Abstract
Mendelian neurodevelopmental disorders caused by variants in genes encoding chromatin modification can be categorized as Mendelian disorders of the epigenetic machinery (MDEMs). These disorders have significant overlap in molecular pathways and phenotypes including intellectual disability, short stature, and obesity. Among the MDEMs is Kleefstra syndrome (KLFS), which is caused by haploinsufficiency of EHMT1. Preclinical studies have identified metabolic dysregulation and obesity in KLFS models, but proper clinical translation lacks. In this study, we aim to delineate growth, body composition, and endocrine-metabolic characteristics in a total of 62 individuals with KLFS. Our results revealed a high prevalence of childhood-onset overweight/obesity (60%; 28/47) with disproportionately high body fat percentage, which aligns perfectly with previous preclinical studies. Short stature was common (33%), likely due to advanced skeletal maturation. Endocrine-metabolic investigations showed thyroid dysregulation (22%; 9/41), elevated triglycerides, and decreased blood ammonia levels. Moreover, hand radiographs identified decreased bone mineralization (57%; 8/14) and negative ulnar variance (71%; 10/14). Our findings indicate a high (cardio)metabolic risk in KLFS. Therefore, we recommend monitoring of weight and endocrine-metabolic profile. Supporting a healthy lifestyle and screening of bone mineralization is advised. Our comprehensive results support translational research and contribute to a better understanding of MDEM-associated phenotypes.
Keywords: 9q34.3, body composition, EHMT1, growth, Kleefstra syndrome, MDEM, metabolism
1. Introduction
The understanding of genetic variants underlying neurodevelopmental disorders (NDDs) has improved tremendously over the past decade and the number of diagnosed patients still increases. Over 1500 genes have been identified as related to Mendelian NDDs (Kochinke et al., 2016; Maia et al., 2021). Among these are two prominent groups based on pathophysiology: those involved in gene expression regulation (such as chromatin remodelers and transcription factors) and those involved in neuronal communication (Satterstrom et al., 2020). To the first class belong the Mendelian disorders of the epigenetic machinery (MDEMs), caused by gene variants that affect chromatin modification. These disorders share phenotypic characteristics with each other and with some of the classical imprinting disorders, including intellectual disability, developmental delay, autism spectrum disorder, short stature, and obesity (Fahrner & Bjornsson, 2014; Kochinke et al., 2016).
Clinical data collection in MDEMs so far has been primarily focused on neurodevelopmental features and somatic malformations. However, features that are less clinically evident and require dedicated and specialized measurements (e.g., biochemical assays to diagnose insulin resistance) are still largely uncharacterized. Comprehensive and longitudinal examinations are rarely done due to the fragmented follow up of individuals with such rare disorders, resulting in limited systematic longitudinal data.
Kleefstra syndrome (KLFS; OMIM #610253) is among the MDEMs (Fahrner & Bjornsson, 2014) and is caused by haploinsufficiency of the gene euchromatin histone methyltransferase 1 (EHMT1), resulting from either 9q34.3 microdeletions or intragenic variants (Kleefstra et al., 2006). Key characteristics of KLFS include intellectual disability, developmental delay, and autism spectrum disorder. Short stature and obesity are commonly observed in KLFS (Kleefstra & de Leeuw, 2010; Willemsen et al., 2012) as well as in other MDEMs, such as Kabuki syndrome and Wiedemann-Steiner syndrome (Fahrner & Bjornsson, 2014). However, the exact prevalence of these features in KLFS and how they progress over time remain uncertain, as no significantly representative cohort has been studied.
In preclinical models of KLFS including mouse and Drosophila, both the EHMT1 ortholog termed G9a-like protein (GLP) and the EHMT2 ortholog G9a, play a crucial role in fat homeostasis (Kramer, 2016; Ohno et al., 2013). Studies in mouse have shown that targeted knockout of GLP in differentiating brown adipocytes impairs brown fat differentiation and leads to obesity and insulin resistance (Ohno et al., 2013). Drosophila G9a mutants also have increased fat tissue and triglyceride levels, illustrating that metabolic regulation by the evolutionary conserved EHMT1/GLP/G9a protein family is not limited to brown fat (Riahi et al., 2019). G9a mutants also have increased glycogen stores, however, upon stress such as oxidative stress of infection, the Kleefstra fly model experiences an exaggerated metabolic shift that quickly exhausts sugar stores while fat stores cannot be assessed efficiently, causing reduced stress tolerance (Riahi et al., 2019). Whereas the metabolic dysregulation in response to stressors appears to be conserved in mammalian systems (Riahi et al., 2021), the clinical relevance of these findings remains unclear since no corresponding studies in a representative patient cohort have been performed to date.
Our aim therefore was to provide comprehensive insights into growth, body composition, and endocrine-metabolic profiles in KLFS. This systematic data collection is crucial for guiding patient management and studying potential clinical biomarkers. These biomarkers can also serve as outcome measures contributing to better diagnoses and as readouts for clinical trials. Ultimately, our study could guide both preclinical and clinical work into other MDEMs and contributes to the understanding of common phenotypes and underlying pathophysiological mechanisms in other MDEMs.
2. Methods
Prospective and retrospective data on growth, body composition, and biochemical investigations were collected from individuals with a molecular confirmed diagnosis of KLFS, known in the Radboud University Medical Center (Radboudumc) expert center for rare genetic NDDs. Individuals born before 32 weeks of gestational age were excluded. The project was approved by the Medical Ethics Committee of the Radboudumc, Nijmegen, The Netherlands (#2021-13018; #2021-13114; #2023-16406). Retrospective data were extracted from the Radboudumc biobank Genetics and Rare disease (#2018-4985).
2.1. Growth parameters
Growth parameters were measured during individuals' Radboudumc outpatient clinic visit and were retrospectively assessed by review of medical records and growth booklets of the Dutch municipal health service, known as GGD. Included parameters were height, weight, head circumference (HC), mid-upper arm circumference (MUAC), waist circumference (WC), and hip circumference (HipC). During participants' outpatient visit, a comprehensive medical history and physical examination was conducted.
Only individuals of Dutch, Moroccan, Turkish, and Hindustani descent were included in the analyses to allow for comparison with sex-, age-, and ethnicity-matched references. The references include the outcomes from the latest Fifth Dutch Growth study: height for Dutch descent (Schonbeck et al., 2013), height for Moroccan and Turkish descent (Schonbeck et al., 2015), height for Hindustani descent (de Wilde et al., 2015), weight and body mass index (BMI, kg/m2) (Schonbeck et al., 2011), HC (Fredriks et al., 2000), MUAC, WC, and HipC (Gerver & Bruin, 2001). Z-scores of measurements taken before 24 months of age were corrected for gestational age if born before 37 weeks (Bocca-Tjeertes et al., 2012). We collected parental height information anamnestically through our survey (Supplementary File 1). Target height was calculated, accounting for sex and ethnicity, when the height of both parents was known (van Zoonen, 2019).
Data on clinical conditions that can affect growth, such as prenatal stressors, congenital disorders, food-related behavior, sports, and familial factors, were collected through a parent survey. This survey also included the request and explanation for performing body circumference measurements (HC, MUAC, WC, and HipC) (Supplementary File 1). HC was measured at the broadest point of the head, just above the eyebrows and ears. MUAC was measured with the arm in relaxed state, at the midpoint between the shoulder and elbow. WC was measured in an upright standing position, taken between the lower rib and the top of the iliac crest following a normal exhalation. HipC was determined as the widest circumference at the hip level. Body circumference measurements provided by parents were included only when measurements performed by medical professionals were not available. Overall, growth measurements were conducted by medical professionals at 89% (448/503) of the time points.
Up to one growth measure per month was included from birth until the age of 1 year. From age 1 until 21 years, a maximum of one measure per 6 months was included. If multiple measurements were available per defined period, the first one was included. For individuals above 21 years of age, only the most recent measurement was included. Measurements taken while using antipsychotic drugs were excluded for weight and BMI calculations. The following definitions were used: short stature = height Z ≤ −2, microcephaly = HC Z ≤ −2, overweight = BMI Z ≥ 1, obesity = BMI Z ≥ 2.
2.2. Body composition
Body composition was evaluated using bioelectrical impedance analysis (BIA; InBody 770 Body Composition Analyzer). BIA measurement was performed while lightly dressed, after at least 3 h of fasting, and after toileting. BIA measurement and/or dual-energy X-ray absorptiometry scan results from routine clinical care (DEXA; Hologic Discovery A, software Horizon A 13.6.1.2) were used to evaluate body fat percentage (BF%), skeletal muscle mass (SMM), bone mineral density, and basal metabolic rate. BF% was considered to be in the normal range when body fat (kg) was above the 15%–23% of body weight (kg) (-Malina, 1988; Tahara et al., 2002; Wohlfahrt-Veje et al., 2014). Appendicular SMM of our individuals were compared to sex- and age-matched controls (McCarthy et al., 2014). Basal metabolic rate was estimated by use of the Schofield equation (Schofield, 1985).
2.3. Radiological characteristics
As our findings showed striking trends in growth and body composition, we decided to conduct additional posteroanterior hand radiographs. This was done to enhance the interpretation and understanding of growth patterns and bone metabolism. Hand radiographs were made during patients' regular outpatient visit and retrospectively collected from medical records. Skeletal age and bone health index (BHI) were evaluated using the software BoneXpert 3.2.2 which includes sex- and age-matched reference data (BoneXpert, n.d.; Thodberg et al., 2016). Radiologist WK measured ulnar variance using the Hafner DIDI method below age 14 years and the adapted perpendicular method above age 14 years (Kox et al., 2020) and evaluated if any further ossal abnormalities were present.
2.4. Endocrine-metabolic investigations
Biochemical investigations were performed during routine clinical care, or retrospective data were collected from medical records if blood sampling was not done at the Radboudumc in years 2021/2022. Only individuals' most recent measure was included, excluding measurements taken while using vitamin D, B12, or folic acid supplementation. All measurements were compared to sex- and age-matched controls (Supplementary File 2) (de Vries, 2015).
The analyzed biochemical markers were selected for their association with endocrine-metabolic dysregulation or their potential to affect endocrine-metabolic function. Sampling was performed through venipuncture. Investigations included hemoglobin (mmol/L), ALAT (U/L), thyroid function (free thyroxine 4 [fT4] pmol/L, thyroid-stimulating hormone [TSH] mIU/L), lipid profile (triglycerides mmol/L, cholesterol mmol/L, high-density lipoprotein cholesterol [HDL] mmol/L, low-density lipoprotein cholesterol [LDL] mmol/L), glucose (mmol/L), HbA1c (mmol/mol), lactate (mmol/L), pyruvate (μmol/L), LDH (U/L), 25-hydroxy vitamin D (25(OH)D, nmol/L), vitamin B12 (pmol/L), folic acid (nmol/L), ammonia (μmol/L), and creatinine (μmol/L). Samples of lactate, pyruvate, and ammonia were stored on ice until processing. Supplementary File 2 shows the used biochemical equipment.
Biochemical results of individuals with KLFS were compared to sex- and age-matched healthy controls who received a blood sampling in the Radboudumc in the past. The controls were anonymously identified and their biochemical results were collected using the software CTcue and Cliniquest.
Statistical analyses were performed using SPSS version 25 and GraphPad Prism 9, including unpaired T-tests, one-way ANOVA, and regression analyses. A p-value <0.05 was considered as significant.
3. Results
In this study, we included 62 individuals (23 males, 39 females) with KLFS. The mean age at inclusion was 18.96 years (range 2.0–57.0 years). KLFS was caused by a pathogenic variant in the EHMT1 gene in 38 individuals and by a 9q34.3 deletion in 24 individuals. Patient characteristics and genotypes are included in Supplementary File 3.
3.1. Growth parameters
Gestational age was known for 51 individuals, with a median gestational age of 40.0 weeks (IQR 3.57). Premature birth (<37 weeks) occurred in 10% of individuals (5/51), while 16% were born post-term (≥42 weeks, 8/51).
To optimize comparability with national growth charts, growth parameters were analyzed in Dutch individuals with a EHMT1 pathogenic variant or 9q34.3 deletion smaller than 1.3 Mb. A cut-off of 1.3 Mb was chosen since no individuals in this study had a deletion between 1.3 and 2 Mb.
Subsequently, a cohort of 48 individuals (17 males, 31 females) was established, with a mean age of 20.5 years (range 2.3–57.0 years) at inclusion. At birth, mean weight Z-score of this cohort was −0.35 ± 1.01, corrected for gestational age. At the time of inclusion, mean height Z-score was −0.69 ± 1.37 (N = 48), mean BMI Z-score was 1.22 ± 1.35 (N = 47), and mean HC Z-score was −1.34 ± 1.29 (N = 42). Growth parameters at birth and throughout life are shown in Table 1, Figure 1, and Figure S1.
Table 1. Overview of longitudinal growth data of Dutch participants with a EHMT1 pathogenic variant or 9q34.3 deletion <1.3 Mb.
| Age (years) |
Height Z- score ± SD |
N | Head circumference Z- score ± SD |
N | Weight Z- score ± SD |
N | BMI Z- score ± SD |
% overweight |
% obesity |
N | Waist circumference Z- score ± SD |
N | Hip circumference Z-score ± SD |
N |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Birth | –0.99 ± 0.80 | 24 | –0.64 ± 1.51 | 11 | –0.19 ± 1.11 | 37 | - | - | - | - | - | - | - | - |
| 0–2 | –0.27 ± 0.95 | 173 | –1.11 ± 1.24 | 119 | –0.20 ± 1.26 | 167 | –0.03 ± 1.02 |
11 | 3 | 157 | - | - | - | - |
| 2–6 | –0.15 ± 0.94 | 76 | –0.85 ± 1.16 | 21 | 0.34 ± 1.11 | 70 | 0.64 ± 1.08 |
34 | 6 | 68 | 1.54 ± 0.70 | 6 | 1.20 ± 0.57 | 6 |
| 6–10 | –0.08 ± 1.01 | 35 | –0.18 ± 1.27 | 15 | 0.98 ± 1.50 | 38 | 1.17 ± 1.23 |
37 | 23 | 30 | 1.60 ± 1.91 | 5 | 0.91 ± 1.89 | 5 |
| 10–14 | –0.38 ± 1.33 | 31 | –0.66 ± 1.03 | 14 | 0.88 ± 1.59 | 27 | 1.35 ± 1.29 |
38 | 31 | 26 | 2.34 ± 1.49 | 5 | 1.39 ± 1.25 | 5 |
| 14–18 | –0.65 ± 1.32 | 26 | –0.94 ± 1.14 | 4 | 1.30 ± 1.75 | 21 | 1.90 ± 1.34 |
19 | 52 | 21 | 1.87 ± 1.24 | 3 | 0.72 ± 0.71 | 3 |
| 18–21 | –1.02 ± 1.22 | 13 | –1.95 ± 0.81 | 6 | 1.60 ± 2.19 | 14 | 1.89 ± 1.71 |
8 | 58 | 12 | 2.96 ± 1.81 | 3 | 1.23 ± 1.86 | 3 |
| >21 | –1.59 ± 1.17 | 18 | –2.14 ± 1.41 | 16 | –0.03 ± 1.58 | 18 | 0.83 ± 1.41 |
39 | 17 | 18 | 1.61 ± 1.64 | 14 | 0.59 ± 1.25 | 14 |
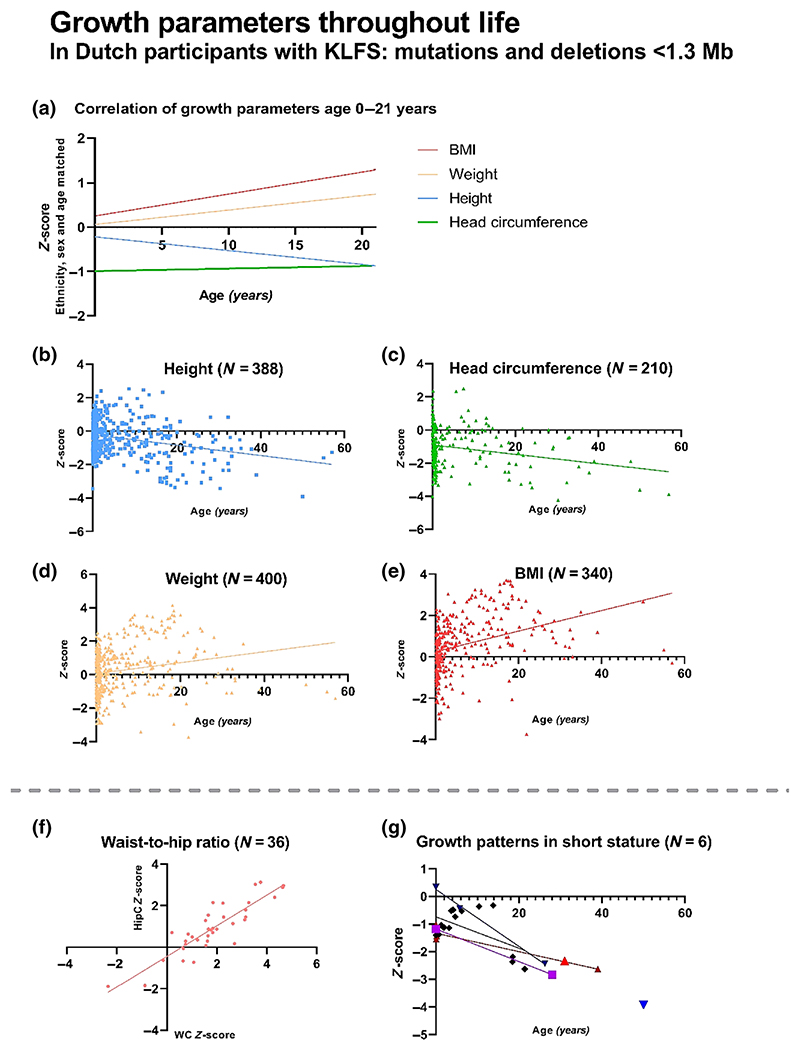
Figure 1.
Growth parameters in 48 Dutch participants with Kleefstra syndrome (KLFS) caused by a EHMT1 pathogenic variant or 9q34.3 deletion <1.3Mb. Graphs A. and B. show decrease in height Z-scores throughout childhood and adolescence. Graphs A. and C. show that the mean head circumference is lower than the population average, but remains stable over time. Graphs A., D., and E. show an increase in weight and BMI throughout childhood and adolescence. Graph F. shows a significant increase in waist circumference (WC) compared to hip circumference (HipC). Graph G. shows a large decrease in height Z-score in individuals who have a short stature above the age of 21 years.
Mean height at inclusion of the cohort was smaller than average. This is also observed by comparison with the individuals' target height. Parental height was available in 37 individuals, showing that 68% of KLFS individuals had a height below their expected target height (25/37) at their last measure. Additionally, height was below the target height range in 11% of individuals (4/37). Height measurements in individuals above 21 years old indicated a short stature in 33% (6/18).
BMI gradually increased during childhood and adolescence. At inclusion, 38% of individuals had a normal weight (18/47), 32% were over-weight (15/47), and 28% were obese (13/47); one individual was underweight (2%, age 21.9 years). The mean BMI Z-score of males and females was 1.11 and 1.27, respectively (p = 0.70). In the age category of 2–12 years, 33% were overweight (10/30) and 23% were obese (7/30). Three individuals below 21 years were using antipsychotic drugs at the time of one measurement, at which point they all had a normal BMI.
Head circumference was generally decreased compared to sex- and age-matched controls. Above the age of 21 years, 56% of individuals (9/16) had a microcephaly.
In correlation analyses, no significant differences were found for birthweight, height, BMI, and HC between males and females or between individuals with EHMT1 variants and deletions smaller than 1.3 Mb. However, when this cohort was compared to individuals with deletions over 2 Mb (N = 6, range 2.1–3.2 Mb), the results indicated smaller height (mean Z-score −2.04 ± 1.62) and lower BMI (mean Z-score 0.39 ± 1.72) within the larger deletion group (height p = 0.03; BMI p = 0.17).
Several clinical conditions that might affect growth were identified (all results are included in Supplementary File 4). A congenital heart defect was present in 34% (13/38) of the individuals. Hyperphagia (50%; 16/32) and appetite for non-food substances (31%; 10/32) were common. Only a minority of the individuals exercised at least 1 h a day (33%; 12/36). Interestingly, none of the individuals’ siblings were overweight or obese. Hypertension, diabetes, or menstruation occurring before the age of 10 years were not reported.
3.2. Body composition
Body circumference measurements were performed in 36 individuals and compared to sex- and age-matched controls. As shown in Table 1 and Figure 1, the results indicate significantly increased waist circumference (WC, Z-score 1.84 ± 1.49) compared to hip circumference (HipC, Z-score 0.91 ± 1.24), p = 0.006. The mean WC/height ratio was increased (0.54 ± 0.07). Mid-upper arm circumference (MUAC) measurements compared to sex- and age-matched controls were above the p90 in 72% of our individuals (26/36), while 6% (2/36) had an MUAC below the p10.
A BIA and/or DEXA scan was performed in seven female individuals to evaluate body fat percentage (BF%), skeletal muscle mass (SMM), and basal metabolic rate (Table 2). The amount of body fat (kg) was above the 15%–23% of body weight (kg) in all these individuals, resulting in a disproportionally high BF% for weight. Fat percentage was also above average in the three (3/7) individuals with a normal BMI.
Table 2. Results of body composition measurements, investigated by BIA and/or DEXA scan.
| Individual (study ID) | 19 | 32 | 45 | 52 | 53 | 58 | 99 | |
|---|---|---|---|---|---|---|---|---|
| Baseline | Age | 15.4 | 12.8 | 4.9 | 7.5 | 11.4 | 8.8 | 13.3 |
| Weight (kg) | 55.7 | 44.2 | 18.4 | 37 | 62.8 | 50.3 | 72.1 | |
| BMI (kg/m2) | 22.19 | 18.44 | 16.1 | 21.6 | 26.6 | 24.6 | 27.68 | |
| BMI Z-score | 0.98 | 0.23 | 0.2 | 2.8 | 2.8 | 3.23 | 2.65 | |
| BIA | BF% | 33.5 | - | - | 35.5 | 38 | 42.7 | 35.6 |
| Visceral fat area (cm2) | 88.4 | - | - | 69.6 | 123.9 | 128.8 | 125.8 | |
| SMM total (kg) | 20 | - | - | 12 | 20 | 15.1 | 25.6 | |
| SMMa(kg)a | 14.15 | - | 7.71 | 11.97 | 13.77 | 18.79 | ||
| SMMa (kg) in healthy individualsb | 16.2 ± 2.6 | - | - | 6.1 ± 1.4 | 12.8 ± 3.0 | 9.1 ± 2.5 | 12.8 ± 3.0 | |
| SMMa (% SMMa of weight) | 25.4 | - | - | 20.8 | 19.1 | 27.4 | 26.1 | |
| SMMa (%) in healthy individualsb | 29.6 ± 3.8 | - | - | 27.1 ± 2.4 | 28.3 ± 4.3 | 27.1 ± 4.2 | 28.3 ± 4.3 | |
| Skeletal muscle index (kg/m2) | 5.7 | 4.5 | 6.1 | 5.8 | 7.2 | |||
| DEXA | BF% | - | 37.6 | 39 | - | 44.2 | - | - |
| Visceral fat area (cm2) | - | 64.9 | 21.5 | - | 107 | - | - | |
| Lean mass/height2 Z-score | - | –2.3 | - | - | 0.4 | - | - | |
| Skeletal muscle index (kg/m2) | 4.37 | 2.64 | 5.91 |
Abbreviations: BF%, body fat percentage; BIA, bioelectrical impedance analysis; DEXA, dual-energy X-ray absorptiometry; SMMa, appendicular skeletal muscle mass.
Appendicular skeletal muscle mass: skeletal muscle mass of arms and legs.
Sex- and age-matched healthy controls, based on results of McCarthy et al. (2014).
Moreover, appendicular skeletal muscle mass (SMMa) was increased in 3/5 individuals with KLFS compared to matched controls. However, when SMMa was calculated as a percentage of weight, SMMa was as average in 2/5 individuals and below average in 3/5 individuals. One individual had a decreased lean mass (Z-score −2.3) as identified by DEXA scan. In individuals who underwent BIA-measurement, the basal metabolic rate (mean 1121 kcal, range 886–1374 kcal/day) was below the estimations of the Schofield equation.
3.3. Radiological characteristics
A radiograph of the left hand was performed in 14 individuals who all had a height SDS in the normal range, with the aim to assess skeletal age, BHI, and ulnar variance (Table 3). The mean calendar age of the individuals was 9.2 years (range 3.0–20.0 years). Skeletal age was increased compared to calendar age, with a mean standard deviation score (SDS) of skeletal age of 0.5 ± 1.2.
Table 3. Radiological bone parameters investigated by X-hand, BIA, and DEXA scan.
| X-hand | BIA | DEXA | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Age | Sex | Height SDS |
Skeletal age |
Skeletal age SDS (compared to calendar age) |
Bone health index |
Bone health index SDS |
Negative ulnar variance, yes/no (mm) |
Ulnar variance (mm) in healthy individualsa |
Bone mineral content (kg) compared to BIA reference range for sex and age |
Bone mineral density: lumbar vertebrae (Z-score) |
Bone mineral density: femoral neck (Z-score) |
||
| 3.0 | M | –1.15 | 2.9 | –0.3 | 3 | –1.5 | No (–3.5) | –2.0 ± 1.8 | - | - | - | ||
| 4.1 | F | –1.12 | 3.3 | –1.3 | 3.4 | –1.2 | Yes (–3.6) | –2.1 ± 1.4 | - | –2.6 | –4.7 | ||
| 4.2 | F | –0.57 | 5.1 | 1.3 | 3.6 | –1.7 | Yes (–5.7) | –2.1 ± 1.4 | - | - | - | ||
| 4.6 | F | –0.22 | 4.4 | –0.5 | 4.2 | 0.0 | Yes (–4.6) | –2.1 ± 1.4 | - | - | - | ||
| 4.9 | F | –0.99 | 4.7 | –0.4 | 3.4 | –2.2 | No (–3.4) | –2.1 ± 1.4 | - | 0.2 | - | ||
| 5.0 | F | 0.07 | - | - | - | - | - | - | –0.4 | - | |||
| 7.5 | F | 0.44 | 8.1 | 0.7 | 4.4 | –0.3 | Yes (–6.1) | –2.3 ± 1.3 | 1.4 (high) | 1.1 | -0.3 | ||
| 8.8 | F | 1.22 | 10.4 | 1.9 | 4.3 | –2.8 | Yes (–7.9) | –2.7 ±2.5 | 1.4 (low) | - | - | ||
| 9.5 | F | 0.07 | 11.9 | 2.9 | 4.4 | –1.4 | Yes (–4.8) | – 1.9 ± 1.4 | - | - | - | ||
| 9.7 | M | –1.05 | 10.3 | 0.9 | 4.2 | –1 | Yes (–3.8) | –1.6 ± 1.8 | - | - | - | ||
| 10.4 | F | –0.02 | 9.4 | –0.6 | 4.5 | –0.2 | Yes (–8.0) | –1.9 ± 1.5 | - | - | - | ||
| 11.0 | F | 0.00 | - | - | - | - | - | 2.2 (high) | 1.8 | 2.4 | |||
| 12.8 | F | –0.70 | 12.1 | –0.5 | 5.3 | 0.1 | Yes (–4.4) | –1.0 ±2.0 | - | –0.2 | –1.6 | ||
| 13.7 | F | –0.15 | 14.9 | 1.3 | 5.0 | –1.0 | No (–2.3) | – 1.8 ± 1.7 | 2.6 (high) | - | - | ||
| 15.5 | F | –0.17 | 16.4 | 1.0 | 5.9 | 1.3 | No (–2.6) | –1.0 ± 1.8 | 2.3 (normal) | ||||
| 20.0 | F | –0.07 | 17.5 | N/A | 5.7 | 0.1 | Yes (–5.0) | – 1.4 ± 1.2 | - | - | - | ||
Abbreviations: BIA, bioelectrical impedance analysis; DEXA, dual-energy X-ray absorptiometry; SDS, standard deviation score.
Matched for method, sex, and skeletal age (Kox et al., 2020).
The BHI SDS was in 57% of individuals below −1 SDS (8/14; mean −0.8 ± 1.1 SDS). In a 4-year-old female with a BHI SDS of −1.2 and sclerotic bone lesions based on the X-hand, a DEXA scan was performed which revealed osteoporosis.
Negative ulnar variance (shortness/hypoplastic aspect of the distal ulna) was present in 71% of individuals (10/14). No other ossal abnormalities were observed in any of the individuals.
3.4. Endocrine-metabolic investigations
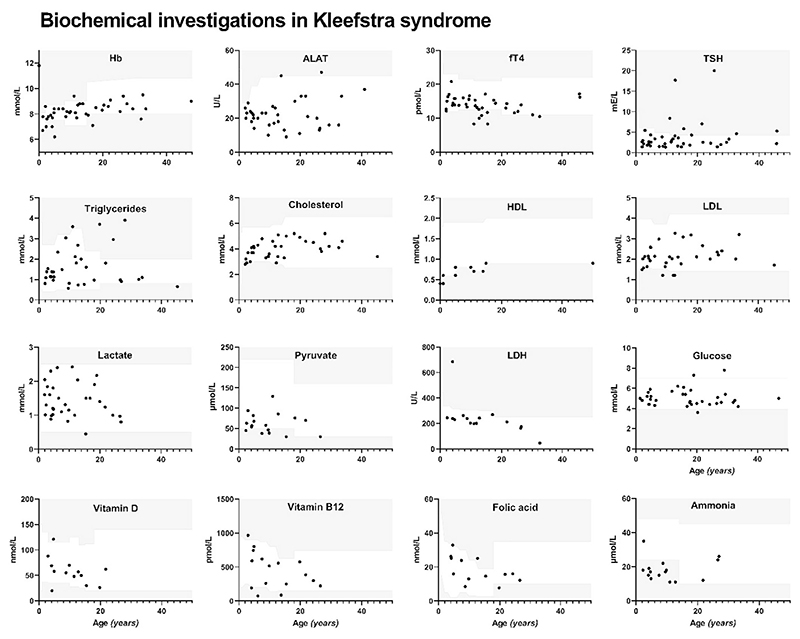
Biochemical investigations were conducted in 44 individuals with KLFS, including tests for hemoglobin, ALAT, thyroid function, lipid profile, lactate, pyruvate, LDH, glucose, HbA1c, 25(OH)D, vitamin B12, folic acid, ammonia, and creatinine. Results are presented in Table 4 and Figure 2.
Table 4. Comparison of endocrine-metabolic biochemical results between our cohort and healthy controls.
| Kleefstra syndrome | Healthy controls | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Measurement | N (male %) | Mean age (range) | Mean ± SD | % increase (N)a | % decrease (N)a | N (male %) | Mean age (range) | Mean ± SD | |
| Hemoglobin | 41 (39%) | 13.8 (0–48) | 8.3 ± 0.9 mmol/L | 2% (1/41) | 7% (3/41) | 33 (45%) | 15.5 (5–25) | 8.5 ± 2.5 mmol/L | |
| ALAT | 36 (36%) | 14.6 (2–41) | 23 ± 9 U/L | 3% (1/36) | - | 26 (50%) | 16.0 (2–25) | 19 ± 15 U/L | |
| fT4 | 39 (44%) | 13.9 (2–46) | 13.9 ± 2.6 μmol/L | - | 18% (7/39) | ||||
| TSH | 41 (41%) | 14.6 (2–46) | 3.81 ± 3.80 mIU/L | 20% (8/41) | - | 31 (48%) | 14.9 (4–25) | 2.66 ± 1.49 mE/L | |
| Triglycerides** | 33 (36%) | 13.9 (2–45) | 1.62 ± 0.93 mmol/L | 15% (5/33) | 3% (1/33) | 34 (47%) | 14.3 (2–25) | 1.01 ± 0.49 mmol/L | |
| Cholesterol | 34 (35%) | 14.4 (2–45) | 4.0 ± 0.7 mmol/L | - | 6% (2/34) | 31 (42%) | 14.5 (2–25) | 3.75 ± 0.71 mmol/L | |
| HDL | 33 (36%) | 13.9 (2–45) | 1.16 ± 0.24 mmol/L | - | - | 33 (42%) | 14.4 (2–25) | 1.23 ± 0.34 mmol/L | |
| LDL | 33 (36%) | 13.9 (2–45) | 2.13 ± 0.57 mmol/L | - | 12% (4/33) | 33 (42%) | 14.4 (2–25) | 2.04 ± 0.70 mmol/L | |
| Lactate (venous blood gas) | 13 (54%) | 11.3 (2–27) | 1.51 ± 0.47 mmol/L | - | - | 35 (60%) | 16.5 (9–25) | 1.76 ± 0.97 mmol/L | |
| Lactate | 19 (26%) | 9.9 (2–27) | 1.36 ± 0.54 mmol/L | - | 5% (1/19) | ||||
| Pyruvate | 17 (24%) | 9.8 (2–27) | 63 ± 26 μmol/L | - | 29% (5/17) | 35 (63%) | 17.0 (9–25) | 61 ± 39 μmol/L | |
| L/P ratio | 17 (24%) | 9.8 (2–27) | 20.64 ± 5.67 | 29% (5/17) | - | ||||
| LDH | 15 (27%) | 13.4 (2–33) | 241 ± 134 U/L | 7% (1/15) | - | 23 (57%) | 17.8 (9–25) | 182 ± 51 U/L | |
| Fasting glucose | 14 (29%) | 23.4 (1–47) | 4.9 ± 0.7 mmol/L | - | 7% (1/14) | ||||
| Non-fasting glucose | 22 (32%) | 12.4(1–32) | 5.2 ± 0.9 mmol/L | 34 (53%) | 15.6 (2–25) | 5.37 ± 2.6 mmol/L | |||
| HbAlC | 20 (30%) | 13.7 (2–34) | 34 ± 3 mmol/moL | - | - | ||||
| 25(OH)D | 12 (25%) | 10.8 (3–22) | 53 ± 20 nmol/L | - | 25% (3/12) | 35 (49%) | 14.0 (2–25) | 63.4 ± 31.8 nmol/L | |
| Vitamin B12 | 16(19%) | 11.7 (3–27) | 446 ± 265 pmol/L | 13% (2/16) | - | 40 (53%) | 14.2 (3–25) | 440 ± 220 pmol/L | |
| Folic acid | 13 (8%) | 12.7 (4–27) | 18.2 ± 7.7 nmol/L | - | 8% (1/13) | 39 (54%) | 13.9 (3–25) | 24.0 ± 13.7 nmol/L | |
| Ammonia**** | 15 (20%) | 10.5 (2–27) | 18 ± 6 μmol/L | - | 73% (11/15) | 34 (44%) | 14.8 (2–25) | 34 ± 12 μmol/L | |
| Creatinine | 26 (38%) | 17.4 (2–45) | 59 ± 23 μmol/L | 15% (4/26) | 34 (47%) | 15.7 (5–25) | 50 ± 25 μmol/L | ||
Note: This table shows the results of biochemical endocrine-metabolic investigations in Kleefstra syndrome (KLFS) compared to sex- and age-matched healthy controls. The whole standardized set of biochemical investigations could not be evaluated in all participants; the second column shows the number of studied KLFS participants. In KLFS, triglycerides were significantly increased (p < 0.005) and ammonia was significantly decreased (p < 0.0001) compared to matched controls.
Compared to sex- and age-matched reference values (Supplementary File 3).
p < 0.005.
p < 0.0001.
Figure 2.
Results of biochemical endocrine-metabolic evaluations and its distribution per age in Kleefstra syndrome (KLFS). The white area in the graphs shows the reference range for sex and age in the healthy population.
Thyroid investigations were performed in 41 individuals (17 males, 24 females) with the mean age of 14.6 years (range 2.0–45.8 years). Mean fT4 was 13.9 ± 2.6 pmol/L (N = 39) and mean TSH was 3.81 ± 3.80 mIU/L (N = 41). Thyroid dysregulation was observed in 22% (9/41) of individuals and classified as follows:
1. Subclinical hypothyroidism
Six individuals had (sub)normal fT4 levels (10.5–16.2 pmol/L) and elevated TSH levels (4.29–7.46 mIU/L). Four of the six individuals had a normal BMI, while two had obesity. Levothyroxine treatment was initiated in two individuals at ages 2.5 and 32.7 years.
2. Thyroiditis
One individual, aged 12.8 years, was diagnosed with thyroiditis. TSH was elevated (17.7 mIU/L) while fT4 was in the low-normal range (10 pmol/L). TPO and TSH-receptor antibodies were absent. Thyroid ultrasound showed a pattern suggestive for thyroiditis (granulomatous reflection pattern with lobulated contours and some hyperemia). Levothyroxine treatment was started.
3. Auto-immune thyroiditis
One individual, aged 15.5 years, was diagnosed with auto-immune thyroiditis with TPO (thyroid peroxidase) antibodies. Levothyroxine treatment was prescribed, but the individual showed persistent hypothyroidism at age 20 (fT4 5.4 pmol/L, TSH 17.3 mIU/L, TPO antibodies 224 U/mL), likely due to non-compliance with therapy.
4. Central hypothyroidism
One individual, aged 11.0 years, was diagnosed with isolated central hypothyroidism based on low fT4 (8.3 pmol/L) and elevated TSH (8.38 mIU/L). MRI ruled out pituitary gland malformation. A subsequent measure showed comparable values after which levothyroxine was prescribed and values normalized (fT4 16.8 pmol/L, TSH 1.3 mIU/L).
We retrospectively analyzed MRI cerebrum results of 19 individuals from prior clinical care. While a pituitary cyst was identified in one individual, no pituitary gland abnormalities were found in the remaining individuals.
Lipid spectrum analyses were performed in 33 individuals, including 23 children. Triglycerides were significantly increased in KLFS (mean 1.62 ± 0.93 mmol/L) compared to healthy controls (mean 1.01 ± 0.49 mmol/L), p < 0.005. Two children with a normal BMI showed triglyceride levels above the reference range for age and sex (6.81 and 3.59 mmol/L, respectively), which persisted during follow-up.
Simultaneous measurements of fasting glucose and insulin levels were obtained from three females (ages 26.6, 17.7, and 21.8 years). All participants had normal glucose levels, but the last two individuals showed elevated insulin levels (15.4 mIU/L with a glucose level of 4.6 mmol/L, and 24 mIU/L with a glucose level of 4.7 mmol/L). These findings are indicative of insulin resistance, as revealed by HOMA-IR values of 3.2 and 5.0, respectively.
Blood ammonia levels were found to be significantly lower in KLFS compared to healthy controls (18 ± 6 μmol/L and 34 ± 12 μmol/L respectively, p < 0.0001), with 73% (11/15) of individuals with KLFS exhibiting results below the reference range. Ammonia levels were decreased independent of age (r = 0.005).
Vitamin D levels were evaluated in 12 individuals and 25% (3/12) were found to have a vitamin D level ≤30 nmol/L (females, aged 4.2, 15.4, and 19.8 years) after which supplementation was started. Two out of three individuals with vitamin D deficiency had a BMI within the normal range (BMI Z-score between −1 and 1). Five individuals above the age of 4 years were excluded from analyses due to vitamin D supplementation.
One participant had a folic acid deficiency (1/13; female, aged 19.8 years). In another participant (female, aged 26.6 years) the results of homocysteine and methylmalonic acid indicated a folate deficiency, despite having normal folic acid levels.
4. Discussion
In this first comprehensive study on growth, body composition, and endocrine-metabolic status in Kleefstra syndrome (KLFS), we collected data from a relatively large rare disease cohort comprising 62 individuals. Our obtained insights might be relevant to optimize health surveillance, understand pathogenic mechanisms, support translational research, and determine treatment targets (McPartland, 2016). We introduced comprehensive study methods that can be performed within regular clinical care and applied to characterize other MDEMs as well.
4.1. BMI and body composition
We collected growth parameters of Dutch individuals with KLFS, which allowed comparison with ethnicity-matched controls. Our results showed a high prevalence of individuals who were overweight or obese (overall 59%), consistent with previous reports on KLFS (Kleefstra & de Leeuw, 2010; Willemsen et al., 2012) and other MDEMs (Fahrner & Bjornsson, 2014). Interestingly, we found gradual increase in BMI during childhood and adolescence and 56% of individuals who were overweight or obese in the age category of 2–12 years. This 56% prevalence greatly exceeds the 15% prevalence in the age-matched Dutch population (Centraal Bureau voor de Statistiek, 2022). Furthermore, although a previous Dutch study found increased risk and high rates of overweight (23%–25%) and obesity (10%–15%) in children aged 2–18 years with intellectual disability (Wouters et al., 2020), our study also shows significantly higher rates in individuals with KLFS compared to this population.
Interestingly, hyperphagia was frequently present (50%), while overweight in siblings was absent. Moreover, our results showed disproportionally high body fat percentages (Wohlfahrt-Veje et al., 2014), truncal fat distribution and a waist circumference/height ratio above 0.5. Taken together, these results mark a high (cardio)metabolic risk in KLFS (Browning et al., 2010) and indicate that overweight/obesity in KLFS is a consequence of haploinsufficiency of the chromatin regulator EHMT1.
The high body fat percentage perfectly matches previous preclinical studies in mouse and Drosophila models of KLFS (Kramer, 2016; Ohno et al., 2013; Riahi et al., 2019). In the latter, the authors demonstrated normal food intake and limited access to lipid stores upon conditions of increased energy demands (Riahi et al., 2019), strongly suggesting that the high prevalence of obesity is a direct consequence of evolutionary conserved transcriptional and metabolic dysregulation that occurs upon deficiency of members of the EHMT protein family (Riahi et al., 2019; Riahi et al., 2021).
Body composition analyses showed that appendicular skeletal muscle mass (SMMa) in KLFS was either as average or below average when compared to controls (McCarthy et al., 2014). Previous findings in the Kleefstra mouse model (Ohno et al., 2013) have demonstrated that the loss of EHMT1 in brown adipose tissue and its consequent H3K9 demethylation of the muscle-selective gene promoters induce muscle differentiation (Ohno et al., 2013). Whether this mechanism is also present in brown adipose tissue derived from KLFS individuals needs to be confirmed but our initial data does not support this observation. Our findings are in line with the typically observed correlation between increased BMI and decreased SMM in healthy individuals, Down syndrome, and Prader-Willi syndrome (Abramowitz et al., 2018; Khan et al., 2018; Lin & Wuang, 2012). Therefore, decreased muscle mass in KLFS could serve as an additional risk factor for the development of overweight.
4.2. Growth and radiological characteristics
At maturity, short stature and microcephaly were present in 33% and 56% of individuals, respectively. These findings are consistent with previous reports on MDEMs, associating short stature and/or microcephaly with 58% (41/70) of the MDEMs, including Kabuki syndrome and Wiedemann-Steiner syndrome (Fahrner & Bjornsson, 2014, 2019). Although in some MDEMs the mechanisms underlying growth retardation have been explored (e.g., altered balance between cellular proliferation and differentiation), the exact mechanisms are unclear (Fahrner & Bjornsson, 2019).
In KLFS, various mechanisms may contribute to short stature. Our findings indicate that individuals with a higher BMI during childhood were more likely to have an advanced skeletal age, resulting in adult height being reached at younger ages. Conversely, individuals with a normal BMI could reach average height. Based on our growth data, individual growth patterns, and skeletal ages, we conclude that advanced skeletal maturation appears likely to be the primary cause of short stature in KLFS.
In several MDEMs, short stature is associated with an insufficient growth spurt (Schott, Blok, et al., 2016) and/or growth hormone deficiency (Schott, Blok, et al., 2016; Schott, Gerver, & Stumpel, 2016; Sheppard et al., 2021). However, we observed a sufficient growth spurt in most individuals with KLFS. Based on the individual growth charts of our participants, we consider IGF-1 deficiency unlikely in KLFS. Further work is crucial to expand data collection on growth parameters to validate if our results are internationally consistent and to further explore underlying mechanisms of short stature. Additionally, it is essential to develop syndrome-specific growth charts to monitor health and disease in clinical practice.
Radiological investigations showed decreased BHI at all ages in 57% of individuals, suggestive of decreased bone mineral density (Leijten et al., 2019). Anti-epileptic drugs (affecting calcium and bone metabolism) were not used in any of the participants. While most participants were walking independently, the majority of them had daily exercise times below the Dutch recommendations of at least 1 h a day (Gezondheidsraad, 2017), which could potentially have a negative impact their bone quality (Mergler et al., 2016). The decreased bone mineralization in KLFS has not been observed before. However, EHMT1 is expressed in osteocytes and osteoblasts (Balemans et al., 2014), supporting a role for the chromatin remodeler in bone mineralization. Since decreased bone mineral density is clinically relevant and sufficient treatment is available, this clinical aspect required further attention and investigation in KLFS.
Furthermore, negative ulnar variance was found in 71% of individuals. Although ulnar variance is rarely studied in NDDs, negative variance has been reported in Rett syndrome with a prevalence of 41%–79% (Glasson et al., 1998; Leonard et al., 1995). The clinical relevance and underlying mechanism of this finding is unclear but it appears to be a clinical feature of KLFS as well.
4.3. Endocrine-metabolic investigations
In endocrine biochemical studies, we observed thyroid dysregulation in KLFS in 22% of individuals. This prevalence exceeds the population prevalence of 5% (Chiovato et al., 2019). We identified various factors that can contribute to thyroid dysregulation, highlighting the importance of comprehensive diagnostic screening. One individual had isolated central hypothyroidism without pituitary gland abnormalities. Central hypothyroidism has so far not been associated with KLFS, however, some case reports have described hypothalamic dysfunction (e.g., hypogonadotropic hypogonadism) (Higuchi et al., 2018; Torga et al., 2018). Therefore, further research is required to evaluate pituitary etiology and its implications in KLFS.
Triglycerides were significantly increased in KLFS compared to sex- and age-matched controls, while there were no differences in cholesterol, HDL, and LDL. Triglycerides remain significantly increased compared to an age- and BMI-matched Kabuki syndrome cohort (van Montfort et al., 2021) (Supplementary File 5). With the inclusion of individuals having a BMI Z-score within the range of −1 to 1, the mean triglyceride levels in the KLFS group are 1.22 ± 0.65 mmol/L, whereas they are 0.61 ± 0.19 mmol/L in the Kabuki syndrome (p < 0.03). Interestingly, increased triglycerides in KLFS align with findings obtained in the Drosophila KLFS model, which shows elevated triglyceride levels at steady state and impaired triglyceride mobilization under oxidative stress (Riahi et al., 2019). Impaired mobilization of fat stores, together with altered expression of genes involved in lipid metabolism, could contribute to obesity in KLFS (Riahi et al., 2019).
Fasting insulin and glucose measurements were performed in three individuals, of whom two individuals showed a high HOMA-IR, indicating insulin resistance. These individuals had normal blood glucose levels and no medical history of diabetes mellitus. In none of our other participants diabetes mellitus was present. Insulin resistance has been previously observed in mouse models with EHMT1 deficiency in brown adipose tissue (Ohno et al., 2013). Although this previous study also associated EHMT1 haploinsufficiency with liver steatosis (Ohno et al., 2013), we could not confirm this association with liver steatosis based on normal ALAT levels in 94% of our participants.
A noteworthy finding from our biochemical evaluations is significantly decreased ammonia in KLFS, which has not been previously associated with the condition. The clinical relevance of hypoammonia in general and in KLFS specific remains unknown, and we do not expect a different dietary protein intake in these individuals. Additional studies on amino acid metabolism and urea cycle metabolism are necessary to determine the significance of decreased ammonia.
To illustrate the clinical importance of endocrine-metabolic dysregulation, we present the case of a 20-year-old female participant (BMI Z-score 0.50 at last examination aged 15.5 years) who experienced major depression and severe fatigue, commonly associated with KLFS (Vermeulen et al., 2017). Results of biochemical investigations revealed auto-immune thyroiditis (fT4 5.4 pmol/L), folate deficiency (7.71 nmol/L), and vitamin D deficiency (26 nmol/L), which may explain her symptoms. Considering our findings of a high prevalence of thyroid dysregulation, folate deficiency, and vitamin D deficiency independently of BMI, we recommend routine biochemical assessments in these patients, as they might report their complaints differently due to cognitive impairment.
To what extent growth and endocrine-metabolic profiles contribute to neurodevelopmental and cognitive phenotypes associated with KLFS remains an important question for future research.
5. Conclusion and Recommendations
In conclusion, this study has shown that KLFS is associated with being overweight/obese, a high body fat percentage, insulin resistance, and increased triglycerides, resulting in a high risk for (cardio)metabolic disease. Further biochemical investigations highlighted significantly decreased ammonia levels, thyroid dysregulation, and vitamin deficiencies. Based on hand radiographs and DEXA scans, the bone density was generally decreased. This research contributes to a detailed phenotypic characterization of KLFS, provides novel biomarkers, and emphasizes the potential of clinical biomarkers in the translation of preclinical studies, both in KLFS and in the broader group of MDEMs.
Based on our findings, we have established five key recommendations: (1) We recommend lifelong surveillance of weight and waist circumference and the support of a healthy lifestyle. (2) We suggest to evaluate bone mineralization through hand radiograph or DEXA scan at the onset of puberty and when multiple or unexpected fractures occur, with follow-up according to local protocols. (3) Moreover, we recommend screening of thyroid function at puberty onset and awareness of the potential central origin of hypothyroidism. Therefore, evaluate both fT4 and TSH levels and consider an MRI of the pituitary gland when central hypothyroidism is suspected. (4) In addition, based on our biochemical findings, we advise screening for glucose metabolism (glucose, insulin), lipid spectrum, and vitamin status (vitamin D, B12, and folic acid) at the onset of puberty. For individuals with obesity starting from age 8 to 10 years, regular monitoring of glucose metabolism (glucose, insulin), lipid spectrum, and vitamin status (vitamin D, B12, and folic acid) is advised, with a frequency set at once every 1–3 years. (5) Lastly, consider the possible coexistence of somatic and psychiatric morbidity, including screening on hypothyroidism, and vitamin status before prescribing psychiatric drug treatment.
Supplementary Material
Additional supporting information can be found online in the Supporting Information section at the end of this article.
Acknowledgments
The authors are grateful to all patients and their caregivers for participation. We thank Bregina Kersten for the availability of the InBody 770 body composition analyzer. We also thank Dr. Nina Schott for the availability of lipid spectra and BMI of the Kabuki syndrome study cohort.
Funding Information
AB and TK received funding from the Netherlands Organisation for Health Research and Development PSIDER—project BRAINMODEL (10250022110003). TK received a grant from the Dutch Research Council (015.014.036 and 1160.18.320), and the Netherlands Organisation for Health Research and Development (91718310). AS was supported by a Dutch Research Council grant (09150181910022). This work was funded by a Catalyst Grant from United for Metabolic Diseases (UMD-CG-2021-014) which is funancially supported by Metakids.
Nederlandse Organisatie voor Wetenschappelijk Onderzoek, Grant/Award Numbers: 10250022110003, 91718310; ZonMw; Dutch, Research Council, Grant/Award Numbers: 09150181910022, 1160.18.320, 015.014.036
Abbreviations
- BF%
body fat percentage
- BHI
bone health index
- BIA
bioelectrical impedance analysis
- BMI
body mass index (kg/m2)
- DEXA
dual-energy X-ray absorptiometry
- HC
head circumference (cm)
- HipC
hip circumference (cm)
- KLFS
Kleefstra syndrome
- MDEM
Mendelian disorder of the epigenetic machinery
- MUAC
mid-upper arm circumference
- NDD
neurodevelopmental disorder
- SDS
standard deviation score
- SMM(a)
(appendicular) skeletal muscle mass
- WC
waist circumference (cm)
Footnotes
Author Contributions
Conceptualization and methodology: Arianne Bouman, Joyce M. Geelen, and Tjitske Kleefstra. Recruitment of participants: Arianne Bouman, Joost Kummeling, and Tjitske Kleefstra. Retrospective data collection: Arianne Bouman and Joost Kummeling. Growth and biochemical investigations: Arianne Bouman, Joyce M. Geelen, Yvonne G. van der Zwan, and Tjitske Kleefstra. Radiological investigations: Arianne Bouman and Willemijn M. Klein. Writing—original draft: Arianne Bouman, Joyce M. Geelen, and Tjitske Kleefstra. Writing—review and editing: Arianne Bouman, Joyce M. Geelen, Joost Kummeling, Annette Schenck, Yvonne G. van der Zwan, Willemijn M. Klein, and Tjitske Kleefstra.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationship that would be construed as a potential conflict of interest.
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
References
- Abramowitz MK, Hall CB, Amodu A, Sharma D, Androga L, Hawkins M. Muscle mass, BMI, and mortality among adults in the United States: A population-based cohort study. PLoS One. 2018;13(4):e0194697. doi: 10.1371/journal.pone.0194697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balemans MC, Ansar M, Oudakker AR, van Caam AP, Bakker B, Vitters EL, van der Kraan PM, de Bruijn DR, Janssen SM, Kuipers AJ, Huibers MM, et al. Reduced euchromatin histone methyltransferase 1 causes developmental delay, hypotonia, and cranial abnormalities associated with increased bone gene expression in Kleefstra syndrome mice. Developmental Biology. 2014;386(2):395–407. doi: 10.1016/j.ydbio.2013.12.016. [DOI] [PubMed] [Google Scholar]
- Bocca-Tjeertes IF, van Buuren S, Bos AF, Kerstjens JM, Ten Vergert EM, Reijneveld SA. Growth of preterm and full-term children aged 0-4 years: Integrating median growth and variability in growth charts. The Journal of Pediatrics. 2012;161(3):460–465.:E1. doi: 10.1016/j.jpeds.2012.03.016. [DOI] [PubMed] [Google Scholar]
- BoneXpert. Bone health index. (n.d.) https://bonexpert.com/what-is-bone-age/#bonehealthindex.
- Browning LM, Hsieh SD, Ashwell M. A systematic review of waist-to-height ratio as a screening tool for the prediction of cardio-vascular disease and diabetes: 0.5 could be a suitable global boundary value. Nutrition Research Reviews. 2010;23(2):247–269. doi: 10.1017/S0954422410000144. [DOI] [PubMed] [Google Scholar]
- Centraal Bureau voor de Statistiek. Jaarrapport Landelijke Jeugdmonitor 2022. Welzijn en Sport: Ministerie van Volksgezondheid; 2022. [Google Scholar]
- Chiovato L, Magri F, Carle A. Hypothyroidism in context: Where we've been and where we're going. Advances in Therapy. 2019;36(Suppl 2):47–58. doi: 10.1007/s12325-019-01080-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Vries T. Laboratoriumdiagnostiek bij kinderen. Een praktische handleiding. 2015 [Google Scholar]
- de Wilde JA, van Dommelen P, van Buuren S, Middelkoop BJ. Height of South Asian children in the Netherlands aged 0-20 years: Secular trends and comparisons with current Asian Indian, Dutch and WHO references. Annals of Human Biology. 2015;42(1):38–44. doi: 10.3109/03014460.2014.926988. [DOI] [PubMed] [Google Scholar]
- Fahrner JA, Bjornsson HT. Mendelian disorders of the epigenetic machinery: Tipping the balance of chromatin states. Annual Review of Genomics and Human Genetics. 2014;15:269–293. doi: 10.1146/annurev-genom-090613-094245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fahrner JA, Bjornsson HT. Mendelian disorders of the epigenetic machinery: Postnatal malleability and therapeutic prospects. Human Molecular Genetics. 2019;28(R2):R254–R264. doi: 10.1093/hmg/ddz174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fredriks AM, van Buuren S, Burgmeijer RJ, Meulmeester JF, Beuker RJ, Brugman E, Roede MJ, Verloove-Vanhorick SP, Wit JM. Continuing positive secular growth change in the Netherlands 1955-1997. Pediatric Research. 2000;47(3):316–323. doi: 10.1203/00006450-200003000-00006. [DOI] [PubMed] [Google Scholar]
- Gerver WJM, Bruin R. Paediatric morphometrics: A reference manual. Vol. 2. Datawyse/Universitaire Pers Maastricht; 2001. [Google Scholar]
- Gezondheidsraad. Beweegrichtlijnen. 2017/08. Gezondheidsraad; 2017. [Google Scholar]
- Glasson EJ, Bower C, Thomson MR, Fyfe S, Leonard S, Rousham E, Christodoulou J, Ellaway C, Leonard H. Diagnosis of Rett syndrome: Can a radiograph help? Developmental Medicine and Child Neurology. 1998;40(11):737–742. doi: 10.1111/j.1469-8749.1998.tb12341.x. [DOI] [PubMed] [Google Scholar]
- Higuchi S, Takagi M, Takeda R, Yoshihashi H, Narumi S, Hasegawa T. An association with hypopituitarism and 9q subtelomere deletion syndrome. Clinical Case Reports. 2018;6(12):2371–2375. doi: 10.1002/ccr3.1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan MJ, Gerasimidis K, Edwards CA, Shaikh MG. Mechanisms of obesity in Prader-Willi syndrome. Pediatric Obesity. 2018;13(1):3–13. doi: 10.1111/ijpo.12177. [DOI] [PubMed] [Google Scholar]
- Kleefstra T, Brunner HG, Amiel J, Oudakker AR, Nillesen WM, Magee A, Genevieve D, Cormier-Daire V, van Esch H, Fryns JP, Hamel BC, et al. Loss-of-function mutations in euchromatin histone methyl transferase 1 (EHMT1) cause the 9q34 subtelomeric deletion syndrome. American Journal of Human Genetics. 2006;79(2):370–377. doi: 10.1086/505693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleefstra T, de Leeuw N. In: GeneReviews(®) Adam MP, Ardinger HH, Pagon RA, Wallace SE, Bean LJH, Stephens K, Amemiya A, editors. University of Washington; 2010. Kleefstra syndrome. [Google Scholar]
- Kochinke K, Zweier C, Nijhof B, Fenckova M, Cizek P, Honti F, Keerthikumar S, Oortveld MA, Kleefstra T, Kramer JM, Webber C, et al. Systematic phenomics analysis deconvolutes genes mutated in intellectual disability into biologically coherent modules. American Journal of Human Genetics. 2016;98(1):149–164. doi: 10.1016/j.ajhg.2015.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kox LS, Jens S, Lauf K, Smithuis FF, van Rijn RR, Maas M. Well-founded practice or personal preference: A comparison of established techniques for measuring ulnar variance in healthy children and adolescents. European Radiology. 2020;30(1):151–162. doi: 10.1007/s00330-019-06354-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer JM. Regulation of cell differentiation and function by the euchromatin histone methyltransferases G9a and GLP. Biochemistry and Cell Biology. 2016;94(1):26–32. doi: 10.1139/bcb-2015-0017. [DOI] [PubMed] [Google Scholar]
- Leijten AD, Hampsink B, Janssen M, Klein WM, Draaisma JMT. Can digital X-ray radiogrammetry be an alternative for dual-energy X-ray absorptiometry in the diagnosis of secondary low bone quality in children? European Journal of Pediatrics. 2019;178(9):1433–1441. doi: 10.1007/s00431-019-03425-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonard H, Thomson M, Bower C, Fyfe S, Constantinou J. Skeletal abnormalities in Rett syndrome: Increasing evidence for dysmorphogenetic defects. American Journal of Medical Genetics. 1995;58(3):282–285. doi: 10.1002/ajmg.1320580316. [DOI] [PubMed] [Google Scholar]
- Lin HC, Wuang YP. Strength and agility training in adolescents with Down syndrome: A randomized controlled trial. Research in Developmental Disabilities. 2012;33(6):2236–2244. doi: 10.1016/j.ridd.2012.06.017. [DOI] [PubMed] [Google Scholar]
- Maia N, Nabais Sa MJ, Melo-Pires M, de Brouwer APM, Jorge P. Intellectual disability genomics: Current state, pitfalls and future challenges. BMC Genomics. 2021;22(1):909. doi: 10.1186/s12864-021-08227-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malina RM. Human growth: Selected aspects of current research on well nourished children. Annual Review of Anthropology. 1988;17:187–219. [Google Scholar]
- McCarthy HD, Samani-Radia D, Jebb SA, Prentice AM. Skeletal muscle mass reference curves for children and adolescents. Pediatric Obesity. 2014;9(4):249–259. doi: 10.1111/j.2047-6310.2013.00168.x. [DOI] [PubMed] [Google Scholar]
- McPartland JC. Considerations in biomarker development for neurodevelopmental disorders. Current Opinion in Neurology. 2016;29(2):118–122. doi: 10.1097/WCO.0000000000000300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mergler S, de Man SA, Boot AM, Heus KG, Huijbers WA, van Rijn RR, Penning C, Evenhuis HM. Automated radio-grammetry is a feasible method for measuring bone quality and bone maturation in severely disabled children. Pediatric Radiology. 2016;46(7):1017–1022. doi: 10.1007/s00247-016-3548-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohno H, Shinoda K, Ohyama K, Sharp LZ, Kajimura S. EHMT1 controls brown adipose cell fate and thermogenesis through the PRDM16 complex. Nature. 2013;504(7478):163–167. doi: 10.1038/nature12652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riahi H, Brekelmans C, Foriel S, Merkling SH, Lyons TA, Itskov PM, Kleefstra T, Ribeiro C, van Rij RP, Kramer JM, Schenck A. The histone methyltransferase G9a regulates tolerance to oxidative stress-induced energy consumption. PLoS Biology. 2019;17(3):e2006146. doi: 10.1371/journal.pbio.2006146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riahi H, Fenckova M, Goruk KJ, Schenck A, Kramer JM. The epigenetic regulator G9a attenuates stress-induced resistance and metabolic transcriptional programs across different stressors and species. BMC Biology. 2021;19(1):112. doi: 10.1186/s12915-021-01025-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satterstrom FK, Kosmicki JA, Wang J, Breen MS, De Rubeis S, An JY, Peng M, Collins R, Grove J, Klei L, Stevens C, et al. Large-scale exome sequencing study implicates both developmental and functional changes in the neurobiology of autism. Cell. 2020;180(3):568–584.:e23. doi: 10.1016/j.cell.2019.12.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schofield WN. Predicting basal metabolic rate, new standards and review of previous work. Human Nutrition Clinical Nutrition. 1985;39(Suppl 1):5–41. [PubMed] [Google Scholar]
- Schonbeck Y, Talma H, van Dommelen P, Bakker B, Buitendijk SE, Hirasing RA, van Buuren S. Increase in prevalence of overweight in Dutch children and adolescents: A comparison of nationwide growth studies in 1980, 1997 and 2009. PLoS One. 2011;6(11):e27608. doi: 10.1371/journal.pone.0027608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schonbeck Y, Talma H, van Dommelen P, Bakker B, Buitendijk SE, HiraSing RA, van Buuren S. The world's tallest nation has stopped growing taller: The height of Dutch children from 1955 to 2009. Pediatric Research. 2013;73(3):371–377. doi: 10.1038/pr.2012.189. [DOI] [PubMed] [Google Scholar]
- Schonbeck Y, van Dommelen P, HiraSing RA, van Buuren S. Trend in height of Turkish and Moroccan children living in The Netherlands. PLoS One. 2015;10(5):e0124686. doi: 10.1371/journal.pone.0124686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schott DA, Blok MJ, Gerver WJ, Devriendt K, Zimmermann LJ, Stumpel CT. Growth pattern in Kabuki syndrome with a KMT2D mutation. American Journal of Medical Genetics Part A. 2016;170(12):3172–3179. doi: 10.1002/ajmg.a.37930. [DOI] [PubMed] [Google Scholar]
- Schott DA, Gerver WJ, Stumpel CT. Growth hormone stimulation tests in children with Kabuki syndrome. Hormone Research in Pædiatrics. 2016;86(5):319–324. doi: 10.1159/000449221. [DOI] [PubMed] [Google Scholar]
- Sheppard SE, Campbell IM, Harr MH, Gold N, Li D, Bjornsson HT, Cohen JS, Fahrner JA, Fatemi A, Harris JR, Nowak C, et al. Expanding the genotypic and phenotypic spectrum in a diverse cohort of 104 individuals with Wiedemann-Steiner syndrome. American Journal of Medical Genetics Part A. 2021;185(6):1649–1665. doi: 10.1002/ajmg.a.62124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tahara Y, Moji K, Aoyagi K, Tsunawake N, Muraki S, Mascie-Taylor CG. Age-related pattern of body density and body composition of Japanese men and women 18-59 years of age. American Journal of Human Biology. 2002;14(6):743–752. doi: 10.1002/ajhb.10091. [DOI] [PubMed] [Google Scholar]
- Thodberg HH, Bottcher J, Lomholt J, Kreiborg S, Wolf G, Pfeil A. A new implementation of digital X-ray radiogrammetry and reference curves of four indices of cortical bone for healthy European adults. Archives of Osteoporosis. 2016;11:17. doi: 10.1007/s11657-016-0267-2. [DOI] [PubMed] [Google Scholar]
- Torga AP, Hodax J, Mori M, Schwab J, Quintos JB. Hypogonadotropic hypogonadism and Kleefstra syndrome due to a pathogenic variant in the EHMT1 gene: An underrecognized association. Case Reports in Endocrinology. 2018;2018:4283267. doi: 10.1155/2018/4283267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Montfort L, Gerver WJM, Kooger BLS, Plat J, Bierau J, Stumpel C, Schott DA. Follow-up study of growth hormone therapy in children with Kabuki syndrome: Two-year treatment results. Hormone Research in Pædiatrics. 2021;94(7–8):285–296. doi: 10.1159/000519963. [DOI] [PubMed] [Google Scholar]
- van Zoonen R. JGZ-richtlijn lengtegroei. TNO; 2019. http://resolver.tudelft.nl/uuid:ec6a3440-93d5-4cbc-9f3b-45513a1ca520 . [Google Scholar]
- Vermeulen K, de Boer A, Janzing JGE, Koolen DA, Ockeloen CW, Willemsen MH, Verhoef FM, van Deurzen PAM, van Dongen L, van Bokhoven H, Egger JIM, et al. Adaptive and maladaptive functioning in Kleefstra syndrome compared to other rare genetic disorders with intellectual disabilities. American Journal of Medical Genetics Part A. 2017;173(7):1821–1830. doi: 10.1002/ajmg.a.38280. [DOI] [PubMed] [Google Scholar]
- Willemsen MH, Vulto-van Silfhout AT, Nillesen WM, Wissink-Lindhout WM, van Bokhoven H, Philip N, Berry-Kravis EM, Kini U, van Ravenswaaij-Arts CM, Delle Chiaie B, Innes AM, et al. Update on Kleefstra syndrome. Molecular Syndromology. 2012;2(3–5):202–212. doi: 10.1159/000335648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wohlfahrt-Veje C, Tinggaard J, Winther K, Mouritsen A, Hagen CP, Mieritz MG, de Renzy-Martin KT, Boas M, Petersen JH, Main KM. Body fat throughout childhood in 2647 healthy Danish children: Agreement of BMI, waist circumference, skinfolds with dual X-ray absorptiometry. European Journal of Clinical Nutrition. 2014;68(6):664–670. doi: 10.1038/ejcn.2013.282. [DOI] [PubMed] [Google Scholar]
- Wouters M, Evenhuis HM, Hilgenkamp TIM. Physical fitness of children and adolescents with moderate to severe intellectual disabilities. Disability and Rehabilitation. 2020;42(18):2542–2552. doi: 10.1080/09638288.2019.1573932. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.