Abstract
Objectives
The fetal thymus gland has been shown to involute in response to intrauterine infection, and therefore could be used as a non-invasive marker of fetal compartment infection. The objective of this study was to evaluate how accurately 2D ultrasound-derived measurements of the fetal thymus reflect the 3D volume of the gland derived from motion corrected MRI images.
Study design
A retrospective study was performed using paired ultrasound and MRI datasets from the iFIND project (http://www.ifindproject.com). To obtain 3D volumetry of the thymus gland, T2-weighted single shot turbo spin echo (ssTSE) sequences of the fetal thorax were acquired. Thymus volumes were manually segmented from deformable slice-to-volume reconstructed images. To obtain 2D ultrasound measurements, previously stored fetal cine loops were used and measurements obtained at the 3-vessel-view (3VV) and 3-vessel-trachea view (3VT): anterior-posterior diameter (APD), intrathoracic diameter (ITD), transverse diameter (TD), perimeter and 3-vessel-edge (3VE). Inter-observer and intra-observer reliability (ICC) was calculated for both MRI and ultrasound measurements. Pearson correlation coefficients (PCC) were used to compare 2D-parameters with acceptable ICC to TV.
Results
38 participants were identified. Adequate visualisation was possible on 37 MRI scans and 31 ultrasound scans. Of the 30 datasets where both MRI and ultrasound data were available, MRI had good interobserver reliability (ICC 0.964) and all ultrasound 3VV 2D-parameters and 3VT 3VE had acceptable ICC (>0.75). Four 2D parameters were reflective of the 3D thymus volume: 3VV TD r = 0.540 (P = 0.002); 3VV perimeter r = 0.446 (P = 0.013); 3VV APD r = 0.435 (P = 0.110) and 3VT TD r = 0.544 (P = 0.002).
Conclusions
MRI appeared superior to ultrasound for visualization of the thymus gland and reproducibility of measurements. Three 2D US parameters, 3VV TD, perimeter and 3VT APD, correlated well with TV. Therefore, these represent a more accurate reflection of the true size of the gland than other 2D measurements, where MRI is not available.
Keywords: Fetal thymus, Magnetic resonance imaging, Obstetric ultrasound thymus, Volume
Introduction
The fetal thymus is a primary lymphoid organ involved in the development and differentiation of T-cells as part of the fetal immune system [1]. The fetal thymus has also been demonstrated to involute in response to intrauterine infection in pregnancies affected by preterm premature rupture of membranes (PPROM) [2,3]. These findings suggest that the fetal thymus gland may be an early, non-invasive indicator of fetal compartment infection in high-risk pregnancies.
Currently, there is no direct, non-invasive method to identify fetal compartment infection and clinical markers, including elevated maternal temperature, maternal and fetal tachycardia and uterine tenderness, are used in conjunction with raised maternal inflammatory markers to make this diagnosis [2,4]. However, cases of fetal compartment infection, such as those associated with PPROM, have been shown to present without overt clinical signs [5,6], suggesting that by the time of presentation, a fetal infection may already be established. In addition, adverse infant outcomes including bronchopulmonary dysplasia [2,7,8], cerebral palsy [9], intraventricular haemorrhage [10] and neonatal sepsis and pneumonia [2] have been associated with the presence of intrauterine infection. The most significant adverse neonatal outcomes occur in fetuses that deliver very prematurely (before 32 weeks gestation) [8,10].
Although antenatal ultrasound (US) has been successfully used to identify the fetal thymus gland [11–13], US data is often limited by adverse fetal positioning [12,13], increased maternal body habitus [14] and oligohydramnios [15]. Furthermore, the two-dimensional (2D) measurements used in these studies may not account for the inherent variability of the three-dimensional (3D) thymus gland.
3D imaging of the fetal thymus on antenatal US has also been attempted and has been shown to provide improved visualisation of its borders [16]. However, the technique is time-consuming [17], limited by both acoustic shadowing and movement artefact [18] and has poor inter-observer reliability [19]. MRI is less sensitive to fetal lie and increased maternal habitus, and slice-to-volume reconstruction methods can account for unpredictable fetal motion [20] providing a more accurate representation of true fetal thymic size [21]. Fetal thymus volume (TV) has been successfully measured on fetal magnetic resonance imaging (MRI) in both normal and growth-restricted fetuses [22] and those at high risk of preterm birth [21]. However, the cost of MRI in comparison to conventional ultrasound may preclude its use in routine clinical practice.
This study therefore aims to assess the suitability of two-dimensional US-derived measures of thymus size as a proxy marker for overall size of the gland, measured from motion corrected MRI datasets, in fetuses between 20+0 and 32+0 weeks gestation by:
Assessing the reproducibility of 2D US and 3D MRI measurements of the fetal thymus
Comparing 2D US measurements with 3D MRI-derived TV
Materials and methods
Participants
Datasets had previously been acquired as part of the intelligent fetal imaging and diagnosis (iFIND) project (http://www.ifindproject.com). Cases were selected in singleton pregnancies when: both US and MRI scanning had been undertaken between 20+0 and 32+0 weeks gestation; MRI scanning had occurred on a 1.5 T MRI system; no antenatal complications had occurred; and pregnancies were delivered after 37+0 weeks gestation. All women had undergone US and MRI scanning within a 3-day period. Maternal demographics were recorded.
MRI scans
All women had given informed, written consent (Ethics reference 14/LO/1806). All fetal MRI were performed on a 1.5 T Philips Ingenia MRI system (Philips Medical systems, Best, the Netherlands) with a 28-channel Torso-coil placed on the mother’s abdomen. The mother was scanned in the “left lateral tilt” position. Imaging of the fetus was performed using T2-weighted single shot turbo spin echo (ssTSE), obtained in three orthogonal planes. The following scanning parameters were followed: TR = 25,991 ms, TE = 80 ms, slice thickness = 2.5 mm, slice overlap = 1.25 mm and flip angle 90°. Medical cover, by either an obstetrician or midwife, frequent verbal interaction with mother and continuous monitoring of oxygen saturation and heart rate was provided throughout the scan. Scanning time was limited to one hour.
The fetal thorax was reconstructed in order to correct motion-artefacts. Slice-to-volume reconstruction tool (SVR) was performed, using 6–8 MRI stacks acquired in different directions through the fetal thorax [23,24]. Using the software ITK-SNAP version 3.6.0 [25], the fetal thymus was manually segmented from the SVR volumes, enabling the acquisition of thymus volume from the fetal thorax. All segmentations were undertaken by one operator (LS). Good inter observer variability had previously been confirmed with a further operator (AE) [21].
Ultrasound scans
All fetal ultrasounds were performed on a Philips EPiQ ultrasound system (Best, Netherlands) with a high frequency (5–9 mHz) curvilinear probe. A dedicated fetal cardiac preset was used. The acquisition plane was achieved by an experienced fetal cardiologist or senior obstetric sonographer [26]. Five second cine loops were stored of all cardiac imaging when the plane of interest was achieved.
Previously cited 2D measurements, were obtained from the recorded cine loops using Medical Imaging Interaction Toolkit (MITK) Workbench software (version 2018.04.2) [27] at two anatomical levels: the 3-vessel-view (3VV) shown in Fig. 1, and the 3-vessel-trachea view (3VT) shown in Fig. 2 [26].
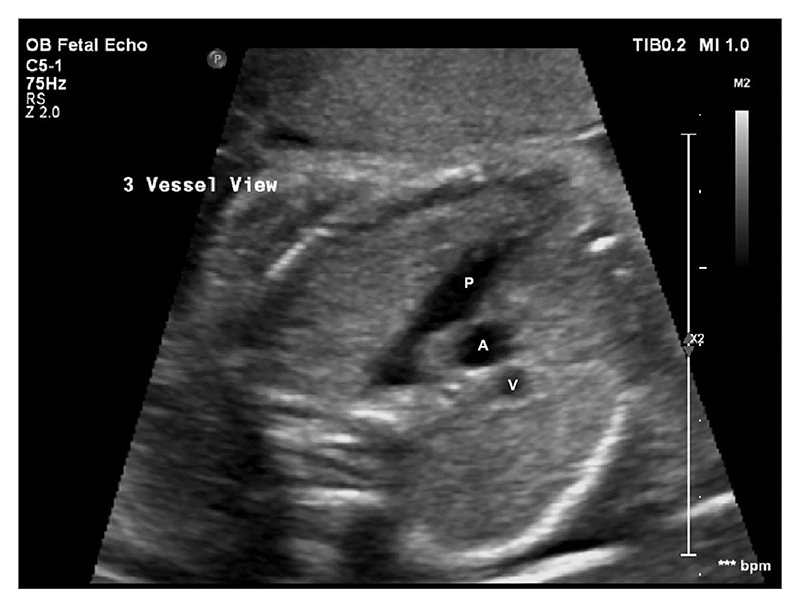
Fig. 1.
Obstetric ultrasound of fetal thorax showing the 3-vessel-view (3VV). Pulmonary Artery (P), ascending aorta (A) and superior vena cava (V).
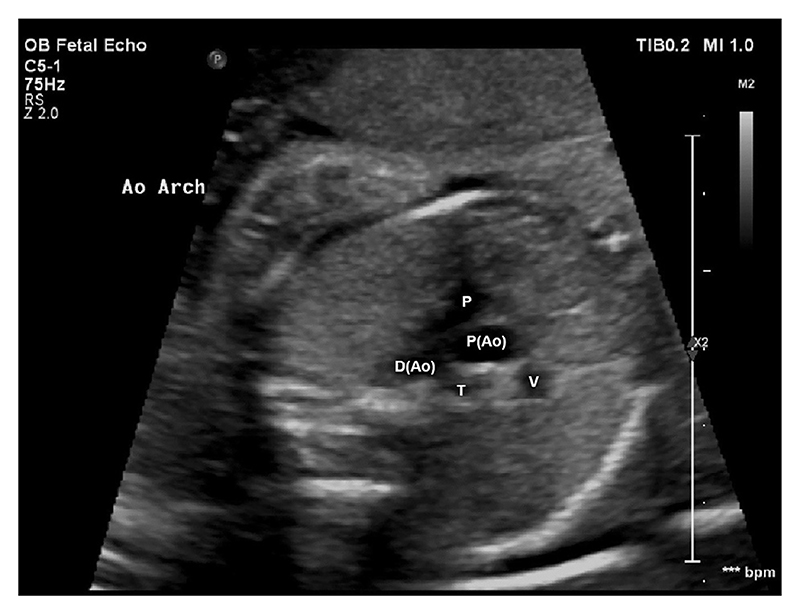
Fig. 2.
Obstetric ultrasound of fetal thorax in the 3-vessel-trachea view (3VT). Pulmonary artery (P), proximal aorta (P(Ao)), distal aorta (D(Ao)), superior vena cava (V), trachea (T).
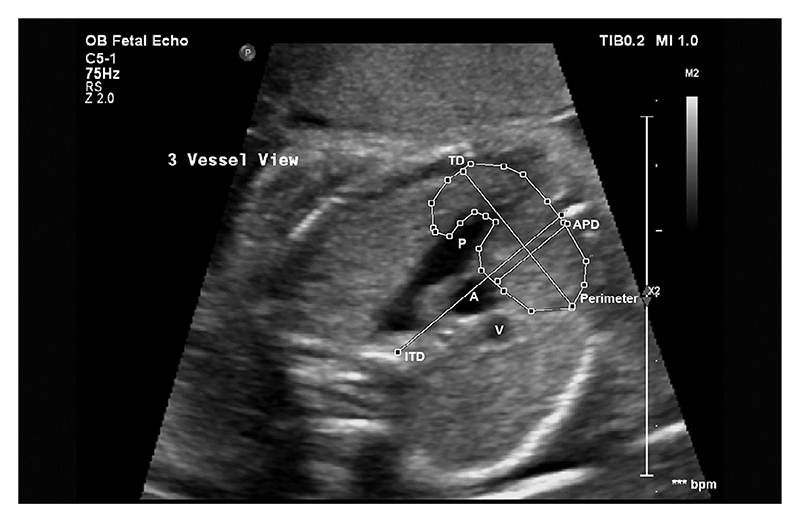
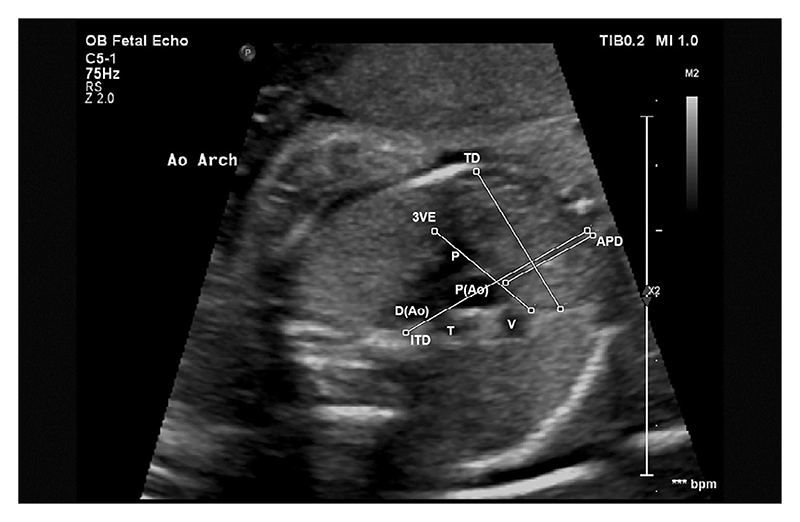
At the 3VV, as in Chaoui et al [28], the thymic-thoracic ratio (TTR) was calculated by dividing the anterior-posterior diameter (APD) of the thymus by the intrathoracic diameter (ITD). The maximum transverse diameter (TD) was then measured perpendicularly to the ITD at the widest point of the thymus gland, as in Cho et al [13]. Perimeter was measured through manual tracing of the thymic borders, as described in Zalel et al [12]. This is shown in Fig. 3. At the 3VT, APD, ITD and TD were measured, as well as 3-vessel-edge (3VE), which was measured by drawing a straight line through the anterior borders of the superior vena cava (SVC), proximal aortic arch and pulmonary artery (PA), as shown by Diemert et al [17]. This is shown in Fig. 4.
Fig. 3.
Obstetric ultrasound of fetal thorax showing the 3-vessel-view (3VV). Pulmonary Artery (P), ascending aorta (A) and superior vena cava (V), Anterior-Posterior Diameter (APD), Intrathoracic Diameter (ITD), Transverse Diameter (TD) and Perimeter.
Fig. 4.
Obstetric ultrasound of fetal thorax showing the 3-vessel-trachea view (3VT). Pulmonary artery (P), proximal aorta (P(Ao)), distal aorta (D(Ao)), superior vena cava (V), trachea (T), Anterior-Posterior Diameter (APD), Intrathoracic Diameter (ITD), Transverse Diameter (TD) and 3-vessel-edge (3VE).
For all 2D measurements, inter observer reliability was determined between four operators, including a fetal medicine consultant, senior radiographer and fetal cardiologist (SN, JM, DL and RM) across 5 control scans. Intra-observer reliability was also confirmed (RM). Maternal demographics and neonatal parameters were recorded. All datasets were then analysed by one operator (RM).
Statistical analysis
Intraclass correlation coefficients (ICC) were calculated to obtain the intra- and inter-observer reliability for 2D US-derived measurements of the thymus gland in each of the above views. Intra- and inter-observer variability of MRI-derived TV had previously been ascertained [21]. Pearson correlation coefficients (PCC) (r) were then used to compare US-derived measurements with the 3D MRI-derived TV. Analyses were conducted using IBM SPSS Statistics Version 26.0.
Results
Participants
During the period of the study, 38 cases were selected from existing datasets (iFIND project). On fetal MRI, 3D reconstruction of the fetal thorax was completed in all women. The fetal thymus could not be visualised in one case. In US imaging, visualisation of the thymus gland was not possible in seven cases (18%) due to acoustic shadowing impeding visualisation of the thymus borders. This left 30 cases with both US and MRI images suitable for comparison. All fetuses had normal liquor volume on scan.
Maternal and neonatal parameters were recorded. Mean maternal age was 32.83 years (range (R) = 20–39 years) and mean maternal BMI was 22.9 kg/m2 (R = 19–33 kg/m2).
Thymic measurements
Intra and inter-observer reliability was calculated using ICC are presented in Table 1. Coefficients > 0.75 were accepted as an indicator of good reliability [29]. Intra-observer reliability for MRI-derived TV was confirmed previously between two expert operators (AE and LS), ICC = 0.964, P<=0.0001) [21].
Table 1.
Intraclass Correlation Coefficients for Intra and Inter-reliability for thymic parameters measured on ultrasound scan. 3VV (3 vessel-view), 3VT (3 vessel-trachea view), APD (anterior-posterior diameter), ITD (intrathoracic diameter), TTR (thymic: thoracic ratio), TD (transverse diameter), 3VE (3-vessel-edge). Acceptable ICC values (>0.75 [29]) that are clinically significant (P< = 0.05) are in bold.
| Thymus Measurement | Intra-observer reliability (n = 5) | Inter-observer reliability (n = 5) | ||||||
|---|---|---|---|---|---|---|---|---|
| ICC (95% CI) | P value | ICC (95% CI) | P value | |||||
| 3VV | APD | 0.984 (0.851-0.998) | 0.001 | 0.913 (0.35-0.99) | 0.012 | |||
| ITD | 0.981 (0.860-0.998) | 0.001 | 0.931 (0.37-0.99) | 0.015 | ||||
| TTR | 0.903(0.232-0.990) | 0.024 | 0.771(−0.256-0.968) | 0.052 | ||||
| TD | 0.954 (0.516-0.995) | 0.008 | 0.872 (0.700-0.986) | 0.035 | ||||
| Perimeter | 0.962 (0.648-0.996) | 0.002 | 0.788 (0.264-0.976) | 0.041 | ||||
| 3VT | APD | 0.894(0.256-0.989) | 0.022 | 0.731(−0.358-0.782) | 0.052 | |||
| ITD | 0.989 (0.917-0.999) | <0.001 | 0.210(−0.072-0.782) | 0.077 | ||||
| TTR | 0.996(0.966-1.000) | <0.001 | 0.705(0.099-0.963) | 0.015 | ||||
| TD | 0.852(−0.200-0.984) | 0.049 | 0.790(−0.590-0.828) | 0.375 | ||||
| 3VE | 0.988 (0.881-0.999) | 0.001 | 0.901 (−0.191-0.990) | 0.032 |
Pearson correlation coefficients (PCC) (r and r2) were used to compare 2D US measurements with TV and are presented in Table 2. 3VV APD, TD, and perimeter, as well as 3VT TD correlated with TV and were statistically significant (P<=0.05), whereas 3VV ITD, TTR and 3VT ITD, TTR and 3VE were neither well correlated nor statistically significant [30].
Table 2.
Table showing Pearson Correlation Coefficients (r and r2) of 2D ultrasound measurements compared to Thymus Volume (TV). 3VV (3 vessel-view), 3VT (3 vessel-trachea view), APD (anterior-posterior diameter), ITD (intrathoracic diameter), TTR (thymic: thoracic ratio), TD (transverse diameter), 3VE (3-vessel-edge). Clinically significant P values are in bold.
| Measurement | Thymus Volume (n = 30) | |||
|---|---|---|---|---|
| r | r 2 | P value | ||
| 3VV | APD | 0.435 | 0.189 | 0.016 |
| ITD | 0.342 | 0.117 | 0.064 | |
| TTR | 0.083 | 0.007 | 0.664 | |
| TD | 0.540 | 0.292 | 0.002 | |
| Perimeter | 0.446 | 0.199 | 0.013 | |
| 3VT | APD | 0.409 | 0.167 | 0.025 |
| ITD | 0.325 | 0.105 | 0.080 | |
| TTR | 0.123 | 0.015 | 0.518 | |
| TD | 0.544 | 0.296 | 0.002 | |
| 3VE | 0.298 | 0.089 | 0.110 |
Discussion
Reproducibility of thymic measurements
MRI proved superior for visualisation of the thymus gland, with adequate visualisation occurring in 97% of cases, compared to 80% (30/38) on ultrasound. MRI-derived TV also provided the most reproducible method of assessment of thymus size (0.964, P < 0.01) [21].
Few studies have assessed the fetal thymus using MRI, however a previous study from our research group evaluated the gland in 39 fetuses with the gland visualised in all cases [22]. Although some studies have reported poor visualisation of the fetal thymus on ultrasound [11,18], our finding that adequate visualisation occurred in only 80% of cases on ultrasound images is lower than previously reported studies where rates of up to 100% have been described [12,19]. All previous studies were conducted by specialists specifically assessing the thymus gland. However, our datasets were evaluated retrospectively from stored cine loops, with measurements from two planes (3VV and 3VT-view), which have been previously described as necessary for obtaining optimal thymus images to ensure standardization of measurements [12,13,17,26,28]. Our results may therefore be more reflective of practitioners assessing the thymus in clinical practice.
In the present study, 2D measurements with acceptable inter-observer reliability (ICC > 0.75, P<=0.05) were 3VV APD (0.913), ITD (0.931), TD (0.872) and perimeter (0.788), as well as 3VT 3VE (0.913), with only 3VV APD and 3VT 3VE with excellent inter-observer reliability (>0.9) [29]. Multiple previous studies have reported good agreement for all 2D parameters [12,13,17,28], although Diemert et al found poor inter-observer reliability for thymic perimeter [17]. There are difficulties in obtaining standardised planar views on ultrasound imaging; 2D measurements are angle-dependent, meaning that only a few degrees of error can have significant effects on the overall measurement. Furthermore, ultrasound data was stored as cine loops and not observed in real time, meaning that investigators had a limited number of views to measure from. Therefore, these differences may be exacerbated in clinical practice without a preset number of frames to view. However, it is likely that US may perform better with prospective measurements as individual adjustments can be made to allow for better visualisation.
Furthermore, although acceptable ICC for MRI-derived TV has been identified in growth restricted fetuses previously [22], the high ICC obtained in the present study (0.964, P<=0.0001) is likely due to improvements in slice-to-volume reconstruction techniques [20].
Comparison of 2D-ultrasound and 3D-MR thymus measurements
Results from the study found only four 2D parameters correlated with MR-derived thymus volume [30]: 3VV TD, APD and perimeter, and 3VT TD. The variation in correlation between different 2D measurements and TV may be attributable to significant variability in thymus shape even within healthy fetuses [31], where some thymuses have a more globular appearance and others more diffuse. This is demonstrated in Figs. 5, 6 and 7. Furthermore, r2 values for the 2D-parameters that best correlate with MRI-derived TV remain low. This indicates that approximately only 20 to 30% of the variance in MRI TV can be explained, highlighting that the variable thymus shape is poorly captured by linear measurements.
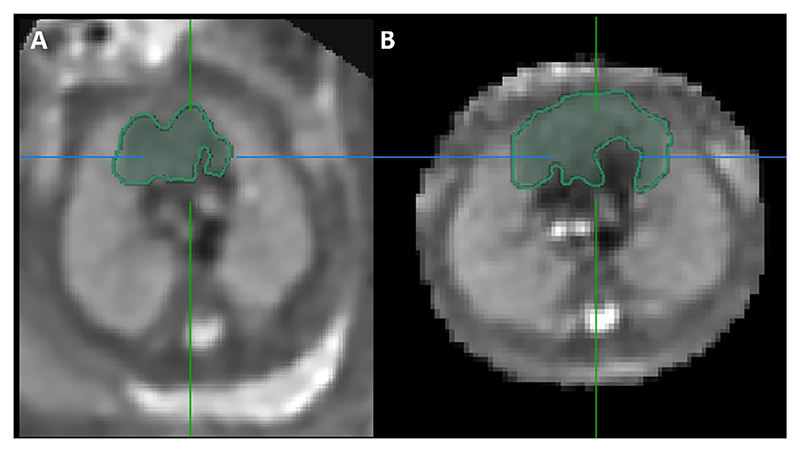
Fig. 5.
Slice-to-volume reconstructed MRI images of the fetal thorax from two fetuses showing the segmented fetal thymus gland in the axial plane. Scan A conducted at 24 + 6 weeks gestation, Scan B conducted at 24 + 2 weeks gestation.
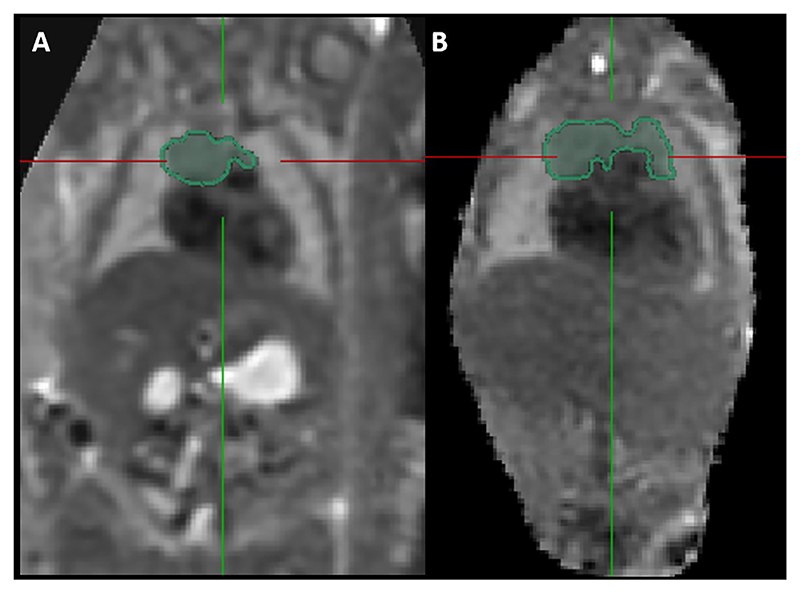
Fig. 6.
Slice-to-volume reconstructed MRI images of the fetal thorax from two fetuses showing the segmented fetal thymus gland in the coronal plane. Scan A conducted at 24 + 6 weeks gestation. Scan B conducted at 24 + 2 weeks gestation.
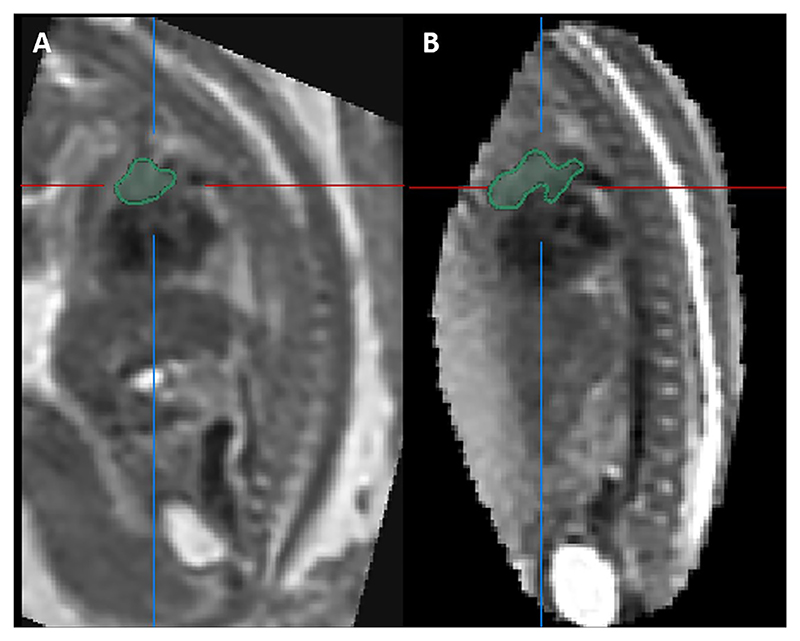
Fig. 7.
Slice-to-volume reconstructed MRI images of the fetal thorax from two fetuses showing the segmented fetal thymus gland in the sagittal plane. Scan A conducted at 24 + 6 weeks gestation. Scan B conducted at 24 + 2 weeks gestation.
These findings are supported by Li et al who previously compared the size and volume of the thymus by 2D (maximum transverse diameter (TD), maximum transverse area (A) and anterior-posterior diameter (APD) and superior-inferior diameter (SID)) and 3D ultrasound in 321 fetuses [16]. Results found that the correlation between thymus volume and gestation age was stronger than between each of the 2D measurements individually (r(TV) = 0.99 and r(TA) = 0.92, r(TD) = 0.88, r(APD) = 0.85 and r(SID) = 0.82)), suggesting that TV is more accurate than 2D measurements in determining thymic size.
Furthermore, Tonni et al compared ultrasound-derived transverse diameter and TV, identifying the thymus gland with the use of Doppler ultrasound to identify perithymic vessels in order to improve visualisation of the gland [19]. Across 100 women, a good correlation between transverse diameter and TV was reported (r = 0.58p < 0.001). However, the inter-observer reliability for TV was low (ICC = 0.57), suggesting some difficulty obtaining accurate US-derived 3D measurements.
With US being a quick, non-resource intensive imaging technique, questions of accessibility to fetal MRI and difficulties in post-imaging processing have been raised; however a recent study demonstrating the advances in MRI post-acquisition techniques ensures processing can be completed with relative ease in a less time-consuming manner [32].
Limitations
Limitations of the present study include a small sample size (n = 30) which was retrospective in nature. Additionally, all patients had normal liquor volume and a normal maternal BMI (R = 19–33 kg/m2). As both oligohydramnios [15] and raised maternal BMI [14] reduce visualisation of fetal structures on ultrasound, quantification of the fetal thymus may be even more challenging in these scenarios. Finally, confidence intervals for intra and inter-reliability were wide, which may relate to the small sample number (n = 5), that these values were determined from.
In the future, further 2D US measurements of the fetal thymus should be evaluated in women at high-risk of preterm birth so as to assess whether accurate visualisation of the gland occurs in the presence of oligohydramnios and whether MRI-derived TV may prove superior in such cases. In addition, exactly how the shape changes in the presence of infection also needs to be evaluated. This is of importance as this subgroup of patients who are at significant risk of fetal infection. Finally, larger studies investigating 2D measurements are representative of true thymus size at different gestational ages should be undertaken.
Conclusions
Results have demonstrated that thymus volume is a more reproducible measure of thymic size compared to 2D parameters. However, where US is used, the measures that best correlated to thymus volume were 3VV TD, APD and perimeter, making these the more suitable measures in clinical practice where fetal MR scanning may not be feasible due to cost and scanner availability.
Acknowledgements
Dr Jana Hutter is a UKRI Future Leaders fellow and Dr Lisa Story is an NIHR funded Clinical lecturer. This work was supported by the Wellcome Trust IEH Award [102431] and by core funding from the Wellcome/EPSRC Centre for Medical Engineering [WT203148/Z/16/Z]. The research was funded/supported by the National Institute for Health Research (NIHR) Biomedical Research Centre based at Guy’s and St Thomas’ NHS Foundation Trust and King’s College London and supported by the NIHR Clinical Research Facility (CRF) at Guy’s and St Thomas’. The views expressed are those of the author(s) and not necessarily those of the NHS, the NIHR or the Department of Health.
Biographies
Rebecca Myers is a penultimate year medical student, currently studying at St George’s University of London. She completed her intercalated degree in Women’s Health at Kings College London.
Jana Hutter is a Researcher in the field of MRI at King’s College London focusing on novel efficient functional techniques applied to clinical and research questions around the time of birth.
Jacqueline Matthew is a research sonographer and radiographer with an interest in multimodal studies (MRI/US) for improved fetal anomaly detection and diagnosis, placental and craniofacial imaging, image analysis, machine learning and automation.
Dr Tong Zhang is an Assistant Professor at Artificial Intelligence Research Center, Peng Cheng Laboratory, Shenzhen, China. Her research focuses on intelligent fetal imaging and image analysis.
Dr Alena Uus is a Research Associate at the Department of Biomedical Engineering at King’s College London. Her research focuses on software development for motion correction and analysis of fetal and neonatal MRI datasets.
Dr David Lloyd is a Consultant in paediatric and fetal cardiology at the Evelina London Children’s Hospital and a Clinical Lecturer in Child Health and King’s College London. His research interests include novel fetal cardiac MRI techniques, advanced fetal ultrasound and artificial intelligence.
Dr Alexia Egloff is a Consultant radiologist with subspecialties in paediatric radiology, paediatric neuroradiology and fetal imaging.
Dr Maria Deprez is a Lecturer in Medical Imaging at King’s College London. She focuses on image analysis and AI methods for motion-corrected fetal MRI and quantification of fetal and neonatal development.
Dr Surabhi Nanda is a Consultant in Maternal Fetal Medicine at Guy’s and St Thomas’ NHS Foundation Trust, and an honorary senior lecture at King’s College London. Her research interests include multiple pregnancy, placental imaging, and developing ways to bridge the gap between advanced MR imaging and mainstream clinical fetal medicine.
Mary Rutherford trained as a paediatrician, specialising in neonatal neurology. She has worked with magnetic resonance imaging (MRI) for 30 years. Her expertise is in the acquisition and interpretation of fetal and neonatal MRI of the brain. Her research interests include optimising MR sequences to allow objective quantification of both normal and abnormal brain development. She is Professor of Perinatal Imaging at Kings College London and has an Honorary Contract with Guys and St Thomas’ Trust (GSTT). She also holds an Honorary Professor Post in the Department of Paediatrics, University of Stellenbosch, South Africa.
Dr Lisa Story is a NIHR funded Clinical Lecturer and Subspecialty Trainee at King’s College London/King’s College London.
Footnotes
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- [1].Gordon J, Manley NR. Mechanisms of thymus organogenesis and morphogenesis. Development. 2011;138:3865–78. doi: 10.1242/dev.059998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Ramsey PS, Lieman JM, Brumfield CG, Carlo W. Chorioamnionitis increases neonatal morbidity in pregnancies complicated by preterm premature rupture of membranes. Am J Obstet Gynecol. 2005;192(4):1162–6. doi: 10.1016/j.ajog.2004.11.035. [DOI] [PubMed] [Google Scholar]
- [3].Toti P, De Felice C, Stumpo M, Schürfeld K, Leo LD, Vatti R, et al. Acute thymic involution in fetuses and neonates with chorioamnionitis. Hum Pathol. 2000;31(9):1121–8. doi: 10.1053/hupa.2000.16676. [DOI] [PubMed] [Google Scholar]
- [4].NICE. Preterm labour and birth. 2019. [cited 2021 Apr 18]. [Internet]. Available from: https://www.nice.org.uk/guidance/ng25.
- [5].Yoon BH, Romero R, Park JS, Kim M, Oh S-Y, Kim CJ, et al. The relationship among inflammatory lesions of the umbilical cord (funisitis), umbilical cord plasma interleukin 6 concentration, amniotic fluid infection, and neonatal sepsis. Am J Obstet Gynecol. 2000;183(5):1124–9. doi: 10.1067/mob.2000.109035. [DOI] [PubMed] [Google Scholar]
- [6].Rovira N, Alarcon A, Iriondo M, Ibañez M, Poo P, Cusi V, et al. Impact of histological chorioamnionitis, funisitis and clinical chorioamnionitis on neurodevelopmental outcome of preterm infants. Early Hum Dev. 2011;87(4):253–7. doi: 10.1016/j.earlhumdev.2011.01.024. [DOI] [PubMed] [Google Scholar]
- [7].Yoon BH, Romero R, Kim KS, Park JS, Ki SH, Kim B, et al. Am J Obstet Gynecol. Vol. 181. Mosby Inc; 1999. A systemic fetal inflammatory response and the development of bronchopulmonary dysplasia; pp. 773–9. [DOI] [PubMed] [Google Scholar]
- [8].De Felice C, Latini G, Del Vecchio A, Toti P, Bagnoli F, Petraglia F. Small thymus at birth: A predictive radiographic sign of bronchopulmonary dysplasia. Pediatrics. 2002;110(2):386–8. doi: 10.1542/peds.110.2.386. [DOI] [PubMed] [Google Scholar]
- [9].Yoon BH, Romero R, Park JS, Kim CJ, Kim SH, Choi J-H, et al. Fetal exposure to an intra-amniotic inflammation and the development of cerebral palsy at the age of three years. Am J Obstet Gynecol. 2000;182(3):675–81. doi: 10.1067/mob.2000.104207. [DOI] [PubMed] [Google Scholar]
- [10].Villamor-Martinez E, Fumagalli M, Mohammed Rahim O, Passera S, Cavallaro G, Degraeuwe P, et al. Chorioamnionitis is a risk factor for intraventricular hemorrhage in preterm infants: A systematic review and meta-analysis. Front Physiol. 2018;9 doi: 10.3389/fphys.2018.0125310.3389/fphys.2018.01253.s001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Felker RE, Cartier MS, Emerson DS, Brown DL. Ultrasound of the fetal thymus. J Ultrasound Med. 1989;8:669–73. doi: 10.7863/jum.1989.8.12.669. [DOI] [PubMed] [Google Scholar]
- [12].Zalel Y, Gamzu R, Mashiach S, Achiron R. The development of the fetal thymus: An in utero sonographic evaluation. Prenat Diagn. 2002;22(2):114–7. doi: 10.1002/pd.257. [DOI] [PubMed] [Google Scholar]
- [13].Cho JY, Min JY, Lee Y-H, McCrindle B, Hornberger LK, Yoo S-J. Diameter of the normal fetal thymus on ultrasound. Ultrasound Obstet Gynecol. 2007;29(6):634–8. doi: 10.1002/uog.3979. [DOI] [PubMed] [Google Scholar]
- [14].Dashe JS, McIntire DD, Twickler DM. Maternal obesity limits the ultrasound evaluation of fetal anatomy. J Ultrasound Med. 2009;28:1025–30. doi: 10.7863/jum.2009.28.8.1025. [DOI] [PubMed] [Google Scholar]
- [15].Levine D, Goldstein RB, Callen PW, Damato N, Kilpatrick S. The effect of oligohydramnios on detection of fetal anomalies with sonography. Am J Roentgenol. 1997;168(6):1609–11. doi: 10.2214/ajr.168.6.9168737. [DOI] [PubMed] [Google Scholar]
- [16].Li L, Bahtiyar MO, Buhimschi CS, Zou L, Zhou Q-C, Copel JA. Assessment of the fetal thymus by two- and three-dimensional ultrasound during normal human gestation and in fetuses with congenital heart defects. Ultrasound Obstet Gynecol. 2011;37(4):404–9. doi: 10.1002/uog.8853. [DOI] [PubMed] [Google Scholar]
- [17].Diemert A, Hartwig I, Pagenkemper M, Mehnert R, Hansen G, Tolosa E, et al. Fetal thymus size in human pregnancies reveals inverse association with regulatory T cell frequencies in cord blood. J Reprod Immunol. 2016;113:76–82. doi: 10.1016/j.jri.2015.12.002. [DOI] [PubMed] [Google Scholar]
- [18].Re C, Bertucci E, Weissmann-Brenner A, Achiron R, Mazza V, Gindes L. Fetal thymus volume estimation by virtual organ computer-aided analysis in normal pregnancies. J Ultrasound Med. 2015;34:847–52. doi: 10.7863/ultra.34.5.847. [DOI] [PubMed] [Google Scholar]
- [19].Tonni G, Rosignoli L, Cariati E, Martins WP, Miyague AH, Bruns RF, et al. Fetal thymus: Visualization rate and volume by integrating 2D- and 3D-ultrasound during 2nd trimester echocardiography. J Matern Neonatal Med. 2016;29(14):2223–8. doi: 10.3109/14767058.2015.1081892. [DOI] [PubMed] [Google Scholar]
- [20].Uus A, Zhang T, Jackson LH, Roberts TA, Rutherford MA, Hajnal JV, et al. Deformable Slice-to-Volume Registration for Motion Correction of Fetal Body and Placenta MRI. IEEE Trans Med Imaging. 2020;39(9):2750–9. doi: 10.1109/TMI.4210.1109/TMI.2020.2974844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Story L, Zhang T, Uus A, Hutter J, Egloff A, Gibbons D, et al. Antenatal thymus volumes in fetuses that delivered <32 weeks gestation: An MRI pilot study. Acta Obstet Gynecol Scand. 2020:aogs.13983. doi: 10.1111/aogs.13983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Damodaram MS, Story L, Eixarch E, Patkee P, Patel A, Kumar S, et al. Foetal volumetry using Magnetic Resonance Imaging in intrauterine growth restriction. Early Hum Dev. 2012;88:S35–40. doi: 10.1016/j.earlhumdev.2011.12.026. [DOI] [PubMed] [Google Scholar]
- [23].Kuklisova-Murgasova M, Quaghebeur G, Rutherford MA, Hajnal JV, Schnabel JA. Reconstruction of fetal brain MRI with intensity matching and complete outlier removal. Med Image Anal. 2012;16(8):1550–64. doi: 10.1016/j.media.2012.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Kainz B, Steinberger M, Wein W, Kuklisova-Murgasova M, Malamateniou C, Keraudren K, et al. Fast Volume Reconstruction From Motion Corrupted Stacks of 2D Slices. IEEE Trans Med Imaging. 2015;34(9):1901–13. doi: 10.1109/TMI.2015.2415453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Yushkevich PA, Piven J, Hazlett HC, Smith RG, Ho S, Gee JC, et al. User-guided 3D active contour segmentation of anatomical structures: Significantly improved efficiency and reliability. Neuroimage. 2006;31(3):1116–28. doi: 10.1016/j.neuroimage.2006.01.015. [DOI] [PubMed] [Google Scholar]
- [26].The International Society of Ultrasound in Obstetrics. ISUOG Practice Guidelines (updated): sonographic screening examination of the fetal heart. Ultrasound Obstet Gynecol. 2013;41:348–59. doi: 10.1002/uog.12403. [DOI] [PubMed] [Google Scholar]
- [27].Wolf I, Vetter M, Wegner I, Nolden M, Bottger T, Hastenteufel M, et al. The medical imaging interaction toolkit (MITK): a toolkit facilitating the creation of interactive software by extending VTK and ITK. Med Imaging 2004 Vis Image-Guided Proced Disp. 2004;5367:16. doi: 10.1117/12.535112. [DOI] [Google Scholar]
- [28].Chaoui R, Heling K-S, Sarut Lopez A, Thiel G, Karl K. The thymic-thoracic ratio in fetal heart defects: A simple way to identify fetuses at high risk for microdeletion 22q11. Ultrasound Obstet Gynecol. 2011;37(4):397–403. doi: 10.1002/uog.8952. [DOI] [PubMed] [Google Scholar]
- [29].Koo TK, Li MY. A Guideline of Selecting and Reporting Intraclass Correlation Coefficients for Reliability Research. J Chiropr Med. 2016;15(2):155–63. doi: 10.1016/j.jcm.2016.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Schober P, Boer C, Schwarte LA. Correlation coefficients: Appropriate use and interpretation. Anesth Analg. 2018;126(5):1763–8. doi: 10.1213/ANE.0000000000002864. [DOI] [PubMed] [Google Scholar]
- [31].Yuan L, Cao J, Wang Z, Zhang L, Wang X, Wu Y, et al. Fetal thymus in the middle and late trimesters: Morphometry and development using post mortem 3.0T MRI. Exp Ther Med. 2020;20:1–1. doi: 10.3892/etm.2020.9172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Davidson JR, Uus A, Matthew J, Egloff AM, Deprez M, Yardley I, et al. Fetal body MRI and its application to fetal and neonatal treatment: an illustrative review. Lancet Child Adolesc Heal. 2021;5(6):447–58. doi: 10.1016/S2352-4642(20)30313-8. [DOI] [PMC free article] [PubMed] [Google Scholar]