Abstract
Conventional MRI studies have not provided definitive evidence of progressive loss of brain volume in the early stages of schizophrenia, although more subtle changes may have gone undetected. We have looked for such subtle changes using volumetric MRI and magnetization transfer imaging (MTI), an advanced MRI technique sensitive to subtle neuropathological abnormalities. Magnetization transfer images and high-resolution volumetric T1-weighted images were acquired from 16 patients with first-episode schizophrenia at the start of the study and 3.7 years later. A group of 12 healthy controls were also scanned on two occasions. Images were processed using a voxel-based approach that allows whole-brain analysis. There was a group difference with a significant volume loss in the patients' white matter adjacent to the lateral ventricles in the right and left temporal lobes, in medial temporal gyrus, and in the white matter in and around the right middle frontal gyrus. No cortical differences were detected between the groups using MTI or volumetric MRI. The absence of any time-by-group interaction suggests that these abnormalities do not progress in the early stages of the disease. The results of the study need to be interpreted in the light of the small sample size and of the limitations of current image analysis methods.
1. Introduction
The most consistent finding in magnetic resonance imaging (MRI) studies in schizophrenia is a reduction in brain volume (Lawrie and Abukmeil, 1998, Shenton et al., 2001) and enlargement of lateral ventricles (Shenton et al., 2001). However, the time course of these and other brain changes is debatable. It has also been shown that certain brain MRI abnormalities are already evident by the first episode of illness (Gur et al., 2000, Pantelis et al., 2003) and may be present in unaffected relatives (McDonald et al., 2004). Recent studies using magnetization transfer imaging (MTI), an MRI technique sensitive to subtle neuropathological changes, have reported diffuse cortical abnormalities in people with chronic schizophrenia (Foong et al., 2001) and focal abnormalities in the medial prefrontal cortex, insula, and fasciculus uncinatus in first-episode patients in the absence of detectable atrophy (Bagary et al., 2003).
The histological abnormalities that underlie these imaging changes are subtle. Decreases in neuronal size in hippocampus and neocortex with reduced dendritic arborisation and synaptic abnormalities (Blennow et al., 1996), together with reduction in the number of oligodendrocytes, have been described (Uranova et al., 2004). These abnormalities may be genetically determined (see Harrison and Weinberger, 2005, for a review), and may represent the structural basis of the functional “disconnectivity” that may explain the symptoms and cognitive impairment of schizophrenia (Harrison and Eastwood, 2001).
The natural history of the neuropathology of schizophrenia is still unclear. Longitudinal imaging studies have not provided clear evidence of progression (see Shenton et al., 2001 for a review). However, the apparent clinical deterioration of some patients over time and reports suggesting progressive volumetric loss (Woods et al., 1990, DeLisi et al., 1995, Rapoport et al., 1999, Ho et al., 2003) have rekindled interest in the notion of neurodegeneration as an alternative to a non-progressive neurodevelopmental pathology, even if neuropathological studies have so far failed to support such an hypothesis (Arnold et al., 1998, Purohit et al., 1998, Falke et al., 2000).
Here we present data from a volumetric MRI and MTI follow-up study of first-episode schizophrenia patients. MTI is based on the interaction between protons bound to macromolecular structures (e.g. myelin and cell membranes) and free protons in tissue water (Wolff and Balaban, 1989). If the bound protons (which are invisible to conventional MRI) are preferentially saturated using an off-resonance radio-frequency pulse, this saturation is transferred to the free pool by cross-relaxation and chemical exchange, resulting in decreased signal from the MR-visible free water. The amount of signal loss, measured in percentage units, is the magnetization transfer ratio (MTR) which is highly reproducible in normal subjects rescanned at intervals (Barker et al., 1996). MTR depends on the density of macromolecules in a given tissue and pathological processes that damage the macromolecular structure will result in MTR reductions. We used voxel-based morphometry (VBM) (Ashburner and Friston, 2000, Good et al., 2001) to analyze the T1-weighted data sets and a similar voxel-based method for the MTR data sets. VBM is an automated method that avoids operator bias and yields information about the whole brain without an a priori selection of possibly abnormal brain regions. It has recently been applied to the study of brain abnormalities in schizophrenia and other psychiatric disorders (Kubicki et al., 2002, Moorhead et al., 2004).
2. Methods
2.1. Subjects
The subjects participating in this follow-up study had taken part in a previously reported cross sectional study of first-episode schizophrenia (Bagary et al., 2003). The patients were initially recruited and scanned within 1 month of their first presentation to psychiatric services with a first-episode of psychosis with the diagnosis of schizophrenia according to DSM-IV criteria subsequently being confirmed in all patients. The mean interval between the initial and the follow-up examination was 3.7 years. Of the 30 patients who were part of the original study, 12 were not available for follow-up: 3 had moved away and could not be contacted, 8 refused to participate, and one had died. Eighteen patients agreed to undergo scanning but two could not tolerate the procedure. This resulted in a final group of 16 patients (12 males and 4 females), with a mean age of 26.3 years at baseline (range 16–45) and 29.9 years (range 19–49) at follow-up. All participants were reviewed by experienced clinicians (EJ and TREB) after recruitment. Patients' symptoms were also assessed with the Scales for the Assessment of Positive Symptoms (SAPS) (Andreasen, 1981) and Negative Symptoms (SANS) (Andreasen, 1983). Scores for positive, disorganisation and negative syndromes of schizophrenia (Liddle and Barnes, 1990) were calculated for each patient by summing the SAPS and SANS global sub-scale scores relevant to each factor. Antipsychotic medication being taken at the time of both scans was recorded.
Thirty healthy volunteers had taken part in the original study (Bagary et al., 2003) and 12 returned for follow-up (4 males and 8 females); their mean ages were 31.6 years at baseline (range 21–48 years) and 36 years at follow-up (range 26–50 years). The mean interval between scans was 4.4 years. The criteria for exclusion for patients and controls were a history of head injury, neurological or systemic illness, a history of substance abuse during the study, or alcohol intake above 210 g/week during the study period. Controls with a history of psychiatric illness were also excluded. The study was approved by the relevant research Ethics Committees and written informed consent was obtained from all subjects.
2.2. MRI
MRI scans for all subjects were obtained using a standard quadrature head coil on a GE Signa 1.5 Tesla scanner (General Electric, Milwaukee, WI), which underwent regular quality-control checks. The same MRI scanner and an identical scanning protocol to that used in the original study (Bagary et al., 2003) were used at follow-up. A preliminary sagittal localizing scan was acquired, which was also used to check head position for the follow-up scan. High-resolution coronal volumetric images were acquired using a 3-dimensional inversion-recovery prepared T1-weighted spoiled gradient recalled (SPGR) echo sequence generating 124 contiguous, 1.5-mm coronal slices (echo time [TE] 4.2 ms, repetition time [TR] 15 ms, inversion time [TI] 450 ms, field of view [FOV] 24 cm2, 256 × 192 matrix, flip angle 20°). The 1 × 1.2 × 1.5-mm voxel size at acquisition was determined by scan time constraints, and images were automatically reconstructed by the scanner at a 1 × 1 × 1.5 mm voxel size. Axial magnetization transfer (MT) images were acquired using a dual spin-echo-based MT sequence (TE 30/80 ms, TR 1720 ms, 28 contiguous 5-mm axial slices, 256 × 128 pixel image matrix, 24 cm2 FOV) with and without a saturation pulse (16 ms, 23.2 μT Hamming appodized 3-lobe sinc pulse applied 1 KHz from water resonance). The 1 × 2 × 5 mm voxel size was again determined by scan time constraints, and the scanner automatically reconstructed the images at a 1 × 1 × 5 mm voxel size. The MT sequence (Barker et al., 1996) generates proton-density and T2-weighted images along with MT-weighted images. These images are all inherently registered to each other, and to the calculated MTR image created from proton-density and MT-weighted images.
2.3. Image processing
Data were processed on a Sun workstation (Sun Microsystems, Santa Clara, CA). First MTR maps were calculated on a pixel-by-pixel basis from the formula: MTR = {[Mo − Ms]/Mo} × 100, where Ms and Mo are mean signal intensities with and without the saturation pulse, respectively. MTR is not calculated in voxels where values in source images are below a threshold determined by the mean background noise level. We used the 30-ms (first) echo for MT calculation because the resulting map has higher signal-to-noise ratio than that from the 80-ms echo. For our spin-echo sequence, typical MTR values were approximately 40 pu (percentage units) for white matter, 35 pu for subcortical grey matter, 32 pu for cortical grey matter, and 2 pu (demonstrating the minimal direct saturation effects) for CSF.
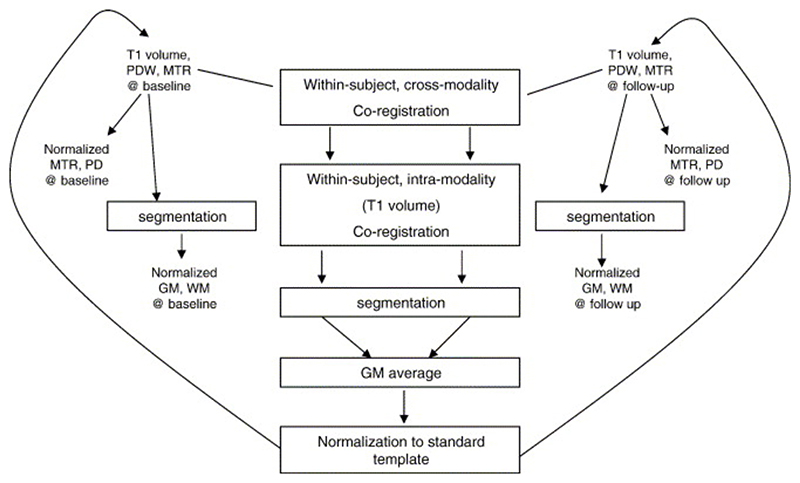
The following steps of the processing were performed using SPM2 (Wellcome Department of Cognitive Neurology, London, UK) in MATLAB (MathWorks, Natick, MA) (Fig. 1). First, the proton-density weighted scans from the MT sequences at baseline and at follow-up were each coregistered to the corresponding SPGR volume, using normalized mutual information as the cost function (Studholme et al., 1996). The SPGR volume at follow-up was then coregistered to the SPGR volume at baseline, and the transformation determined was applied to the follow-up MTR scan. At this stage all images were in the space of the baseline SPGR volume. The SPGR volumes were then segmented to produce images of the grey matter ‘shell’, both at baseline and follow-up (Ashburner and Friston, 2000). An average of the baseline and follow-up grey matter shells was then normalized to the SPM grey matter template, and the transformation thus determined was applied to the coregistered SPGR and MTR images. This approach was chosen, instead of normalizing each time point separately, in order to preserve small changes that might have occurred over the follow-up period. Finally, the normalized SPGRs were then segmented to produce images of grey matter and white matter volume in normalized space.
Fig. 1. Image processing steps using SPM2.
GM, grey matter; MTR, magnetic transfer ratio; PD, Proton Density; PDW, Proton Density Weighted; WM, white matter.
2.4. Smoothing
To improve signal-to-noise ratio, the segmented grey matter and white matter images were smoothed using a 6-mm full-width-half-maximum (FWHM) Gaussian filter. This procedure also allows for anatomical variability between subjects and has the effect of normalizing the distribution of the error terms in the data. After smoothing, the individual voxel intensity becomes the locally weighted average of grey matter density (Ashburner and Friston, 2000). Smoothing ensures the validity of inferences based on parametric tests according to the central limit theorem (Salmond et al., 2002) and sensitizes the analysis to features of a size determined by the smoothing kernel width (the ‘matched filter theorem’). Thus the size of the kernel can have a significant effect on the results of the analysis (as demonstrated, albeit for a different type of data, by Jones et al., 2005). We selected a priori a small smoothing kernel (6 mm) to match the expected small extent of changes taking place during the follow-up period (see Shenton et al., 2001). A similar smoothing procedure using 6-mm FWHM was performed on the MTR images.
Since we were interested in estimating group differences over time, as well as differences between groups at baseline, for each image type (MTR, white matter and grey matter maps) we computed a ‘sum’ and a ‘difference’ image between the normalized and smoothed image at follow-up and the normalized and smoothed image at baseline. As this step was performed for each subject, this gave a total of 28 ‘difference’ images and 28 ‘summed’ images for each grey matter, white matter and MTR image set. These combined images were used for the statistical comparison as explained below.
2.5. Statistical analysis
Statistical comparisons of both grey and white matter volumes, and of MTR maps, were performed using SPM2, which is based upon the general linear model and the theory of Gaussian random fields (Friston et al., 1994). Two ANCOVA models were used to compare the ‘difference’ and the ‘sum’ images between patients and controls:
(1) The ‘difference’ images were used to assess the main effect of time, by means of the T contrast [1, 1], as well as the group-by-time interaction, by means of the T contrast [1, − 1]. (2)The ‘sum’ images were used to assess the group effect, by means of the T contrast [1, − 1].
This type of analysis, recommended as a way of implementing a repeated-measure ANCOVA in SPM (http://www.jiscmail.ac.uk/lists/spm.htm), also prevents the differences already present at baseline between patients and controls from masking subsequent changes.
In both comparisons we accounted for age, sex and scan interval for each subject as nuisance variables. The choice of age and sex as nuisance variables was based on previous studies demonstrating regional anatomical differences in normal subjects related to these variables (Xu et al., 2000, Good et al., 2001).
3. Results
There were no significant differences in age between the patient and control groups (t(26) = 0.076). However, there were significant differences in gender, with more males in the patient group (χ2(1) = 0.027) (Table 1). When patients in the current study were compared with those from the original study who were not available for follow-up, there were no baseline differences in age (t(28) = − 0.52), gender ratio (χ2(1) = 0.05), premorbid IQ (t(27) = − 0.79), or duration of untreated psychosis (t(28) = − 0.79). An assessment of baseline symptoms scores revealed that there were no significant differences in negative symptoms (t(28) = 0.382), positive symptoms (t(28) = 0.167) or disorganization symptoms (t(28) = 0.053) between these two patient groups. One year from the onset of the study, quantitative clinical data were available in 10 of the 14 patients who failed to participate in the follow-up imaging study and in all 16 who participated. Comparisons between the two groups of patients revealed no significant differences on the scores for negative symptoms (t(24) = 0.426), positive symptoms (t(24) = 0.827) and disorganization symptoms (t(24) = 0.075). These comparisons suggest that the subgroup that took part in the follow-up imaging study is representative of the original sample, at least in the early stages of the disease. Unfortunately, at 3-year follow-up, quantitative clinical data were available for only 3 of the 14 patients who did not take part in the follow-up imaging study.
Table 1. Demographic data of follow-up patients and controls.
| Demographic data | Patients (n = 16) | Controls (n = 12) |
|---|---|---|
| Age at baseline [mean (range)] | 26.3 (16–45) years | 31.6 (21–48) years |
| Age at follow-up [mean (range)] | 29.9 (19–49) years | 36 (26–50) years |
| Males:females | 3:1 | 1:2 |
| *NART IQ (mean ± range) at baseline | 100.3 (75–121) | 110.9 (93–126) |
| Mean follow-up period (range in days) | 1329 days | 1606 days |
| 3.64 years | 4.4 years |
The clinical outcome of the patients reported in this study was variable. Eight had been continuously symptomatic in the intervening period despite medication, four had relapsed at least once or exhibited mild residual symptoms and were receiving medication, and the remaining four had been in full remission and medication-free throughout. When first scanned, 7 of these patients were receiving first-generation (typical) and 9 second-generation (atypical) antipsychotics. Of the 12 patients who were taking medication on both occasions, 8 had remained on the same type of antipsychotic (3 on first-generation and 5 on second-generation antipsychotics), and 4 had switched to a different type of drug (3 from first to second-generation antipsychotics). No gross structural brain abnormalities were reported at follow-up in any of the subjects when the MRI images were visually inspected by an experienced neuroradiologist.
3.1. Longitudinal changes in MTR and brain volume (time effect)
When patients and controls were considered as a single group, there were no significant changes between baseline and follow-up in MTR, white matter and grey matter volumes.
3.2. Comparisons of MTR and brain volumes between patients and controls (group effect)
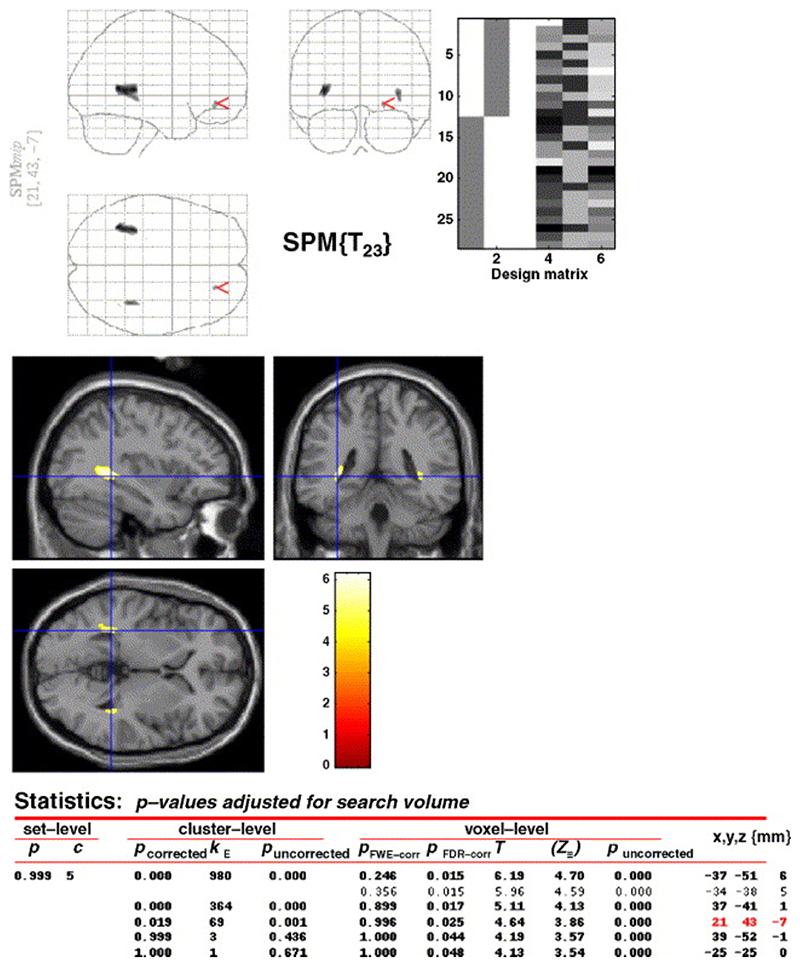
When the ‘sum’ images (initial and follow-up scans) of patients and controls were compared, there were no significant between-group differences in MTR or grey matter volume that survived corrections for multiple comparisons. On the other hand, there were significant volume losses (p < 0.05) in the patients in the white matter adjacent to the lateral ventricles in the right and left temporal lobes, in medial temporal gyrus, and in the white matter in and around the right middle frontal gyrus, and this finding survived the false discovery rate (FDR) correction for multiple comparisons (Fig 2).
Fig. 2. SPM map of white matter reductions in patients vs. controls at baseline.
3.3. Comparisons of change in MTR and brain volumes over time between patients and controls (group-by-time interaction)
There were no significant differences between the groups in the magnitude of changes in MTR, white or grey matter volumes during the follow-up period, suggesting that the changes in these parameters (i.e. white matter volume) were already present at baseline in the patient group and did not change over time.
4. Discussion
In this small follow-up study of patients with first-episode schizophrenia using both conventional and advanced MRI techniques, we found no evidence to suggest a progression of brain abnormalities in the early stages of the disease. Volumetric losses in white matter were present in patients with schizophrenia early in the disease, mostly in an area adjacent to the lateral ventricles and in and around the right middle fontal gyrus, areas that have previously been implicated in the illness (Shenton et al., 2001). Our findings are in keeping with those of other studies showing reductions in white matter volume (Hulshoff Pol et al., 2004, Antonova et al., 2005), and in particular with those of voxel based morphometric studies reporting white matter volume loss in frontal regions (Paillere-Martinot et al., 2001) and in areas adjacent to the left frontal and temporal cortex (Spalletta et al., 2003). Our findings are also in keeping with one of the most robust MRI findings in schizophrenia research, namely ventricular enlargement (Shenton et al., 2001, DeLisi et al., 1997, Rapoport et al., 1997, Lieberman et al., 2001), which is likely to be related to a reduction of white matter adjacent to the ventricles (Christensen et al., 2004).
In our original first-episode study (Bagary et al., 2003) MTI proved a valuable tool to detect grey matter abnormalities at baseline in a larger group of patients that included those reported here. The sample attrition at follow-up is likely to explain our failure to detect such abnormalities here, although the image analysis methodology used in this longitudinal study may also have contributed to our negative findings.
The lack of evidence for progressive brain abnormalities in our study echoes the findings of others in the early stages of the disease (Puri et al., 2001, Dickey et al., 2004, DeLisi and Hoff, 2005) and may be related to the characteristics of our sample, as previous follow-up studies have suggested that the evolution of brain abnormalities detected during the first episode is variable and to some extent related to clinical outcome and treatment, and that progressive changes, if present, may occur at different times and affect some brain areas preferentially. The results of these studies, including our own, have to be considered in the light of their limitations, among which the small sample sizes and methodological variability are paramount. DeLisi and collaborators, following a group of patients over a 10-year period, did not detect changes in ventricular, hippocampal or temporal lobe volume in the first 5 years of the study, although ventricular enlargement occurred in the subsequent 5 years in those with a poor clinical outcome (DeLisi et al., 1992, DeLisi et al., 1995, DeLisi et al., 1997, DeLisi et al., 2004). Others (Nair et al., 1997) have described early ventricular enlargement with later stabilization. Lieberman et al. (2001), in a 6-year follow-up study, also described progressive ventricular enlargement in relation to the course of illness, while cortical and hippocampal volumes remained unchanged. Several studies (Keshavan et al., 1998, Lieberman et al., 2001, Lieberman et al., 2005, Chakos et al., 2005) have suggested that antipsychotic drugs, in particular the second-generation drugs, may prevent some of these changes. More recent studies have also reported decreased frontal white matter (Ho et al., 2003) in the early stages of the illness, and other studies have revealed progressive grey matter changes (Mathalon et al., 2001, Shenton et al., 2001, Pantelis et al., 2003). A series of related studies of childhood-onset schizophrenia (Thompson et al., 2001, Gogtay et al., 2004, Whitworth et al., 2005, Vidal et al., 2006) have found severe cortical volume loss in this subgroup of patients with a particularly bad prognosis.
Differences in image analysis are also important in explaining the conflicting results of different follow-up studies. We used a voxel-based method, because it has the advantage of exploring the whole brain, without any a priori hypothesis on the location of abnormalities, but there are shortcomings to this method that limited its sensitivity to small changes. In particular, individual anatomical variability (Rajkowska and Goldman-Rakic, 1995), difficulty of coregistration (Ashburner and Friston, 2001, Bookstein, 2001) and overstringent statistical corrections for multiple comparisons (Worsley et al., 1996) which have all been discussed by others. In this context it is also important to mention that the choice of the size of the smoothing kernel (6 mm in our case), dictated by the magnitude of the expected changes, determines in turn the sensitivity of the method and, therefore, our results should be interpreted as showing lack of evidence for longitudinal changes of a spatial extent comparable to the size of our smoothing kernel, while more subtle changes would have gone undetected. We cannot exclude the possibility that the use of a different smoothing kernel, or a less stringent analysis (e.g. region of interest methodology), might have led to a different result.
Aside from these methodological considerations, there are other potential shortcomings of this study, such as the small sample size and the imperfect matching between patients and controls. Both of these problems resulted from the difficulty in tracing some of the subjects from the original study (Bagary et al., 2003). The small sample size has prevented us from exploring whether brain abnormalities evolve differently in patients exposed to different types of neuroleptics or with different clinical outcome. However, the size of our sample does not differ greatly from that of many follow-up studies (Chakos et al., 1994, DeGreef et al., 1991, Jacobsen et al., 1998, Keshavan et al., 1998, Nair et al., 1997, Rapoport et al., 1997, Rapoport et al., 1999, Kasai et al., 2003) and we have, as far as possible, controlled for the differences in age and gender between the groups by statistical means.
Finally, our study does not rule out the possibility that progressive brain abnormalities may occur at a later stage in the disease in relation to poor clinical outcome or cognitive impairment, and a longer follow-up study of a larger sample of patients will be required to answer these questions.
Acknowledgements
This study was supported by a programme grant from the Wellcome Trust.
We are grateful to Professor David Miller and other members of the NMR Unit, Clare Foster for her assistance and Dr. Dan Altmann for his statistical advice. We thank all the subjects who participated in this study and the consultants of West London and South West London and St George's Mental Health NHS Trusts.
References
- Andreasen NC. Scale for the Assessment of Negative Symptoms (SANS) University of Iowa; Iowa City, IA: 1981. [Google Scholar]
- Andreasen NC. Scale for the Assessment of Positive Symptoms (SAPS) University of Iowa; Iowa City, IA: 1983. [Google Scholar]
- Antonova E, Kumari V, Morris R, Halari R, Anilkumar A, Mehrotra R, Sharma T. The relationship of structural alterations to cognitive deficits in schizophrenia: a voxel-based morphometry study. Biol Psychiatry. 2005;58(6):457–467. doi: 10.1016/j.biopsych.2005.04.036. [DOI] [PubMed] [Google Scholar]
- Arnold SE, Trojanowski JQ, Gur RE, Blackwell P, Han LY, Choi C. Absence of neurodegenera-tion and neural injury in the cerebral cortex in a sample of elderly patients with schizophrenia. Arch Gen Psychiatry. 1998;55:225–232. doi: 10.1001/archpsyc.55.3.225. [DOI] [PubMed] [Google Scholar]
- Ashburner J, Friston KJ. Voxel based morphometry—the methods. Neuroimage. 2000;11:805–821. doi: 10.1006/nimg.2000.0582. [DOI] [PubMed] [Google Scholar]
- Ashburner J, Friston KJ. Why voxel-based morphometry should be used. Neuroimage. 2001;14(6):1238–1243. doi: 10.1006/nimg.2001.0961. [DOI] [PubMed] [Google Scholar]
- Bagary MS, Symms MR, Barker GJ, Mutsatsa SH, Joyce EM, Ron MA. Grey and white matter brain abnormalities in first-episode schizophrenia inferred from magnetization transfer imaging. Arch Gen Psychiatry. 2003;60:779–788. doi: 10.1001/archpsyc.60.8.779. [DOI] [PubMed] [Google Scholar]
- Barker GJ, Tofts PS, Gass A. An interleaved sequence for accurate and reproducible clinical measurement of magnetization transfer ratio. Magn Reson Imaging. 1996;14:403–411. doi: 10.1016/0730-725x(96)00019-7. [DOI] [PubMed] [Google Scholar]
- Blennow K, Davidsson P, Gottfries C-G, Ekman R, Heilig M. Synaptic degeneration in thalamus in schizophrenia [letter] Lancet. 1996;348:692–693. doi: 10.1016/S0140-6736(05)65124-0. [DOI] [PubMed] [Google Scholar]
- Bookstein FL. “Voxel-based morphometry” should not be used with imperfectly registered images. Neuroimage. 2001;14(6):1454–1462. doi: 10.1006/nimg.2001.0770. [DOI] [PubMed] [Google Scholar]
- Chakos MH, Lieberman JA, Bilder RM, Borenstein M, Lerner G, Bogerts B, Wu H, Kinon B, Ashtari M. Increase in caudate nuclei volumes of first-episode schizophrenic patients taking antipsychotic drugs. Am J Psychiatry. 1994;151:1430–1436. doi: 10.1176/ajp.151.10.1430. [DOI] [PubMed] [Google Scholar]
- Chakos MH, Schobel SA, Gu H, Gerig G, Bradford D, Charles C, et al. Duration of illness and treatment effects on hippocampal volume in male patients with schizophrenia. Br J Psychiatry. 2005;186:26–31. doi: 10.1192/bjp.186.1.26. [DOI] [PubMed] [Google Scholar]
- Christensen J, Holcomb J, Garver DL. State-related changes in cerebral white matter may underlie psychosis exacerbation. Psychiatry Res. 2004;130(1):71–78. doi: 10.1016/j.pscychresns.2003.08.002. [DOI] [PubMed] [Google Scholar]
- DeGreef G, Ashtari M, Wu HW, Borenstein M, Geisler S, Lieberman J. Follow up MRI study in first episode schizophrenia. Schizophr Res. 1991;5:204–206. doi: 10.1016/0920-9964(91)90075-3. [DOI] [PubMed] [Google Scholar]
- DeLisi LE, Hoff AL. Failure to find progressive temporal lobe volume decreases 10years subsequent to a first episode of schizophrenia. Psychiatry Res. 2005;138:265–268. doi: 10.1016/j.pscychresns.2005.02.005. [DOI] [PubMed] [Google Scholar]
- DeLisi LE, Stritzke P, Riordan H, Holan V, Boccio A, Kushner M, McClelland J, Van Eyl O, Anand A. The timing of brain morphological changes in schizophrenia and their relationship to clinical outcome. Biol Psychiatry. 1992;31:241–254. doi: 10.1016/0006-3223(92)90047-4. [DOI] [PubMed] [Google Scholar]
- DeLisi LE, Tew W, Xie S, Hoff AL, Sakuma M, Kushner M, et al. A prospective follow-up study of brain morphology and cognition in first-episode schizophrenic patients: preliminary findings. Biol Psychiatry. 1995;38:349–360. doi: 10.1016/0006-3223(94)00376-e. [DOI] [PubMed] [Google Scholar]
- DeLisi LE, Sakuma M, Tew W, Kushner M, Hoff AL, Grimson R. Schizophrenia as a chronic active brain process: a study of progressive brain structural change subsequent to the onset of schizophrenia. Psychiatry Res. 1997;74:129–140. doi: 10.1016/s0925-4927(97)00012-7. [DOI] [PubMed] [Google Scholar]
- DeLisi LE, Sakuma M, Maurizio AM, Relja M, Hoff AL. Cerebral ventricular change over the first 10 years after the onset of schizophrenia. Psychiatry Res. 2004;130:57–70. doi: 10.1016/j.pscychresns.2003.08.004. [DOI] [PubMed] [Google Scholar]
- Dickey CC, Salisbury DF, Nagy AI, Hirayasu Y, Lee CU, McCarley RW, Shenton ME. Follow-up MRI study of prefrontal volumes in first-episode psychotic patients. Schizophr Res. 2004;71:349–351. doi: 10.1016/j.schres.2004.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falke E, Han LY, Arnold SE. Absence of neurodegeneration in the thalamus and caudate of elderly patients with schizophrenia. Psychiatry Res. 2000;93:103–110. doi: 10.1016/s0165-1781(00)00104-9. [DOI] [PubMed] [Google Scholar]
- Foong J, Symms MR, Barker GJ, Maier M, Woermann FG, Miller DH, et al. Neuropathological abnormalities in schizophrenia: evidence from magnetization transfer imaging. Brain. 2001;124:882–892. doi: 10.1093/brain/124.5.882. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Worsley K, Frackowiak RS, Maziotta JC, Evans AC. Assessing the significance of focal activation using their spatial extent. Hum Brain Mapp. 1994;1:210–220. doi: 10.1002/hbm.460010306. [DOI] [PubMed] [Google Scholar]
- Gogtay N, Sporn A, Clasen LS, Nugent TF, III, Greenstein D, Nicolson R, Giedd JN, Lenane M, Gochman P, Evans A, Rapoport JL. Comparison of progressive cortical gray matter loss in childhood-onset schizophrenia with that in childhood-onset atypical psychoses. Arch Gen Psychiatry. 2004;61:17–22. doi: 10.1001/archpsyc.61.1.17. [DOI] [PubMed] [Google Scholar]
- Good CD, Johnsrude IS, Ashburner J, Henson RN, Friston KJ, Frackowiak RS. A voxel-based morphometric study of ageing in 465 normal adult human brains. Neuroimage. 2001;14:21–36. doi: 10.1006/nimg.2001.0786. [DOI] [PubMed] [Google Scholar]
- Gur RE, Turetsky BI, Cowell PE, Finkelman C, Maany V, Grossman RI, et al. Temporolimbic volume reductions in schizophrenia. Arch Gen Psychiatry. 2000;57:769–775. doi: 10.1001/archpsyc.57.8.769. [DOI] [PubMed] [Google Scholar]
- Harrison PJ, Eastwood SL. Neuropathological studies of synaptic connectivity in the hippocampal formation in schizophrenia. Hippocampus. 2001;11:508–519. doi: 10.1002/hipo.1067. [DOI] [PubMed] [Google Scholar]
- Harrison PJ, Weinberger DR. Schizophrenia genes, gene expression, and neuropathology: on the matter of their convergence. Mol Psychiatry. 2005;10:40–68. doi: 10.1038/sj.mp.4001558. [DOI] [PubMed] [Google Scholar]
- Ho BC, Andreasen NC, Nopoulos P, Arndt S, Magnotta V, Flaum M. Progressive structural brain abnormalities and their relationship to clinical outcome a longitudinal magnetic resonance imaging study early in schizophrenia. Arch Gen Psychiatry. 2003;60:585–594. doi: 10.1001/archpsyc.60.6.585. [DOI] [PubMed] [Google Scholar]
- Hulshoff Pol HE, Brans RG, van Haren NE, Schnack HG, Langen M, Baare WF, van Oel CJ, Kahn RS. Gray and white matter volume abnormalities in monozygotic and same-gender dizygotic twins discordant for schizophrenia. Biol Psychiatry. 2004;55(2):126–130. doi: 10.1016/s0006-3223(03)00728-5. [DOI] [PubMed] [Google Scholar]
- Jacobsen LK, Giedd J, Castellanos FX, Vaituzis AC, Hamburger SD, Kumra S, et al. Progressive reduction of temporal lobe structures in childhood-onset schizophrenia. Am J Psychiatry. 1998;155:678–685. doi: 10.1176/ajp.155.5.678. [DOI] [PubMed] [Google Scholar]
- Jones DK, Symms MR, Cercignani M, Howard RJ. The effect of filter size on VBM analyses of DT-MRI data. Neuroimage. 2005;26:546–554. doi: 10.1016/j.neuroimage.2005.02.013. [DOI] [PubMed] [Google Scholar]
- Kasai K, Shenton ME, Salisbury DF, Hirayasu Y, Onitsuka T, Spencer MH, et al. Progressive decrease of left Heschl gyrus and planum temporale grey matter volume in first-episode schizophrenia: a longitudinal magnetic resonance imaging study. Arch Gen Psychiatry. 2003;60:766–775. doi: 10.1001/archpsyc.60.8.766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keshavan MS, Haas GL, Kahn CE, Aguilar E, Dick EL, Schooler NR, Sweeney JA, Pettegrew JW. Superior temporal gyrus and the course of early schizophrenia: progressive, static, or reversible? J Psychiatr Res. 1998;32:161–167. doi: 10.1016/s0022-3956(97)00038-1. [DOI] [PubMed] [Google Scholar]
- Kubicki M, Shenton ME, Salisbury DF, Hirayasu Y, Kasai K, Kikinis R, et al. Voxel-based morphometric analysis of grey matter in first episode schizophrenia. Neuroimage. 2002;17:1711–1719. doi: 10.1006/nimg.2002.1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrie SM, Abukmeil SS. Brain abnormality in schizophrenia. A systematic and quantitative review of volumetric magnetic resonance imaging studies. Br J Psychiatry. 1998;172:110–120. doi: 10.1192/bjp.172.2.110. [DOI] [PubMed] [Google Scholar]
- Liddle PF, Barnes TR. Syndromes of chronic schizophrenia. Br J Psychiatry. 1990;157:558–561. doi: 10.1192/bjp.157.4.558. [DOI] [PubMed] [Google Scholar]
- Lieberman J, Chakos M, Wu Hu, Alvir J, Hoffman E, Robinson D, et al. Longitudinal study of brain morphology in first episode schizophrenia. Biol Psychiatry. 2001;49:487–499. doi: 10.1016/s0006-3223(01)01067-8. [DOI] [PubMed] [Google Scholar]
- Lieberman JA, Tollefson GD, Charles C, Zipursky R, Sharma T, Kahn RS, et al. Antipsychotic drug effects on brain morphology in first-episode psychosis. Arch Gen Psychiatry. 2005;62:361–370. doi: 10.1001/archpsyc.62.4.361. [DOI] [PubMed] [Google Scholar]
- Mathalon DH, Sullivan EV, Lim KO, Pfefferbaum A. Progressive brain volume changes and the clinical course of schizophrenia in men: a longitudinal magnetic resonance imaging study. Arch Gen Psychiatry. 2001;58:148–157. doi: 10.1001/archpsyc.58.2.148. [DOI] [PubMed] [Google Scholar]
- McDonald C, Bullmore ET, Sham PC, Chitnis X, Wickham H, Bramon E, et al. Association of genetic risks for schizophrenia and bipolar disorder with specific and generic brain structural endophenotypes. Arch Gen Psychiatry. 2004;61:974–984. doi: 10.1001/archpsyc.61.10.974. [DOI] [PubMed] [Google Scholar]
- Moorhead TW, Job DE, Whalley HC, Sanderson TL, Johnstone EC, Lawrie SM. Voxel-based morphometry of comorbid schizophrenia and learning disability: analyses in normalized and native spaces using parametric and nonparametric statistical methods. Neuroimage. 2004;22:188–202. doi: 10.1016/j.neuroimage.2003.12.012. [DOI] [PubMed] [Google Scholar]
- Nair TR, Christensen JD, Kingsbury SJ, Kumar NG, Terry WM, Garver DL. Progression of cerebroventricular enlargement and the subtyping of schizophrenia. Psychiatry Res. 1997;74:141–150. doi: 10.1016/s0925-4927(97)00013-9. [DOI] [PubMed] [Google Scholar]
- Nelson HE. The National Adult Reading Test. NFER-Nelson Publishing; Windsor, Berks, UK: 1982. [Google Scholar]
- Pantelis C, Velakoulis D, McGorry PD, Wood SJ, Suckling J, Phillips LJ, et al. Neuroanatomical abnormalities before and after onset of psychosis: a cross-sectional and longitudinal MRI comparison. Lancet. 2003;361:281–288. doi: 10.1016/S0140-6736(03)12323-9. [DOI] [PubMed] [Google Scholar]
- Paillere-Martinot M, Caclin A, Artiges E, Poline JB, Joliot M, Mallet L, Recasens C, Attar-Levy D, Martinot JL. Cerebral gray and white matter reductions and clinical correlates in patients with early onset schizophrenia. Schizophr Res. 2001;50(1-2):19–26. doi: 10.1016/s0920-9964(00)00137-7. [DOI] [PubMed] [Google Scholar]
- Puri BK, Hutton SB, Saeed N, Oatridge A, Hajnal JV, Duncan L, et al. A serial longitudinal quantitative MRI study of cerebral changes in first-episode schizophrenia using image segmentation and subvoxel registration. Psychiatry Res. 2001;106:141–150. doi: 10.1016/s0925-4927(01)00072-5. [DOI] [PubMed] [Google Scholar]
- Purohit DP, Perl DP, Haroutunian V, Powchik P, Davidson M, Davis KL. Alzheimer disease and related neurodegenerative diseases in elderly patients with schizophrenia: a postmortem neuropathologic study of 100 cases. Arch Gen Psychiatry. 1998;55:205–211. doi: 10.1001/archpsyc.55.3.205. [DOI] [PubMed] [Google Scholar]
- Rajkowska G, Goldman-Rakic PS. Cytoarchitectonic definition of prefrontal areas in the normal human cortex: II. Variability in locations of areas 9 and 46 and relationship to the Talairach Coordinate System. Cereb Cortex. 1995;5(4):323–337. doi: 10.1093/cercor/5.4.323. [DOI] [PubMed] [Google Scholar]
- Rapoport JL, Giedd J, Kumra S, Jacobsen L, Smith A, Lee P, Nelson J, Hamburger S. Childhood-onset schizophrenia. Progressive ventricular change during adolescence. Arch Gen Psychiatry. 1997;54:897–903. doi: 10.1001/archpsyc.1997.01830220013002. [DOI] [PubMed] [Google Scholar]
- Rapoport JL, Giedd JN, Blumenthal J, Hamburger S, Jeffries N, Fernandez T, et al. Progressive cortical change during adolescence in childhood-onset schizophrenia. A longitudinal magnetic resonance imaging study. Arch Gen Psychiatry. 1999;56:649–654. doi: 10.1001/archpsyc.56.7.649. [DOI] [PubMed] [Google Scholar]
- Salmond CH, Ashburner J, Vargha-Khadem F, Connelly A, Gadian DG, Friston KJ. Distributional assumptions in voxel-based morphometry. Neuroimage. 2002;17(2):1027–1030. [PubMed] [Google Scholar]
- Shenton ME, Dickey CC, Frumin M, McCarley RW. A review of MRI findings in schizophrenia. Schizophr Res. 2001;49:1–52. doi: 10.1016/s0920-9964(01)00163-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spalletta G, Tomaiuolo F, Marino V, Bonaviri G, Trequattrini A, Caltagirone C. Chronic schizophrenia as a brain mis-connection syndrome: a white matter voxel-based morphometry study. Schizophr Res. 2003;64(1):15–23. doi: 10.1016/s0920-9964(03)00010-0. [DOI] [PubMed] [Google Scholar]
- Studholme C, Hill DLG, Hawkes DJ. Automated three-dimensional registration of magnetic resonance and positron emission tomography brain images by multiresolution optimization of voxel similarity measures. Med Phys. 1996;24:25–35. doi: 10.1118/1.598130. [DOI] [PubMed] [Google Scholar]
- Thompson PM, Vidal C, Giedd JN, Gochman P, Blumenthal J, Nicolson R, et al. Mapping adolescent brain change reveals dynamic wave of accelerated gray matter loss in very earlyonset schizophrenia. Proc Natl Acad Sci U S A. 2001;98:11650–11655. doi: 10.1073/pnas.201243998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uranova NA, Vostrikov VM, Orlovskaya DD, Rachmanova VI. Oligodendroglial density in the prefrontal cortex in schizophrenia and mood disorders: a study from the Stanley Neuropathology Consortium. Schizophr Res. 2004;67:269–275. doi: 10.1016/S0920-9964(03)00181-6. [DOI] [PubMed] [Google Scholar]
- Vidal CN, Rapoport JL, Hayashi KM, Geaga JA, Sui Y, McLemore LE, Alaghband Y, Giedd JN, Gochman P, Blumenthal J, et al. Dynamically spreading frontal and cingulate deficits mapped in adolescents with schizophrenia. Arch Gen Psychiatry. 2006;63:25–34. doi: 10.1001/archpsyc.63.1.25. [DOI] [PubMed] [Google Scholar]
- Whitworth AB, Kemmler G, Honeder M, Kremser C, Felber S, Hausmann A, Walch T, Wanko C, Weiss EM, Stuppaeck CH, Fleischhacker WW. Longitudinal volumetric MRI study in first-and multiple-episode male schizophrenia patients. Psychiatry Res. 2005;140:225–237. doi: 10.1016/j.pscychresns.2005.07.006. [DOI] [PubMed] [Google Scholar]
- Wolff SD, Balaban RS. Magnetization transfer contrast (MTC) and tissue water proton relaxation in vivo. Magn Reson Med. 1989;10:135–144. doi: 10.1002/mrm.1910100113. [DOI] [PubMed] [Google Scholar]
- Woods BT, Yurgelun-Todd D, Benes FM, Frankenburg FR, Pope HG, Jr, McSparren J. Progressive ventricular enlargement in schizophrenia: comparison to bipolar affective disorder and correlation with clinical course. Biol Psychiatry. 1990;27:341–352. doi: 10.1016/0006-3223(90)90008-p. [DOI] [PubMed] [Google Scholar]
- Worsley KJ, Marrett S, Neelin P, Vandal AC, Friston KJ, Evans AC. A unified statistical approach for determining significant signals in images of cerebral activation. Hum Brain Mapp. 1996;4(1):58–73. doi: 10.1002/(SICI)1097-0193(1996)4:1<58::AID-HBM4>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- Xu J, Kobayashi S, Yamaguchi S, Iijima K, Okada K, Yamashita K. Related Articles, 2000; Gender effects on age-related changes in brain structure. AJNR Am J Neuroradiol. 2000 Jan;21(1):112–118. [PMC free article] [PubMed] [Google Scholar]