Abstract
Objective
The diagnosis of pulmonary tuberculosis presents challenges in children, because symptoms are nonspecific, sputa are not accessible, and Mycobacterium tuberculosis cultures and smears often are negative. The Microscopic Observation Drug Susceptibility technique is a simple, inexpensive method for Mycobacterium tuberculosis isolation with superior speed and sensitivity over Lowenstein-Jensen culture in studies of adults with pulmonary tuberculosis. The objective of this study was to determine whether Microscopic Observation Drug Susceptibility culture can improve the sensitivity and the speed of Mycobacterium tuberculosis recovery among Peruvian children with symptoms suggestive of pulmonary tuberculosis.
Methods
Two specimens of each type (gastric aspirate, nasopharyngeal aspirate, and stool specimens) were collected from each patient, examined by auramine stain, and cultured by Microscopic Observation Drug Susceptibility and Lowenstein-Jensen techniques. Patients (n = 165) were enrolled between April 2002 and February 2004 at the Instituto de Salud del Niño, the major pediatric hospital in Lima, Peru. Inclusion criteria were age ≤12 years, Stegen-Toledo clinical score ≥5 points, and absence of antituberculous therapy. The main outcome measurements were (1) proportion of specimens that were culture positive by Microscopic Observation Drug Susceptibility versus Lowenstein-Jensen and (2) days required for positive culture result, stratified by specimen type and auramine stain result.
Results
Fifteen (9%) patients had at least 1 positive Mycobacterium tuberculosis culture (from stool in 3 cases, nasopharyngeal aspirate in 8 cases, and gastric aspirate in 15 cases). Thirty-eight culture-positive specimens were obtained (22 gastric aspirate, 12 nasopharyngeal aspirates, and 4 stools). Microscopic Observation Drug Susceptibility provided significantly more positive cultures than Lowen-stein-Jensen (33 of 38 specimens culture positive by Microscopic Observation Drug Susceptibility vs 21 of 38 by Lowenstein-Jensen). This was attributed to enhanced recovery of Mycobacterium tuberculosis from auramine-negative specimens (19 of 23 by Microscopic Observation Drug Susceptibility vs 9 of 23 by Lowenstein-Jensen), in contrast to similar detection rates for the 2 tests with auramine-positive samples. Similar results were found for analyses that were limited to gastric aspirates. Isolation was faster by Microscopic Observation Drug Susceptibility than Lowenstein-Jensen.
Conclusions
Isolation of Mycobacterium tuberculosis from children with suspected pulmonary tuberculosis by Microscopic Observation Drug Susceptibility demonstrated greater yield and faster recovery than by Lowenstein-Jensen method, significantly improving local capabilities to detect pediatric tuberculosis in resource-poor settings.
Keywords: tuberculosis, isolation, diagnosis, children, Peru
TUBERCULOSIS (TB) KILLS almost 2 million people per year, principally in developing countries, but the burden of tuberculous disease in children often is underappreciated. In 1990, the World Health Organization (WHO) estimated that there were ∼7.5 million cases of TB worldwide, 650 000 of which occurred in children.1 More recent estimates of the global burden of TB have increased to 8.8 million cases in 2002, although pediatric TB is greatly underreported as a result of WHO methods that include only acid-fast bacillus smear-positive cases, which excludes >95% of TB cases among children who are younger than 12 years.2 The diagnosis of pulmonary TB (PTB) in children is challenging.3 Adequate clinical diagnostic specimens often are difficult to obtain in children because of lack of sputum production and because clinical specimens frequently have low concentrations of organisms. Furthermore, the best available diagnostic tests are costly, and traditional methods are slow and insensitive. Even under the best circumstances Mycobacterium tuberculosis (MTB) is isolated in <50% of pediatric cases.4
The Microscopic Observation Drug Susceptibility (MODS) technique is an easy and transferable method for MTB isolation and is well suited to laboratories in developing countries. The concept is simple: it uses direct microscopic observation of early MTB colony formation in Middlebrook 7H9 broth in the wells of tissue culture plates.5 The “cording” growth appearance of MTB in liquid medium is characteristic, preventing confusion with bacteria or fungi.6–8 M avium–intracellulare complex and M kansasii are readily differentiated from MTB using MODS on the basis of their characteristic noncorded appearance. The essential equipment that is necessary for carrying out the MODS assay are a P2 biosafety cabinet, 37°C incubator, and an inverted light microscope. In auramine stain–positive specimens from adults, the technique also permits initial screening for antimicrobial sensitivity. This simple method results in faster isolation and susceptibility results than conventional methods for adults with TB.9,10
The purpose of this study was to compare the sensitivity and the speed of MODS and the Lowenstein-Jensen agar method (LJ) for culturing MTB from clinical specimens that were collected from Peruvian children with clinical characteristics suggestive of PTB. Specimens were cultured in parallel by MODS and LJ, the traditional method used in most of the developing world. Because the goal of the study was to compare methods that would be cost-effective and practical in a developing country, we did not evaluate more expensive rapid techniques that would be too expensive to use in that setting. Preliminary data that were published previously by our group suggest that nasopharyngeal aspirates (NPAs) are a useful alternative to gastric aspirates (GAs) for diagnosis of TB in children.11 Stool culture for MTB, although relatively insensitive and technically challenging, has been used successfully in many developing countries12,13 and deserves consideration because the specimen is so easily obtainable.
Methods
Patients
Patients with clinical suspicion of TB (as defined below) were enrolled consecutively between April 2002 and February 2004 at the Instituto de Salud del Niño of Lima, Peru. Approximately 20% of patients were inpatients, and 80% were outpatients. Patients were classified according to the clinical criteria of Stegen et al14 for diagnosis of pediatric TB as modified by Toledo et al15 in 1979. These clinical criteria for pediatric TB are called the Stegen-Toledo criteria (ST criteria) and are used widely throughout Latin America. ST criteria are used to classify patients into 4 risk categories (Table 1). However, the ST criteria were used only as an initial patient selection tool, and the purpose of our study was to compare culture methods and not to validate the use of ST criteria for diagnosis of pediatric TB. Inclusion criteria were (1) 12 years and younger, (2) ST clinical score ≥5 points, and (3) no previous diagnosis of TB and absence of previous antituberculous therapy. Although positive MTB culture is 1 of the Stegen-Toledo clinical criteria (Table 1), culture results are the primary outcome parameter of the study, and these were not available at enrollment, so this criterion was not used to determine patient eligibility. Patients were enrolled by parental informed consent. Concurrent with the collection of specimens, patient were evaluated by infectious diseases specialists, and empiric treatment for TB was started immediately in all cases using standard guidelines provided by WHO and the Peruvian Ministry of Health. Antituberculous treatment was based on the presence of the clinical criteria that were necessary to enroll in the study, so treatment was given to all patients regardless of MTB culture results. A screening test using an enzyme-linked immunosorbent assay for HIV was requested but not required if parents declined the test. The study protocol and consents were approved by the institutional review boards at Tulane Medical Center, Johns Hopkins Bloomberg School of Public Health, Asociacion Benefica PRISMA, and the Instituto de Salud del Niño of Lima, Peru. Written informed consent was obtained from all patients or their parents or guardians, and the human experimentation guidelines of the U.S. Department of Health and Human Services were followed in conducting this research. The protocol and consent forms were approved by the Institutional Review Boards of Tulane Medical Center, the Johns Hopkins Bloomberg School of Public Health, the Instituto de Salud del Niño, and the Asociacion Benefica PRISMA.
Table 1. ST Criteria for the Diagnosis of TB in Children14,15.
| Finding | Score |
|---|---|
| Positive culture | 7 |
| TB granuloma | 4 |
| Positive purified protein derivative >10 mm | 3 |
| Known contact in the last 2 y | 2 |
| Suggestive radiograph | 2 |
| Suggestive clinical picture (cough >2 wk) | 2 |
| Clinical criteria | |
| Highly probable TB: score ≥7 | |
| Probable TB: score 5–6 | |
| Suspicious TB: score 3–4 | |
| Unlikely TB: score 0–2 |
Clinical Specimens
Specimens were collected from patients for TB cultures before initiation of treatment or no more than 5 days after start of TB treatment and included the following:
NPAs, collected daily for 2 consecutive days by insertion of a soft, flexible nasopharyngeal tube into the nasopharynx, lavage with 5 mL of saline, and aspiration of the respiratory secretions into a container with an electrical suction device or hand-held aspirator.
GAs, collected on 2 successive mornings by nasogastric intubation. The volume of GAs was augmented as needed by injection of 5 mL of sterile water and aspiration back.
Stool specimens, collected daily for 2 days
Culture Techniques
For stool specimens, 0.1 g of stool was resuspended in 6 mL of sterile distilled water, mixed, and left for 15 minutes to separate, after which 2 mL of the supernatant was processed like other samples. When NPA or GA samples exceeded 2 mL of volume, they were centrifuged and the excess upper supernatant was discarded, leaving a 2-mL sample. Specimens were decontaminated by the addition of an equal volume of 0.5% N-acetyl-L-cysteine, 2% NaOH, and 1.45% sodium citrate for 15 minutes, as previously described,4 and centrifuged, and the pellet was resuspended in 2 mL of 0.2% bovine serum albumin in 0.9% saline and tested for the presence of MTB in a blinded manner by the following methods:
-
MODS method: Detailed methods have been published previously.4,5 A total of 500 mL of each decontaminated sample was inoculated into 4.8 mL of modified Middlebrook 7H9 medium that contained 5.9 g/L Midlebrook 7H9 broth base, 0.31% glycerol, 1.25 g/L Bacto Casitone (Difco, Sparks, MD), 10% oleic acid-albumin-dextrose-catalase enrichment medium, and 160 μL of PANTA antimicrobic supplement stock.
This mixture was separated into four 1.2-mL aliquots in a sterile 24-well plate. Each run also included a positive control of MTB H37Rv. Plates were sealed in a plastic resealable bag, incubated at 37°C for up to 30 days, and examined every other day by inverted light microscopy at ×40 for 30 to 45 seconds per well. Presumptive MTB isolates were confirmed by standard biochemical tests (eg, niacin reduction) and/or by a heminested IS 6110 MTB polymerase chain reaction assay.
LJ: A total of 250 μL of each decontaminated sample was inoculated onto an LJ slant, incubated at 37°C, and examined in a blinded manner twice weekly from the first to eighth week after inoculation.
Auramine stain (Smear test): One drop of each decontaminated sample was placed on a microscope slide, stained with 0.1% Auramine O, and examined at ×100 magnification. The test was positive when it contained at least 5 bacilli per 300 fields.
Data Analysis
Data analysis was performed at Tulane using SPSS version 7.5 (SPSS Inc, Chicago, IL) and EpiInfo Version 6 (Centers for Disease Control and Prevention, Atlanta, GA) software programs. Recovery rates for MTB were compared by culture method for specimens stratified by specimen type (GA, NPA, and stool) and by auramine stain result. Separate analyses were conducted using both individual specimens and individual patients as the unit of analysis; in the latter analyses, patients with at least 1 clinical specimen that was culture-positive for MTB by any method were classified as true TB cases. Duplicate specimens of the same specimen type were analyzed individually in comparisons using specimens as the unit of analysis; results from duplicate specimens were not pooled. Concordance was determined by κ test analysis. χ2 and, when necessary, McNemar’s tests were used to measure strengths of association between categorical variables. The 2-tailed t test or Wilcoxon signed rank sum test was used to compare continuous variables.
Results
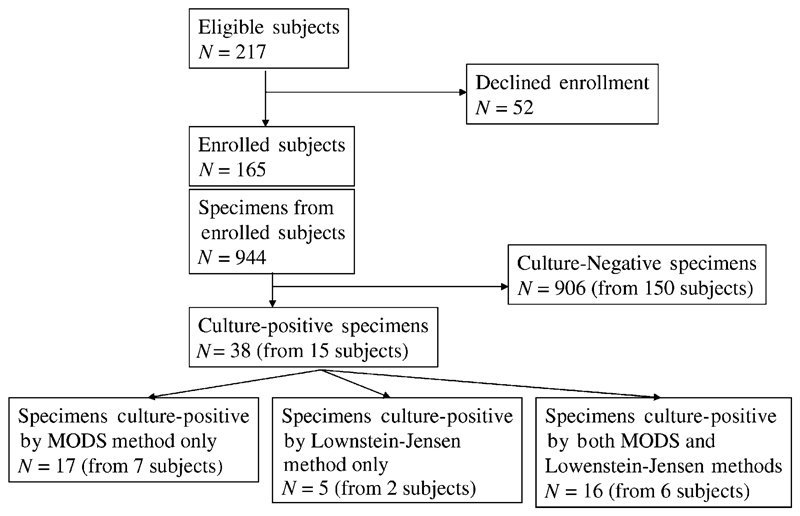
The distribution of patients and specimens by culture result is shown in Fig 1. Patients included 93 boys (56%) and 72 girls (N = 165). Mean age of all patients was 4.6 years, and the age distribution of each group was as follows: ≤1 year, 21 (12%) patients; 1 to 5 years, 72 (44%) patients; and >5 years, 72 (44%) patients. Fiftynine (36%) patient had an ST clinical score of 7 points or higher (highly probable TB). HIV enzyme-linked immunosorbent assay results were negative in 136 (82%) patients, and the test was declined in 29 (18%) patients, none of whom had clinical evidence of HIV infection. From these 165 patients, a total of 324 GAs, 319 NPAs, and 301 stools were cultured.
Figure 1.
Patient and specimen Standards for Reporting of Diagnostic Accuracy flow chart.
Fifteen (9%) patient had at least 1 confirmed positive culture for MTB in a clinical specimen. These patients were similar to culture-negative patients on the basis of gender distribution (9 [60%] boys) and mean age (4.2 years). Mean ST score of patients with a positive culture for MTB (7.1 points; range: 5–11) was significantly greater than the mean ST score of the culture-negative patients (5.86 points; range: 5–14; P < .03). When stratified by ST score, 5 (4.7%) of 106 patients with ST scores of 6 or less were culture positive and 10 (16.9%) of 59 patients with ST scores of 7 or greater were culture positive (odds ratio: 4.12; P = .01). The proportion of culture-positive patients with ST scores ≥7 (10 [66%] of 15) also was significantly higher than among the culture-negative patients (P < .02). Whereas 2 specimens of each type were cultured in each of the 15 patients (for a total of 30 GAs, 30 NPAs, and 30 stools from these 15 patients), 5 patients were culture positive in a single (GA) specimen only. In all, 38 culture-positive specimens were obtained: 22 GAs, 12 NPAs, and 4 stool specimens. Among the 15 culture-positive patients, culture-positive specimens included stools in 3 (20%) cases, NPAs in 8 (53%) cases, and GAs in all 15 (100%) cases.
The comparison between LJ and MODS cultures is shown in Table 2. Among 22 culture-positive GAs, 3 were positive by LJ only, 11 by MODS only, and 8 by both. For auramine stain–negative GAs, recovery by MODS (12 of 14 specimens) was greater than by LJ (4 of 14 specimens; P = .04). Among 12 culture-positive NPAs, 1 was positive by LJ only, 4 by MODS only, and 7 by both. The 4 auramine stain–positive specimens all were culture positive by both methods. For auramine stain–negative NPAs, recovery by MODS (7 of 8 specimens) was greater than recovery by LJ (4 of 8 specimens; P = .3).
Table 2. Culture Results by Method From Specimens Tested, Grouped by Specimen Type and Auramine Stain Result.
| Specimen Type | Total No. Tested | Culture-Positive Specimensa | No. Culture Positive by MODS | No. Culture Positive by LJ | P b | κ Score |
|---|---|---|---|---|---|---|
| GA (all) | 321 | 22 | 19 (11 by MODS only) | 11 (3 by LJ only) | .06 | 0.51 |
| GA auramine positive | 8 | 8 | 7 (1 by MODS only) | 7 (1 by LJ only) | 1.00 | NA |
| GA auramine negative | 313 | 14 | 12 (10 by MODS only) | 4 (2 by LJ only) | .04 | 0.24 |
| NPA (all) | 314 | 12 | 11 (4 by MODS only) | 8 (1 by LJ only) | .04 | 0.73 |
| NPA auramine positive | 4 | 4 | 4 | 4 | 1.00 | 1.00 |
| NPA auramine negative | 310 | 8 | 7 (4 by MODS only) | 4 (1 by LJ only) | .38 | 0.54 |
| Stool (all) | 292 | 4 | 3 (2 by MODS only) | 2 (1 by LJ only) | 1.00 | 0.40 |
| Stool auramine positive | 4 | 3 | 3 (2 by MODS only) | 1 | .50 | 0.20 |
| Stool auramine negative | 288 | 1 | 0 | 1 (1 by LJ only) | NA | NA |
| All specimens | 927 | 38 | 33 (17 by MODS only) | 21 (5 by LJ only) | .02 | 0.58 |
| All auramine positive | 16 | 15 | 14 (3 by MODS only) | 12 (1 by LJ only) | .63 | 0.20 |
| All auramine negative | 911 | 23 | 19 (14 by MODS only) | 9 (4 by LJ only) | .03 | 0.35 |
Figures in parentheses show the number of specimens that were culture positive by the indicated method only, ie, not culture positive by the other method used. NA indicates not applicable.
Number of specimens of the indicated type that were culture positive by at least 1 of the 2 culture methods used.
McNemar’s test, comparing isolation rate by MODS and isolation rate by LJ.
Of the 19 specimens (of any type) that were culture positive by 1 method but not the other, 4 (21%) of 19 were culture positive by LJ only and 15 (79%) of 19 were culture positive by MODS only (P < .001). When all specimens that were culture positive by at least 1 method were analyzed, the overall detection rate for MODS cultures (33 [87%] of 38) was significantly greater than by LJ culture (21 [55%] of 38; P = .02, McNemar, 2-sided test).
Recovery by MODS and by LJ were compared in Table 2 by 2 methods. The proportion of all culture-positive specimens of each type that were detected by each of the 2 methods (MODS versus LJ) were compared statistically (P value), and concordance of the 2 methods is compared by calculation of κ scores. Relative recovery of MTB by culture method (P values) demonstrated significantly greater recovery by MODS as compared with LJ (P < .05) for all specimens, all NPAs, all auramine-negative specimens, and auramine-negative GAs. Auramine-positive specimens of all types were detected at high rates by both methods, but greater concordance was found for auramine-negative specimens as compared with auramine-positive specimens of the same type as a result of the large number of specimens that were culture negative by both methods. Lower concordance in some specimen types is attributable primarily to greater detection of MTB by MODS culture (Table 2).
When the proportion of the 15 culture-positive patients who were detected was analyzed by culture method, 8 (53%) of 15 were detected by LJ method with at least 1 culture-positive specimen, and 13 (86%) of 15 were detected by MODS with at least 1 culture-positive specimen (P = .1, McNemar 2-sided test). Overall, MODS detected positive cultures that were missed by LJ cultures in 7 (47%) of the 15 cases, LJ detected positive cultures that were missed by MODS cultures in 2 (13%) of the 15 cases, and at least 1 specimen was culture-positive by both methods in 6 (40%) of the 15 cases. MODS cultures were uninterpretable because of bacterial/fungal contamination significantly less often than LJ (1.8% vs 16% respectively; P < .001), and there were no significant differences between these contamination rates for stool, NPA, or GA specimens for either culture method.
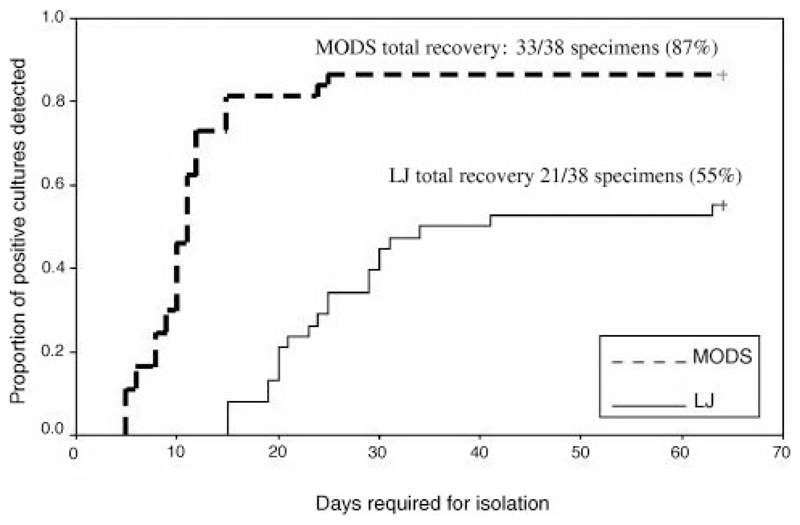
The proportion of all positive cultures that were isolated (n = 38), at specific points in time, is shown by culture method in Fig 2. Because neither MODS nor LJ culture identified all culture-positive specimens, neither line reaches the 100% detected mark. The figure illustrates the additional speed of recovery by MODS as compared with LJ culture technique. Mean time from inoculation of plates to isolation of MTB was significantly shorter (ie, faster isolation) in the case of MODS cultures (mean ± SD: 25.9 ± 10.5 days for LJ medium [median: 24 days; low: 15 days; high: 63 days] and 10.7 ± 4.5 days for MODS [median: 10 days; low: 5 days; high: 25 days]; P < .001). Time to positive culture analyses was adjusted by subtracting 3.5 days from the time required for positive detection on LJ medium to correct for differences in the frequency of checking for growth by LJ and MODS techniques (this is the maximum possible time that LJ cultures could have been positive but not detected as a result of the frequency of checking for growth). When reanalyzed with this adjustment and by selecting only specimens that were culture positive by both methods, time to detection by MODS still was significantly shorter than by the LJ method (mean: 18.7 days LJ vs 10 days MODS; P < .001, Wilcoxon signed rank sum test).
Figure 2.
The proportion of all positive cultures that were isolated (n 38), at specific points in time, is shown by culture method.
Discussion
These data are in agreement with results of our previous studies in adults,4,5 demonstrating better and faster recovery of MTB by MODS as compared with traditional LJ agar. MODS performed significantly better than the LJ method for isolation of MTB from auramine stain–negative specimens, which are of particular interest because most pediatric specimens are paucibacillary and consequently auramine negative. Furthermore, auramine-negative specimens do not routinely prompt empiric antituberculous therapy in the way that auramine-positive specimens would under similar circumstances. MODS demonstrated significantly greater yield as compared with LJ in the following specimen subsets: all GAs, auramine-negative GAs, and all auramine-negative specimens. Overall, MODS culture techniques resulted in detection of ∼50% more cases of confirmed pediatric TB than LJ culture alone, and all of these cases were auramine negative in all specimens. Both MODS and LJ methods demonstrated similar yield from auramine-positive specimens, so the improved yield from MODS culture as compared with LJ in all specimens was entirely attributable to significantly improved yield from smear-negative specimens. Because pediatric TB usually is paucibacillary, as in our study, this has tremendous implications for improving the yield of TB cultures in children. Although the yield of MTB cultures of NPA and stool specimens by MODS was superior to LJ as well, the smaller sample size of culture-positive specimens in these categories limited our ability to detect significant differences.
Bacterial isolation by MODS was significantly faster than LJ culture. The average time from inoculation to isolation of MTB by MODS (10.7 ± 4.5 days) is similar to the speed of isolation of much costlier culture techniques, such as the BACTEC (BD Diagnostics, Sparks, MD) and mycobacteria growth indicator tube (MGIT; BD Diagnostics) methods. At $1.26 US per sample tested, the cost of MTB isolation by MODS is much less than that for the BACTEC ($10.00 per sample, after purchase of the BACTEC machine [approximately $70 000]) or MGIT ($8.00–$10.00 per sample), and given the speed of isolation, it is likely to be more cost-effective than LJ media ($0.85 per sample). The implications of these results are enormous, because laboratories in many developing countries can reduce the time required for MTB isolation by >50% and improve the yield from paucibacillary specimens with little to no additional investment. MODS also may improve the yield of mycobacterial cultures in other populations with paucibacillary pulmonary disease, such as individuals with HIV infection.
In this group of pediatric patients, recovery from gastric aspirates (15 of 15 cases) clearly was superior to recovery from stool (3 of 15 cases) by culture and also seems to be superior to recovery from NPA specimens (8 of 15 cases), although additional cases are needed to confirm this difference with 95% confidence. Our data suggest that GAs still are the most reliable source for recovery of MTB in high-risk children. Sensitivity of detection by stool culture may be increased by culturing a larger volume of specimen and improving decontamination/concentration techniques.
Rates of MTB detection from GA specimens from children with presumptive TB range from 1% to 50% in the medical literature.16–19 The inherent problem in these figures is the case definition for pediatric TB, which greatly affects the denominator value and hence the reported recovery rate. Because TB in children can be proved definitively only by recovery of MTB from a clinical specimen and the specificity of clinical case definitions varies greatly on the basis of the stringency of criteria applied, use of bacille Calmette-Guérin vaccine in the population, and prevalence of TB in the population, any assessment of the sensitivity of an MTB culture method using a non–culture-based gold standard for comparison is problematic. Our recovery rate of 16.9% from patients with ST scores of 7 or more certainly is within this broad range of reported values and similar to recovery rates that were reported from studies in other developing countries. However, that we also could recover MTB from almost 5% of children who had some degree of clinical suspicion (ST scores of 5 or 6) but did not meet the more stringent case definition illustrates the inherent limitations of clinical case definitions for pediatric TB. Studies with reported recovery rates of close to 50% were mostly from the United States and other developed countries and based on much smaller sample sizes and used strict clinical criteria in an environment where bacille Calmette-Guérin vaccine is not used and the diagnostic value of purified protein derivative reactions are increased. These factors have a major impact on the performance of diagnostic tests.
Because of these difficulties in interpreting sensitivity and specificity, we chose to compare concordance between MODS and LJ culture as a measure of relative test performance. This assessment also poses challenges, because our study and others that were conducted in adults suggest that MTB recovery by MODS is significantly greater than by LJ. Nevertheless, concordance between MODS and LJ results was greatest for NPA specimens (κ = 0.73), and κ values for all specimens and other specimen types (stools and GAs) all were in the fair to good reliability range (κ = 0.4–0.75). Lower concordance in some specimen types is attributable primarily to greater detection of MTB by MODS culture.
The improved yield of MTB recovery by MODS culture in these children is impressive, but certain limitations in interpreting these results should be acknowledged. Although our study demonstrated significant improvement in MTB detection from pediatric patients using the MODS method, the lack of a true and indisputable gold standard reference test means that we cannot determine accurately how many cases of TB disease were missed by culture. The increased yield of MTB by MODS culture will improve our rate of case detection, but the negative predictive value of MODS cultures (ie, our ability to exclude TB disease on the basis of a negative culture result) remains impossible to determine by currently available methods. Limited numbers of culture-positive specimens were available from 15 culture-positive patients, so additional studies with larger samples also would be useful. The patients who were included here did not include known HIV-infected children, and recovery rates in these children may be very different. Induced sputa also have been investigated recently as an alternative clinical specimen for TB cultures in children, but these specimens were not studied in this group.20 We did not compare recovery by MODS with other, more expensive culture techniques that are used in more developed countries, such as MIGIT, MBBact (Organon Teknika, Cambridge, United Kingdom), or BACTEC. Although these comparisons would have provided interesting information, this was not possible because of cost. Our primary intention was to show that the MODS technique is superior to the standard approach that usually is the only available method in developing countries where pediatric TB poses the greatest threat. Although the MODS technique provides a major step forward in our ability to diagnose TB disease in children using methods that are economically feasible in developing countries, additional work still is needed to define the most sensitive and accurate tests for case detection in this elusive disease.
Acknowledgments
This study was supported in part by the following grants: National Institutes of Health (NIH) 1 RO1 AI-49139, United States Agency for International Development Tuberculosis Award HRN-5986-A-00-6006-00, NIH International Training and Research Program in Emerging Infectious Diseases grant 5D43-TW00910, the Fogarty-NIH AIDS training program 3T22-TW00016-05S3, and the National Institute of Allergy and Infectious Diseases tutorial training grant 5T35-AI07646-02.
Abbreviations
- TB
tuberculosis
- WHO
World Health Organization
- PTB
pulmonary tuberculosis
- MTB
Mycobacterium tuberculosis
- MODS
Microscopic Observation Drug Susceptibility
- LJ
Lowenstein-Jensen culture
- NPA
nasopharyngeal aspirate
- GA
gastric aspirate
- ST
Stegen-Toledo criteria
Biography
Dr Oberhelman was the principal investigator and provided study design, enrollment, data collection, laboratory analysis, data analysis, and manuscript preparation. Dr Soto-Castellares was the physician study coordinator and provided enrollment, data collection, data analysis, and manuscript preparation.MsCaviedes was laboratory coordinator and provided laboratory analysis and manuscript preparation. Dr Castillo was an investigator and provided enrollment and data collection. Dr Kissinger was the epidemiologist/statistician and provided data analysis and manuscript preparation. Dr Moore was an investigator and provided data analysis and manuscript preparation. Dr Evans was an investigator and provided data analysis and manuscript preparation. Dr Gilman was an investigator and provided study design, laboratory analysis, data analysis, and manuscript preparation.
References
- 1.Dolin P, Raviglione MC, Kochi A. Global tuberculosis incidence and mortality during 1990–2000. Bull World Health Organ. 1994;72:213–220. [PMC free article] [PubMed] [Google Scholar]
- 2.World Health Organization. Global Tuberculosis Control: Surveillance, Planning, Financing. WHO Report 2004. Geneva, Switzerland: World Health Organization; 2004. [Google Scholar]
- 3.Starke JR, Taylor-Watts KT. Tuberculosis in the pediatric population of Houston, Texas. Pediatrics. 1989;84:28–35. [PubMed] [Google Scholar]
- 4.Abadco D, Steiner P. Gastric lavage is better than bronchoalveolar lavage for isolation of Mycobacterium tuberculosis in childhood pulmonary tuberculosis. Pediatr Infect Dis J. 1992;11:735–738. doi: 10.1097/00006454-199209000-00013. [DOI] [PubMed] [Google Scholar]
- 5.Caviedes L, Lee TS, Gilman RH, et al. Rapid, efficient detection and drug susceptibility testing of Mycobacterium tuberculosis in sputum by microscopic observation of broth cultures. J Clin Microbiol. 2000;38:1203–1208. doi: 10.1128/jcm.38.3.1203-1208.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Koksalan OK, Aydin MD, Eraslan S, Bekiroglu N. Reliability of cord formation in BACTEC 12B/13A media for presumptive identification of Mycobacterium tuberculosis complex in laboratories with a high prevalence of Mycobacterium tuberculosis. Eur J Clin Microbiol Infect Dis. 2002;21:314–317. doi: 10.1007/s10096-002-0701-9. [DOI] [PubMed] [Google Scholar]
- 7.McCarter YS, Ratkiewicz IN, Robinson A. Cord formation in BACTEC medium is a reliable, rapid method for presumptive identification of Mycobacterium tuberculosis complex. J Clin Microbiol. 1998;36:2769–2771. doi: 10.1128/jcm.36.9.2769-2771.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Morris AJ, Reller LB. Reliability of cord formation in BACTEC media for presumptive identification of mycobacteria. J Clin Microbiol. 1993;31:2533–2534. doi: 10.1128/jcm.31.9.2533-2534.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Moore DAJ, Mendoza D, Gilman RH, et al. MODS: a rapid, reliable diagnostic test for MDR TB suitable for resource-poor settings. J Clin Microbiol. 2004;42:4432–4437. doi: 10.1128/JCM.42.10.4432-4437.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Park WG, Bishai WR, Chaisson RE, Dorman SE. Performance of the microscopic observation drug susceptibility assay in drug susceptibility testing of Mycobacterium tuberculosis. J Clin Microbiol. 2002;40:4750–4752. doi: 10.1128/JCM.40.12.4750-4752.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Franchi LM, Cama RI, Gilman RH, Montenegro-James S, Sheen P. Detection of Mycobacterium tuberculosis in nasopharyngeal aspirate samples in children. Lancet. 1998;352:1681–1682. doi: 10.1016/s0140-6736(05)61454-7. [DOI] [PubMed] [Google Scholar]
- 12.Donald PR, Schaaf HS, Gie RP, Beyers N. Stool microscopy and culture to assist the diagnosis of pulmonary tuberculosis in children. J Trop Pediatr. 1996;42:311–312. doi: 10.1093/tropej/42.5.311. [DOI] [PubMed] [Google Scholar]
- 13.Murcia-Aranguren MI, Gomez-Marin JE, Alvarado FS, et al. Frequency of tuberculous and non-tuberculous mycobacteria in HIV infected patients from Bogota, Colombia. BMC Infect Dis. 2001;1:21–27. doi: 10.1186/1471-2334-1-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stegen G, Jones K, Kaplan P. Criteria for guidance in the diagnosis of tuberculosis. Pediatrics. 1969;43:260–263. [PubMed] [Google Scholar]
- 15.Toledo A, Katz F, Montiel J, Rico FG. Criterios de diagnóstico en tuberculosis infantil [Diagnostic criteria for pediatric tuberculosis] Rev Mex Pediatr. 1979;46:239–243. [Google Scholar]
- 16.Feja K, Saiman L. Tuberculosis in children. Clin Chest Med. 2005;26:295–312. doi: 10.1016/j.ccm.2005.02.010. [DOI] [PubMed] [Google Scholar]
- 17.Walls T, Shingadia D. Global epidemiology of paediatric tuberculosis. J Infect. 2004;48:13–22. doi: 10.1016/s0163-4453(03)00121-x. [DOI] [PubMed] [Google Scholar]
- 18.Lobato MN, Loeffler AM, Furst K, Cole B, Hopewell PC. Detection of Mycobacterium tuberculosis in gastric aspirates collected from children: hospitalization is not necessary. Pediatrics. 1998;102(4) doi: 10.1542/peds.102.4.e40. Available at: www.pediatrics.org/cgi/content/full/102/4/e40. [DOI] [PubMed] [Google Scholar]
- 19.Osborne CM. The challenge of diagnosing childhood tuberculosis in a developing country. Arch Dis Child. 1995;72:369–374. doi: 10.1136/adc.72.4.369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zar HJ, Hanslo D, Apolles P, Swingler G, Hussey G. Induced sputum versus gastric lavage for microbiological confirmation of pulmonary tuberculosis in infants and young children: a prospective study. Lancet. 2005;365:130–134. doi: 10.1016/S0140-6736(05)17702-2. [published correction appears in Lancet. 2005;365:1926] [DOI] [PubMed] [Google Scholar]