Abstract
Neurocysticercosis, infection with larval Taenia solium, is a common, serious neuroparasitic infection. Larval degeneration results in inflammatory cell influx and granuloma formation which leads to clinical symptomatology. The role of chemokines in such cell influx is unknown. We demonstrate that monocyte stimulation by T. solium larval antigen (TsAg) results in a differential profile of CXCL8/IL-8 (146.5 ± 8.5 ng/ml after 24 h), CCL2/MCP-1 (267 ± 4 ng/ml after 48 h) and CCL3/MIP-1α (1.72 ± 0.43 ng/ml after 8 h) secretion. There was coordinate mRNA accumulation reaching maximum at 1 h for CCL3 and 2 h for CXCL8 and CCL2. TsAg induced maximal nuclear binding of p65, p50 and c-rel subunits of the transcriptional regulator NF-κB by 2 h. IκBα but not IκBβ was degraded within 10 min before resynthesis by 2 h. Pre-treatment with the broad-spectrum NF-κB inhibitor pyrrolidine dithiocarbamate caused complete abrogation of TsAg-induced CCL2 secretion (p = 0.005) and 91% reduction of CXCL8 secretion (p = 0.0003). TsAg was unable to induce CXCL8 promoter activity in Toll-like receptor (TLR)-2 or TLR-4/MD-2 transfected HeLa cells in the absence of lectins or other adaptor molecules. In summary, our data demonstrate that TsAg induces chemokine secretion via specific pathways dependent on NF-κB but not TLR-4/TLR-2, and indicate a potential mechanism whereby larval degeneration results in brain inflammation.
Keywords: Cysticercosis, CXCL8, CCL2, Toll-like receptor, NF-κB, Monocyte
1. Introduction
Cysticercosis is a disease caused by infection with the larval stage of the pork tapeworm Taenia solium. Human disease is almost exclusively acquired via the faecal–oral route following ingestion of microscopic T. solium eggs. These penetrate the mucosal lining of the gut and enter the general circulation, from where they migrate and can encyst in almost all tissues and organs of the body. Larval dissemination in the central nervous system (CNS), known as neurocysticercosis (NCC) affects an estimated 50 million people worldwide. The disease is endemic in South America, sub-Saharan Africa and many parts of Asia, with increasing rates of prevalence in countries such as USA, where there is immigration from endemic areas [1]. Symptomatic NCC usually follows a long asymptomatic period with little or no evident inflammation around cysticerci lodged in the brain. Larval degeneration, which may be spontaneous or secondary to anti-parasitic therapy, results in tissue damage, which then manifests most frequently as seizures but can result in almost any neurological disorder including meningitis, cerebral infarction, intracranial hypertension and psychiatric disturbance [1].
The nature of the immune response around the degenerating cysticerci involving a potentially CNS-specific response is key to understanding the underlying pathology in NCC. Little is known about the immunology of human NCC, although there have been useful studies in animal models of disease which involve organisms related to T. solium. Various strategies are thought to be employed by the viable parasite to maintain a Th2 permissive environment [2]. Larval degeneration may be initiated by a switch to Th1-type cytokine secretion profile, resulting in the influx of host inflammatory cells including monocytes, which ultimately results in the formation of granulomas consisting of mononuclear cells and granulocytes [3]. Chemokines are key regulators of leukocyte recruitment and activation, but relatively little is known of their role in mediating cell influx in NCC.
Chemokines are divided into four families, depending on the arrangement of the first two NH2-terminal cysteine residues. The CXC and CC chemokines, typified by CXCL8/IL-8 and CCL2/MCP-1, respectively, are the largest of families. Chemokines have a wide array of biological functions but are key in leukocyte trafficking in response to inflammatory and infectious stimuli. In the context of granulomatous and parasitic infection, CXCL8, a key attractant for neutrophils, monocytes and T cells [4,5], has a central role in Mycobacterium tuberculosis-induced granuloma formation [6] as well as being involved in the immune response to Leishmania major and Plasmodium falciparum [7,8]. CCL3 and CCL2, predominantly mononuclear cell chemoattractants, are important determinants of granuloma size and composition in inflammation induced by larval Schistosoma mansoni [9]. In mice, CCL2 may shape adaptive immune responses by controlling Th2 cell polarisation and may be essential for monocyte recruitment in vivo [10].
Chemokine gene expression is regulated by transcription factors such as NF-κB, which bind to sequence-specific sites on gene promoters. NF-κB dimers translocate from the cytoplasm, where they are associated with IκB inhibitory proteins, which on cell activation, are phosphorylated, ubiquitinylated and proteolytically degraded. Activation of NF-κB-dependent pathways by microbial stimuli is critically regulated by Toll-like receptors (TLR) initially identified in the fruit fly Drosophila. TLRs recognise pathogen-associated molecular patterns (PAMP) before initiating activation of transcription factors including NF-κB and activation of innate immune responses. TLR-2 and TLR-4 are the best characterised members of the family. TLR-4 recognises Gram-negative bacterial products such as LPS, a process dependent on the accessory molecule MD-2 [11]. TLR-2 is the receptor responsible for transmitting signals initiated by Gram-positive bacterial products and mycobacterial lipoproteins and lipoarabinomannan [12]. In addition, these receptors are important in the initiation of innate immune responses to viruses, fungi and parasites [13–15].
Although much is known about chemokines in granulomatous inflammation, there are few data on chemokines in NCC. We found elevated serum CCL11/eotaxin concentrations in patients in vivo [16], and CCL2, CCL3, CCL4, CCL5 and CCL11 are expressed in the brain of mice infected with Mesocestoides corti [17]. In the present study, we have investigated mechanisms regulating human monocyte chemokine production in response to stimulation with T. solium larval antigens (TsAg). We show for the first time that cysticercal antigens have the ability to drive CXCL8/IL-8, CCL2/MCP-1 and CCL3/MIP-1α secretion from human monocytes. We demonstrate that secretion of these chemokines is critically regulated transcriptionally via an NF-κB-dependent and TLR-2/4-independent pathway.
2. Materials and methods
2.1. Media, reagents and Abs
RPMI-1640 media, HBSS and L-glutamine were obtained from Life Technologies (Paisley, UK). Foetal calf serum was obtained from Labtech International (Ringmer, UK). The Limulus Amebocyte Lysate (LAL) assay kit was obtained from Associates of Cape Cod (Liverpool, UK). Ficoll-Paque, nitrocellulose membrane (Hybond-C), nylon membrane (Hybond-N), ECL Hyperfilm and [γ-32P] ATP were obtained from Amersham Biosciences (Little Chalfont, UK). The RNase Protection Assay (RPA) system was obtained from BD Pharmingen (San Diego, CA) and the DIG Wash and Blot set and CDP-Star were from Roche (Lewes, UK). The Avidin-Phosphatase was obtained from Tropix-Applied Biosystems (Warrington, UK). The SP-1 and NF-κB consensus oligos were purchased from Promega, as was the T4 polynucleotide kinase (Promega, Southampton, UK). Pre-made 30% Bis-acrylamide stock used for PAGE was obtained from Anachem (Luton, UK). Rabbit anti-human p65, p50, p52, c-rel, rel-B and c-fos antibodies used in supershift assays along with rabbit anti-human IκBα/β antibodies used for westerns were obtained from Santa Cruz Biotechnology (Santa Cruz, CA). All other materials and reagents were purchased from Sigma (Poole, UK).
2.2. Preparation of TsAg
T. solium metacestodes (cysticerci/larvae) were dissected at post-mortem from naturally infected pigs obtained from endemic areas in the Peruvian highlands. Metacestodes were homogenised in cold-buffered PBS using a glass homogeniser. Antigen suspensions were subsequently prepared by sonicating at 70 Hz for 3 min before centrifugation at 28,000g for 30 min at 4 °C. They were then stored at −70 °C until required for experiments. This preparation, when run on a Western blot under non-reducing conditions using gradient polyacrylamide gels exposed to sera from vaccine-protected pigs, was shown to contain a range of proteins from 8 kDa to 100 kDa in size. Concentration of protein in the antigen preparation was quantified after homogenisation and sonication using the Bradford technique. In the preparation used in experiments for this study, the protein concentration was 5.2 mg/ml. Endotoxin contamination, measured using the LAL assay, was found to be minimal (between 0.11 and 5.46 pg/ml) and did not, when assayed in isolation, stimulate chemokine secretion in vitro.
2.3. Human primary monocyte isolation
Human monocytes were prepared from pooled buffy coat residues, obtained from healthy donors (North London Blood Transfusion Service, Colindale, UK). Briefly, mononuclear cells were isolated by density gradient centrifugation over Ficoll-Paque. Monocytes were adhesion purified on tissue culture plastic for 1 h before being washed three times with sterile HBSS to remove non-adherent lymphocytes. Purified monocytes were maintained in RPMI-1640 media supplemented with 10% FCS (endotoxin level <0.06 ng/ml), 2 mM L-glutamine and 100 μg/ml ampicillin at 37 °C in a humidified 5% CO2 atmosphere. Cell preparations obtained using this methodology were at least 95% monocytes as analysed by FACS using CD14 staining.
2.4. Experimental protocol
Purified monocytes were seeded at a density of 1 × 105 cells/cm2 in standard 6-well tissue culture plates, 100-mm- or 150-mm-diameter dishes as appropriate for preparation of cell supernatants, RNA or nuclear proteins. Cells were stimulated in triplicate for each experimental condition with 100 μg/ml TsAg or 10 μg/ml LPS (positive control from Escherichia coli serotype 0127:B8) for defined time points. Cell-free supernatants, cellular RNA, whole cell lysates or nuclear extracts were subsequently collected and stored at either −20 °C or −80 °C (as appropriate) before being assayed. In NF-κB blocking experiments, monocytes were pre-incubated with 1, 10 or 100 μM pyrrolidine dithiocarbamate (PDTC—a broad-spectrum NF-κB inhibitor) for 2 h before being stimulated by either TsAg or LPS for 24 h.
2.5. Chemokine ELISAs
CXCL8/IL-8 and CCL2/MCP-1 protein concentrations in cell culture supernatants were measured using standard ELISAs based on matched antibody pairs (R&D Systems, Abingdon, UK). The lower limit of sensitivity of the CCL2 and CXCL8 assays was 15 pg/ml. The level of CCL3/MIP-1α was measured using cytosets, according to the manufacturer’s protocols (Bio-source). The lower limit of detection of this assay was 12 pg/ml.
2.6. RNA extraction and RNase protection assays (RPA)
At specific time points after stimulation with TsAg, total cellular RNA was extracted from 1 × 107 monocytes. Cells were homogenised in a guanidine thiocyanate and phenol mixture (Tri-reagent, Sigma) and frozen at −80 °C. RNA was precipitated with isopropanol and washed with 70% ethanol before being dissolved in RNase-free water containing 0.1% diethyl pyrocarbonate. Determination of chemokine mRNA was carried out using a modified version of the RiboQuant multi-probe RPA protocol (BD Pharmingen). RNA (15 μg) was hybridised overnight with a multi-probe set containing cDNA templates for human XCL1, CCL5, CXCL10, CCL4, CCL3, CCL2, CXCL8, CCL1 and housekeeping genes L32 and GAPDH (hCK-5, BD Pharmingen), which had been biotinylated and in vitro transcribed using T7 RNA polymerase. After hybridisation, RNA mixtures were treated with an RNase cocktail at 30 °C for 45 min, and protected mRNA species were then precipitated and separated on an 8 M urea/5% polyacrylamide gel before being transferred to Hybond-N by electroblotting and fixed by UV exposure (UV Stratalinker 1800, Stratagene, La Jolla, CA). Blots were blocked overnight prior to incubation with alkaline phosphatase. Following three washes, the substrate CDP-Star™ was added, and blots were exposed to ECL hyperfilm for up to 1 h. Images were scanned and analysed using Scion Image vBeta 4.0.2 (Scion Corporation, Frederick, MD). Chemokine mRNA densitometry values were normalised to L32:GAPDH levels and results expressed as percentage increase in mRNA compared to levels at time 0 h.
2.7. Nuclear extraction and electromobility shift assays (EMSA)
Nuclear extracts were prepared from 2 × 107 monocytes grown in 150-mm-diameter tissue culture dishes, according to Clarke et al. [18]. Briefly, cells were washed with ice-cold HBSS prior to extraction with a hypotonic buffer (5 mM HEPES (pH 7.9), 10 mM KCl, 1.5 mM MgCl2 containing protease inhibitors leupeptin, aprotinin, pepstatin, bestatin and PMSF, all at 1 μg/ml). Following addition of 0.25% NP-40, the nuclei were pelleted, supernatant removed and resuspended in a hypertonic buffer (5 mM HEPES (pH 7.9) 0.5 M NaCl, 1.5 mM MgCl2, 0.2 mM EDTA, 25% glycerol). After equilibration for 2 h at 4 °C, nuclei were pelleted, and the soluble nuclear extract was aspirated and stored at −80 °C. After quantifying protein concentrations by Bradford assay, EMSAs were performed as follows. First, a double-stranded NF-κB consensus oligonucleotide was end-labelled using [γ-32P]-ATP and T4 polynucleotide kinase. Nuclear extracts (7 μg) and labelled oligo probes (sp. Act. > 1 × 108 cpm) were mixed in binding buffer (20% glycerol, 5 mM MgCl2, 2.5 mM EDTA, 2.5 mM DTT, 250 mM NaCl, 50 mM Tris–HCl (pH 7.5), 0.25 mg/ml poly dI:dC:poly dI:dC) for 20 min at room temperature before being subjected to 5% non-denaturing PAGE and autoradiography. Probe binding specificity was confirmed in competition assays using a 50-fold molar excess of cold, unlabelled NF-κB or SP-1 oligos. Supershift analysis was performed by adding 1 μg of NF-κB subunit-specific Abs raised against human p65, p50, p52, c-rel, rel-B or c-fos (a subunit of the unrelated AP-1 transcription factor), to the binding mixture 40 min before addition of radiolabelled oligo.
2.8. Western blot analysis for IκBα/β
Western blot analysis was performed according to standard procedures [19]. Briefly, cell lysates were prepared by adding ice-cold lysis buffer (PBS containing 0.1% SDS, 0.1% NP-40, 0.5% deoxycholate, 10 mM NaF, 1 mM sodium orthovanadate, 170 μg/ml PMSF and protease inhibitors; leupeptin, pepstatin, bestatin and aprotinin all at 1 μg/ml) to 5 × 106 cells, followed by centrifugation at 800g for 5 min at 4 °C. Protein concentration was determined by Bradford assay. Equal volumes of loading buffer (containing 50 mM HEPES, 10% glycerol, 5% DTT, 2% SDS and bromophenol blue) was added to 50 μg of protein and samples were boiled for 5 min prior to being frozen at −80 °C. Proteins were resolved on a 10% SDS-PAGE gel, transferred by electroblotting to a nitrocellulose membrane and probed with 0.5 μg of rabbit anti-human IκBα or 0.8 μg of rabbit anti-human IκBβ. This was followed by incubation with peroxidase-conjugated goat anti-rabbit IgG (Sigma) followed by visualisation of protein bands by chemiluminescence.
2.9. Transient transfection and luciferase assays
Transfection and luciferase assays were performed as described [20]. Plasmids containing reporter vectors (pTK-rLUC, human pIL-8-pLUC, which contains the full-length of the IL-8 promoter and pCDM8-CD14) and expression vectors (TLR-2 or MD-2 amplified from human peripheral blood leukocyte cDNA [20]), were prepared using endotoxin-free kits (EndoFree Maxi, Qiagen, Crawley, UK). HeLa cells do not express TLR-2, but do express TLR-4; however, there is no signal via this receptor, because of a lack of the MD-2 accessory molecule. HeLa cells (1.5 × 104/well) were seeded into 96-well tissue culture plates. When cells were approximately 80% confluent, they were transfected according to manufacturers’ protocols using SuperFect™ transfection reagent. Each well of HeLa cells received 400 ng of pIL-8-pLUC, 25 ng of pCDM8-CD14, 100 ng of pTK-rLUC and 50 ng of mock (vector control), TLR-2 or MD-2 expression vectors. After transfection, cells were washed and left to equilibrate for 24 h before addition of either 100 μg/ml TsAg, 1 μg/ml ultra-pure, or E. coli-derived highly purified LPS (a gift from Dr. S. N. Vogel, University of Maryland, Baltimore, MD) or 1 μg/ml Staphylococcus aureus-derived peptidoglycan for 6 h. Reporter activity was measured using the Dual-Luciferase assay system and CXCL8 promoter activity normalised to Renilla luciferase activity. All experiments were performed in triplicate.
2.10. Statistics and presentation of data
Results are expressed as mean ± SEM. Data were analysed using unpaired t-tests, and p < 0.05 was taken as significant.
3. Results
3.1. TsAg stimulates secretion of CXCL8, CCL2 and CCL3 from human monocytes
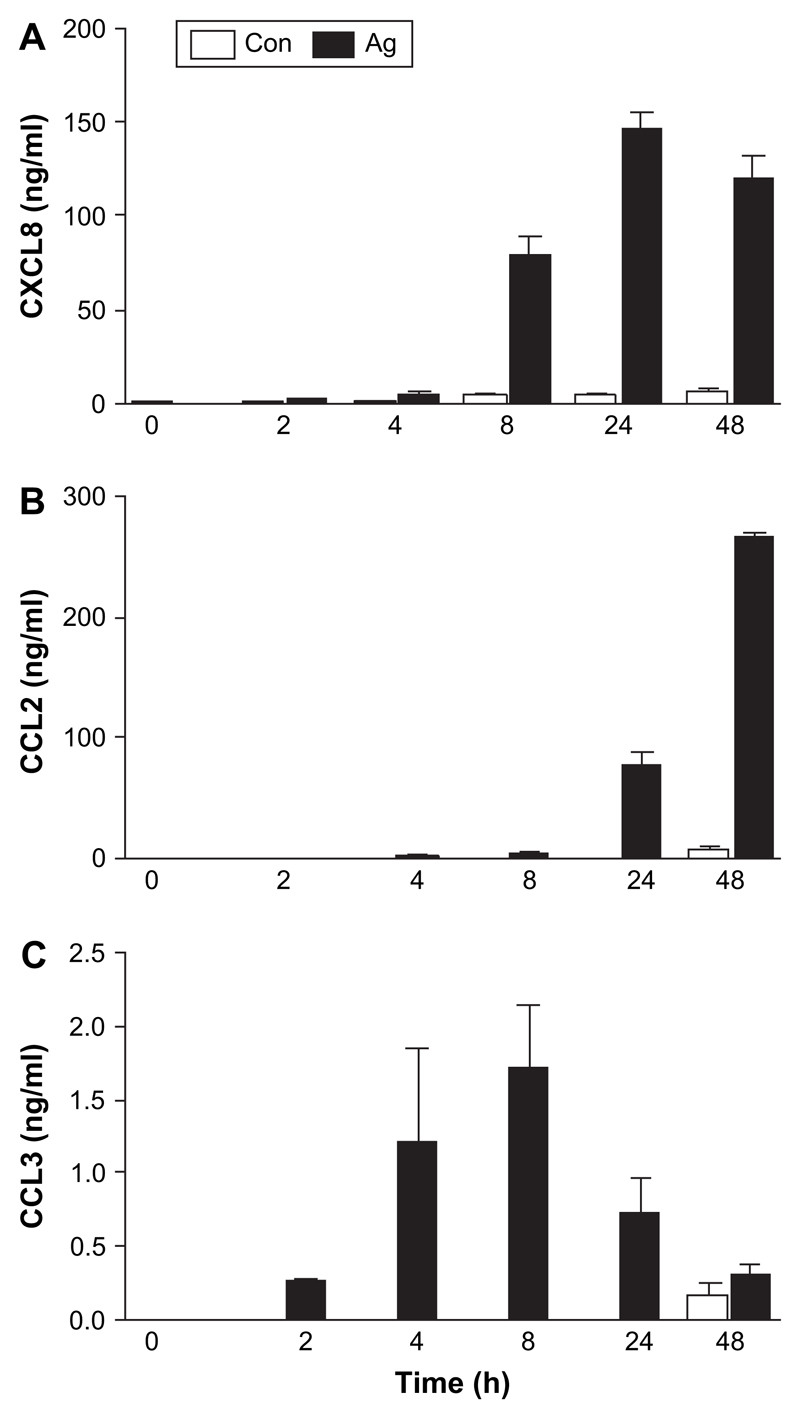
We first determined whether TsAg could drive the secretion of prototypic CXC and CC chemokines from monocytes. TsAg caused early secretion of CXCL8 at 2 h (a 2.5-fold increase above control, unstimulated cells) which increased at 4 and 8 h. However, the major increase in CXCL8 secretion was delayed, and maximal concentrations of 146.5 ± 8.5 ng/ml were reached at 24 h (a 31-fold increase above unstimulated cells, Fig. 1A). CCL2 secretion was initially detected after 8 h and increased to a much greater extent, by up to 38-fold above unstimulated cells at 48 h (Fig. 1B). Increased secretion of CCL3 occurred within 2 h reaching maximal concentrations of 1.72 ± 0.43 ng/ml at 8 h (Fig. 1C). Chemokine secretion after TsAg-stimulation was dose dependent (data not shown). Cytokine secretion was not due to LPS contamination, since the endotoxin concentrations in TsAg (0.11–5.46 pg/ml) did not, when assayed in isolation, stimulate chemokine secretion. Furthermore, the kinetic profile of chemokine secretion in response to LPS was different. For example, LPS-induced CXCL8 secretion increased throughout the 48-h time course (LPS data not shown). In addition, THP-1 cells stimulated with TsAg did not secrete CCL2, CCL3 or CXCL8, even though they were to a limited extent responsive to LPS (data not shown).
Fig. 1.
Kinetics of TsAg-induced CXCL8, CCL2 and CCL3 from monocytes. Purified human monocytes (1 × 105/cm2) were stimulated with TsAg (100 μg/ml) or media (Con) for 0, 2, 4, 8, 24 and 48 h. Cell-free culture supernatants were subsequently collected, and protein levels of (A) CXCL8, (B) CCL2, and (C) CCL3 were measured using specific ELISAs. Results are expressed as mean ± SEM of a triplicate experiment, representative of three independent experiments.
3.2. TsAg induces early chemokine mRNA accumulation in human monocytes
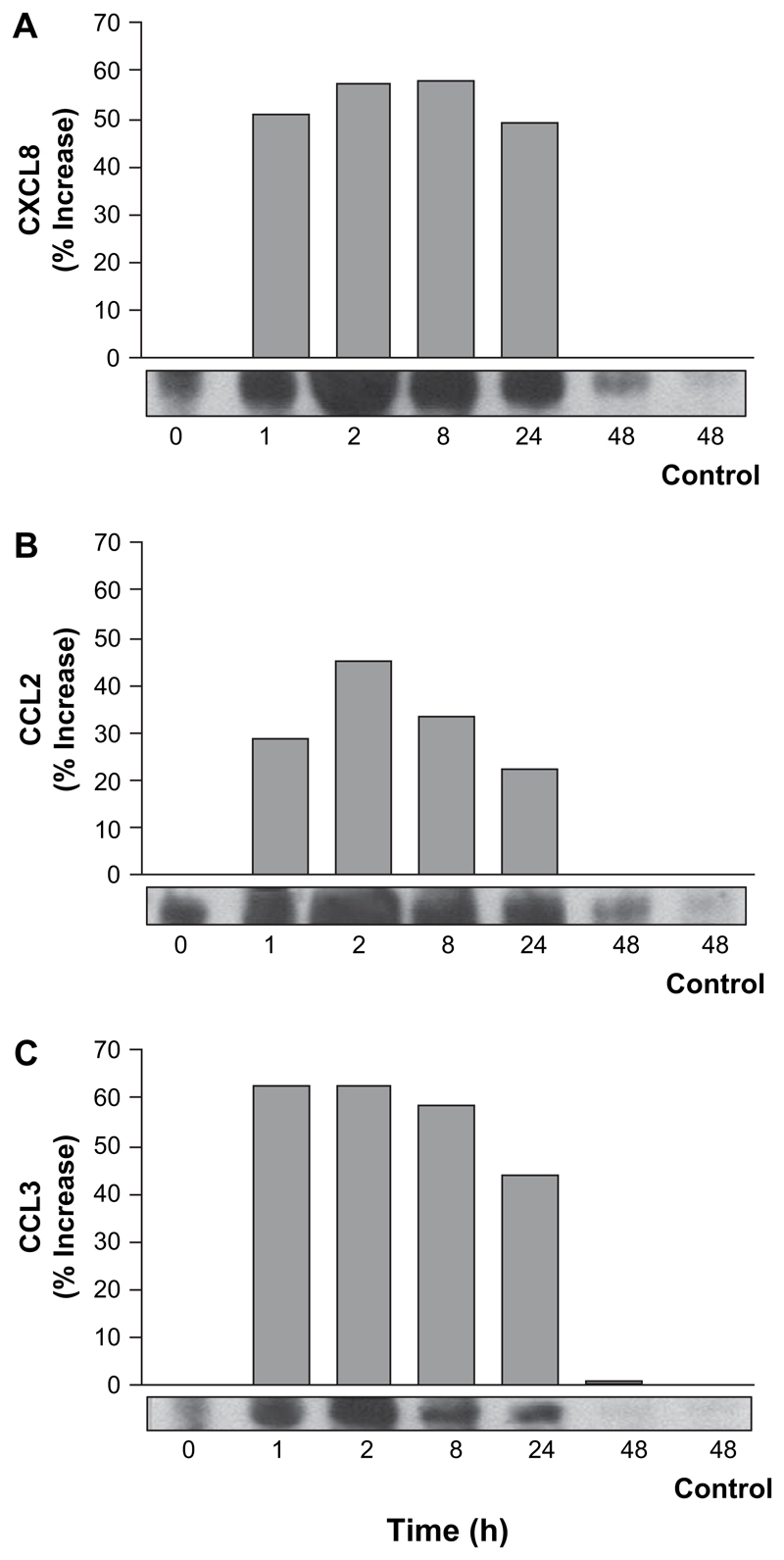
Since chemokine secretion is often transcriptionally regulated, we next investigated the effect of TsAg on chemokine gene expression by multi-probe RPA. Although there was variability in probing of RNA for specific chemokines (it is possible that the TsAg solution affects RNA processing), the more accurate densitometric analysis of all data is presented where all values have been normalised relative to housekeeping genes. Induction of CXCL8, CCL2 and CCL3 mRNA was detected as early as 1 h (Fig. 2). CXCL8 mRNA reached maximal levels of expression by 2 h, with similar levels of transcription being maintained at 8 h, followed by a sharp fall after 24 h (Fig. 2A). All mRNA species were constitutively expressed. CCL2 was upregulated to a slightly lesser extent than CXCL8 or CCL3 (Fig. 2B). CCL3 mRNA expression was upregulated maximally at 1 h, and the kinetics were similar to those of CXCL8, even though the kinetics of secretion of these chemokines were distinct (Fig. 2C). All chemokine mRNA expression returned to baseline by 48 h. XCL1/Lymphotactin, CCL5/RANTES and CXCL10/IP-10 mRNA expression did not significantly change over 48 h (data not shown). Transcription of CCL4/MIP-1β mRNA paralleled that seen for CCL3/MIP-1α.
Fig. 2.
TsAg-induced rapid CXCL8, CCL2 and CCL3 mRNA accumulation in monocytes. Purified human monocytes (1 × 105/cm2) were cultured for 0, 1, 2, 8, 24 and 48 h with 100 μg/ml TsAg or under control conditions for 48 h. Total cellular RNA was extracted, purified and chemokine mRNA was assessed using a biotinylated multi-probe RNase protection assay. Following densitometric analysis, mRNA levels were normalised for total mRNA using L32/GAPDH densitometry. Shown are representative densitometry and autoradiography showing (A) CXCL8, (B) CCL2 and (C) CCL3 mRNA levels, expressed as percentage increase above t = 0. Data shown are representative of three experiments.
3.3. TsAg-induced NF-κB activation and IκBα degradation
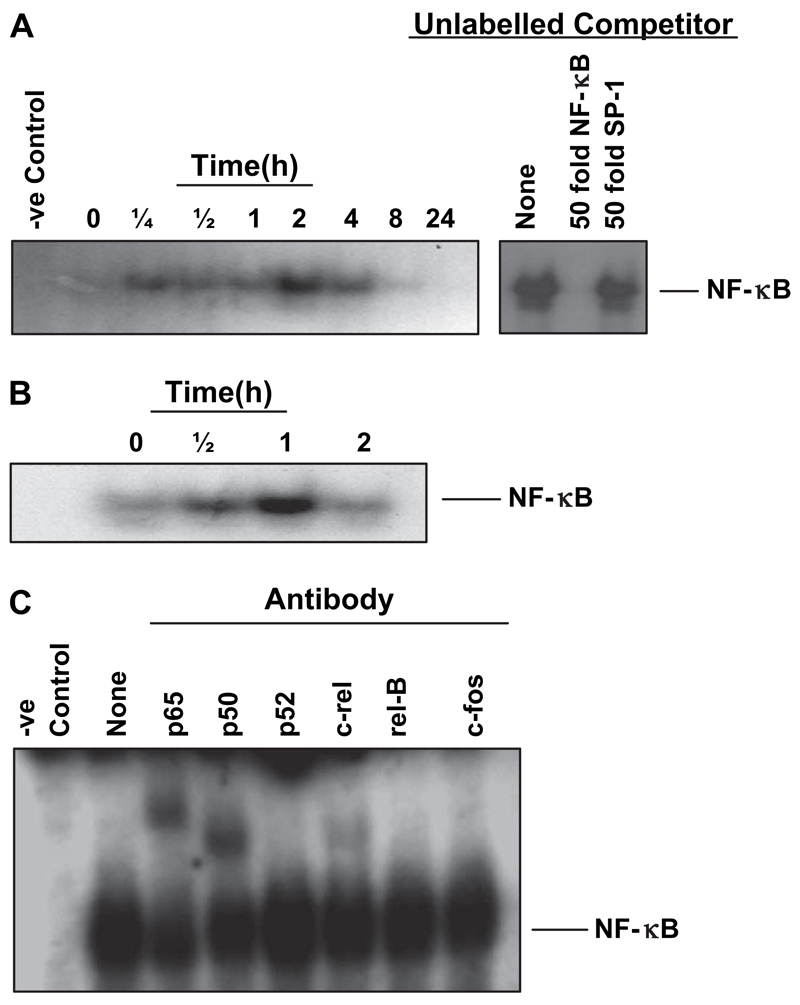
In order to investigate mechanisms regulating transcription, the next experiments focussed on the role of NF-κB, a key activator of many chemokines. TsAg induced early NF-κB nuclear binding within 15 min on electromobility gel shift assay, which was maintained up to 4 h, peaking at 2 h and still detectable at 8 h (Fig. 3A). NF-κB activation due to TsAg was specific, being effectively competed with a 50-fold excess of unlabelled NF-κB oligonucleotide, but not with an unrelated SP-1 oligonucleotide (Fig. 3A). Such NF-κB activation was relatively delayed and prolonged compared to that induced by LPS, which peaked at 1 h (Fig. 3B). Supershift analysis revealed that the TsAg-induced DNA—protein complexes were shifted with anti-p65, p50 and c-rel antibodies (Fig. 3C), whereas LPS only activated the p65 and p50 components of NF-κB (data not shown). No shift was detected with other Rel-family antibodies p52 and rel-B, or in response to the irrelevant c-fos antibody (Fig. 3C).
Fig. 3.
TsAg stimulates NF-κB nuclear translocation in primary human monocytes. Nuclear extracts were prepared from human monocytes at 0, 1/4, 1/2, 1, 2, 4, 8 and 24 h post-stimulation with TsAg (100 μg/ml, (A)) or 0, 1/2, 1 and 2 h post-stimulation with LPS (10 μg/ml, (B)). Equal amounts of extract were mixed with 32P-end labelled NF-κB consensus oligonucleotides and complexes were analysed by PAGE and autoradiography. Competition assays were performed on extracts prepared 2 h post-stimulation with TsAg to demonstrate binding specificity (A). NF-κB binding to labelled probe was competed out with a 50-fold molar excess of cold, unlabelled NF-κB probe but not with a 50-fold excess of the unlabelled, irrelevant SP-1 probe. (C) Super-shift assays were performed on extracts prepared 2 h post-stimulation to investigate specific subunits involved in TsAg-induced NF-κB nuclear binding. Extracts were incubated for 40 min with 1 μg of an antibody specific to NF-κB subunits p65, p50, p52, c-rel and rel-B (or c-fos—an unrelated antibody to the AP-1 subunit) prior to addition of 32P-labelled NF-κB probe. Negative control samples relate to cells grown unstimulated in tissue culture medium alone. Autoradiographs shown are representative of experiments performed on at least two separate occasions.
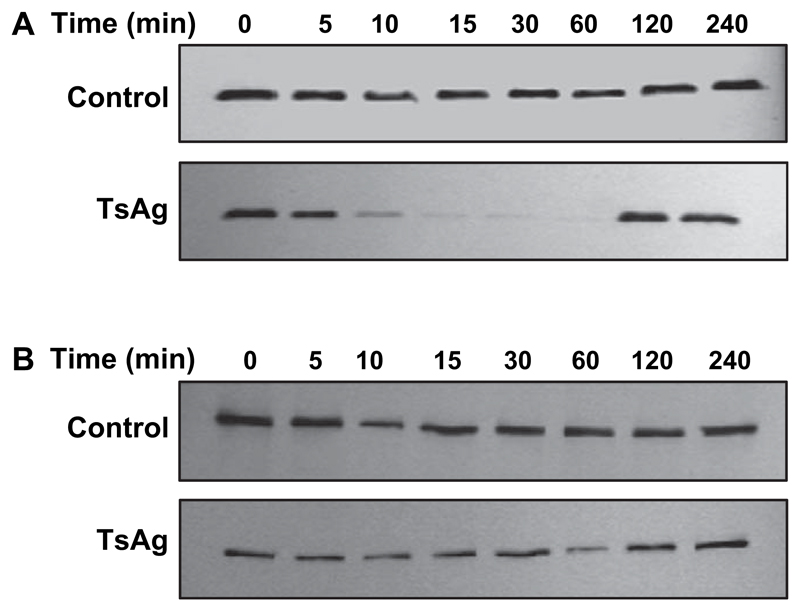
Activation of NF-κB requires sequential phosphorylation-dependent degradation of IκBα. Therefore, cell lysates were obtained from monocytes at specific time points after exposure to TsAg and analysed by Western blotting using IκBα or β specific antibodies. IκBα was degraded within 10 min before resynthesis by 2 h (Fig. 4A). In contrast, there was no change in IκBβ stability in either TsAg-stimulated or control cells (Fig. 4B). These results confirm that TsAg activates NF-κB by inducing IκBα degradation.
Fig. 4.
Kinetics of IκBα and β expression in TsAg-stimulated human monocytes. Human monocytes were stimulated with either media (control) or TsAg (100 μg/ml) for 0, 5, 10, 15, 30, 60, 120 and 240 min. Cytoplasmic lysates were extracted and equal amounts of protein separated by SDS-PAGE before being analysed by Western blotting using antibodies specific to (A) IκBα and (B) IκBβ. Blots shown are representative of experiments performed on three separate occasions.
3.4. TsAg-induced CXCL8 and CCL2 secretion is dependent on NF-κB
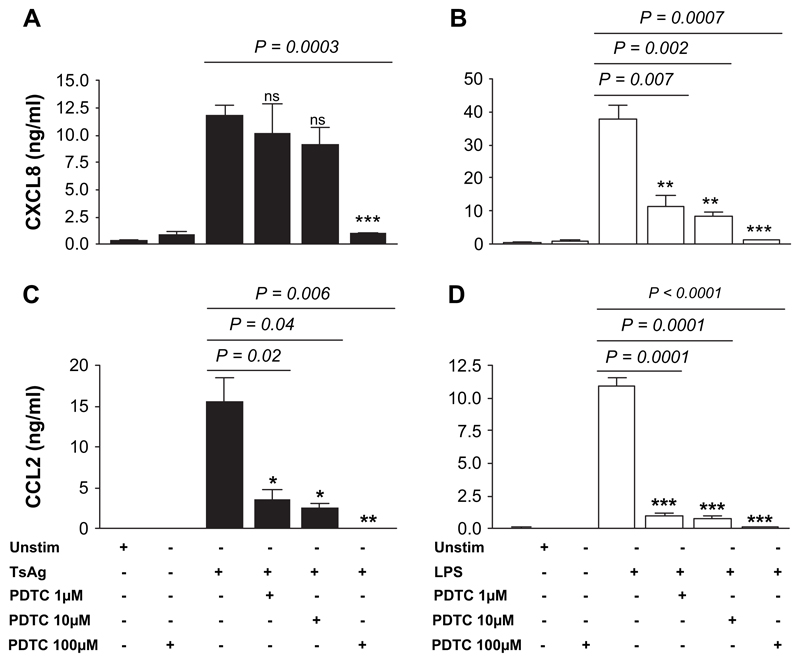
To determine if TsAg-induced, transcription-dependent chemokine secretion was due to NF-κB nuclear binding, monocytes were pre-treated with the specific NF-κB inhibitor PDTC, which inhibits dissociation of IκBα from cytoplasmic NF-κB. The inhibitory activity of PDTC does not extend to other transcription factors such as AP-1 whose activity may be enhanced rather than suppressed [21]. PDTC treatment significantly inhibited CXCL8 and CCL2 secretion induced by TsAg in a dose-dependent manner (Fig. 5A/C). TsAg-induced CXCL8 secretion was relatively insensitive to PDTC treatment, with no significant decrease at concentrations of 1 and 10 μM, although 91% inhibition of secretion with 100 μM PDTC was observed (p = 0.0003, Fig. 5A). In contrast, LPS-induced CXCL8 secretion was highly PDTC sensitive, further indicating the specific nature of the response to TsAg. CCL2 secretion in response to either TsAg or LPS was very sensitive to PDTC treatment, with complete blockade at a dose of 100 μM (p < 0.01, Fig. 5C/D).
Fig. 5.
PDTC inhibits TsAg- and LPS-induced CXCL8 and CCL2 secretion in monocytes. Monocytes were pre-incubated for 2 h with 1, 10, or 100 μM PDTC prior to stimulation with either 100 μg/ml TsAg (black bars) or 10 μg/ml LPS (open bars) for 24 h. Cell-free culture supernatants were subsequently collected and analysed for (A,B) CXCL8 and (C,D) CCL2 by ELISA. PDTC alone (100 μM) has no effect on either CXCL8 or CCL2 secretion. Data are expressed as mean SEM of a triplicate experiment, which is representative of two independent experiments. Differences between groups were analysed for statistical significance using Student’s t-test.
3.5. TsAg does not induce CXCL8 promoter activation via TLR-2 or TLR-4
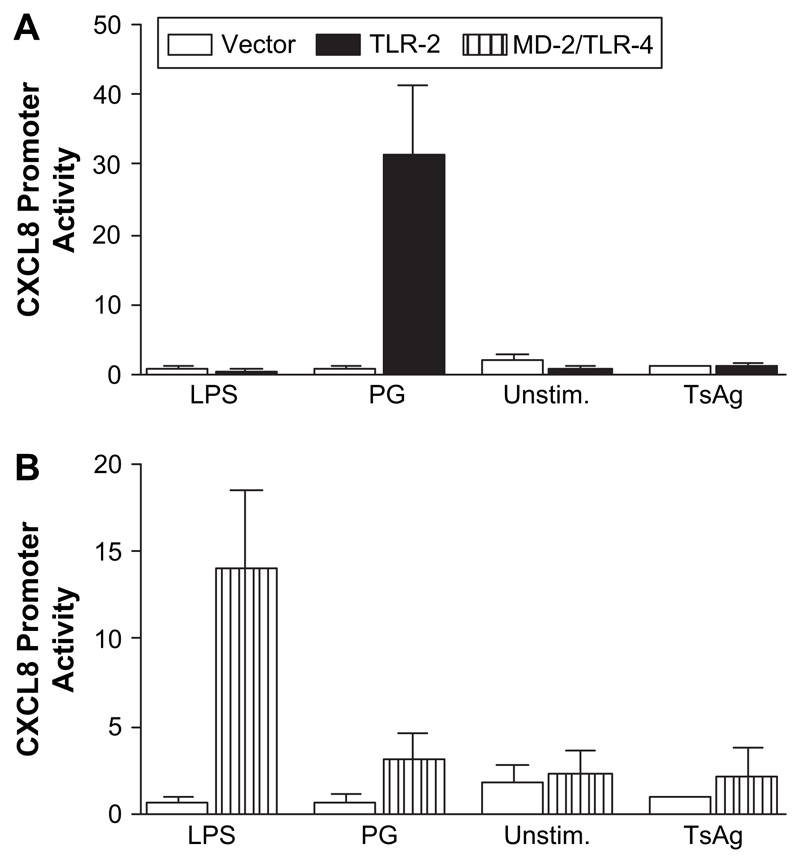
We next investigated using promoter–reporter assays, whether TsAg-induced CXCL8 gene expression was regulated by upstream signals initiated via TLR-2 or TLR-4 activation. TLR-2/4 are involved in host responses to bacterial lipopolysaccharide and to a broad range of pathogens and may regulate CXCL8 gene expression. However, there are no previous data investigating whether TLR-2/4 have any role in regulating expression of any gene activated by cysticercosis. HeLa cells were co-transfected with plasmids containing CD14, a luciferase-CXCL8 promoterereporter and either TLR-2, MD-2 or vector control plasmids 24 h prior to stimulation with TsAg for 6 h. TsAg, at a concentration shown to stimulate chemokine release from human monocytes, did not alter CXCL8 promoter activity above that seen in vector control or unstimulated cells, regardless of whether cells expressed TLR-2 or MD-2/TLR-4 (Fig. 6). In contrast, compared to vector controls, peptidoglycan (PG), a positive control, caused a 51-fold increase in CXCL8 promoter activity in cells expressing TLR-2 (Fig. 6A), and ultra-pure LPS elicited a 21-fold increase in CXCL8 promoter activity in MD-2/TLR-4 transfectants (Fig. 6B). The lack of effect of TsAg on the TLR-4 pathway is consistent with the differences in control of chemokine gene expression in monocytes stimulated with this antigen when compared to LPS-stimulated cells. However, we did not investigate the possibility that lectins or other molecules may cooperate with TLRs in primary cells or undertake studies with siRNA in primary cells in this study.
Fig. 6.
TLR-2 and MD-2/TLR-4 are not required for TsAg-induced CXCL8 promoter activity. HeLa cells were grown to approximately 80% confluency and then co-transfected with plasmids containing CXCL8 promoter-linked to a Firefly luciferase reporter and either (A) TLR-2 or (B) MD-2 constructs together with a control plasmid, pTK-rLUC, which constitutively expresses low levels of Renilla luciferase. Cells were stimulated the next day with either TsAg (100 μg/ml), peptidoglycan (PG, 1 μg/ml) or LPS (1 μg/ml) and lysates assayed for luciferase activity. Data shown are CXCL8 promoter activity after normalisation of Firefly luciferase activity for control Renilla luciferase activity. Results are the mean ± SEM of a triplicate experiment. Open bars are data from cells transfected with a vector control construct, black bars represent cells transfected with the TLR-2 construct, and hatched bars represent cells transfected with the MD-2 construct.
4. Discussion
In this study, we have started to dissect the mechanisms regulating chemokine secretion during the host response to T. solium larval antigens. We have shown for the first time that monocytes responding to stimulation with TsAg secrete significant amounts of the CXC chemokine CXCL8 and CC chemokines CCL2 and CCL3. Chemokine secretion was transcriptionally regulated, and we have shown that NF-κB has a pivotal role in regulating the activation of monocyte chemokine gene expression, in response to TsAg. However, in contrast to many other pathogens and to LPS, TsAg-dependent CXCL8 activity does not seem to be mediated via signalling pathways activated by TLR-2 or TLR-4.
Monocytes/macrophages play key roles in immunity against many pathogens, both in their ability to phagocytose and release specific mediators which act either directly or indirectly to modulate protective immunity. Under physiological conditions there are relatively few peripheral immune cells in the CNS. However, there is a baseline physiological influx of monocytes/macrophages and T-lymphocytes across the BBB, a process which may be important in local immune surveillance. In addition, microglial cells are the resident macrophage-like phagocytic cells of the CNS. They are difficult to distinguish from peripheral monocytes/macrophages in many respects, since they originate from the same mesodermal lineage and express many common cell surface markers such as CD68 [22]. Many investigators have used monocytes to represent brain microglial cells. We have shown that peripheral monocytes stimulated by TsAg, secrete CCL2, CCL3 and CXCL8. Such chemokine secretion may be important in cerebral monocyte and neutrophil recruitment occurring after larval degeneration and antigenic stimulation of microglia and circulating monocytes. Our data are consistent with reports which have shown that macrophages and granulocytes are present in brain granulomas associated with NCC [23]. However, relatively little has been reported about the mechanisms of in vivo migration of leukocytes in animal model systems.
The pattern of chemokine secretion and gene expression from TsAg-stimulated monocytes was coordinated, with CCL3 being upregulated before CXCL8, followed by a later secretion of CCL2. In part, this may be regulated by post-transcriptional mechanisms, since there were greater differences in chemokine secretion than gene expression; further research is required to obtain direct evidence of such regulatory mechanisms. Such coordinate chemokine secretion may be involved in shaping and sustaining granulomas in NCC. Similar temporal patterns of chemokine expression have been shown to be important in formation, progression and resolution of schistosomal egg-antigen induced granulomatous inflammation [24]. Chemokines and their receptors are associated with driving Th1 or Th2 polarisation [10]. CCL3 promotes Th1 cell polarisation, whereas CCL2 favours development of a Th2 phenotype, processes regulated by STAT-4 and -6, respectively [25]. Th1 responses are associated with both larval degeneration and symptomatic disease in human and in murine models of NCC [3,26]. In addition, STAT-6 −/− mice lacking Th2 responses are less susceptible to infection with Taenia crassiceps and effectively induce larval degeneration and control infection via Th1-mediated pathways [27]. Although in our in vitro system, TsAg-dependent CCL3 secretion was transient and low level, it may be important in the development of Th1-type immune responses in the CNS.
Many genes including chemokine genes activated in monocytes after microbial stimuli, are target genes of the ubiquitous transcription factor NF-κB. In the present study, TsAg stimulated NF-κB nuclear translocation in monocytes following IκBα degradation and this was associated with chemokine gene expression. Blocking studies with PDTC show that NF-κB binding is necessary for TsAg-induced chemokine secretion acting at very low concentrations in the context of CCL2. However, the kinetics of NF-κB activation, the subunits involved and the responses to PDTC in TsAg-stimulated cells were distinct from those in LPS-activated monocytes, indicating involvement of different signal transduction pathways. TsAg activated p50, p65 and c-rel NF-κB subunits. Both p65 and c-rel NF-κB subunits contain transactivation domains necessary for gene induction, whereas p50 does not, and homodimers of p50 may repress gene transcription. It is therefore likely that TsAg-induced chemokine gene expression is regulated by heterodimers, p65/p50 or p65/c-rel, or alternatively, homodimers and monomers may be involved. Selective activation of NF-κB proteins is important in providing site and event-specificity in responses to inflammatory stimuli. Furthermore, recent evidence suggests that specific NF-κB subunits have potentially important roles in controlling intestinal helminth infection via the modulation of Th2 cytokine responses. Mice deficient in NF-κB1 (p105/p50) but not c-rel were unable to clear intestinal Trichuris muris infection and developed destructive colitis-like pathology [28].
NF-κB activation in response to microbial stimuli and LPS is regulated by upstream signalling elements initiated as a result of PAMP recognition by TLRs. However, in this study we did not show any functional role for TLR-2 or TLR-4 in control of TsAg-stimulated chemokine secretion. The apparent lack of association between TLR-4 and upregulation of chemokine activity is consistent with the divergence in responses between TsAg-activated and LPS-stimulated monocytes. However, the findings do not exclude a role for other members of the TLR family in TsAg-induced, NF-κB-dependent chemokine secretion. In addition, cooperative interaction between different TLRs may also be important in PAMP recognition. Furthermore, there are TLR-independent pathways activating NF-κB [29]. Such alternative pattern-recognition pathways may play a part in TsAg-induced signal transduction. It is also possible that TLR-2/4 may cooperate with additional receptors in the target cells such as lectins which are not present in HeLa cells used for reporter assays.
In summary, the data show that TsAg has the ability to induce coordinate chemokine production from monocytes, acting via an NF-κB-dependent pathway which is not activated via TLR-2 or TLR-4-mediated signalling. Stimulation of monocyte-derived chemokine gene expression and secretion is likely to have a key role in coordinating leukocyte recruitment to areas of T. solium larval degeneration, working in concert with monocyte—astrocyte networks, which we have shown are important in the regulation of chemokine secretion in NCC [30]. This may shape adaptive immune responses through modulation of Th1/Th2 phenotypes and ultimately have a critical role in mediating tissue damage leading to symptomatic NCC.
Acknowledgements
We thank Dr. José A. Chabalgoity for critical reading and helpful comments. This work was supported by grant numbers AI-42037-01 and AI-35894 from the National Institutes of Health, USA, the ITREID training grant TW00910 and by The Wellcome Trust.
References
- [1].White AC., Jr Neurocysticercosis: updates on epidemiology, pathogenesis, diagnosis, and management. Annu Rev Med. 2000;51:187–206. doi: 10.1146/annurev.med.51.1.187. [DOI] [PubMed] [Google Scholar]
- [2].Villa OF, Kuhn RE. Mice infected with the larvae of Taenia crassiceps exhibit a Th2-like immune response with concomitant anergy and downregulation of Th1-associated phenomena. Parasitology. 1996;112:561–570. doi: 10.1017/s0031182000066142. [DOI] [PubMed] [Google Scholar]
- [3].Restrepo BI, Llaguno P, Sandoval MA, Enciso JA, Teale JM. Analysis of immune lesions in neurocysticercosis patients: central nervous system response to helminth appears Th1-like instead of Th2. J Neuroimmunol. 1998;89:64–72. doi: 10.1016/s0165-5728(98)00112-x. [DOI] [PubMed] [Google Scholar]
- [4].Larsen CG, Anderson AO, Appella E, Oppenheim JJ. Matsushima, The Neutrophil-Activating Protein (NAP-1) is also chemotactic for lymphocytes. Science. 1989;243:1464–1466. doi: 10.1126/science.2648569. [DOI] [PubMed] [Google Scholar]
- [5].Gerszten RE, Garcia-Zepeda EA, Lim YC, Yoshida M, Ding HA, Gimbrone MA, Luster AD, Luscinskas FW, Rosenzweig A. MCP-1 and IL-8 trigger firm adhesion of monocytes to vascular endothelium under flow conditions. Nature. 1999;398:718–723. doi: 10.1038/19546. [DOI] [PubMed] [Google Scholar]
- [6].Larsen CG, Thomsen MK, Gesser B, Thomsen PD, Deleuran BW, Nowak J, Skodt V, Thomsen HK, Deleuran M, Therstrup-Pedersen K, Harada A, et al. The delayed-type hypersensitivity reaction is dependent on IL-8. J Immunol. 1995;155:2151–2157. [PubMed] [Google Scholar]
- [7].Burgmann H, Hollenstein U, Wenisch C, Thalhammer F, Looareesuwan S, Graninger W. Serum concentrations of MIP-1 alpha and interleukin-8 in patients suffering from acute Plasmodium falciparum malaria. Clin Immunol Immunopathol. 1995;76:32–36. doi: 10.1006/clin.1995.1084. [DOI] [PubMed] [Google Scholar]
- [8].Badolato R, Sacks DL, Savoia D, Musso T. Leishmania major: infection of human monocytes induces expression of IL-8 and MCAF. Exp Parasitol. 1996;82:21–26. doi: 10.1006/expr.1996.0003. [DOI] [PubMed] [Google Scholar]
- [9].Chensue SW, Warmington KS, Ruth JH, Sanghi PS, Lincoln P, Kunkel SL. Role of Monocyte Chemoattractant Protein-1 (MCP-1) in Th1 (mycobacterial) and Th2 (schistosomal) antigen-induced granuloma formation. J Immunol. 1996;157:4602–4608. [PubMed] [Google Scholar]
- [10].Gu L, Tseng S, Horner RM, Tam C, Loda M, Rollins BJ. Control of TH2 polarization by the chemokine monocyte chemoattractant protein-1. Nature. 2000;404:407–411. doi: 10.1038/35006097. [DOI] [PubMed] [Google Scholar]
- [11].Shimazu R, Akashi S, Ogata H, Nagai Y, Fukudome K, Miyake K, Kimoto M. MD-2, a molecule that confers lipopolysaccharide responsiveness on toll-like receptor 4. J Exp Med. 1999;189:1777–1782. doi: 10.1084/jem.189.11.1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Brightbill HD, Libraty DH, Krutzik SR, Yang RB, Belisle JT, Bleharski JR, Maitland M, Norgard MV, Plevy SE, Smale ST, Brennan PJ, et al. Host defense mechanisms triggered by microbial lipoproteins through toll-like receptors. Science. 1999;285:732–736. doi: 10.1126/science.285.5428.732. [DOI] [PubMed] [Google Scholar]
- [13].Kurt-Jones EA, Popova L, Kwinn L, Haynes LM, Jones LP, Tripp RA, Walsh EE, Freeman MW, Golenbock DT, Anderson LJ, Finberg RW. Pattern recognition receptors TLR4 and CD14 mediate response to respiratory syncytial virus. Nat Immunol. 2000;1:398–401. doi: 10.1038/80833. [DOI] [PubMed] [Google Scholar]
- [14].Campos MA, Almeida IC, Takeuchi O, Akira S, Valente EP, Procopio DO, Travassos LR, Smith JA, Golenbock DT, Gazzinelli RT. Activation of toll-like receptor-2 by glycosylphosphatidylinositol anchors from a protozoan parasite. J Immunol. 2001;167:416–423. doi: 10.4049/jimmunol.167.1.416. [DOI] [PubMed] [Google Scholar]
- [15].Wang JE, Warris A, Ellingsen EA, Jorgensen PF, Flo TH, Espevik T, Solberg R, Verweij PE, Aasen AO. Involvement of CD14 and toll-like receptors in activation of human monocytes by Aspergillus fumigatus hyphae. Infect Immun. 2001;69:2402–2406. doi: 10.1128/IAI.69.4.2402-2406.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Evans CA, Garcia HH, Hartnell A, Gilman RH, Jose PJ, Martinez M, Remick DG, Williams TJ, Friedland JS. Elevated concentrations of eotaxin and interleukin-5 in human neurocysticercosis. Infect Immun. 1998;66:522–4525. doi: 10.1128/iai.66.9.4522-4525.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Cardona AE, Gonzalez PA, Teale JM. CC chemokines mediate leukocyte trafficking into the central nervous system during murine neurocysticercosis: role of gamma delta T cells in amplification of the host immune response. Infect Immun. 2003;71:2634–2642. doi: 10.1128/IAI.71.5.2634-2642.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Clarke CJP, Hales A, Hunt A, Foxwell BMJ. IL-10-mediated suppression of TNF-α production is independent of its ability to inhibit NF-κB activity. Eur J Immunol. 1998;28:1719–1726. doi: 10.1002/(SICI)1521-4141(199805)28:05<1719::AID-IMMU1719>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- [19].Thomas LH, Wickremasinghe MI, Sharland M, Friedland JS. Synergistic upregulation of IL-8 secretion from pulmonary epithelial cells by direct and monocyte-dependent effects of respiratory syncytial virus infection. J Virol. 2000;74:8425–8433. doi: 10.1128/jvi.74.18.8425-8433.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Pridmore AC, Wyllie DH, Abdillahi F, Steeghs L, van der LP, Dower SK, Read RC. A lipopolysaccharide-deficient mutant of Neisseria meningitidis elicits attenuated cytokine release by human macrophages and signals via toll-like receptor (TLR) 2 but not via TLR4/MD2. J Infect Dis. 2001;183:89–96. doi: 10.1086/317647. [DOI] [PubMed] [Google Scholar]
- [21].Schenk H, Klein M, Erdbrugger W, Droge W, Schulze-Osthoff K. Distinct effects of thioredoxin and antioxidants on the activation of transcription factors NF-kappa B and AP-1. Proc Natl Acad Sci USA. 1994;91:1672–1676. doi: 10.1073/pnas.91.5.1672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Gehermann J, Matsumoto Y, Kreutzberg GW. Microglia: intrinsic immuneffector cell of the brain. Brain Res Rev. 1995;20:269–287. doi: 10.1016/0165-0173(94)00015-h. [DOI] [PubMed] [Google Scholar]
- [23].Restrepo BI, Alvarez JI, Castano JA, Arias LF, Restrepo M, Trujillo J, Colegial CH, Teale JM. Brain granulomas in neurocysticercosis patients are associated with a Th1 and Th2 profile. Infect Immun. 2001;69:4554–4560. doi: 10.1128/IAI.69.7.4554-4560.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Chiu B, Chensue SW. Chemokine responses in schistosomal antigen-elicited granuloma formation. Parasite Immunol. 2002;24:285–294. doi: 10.1046/j.1365-3024.2002.00466.x. [DOI] [PubMed] [Google Scholar]
- [25].Zhang S, Lukacs NW, Lawless VA, Kunkel SL, Kaplan MH. Cutting edge: differential expression of chemokines in Th1 and Th2 cells is dependent on Stat6 but not Stat4. J Immunol. 2000;165:10–14. doi: 10.4049/jimmunol.165.1.10. [DOI] [PubMed] [Google Scholar]
- [26].Cardona AE, Restropo BI, Jaramillo JM, Teale JM. Development of an animal model for neurocysticercosis: immune response in the central nervous system is characterized by a predominance of γδ T cells. J Immunol. 1999;162:995–1002. [PubMed] [Google Scholar]
- [27].Rodriguez-Sosa M, David JR, Bojalil R, Satoskar AR, Terrazas LI. Cutting edge: susceptibility to the larval stage of the helminth parasite Taenia crassiceps is mediated by Th2 response induced via STAT6 signaling. J Immunol. 2002;168:3135–3139. doi: 10.4049/jimmunol.168.7.3135. [DOI] [PubMed] [Google Scholar]
- [28].Artis D, Shapira S, Mason N, Speirs KM, Goldschmidt M, Caamano J, Liou HC, Hunter CA, Scott P. Differential requirement for NF-kappa B family members in control of helminth infection and intestinal inflammation. J Immunol. 2002;169:4481–4487. doi: 10.4049/jimmunol.169.8.4481. [DOI] [PubMed] [Google Scholar]
- [29].Inohara N, Nunez G. NODs: intracellular proteins involved in inflammation and apoptosis. Nat Rev Immunol. 2003;3:371–382. doi: 10.1038/nri1086. [DOI] [PubMed] [Google Scholar]
- [30].Uddin J, Garcia HH, Gilman RH, Gonzalez AE, Friedland JS. Monocyte–astrocyte networks and the regulation of chemokine secretion in neurocysticercosis. J Immunol. 2005;175:3273–3281. doi: 10.4049/jimmunol.175.5.3273. [DOI] [PubMed] [Google Scholar]