Summary
Background
A study of the cause of the epidemic microcephaly in Brazil, including congenital Zika virus infection and other potential causes.
Methods
Prospective case-control study in Recife/Brazil. Cases: neonates with microcephaly born in public maternities. Controls (without microcephaly) matched by expected date of delivery and area of residence. Laboratory tests: Serum of cases/ controls and cerebrospinal fluid of cases (qRT-PCR and anti-Zika-IgM); tissues of stillbirth cases qRT-PCR). Maternal serum (PRNT for Zika and dengue). The association between microcephaly and ZIKV infection quantified using exact conditional logistic regression.
Findings
We included 91 cases and 173 controls. Congenital ZIKV infection was confirmed in 32/91 cases compared to 0/173 controls. 83%(68/83) of cases were small for gestational age; 5%(8/173) of controls. The overall matched odds ratio (mOR) was 87 (95%CI:15·6-∞), and 73·1(95%CI:13·0-∞) when adjusted. No controls had laboratory confirmed Zika. Neither vaccinations during pregnancy nor larvicide use was associated with microcephaly. mORs in subgroups were: severe microcephay 52·4 (95%CI:9·1-∞); less severe 33·7 (95%CI:5·6-∞); abnormal brain image 32·2 (95%CI:5·3-∞), and normal brain image 29·3 (95%CI:4·8-∞). About half cases (49/91) had either laboratory confirmation of Zika or typical brain imaging abnormalities but only 15% (12/79) had both.
Interpretation
Congenital Zika infection caused the microcephaly epidemic in Brazil. Neither larvicides nor vaccinations during pregnancy increased the risk of microcephaly. Neither negative Zika laboratory results nor normal brain image alone is sufficient to discard Zika as a cause of individual cases of microcephaly.
Source of funding
The study was funded by the Brazilian Ministry of Health and the Pan American Health Organization and AREAS. Some of the investigators received partial support from the National Advisory Board of Scientific and Technological Development (Conselho Nacional de Desenvolvimento Cientifico e Tecnologico [CNPq]; scholarship 306708/2014-0 to CMTM, 308818/2013-0 to RAAX, 308590/2013-9 to DBMF, 308491/2013-0 to MFPMA, 304174/2014-9 to CB, and 306222/2013-2 to WVS). LCR is partially funded by the European Union’s Horizon 2020 research and innovation program under Zika-PLAN (grant agreement No. 734584).
Introduction
At the start of the epidemic of microcephaly in Brazil, the main causal hypothesis was Zika infection during pregnancy,1 but other potential causes were proposed, of particular interest, because of the potential implications, use of larvicides in drinking water reservoirs to combat Aedes aegypti and vaccinations during pregnancy.2,3 There has been no investigation of use of larvicide or of vaccines during pregnancy at individual level, although an ecological study found no area level association between the use of larvicide pyriproxyfen and prevalence of microcephaly.3 The evidence for microcephaly as a manifestation of the Congenital Zika Syndrome (CZS) consolidated, and included the preliminary results of a case control study commissioned at the start of the epidemic the Brazilian Ministry of Health to investigate the causes of the epidemic of microcephaly.4 This is the final report of this case control study, presenting the result of the investigation not only of Zika infection but also the other factors suggested as potential causes.
Methods
Study design and participants
The final analysis of a prospective case-control study, conducted to investigate the cause of the microcephaly epidemic in Pernambuco State, Brazil. A preliminary analysis reported on subjects recruited from Jan 15 to May 2, 2016; this final analysis includes subjects recruited up to Nov 30, 2016. The paper reporting the preliminary analysis it includes detailed information on methods.4 We conducted this final analysis before recruting the 200 cases originally proposed for two reasons: first, we reached the necessary power, because the proportion of controls exposed was lower than expected when defining the necessary sample size; second, the epidemic slowed down in Recife and cases became rarer.
Study population consisted of neonates born to women resident in Pernambuco and delivered in eight public maternity. Cases - neonates with microcephaly (liveborn or stillbirth) – had head circumference at least 2 SD smaller than the mean for sex and gestational age in the Fenton growth chart.5 Microcephaly was considered severe when the head circumference at least 3SD smaller than the mean. Exclusion criteria were anencephaly, encephalocele, and phenotype of well-defined congenital syndrome. Controls - live neonates without microcephaly – with no brain abnormalities (at transfontanellar ultrasonography) and no major birth defects at physical examination. Two controls per case were selected in the study hospitals, matched by health region of residence and expected date of delivery (to ensure cases and controls were conceived at the same stage of the epidemic).
Procedures
Details of the procedures for estimating gestational age, to measure head circumference, for radiological brain imaging, to collect blood sample of mothers and neonates (case and controls) at birth, and cerebrospinal fluid for cases, were described elsewhere.4 Serum samples were tested for toxoplasmosis, rubella, and cytomegalovirus IgM antibodies, the main infectious causes of congenital microcephaly.6
The detailed laboratory procedures were described elsewhere.4 Sera of mothers and neonates (cases and controls) and cerebrospinal fluid samples (of cases) were tested by quantitative real time polymerase chain reaction (qRT-PCR) for the detection of Zika virus genome,7 and with capture-IgM enzyme-linked immunosorbent assay (ELISA) for IgM antibodies,8 respectively. Macerated tissues (brain, kidney or pooled organs) of stillbirths cases were tested by qRT-PCR. The presence of ZIKV and DENV (1-4) specific neutralizing antibodies were assessed in sera of mothers and neonates (cases and controls) by Plaque Reduction Neutralization test (PRNT50), with a 50% cut-off value for positivity.
Brain imaging was done by CTscan in cases and transfontanellar ultrasonography in controls and classified as normal or abnormal (including calcification, ventriculomegaly, lissencephaly, and other abnormalities) by physicians specialized in image diagnosis. Mothers were interviewed in the hospital by a trained female nurse, using a structured standardized questionnaire after signing an informed consent form.
Variables: Laboratory-confirmed ZIKV-infection was defined in a neonate as a positive qRT-PCR and/ or IgM result for ZIKV in any biological specimen (serum, CSF or macerated tissues); Neonates were considered to be small for gestational age (SGA) if birth weight was lower than the 10th percentile for gestational age and sex in the Fenton growth chart.
Information was collected on demographic and socio economic factors included: mothers’ age; mother's years of schooling; skin color (self-referenced and categorized nonwhite or white). The purchasing power of individuals and families was defined using the Brazilian Economic Classification Criteria – CCEB 2015,9 which defines classes from A (highest socioeconomic level) to E (lowest socioeconomic level), considering the education level of the head of the household, household assets (i.e. car, washing machine, etc.), presence and number of domestic servants, source of the water used in the household and whether the street in which the house is located is paved. We also collected data on family history of microcephaly or malformations; vaccination, self-report misoprostol ingestion (medical abortion pill), epilepsy treatment or folic acid; and use of recreation drugs, tobacco and alcohol in pregnancy; and on exposure to chemical larvicide (including any domestic water reservoir) and use of insect repellent on skin using structured interview. Vaccination cards were consulted, when available, we only considered vaccination during pregnancy.
Statistical analysis
The outcome was microcephaly and the main exposure of interest was ZIKV infection, defined by laboratory confirmation. We estimated the crude mOR and 95% CI for the association between microcephaly and laboratory confirmation of ZIKV infection in neonates using a median unbiased estimator for binary data (exact conditional logistic regression) for all cases, considering the results in any specimen (serum or cerebrospinal fluid for livebirth or macerated tissues for the stillbirth). Additionally, crude odds ratio were estimated, separately, for cases with and without radiological evidence of brain abnormalities, by sample type (serum or CSF), and microcephaly severity. Since no controls were laboratory positive to Zika, the mORs would be infinity in a traditional logistic regression. The conditional exact model was used to provide an estimate of mOR, taking into account the sample size.10 It respects the matching and allows for controlling variables for which the estimate is not of interest - conditioning variables – “condvars”.11
We investigated the association between microcephaly and other factors, one by one, by conditional logistic regression. The variables associated with microcephaly with a p≤0,10 were taken to multivariate analysis using conditional exact logistic regression model. Thus, we calculated mOR for the association between microcephaly and ZIKV adjusted by smoking during pregnancy, receiving tetanus, diphtheria, and acellular pertussis vaccine (Tdap), recently introduced in Brazil, during pregnancy and skin color. We investigated the agreement between qRT-PCR ZIKV-positivity in serum and cerebrospinal fluid (CSF); and the agreement between the IgM positivity in serum and CSF. Stata version 14.1 software was used for the statistical analyses.
The study was approved by the research ethics committees of the Pan American Health Organization (protocol number PAHO-2015-12-0075) and Fiocruz Pernambuco (protocol number CAAE: 51849215.9.0000.5190). All mothers provided written informed consent to participate in the study.
Role of the funding source
The funders of the study were involved in the data interpretation and writing of the report. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Results
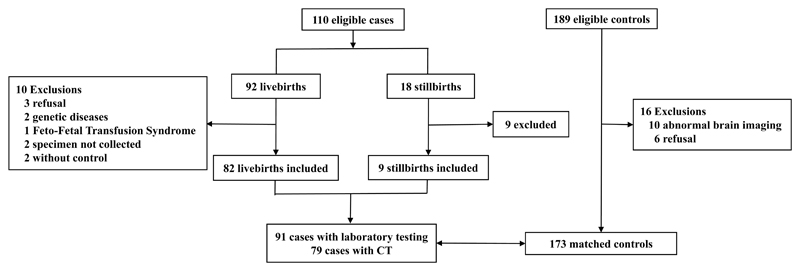
The flowchart describes the cases and controls screened (Figure1). There were 110 eligible cases: 92 livebirths and 18 stillbirths. There were ten exclusions among livebirths: three refusals; two cases were excluded because they had a genetic cause for the microcephaly; one was excluded because of Feto-Fetal Transfusion Syndrome; two because specimens could not be collected and two because a matched control could not be found. Among the 18 eligible stillbirths, nine were excluded: five were not necropsied in time and four had no laboratory specimens collected. So, this analysis included 91 cases: 82 livebirths and nine stillbirths. Of 189 eligible controls, there were 16 exclusions: ten due to abnormal brain imaging (one ventriculomegaly, one with calcification, two with hydrocephaly and six with other abnormalities) and six refusals. Our final analyses included 91 cases of microcephaly and 173 controls.
Figure 1.
Flow chart of participants of the case-control study of microcephaly and Zika virus infection in pregnancy. Pernambuco - Brazil, 2016.
Cases were more frequently than controls, female, small for gestational age (SGA), and premature. Around forty percent of cases had abnormal brain imaging. 29% (26/91) of cases had severe microcephaly. There was no difference in age or schooling of mothers of cases and controls. Mothers of cases were more likely to have serological markers of previous ZIKA infection by PRNT50 (70%) than the mothers of controls (57%) (p=0·05) (Table1). Interestingly, of 28 negative PRNT50 mothers of cases, 6 cases were Zika IgM seropositive and 8 had abnormal image (data not shown).
Table 1. Characteristics of mothers and neonates.
| Cases (n=91) |
Controls (n=173) |
p value | |
|---|---|---|---|
| Mothers | |||
| Age, years | |||
| 13-24 | 44 (48%) | 95 (55%) | 0·11 |
| 25-34 | 29 (32%) | 60 (35%) | |
| ≥35 | 18 (20%) | 18 (10%) | |
| Number of years in education | |||
| <4 | 17 (19%) | 20 (11%) | 0·13 |
| 5-9 | 36 (40%) | 60 (35%) | |
| 10-12 | 33 (36%) | 87 (50%) | |
| ≥ 13, higher education | 5 (5%) | 6 (4%) | |
| Reported rash during pregnancy | |||
| No rash | 66 (73%) | 139 (80%) | 0·10 |
| First trimester | 7 (8%) | 10 (6%) | |
| Second trimester | 13 (14%) | 10 (6%) | |
| Third trimester | 5 (5%) | 14 (8%) | |
| Specific antibodies, PRNT50 | |||
| Zika virus positive | 62 (70%) | 99 (57%) | 0·05 |
| Zika virus negative | 27 (30%) | 74 (43%) | |
| Not done | 2 | 0 | |
| Neonates | |||
| Sex | |||
| Girls | 61 (67%) | 84 (49%) | <0·01 |
| Boys | 29 (32%) | 89 (51%) | |
| Intersex | 1 (1%) | 0 | |
| Gestational age | |||
| Term (≥ 37 weeks) | 66 (72%) | 153 (88%) | <0·01 |
| Premature (≤36 weeks) | 25 (28%) | 20 (12%) | |
| Birthweight, g | |||
| ≥2500 | 21 (23%) | 159 (92%) | <0·01 |
| 1500-2499 | 52 (57%) | 14 (8%) | |
| <1500 | 18 (20%) | 0 | |
| Weight for gestational age | |||
| Normal | 14 (17%) | 165 (95%) | < 0·01 |
| Small for gestational age | 69 (83%) | 8 (5%) | |
| Not done† | 8 | 0 |
Data are n (%). PRNT50=plaque reduction neutralization test.
Not available in 8 stillbirths.
One third of cases were laboratory confirmed; confirmation in CSF was more frequently detected than in serum, and more cases were confirmed by qRT-PCR than by IgM. There was a good agreement between Zika IgM positivity in CSF and in serum (Kappa= 0·94, 95% CI 0·82-1·00).(Table2) No neonate tested IgM positive for cytomegalovirus, toxoplasmosis or rubella (data not showed). Of the nine stillbirths, seven were laboratory positive for ZIKV, and six had severe microcephaly. There were three neonatal deaths and all died in intensive care unit; CTscan imaging was not performed. Two were laboratory positive for ZIKV and severe microcephaly; one was laboratory negative for ZIKV (data not shown).
Table 2. Cases by laboratory confirmation by qRT-PCR or Zika specific IgM in cerebrospinal fluid, serum samples or tissue macerate.
| qRT-PCR Positive/Total (%) |
Zika Virus-specific IgM Positive/Total (%) |
Both Tests Positive/Total (%) |
|
|---|---|---|---|
| Cerebrospinal Fluid | 17/70 (24%) | 10/70 (14%) | 25/71 (35%) |
| Serum | 1/78 (1%) | 9/79 (11%) | 10/79 (13%) |
| Tissue macerate | 7/9 (78%) | - | - |
Overall ZIKV positive by any specimen: 32/91 (35%).
CSF or Serum not collected for 9 stillbirths, 2 neonatal deaths and 11 for other reasons.
Of the 79 cases with brain image and laboratory tests performed, 70% (39/56) of cases who tested negative for ZIKV had a normal brain image. 52% (12/23) of cases with laboratory confirmation of Zika had an abnormal brain image and 41% (12/29) of cases with abnormal brain image had laboratory confirmation of Zika. A third (17/56) of cases with negative laboratory tests had abnormal brain image. (Table 3)
Table 3. Cases* by laboratory confirmation of Zika infection (qRT-PCR or specific IgM) and brain imaging findings.
| Laboratory confirmation | |||
|---|---|---|---|
|
| |||
| ZIKV positive | ZIKV negative | p value | |
| Brain imaging | |||
| Abnormal | 12/23 (52%) | 17/56 (30%) | 0·068 |
| Normal | 11/23 (48%) | 39/56 (70%) | Chi2 =3·34 |
Only for cases with brain imaging and laboratory results.
Most mothers of cases and controls lived in poverty; around half were classified in the two bottom levels of the socioeconomic scale. There was no association between skin color and socioeconomic condition (data not shown). Of 18 factors investigated, only two factors were associated with microcephaly (p<0·05) on the conditional analysis: Smoking (OR 3·2; 95% CI: 1·5-7·0; p=0·004); Being non white (OR 0·3, 95%CI 0·1-0·7; p=0·01); Having received Tdap in pregnancy (OR 0·6, 95%CI 0·3-1·0; p=0·06) (Table 4). Only two mothers of controls and no mothers of cases reported having taken misoprostol in pregnancy. Of particular interest, there was no increase in risk of microcephaly with MMR (measles/rubella/mumps) or MR (measles/rubella) vaccines. When controlling for laboratory confirmation of Zika, the association between microcephaly and smoking, not being white and Tdap vaccine lost significance (p-values between 0·07 and 0·10).
Table 4. Association between microcephaly and co-factors investigated.
| Case (n=91) |
Control (n=173) |
OR | 95% CI | p value | |
|---|---|---|---|---|---|
| Mother race/skin color | |||||
| Not White | 84 (92%) | 141 (82%) | 1·0 | - | - |
| White | 7 (8%) | 32 (19%) | 0·3 | 0·1 – 0·7 | 0·01 |
| Family per capita income (US Dollar) | |||||
| ≤ 56·0 | 24 (29%) | 43 (25%) | 1·0 | - | - |
| 56·0 – 96·9 | 22 (26%) | 39 (23%) | 1·0 | 0·5 – 2·1 | 0·97 |
| 97·0 – 168·6 | 19 (23%) | 43 (25%) | 0·8 | 0·4 – 1·7 | 0·63 |
| > 168·6 | 19 (23%) | 44 (26%) | 0·8 | 0·4 – 1·6 | 0·47 |
| Ignored | 7 | 4 | |||
| Economic Class (ABEP) | |||||
| D-E | 52 (57%) | 83 (48%) | 1·0 | - | - |
| C2 | 28 (31%) | 60 (35%) | 0·7 | 0·4 – 1·3 | 0·26 |
| B2-C1 | 11 (12%) | 30 (17%) | 0·6 | 0·3 – 1·3 | 0·17 |
| Siblings with malformation (included microcephaly) |
|||||
| Not | 53 (96%) | 89 (98%) | 1.0 | ||
| Yes | 2 (4%) | 2 (2%) | 1·7 | 0·2 – 16·4 | 0·62 |
| Had no siblings | 36 | 82 | |||
| Parents familiar history of microcephaly or other malformation |
|||||
| Not | 65 (71%) | 124 (72%) | 1·0 | - | - |
| Yes | 26 (29%) | 49 (28%) | 1·0 | 0·6 – 1·8 | 0·96 |
| Mother use of folic acid in pregnancy (self-reported) | |||||
| Yes, regularly | 56 (63%) | 120 (69%) | 1·0 | - | - |
| Yes, eventually | 15 (17%) | 18 (10%) | 1·7 | 0·8 – 3·4 | 0·18 |
| Not | 18 (20%) | 35 (20%) | 1·1 | 0·6 – 2·1 | 0·79 |
| Not known | 2 | 0 | - | - | - |
| Mother use of medication for epilepsy (self-reported) | |||||
| Not | 87 (96%) | 164 (95%) | 1·0 | - | - |
| Yes | 4 (4%) | 9 (5%) | 0·8 | 0·3 - 2·5 | 0·77 |
| Vaccines | |||||
| Tdap | |||||
| Not | 34 (43%) | 45 (30%) | 1·0 | - | - |
| Yes | 45 (57%) | 107 (70%) | 0·6 | 0·3 – 1·0 | 0·06 |
| Not known | 12 | 21 | |||
| MR (Measles/rubella) | |||||
| Not | 69 (96%) | 130 (96%) | 1·0 | - | - |
| Yes | 3 (4%) | 6 (4%) | 0·9 | 0·2 – 3·3 | 0·90 |
| Not known | 19 | 37 | |||
| MMR (measles/rubella/mumps) | |||||
| Not | 69 (96%) | 131 (96%) | 1·0 | - | - |
| Yes | 3 (4%) | 5 (4%) | 1·1 | 0·3 – 5·0 | 0·88 |
| Not known | 19 | 37 | |||
| Mothers risk behavior in pregnancy | |||||
| Smoking | |||||
| No | 73 (80%) | 161 (93%) | 1·0 | ||
| Yes | 18 (20%) | 12 (7%) | 3·2 | 1·5 – 7·0 | <0·01 |
| Drink alcohol | |||||
| Not | 75 (82%) | 151 (87%) | 1·0 | - | - |
| Yes | 16 (18%) | 22 (13%) | 1·6 | 0·8– 3·3 | 0·21 |
| Recreational drugs | |||||
| Not | 88 (97%) | 172 (99%) | 1·0 | - | - |
| Yes | 3 (3%) | 1 (1%) | 5·2 | 0·5 – 50·3 | 0·16 |
| Exposure to other substances | |||||
| Larvicides at the water storage site | |||||
| Not | 42 (46%) | 81 (47%) | 1·0 | - | - |
| Yes | 49 (54%) | 92 (53%) | 1·0 | 0·6 – 1·8 | 0·89 |
| Larvicida elsewhere in the house | |||||
| Not | 77 (85%) | 151 (87%) | 1·0 | - | - |
| Yes | 14 (15%) | 22 (13%) | 1·3 | 0·6 – 2·8 | 0·45 |
| Body Repellent | |||||
| Not | 82 (90%) | 160 (92%) | 1·0 | - | - |
| Yes. Daily | 9 (10%) | 13 (8%) | 0·9 | 0·4 – 2·0 | 0·83 |
| Exposure to pesticides at work | |||||
| Not | 87 (96%) | 169 (98%) | 1·0 | - | - |
| Yes | 4 (4%) | 4 (2%) | 1·9 | 0·5 – 7·7 | 0·39 |
We further explored the association of reported smoking and skin color with economic class. Smoking was more common among the poorest (2% in B2-C1, 3% in C2, 19% in D-E), both between the cases and controls, but the proportion of reported smoking during pregnancy in the D-E category was higher among cases (29%) than controls (13%). Skin color was not associated with economic class (p=0.51).
The matched association between microcephaly and laboratory confirmation of ZIKV infection was extremely strong (mOR 87); no controls had laboratory confirmed ZIKV infection. The association remained strong (mOR 73·1) and significant when adjusted by confounders (smoking during pregnancy, receiving Tdap during pregnancy and skin color). By sub-groups: severe cases (mOR 52·4); less severe cases (mOR 33·7); abnormal brain imaging (mOR 32·2), and normal brain imaging (mOR 29·3). (Table 5)We would like to point out that the smaller mOR estimated in the subgroups do not indicate weaker association and is a function of numbers. The interpretation is more complex because we used exact methods to avoid mORs of infinity.
Table 5. Association between microcephaly and laboratory confirmation of Zika virus infection.
| Cases Positive/Total |
Controls Positive/Matched |
Odds ratio 95% CI) |
|
|---|---|---|---|
| Serum or cerebrospinal fluid samples or macerated tissue* | |||
| All cases | 32/91 (35%) | 0/173 | 87·0 (15·6 to ∞ ) |
| All cases adjusted† | - | - | 73·1 (13·0 to ∞ ) |
| Cases categorized by severity‡ | |||
| Severe (<-3SD) | 19/26 (73%) | 0/51 | 52·4 (9·1 to ∞ ) |
| Not (between -2SD and lower than -3SD) | 13/65 (20%) | 0/122 | 33·7 (5·6 to ∞ ) |
| Cases categorized by brain imaging findings‡ | |||
| Abnormal | 12/29 (41%) | 0/53 | 32·2 (5·3 to ∞ ) |
| Normal | 11/50 (22%) | 0/97 | 29·3 (4·8 to ∞ ) |
Positive at qRT-PCR or Zika specific IgM.
ORs adjusted by smoking during pregnancy, acellular pertussis vaccine (Tdap) during pregnancy and skin color.
ORs are crude in subgroup analysis due to small numbers.
Discussion
The association between microcephaly and Zika laboratory confirmation by qRT-PCR and/or IgM was strong after controlling for confounders. The association was stronger with severe and no severe microcephaly, in cases with and without brain abnormalities by imaging. None of the risk factors investigated (other than Zika) was associated with cases in multivariate analysis; these include use of the larvicide pyriproxyfen and vaccines during pregnancy. We confirm our preliminary analysis that the microcephalic epidemic in the Northeast of Brazil was caused by congenital Zika infection.1
The study has limitations, and some aspects of interpretation need to be clear: The study investigated the causes of the microcephaly epidemic, and cases had microcephaly. Inevitably, the very few cases that would have been born with microcephaly in the absence of a Zika epidemic would have been recruited in the study. Some cases in the study might not have been caused by CZS but these would be few: the pre-epidemic level In the Northeast was 1·65 per 10,000 livebirths,12 corresponding to 1·5 cases a year. Exclusion of any case would have increased the OR for the association. Because we only included microcephaly cases, conclusions are not necessarily generalizable to the full spectrum of CZS.
We used CT scan not MRI to investigate brain images, because of the risk to neonates of sedation and additional radiation. It is possible that a few more cases will have shown alterations had we used MRI. We investigated the brain image of controls using USG for controls and CT for cases, for ethical reasons, as there was no justification for exposing healthy babies to radiation. It is possible that we might have missed small calcifications in some controls. We collected CSF of cases but not of controls, and might have missed laboratory confirmation of infection for controls. Nine stillbirths were not included in the analysis as they had not biologic material collected. But the proportion positive was higher than in livebirths (7/9 or 77·8%) so this exclusion could only had reduced the strength of the association.
The timing of the maternal infection indicated by neutralization cannot be identified in a case-control study and therefore not interpretable as relates to the association investigated. The information is however useful as the presence of typical CZS microcephaly in neonates of negative maternal PRNT50 shows limitation of maternal serology. A case control study, by its nature, can not estimate the risk of CZS. The risk, and the full spectrum, and any effect of previous dengue exposure will only be provided by the ongoing cohorts of pregnant women with Zika.
At the end of the case-control study the proportion of laboratory confirmed case was similar to the published preliminary results.4 Even increasing the number of controls from 62 to 173, none was positive. The magnitude of the mOR remained extremely strong and asymptotically infinite. The mOR point estimate was higher in the final analysis (mOR 87) since the fact that no control had a laboratory confirmation of Zika even with increased numbers decreased the probability of having missed a positive control. Our study found a very high proportion of cases were small for gestational age (SGA). A cohort study of mothers who had Zika during pregnancy reported, four neonates born with microcephaly, of which three were SGA. four neonates born with microcephaly, of which three were SGA.1 We expect one component of the congenital Zika syndrome to be intrauterine growth restriction.
In our case-control study of 189 recruited controls (normal head circumference) ten (5%) were excluded by abnormal brain imaging, according to the protocol; it is possible that one or more of these had congenital Zika Syndrome since microcephaly is only a part of the whole spectrum.1,13
Consistently with the preliminary analysis, around 52% of Zika laboratory confirmed cases had abnormal brain imaging; and 41% of cases with abnormal brain image had laboratory confirmation. The initial descriptions of children with microcephaly during the early days of the epidemic showed abnormal brain imaging for all cases but this may have result from abnormal imaging being part of the inclusion criteria in the first case series.14,15 Although one typical phenotype of Zika microcephaly has been described,16 it is accepted that not all cases of CZS with microcephaly will have that phenotype, and that the spectrum of CZS is not restricted to Microcephaly.1,13,17 An early description of the spectrum of abnormalities found microcephaly with normal imaging and abnormal image without microcephaly.1 An important finding is that microcephaly with congenital ZIKV syndrome can be present with normal brain imaging and that cases with typical brain image can be laboratory negative. The low proportion of neonates with laboratory confirmation is not surprising: Zika qRT-PCR is very specific but less sensitive, especially if the virus has disappeared from serum at the end of pregnancy. IgM is more sensitive as it is a marker of recent infection, only synthesized by the fetus; however, the duration of persistence is unknown and might have also disappeared at birth.18
Our finding of a higher ZIKV positivity in CSF then in serum (both qRT-PCR and capture-IgM ELISA) highlights the relevance of this biological specimen to confirm the diagnosis of ZIKV infection in neonates with microcephaly. However, the good agreement between ZIKV positive IgM in CSF and serum observed places IgM in serum as an alternative to the diagnosis. The qRT-PCR still positive in neonates suggested either the infection occurred late in pregnancy or the persistence of the virus for a long period.19 It appears that ZIKV might persist in CSF for longer than in serum.
Our study confirms the ZIKV PRNT50 seropositivity (63%) among mothers of controls found in the preliminary analysis – which represents the population- indicating that by December 2016 a large part of the population of Recife had been exposed to ZIKV infection. Similar frequencies were observed in Yap Island,20 and in the French Polynesia after their outbreaks of ZIKV.21 Population-based sero-surveys are needed to better estimate the stage of the epidemic in different parts of Brazil, and in different countries. Mothers of cases and controls were mostly young and immune to multi-types Flavivirus infections, mainly to the combination of ZIKV, DENV3 and DENV4. This maternal DENV immune reflects the epidemiology of DENV circulation in the area in the last 30 years investigation of the effect of previous dengue.
The similarity of socio economic conditions between cases and controls is not surprising because they were matched by area of residence.22 This procedure was based on a rapid survey of the Aedes aegypti infestation (Levantamento Rapido de Indice para Aedes aegypti–LIRAa), performed by the National Dengue Control Program to estimate dengue vector densities. In addition, the study only included women delivering in the public health system. Overall the surveillance of microcephaly data from National Information System on Live Births (SINASC) indicated that the majority of the microcephaly were from low socioeconomic status.12
At the beginning of the microcephaly epidemic the hypothesis was raised that the microcephaly was due to larvicides added to water domestic reservoirs for vector control,2,3 common in areas of water shortage (a new larvicide, Pyriproxyfen was introduced in 2014, by the Brazilian Ministry of Health),2,23 and vaccines during pregnancy,23 (the microcephaly overlapped followed the introduction of Tdap to pregnant women to reduce the risk of pertussis in the first months of life). Our results provides evidence rejecting both hypothesis, confirming the findings of an ecological study in the Pernambuco State3 and previous studies on the safety of Tdap vaccine administration during pregnancy.24,25
The association between reported smoking during pregnancy and microcephaly was explained by confounding as smoking was associated with poverty and poverty with Zika. Smoking causes other adverse perinatal outcomes (such as stillbirth, preterm birth, low birth weight and SGA),26 including other birth defects, none related to microcephaly.27
In our data skin color was not statistically associated with socioeconomic conditions, although this association is well documented28 probably to relatively homogeneous conditions of the study population. Areas of low socioeconomic conditions suffer more environmental degradation and favorable conditions for mosquito breeding and, consequently, infection transmission.
The study population was restricted to babies delivered in maternity hospitals of the Public Health System (SUS), however approximately 62% of the total number of births in Recife occur in the SUS, according to the National Information System on Live Births (SINASC).29 In the state of Pernambuco, in 2014, the proportion of deliveries in hospital was 99%.29
Although neonates were examined at birth, because of the nature of the case-control design, information on exposures during gestational period are reported by the mother. Therefore, presence of rash, timing of the rash, other infections during pregnancy as well as exposure to the other potential risk factors such as larvicides may be subject to recall bias. Cohorts of pregnant women currently being conducted (ZIKA-Plan and others) will be able to assess the timing of the onset of ZIKV infection, whether co-factors increase the risk of microcephaly and describe the full spectrum of the adverse outcome of pregnancy.
The recruitment of neonates and collection of samples at birth ensures that laboratory confirmation resulted from intrauterine, rather than post-natal, ZIKV infection. We used the best available assays for recent Zika virus infection, however at birth neonates and mothers may not have detectable viral RNA or IgM antibodies.
In conclusion, our data shows evidence that microcephaly epidemic is a result of Zika virus infection during pregnancy. No other potential risk factors were implicated with the microcephalic cases. Neither a negative brain image or a negative laboratory result alone is grounds for discarding Zika as a cause of individual cases of microcephaly.
Supplementary Material
Acknowledgments
We are grateful to the University of Pernambuco, the Federal University of Pernambuco, the London School of Hygiene & Tropical Medicine, and Fiocruz Pernambuco for giving the investigators time to work in the study (RAAX, TVBA, DBM-F, LCR, CMTM, WVS, MFPMA, CB, SV, APLM, ETAM-J, RD, MTC, and URM). We thank the director and staff of the participating hospitals. We thank the mothers for their collaboration and generosity.
Footnotes
Contributors
TVBA, CMTM, LCR, RAAX, and DBM-F participated in all phases of the study. All other authors participated in data interpretation and critical revision of the manuscript. All authors approved the final version and agree to be accountable for all aspects of the work.
Declaration of interests
We declare no competing interests.
Contributor Information
Thália V. Barreto de Araújo, Department of Social Medicine, Federal University of Pernambuco, Recife, Brazil
Professor Ricardo A. A. Ximenes, Department of Tropical Medicine, Federal University of Pernambuco, Recife, Brazil; University of Pernambuco, Recife, Brazil.
Demócrito B Miranda-Filho, Department of Tropical Medicine, University of Pernambuco, Recife, Brazil.
Wayner V Souza, The Research Center Aggeu Magalhães (CPqAM) and Oswaldo Cruz Foundation (Fiocruz), Recife, Brazil.
Ulisses R Montarroyos, University of Pernambuco, Recife, Brazil.
Ana Paula L Melo, Federal University of Pernambuco, Recife, Brazil; Department of Community Health, Vitória de Santo Antão, Brazil.
Sandra Valongueiro, Department of Social Medicine, Federal University of Pernambuco, Recife, Brazil.
Maria de Fátima M Albuquerque, The Research Center Aggeu Magalhães (CPqAM) and Oswaldo Cruz Foundation (Fiocruz), Recife, Brazil.
Cynthia Braga, The Research Center Aggeu Magalhães (CPqAM) and Oswaldo Cruz Foundation (Fiocruz), Recife, Brazil.
Sinval P Brandao, The Research Center Aggeu Magalhães (CPqAM) and Oswaldo Cruz Foundation (Fiocruz), Recife, Brazil.
Marli T Cordeiro, The Research Center Aggeu Magalhães (CPqAM) and Oswaldo Cruz Foundation (Fiocruz), Recife, Brazil.
Enrique Vazquez, Pan American Health Organization, Brasília, Brazil.
Danielle C Cruz, Instituto Materno Infantil Fernando Figueira, Recife, Brazil.
Claudio M Henriques, Fiocruz Brasília, Brasília, Brazil.
Luciana C A Bezerra, Executive Secretariat of Health Surveillance. Pernambuco State Health Department, Recife, Brazil.
Priscila M Castanha, The Research Center Aggeu Magalhães (CPqAM) and Oswaldo Cruz Foundation (Fiocruz), Recife, Brazil.
Rafael Dhalia, The Research Center Aggeu Magalhães (CPqAM) and Oswaldo Cruz Foundation (Fiocruz), Recife, Brazil.
Ernesto T Marques-Junior, The Research Center Aggeu Magalhães (CPqAM) and Oswaldo Cruz Foundation (Fiocruz), Recife, Brazil.
Professor Celina M Martelli, The Research Center Aggeu Magalhães (CPqAM) and Oswaldo Cruz Foundation (Fiocruz), Recife, Brazil.
Professor Laura C Rodrigues, Department of Infectious Disease Epidemiology, London School of Hygiene & Tropical Medicine, London, UK.
References
- 1.Brasil P, Pereira JP, Moreira ME, et al. Zika Virus Infection in Pregnant Women in Rio de Janeiro. Massachusetts Medical Society. 2016 doi: 10.1056/NEJMoa1602412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Evans D, Nijhout F, Parens R, Morales AJ, Bar-Yam Y. A Possible Link Between Pyriproxyfen and Microcephaly. bioRxiv. 2016 doi: 10.1371/currents.outbreaks.5afb0bfb8cf31d9a4baba7b19b4edbac. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.de Albuquerque MDFMP, De Souza WV, Da Cruz A, et al. Pyriproxyfen and the microcephaly epidemic in Brazil - an ecological approach to explore the hypothesis of their association. Mem Inst Oswaldo Cruz. 2016;111:1–3. doi: 10.1590/0074-02760160291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Araujo TVB, Rodrigues LC, de Alencar Ximenes RA, et al. Association between Zika virus infection and microcephaly in Brazil, January to May, 2016: Preliminary report of a case-control study. Lancet Infect Dis. 2016;16:1356–63. doi: 10.1016/S1473-3099(16)30318-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fenton TR, Kim J. A systematic review and meta-analysis to revise the Fenton growth chart for preterm infants. BMC Pediatr. 2013;13 doi: 10.1186/1471-2431-13-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Panchaud A, Stojanov M, Ammerdorffer A, Vouga M, Baud D. Emerging role of Zika virus in adverse fetal and neonatal outcomes. Clin Microbiol Rev. 2016;29:659–94. doi: 10.1128/CMR.00014-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lanciotti RS, Kosoy OL, Laven JJ, et al. Genetic and serologic properties of Zika virus associated with an epidemic, Yap State, Micronesia, 2007. Emerg Infect Dis. 2008;14:1232–9. doi: 10.3201/eid1408.080287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cordeiro MT, Pena LJ, Brito CA, Gil LH, Marques ET. Positive IgM for Zika virus in the cerebrospinal fluid of 30 neonates with microcephaly in Brazil. Lancet. 2016;387:1811–2. doi: 10.1016/S0140-6736(16)30253-7. [DOI] [PubMed] [Google Scholar]
- 9.Associação Brasileira de Empresas de Pesquisa (ABEP) Critério Brasil. 2015. [accessed April 6, 2017]. http://www.abep.org/criterio-brasil .
- 10.Hirji KF, Tsiatis AA, Mehta CR. Median unbiased estimation for binary data. Am Stat. 1989;43:7–11. [Google Scholar]
- 11.STATA. Exact logistic regression Reference Manual. Release 14. A Stata Press Publication. Stata Corporation, College Station; TX, USA: pp. 507–28. [Google Scholar]
- 12.Marinho F, de Araújo V Etelvina Miranda, Porto D Lopes, et al. Microcephaly in Brazil: prevalence and characterization of cases from the Information System on Live Births (Sinasc), 2000-2015. Epidemiol e Serviços Saúde. 2016;25 doi: 10.5123/S1679-49742016000400004. [DOI] [PubMed] [Google Scholar]
- 13.Reynolds MR, Jones AM, Petersen EE, et al. Vital Signs: Update on Zika Virus–Associated Birth Defects and Evaluation of All U.S. Infants with Congenital Zika Virus Exposure — U.S. Zika Pregnancy Registry, 2016. MMWR Morb Mortal Wkly Rep. 2017;66 doi: 10.15585/mmwr.mm6613e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Microcephaly Epidemic Research Group. Microcephaly in Infants, Pernambuco State, Brazil, 2015. Emerg Infect Dis. 2016;22:1090–3. doi: 10.3201/eid2206.160062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schuler-Faccini L, Ribeiro EM, Feitosa IML, et al. Possible Association Between Zika Virus Infection and Microcephaly — Brazil, 2015. MMWR Morb Mortal Wkly Rep. 2016;65:59–62. doi: 10.15585/mmwr.mm6503e2. [DOI] [PubMed] [Google Scholar]
- 16.Moore CA, Staples JE, Dobyns WB, et al. Characterizing the Pattern of Anomalies in Congenital Zika Syndrome for Pediatric Clinicians. JAMA Pediatr. 2016;171:1–8. doi: 10.1001/jamapediatrics.2016.3982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Honein MA, Dawson AL, Petersen EE, et al. Birth Defects Among Fetuses and Infants of US Women With Evidence of Possible Zika Virus Infection During Pregnancy. Jama. 2016;317:59. doi: 10.1001/jama.2016.19006. [DOI] [PubMed] [Google Scholar]
- 18.Schaub B, Vouga M, Najioullah F, et al. Analysis of blood from Zika virus-infected fetuses: a prospective case series. Lancet Infect Dis. 2017;17:520–7. doi: 10.1016/S1473-3099(17)30102-0. [DOI] [PubMed] [Google Scholar]
- 19.Paz-Bailey G, Rosenberg ES, Doyle K, et al. Persistence of Zika Virus in Body Fluids — Preliminary Report. N Engl J Med. 2017 doi: 10.1056/NEJMc1814416. NEJMoa1613108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Duffy MR, Chen T-HH, Hancock WT, et al. Zika virus outbreak on Yap Island, federated states of Micronesia. N Engl J Med. 2009;360:2536–43. doi: 10.1056/NEJMoa0805715. [DOI] [PubMed] [Google Scholar]
- 21.Aubry M, Teissier A, Huart M, et al. Zika Virus Seroprevalence, French Polynesia, 2014-2015. Emerg Infect Dis. 2017;23:669–72. doi: 10.3201/eid2304.161549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Breslow N. Design and analysis of case-control studies. Annu Rev Public Health. 1982;3:29–54. doi: 10.1146/annurev.pu.03.050182.000333. [DOI] [PubMed] [Google Scholar]
- 23.Ministério da Saúde, Secretaria de Vigilância em Saúde, Departamento de Vigilância Epidemiológica, Coordenação Geral do Programa Nacional de Imunizações. Informe Técnico para Implantação da Vacina Adsortiva Difteria, Tétano e Coqueluche (Pertussis Acelular) Tipo adulto - dTpa. 2014:1–22. [Google Scholar]
- 24.Munoz FM, Bond NH, Maccato M, et al. Safety and immunogenicity of tetanus diphtheria and acellular pertussis (Tdap) immunization during pregnancy in mothers and infants: a randomized clinical trial. Jama. 2014;311:1760–9. doi: 10.1001/jama.2014.3633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Walls T, Graham P, Petousis-Harris H, Hill L, Austin N. Infant outcomes after exposure to Tdap vaccine in pregnancy: an observational study. BMJ Open. 2016;6:e009536. doi: 10.1136/bmjopen-2015-009536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ko T-J, Tsai L-Y, Chu L-C, et al. Parental smoking during pregnancy and its association with low birth weight, small for gestational age, and preterm birth offspring: a birth cohort study. Pediatr Neonatol. 2014;55:20–7. doi: 10.1016/j.pedneo.2013.05.005. [DOI] [PubMed] [Google Scholar]
- 27.Hackshaw A, Rodeck C, Boniface S. Maternal smoking in pregnancy and birth defects: A systematic review based on 173 687 malformed cases and 11.7 million controls. Hum Reprod Update. 2011;17:589–604. doi: 10.1093/humupd/dmr022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.IPEA. Situação social da população negra por estado. 2014. [accessed April 6, 2017]. http://www.ipea.gov.br/portal/index.php?option=com_content&view=article&id=24121 .
- 29.Ministério da Saúde. TabNet Win32 3.0: Nascidos vivos - Brasil. [accessed April 6, 2017]. http://tabnet.datasus.gov.br/cgi/deftohtm.exe?sinasc/cnv/nvuf.def .
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.