Abstract
Although suppressed cAMP levels have been linked to cancer for nearly five decades, the molecular basis remains uncertain. Here, we identify endosomal pH as a novel regulator of cytosolic cAMP homeostasis and a promoter of transformed phenotypic traits in colorectal cancer (CRC). Combining experiments and computational analysis, we show that the Na+/H+ exchanger NHE9 contributes to proton leak and causes luminal alkalinization, which induces resting [Ca2+], and in consequence, represses cAMP levels, creating a feedback loop that echoes nutrient deprivation or hypoxia. Higher NHE9 expression in cancer epithelia is associated with a hybrid epithelial-mesenchymal (E/M) state, poor prognosis, tumour budding, and invasive growth in vitro and in vivo. These findings point to NHE9-mediated cAMP suppression as a pseudo-starvation-induced invasion state and potential therapeutic vulnerability in CRC. Our observations lay the groundwork for future research into the complexities of endosome-driven metabolic reprogramming and phenotype switching and the biology of cancer progression.
Keywords: colorectal cancer, NHE9, endosomal pH, Na+/H+ exchanger, cyclic AMP, hybrid epithelial/mesenchymal, tumour progression
Introduction
Cancers of the colon and rectum (CRC) contribute majorly to the mortality and morbidity associated with malignancies, and novel treatment approaches are urgently needed (1). To overcome the bottleneck in cancer therapy, translating our rapidly growing knowledge of epithelial to mesenchymal transition (EMT) in human cancers and targeting specific genes and pathways in individual patients is crucial as a step towards personalised medicine (2–4). This has been the driving force behind the development of several transcriptomics-based scoring metrics for rigorous classification of EMT status, which are now being widely used to identify novel therapeutic targets and improve the prospect of cure or progression-free survival (3,4). Building on these discoveries, we set out to leverage this approach to develop prognostic markers for progression of CRC from primary to metastatic disease.
Endosomes have emerged as a pathogenic driver in human diseases and an underappreciated cache of novel therapies (5). Studies on linkage, single nucleotide polymorphism, and mutation analysis (1,6,7), indicate an association of CRC incidence with the SLC9A9 gene, which encodes the Na+/H+ exchanger NHE9, an endosomal pH regulator first discovered for its role in autism (8). Clinically, NHE9 expression is correlated with the metastatic burden of CRC (9), highlighting its potential role as an important regulator of cancer progression and metastasis. Previously, we reported that the loss-of-function of related NHE6 causes endosomal hyperacidification and amyloid clearance defects associated with Alzheimer’s disease (5). More recently, we elucidated a link between NHE9 and coronavirus disease (COVID-19) and how these findings can be translated into potential antiviral therapies (10).
Garca-Jiménez and Goding recently proposed a unifying theme to explain why cancer cells adopt an invasive phenotype based on lessons learned from unicellular organisms that undergo phenotypic transitions to resolve a nutrient supply-demand imbalance (11). Previously, we showed that nutrient restriction-dependent upregulation of endosomal NHE in yeast, fruit fly, and mammalian models is an ancient response for cell survival (12). Indeed, when starved of nutrients, Saccharomyces cerevisiae upregulates expression of Nhx1, the ortholog of human NHE9, and switches from budding to an invasive pseudo-hyphal growth (12,13). Induction of NHE9 may thus be regarded as an evolutionarily conserved hallmark of a starvation response, and its higher expression in cancer cells even under nutrient-rich conditions may represent a pseudo-starvation state, triggering phenotypic transition and invasion. How can pH changes within the limited confines of an endosomal space mimic the starvation response and confer invasive and proliferative properties on cancer cells?
In this study, we demonstrate that NHE9 levels in CRC cells drive proliferation, cell-matrix adhesion, expansive collective invasion, and tumour budding in in vitro and in vivo mouse xenograft models. NHE9 expression was associated with a consistent transcriptomic shift that broadly indicated metabolic reprogramming and may be an important mediator of multiple steps in the invasion-metastasis cascade. Mechanistically, NHE9 induces resting [Ca2+], and in consequence, represses cAMP levels, echoing a nutrient deficient state. With the help of dynamic mathematical modelling, we demonstrate that the NHE9/cAMP axis-mediated feedback regulation of endosomal pH conserves resources and ensures system robustness.
Although EMT has been traditionally described as a binary phenomenon, we and others have recently provided conceptual and experimental evidence that it is associated with more stable intermediate phenotypes (2–4). Our current study supports this model, in which specific changes in endosomal pH contributed to the generation of hybrid E/M states in vitro and were validated in a nude mouse model. Taken together, these findings point to an important, hitherto underappreciated role for endosomal pH in CRC progression and allow us to propose that exploiting metabolic vulnerabilities rendered by NHE9 as a promising therapeutic approach for cancer.
Materials and Methods
1. EMT Score Calculation
Three independent transcriptomics-based EMT scoring metrics were used to calculate EMT scores for all samples and/or datasets mentioned as previously described (3,4), and briefly discussed below.
GS76
The 76-gene EMT scoring method (GS76) was developed using transcriptomic data from non-small cell lung cancer cell lines and patient samples, and it employs 76 gene signatures. For each sample, the weighted sum of gene expression values from these 76 genes is calculated, with the weight being the correlation coefficient of a given gene with E-cadherin expression levels. The EMT score for each sample is subtracted from the mean obtained from all samples, resulting in a mean score of zero. A higher negative score indicates a mesenchymal (M) phenotype, while a higher positive score indicates an epithelial (E) phenotype.
MLR
The Multinomial Logistic Regression (MLR) method is based on an iterative algorithm that performs probabilistic categorization of samples into epithelial (E), hybrid (E/M), and mesenchymal (M). On a scale of 0-2, this model quantifies the degree of EMT in a given sample. This method focuses on characterizing a hybrid E/M phenotype using the expression levels of 23 genes (3 predictors and 20 normalizers) identified through NCI-60 gene expression data. It then computes the likelihood that a given sample falls into the E, M, or hybrid E/M categories. Based on those probabilities, an EMT score is assigned; the higher the score, the more M the sample.
KS
The two-sample Kolmogorov Smirnov test (KS) calculates EMT scores for cell lines and tumor samples on a scale of -1 to 1. Cumulative distribution functions (CDFs) are generated for each of the two signatures (E and M), and the greatest difference between these CDFs is used as the test statistic for a two-sample KS test. A sample with a positive EMT score has the M phenotype, whereas a sample with a negative EMT score has the E phenotype.
2. Clinical Data Analysis
Patient data were accessed via the R2 genomics analysis and visualisation platform (https://r2.amc.nl) to screen for survival correlations with NHE isoform expression, as previously described (14). CRC patient and mouse data for analysis were obtained from the following databases: GSE39582, GSE15960, GSE3629, GSE4107, GSE41568, GSE16125, GSE28722, GSE6383, and human protein atlas. Data from the TCGA, CCLE, and NCI-60 databases were extracted from the cBioPortal, UALCAN, and DepMap portals. Phenotype categories (E, E/M, and M) of NCI-60 cell lines were obtained from George et al (3). Data on survival were obtained from the TCGA and the online Kaplan-Meier plotter platform (https://kmplot.com). The data regarding DNA methylation from the TCGA database was accessed using the UALCAN portal (15).
3. ssGSEA Score Calculation
The Single-sample Gene Set Enrichment Analysis (ssGSEA) determines the degree to which genes in a specific gene set are co-ordinately up- or down-regulated in a sample. A normalized enrichment score was obtained by performing an analysis on a specific gene set. All calculations were performed using the GSEApy module. We used KEGG_CALCIUM_SIGNALING_PATHWAY, GOBP_CALCIUM_ION_TRANSPORT, GOBP_CALCIUM_ION_IMPORT, and CAMP_UP.V1_UP gene sets from the Molecular Signatures Database (MSigDB) database.
4. Transcriptomic Profiling
RNA was extracted from Caco-2 cells expressing NHE9 or the vector control using the Qiagen RNeasy Mini kit (#74104). RNA purity and integrity were determined. The raw sequence data using Illumina HiSeq and FastQC and MultiQC software were used to check data quality. Using the STAR v2 (RRID:SCR_004463) aligner, the QC-passed reads were mapped onto an indexed Human reference genome (GRCh38.p7). Picard tools were used to mark and remove the PCR and optical duplicates. Using the featureCounts software, read counts were used to calculate gene expression levels. The edgeR (RRID:SCR_012802) package was used for differential expression analysis. Significant genes were those with an absolute log2 fold change of ≥2 and an adjusted p-value of ≤0.05. For the transcriptomic analysis of cells treated with forskolin, Caco-2 monolayers were incubated with 500μM IBMX (isobutylmethylxanthine) for 30 minutes before being incubated with or without 25μM forskolin for 3 hours. Total RNA was extracted from cells using RNeasy Mini kit (#74104, Qiagen) as per manufacturer’s instructions. The purity and integrity of RNA were assessed and hybridised on an Affymetrix Clariom S human array, which covered over 20,000 genes. Data from the two experiments were analysed using Ingenuity Pathway Analysis (RRID:SCR_008653) (Qiagen). Pathway analysis was carried out using the default settings, and the z-scores of Upstream Regulators were examined. Using IPA Comparison Analysis, Caco-2 cells expressing NHE9 or vector control were compared to Caco-2 cells treated with forskolin or vector control, and Upstream Regulators from the two experiments were examined.
5. Animal Experiments
All procedures were carried out with the approval of the Institutional Animal Ethics Committee (IAEC). For the murine inflammatory carcinogenesis model, 8-week-old male C57BL/6 mice weighing 19-22 g were injected intraperitoneally with 12.5 mg/kg azoxymethane (AOM) (Sigma-Aldrich). Following a 5-day recovery period, the animals began the first of three cycles of 1.5% Dextran Sodium Sulfate (DSS) ad libitum. Each cycle lasted 5 days and was followed by a 16-day recovery period. Following 38 days of recovery after the third cycle, animals were sacrificed. The colon was removed, flushed with ice-cold PBS, and longitudinally opened. Tumour and normal colon samples were collected and snapfrozen in TRI Reagent (RNAiso Plus; TaKaRa) for RNA extraction or fixed in formalin overnight and sectioned for histological analysis. To develop an in vivo model of tumour growth, 2.5X106 cells in reconstituted basement membrane matrix were injected subcutaneously in the right flank of each mouse above the hind limb of 5-6 weeks old male nude mice weighing 20-22 g. Tumours were discovered by day 11, and tumour size was measured every three days. The tumour was assumed to be ellipsoidal, and a digital Vernier calliper was used to measure its size in two perpendicular directions (L and W), and the volume of the tumour was calculated using the formula V = (W(2) × L)/2. Mice were sacrificed three weeks after tumour injection for evaluation. Haematoxylin and eosin (H&E), Masson’s trichrome, and immunohistochemistry were used to stain formalin-fixed paraffin-embedded sections.
6. Cell Culture, Plasmids, and Reagents
Human colorectal adenocarcinoma cells, Caco-2 (HTB-37), were purchased from the American Type Culture Collection. Cells were cultured in Dulbecco’s Minimal Essential Medium (DMEM) high glucose supplemented with 10% foetal bovine serum (FBS), 100 U/mL penicillin, 100 g/mL streptomycin, 2 mM glutamine, 10 mM nonessential amino acids, and 1 mM sodium pyruvate. HEK293FT (CVCL_6911) cells were cultured in DMEM supplemented with 10% FBS, 100 U/mL penicillin, 100 g/mL streptomycin, and 8mM glutamine. Cell lines were grown for no more than six passages. We found no obvious contamination from mycoplasma; however, no testing was performed. Cells were grown in a humidified incubator at 37°C and 5% CO2. All chemicals were purchased from Sigma-Aldrich. NHE9-GFP was cloned into the lentiviral vector FUGW using the BamHI site. Lentiviral packaging and transduction enabled stable expression. NHE9 with the desired D244N mutation was created using two primers, 5′ TGGCCACCGCATTATTCAGTACACTCTCTCCAAAC 3′ and 5′ GTTTGGAGAGAGTGTACTGAATAATGCGGTGGCCA 3′, and the resulting plasmid was confirmed by sequencing. Cellular metabolic activity/viability was measured using the Celltiter 96 Aqueous One Solution Cell Proliferation Assay (# G3580, Promega). Briefly, 1.5 X 104 cells/well were seeded in a 96-well plate. At each time point, 20 μl of Celltiter 96 Aqueous One Solution reagent was added to each well containing 200µl culture medium and incubated at 37 °C for 2 hours. The absorbance was measured at 490nM using a microplate reader (Tecan Infinite M200 PRO). Cell cycle analysis was performed using propidium iodide (# P1304MP, Thermo Fisher Scientific) labelling and flow cytometry according to manufacturer’s instructions. The apoptosis assay was carried out by labelling cells with Annexin V tagged to Alexa Fluor 647 (# A23204, Thermo Fisher Scientific) and propidium iodide (# P1304MP, Thermo Fisher Scientific) and analysing them using flow cytometry according to the manufacturer’s instructions. Caco-2 cells grown in monolayer spontaneously form hemicysts as a functional assay for cAMP levels due to vectorial transepithelial transport and fluid accumulation in focal regions beneath the cell layer(16). For transepithelial electrical resistance (TEER) measurement, Caco-2 cells were seeded at a density of 1 X 105 cells/well in 12-well trans-well inserts. The medium was changed every 2-3 days for both the apical and basal sides of the trans-well inserts. TEER development was monitored using an Epithelial Volt/Ohm Meter (Millicell ERS-2).
7. Endosomal pH
Endosomal pH was measured as we previously described (5), with some modifications. In brief, cells were rinsed with PBS and incubated in serum-free medium at 37°C for 30 minutes to remove residual transferrin before being incubated with pH-sensitive pHrodo Red-Transferrin (#P35376, Thermo Fisher Scientific) (75μg/ml) and pH non-sensitive Alexafluor 633-Transferrin (#T23362, Thermo Fisher Scientific) (25μg/ml) at 37°C for 1 hour. Transferrin endocytosis was halted by placing cells on ice. Excess transferrin was washed away with ice-cold serum-free DMEM, whereas bound transferrin was washed away with ice-cold PBS at pH 5.0 and pH 7.0. Cells were trypsinized and filtered through a cell strainer and endosomal pH was determined by flow cytometry analysis of ~10,000 cells using the BD Influx cell sorter (BD Biosciences). Intracellular pH Calibration Buffer Kit (#P35379, Thermo Fisher Scientific) was used to generate a four-point calibration curve with different pH values (4.5, 5.5, 6.5, and 7.5) in the presence of 10µM K+/H+ ionophore nigericin and 10μM K+ ionophore valinomycin.
8. Lysosomal pH
Lysosomal pH was measured as we previously described (5), with some modifications. In brief, cells were incubated for 12 hours with pH-sensitive pHrodo Red-Dextran (#P10361, Thermo Fisher Scientific) (5μg/ml) and pH non-sensitive Alexa Fluor 647-Dextran (#D22914, Thermo Fisher Scientific) (10μg/ml), washed, and chased in dye-free media for an additional 6 hours at 37°C. Cells were trypsinized and filtered through a cell strainer and lysosomal pH was determined by flow cytometry analysis of ~10,000 cells using the BD Influx cell sorter (BD Biosciences). In the presence of 10µM K+/H+ ionophore nigericin and 10μM K+ ionophore valinomycin, a four-point calibration curve with different pH values (3.5, 4.5, 5.5, and 6.5) was generated.
9. Cytoplasmic pH
Cytoplasmic pH was measured as previously described (14), with some modifications. In brief, cells were incubated at 37°C for 30 minutes with pH-sensitive pHrodo Red AM Intracellular pH Indicator (#P35372, Thermo Fisher Scientific) (5μM), washed, and replaced with dye-free media. Fluorescence intensity was measured using a microplate reader (Tecan Infinite M200 PRO) or the InCell Analyzer-6000 (GE Healthcare) for live cell imaging, and the fluorescence intensities of individual cells were calculated using ImageJ (RRID:SCR_003070) software. A five-point calibration curve with different pH values (5.5, 6.0, 6.5, 7.0, and 7.5) was generated in the presence of 10μM K+/H+ ionophore nigericin and 10μM K+ ionophore valinomycin.
10. Scaffold Based 3D Spheroidogenesis Assay
Cells were trypsinized and plated in a 10% reconstituted basement membrane (rBM) (#354230, Corning) scaffold layered on top of 100% rBM and grown in defined medium that was changed every 3 days. For confocal imaging, spheroids were fixed with 1% formaldehyde for 30 minutes at 37°C. To neutralize formaldehyde traces, 2% glycine in PBS was used. Blocking was performed at room temperature for 1 hour with 5% BSA in PBS and 0.1% Triton X-100. The clusters were stained overnight at 4°C with hoechst and rhodamine-phalloidin (#R415, Thermo Fisher Scientific). The cultures were washed three times with PBS containing 0.1% Triton X-100 for 10 minutes each and later imaged with a Zeiss LSM 880 microscope.
11. Cell-Matrix Adhesion
Cell-matrix adhesion assay was performed as we previously described (17), with some modifications. In brief, 96-well plates were coated overnight at 37°C with 50μg/mL reconstituted basement membrane (rBM) (#354230, Corning) or 50μg/mL neutralized rat tail collagen type 1 (#A1048301, Gibco) or 0.5% bovine serum albumin (BSA). Following coating, excess matrix was aspirated, and plates were allowed to dry for 30 minutes at 37°C before being blocked with 0.5% BSA for 2 hours at 37°C and used for the adhesion assay. After trypsinization, 30,000 cells/well were incubated for 30 minutes at 37°C in BSA- and matrix-coated wells. Unadhered cells were aspirated, and wells were carefully washed three times with PBS. Cells were fixed in 100% methanol for 10 minutes at room temperature and washed three times with PBS. Cells were stained for 30 minutes at room temperature with 50μg/mL propidium iodide and washed three times with PBS. Fluorescence was measured using a microplate reader at excitation at 535nm and emission at 617nm (Tecan Infinite M200 PRO).
12. Clonogenic Assay
Cells were trypsinized and 1,000 cells/well was plated in a 6 well plate and grown in humidified incubator at 37°C with 5% CO2 for 10 days. Colonies were washed with PBS, fixed with methanol for 20 minutes at room temperature, stained with crystal violet (0.5% w/v), and images were captured and quantified using ImageJ (RRID:SCR_003070).
13. Wound Healing Assay
Cells were trypsinized and plated in a 12-well culture plate. When the cells reached 70% confluence, they were placed in 0.5% FBS medium for 16 hours. An autoclaved tooth pick was used to make a uniform horizontal scratch across the diameter of the well. The monolayer was washed several times with PBS to remove any floating cells caused by the scratching process, and fresh media containing 0.5% FBS was added. Images of individual scratches were captured immediately and sequentially as different time points using an inverted microscope (Zeiss Observer Z1), and ImgaeJ was used to determine the percentage of scratch coverage.
14. Cytoplasmic Ca2+ Changes
Cytosolic Ca2+ was assessed by ratiometric Fura-2 assay. In brief, cells were washed with PBS before being loaded with Fura-2 AM (#F1221, Thermo Fisher Scientific) at 1μg/mL in calcium-containing buffer (2mM CaCl2, 126mM NaCl, 4.5mM KCl, 2mM MgCl2, 10mM glucose, 20mM HEPES, pH 7.4) for 30 minutes at room temperature. After washing the cells in calcium-containing buffer without Fura-2 AM, they were incubated for another 30 minutes to allow Fura-2 AM de-esterification. Resting calcium levels were measured by exciting cells at 340nm and 380nm and capturing emission at 510nm using a microplate reader (Tecan Infinite M200 PRO) in a calcium-free buffer (126mM NaCl, 4.5mM KCl, 2mM MgCl2, 10mM glucose, 20mM HEPES, pH 7.4). The ratios of 340/380 nm emissions proportional to intracellular [Ca2+] were calculated and plotted.
15. Transferrin Uptake
Transferrin uptake was measured as previously described (18), with some modifications. In brief, cells were rinsed with PBS and incubated in serum-free medium at 37°C for 30 minutes to remove residual transferrin before being incubated with Alexafluor 633-Transferrin (#T23362, Thermo Fisher Scientific) (25μg/ml) at 37°C for 1 hour. Transferrin endocytosis was halted by placing cells on ice. Excess transferrin was washed away with ice-cold serum-free DMEM, whereas bound transferrin was washed away with ice-cold PBS at pH 5.0 and pH 7.0. Cells were trypsinized and filtered through a cell strainer and transferrin uptake was determined by flow cytometry analysis of ~10,000 cells using the BD Influx cell sorter (BD Biosciences).
16. Radio-iodination and Radioimmunoassay
Radio-iodination and radioimmunoassay of cAMP and cGMP was performed. In brief, radio-iodination was carried out using the chloramine T technique. Subsequently, a competition between unlabelled cyclic nucleotide and corresponding iodinated monosuccinyl cyclic nucleotide tyrosine methyl ester was set up in a total volume of 300μl of 50mM sodium acetate buffer of pH 4.75 containing 5mg/mL BSA and allowed to equilibrate overnight at 4°C. Free cyclic nucleotides were separated from antibody-bound nucleotides by adding activated charcoal and BSA in a 50mM potassium phosphate buffer. Charcoal was recovered by centrifugation and radioactivity associated with it was measured in a γ counter (Perkin-Elmer, USA). A standard curve consisting of 12 known concentrations of cAMP or cGMP was used to calculate concentration in unknown samples.
17. Quantitative PCR
RNA was extracted from tissue or cells using the RNeasy Mini kit (#74104, Qiagen) according to the manufacturer’s instructions. RevertAid RT Reverse Transcription Kit (#K1691, Thermo Fisher Scientific) was used to reverse transcribe 4μg of RNA to cDNA. Real-time PCR was carried out with TB Green Premix Ex Taq II (Tli RNase H Plus) (#RR820A, Takara Bio) on CFX96 Touch Real-Time PCR Detection System (Bio-Rad). The qPCR data were normalised using the housekeeping gene glyceraldehyde 3-phosphate dehydrogenase.
Mouse primers included in the study are:
Slc9a9_Fwd 5’ CGATTCCGCTTCTTGCATGAG 3’
Slc9a9_Rev 5’ ACTTGGTCGGTGATGTTGACT 3’
Cdh1_Fwd 5’ CAGGTCTCCTCATGGCTTTGC 3’
Cdh1_Rev 5’ CTTCCGAAAAGAAGGCTGTCC 3’
Cldn7_Fwd 5’ GGCCTGATAGCGAGCACTG 3’
Cldn7_Rev 5’ GTGACGCACTCCATCCAGA 3’
Zeb1_Fwd 5’ GCTGGCAAGACAACGTGAAAG 3’
Zeb1_Rev 5’ GCCTCAGGATAAATGACGGC 3’
Snail1_Fwd 5’ CACACGCTGCCTTGTGTCT 3’
Snail1_Rev 5’ GGTCAGCAAAAGCACGGTT 3’
Gapdh_Fwd 5’ CAACTCCCTCAAGATTGTCAGCAA 3’
Gapdh_Rev 5’ GGCATGGACTGTGGTCATGA 3’
Human primers included in the study are:
SLC9A9_Fwd 5’ GTGGAGCTGCTTGTCTTCAAT 3’
SLC9A9_Rev 5’ GCAGAGTTGATGGACTGAAAGTT 3’
HSPA4L_Fwd 5’ GAGAAGTGGCGGCATCGAGA 3’
HSPA4L_Rev 5’ TGCTGCATTTCCAATGGCTCG 3’
ID3_Fwd 5’ GAGAGGCACTCAGCTTAGCC 3’
ID3_Rev 5’ TCCTTTTGTCGTTGGAGATGAC 3’
NR4A2_Fwd 5’ TGAGGTGTCCGGGTTCCCT 3’
NR4A2_Rev 5’ AATGGGGAGTCCAGCCTGTC 3’
MMP3_Fwd 5’ AGTCTTCCAATCCTACTGTTGCT 3’
MMP3_Rev 5’ TCCCCGTCACCTCCAATCC 3’
MMP10_Fwd 5’ TGCTCTGCCTATCCTCTGAGT 3’
MMP10_Rev 5’ TCACATCCTTTTCGAGGTTGTAG 3’
CARMIL1_Fwd 5’ GAGATTCATGGCGTCGTTTGC 3’
CARMIL1_Rev 5’ CACCTCACTCACGTCCTCG 3’
MMP1_Fwd 5’ CTCTGGAGTAATGTCACACCTCT 3’
MMP1_Rev 5’ TGTTGGTCCACCTTTCATCTTC 3’
GAPDH_Fwd 5’ GGAGCGAGATCCCTCCAAAAT 3’
GAPDH_Rev 5’ GGCTGTTGTCATACTTCTCATGG 3’.
18. Western Blotting
Cells were lysed in lysis buffer containing Protease/Phosphatase Inhibitor Cocktail (#5872, Cell Signallig Technology). Cells were sonicated before being centrifuged for 15 minutes at 14,000 rpm at 4°C. The protein concentration was determined using the Bradford assay. The membranes were blocked with 5% milk, then incubated overnight with primary antibodies and 1 hour with HRP-conjugated secondary antibodies. Immobilon reagent (Millipore) was used for detection, and the Chemidoc XRS+ imaging system (Bio-Rad) was used to capture images. Antibodies used were anti-NHE9 from ProteinTech (#13718-1-AP), anti-MMP1 from Thermo Fisher Scientific (#MA5-15872; RRID:AB_11154007), anti-β-actin from Cell Signalling Technology (#4970S), and anti-tubulin from Cell Signalling Technology (#2148).
19. Collagen Zymography
Acid-extracted rat tail collagen type 1 (#A1048301, Gibco) was neutralised on ice with 10X DMEM and 0.1N NaOH to a final concentration of 1mg/ml. Collagen (0.3mg/mL) was copolymerized with SDS-PAGE gels (10% polyacrylamide). For zymographic analyses, cells were cultured in serum-free medium for 24 hours in a humidified incubator at 37°C with 5%CO2. The conditioned medium obtained was loaded onto SDS-PAGE with collagen. After the run, the gel was incubated in 2.5% Triton X-100 for 40 minutes before being incubated in an incubation buffer (10mM Tris-Cl buffer pH 7.5, 1.25% Triton X-100, 5mM CaCl2, 1μM ZnCl2) for 6 hours at 5% CO2. The gel was stained with Coomassie brilliant blue for 2 hours and then destained for 1 hour before images were taken and collagenolytic bands were quantified using ImageJ.
20. Three-Dimensional Invasion Assay
Three-dimensional invasion assay was performed as we previously described (19), with some modifications. In brief, cells were trypsinized and 20,000 cells were plated in a 96-well plate coated overnight with 3% polyHEMA (#P3932, Sigma). Cell clusters were developed in defined medium supplemented with 4% reconstituted basement membrane (rBM) (#354230, Corning) and cultured for 24 to 48 hours in a humidified incubator at 37°C with 5% CO2. The rBM-coated cell clusters were then collected into a 1.5-ml microcentrifuge tube, spun down, and the supernatant was removed. Acid-extracted rat tail collagen type 1 (#A1048301, Gibco) was neutralized on ice with 10X DMEM and 0.1N NaOH to a final concentration of 1mg/ml. Pelleted cell clusters were resuspended in 50μl of neutralized collagen, and 3D cultures were grown in a humidified incubator at 37°C with 5% CO2. Invaded cells were imaged and quantified using ImageJ by determining the reciprocal of circularity of cell clusters.
21. Data Analysis
The data was analysed with GraphPad Prism (RRID:SCR_002798), and the statistical analyses used for individual experiments are listed in the figure legends. No power analysis was performed. All values in the bar plots represent the mean ± SD.
22. Mathematical model of endosomal pH regulation
To develop a mathematical model of endosomal pH regulation, we adapted work on lysosomal acidification by Ishida et al and incorporated the endosomal Na+/H+ exchanger NHE9, as well as other salient ion regulating elements (20). The transport of each ion is governed by a differential equation that describes its rate of entry and exit from the endosome. Independent modules can be modelled and then combined to form the system of differential equations. For example, the differential equation for H+ would include one component (V-ATPase) that brings H+ ions into the endosome and three components that move H+ ions out of the endosome (NHE9 antiporter, CLC antiporter, and passive leak). All parameter values are given in Supplementary Table 1.
V-ATPase proton pump
The pumping rate of protons across the membrane of a single V-ATPase pump (positive when H+ ions move into the endosome), JV, is calculated using experimental current-voltage data from Grabe et al (21). The proton flux due to V-ATPase depends on the endosomal pH (pHe) and the membrane potential due to charge imbalance in the endosome (ΔΨ). The total proton flux based on V-ATPase is calculated by multiplying the flux of a single pump by the number of pumps (NV) present on the membrane.
NHE9 antiporter
An acidic load inside the endosome causes high activity of the NHE9, which acts as a brake and leaks excess protons to restore basal pH levels. The activity is heavily influenced by the endosomal pH value. The exchanger is assumed to be inactive at higher pH values inside the endosome. The flux is given by the equation:
The NHE model from Marcoline et al was modified here (22). The PNHE9(pHe) modelling was changed from a linear drop from 1 to 0 between pH values of 6.55 and 7.25 by using the following equation for drop values with parameters chosen to produce sigmoidal behaviour as a reasonable approximation of NHE transport.
VNHE9 (maximum single exchanger turnover) is set to 1500/s, based on rates for the bacterial transporter NapA (23). KNHE9 is set to 3 kBT, which was based on NHE model by Marcoline et al (22). The total flux is calculated by multiplying the activity of a single exchanger by the total number of exchangers.
CLC Cl-/H+ antiporter
Endosomal CLC Cl-/H+ antiporters, like their lysosomal counterpart CLC7, have a 2:1 Cl-/H+ stoichiometric ratio. Ishida et al previously calibrated the activity of a single CLC antiporter against electrophysiological recordings, yielding the following analytical expression, which depends on the difference in cytoplasmic and endosomal pH as well as cytoplasmic and endosomal Cl- ion concentrations (20):
where a is 0.3, b is 1.5 × 10−5, ΔpCIC is, is the driving force:
and x is a simple switching function equal to
Total fluxes of Cl- inflow and H+ outflow are calculated by multiplying the performance of a single antiporter by the total number of antiporters (Ncl) as well as a stoichiometric coefficient corresponding to the ion exchange ratio (2 for Cl- and 1 for H+).
Membrane potential
We used a physical model of the membrane potential adapted by Ishida et al (20), which is dependent on the net accumulated charge inside the endosome:
Where C0 is the membrane capacitance per unit area, F is the Faraday’s constant and S and V are the surface area and the volume of the endosome. The terms under summation correspond to the concentrations of the specific ions. The term corresponding to the concentration of impermeant ions (B), known as Donnan particles, reflects the effect of negatively charged proteins over the distribution of permeant ions inside the endosome. The convention follows that a positive membrane potential indicates excess of positive charge inside the endosome relative to the cytoplasm.
Passive Leaks for H+, Cl-, K+, and Na+ ions
Channels are present over the endosomal membrane through which every ion leak or enters the endosome with certain permeability in a passive manner. We model passive channels with the following generic form46:
where q is the charge carried on the corresponding ion, S is the membrane surface area, U is the reduced membrane potential (U = eΨ/kBT), and [X] is the concentration of ion X.
The differential equations describing the system are given as the following:
Results
CRC displays increased NHE9 expression that affects patient survival
To ascertain the clinical and molecular relevance of NHEs in CRC, we interrogated publicly available patient databases. We found that among the NHE isoforms, increased expression of NHE9 (SLC9A9) consistently correlated with poor prognosis (Fig.1a). NHE9 transcript levels were indeed significantly higher in the AOM/DSS induced-CRC mouse model, colorectal adenoma and carcinoma, relative to nonneoplastic tissue (Fig.1b-c). Immunohistochemistry revealed that approximately 30% of CRC showed upregulation of NHE9 protein expression as compared to normal colon and rectal samples from the Human Protein Atlas (Supplementary Fig.1a). Notably, high-grade CRC exhibited strong staining of NHE9, which was mostly found on membranes, whereas low-grade CRC showed weak or no staining. Higher NHE9 expression was correlated with poorer overall- and disease-free-survival (Fig.1d). There were no gender differences in patient survival based on NHE9 expression (Supplementary Fig.1b-f). In addition, CRC subtype analysis revealed that NHE9 expression was highest in the most aggressive molecular subtypes (Fig.1e). Specifically, aggressive subtypes C4 (cancer stem cell) and C6 (normal cell-like tumours) with up-regulation of the epithelial-mesenchymal transition/motility pathways had higher NHE9 levels than low risk molecular subtypes C1 (down-regulated immune pathways), C2 (deficient mismatch repair), C3 (KRAS-mutant), and C5 (up-regulated Wnt pathway) (24).
Figure 1. NHE9 affects patient survival and is upregulated in CRC.
(a) Clinical correlation between expression of NHE isoforms and prognosis in CRC patients determined using the R2 genomics analysis and visualization platform (http://r2.amc.nl). (b-c) Experimental timeline and representative images of haematoxylin and eosin-stained sections from normal colon and colon of AOM/DSS-treated mice. Features include hyperchromasia, increased nuclear pleomorphism, and loss of cell polarity, indicating low- to high-grade dysplasia (left). NHE9 transcript levels in AOM/DSS-induced CRC mouse model compared to normal tissue (right) (b). NHE9 transcript levels in colorectal adenoma (GSE15960) and carcinoma (GSE3629) compared with nonneoplastic tissue (c). Expression levels are represented as scatter plots with bar charts displaying mean±s.d. P values were calculated by unpaired, two-tailed t test. (d) Overall survival (left) and disease-free survival (right) in patients with CRC with low and high NHE9 expression in the TCGA dataset. P values were calculated by the log-rank test. (e) NHE9 transcript levels in CRC molecular subtypes (GSE39582). Bars represent mean±s.d. High risk subtypes are marked in red. CPN, conventional precursor neoplasia; SPN, serrated precursor neoplasia. (f) Schematic overview and volcano plot depicting differentially expressed proteins in CRC with low and high NHE9 expression in the TCGA dataset. (g) Transcript levels of epithelial (CDH1, CLDN7) and mesenchymal (ZEB1, SNAIL1) in AOM/DSS-induced CRC mouse model compared to normal tissue. Expression levels are represented as scatter plots with bar charts displaying mean±s.d. P values were calculated by unpaired, two-tailed t test. (h) Hierarchical clustering and heat map of the correlation coefficient matrix of expression pattern of NHE9 with epithelial and mesenchymal genes in CRC samples from TCGA (left) and an early onset CRC dataset (GSE4107) (right) (low correlation, blue; high correlation, red). Note that epithelial and mesenchymal genes are separated as two tightly distinct and mutually correlated clusters, with NHE9 showing strong association with mesenchymal genes (arrow). (i) Heatmap of normalised expression of NHE isoforms in intestine epithelial and mesenchymal fractions (GSE6383). P values calculated by unpaired, two-tailed t test are shown on the right. Scale bar, 200μm (b). See related Supplementary Fig.1-2.
Next, we examined high-throughput reverse phase protein array proteomics data extracted from the Cancer Genome Atlas (TCGA) to infer causal mechanisms. Interestingly, an E- to N-cadherin switch in adhesion molecules was observed in CRC with high NHE9 expression (Fig.1f). Notably, we found that NHE9 upregulation was associated with the acquisition of mesenchymal characteristics in the AOM/DSS induced-CRC mouse model (Fig.1g). A more thorough examination of the TCGA dataset revealed a strong correlation of NHE9 gene expression pattern with mesenchymal genes such as ZEB1, ZEB2, VIM, and MSN, and anticorrelation with epithelial specific genes, namely CDH1, CLDN7, CLDN3, and CLDN4 (Fig.1h). Similar findings were also observed in early-onset CRC, a clinically distinct form that is frequently associated with a poor prognosis (Fig.1h).
We validated the performance of three independent EMT scoring metrics, GS76, MLR, and KS, by analysing transcriptomes of the epithelial and mesenchymal population in the normal intestine (Supplementary Fig.2a). The observed consistency and resolvability of calculated EMT scores suggest that all metrics resulted in a similar classification. Importantly, calculated EMT scores were highly correlated with NHE9 expression levels, consistent with enrichment of NHE9 in the mesenchymal fraction (Fig.1i and Supplementary Fig.2a). Similar findings in the Cancer Cell Line Encyclopaedia data (n=1376) demonstrate the robustness of these metrics (Supplementary Fig.2b). An independent analysis of the NCI-60 panel validated NHE9 as a mesenchymal marker (Supplementary Fig.2c). Taken together, the observations suggest that NHE9 expression levels may be useful in predicting clinical outcomes in patients with CRC.
NHE9 confers oncogenic properties on CRC cells
The precise balance of proton pump and leak pathways, mediated by V-ATPase and NHE9, determines the luminal pH of endosomes (8,25–27). We mutated the strictly conserved aspartate essential for ion transport to asparagine at position 244 in NHE9 (28) to abrogate the molecular activity/function of NHE9 (Fig.2a). To assess the role of NHE9 at the cellular level, we screened a panel of 54 established CRC cell lines for an appropriate cell system by extracting transcriptomic, mutation, and copy number profiles. Caco-2, a CRC cell line with the lowest NHE9 expression, was found to be suitable for answering the question of oncogenic properties conferred by ectopic NHE9 expression (Supplementary Fig.2d).
Figure 2. NHE9 regulates endosomal pH in colorectal cancer cells.
(a) Schematic and gene orthologue alignments showing strict conservation of the D244N substitution in various species (left). Side view of NHE9 dimer composed of transport domain and dimerization domain with Asp244 (required for ion transport) depicted and coloured according to the degree of ConSurf conservation (colour scale on the bottom) (right). (b) NHE9 wild-type and D244N mutant expression levels in CRC cells as assessed by Western blot analysis. (c) Schematic and representative fluorescence-activated cell sorting histograms (left) and quantification (right) demonstrating endosomal alkalinization in cells expressing NHE9 versus the empty vector or the D244N mutant. (d) Bar plots of cytoplasmic pH (left) lysosomal pH (right) in cells transduced with indicated plasmids. Error bar, s.d. P values were calculated by and one way ANOVA (c,d). The illustration was created with Biorender. See related Supplementary Fig.3.
First, we expressed wild type and D244N mutant NHE9 in CRC cells and confirmed equivalent expression (Fig.2b). Second, to test whether NHE9 regulates the luminal pH of the endosomes, we measured endosomal pH by incubations with pH-sensitive pHrodo Red-transferrin together with pH-nonsensitive Alexa Fluor 633-transferrin. Relative to the empty vector control cells (pH 5.49 ± 0.11), endosomal pH in NHE9-expressing cells was more alkaline (pH 7.12 ± 0.08) (Fig.2c and Supplementary Fig.3a-b). Cells expressing the D244N mutant failed to alkalinize the endosomal lumen (Fig.2c). Notably, there was no change in cytosolic or lysosomal pH in response to NHE9 expression (Fig.2d).
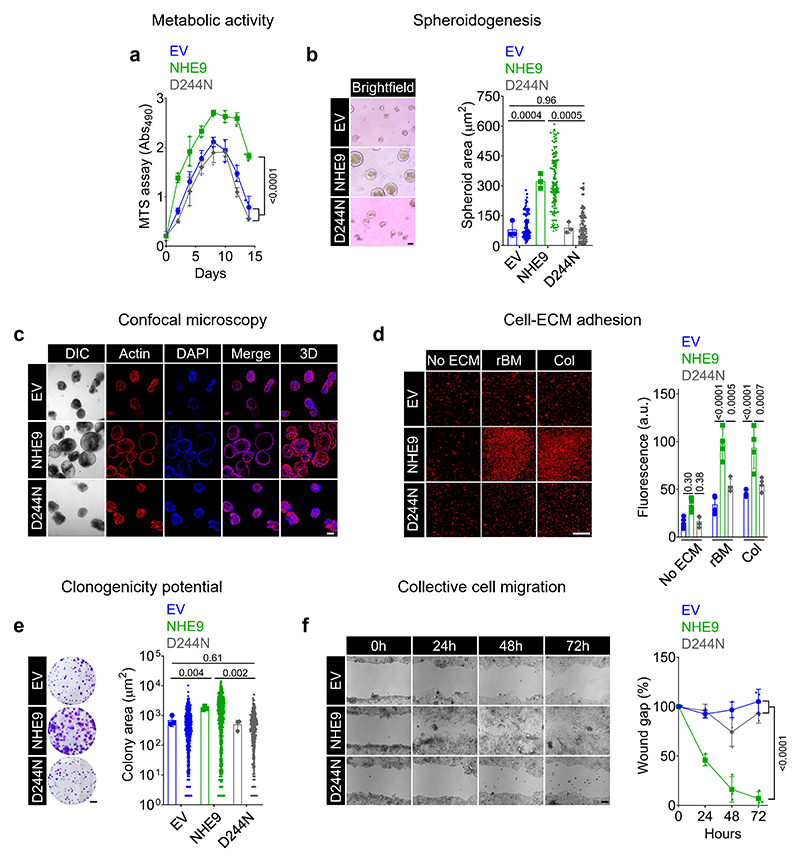
To probe the oncogenic potential of NHE9 in CRC cells, we used the tetrazolium reduction assay to compare cellular metabolic activity over a two-week period. Consistent with the tumour-promoting role of NHE9, we found that cells ectopically expressing NHE9 had significantly higher viability/metabolic activity (Fig.3a). Furthermore, consistent with previous findings in oesophageal squamous cell carcinoma (29), NHE9 expression inhibited cell apoptosis, but had no impact on cell cycle progression (Supplementary Fig.4a-c). Notably, cells expressing the D244N mutant showed a modest but significant reduction of cells in the early apoptosis stage when compared to the EV control, albeit significantly higher than cells expressing wild-type NHE9 (Supplementary Fig.4b), pointing to a potential scaffolding function of the disordered NHE9 C-terminal tail (30). Second, a scaffold-based 3D spheroidogenesis assay that can mimic the structural character of microtumours revealed that cells expressing NHE9 grown in reconstituted basement membrane (rBM) matrix had ~4-fold increase in spheroid area (Fig.3b). Confocal microscopy revealed that spheroids grown from empty vector- and D244N mutant-expressing cells did not cavitate and formed smaller and solid multicellular clusters, in contrast to the bigger spheroids derived from NHE9-expressing cells (Fig.3c).
Figure 3. NHE9 confers oncogenic properties in colorectal cancer cells.
(a) Metabolic activity of CRC cells after transduction with the indicated plasmids, as assessed by MTS assay. (b-c) Spheroidogenesis assay in reconstituted basement membrane (rBM) matrix after transduction with the indicated plasmids. Representative images on day 10 of culture are shown, and quantification was plotted (b). Representative confocal micrographs of spheroids on day 10 of culture stained for F-actin with phalloidin (red) and nucleus with DAPI (blue). Differential interference contrast (DIC) and 3D reconstruction of confocal z-stack images are also shown (c). (d) Cell-matrix adhesion assay on rBM or collagen versus bovine serum albumin (no extracellular matrix (ECM) control) coating after transduction with the indicated plasmids. Representative propidium iodide-stained images are shown, and quantification was plotted. (e) Clonogenic assay performed in six-well plates 10 days after plating, with clones produced by CRC cells with indicated transductions and stained with 0.5% crystal violet. Representative images are shown, and quantification was plotted. (f) Wound healing assay of CRC cells following indicated transductions imaged over 72h. Representative images are shown, and quantification was plotted. Data are presented as the mean±s.d. P values were calculated by two-way ANOVA (a) or one-way ANOVA (b,d,e,f). Scale bar, 10μm (b); 100μm (c,d,f); 5mm (e). See related Supplementary Fig.4.
Third, we asked if NHE9 levels correlate with a greater ability of cancer cells to adhere to extra-cellular matrices (ECMs) present within the parenchymal and stromal ECMs (17). While NHE9 expression had no effect on cell adhesion to a bovine serum albumin-coated surface, it did show greater adhesion to both nonfibrillar rBM and fibrillar type 1 collagen matrices (Fig.3d). We observed that a greater number of single cells with overexpression of NHE9 showed the ability to form multicellular colonies and of greater sizes than cells expressing the empty vector or the D244N mutant NHE9 (Fig.3e). Finally, a wound healing assay revealed that cells expressing NHE9 had accelerated collective cell migration and essentially complete wound closure at 48-72 hours, whereas cells expressing either the empty vector or the D244N mutant showed poor migration (Fig.3f). Taken together, these findings suggest a causal relationship between NHE9 transport function and oncogenic signalling in CRC and serve as a necessary starting point for deciphering the mechanistic underpinnings of tumour NHE9 expression.
NHE9 associates with a hybrid E/M state in CRC
To obtain an unbiased view of the contribution of NHE9 in CRC, we used RNA sequencing to compare the global transcriptional changes induced by NHE9 expression in CRC cells. Remarkably and unexpectedly, NHE9 expression was linked to profound changes in gene expression profiling, indicating that subtle changes in endosomal acid base homeostasis could alter gene expression and changes in cell physiology. Strikingly, in response to NHE9 expression, 2,079 genes were downregulated and 310 genes were upregulated, suggesting widespread transcriptional adaptation to tumour NHE9 upregulation (Fig.4a). No differences in the development of transepithelial electrical resistance were observed (Fig.4b).
Figure 4. NHE9 induces resting [Ca2+] and maintains a hybrid E/M state.
(a) Volcano plot (right) and Heatmap (left) depicting the total number of significantly differentially expressed genes (absolute log 2 [fold change] ≥ 2, FDR/adjusted p value < 0.05) in CRC cells expressing NHE9 versus empty vector (EV) control. (b) CRC cells transduced with the indicated plasmids were cultured in transwell membrane inserts, and transepithelial electrical resistance (TEER) was measured and plotted. (c) Plots of EMT score of CRC cells with NHE9 expression versus EV control. Lower GS76 scores and higher MLR and KS scores upon NHE9 expression indicate a hybrid E/M state. (d) Pairwise Pearson’s correlation of NHE9 and EMT regulatory genes in CRC cells with expression of NHE9 and EV control. Correlation analysis was performed across all 8 samples from EV control and NHE9 groups. Pearson’s correlation value for each gene pair is represented as the size of the circle and filled with the corresponding colour from the colour scale ranging from -1 (red) to +1 (blue), shown below. *P<0.05, **P<0.01, ***P<0.001. (e) Plots of ssGSEA scores for Ca2+ signalling, Ca2+ import and Ca2+ transport in CRC cells with NHE9 expression versus EV control. (f) Scatter plots illustrating the relationship between NHE9 expression and ssGSEA scores for Ca2+ signalling in primary (left) and metastatic (right) CRC samples (GSE41568). The linear fit and 95% CI, Pearson’s correlation coefficient (R), and P-value are all shown. (g) Plot of resting [Ca2+] in CRC cells after transduction with the indicated plasmids, as determined by the Fura-2 ratiometric assay. (h) Representative flow cytometry histograms (left) and quantification of mean fluorescence intensity (right) demonstrating transferrin (TFN) endocytosis after 60 minutes of uptake by CRC cells after monensin treatment versus vehicle control. The x axis of the histograms represents TFN uptake on a logarithmic scale, and the vertical dashed line represents the median fluorescence intensity. (i) Endosomal pH of CRC cells after monensin treatment compared to vehicle control. Bars represent mean±s.d. (j) Plot of resting [Ca2+] in CRC cells after treatment with monensin in comparison to the vehicle control. P values were calculated by unpaired, two-tailed t test (c,e,h,i), Pearson’s correlation (d,f), and one-way ANOVA (b,g,j). See related Supplementary Fig.5-6.
We calculated EMT scores and used Ingenuity Pathway Analysis (IPA) to identify enriched molecular and cellular functions. Strikingly, EMT scores from three independent metrics (GS76, KS, MLR) consistently suggested that cells expressing NHE9 gained mesenchymal traits, as denoted by increased MLR and KS scores but lower GS76 scores (Fig.4c). Importantly, the magnitude of change in any of these scores suggested that NHE9 does not force cells to an extreme mesenchymal state. Thus, rather than complete EMT, NHE9 prompted CRC cells to maintain a hybrid E/M state, which is predicted to increase invasiveness and overall metastatic potential (2) (Fig.4c). Indeed, when compared to primary tumours, NHE9 levels were significantly higher in metastatic CRC and strongly correlated with EMT scores (Supplementary Fig.5a-b). Remarkably, pairwise comparisons revealed a significant positive correlation between NHE9 and mesenchymal genes in CRC cells and patient samples (Fig.4d and Supplementary Fig.5c). Pathway analyses using IPA identified “cellular movement” as the top molecular and cellular function regulated by NHE9 expression in CRC cells, which is consistent with the wound healing assay finding of greater collective cell migration in cells expressing NHE9 (Fig.3f and Supplementary Fig.6a).
How do specific and localised pH changes within endosomes cause marked changes in global gene expression and drive the acquisition of a hybrid E/M state? Cellular pH and calcium homeostasis are inextricably linked, and recent years have seen a renewed appreciation for the role resting [Ca2+] plays in tumour progression by driving a hybrid E/M state (31,32). It is conceivable that luminal alkalinization could trigger Ca2+ release from acidic endosomal stores and raises resting [Ca2+] levels (33). Consistent with this model, single-sample Gene Set Enrichment Analysis (ssGSEA) revealed that CRC cells expressing NHE9 were highly enriched for calcium signalling signatures (Fig.4e). Notably, there was no difference in ssGSEA scores for Ca2+ import and transport, corroborating the hypothesis that NHE9 modulates acidic endosomal Ca2+ stores and regulates resting cytosolic [Ca2+] as compared to Ca2+ influx across the plasma membrane (Fig.4e). NHE9 levels correlated strongly and positively with Ca2+ signalling scores in both primary and metastatic CRC samples (Fig.4f). An independent analysis of the TCGA data validated the link between NHE9 and intracellular Ca2+ remodelling in CRC (Supplementary Fig.6b). Experimental validation of these results using the Fura-2 AM ratiometric calcium assay revealed a significant increase in resting [Ca2+] in CRC cells expressing NHE9 (Fig.4g). To establish causation, we tested monensin, a known chemical mimic of NHE9 activity, which increased transferrin uptake (18) (Fig.4h). Notably, monensin treatment increased endosomal pH and resting [Ca2+] to a similar extent as cells expressing NHE9 (Fig.4i-j). Notably, monensin treatment increased stemness gene expression and stabilised receptor tyrosine kinases by preventing lysosomal degradation (34). These data collectively suggest that NHE9 may elevate resting [Ca2+] and is a critical regulator of phenotypic identity of cancer cells.
Endosomal alkalinization by NHE9 reprograms cAMP metabolism in CRC cells
We next investigated the mechanisms underlying NHE9-mediated oncogenic signalling. To this end, we performed IPA upstream regulator analysis to explain the observed global gene expression changes in CRC cells with NHE9 expression (Fig.5a and Supplementary Fig.7a). A thorough examination revealed that the top four upstream regulators inhibited by NHE9 (NECA, PKA, cyclic AMP, and 8-bromo-cyclic AMP) were funnelled into a single pathway: the cAMP signalling axis, a putative negative regulator of tumorigenicity in CRC (35,36) (Supplementary Fig.7b). In line with our hypothesis, overall cAMP signalling was significantly downregulated in CRC patients with worse prognosis and metastasis (Fig.5b). Furthermore, gene ontology analysis revealed significant repression of cAMP-regulated genes in CRC cells with NHE9 expression (Fig.5c).
Figure 5. NHE9 regulates cytosolic cAMP homeostasis.
(a) Bar chart with top IPA upstream regulators of CRC cells with NHE9 expression versus EV control displayed on the x-axis and the activation Z-score plotted on the y-axis. Four of the most inhibited upstream regulators (shown in bold) were funnelled into a single pathway: the cAMP signalling axis (left). Gene network depicting up-regulated (red) or down-regulated (green) genes that determine the activation state of cAMP in CRC cells in response to NHE9 expression, with their relationships to cAMP colour coded to indicate whether they lead to activation (orange) or inhibition (blue), and those that are inconsistent with the prediction highlighted in yellow (right). (b) Box-plot indicating downregulation of cAMP signalling in CRC patients with poor prognosis (survival ≤10 months) (GSE16125) (left) and with metastasis (GSE28722) (right). (c) Heatmap of normalised expression of cAMP regulated genes in CRC cells following transduction with the indicated plasmids. (d) Plots of intracellular cAMP levels (left) and cGMP levels (right) in CRC cells after transduction with the indicated plasmids, as determined by the radioimmunoassay. (e) Representative images of hemicysts with fluid accumulation focally beneath the epithelium (arrow) are shown, and quantification was plotted. (f) Schematic of the experimental protocol. (g) Heatmap of activation Z-scores from IPA comparison analysis demonstrating that upstream regulators activated by NHE9 were found to be inhibited by forskolin and vice versa (colour scale on the bottom). (h) Venn diagrams depicting overlap genes that are regulated reciprocally in CRC cells by NHE9 expression and forskolin treatment (left). Graph depicting the expression of predicted cAMP-regulated genes downstream of NHE9 in colon and rectal cancer, with fold change on the x-axis and -Log10 (P-value) on the y-axis. Dashed horizontal line corresponds to P=0.05 (right). (i) Western blot analysis of MMP1 protein in cells after transduction with the indicated plasmids. Collagen zymography examining MMP1 secretion and activity. The upper bands in the zymograms are pro-MMP1, and the lower bands are active MMP1. The densitometric analysis of collagenolytic activities was plotted on the right. P values were calculated by unpaired, two tailed t test (b), and one-way ANOVA (d,e,i). See related Supplementary Fig.7-9.
Although suppression of cAMP levels has been linked to variety of cancers, including CRC, since the early 1970s, whether and how it plays a pivotal role remains uncertain (36–38). We therefore hypothesized that the reduction of cAMP levels may be important for NHE9-mediated oncogenic signalling. To test this, we measured cyclic nucleotides using radioimmunoassay and found markedly diminished intracellular cAMP (~80% lower) in cells expressing NHE9. No changes in cGMP were observed (Fig.5d). As a functional assay for low intracellular cAMP levels, we observed that cells expressing NHE9 had a reduced ability to form hemicysts (16) (Fig.5e). Of note, while basal cAMP levels in cells expressing NHE9 were significantly lower, forskolin-stimulated cAMP production in these cells was not different from cells expressing the empty vector or the D244N mutant, suggesting that forskolin-mediated simulation of a subset of membrane associated adenylyl cyclases may be independent of NHE9 expression (Supplementary Fig.8a). Finally, to establish causality, we tested the effect of monensin, which, like NHE9, reduced cAMP in CRC cells but had no effect on cGMP levels (Supplementary Fig.8b).
We next sought to determine the mechanisms by which NHE9 downregulates cAMP levels. Since intracellular acidification is a well-known inhibitor of pH-sensing soluble adenylyl cyclase (sAC) (39), we first considered if the effect of NHE9 is mediated by cytoplasmic pH regulation. However, no changes in cytoplasmic pH were detected (Fig.2d), though we cannot rule out changes in pH-microclimate in the periendosomal area due to NHE9-mediated H+ leak from endosomes (39). Studies have shown that adenylyl cyclase-mediated cAMP generation is either dampened or driven by [Ca2+] changes, highlighting the context-dependent effects in different cell types (40). Given that NHE9 increased resting [Ca2+] while decreasing cAMP levels, we examined whether pharmacologically induced [Ca2+] rises recapitulated the effect of NHE9 on cAMP levels. Intracellular calcium mobilisation induced by ionomycin, a Ca2+ ionophore, and thapsigargin, a sarcoplasmic reticulum Ca2+ pump inhibitor, was found to significantly reduce cAMP but not cGMP levels in CRC cells (Supplementary Fig.8c). We confirmed that the effect of [Ca2+] on cAMP levels occurred downstream of endosomal function by treating CRC cells with ionomycin, which caused modest changes in transferrin endocytosis but had no effect on endosomal pH (Supplementary Fig.8d-e).
Notably, in both primary and metastatic CRC, NHE9 expression and cAMP signalling scores demonstrated a strong negative correlation (Supplementary Fig.8f). Similar findings in the TCGA data suggest that elevated NHE9 expression may antagonise cAMP levels in CRC (low NHE9>intermediate NHE9>>high NHE9) (Supplementary Fig.8g). In line with this, Ca2+ signalling scores showed a strong negative association with cAMP signalling scores across primary and metastatic CRC samples, as well as in the TCGA data (Supplementary Fig.8h-i). Consistent with these findings, activation of calcium signalling by ionomycin treatment promoted the acquisition of a hybrid epithelial-mesenchymal state in cancer cells (32). Altogether, these data demonstrate that NHE9-mediated endosome alkalinization contributes to the maintenance of suppressed cAMP levels in the cytosol in CRC cells.
NHE9 induces expansive collective invasion in vitro and tumour formation in vivo
To pursue the effector mechanisms by which the NHE9-cAMP axis affects cellular physiology and modifies CRC risk, we first performed transcriptome profiling on CRC cells treated with forskolin (Fig.5f and Supplementary Fig.9a-b). We conducted an IPA comparison analysis of upstream regulators and observed that those activated by NHE9 were inhibited by forskolin and vice versa (Fig.5g). Next, we used a combinatorial approach to overlap genes that show reciprocal regulation between NHE9 expression and forskolin treatment (Fig.5h). These genes included the matrix metallopeptidases MMP1 and MMP10, which are upregulated by NHE9 expression, as well as several genes that are downregulated by NHE9 expression (KLHL30, NR4A2, NR4A3, PCK1, SLC26A4, and ZNF331) (Fig.5h). MMP1 was found to exhibit consistent changes in both colon and rectal cancers (Fig.5h), and ranked among the top 25 overexpressed genes in CRC (15). Importantly, CRC patient samples with NHE9 upregulation had significantly higher MMP1 levels (Supplementary Fig.9c). We therefore hypothesized that the induction of MMP1 may be important for NHE9-mediated promotion of metastasis in CRC. To test this, we first confirmed that, while NHE9 expression increases MMP1 expression, forskolin treatment downregulated it (Supplementary Fig.9d). This was verified by Western blot analysis, which revealed higher MMP1 protein levels in cells expressing NHE9 compared to empty vector or D244N mutant expression (Fig.5i). We further examined the secretion and activity of MMP1 by collagen zymography. The mature form of MMP1 detected in the conditioned media showed ~2-fold higher collagenolytic activity in cells expressing NHE9 (Fig.5i). These data corroborate the involvement of NHE9 in matrisome remodelling and the promotion of tumour invasion and metastasis.
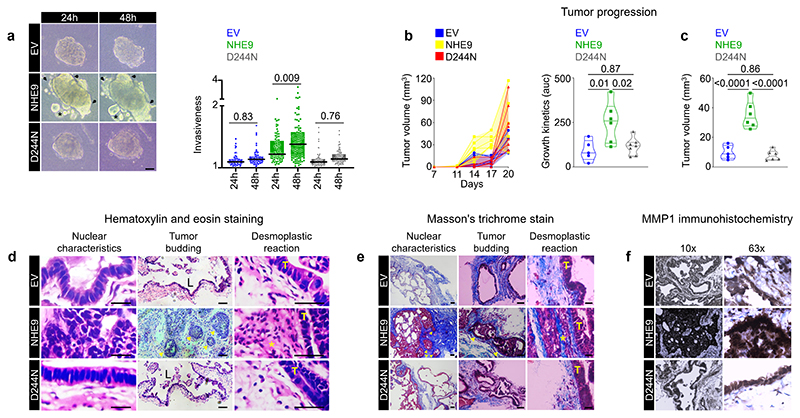
We next developed a three-dimensional tumour invasion assay to test if NHE9 drives cells at the tumour front to breach the basement membrane and invade the stroma, a rate-limiting step in the metastatic cascade (19). Intriguingly, CRC cell clusters harbouring the empty vector or the D244N mutant exhibited a round phenotype, whereas clusters expressing NHE9 exhibited a stellate phenotype or irregular shape with blurred contours, as well as the formation of tumour buds (Fig.6a). These findings suggest that NHE9 increases the propensity of cells to actively migrate centrifugally breaching the basement membrane into the stroma in a collective manner rather than solitary cell invasion.
Figure 6. NHE9 induces collective invasion in vitro and tumour formation in vivo.
Representative images of three-dimensional tumour invasion assay in CRC cells transduced with the indicated plasmids and quantification was plotted. Arrows indicated invading tumour cells and asterisk denotes tumour buds. (b-c) Graph on the left depicts the evolution of tumour volumes in individual mice until day 20. Area under the curve, a measure of tumour growth kinetics in individual mice, was plotted on the right (b). Plot depicting differences in tumour volume in individual mice on day 17 post-transplantation (c). (d-e) Representative images of haematoxylin and eosin-stained (d) and Masson’s trichrome-stained (e) tumour sections demonstrating nuclear characteristics, tumour budding, and desmoplastic reaction. T denotes the tumour, L denotes gland-like structures, arrows denote tumour buds, and an asterisk denotes a prominent desmoplastic reaction. (f) Representative images (10X and 63X) of tumour sections immunohistochemically stained for MMP1 revealed prominent expression in tumours expressing NHE9, particularly at the invasive front.
To determine whether NHE9 was sufficient for promoting tumour growth in vivo, we transplanted CRC cells subcutaneously in a nude mouse model. Quantitative analysis of tumour volume using area under the curve as a measure of growth kinetics revealed that NHE9 results in statistically significant tumour burden, with the greatest difference occurring on day 17 post-transplantation (Fig.6b). Mice were sacrificed three weeks after tumour injection for evaluation. Haematoxylin and eosin staining revealed that cells expressing the empty vector or the D244N mutant resulted in cystic tumours with fluid-filled lumens (Fig.6c), explaining the dramatic late increase in tumour volume (Fig.6b). Strikingly, cells expressing NHE9 exhibited solid growth in nested or trabecular patterns, as well as several defining features of tumour aggressiveness, such as collective cell migration and infiltration resulting in finger-like projections, which is typical of invasive tumours (2) (Fig.6d). Additionally, tumours expressing NHE9 displayed remarkable nuclear polymorphism, stromal extensions into the tumour, and the formation of tumour buds in the invasive front (Fig.6d).
Notably, tumours expressing NHE9 displayed an abundant desmoplastic stroma reaction, which could be important for aggressive tumour biology (Fig.6d). Masson’s trichrome staining independently validated features of tumour aggressiveness associated with NHE9 expression (Fig.6e). Immunohistochemical examination revealed prominent expression of MMP1 in tumours expressing NHE9, particularly at the leading front of leader cells, which was consistent with a causal effect (19) (Fig.6f). Given that tumour buds are considered adverse prognosticators and reflect a hybrid E/M state, their formation in vivo in tumours expressing NHE9 provides strong independent support to its role as a phenotypic stability factor in CRC cells (2). Collectively, both in vitro and in vivo findings support a critical role for NHE9 in tumour progression and invasion.
Mathematical and physiological basis of NHE9 transport function in CRC
We hypothesised that NHE9 upregulation might be involved in dampening cAMP signalling and thus play a role in invasion and metastasis (Fig.7a). We developed a mathematical model for endosomal pH regulation based on the assumption that the acidification process requires V-ATPase to pump protons into the lumen, NHE9 to leak protons out of the lumen in exchange for Na+ ions, Cl-/H+ antiporter mediated counterion flux to neutralise the membrane potential caused by proton accumulation, and other salient ion regulating elements. We adapted work on lysosomal acidification by Ishida et al and individual transporter properties are calibrated using available data, including electrophysiological measurements to estimate protein copy numbers, and endosome physical properties, such as surface area, volume, and buffering capacity, are estimated using available data (20–22) (Supplementary Table 1, and Methods). Early experiments in yeast, plant, and mammalian models suggested that intracellular NHEs function as an H+ leak pathway (8), but more recently an alternative H+ loading mechanism has been also proposed (14). The model shows that the results of in vitro endosome experiments can be explained by the proton-leak function of NHE9 (Fig.7b). We also show that loss of this leak pathway, as seen in autism-associated NHE9 mutations, causes endosome overacidification, which is consistent with experimental data (8) (Fig.7c).
Figure 7. Model for feedback regulation of endosomal pH by NHE9.
(a) Proposed role for NHE9 in CRC. Pathological endosomal alkalinization caused by NHE9 upregulation induces resting [Ca2+], which maintains a hybrid E/M state while suppressing cAMP levels, all of which may drive tumour migration and invasion. The illustration was created with Biorender. (b) Dynamics of endosomal acidification considering proton loading mechanism for NHE9 function. Endosomes, according to this model, would have an abnormally low steady-state pH (pH<5), approaching that of lysosomes (left). Dynamics of endosomal acidification considering proton leak mechanism for NHE9 function. Endosomes, according to this model, would have a steady-state pH (pH~5.5) consistent with experimental observations (right). (c) Loss of the proton leak pathway, as seen in autism-associated NHE9 mutations, cause endosome overacidification (pH~5.0). (d) Model for feedback regulation of endosomal pH. Upregulation of NHE9, as seen in CRC, suppresses cAMP levels, creating a feedback loop for modulation of V-ATPase function. (e) Dynamics of endosomal acidification caused by a modest 2-fold (left) to 5-fold (right) increase in NHE9 levels show dramatic luminal alkalinization (pH~7.0). The incorporation of feedback mediated by the NHE9/cAMP axis into the model resulted in a slower initial rate of endosomal acidification (see inserts) while maintaining a comparable steady-state pH. See related Supplementary Table 1.
We apply our approach to experimental findings that showed that cAMP levels could regulate V-ATPase activity and endosomal pH (41–43) (Fig.7d). The model predicted that it can explain the perplexing clinical phenotypes and experimental data on the large magnitude of endosomal alkalinization observed with NHE9 expression, as well as make verifiable predictions. Our data and mathematical models suggest that even a modest 2-5-fold increase in NHE9 levels, as seen in CRC patients, can result in dramatic luminal alkalinization, because of its exceptionally high transport rates of ~1,500 ions/s (23) (Fig.7e). Importantly, the suppression of cAMP levels by NHE9, which dampens V-ATPase activity (41,42), would recapitulate the design principles of a negative feedback mechanism (Fig.7d). This is a ubiquitous feature of biological networks that confers robustness and aids in the establishment of multistable stationary states (44). Incorporating this feedback into our model resulted in a slower initial rate of endosomal acidification, which could help conserve ATP stores while achieving a similar steady-state pH (Fig.7e). Together, these findings lead to a model in which a decrease in cytosolic cAMP levels, which is typically seen in starvation or hypoxia due to decreased cellular ATP levels, can be hijacked by signals such as NHE9 expression, thereby promoting invasion by putting cells in a pseudo-starvation state.
Discussion
Perturbation of cytoplasmic pH dynamics has long been recognised as a pathophysiological hallmark of cancers (45). In contrast, despite emerging evidence linking NHE9 to a variety of cancers (9,18,29), the role of endosomal pH in oncogenesis has received less attention. In this context, our discovery of an NHE9-Ca2+-cAMP signalling axis that accelerates colorectal tumorigenesis and invasion has several important implications (Fig.7a).
First, it reveals a novel pathophysiological function of endosomes. Experiments conducted by us and others have shown that in response to nutrient deprivation, the non-motile yeast Saccharomyces cerevisiae, upregulates Nhx1, the human NHE9 ortholog, alkalinizes endosomes, promotes endocytosis, and switches to invasive growth (12,13,46). Accordingly, Nhx1 induction may complement supply and demand adjustments by increasing nutrient uptake and enabling yeast cells to escape an environment of scarcity and forage for new resources. Thus, it appears likely that endosomal reprogramming in response to starvation or pseudo-starvation is an evolutionary conserved mechanism that may serve as a universal driver of tumorigenesis and metastatic dissemination (11). Second, our findings that NHE9-mediated subtle changes in endosomal pH can regulate cytosolic cAMP metabolism could be part of a feedback mechanism to control V-ATPase and reallocate resources away from energy-intensive H+ pumping during starvation. When there is an energy surplus, cAMP levels rise, promoting V-ATPase activation and endosomal-lysosomal acidification, which acts as a sink to prevent cytosolic proton build-up caused by high glycolysis rates (41). We suggest that NHE9 upregulation in cancer caused by (epi)genetic lesions may hijack the response to nutrient limitation and impose a dialled-down cAMP signalling state, a cell-intrinsic mechanism that could echo nutrient supply-demand imbalance and contribute to phenotypic switching in vivo (9,18,29). These lessons go far beyond the realm of cancer. In particular, NHE9-mediated cAMP regulation may be crucial in the functioning of the nervous and immune systems, as well as in pathologies such as autism and COVID-19 (8,10,47,48).
Third, together with studies investigating the effects of cytoplasmic Ca2+ and cAMP levels on hybrid E/M state (32,49), our findings suggest a model whereby tumour-specific NHE9 expression patterns determine the degree of phenotypic heterogeneity and contribute to leader–follower organization. In vivo, NHE9 upregulation resulted in collective invasion, tumour podia, and tumour buds at the protrusive front, all of which are morphological hallmarks of the hybrid E/M state (2). This model raises the possibility that developing high affinity, selective NHE9 inhibitors that work synergistically with surgery, chemotherapy, or immunotherapy could lead to better cancer treatment outcomes. Fourth, our findings bring new focus to endosome biology, an underrepresented field in cancer research, and may stimulate further studies into the mechanistic basis for transcriptional up-regulation of NHE9. Aberrant DNA methylation of NHE9 promoter in colon cancer has been demonstrated, ranking among the top 10 hypomethylated promoters, and could act as a potential DNA methylation biomarker in CRC (15) (Supplementary Fig.10a-c). The mechanism of induction of NHE9 expression by DNA hypomethylation facilitating invasive growth of tumour cells might be further elucidated.
Finally, our study demonstrates that tumour cells have superior fitness at higher endosomal pH, but their reliance on an alkaline endosomal milieu confers vulnerabilities that can be exploited to develop therapeutic strategies. Studies have shown that metformin, which is being explored for cancer prevention and treatment, functions via endosomal pH and reduces intracellular cAMP levels, and that upregulation of NHE9 may sensitise cells to its anticancer effect (50–52). Additionally, our study supports a rational, mechanistic basis for the therapeutic use of cAMP analogues such as 8-Cl-cAMP (tocladesine) in CRC, which have already entered clinical trials (NCT00021268). We suggest that the dependency of CRC cells on NHE9 is in part a result of high biosynthetic activity, which necessitates enhanced nutrient uptake. This distinct survival reliance on increased endocytosis can be harnessed to develop pH-rebalancing or cytotoxic nanoparticle-based strategies that are selectively targeted to these cells while causing minimal toxicity to normal tissue.
Supplementary Material
Implications.
Endosomal pH regulator NHE9 actively controls cytosolic Ca2+ levels to downregulate the adenylate cyclase-cAMP system, enabling colorectal cancer cells to acquire hybrid epithelial-mesenchymal characteristics and promoting metastatic progression.
Acknowledgements
This work was supported by the following grants: IA/E/17/1/503665 (H.P.), IA/I/17/2/503312 (R.B.), and IA/M/16/1/502 (S.S.V.) from the DBT-Wellcome Trust India Alliance, and SB/S2/RJN-049/2018 (M.K.J.) from the Science and Engineering Research Board (SERB), Department of Science and Technology (DST), Government of India. S.S.V. is a JC Bose National Fellow (SB/S2/JCB-18/2013). We thank Professor Rajini Rao (Johns Hopkins University School of Medicine, Baltimore) for the gift of NHE9-GFP plasmid.
Footnotes
Conflict of interest statement: The authors declare no competing interest.
23. Data Availability Statement
Transcriptomic data have been deposited in ArrayExpress with accession numbers E-MTAB-13742 and E-MTAB-13746.
References
- 1.Cancer Genome Atlas Network. Comprehensive molecular characterization of human colon and rectal cancer. Nature. 2012;487(7407):330. doi: 10.1038/nature11252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vilchez Mercedes SA, Bocci F, Levine H, Onuchic JN, Jolly MK, Wong PK. Decoding leader cells in collective cancer invasion. Nat Rev Cancer. 2021;21(9):592–604. doi: 10.1038/s41568-021-00376-8. [DOI] [PubMed] [Google Scholar]
- 3.George JT, Jolly MK, Xu S, Somarelli JA, Levine H. Survival Outcomes in Cancer Patients Predicted by a Partial EMT Gene Expression Scoring Metric. Cancer Res. 2017;77(22):6415–28. doi: 10.1158/0008-5472.CAN-16-3521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tan TZ, Miow QH, Miki Y, Noda T, Mori S, Huang RY, et al. Epithelial-mesenchymal transition spectrum quantification and its efficacy in deciphering survival and drug responses of cancer patients. EMBO Mol Med. 2014;6(10):1279–93. doi: 10.15252/emmm.201404208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Prasad H, Rao R. Amyloid clearance defect in ApoE4 astrocytes is reversed by epigenetic correction of endosomal pH. Proc Natl Acad Sci U S A. 2018;115(28):E6640-E9. doi: 10.1073/pnas.1801612115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Picelli S, Vandrovcova J, Jones S, Djureinovic T, Skoglund J, Zhou XL, et al. Genome-wide linkage scan for colorectal cancer susceptibility genes supports linkage to chromosome 3q. BMC Cancer. 2008;8:87. doi: 10.1186/1471-2407-8-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee AR, Park J, Jung KJ, Jee SH, Kim-Yoon S. Genetic variation rs7930 in the miR-4273-5p target site is associated with a risk of colorectal cancer. Onco Targets Ther. 2016;9:6885–95. doi: 10.2147/OTT.S108787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Prasad H, Rao R. Endosomal Acid-Base Homeostasis in Neurodegenerative Diseases. Rev Physiol Biochem Pharmacol. 2023;185:195–231. doi: 10.1007/112_2020_25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ueda M, Iguchi T, Masuda T, Komatsu H, Nambara S, Sakimura S, et al. Up-regulation of SLC9A9 Promotes Cancer Progression and Is Involved in Poor Prognosis in Colorectal Cancer. Anticancer Res. 2017;37(5):2255–63. doi: 10.21873/anticanres.11562. [DOI] [PubMed] [Google Scholar]
- 10.Prasad H. Protons to Patients: targeting endosomal Na(+) /H(+) exchangers against COVID-19 and other viral diseases. FEBS J. 2021;288(17):5071–88. doi: 10.1111/febs.16163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Garcia-Jimenez C, Goding CR. Starvation and Pseudo-Starvation as Drivers of Cancer Metastasis through Translation Reprogramming. Cell Metab. 2019;29(2):254–67. doi: 10.1016/j.cmet.2018.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Prasad H, Rao R. Histone deacetylase-mediated regulation of endolysosomal pH. J Biol Chem. 2018;293(18):6721–35. doi: 10.1074/jbc.RA118.002025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gimeno CJ, Ljungdahl PO, Styles CA, Fink GR. Unipolar cell divisions in the yeast S. cerevisiae lead to filamentous growth: regulation by starvation and RAS. Cell. 1992;68(6):1077–90. doi: 10.1016/0092-8674(92)90079-r. [DOI] [PubMed] [Google Scholar]
- 14.Galenkamp KMO, Sosicka P, Jung M, Recouvreux MV, Zhang Y, Moldenhauer MR, et al. Golgi Acidification by NHE7 Regulates Cytosolic pH Homeostasis in Pancreatic Cancer Cells. Cancer Discov. 2020;10(6):822–35. doi: 10.1158/2159-8290.CD-19-1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chandrashekar DS, Karthikeyan SK, Korla PK, Patel H, Shovon AR, Athar M, et al. UALCAN: An update to the integrated cancer data analysis platform. Neoplasia. 2022;25:18–27. doi: 10.1016/j.neo.2022.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Valentich JD, Tchao R, Leighton J. Hemicyst formation stimulated by cyclic AMP in dog kidney cell line MDCK. J Cell Physiol. 1979;100(2):291–304. doi: 10.1002/jcp.1041000210. [DOI] [PubMed] [Google Scholar]
- 17.Pally D, Pramanik D, Hussain S, Verma S, Srinivas A, Kumar RV, et al. Heterogeneity in 2,6-Linked Sialic Acids Potentiates Invasion of Breast Cancer Epithelia. ACS Cent Sci. 2021;7(1):110–25. doi: 10.1021/acscentsci.0c00601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kondapalli KC, Llongueras JP, Capilla-Gonzalez V, Prasad H, Hack A, Smith C, et al. A leak pathway for luminal protons in endosomes drives oncogenic signalling in glioblastoma. Nat Commun. 2015;6:6289. doi: 10.1038/ncomms7289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pally D, Pramanik D, Bhat R. An Interplay Between Reaction-Diffusion and Cell-Matrix Adhesion Regulates Multiscale Invasion in Early Breast Carcinomatosis. Front Physiol. 2019;10:790. doi: 10.3389/fphys.2019.00790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ishida Y, Nayak S, Mindell JA, Grabe M. A model of lysosomal pH regulation. J Gen Physiol. 2013;141(6):705–20. doi: 10.1085/jgp.201210930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Grabe M, Wang H, Oster G. The mechanochemistry of V-ATPase proton pumps. Biophys J. 2000;78(6):2798–813. doi: 10.1016/S0006-3495(00)76823-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Marcoline FV, Ishida Y, Mindell JA, Nayak S, Grabe M. A mathematical model of osteoclast acidification during bone resorption. Bone. 2016;93:167–80. doi: 10.1016/j.bone.2016.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee C, Kang HJ, von Ballmoos C, Newstead S, Uzdavinys P, Dotson DL, et al. A two-domain elevator mechanism for sodium/proton antiport. Nature. 2013;501(7468):573–7. doi: 10.1038/nature12484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Marisa L, de Reynies A, Duval A, Selves J, Gaub MP, Vescovo L, et al. Gene expression classification of colon cancer into molecular subtypes: characterization, validation, and prognostic value. PLoS Med. 2013;10(5):e1001453. doi: 10.1371/journal.pmed.1001453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Donowitz M, Ming Tse C, Fuster D. SLC9/NHE gene family, a plasma membrane and organellar family of Na(+)/H(+) exchangers. Mol Aspects Med. 2013;34(2-3):236–51. doi: 10.1016/j.mam.2012.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fuster DG, Alexander RT. Traditional and emerging roles for the SLC9 Na+/H+ exchangers. Pflugers Arch. 2014;466(1):61–76. doi: 10.1007/s00424-013-1408-8. [DOI] [PubMed] [Google Scholar]
- 27.Pedersen SF, Counillon L. The SLC9A-C Mammalian Na(+)/H(+) Exchanger Family: Molecules, Mechanisms, and Physiology. Physiol Rev. 2019;99(4):2015–113. doi: 10.1152/physrev.00028.2018. [DOI] [PubMed] [Google Scholar]
- 28.Winklemann I, Matsuoka R, Meier PF, Shutin D, Zhang C, Orellana L, et al. Structure and elevator mechanism of the mammalian sodium/proton exchanger NHE9. EMBO J. 2020;39(24):e105908. doi: 10.15252/embj.2020105908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen J, Yang H, Wen J, Luo K, Liu Q, Huang Y, et al. NHE9 induces chemoradiotherapy resistance in esophageal squamous cell carcinoma by upregulating the Src/Akt/beta-catenin pathway and Bcl-2 expression. Oncotarget. 2015;6(14):12405-20. doi: 10.18632/oncotarget.3618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Prasad H, Osei-Owusu J, Rao R. Functional analysis of Na(+)/H(+) exchanger 9 variants identified in patients with autism and epilepsy. Matters (Zur) 2017:2017. doi: 10.19185/matters.201704000009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ullman JC, Yang J, Sullivan M, Bendor J, Levy J, Pham E, et al. A mouse model of autism implicates endosome pH in the regulation of presynaptic calcium entry. Nat Commun. 2018;9(1):330. doi: 10.1038/s41467-017-02716-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Norgard RJ, Pitarresi JR, Maddipati R, Aiello-Couzo NM, Balli D, Li J, et al. Calcium signaling induces a partial EMT. EMBO Rep. 2021;22(9):e51872. doi: 10.15252/embr.202051872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Patel S, Docampo R. Acidic calcium stores open for business: expanding the potential for intracellular Ca2+ signaling. Trends Cell Biol. 2010;20(5):277–86. doi: 10.1016/j.tcb.2010.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ko M, Makena MR, Schiapparelli P, Suarez-Meade P, Mekile AX, Lal B, et al. The endosomal pH regulator NHE9 is a driver of stemness in glioblastoma. PNAS Nexus. 2022;1(1):pgac013. doi: 10.1093/pnasnexus/pgac013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bradbury AW, Carter DC, Miller WR, Cho-Chung YS, Clair T. Protein kinase A (PK-A) regulatory subunit expression in colorectal cancer and related mucosa. Br J Cancer. 1994;69(4):738–42. doi: 10.1038/bjc.1994.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.DeRubertis FR, Chayoth R, Field JB. The content and metabolism of cyclic adenosine 3’, 5’-monophosphate and cyclic guanosine 3’, 5’-monophosphate in adenocarcinoma of the human colon. J Clin Invest. 1976;57(3):641–9. doi: 10.1172/JCI108320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Polgar P, Vera JC, Kelley PR, Rutenburg AM. Adenylate cyclase activity in normal and leukemic human leukocytes as determined by a radioimmunoassay for cyclic AMP. Biochim Biophys Acta. 1973;297(2):378–83. doi: 10.1016/0304-4165(73)90085-8. [DOI] [PubMed] [Google Scholar]
- 38.Safitri D, Harris M, Potter H, Yan Yeung H, Winfield I, Kopanitsa L, et al. Elevated intracellular cAMP concentration mediates growth suppression in glioma cells. Biochem Pharmacol. 2020;174:113823. doi: 10.1016/j.bcp.2020.113823. [DOI] [PubMed] [Google Scholar]
- 39.Caldieri G, Sigismund S. Spatial resolution of cAMP signaling by soluble adenylyl cyclase. J Cell Biol. 2016;214(2):125–7. doi: 10.1083/jcb.201606123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Halls ML, Cooper DM. Regulation by Ca2+-signaling pathways of adenylyl cyclases. Cold Spring Harb Perspect Biol. 2011;3(1):a004143. doi: 10.1101/cshperspect.a004143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bond S, Forgac M. The Ras/cAMP/protein kinase A pathway regulates glucose-dependent assembly of the vacuolar (H+)-ATPase in yeast. J Biol Chem. 2008;283(52):36513–21. doi: 10.1074/jbc.M805232200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Christensen HL, Paunescu TG, Matchkov V, Barbuskaite D, Brown D, Damkier HH, et al. The V-ATPase is expressed in the choroid plexus and mediates cAMP-induced intracellular pH alterations. Physiol Rep. 2017;5(1) doi: 10.14814/phy2.13072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Van Dyke RW, Root KV, Hsi RA. cAMP and protein kinase A stimulate acidification of rat liver endosomes in the absence of chloride. Biochem Biophys Res Commun. 1996;222(2):312–6. doi: 10.1006/bbrc.1996.0741. [DOI] [PubMed] [Google Scholar]
- 44.Birtwistle MR, Kolch W. Biology using engineering tools: the negative feedback amplifier. Cell Cycle. 2011;10(13):2069–76. doi: 10.4161/cc.10.13.16245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Stock C, Pedersen SF. Roles of pH and the Na(+)/H(+) exchanger NHE1 in cancer: From cell biology and animal models to an emerging translational perspective? Semin Cancer Biol. 2017;43:5–16. doi: 10.1016/j.semcancer.2016.12.001. [DOI] [PubMed] [Google Scholar]
- 46.Lang MJ, Martinez-Marquez JY, Prosser DC, Ganser LR, Buelto D, Wendland B, et al. Glucose starvation inhibits autophagy via vacuolar hydrolysis and induces plasma membrane internalization by down-regulating recycling. J Biol Chem. 2014;289(24):16736–47. doi: 10.1074/jbc.M113.525782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yang Q, Tang J, Cao J, Liu F, Fu M, Xue B, et al. SARS-CoV-2 infection activates CREB/CBP in cellular cyclic AMP-dependent pathways. J Med Virol. 2023;95(1):e28383. doi: 10.1002/jmv.28383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kelley DJ, Bhattacharyya A, Lahvis GP, Yin JC, Malter J, Davidson RJ. The cyclic AMP phenotype of fragile X and autism. Neurosci Biobehav Rev. 2008;32(8):1533–43. doi: 10.1016/j.neubiorev.2008.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kolosionek E, Savai R, Ghofrani HA, Weissmann N, Guenther A, Grimminger F, et al. Expression and activity of phosphodiesterase isoforms during epithelial mesenchymal transition: the role of phosphodiesterase 4. Mol Biol Cell. 2009;20(22):4751–65. doi: 10.1091/mbc.E09-01-0019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kim J, Lee HY, Ahn J, Hyun M, Lee I, Min KJ, et al. NHX-5, an Endosomal Na+/H+ Exchanger, Is Associated with Metformin Action. J Biol Chem. 2016;291(35):18591–9. doi: 10.1074/jbc.C116.744037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Miller RA, Chu Q, Xie J, Foretz M, Viollet B, Birnbaum MJ. Biguanides suppress hepatic glucagon signalling by decreasing production of cyclic AMP. Nature. 2013;494(7436):256–60. doi: 10.1038/nature11808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pollak M. Overcoming Drug Development Bottlenecks With Repurposing: Repurposing biguanides to target energy metabolism for cancer treatment. Nat Med. 2014;20(6):591–3. doi: 10.1038/nm.3596. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Transcriptomic data have been deposited in ArrayExpress with accession numbers E-MTAB-13742 and E-MTAB-13746.