Abstract
Background
Tuberculosis, often undiagnosed, is the major cause of death among HIV-positive people. We tested an algorithm enabling nurses in South African primary healthcare clinics (PHCs) to initiate empirical tuberculosis treatment among adults with advanced HIV disease.
Methods
In an open-label cluster-randomised trial, 24 PHCs were randomised 1:1 to intervention or control (routine care) using computer-generated random numbers. HIV-positive adults were eligible if they had CD4 count ≤150 cells per μL; no antiretroviral therapy (ART) or tuberculosis treatment in the last six or three months respectively; and did not require urgent hospital referral. In intervention clinics, study nurses assessed participants based on tuberculosis symptoms, body mass index (BMI), point-of-care haemoglobin, and urine lipoarabinomannan assay (Determine TB-LAM, Alere). A study algorithm assigned tuberculosis probability as high (positive urine TB-LAM or BMI <18·5 kg/m2 or haemoglobin <100 g/L), to start tuberculosis treatment immediately then ART two weeks later; medium (tuberculosis symptoms, no high probability criteria), to have symptom-guided investigation; or low (no tuberculosis symptoms or high probability criteria), to start ART immediately. The primary outcome was all-cause mortality at six months. (ISRCTN35344604)
Findings
3091 individuals were screened; 3053 assigned a study identifier; and 3022 (1507 intervention, 1515 control; median age 37 years, 55·2% female, median CD4 72 cells per μL) analysed. 930/1507 (61·7%) versus 172/1515 (11·4%) of participants in the intervention versus control arm started tuberculosis treatment by two months. The mortality rate was 19·0 (134 deaths/704 person-years [pyrs]) versus 21·6 (151/699 pyrs) per 100 pyrs in the intervention versus control arm (unadjusted hazard ratio [HR] 0·92, 95% CI 0·67–1·26; adjusted HR 0·87, 95% CI 0·61–1·24, p=0·41). There were 29 versus 11 serious or severe adverse events in the intervention versus control arm.
Interpretation
Our intervention substantially increased coverage of tuberculosis treatment in this high-risk population, but did not reduce mortality.
Funding
Joint Global Health Trials (Medical Research Council, Department for International Development, Wellcome Trust).
Introduction
Despite antiretroviral therapy (ART), early mortality among HIV-positive people with advanced disease remains unacceptably high in low- and middle-income countries.1 Tuberculosis is consistently identified as the leading cause of death among HIV-positive people, and is often undiagnosed prior to death.2 Diagnostic tests for tuberculosis remain unsatisfactory; no available test which is logistically feasible for point-of-care use in primary-level healthcare clinics (PHCs) has adequate sensitivity for use among HIV-positive people, particularly those with advanced disease. As a consequence, empirical tuberculosis treatment, meaning treatment without bacteriological confirmation, is common.3–5 Empirical tuberculosis treatment generally requires a physician’s decision,6 but in many primary care settings physicians are not easily accessible. The process of investigating for tuberculosis may therefore be slow, and may delay ART initiation.7
Wider use of empirical tuberculosis treatment has been discussed;8 advantages include rapid initiation of tuberculosis treatment, with potential mortality benefits for those with active tuberculosis, and protection against reactivation of latent tuberculosis for those without active tuberculosis. Disadvantages, for those without active tuberculosis, include drug toxicity, drug interactions, increased pill burden, and, potentially, failure to identify and treat other co-morbidities.8
South African guidelines in 2010 recommended ART initiation for HIV-positive people in World Health Organization (WHO) stage 3 or 4, or CD4≤350 cells per μL, with "fast-track" (within two weeks) initiation for people with CD4 counts <100 cells per μL. The need for symptom screening, and, if symptomatic, investigation, for tuberculosis prior to initiating ART was emphasised. The first-line test for tuberculosis was sputum smear microscopy, progressively replaced by Xpert MTB/RIF, rolled out nationally between 2011 and 2013. ART start was recommended within two to four weeks of tuberculosis treatment initiation. From March 2013, fast-track (within seven days) ART initiation was recommended for all HIV-positive people with CD4<200 cells per μL, and those with tuberculosis with CD4<50 cells per μL.
We hypothesised that a management algorithm which allowed nurses to triage HIV-positive patients with advanced immunosuppression, identify those with the highest probability of tuberculosis, and start tuberculosis treatment immediately, followed by ART, would reduce early mortality. We reasoned that this reduction in mortality would be achieved by two mechanisms: first, by reducing the number of people with active tuberculosis who remained untreated; second, by removing delays associated with investigation for tuberculosis, we assumed that time to ART initiation would be reduced in all patients (including those treated for tuberculosis), with an additional mortality benefit.9
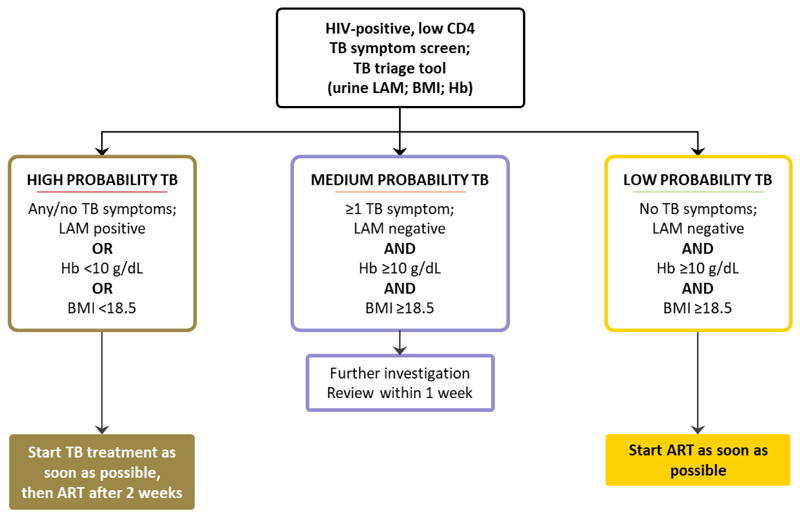
We designed a “TB triage tool” to enable nurses to assess tuberculosis risk, using measures which can give an in-session result in a primary care setting. A candidate component of our TB triage tool was an assay for mycobacterial lipoarabinomannan (LAM), commercially available as a urine-based point-of-care lateral flow assay (Determine™ TB LAM, Alere Inc, Waltham, USA). However, it is too insensitive to be used alone as a screening test,10,11 and other measures were needed to increase triage tool sensitivity. Haemoglobin and body mass index (BMI) are consistently associated with risk of mortality among HIV-positive people, and risk of active tuberculosis.12–14 We constructed a clinical algorithm based on this TB triage tool (figure 1; appendix page 1), to allow nurses to assess HIV-positive patients with advanced disease, and guide them to start tuberculosis treatment immediately, followed by ART two weeks later (in line with national guidelines) for those in the highest probability category; start ART immediately for those in the lowest probability category; and arrange further investigation for those with medium probability, with review within one week so that tuberculosis treatment or ART could be started promptly.
Figure 1.
TB Fast Track intervention management algorithm
ART=antiretroviral therapy. BMI=body mass index. Hb=haemoglobin. LAM=urine lipoarabinomannan. TB=tuberculosis.
The aim of the TB Fast Track trial was to determine whether patients with advanced HIV disease managed according to this clinical algorithm experienced lower six-month mortality than those managed according to routine clinic practice.
Methods
Study design and participants
The TB Fast Track trial was a pragmatic, unmasked, parallel, two-arm cluster-randomised controlled trial (methods described previously;15 protocol available: https://doi.org/10.17037/PUBS.04653158). Because the intervention was a management strategy involving assessment of risk of active tuberculosis, we thought it likely that individual randomisation would result in “contamination” among standard of care (control) arm participants, i.e. that study implementation would alter their clinical management substantially, compared to routine care. After obtaining approval from district health staff and clinic managers, we therefore randomised clusters rather than individuals, where clusters comprised PHCs in Gauteng, Limpopo, and North-West provinces in South Africa. Eligible PHCs delivered both ART and tuberculosis treatment, had ART initiation rates estimated to be high enough to produce enough study participants, and did not have on-site tuberculosis testing facilities; Xpert MTB/RIF (performed at off-site laboratories) became the first-line test for tuberculosis at all clinics during the course of the trial. In most PHCs a doctor was available for morning sessions 3-5 days per week.
Adults (aged ≥18 years) with a recorded HIV-positive test result were eligible if they had a CD4 count ≤150 cells per μL, and had not taken ART in the previous six months, or tuberculosis treatment in the previous three months. We excluded adults at higher risk of adverse events from tuberculosis treatment, specifically those reporting chronic liver disease, or alcohol intake exceeding 28 units (men) or 21 units (women) per week; those with danger signs necessitating urgent referral to secondary care, based on WHO guidelines;6 and those planning to leave their clinic catchment area within six months. South African guidelines effective at study initiation recommended that women in the first trimester of pregnancy should not take efavirenz-based ART. Women of child-bearing potential were therefore initially required to have a negative pregnancy test to be included. After this caution was removed from guidelines, from July 2013 enrolment was open to all women regardless of child-bearing potential. Study staff offered enrolment to all eligible participants until the required sample size for each clinic was reached.
The research ethics committees of the University of the Witwatersrand and the London School of Hygiene & Tropical Medicine, and the South African Medicine Controls Council approved the study. All participants gave written or witnessed verbal informed consent. An independent Trial Steering Committee and Data Monitoring Committee provided trial oversight.
Randomisation and masking
In August 2012, a statistician randomised 20 PHCs in a ratio of 1:1 to intervention or control arms, based on restriction to achieve reasonable balance, separately, for mean CD4 count, peri-urban versus rural clinic location, and total monthly ART initiations. The random allocation was selected at a public ceremony involving clinic representatives.15 Early participant recruitment was slower than anticipated, and therefore in October 2013 we randomised four additional PHCs, giving a total of 24 clusters; we considered these additional four clinics as a separate stratum in the analysis.
Procedures
In intervention clinics, study nurses assessed participants including tuberculosis symptom screening, based on the WHO screening tool (any of cough, weight loss, night sweats, or fever);16 measurement of height and weight to determine BMI; haemoglobin estimation based on a finger-prick blood sample (Hemocue 201+, Hemocue, Angelholm, Sweden); and estimation of urine LAM using the lateral flow assay according to manufacturer’s instructions (Determine™ TB LAM, as above). Study nurses then used the study algorithm (figure 1) to classify participants as high probability of tuberculosis if the urine LAM assay was positive (band of intensity grade 1 or above, as recommended by the company until January 2014), BMI was less than 18·5 kg/m2 or haemoglobin was less than 100 g/L; medium probability if they had no high probability criteria but one or more tuberculosis symptoms; or low probability if they had no high probability criteria and reported no tuberculosis symptoms. For participants classified as high probability, study nurses facilitated the start of tuberculosis treatment as soon as possible, with ART initiation two weeks later, in line with national guidelines, unless there was a contraindication. Those classified as low probability started ART as soon as possible. Those classified as medium probability were further assessed in line with national guidelines for management of sputum smear-negative tuberculosis with chest radiography, sputum for smear and mycobacterial culture (or Xpert MTB/RIF, as it became available), and/or a course of antibiotics, as clinically appropriate. Study staff reviewed medium probability participants within a week wherever possible, aiming to start tuberculosis treatment or ART at that point.
For intervention arm participants, at enrolment, research staff collected a single spot sputum sample for smear, mycobacterial culture, organism identification, and sensitivity testing for isoniazid and rifampicin; these results were fed back to clinic staff. We intended this sample to provide a minimum reference standard to indicate which participants had active tuberculosis, acknowledging that this would have suboptimal sensitivity. Research staff did not collect sputum specimens from control arm participants, as this was not standard of care. After LAM testing, residual urine was frozen at -80°C for mycobacterial culture at the end of the study.
Study staff reviewed intervention arm participants to facilitate early management according to the study algorithm; clinic staff delivered subsequent HIV care (and tuberculosis treatment, if initiated) according to their usual practice.
In standard of care clinics, study staff enrolled participants and collected a urine sample which was frozen and cultured for mycobacteria at the end of the study; all subsequent care was by clinic staff. For all participants in both study arms, laboratory monitoring was per South African guidelines; liver function tests were not routinely performed, and there were no laboratory evaluations for study purposes after the enrolment visit. Six months after enrolment, all participants were reviewed by study nurses, in person where possible, to capture information relevant to study outcomes.
Outcomes
The primary outcome was all-cause mortality at six months after study enrolment, defined as a known death before six months, or known to be alive after 150 days, based on reports from participant-nominated contacts and clinic staff, and data from South African vital status registration. We defined outcomes at six months because the highest risk of death was expected to be in the first three months and because, by six months, participants who started tuberculosis treatment as part of the intervention would be nearing tuberculosis treatment completion. Secondary outcomes, ascertained by self-report or record review, were hospitalisation within six months after enrolment; time from enrolment to ART initiation (within six months); a binary outcome of whether ART was started within 30 days of enrolment; retention in HIV care at six months (defined as any HIV-related visit between four and eight months after enrolment); and adverse events. All-cause 12-month mortality was pre-specified as an exploratory outcome. All outcomes were measured at the individual level.
Research staff undertook case note reviews at two and six months, and conducted a study visit at six months, to determine vital status; tuberculosis treatment and ART start dates; and hospitalisation and other important events. To ascertain possible serious and severe (grade 3 or 4) adverse events in specified categories relevant to the intervention (specifically hepatotoxicity, hypersensitivity, peripheral neuropathy, optic neuritis, and nephrotoxicity), research nurses and research assistants enquired about symptoms and history suggesting possible adverse events at every study visit, including early study review visits for intervention arm participants, and sought relevant data in case note reviews. Given that, in line with the pragmatic design, there were no equivalent early study visits for control arm participants, we anticipated that adverse events would be more completely ascertained in the intervention arm.
Study clinicians classified adverse events, using conventional criteria,17 after detailed review of all available research and routine data, including hospitalisation records, and assigned relationship to the intervention based on likelihood of association with empirical tuberculosis treatment.
As part of a sub-study, reported separately, where possible we conducted minimally-invasive autopsies on study participants who died.18 An independent panel assigned causes of death, based on clinical information from this study along with health facility and autopsy data, where available, to decedents who also had verbal autopsy data available.19
Statistical analysis
Sample size calculations are detailed elsewhere.15 Briefly, assuming ten clinics per arm, a harmonic mean of 175 participants per clinic, 5% with unknown vital status at six months, a mortality of 25 per 100 person-years (pyrs) in the standard of care arm, and coefficients of variation of 0·2 and 0·25, there would be 91% and 85% power to assess a 40% reduction in mortality, respectively. If the coefficient of variation was 0·2, there would be 81% power to assess a 35% reduction in mortality. Six months into enrolment, due to slower than anticipated recruitment, we reassessed sample size calculations. Assuming a harmonic mean of 109 participants per clinic, by randomising an additional four clinics, the study maintained similar power and effect sizes as with the original calculation.
As described previously,15 we based analysis on a cluster-level approach, appropriate for the small number of clusters. For rate or binary outcomes, we calculated the overall rate (or risk) for each cluster. We calculated the rate ratio as the geometric mean of cluster-level rates in the intervention divided by control arm, taking into account the stratified randomisation. We calculated the standard error for each log(effect measure) by regressing the log(rate) on the study arm, strata, and their interaction. We based the 95% confidence interval (CI) and p-value on 20 degrees of freedom. We conducted adjusted analyses if, after visual inspection, we observed imbalance by study arm in individual-level factors, using methods for a small number of clusters.
We conducted pre-specified subgroup analyses for the primary outcome for baseline CD4 count (<50 or ≥50 cells per μL), self-reported tuberculosis history (no previous or previous tuberculosis), baseline BMI (<18·5 or ≥18·5 kg/m2), and baseline haemoglobin (<80 or ≥80 g/L). The study was not powered to detect differences in these subgroups. In two post hoc sensitivity analyses for the primary outcome, i) we assumed those with unknown vital status at six months had died, with date of death the midpoint between date last known alive and six months after enrolment; and ii) we excluded two control clusters in which more than 25% of participants self-reported taking IPT at enrolment.
Role of the funding source
The funder played no role in study design, implementation, data collection, analysis, or decision to publish. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Results
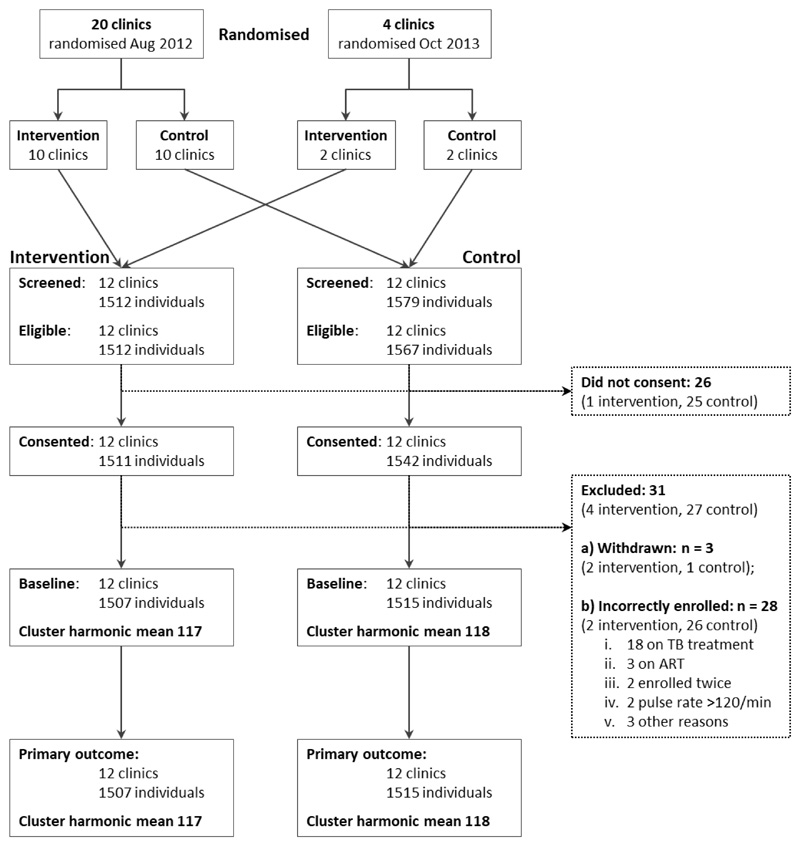
Between 19 December 2012 and 18 December 2014 we screened 3091 people; 26 declined enrolment, 12 were ineligible, and 3053 were enrolled (figure 2). 31 participants were subsequently excluded, leaving 3022 (1507 intervention, 1515 control) in the analysis. The median age was 37 years, 55·2% were female and the median CD4 count was 72 cells per μL (table 1). Baseline variables were similar by study arm except that participants in the intervention arm were more likely to report one or more symptoms compatible with tuberculosis (72·7% intervention versus 65·7% control); to report having had tuberculosis tests done in the six months prior to enrolment (48·9% intervention versus 42·7% control); and less likely to be taking isoniazid preventive therapy (IPT; 3·4% intervention versus 10·4% control). This difference in IPT use was attributable to two control clusters where more than 20% of participants reported taking IPT at enrolment.
Figure 2.
Trial profile
ART=antiretroviral therapy. min=minute. TB=tuberculosis
Table 1. Baseline characteristics of the study population, by study arm (n=3022).
| Intervention (n=1507) | Control (n=1515) | ||
|---|---|---|---|
| Sex, female | n (%) | 820 (54·4%) | 849 (56·0%) |
| Age, years | Median (IQR) | 37 (32–44) | 36 (31–43) |
| CD4*, cells per μL | Median (IQR) | 73 (35–111) | 70 (35–113) |
| CD4* <50 cells per μL | n (%) | 508 (33·7%) | 521 (34·4%) |
| BMI† | Median (IQR) | 21·2 (18·8–24·7) | 21·6 (19·2–24·8) |
| BMI† <18.5 kg/m2 | n (%) | 320 (21.·2%) | 271 (17·9%) |
| Haemoglobin‡ <100 g/L | n (%) | 428 (28·4%) | 322 (29·8%) |
| Urine LAM positive§ | n (%) | 181 (12·0%) | 222 (15·2%) |
| ≥ one TB symptom¶ | n (%) | 1095 (72·7%) | 993 (65·7%) |
| Previous TB|| | n (%) | 151 (10·0%) | 136 (9·0%) |
| Taking IPT** | n (%) | 51 (3·4%) | 158 (10·4%) |
| Taking co-trimoxazole†† | n (%) | 691 (45·9%) | 718 (47·4%) |
| TB tests in last 6 months (self-reported)‡‡ | n (%) | 737 (48·9%) | 645 (42·7%) |
| Sputum test sent in 28 days prior to enrolment (record review) | n (%) | 471 (31·3%) | 412 (27·2%) |
| Among those who had sputum sent, Xpert MTB/RIF result recorded | n (%) | 357/471 (75·8%) | 321/412 (77·9%) |
| Chest radiograph performed in 28 days prior to enrolment (record review) | n (%) | 79 (5·2%) | 38 (2·5%) |
Missing data for one participant in the intervention arm.
Missing data for three participants in the control arm.
Missing data for one participant in the intervention arm and 435 participants in the control arm.
Missing data for two participants in the intervention arm and 52 participants in the control arm.
Missing data for one participant in the intervention arm and four participants in the control arm.
Missing data for one participant in the intervention arm.
Missing data for one participant in each of the intervention and control arms.
Missing data for one participant in each of the intervention and control arms.
Missing data for one participant in the intervention arm and six participants in the control arm.
IQR=interquartile range. BMI=body mass index. LAM=lipoarabinomannan. TB=tuberculosis.
IPT=isoniazid preventive therapy.
TB symptoms defined as any of cough, fever, unintentional weight loss, night sweats.
In the intervention arm, study nurses used the algorithm to assign 689 (45·7%) participants to the high probability category, 475 (31·5%) to medium, and 342 (22·7%) to low probability of tuberculosis (data missing for one participant; baseline characteristics by category shown in appendix page 2; intersection between algorithm components shown in appendix pages 9-11). In the 14 days after enrolment, based on record review, 23·9% control arm participants either gave a sputum sample (17·5%, among whom 195/264 [73·9%] had an Xpert MTB/RIF result recorded) or had a chest radiograph performed (8·6%); investigation was slightly more frequent among those reporting one or more symptoms at enrolment (sputum 19·7%, chest radiograph request 10·5%, either 27·3%) versus those who reported no symptoms (sputum 13·3%, chest radiograph request 5·0%, either 17·5%). Overall, 930/1507 (61·7%) intervention arm participants had started tuberculosis treatment by 60 days after enrolment compared with 172/1515 (11·4%) in the control arm (appendix page 12).
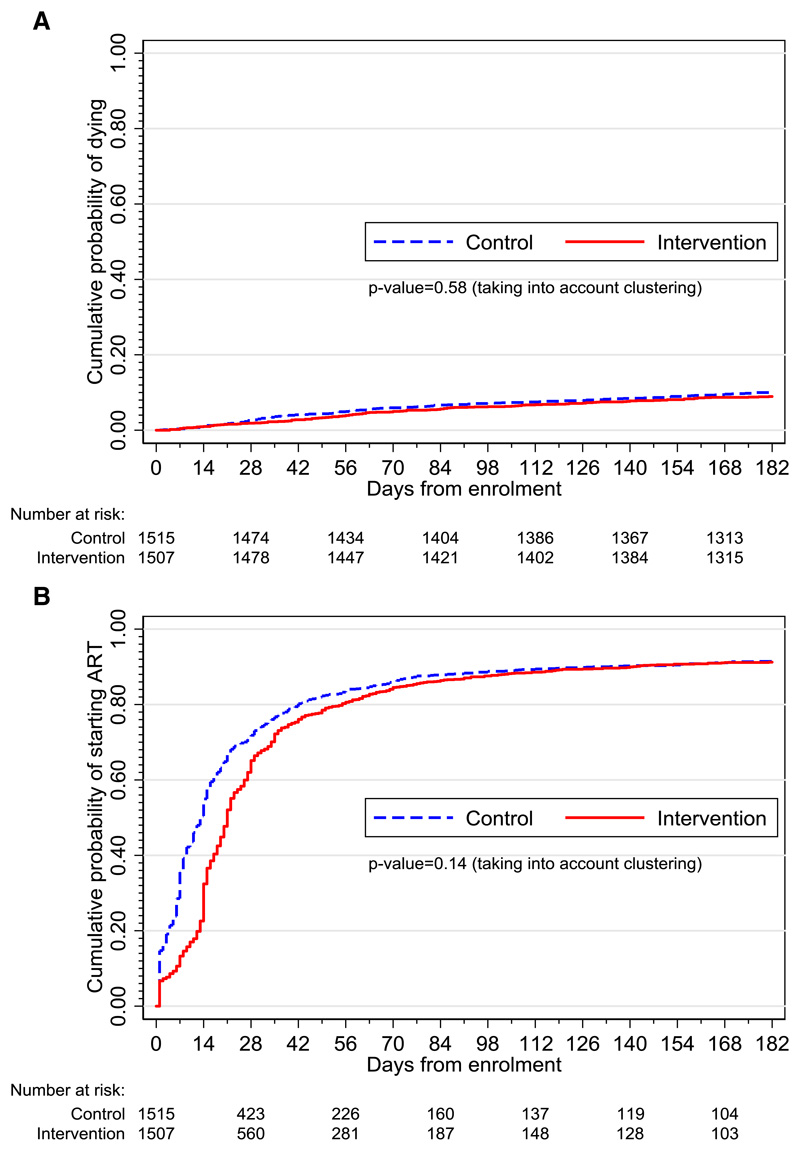
The total follow-up time was 1404 person-years. Vital status at six months was determined for 98·7% intervention and 98·0% standard of care arm participants. The mortality rate was 19·0 (134 deaths/704 pyrs) in the intervention versus 21·6 (151/699 pyrs) per 100 pyrs in the control arm (unadjusted hazard ratio [HR] 0·92, 95% CI 0·67–1·26; adjusted HR 0·87, 95% CI 0·61–1·24, p=0·41, table 2, figure 3, appendix page 3). In a post hoc sensitivity analysis, assuming those with unknown vital status at six months (n=50; 19 intervention, 31 control) had died, the adjusted HR was 0·86 (95% CI 0·61–1·21, p=0·37). Prespecified subgroup analyses for CD4 and BMI strata gave similar results for the intervention effect on mortality (appendix page 4). Estimation of the intervention effect for those with previous tuberculosis and by haemoglobin category was not possible because when data were restricted to these subgroups, six and five clinics respectively had no deaths. The coefficient of variation for the primary outcome in all clusters was 0·09. In a post hoc sensitivity analysis, excluding the two control clusters in which more than 25% of participants self-reported taking IPT at enrolment, the effect estimates for the primary outcome were similar: unadjusted HR 0·95, 95% CI 0·68-1·32; p=0·75 and adjusted HR 0·88, 95% CI 0·61-1·28; p=0·50. The 12-month mortality rate was 13·1 (178 deaths/1314 pyrs) in the intervention versus 14·7 (187/1269 pyrs) per 100 pyrs in the control arm (unadjusted HR 0·97, 95% CI 0·75–1·25; adjusted HR 0·92, 95% CI 0·69–1·24, p=0·57, appendix page 5).
Table 2. Effect of the intervention on primary and secondary outcomes.
| Intervention | Control | |||||||
|---|---|---|---|---|---|---|---|---|
| Primary outcome | Rate/100 py (deaths/py) | Rate/100 py (deaths/py) | Unadjusted HR | 95% CI | P-value | Adjusted HR * | 95% CI | P-value |
|
| ||||||||
| Mortality rate over 6 months | 19·0 (134/704) |
21.6 (151/699) |
0.92 | (0.67, 1.26) | 0.58 | 0.87† | (0.61, 1.24) | 0.41 |
| Rate/100 py (deaths/py) | Rate/100 py (deaths/py) | Unadjusted RD | 95% CI | P-value | Adjusted RD * | 95% CI | P-value | |
|
|
||||||||
| 19·0 (134/704) |
21.6 (151/699) |
-2.14 | (-7.76, 3.49) | 0.44 | -2.19† | (-5.43, 1.05) | 0.17 | |
| Secondary outcomes | % (n/N) | % (n/N) | Unadjusted RR | 95% CI | P-value | Adjusted RR * | 95% CI | P-value |
|
| ||||||||
| Hospital admission over 6 months | 13·3% (201/1507) |
10·4% (158/1515) |
1·19 | (0·94–1·50) | 0·14 | 1·11 | (0·89–1·38) | 0·34 |
| Retention in care by 6 months | 62·0% (934/1507) |
62·4% (945/1515) |
1·01 | (0·82–1·23) | 0·94 | 1·02‡ | (0·83–1·24) | 0·87 |
| Started ART within 30 days of enrolment | 66·4% (1001/1507) |
72·9% (1104/1515) |
0·89 | (0·76–1·04) | 0·13 | 0·91§ | (0·79–1·05) | 0·17 |
| Rate/100 py (deaths/py) | Rate/100 py (deaths/py) | Unadjusted HR | 95% CI | P-value | Adjusted HR * | 95% CI | P-value | |
|
|
||||||||
| Rate of starting ART by 6 months | 834·5 (1338/160·3) |
1034 (1347/130·3) |
0·68 | (0·41–1·15) | 0·14 | 0·69¶ | (0·46–1·04) | 0·07 |
| Post hoc analysis | % (n/N) | % (n/N) | Unadjusted RR | 95% CI | P-value | Adjusted RR * | 95% CI | P-value |
|
| ||||||||
| Risk of starting ART by 6 months | 88·8% (1338/1507) |
88·9% (1347/1515) |
0·99 | (0·94–1·04) | 0·75 | 1·0¶ | (0·95–1·04) | 0·84 |
HR=hazard ratio. RD=rate difference. RR=risk ratio. CI=confidence interval. ART=antiretroviral therapy. py=person-years.
Analyses adjusted for sex, age, BMI, CD4, taking IPT at baseline, TB symptoms, TB tests on last 6 months, previously treated for TB and randomisation strata. Analysis of time to starting ART also adjusted for follow-up time. All adjusted analyses included 3009 participants (1504 intervention and 1505 control).
Includes 284 deaths (134 in intervention and 150 in control).
Includes 1872 outcomes (932 in intervention and 940 in control).
Includes 2098 outcomes (999 in intervention and 1099 in control).
Includes 2677 outcomes (1336 in intervention and 1341 in control).
Figure 3.
A: Kaplan-Meier curves showing time from enrolment to death by study arm
B: Kaplan-Meier curves showing time from enrolment to ART start by study arm
The risk of hospital admission over the six-month follow-up period was similar by arm: 13·3% (201/1507) and 10·4% (158/1515) in the intervention and control arms, respectively, adjusted risk ratio (RR) 1·11 (95% CI 0·89–1·38, p=0·34, table 2). In the intervention versus control arm, a lower percentage of participants started ART within 30 days of enrolment (66·4% [1001/1507] versus 72·9% [1104/1515]), though there was no statistical evidence for a difference by arm (adjusted RR 0·91, 95% CI 0·79–1·05, p=0·17, figure 3B). The median time to ART start was 21 (interquartile range [IQR] 14–39) days in the intervention versus 13 (IQR 6–31) days in the control arm; in the control arm, the median time to ART start was no different among participants who did versus did not report one or more tuberculosis symptoms at enrolment (median [IQR] 13 [6-29] vs. 13 [6-31] days respectively). The incidence rate of starting ART within six months of enrolment was 30% lower in the intervention versus standard of care arm (adjusted HR 0·69, 95% CI 0·46–1·04, p=0·07) though the 95% CI included one; however by six months, the proportion of participants who had started ART was no different by arm (88·8% versus 88·9% for intervention versus control; outcome not pre-specified). Among participants who started tuberculosis treatment followed by ART, the median time between tuberculosis treatment start and ART start was 19 days (IQR 14–32, n=820) in the intervention arm versus 25 days (IQR 15–49, n=131) in the control arm. Retention in HIV care was similar by study arm: 934/1507 (62·0%) intervention versus 945/1515 (62·4%) control, adjusted RR 1·02 (95% CI 0·83–1·24). Among 774 intervention arm participants who started tuberculosis treatment within 60 days of enrolment (thus likely attributable to the intervention), 85·0% were still on tuberculosis treatment 168 days after tuberculosis treatment start.
Table 3 shows serious or severe adverse events, in the pre-specified study categories, that were detected by self-report at study visits and/or by record review. 38 participants (28 intervention, ten control) reported 40 serious or severe adverse events in study-defined categories. Grade 3/4 nausea and vomiting was the category of event most commonly reported (ten intervention, four control); followed by symptoms which could indicate peripheral neuropathy at grade 3 or above (eight intervention, two control); and grade 3 or above skin rash (six intervention, two control). Abnormal liver function tests were recorded in clinic records in four intervention and three control participants. In the intervention arm, 19 of 29 adverse events occurred at least one day after the start of tuberculosis treatment (median 21, range 1–165). In the intervention versus control arm, six versus two adverse events were recorded to have a fatal outcome. Following review, among six deaths in the intervention arm, none appeared to be attributable to the study intervention (see appendix pages 6-8 for further details). Among intervention arm decedents who underwent minimally-invasive autopsy, 0/17 had fulminant hepatitis.18
Table 3. Serious and severe adverse events detected by self-report and record review, by study arm.
| Intervention | Control | |||
|---|---|---|---|---|
| Number of events (# participants) | 29 (28) | 11 (10) | ||
|
| ||||
| Age in years, median (range) | 35 (22–52) | 39 (30–55) | ||
|
| ||||
| Sex, n (%) female | 14 (50%) | 4 (40%) | ||
|
| ||||
| CD4 at enrolment in cells per μL, median (range) | 68 (4–143) | 50·5 (21–142) | ||
|
| ||||
| Type, n (%) | Nausea/vomiting† | 10 (33%) | 4 (36%) | |
|
| ||||
| Suspected peripheral neuropathy | 8 (27%) | 2 (18%) | ||
|
| ||||
| Skin rash/hypersensitivity | 6 (20%) | 2 (18%) | ||
|
| ||||
| Abnormal liver function tests/hepatitis | 4 (13%) | 3 (27%) | ||
|
| ||||
| Other* | 1 (7%) | 0 | ||
|
| ||||
| Days from enrolment to onset of AE | Median; IQR range |
26; 13–79 0–169 |
29; 8–109 7–166 |
|
|
| ||||
| AE occurred after ART start‡, n (%) | At least one day after | 14 (48%) | 5 (45%) | |
| Before/same day as ART start | 12 (41%) | 3 (27%) | ||
| Did not start ART | 3 (10%) | 3 (27%) | ||
|
| ||||
| Days from starting ART to AE, restricted to AEs after ART | Median; range n |
53·5; 3–162 14 |
103; 18–144 5 |
|
|
| ||||
| AE occurred after TB start‡, n (%) | At least one day after | 19 (66%) | 5 (45%) | |
| Before/same day as TB start | 4 (14%) | 1 (9%) | ||
| Did not start TB treatment | 6 (21%) | 5 (45%) | ||
|
| ||||
| Days from starting TB treatment to AE, restricted to AEs after TB | Median; range n |
21; 1–165 19 |
14; 5–125 5 |
|
|
| ||||
| Outcome of AE, n (%) | Resolved | 15 (52%) | 8 (73%) | |
| Resolved with sequelae | 1 (3%) | 0 | ||
| Ongoing | 7 (24%) | 1 (9%) | ||
| Fatal† | 6 (21%) | 2 (18%) | ||
|
| ||||
| Relationship with intervention, n(%) | Not associated | 9 (31%) | 11 (100%) | |
| Probably not associated | 1 (3%) | 0 | ||
| Possibly associated | 7 (24%) | 0 | ||
| Probably associated | 8 (28%) | 0 | ||
| Definitely associated† | 4 (14%) | 0 | ||
generalised body weakness
One adverse event of nausea/vomiting (see appendix page 7: AE5) is recorded as definitely associated with a fatal outcome because there was no documentation of resolution. The patient was lost to follow-up from TB treatment, subsequently admitted to hospital with vomiting and diarrhoea and died 48 days post-enrolment. Hospital-assigned cause of death was meningitis and renal failure. Autopsy: Mycobacterium tuberculosis (sensitive to isoniazid and rifampicin) isolated from liver and spleen but not cerebrospinal fluid. Panel-assigned cause of death was disseminated TB. Therefore, the adverse event (nausea and vomiting) was considered associated, but death was considered not associated with the intervention.
33/38 participants started ART (8/10 in control arm and 25/28 in intervention arm); 28/38 started TB treatment (5/10 in control arm and 23/28 in intervention arm).
Two participants reported two AEs each; one in the control arm – both events (abnormal liver function tests/hepatitis and nausea/vomiting) occurred 5 days after starting TB treatment (patient did not start ART); one in the intervention arm - nausea/vomiting occurred on the same day as starting ART and suspected peripheral neuropathy occurred after starting ART (patient did not start TB treatment).
AE=adverse event. IQR=interquartile range. ART=antiretroviral therapy. TB=tuberculosis.
970/1507 (64·4%) intervention arm participants produced a sputum specimen for mycobacterial culture, among which 36/1507 (2·4%) were smear positive and 102/1507 (6·8%) were culture-positive for Mycobacterium tuberculosis (classifying participants who could not produce sputum as smear- and culture-negative). Eight participants had stored urine which was M. tuberculosis culture positive at the end of the study (seven intervention, one control); of the seven in the intervention arm, four had a bacteriologically-positive sputum result at enrolment (three culture positive, one smear positive but culture negative; the remaining three were unable to provide sputum). The proportions of intervention arm participants with a positive sputum culture by study algorithm-assigned probability of tuberculosis were 11·2%, 4·0% and 1·8% in the high, medium, and low probability categories, respectively. The overlap between sputum smear and culture positivity and urine LAM positivity is shown in appendix page 13. Among 102 participants with M. tuberculosis cultured from sputum, four (3·9%) isolates were isoniazid monoresistant, and one (1·0%) was resistant to both isoniazid and rifampicin.
Six-month mortality among intervention arm participants assigned to high, medium, and low probability of tuberculosis was 14·2%, 5·5%, and 2·9% respectively (appendix page 14); this compared to 14·6%, 7·4%, and 3·7% for control participants when probabilities were applied retrospectively based on LAM testing of stored urine and haemoglobin estimation from routine records, when available (n=1039, appendix page 15).
Discussion
Our study triage tool successfully identified individuals who were at higher risk of tuberculosis and of mortality, and our study algorithm resulted in much higher coverage of tuberculosis treatment in the intervention compared to control arm. However, the intervention did not reduce mortality or improve other clinical outcomes. The large anticipated effect size depended on the assumption that the intervention would, firstly, reduce tuberculosis-specific mortality by increasing coverage of tuberculosis treatment to individuals with active tuberculosis that would not be detected in routine practice, and, secondly, reduce all-cause mortality by accelerating ART initiation. In practice, the intervention did not accelerate ART initiation compared to the control arm, and we found that a substantial increase in coverage of tuberculosis treatment did not result in the large reduction in mortality that the trial was powered to detect.
Possible explanations for the lack of mortality benefit include that that tuberculosis was not a major cause of mortality. This would be inconsistent with evidence from autopsy studies;2 furthermore, in a small subset of deaths (n=34) among study participants where minimally-invasive autopsy was performed, almost half had prevalent tuberculosis.18 Among 259 decedents included in a sub-study validating verbal autopsy, 69 (26·6%) were assigned tuberculosis as cause of death by an independent panel.19 It seems unlikely that the intervention increased mortality due to unnecessary tuberculosis treatment, given the low number of recorded serious and severe adverse events, or that the intervention effect was undermined by undetected drug-resistant tuberculosis, given the low prevalence of drug-resistant tuberculosis among intervention arm participants. However over 40% participants in both study arms reported undergoing tuberculosis tests as part of their routine care in the six months prior to enrolment; around 30% in both arms were recorded to have had a sputum test in the 28 days prior to enrolment, substantially higher than our pilot work suggested, possibly reflecting increased awareness of tuberculosis as a result of Xpert MTB/RIF roll-out, or study activities, or both. This could have reduced the prevalence of active tuberculosis among the enrolled population, especially later in the study as Xpert MTB/RIF; the intervention might have a larger effect in populations where there was less tuberculosis investigation prior to ART initiation.
The lack of mortality benefit could be partly explained by the intervention's failure to accelerate ART initiation. The major reason for slower ART initiation in the intervention versus control arm was the high proportion (62%) of intervention participants starting tuberculosis treatment, combined with faster than expected ART initiation in the control arm. Intervention arm participants given empirical tuberculosis treatment were intended to start ART two weeks later, but in practice the median time to start ART after tuberculosis treatment among intervention arm participants was 19 days, slightly shorter than observed among control arm participants (median 25 days). We do not have detailed data to explain why ART initiation was slower than planned. In some cases there were understandable explanations for delay, such as abnormal renal function, but often the reason was unclear. Our impression was that some clinic staff were reluctant to start ART in people who were perceived to be unwell; further work to understand and correct misconceptions may be needed, and this is particularly relevant in the context of efforts to accelerate ART initiation. The presence of the study team in control clinics might have had an unplanned effect to speed up ART initiation; the median time from enrolment to ART initiation in the TB Fast Track control arm was 13 days compared to 25 days in a pilot study in 21 study clinics in 2011;7 secular change may also have contributed. It seems unlikely that the overall one-week difference in time to ART initiation in our intervention versus control arm would account for a large difference in six-month mortality; however, our assumption that empirical tuberculosis treatment would have an overall effect to accelerate ART initiation proved incorrect. Thus our intervention, in practice, comprised much higher coverage of tuberculosis treatment, without any additional benefit from earlier ART, and the observed intervention effect likely reflects this.
Time to ART initiation among control arm participants was the same for the 67% who reported symptoms consistent with tuberculosis compared to the 33% who did not. Thus, in the control arm, any investigation or treatment for active tuberculosis did not delay ART initiation. This may be partly explained by the unexpectedly high frequency of investigation for tuberculosis prior to enrolment. Nonetheless, symptomatic individuals should have had sputum sent for mycobacterial culture (or, as it was rolled out, Xpert MTB/RIF if not previously sent); in the 14 days after enrolment only 20% symptomatic control arm participants were recorded to have had a sputum sample sent. These data suggest that any adverse outcomes attributable to early ART start without comprehensive tuberculosis investigation among symptomatic control arm participants were not outweighed by empirical tuberculosis treatment in the intervention arm, suggesting that guidelines may overemphasise the requirement to delay ART start until active tuberculosis has been excluded. Strategies for rapid ART initiation among symptomatic people with advanced HIV disease merit further evaluation.
The REMEMBER trial similarly addressed the mortality benefit of empirical tuberculosis treatment.20 The trial showed no benefit of empirical tuberculosis treatment compared to IPT, in a selected population with CD4 counts less than 50 cells per μL, and no evidence of tuberculosis based on clinical presentation and initial investigation, which included chest radiography in 65% and Xpert MTB/RIF in around 91%.20 The PROMPT trial individually randomised individuals in four countries in Africa with CD4 less than 50 cells per μL and no evidence of tuberculosis on sputum smear or chest radiography to presumptive tuberculosis treatment or control; it was stopped early because of slow recruitment.21 The STATIS trial found no difference in death or invasive bacterial disease by 24 weeks among adults with CD4 <100 cells per μL individually randomised to ART plus extensive tuberculosis investigation versus ART and empirical tuberculosis treatment.22 The LAM assay is feasible for PHC-level use but, as elsewhere, its sensitivity in our study was too low to be used in isolation.23 New, high-sensitivity LAM assays hold promise if they can be formulated for point-of-care use in PHCs.
Strengths of the TB Fast Track trial include its pragmatic design, with broad inclusion criteria, maximising generalisability. The intervention algorithm was based on existing, low-cost measures and delivered by nurses, who were integrated into routine PHC care. Completeness of follow-up was excellent. Some limitations of the trial are consequent on its pragmatic design. We performed few non-routine investigations, and thus have limited information from which to determine which participants genuinely had tuberculosis at enrolment, and what caused death. We minimised study-specific follow-up visits, to approximate how the intervention would be delivered in routine conditions. This meant that adverse events were under-ascertained in both arms; additional visits to implement the study algorithm for most participants in the intervention arm likely resulted in more complete recording of possible adverse events in the intervention versus control arm. However, ascertainment of deaths was very high in both arms, and thus we can be confident that fatal adverse events did not outweigh any benefit of the intervention. Hospital admissions may have been under-ascertained in control clinics because of fewer study visits and less interaction with study staff. It is also possible that intervention arm participants had a higher probability of referral to hospital because of increased contact with research and clinic staff.
The TB Fast Track intervention enabled nurses in PHCs to identify HIV-positive adults at high risk of tuberculosis and of death, and achieved high coverage of empirical tuberculosis treatment. However, the intervention did not result in the large mortality benefit which the trial was powered to detect. People presenting for care with advanced HIV disease need a package of care in which tuberculosis diagnosis and treatment are a key component. More sensitive, rapid tuberculosis diagnostic tests suitable for use by nurses in primary care clinics are urgently needed so that people with tuberculosis can be correctly identified and promptly treated. Strategies for rapid ART initiation among symptomatic people with advanced HIV disease merit further evaluation.
Supplementary Material
Research in context panel.
Evidence before this study
Prior to undertaking the study, we searched Medline and Embase to December 2010 using the terms (presumptive or empiric*) adj3 (treatment or therap*) AND AIDS or HIV or acquired immunodeficiency syndrome AND TB or tuberculosis. We also searched "Africa wide information" using equivalent terms, and clinical trials databases. These searches yielded no relevant studies.
Since then, the REMEMBER trial similarly addressed the mortality benefit of empirical tuberculosis treatment, in an individually-randomised trial. The trial showed no benefit of empirical tuberculosis treatment compared to IPT, in a selected population with CD4 counts less than 50 cells per μL, and no evidence of tuberculosis based on clinical presentation and initial investigation, which included chest radiography in 65% and Xpert MTB/RIF in around 91%. The PROMPT trial individually randomised individuals in four countries in Africa with CD4 less than 50 cells per μL and no evidence of tuberculosis on sputum smear or chest radiography to presumptive tuberculosis treatment or control; it was stopped early because of slow recruitment. The STATIS trial found no difference in death or invasive bacterial disease by 24 weeks among adults with CD4 <100 cells per μL individually randomised to ART plus extensive investigation for tuberculosis versus ART and empirical tuberculosis treatment.
Added value of this study
This is the first trial to test an algorithm enabling nurses to initiate empirical tuberculosis treatment in primary healthcare centres using point of care diagnostic tests. The intervention resulted in high coverage of tuberculosis treatment but did not accelerate ART initiation. There was no difference in mortality at six months between participants in the intervention versus control arms. The intervention was designed to use simple, currently-available point-of-care measures to guide empirical tuberculosis treatment, and thus to be feasible to implement in low-income settings.
Implications of all the available evidence
Accumulating evidence suggests that HIV-positive adults with advanced disease are unlikely to experience a large mortality benefit from untargeted empirical TB treatment. More sensitive TB diagnostic tests suitable for use by nurses in primary healthcare clinics in low-income settings remain a key priority.
Acknowledgements
Presented in part at the 23rd Conference on Retroviruses and Opportunistic Infections, Boston, USA, February 2016. We gratefully acknowledge the contribution of our study participants; the work of the study team and the cooperation of Department of Health staff in participating clinics. We thank members of the Trial Steering Committee and Data Monitoring Committee for their time and invaluable advice. The study was funded by Joint Global Health Trials (UK Medical Research Council, UK Department for International Development, Wellcome Trust). This UK-funded award is part of the EDCTP2 programme supported by the European Union. Alere donated materials for quality control of their LAM assay. The autopsy sub-study was funded by the Bill & Melinda Gates Foundation (OPP1083118). GC is supported by ACT4TB/HIV.
Trial registration numbers: ISRCTN registry number ISRCTN35344604; South African National Clinical Trials registry number DOH–27–0812–3902.
Footnotes
Contributors
ADG, SC, SED, CH, SJ, AV, GJC, and KLF conceived, designed, and secured funding for the study. ADG, SC, MT, ASK, SJ, and KLF were responsible for data collection. ADG, SC, MT, and KLF analysed the data. ASK and KLF produced the figures. ADG, SC, and KLF drafted the manuscript. All authors contributed to data interpretation and critically reviewed the manuscript.
Declaration of interests
We declare no competing interests.
Data sharing
Deidentified individual participant data that underlie the results reported in this article, a data dictionary and the study protocol will be available from https://datacompass.lshtm.ac.uk/
ORCID ID: 0000-0002-2437-5195
References
- 1.Boulle A, Schomaker M, May MT, et al. Mortality in patients with HIV-1 infection starting antiretroviral therapy in South Africa, Europe, or North America: a collaborative analysis of prospective studies. PLoS Med. 2014;11(9):e1001718. doi: 10.1371/journal.pmed.1001718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gupta RK, Lucas SB, Fielding KL, Lawn SD. Prevalence of tuberculosis in post-mortem studies of HIV-infected adults and children in resource-limited settings: a systematic review and meta-analysis. AIDS. 2015;29(15):1987–2002. doi: 10.1097/QAD.0000000000000802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Churchyard GJ, Stevens WS, Mametja LD, et al. Xpert MTB/RIF versus sputum microscopy as the initial diagnostic test for tuberculosis: a cluster-randomised trial embedded in South African roll-out of Xpert MTB/RIF. Lancet Glob Health. 2015;3(8):e450–7. doi: 10.1016/S2214-109X(15)00100-X. [DOI] [PubMed] [Google Scholar]
- 4.Cox HS, Mbhele S, Mohess N, et al. Impact of Xpert MTB/RIF for TB Diagnosis in a Primary Care Clinic with High TB and HIV Prevalence in South Africa: A Pragmatic Randomised Trial. PLoS Med. 2014;11(11):e1001760. doi: 10.1371/journal.pmed.1001760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Durovni B, Saraceni V, van den Hof S, et al. Impact of Replacing Smear Microscopy with Xpert MTB/RIF for Diagnosing Tuberculosis in Brazil: A Stepped-Wedge Cluster-Randomized Trial. PLoS Med. 2014;11(12):e1001766. doi: 10.1371/journal.pmed.1001766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.World Health Organization. Improving the diagnosis and treatment of smear-negative pulmonary and extrapulmonary tuberculosis among adults and adolescents: recommendations for HIV-prevalent and resource-constrained settings. 2007. [accessed 11 December 2018]. http://apps.who.int/iris/bitstream/10665/69463/1/WHO_HTM_TB_2007.379_eng.pdf .
- 7.Karat A, Charalambous S, Tlali M, et al. Investigation for TB delays start of antiretroviral therapy in South African primary care clinics; 44th World Conference on Lung Health; Paris, France: 2013. [Google Scholar]
- 8.Lawn SD, Ayles H, Egwaga S, et al. Potential utility of empirical tuberculosis treatment for HIV-infected patients with advanced immunodeficiency in high TB-HIV burden settings. Int J Tuberc Lung Dis. 2011;15(3):287–95. [PubMed] [Google Scholar]
- 9.Hoffmann CJ, Lewis JJ, Dowdy DW, et al. Mortality associated with delays between clinic entry and ART initiation in resource-limited settings: results of a transition-state model. J Acquir Immune Defic Syndr. 2013;63(1):105–11. doi: 10.1097/QAI.0b013e3182893fb4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shah M, Hanrahan C, Wang ZY, et al. Lateral flow urine lipoarabinomannan assay for detecting active tuberculosis in HIV-positive adults. Cochrane Database Syst Rev. 2016;5:CD011420. doi: 10.1002/14651858.CD011420.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.World Health Organization. The use of lateral flow urine lipoarabinomannnan assay (LF-LAM) for the diagnosis and screening of active tuberculosis in people living with HIV. 2015. [accessed 19 April 2019]. Policy guidance. https://www.who.int/tb/publications/use-of-lf-lam-tb-hiv/en/
- 12.Gounder CR, Kufa T, Wada NI, et al. Diagnostic accuracy of a urine lipoarabinomannan enzyme-linked immunosorbent assay for screening ambulatory HIV-infected persons for tuberculosis. J Acquir Immune Defic Syndr. 2011;58(2):219–23. doi: 10.1097/QAI.0b013e31822b75d4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hanifa Y, Fielding KL, Charalambous S, et al. Tuberculosis among adults starting antiretroviral therapy in South Africa: the need for routine case finding. Int J Tuberc Lung Dis. 2012;16(9):1252–9. doi: 10.5588/ijtld.11.0733. [DOI] [PubMed] [Google Scholar]
- 14.Kerkhoff AD, Wood R, Cobelens FG, Gupta-Wright A, Bekker LG, Lawn SD. The predictive value of current haemoglobin levels for incident tuberculosis and/or mortality during long-term antiretroviral therapy in South Africa: a cohort study. BMC Med. 2015;13(1):70. doi: 10.1186/s12916-015-0320-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fielding KL, Charalambous S, Hoffmann CJ, et al. Evaluation of a point-of-care tuberculosis test-and-treat algorithm on early mortality in people with HIV accessing antiretroviral therapy (TB Fast Track study): study protocol for a cluster randomised controlled trial. Trials. 2015;16:125. doi: 10.1186/s13063-015-0650-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Getahun H, Kittikraisak W, Heilig CM, et al. Development of a standardized screening rule for tuberculosis in people living with HIV in resource-constrained settings: individual participant data meta-analysis of observational studies. PLoS Med. 2011;8(1):e1000391. doi: 10.1371/journal.pmed.1000391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.U.S. Department of Health and Human Services NIoH, National Institute of Allergy and Infectious Diseases Division of AIDS. Division of AIDS Table for Grading the Severity of Adult and Pediatric Adverse Events, Version 1.0. 2009. [accessed 15 April 2018]. [Updated August 2009] http://rsc.tech-res.com/docs/default-source/safety/table_for_grading_severity_of_adult_pediatric_adverse_events.pdf.
- 18.Karat AS, Omar T, von Gottberg A, et al. Autopsy Prevalence of Tuberculosis and Other Potentially Treatable Infections among Adults with Advanced HIV Enrolled in Out-Patient Care in South Africa. PLoS One. 2016;11(11):e0166158. doi: 10.1371/journal.pone.0166158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Karat AS, Tlali M, Fielding KL, et al. Measuring mortality due to HIV-associated tuberculosis among adults in South Africa: Comparing verbal autopsy, minimally-invasive autopsy, and research data. PLoS One. 2017;12(3):e0174097. doi: 10.1371/journal.pone.0174097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hosseinipour MC, Bisson GP, Miyahara S, et al. Empirical tuberculosis therapy versus isoniazid in adult outpatients with advanced HIV initiating antiretroviral therapy (REMEMBER): a multicountry open-label randomised controlled trial. Lancet. 2016;387(10024):1198–209. doi: 10.1016/S0140-6736(16)00546-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Manabe YC, Worodria W, van Leth F, et al. Prevention of Early Mortality by Presumptive Tuberculosis Therapy Study: An Open Label, Randomized Controlled Trial. Am J Trop Med Hyg. 2016;95(6):1265–71. doi: 10.4269/ajtmh.16-0239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Blanc FX, Badje AD, Bonnet M, et al. Systematic vs test-guided tuberculosis treatment. Data of the STATIS randomized trial. 25th Conference on Retroviruses and Opportunistic Infections; Boston, USA. 2018. [Google Scholar]
- 23.Tlali M, Fielding K, Charalambous S, et al. Sensitivity of the TB Determine LAM test compared to sputum culture gold standard TB in ambulant HIV positive participants enrolled in the TB fast track study in South Africa; 4th South African TB Conference; Durban, South Africa: 2014. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.