google Scholar]
- 11.Oliveira Melo AS, Malinger G, Ximenes R, Szejnfeld PO, Alves Sampaio S, Bispo De Filippis AM. Zika virus intrauterine infection causes fetal brain abnormality and microcephaly: Tip of the iceberg? Ultrasound Obstet Gynecol. 2016;47:6–7. doi: 10.1002/uog.15831. [DOI] [PubMed] [Google Scholar]
- 12.Musso D, Baud D. Zika virus: time to move from case reports to case control. Lancet Infect Dis. 2016;16 doi: 10.1016/S1473-3099(16)00096-7. [DOI] [PubMed] [Google Scholar]
- 13.Jouannic J-M, Friszer S, Leparc-Goffart I, Garel C, Eyrolle-Guignot D. Zika virus infection in French Polynesia. Lancet. 2015;387:1051–2. doi: 10.1016/S0140-6736(16)00625-5. [DOI] [PubMed] [Google Scholar]
- 14.Meaney-Delman D, Hills SL, Williams C, Galang RR, Iyengar P, Andrew K. Zika Virus Infection Among U.S. Pregnant Travelers — August 2015 – February 2016. MMWR Morb Mortal Wkly Rep. 2016;65:211–4. doi: 10.15585/mmwr.mm6508e1. [DOI] [PubMed] [Google Scholar]
- 15.Martines RB, Bhatnagar J, Kelly Keating M, Silva-Flannery L. Notes from the field: evidence of Zika virus infection in brain and placental tissues from two congenitally infected newborns and two fetal losses—Brazil, 2015. MMWR Morb Mortal Wkly Rep. 2016;65:159–60. doi: 10.15585/mmwr.mm6506e1. [DOI] [PubMed] [Google Scholar]
- 16.Mlakar J, Korva M, Tul N, et al. Zika Virus Associated with Microcephaly. N Engl J Med. 2016;374:1–8. doi: 10.1056/NEJMoa1600651. [DOI] [PubMed] [Google Scholar]
- 17.Cauchemez S, Besnard M, Bompard P, et al. Association between Zika virus and microcephaly in French Polynesia, 2013–15: a retrospective study. Lancet. 2016;387:2125–32. doi: 10.1016/S0140-6736(16)00651-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rasmussen SA, Jamieson DJ, Honein MA, Petersen LR. Zika Virus and Birth Defects — Reviewing the Evidence for Causality. N Engl J Med. 2016;374:1981–7. doi: 10.1056/NEJMsr1604338. [DOI] [PubMed] [Google Scholar]
- 19.Microcephaly Epidemic Research Group. Microcephaly in Infants, Pernambuco State, Brazil, 2015. Emerg Infect Dis. 2016;22 doi: 10.3201/eid2206.160062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schuler-Faccini L, Ribeiro EM, Feitosa IML, et al. Possible Association Between Zika Virus Infection and Microcephaly — Brazil, 2015. MMWR Morb Mortal Wkly Rep. 2016;65:59–62. doi: 10.15585/mmwr.mm6503e2. [DOI] [PubMed] [Google Scholar]
- 21.Fenton TR, Kim JH. A systematic review and meta-analysis to revise the Fenton growth chart for preterm infants. BMC Pediatr. 2013;13 doi: 10.1186/1471-2431-13-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lanciotti RS, Kosoy OL, Laven JJ, et al. Genetic and serologic properties of Zika virus associated with an epidemic, Yap State, Micronesia, 2007. Emerg Infect Dis. 2008;14:1232–9. doi: 10.3201/eid1408.080287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Castanha PMS, Braga C, Cordeiro MT, et al. Placental transfer of dengue-specific antibodies and kinetics of dengue infection enhancing-activity in Brazilian infants. J Infect Dis. 2016:1–22. doi: 10.1093/infdis/jiw143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Panchaud A, Stojanov M, Ammerdorffer A, Vouga M. Emerging Role of Zika Virus in Adverse Fetal and Neonatal Outcomes. Clin Microbiol Rev. 2016;29:659–94. doi: 10.1128/CMR.00014-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Heinze G, Schemper M. A solution to the problem of separation in logistic regression. Stat Med. 2002;21:2409–19. doi: 10.1002/sim.1047. [DOI] [PubMed] [Google Scholar]
- 26.Hirji KF, Tsiatis AA, Mehta CR. Median Unbiased Estimation for Binary Data. Am Stat. 1989;43:7–11. [Google Scholar]
- 27.Miranda-Filho D, Martelli C, Ximenes R, et al. Initial Description of the Presumed Congenital Zika Syndrome. Am J Public Health. 2016;106:598–600. doi: 10.2105/AJPH.2016.303115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Capurro H, Korichzky S, Fonseca O, Caldeiro-Barcia R. A simplified method for diagnosis of gestational age in the newborn enfant. J Pediatr. 1978;93:120–2. doi: 10.1016/s0022-3476(78)80621-0. [DOI] [PubMed] [Google Scholar]
- 29.Duffy M, Chen T, Hancock T, Powers A, Kool J, Lanciotti R, Pretrick M. Zika Virus Outbreak on Yap Island, Federated States of Micronesia. N Engl J Med. 2009;360:2536–43. doi: 10.1056/NEJMoa0805715. [DOI] [PubMed] [Google Scholar]
- 30.U.S. Food and Drug Administration. Zika Virus Emergency Use Authorization. 2016. [accessed Aug 1, 2016]. http://www.fda.gov/MedicalDevices/Safety/EmergencySituations/ucm161496.htm .
- 31.Rabe IB, Staples JE, Villanueva J, et al. Interim Guidance for Interpretation of Zika Virus Antibody Test Results. MMWR Morb Mortal Wkly Rep. 2016;65:543–6. doi: 10.15585/mmwr.mm6521e1. [DOI] [PubMed] [Google Scholar]
- 32.Petersen LR, Jamieson DJ, Powers AM, Honein MA. Zika Virus. N Engl J Med. 2016;374:1552–63. doi: 10.1056/NEJMra1602113. [DOI] [PubMed] [Google Scholar]
- 33.WHO. Screening, assessment and management of neonates and infants with complications associated with Zika virus exposure in utero. Interim guidance update. [accessed Aug 1, 2016]. http://apps.who.int/iris/bitstream/10665/204475/1/WHO_ZIKV_MOC_16.3_eng.pdf?ua=1 .
- 34.Hazin A, Poretti A, Martelli C, et al. Computed Tomographic Findings in Microcephaly Associated with Zika Virus. N Engl J Med. 2016;374:2193–5. doi: 10.1056/NEJMc1603617. [DOI] [PubMed] [Google Scholar]
- 35.Cordeiro MT, Pena LJ, Brito CA, Gil LH, Marques ET. Positive IgM for Zika virus in the cerebrospinal fluid of 30 neonates with microcephaly in Brazil. Lancet. 2016;387:1811–2. doi: 10.1016/S0140-6736(16)30253-7. [DOI] [PubMed] [Google Scholar]
- 36.Hennessey M, Fischer M, Staples JE. Zika Virus Spreads to New Areas - Region of the Americas, May 2015-January 2016. MMWR Morb Mortal Wkly Rep. 2016;65:1–4. doi: 10.15585/mmwr.mm6503e1. [DOI] [PubMed] [Google Scholar]
- 37.Fauci AS, Morens DM. Zika Virus in the Americas - Yet Another Arbovirus Threat. N Engl J Med. 2016;374:601–4. doi: 10.1056/NEJMp1600297. [DOI] [PubMed] [Google Scholar]