Abstract
Background
Overall colorectal cancer (CRC) incidence rates are in decline in many high-income countries, yet recent analyses in the U.S. and elsewhere have reported increasing risks among younger generations. Here, we examined generational changes in colon and rectal cancer incidence rates across seven high-income countries.
Methods
Data on colon and rectal cancer incidence were obtained from 21 population-based cancer registries from Australia, Canada, Denmark, Norway, New Zealand, Ireland and the U.K. for the earliest available year until 2014. Age-period-cohort (APC) modeling was used to assess trends by age group, period and birth cohort.
Findings
An overall decline or stabilization in the incidence of colon and rectal cancer was observed in all studied countries. In the most recent 10-year period however, steep increases were seen in both colon and rectal cancer incidence rates among younger persons (ages <50 years), with the largest increases for colon cancer in Denmark (3.1% per year) and for rectal cancer in Norway (4.0%). This coincided with a decrease in incidence at older ages (50-74 years) in all countries. Trends in CRC incidence in the young were mainly driven by increases in distal (left-sided) tumours of the colon. In all countries, we observed strong non-linear cohort effects were observed, which were more pronounced for rectal than colon cancer.
Interpretation
A considerable increase in the incidence of CRC in younger persons under age 50 can be observed across seven high-income countries included in this study. Strong birth cohort effects imply that a changing distribution and prevalence of risk factors that may exert particular influence on earlier stages of carcinogenesis is affecting recent generations. Evidently future studies are needed to determine the root causes of the upsurge of colorectal cancer incidence among younger persons that aid potential preventive and early detection strategies.
Funding
Canadian Partnership Against Cancer; Cancer Council Victoria; Cancer Institute New South Wales; Cancer Research UK; Danish Cancer Society; National Cancer Registry Ireland; The Cancer Society of New Zealand; NHS England; Norwegian Cancer Society; Public Health Agency Northern Ireland, on behalf of the Northern Ireland Cancer Registry; The Scottish Government; Western Australia Department of Health; Wales Cancer Network. The funders had no role in study design, data collection, data analysis, data interpretation, writing of the report, or the decision to submit.
Introduction
Colorectal cancer (CRC) is the third most common cancer worldwide, with an estimated 1.8 million new cases and 881,000 associated deaths in 2018.6 While the incidence of CRC is increasing rapidly in emerging economies, rates have been stabilising or declining in many highly-resourced settings, including the U.S., Australia, New Zealand and several Western European countries.7,8 Such declines, driven by rates at ages >50, have largely been attributed to the implementation of screening for premalignant polyps,9 though other risk factors may have contributed.2
Despite observed declines in overall CRC incidence, several recent studies have reported increasing CRC rates in adults younger than 50 years – an age group that is typically not targeted by organized screening.1–5 In Australia, colon cancer incidence rates in the 20-29 age range increased by 9.3% per annum from 2004 to 2014. Similarly, rectal cancer incidence increased by 7.1% every year from 1993 to 2014.3 In the U.S., those born circa 1990 were found to have double the risk of colon cancer and quadruple the risk of rectal cancer, relative to their counterparts born around 1950.2 This recent rise in CRC incidence at ages<50 years may imply a role for specific lifestyle factors, including diet or increasing rates of obesity.10
In this study, we characterized and comprehensively assessed time trends in CRC incidence by tumour subsite, tumour location, age at diagnosis, and year of birth using data from seven high income countries (Australia, Canada, Denmark, Ireland, New Zealand, Norway, and the U.K.). By means of age-period-cohort modelling, we analysed time trend by period of diagnosis and year of birth (birth cohort) in order to increase our understanding of factors that affect the population regardless of age at a given time (e.g., changes in medical practice), versus those that vary in generations of individuals as they age (e.g., changing prevalence and distribution of known or putative risk factors).
Methods
Study Design and Data Sources
The second phase of the International Cancer Benchmarking Partnership (ICBP SURVMARK-2) aims to measure international differences in cancer survival, across seven high-income countries with similar access to health care; (1) Australia [New South Wales, Western Australia, Victoria], (2) Canada [Alberta, British Columbia, Manitoba, New Brunswick, Newfoundland, Nova Scotia, Ontario, Prince Edward Island, Saskatchewan], (3) Denmark, (4) Ireland, (5) New Zealand, (6) Norway and (7) the U.K. [England, Northern Ireland, Scotland, and Wales]. According to the ICBP SURVMARK2 data specification protocol, data on primary cancers of the colon and rectum were obtained from 21 population-based cancer registries for cases diagnosed in the period 1995-2014 (with the exception of Australia and Denmark: 1995-2012, and Ireland: 1995-2013). In order to investigate long-term trends, the Cancer Incidence in Five Continents (CI5) database was used, to expand these data series going back in time for Australia: (from) 1983, Denmark: 1978, and Norway: 1953.11 Cases were stratified by tumour subsite as follows: colon (the International Classification of Diseases, 10th edition (ICD-10) codes C18.0, C18.2-C18.9), proximal (right) colon (C18.0, C18.2-C18.4), distal (left) colon (C18.5-C18.7), rectum (C19-C20). Malignant neoplasms of the appendix (C18.1) were excluded, as their aetiology is different to other CRC tumours and changes in the classification of the behaviour of these tumours may render the trends unreliable. The data submitted from registries included information on patient- and tumour-level data including topography, histology, morphology and basis of diagnosis. Datasets were quality-checked and harmonized according to a pre-defined protocol. As incidence trends were similar in males and females (appendix pp 3-4), results are presented for both sexes combined.
Statistical Analysis
Age-standardized incidence rates (ASIR) were calculated for three age groups (0-49 years, 50-74 years, 75+ years) and all ages combined, using the world standard population and presented per 100,000 person-years.12 For the detailed assessment of trends among younger people, these were further stratified by age (20-29 years, 30-39 years, and 40-49 years). Joinpoint regression analysis was used to identify magnitude and direction of temporal trends by anatomic sub-site, and age for varying time periods. This method uses a statistical algorithm to define a best-fitting regression line through incidence data across time to estimate the annual percent change (APC) and average annual percent change (AAPC) (with 95% confidence intervals).13 Trends in incidence rates were quantified by AAPC and the corresponding 95% confidence interval over the most recent 10-year period of available data (Australia: 2003-2012; Canada/ New Zealand/ Norway/U.K.: 2005-2014; Denmark: 2003-2012; Ireland: 2004-2013). Two-sided P values were considered to indicate statistical significance when they were less than 0.05.
Observed trends (in 5-year intervals for age and period, and 10-years for birth cohort) were presented as rates versus birth cohort by age, and rates versus calendar period by age, with the identification of quasi-parallelism between age-specific curves providing an indication of their effects on the temporal pattern. Assuming CRC incidence rates were constant within the 5-year age classes and 5-year periods of diagnosis, an age-period-cohort (APC) model was fitted and goodness-of-fit was evaluated by comparing the deviance for each sub model.14,15 The APC model assumes a linear dependency among age, period, and cohort (cohort = period – age). To solve the non-identifiability problem of the full APC model, the general method of setting constraints on the parameters was used.16 Both period and cohort effects are presented as incidence rate ratios (IRR) relative to their respective medians (with respect to the number of cases for the period and cohort variable). The model analysis and presentation was performed using APCfit in Stata, which uses the splines to estimate each of the three effects that are then combined to give estimated rates.17 The default number of internal knots (five) for each of the spline bases for the three variables (age, period and cohort) was used.
Results
Overall, the incidence of colon cancer in the most recent five year period varied between 24.5 in Norway and 19.0 per 100,000 person-years in the U.K. Rectal cancer incidence was somewhat lower, but showed similar geographical patterns, with the highest and lowest rates observed in the same countries: Norway (13.6) and the U.K. (10.9) (Table 1). Of the 402,376 CRC patients diagnosed during the last five years of available data across countries, 66% were diagnosed with colon cancer and 34% were diagnosed with rectal cancer. An overview of the number of CRC cases diagnosed throughout the study period by sex, age, tumour subsite, year of diagnosis and year of birth by country is provided in the supplementary material (appendix p 1).
Table 1. Absolute number of cases and age standardised incidence rates (ASIR), by age, cancer site and country of the last five years of the study perioda.
| Age groups | Countries | Colon Cancer | Rectal Cancer | ||
|---|---|---|---|---|---|
| Cases | ASIR b | Cases | ASIR b | ||
| Australia | 32,875 | 23·4 | 16,759 | 13·4 | |
| Canada | 53,450 | 20·0 | 26,424 | 11·0 | |
| All ages | Denmark | 13,764 | 22·6 | 7,343 | 13·0 |
| Ireland | 7,973 | 21·6 | 4,225 | 12·3 | |
| New Zealand | 9,870 | 23·2 | 4,945 | 13·0 | |
| Norway | 13,051 | 24·5 | 6,460 | 13·6 | |
| United Kingdom | 135,553 | 19·0 | 69,684 | 10·9 | |
| Australia | 1,851 | 2·7 | 1,646 | 2·3 | |
| Canada | 2,913 | 2·3 | 2,560 | 2·0 | |
| 0-49 years | Denmark | 548 | 2·1 | 384 | 1·4 |
| Ireland | 474 | 2·2 | 354 | 1·6 | |
| New Zealand | 517 | 2·6 | 426 | 2·1 | |
| Norway | 543 | 2·3 | 445 | 1·9 | |
| United Kingdom | 5,963 | 2·0 | 4,280 | 1·4 | |
| Australia | 16,426 | 85·0 | 9,946 | 52·0 | |
| Canada | 27,583 | 72·8 | 16,176 | 42·7 | |
| 50-74 years | Denmark | 7,359 | 83·5 | 4,506 | 52·1 |
| Ireland | 4,179 | 78·6 | 2,580 | 48·9 | |
| New Zealand | 4,730 | 78·8 | 2,922 | 49·4 | |
| Norway | 6,295 | 88·0 | 3,754 | 53·1 | |
| United Kingdom | 65,253 | 69·1 | 39,501 | 42·7 | |
| Australia | 14,598 | 301·2 | 5,167 | 108·4 | |
| Canada | 22,954 | 256·1 | 7,688 | 87·6 | |
| 75+ years | Denmark | 5,857 | 293·9 | 2,453 | 125·7 |
| Ireland | 3,320 | 286·8 | 1,291 | 111·5 | |
| New Zealand | 4,623 | 346·7 | 1,597 | 121·6 | |
| Norway | 6,213 | 343·4 | 2,261 | 129·1 | |
| United Kingdom | 64,337 | 250·0 | 25,903 | 102·2 | |
The last five years of the study period: Australia: 2008-2012, Canada: 2010-2014, Denmark: 2008-2012, Ireland: 2009-2013, New Zealand: 2010-2014, Norway: 2010-2014 and United Kingdom: 2010-2014
Age standardised incidence rate using the world population standard per 100,000 (ASIR)
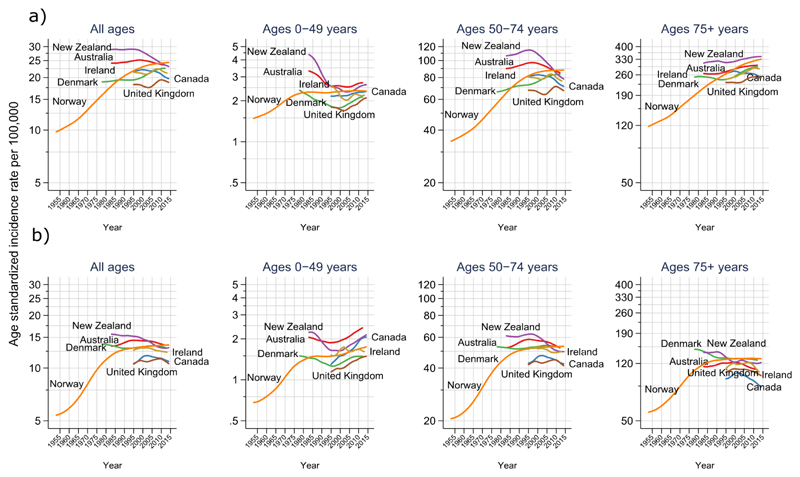
While overall trends in colon cancer incidence stabilized and subsequently decreased in Australia, New Zealand, Canada, and to a lesser extend in the U.K. and Ireland, rates continued to rise in Denmark and Norway (Figure 1). As for recent trends, in the most 10-year period, colon cancer incidence rates for all ages decreased by 1.8% per annum in New Zealand and by 1.3% in Canada, but stabilized in Australia and Ireland and increased in Denmark (Figure 2, appendix p 2). Trends for rectal cancer were similar to colon cancer, except for the U.K., where incidence rates declined by 1.2% every year, similar to decreases observed in Australia and Canada (Figure 2, appendix p 2). No significant changes were observed for rectal cancer in the remaining countries.
Figure 1.
Long-term trends in age-standardized incidence rates of colon (a) and rectal (b) cancer in four age groups (all ages, 0-49 years, 50-74 years, 75+ years) by country
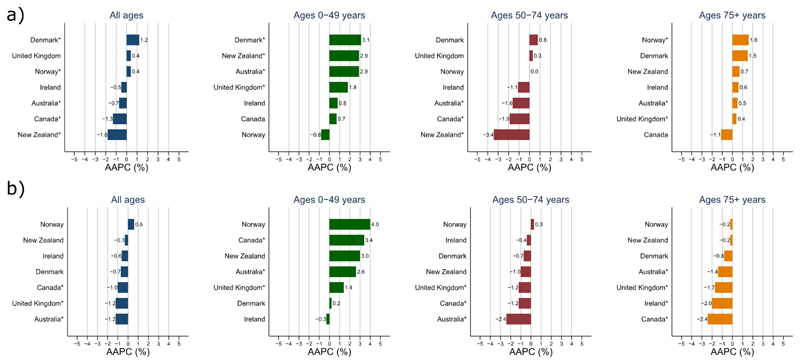
Figure 2.
Average annual percent change (AAPC) in colon (a) and rectal (b) cancer incidence in four age groups (all ages, 0-49 years, 50-74 years, 75+ years) for the most recent 10-year period
*Statistically significant (p <0.05)
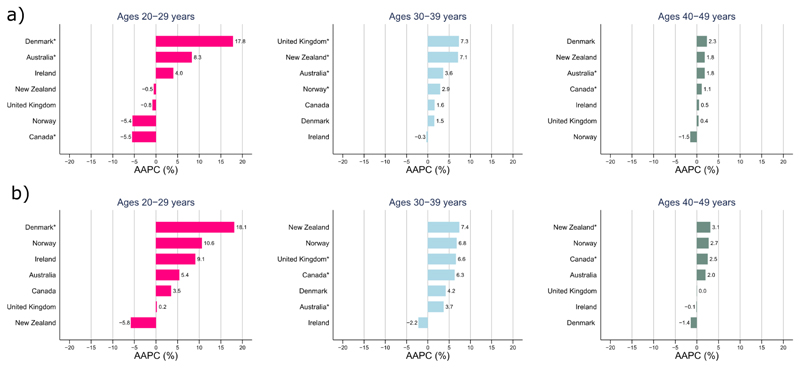
Incidence trends by age group showed diverging patterns (Figures 1 & 2, appendix p 2). In younger persons aged 0-49 years, colon cancer incidence rates increased in all countries except Norway over the most recent 10-year period (AAPC: Denmark +3.1%; New Zealand +2.9%; Australia +2.9%, U.K. +1.8%; Ireland +0.8%; Canada +0.7%, p < 0.05 for all, except Ireland and Canada, Figure 2), with increases more pronounced in females than males (appendix pp 3-4). In this age group, increasing incidence trends were specially seen for tumours occurring in the distal (left) colon (appendix p 8). Similar increases were also observed for rectal cancer across all countries, except Ireland and Denmark (AAPC: Norway +4.0%; Canada +3.4%, New Zealand +3.0%; Australia +2.6%, U.K. +1.4%; p < 0.05 for all, except Norway and New Zealand). Sub-analyses showed that observed increases for both colon and rectal cancer were most pronounced amongst those aged 20-29 years and were driven by increasing incidence rates of rectal and left-sided colon cancer (Figure 3, appendix p 9).
Figure 3.
Average annual percent change (AAPC) in colon (a) and rectal (b) cancer incidence in young age groups (20-29 years, 30-39 years, 40-49 years) for the most recent 10-year period
*Statistically significant (p <0.05)
In the 50-74 age group, colon cancer incidence rates decreased significantly in New Zealand (-3.4% per year) and showed downward trends in Canada (-1.9%), Australia (-1.6%) and Ireland (-1.1%), while rates stabilized or slightly increased in Denmark, the U.K. and Norway. Similarly, decreases or a levelling off of rectal cancer incidence rates were observed in this age group across all countries. In the elderly (ages 75 and over), colon cancer incidence rates increased in Norway and Denmark but were stable elsewhere; whereas rectal cancer incidence decreased or stabilized in all countries (Figures 1 & 2, appendix p 2).
To explore period and cohort effects, age-specific colon and rectal cancer rates are presented by year of birth (birth cohort) and period of diagnosis in all countries (appendix pp 10-11). The parallelisms in the lines are more apparent for birth cohorts than across period of diagnosis and therefore indicative of strong generational effects. Uniform increases in incidence rates of colon and rectal cancer are seen in successive birth cohorts (Australia: those born between 1970-1985, Canada: 1975-1990, Denmark & New Zealand: 1970-1980, Norway: 1930-1980, Ireland: 1980-1990, and the U.K.: 1970-1980), affecting those aged of 20-39 years in the most recent period. In contrast, rates seem to stabilize for those aged 50+ that were born from 1950 onward.
On the basis of the full APC model and a parameterization that allocates drift to birth cohort, an increasing trend in the IRRs can be seen in successive generations for both colon and rectal cancer across all countries (appendix pp 12-13), with more pronounced patterns for rectal relative to colon cancer. For example, compared with those born circa 1925, generations born around 1990 in Norway had a twofold increase in the age-specific risk of colon cancer (IRR = 2.0, 95% CI = 1.6−2.5) and a fivefold increase in the risk of rectal cancer (IRR = 5.1, 95% CI = 4.0− 6.6). Similar trends were observed for recently born cohorts in the U.K., Australia, Canada and New Zealand (appendix pp 5-6). In Ireland and Denmark, increases in IRRs of colon and rectal cancer in younger cohorts did not reach statistical significance.
Discussion
Despite a general decline or stabilization in colon and rectal cancer incidence rates for all ages and at ages 50 years and over, the phenomenon of an increase in incidence in adults below the age of 50 can be observed across several high-income countries, with the rate of change as much as 3.1% per year in Denmark (for colon cancer) and 4.0% per year in Norway (for rectal cancer) over the most recent 10-year period. Our results underscore the presence of strong (non-linear) cohort effects, pointing towards significant upward trends in CRC risk for those born after 1990 in all seven countries under study.
The increasing incidence of CRC in individuals aged below 50 years is in line with previous studies investigating CRC trends at the national level.1–5 In the U.S., Siegel and colleagues2 showed that age-specific CRC risk for recent birth cohorts has declined to the levels seen among generations born circa 1890. Similarly, increasing incidence of CRC in young patients has been reported in studies from Australia,3 Canada,4 and Norway.5 The parallelism of the incidence rates by cohort and the strong significance of non-linear cohort effects in the APC model across countries likely reflects differentials in the changing prevalence and distribution of risk factors in recent generations of men and women.
Such factors may include lifestyle-related risk factors, including population-level changes in excess body weight, consumption of red and processed meat, cooking meats at high temperatures, high fat intake, physical activity, dietary fibre intake, alcohol consumption, and cigarette smoking.18 Recent evidence also suggests that antibiotic use, which alters the gut microbiome, may also be associated with a rise in paediatric inflammatory bowel diseases that may eventually increase the risk of CRC.19 Site-specific effects have been reported for some of these factors that may selectively promote tumorigenesis proximally (high fat consumption), distally (high consumption of processed meat) or the entire colon albeit not the rectum (obesity and low levels of physical activity).20 This study confirmed that the increasing trends among those aged 20-29 years was more pronounced for distal colon and rectal tumours– a finding that has also been previously been reported among those aged <50 years in USA.2
Obesity has become a global pandemic over the past 40 years and compared to older generations, younger populations across the world may experience longer lasting effect of excess adiposity across their life span.21 In the U.S., the risk of obesity-related cancer has been found to be increasing in younger birth cohorts.21 Young-onset CRC may also be a result of cumulative exposure to excess weight over the lifetime of recent birth cohorts.22 Moreover, the complex epigenetic interactions between dietary intake and obesity, on the one hand, and CRC risk on the other can be explained when different components of unhealthy diet such as high–glycaemic load carbohydrate are assessed separately.23 A recent study found that exposure to high-fat and low-fibre diets initiates inflammation and proliferation in the colonic mucosa.24 The marked alterations in the incidence rates of CRC between generations of Asian immigrants in the U.S. and U.K. suggest that Western dietary and lifestyle factors may play a pivotal role in the aetiology of the disease.25,26
In persons aged 50 years and older, we observed a declining incidence of colon and rectal cancer in most of the countries studied, which may be due to interventions leading to removal of adenomatous polyps.27 In several countries, it is recommended for those aged 50-74 years and older to participate in routine population-based screening activities other than considered at high-risk (e.g. patients with a history of familial polyposis, hereditary nonpolyposis CRC, or ulcerative colitis).28 The fact that routine screening is largely confined to individuals within this age range may partly explain the age-related disparities in secular trends in CRC incidence rates.
Indeed, where population-based screening has started earlier, for example in Australia, Canada, and the U.K. (2006), observed overall decreases of CRC seem to be more pronounced.29 On the other hand, in Ireland (2012), Denmark (2014), Norway (only pilot programme, 2012), and New Zealand (2017), where screening started later, rates within the age range of 50-74 years remain more stable.30 Controversies persist as to the role of screening for sporadic cancers in younger populations. Some studies have identified distinctive tumour sites, more advanced stage at diagnosis and histologic features in young-onset CRC.31–35 For example, patients below age 50 less often presented with more proximal colon tumours (age < 50 years, 24% vs age ≥ 50 years, 43%) and had fewer rectal tumours (age < 50 years, 39% vs age ≥ 50 years, 27%).35 Patients with young-onset CRC were also more likely to present with greater spread of disease, with 22% having distant-stage CRC with worst prognosis, relative to patients with later-onset disease.36–38 Moreover, poorly differentiated histologic features and mucinous and signet ring cell features are more common in younger-onset disease - characteristics that are also associated with bad prognosis.39,40
Furthermore, the proportion of high microsatellite instability (MSI-H) among young CRC cases ranges from 19.7% to 41.0% depending on the age of onset, which has been further associated with poor prognosis.41 Given the low CRC rates at younger ages, the cost of population screening per life year gained is high and not presently considered cost-effective.42 Nonetheless, in view of the recent trends, the American Cancer Society adopted screening guidelines recommending CRC screening from the age of 45 years.43 Multiple studies have reported strong associations of cancer history among first-degree relatives in younger onset CRC.44 One in every 6 patients with young onset CRC cases has been reported to present with at least one pathogenic cancer susceptibility gene mutation.45 For example, mismatch repair gene variants linked to Lynch syndrome seems to be much more common than previously reported. As such, screening for genetic susceptibility in primary care through ascertainment of family history may identify high risk individuals who may require referral.
While the incidence of CRC in adults under age 50 years remains low when compared to older age groups, the observed increases are of clear concern and highlight the need for action to counteract the rising burden of CRC in this age group. Some genetic components may play a role; these however cannot fully explain the rapid changes in cancer incidence we reported here. For this reason, primary prevention is a key course of action, and therefore raising awareness of the public and clinicians with respect to a balanced and healthy diet, and maintenance of an ideal weight and lifestyle from an early age are critical measures to control the future burden. National level programs to promote healthy diets and physical activity might be the most efficient approach to ensure population level changes.46 In addition, educational and preventive programs that provide information on symptoms and high-risk populations, as well as additional screening for high risk patients with inflammatory bowel disease or a genetic predisposition to CRC, are needed. Further research is warranted to identify the risk factors for young-onset cancer. Finally, development and validation of risk prediction models could aid clinicians to identify the presence of high risk adenomatous polyps in younger populations.47
The strengths of this study include the use of high quality and the most up-to-date cancer incidence data, and a comprehensive assessment of time trends in the incidence of CRC by subsite across seven high-income countries. The longer time series in some countries, such as Norway, enabled cohort analyses of those born from 1870 to 1995. Our study also has limitations. The calculation of birth cohorts from period and age may cause a linear dependency between the time components and a non-identifiability of their linear slopes.16 As the solution presented is entirely dependent on our choice of allocation of the overall time trend (drift), caution should be applied when interpreting the results. We did not have access to individual-level data on risk factors and screening to examine trends of CRC over time across age groups, in relation to temporal changes in those factors.
In conclusion, the rise of colon and rectal cancer incidence rates among young adults is now evident in a number of high-income countries. This is paralleled by ongoing incidence declines in adults older than 50 years. These results highlight the future need for studies that determine the underlying causes for rapidly altering trends and identify potential preventive and early detection strategies that arrest the escalating rates of young-onset colorectal cancer.
Supplementary Material
Research in context.
Evidence before this study
We searched PubMed for studies containing the terms “young adult”, “colorectal cancer”, “incidence trends”, and “birth cohort” to identify previous studies on colorectal cancer (CRC) incidence with a focus on age- or cohort-specific trends. The final search date for all previous papers published in English was January 03, 2019. Previous studies have shown CRC incidence rates at ages <50 have been rising in the U.S.1,2 and other high-income countries including Australia,3 Canada,4 and Norway.5 Data from the Surveillance, Epidemiology, and End Results (SEER) database covering the period 2000-2013 showed that U.S. CRC rates decreased at ages >55 years, but increased for younger adults aged 20-54 years (with the steepest rise in adults aged 20-29).2 The increase in incidence rates was more pronounced for rectal than colon cancer.
Added value of this study
There are currently no other studies that comprehensively address and compare age-specific trends and cohort effects in CRC incidence across high-income countries. In this study, we used long-term data from the high quality population-based cancer registries in Australia, Canada, Denmark, Ireland, New Zealand, Norway, and the U.K. to assess CRC incidence trends by age and period of diagnosis, and by birth cohort. While marked declines or stabilizations in colon and rectal cancer incidence rates were observed for all ages combined and among older ages in all countries, the incidence rates increased among younger age groups (<50 years). Our results confirm that generational effects were more pronounced for rectal than colon cancer in all countries.
Implications of all the available evidence
These findings suggest that the epidemiology of colorectal cancer is changing, with risk increasing in successive generations born towards the end of the 20th century in high-income countries in different world regions. Research into risk factors such as obesity is warranted to identify the main drivers behind the upsurge in colorectal cancer among recent generations. Sustained monitoring of trends is key to successful cancer control as these findings also have important implications for policy and early detection programs. There is an acute need for innovative approaches to promote sustainable and healthy dietary behaviours to reduce obesity.
Acknowledgements
The authors would like to thank the ICBP management team of Cancer Research UK, the ICBP Programme Board, Clinical Committee, Academic Reference Group and Local Leads for their support and advice. We also thank the cancer registry staff in all jurisdictions, whose sustained efforts in data collection and quality control over many years have enabled this study. The authors alone are responsible for the views expressed in this article and they do not necessarily represent the views, decisions or policies of the institutions with which they are affiliated.
Footnotes
Contributors
MaA, MeA and IS conceived the study and contributed to study design, analysis and wrote the first draft of the manuscript. DSM, PD, HT, PMW, OB, GE, CJ, CMC, RRW, NSJ, EM, DR, VT, BM and SL contributed to data collection and critically reviewed the manuscript. AB, JF and CC contributed to data preparation and analysis. FB and MGG contributed to drafting and finalizing the report. All authors read and approved the final manuscript.
Declaration of interests
We declare no competing interests.
References
- 1.Bailey CE, Hu CY, You N, et al. Increasing Disparities in the Age-Related Incidences of Colon and Rectal Cancers in the United States, 1975-2010. Jama Surg. 2015;150(1):17–22. doi: 10.1001/jamasurg.2014.1756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Siegel RL, Fedewa SA, Anderson WF, et al. Colorectal Cancer Incidence Patterns in the United States, 1974-2013. J Natl Cancer Inst. 2017;109(8) doi: 10.1093/jnci/djw322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Feletto E, Yu XQ, Lew JB, et al. Trends in Colon and Rectal Cancer Incidence in Australia from 1982 to 2014: Analysis of Data on Over 375,000 Cases. Cancer Epidemiol Biomarkers Prev. 2019;28(1):83–90. doi: 10.1158/1055-9965.EPI-18-0523. [DOI] [PubMed] [Google Scholar]
- 4.Brenner DR, Ruan YB, Shaw E, De P, Heitman SJ, Hilsden RJ. Increasing colorectal cancer incidence trends among younger adults in Canada. Prev Med. 2017;105:345–9. doi: 10.1016/j.ypmed.2017.10.007. [DOI] [PubMed] [Google Scholar]
- 5.Larsen IK, Bray F. Trends in colorectal cancer incidence in Norway 1962-2006: an interpretation of the temporal patterns by anatomic subsite. Int J Cancer. 2010;126(3):721–32. doi: 10.1002/ijc.24839. [DOI] [PubMed] [Google Scholar]
- 6.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018 doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 7.Arnold M, Sierra MS, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global patterns and trends in colorectal cancer incidence and mortality. Gut. 2017;66(4):683–91. doi: 10.1136/gutjnl-2015-310912. [DOI] [PubMed] [Google Scholar]
- 8.Siegel RL, Miller KD, Fedewa SA, et al. Colorectal cancer statistics, 2017. CA Cancer J Clin. 2017;67(3):177–93. doi: 10.3322/caac.21395. [DOI] [PubMed] [Google Scholar]
- 9.Edwards BK, Ward E, Kohler BA, et al. Annual report to the nation on the status of cancer, 1975-2006, featuring colorectal cancer trends and impact of interventions (risk factors, screening, and treatment) to reduce future rates. Cancer. 2010;116(3):544–73. doi: 10.1002/cncr.24760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Patel P, De P. Trends in colorectal cancer incidence and related lifestyle risk factors in 15-49-year-olds in Canada, 1969-2010. Cancer Epidemiol. 2016;42:90–100. doi: 10.1016/j.canep.2016.03.009. [DOI] [PubMed] [Google Scholar]
- 11.Forman D, Bray F, Brewster DH, et al. Cancer Incidence in Five Continents, Vol. X (electronic version) Lyon: IARC; 2014. [Google Scholar]
- 12.Doll R, Payne P, Waterhouse JAH. Cancer Incidence in Five Continents, Vol. I Union Internationale Contre le Cancer. Geneva: 1966. [Google Scholar]
- 13.Kim HJ, Fay MP, Feuer EJ, Midthune DN. Permutation tests for joinpoint regression with applications to cancer rates. Stat Med. 2000;19(3):335–51. doi: 10.1002/(sici)1097-0258(20000215)19:3<335::aid-sim336>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 14.Clayton D, Schifflers E. Models for Temporal Variation in Cancer Rates .1. Age Period and Age Cohort Models. Stat Med. 1987;6(4):449–67. doi: 10.1002/sim.4780060405. [DOI] [PubMed] [Google Scholar]
- 15.Clayton D, Schifflers E. Models for Temporal Variation in Cancer Rates .2. Age Period Cohort Models. Stat Med. 1987;6(4):469–81. doi: 10.1002/sim.4780060406. [DOI] [PubMed] [Google Scholar]
- 16.Holford TR. The estimation of age, period and cohort effects for vital rates. Biometrics. 1983;39(2):311–24. [PubMed] [Google Scholar]
- 17.Rutherford MJ, Thompson JR, Lambert PC. Projecting cancer incidence using age-period-cohort models incorporating restricted cubic splines. Int J Biostat. 2012;8(1):33. doi: 10.1515/1557-4679.1411. [DOI] [PubMed] [Google Scholar]
- 18.Huxley RR, Ansary-Moghaddam A, Clifton P, Czernichow S, Parr CL, Woodward M. The impact of dietary and lifestyle risk factors on risk of colorectal cancer: a quantitative overview of the epidemiological evidence. Int J Cancer. 2009;125(1):171–80. doi: 10.1002/ijc.24343. [DOI] [PubMed] [Google Scholar]
- 19.Cao Y, Wu K, Mehta R, et al. Long-term use of antibiotics and risk of colorectal adenoma. Gut. 2018;67(4):672–+. doi: 10.1136/gutjnl-2016-313413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Papagiorgis PC, Oikonomakis I, Delaportas D, et al. Proximal shift of colorectal cancer. A persistent phenomenon with multiple causes, patterns and clinical implications. J BUON. 2014;19(3):605–17. [PubMed] [Google Scholar]
- 21.Sung H, Siegel RL, Rosenberg PS, Jemal A. Emerging cancer trends among young adults in the USA: analysis of a population-based cancer registry. Lancet Public Health. 2019 doi: 10.1016/S2468-2667(18)30267-6. [DOI] [PubMed] [Google Scholar]
- 22.Lee JM, Pilli S, Gebremariam A, et al. Getting heavier, younger: trajectories of obesity over the life course. Int J Obes (Lond) 2010;34(4):614–23. doi: 10.1038/ijo.2009.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ludwig DS. Lifespan Weighed Down by Diet. JAMA. 2016;315(21):2269–70. doi: 10.1001/jama.2016.3829. [DOI] [PubMed] [Google Scholar]
- 24.O'Keefe SJ, Li JV, Lahti L, et al. Fat, fibre and cancer risk in African Americans and rural Africans. Nat Commun. 2015;6:6342. doi: 10.1038/ncomms7342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ali R, Barnes I, Kan SW, Beral V. Cancer incidence in British Indians and British whites in Leicester, 2001-2006. Brit J Cancer. 2010;103(1):143–8. doi: 10.1038/sj.bjc.6605744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rastogi T, Devesa S, Mangtani P, et al. Cancer incidence rates among South Asians in four geographic regions: India, singapore, UK and US. Int J Epidemiol. 2008;37(1):147–60. doi: 10.1093/ije/dym219. [DOI] [PubMed] [Google Scholar]
- 27.Murphy CC, Sandler RS, Sanoff HK, Yang YC, Lund JL, Baron JA. Decrease in Incidence of Colorectal Cancer Among Individuals 50 Years or Older After Recommendations for Population-based Screening. Clin Gastroenterol H. 2017;15(6):903–+. doi: 10.1016/j.cgh.2016.08.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Force USPST. Screening for colorectal cancer: recommendation and rationale. Ann Intern Med. 2002;137(2):129–31. doi: 10.7326/0003-4819-137-2-200207160-00014. [DOI] [PubMed] [Google Scholar]
- 29.Schreuders EH, Ruco A, Rabeneck L, et al. Colorectal cancer screening: a global overview of existing programmes. Gut. 2015;64(10):1637–49. doi: 10.1136/gutjnl-2014-309086. [DOI] [PubMed] [Google Scholar]
- 30.Basu P, Ponti A, Anttila A, et al. Status of implementation and organization of cancer screening in The European Union Member States-Summary results from the second European screening report. Int J Cancer. 2018;142(1):44–56. doi: 10.1002/ijc.31043. [DOI] [PubMed] [Google Scholar]
- 31.Ahnen DJ, Wade SW, Jones WF, et al. The increasing incidence of young-onset colorectal cancer: a call to action. Mayo Clin Proc. 2014;89(2):216–24. doi: 10.1016/j.mayocp.2013.09.006. [DOI] [PubMed] [Google Scholar]
- 32.Connell LC, Mauricio J, Braghiroli MI, Hoff PM. The Rising Incidence of Younger Patients With Colorectal Cancer: Questions About Screening, Biology, and Treatment. Curr Treat Option On. 2017;18(4) doi: 10.1007/s11864-017-0463-3. [DOI] [PubMed] [Google Scholar]
- 33.Domergue J, Ismail M, Astre C, et al. Colorectal-Carcinoma in Patients Younger Than 40-Years-of-Age - Montpellier-Cancer-Institute Experience with 78 Patients. Cancer. 1988;61(4):835–40. doi: 10.1002/1097-0142(19880215)61:4<835::aid-cncr2820610432>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 34.Fairley TL, Cardinez CJ, Martin J, et al. Colorectal cancer in US adults younger than 50 years of age 1998-2001. Cancer. 2006;107(5):1153–61. doi: 10.1002/cncr.22012. [DOI] [PubMed] [Google Scholar]
- 35.Myers EA, Feingold DL, Forde KA, Arnell T, Jang JH, Whelan RL. Colorectal cancer in patients under 50 years of age: a retrospective analysis of two institutions' experience. World J Gastroenterol. 2013;19(34):5651–7. doi: 10.3748/wjg.v19.i34.5651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Abdelsattar ZM, Wong SL, Regenbogen SE, Jomaa DM, Hardiman KM, Hendren S. Colorectal cancer outcomes and treatment patterns in patients too young for average-risk screening. Cancer. 2016;122(6):929–34. doi: 10.1002/cncr.29716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chou CL, Chang SC, Lin TC, et al. Differences in clinicopathological characteristics of colorectal cancer between younger and elderly patients: an analysis of 322 patients from a single institution. Am J Surg. 2011;202(5):574–82. doi: 10.1016/j.amjsurg.2010.10.014. [DOI] [PubMed] [Google Scholar]
- 38.Mauri G, Sartore-Bianchi A, Russo AG, Marsoni S, Bardelli A, Siena S. Early-onset colorectal cancer in young individuals. Mol Oncol. 2018 doi: 10.1002/1878-0261.12417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chang DT, Pai RK, Rybicki LA, et al. Clinicopathologic and molecular features of sporadic early-onset colorectal adenocarcinoma: an adenocarcinoma with frequent signet ring cell differentiation, rectal and sigmoid involvement, and adverse morphologic features. Modern Pathol. 2012;25(8):1128–39. doi: 10.1038/modpathol.2012.61. [DOI] [PubMed] [Google Scholar]
- 40.Schellerer VS, Merkel S, Schumann SC, et al. Despite aggressive histopathology survival is not impaired in young patients with colorectal cancer : CRC in patients under 50 years of age. Int J Colorectal Dis. 2012;27(1):71–9. doi: 10.1007/s00384-011-1291-8. [DOI] [PubMed] [Google Scholar]
- 41.Ballester V, Rashtak S, Boardman L. Clinical and molecular features of young-onset colorectal cancer. World J Gastroenterol. 2016;22(5):1736–44. doi: 10.3748/wjg.v22.i5.1736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zauber AG, Lansdorp-Vogelaar I, Knudsen AB, Wilschut J, van Ballegooijen M, Kuntz KM. Evaluating Test Strategies for Colorectal Cancer Screening: A Decision Analysis for the US Preventive Services Task Force. Annals of Internal Medicine. 2008;149(9):659–+. doi: 10.7326/0003-4819-149-9-200811040-00244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wolf AMD, Fontham ETH, Church TR, et al. Colorectal cancer screening for average-risk adults: 2018 guideline update from the American Cancer Society. CA Cancer J Clin. 2018 doi: 10.3322/caac.21457. [DOI] [PubMed] [Google Scholar]
- 44.Hogan NM, Hanley M, Hogan AM, et al. Awareness and Uptake of Family Screening in Patients Diagnosed with Colorectal Cancer at a Young Age. Gastroent Res Pract. 2015 doi: 10.1155/2015/194931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pearlman R, Frankel WL, Swanson B, et al. Prevalence and Spectrum of Germline Cancer Susceptibility Gene Mutations Among Patients With Early-Onset Colorectal Cancer. Jama Oncol. 2017;3(4):464–71. doi: 10.1001/jamaoncol.2016.5194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Balk EM, Earley A, Raman G, Avendano EA, Pittas AG, Remington PL. Combined Diet and Physical Activity Promotion Programs to Prevent Type 2 Diabetes Among Persons at Increased Risk: A Systematic Review for the Community Preventive Services Task Force. Ann Intern Med. 2015;163(6):437–51. doi: 10.7326/M15-0452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Murphy N, Cross AJ, Abubakar M, et al. A Nested Case-Control Study of Metabolically Defined Body Size Phenotypes and Risk of Colorectal Cancer in the European Prospective Investigation into Cancer and Nutrition (EPIC) Plos Med. 2016;13(4) doi: 10.1371/journal.pmed.1001988. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.