Summary
Background
Nine potentially modifiable risk factors (less childhood education, midlife hearing loss, hypertension, and obesity, and later-life smoking, depression, physical inactivity, social isolation, and diabetes) account for 35% of worldwide dementia, but most data to calculate these risk factors come from high-income countries only. We aimed to calculate population attributable fractions (PAFs) for dementia in selected low-income and middle-income countries (LMICs) to identify potential dementia prevention targets in these countries.
Methods
The study was an analysis of cross-sectional data obtained from the 10/66 Dementia Research surveys of representative populations in India, China, and six Latin America countries (Cuba, Dominican Republic, Mexico, Peru, Puerto Rico, and Venezuela), which used identical risk factor ascertainment methods in each country. Between 2004 and 2006 (and between 2007 and 2010 for Puerto Rico), all residents aged 65 years and older in predefined catchment areas were invited to participate in the survey. We used risk factor prevalence estimates from this 10/66 survey data, and relative risk estimates from previous meta-analyses, to calculate PAFs for each risk factor. To account for individuals having overlapping risk factors, we adjusted PAF for communality between risk factors, and used these values to calculate overall weighted PAFs for India, China, and the Latin American sample.
Findings
The overall weighted PAF for potentially modifiable risk factors for dementia was 39·5% (95% CI 37·5–41·6) in China (n=2162 participants), 41·2% (39·1–43·4) in India (n=2004), and 55·8% (54·9–56·7) in our Latin American sample (n=12 865). Five dementia risk factors were more prevalent in these LMICs than worldwide estimates, leading to higher PAFs for dementia: less childhood education (weighted PAF of 10·8% in China, 13·6% in India, and 10·9% in Latin America vs 7·5% worldwide), smoking (14·7%, 6·4%, and 5·7%, respectively, vs 5·5% worldwide), hypertension (6·4%, 4·0%, and 9·3%, vs 2·0%), obesity (5·6%, 2·9%, and 7·9%, vs 0·8%), and diabetes (1·6%, 1·7%, and 3·2%, vs 1·2%).
Interpretation
The dementia prevention potential in India, China, and this sample of Latin American countries is large, and greater than in high-income countries. Less education in early life, hypertension, hearing loss, obesity, and physical inactivity have particularly high PAFs and could be initial targets for dementia prevention strategies.
Funding
No funding.
Introduction
Around two thirds of people with dementia worldwide live in low-income and middle-income countries (LMICs). The number of people with dementia in these countries is predicted to rise more rapidly than in higher-income countries because an increasing number of people in LMICs are living to an older age.1 However, potentially modifiable risk factors for dementia might drive or moderate this increase, as they have in many higher-income countries where falling age-specific dementia incidence and prevalence have been reported,2,3 related to higher levels of education4 and reduced cardiovascular morbidity.5
Population attributable fractions (PAFs) estimate the proportion of disease cases that would not occur in a population if an individual risk factor were to be eliminated. We previously reported the overall PAF for dementia using the nine potentially modifiable risk factors for dementia that had been identified by the UK National Institute for Health and Care Excellence6 and the US National Institutes of Health.7 We found that 35% (95% CI 34·1–35·9) of dementia was theoretically preventable through elimination of these risk factors;2 namely, less education in childhood, hearing loss, hypertension, obesity in midlife (age 45–64 years), depression, social isolation, physical inactivity, diabetes, and smoking in later life (age >65 years). To calculate this percentage, we used meta-analyses of relative risk (RR) for dementia associated with each risk factor and prevalence of that risk based on summary global prevalence estimates,8 which were studied predominantly in high-income countries. Whether PAF estimates are applicable to LMICs is therefore unclear, given that the prevalence of some risk factors are likely to differ in these regions because of different educational policies, health behaviours, genetic predisposition, and health-care practice. PAF in LMICs might therefore be higher or lower than in high-income countries, resulting in specific priority targets for dementia prevention in particular countries. In this study, we aimed to estimate the PAF of potentially modifiable risk factors for dementia in LMICs for which we could obtain population-level data.
Methods
Data
The study was an analysis of cross-sectional data obtained from the 10/66 Dementia Research Group comprehensive one-phase prevalence surveys,9 given that these surveys reported data on the nine identified potentially modifiable risk factors for dementia. The 10/66 Dementia Research Group aimed to interview all residents aged at least 65 years in geographically defined catchment areas in selected LMICs, and used cross-culturally validated assessments for dementia.9 Participants were interviewed between 2004 and 2006 (and between 2007 and 2010 in Puerto Rico). These data are available for 17 031 participants in eight countries (Cuba, Dominican Republic, Mexico, Peru, Puerto Rico and Venezuela [grouped hereafter as the Latin American sample]; China; and India). Sample size varied between 1900 and 3000 for each country and more than 80% of the target population responded in all areas surveyed.
For more on the 10/66 Dementia Research Group see https://www.alz.co.uk/1066/
Risk factor prevalence
We calculated prevalence for the risk factors of interest using 10/66 Dementia Research Group data,9 aiming to group them into early-life (age <45 years), midlife (age 45–64 years), and later-life (age ≥65 years) risk factors on the basis of previous literature10 that linked these factors to these particular age groups. Definitions of each risk factor are presented in panel 1. We dealt with missing data by case-wise deletion given that the proportion of missing data was very low (0–2%) for all variables, except social contact, which had 10–12·7% missing data.
Some factors that are risks in middle age (eg, hyper-tension and obesity) decrease before the onset of dementia as part of the developing illness, and therefore attributing risk factors to specific time periods within the lifecourse is essential.2 However, for hypertension and obesity, prevalence data for midlife were not available. We therefore used the prevalence of pre-existing hypertension and obesity at the time of the survey as an estimate of mid-life prevalence. Although the 10/66 Dementia Research Group sample contained individuals who were older than 65 years, 60% of those were younger than 75 years, and so for a substantial proportion of the sample, the diagnosis of hypertension and the development of obesity are likely to have occurred during midlife. Obesity was measured by waist circumference (which is considered to be a more valid measure of obesity-related risk than body-mass index)13 and was favoured over waist-to-hip ratio because of the relative ease of obtaining this measurement.11
The PAF for a risk factor is defined as the percentage of cases of a disease that would be eliminated if that particular risk factor was eliminated. The value of the PAF depends on the prevalence of the risk factor and the strength of its association (RR) with the disease.
Statistical analysis
We calculated the prevalence estimates of the nine risk factors for each of the eight countries using 10/66 Dementia Research Group data. Given that the prevalence estimates were similar in each Latin American country, we grouped these into one estimate for the Latin American sample by calculating the prevalence from the combined data. As in our previous study, we used previously published meta-analyses of RR for individual risk factors.8 We then adjusted the results for communality (ie, overlap between risk factors).
Panel 1: Definitions of risk factors from 10/66 Dementia Research Group data9.
Less education
Not receiving more than primary education in early life
Hearing loss
Self-reported hearing impairment
Hypertension
Self-reported known diagnosis of hypertension
Obesity
Waist circumference measured by the 10/66 research team, of at least 88 cm in women and more than 102 cm in men, according to WHO guidelines11
Smoking
Self-reported smoking in later life
Depression
Diagnosis of depression (according to the fourth edition of the Diagnostics and Statistical Manual of Mental Disorders) in later life following a structured Geriatric Mental State12 interview or self-report of previous depression
Physical inactivity
Self-report of being either not at all or not very physically active in later life on a four-point Likert scale of level of physical activity (categories are not at all, not very, fairly, and very physically active)
Social isolation
Social contact occurring less than once per month in later life,2 calculated using pooled self-reported contact frequency with friends, relatives, and neighbours or attendance at social clubs
Diabetes
Self-report of known diagnosis of diabetes in later life
The formula used to calculate PAF is available in panel 2. We chose to use RRs for risk factors on the basis of previous meta-analyses.2,8,10 Details of how we calculated overall PAF are shown in panel 2. People might have several risk factors and individual PAFs cannot therefore be summed to get the total PAF; thus, it is important to consider communality and to calculate a weighted PAF, taking communality into account. Individual weighted PAFs were calculated with the following formula:
Panel 2: Standard method for the calculation of population attributable fractions and communality10.
Formula for individual population attributable fraction
Population attributable fraction (PAF)=Pe(RRe – 1) / (1 + Pe[RRe – 1]), in which Pe is the prevalence of the exposure and RRe the relative risk of disease because of that exposure.
Calculation of communality
Input data for all nine risk factors into our model.
Calculate the tetrachoric correlation to generate correlation coefficients and a correlation matrix. This calculation establishes the correlation between unobserved and latent variables and observed dichotomous variables.
Do a principal component analysis on the correlation matrix to generate eigenvectors, which are directions mapped onto the datapoints from which variance to the data is measured. These eigenvectors represent unobserved factors underlying all the variables that explain the variance observed.
Components with eigenvalues of at least 1 were retained in the model, as is standard practice, so that only eigenvectors that hold the most information about the data distribution are retained.
Communality was calculated as the sum of the square of all factor loadings (ie, how much each unobserved component explained each measured variable).
Calculation of overall PAF
We then calculated overall PAF: PAF=1 – [(1 – PAF1)(1 – PAF2)(1 – PAF3)…]
Each individual risk factor’s PAF was weighted according to its communality using the formula: Weight (w)=1 – communality
Weighting was included in the calculation of overall PAF using the formula: PAF=1 – [(1 – w*PAF1)(1 – w*PAF2)(1 – w*PAF3)...]
We did sensitivity analyses to estimate variability in PAF depending on prevalence of each risk factor, by using estimates from other high-quality studies (appendix) of risk factor prevalence. We did a literature search in PubMed for articles published from database inception to June 8, 2018, using the search terms for each risk factor, the country or region of interest, and the age group of interest—eg, “China”, “older adults”, and “hypertension” or “high blood pressure”. We did not restrict our searches by language or date of publication. In line with guidance14 for evaluating prevalence studies, we defined a prevalence study as one that used probability sampling with at least 70% response and reported a prevalence for the specified risk factor from the relevant countries, using valid tools for measuring each risk factor.14 We used the lowest and highest prevalence figures found to calculate possible lowest and highest PAFs. In cases in which we found no differing prevalence estimates for a specific risk factor from population surveys, we used 10/66 Dementia Research Group prevalence figures in the sensitivity analyses to calculate the overall PAF. Therefore, in these calculations the weighted prevalence still changed in our sensitivity analyses despite using the same prevalence figures, because of the weighting adjustment. If other factors were less prevalent, then a risk factor with unchanged prevalence contributed a relatively larger risk to the overall PAF (and the inverse for the highest PAF estimate).
Role of the funding source
The funder of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Results
In this analysis, we used data from 12 865 respondents in Latin America, 2162 respondents in China, and 2004 respondents in India to calculate PAFs. The median age was 73 years (range 65 to 110) and 62·5% of the sample was female.
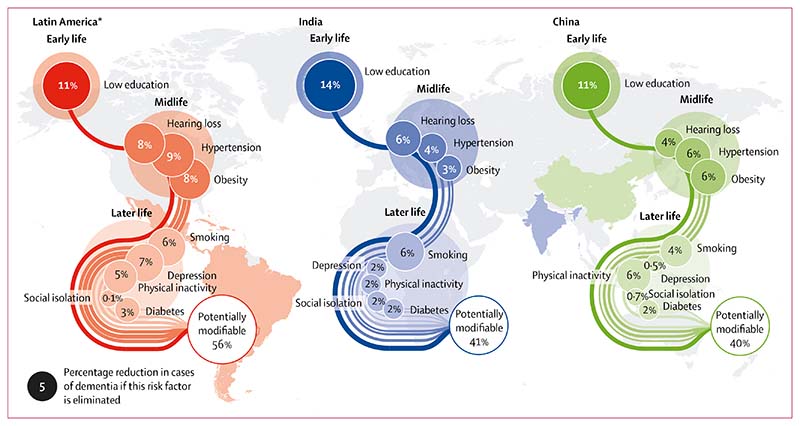
The proportion of dementia cases that were theoretically preventable through elimination of the nine identified risk factors (ie, overall weighted PAF) was 55·8% (95% CI 54·9–56·7) in Latin America, 39·5% (37·5–41·6) in China, and 41·2% (39·1–43·4) in India (figure). Risk factor prevalence, communality, and weighted PAFs for each region are presented in tables 1–3. For comparison, the previously published worldwide risk factor prevalence, communality, and weighted PAF are presented in table 4. We have provided risk factor prevalence estimates for each individual Latin American country and the associated PAFs in the appendix. The PAFs for the Latin American countries in our sample were: 52·7 (95% CI 50·9–54·5) for Cuba, 54·3 (52·1–56·4) for the Dominican Republic, 50·2 (47·9–52·4) for Peru, 54·8 (52·6–57·0) for Venezuela, 55·5 (53·5–57·7) for Mexico, and 53·2 (50·9–55·3) for Puerto Rico. Less childhood education, smoking, hypertension, obesity, and diabetes were more common than worldwide estimates across all three regions, leading to higher PAF.
Figure. Population attributable fractions for potentially modifiable risk factors in low-income and middle-income countries.
*Our data for Latin America include the data for Cuba, Dominican Republic, Mexico, Peru, Puerto Rico, and Venezuela.
Table 1. PAF for dementia risk factors in China (n=2l62).
| RR for dementia (95% CI) | Risk factor prevalence2,8,10 | Communality | PAF | Weighted PAF* | |
|---|---|---|---|---|---|
| Early life (<45 years) | |||||
| Low education | 1.6 (1.3–2.0) | 75.9% | 72% | 31.3% (29.4–33.3) | 10.8% (9.5–12.2) |
| Midlife (45–64 years) | |||||
| Hearing loss | 1.9 (1.4–2.7) | 14.3% | 46% | 11.4% (10.1–12.8) | 3.9% (3.2–4.8) |
| Hypertension | 1.6 (1.2–2.7) | 38.1% | 52% | 18.6% (17.0–20.3) | 6.4% (5.4–7.5) |
| Obesity | 1.6 (1.3–1.9) | 32.0% | 65% | 16.1% (14.6–17.7) | 5.6% (4.7–6.7) |
| Later life (≥65 years) | |||||
| Smoking | 1.6 (1.2–2.2) | 23.0% | 34% | 14.7% (13.3–16.3) | 4.2% (3.4–5.1) |
| Depression | 1.9 (1.6–2.3) | 1.5% | 55% | 1.3% (0.9–1.9) | 0.5% (0.2–0.9) |
| Physical inactivity | 1.4 (1.2–1.7) | 50.7% | 55% | 23.3% (21.6–25.1) | 5.8% (4.9–6.8) |
| Low social contact | 1.6 (1.3–1.9) | 3.4% | 62% | 1.3% (0.9–1.9) | 0.7% (0.4–1.1) |
| Diabetes | 1.5 (1.3–1.8) | 9.4% | 52% | 4.5% (3.7–5.5) | 1.6% (1.2–2.2) |
| Overall weighted PAF | .. | .. | .. | .. | 39.5% (37.5–41.6) |
PAF=population attributable fraction. RR=relative risk.
Weighted PAF is the relative contribution of each risk factor to the overall PAF when adjusted for communality.
Table 3. PAF for dementia risk factors in the Latin American sample (n=12 865).
| RR for dementia (95% CI) | Risk factor prevalence | Communality | PAF | Weighted PAF* | |
|---|---|---|---|---|---|
| Early life (<45 years) | |||||
| Low education | 1.6 (1.3–2.0) | 68.8% | 36% | 29.2% (28.4–30.0) | 10.9% (10.4–11.5) |
| Midlife (45–64 years) | |||||
| Hearing loss | 1.9 (1.4–2.7) | 28.8% | 48% | 20.6% (19.9–21.3) | 7.7% (7.3–8.2) |
| Hypertension | 1.6 (1.2–2.2) | 55.6% | 59% | 25.0% (24.3–25.8) | 9.3% (8.8–9.8) |
| Obesity | 1.6 (1.3–1.9) | 44.8% | 53% | 21.2% (20.5–21.9) | 7.9% (7.5–8.4) |
| Later life (≥65 years) | |||||
| Smoking | 1.6 (1.2–2.2) | 30.0% | 60% | 17.8% (17.2–18.5) | 5.7% (5.3–6.1) |
| Depression | 1.9 (1.6–2.3) | 23.9% | 55% | 17.7% (17.1–18.4) | 6.6% (6.2–7.0) |
| Physical inactivity | 1.4 (1.2–1.7) | 34.2% | 37% | 17.0% (16.4–17.7) | 4.5% (4.2–4.9) |
| Low social contact | 1.6 (1.3–1.9) | 0.5% | 69% | 0.2% (0.1–0.3) | 0.1% (0–0.2) |
| Diabetes | 1.5 (1.3–1.8) | 18.5% | 35% | 8.5% (8.0–8.9) | 3.2% (2.9–3.5) |
| Overall weighted PAF | .. | .. | .. | .. | 55.8% (54.9–56.7) |
PAF=population attributable fraction. RR=relative risk.
Weighted PAF is the relative contribution of each risk factor to the overall PAF when adjusted for communality.
Table 4. Worldwide PAFs for dementia risk factors.
| RR for dementia (95% CI) | Risk factor prevalence | Communality | PAF | Weighted PAF* | |
|---|---|---|---|---|---|
| Early life (<45 years) | |||||
| Low education | 1.6 (1.3–2.0) | 40.0% | 64% | 19.1% (18.3–19.9) | 7.5% (7.0–8.0) |
| Midlife (45–64 years) | |||||
| Hypertension | 1.6 (1.2–2.2) | 8.9% | 57% | 5.1% (2.9–3.6) | 2.0% (0.6–0.9) |
| Obesity | 1.6 (1.3–1.9) | 3.4% | 60% | 2.0% (1.7–2.3) | 0.8% (2.9–3.6) |
| Hearing loss | 1.9 (1.4–2.7) | 31.7% | 46% | 23.0% (22.2–23.8) | 9.1% (8.6–9.7) |
| Later life (≥65 years) | |||||
| Smoking | 1.6 (1.2–2.2) | 27.4% | 51% | 13.9% (13.2–14.6) | 5.5% (4.1–6.0) |
| Depression | 1.9 (1.6–2.3) | 13.2% | 59% | 10.1% (9.5–10.7) | 4.0% (3.6–4.4) |
| Physical inactivity | 1.4 (1.2–1.7) | 17.7% | 27% | 6.5% (6.0–7.0) | 2.6% (2.3–2.9) |
| Low social contact | 1.6 (1.3–1.9) | 11.0% | 46% | 5.9% (5.5–6.4) | 2.3% (2.0–2.6) |
| Diabetes | 1.5 (1.3–1.8) | 6.4% | 70% | 3.2% (2.9–3.6) | 1.2% (1.0–1.4) |
| Total adjusted for communality | .. | .. | .. | .. | 35.0% (34.1–35.9) |
Data were obtained from Livingston and colleagues.2 PAF=population attributable fraction. RR=relative risk.
Weighted PAF is the relative contribution of each risk factor to the overall PAF when adjusted for communality.
The range of estimates for the overall PAF combining all nine risk factors and using highest and lowest risk factor prevalence estimates was 31–55% in China, 34–45% in India, and 44–58% in the Latin American sample. In particular, we found a trend for better educational attainment over time, which reduces the PAF for less education in each region. Variability in prevalence estimates were due in part to variations in measurement (eg, in hearing loss) and in part due to the subjective nature of some assessments, such as physical activity. Details of the studies that were used to calculate the sensitivity analyses are shown in the appendix.
Discussion
To our knowledge, this study is the first to estimate the proportion of dementia cases that are attributable to nine risk factors (ie, the PAF) in LMICs. Overall PAFs for dementia were higher in China and India than our previous worldwide estimate of 35%, and higher still in our Latin American sample at 56%. The potential for prevention of dementia is therefore even greater in these countries than in higher-income countries. The highest PAF in each region was for less education in early life, but smoking, hypertension, obesity, and physical inactivity PAFs were also high, which highlights the potential priorities for prevention of dementia in these regions.
Dementia is more prevalent overall in India and Latin America than in developed countries (eg, the UK, when culturally appropriate screening tools are used), with prevalence estimates of 8·5% in India, 6·4% in China, and 8·6% in our Latin American sample,15 as compared with 6·4% in the EURODEM study16 of 11 European countries (to which the 10/66 data were directly standardised for age, sex, and education).
The estimation of PAFs are dependent on accurate prevalence data, which in turn are dependent on accurate measurement of a risk factor in the population. We did sensitivity analyses to identify whether alternative prevalence data changed our PAF estimates. We found that all regions had a similar or higher overall PAF than our previous worldwide estimates, even when the lowest prevalence figures were used, indicating the potential for greater targeting of dementia risk factors in these regions. In general, the prevalence estimates of low education, hypertension, obesity, and diabetes were higher than worldwide estimates across all three LMIC regions (although the diabetes estimate was similar to the US prevalence),10 leading to higher PAFs for all of these risk factors than previously calculated PAFs, which were mostly based on data from higher-income countries. The relative importance of each risk factor, as indicated by their individual PAF, also remained the same in most sensitivity estimates.
Previous estimates of the proportion of people with low education17 found that, in the 1960s, 57% of people older than 15 years in Latin America, 80·3% in south Asia, and 68·5% in east Asia had no education or only completed primary school. The 10/66 population sample, who were 65 years or older, would therefore have been older than 15 years at the time these data were collected in the 1960s, and included in these schooling estimates. Estimates of low education from 2010 are lower in all regions than the data from the 1960s,17 which suggests that progress to improve education over the past 50 years might lead to a reduction in the prevalence of dementia, and the relative importance of this risk factor is likely to be lower for future generations than the present generation.
Lifecourse analysis is important, given that, for example, hypertension and obesity, which are risk factors in midlife, decrease as part of the development of dementia; therefore, tackling these risk factors in midlife years before the probable development of dementia is appropriate and important as a management strategy.18,19 Although the 10/66 prevalence estimate might have included some people with later-life hypertension, it does not include those with undiagnosed hypertension or those who did not report their diagnosis. Sensitivity analyses based on other available prevalence estimates gave similar PAFs for all regions, indicating that hypertension is an important potentially modifiable risk factor, and so public health programmes to increase identification and management of hypertension in midlife might reduce dementia prevalence.
Depression was relatively uncommon in China and India but more prevalent in Latin America than in higher-income countries. Our prevalence estimates for depression were similar to previous findings for India20 and China,21 but for our Latin American sample were much higher than those reported in a WHO survey20 (although only Mexico was included in their Latin American sample). Notably, the prevalence estimates for depression vary substantially between these regions (1·5% in China vs 23·9% in our Latin American sample), which is in keeping with a meta-analyses that showed a higher prevalence of depression in South America than in Asia.22 These results possibly reflect a true difference in prevalence. However, previous studies that made use of 10/66 data have highlighted that the low prevalence of depression reported in China might in part be due to contextual and cultural factors.23 If, as previous authors have suggested, the divergent prevalence of depression in these cultures reflect different cultural expressions of the same underlying depressive process, which is inadequately captured by traditional diagnostic approaches, then the PAF for depression in China might be an underestimate and the Latin American PAF might be an overestimate.
Social isolation was very uncommon in China and our Latin American sample, but in India was similar in prevalence to our UK estimates.2 This finding led to social isolation having the lowest PAF out of all the risk factors across the three regions, with a value of close to zero in China and our Latin American sample. In Health Survey England, 11% of people had less than monthly social contact.24 The higher amount of social support might be a strength of LMICs, and could be a useful model for how to build better connected communities in higher-income countries. Physical inactivity was more common in China and less common in India than worldwide estimates. Self-reported prevalence of physical inactivity was also higher in all countries than estimates from other cross-sectional representative surveys,25 but lower than the studies in the sensitivity analyses,26,27 which could reflect the inaccuracy of self-reported activity.28
The prevalence of diabetes in the 10/66 surveys was similar to other cross-sectional surveys in India29 and China,30 but higher for Latin America.31 The PAF for diabetes was relatively low in all three regions, but the fact that, in previous surveys, almost 50% of people surveyed in India,29 nearly 40% in China,30 and 20% in Latin America32 were unaware that they had diabetes indicates that more needs to be done to diagnose and treat diabetes, to further mitigate any risk to dementia development it might pose.
Hearing loss prevalence was lower in China and India than worldwide estimates and our estimates from the UK.2 The 10/66 survey data only documented self-reported hearing impairment and not hearing impairment from audiometry, as done in cohort studies of hearing loss. Around two thirds of people with mild hearing loss and a third of those with severe hearing loss on audiometry report normal hearing.33 This might have led to an underestimate of hearing loss in the current study, given that a substantial proportion of older people (aged ≥65 years) with hearing loss are unaware of their impairment.34 Hearing loss has been previously classed as a midlife risk factor given that the lowest mean age of participants in studies that showed this link was 55 years. However, hearing loss is also a risk factor in adults aged 65 years or older, and preliminary evidence suggests that hearing aid use can mitigate the risk of dementia from hearing loss, indicating some potential for prevention.35
To our knowledge, this study is the first to estimate PAFs for the widely accepted dementia risk factors in LMICs. We used data from a study whose methodology was identical in the eight countries it surveyed, making these figures comparable between countries. Our study has some limitations. Because the quality of the 10/66 survey’s data on the prevalence of some risk factors, such as hearing loss, was uncertain, we did sensitivity analyses to model the potential effect of different risk factor prevalence estimates. The population sample was aged 65 years and older, so we could not measure midlife hypertension or obesity. We were also unable to measure exercise in midlife, but our calculations are based on the risk of physical inactivity in later life. The data relied on self-report for some of the risk factors, which is not ideal (eg, previous studies29,30,32 have shown that awareness of diabetes can be very low). Self-report is likely to have led to the underestimation of PAFs. Although 10/66 study participants had their blood pressure measured, we used self-report data, given that blood pressure might decrease in people who are developing dementia, which could lead to the underestimation of its prevalence. We did not use country-specific studies for risk associated with factors but rather available meta-analysis data, more of which was from higher-income countries than from LMICs. However, these meta-analysis-based estimates are likely to be more precise than individual studies in specific countries, given that they combine the findings from several studies, and they make the PAF more directly comparable with previous estimates.2 There is also no consistent evidence of gene–environment interaction with regard to dementia, which might cause the effect of a risk factor on dementia to vary globally.36–38 The causal direction underlying the association between these risk factors and dementia is uncertain, especially for associations found in later life. For example, depression might be a prodromal feature or a consequence rather than a cause of dementia.39 Additionally, a PAF is a theoretical construct that assumes a reduction in risk on the basis of elimination of the risk factor. Risk factors are unlikely to be completely eliminated; however, reduction in risk factors is still likely to delay the onset of dementia, and thereby reduce the number of dementia cases.40 However, even a partial reduction in some of the factors could, at a population level, make an enormous difference in the future prevalence of dementia in LMICs, and we have indicated which factors could potentially have the largest effect. Finally, the data collected were recorded more than 10 years ago, and the current prevalence of some risk factors might have changed. This limitation is, however, common within research that involves large amounts of data collection and periods of time between analyses.
Although overall numbers of people with dementia are increasing globally, the age-specific incidence and prevalence of dementia has reduced in many high-income countries over the past two decades.2,3,41 This reduction has been attributed to reduced frequency of dementia risk factors, particularly low education and cardiovascular risk, in successive generations of older people. Our study suggests that, because these risk factors are more common in Asia and Latin America, there is greater dementia prevention potential in these LMICs than in high-income countries. Low education, hearing loss, obesity, and physical activity had particularly high PAFs and so might be initial targets for policy makers devising dementia prevention strategies. Public health strategies are likely to need to be specific to the setting in which they are used, and future research should establish whether such strategies affect the prevalence of risk factors and subsequent dementia prevalence. As dementia is forecast to become the leading public health challenge globally, capitalising on the potential for prevention is an urgent priority.
Supplementary Material
Research in context.
Evidence before this study
We searched PubMed on March 21, 2018, for any studies investigating population attributable fractions (PAFs) or preventable dementia in low-income and middle-income countries (LMICs) using the search terms “dementia”, “preventable”, and “low income”, with no limits on language or date of publication. We found no papers investigating overall dementia risk, although there were some articles investigating individual risk factors and their link with dementia in LMICs. We have previously published estimates of PAFs for dementia risk factors based on worldwide prevalence estimates from meta-analyses (primarily those that included studies from higher-income countries), and found that up to 35% of dementia cases are potentially preventable worldwide.
Added value of this study
We used data from the 10/66 Dementia Research Group from eight LMICs to estimate the prevalence of nine risk factors for dementia and then we calculated the percentage of potentially preventable dementia cases in these regions. To the best of our knowledge, this is the first such calculation. We searched the literature for other studies of risk factor prevalence for dementia and used the studies with the highest and lowest prevalence estimates in sensitivity analyses to calculate highest and lowest possible PAF estimates.
Implications of all the available evidence
The PAF percentage is higher in India, China, and our Latin American sample of countries than worldwide estimates, indicating greater potential for dementia prevention in these regions. Low education was the risk factor with the highest PAF in all three regions, but other priority risk factors are hypertension in China and Latin America and smoking in India.
Table 2. PAF for dementia risk factors in India (n=2004).
| RR for dementia (95% CI) | Risk factor prevalence2,8,10 | Communality | PAF | Weighted PAF* | |
|---|---|---|---|---|---|
| Early life (<45 years) | |||||
| Low education | 1.6 (1.3–2.0) | 92.2% | 44% | 35.6% (33.5–37.7) | 13.6% (12.2–15.2) |
| Midlife (45–64 years) | |||||
| Hearing loss | 1.9 (1.4–2.7) | 22.3% | 37% | 16.7% (15.2–18.4) | 6.4% (5.4–7.6) |
| Hypertension | 1.6 (1.2–2.2) | 19.3% | 60% | 10.4% (9.1–11.8) | 4.0% (3.2–4.9) |
| Obesity | 1.6 (1.3–1.9) | 13.7% | 64% | 7.6% (6.5–8.8) | 2.9% (2.2–3.7) |
| Later life (≥65 years) | |||||
| Smoking | 1.6 (1.2–2.2) | 39.9% | 68% | 19.3% (17.6–21.1) | 6.4% (5.4–7.6) |
| Depression | 1.9 (1.6–2.3) | 5.2% | 72% | 4.5% (3.7–5.5) | 1.7% (1.2–2.4) |
| Physical inactivity | 1.4 (1.2–1.7) | 15.3% | 78% | 8.4% (7.3–9.7) | 2.2% (1.6–2.9) |
| Low social contact | 1.6 (1.3–1.9) | 10.4% | 57% | 4.0% (3.2–4.9) | 2.3% (1.7–3.1) |
| Diabetes | 1.5 (1.3–1.8) | 9.3% | 52% | 4.4% (3.6–5.4) | 1.7% (1.2–2.4) |
| Overall weighted PAF | .. | .. | .. | .. | 41.2% (39.1–43.4) |
PAF=population attributable fraction. RR=relative risk.
Weighted PAF is the relative contribution of each risk factor to the overall PAF when adjusted for communality.
Acknowledgments
This is a secondary analysis of data collected by the 10/66 Dementia Research Group. NM and JH are funded by the University College London Hospitals (UCLH) National Institutes of Health Research (NIHR) Biomedical Research Centre. AS is funded by the Wellcome Trust. GL is supported by the Economic and Social Research Council and NIHR (ES/L001780/1) and the UCLH NIHR Biomedical Research Centre, and receives funding from the NIHR Collaboration for Leadership in Applied Health Research and Care, North Thames at Bart’s Health National Health Service Trust, and through an NIHR Senior Investigator Award.
Footnotes
Contributors
NM obtained and analysed the data. All authors designed the study, interpreted the data, and wrote the manuscript.
Declaration of interests
We declare no competing interests.
References
- 1.Prince M, Wimo A, Guerchet M, Ali GC, Wu YT, Prina M. World Alzheimer report 2015. The global impact of dementia. An analysis of prevalence, incidence, cost and trends. London: Alzheimer’s Disease International; 2015. [Google Scholar]
- 2.Livingston G, Sommerlad A, Orgeta V, et al. Dementia prevention, intervention, and care. Lancet. 2017;390:2673–734. doi: 10.1016/S0140-6736(17)31363-6. [DOI] [PubMed] [Google Scholar]
- 3.Matthews FE, Stephan BC, Robinson L, et al. A two decade dementia incidence comparison from the Cognitive Function and Ageing Studies I and II. Nat Commun. 2016;7:11398. doi: 10.1038/ncomms11398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Langa KM, Larson EB, Crimmins EM, et al. A comparison of the prevalence of dementia in the United States in 2000 and 2012. JAMA Intern Med. 2017;177:51–58. doi: 10.1001/jamainternmed.2016.6807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ahmadi-Abhari S, Guzman-Castillo M, Bandosz P, et al. Temporal trend in dementia incidence since 2002 and projections for prevalence in England and Wales to 2040: modelling study. BMJ. 2017;358:j2856. doi: 10.1136/bmj.j2856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.National Institue for Health Care and Excellence. Dementia, disability and frailty in later life: mid-life approaches to delay or prevent onset (NG16) London: National Institute for Health Care and Excellence; 2015. [Google Scholar]
- 7.Daviglus ML, Bell CC, Berrettini W, et al. NIH state-of-the-science conference statement: preventing Alzheimer’s disease and cognitive decline. NIH Consens State Sci Statements. 2010;27:1–30. [PubMed] [Google Scholar]
- 8.Barnes DE, Yaffe K. The projected effect of risk factor reduction on Alzheimer’s disease prevalence. Lancet Neurol. 2011;10:819–28. doi: 10.1016/S1474-4422(11)70072-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Prince M, Ferri CP, Acosta D, et al. The protocols for the 10/66 Dementia Research Group population-based research programme. BMC Public Health. 2007;7:165. doi: 10.1186/1471-2458-7-165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Norton S, Matthews FE, Barnes DE, Yaffe K, Brayne C. Potential for primary prevention of Alzheimer’s disease: an analysis of population-based data. Lancet Neurol. 2014;13:788–94. doi: 10.1016/S1474-4422(14)70136-X. [DOI] [PubMed] [Google Scholar]
- 11.WHO. Report of a WHO expert consultation. Geneva, 8–11 December 2008. Geneva: World Health Organization; 2011. Waist circumference and waist-hip ratio. [Google Scholar]
- 12.Copeland J, Dewey ME, Griffiths-Jones H. A computerized psychiatric diagnostic system and case nomenclature for elderly subjects: GMS and AGECAT. Psychol Med. 1986;16:89–99. doi: 10.1017/s0033291700057779. [DOI] [PubMed] [Google Scholar]
- 13.Janssen I, Katzmarzyk PT, Ross R. Waist circumference and not body mass index explains obesity-related health risk. Am J Clin Nutr. 2004;79:379–84. doi: 10.1093/ajcn/79.3.379. [DOI] [PubMed] [Google Scholar]
- 14.Boyle MH. Guidelines for evaluating prevalence studies. Evid Based Ment Health. 1998;1:37–39. [Google Scholar]
- 15.Rodriguez JJL, Ferri CP, Acosta D, et al. Prevalence of dementia in Latin America, India, and China: a population-based cross-sectional survey. Lancet. 2008;372:464–74. doi: 10.1016/S0140-6736(08)61002-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lobo A, Launer LJ, Fratiglioni L, et al. Prevalence of dementia and major subtypes in Europe: a collaborative study of population-based cohorts. Neurology. 2000;54:S4. [PubMed] [Google Scholar]
- 17.Barro RJ, Lee JW. A new data set of educational attainment in the world, 1950–2010. J Dev Econ. 2013;104:184–98. [Google Scholar]
- 18.Kivimäki M, Luukkonen R, Batty GD, et al. Body mass index and risk of dementia: analysis of individual-level data from 1.3 million individuals. Alzheimers Dement. 2018;14:601–09. doi: 10.1016/j.jalz.2017.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Singh-Manoux A, Dugravot A, Shipley M, et al. Obesity trajectories and risk of dementia: 28 years of follow-up in the Whitehall II Study. Alzheimers Dement. 2018;14:178–86. doi: 10.1016/j.jalz.2017.06.2637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kessler RC, Birnbaum HG, Shahly V, et al. Age differences in the prevalence and co-morbidity of DSM-IV major depressive episodes: results from the WHO World Mental Health Survey Initiative. Depress Anxiety. 2010;27:351–64. doi: 10.1002/da.20634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gu L, Xie J, Long J, et al. Epidemiology of major depressive disorder in mainland China: a systematic review. PLoS One. 2013;8:e65356. doi: 10.1371/journal.pone.0065356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lim GY, Tam WW, Lu Y, Ho CS, Zhang MW, Ho RC. Prevalence of depression in the community from 30 countries between 1994 and 2014. Sci Rep. 2018;8:2861. doi: 10.1038/s41598-018-21243-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Guerra M, Ferri CP, Sosa AL, et al. Late-life depression in Peru, Mexico and Venezuela: the 10/66 population-based study. Br J Psychiatry. 2009;195:510–15. doi: 10.1192/bjp.bp.109.064055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Scholes S, Mindell J, Craig R. Health Survey for England 2014: health, social care and lifestyles. Leeds: Health and Social Care Information Centre; 2014. [Google Scholar]
- 25.Guthold R, Ono T, Strong KL, Chatterji S, Morabia A. Worldwide variability in physical inactivity. A 51-country survey. Am J Prevent Med. 2008;34:494. doi: 10.1016/j.amepre.2008.02.013. [DOI] [PubMed] [Google Scholar]
- 26.Poggio R, Serón P, Calandrelli M, et al. Prevalence, patterns, and correlates of physical activity among the adult population in Latin America: cross-sectional results from the CESCAS I study. Glob Heart. 2016;11:81–8.e1. doi: 10.1016/j.gheart.2015.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Anjana RM, Pradeepa R, Das AK, et al. Physical activity and inactivity patterns in India–results from the ICMR-INDIAB study (Phase-1)[ICMR-INDIAB-5] Int J Behav Nutr Phys Act. 2014;11:26. doi: 10.1186/1479-5868-11-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Visser M, Brychta RJ, Chen KY, Koster A. Self-reported adherence to the physical activity recommendation and determinants of misperception in older adults. J Aging Phys Act. 2014;22:226–34. doi: 10.1123/japa.2012-0219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Anjana RM, Deepa M, Pradeepa R, et al. Prevalence of diabetes and prediabetes in 15 states of India: results from the ICMR–INDIAB population-based cross-sectional study. Lancet Diabetes Endocrinol. 2017;5:585–96. doi: 10.1016/S2213-8587(17)30174-2. [DOI] [PubMed] [Google Scholar]
- 30.Wang L, Gao P, Zhang M, et al. Prevalence and ethnic pattern of diabetes and prediabetes in China in 2013. JAMA. 2017;317:2515–23. doi: 10.1001/jama.2017.7596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Aschner P, Aguilar-Salinas C, Aguirre L, et al. Diabetes in South and Central America: an update. Diabetes Res Clin Pract. 2014;103:238–43. doi: 10.1016/j.diabres.2013.11.010. [DOI] [PubMed] [Google Scholar]
- 32.Irazola V, Rubinstein A, Bazzano L, et al. Prevalence, awareness, treatment and control of diabetes and impaired fasting glucose in the Southern Cone of Latin America. PLoS One. 2017;12:e0183953. doi: 10.1371/journal.pone.0183953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kim SY, Kim H-J, Kim M-S, Park B, Kim J-H, Choi HG. Discrepancy between self-assessed hearing status and measured audiometric evaluation. PLoS One. 2017;12:e0182718. doi: 10.1371/journal.pone.0182718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rawool VW, Keihl JM. Perception of hearing status, communication, and hearing aids among socially active older individuals. J Otolaryngol. 2008;37:27–42. [PubMed] [Google Scholar]
- 35.Amieva H, Ouvrard C, Meillon C, Rullier L, Dartigues J-F. Death, depression, disability and dementia associated with self-reported hearing problems: a 25-year study. J Gerontol A Biol Sci Med Sci. 2018;73:1383–89. doi: 10.1093/gerona/glx250. [DOI] [PubMed] [Google Scholar]
- 36.Ghebranious N, Mukesh B, Giampietro PF, et al. A pilot study of gene/gene and gene/environment interactions in Alzheimer disease. Clin Med Res. 2011;9:17–25. doi: 10.3121/cmr.2010.894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wirth M, Villeneuve S, La Joie R, Marks SM, Jagust WJ. Gene–environment interactions: lifetime cognitive activity, APOE genotype, and beta-amyloid burden. J Neurosci. 2014;34:8612–17. doi: 10.1523/JNEUROSCI.4612-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Quinn J. Comment: gene-environment interactions in dementia— not just another fish story. Neurology. 2016;86:2069. doi: 10.1212/WNL.0000000000002728. [DOI] [PubMed] [Google Scholar]
- 39.Singh-Manoux A, Dugravot A, Fournier A, et al. Trajectories of depressive symptoms before diagnosis of dementia: a 28-year follow-up study. JAMA Psychiatry. 2017;74:712–18. doi: 10.1001/jamapsychiatry.2017.0660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jorm AF, Korten AE, Henderson AS. The prevalence of dementia–a quantitative integration of the literature. Acta Psychiatr Scand. 1987;76:465–79. doi: 10.1111/j.1600-0447.1987.tb02906.x. [DOI] [PubMed] [Google Scholar]
- 41.Matthews FE, Arthur A, Barnes LE, et al. A two-decade comparison of prevalence of dementia in individuals aged 65 years and older from three geographical areas of England: results of the Cognitive Function and Ageing Study I and II. Lancet. 2013;382:1405–12. doi: 10.1016/S0140-6736(13)61570-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.