Abstract
Background
Patients with brain injury who are unresponsive to command may perform cognitive tasks that are detected by functional magnetic resonance imaging (fMRI) and electroencephalography (EEG). This phenomenon, known as cognitive motor dissociation, has not been systematically studied in a large cohort of patients with disorders of consciousness.
Methods
In this prospective cohort study conducted at six international centers, we collected clinical, behavioral, and task-based fMRI and EEG data on a convenience sample of 353 adults with disorders of consciousness. Sixty-six percent of participants had only fMRI or EEG and 34% had both. We determined the proportion of participants with and without observable responses to verbal commands who had responses to command on task-based fMRI or EEG.
Results
Participants’ median age was 37.9 years, median time from injury was 7.9 months (26% within 28 days of injury), and 50% had a traumatic etiology. Of 241 participants without observable response to commands (i.e., behavioral diagnosis of coma, vegetative state, or minimally conscious state minus), we detected cognitive motor dissociation in 60 (25%; n=11 assessed with fMRI only, n=13 with EEG, and n=36 with both methods). Cognitive motor dissociation was associated with younger age, longer chronicity, and traumatic etiology. In contrast, of 112 participants with observable response to commands, task-based fMRI or EEG responses were present in 43 (38%).
Conclusions
Approximately one in four participants without observable response to commands performed a cognitive task on fMRI or EEG, compared with one in three participants with observable response to commands.
Introduction
Cognitive motor dissociation1 is an established phenomenon2–4 that describes individuals with severe brain injury who are observed to be behaviorally unresponsive to commands, yet demonstrate brain activation on functional magnetic resonance imaging (fMRI) or electroencephalography (EEG) when presented with cognitive tasks, such as motor imagery commands. Failing to identify cognitive motor dissociation in patients with disorders of consciousness could affect decisions related to withdrawing life-sustaining treatment, goals of care, and clinical management. Evidence of cognitive motor dissociation may prompt more thorough investigation of subtle behaviors that are under volitional control,5 uncovering potential avenues for communication and patient autonomy.
In prior studies, cognitive motor dissociation was observed in 10-20% of persons with a disorder of consciousness,3,6–9 a finding demonstrated in both the acute10,11 and chronic12 stages of recovery as well as across etiologies.9 Detection of cognitive motor dissociation has been associated with more rapid recovery and better outcome at 1-year post-injury.11,13 To be detected on fMRI or EEG, responses to command must be sustained and require not only language comprehension but likely more cognitive processing (e.g., short-term memory, attention, persistence; Supplementary Appendix Table S1) than responding to a single command at the bedside. Identifying that a patient who otherwise appears unconscious has the capacity for cognitive processing may mitigate emotional suffering when their clinical team and family recognizes that they are aware and treat them as such. The harm in assuming an unresponsive patient is also unaware has been previously described.14 Recent international clinical guidelines vary in their level of endorsement of fMRI and EEG for detecting cognitive motor dissociation, from supporting their use15,16 to proposing that these techniques should be further studied prior to their application to routine medical practice.17
Most prior cognitive motor dissociation studies were conducted at a single site with relatively small cohorts.3,6,7,9–11,18,19 Our consortium determined the proportion of cognitive motor dissociation in a multi-center, multi-national cohort of participants with disorders of consciousness who were assessed at specialized centers that have the capability of studying this phenomenon.
Methods
Sites and Participants
Six multi-national sites contributed behavioral and task-based fMRI and/or EEG data to a centrally-curated database from 2006 to 2023. Participants were adults (≥18 years) with a disorder of consciousness recruited from intensive care units, hospital wards, rehabilitation facilities, nursing homes, and the community. Exclusion criteria at all sites were: 1) prior neurological or psychiatric disease, and 2) contraindication for MRI/EEG (as appropriate based on modalities used at each site; e.g. for fMRI, inability to lay flat or ferrous metal implants). References for inclusion and exclusion criteria for each site are available in Supplementary Appendix Table S2 and criteria are further detailed in Supplementary Appendix Table S3. Sites received approval from local ethics review boards and followed local regulations to obtain surrogate consent for study participation. Participants may have been included in prior studies aimed at testing specific methodologies or answering different research questions (Supplementary Appendix Figure S1).
NS (Administrative Principal Investigator), AO, and SL planned the initial phase of the study in 2008; NS, AO, SL, LN, JC, ES, OG, BE, JA, JP, and JG were site Principal Investigators from 2011 to 2023. The REDCap multi-center database and analyses were designed by NS, EB, YB, JG, JA, ES, LN, JC, JP, DM, and OG. EB carried out the data analyses and had no role in data collection. All other authors participated in data acquisition and supported local site infrastructure. YB and NS wrote the first draft of the paper, which was further developed in discussion with JA, PC, JC, OG, DM, LN, JP, ES, JG, and EB. All authors reviewed the paper.
Procedures
Trained study staff conducted behavioral assessments by administering the Coma Recovery Scale-Revised (CRS-R, Supplementary Appendix Table S4),20,21 a standardized measure with high interrater and test-retest reliability20 that is validated in multiple languages.22,23 The CRS-R is the preferred measure for assessment of level of consciousness across international guidelines, and was the means by which we assigned patients a disorders of consciousness diagnosis.15–17 CRS-R examiners were blind to fMRI and EEG assessment results.
Each of the six sites has experience designing fMRI and EEG studies for patients with disorders of consciousness and followed local, previously published, validated procedures for acquiring, analyzing, and interpreting these data (Supplementary Appendix Table S2). fMRI and EEG data processing and interpretation procedures were automated to minimize bias associated with subjective discrimination of positive from negative responses. fMRI data used established statistical cut-points and cluster-correction for multiple comparisons to reduce the potential for spurious activations to appear in the a priori established regions of interest. EEG analysis utilized either a comparison of power spectral density at each channel (corrected for multiple comparisons) or a machine learning algorithm. Trained study staff who were masked to the participants’ behavioral assessment conducted EEG artifact rejection. Prior to evaluating participants with disorders of consciousness, sites tested the fMRI3,8,10 and EEG10,11,24,25 acquisition and analytic methods in healthy participants to ensure positive responses were obtained in individuals with intact cognitive processing; across these studies, which included 5 - 16 healthy participants, 70-100% demonstrated responses to command on task-based fMRI or EEG.
We included participants who had: 1) at least one CRS-R score and 2) assessment of command-following via task-based fMRI and/or EEG (e.g., “imagine playing tennis”, “imagine opening and closing your hand”, “open and close your hand”, or visual/auditory discrimination; see Supplementary Appendix Table S2 and Table S3 for complete task-based query) within seven days of the CRS-R. If participants were tested across multiple days with either fMRI or EEG, we included only the best performance on the first day in our analyses. We also documented the number of participants for whom it was not possible to analyze or interpret any fMRI or EEG sessions (e.g., due to motion artifact). Study staff from each site entered data into a central REDCap (Research Electronic Data Capture)26 database housed at Icahn School of Medicine at Mount Sinai, the Data Coordinating Center. REDCap variables included: demographic and clinical characteristics, CRS-R subscale (auditory, visual, motor, oromotor/verbal, communication, and arousal) and total scores (Supplementary Appendix Table S4), number of task-based fMRI and/or EEG sessions attempted, and number of task-based fMRI and/or EEG sessions with a positive or negative result.
Analysis
We divided participants into two groups based on whether or not responses to verbal commands or intelligible speech was observed on the CRS-R examination. Cognitive motor dissociation was operationally-defined as the absence of command-following and intelligible speech on the CRS-R (i.e., auditory subscale score <3, and visual subscale <5, and oromotor/verbal subscale <3, and communication subscale <1; Supplementary Appendix Table S4) in the setting of a positive response to at least one task-based fMRI and/or EEG paradigm.1 Applying this definition, only participants with a CRS-R diagnosis of coma, vegetative state (also referred to as unresponsive wakefulness syndrome), or minimally conscious state minus (i.e., participants with signs of conscious awareness, such as visual pursuit, but without responses to commands or intelligible verbal output, Supplementary Appendix Table S4)27 can be classified as having cognitive motor dissociation. We combined the diagnostic categories of coma and vegetative state as both indicate an unconscious state. We also evaluated task-based fMRI and EEG responses in participants with observable response to commands (a behavioral diagnosis of minimally conscious state plus [participants with signs of conscious awareness that include following commands or intelligible verbal output] or emerged from minimally conscious state [participants who use common objects in a functional manner or correctly respond to basic yes/no situational orientation questions]).
The preservation or recovery of multiple complex cognitive functions required to perform the fMRI/EEG tasks over minutes of sustained engagement (Supplementary Appendix Table S1) minimizes spurious responses on fMRI28 and EEG.29,30 This methodological approach results in a high rate of test failure (i.e., no fMRI or EEG response in patients with observable command-following, or healthy participants, i.e., “false negative”).3,10,31,32 Given this context, we interpret positive fMRI and EEG results in participants without observable response to commands (a behavioral diagnosis of coma, vegetative state, or minimally conscious state minus) as specific for cognitive motor dissociation, but at a potential cost of sensitivity.
We report descriptive characteristics of the sample and the proportion of all participants who demonstrated cognitive motor dissociation. We describe differences in cognitive motor dissociation rates by age, chronicity, CRS-R diagnosis, etiology, and site. We calculated kappa coefficients to determine the agreement between the behavioral diagnosis, task-based fMRI, and task-based EEG results. Confidence intervals are not adjusted for multiplicity and cannot be used in place of hypothesis testing.
Results
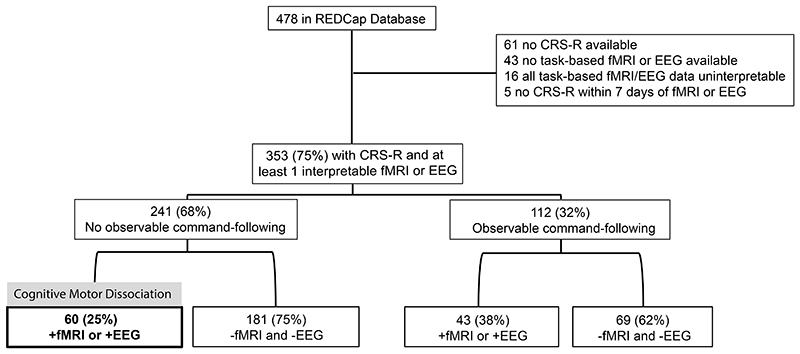
The central database included 478 participants of whom 125 were excluded from the current study (n=61 with no CRS-R score, n=43 with no task-based fMRI or EEG data, n=16 with uninterpretable fMRI and/or EEG data, and n=5 with a CRS-R score that was obtained more than 7 days before or after fMRI/EEG; Figure 1). Characteristics of included participants (n=353) are provided in Table 1 and Supplementary Appendix Figure S2. Supplementary Appendix Figure S1 describes the 232 (66%) participants who were included in prior studies addressing different research questions. All participants had at least one fMRI (n=215, 60.9%) or EEG (n=260, 73.7%) assessment. Both fMRI and EEG were performed in 122 (34.6%) participants. The median [IQR] days between the CRS-R assessment and fMRI or EEG was 1 [0-2] and 0 [0-1] days, respectively. The CRS-R was performed within one day of fMRI or EEG in approximately 70% of participants, (Supplementary Appendix Figure S3). The demographic representativeness of our sample is addressed in Supplementary Appendix Table S5.
Figure 1. Participant Enrollment and Proportion with Cognitive Motor Dissociation.
Of 478 participants in the REDCap database, 353 were assessed with the CRS-R and with at least one command-following paradigm on fMRI or EEG within 7 days. Cognitive motor dissociation was observed in 25% of participants with no observable evidence of command-following (i.e., behavioral diagnosis of coma/vegetative state, [unconscious], or minimally conscious state minus [minimally conscious state without command-following], left branch). In participants with observable command-following (i.e., behavioral diagnosis of minimally conscious state plus [minimally conscious state with command-following] or emerged from minimally conscious state, right branch), a response to task-based fMRI or EEG was not detected in more than 60%. “+fMRI or +EEG” indicates that at least one assessment (either fMRI or EEG regardless of whether participants had one or both of these assessments) was positive. “-fMRI and -EEG” indicates that for participants with fMRI only, the fMRI assessment was negative; for participants with EEG only, the EEG assessment was negative; for participants with both fMRI and EEG, both assessments were negative.
Abbreviations: CRS-R Coma Recovery Scale-Revised, EEG electroencephalography, fMRI functional magnetic resonance imaging
Table 1. Participant Demographic and Injury Characteristics.
| Variable | Total Sample N=353a |
|---|---|
| Age at injury, median [IQR] | 37.9 [23.8, 55.8] |
| Sex no. (%) | |
| Male | 226 (64.4%) |
| Female | 125 (35.6%) |
| Missing | 2 (0.6%) |
| Months between injury and Coma Recovery Scale-Revised assessment, median [IQR] | 7.9 [1.0, 22.1] |
| < 28 days post injury no. (%) | 90 (25.5%) |
| Etiology no. (%) | |
| Traumatic brain injury | 176 (49.9%) |
| Cardiac arrest/anoxia | 57 (16.1%) |
| SAH, IVH, ICH, stroke, aneurysm | 65 (18.4%) |
| Other | 55 (15.6%) |
| Diagnosis (based on the Coma Recovery Scale-Revised), no. (%) | |
| Unconscious (coma/vegetative state) | 140 (39.7%) |
| Minimally conscious state minus | 101 (28.6%) |
| Minimally conscious state plus | 77 (21.8%) |
| Emerged from minimally conscious state | 35 (9.9%) |
all proportions are calculated from the number of participants indicated in the column heading (n=353); Minimally conscious state minus = minimally conscious state without command-following, Minimally conscious state plus = minimally conscious with command-following
Abbreviations: ICH intracerebral hemorrhage; IVH intraventricular hemorrhage; SAH subarachnoid hemorrhage
Cognitive Motor Dissociation in Participants without Observable Response to Commands
Of 241 participants with a CRS-R diagnosis of coma/vegetative state (i.e., unconscious), or minimally conscious state minus, 60 (25%) responded to the command-following task on fMRI, EEG, or both (Figure 1). Supplementary Appendix Figures S4, S5 and Supplementary Appendix Table S6 provide the distribution of cognitive motor dissociation by CRS-R total score. Compared to participants without cognitive motor dissociation, participants with cognitive motor dissociation were younger (median [IQR] 30.5 [20.4] versus 45.3 [32.6] years), more likely to have a traumatic etiology (65% versus 38%), and more likely to have a CRS-R diagnosis of minimally conscious state minus (53% versus 38%). Participants with cognitive motor dissociation were also evaluated later post-injury or illness (10.7 [20.6] versus 4.3 [13.8] months, Table 2). Among participants with cognitive motor dissociation, 18% were assessed with fMRI only, 22% with EEG only, and 60% with both fMRI and EEG. The frequency of cognitive motor dissociation varied across sites (Supplementary Appendix Table S7). Supplementary Appendix Table S8 provides the proportion of participants with CRS-R diagnoses of coma/vegetative state and minimally conscious state minus who have positive and negative fMRI/EEG responses.
Table 2. Demographics, Clinical Characteristics, and fMRI/EEG Results in Participants Without Observable Command-following.
| Variable | All Participants Without Observable Command-following N=241a |
+fMRI or +EEG (i.e., cognitive motor dissociation) N=60 |
-fMRI and -EEG N=181 |
|---|---|---|---|
| Diagnosis (based on the Coma Recovery Scale-Revised), no. (%) |
|||
| 140 (58.1%) | 28 (46.7%) | 112 (61.9%) | |
| Unconscious (coma/vegetative state) | 101 (41.9%) | 32 (53.3%) | 69 (38.1%) |
| Minimally conscious state minus | |||
| Assessed with fMRI only no. (%) | 61 (25.3%) | 11 (18.3%) | 50 (27.6%) |
| Assessed with EEG only no. (%) | 101 (41.9%) | 13 (21.7%) | 88 (48.6%) |
| Assessed with fMRI and EEG no. (%) | 79 (32.8%) | 36 (60.0%) | 43 (23.8%) |
| Age at injury, median [IQR] | 40.2 [15.0] | 30.5 [20.4] | 45.3 [32.6] |
| Sex no. (%) | |||
| Male | 146 (60.6%) | 39 (65.0%) | 107 (59.1%) |
| Female | 93 (38.6%) | 21 (35.0%) | 72 (39.8%) |
| Missing | 2 (0.8%) | 0 (0%) | 2 (1.1%) |
| Months between injury and Coma Recovery Scale-Revised assessment, median [IQR] | 6.3 [16.3] | 10.7 [20.6] | 4.3 [13.8] |
| < 28 days post injury/illness no. (%) | 72 (29.9%) | 12 (20.0%) | 60 (33.1%) |
| ≥ 28 days post injury/illness no. (%) | 169 (70.1) | 48 (80.0%) | 121 (66.9%) |
| Etiology no. (%) | |||
| Traumatic brain injury | 108 (44.8%) | 39 (65.0%) | 69 (38.1%) |
| Cardiac arrest/anoxia | 45 (18.6%) | 4 (6.7%) | 41 (22.7%) |
| SAH, IVH, ICH, stroke, aneurysm | 48 (19.9%) | 9 (15.0%) | 39 (21.6%) |
| Other | 40 (16.6%) | 8 (13.3%) | 32 (17.7%) |
all proportions are calculated from the number of participants indicated in the column heading; for example, of 241 participants with a Coma Recovery Scale-Revised (CRS-R) behavioral diagnosis of coma or vegetative state (unconscious) or minimally conscious state minus (minimally conscious state without command-following), 140 (58.1%) were unconscious. “+fMRI or +EEG” indicates that at least one assessment (either fMRI or EEG regardless of whether participants had one or both of these assessments) was positive. “-fMRI and -EEG” indicates that for participants with fMRI only, the fMRI assessment was negative; for participants with EEG only, the EEG assessment was negative; for participants with both fMRI and EEG, both assessments were negative.
Abbreviations: ICH intracerebral hemorrhage; IVH intraventricular hemorrhage; EEG electroencephalography; fMRI functional magnetic resonance imaging; SAH subarachnoid hemorrhage
Task-based fMRI and EEG responses in Participants with Observable Response to Commands
Of 112 participants with a CRS-R diagnosis of minimally conscious state plus or emerged from minimally conscious state, 43 (38%, Table 3) demonstrated command-following on task-based fMRI, task-based EEG, or both assessments. Among participants in this group, 23% were assessed with fMRI only, 19% with EEG only, and 58% with both fMRI and EEG. Responses to fMRI and EEG command-following tests were absent in more than 60% of participants who demonstrated evidence of command-following via behavioral responses on bedside assessment. Supplementary Appendix Table S9 provides the proportion of participants who demonstrated command-following on task-based fMRI or EEG stratified by CRS-R diagnosis, chronicity, and etiology.
Table 3. Demographics, Clinical Characteristics, and fMRI/EEG Results in Participants With Observable Command-following.
| Variable | All Participants With Observable Command-following N=112a |
+fMRI or +EEG N=43 |
-fMRI and -EEG N=69 |
|---|---|---|---|
| Diagnosis (based on the Coma Recovery Scale-Revised) no. (%) | 77 (68.8%) 35 (31.3%) |
26 (60.5%) 17 (39.5%) |
51 (73.9%) 18 (26.1%) |
| Minimally conscious state plus | |||
| Emerged from the minimally conscious state | |||
| Assessed with fMRI only no. (%) | 32 (28.6%) | 10 (23.3%) | 22 (31.9%) |
| Assessed with EEG only no. (%) | 37 (33.0%) | 8 (18.6%) | 29 (42.0%) |
| Assessed with fMRI and EEG no. (%) | 43 (38.4%) | 25 (58.1%) | 18 (26.1%) |
| Age at injury, median [IQR] | 33.8 [32.4] | 29.4 [24.7] | 38.6 [33.0] |
| Sex no. (%) | |||
| Male | 80 (71.4%) | 30 (69.8%) | 50 (72.5%) |
| Female | 32 (28.6%) | 13 (30.2%) | 19 (27.5%) |
| Months between injury and Coma Recovery Scale-Revised assessment, median [IQR] | 12.9 [45.3] | 12.6 [51.9] | 12.9 [40.7] |
| < 28 days post injury/illness no. (%) | 18 (16.1%) | 10 (23.3%) | 8 (11.6%) |
| ≥ 28 days post injury/illness no. (%) | 94 (83.9%) | 33 (76.7%) | 61 (88.4%) |
| Etiology no. (%) | |||
| Traumatic brain injury | 68 (60.7%) | 30 (69.8%) | 38 (55.1%) |
| Cardiac arrest/anoxia | 12 (10.7%) | 1 (2.3%) | 11 (15.9%) |
| SAH, IVH, ICH, stroke, aneurysm | 17 (15.2%) | 9 (20.9%) | 8 (11.6%) |
| Other | 15 (13.4%) | 3 (7.0%) | 12 (17.4%) |
all proportions are calculated from the number of participants indicated in the column heading; for example, of 112 patients with a Coma Recovery Scale-Revised (CRS-R) behavioral diagnosis of minimally conscious state plus (minimally conscious state with command-following) or emerged from the minimally conscious state, 77 (68.8%) had a CRS-R diagnosis of minimally conscious state plus. “+fMRI or +EEG” indicates that at least one assessment (either fMRI or EEG regardless of whether participants had one or both of these assessments) was positive. “-fMRI and -EEG” indicates that for participants with fMRI only, the fMRI assessment was negative; for participants with EEG only, the EEG assessment was negative; for participants with both fMRI and EEG, both assessments were negative.
Abbreviations: ICH intracerebral hemorrhage; IVH intraventricular hemorrhage; EEG electroencephalography; fMRI functional magnetic resonance imaging; SAH subarachnoid hemorrhage; TBI traumatic brain injury
Kappa coefficients indicating level of agreement between the behavioral diagnosis, fMRI, and EEG were low (0.09-0.15 for agreement between CRS-R and fMRI or EEG; 0.02-0.04 for agreement between fMRI and EEG; see Supplementary Appendix Tables S10 and S11).
Discussion
In this multi-national investigation of a convenience sample of patients with disorders of consciousness, we detected cognitive motor dissociation on task-based fMRI or EEG in approximately 25%. This proportion is higher than previous estimates3,6,9–11. While standardized behavioral evaluation remains the reference standard for detecting command-following at the bedside, task-based fMRI and EEG can improve the detection rate, and performing both appears to be a more sensitive method.
The proportion of participants with cognitive motor dissociation in our study is 5-10 percent higher than previously reported.3,6,9–11 This finding may be due to our multi-modal approach, which classified participants based on responses to either fMRI or EEG in the 30% who had both assessments. The rate of cognitive motor dissociation may have been even higher if all participants were assessed with both modalities. Consistent with prior research, we found that cognitive motor dissociation is most common in patients with TBI,3,6,9,11 chronic disorders of consciousness,9 and a behavioral diagnosis of minimally conscious state minus.11 However, cognitive motor dissociation was also detected in participants with non-traumatic etiologies such as stroke and cardiac arrest, as well as in acute disorders of consciousness, and in patients who were behaviorally unconscious (coma/vegetative state).
The frequency of cognitive motor dissociation may be underestimated in prior studies and in ours for multiple reasons. First, the tasks used in active fMRI and EEG studies may require more cognitive resources (e.g., short term memory, selective attention, mental persistence) than typical command-following trials performed at the bedside. Although this hypothesis has not been proven (Supplementary Appendix Table S1)33, it is supported by our finding that fMRI and EEG responses were detected in only 38% of participants who demonstrated command-following behaviorally at the bedside. Second, the fMRI and EEG analytic techniques employed by the study sites are intentionally designed to minimize the potential for a false positive result, which may increase the likelihood of a false negative finding. Third, most studies assess participants with either fMRI or EEG. We found that participants assessed with both modalities were more likely to demonstrate cognitive motor dissociation. Finally, behavioral fluctuation is common in patients across all disorder of consciousness categories, which may contribute to negative fMRI or EEG findings or to disparate results between these two modalities.34–36
Several limitations should be considered when interpreting the results of this study. Participants were recruited using a variety of methods, including critically ill patients enrolled consecutively from the intensive care unit and those with chronic illness or injury enrolled by caregivers during the post-acute phase of recovery. All participants in the chronic group survived their initial illness or injury and had access to a research facility with advanced fMRI and EEG capabilities. This survival bias may reflect greater cognitive reserve and resilience over time. As such, our results may not be representative of global cognitive motor dissociation prevalence (see Supplementary Appendix Table S5). In the absence of standardized approaches to evaluate for cognitive motor dissociation, participating sites used heterogeneous strategies to acquire, analyze, and interpret data, leading to differences in the number, type, and ordering of the tasks. These differences, along with variations in recruitment strategies and participant characteristics, may contribute to the unequal proportion of cognitive motor dissociation observed at each site (ranging from 2% to 45%). Our findings may therefore not generalize across all centers. Large-scale validation studies are needed to optimize data acquisition and analysis for clinical translation. Statistical analyses conducted as part of this study were univariate and descriptive. Thus, we are unable to evaluate the independent contribution of any one variable in predicting cognitive motor dissociation. Agreement between cognitive motor dissociation detected by fMRI versus EEG was low which may result from fluctuations in awareness or differences in the underlying construct measured by each technique. Although participants were evaluated with CRS-R, fMRI, and EEG a variable number of times, for consistency, we analyzed the best performance from each modality and are unable to determine the number of assessments that were excluded due to poor performance. Serial CRS-R, fMRI, and EEG assessments may improve detection of cognitive motor dissociation but requires that these techniques be readily available. Finally, access to both the specially-trained personnel and technical assessments needed to assess for cognitive motor dissociation is presently available in only a few academic medical centers around the world, limiting the feasibility of performing these assessments in general practice.
Our results confirm, using neuroimaging and electrophysiologic methods that cognitive motor dissociation is more common than currently realized. Although task-based fMRI and EEG are not yet widely available for clinical assessment of disorders of consciousness, the knowledge that cognitive motor dissociation is not a rare occurrence should prompt further study to explore whether its detection can improve outcomes. Additionally, standardization, validation, and simplification of task-based fMRI and EEG methods used to detect cognitive motor dissociation is needed to prompt widespread clinical integration of these techniques and investigation of the bioethical implications of the findings.37
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org.
Supplementary Material
Acknowledgements
We acknowledge the seminal contributions of Dr. Martin Coleman (1975 – 2011) who died while rescuing others from an avalanche in Snowdonia.
We thank our patients, their families, and caregivers for their participation in these studies.
We thank the many authors of contributing studies (see references), hospital staff, and personnel who supported these studies at Weill Cornell Medicine; Rockefeller University Hospital; Massachusetts General Hospital; Addenbrookes Hospital Cambridge, UK; University Hospital of Liege, Belgium; and Pitié-Salpêtrière University Hospital (APHP, SU, Paris, France).
We also thank the team at the Royal Hospital for Neurodisability, Putney, London UK for referring a substantial proportion of the participants included in the University of Cambridge cohort.
Funding
Primary funding for all sites in this study was generously provided by the James S. McDonnell Foundation “Collaborative study of recovery of consciousness after severe brain injury”, 2008-2024. No part of this study was supported or funded by vendors of fMRI or EEG companies.
Funding was also provided to individual investigators:
AT: Research Associate at the Belgian National Fund for Scientific Research (F.R.S-FNRS)
BLE: NIH Director’s Office (DP2HD101400), Center for Integration of Medicine and Innovative Technology, Chen Institute MGH Research Scholar Award
BR, LN, JDS, MV: Paris Brain Institute (France) and the program “Investissements d’avenir” (ANR-10-IAIHU-06)
DKM: National Institute for Heath and Care Research (NIHR) Biomedical Research Centre, Cambridge (Neuroscience Theme; Brain Injury and Repair Theme), an NIHR Senior Investigator Award (NF-SI-0512-10090), Canadian Institute for Advanced Research
ES: The Stephen Erskine Fellowship (Queens’ College, Cambridge), the Canadian Institute for Advanced Research (CIFAR; grant RCZB/072 RG93193)
JA: The Evelyn trust, Neurorehabilitation Project, East of England CLARHC (NIHR) Research Fellowship, Cambridge, UK
JC: NINDS (R01NS106014, R03 NS112760, and the DANA Foundation))
JDS: ERA PerMed JTC2019 “PerBrain” and FLAG-ERA project “ModelDXConsciousness”.
JTG: National Institute on Disability and Rehabilitation Research (H133A120085, 90DPTB0011, 90DPTB0027), The Barbara Epstein Foundation
JP: MRC G0001237, G9439390, G0600986; NIHR-IS-HTC-0112-10165, MIC-2016-009.
LN: UNIM and ‘Equipe FRM 2015’
NDS, MC, HUV, JDV, JJF: R01 HD051912-02, Jerold B. Katz Foundation, Lenny C. Katz Foundation, Award numbern: UL1TR002384 and UL1 TR001866 from the National Center for Advancing Translational Sciences (NCATS, National Institutes of Health (NIH) Clinical and Translational Science Award (CTSA) program
NDS, JJF: Charles A. Dana Foundation; Richard Lounsbery Foundation
OG: Research Associate at the Belgian National Fund for Scientific Research (F.R.S-FNRS)
PC: Research Fellow at the Belgian National Fund for Scientific Research (F.R.S-FNRS)
SL: National Natural Science Foundation of China (Grant no. 81920108023), European Foundation of Biomedical Research FERB Onlus, fund Generet of King Baudouin Foundation, Mind Care International Foundation. SL is Chairholder of the Canada Excellence Research Chair (CERC) in Neuroplasticity, Laval University, CERVO Brain Research Centre, Quebec, Canada, Research Director at the Belgian National Fund for Scientific Research, and Research Director at the at the Belgian National Fund for Scientific Research (F.R.S-FNRS).
YGB: National Institute on Disability and Rehabilitation Research (H133A120085), National Institute on Disability, Independent Living, and Rehabilitation Research (90DPTB0011, 90DPTB0027)
References
- 1.Schiff ND. Cognitive motor dissociation following severe brain injuries. JAMA Neurol. 2015;72:1413–5. doi: 10.1001/jamaneurol.2015.2899. [DOI] [PubMed] [Google Scholar]
- 2.Owen AM, Coleman MR, Boly M, Davis MH, Laureys S, Pickard JD. Detecting awareness in the vegetative state. Science. 2006;313:1402. doi: 10.1126/science.1130197. [DOI] [PubMed] [Google Scholar]
- 3.Monti MM, Vanhaudenhuyse A, Coleman MR, et al. Willful modulation of brain activity in disorders of consciousness. N Engl J Med. 2010;362:579–89. doi: 10.1056/NEJMoa0905370. [DOI] [PubMed] [Google Scholar]
- 4.Edlow BL, Claassen J, Schiff ND, Greer DM. Recovery from disorders of consciousness: mechanisms, prognosis and emerging therapies. Nat Rev Neurol. 2021;17:135–56. doi: 10.1038/s41582-020-00428-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Whyte J, DiPasquale MC, Vaccaro M. Assessment of command-following in minimally conscious brain injured patients. Archives of Physical Medicine & Rehabilitation. 1999;80:653–60. doi: 10.1016/s0003-9993(99)90168-5. [DOI] [PubMed] [Google Scholar]
- 6.Kondziella D, Friberg CK, Frokjaer VG, Fabricius M, Møller K. Preserved consciousness in vegetative and minimal conscious states: systematic review and meta-analysis. Journal of Neurology, Neurosurgery & Psychiatry. 2016;87:485–92. doi: 10.1136/jnnp-2015-310958. [DOI] [PubMed] [Google Scholar]
- 7.Curley WH, Forgacs PB, Voss HU, Conte MM, Schiff ND. Characterization of EEG signals revealing covert cognition in the injured brain. Brain. 2018;141:1404–21. doi: 10.1093/brain/awy070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bardin JC, Fins JJ, Katz DI, et al. Dissociations between behavioural and functional magnetic resonance imaging-based evaluations of cognitive function after brain injury. Brain. 2011;134:769–82. doi: 10.1093/brain/awr005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schnakers C, Hirsch M, Noé M, et al. Covert cognition in disorders of consciousness: a meta-analysis. Brain Sci. 2020;10 doi: 10.3390/brainsci10120930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Edlow BL, Chatelle C, Spencer CA, et al. Early detection of consciousness in patients with acute severe traumatic brain injury. Brain. 2017;140:2399–414. doi: 10.1093/brain/awx176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Claassen J, Doyle K, Matory A, et al. Detection of brain activation in unresponsive patients with acute brain injury. N Engl J Med. 2019;380:2497–505. doi: 10.1056/NEJMoa1812757. [DOI] [PubMed] [Google Scholar]
- 12.Stender J, Gosseries O, Bruno M-A, et al. Diagnostic precision of PET imaging and functional MRI in disorders of consciousness: a clinical validation study. The Lancet. 2014;384:514–22. doi: 10.1016/S0140-6736(14)60042-8. [DOI] [PubMed] [Google Scholar]
- 13.Egbebike J, Shen Q, Doyle K, et al. Cognitive-motor dissociation and time to functional recovery in patients with acute brain injury in the USA: a prospective observational cohort study. Lancet Neurol. 2022;21:704–13. doi: 10.1016/S1474-4422(22)00212-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.La Puma J, Schiedermayer DL, Gulyas AE, Siegler M. Talking to comatose patients. Arch Neurol. 1988;45:20–2. doi: 10.1001/archneur.1988.00520250026012. [DOI] [PubMed] [Google Scholar]
- 15.Kondziella D, Bender A, Diserens K, et al. European Academy of Neurology guideline on the diagnosis of coma and other disorders of consciousness. European Journal of Neurology. 2020;27:741–56. doi: 10.1111/ene.14151. [DOI] [PubMed] [Google Scholar]
- 16.Giacino JT, Katz DI, Schiff ND, et al. Practice guideline update recommendations summary: Disorders of consciousness. Neurology. 2018;91:450. doi: 10.1212/WNL.0000000000005926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Royal College of Physicians. Prolonged disorders of consciousness following sudden onset brain injury: National clinical guidelines. RCP; London: 2020. [Google Scholar]
- 18.Naci L, Sinai L, Owen AM. Detecting and interpreting conscious experiences in behaviorally non-responsive patients. Neuroimage. 2017;145:304–13. doi: 10.1016/j.neuroimage.2015.11.059. [DOI] [PubMed] [Google Scholar]
- 19.Sitt JD, King JR, El Karoui I, et al. Large scale screening of neural signatures of consciousness in patients in a vegetative or minimally conscious state. Brain. 2014;137:2258–70. doi: 10.1093/brain/awu141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Giacino JT, Kalmar K, Whyte J. The JFK Coma Recovery Scale-Revised: measurement characteristics and diagnostic utility. Arch Phys Med Rehabil. 2004;85:2020–9. doi: 10.1016/j.apmr.2004.02.033. [DOI] [PubMed] [Google Scholar]
- 21.Bodien YG, Chatelle C, Taubert A, Uchani S, Giacino JT, Ehrlich-Jones L. Updated measurement characteristics and clinical utility of the Coma Recovery Scale-Revised among individuals with acquired brain injury. Arch Phys Med Rehabil. 2021;102:169–71. [Google Scholar]
- 22.Schnakers C, Majerus S, Giacino J, et al. A French validation study of the Coma Recovery Scale-Revised (CRS-R) Brain Inj. 2008;22:786–92. doi: 10.1080/02699050802403557. [DOI] [PubMed] [Google Scholar]
- 23.Tamashiro M, Rivas ME, Ron M, Salierno F, Dalera M, Olmos L. A Spanish validation of the Coma Recovery Scale-Revised (CRS-R) Brain Inj. 2014;28:1744–7. doi: 10.3109/02699052.2014.947621. [DOI] [PubMed] [Google Scholar]
- 24.Goldfine AM, Victor JD, Conte MM, Bardin JC, Schiff ND. Determination of awareness in patients with severe brain injury using EEG power spectral analysis. Clin Neurophysiol. 2011;122:2157–68. doi: 10.1016/j.clinph.2011.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cruse D, Chennu S, Chatelle C, et al. Bedside detection of awareness in the vegetative state: a cohort study. Lancet. 2011;378:2088–94. doi: 10.1016/S0140-6736(11)61224-5. [DOI] [PubMed] [Google Scholar]
- 26.Harris PA, Taylor R, Minor BL, et al. The REDCap consortium: Building an international community of software platform partners. Journal of Biomedical Informatics. 2019;95:103208. doi: 10.1016/j.jbi.2019.103208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Thibaut A, Bodien YG, Laureys S, Giacino JT. Minimally conscious state “plus”: diagnostic criteria and relation to functional recovery. Journal of Neurology. 2020;267:1245–54. doi: 10.1007/s00415-019-09628-y. [DOI] [PubMed] [Google Scholar]
- 28.Eklund A, Nichols TE, Knutsson H. Cluster failure: Why fMRI inferences for spatial extent have inflated false-positive rates. Proc Natl Acad Sci U S A. 2016;113:7900–5. doi: 10.1073/pnas.1602413113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bokil H, Purpura K, Schoffelen JM, Thomson D, Mitra P. Comparing spectra and coherences for groups of unequal size. J Neurosci Methods. 2007;159:337–45. doi: 10.1016/j.jneumeth.2006.07.011. [DOI] [PubMed] [Google Scholar]
- 30.Noirhomme Q, Brecheisen R, Lesenfants D, Antonopoulos G, Laureys S. “Look at my classifier’s result”: Disentangling unresponsive from (minimally) conscious patients. Neuroimage. 2017;145:288–303. doi: 10.1016/j.neuroimage.2015.12.006. [DOI] [PubMed] [Google Scholar]
- 31.Boly M, Coleman MR, Davis MH, et al. When thoughts become action: an fMRI paradigm to study volitional brain activity in non-communicative brain injured patients. Neuroimage. 2007;36:979–92. doi: 10.1016/j.neuroimage.2007.02.047. [DOI] [PubMed] [Google Scholar]
- 32.Peterson A, Cruse D, Naci L, Weijer C, Owen AM. Risk, diagnostic error, and the clinical science of consciousness. Neuroimage Clin. 2015;7:588–97. doi: 10.1016/j.nicl.2015.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Glover S, Baran M. The motor-cognitive model of motor imagery: Evidence from timing errors in simulated reaching and grasping. J Exp Psychol Hum Percept Perform. 2017;43:1359–75. doi: 10.1037/xhp0000389. [DOI] [PubMed] [Google Scholar]
- 34.Wannez S, Heine L, Thonnard M, Gosseries O, Laureys S, Group CS. The repetition of behavioral assessments in diagnosis of disorders of consciousness. Ann Neurol. 2017;81:883–9. doi: 10.1002/ana.24962. [DOI] [PubMed] [Google Scholar]
- 35.Papadimitriou C, Weaver JA, Guernon A, Walsh E, Mallinson T, Pape TLB. “Fluctuation is the norm”: Rehabilitation practitioner perspectives on ambiguity and uncertainty in their work with persons in disordered states of consciousness after traumatic brain injury. PLoS One. 2022;17:e0267194. doi: 10.1371/journal.pone.0267194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Giacino J, Ashwal S, Childs N, et al. The minimally conscious state: definition and diagnostic criteria. Neurology. 2002;58:349–53. doi: 10.1212/wnl.58.3.349. [DOI] [PubMed] [Google Scholar]
- 37.Fins JJ. Rights Come to Mind: Brain injury, ethics, and the struggle for consciousness. Cambridge University Press; New York, NY: 2015. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.