Abstract
Splicing modifiers promoting SMN2 exon 7 inclusion have the potential to treat spinal muscular atrophy, the leading genetic cause of infantile death. The small molecules are SMN2 exon 7 selective and act during the early stages of spliceosome assembly. Here, we show at atomic resolution how the drug selectively promotes the recognition of the weak 5’-splice site of SMN2 exon 7 by U1 snRNP. The solution structure of the RNA duplex formed upon 5’-splice site recognition in the presence of the splicing modifier revealed that the drug specifically stabilizes a bulged adenine at this exon-intron junction and converts the weak 5’-splice site of SMN2 exon 7 into a stronger one. The small molecule acts as a specific splicing enhancer cooperatively with the splicing regulatory network. Our investigations uncovered a novel concept for gene-specific alternative splicing correction that we coined 5’-splice site bulge repair.
Introduction
Spinal muscular atrophy (SMA) is an autosomal recessive neuromuscular disease that represents the leading genetic cause of infant mortality. The disorder is characterized by progressive degeneration of motor neurons from the spinal cord resulting in muscle weakness and atrophy1. SMA is caused by the genetic homozygous inactivation of the survival of motor neuron-1 gene (SMN1), which encodes SMN, a multifunctional protein important for proper assembly of small nuclear ribonucleoprotein particles2. Although a paralog gene SMN2 is also present in the human genome, it differs by several silent mutations and mainly produces a different mRNA isoform lacking exon 7 which encodes for an unstable protein3–5. The reduced level of functional SMN protein alters motor neuron functions, however, the detailed mechanism leading to motor neuron degeneracy remains elusive6. All SMA patients have at least one functional copy of the SMN2 gene that still produces small amounts of functional SMN protein but not enough to compensate the SMN1 loss7. Accordingly, a promising therapeutic strategy to treat SMA consists in promoting SMN2 exon 7 (E7) inclusion to produce a larger amount of functional SMN protein from SMN2.
Several approaches have been developed to promote SMN2 E7 inclusion8–13 and, recently, a treatment has been approved. It targets pre-mRNA splicing regulation using an anti-sense oligonucleotide (ASO) derivative (Spinraza™) that displaces the main splicing repressor hnRNP A1 from the cis-RNA silencer flanking the 5’-splice site9,14–16. Spinraza induces SMN2 E7 inclusion in a dose dependent manner as well as the production of functional SMN protein. One main disadvantage of the ASO approach is that it requires frequent intrathecal injections17. Two alternative strategies have since led to promising preliminary clinical evaluations: a gene therapy18 and a small molecule approach12,13,19,20. The gene therapy strategy consists in delivering a copy of the SMN1 allele to patients using an adeno-associated virus. Initial clinical trials revealed the potential of this approach since a large majority of type I SMA patients responded efficiently after a single injection21,22. However, the long-term safety and expression of the transgene still needs to be evaluated. Several classes of SMN2 specific splicing modifiers have been described and have the potential to be used for SMA treatment. These compounds promote SMN2 E7 inclusion, induce the production of functional SMN protein and increase the motor function as well as the survival of a mouse model with severe SMA in a dose dependent manner12,13. Two such compounds (ridisplam and branaplam) are currently under clinical evaluation. They offer the benefit of oral administration and distribute systematically23. Their mechanism of action is still under investigation and understanding the small molecule selectivity is of main fundamental interest since it could potentially help with development of innovative RNA-based therapeutic strategies.
Genome-wide analysis of alternative splicing changes induced by the small molecule revealed that only a limited set of exons are regulated (including SMN2 E7). They are enriched for a nGA motif at their 3’-ends13. Transcriptomic analysis also revealed that two closely related exons experienced strong changes, the SMN2 E7 and the exon 8 of the STRN3 pseudogene24. Both exons have the same 5’-splice site (5’-SS) and contain a purine rich element (named ESE2 in SMN2) known to recruit splicing modulators like Tra2β1, SRSF9 and hnRNP G in the case of the SMN2 pre-mRNA25–28. Deletion of ESE2 within SMN2 E7 reduced the exon sensitivity to the splicing modifier24, however, the splicing switch was still observed at higher doses (~50-times higher). Moreover, the small molecule selectivity seems to be provided by the 5’-SS since introducing the SMN2 E7 5’-SS downstream of the BRCA1 exon 18 makes this chimeric construct sensitive to the drug13. We could confirm that the splicing modifier indeed recognizes the exon-intron junction in vitro24. Although the specificity of the splicing switch seems to be triggered by the binding to the 5’-SS, the molecule is also sensitive to the upstream exon sequence and it was even proposed to act directly at ESE229. Altogether, previous experimental data support that the small molecule acts in concert with the splicing regulatory network to stabilize a specific enhancer complex at the weak SMN2 E7 5’-SS13,24. However, the exact mechanism of action of the drug remains unclear.
To understand how the splicing switch is driven at atomic level, the molecular mechanisms of the SMN2 specific splicing correction need to be investigated. Here, we first demonstrated that the drug is selective for U1 snRNP once in complex with the E7 5’-SS and solved the structures of the intermolecular RNA helix (U1 snRNA:5’-SS) with and without the small molecule. The structures show that the splicing modifier stabilizes an unpaired adenine at the exon-intron junction in the RNA helix base stack and allosterically promotes the binding of the U1-C zinc finger and U1 snRNP. The splicing modifier therefore specifically transforms the weak 5’-SS of E7 SMN2 into a stronger one.
Results
SMN-C5 targets the 5’-SS/U1 snRNP interface
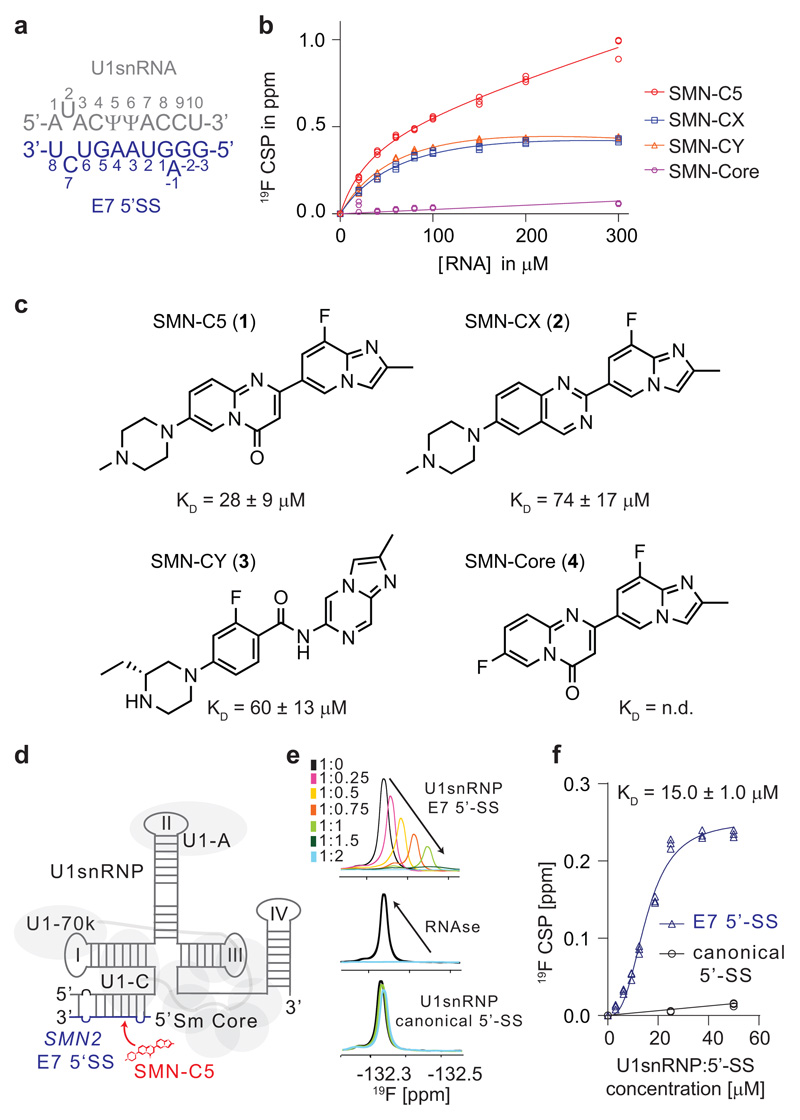
We previously showed that the SMN2 splicing modifier SMN-C524, a compound from the same chemical class as risdiplam23,30 which is currently undergoing pivotal clinical trials, binds to the RNA duplex formed by the U1 snRNA 5’-end and the E7 5’-SS (Fig. 1a). To analyze the relationship between the biological activity and the molecule’s ability to recognize the RNA duplex, four molecules31, including two highly potent molecules in vivo SMN-C5 (1) and SMN-CX (2), one molecule that is only active in vitro SMN-CY (3) and one inactive molecule SMN-Core (4), were tested for their ability to recognize the RNA duplex using NMR spectroscopy (Fig. 1b and 1c). The three active compounds interacted with the intermolecular RNA helix at the same binding site located at the exon-intron junction, as evidenced by similar chemical shift changes in this region (Supplementary Fig. 1). SMN-C5, SMN-CX and SMN-CY bind the RNA duplex with affinity constants of 28 ± 9 μM, 74 ± 17 μM and 60 ± 13 μM, respectively. Since SMN-C5 was the best RNA duplex binder, we then investigated its selectivity in the context of the entire U1 snRNP (Fig. 1d). We therefore in vitro reconstituted U1 snRNP bound to the canonical 5’-SS (strong) or the SMN2 E7 5’SS (weak) and monitored the direct binding of the small molecule using 19F NMR spectroscopy (Fig. 1e and Supplementary Fig. 2). Upon addition of the U1 snRNP: E7 5’-SS complex, the SMN-C5 19F signal was perturbed and almost disappeared as a consequence of binding to a large molecular weight complex of ~220 kDa. The binding affinity of SMN-C5 for the ribonucleoparticle could be estimated to 15.0 ± 1.0 μM, meaning that the splicing modifier binds the RNA duplex more strongly in the context of U1 snRNP (Fig. 1f). Addition of ribonucleases restored the initial SMN-C5 19F signal, indicating that the interaction is RNA dependent. Replacement of the weak E7 5’-SS by a strong and canonical 5’-SS impairs the interaction, confirming the SMN-C5 selectivity for the RNA duplex formed between the E7 5’-SS and the 5’-end of U1 snRNA in the context of the ribonucleoparticle (Fig. 1e). To better understand the SMN-C5 mode of action, the structural basis for the splicing modifier selectivity was further investigated.
Fig. 1. SMN-C5 recognizes the interface between U1 snRNP and the SMN2 E7 5’-SS.
a, Primary sequence of the RNA duplex. In all the figures, the U1 snRNA and the E7 5’-SS are grey and blue, respectively. b, Plot of the 19F chemical shift change (CSP) observed for the four different SMN2 splicing modifiers as a function of the RNA duplex concentrations. All the experimental points of the three technical replicates are shown on the plot. c, Planar structures of SMN-C5 (1), SMN-CX (2), SMN-CY (3) and SMN-Core (4). Below each structure, the binding affinities of the molecule for the RNA duplex is indicated. d, Schematic of U1 snRNP bound to the E7 5’-SS. The U1 snRNP proteins are depicted in grey. e, Overlay of the 1D 19F NMR spectra of SMN-C5 as a function of the U1 snRNP:5’-SS E7 concentrations. Spectra are colored according to the SMN-C5:U1 ratios indicated on the panel. Addition of ribonuclease activities (RNAses) restores the initial 19F NMR signal (middle panel). At the bottom, overlay of the 1D 19F NMR spectra of SMN-C5 as a function of the U1 snRNP: consensus 5’-SS concentrations. f, Plot of the 19F CSP as a function of the U1 snRNP:5’-SS concentrations. All the experimental points of the three technical replicates are shown on the plot.
Structural basis for SMN-C5 5’-SS selectivity
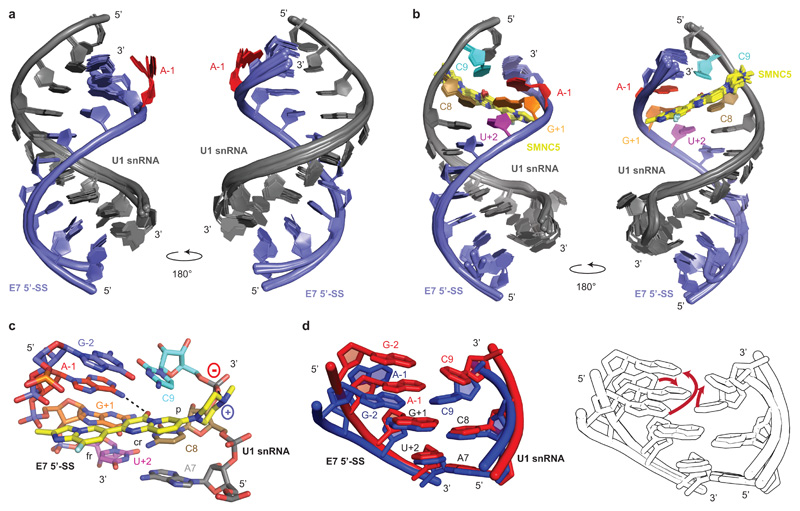
The E7 5’-SS is atypical for three reasons: (i) it contains an adenine at the position -1 that deviates from the consensus 5’-SS; (ii) it is embedded into a secondary structure element called the inhibitory terminal stem loop 2 or TSL211 and (iii) it is flanked by a splicing silencer (ISS-N1) that recruits hnRNP A116. All these features modulate this versatile splicing event. However, beside the inhibitory secondary structure and the effect of the splicing silencers, the SMN2 E7 5’-SS is intrinsically weak. We solved the NMR solution structure of the apo RNA duplex (Fig. 2a and Supplementary Table 1) that revealed an RNA helix containing an unpaired adenine at position -1 which lies in the minor groove (Supplementary movie 1). We next investigated the complex with SMN-C5. Several SMN-C5 resonances experienced large chemical shift changes (Supplementary Fig. 3) and sixteen intermolecular NOEs between SMN-C5 and RNA resonances could unambiguously define a precise structure and the binding interface of the drug-RNA complex (Supplementary Fig. 4). The structure of the complex revealed that SMN-C5 contacts the exon-intron junction and positions itself across the entire major groove (Fig. 2b and Supplementary Table 1). SMN-C5 anchors to the U1 snRNA phosphate group of C9 via its piperazine group that is positively charged at neutral pH. SMN-Core which does not contain this chemical group is inactive and does not bind to the RNA, suggesting that this electrostatic interaction is essential for the biological activity (Fig. 1). At the other extremity of the molecule, the second aromatic cycle interacts exclusively with the 5’-SS. The structure also revealed the crucial role of the central aromatic ring which forms a direct hydrogen bond with the amino group of the unpaired A-1 and inserts between C8 and C9 of U1 snRNA (Fig. 2c and Supplementary movie 2). In agreement with the structure, strong chemical shift changes were observed for the NMR signals of A-1 and C8 aromatic protons upon addition of SMN-C5 (Supplementary Fig. 5) and it was previously observed that the central ring of the molecule must be planar and aromatic to be functional31. The structure shows that the splicing modifier bridges U1 snRNA and the 5’-SS and acts as a bona fide splicing factor by increasing the thermal stability of the RNA duplex by almost 1°C (Supplementary Fig. 5). The single hydrogen bond observed between the central ring and the unpaired adenine explains the specificity of SMN-C5 for E7 5’-SS. Indeed, if the adenine is replaced by a guanine, the bulge disappears and the SMN-C5 binding pocket does not exist anymore. Furthermore, as the hydrogen bond donor involves the N6 atom of the adenine, the potential hydrogen bond donors of pyrimidine (N4 or N3 atoms for cytosine or uracil) would be too far from the SMN-C5 carbonyl group to form a direct hydrogen bond (Fig. 2c). In conclusion, the structure shows at atomic resolution that the SMN-C5 specificity for SMN2 E7 is governed by the sequence of the 5’-SS and the presence of an unpaired adenine in position -1.
Fig. 2. Structural basis for SMN-C5 selectivity.
a, Overlay of the 20 NMR structures of the RNA duplex. The U1 snRNA and the E7 5’-SS are shown in grey and blue, respectively. The unpaired A-1 is highlighted in red. b, Overlay of the 20 NMR structures of the complex formed by the RNA duplex and SMN-C5. The U1 snRNA, the E7 5’-SS and SMN-C5 are shown in grey, blue and yellow, respectively. c, Closed up view of the SMN-C5 binding pocket. Black dashed lines illustrate the direct hydrogen bonds between SMN-C5 and the RNA. d, On the left, superimposition of the structures of the SMN-C5 binding pockets in the presence (red) or in the absence (blue) of SMN-C5. On the right, the same structures are represented colorless and red arrows indicate the RNA conformational change induced by SMN-C5.
Mechanism of action of the splicing modifier
The comparison of the structures with and without SMN-C5 pinpoints to an RNA conformational switch induced by SMN-C5 resulting in a stabilization of the bulged adenine into the RNA helix base stack (Fig. 2d and Supplementary movie 3). The RNA conformational change was detected in the NMR spectra since addition of SMN-C5 triggered the broadening of the G-2 imino proton signal (Supplementary Fig. 1). Indeed, in the apo-structure, the base pair G-2-C8 stacks against G+1-C8 since A-1 is primarily excluded from the RNA base stack. However, the binding of SMN-C5 locks A-1 in the RNA base stack and, consequently, the base pairs G-2-C9 and G+1-C8 come apart and become more solvent exposed, explaining the broadening of G-2 (Supplementary movie 3). Previous structures of U1 snRNP have shown that the U1-C zinc finger stabilized the minor groove at the exon-intron junction during 5’-SS recognition32,33. The structure of the apo-RNA duplex revealed that the unpaired adenine at position -1 lies in the minor groove at the exon-intron junction and could interfere with U1-C binding. Modelling of the U1-C zinc finger recognition with our two structures reveals that the position of A-1 induced a major steric clash with the protein in the absence of the drug (Supplementary Fig. 6). However, the addition of SMN-C5 makes the minor groove accessible for optimal interactions with U1-C (Supplementary movie 4 and Supplementary Fig. 6). By mimicking a regular and stronger 5’-SS, SMN-C5 modifies the position of A-1 and could increase the binding of U1-C zinc finger to the RNA. Consistent with this hypothesis, we measured a 3-fold affinity increase of U1-C when SMN-C5 is present (Supplementary Fig. 7), that explains the difference we observed when we compared the binding of SMN-C5 to U1 snRNP-SMN2 5’-SS or to the RNA duplex alone (Fig. 1). Altogether, by stabilizing the bulge adenine -1 into the RNA helix base stack, SMN-C5 strengthens the interaction between the U1 snRNA and the weak 5’-SS of SMN2 E7 and allosterically promotes the binding of U1-C zinc finger.
Validation in cellular models
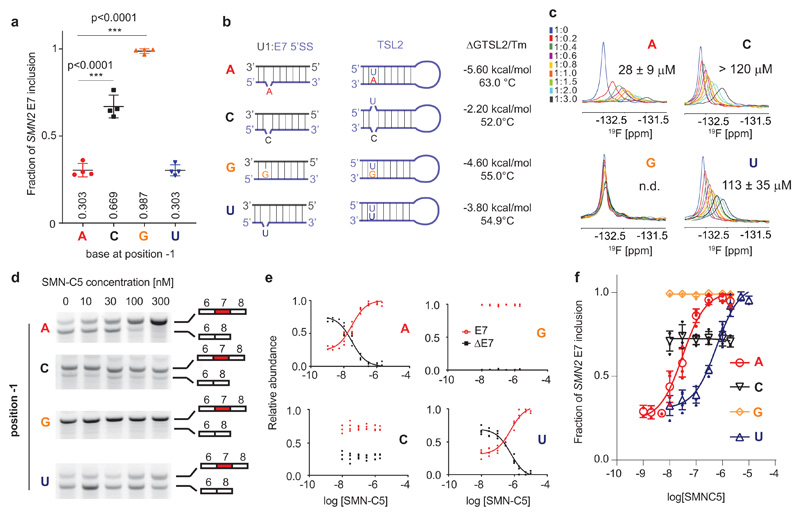
The recognition of the 5’-SS by U1 snRNP has been shown to be flexible; bulges are often created by the pairing of U1 snRNA with the 5’-SS and shifted base pairing is commonly observed in weak 5’-SS34,35. To probe the effect of the bulged nucleotide, we then evaluated the role of the position -1 in the splicing regulation of SMN2 E7. The adenine base was substituted in the SMN2 mini-gene and the effect of these mutations was evaluated in human cellular models (Fig. 3a). Both substitutions A-1G and A-1C promoted E7 inclusion in HEK293T cells. The A-1G substitution closes the bulge, boosts E7 inclusion and transforms the weak E7 5’-SS into a strong 5’-SS. Moreover, we could show that the inhibitory secondary structure TLS2 is destabilized by the mutation since the melting temperature of TLS2 A-1G is reduced by 8°C when compared with the wild-type TLS2 (Supplementary Fig. 8). In contrast, the A-1C mutation does not change the RNA duplex base pairing but reduces the TLS2 stability even further since its melting temperature is reduced by 11°C when compared to the wild-type (Fig. 3b). These experiments confirm previous observations showing that both the TLS2 stability and the complementarity between U1 snRNA and E7 5’-SS control E7 inclusion36,37. Also consistent with those, the A-1U substitution has a level of splicing similar to the wild-type SMN2. Although the TLS2 stability is reduced by 8°C and we could observe the formation of the U:U base pair (Supplementary Fig. 8) and a bulge U is likely to be present in the 5’SS duplex (Fig. 1). Taken together, these data confirmed that the position -1 of the 5’SS modulates the E7 inclusion level. Next, we evaluated the binding of SMN-C5 on the mutated RNA duplexes using 19F NMR spectroscopy (Fig. 3c). All three mutants A-1G, A-1C and A-1U show reduced binding affinity for the drug compared to the wild-type RNA duplex consistent with the specificity of the drug for an adenine at the -1 position. Then, we tested how the splicing modifier would act on these RNA mutants in cellular models (Fig. 3d and Supplementary Fig. 9). In this context, SMN-C5 acts very efficiently on the wild-type SMN2 mini-gene with an EC50 of 31 ± 10 nM. Consistent with the weak binding to A-1G and A-1C RNA duplexes, SMN-C5 does not change the splicing of A-1G and A-1C mini-genes (Fig. 3e). For the A-1U mutation, SMN-C5 can increase splicing inclusion of SMN2 E7, but at a much higher concentration (20-fold) with an EC50 = 598 ± 112 nM which is in agreement with the 4-fold lower affinity of SMN-C5 for the A-1U RNA duplex (Fig. 3f). Altogether, our experiments demonstrate that the adenine at position -1 is crucial for the optimal SMN-C5 activity in cellular models validating the mechanism of action proposed based on our structures.
Fig. 3. Validation of the SMN-C5 mode of action in cellular models.
a, Effect of base substitution at position -1 on SMN2 E7 splicing. All the experimental points are shown on the plot (symbols) as well as the mean and standard deviation (lines). One-way ANOVA test was performed to compare the effect of position -1 substitutions with respect to wild-type (N=4). b, Predictions for base pairing at the RNA duplex and TSL2. The predicted free energies and the measured melting temperatures of the TLS2 variants are given. Each melting curve was performed in duplicate. c, Overlay of the 19F NMR spectra of SMN-C5 as a function of the RNA duplex wild type, A-1C, A-1G and A-1U concentrations. The NMR spectra are colored according the SMN-C5:RNA ratios indicated on the panel. Each experiment was performed in duplicates. d, Evaluation of the effect of SMN-C5 on the splicing of SMN2 mini-genes that differs at the position -1. Pictures of the 2% agarose gels showing both RNA isoforms of SMN2 as a function of the SMN-C5 concentrations (N=4). Non-cropped pictures are shown in Supplementary Fig. 9. e, Plot showing the relative abundance of both RNA isoforms as a function of the logarithm of the SMN-C5 concentration. Experimental points are shown on plots and non-linear fits. f, The fraction of SMN2 E7 inclusion is plotted as a function of the logarithm of the SMN-C5 concentration. Experimental points and means are shown as plain and open symbols. Error bars represent standard errors (N=4).
SMN-C5 cooperates with the splicing regulatory network
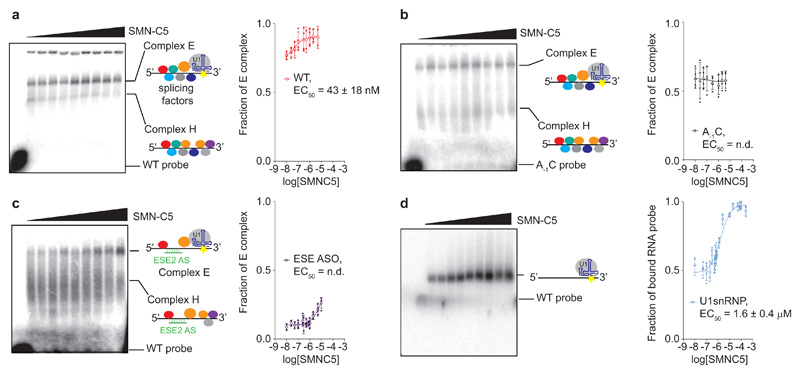
In this study, we observe an important difference between the binding affinity of SMN-C5 for the U1 snRNP in vitro (Kd ~ 15 μM) and the biological activity of the molecule in cellular models (EC50 ~ 30 nM). To understand the origins of this difference, we investigated the effect of the splicing regulatory network on the SMN-C5 activity. Although the SMN2 splicing modifiers were initially proposed to stabilize an enhancer complex on the SMN2 E724, the stabilized ribonucleoparticle was never clearly observed. We therefore prepared a radiolabelled SMN2 E7 pre-mRNA fragment (that extends from the 5’-end of the exon 7 until the 3’-end of the ISSN-1 silencer, see Methods) and monitored the formation of early spliceosomal complexes in human nuclear extracts using native gel electrophoresis. In absence of the splicing modifier, two protein-RNA complexes are observed. The most retarded complex disappears when the mixture is incubated at 4°C, upon addition of heparin or cold SMN2 E7 5’-SS oligonucleotide (Supplementary Fig. 10). Accordingly, the most retarded complex corresponds to the spliceosomal E complex38 in which U1 snRNP is bound to the 5’-SS together with splicing regulators. By increasing the SMN-C5 concentration, we observe that this E complex accumulates while the H complex disappears, confirming that the splicing modifier can also promote the recruitment of U1 snRNP on the SMN2 pre-mRNA in vitro (Fig. 4a). The E complex accumulation is SMN-C5 dose-dependent with an EC50 of 43 ± 18 nM, recapitulating well the EC50 determined in cellular models. The same experiment was performed with an RNA fragment harbouring a single point mutation at the 5’-SS outside of the conserved GU dinucleotide, the mutation A-1C. In absence of SMN-C5, we observed both the E and H complexes at ratio 60:40. However, addition of SMN-C5 does not promote E complex accumulation as observed with the wild type RNA (Fig 4b). This experiment demonstrates that the adenine in position -1 is an essential determinant for the SMN-C5 activity. To evaluate how the splicing regulatory network influences the splicing modifier activity, we performed the same experiment with an anti-sense oligonucleotide complementary to ESE2 (ESE2 ASO), a specific splicing enhancer previously shown to be important for SMN-C5 activity24. In presence of the ESE2 ASO, the proportion of E complex is strongly reduced and the H complex signal broadens, suggesting that the H complex is more heterogeneous. Upon addition of the splicing modifier, the E complex starts to accumulate at higher concentrations (micromolar doses) of SMN-C5. The splicing correction still occurs but it requires a larger amount of the small molecule. Since a SMN-C5 concentration higher than 10 μM induces nuclear extract precipitation, we were not able to determine a precise EC50 in presence of ESE2 ASO, however, the splicing correction is strongly shifted towards higher doses of splicing modifier (Fig. 4c). Altogether, the cis RNA element ESE2 modulates the splicing modifier activity. Finally, a similar experiment was performed using in vitro reconstituted U1 snRNP but without nuclear extracts. The same RNA probe was then incubated with increasing amounts of U1 snRNP to determine an optimal concentration of U1 snRNP in which both the free and bound probes are observed (Supplementary Fig. 8). Under these conditions, SMN-C5 promotes the binding of U1 snRNP on SMN2 E7 pre-mRNA in a dose dependent manner with a much higher EC50 of 1.6 ± 0.4 μM (Fig. 4d), which is in a similar range as the affinity constant of SMN-C5 to the U1 snRNP:5’SS complex using 19F NMR spectroscopy (Fig. 1f). These experiments strongly suggest that the nanomolar effect of SMN-C5 is due to a positive cooperativity between the splicing regulatory network and the splicing modifier39. SMN-C5 could promote the recruitment of U1snRNP and the splicing factors for efficient assembly of the splicing enhancer complex at nanomolar concentrations. The splicing modifier would act as an allosteric enhancer of the splicing reaction40,41. However, we cannot rule out that the splicing regulatory network positions U1 snRNP on the E7 5’-SS in a conformation prone to bind the splicing modifier with nanomolar affinity. This last scenario would be similar to a conformation selection process42,43. To conclude, the positive cooperativity between SMN-C5 and the splicing regulatory network results in optimal SMN2 splicing correction and explains the difference between the binding affinity of SMN-C5 for the U1 snRNP in vitro and the biological activity of the molecule in cellular models.
Fig. 4. Positive cooperativity between SMN-C5 and the splicing regulatory network.
a, Autoradiograph of the native gel showing the effect of SMN-C5 (from 10 nM to 2 μM) on the formation of early spliceosomal complexes in nuclear extracts. The free RNA probe, the H and E complexes are indicated. The colored circles on the scheme represent the splicing factors. The fraction of E complex was plotted as a function of the logarithm of the SMN-C5 concentration. b, Same experiment performed with the A-1C SMN2 E7 pre-mRNA fragment. The fraction of E complex was plotted as a function of the logarithm of the SMN-C5 concentration. c, Same experiment than a performed in the presence of the anti-sense oligonucleotide complementary to ESE2 (ESE2 ASO). The fraction of E complex was plotted as a function of the logarithm of the SMN-C5 concentration. d, Autoradiograph of the native gel showing the effect of SMN-C5 (from 800 nM to 250 μM) on the binding of in vitro reconstituted U1 snRNP on the SMN2 E7 pre-mRNA fragment. The positions of the free and bound RNA probes are indicated. The fraction of bound RNA probe was plotted as a function of the logarithm of the SMN-C5 concentration. For the four plots, experimental points and means are shown as plain and open symbols. The error bars represent the standard deviations. The experiment was performed four times (N=4) using the same batch of nuclear extracts.
Discussion
Pre-mRNA processing is an essential step of gene expression regulation and it has been estimated that 14% of all disease-associated point mutations affect splice site selection44,45. Such mutations cause aberrant splicing of relevant genes in numerous diseases and the identification of small molecules acting on RNA processing constitutes an emerging field of drug discovery. Several general inhibitors of the splicing cycle have been previously identified as isoginkgetin which blocks the transition between A and B spliceosomal complexes46,47. More recently, a general splicing modulator with antitumor activity, called pladienolide B, was shown to stall SF3B, a component of U2 snRNP, in an open conformation, therefore inhibiting the recruitment of U2 snRNP at the branch point48. Blocking the general mechanism of pre-mRNA splicing could induce cancer cell death, but targeting pre-mRNA and gene expression at gene resolution to restore specific function in the cell still remains challenging using small molecules. SMN2 splicing modifiers represent the first example of gene-specific RNA-processing drug12,13.
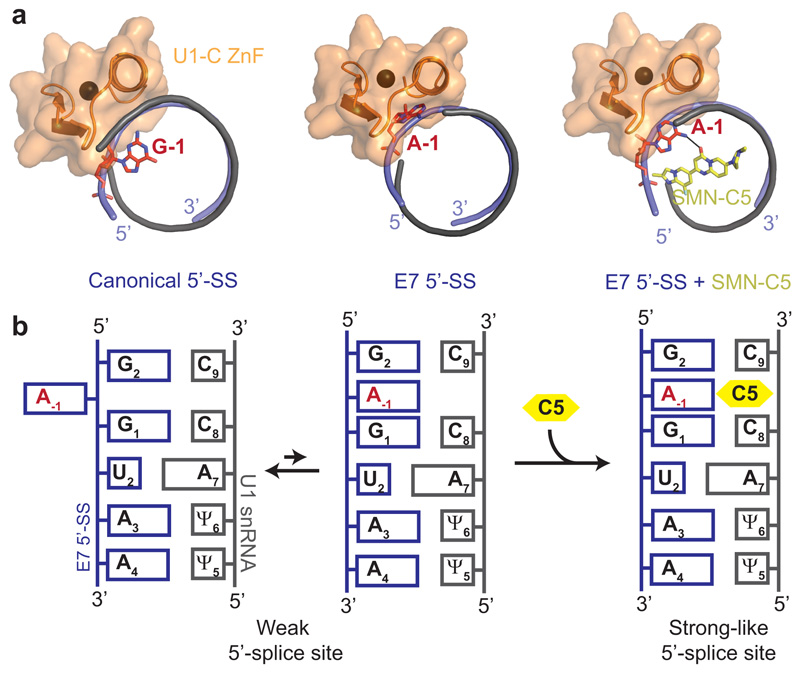
In this report, we could show that the SMN2 splicing modifier SMN-C5 stabilizes U1 snRNP on the SMN2 E7 5’-SS by binding the intermolecular RNA helix in a 5’-SS specific manner. The solution structure of the complex formed by the RNA duplex and SMN-C5 revealed that SMN-C5 binds the RNA in the major groove at the exon-intron junction, as we previously proposed from sparse NMR data24. In the structure, SMN-C5 contacts the ribose-phosphate backbone of the U1 snRNA through its positively charged piperazine moiety while the central ring of the molecule inserts between C8 and C9 to form a direct hydrogen bond with the adenine -1 of the SMN2 exon 7. The drug is selective to the bulged formed by the unpaired A-1 located at the last position of the exon and functions as a bona fide splicing factor. Indeed, SMN-C5 creates additional contacts between U1 snRNP and the pre-mRNA and increases the stability of the intermolecular RNA helix formed upon 5’-SS recognition. By understanding the mode of action of the first gene-specific RNA-processing drug at the atomic level, we uncovered a novel concept for splicing correction that we coined 5’-splice site bulge repair (Fig. 5). This concept demonstrates that helping U1 snRNP to land on weak 5’-SS is a major avenue to help curing splicing disorders induced diseases.
Fig. 5. The concept of 5’-SS bulge repair.
a, Ribbon representations of different RNA duplexes bound to U1-C zinc finger. On the left side, the structure was extracted from the crystal structure of U1 snRNP containing a canonical 5’-SS33. On the middle and right panels, the position of U1-C zinc finger was modelled on the solution structures of the RNA duplex or the RNA duplex bound to SMN-C5. b, Schematic representation of the 5’-SS bulge repair concept.
Using in cell SMN2 splicing assays and spliceosome assembly experiments in nuclear extracts, we could validate the SMN-C5 mechanism of action since an adenine in position -1 is essential for SMN-C5 optimal activity. However, due to the large difference between the in vitro RNA binding activity (micromolar range) and the molecule biological activity (nanomolar range), we further investigated the effect of the splicing regulatory network on the SMN-C5 activity. A single point mutation on the adenine -1 abolishes the activity of SMN-C5, confirming that the 5’-SS is the primary SMN-C5 target. We and others24,29 previously observed that SMN-C5 is sensitive to the presence of ESE2, a purine rich element known to recruit specific splicing enhancers. However, by blocking the accessibility of ESE2 to splicing factors using an anti-sense oligonucleotide, the formation of the E complex was still sensitive to the splicing modifier but required larger concentrations of SMN-C5 (micromolar range). Altogether, our analysis confirmed the dominant role of the 5’-SS for SMN-C5 activity and that the splicing modifier cooperates with the splicing regulatory network to achieve optimal activity. Although we could show that SMN-C5 allosterically promotes the binding of U1-C to trigger the splicing correction, we propose that SMN-C5 positively cooperates with splicing enhancers to help U1 snRNP recognizing the weak SMN2 E7 5’-SS. However, we cannot rule out that additional specific splicing factors could also contribute to additional direct interactions with the splicing modifier. Notably, the recent cryo-electron microscopy structure of the human pre-B spliceosome complex captured before U1 snRNP dissociation revealed that another protein called Prp28 also contacts the minor groove of the intermolecular helix formed upon 5’-splice site recognition49. Prp28 binding could also contribute to the difference in SMN-C5 activity we observed between in vitro and in cell activities. Solving the structure of the E complex assembled on the SMN2 exon 7 RNA in presence of SMN-C5 could answer all these questions.
To conclude, this study uncovered the mode of action of SMN2 splicing modifiers at atomic resolution and will stimulate the optimization of the current clinical molecules to increase their potency and specificity. Furthermore, as numerous bulges similar to this one are created in several disease-causing mutations34, the mechanism of 5’-SS bulge repair should help the design of novel drugs to correct other splicing defects.
Online Methods
Cloning, expression and purification of the U1 snRNP protein components
The U1-A open reading frame (ORF) was cloned into pET26bII in fusion with a hexa-histidine tag cleavable by thrombin. The U1-C and U1-70K (1-216) ORFs were cloned in fusion with a C-terminal hexa-histidine tag using the pET28a plasmid (Novagen). U1-A, U1-C and U1-70K were expressed in Escherichia coli BL21 DE3 at 37°C, 25°C and 20°C, respectively. The Sm protein ORFs were combined into polycistronic genes and inserted into pET26bII to express the following combinations: Sm B-D3, Sm D1-D2 and Sm E-F-G. The expression of Sm B-D3, Sm D1-D2 and Sm E-F-G have been performed in Escherichia coli BL21 DE3 at 20°C, 25°C and 37°C during 12h, 12h and 24h, respectively.
Sm B-D3 was solubilized in buffer A (Hepes 20 mM pH 7.8, NaCl 1 M, Urea 2 M, β-mercapto-ethanol 2.8mM). The clarified cell lysate was loaded at 0.5 ml/min on a 5 ml HisTrap column (GE Healthcare) at 4°C, washed with buffer A and eluted with buffer B (Hepes 20 mM pH 7.8, NaCl 1 M, imidazole 500 mM, β -mercapto-ethanol 2.8 mM). The heterodimer Sm B-D3 was cleaved by thrombin (Sigma) and further purified by size exclusion chromatography (SEC) in buffer C (Na/K phosphate buffer 10 mM pH 5.5, DTT 5 mM). Sm D1-D2 was solubilized in buffer E (Hepes 20 mM pH 7.4, NaCl 0.5 M, Urea 0.5 M, DTT 10 mM, EDTA 1mM). The clarified cell lysate was loaded at 2 ml/min on a 5 ml HiTrap SP column (GE Healthcare) at 4°C, washed with buffer E and eluted with buffer F (Hepes 20 mM pH 7.4, NaCl 2 M, DTT 10 mM, EDTA 1mM). Sm D1-D2 was further purified by SEC in buffer G (sodium-phosphate buffer 10 mM pH 6.8, NaCl 50 mM, DTT 5 mM). Sm E-F-G was solubilized in buffer H (Hepes 20 mM pH 7.5, NaCl 0.5 M, Urea 0.5 M, imidazole 10 mM, β -mercapto-ethanol 2.8 mM). The clarified cell lysate was loaded at 2 ml/min on a 5 ml HisTrap column (GE Healthcare) at room temperature, washed with buffer H and eluted with buffer I (Hepes 20 mM pH 7.5, NaCl 0.5 M, imidazole 500 mM, β-mercapto-ethanol 2.8 mM). The heterotrimer was cleaved by thrombin (Sigma) and further purified by sec in buffer J (Hepes 20 mM pH 7.5, NaCl 0.5 M, DTT 5 mM). U1-70k (1-216) was solubilized in buffer K (Hepes 20 mM pH 7.5, NaCl 1 M, Urea 0.5 M, imidazole 10 mM, β-mercapto-ethanol 2.8 mM). The clarified cell lysate was loaded at 2 ml/min on a 5 ml HisTrap column (GE Healthcare) at room temperature previously equilibrated with buffer K. The column was extensively washed with buffer K and eluted with a linear gradient of buffer L (Hepes 20 mM pH 7.5, NaCl 0.5 M, imidazole 300 mM, β-mercapto-ethanol 2.8 mM). Fractions enriched for U1-70k were flash frozen in liquid nitrogen and stored at -80°C. U1-A was solubilized in buffer M (Hepes 20 mM pH 7.5, NaCl 0.5 M, Urea 0.5 M). The clarified cell lysate was loaded at 2 ml/min on a 5 ml HisTrap column (GE Healthcare) at room temperature, washed with buffer M and eluted with buffer N (Hepes 20 mM pH 7.5, NaCl 0.5 M, imidazole 300 mM). The protein was cleaved by thrombin (Sigma) at room temperature and diluted 5 times to reduce the NaCl concentration to 100 mM. The cleavage reaction was loaded on a 5 ml HiTrap SP column (GE Healthcare) at room temperature previously equilibrated with buffer O (Hepes 20 mM pH 7.5, NaCl 0.1 M), washed with buffer O and U1-A was eluted with buffer P (Hepes 20 mM pH 7.5, NaCl 2 M), concentrated and further purified by sec in buffer Q (sodium-phosphate buffer 10 mM pH 6.8, NaCl 50 mM, EDTA 0.5 mM, DTT 5 mM). U1-C was solubilized in buffer R (Hepes 10 mM pH 7.8, NaCl 0.1 M, Urea 0.5 M, β-mercapto-ethanol 2.8 mM). The clarified cell lysate was loaded on a 5 ml HisTrap column (GE Healthcare) at 4°C. The column was washed with buffer R, 10% buffer S (Hepes 10 mM pH 7.8, NaCl 0.1 M, Urea 0.5 M, Imidazole 250 mM, β-mercapto-ethanol 2.8 mM) and U1-C was eluted with 100% buffer S. The protein was concentrated at 4°C and further purified by sec in buffer T (sodium-phosphate buffer 10 mM pH 6.8, NaCl 50 mM, DTT 5 mM). Elution fractions were flash frozen in liquid nitrogen and stored at -80°C. The GST-U1-C zinc finger (1-61) was expressed from pGEX4T3-U1C and solubilized in buffer U (Tris 0.1 M pH 7.5, NaCl 0.1M, β-mercapto-ethanol 2.8 mM) and bound on a 5ml GSTrap column (GE Healthcare) at 4°C. The column was washed extensively with buffer U and eluted with a gradient of reduced glutathione. The protein was then cleaved by thrombin (Sigma) and the U1-C zinc finger was further purified by sec in buffer V (Tris 10 mM pH 7.2, NaCl 50mM, MgSO4 50 mM, DTT 5 mM). Isotopically labelled proteins were expressed in M9 medium complemented with 1 g/L of 15NH4Cl (Cambridge Isotopes).
RNA preparation
The U1 snRNA was transcribed in vitro from pUC19-U1 snRNA previously linearized by Sal1 using homemade T7 RNA polymerase. pUC19-U1 snRNA contains a T7 promoter followed by the hammerhead ribozyme in fusion with the sequence coding for the U1 snRNA and a Sal1 cleavage site. To prepare the RNA probes used for EMSA, we designed and ordered (General Biosystem) a plasmid (pUC57-SMN2 E7 RNA) encoding for the hammerhead ribozyme fused to the entire SMN2 exon 7 followed by the 5’-SS and the ISSN-1 (SMN2 E7 RNA sequence: 5’-GGUUUUAGACAAAAUCAAAAAGAAGGAAGGUGCUCACAUUCCUUAAAUUAAGGAGUAAGUCUGCCAGCAUUAUGAAAGUGAAUCA-3’; position -1 is highlighted in bold). The plasmid was linearized by HindIII and used as template for T7 in vitro transcription. The transcription mixtures were applied to High Performance Liquid Chromatography (HPLC) system that allows the RNA separation on an anion exchange column at 85°C and in the presence of 6M Urea. Fractions containing the U1 snRNA or the SMN2 E7 RNA were precipitated using butanol and dissolved in water (3 times) to remove urea. The RNA were then refolded, lyophilized and stored at -20°C. The 5’-splice site of SMN2 exon 7 (5'-GGAGUAAGUCU-3'), the regular 5’-splice site (5'-GAGGUAAGUAU-3') and the U1 snRNA 5’-end (5’-AUACψψACCUG-3’) were purchased (Dharmacon). The ESE2 ASO was chemically synthetized (Microsynth) as a 2’-O-methyl RNA to avoid rapid degradation in nuclear extract. The ESE2 ASO has the following sequence: 5’-CCUUCCUUCUUUUU-3’. To analyze the stability of TLS2 variants, synthetic RNA oligonucleotides were used as DNA template for in vitro transcription. A solution of 5μM of each RNA variants was then used to monitor the thermal denaturation by UV using a CARY300 spectrophotometer. The free energy predictions of TLS2 variants were performed on the RNA-fold webserver (https://rna@tbi.univie.ac.at).
Reconstitution and purification of U1 snRNP particles
In a prewarmed tube (37°C), 0.1 volume of 10X reconstitution buffer (sodium-phosphate buffer 0.1 M pH 6.8, NaCl 0.5 M, DTT 20 mM) was mixed with 1 molar equivalent of all the Sm proteins. The mixture was incubated 5 minutes at 37°C before addition 1 molar equivalent of the refolded U1 snRNA. After 5 minutes at 37°C, 1 molar equivalent of U1-70k was added. The particle was further decorated with U1-A prior the addition of the 5’-splice site. Finally, U1-C was added in equimolar amount and the particles were concentrated at 18°C. Reconstituted particles were further purified using SEC (S200, GE Healthcare) in the buffer T (Hepes 10 mM pH 7.4, NaCl 50 mM, DTT 1 mM).
Splicing modifier preparation
The splicing modifiers were prepared as already described31,50. The splicing modifiers were resuspended in buffer T (Hepes 10 mM pH 7.4, NaCl 50 mM, DTT 1 mM) or in the NMR buffer (MES d-4 5 mM pH 5.5, NaCl 50 mM) complemented with 6% d8-glycerol (Cambridge Isotopes). For SMN-Core, 5% d6-DMSO (Cambridge Isotopes) was added to increase compound solubility.
Electrophoretic mobility shift assays
The RNA probes were radiolabeled directly using γ-32P ATP (Hartmann chemical) and diluted to 21 fmoles/μl. To observed the formation of the E complex, the RNA probes were incubated with human Hela nuclear extract (Ipracell) at ~ 0,6 mg/ml (final concentration) in presence of increasing amount of SMN-C5 (from 10 nM to 10 μM), yeast tRNA (0.025 μg/ml), Polyvinyl-alcohol (1.3%), RNAsin (Promega; diluted 40-times) at 30°C during 30 minutes. Alternatively, the ESE ASO was added at a final concentration of 2 μM. To observe the association between U1 snRNP, SMN-C5 and the pre-mRNA, the same strategy was followed and the nuclear extract was replaced by in vitro reconstituted U1 snRNP. We first determined the U1 snRNP concentration required to upshift half of the RNA (500 nM, see Supplementary Fig. 8). Using these conditions, the effect of SMN-C5 on the binding of U1 snRNP to the pre-mRNA fragment was further investigated. The mixture was then loaded onto a 1%-Agarose gel and separated in Tris-Glycine buffer (50 mM each, pH 8) at room temperature for 1 hour at 100V. Gel were exposed and revealed with a phosphor imager. Autoradiographs were analyzed using Image Studio Lite (LI-COR) and the data were analyzed with GraphPad (GraphPad software).
In vitro binding assays followed by NMR spectroscopy
To test the binding of SMN-C5 on U1 snRNP particles, SMN-C5 was dissolved at 50 μM in buffer T in the presence of 10% D2O and reference spectra (1D 1H and 1D 19F) were recorded using a cryo-probed AV IIIHD 600 MHz NMR spectrometer (Bruker) at 298K. The U1 snRNP solution (250 μM) was progressively added to the SMN-C5 sample and after each addition; 1D 1H and 1D 19F spectra were recorded. After the completion of the titration, 10 μl of RNAse A (1 mg/ml), 5 μl of RNAse T1 (1U/μl) and 5 μl of Benzonase (1U/μl) were added to the complex and series of 1D 1H and 1D 19F spectra were recorded.
To test the binding of the splicing modifiers on the RNA duplex, the different splicing modifiers (SMN-C5, SMN-CX, SMN-CY and SMN-Core) were solubilized at 2.5 mM in the NMR buffer complemented with 6% d8-glycerol (Cambridge Isotopes) and 5% d6-DMSO (Cambridge Isotopes) in the case of SMN-Core. The RNA duplex was dissolved at 250 μM in the NMR buffer containing 10% D2O. The NMR resonances of the RNA duplex were followed by 1D 1H, 1D 31P and 2D 1H-1H TOCSY using a cryo-probed AV III 600 MHz NMR spectrometer (Bruker) at 293K. The binding of the RNA duplexes variants to SMN-C5 was performed by following the NMR resonances of the splicing modifiers with 1D 1H and 1D 19F experiments using a cryo-probed AV IIIHD 600 MHz NMR spectrometer (Bruker) at 293K.
To investigate the effect of the splicing modifier on the interaction between the U1-C zinc finger and the RNA duplex, 15N-labelled U1-C zinc finger was prepared at 0.2 mM in Hepes 10 mM pH 7.0, NaCl 100 mM, MgCl2 1mM and β-mercapto-ethanol 2.5 mM complemented with 10% D2O. The RNA duplex with or without SMN-C5 (3 times excess compared to the RNA) was added to the protein sample progressively. For the ratio U1C:RNA (1:0; 1:0.5; 1:1; 1:2; 1:3; 1:4 and 1:5); 1D 1H and 2D 1H-15N HSQC were recorded after each SMN-C5 addition using a cryo-probed AV IIIHD 600 MHz NMR spectrometer (Bruker) at 293K.
All the NMR spectra were processed using Topspin (Bruker) and peak lists were generated using CARA51. Chemical shift changes were calculated and normalized using Excel. Curve fit was performed using GraphPad Prism 7.04.
NMR resonance assignment
In order to assign the resonances of the SMN-C5 splicing modifier, a 400 μM sample of SMN-C5 prepared in the NMR buffer (100% D2O) has been used to record 2D homonuclear experiments (2D 1H-1H TOCSY and 2D 1H-1H NOESY) on the cryo-probed 500 MHz AVIII NMR spectrometer (Bruker) at 293K. Spectra were processed using Topspin (Bruker) and analyzed using CARA. In order to facilitate the resonance assignment process, chemical shifts of SMN-C5 were predicted on the nmrdb.org server52.
The RNA duplex resonance assignment was performed by combining 2D homonuclear NMR spectroscopy experiments (2D 1H-1H TOCSY and 2D 1H-1H NOESY) together with natural abundance 2D 1H-13C HSQC. Most experiments were recorded on the cryo-probed AVIIIHD 900 MHz NMR spectrometer at 293K. In addition, a 5’-SS E7 RNA strand was chemically synthesized using 13C-labeled adenine-phosphoramidate precursors and combined with an unlabeled U1 snRNA 5’-end oligonucleotide to form an RNA duplex sample on which the adenine nucleotides from the 5’-SS E7 are 13C-labeled on their ribose moieties. Using this sample, a 3D 1H-13C HSQC NOESY has been recorded (mixing time of 150 ms) to help assignment.
The RNA duplex-SMN-C5 (ratio 1:2) resonance assignment was performed by combining 2D homonuclear NMR spectroscopy experiments (2D 1H-1H TOCSY and 2D 1H-1H NOESY) together with natural abundance 2D 1H-13C HSQC. The intermolecular NOE were identified on the 2D 1H-1H NOESY. As previously described for the RNA duplex alone, an RNA duplex sample on which the adenine nucleotides from the 5’-SS E7 are 13C-labeled on the ribose moieties was used to record a 3D 1H-13C HSQC NOESY on which intramolecular and intermolecular connections were identified. All experiments were recorded on the cryo-probed AVIIIHD 900 MHz NMR spectrometer at 293K.
Structure calculation
In order to determine the structure of the RNA duplex, the base pairing was first deduced from the analysis of the 2D 1H-1H NOESY recorded in 90% H2O. All the hydrogen bonds have been identified via unambiguous NMR signals except for both terminal base pairs. The Watson-Crick base pairing information was used as a source of restraints. Based on the analysis of the 2D 1H-1H TOCSY, most of the ribose conformations have been defined as C2’-endo and angular restraints have been defined. The base pairing and the angular restraints were combined with the chemical shifts and the experimental NOESY spectra recorded in 100% D2O (2D 1H-1H NOESY and 3D 1H-13C HSQC NOESY) for automatic NOE assignment and structure calculation using CYANA 3.253. The resulting structure was further refined using the SANDER module of AMBER1254. The AMBER parameters for the pseudo-uridines (http://research.bmh.manchester.ac.uk/bryce/amber) were updated to current amber RNA default (bsc0chiOL3). Analysis of the calculation was performed using AMBER and structures were aligned and visualized using UCSF Chimera55 and PyMol (Schrödinger), respectively.
To determine the structure of the RNA duplex-SMN-C5 complex, we first determined the structure of the RNA within the complex. As described for the free RNA duplex, the base pairing was analyzed based on the 2D 1H-1H NOESY recorded in 90% H2O. Angular restraints for the sugar pucker were derived from the analysis of the 2D 1H-1H TOCSY. Then, we combined this information with the chemical shifts and the experimental NOESY spectra recorded in 100% D2O (2D 1H-1H NOESY and 3D 1H-13C HSQC NOESY) for automatic NOE assignment and structure calculation using CYANA 3.253. A set of intramolecular NOE-derived distances was derived from the automatic analysis. In order to include SMN-C5 in the CYANA calculation, a coordinate file together with a definition file for CYANA were generated using the Win!P software (http://www.biochem-caflisch.uzh.ch/download). Due to the excess of SMN-C5 compared to the RNA duplex, strong NMR signals due to the presence of free SMN-C5 impair the automatic analysis of the intermolecular NOEs. Therefore, intermolecular NOE correlations were identified, integrated and calibrated manually. Initial structure calculations of the RNA duplex-SMN-C5 complex were performed using CYANA in the torsion-angle space. The structures were further refined in the Cartesian space using the sander module of AMBER12. The AMBER parameters for the SMN-C5 were generated using the Antichamber module of AMBER12. These files are available upon request to S.C. Analysis of the calculation was performed using AMBER and structures were aligned and visualized using UCSF Chimera55 and PyMol (Schrödinger), respectively.
Molecular modeling
To model how U1-C zinc finger could recognize the RNA duplex with or without SMN-C5, the NMR structures of the RNA duplexes (model 1 of each ensemble) were aligned on the structure of U1-C zinc finger bound to the RNA duplex formed with the canonical 5’-SS33 using PyMol (Schrödinger).
Cell culture and plasmids
HEK293T (human embryonic kidney) cells were obtained from the European Collection of Cell Cultures (ECACC No. 85120602) and were cultured in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% fetal bovine albumin (FBS) and 1% streptomycin/penicillin. The pCI-SMN2 plasmid containing the SMN2 mini-gene was previously described15. The SMN2 mini-gene mutants were generated by side-directed mutagenesis using specific primers.
SMN2 splicing assays
One microgram of pCI-SMN2 (wild-type or mutant) was transfected in HEK293T cells using the Lipofectamine 2000 Reagent (Lifetechnologies) according to the manufacturers protocol. The cells were previously counted and seeded 24 hours in advance at 400’000 cells/well. After 24 hours, the medium was replaced by fresh medium containing SMN-C5 at the following concentrations (0, 1, 2, 5, 10, 30, 100, 300, 1000, 2000, 5000 or 10000 nM). The cells were kept at 37°C for 7 hours after which cells were harvested. Total RNA was extracted and 1 μg was used for reverse transcription using Oligo(dT)15 primer and M-MLV Reverse Transcriptase RNase (H-) (Promega). 10% of the resulting cDNA was then used for semiquantitative PCR using a vector specific forward primer (pCI-fwd 5´-GGTGTCCACTCCCAGTTCAA-3´) and a SMN2 specific reverse primer (SMN2rev 5´-GCCTCACCACCGTGCTGG-3). The PCR products were separated on a 2%-TBE Agarose gel migrated at 80V during 3 hours and stained with GelRed (Biotium). Band integration was performed Image Studio Lite (LI-COR) and the ratio between both isoforms for each condition was determined. Experiments were repeated four times independently allowing for the calculation of the mean and standard error of the mean for each assay. Data were analyzed using GraphPad Prism 7.04 (GraphPad Software).
Supplementary Material
Acknowledgments
We thank F. Tessaro, A. Gossert and M. Krepl for their assistance with molecular dynamics set up, K. McCarthy and F. Metzger for useful discussions and the RNA synthesis platform of the NCCR RNA and Diseases. This work was supported by the Swiss National Science Foundation, the NCCR RNA and Diseases, cure-SMA and SMA-Europe.
Footnotes
Data availability. The chemical shifts and the atomic coordinates of the RNA duplex with or without SMN-C5 have been deposited in the Biological Magnetic Resonance Bank (BMRB ID: 34311 and 34312) and in the Protein Data Bank (PDB ID: 6HMI and 6HMO). Other data and materials are available from the authors upon reasonable request.
Authors contributions:
S.C, A.C. and F.A. designed the research. S.C. and L.G. performed gel shift assays. S.C., S.B. and A. M. performed the splicing assays in cellular models. S.C. and S.R. performed NMR data collection. A.K. and J.H. synthetized the isotopically labelled RNA. H.R. furnished the splicing modifiers. S.C. analyzed the data and solved the structures. S.C. and F.A. wrote the manuscript.
Competing interests:
Hasane Ratni is an employee of F. Hoffmann-La Roche Ltd.
References
- 1.Pearn J. Classification of spinal muscular atrophies. Lancet. 1980;1:919–922. doi: 10.1016/s0140-6736(80)90847-8. [DOI] [PubMed] [Google Scholar]
- 2.Paushkin S, Gubitz AK, Massenet S, Dreyfuss G. The SMN complex, an assemblyosome of ribonucleoproteins. Curr Opin Cell Biol. 2002;14:305–312. doi: 10.1016/s0955-0674(02)00332-0. [DOI] [PubMed] [Google Scholar]
- 3.Rochette CF, Gilbert N, Simard LR. SMN gene duplication and the emergence of the SMN2 gene occurred in distinct hominids: SMN2 is unique to Homo sapiens. Hum Genet. 2001;108:255–266. doi: 10.1007/s004390100473. [DOI] [PubMed] [Google Scholar]
- 4.Cartegni L, Krainer AR. Disruption of an SF2/ASF-dependent exonic splicing enhancer in SMN2 causes spinal muscular atrophy in the absence of SMN1. Nat Genet. 2002;30:377–384. doi: 10.1038/ng854. [DOI] [PubMed] [Google Scholar]
- 5.Kashima T, Manley JL. A negative element in SMN2 exon 7 inhibits splicing in spinal muscular atrophy. Nat Genet. 2003;34:460–463. doi: 10.1038/ng1207. [DOI] [PubMed] [Google Scholar]
- 6.Burghes AH, Beattie CE. Spinal muscular atrophy: why do low levels of survival motor neuron protein make motor neurons sick? Nat Rev Neurosci. 2009;10:597–609. doi: 10.1038/nrn2670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mailman MD, et al. Molecular analysis of spinal muscular atrophy and modification of the phenotype by SMN2. Genet Med. 2002;4:20–26. doi: 10.1097/00125817-200201000-00004. [DOI] [PubMed] [Google Scholar]
- 8.Marquis J, et al. Spinal muscular atrophy: SMN2 pre-mRNA splicing corrected by a U7 snRNA derivative carrying a splicing enhancer sequence. Mol Ther. 2007;15:1479–1486. doi: 10.1038/sj.mt.6300200. [DOI] [PubMed] [Google Scholar]
- 9.Hua Y, Vickers TA, Baker BF, Bennett CF, Krainer AR. Enhancement of SMN2 exon 7 inclusion by antisense oligonucleotides targeting the exon. PLoS Biol. 2007;5:e73. doi: 10.1371/journal.pbio.0050073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rogalska ME, et al. Therapeutic activity of modified U1 core spliceosomal particles. Nat Commun. 2016;7:11168. doi: 10.1038/ncomms11168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Garcia-Lopez A, et al. Targeting RNA structure in SMN2 reverses spinal muscular atrophy molecular phenotypes. Nat Commun. 2018;9:2032. doi: 10.1038/s41467-018-04110-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Naryshkin NA, et al. Motor neuron disease. SMN2 splicing modifiers improve motor function and longevity in mice with spinal muscular atrophy. Science. 2014;345:688–693. doi: 10.1126/science.1250127. [DOI] [PubMed] [Google Scholar]
- 13.Palacino J, et al. SMN2 splice modulators enhance U1-pre-mRNA association and rescue SMA mice. Nat Chem Biol. 2015;11:511–517. doi: 10.1038/nchembio.1837. [DOI] [PubMed] [Google Scholar]
- 14.Hua Y, et al. Peripheral SMN restoration is essential for long-term rescue of a severe spinal muscular atrophy mouse model. Nature. 2011;478:123–126. doi: 10.1038/nature10485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hua Y, Vickers TA, Okunola HL, Bennett CF, Krainer AR. Antisense masking of an hnRNP A1/A2 intronic splicing silencer corrects SMN2 splicing in transgenic mice. Am J Hum Genet. 2008;82:834–848. doi: 10.1016/j.ajhg.2008.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Beusch I, Barraud P, Moursy A, Clery A, Allain FH. Tandem hnRNP A1 RNA recognition motifs act in concert to repress the splicing of survival motor neuron exon 7. Elife. 2017;6 doi: 10.7554/eLife.25736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hache M, et al. Intrathecal Injections in Children With Spinal Muscular Atrophy: Nusinersen Clinical Trial Experience. J Child Neurol. 2016;31:899–906. doi: 10.1177/0883073815627882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Foust KD, et al. Rescue of the spinal muscular atrophy phenotype in a mouse model by early postnatal delivery of SMN. Nat Biotechnol. 2010;28:271–274. doi: 10.1038/nbt.1610. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 19.Vigevani L, Valcarcel J. Molecular biology. A splicing magic bullet. Science. 2014;345:624–625. doi: 10.1126/science.1258444. [DOI] [PubMed] [Google Scholar]
- 20.Cully M. Neuromuscular disorders: Beefing up the right splice variant to treat spinal muscular atrophy. Nat Rev Drug Discov. 2014;13:725. doi: 10.1038/nrd4445. [DOI] [PubMed] [Google Scholar]
- 21.Mendell JR, et al. Single-Dose Gene-Replacement Therapy for Spinal Muscular Atrophy. N Engl J Med. 2017;377:1713–1722. doi: 10.1056/NEJMoa1706198. [DOI] [PubMed] [Google Scholar]
- 22.Schneider R. CIM Journal Club: Gene therapy for spinal muscular atrophy Comment on Mendell et al. N Engl J Med 2017;377:1713-22. Clin Invest Med. 2018;41:E31–E33. doi: 10.25011/cim.v41i1.29461. [DOI] [PubMed] [Google Scholar]
- 23.Sturm S, et al. A phase 1 healthy male volunteer single escalating dose study of the pharmacokinetics and pharmacodynamics of risdiplam (RG7916, RO7034067), a SMN2 splicing modifier. Br J Clin Pharmacol. 2019;85:181–193. doi: 10.1111/bcp.13786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sivaramakrishnan M, et al. Binding to SMN2 pre-mRNA-protein complex elicits specificity for small molecule splicing modifiers. Nat Commun. 2017;8:1476. doi: 10.1038/s41467-017-01559-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Clery A, et al. Molecular basis of purine-rich RNA recognition by the human SR-like protein Tra2-beta1. Nat Struct Mol Biol. 2011;18:443–450. doi: 10.1038/nsmb.2001. [DOI] [PubMed] [Google Scholar]
- 26.Moursy A, Allain FH, Clery A. Characterization of the RNA recognition mode of hnRNP G extends its role in SMN2 splicing regulation. Nucleic Acids Res. 2014;42:6659–6672. doi: 10.1093/nar/gku244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hofmann Y, Wirth B. hnRNP-G promotes exon 7 inclusion of survival motor neuron (SMN) via direct interaction with Htra2-beta1. Hum Mol Genet. 2002;11:2037–2049. doi: 10.1093/hmg/11.17.2037. [DOI] [PubMed] [Google Scholar]
- 28.Young PJ, et al. SRp30c-dependent stimulation of survival motor neuron (SMN) exon 7 inclusion is facilitated by a direct interaction with hTra2 beta 1. Hum Mol Genet. 2002;11:577–587. doi: 10.1093/hmg/11.5.577. [DOI] [PubMed] [Google Scholar]
- 29.Wang J, Schultz PG, Johnson KA. Mechanistic studies of a small-molecule modulator of SMN2 splicing. Proc Natl Acad Sci U S A. 2018;115:E4604–E4612. doi: 10.1073/pnas.1800260115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ratni H, et al. Discovery of Risdiplam, a Selective Survival of Motor Neuron-2 (SMN2) Gene Splicing Modifier for the Treatment of Spinal Muscular Atrophy (SMA) J Med Chem. 2018;61:6501–6517. doi: 10.1021/acs.jmedchem.8b00741. [DOI] [PubMed] [Google Scholar]
- 31.Pinard E, et al. Discovery of a Novel Class of Survival Motor Neuron 2 Splicing Modifiers for the Treatment of Spinal Muscular Atrophy. J Med Chem. 2017;60:4444–4457. doi: 10.1021/acs.jmedchem.7b00406. [DOI] [PubMed] [Google Scholar]
- 32.Pomeranz Krummel DA, Oubridge C, Leung AK, Li J, Nagai K. Crystal structure of human spliceosomal U1 snRNP at 5.5 A resolution. Nature. 2009;458:475–480. doi: 10.1038/nature07851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kondo Y, Oubridge C, van Roon AM, Nagai K. Crystal structure of human U1 snRNP, a small nuclear ribonucleoprotein particle, reveals the mechanism of 5' splice site recognition. Elife. 2015;4 doi: 10.7554/eLife.04986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Roca X, et al. Widespread recognition of 5' splice sites by noncanonical base-pairing to U1 snRNA involving bulged nucleotides. Genes Dev. 2012;26:1098–1109. doi: 10.1101/gad.190173.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Roca X, Krainer AR. Recognition of atypical 5' splice sites by shifted base-pairing to U1 snRNA. Nat Struct Mol Biol. 2009;16:176–182. doi: 10.1038/nsmb.1546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Singh NN, Lee BM, Singh RN. Splicing regulation in spinal muscular atrophy by an RNA structure formed by long-distance interactions. Ann N Y Acad Sci. 2015;1341:176–187. doi: 10.1111/nyas.12727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Singh NN, Singh RN, Androphy EJ. Modulating role of RNA structure in alternative splicing of a critical exon in the spinal muscular atrophy genes. Nucleic Acids Res. 2007;35:371–389. doi: 10.1093/nar/gkl1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rhode BM, Hartmuth K, Urlaub H, Luhrmann R. Analysis of site-specific protein-RNA cross-links in isolated RNP complexes, combining affinity selection and mass spectrometry. RNA. 2003;9:1542–1551. doi: 10.1261/rna.5175703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stefan MI, Le Novere N. Cooperative binding. PLoS Comput Biol. 2013;9:e1003106. doi: 10.1371/journal.pcbi.1003106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ehlert FJ. Estimation of the affinities of allosteric ligands using radioligand binding and pharmacological null methods. Mol Pharmacol. 1988;33:187–194. [PubMed] [Google Scholar]
- 41.Christopoulos A, Kenakin T. G protein-coupled receptor allosterism and complexing. Pharmacol Rev. 2002;54:323–374. doi: 10.1124/pr.54.2.323. [DOI] [PubMed] [Google Scholar]
- 42.Jansen JM, et al. Inhibition of prenylated KRAS in a lipid environment. PLoS One. 2017;12:e0174706. doi: 10.1371/journal.pone.0174706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hammes GG, Chang YC, Oas TG. Conformational selection or induced fit: a flux description of reaction mechanism. Proc Natl Acad Sci U S A. 2009;106:13737–13741. doi: 10.1073/pnas.0907195106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Krawczak M, et al. Human gene mutation database-a biomedical information and research resource. Hum Mutat. 2000;15:45–51. doi: 10.1002/(SICI)1098-1004(200001)15:1<45::AID-HUMU10>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 45.Soemedi R, et al. Pathogenic variants that alter protein code often disrupt splicing. Nat Genet. 2017;49:848–855. doi: 10.1038/ng.3837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Disney MD. Short-circuiting RNA splicing. Nat Chem Biol. 2008;4:723–724. doi: 10.1038/nchembio1208-723. [DOI] [PubMed] [Google Scholar]
- 47.O'Brien K, Matlin AJ, Lowell AM, Moore MJ. The biflavonoid isoginkgetin is a general inhibitor of Pre-mRNA splicing. J Biol Chem. 2008;283:33147–33154. doi: 10.1074/jbc.M805556200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cretu C, et al. Structural Basis of Splicing Modulation by Antitumor Macrolide Compounds. Mol Cell. 2018;70:265–273 e268. doi: 10.1016/j.molcel.2018.03.011. [DOI] [PubMed] [Google Scholar]
- 49.Charenton C, Wilkinson ME, Nagai K. Mechanism of 5' splice site transfer for human spliceosome activation. Science. 2019;364:362–367. doi: 10.1126/science.aax3289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ratni H, et al. Specific Correction of Alternative Survival Motor Neuron 2 Splicing by Small Molecules: Discovery of a Potential Novel Medicine To Treat Spinal Muscular Atrophy. J Med Chem. 2016;59:6086–6100. doi: 10.1021/acs.jmedchem.6b00459. [DOI] [PubMed] [Google Scholar]
- 51.Keller R. The Computer-aided Resonance Assignment Tutorial. Goldau, Switzerland: Cantina Verlag; 2004. [Google Scholar]
- 52.Binev Y, Marques MM, Aires-de-Sousa J. Prediction of 1H NMR coupling constants with associative neural networks trained for chemical shifts. J Chem Inf Model. 2007;47:2089–2097. doi: 10.1021/ci700172n. [DOI] [PubMed] [Google Scholar]
- 53.Guntert P. Automated NMR structure calculation with CYANA. Methods Mol Biol. 2004;278:353–378. doi: 10.1385/1-59259-809-9:353. [DOI] [PubMed] [Google Scholar]
- 54.Case DA, et al. The Amber biomolecular simulation programs. J Comput Chem. 2005;26:1668–1688. doi: 10.1002/jcc.20290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pettersen EF, et al. UCSF Chimera--a visualization system for exploratory research and analysis. J Comput Chem. 2004;25:1605–1612. doi: 10.1002/jcc.20084. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.