Abstract
Metal-organic framework (MOF) materials are gaining significant interest in biomedical research, owing to their high porosity, crystallinity, and structural and compositional diversity. Their versatile hybrid organic/inorganic chemistry endows MOFs with the capacity to retain organic (drug) molecules, metals, and gases, to effectively channel electrons and photons, to survive harsh physiological conditions such as low pH, and even to protect sensitive biomolecules. Extensive preclinical research has been carried out with MOFs to treat several pathologies and, recently, their integration with other biomedical materials such as stents and implants has demonstrated promising performance in regenerative medicine. However, there remains a significant gap between MOF preclinical research and translation into clinically and societally relevant medicinal products. Here, we outline the intrinsic features of MOFs and discuss how these are suited to specific biomedical applications like detoxification, drug and gas delivery, or as (combination) therapy platforms. We furthermore describe relevant examples of how MOFs have been engineered and evaluated in different medical indications, including cancer, microbial, and inflammatory diseases. Finally, we critically examine the challenges facing their translation into the clinic, with the goal of establishing promising research directions and more realistic approaches that can bridge the translational gap of MOFs and MOF-containing (nano)materials.
Keywords: metal-organic frameworks, porous materials, nanoparticles, biomedicine, metallotherapy
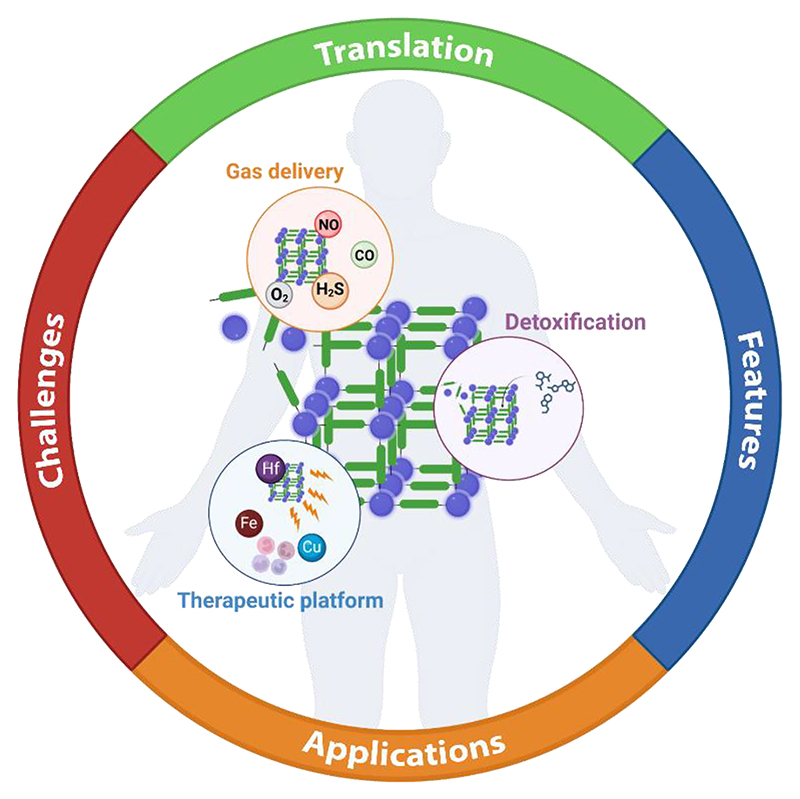
Graphical abstract.
Translational challenges and perspectives of metal-organic framework materials in biomedicine: the state-of-the-art in the medicinal application is reviewed and potential barriers and avenues to clinical translation are explored.
1. Introduction
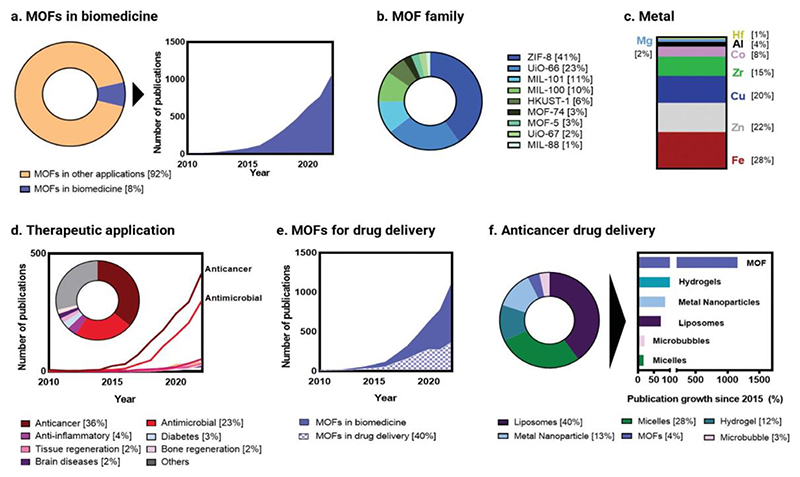
Porous materials are increasingly relevant in biomedicine. Porosity is an important feature employed in medical implants and tissue scaffolds, and porous materials have already been used as adsorbents to remove toxins and treat (drug) overdose.[1–3] Since the first reports on aluminosilicates (1930s), microporous carbons (1945s), and synthetic zeolites (1950s), a vast array of different organic, inorganic, and hybrid porous structures have been developed.[3–6] Among all of them, metal-organic frameworks (MOFs), a relatively new class of porous coordination polymers (1990s),[7,8] have grown particularly popular in biomedical research in the last decade, with the field representing about 10% of all the publications on MOFs (Figure 1a).[9–14] This interest is reflected by the exponential increase in the number of related publications per year with regard to biomedical applications, both for therapy and diagnosis: from a few hundred to over a thousand each year (Figure 1a).
Figure 1. Publication record for metal-organic frameworks in biomedicine.
(a) Proportion of all metal-organic framework (MOF) publications for biomedical applications as compared to all other applications (left), and their growth (right). (b) The most prominent MOF families explored in biomedical research. (c) Most common metals used in MOF biomedical research (this includes metal nodes in the framework, metals doped into the framework and metals which are part of other materials that have been explored in combination with MOFs). (d) Distribution of MOF papers by therapeutic indication, showing the total number of publications per indication as well as the growth of each major application since 2010. (e) Publication numbers of MOFs for drug delivery, showing the significant proportion of MOFs used as drug carriers. (f) Proportion (left) and growth (right) of MOF research in anticancer drug delivery since 2015, as compared to other carrier (nano)materials. Data, comprising the period 2010-2022 (except for panel f, which comprises 2015-2022), was obtained by systematic search of the SCOPUS database of journal publication[22] (NB: details of search terms are also provided in the corresponding reference 22).
This growth has also been mirrored by the increasing diversity of metals and organic ligands that can be used to design MOFs and, consequently, by the breadth of achieved topologies and structures.[15] Since the first reports of MOFs in the 1990s,[16] more than 100,000 different structures have been published, highlighting the structural and chemical diversity of these materials. Fe (MOF-74, MIL-88, MIL-100 and MIL-101), Zn (ZIF-8, MOF-74), Cu (HKUST-1) and Zr (UiO-66 and UiO-67) are among the most common metals and families of MOF structures explored in MOF biomedical research (Figure 1b-c), most notably for cancer, microbial infections, and inflammatory diseases (Figure 1d). In general, and particularly in oncology, MOFs have been extensively studied for drug delivery (Figure 1e), with a research interest that has been drastically accelerated in the last five years. Although lipid- and polymer-based materials are still playing major roles in drug delivery (Figure 1f, left), MOF-related publications for anticancer drug delivery are indeed growing at a much faster rate than conventional drug delivery systems like liposomes, micelles, and/or hydrogels (Figure 1f, right).
Significant progress in MOFs and MOF-based chemistry has been made in both academic and industrial settings.[17] Advanced methods to prepare and scale-up these materials for industrial production have been developed, including strategies to control their synthesis and specific properties. These research achievements have already resulted in the foundation of more than 30 companies aiming to commercialize MOFs for gas storage,[18] catalysis,[19] environmental remediation,[20] and even water harvesting from air.[17,21] Despite their benchtop success in different areas of application, and although there have been many reports on the biomedical potential and clinical promise of MOFs, only one MOF platform has reached clinical trials (NCT05838729 and NCT03444714), highlighting the wide gap between the preclinical performance and their clinical feasibility.
In this review, we summarize the distinctive multifunctional features of MOFs and MOF-containing (nano)materials for biomedical applications and critically analyze the potential of these features to address current medical needs. We furthermore discuss the challenges and translational barriers MOFs face and finally (re)define therapeutic opportunities for MOFs to generate clinical impact. By identifying current research gaps and future directions, we aim to contribute to the rational design of MOF-based materials with enhanced translational and societal value.
2. Properties of metal-organic frameworks for biomedical applications
MOFs are crystalline porous structures constructed by connecting metal-containing clusters and organic linkers via coordination bonds. The inorganic and organic building units act as ‘nodes’ and ‘struts’ that form extended one-, two- and three-dimensional periodic crystalline networks with molecularly-defined directionality. One of the most unique and useful properties of MOFs is their ultra-high porosity. They boast among the highest porosity ever measured in synthetic materials to date (up to 10,400 m2/g),[23] with remarkable adsorption capacities. Having the ability to adsorb more than 2,870 mg of CO2 per gram of MOF,[23] they clearly outperform the CO2 adsorption capacities of zeolites (up to 310 mg/g),[24] or ultra-microporous graphitic carbon (345 mg/g).[25] The structural rigidity of the building units and robust network connectivity ensure permanent porosity and prevent the framework from collapsing during functionalization or guest exchange.
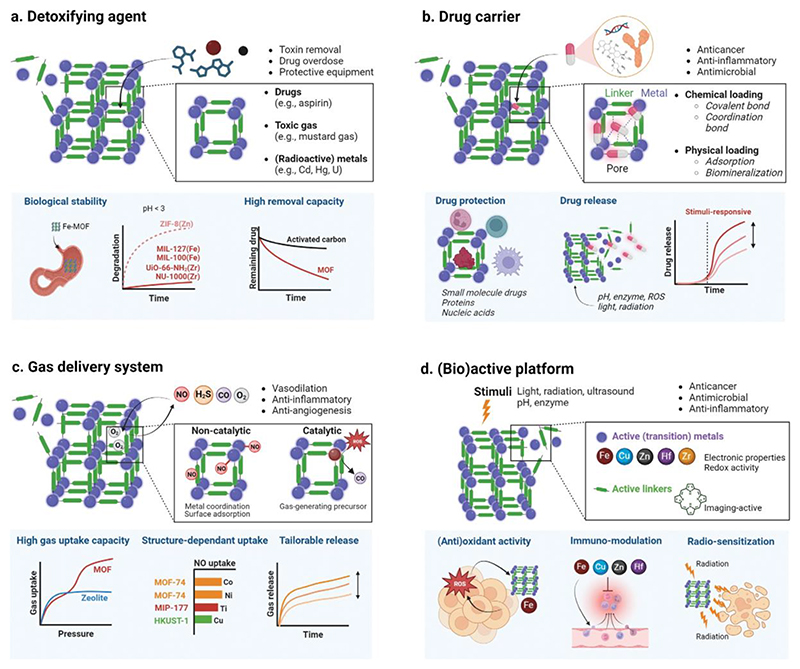
The chemistry of MOFs is highly versatile. Structures can be made with almost any combination of organic linkers, including macro- and bio-molecules, and metal nodes (or even multiple metal nodes),[26,27] to produce a wide range of pore dimensions in structures of myriad topologies. The porous structure provides MOFs, particularly nanoscale MOFs, with distinctive functional properties that are useful in diverse biomedical applications. These functional features allow MOFs to be used as: (a) detoxifying/capturing agents, (b) drug carriers, (c) gas delivery systems, and (d) (bio)active platforms (Figure 2).
Figure 2. Metal-organic framework properties for biomedical applications.
The high porosity, surface area and metal-organic composition endows metal-organic frameworks (MOFs) with potential use as detoxifying agents, drug and gas carriers, and as active therapeutic platforms. (a) The adsorptive capacity of the framework can be exploited to capture harmful substances such as radioisotopes and excess drugs, facilitating removal from the body. (b) The high porosity and structural versatility of MOFs allow them to load a wide variety of drug cargoes, from low molecular weight compounds to biomacromolecules. (c) Gases are a therapeutic cargo that MOFs have demonstrated a unique ability to load and deliver with high efficiency. They can be catalytically generated by the MOF or directly loaded into the structure. (d) Both the metal and the organic linker of the framework can act as active therapeutic entities, often in response to endogenous (e.g., pH and enzyme) or exogenous stimuli (e.g., light, radiation and ultrasound), and potentially in combination with other treatment strategies.
a. Detoxifying/capturing agent
Porous materials such as charcoal (activated carbon) have been used as detoxifying agents for thousands of years.[3] The ultra-high porosity, large specific surface area, and the polar/non-polar nature of metal-organic constituents endow MOFs with the capacity to capture and store large amounts of chemically diverse substrates,[26] including (small molecule) drugs, toxic metals, and gases (Figure 2a). The mechanism of substrate uptake, which varies among MOFs, are primarily chemisorption (e.g., via coordination bonds[31]), physisorption (via Van der Waals interactions[30]), physicochemical phenomena such as size exclusion of the adsorbate, and combinations of these mechanisms.[32] The pore size of the framework can, in principle, be tailored to accommodate targeted compounds, as illustrated in a landmark study where a series of MOF-74 derivatives were made with pores ranging in size from 14 to 98 Å by using a series of linkers of increasing length.[28] Many MOFs have already been successfully used in environmental studies to capture analytes from complex matrices, exemplifying their adsorptive capacity. Environmental chemists have shown that MOFs are capable of capturing heavy metals in heterogenous samples,[29] pharmaceuticals in wastewater,[30,31] radioactive species in runoff,[32] and many other pollutants.[20,33] Although there are many hurdles to adapting a material designed for large-scale filtration to be a small-scale pharmaceutical, there is already a solid foundation for using MOFs as detoxifying/capturing agent.
The removal of toxins from organisms is important in multiple clinical indications, and it is particularly crucial when endogenous mechanisms and organs cannot detoxify and excrete harmful compounds. These include not only exogenous toxins (e.g., excess or off-target accumulation of drugs and poisonous gases) but also endogenous toxins (e.g., ammonia, bilirubin, or uremic toxins) left in circulation in certain metabolic conditions like hyperammonemia or upon kidney and liver dysfunction.[34,35] A handful of MOFs have been exploited for their porous nature in this regard. Some studies have shown the intrinsic capacity of MOFs to adsorb (and potentially remove) endogenous toxins from blood. These include uremic toxins (e.g., Zr-MOFs such as NU-901(Zr) and NU-1000(Zr)),[36] which often accumulate in patients with kidney failure, or bilirubin (e.g., PCN-333(Al)), which is found at harmful levels in the blood of patients suffering from acute liver failure.[37] Substantial preclinical evidence has already demonstrated that different MOF structures can adsorb a variety of exogenous toxins, including drugs such analgesics like aspirin (e.g., MIL-127(Fe)[38] and MIL-125-NH2(Ti)[39]), or radioactive substances such as uranium (e.g., UiO-66-(COOH)4-180(Zr)[40]); and remove them from the body in the event of acute drug overdose or radiation exposure. MOFs have also been used to capture external toxins when embedded in protective equipment. There are several examples of masks impregnated with MOFs to protect against chemical warfare agents (e.g., UiO-66-NH2(Zr))[41,42] and microbial diseases (e.g., ZIF-8(Zn)).[43,44] MOFs (and related porous frameworks[45]) are highly chemically versatile and can therefore be designed to be more sensitive and selective removal platforms than other existing materials. But despite this intrinsic advantage, their translational value will depend on whether they can improve the balance between detoxification efficacy and innate toxicity as compared to current treatments (such as sodium bicarbonate for the treatment of aspirin overdose[46] or chelating agents like diethylenetriamine pentaacetate (DTPA) for the treatment of uranium exposure[47]).
b. Drug carrier
This is by far one of the most explored applications of (nano)porous materials, including MOFs, and there are several comprehensive reviews on this topic.[48–51] As in other drug delivery systems, drug encapsulation in MOFs can improve drug solubility[52] and/or alter pharmacokinetics and pharmacodynamics.[53] The high surface area and porous structure of MOFs make them suitable to encapsulate and transport drugs, either (i) entrapped within the pores (i.e., inner surface area adsorption), (ii) through external surface adsorption, or (iii) chemically bound to the metal nodes (via coordination bonds) or organic linkers (via covalent bonds) (Figure 2b). Several MOF structures, such as ZIF-8(Zn),[54–57] MIL-100(Fe),[58–60] NU-1000(Zr),[61,62] and MIL-125(Ti)[63] have reportedly encapsulated a variety of different therapeutics, including biologics. The tunability and the amphiphilic nature of the pores, where the metals and organic linkers can be tailored to modify the degree of hydrophobicity of the inner framework cavities, enable the loading of a variety of polar and non-polar compounds. These cargoes traditionally encompass small molecule drugs such as anticancer agents (e.g., doxorubicin,[64] paclitaxel,[65] pemetrexed,[66] zoledronate,[67] and methotrexate[68]) and antimicrobial agents (e.g., favipiravir[58] and acriflavine[62]), among others.
More recently, several reports have demonstrated successful encapsulation and stabilization of larger functional biomolecules, including nucleic acids like siRNA[69] or GFP plasmid;[56] as well as proteins such as bovine serum albumin,[70] myoglobin,[71] insulin,[72] Cas9/sgRNA ribonucleoproteins,[70] and even enzymes like DNAzymes,[55] urease[73] and horseradish peroxidase.[70,73] Because (bio)macromolecules are relatively large cargoes, they can be encapsulated through biomineralization: a process that builds a MOF around the biotherapeutic agent.[56,74] Biomacromolecules tend to be highly sensitive to specific biological conditions and, therefore, at risk of degradation in the body before reaching their intended target, so encapsulation is used to protect and stabilize them.[75] For example, ZIF-8(Zn) has successfully encapsulated the enzyme horseradish peroxidase, increasing its shelf-life and preserving the structure even after boiling in DMF.[73] Another study found that ZIF-8(Zn) could be loaded with the gene-editing CRISPR/Cas9 plasmid through biomimetic mineralization, facilitating endosomal escape of the cargo and improving therapeutic efficacy.[54,76] Interestingly, a sustained release system for proteins, DNA and RNA was created using ZIF-8(Zn) which could be transdermally delivered via needle-free administration approaches (e.g., biolistic delivery).[77,78] This approach used a gas propellant which enabled drug administration and could control the rate of payload release from the MOF. While the use of CO2 gas caused local acidity that drove rapid MOF degradation and payload release, compressed air (i.e., about 80% N2 and 20% O2 gas) allowed for over one week of sustained release.
Many MOF structures for drug delivery applications are designed to be responsive to stimuli, potentially allowing for controlled release under endogenous triggers such as pH, shear stress or enzymes, and exogenous triggers such as ultrasound, magnetism, and radiation.[57,79,80] This feature has been demonstrated in many different MOFs, including UiO-66-NH2(Zr),[81] MIL-100(Fe)[82] and ZIF-8(Zn),[57,83] which have been designed to be responsive to light, glutathione/ATP, and radiation or pH, respectively. Developing biomedical materials that can respond to specific cues has the potential to produce targeted and autonomous medicines. For instance, a recent study reported a Zn-based MOF loaded with either insulin or the aptamer VEGF for treating diabetes.[84] The framework was selectively able to release its cargo in the presence of high glucose levels by incorporating a glucose oxidase “gatekeeper”, paving the way towards the design of alternative treatments to the regular insulin injections used by diabetics. But designing stimuli-responsive materials can add additional layers of complexity to production, increase manufacturing costs, and complicate clinical procedures.[85] This is especially true in the case of nano-therapeutics that respond to external stimuli and are systemically administered (e.g., via intravenous, subcutaneous, and oral routes). In these cases, the external stimulus controls targeted drug release and must therefore be applied with high spatiotemporal accuracy. To illustrate it, no externally triggered drug-loaded anticancer nanomedicine has been approved to date, despite more than 30 years of intensive preclinical and clinical research.[86,87]
c. Gas delivery system
Gases like nitrogen monoxide (NO), hydrogen sulfide (H2S), carbon monoxide (CO), and oxygen (O2) play pivotal roles as signaling molecules (gasotransmitters), regulating many physiological and pathological processes.[88] There is mounting evidence that they can have therapeutic effects in treating many diseases. They have shown anticancer,[89–91] wound healing,[92,93] and antimicrobial properties,[94–96] and can also support vasodilation,[14] reduce inflammation,[97] overcome drug resistance in combination therapies,[98] and treat radiation damage.[97] However, their clinical potential has been limited by the difficulty of safe and controlled delivery. Unlike solid or even liquid medications, gases are notoriously challenging to encapsulate and selectively release. Besides having a short half-life under physiological conditions,[99,100] gases such as H2S, CO, and NO are known to be highly toxic if released off-target, producing side effects such as pulmonary edema, convulsions, loss of consciousness, and even death.[101–103] While several gas-releasing (organometallic) compounds (e.g., MnBr(CO)5) have been loaded in nanocarriers for improved gas transport, the gas-carrying capacity of the compounds is limited by their structural and spatial constraints, as well as their chemical compatibility with different gases. In short, there is still a clear medical need and translational opportunity for gas-entrapping materials that can perform safe and efficient gas delivery.[97]
Porous materials like MOFs not only have high porosities and surface areas but also incorporate multiple functionalities within the framework that make them attractive materials for gas delivery[14] (Figure 2c). MOFs deliver gases mostly by two different mechanisms: catalytic gas generation (indirect delivery) or non-catalytic release (direct delivery). In indirect delivery, the metals of the framework[104] (and occasionally metals doped into the framework[105]) can trigger the generation of gas after reacting with substrates present in tissues. For example, Cu can be used to catalyze the production of NO gas from S-nitrosoglutathione or S-nitrosocysteine either when introduced as the constituent metal of the frameworks HKUST-1(Cu)[104] or CuBTTri(Cu),[106] or as Cu(II) ions doped into ZIF-8(Zn).[105] In direct gas delivery, the framework is loaded with gas, transports it into the body, and eventually releases it at the target site. MOFs are typically loaded by direct adsorption of gases at high pressure, taking advantage of their extremely large surface area. They can be further optimized to better entrap and retain gases by engineering the pore size and MOF morphology[107] in order to maximize MOF-gas interaction.[108] Non- or under-coordinated metals embedded within the framework and chemical functional groups (e.g., amino groups) in the pores are especially useful for coordinating and adsorbing gases, properties not available to other porous materials such as zeolites and/or mesoporous silica particles.[24,109,110] Several MOF structures (e.g., MOF-74(Co,[111] Ni,[111] Mg,[112] or Zn[112]) and ZIF-8(Zn)[113–115]) have been used for direct gas delivery. For example, H2S gas has been delivered non-catalytically by MOF-74(Ni) to promote vasodilation,[116] as has NO by PCN-223(Zr) in preclinical anticancer research.[117] While it is usually complicated to control the delivery of the gas, some MOFs, such as PCN-223(Zr), have been shown to release NO via a near-infrared (NIR) light trigger, which cleaves the NO-sulfur bond holding it inside the framework and freeing it to inhibit tumor growth.[117] Even though the control over gas delivery and the translational obstacles associated with complex delivery materials remain unaddressed, MOFs show promise for these applications.
d. (Bio)active platform
Unlike other (bio)material classes, MOFs do not need to be loaded with active pharmaceutical ingredients, and the framework itself can be therapeutically active. Their metallic nature and the metal-organic interplay offers unique (electronic) properties and (re)activity.[118–121] By tailoring the metal type, oxidation state, and nature of the linker, MOFs can be engineered in a way that they are able to trigger certain reactions in several (sub-)cellular targets, alter metabolic pathways, and/or react to exogenous or endogenous stimuli (Figure 2d). Exploiting the (re)active nature of metals for therapeutic purposes has already been successfully used in other clinically approved inorganic (nano)materials, such as Hensify (Hf-based radiosensitizing nanoparticles),[122] NanoTherm (magnetically activated Fe-oxide nanoparticles)[123] or AGuIX (Gd-based ultrasmall nanoparticles for radiosensitization) for cancer therapy;[124] but also Thermazene (Silvadene or silver sulfadiazine, an antibiotic containing Ag nanoparticles) for antimicrobial purposes;[125,126] and Alum (Al sulfate salt particles) as a vaccine adjuvant.[127,128] They all exploit the electronic or therapeutic properties of metals like Hf, Fe, Gd, Ag, and Al to perform specific activities that otherwise may not be easily achieved via purely organic entities.
The metals and organic ligands in MOFs can serve as therapeutic entities, alone or as part of combination therapies, and there are numerous reports of MOFs that reliably decompose in biological environments and release therapeutic metal ions. An example of this is the release of Cu(II) or Fe(II/III) ions[59,129–134] to catalyze Fenton-like and other similar redox reactions, thereby increasing levels of cytotoxic reactive oxygen species (ROS) at pathological sites (e.g., tumors). Other metals like Zn (in ZIF-8 frameworks) have been used to sterilize wound sites and reduce inflammation by disrupting bacterial membranes and inhibiting nucleic acid expression.[135] Metal ions play a crucial role in the modulation of the immune system and consequently in the prognosis of multiple diseases.[136–138] Many recent studies have explored the use of metal and metal-containing materials in immunotherapy and combination immunotherapy; a concept dubbed metalloimmunotherapy. Metals have already been used in vivo to activate immune responses and boost therapeutic outcomes. For example, Mn, a known adjuvant, acts as a damage-associated molecular pattern (DAMP), and it is commonly explored as a STING pathway activator, promoting interferon I production for improved antitumor immune response.[120,139] Other metals (such as Zn, Mg or Al) impact specific and non-specific immune responses, including T cell development and activation.[128,137,140,141] The chemical versatility of MOFs can tailor metal-induced immune system pathways, which can be exploited to generate therapeutic benefits.[142] Fe-MOFs such as MIL-100(Fe) have been used to induce Fe-mediated immunogenic cell-death pathways such as ferroptosis or pyroptosis, which can boost responses to immunotherapy.[82] Overall, MOFs can therefore be exploited as a platform for metalloimmunotherapy, including in combination therapy (e.g., adjuvant metals can be embedded in the framework and active therapeutic agents such as gases loaded within).
In addition to directly acting as a therapeutic entity themselves, MOFs can mediate and amplify the therapeutic responses of other treatments, for example by acting as radiosensitizing agents. Metals such as Hf (as in Hensify[122]), have proven to be excellent radiation therapy enhancers, and consequently Hf-MOFs (e.g., Hf-BPY-Fe,[132] Hf6-DBA,[143] and Hf12-DBA[143]) have been preclinically and clinically evaluated in tandem with radio- and radio-dynamic therapy. Many studies have shown how MOF structures can be used to mediate photodynamic therapy (PDT, which uses a light-activated drug that in the presence of O2 produces ROS). Porphyrin-based MOFs have been extensively explored for PDT because porphyrin is a photosensitizer that can be used as the organic linker of the MOF (often also loaded with other therapeutic agents).[144,145]
Finally, MOFs have also been thoroughly investigated for imaging using imaging-active metals or ligands. Fe-based MOFs have, for instance, been used in magnetic resonance imaging (MRI),[131,146–148] and UiO-66(Zr) has been used as a radiation source for in vivo positron emission tomography (PET) by labeling the MOF structure with 89Zr.[149] While substantial research has been carried out for both diagnostic and theranostic purposes, the translational success rate of MOFs or any other nanoparticle as diagnostic agents has thus far been scarce, often because of the availability, clinical feasibility, and cost-effectiveness of other existing imaging probes and protocols.[150–152]
Collectively, MOFs have been highly exploited for drug delivery, largely because their structures have the capacity to encapsulate a wide variety of different compounds within their pores. However, their metallic nature can increase toxicity[153] and decrease tolerability compared to other more conventional drug delivery systems in clinical use such as liposomes, lipid nanoparticles, or hydrogels, which are constituted by biomolecules (e.g., lipids and cholesterol) or of other materials already proven to be biocompatible (e.g., PLGA polymers). Toxicity, production difficulties, and colloidal/drug stability issues, further discussed in Section 4, are some of the challenges that make it difficult for these materials to compete against clinically approved carrier materials;[154–156] thereby widening the translational gap and narrowing the market niche for MOFs in drug delivery. Instead, emphasizing (pre)clinical work that exploits the unique properties of MOFs and MOF-based chemistry may reveal opportunities beyond what is offered by other (nano)materials. Thus, works that focus on leveraging the porosity and active nature of MOFs (alone and/or integrated into other materials to potentiate their properties) for gas delivery, detoxification applications and direct (immuno)therapeutics, and even non-systemic/local (biomacromolecule) delivery, hold increased translational potential, as well as opportunities to find niches in the market gaps left by current (nano)materials and treatment strategies.
3. Metal-organic framework formulation and therapeutic applications
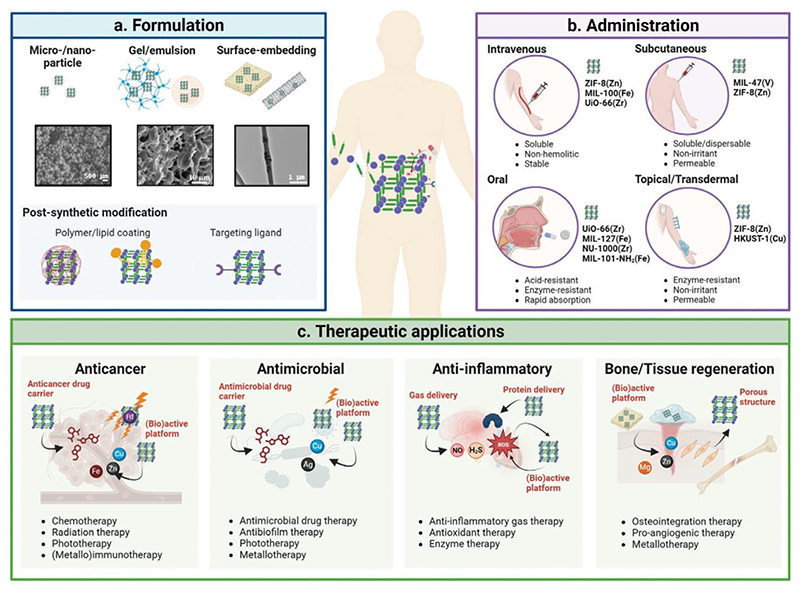
With an understanding of the intrinsic functionalities that make MOFs applicable as (nano-) medicinal platforms, they have consequently seen significant preclinical development. Building upon these functionalities, we explore in this section the biomedical application of MOFs with a focus on post-synthetic modifications and the formulation methods to make their administration possible. We then provide an overview of the main medical indications and contexts for which MOFs have been therapeutically assessed, thus far, mostly preclinically (Figure 3).
Figure 3. Metal-organic framework formulation, administration, and therapeutic applications.
(a) Metal-organic framework (MOF) formulation and post-synthetic strategies used to enable and enhance preclinical and clinical application of MOFs. (b) Main routes of administration of MOF formulations, the major formulation requirements for each form of administration, and examples of MOFs that have been shown to be compatible with each route. (c) Four of the most common therapeutic applications of MOFs showing different treatment approaches and how the intrinsic functionalities of MOFs are applied for that indication. Microscopy images for gel/emulsion and surface-embedding (panel 3a) were reproduced with permission (Copyright 2019, Royal Society of Chemistry;[211] and Copyright 2021, Elsevier[212]).
3.1. Post-synthetic modifications, formulation and administration
Translation of (nano)therapeutics requires control over the reproducibility, stability, administrability and safety of the formulation, while also ensuring improved efficacy (often by reaching and engaging with the key target(s)).[157] In addition, the metallic structure and active nature of MOFs can result in strong interactions with biological systems such as serum proteins,[158] the immune system and tissues, and their stability and properties can be altered by physiological conditions such as changes in pH, temperature and presence of ions and competitive (bio)molecules with coordinating capacity.[153,158] Therefore, control over these parameters and over the interactions at the material-biological interface are key to maximize therapeutic performance and streamline pharmaceutical development.[159]
While there have been many different MOF structures reported, their translational success is limited by the complications encountered when transforming lab-scale crystalline powders into administrable formulations. MOFs in biomedical research have been mostly formulated at the nanoscale (nanoparticles) with varying compositions, particle and pore sizes, to which different functionalities can be embedded to tailor physicochemical and pharmaceutical attributes (Figure 3a). In some cases, MOFs have also been added onto/integrated into other (nano)materials in order to achieve synergistic performances by exploiting the properties of both. For example, central nervous system (CNS)-directed antioxidant CeO2 nanoparticles were coated with ZIF-8(Zn) MOFs, allowing for greater control over size, surface charge and shape, and hence prolonging blood circulation and optimizing delivery upon intravenous administration.[160] Other examples include the use of MOF coatings for various medical implants such as stents[161] and bone replacements[162] in regenerative medicine, to improve biocompatibility and inhibit post-implantation infection. Pushing the boundaries of MOF application, biohybrid materials have also been formed by constructing MOF shells around living cells, such as red blood cells, protecting them from extreme conditions and cryopreserving them.[163–165] While there are other procedures to cryopreserve cells, the possibility of achieving similar performances with MOFs is remarkable, at least from a material engineering perspective. In a similar vein, liposomes were encapsulated into ZIF-8(Zn) nano-shells, which protected the liposomes from thermal and shear stress. This enabled needle-free biolistic vaccine delivery by firing the vaccine liposomes at high velocities through the skin,[78] an application that could be useful to develop safer, more accepted and more suitable global immunization practices, especially in under-developed countries.[166]
However, in order to prepare suitable administrable MOF formulations, post-synthetic modifications that tailor the properties of the MOF platform are often needed (Figure 3a). These modifications can include surface conjugation, embedding or encapsulating the MOF in protective materials and even loading them into living microorganisms. In addition, some of these modifications have been used to address compatibility issues, improve pharmaceutical properties (e.g., loading of active pharmaceutical ingredients), increase long-term stability, enable administration and/or enhance biological targeting, among others.
a. Biocompatibility and (bio)stability
The external surface of nanoparticles (including nano-MOFs) plays a large role in determining their biological interactions, and therefore influences the final pharmacokinetic and pharmacodynamic properties.[167] For example, Fe-MOFs based on MIL-101 family have been shown to readily hydrolyze, often making them unstable in biological conditions without modification.[168,169] Thus, MOFs are often coated with another material, typically biomolecules like lipids[82,170] (e.g., DOPC) and biocompatible polymers (e.g., polyethylene glycol (PEG)), to increase biocompatibility and biostability. PEG and other alternative (stealth) polymers (e.g., polyoxazoline)[171] are commonly used to modify the surface of the nanoparticles, enabling superior water solubility, decreased protein adsorption[172] and thus reduced clearance by circulating immune cells (i.e., longer circulation time). Likewise, several groups have explored PEGylation with MOFs, improving physicochemical and colloidal stability, preventing aggregation[173] and providing further sites for additional functionalization (e.g., with targeting ligands).[148] Integration of MOFs into other materials has also achieved similar outcomes, as illustrated by a study where a HKUST-1(Cu)-based wound-dressing was developed by embedding MOF nanoparticles into a PEG-coated isopropylacrylamide-based hydrogel to control the release of Cu(II) ions. This allowed for greater stability within the wound, reducing toxicity and improving wound closure over free MOF particles.[174]
b. Drug formulation/payload control
MOFs can also be coated with different polymers[12,148] (including hydrogels) and lipids[170,175] to improve the loading efficiency of the payload and its retention in the framework. For example, ZIF-8(Zn) particles were coated with polyethyleneimine (PEI) to increase the loading of plasmid DNA, while also enabling pH sensitive release.[176] This coating was demonstrated to significantly increase plasmid expression and transfection efficiency due to improved cell uptake and endosomal escape. Meanwhile, a nucleic acid-based hydrogel was used to control the release of doxorubicin from UiO-68(Zr). The hydrogel included an ATP-responsive aptamer which caused MOF degradation upon ATP exposure, liberating doxorubicin.[80] Lipid coatings have been used to cap drug-loaded MOFs, reducing passive drug leakage[177] and allowing for triggered release of the drug payload under endogenous and exogenous stimuli such as pH and ultrasound.[178] Lipid coatings derived from cells (including exosomes) have similarly been applied to cap MOFs and control the release of the loaded cargo,[170] although these incur additional translational barriers such as upscaling limitations, more complex manufacturing, and purification over synthetic lipid coatings.[179]
c. Administration
Formulation and post-synthetic modifications of MOFs are also used to enable their administration through different routes. The route of administration determines the barriers and interfaces that a therapeutic agent will encounter en route to the target,[159] with MOFs being explored for e.g., intravenous, subcutaneous, oral, nasal, and dermal administration[10] (Figure 3b and Table 1). Thus, various strategies have been taken to adapt MOF micro- and nano-scale particles accordingly. The physicochemical properties of the particles play a strong role in determining their administrability through any given route.[155] For inhaled nanoparticles, it has been previously demonstrated that particle sizes below 1 μm achieve superior alveolar deposition, while sub-20 nm particles tend to rapidly enter the blood stream rather than be retained in the lung tissue.[180] For oral administration, nanoparticle uptake and transport across the intestinal cells was improved as particle size decreased (1 μm – 50 nm).[181] Similarly, in subcutaneous and transdermal administration smaller nanoparticles (∼ 25 nm) tended to travel more efficiently from the injection site compared to larger particles (∼100 nm).[182] Once in circulation, size also impacts on the biodistribution and tissue uptake of the nanoparticles, with particles below 5-10 nm size showing clearance by the kidneys, and larger particles (> 200 nm) being predominantly accumulated in the liver, spleen and lungs.[155,183] Therefore, nanoparticles around 100 nm have been found to exhibit longer circulation times and increased specific tissue accumulation, particularly when the enhanced permeability and retention (EPR) effect can be exploited. Despite being highly heterogenous,[184] this phenomenon has been generally found to occur in inflamed (pathological) tissues, such as tumors, where the increased vascular leakiness and impaired lymphatic drainage result in an increased nanoparticle accumulation and retention. Other physicochemical properties like shape have also been shown to be a critical factor in biodistribution, with non-spherical particles being found to tumble in circulation, thereby increasing the interaction with the vessel walls and improving extravasation into the tissue. Likewise, nanoparticle surface charge modulates the interaction with bloodstream proteins and circulating cells. As a general trend, neutral or slightly negative surface charge show reduced protein interactions and are known to exhibit longer blood circulation times than strongly (positively) charged particles.[185]
Table 1. Selected examples of preclinical therapeutic metal-organic frameworks for different indications.
| Indication | Routea | Mb | MOF | Formulationc | Functiond | Ref. |
|---|---|---|---|---|---|---|
| Cancer | IV | Cu | Nano-CuTz-1 | Pluronic F-127 coating | Gas delivery (O2), Catalysis (ROS), Depletion (GSH), PDT | [213] |
| Zr | Nano-PCN-224 | 4T1 breast cancer cell membrane coating | Drug delivery (tirapazamine), PDT | [202] | ||
| Fe | Nano-MIL-101 | Shewanella oneidensis bacteria loaded | Drug delivery (Dox), Catalysis (ROS), Depletion (lactate, P-gp) | [60] | ||
| Hf | Nano-PCN-224 | PEGylation | Radiotherapy, PDT | [173] | ||
| Zr | Nano-PCN-223, Mn doped | S-nitrosothiol conjugate | Imaging (MRI), Gas delivery (NO), PTT | [117] | ||
| Zn | Nano-ZIF-8 | Hyaluronic acid ligand | PTT (ICG photosensitizer) | [194] | ||
| Mannan targeting ligand | Vaccine (imiquimod and 1-MT ICI) | |||||
| Zn | Nano-ZIF-8 | HeLa cervical cancer cell coating | Protein delivery (catalase), PDT (phthalocyanine photosensitizer) | [253] | ||
| SC | Zn | Nano-ZIF-8 | - | Vaccine (ovalbumin antigen, CpG ODN) | [220] | |
| IT | Hf | Nano-di(p-benzoato) aniline | - | Radiotherapy, Immunotherapy (ICD) | [143] | |
| Hf | Nano-PCN-222 | - | Radiotherapy, Immunotherapy (epacadostat and IDO1 inhibitor) | [219] | ||
| Nano-tetra(p-benzoato)porphyrin | - | Radiotherapy, Immunotherapy (ICD) | ||||
| Hf | Nano-di(4-benzoato)-bipyridine | Ir doped | Radiotherapy, Immunotherapy (ICD), Vaccine (adjuvant) | [254] | ||
| Microbial Diseases | IV | Fe | Nano-MIL-100 | Pluronic-127 coating | Drug delivery (3-azido-d-alanine) | [228] |
| Topical | Zn | Nano-ZIF-8 | Carbonized MOF, Ag nanoparticle coating | PTT, Antimicrobial (Zn2+, Ag+) | [226] | |
| IP | Zn | Nano-ZIF-8 | Hyaluronic acid ligand | Tetracycline | [193] | |
| (in vitro) | Ni | Micro-MOF-74 | MOF-embedded in polyurethane film | Gas delivery (NO) | [230] | |
| Implant | Mg, Zn | MOF-74 | MOF coated in Ti implant | Bone regeneration, Antimicrobial (Zn2+, Mg2+) | [229] | |
| Tissue/Bone Injury | Implant | Zn | Nano-ZIF-8 | MOF-embedded PCL-dicalcium phosphate dihydrate scaffold | Bone regeneration | [238] |
| Topical/Transdermal | Cu | Nano-HKUST-1 | PPCN hydrogel embedded | Diabetic wound healing (Cu2+) | [174] | |
| Implant | Cu | Nano-HKUST-1 | Polydopamine coating, MOF coated in Ti stent | Anti-thrombosis, Anti-restenosis | [161] | |
| SC | V | Nano-MIL-47 | - | Catalysis (H2O2 reduction), Anti-inflammatory | [231] | |
| Inflammation | Oral | Zr | Nano-UiO-66 | HPMCAS polymer coating | Gas scavenging (H2S), Imaging (fluorescence) | [189] |
| Fe | Nano-MIL-101-NH2 | Gelatin capsule, NIPAM-MPDMSA Hydrogel coating | Protein delivery (exendin-4) | [190] | ||
| Diabetes | SC | Zn | Nano-ZIF-8 | - | Protein (insulin and GOx) | [248] |
| Oral | Fe | Nano-MIL-100 | SDS ligand, mPEG-PLLA microsphere loaded | Protein (insulin) | [247] | |
| Cardiovascular Diseases | IV | Zn | Nano-ZIF-8 | Carbon nanosphere loaded, RGD ligand | PTT, PDT (thrombolysis) | [195] |
| CNS Diseases | IV | Zn | Nano-ZIF-8 | MOF-coated CeO2 nanoparticles | Antioxidant, neuroprotection (ischemic stroke) | [160] |
| Pulmonary Diseases | Nasal | Zr | Nano-UiO-66 | - | Drug delivery (dexamethasone and rhodamine B) | [250] |
| Other | Oral | Fe | MIL-127 | - | Overdose detoxification (aspirin) | [38] |
| SC | Zn | Nano-ZIF-8 | MOF-coated tobacco mosaic virus | Viral vector (vaccination) | [255] | |
| SC | Zn | ZIF-8 | MOF-coated bacteria | Whole-cell bacteria (vaccination against Urinary Tract Infection) | [252] |
Abbreviations: SC – Subcutaneous; IT – Intratumoral; IV – Intravenous; IP – intraperitoneal;
M refers to metal;
Abbreviations: PCL – Polycaprolactone; PPCN – Poly-(polyethylene glycol citrate-co-N-isopropylacrylamide); HPMCAS – Hydroxypropylmethylcellulose acetate succinate; NIPAM – N-isopropylacrylamide; MPDMSA – [3-(methacryloylamino)propyl]dimethyl(3-sulfopropyl)ammonium hydroxide; SDS – Sodium dodecyl sulfate; mPEG-PLLA – Methoxy poly(ethylene glycol)-block-poly(L-lactide); RGD – Arginylglycylaspartic acid;
Abbreviations: 1-MT – 1-Methyl Tryptophan; CpG ODN – CpG Oligodeoxynucleotide; Dox – Doxorubicin; P-gp – P-glycoprotein 1; GOx – Glucose Oxidase; GSH – Glutathione; ICD – Immunogenic Cell Death; ICG – Indocyanine Green; ICI – Immune Checkpoint Inhibitor; MRI – Magnetic Resonance Imaging; NO – Nitric Oxide; PDT – Photodynamic Therapy; PTT – Photothermal Therapy; ROS – Reactive Oxygen Species.
As described above, PEGylation is commonly used to improve water solubility and is thus often employed to enable parenteral (e.g., intravenous or subcutaneous) administration of MOF nanoparticles. Many nano-MOFs are intrinsically unstable in physiological conditions, where they tend to collapse into large aggregates in biological media.[186] These aggregates can lead to serious health risks like emboli, and as such, solubilizing coatings are critical to safe and effective systemic administration. While intravenous administration has been an extensive research focus for MOFs, oral delivery is often the preferred route due to increased patient compliance and lower invasiveness. It requires that the platform survives the harsh conditions of the digestive system, including wide pH swings, enzymatic degradation,[187] and liver filtration.[188] For example, UiO-66(Zr) was coated with hydroxypropyl methylcellulose acetate succinate using microfluidics to produce an orally available formulation that protects the MOF core from the acidic conditions in the stomach.[189] In other cases, pH-responsive polymer-coated gelatin capsules or zwitterionic N-isopropylacrylamide-based hydroxide (NIPAM-MPDMSA) coatings were employed to protect the MOF nanomaterial and/or ensure bioavailability upon oral administration.[190] MOFs have also enabled long-lasting sustained drug delivery systems for local application, such as the case with the corticosteroid dexamethasone in ZIF-8(Zn).[191]
d. Biological targeting
Targeting moieties have also been added to MOFs, allowing therapeutic entities to more selectively direct them towards tissues that overexpress a specific marker. This decreases off-target accumulation (and thereby toxicity) and improves on-target dosing by increasing the number of MOF particles that reach the target site. As in other biomedical nanoparticles, multiple targeting ligands have been explored with MOFs, including folate,[67,192] hyaluronic acid,[193] mannan,[194] RGD peptides,[195] and antibodies.[196] The benefit of actively targeting nanoparticles by adding ligands on the surface remains controversial, however. While it has found some success, particularly in the targeting of the immune system,[197,198] it has also been shown to promote protein corona formation or interaction with macrophages that might result in faster clearance from the body, and hence, not always leading to improved therapeutic efficacy (as is the case of active tumor targeting strategies).[199–201] Therefore, the addition of targeting ligands requires careful consideration of the intended target and the potential side interactions. Finally, biological coatings have also been explored for targeting purposes, such as cancer cell membranes, taking advantage of the natural biocompatibility of lipids and of the specific tropisms to increase stability, circulation time and cancer cell specificity. Examples of this include 4T1 breast and HeLa cervical cancer cells[202] or dental pulp mesenchymal stem cells,[203] where the targeting moieties naturally expressed by the cancer cells trafficked cell membrane-coated MOFs towards other cancer cells while also inhibiting uptake by circulating immune cells. Interestingly, doxorubicin-containing Fe-based MIL-101 nanoparticles were stably loaded into the bacteria Shewanella oneidensis to exploit its tumor tropism and metabolism. The bacterial metabolism of lactate triggered the degradation of the scaffold in the tumor, resulting in release of the drug doxorubicin, combined with the oxidative stress induced by the Fe(II) ions generated upon reduction of the Fe(III) of the MOF.[60]
3.2. Medical indications for therapeutic metal-organic frameworks
MOFs and their properties have been explored for several medical indications, particularly cancer and microbial infections (as shown in Figure 1). Many platforms have entered preclinical (and very few clinical) development, with an emphasis on nanoscale MOFs for therapeutic and diagnostic applications.[11] While MOFs hold potential for therapeutic applications, diagnostic nanoparticles, including nano-MOFs, face several challenges in clinical translation due to stiff competition from available traditional probes and methods.[13,204] Yet, MOFs have shown promise for biosensing purposes.[205,206] Various molecules (mostly gases) have been detected using MOFs, including acetone vapours,[207] oxygen,[208] and hydrogen sulfide,[209] as have biologics like miRNA.[210] By modulating the electronic properties of the structure, MOFs can enhance analyte adsorption affinity, pre-concentrate it, and thereby increase sensing performance. Interactions between the substrate and any component of the MOF (metal, ligand, or assorted functional groups) can create or amplify signals, so alterations in the structure can tune the sensitivity to or selectivity of the analyte.[207]
In this section, we do not aim to comprehensively analyze MOF research on every therapeutic application, but instead to overview and illustrate some examples of the recent research carried out with therapeutic MOFs (mostly MOF nanoparticles) for some medical indications, starting with cancer (the most widely studied disease), but also beyond (Figure 3c and Table 1).
a. Cancer
As for nearly all nanomaterials, cancer is the most common indication targeted by MOFs in clinical and preclinical research. MOFs have most commonly been used as multifunctional and multimodal anticancer drug (and gas) carriers while frequently exploiting combination therapies with the intrinsic activity of the platform, often derived from the reactivity of the metals. The intrinsic activity of MOFs presents a distinctive feature over other nanocarriers, as exemplified by a Cu-based triazolate MOF (CuTz-1) loaded with O2 gas for photodynamic therapy (PDT). Under near-infrared (NIR) light, the MOF triggered the formation of toxic reactive oxygen species (ROS), killing cancer cells through oxidative stress. Additionally, the MOF adsorbed the overexpressed enzyme glutathione (GSH) and alleviated tumor hypoxia through O2 delivery, potentiating the effects of the PDT.[213] In another work, camptothecin-loaded Zr-TCCP MOF was coated onto Au nanorods. The Au nanorods acted as a photosensitizer to enable localized tissue heating, called photothermal therapy (PTT), combined with MOF-catalyzed PDT and controlled release of the chemotherapeutic under NIR light.[214] Similarly, the enzymes glucose oxidase (GOx) and catalase were delivered in combination with PDT, enhanced by the intrinsic photosensitizing effect of the PCN-224 MOF, generating ROS overload and starving the tumor of glucose.[215] PTT was in fact used as a trigger, as well as a therapy, to induce the release of NO gas from PCN-223(Zr/Mn) loaded with the heat-sensitive NO-donor S-nitrosothiol.[117] The active gas potentiated the antitumor effects of the PTT in vivo. Overall, these works show that much attention has been focused on MOFs as multifunctional phototherapy systems, in no small part due to the effectiveness of phototherapies in vitro and in vivo. However, besides the increased engineering complexity of the system, caution should be taken regarding their translational value as, while light penetrates with sufficient energy to stimulate phototherapy in small animal models such as mice, it is much less effective (and more challenging) in large systems like the human body, where there is a much greater mass of tissue that hinders effective light penetration.[216]
Immunotherapy has become a cornerstone of modern cancer therapy, and MOFs have also been developed to potentiate (chemo-)immunotherapy.[143,217–219] For example, a model cancer antigen, ovalbumin, was co-loaded with cytosine-phosphate-guanine oligodeoxynucleotide (CpG ODN) TLR9 agonist in a ZIF-8(Zn) MOF to produce a cancer vaccine.[220] As previously discussed in Section 2d, metalloimmunotherapy approaches have also been recently exploited, for example, in an Fe-based dithiodiglycolic acid MOF loaded with doxorubicin and glucose oxidase (GOx). Fe(II) ions from the MOF catalyzed ROS generation, amplified by glutathione depletion and GOx, and induced ferroptosis in cancer cells, which combined with the doxorubicin-induced immunogenic cell death (ICD), lead to a strong anticancer immune response.[221] In another study, a complex dual MOF platform injected a tumor-targeted dye-loaded ZIF-8(Zn) for PTT, which induced ICD and generated damage-associated molecular patterns (DAMPs), boosting immune responses.[194] This was followed by a second injection of a dendritic cell targeted ZIF-8(Zn) that was loaded with the adjuvant imiquimod and indoleamine dioxygenase (IDO) immune checkpoint inhibitor (1-methyl-d-tryptophan (1-MT)) to stimulate robust immune response against the generated DAMPs. Similarly, a mesoporous silica nanoparticle was coated with ZIF-8(Zn) and co-loaded with the tumor antigen ovalbumin and polyinosinic-polycytidylic acid as an adjuvant. The MOF acted to control the release of the payload and increase delivery to antigen presenting cells and the lymph nodes. The resulting platform boosted chemo-immunotherapeutic outcomes upon combination with PD-1 checkpoint blockade.[217]
One of the most notable MOF works in cancer therapy is the development of MOFs for radiosensitization and cancer immunotherapy. Leveraging the radiation enhancing properties of Hf, Hf-based MOFs are used to absorb low dose X-rays and then generate ROS and O2, enhancing the oxidative damage to DNA caused by the X-rays. Notably, the MOF is not used for drug-loading, but for its intrinsic properties as a therapeutic instead. This also solves the key issue with phototherapies described above, as high-energy radiation does not suffer the same attenuation when passing through human tissue, allowing for much greater penetration in the body. This Hf-MOF was combined with immune checkpoint inhibitors to exploit the production of immunogenic DAMPs from the initial radiotherapy and potentiate antitumor efficacy. This (or an equivalent) Hf-MOF platform is, to the best of our knowledge, the only known MOF to proceed beyond preclinical development into Phase 1 clinical trials (NCT03444714 and NCT05838729), under the name RiMO-301.[222,223]
b. Non-cancer
Beyond cancer, MOFs have been explored as therapeutics to treat several other indications including (i) microbial, (ii) inflammation and oxidation, (iii) bone and tissue regeneration, (iv) diabetes and (v) other diseases; with antimicrobial among the most popular therapeutic uses, as shown previously in Figure 1. In the following, we illustrate the potential versatility of MOFs, particularly the intrinsic properties highlighted in Section 2, being applied to a wide range of different medical conditions.
(i). Antimicrobial
In this area, MOFs have also been primarily used as drug delivery systems. For example, ZIF-8(Zn) was used to control the delivery of ceftazidime for therapy against gram-negative bacteria.[224] However, metals and metal-based compounds are increasingly gaining relevance as potent antimicrobial agents.[125,225] Hence, a Ag-doped carbonized ZIF-8(Zn) MOF was used in phototherapy and light-stimulated release of bactericidal Zn(II) and Ag(I) ions.[226] Another formulation instead aimed to starve bacteria of glucose and induce toxic oxidative stress by delivering the enzyme glucose oxidase via Cu(II)-doped ZIF-8(Zn). The enzyme consumed glucose to produce H2O2, which was then converted to toxic ROS via Cu(II) catalyzed Fenton-like reactions, killing the bacteria.[227] Unlike other nanoparticles, MOFs can be more readily engineered for phototherapies like PTT or PDT, therapies also explored for the treatment of cancer. One particularly intricate system loaded MIL-100(Fe) MOFs with 3-azido-d-alanine, which was taken up by bacteria and expressed on their outer membrane. A photosensitizing nanoparticle functionalized to bind to the exposed 3-azido-d-alanine was subsequently injected, allowing for highly selective PDT of bacteria.[228] MOFs are also explored as antibacterial coatings on medical devices such as bone implants,[229] or catheters,[230] as illustrated by a hybrid Mg/Zn MOF-74 that was coated onto the Ti-surface of a bone implant to prevent bacterial infection and promote new bone formation. Under the acidic conditions produced by the bacteria, the MOF layer degraded into free metal ions, which enhanced osteogenesis, and into the organic ligand 2,5-dihydroxyterephthalic acid, which raised local pH to levels that are toxic for bacteria.[229]
(ii). Anti-inflammatory and Antioxidants
MOFs have also seen considerable development in inflammatory diseases such as neuron damage after ischemic stroke, mostly by taking advantage of the redox and catalytic activity of the metal and linkers. For instance, a ZIF-8(Zn) MOF was used to cap CeO2 nanoparticles, providing not only enhanced physicochemical properties and penetration across the blood brain barrier but also enhanced therapeutic benefit. The degradation products of the MOFs acted synergistically with CeO2 to scavenge ROS and suppress inflammation in brain tissue after an ischemic stroke.[160] Another study found that MIL-47(V) derivatives could be used as a replacement for the enzyme glutathione peroxidase to suppress harmful ROS such as H2O2, thereby alleviating inflammation.[231] This approach was also taken with Pt nanoparticle-functionalized PCN222(Mn) for ulcerative colitis and Crohn’s disease, using the superoxide dismutase-like activity of Mn(II) and the catalase-like activity of the Pt nanoparticles to scavenge ROS.[232] Early preclinical work has also been done on using MOFs for enzyme delivery or replacement. An example was already discussed previously for GSH replacement as part of the anticancer treatment;[213] however, other examples include PCN-333(Al) as a platform for enzyme (superoxide dismutase and catalase) delivery to treat inflammation[233] where the MOFs notably enhanced uptake of the enzyme compared to the free protein.
(iii). Bone and Tissue Regeneration
In the field of regenerative medicine, again, the role of metal ions interacting with biological processes is essential, along with the high porosity of MOFs, both of which enhance cell engagement.[234] MOFs are frequently studied as coatings for bone implants, both to prevent post-implantation infection[229] as well as promote osteointegration and tissue regeneration.[235] Ti-bone implants were also modified with ZIF-8(Zn) MOFs and loaded with the corticosteroid dexamethasone, forming a long-release drug depot that promoted osteointegration[191], while further modifications with hyaluronic acid prolonged drug release to four weeks.[236] Likewise, a ZIF-8(Zn) MOF-reinforced PLLA scaffold was designed to promote bone regeneration.[237] It was found that cell proliferation and maturation were enhanced by the scaffold due to its high porosity and the release of Zn(II) ions as it was degraded. Similar results were also reported with another ZIF-8(Zn) coated polymer scaffold,[238] while other groups have reported MOF coatings such as the Zn-based bio-MOF-1.[239] MOFs have also found potential applications in wound healing,[240] utilizing many of the same principles as for bone regeneration. Here, HKUST-1(Cu) was used to promote diabetic wound healing through the release of Cu(II) ions,[174,241] stimulating critical processes such as angiogenesis, collagen deposition, and cellular integration and migration. In this regard, Mg(II) delivery by Mg-based MOFs has been suggested as a potentially less toxic alternative to Cu for diabetic wound healing. For instance, Mg-based MOF microspheres were embedded into a hydrogel, which was then coated onto a graphene oxide-Ag nanocomposite microneedle array.[242] When applied dermally to the wounded tissue, the release of Mg(II) ions promoted cellular regeneration, supported by antioxidant activity from the gallic acid linker and antimicrobial activity of the Ag layer, while being less cytotoxic than equivalent Cu(II)-MOFs.
(iv). Diabetes
Efficient protein delivery has been a long-standing challenge in the treatment of many metabolic diseases.[243] Presently, insulin is administered via intramuscular injections, often performed by the patients themselves, or via implanted (closed loop) insulin pumps. While these approaches, especially the latter, have overcome significant obstacles in the delivery of insulin, they still pose certain risks to patients[244] and are less tolerable and convenient than oral formulations could be. The robustness of MOF structures hold potential for protein delivery,[245] and they have been shown to enable oral insulin administration by using their porosity to load and protect biomacromolecules. For instance, an acid-resistant NU-1000(Zr) MOF was loaded with insulin to create an orally available formulation of the sensitive protein.[246] Similarly, insulin-loaded MIL-100(Fe) was made orally available by encapsulating the MOF nanoparticles in a mPEG-PLLA microsphere, and the formulation was shown to enhance delivery over the free peptide.[247] Alternatively, MOFs could be used to improve the quality of insulin injections, as shown by a long-lasting ZIF-8(Zn) MOF that was loaded with glucose oxidase and insulin to create a glucose-responsive biosensing and therapy platform. The activity of glucose oxidase induced a pH-dependent degradation of the MOF, triggering the release of the drug to promote glucose regulation up to 72 hours after injection.[248] Interestingly, glucose oxidase and insulin were also loaded into Co-doped ZIF-8(Zn) microneedles that could penetrate the skin and release the payload under glucose exposure, allowing for prolonged diabetes management via topical administration.[249] A similar strategy was taken with a ZIF-8(Zn) loading a VEGF aptamer to inhibit glucose-driven angiogenesis in the eye.[84]
(v). Other Diseases
While not as deeply explored, MOFs have also been evaluated for other indications, including drug overdose, vaccines against infectious diseases, and respiratory and cardiovascular diseases. MIL-127(Ti) MOFs were used to scavenge acetylsalicylic acid from blood[38] in drug overdose, and an inhalable UiO-66(Zr) MOF formulation was explored for pulmonary diseases, delivering the corticosteroid dexamethasone.[250] UiO-66(Zr) MOFs demonstrated prolonged localization in the lungs, and interestingly, it was shown that linker defects could be used to control the aerodynamic size of the MOFs but did not affect drug delivery performance. Other groups have also worked on NO delivery in various diseases, studying direct gas storage and delivery in HKUST-1(Cu) to inhibit platelet aggregation.[251] Recently, MOFs have also shown preclinical promise as adjuvants in vaccine formulations, putatively by better preserving antigen structure and providing a slow-release depot, which promotes B-cell development in the local lymph nodes. In one example, ZIF-8(Zn) was biomimetically grown over the surface of the uropathogenic E. coli bacteria.[252] This MOF coating served the dual purpose of protecting the surface antigens of the bacteria from denaturation while also inactivating it, making it safe for vaccine use. The ZIF-coated bacteria formulation resulted in a significant increase of antigen-specific antibodies production, specifically IgG, and protected 90% of the mice against a lethal sepsis challenge. In comparison, traditional, heat-inactivated or formalin-fixed whole-cell bacteria formulations showed only 0-10% of protection. In cardiovascular therapy, a nanoparticle HKUST-1(Cu) was coated with a polydopamine matrix, allowing it to be bound onto the Ti-surface of cardiovascular stents, preventing restenosis by releasing Cu(II) ions during degradation and catalyzing NO gas generation[161] from local NO-donor biological species.
In summary, MOF research has been highly focused on cancer, particularly using the framework as a drug delivery system. However, MOFs and MOF-nanoparticle formulations face strong competition from other already approved drug delivery materials[256] with proven safety and therapeutic benefit. Many MOF formulations under preclinical investigation are engineered to be multifunctional, multimodal therapeutics aiming to combine multiple properties in one scaffold to achieve synergistic therapeutic effects. However, these often lead to the need for complex materials, thereby increasing the cost and risk to develop the platform, including larger and more costly preclinical testing to convincingly demonstrate the added value over the standard-of-care. By comparison, and to the best of our knowledge, only one MOF platform has entered clinical trials, the Hf-MOF RiMO-301 for cancer radio-immunotherapy. This MOF is not used for drug delivery but rather exploits the intrinsic reactivity of the framework. Indeed, platforms that can potentiate (metallo-)immunotherapies and rational combination therapies (particularly in synergy with therapeutic gases or protein depletion/replacement) can provide a unique value over other existing biomedical (nano)materials.
Additionally, much like cancer, the translational potential of MOFs as drug carriers in other diseases is limited because of the existence of other established and approved delivery systems. Instead, using metals for tissue regeneration and to combat antimicrobial resistance has become increasingly relevant,[125] and are potential niches for both nano-MOFs and MOF-containing (implantable) medical devices. MOFs may even find a niche for the delivery of large macromolecules such as proteins, particularly through non-parenteral routes of administration. Similarly, while diabetes is usually treated with already approved insulin formulations and dietary and lifestyle management, the treatment of diabetic wound healing[257] remains underserved by current technology, demanding innovative solutions that can address this high medical need. Even in tissue regeneration, MOFs see strong competition from hydrogel wound dressings[258] and various regenerative scaffolds.[259] However, synergistic properties could arise from combining these materials with MOFs, albeit taking into account the associated production challenges and increased costs of complex pharmaceuticals. Finally, exploiting MOFs to make formulations that can be administered via more convenient and less burdensome routes, such as oral or nasal, could increase the value and attractiveness of MOF-based platforms to stakeholders. In this context, MOF-based drug delivery systems have been further expanded into long-lasting, local drug depots designed to sustain clinically relevant levels of therapeutics. Such systems may enable more effective and patient-friendly therapy, requiring fewer hospital visits.[260]
4. Challenges and translational barriers of metal-organic frameworks
As discussed above, MOFs have the potential to find a market niche and distinguish themselves from their competitors, especially when exploiting their intrinsic and more unique features such as porosity and framework reactivity. However, their design, production, and translation still face several challenges (Figure 4).[261] In recent years, significant strides have been made in MOF design and property control, including the development of simpler and more robust synthetic and characterization methods,[262,263] improved drug loading strategies and post-synthetic modifications to process MOFs for medicinal applications. While research efforts devoted to the design and synthesis of such (complex) multifunctional systems have provided valuable knowledge for fundamental reticular (nano-)chemistry[264,265] and biomedical engineering, there is a risk of overfocusing on the chemical and materials science aspects and, hence, ignoring practical concerns related to market, disease pathology, and patient needs.
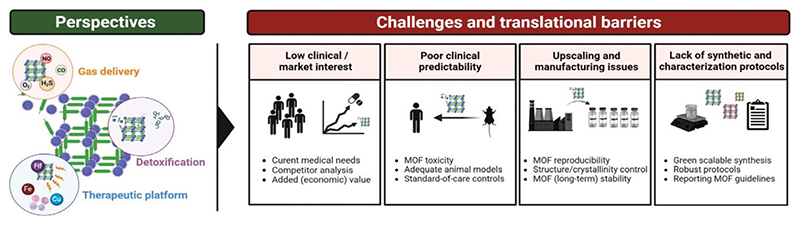
Figure 4. Challenges in metal-organic framework pharmaceutical development and clinical translation.
The perspective on metal-organic framework (MOFs) in biomedicine and the translational challenges that remain. MOFs hold promise in niche areas where their properties have the potential to allow them to outperform current therapeutic materials; most notably for gas delivery, detoxification purposes and as active (immuno)therapeutic entities. However, MOF-based materials face considerable difficulty in attracting interest from stakeholders outside of academia, such as clinicians and the pharmaceutical industry. This is, in part, due to strong competition with currently used biomedical materials, but also due to the lack of rigorous preclinical data, challenging manufacturing protocols, and non-standardized characterization and quality control. Addressing these challenges is expected to help drive MOFs across the bench-to-bedside gap in the right indications.
a. Low clinical and/or pharmaceutical interest
One of the first aspects to consider when developing any therapeutic/diagnostic material, including MOFs, is the envisioned medical, societal, and economic value over currently available solutions for the same medical indication (i.e., potential competitors). Porous materials, coordination polymers, and particularly MOFs are highly attractive from a chemical and material science perspective, and they have already been shown as useful industrial solutions for applications such as gas storage, catalysis, and water harvesting.[17,266–269] MOFs belong to a relatively young field of research and have yet to demonstrate a clear medical benefit over approved materials, so clinicians and pharmaceutical partners are hesitant to invest in their development.
Many justifications exist for this apparently low pharmaceutical and clinical interest, including sub-optimal preclinical design and unrealistic target applications. As evidenced by the publication numbers (Figure 1), a significant proportion of MOF research has focused on their use as drug delivery systems and often as multimodal, multifunctional all-in-one materials or combined with other treatments. Beyond the increase in complexity of material production (discussed in detail below), carefully implementing the treatment regimen in routine clinical practice (both from the medical and patient perspective) is costly and challenging.[270–272] Thus, robust and solid evidence of therapeutic benefit is required to attract the interest needed for new (and sometimes more complex) materials to be translated. Many claim that MOFs hold great biomedical promise, especially for drug delivery, because of their “high chemical versatility, pore tunability, drug loading capacities and stimuli/controlled drug release”. While these could definitely be valuable properties in concrete medical applications, we should first ask ourselves whether such features are really the key attributes driving clinical translation of (nano)medicines.[156,273,274] As stated above, to eventually use MOFs for drug delivery, these need to outperform (and generate higher market interest over) already clinically used materials.[275,276] Approved formulations like the protein-based paclitaxel nanoparticle Abraxane (cancer), the liposomal doxorubicin Doxil (cancer), the mRNA-LNP based COVID-19 vaccines Comirnaty/Spikevax (viral infections) or the liposomal amphotericin B (fungal infections), to name a few, have not been approved because of their high drug loading capacities or high tunability, but rather because of their improved administrability, safety and/or therapeutic performance over the standard of care at the time. It is important, therefore, to identify the distinct features of reticular porous materials, and specifically MOFs, that hold the potential to address current medical and societal needs and that cannot be covered by existing materials.
b. Poor clinical performance predictability
Safety, toxicology, and efficacy can be only partly evaluated in preclinical animal models. In addition to the poor animal-to-human data correlation,[277–279] preclinical evaluation of many (nano)medicinal products is mostly confined to lab-specific experimental conditions, which hinder adequate assessment of real clinical potential. Owing to the novelty and chemical richness of MOFs, many structures have been reported in the last years, whose therapeutic and safety profiles have ‘only’ been evaluated in relatively simple animal models, usually immunocompromised or non-humanized rodents, and hardly ever against adequate standard-of-care controls. While it is obvious that exhaustive assessment in multiple relevant animal models is not realistic or feasible in early-stage research, focus on fewer, better models along with critical and realistic claims, rather than publication-driven overstatements, would more accurately devise the added clinical value of MOFs.
MOF toxicity. More concretely, safety is one of the pivotal aspects evaluated in clinical trials, specifically in phase I clinical trials.[156] The preclinical models used to predict patient tolerability are still limited, and toxicological profiles of MOFs and other materials cannot be accurately evaluated at the preclinical stage.[280] Preclinical toxicity assessments, particularly for novel materials, tend to be limited to a few parameters such as body weight loss and histopathological analysis of tissue damage, typically in the liver, heart, and kidneys. Extensive histopathological analysis at different doses and with different dosage schedules, as well as hypersensitivity reactions and other immunological side-effects are not commonly evaluated at early stages, which can limit projections of human safety. Unlike approved lipid- or polymer-based constructs, metal-containing (nano)materials are not as inert, and they have been shown to actively engage with different cells and proteins, resulting in ancillary effects and unpredictable toxicity profiles.[118] While the reactive nature of metal-based (nano)materials can be exploited to create stand-alone therapeutics, metal/organic-biomolecule interactions can also induce side effects, thereby lowering tolerability and complicating translation. A remarkable case is the MOF ZIF-8(Zn). Despite being wildly popular in MOF biomedical research, especially for drug delivery due to its chemical versatility and drug loading flexibility, its associated toxicity remains controversial.[153,281] Metal-dependent cytotoxicity has been illustrated by MOF-74, increasing in cytotoxicity from Co to Mg to Ni to Cu to Mn.[153] The use of biocompatible building blocks (e.g., in bio-MOFs) can partly contribute to decreased toxicity after framework degradation, although materials constituted by biologically-friendly components can still trigger undesired long-term (immune) responses (as e.g., PEGylation does).[282] Additionally, other factors such as topology, phase, crystallinity, particle size, pore size, external coating, and many other physicochemical and formulation parameters have proven crucial to defining the interaction of these materials with the body, including their tolerability in humans.[153]
c. Upscaling and manufacturing issues
Establishing optimal large-scale production and manufacturing protocols that ensure control over batch-to-batch reproducibility is key to pharmaceutical development. This is particularly true for MOFs, whose properties are strongly linked to their structure, crystallinity, and physicochemical attributes. Procedures to scale-up MOF synthesis are still being optimized, and although there are some protocols explored for other applications,[283–285] methods developed to date for biomedical MOFs are still expensive and tend to result in MOF amorphization, loss of functionality, and physiological instability.[168,169] Maximizing processability while ensuring optimal performance requires technology and manufacturing protocols that integrate both bottom-up and top-down strategies.[286] Several fabrication methods, such as laser writing,[287] have been developed in recent years, but they are still sub-optimal and have limited applicability. More recently, significant improvements have also been made regarding continuous production of MOFs using micro- and milli-fluidic platforms,[288–290] enhancing control over batch-to-batch reproducibility and, therefore, over their properties.
In addition to manufacturing issues, translating optimized protocols to industrial scales is another key challenge.[291,292] The economics of MOF manufacturing is another important consideration for their commercialization, and is primarily driven by the cost of raw materials as well as the complexity and duration of synthesis. The manufacturing scale is also a major concern. Larger scale production generally reduces the cost per unit weight, while the comparatively small quantities demanded by medical applications are likely to raise the cost per kg considerably.[291] Most of the optimized lab-scale synthetic protocols do not account for production time, cost, or environmental and safety risks, instead prioritizing high yield and crystallinity. Consequently, established lab-scale procedures can include the use of solvents (e.g., amide-based or hydrocarbons like N,N-dimethylformamide, DMF), as well as reaction conditions (e.g., high pressure and/or temperature) that pose safety, production, economic, and regulatory limitations at larger-scales. Efforts to establish scalable synthetic protocols (e.g., with “safer” solvents) without significantly reducing product quality should be more seriously considered at early research stages.[293]
MOF (in)stability
Notably, instability is a prevalent issue for many MOF structures, not only in manufacturing/long-term storage but also physiologically.[294] Controlling MOF disintegration and release of metals and/or organic ligands could provide beneficial therapeutic effects, but the lack of stability control is often a key obstacle in pharmaceutical development. A relevant example is the MIL-101 MOF family, in which some of the Fe-based MOFs tend to be chemically and colloidally unstable and, therefore, require functionalization to be used in physiological conditions.[295] In other cases, different MOF structures and metals have shown pH-dependent stability (e.g., ZIF-8(Zn) is unstable below pH 6, while UiO-66(Zr) is stable, even below pH 4).[153] Formulating MOF-based (nano)medicines entails more than accurately controlling and customizing the framework structure (with/without the drug), but rather also preparing them for manufacturing and administration. As with many other drug formulations, using excipients to increase colloidal stability that preserve their properties for long-term storage and/or enable administration (e.g., increase water solubility and reduce local injection toxicity) is often needed.[296] Particularly for MOFs, structural and colloidal instability is usually addressed via post-synthetic modifications such as external functionalization and/or coating with lipids and polymers.[148,297] These minimize aggregation and side reactions such as blood vessel clogging upon injection. However, it is important to note that all these post-synthetic and formulation processing steps increase material complexity, and thus production, safety risks, and costs, indirectly decreasing the appetite of industry partners. Careful choice of such modifications at early stages can minimize risks in subsequent pharmaceutical and translational phases.[298]
d. lack of standardized synthetic and characterization protocols
Most synthetic procedures are tailored for each specific MOF structure, size and topology. Hence, developing and optimizing more robust and translatable (green) synthesis protocols in mild conditions can substantially facilitate transferring them into large-scale production. Additionally, the lack of standardized protocols, which results from the fast-growing research interest and endeavors in the multidisciplinary field of MOFs, has also led to a high diversity of characterization methods and has complicated data standardization, trend analysis, and eventual structure-property-performance correlations. Implementing synthetic characterization standards and guidelines[262] will make the data more robust, improve lab-to-lab reproducibility, and increase value of published data, which collectively promote more efficient and realistic assessment of technological and pharmaceutical potential. Besides establishing general guidelines for MOF production and structural characterization, it is crucial to define specific guidelines and minimum reporting information for each specific application that (far from becoming a publication requirement) should guide the field to maximal impact.[299,300] This can also lay the foundation for the use of data mining approaches and machine learning-assisted methods to tabulate, unify and distill relevant information and structure-performance trends among the constant enormous amount of reported data.[301–303]
5. Outlook
The chemistry of MOFs has revolutionized material science in the last three decades, with breakthrough research advances, such as the capacity to efficiently store gas or to harvest water from air, which promise to impact society in the coming years. Many different structures with intrinsic potential to be used for many different applications, including medicine, have been reported. The hybrid organic-inorganic crystalline structures can be tailor-made to integrate various properties such as high surface area, tuneable pore openings and sizes, accessible binding sites with the desired and required chemical stability, and responsivity to selected stimuli. These features have propelled the research of MOFs as potential detoxifying agents, drug and gas delivery systems, as well as (therapeutically) active platforms for a wide range of medical indications, especially cancer and microbial diseases. In particular, enormous research effort has been invested in promoting the use of MOFs for anticancer drug delivery, sometimes at the risk of overlooking medical and patient needs as well as market interests.
To date, only one MOF platform has managed to cross the bench-to-bedside gap into early Phase I clinical trials. The translation of new therapeutic platforms is an extremely slow and costly endeavour, making the support of academia, industry, financers, regulatory agencies, and society (including end-user acceptance) indispensable. Key stakeholders are much more likely to invest in platforms that target unmet needs and that generate societal and economical benefit. Liposomes, lipid nanoparticles, hydrogels, and polymers are drug delivery platforms that are already approved and used in clinical practice, and therefore, stand as key translational barriers. These can only be overcome by MOFs if they compile strong preclinical evidence in addressing medical needs that cannot be covered by existing materials. Additionally, issues related to scaling up MOFs from the benchtop to an industrial scale, as well as to efficient and cost-effective manufacturing, need to be solved to ensure MOFs pharmaceutical development as marketable products.
While the chemistry of MOFs is rich and attractive, and promises (theoretically) unlimited structural opportunities, the field needs to start approaching MOF research from a more disease-driven perspective, and exploit the unique properties offered by MOFs to treat diseases that cannot be addressed by the current medical toolset. These can include their high adsorption capacities for detoxification purposes, their ability to efficiently load and deliver gases, and their intrinsic metallo-(immuno)therapeutic activity, either alone, in combination therapy, or even synergistically integrated into other materials to potentiate their performances. In addition, more rigorous characterization of MOF safety and toxicity at the preclinical stage, as well as standardization of protocols, guidelines and data reporting are crucial to ensure high-quality data that promotes MOF technology transfer; a process which can be streamlined with emerging methodologies and technologies such as data mining and machine learning. After more than three decades of MOF research, there has been already significant empirical and preclinical evidence collected on the performance of these materials. We believe that it is hence time to start (re)thinking and (re)defining the future directions of MOF research while embracing a more holistic approach that contributes to unlocking their true biomedical potential; altogether with more realistic aims and claims that help bridge the bench-to-bedside gap for MOF materials and, indirectly, for other porous (nano)materials.
Acknowledgments
The authors gratefully acknowledge financial support from the German Research Foundation (DFG: LA2937/4-1; SH1223/1-1; SFB 1066; GRK/RTG 2735 (project number 331065168)), the German Federal Ministry of Research and Education (BMBF: Gezielter Wirkstofftransport, PP-TNBC, Project No. 16GW0319K) and the European Research Council (ERC: Meta-Targeting (864121)). The financial support from Welch Foundation (AT-1989-20220331) and from the Human Frontier Science Program (HFSP, within the project RGP0047/2022) are also acknowledged. The authors thank the European Union (European Cooperation in Science and Technology) for the COST Action EU4MOFs (CA22147). Figures were created using Biorender.
References
- [1].Horcajada P, Chalati T, Serre C, Gillet B, Sebrie C, Baati T, Eubank JF, Heurtaux D, Clayette P, Kreuz C, Chang J-S, et al. Nat Mater. 2010;9:172. doi: 10.1038/nmat2608. [DOI] [PubMed] [Google Scholar]
- [2].Hernandez JL, Woodrow KA. Advanced Healthcare Materials. 2022;11:2102087. doi: 10.1002/adhm.202102087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Day GS, Drake HF, Zhou H-C, Ryder MR. Commun Chem. 2021;4:114. doi: 10.1038/s42004-021-00549-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Bennett TD, Coudert F-X, James SL, Cooper AI. Nat Mater. 2021;20:1179. doi: 10.1038/s41563-021-00957-w. [DOI] [PubMed] [Google Scholar]
- [5].Davis ME. Nature. 2002;417:813. doi: 10.1038/nature00785. [DOI] [PubMed] [Google Scholar]
- [6].Huang N, Wang P, Jiang D. Nat Rev Mater. 2016;1:1. [Google Scholar]
- [7].Li H, Eddaoudi M, O’Keeffe M, Yaghi OM. Nature. 1999;402:276. [Google Scholar]
- [8].Li H, Eddaoudi M, Groy TL, Yaghi OM. J Am Chem Soc. 1998;120:8571. [Google Scholar]
- [9].Wuttke S, Lismont M, Escudero A, Rungtaweevoranit B, Parak WJ. Biomaterials. 2017;123:172. doi: 10.1016/j.biomaterials.2017.01.025. [DOI] [PubMed] [Google Scholar]
- [10].Horcajada P, Gref R, Baati T, Allan PK, Maurin G, Couvreur P, Férey G, Morris RE, Serre C. Chem Rev. 2012;112:1232. doi: 10.1021/cr200256v. [DOI] [PubMed] [Google Scholar]
- [11].Giménez-Marqués M, Hidalgo T, Serre C, Horcajada P. Coordination Chemistry Reviews. 2016;307:342. [Google Scholar]
- [12].Fu D-Y, Liu X, Zheng X, Zhou M, Wang W, Su G, Liu T, Wang L, Xie Z. Coordination Chemistry Reviews. 2022;456:214393 [Google Scholar]
- [13].Zhao Y, Zeng H, Zhu X-W, Lu W, Li D. Chem Soc Rev. 2021;50:4484. doi: 10.1039/d0cs00955e. [DOI] [PubMed] [Google Scholar]
- [14].Zhou Y, Yang T, Liang K, Chandrawati R. Advanced Drug Delivery Reviews. 2021;171:199. doi: 10.1016/j.addr.2021.02.005. [DOI] [PubMed] [Google Scholar]
- [15].Moosavi SM, Nandy A, Jablonka KM, Ongari D, Janet JP, Boyd PG, Lee Y, Smit B, Kulik HJ. Nat Commun. 2020;11:4068. doi: 10.1038/s41467-020-17755-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Freund R, Canossa S, Cohen SM, Yan W, Deng H, Guillerm V, Eddaoudi M, Madden DG, Fairen-Jimenez D, Lyu H, Macreadie LK, et al. Angewandte Chemie International Edition. 2021;60:23946. doi: 10.1002/anie.202101644. [DOI] [PubMed] [Google Scholar]
- [17].Freund R, Zaremba O, Arnauts G, Ameloot R, Skorupskii G, Dincă M, Bavykina A, Gascon J, Ejsmont A, Goscianska J, Kalmutzki M, et al. Angewandte Chemie International Edition. 2021;60:23975. doi: 10.1002/anie.202106259. [DOI] [PubMed] [Google Scholar]
- [18].Banerjee R, Furukawa H, Britt D, Knobler C, O’Keeffe M, Yaghi OM. J Am Chem Soc. 2009;131:3875. doi: 10.1021/ja809459e. [DOI] [PubMed] [Google Scholar]
- [19].Bavykina A, Kolobov N, Khan IS, Bau JA, Ramirez A, Gascon J. Chem Rev. 2020;120:8468. doi: 10.1021/acs.chemrev.9b00685. [DOI] [PubMed] [Google Scholar]
- [20].Rojas S, Horcajada P. Chem Rev. 2020;120:8378. doi: 10.1021/acs.chemrev.9b00797. [DOI] [PubMed] [Google Scholar]
- [21].Xu W, Yaghi OM. ACS Cent Sci. 2020;6:1348. doi: 10.1021/acscentsci.0c00678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Scopus database - Document search, under scopus.com. [accessed on 26th April 2023]. Scopus search details: the search was limited to journal articles and the selected keywords (in title, abstract or keywords, between 2010-2022, except Fig. 1f) are as follows. (a) Fig. 1a - for MOFs: “metal-organic framework” OR “metal organic framework”; for MOFs in biomedicine: “metal-organic framework” OR “metal organic framework” AND (biomedicine OR cancer OR cardiovascular OR brain OR lung OR heart OR microbial OR inflammation OR eye OR wound OR tissue OR bone OR delivery OR disease OR antiinflammatory OR anemia OR overdose OR diabetes). (b) Fig. 1b - MOFs in biomedicine keywords (from Fig 1a) AND (each MOF family). (c) Fig. 1c - MOFs in biomedicine keywords (from Fig 1a) AND (each metal). (d) Fig. 1d - MOFs in biomedicine keywords (from Fig 1a) AND (cancer / microbial OR bacterial / inflammation / wound OR tissue AND regeneration / bone AND regeneration OR osteogenic / diabetes / “brain disease” OR CNS). (e) Fig. 1e - MOFs in biomedicine keywords (from Fig 1a) AND drug delivery. (f) Fig. 1f (from 2015-2022)-drug delivery AND cancer AND (liposo* / hydrogel OR nanogel / micell* / “metal nanoparticle” / microbubble / “metal-organic framewok” OR “metal organic framework”.
- [23].Furukawa H, Ko N, Go YB, Aratani N, Choi SB, Choi E, Yazaydin AÖ, Snurr RQ, O’Keeffe M, Kim J, Yaghi OM. Science. 2010;329:424. doi: 10.1126/science.1192160. [DOI] [PubMed] [Google Scholar]
- [24].Kumar S, Srivastava R, Koh J. Journal of CO2 Utilization. 2020;41:101251 [Google Scholar]
- [25].Mehra P, Paul A. ACS Omega. 2022;7:34538. doi: 10.1021/acsomega.2c04269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Furukawa H, Cordova KE, O’Keeffe M, Yaghi OM. Science. 2013;341:1230444. doi: 10.1126/science.1230444. [DOI] [PubMed] [Google Scholar]
- [27].Andreo J, Ettlinger R, Zaremba O, Peña Q, Lächelt U, De Luis RF, Freund R, Canossa S, Ploetz E, Zhu W, Diercks CS, et al. J Am Chem Soc. 2022;144:7531. doi: 10.1021/jacs.1c11507. [DOI] [PubMed] [Google Scholar]
- [28].Deng H, Grunder S, Cordova KE, Valente C, Furukawa H, Hmadeh M, Gándara F, Whalley AC, Liu Z, Asahina S, Kazumori H, et al. Science. 2012;336:1018. doi: 10.1126/science.1220131. [DOI] [PubMed] [Google Scholar]
- [29].Peng Y, Huang H, Zhang Y, Kang C, Chen S, Song L, Liu D, Zhong C. Nat Commun. 2018;9:187. doi: 10.1038/s41467-017-02600-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Hanikel N, Yaghi OM. Nat Water. 2023;1:418. [Google Scholar]
- [31].Svensson Grape E, Chacón-García AJ, Rojas S, Pérez Y, Jaworski A, Nero M, Åhlén M, Martínez-Ahumada E, Galetsa Feindt AE, Pepillo M, Narongin-Fujikawa M, et al. Nat Water. 2023;1:433. [Google Scholar]
- [32].Peng Y, Huang H, Liu D, Zhong C. ACS Appl Mater Interfaces. 2016;8:8527. doi: 10.1021/acsami.6b00900. [DOI] [PubMed] [Google Scholar]
- [33].Ahmadijokani F, Molavi H, Bahi A, Fernández R, Alaee P, Wu S, Wuttke S, Ko F, Arjmand M. Advanced Functional Materials. 2022;32:2207723 [Google Scholar]
- [34].Auron A, Brophy PD. Pediatr Nephrol. 2012;27:207. doi: 10.1007/s00467-011-1838-5. [DOI] [PubMed] [Google Scholar]
- [35].Meyer TW, Hostetter TH. J Am Soc Nephrol. 2014;25:2151. doi: 10.1681/ASN.2013121264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Kato S, Otake K, Chen H, Akpinar I, Buru CT, Islamoglu T, Snurr RQ, Farha OK. J Am Chem Soc. 2019;141:2568. doi: 10.1021/jacs.8b12525. [DOI] [PubMed] [Google Scholar]
- [37].Li Q, Zhao W, Guo H, Yang J, Zhang J, Liu M, Xu T, Chen Y, Zhang L. ACS Appl Mater Interfaces. 2020;12:25546. doi: 10.1021/acsami.0c03859. [DOI] [PubMed] [Google Scholar]
- [38].Rojas S, Baati T, Njim L, Manchego L, Neffati F, Abdeljelil N, Saguem S, Serre C, Najjar MF, Zakhama A, Horcajada P. J Am Chem Soc. 2018;140:9581. doi: 10.1021/jacs.8b04435. [DOI] [PubMed] [Google Scholar]
- [39].Rojas S, Guillou N, Horcajada P. ACS Applied Materials and Interfaces. 2019;11:22188–22193. doi: 10.1021/acsami.9b06472. [DOI] [PubMed] [Google Scholar]
- [40].Wang X, Chen L, Bai Z, Zhang D, Guan J, Zhang Y, Shi C, Diwu J. Angewandte Chemie. 2021;133:1670. doi: 10.1002/anie.202012512. [DOI] [PubMed] [Google Scholar]
- [41].Cheung YH, Ma K, van Leeuwen HC, Wasson MC, Wang X, Idrees KB, Gong W, Cao R, Mahle JJ, Islamoglu T, Peterson GW, et al. J Am Chem Soc. 2021;143:16777. doi: 10.1021/jacs.1c08576. [DOI] [PubMed] [Google Scholar]
- [42].Yao A, Jiao X, Chen D, Li C. ACS Appl Mater Interfaces. 2020;12:18437. doi: 10.1021/acsami.9b22242. [DOI] [PubMed] [Google Scholar]
- [43].Kumar A, Sharma A, Chen Y, Jones MM, Vanyo ST, Li C, Visser MB, Mahajan SD, Sharma RK, Swihart MT. Advanced Functional Materials. 2021;31:2008054. doi: 10.1002/adfm.202008054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Jabbour CR, Parker LA, Hutter EM, Weckhuysen BM. Nat Rev Chem. 2021;5:370. doi: 10.1038/s41570-021-00275-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Zhao R, Ma T, Cui F, Tian Y, Zhu G. Advanced Science. 2020;7:2001899. doi: 10.1002/advs.202001899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Runde TJ, Nappe TM. StatPearls. StatPearls Publishing; Treasure Island (FL): 2023. [Google Scholar]
- [47].CDC Radiation Emergencies. Treatments for Radiation Exposure and Contamination. 2022.
- [48].Horcajada P, Serre C, Vallet-Regí M, Sebban M, Taulelle F, Férey G. Angew Chem Int Ed. 2006;45:5974. doi: 10.1002/anie.200601878. [DOI] [PubMed] [Google Scholar]
- [49].Lawson HD, Walton SP, Chan C. ACS Appl Mater Interfaces. 2021;13:7004. doi: 10.1021/acsami.1c01089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].He S, Wu L, Li X, Sun H, Xiong T, Liu J, Huang C, Xu H, Sun H, Chen W, Gref R, et al. Acta Pharmaceutica Sinica B. 2021;11:2362. doi: 10.1016/j.apsb.2021.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Wuttke S, Lismont M, Escudero A, Rungtaweevoranit B, Parak WJ. Biomaterials. 2017;123:172. doi: 10.1016/j.biomaterials.2017.01.025. [DOI] [PubMed] [Google Scholar]
- [52].Suresh K, Matzger AJ. Angew Chem Int Ed. 2019;58:16790. doi: 10.1002/anie.201907652. [DOI] [PubMed] [Google Scholar]
- [53].He Y, Zhang W, Guo T, Zhang G, Qin W, Zhang L, Wang C, Zhu W, Yang M, Hu X, Singh V, et al. Acta Pharmaceutica Sinica B. 2019;9:97. doi: 10.1016/j.apsb.2018.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Alyami MZ, Alsaiari SK, Li Y, Qutub SS, Aleisa FA, Sougrat R, Merzaban JS, Khashab NM. J Am Chem Soc. 2020;142:1715. doi: 10.1021/jacs.9b11638. [DOI] [PubMed] [Google Scholar]
- [55].Wang H, Chen Y, Wang H, Liu X, Zhou X, Wang F. Angew Chem Int Ed. 2019;58:7380. doi: 10.1002/anie.201902714. [DOI] [PubMed] [Google Scholar]
- [56].Poddar A, Conesa JJ, Liang K, Dhakal S, Reineck P, Bryant G, Pereiro E, Ricco R, Amenitsch H, Doonan C, Mulet X, et al. Small. 2019;15:1902268. doi: 10.1002/smll.201902268. [DOI] [PubMed] [Google Scholar]
- [57].Teng W, Zhang Z, Wang Y, Ye Y, Yinwang E, Liu A, Zhou X, Xu J, Zhou C, Sun H, Wang F, et al. Small. 2021;17:2102315. doi: 10.1002/smll.202102315. [DOI] [PubMed] [Google Scholar]
- [58].Xu M, Li X, Zheng H, Chen J, Ye X, Liu T. Molecules. 2022;27:2288. doi: 10.3390/molecules27072288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Yao L, Tang Y, Cao W, Cui Y, Qian G. ACS Biomater Sci Eng. 2021;7:4999. doi: 10.1021/acsbiomaterials.1c00874. [DOI] [PubMed] [Google Scholar]
- [60].Wang J-W, Chen Q-W, Luo G-F, Han Z-Y, Song W-F, Yang J, Chen W-H, Zhang X-Z. ACS Nano. 2021;15:17870. doi: 10.1021/acsnano.1c06123. [DOI] [PubMed] [Google Scholar]
- [61].Liu W, Ma R, Lu S, Wen Y, Li H, Wang J, Sun B. ACS Appl Mater Interfaces. 2022;14:55447. doi: 10.1021/acsami.2c18452. [DOI] [PubMed] [Google Scholar]
- [62].Jodłowski PJ, Dymek K, Kurowski G, Jaśkowska J, Bury W, Pander M, Wnorowska S, Targowska-Duda K, Piskorz W, Wnorowski A, Boguszewska-Czubara A. ACS Appl Mater Interfaces. 2022;14:28615. doi: 10.1021/acsami.2c06420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Bagherzadeh M, Safarkhani M, Kiani M, Radmanesh F, Daneshgar H, Ghadiri AM, Taghavimandi F, Fatahi Y, Safari-Alighiarloo N, Ahmadi S, Rabiee N. Sci Rep. 2022;12:12105. doi: 10.1038/s41598-022-16058-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].He Z, Huang X, Wang C, Li X, Liu Y, Zhou Z, Wang S, Zhang F, Wang Z, Jacobson O, Zhu JJ, et al. Angewandte Chemie International Edition. 2019;58:8752. doi: 10.1002/anie.201902612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Bikiaris ND, Ainali NM, Christodoulou E, Kostoglou M, Kehagias T, Papasouli E, Koukaras EN, Nanaki SG. Nanomaterials (Basel) 2020;10:E2490. doi: 10.3390/nano10122490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Steinborn B, Hirschle P, Höhn M, Bauer T, Barz M, Wuttke S, Wagner E, Lächelt U. Advanced Therapeutics. 2019;2:1900120 [Google Scholar]
- [67].Au KM, Satterlee A, Min Y, Tian X, Kim YS, Caster JM, Zhang L, Zhang T, Huang L, Wang AZ. Biomaterials. 2016;82:178. doi: 10.1016/j.biomaterials.2015.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Huxford RC, deKrafft KE, Boyle WS, Liu D, Lin W. Chem Sci. 2011;3:198. doi: 10.1039/C1SC00499A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Zhuang J, Gong H, Zhou J, Zhang Q, Gao W, Fang RH, Zhang L. Sci Adv. 2020;6:eaaz6108. doi: 10.1126/sciadv.aaz6108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Mirzazadeh Dizaji N, Lin Y, Bein T, Wagner E, Wuttke S, Lächelt U, Engelke H. Chem Mater. 2022;34:8684. doi: 10.1021/acs.chemmater.2c01736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Cases Díaz J, Lozano-Torres B, Giménez-Marqués M. Chem Mater. 2022;34:7817. doi: 10.1021/acs.chemmater.2c01338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Wang S, Chen Y, Wang S, Li P, Mirkin CA, Farha OK. J Am Chem Soc. 2019;141:2215. doi: 10.1021/jacs.8b12705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Liang K, Ricco R, Doherty CM, Styles MJ, Bell S, Kirby N, Mudie S, Haylock D, Hill AJ, Doonan CJ, Falcaro P. Nat Commun. 2015;6:7240. doi: 10.1038/ncomms8240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Cao J, Zaremba OT, Lei Q, Ploetz E, Wuttke S, Zhu W. ACS Nano. 2021;15:3900. doi: 10.1021/acsnano.0c10144. [DOI] [PubMed] [Google Scholar]
- [75].Sun Y, Zheng L, Yang Y, Qian X, Fu T, Li X, Yang Z, Yan H, Cui C, Tan W. Nano-Micro Lett. 2020;12:103. doi: 10.1007/s40820-020-00423-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Alsaiari SK, Patil S, Alyami M, Alamoudi KO, Aleisa FA, Merzaban JS, Li M, Khashab NM. J Am Chem Soc. 2018;140:143. doi: 10.1021/jacs.7b11754. [DOI] [PubMed] [Google Scholar]
- [77].Wijesundara YH, Herbert FC, Trashi O, Trashi I, Brohlin OR, Kumari S, Howlett T, Benjamin CE, Shahrivarkevishahi A, Diwakara SD, Perera SD, et al. Chem Sci. 2022;13:13803. doi: 10.1039/d2sc04982a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Kumari S, Wijesundara YH, Howlett TS, Waliullah M, Herbert FC, Raja A, Trashi I, Bernal RA, Gassensmith JJ. Proc Natl Acad Sci USA. 2023;120:e2218247120. doi: 10.1073/pnas.2218247120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].An J, Geib SJ, Rosi NL. J Am Chem Soc. 2009;131:8376. doi: 10.1021/ja902972w. [DOI] [PubMed] [Google Scholar]
- [80].Chen W-H, Liao W-C, Sohn YS, Fadeev M, Cecconello A, Nechushtai R, Willner I. Advanced Functional Materials. 2018;28:1705137 [Google Scholar]
- [81].Zhu Y, Huang Y, Yan T-H, Li J, Li Y, Drake HF, Zhong H, Jin Y, Zhao R, Zhou HC. Advanced Healthcare Materials. 2022;11:2200004. doi: 10.1002/adhm.202200004. [DOI] [PubMed] [Google Scholar]
- [82].Ploetz E, Zimpel A, Cauda V, Bauer D, Lamb DC, Haisch C, Zahler S, Vollmar AM, Wuttke S, Engelke H. Advanced Materials. 2020;32:1907267. doi: 10.1002/adma.201907267. [DOI] [PubMed] [Google Scholar]
- [83].Zheng H, Zhang Y, Liu L, Wan W, Guo P, Nyström AM, Zou X. J Am Chem Soc. 2016;138:962. doi: 10.1021/jacs.5b11720. [DOI] [PubMed] [Google Scholar]
- [84].Chen W-H, Luo G-F, Vázquez-González M, Cazelles R, Sohn YS, Nechushtai R, Mandel Y, Willner I. ACS Nano. 2018;12:7538. doi: 10.1021/acsnano.8b03417. [DOI] [PubMed] [Google Scholar]
- [85].Mura S, Nicolas J, Couvreur P. Nature Mater. 2013;12:991. doi: 10.1038/nmat3776. [DOI] [PubMed] [Google Scholar]
- [86].Dou Y, Hynynen K, Allen C. Journal of Controlled Release. 2017;249:63. doi: 10.1016/j.jconrel.2017.01.025. [DOI] [PubMed] [Google Scholar]
- [87].Regenold M, Bannigan P, Evans JC, Waspe A, Temple MJ, Allen C. Nanomedicine: Nanotechnology, Biology and Medicine. 2022;40:102484. doi: 10.1016/j.nano.2021.102484. [DOI] [PubMed] [Google Scholar]
- [88].Szabo C. Nat Rev Drug Discov. 2016;15:185. doi: 10.1038/nrd.2015.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Jing Y-Z, Li S-J, Sun Z-J. J Mater Chem B. 2021;9:8541. doi: 10.1039/d1tb01661j. [DOI] [PubMed] [Google Scholar]
- [90].Kawahara B, Moller T, Hu-Moore K, Carrington S, Faull KF, Sen S, Mascharak PK. J Med Chem. 2017;60:8000. doi: 10.1021/acs.jmedchem.7b00476. [DOI] [PubMed] [Google Scholar]
- [91].Li Y, Dang J, Liang Q, Yin L. ACS Cent Sci. 2019;5:1044. doi: 10.1021/acscentsci.9b00216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Wu M, Lu Z, Wu K, Nam C, Zhang L, Guo J. J Mater Chem B. 2021;9:7063. doi: 10.1039/d1tb00847a. [DOI] [PubMed] [Google Scholar]
- [93].Thackham JA, McElwain DLS, Long RJ. Wound Repair Regen. 2008;16:321. doi: 10.1111/j.1524-475X.2008.00372.x. [DOI] [PubMed] [Google Scholar]
- [94].Deppisch C, Herrmann G, Graepler-Mainka U, Wirtz H, Heyder S, Engel C, Marschal M, Miller CC, Riethmüller J. Infection. 2016;44:513. doi: 10.1007/s15010-016-0879-x. [DOI] [PubMed] [Google Scholar]
- [95].Lisi F, Zelikin AN, Chandrawati R. Adv Sci. 2021;8:2003895. doi: 10.1002/advs.202003895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Wang T-Y, Zhu X-Y, Wu F-G. Bioact Mater. 2022;23:129. doi: 10.1016/j.bioactmat.2022.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Byrne JD, Gallo D, Boyce H, Becker SL, Kezar KM, Cotoia AT, Feig VR, Lopes A, Csizmadia E, Longhi MS, Lee JS, et al. Science Translational Medicine. 2022;14:eabl4135. doi: 10.1126/scitranslmed.abl4135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Chen L, Zhou S-F, Su L, Song J. ACS Nano. 2019;13:10887. doi: 10.1021/acsnano.9b04954. [DOI] [PubMed] [Google Scholar]
- [99].Hakim TS, Sugimori K, Camporesi EM, Anderson G. Physiol Meas. 1996;17:267. doi: 10.1088/0967-3334/17/4/004. [DOI] [PubMed] [Google Scholar]
- [100].Thomas DD, Liu X, Kantrow SP, Lancaster JR. Proc Natl Acad Sci USA. 2001;98:355. doi: 10.1073/pnas.011379598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Woodall GM, Smith RL, Granville GC. Inhalation Toxicology. 2005;17:593. doi: 10.1080/08958370591000618. [DOI] [PubMed] [Google Scholar]
- [102].Cooper CE, Brown GC. J Bioenerg Biomembr. 2008;40:533. doi: 10.1007/s10863-008-9166-6. [DOI] [PubMed] [Google Scholar]
- [103].Ernst A, Zibrak JD. New England Journal of Medicine. 1998;339:1603. doi: 10.1056/NEJM199811263392206. [DOI] [PubMed] [Google Scholar]
- [104].Harding JL, Reynolds MM. J Am Chem Soc. 2012;134:3330. doi: 10.1021/ja210771m. [DOI] [PubMed] [Google Scholar]
- [105].Zhou Y, Yang T, Namivandi-Zangeneh R, Boyer C, Liang K, Chandrawati R. J Mater Chem B. 2021;9:1059. doi: 10.1039/d0tb02709j. [DOI] [PubMed] [Google Scholar]
- [106].Melvin AC, Tuttle RR, Mohnike M, Reynolds MM. ACS Materials Lett. 2022;4:2434. [Google Scholar]
- [107].Peng Y, Li Y, Ban Y, Jin H, Jiao W, Liu X, Yang W. Science. 2014;346:1356. doi: 10.1126/science.1254227. [DOI] [PubMed] [Google Scholar]
- [108].Li H, Wang K, Sun Y, Lollar CT, Li J, Zhou H-C. Materials Today. 2018;21:108. [Google Scholar]
- [109].Yakin FE, Barisik M, Sen T. J Phys Chem C. 2020;124:19579 [Google Scholar]
- [110].Wheatley PS, Chlubná-Eliášová P, Greer H, Zhou W, Seymour VR, Dawson DM, Ashbrook SE, Pinar AB, McCusker LB, Opanasenko M, Čejka J, et al. Angewandte Chemie. 2014;53:134210. doi: 10.1002/anie.201407676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].McKinlay AC, Xiao B, Wragg DS, Wheatley PS, Megson IL, Morris RE. J Am Chem Soc. 2008;130:10440. doi: 10.1021/ja801997r. [DOI] [PubMed] [Google Scholar]
- [112].Cattaneo D, Warrender SJ, Duncan MJ, Kelsall CJ, Doherty MK, Whitfield PD, Megson IL, Morris RE. RSC Adv. 2016;6:14059. doi: 10.1039/c5ra24023a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Feng J, Yu W, Xu Z, Wang F. Chem Sci. 2020;11:1649. doi: 10.1039/c9sc06337d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Zhang W, Li S, Liu X, Yang C, Hu N, Dou L, Zhao B, Zhang Q, Suo Y, Wang J. Advanced Functional Materials. 2018;28:1706375 [Google Scholar]
- [115].An J, Hu Y-G, Li C, Hou X-L, Cheng K, Zhang B, Zhang R-Y, Li D-Y, Liu S-J, Liu B, Zhu D, et al. Biomaterials. 2020;230:119636. doi: 10.1016/j.biomaterials.2019.119636. [DOI] [PubMed] [Google Scholar]
- [116].Allan PK, Wheatley PS, Aldous D, Mohideen MI, Tang C, Hriljac JA, Megson IL, Chapman KW, De Weireld G, Vaesen S, Morris RE. Dalton Trans. 2012;41:4060. doi: 10.1039/c2dt12069k. [DOI] [PubMed] [Google Scholar]
- [117].Zhang H, Tian X-T, Shang Y, Li Y-H, Yin X-B. ACS Appl Mater Interfaces. 2018;10:28390. doi: 10.1021/acsami.8b09680. [DOI] [PubMed] [Google Scholar]
- [118].Stater EP, Sonay AY, Hart C, Grimm J. Nat Nanotechnol. 2021;16:1180. doi: 10.1038/s41565-021-01017-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [119].Anthony EJ, Bolitho EM, Bridgewater HE, Carter OWL, Donnelly JM, Imberti C, Lant EC, Lermyte F, Needham RJ, Palau M, Sadler PJ, et al. Chem Sci. 2020;11:12888. doi: 10.1039/d0sc04082g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [120].Li J, Ren H, Qiu Q, Yang X, Zhang J, Zhang C, Sun B, Lovell JF, Zhang Y. ACS Nano. 2022;16:16909. doi: 10.1021/acsnano.2c06926. [DOI] [PubMed] [Google Scholar]
- [121].Peña Q, Wang A, Zaremba O, Shi Y, Scheeren HW, Metselaar JM, Kiessling F, Pallares RM, Wuttke S, Lammers T. Chem Soc Rev. 2022;51:2544. doi: 10.1039/d1cs00468a. [DOI] [PubMed] [Google Scholar]
- [122].Bonvalot S, Rutkowski PL, Thariat J, Carrère S, Ducassou A, Sunyach M-P, Agoston P, Hong A, Mervoyer A, Rastrelli M, Moreno V, et al. Lancet Oncol. 2019;20:1148. doi: 10.1016/S1470-2045(19)30326-2. [DOI] [PubMed] [Google Scholar]
- [123].Maier-Hauff K, Ulrich F, Nestler D, Niehoff H, Wust P, Thiesen B, Orawa H, Budach V, Jordan A. J Neurooncol. 2011;103:317. doi: 10.1007/s11060-010-0389-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [124].Lux F, Tran VL, Thomas E, Dufort S, Rossetti F, Martini M, Truillet C, Doussineau T, Bort G, Denat F, Boschetti F, et al. Br J Radiol. 2019;92:20180365. doi: 10.1259/bjr.20180365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [125].Frei A, Verderosa AD, Elliott AG, Zuegg J, Blaskovich MAT. Nat Rev Chem. 2023;7:202. doi: 10.1038/s41570-023-00463-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [126].Venkataraman M, Nagarsenker M. AAPS PharmSciTech. 2012;14:254. doi: 10.1208/s12249-012-9914-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [127].Pulendran B, Arunachalam PS, O’Hagan DT. Nat Rev Drug Discov. 2021;20:454. doi: 10.1038/s41573-021-00163-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [128].Reed SG, Orr MT, Fox CB. Nat Med. 2013;19:1597. doi: 10.1038/nm.3409. [DOI] [PubMed] [Google Scholar]
- [129].Yang B, Ding L, Yao H, Chen Y, Shi J. Advanced Materials. 2020;32:1907152. doi: 10.1002/adma.201907152. [DOI] [PubMed] [Google Scholar]
- [130].Li A, Yang X, Chen J. RSC Adv. 2021;11:10540. doi: 10.1039/d0ra09915e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [131].Meng Y, Zhang D, Chen X, Dai Z, Yao X, Cui P, Yu D, Zhang G, Zheng X. ACS Appl Nano Mater. 2020;3:4494. [Google Scholar]
- [132].Gong T, Li Y, Lv B, Wang H, Liu Y, Yang W, Wu Y, Jiang X, Gao H, Zheng X, Bu W. ACS Nano. 2020;14:3032. doi: 10.1021/acsnano.9b07898. [DOI] [PubMed] [Google Scholar]
- [133].Ji HB, Kim CR, Min CH, Han JH, Kim S-N, Lee C, Choy YB. Bioengineering & Translational Medicine. 2023;8:e10477. doi: 10.1002/btm2.10477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [134].Tang J, Wang J. Chemosphere. 2020;241:125002. doi: 10.1016/j.chemosphere.2019.125002. [DOI] [PubMed] [Google Scholar]
- [135].Wan Y, Fang J, Wang Y, Sun J, Sun Y, Sun X, Qi M, Li W, Li C, Zhou Y, Xu L, et al. Advanced Healthcare Materials. 2021;10:2101515. doi: 10.1002/adhm.202101515. [DOI] [PubMed] [Google Scholar]
- [136].Ge EJ, Bush AI, Casini A, Cobine PA, Cross JR, DeNicola GM, Dou QP, Franz KJ, Gohil VM, Gupta S, Kaler SG, et al. Nat Rev Cancer. 2022;22:102. doi: 10.1038/s41568-021-00417-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [137].Wang C, Zhang R, Wei X, Lv M, Jiang Z. Adv Immunol. 2020;145:187. doi: 10.1016/bs.ai.2019.11.007. [DOI] [PubMed] [Google Scholar]
- [138].Murdoch CC, Skaar EP. Nat Rev Microbiol. 2022;20:657. doi: 10.1038/s41579-022-00745-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [139].Sun X, Zhang Y, Li J, Park KS, Han K, Zhou X, Xu Y, Nam J, Xu J, Shi X, Wei L, et al. Nat Nanotechnol. 2021;16:1260. doi: 10.1038/s41565-021-00962-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [140].Jia Y, Hu J, Zhu C, Li Z, Yang X, Liu R, Zeng L, Zhang L. Journal of Controlled Release. 2023;354:770. doi: 10.1016/j.jconrel.2023.01.043. [DOI] [PubMed] [Google Scholar]
- [141].Bird L. Nat Rev Immunol. 2022;22:144. doi: 10.1038/s41577-022-00688-2. [DOI] [PubMed] [Google Scholar]
- [142].Hidalgo T, Simón-Vázquez R, González-Fernández A, Horcajada P. Chem Sci. 2022;13:934. doi: 10.1039/d1sc04112f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [143].Ni K, Lan G, Chan C, Quigley B, Lu K, Aung T, Guo N, La Riviere P, Weichselbaum RR, Lin W. Nat Commun. 2018;9:2351. doi: 10.1038/s41467-018-04703-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [144].Wang C, Xiong C, Li Z, Hu L, Wei J, Tian J. Chem Commun. 2021;57:4035. doi: 10.1039/d0cc07903k. [DOI] [PubMed] [Google Scholar]
- [145].Xu D, Duan Q, Yu H, Dong W. Journal of Materials Chemistry B. 2023;11:5976. doi: 10.1039/d2tb02789e. [DOI] [PubMed] [Google Scholar]
- [146].Böll K, Zimpel A, Dietrich O, Wuttke S, Peller M. Advanced Therapeutics. 2020;3:1900126 [Google Scholar]
- [147].Böll K, Hirschle P, Klingl K, Zimpel A, Hirschle C, Ingrisch M, Dietrich O, Wuttke S, Peller M. Chemistry of Materials. 2022 [Google Scholar]
- [148].Zimpel A, Preiß T, Röder R, Engelke H, Ingrisch M, Peller M, Rädler JO, Wagner E, Bein T, Lächelt U, Wuttke S. Chem Mater. 2016;28:3318. [Google Scholar]
- [149].Chen D, Yang D, Dougherty CA, Lu W, Wu H, He X, Cai T, Van Dort ME, Ross BD, Hong H. ACS Nano. 2017;11:4315. doi: 10.1021/acsnano.7b01530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [150].Pallares RM, Kiessling F, Lammers T. Nanomedicine. 2023;18:413. doi: 10.2217/nnm-2023-0067. [DOI] [PubMed] [Google Scholar]
- [151].Pallares RM, Mottaghy FM, Schulz V, Kiessling F, Lammers T. J Nucl Med. 2022;63:1802. doi: 10.2967/jnumed.122.263895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [152].Kiessling F, Mertens ME, Grimm J, Lammers T. Radiology. 2014;273:10. doi: 10.1148/radiol.14131520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [153].Ettlinger R, Lächelt U, Gref R, Horcajada P, Lammers T, Serre C, Couvreur P, Morris RE, Wuttke S. Chem Soc Rev. 2022;51:464. doi: 10.1039/d1cs00918d. [DOI] [PubMed] [Google Scholar]
- [154].Manshian BB, Jiménez J, Himmelreich U, Soenen SJ. Biomaterials. 2017;127:1. doi: 10.1016/j.biomaterials.2017.02.039. [DOI] [PubMed] [Google Scholar]
- [155].Mitchell MJ, Billingsley MM, Haley RM, Wechsler ME, Peppas NA, Langer R. Nat Rev Drug Discov. 2021;20:101. doi: 10.1038/s41573-020-0090-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [156].Metselaar JM, Lammers T. Drug Deliv and Transl Res. 2020;10:721. doi: 10.1007/s13346-020-00740-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [157].Cook D, Brown D, Alexander R, March R, Morgan P, Satterthwaite G, Pangalos MN. Nat Rev Drug Discov. 2014;13:419. doi: 10.1038/nrd4309. [DOI] [PubMed] [Google Scholar]
- [158].Zimpel A, Al Danaf N, Steinborn B, Kuhn J, Höhn M, Bauer T, Hirschle P, Schrimpf W, Engelke H, Wagner E, Barz M, et al. ACS Nano. 2019;13:3884. doi: 10.1021/acsnano.8b06287. [DOI] [PubMed] [Google Scholar]
- [159].Desai P, Dasgupta A, Sofias AM, Peña Q, Göstl R, Slabu I, Schwaneberg U, Stiehl T, Wagner W, Jockenhövel S, Stingl J, et al. Advanced Healthcare Materials. :2301062. doi: 10.1002/adhm.202301062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [160].He L, Huang G, Liu H, Sang C, Liu X, Chen T. Sci Adv. 2020;6:eaay9751. doi: 10.1126/sciadv.aay9751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [161].Fan Y, Zhang Y, Zhao Q, Xie Y, Luo R, Yang P, Weng Y. Biomaterials. 2019;204:36. doi: 10.1016/j.biomaterials.2019.03.007. [DOI] [PubMed] [Google Scholar]
- [162].Yu M, You D, Zhuang J, Lin S, Dong L, Weng S, Zhang B, Cheng K, Weng W, Wang H. ACS Appl Mater Interfaces. 2017;9:19698. doi: 10.1021/acsami.7b05296. [DOI] [PubMed] [Google Scholar]
- [163].Zhu W, Guo J, Amini S, Ju Y, Agola JO, Zimpel A, Shang J, Noureddine A, Caruso F, Wuttke S, Croissant JG, et al. Advanced Materials. 2019;31:1900545. doi: 10.1002/adma.201900545. [DOI] [PubMed] [Google Scholar]
- [164].Guo J, Yu Y, Zhu W, Serda RE, Franco S, Wang L, Lei Q, Agola JO, Noureddine A, Ploetz E, Wuttke S, et al. Advanced Functional Materials. 2021;31:2005935 [Google Scholar]
- [165].Zhu W, Guo J, Agola JO, Croissant JG, Wang Z, Shang J, Coker E, Motevalli B, Zimpel A, Wuttke S, Brinker CJ. J Am Chem Soc. 2019;141:7789. doi: 10.1021/jacs.9b00992. [DOI] [PubMed] [Google Scholar]
- [166].Levine MM. Nat Med. 2003;9:99. doi: 10.1038/nm0103-99. [DOI] [PubMed] [Google Scholar]
- [167].Albanese A, Tang PS, Chan WCW. Annual Review of Biomedical Engineering. 2012;14:1. doi: 10.1146/annurev-bioeng-071811-150124. [DOI] [PubMed] [Google Scholar]
- [168].Vinogradov VV, Drozdov AS, Mingabudinova LR, Shabanova EM, Kolchina NO, Anastasova EI, Markova AA, Shtil AA, Milichko VA, Starova GL, Precker RLM, et al. J Mater Chem B. 2018;6:2450. doi: 10.1039/c8tb00072g. [DOI] [PubMed] [Google Scholar]
- [169].Vallés-García C, Gkaniatsou E, Santiago-Portillo A, Giménez-Marqués M, Álvaro M, Greneche JM, Steunou N, Sicard C, Navalón S, Serre C, García H. J Mater Chem A. 2020;8:17002 [Google Scholar]
- [170].Illes B, Hirschle P, Barnert S, Cauda V, Wuttke S, Engelke H. Chem Mater. 2017;29:8042. [Google Scholar]
- [171].Fam SY, Chee CF, Yong CY, Ho KL, Mariatulqabtiah AR, Tan WS. Nanomaterials. 2020;10:787. doi: 10.3390/nano10040787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [172].Veronese FM, Pasut G. Drug Discovery Today. 2005;10:1451. doi: 10.1016/S1359-6446(05)03575-0. [DOI] [PubMed] [Google Scholar]
- [173].Liu J, Yang Y, Zhu W, Yi X, Dong Z, Xu X, Chen M, Yang K, Lu G, Jiang L, Liu Z. Biomaterials. 2016;97:1. doi: 10.1016/j.biomaterials.2016.04.034. [DOI] [PubMed] [Google Scholar]
- [174].Xiao J, Chen S, Yi J, Zhang HF, Ameer GA. Advanced Functional Materials. 2017;27:1604872. doi: 10.1002/adfm.201604872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [175].Zhuang J, Young AP, Tsung CK. Small. 2017;13:1700880. doi: 10.1002/smll.201700880. [DOI] [PubMed] [Google Scholar]
- [176].Li Y, Zhang K, Liu P, Chen M, Zhong Y, Ye Q, Wei MQ, Zhao H, Tang Z. Advanced Materials. 2019;31:1901570. doi: 10.1002/adma.201901570. [DOI] [PubMed] [Google Scholar]
- [177].Wuttke S, Braig S, Preiß T, Zimpel A, Sicklinger J, Bellomo C, Rädler JO, Vollmar AM, Bein T. Chem Commun. 2015;51:15752. doi: 10.1039/c5cc06767g. [DOI] [PubMed] [Google Scholar]
- [178].Karami A, Ahmed A, Sabouni R, Husseini GA, Sharabati MA, AlSawaftah N, Paul V. Colloids and Surfaces B: Biointerfaces. 2022;217:112599. doi: 10.1016/j.colsurfb.2022.112599. [DOI] [PubMed] [Google Scholar]
- [179].Li Y-J, Wu J-Y, Liu J, Xu W, Qiu X, Huang S, Hu X-B, Xiang D-X. Journal of Nanobiotechnology. 2021;19:242. doi: 10.1186/s12951-021-00986-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [180].Chenthamara D, Subramaniam S, Ramakrishnan SG, Krishnaswamy S, Essa MM, Lin F-H, Qoronfleh MW. Biomaterials Research. 2019;23:20. doi: 10.1186/s40824-019-0166-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [181].Banerjee A, Qi J, Gogoi R, Wong J, Mitragotri S. Journal of Controlled Release. 2016;238:176. doi: 10.1016/j.jconrel.2016.07.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [182].Reddy ST, van der Vlies AJ, Simeoni E, Angeli V, Randolph GJ, O’Neil CP, Lee LK, Swartz MA, Hubbell JA. Nat Biotechnol. 2007;25:1159. doi: 10.1038/nbt1332. [DOI] [PubMed] [Google Scholar]
- [183].Azarmi S, Roa WH, Löbenberg R. Advanced Drug Delivery Reviews. 2008;60:863. doi: 10.1016/j.addr.2007.11.006. [DOI] [PubMed] [Google Scholar]
- [184].Maeda H. Advanced Drug Delivery Reviews. 2015;91:3. doi: 10.1016/j.addr.2015.01.002. [DOI] [PubMed] [Google Scholar]
- [185].Blanco E, Shen H, Ferrari M. Nat Biotechnol. 2015;33:941. doi: 10.1038/nbt.3330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [186].McKinlay AC, Morris RE, Horcajada P, Férey G, Gref R, Couvreur P, Serre C. Angewandte Chemie International Edition. 2010;49:6260. doi: 10.1002/anie.201000048. [DOI] [PubMed] [Google Scholar]
- [187].Homayun B, Lin X, Choi H-J. Pharmaceutics. 2019;11:129. doi: 10.3390/pharmaceutics11030129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [188].Benet LZ, Wu C-Y, Hebert MF, Wacher VJ. Journal of Controlled Release. 1996;39:139. [Google Scholar]
- [189].Dou Y, Liu P, Ding Z, Zhou Y, Jing H, Ren Y, Heger Z, Adam V, Li N. Advanced Materials. 2023;35:2210047. doi: 10.1002/adma.202210047. [DOI] [PubMed] [Google Scholar]
- [190].Zhou Y, Chen Z, Zhao D, Li D, He C, Chen X. Advanced Materials. 2021;33:2102044. doi: 10.1002/adma.202102044. [DOI] [PubMed] [Google Scholar]
- [191].Ran J, Zeng H, Cai J, Jiang P, Yan P, Zheng L, Bai Y, Shen X, Shi B, Tong H. Chemical Engineering Journal. 2018;333:20. [Google Scholar]
- [192].Chowdhuri AR, Das B, Kumar A, Tripathy S, Roy S, Sahu SK. Nanotechnology. 2017;28:095102. doi: 10.1088/1361-6528/aa57af. [DOI] [PubMed] [Google Scholar]
- [193].Zhang X, Liu L, Huang L, Zhang W, Wang R, Yue T, Sun J, Li G, Wang J. Nanoscale. 2019;11:9468. doi: 10.1039/c9nr01284b. [DOI] [PubMed] [Google Scholar]
- [194].Zhang H, Zhang J, Li Q, Song A, Tian H, Wang J, Li Z, Luan Y. Biomaterials. 2020;245:119983. doi: 10.1016/j.biomaterials.2020.119983. [DOI] [PubMed] [Google Scholar]
- [195].Zhang F, Liu Y, Lei J, Wang S, Ji X, Liu H, Yang Q. Advanced Science. 2019;6:1901378. doi: 10.1002/advs.201901378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [196].Alt K, Carraro F, Jap E, Linares-Moreau M, Riccò R, Righetto M, Bogar M, Amenitsch H, Hashad RA, Doonan C, Hagemeyer CE, et al. Advanced Materials. 2022;34:2106607. doi: 10.1002/adma.202106607. [DOI] [PubMed] [Google Scholar]
- [197].Dilliard SA, Siegwart DJ. Nat Rev Mater. 2023;8:282. doi: 10.1038/s41578-022-00529-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [198].Huang B, Abraham WD, Zheng Y, Bustamante López SC, Luo SS, Irvine DJ. Science Translational Medicine. 2015;7:291ra94. doi: 10.1126/scitranslmed.aaa5447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [199].van der Meel R, Sulheim E, Shi Y, Kiessling F, Mulder WJM, Lammers T. Nat Nanotechnol. 2019;14:1007. doi: 10.1038/s41565-019-0567-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [200].Kunjachan S, Pola R, Gremse F, Theek B, Ehling J, Moeckel D, Hermanns-Sachweh B, Pechar M, Ulbrich K, Hennink WE, Storm G, et al. Nano Lett. 2014;14:972. doi: 10.1021/nl404391r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [201].Tsvetkova Y, Beztsinna N, Baues M, Klein D, Rix A, Golombek SK, Al Rawashdeh W, Gremse F, Barz M, Koynov K, Banala S, et al. Nano Lett. 2017;17:4665. doi: 10.1021/acs.nanolett.7b01171. [DOI] [PubMed] [Google Scholar]
- [202].Li S-Y, Cheng H, Qiu W-X, Zhang L, Wan S-S, Zeng J-Y, Zhang X-Z. Biomaterials. 2017;142:149. doi: 10.1016/j.biomaterials.2017.07.026. [DOI] [PubMed] [Google Scholar]
- [203].Zhou D, Chen Y, Bu W, Meng L, Wang C, Jin N, Chen Y, Ren C, Zhang K, Sun H. Journal of Colloid and Interface Science. 2021;601:650. doi: 10.1016/j.jcis.2021.05.126. [DOI] [PubMed] [Google Scholar]
- [204].Carrasco S. Biosensors. 2018;8:92. doi: 10.3390/bios8040092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [205].Yang J, Yang Y-W. Small. 2020;16:1906846 [Google Scholar]
- [206].Stassen I, Burtch N, Talin A, Falcaro P, Allendorf M, Ameloot R. Chem Soc Rev. 2017;46:3185. doi: 10.1039/c7cs00122c. [DOI] [PubMed] [Google Scholar]
- [207].Cao S, Xu Y, Yu Z, Zhang P, Xu X, Sui N, Zhou T, Zhang T. Small. 2022;18:2203715. doi: 10.1002/smll.202203715. [DOI] [PubMed] [Google Scholar]
- [208].Xu R, Wang Y, Duan X, Lu K, Micheroni D, Hu A, Lin W. J Am Chem Soc. 2016;138:2158. doi: 10.1021/jacs.5b13458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [209].Ma Y, Su H, Kuang X, Li X, Zhang T, Tang B. Anal Chem. 2014;86:11459. doi: 10.1021/ac503622n. [DOI] [PubMed] [Google Scholar]
- [210].Shao H, Lu J, Zhang Q, Hu Y, Wang S, Guo Z. Sensors and Actuators B: Chemical. 2018;268:39. [Google Scholar]
- [211].Liu H, Peng H, Xin Y, Zhang J. Polym Chem. 2019;10:2263. [Google Scholar]
- [212].Zhang X, Wang Y, Liu J, Shi J, Mao D, Midgley AC, Leng X, Kong D, Wang Z, Liu B, Wang S. Chemical Engineering Journal. 2021;421:129577 [Google Scholar]
- [213].Cai X, Xie Z, Ding B, Shao S, Liang S, Pang M, Lin J. Advanced Science. 2019;6:1900848. doi: 10.1002/advs.201900848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [214].Zeng J-Y, Zhang M-K, Peng M-Y, Gong D, Zhang X-Z. Advanced Functional Materials. 2018;28:1705451 [Google Scholar]
- [215].Li S-Y, Cheng H, Xie B-R, Qiu W-X, Zeng J-Y, Li C-X, Wan S-S, Zhang L, Liu W-L, Zhang X-Z. ACS Nano. 2017;11:7006. doi: 10.1021/acsnano.7b02533. [DOI] [PubMed] [Google Scholar]
- [216].Xie Z, Fan T, An J, Choi W, Duo Y, Ge Y, Zhang B, Nie G, Xie N, Zheng T, Chen Y, et al. Chem Soc Rev. 2020;49:8065. doi: 10.1039/d0cs00215a. [DOI] [PubMed] [Google Scholar]
- [217].Li X, Wang X, Ito A, Tsuji NM. Nat Commun. 2020;11:3858. doi: 10.1038/s41467-020-17637-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [218].Shao Y, Liu B, Di Z, Zhang G, Sun L-D, Li L, Yan C-H. J Am Chem Soc. 2020;142:3939. doi: 10.1021/jacs.9b12788. [DOI] [PubMed] [Google Scholar]
- [219].Lu K, He C, Guo N, Chan C, Ni K, Lan G, Tang H, Pelizzari C, Fu Y-X, Spiotto MT, Weichselbaum RR, et al. Nat Biomed Eng. 2018;2:600. doi: 10.1038/s41551-018-0203-4. [DOI] [PubMed] [Google Scholar]
- [220].Zhang Y, Wang F, Ju E, Liu Z, Chen Z, Ren J, Qu X. Advanced Functional Materials. 2016;26:6454. [Google Scholar]
- [221].Yang J, Ma S, Xu R, Wei Y, Zhang J, Zuo T, Wang Z, Deng H, Yang N, Shen Q. Journal of Controlled Release. 2021;334:21. doi: 10.1016/j.jconrel.2021.04.013. [DOI] [PubMed] [Google Scholar]
- [222].Phase I Study of RiMO-301 With Radiation in Advanced Tumors, NCT03444714. Under clinicaltrialsgov.
- [223].Phase I Study of RiMO-301 and Radiotherapy With PD-1 Inhibitor for the Treatment of Head-Neck Cancer NCT05838729. Under clinicaltrialsgov.
- [224].Sava Gallis DF, Butler KS, Agola JO, Pearce CJ, McBride AA. ACS Appl Mater Interfaces. 2019;11:7782. doi: 10.1021/acsami.8b21698. [DOI] [PubMed] [Google Scholar]
- [225].Lemire JA, Harrison JJ, Turner RJ. Nat Rev Microbiol. 2013;11:371. doi: 10.1038/nrmicro3028. [DOI] [PubMed] [Google Scholar]
- [226].Yang Y, Wu X, He C, Huang J, Yin S, Zhou M, Ma L, Zhao W, Qiu L, Cheng C, Zhao C. ACS Appl Mater Interfaces. 2020;12:13698. doi: 10.1021/acsami.0c01666. [DOI] [PubMed] [Google Scholar]
- [227].Yang H, Gu X, Li Y, Zhang K, Liu X, Huang C, Ren Y, Qi C, Cai K. J Mater Chem B. 2022;10:8664. doi: 10.1039/d2tb01436j. [DOI] [PubMed] [Google Scholar]
- [228].Mao D, Hu F, Ji S, Wu W, Ding D, Kong D, Liu B. Advanced Materials. 2018;30:1706831. doi: 10.1002/adma.201706831. [DOI] [PubMed] [Google Scholar]
- [229].Shen X, Zhang Y, Ma P, Sutrisno L, Luo Z, Hu Y, Yu Y, Tao B, Li C, Cai K. Biomaterials. 2019;212:1. doi: 10.1016/j.biomaterials.2019.05.008. [DOI] [PubMed] [Google Scholar]
- [230].Duncan MJ, Wheatley PS, Coghill EM, Vornholt SM, Warrender SJ, Megson IL, Morris RE. Mater Adv. 2020;1:2509. [Google Scholar]
- [231].Wu J, Yu Y, Cheng Y, Cheng C, Zhang Y, Jiang B, Zhao X, Miao L, Wei H. Angewandte Chemie International Edition. 2021;60:1227. doi: 10.1002/anie.202010714. [DOI] [PubMed] [Google Scholar]
- [232].Liu Y, Cheng Y, Zhang H, Zhou M, Yu Y, Lin S, Jiang B, Zhao X, Miao L, Wei C-W, Liu Q, et al. Science Advances. 2020;6:eabb2695. doi: 10.1126/sciadv.abb2695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [233].Lian X, Erazo-Oliveras A, Pellois J-P, Zhou H-C. Nat Commun. 2017;8:2075. doi: 10.1038/s41467-017-02103-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [234].Guo B, Dong R, Liang Y, Li M. Nat Rev Chem. 2021;5:773. doi: 10.1038/s41570-021-00323-z. [DOI] [PubMed] [Google Scholar]
- [235].Chen J, Zhang X, Huang C, Cai H, Hu S, Wan Q, Pei X, Wang J. Journal of Biomedical Materials Research Part A. 2017;105:834. doi: 10.1002/jbm.a.35960. [DOI] [PubMed] [Google Scholar]
- [236].Sarkar C, Chowdhuri AR, Garai S, Chakraborty J, Sahu SK. Cellulose. 2019;26:7253. [Google Scholar]
- [237].Yang Y, Zan J, Yang W, Qi F, He C, Huang S, Peng S, Shuai C. Mater Chem Front. 2020;4:973. [Google Scholar]
- [238].Zhong L, Chen J, Ma Z, Feng H, Chen S, Cai H, Xue Y, Pei X, Wang J, Wan Q. Nanoscale. 2020;12:24437. doi: 10.1039/d0nr06297a. [DOI] [PubMed] [Google Scholar]
- [239].Liu W, Yan Z, Zhang Z, Zhang Y, Cai G, Li Z. Journal of Alloys and Compounds. 2019;788:705. [Google Scholar]
- [240].Xiong Y, Feng Q, Lu L, Qiu X, Knoedler S, Panayi AC, Jiang D, Rinkevich Y, Lin Z, Mi B, Liu G, et al. Advanced Materials. 2023 doi: 10.1002/adma.202302587. [DOI] [PubMed] [Google Scholar]
- [241].Xiao J, Zhu Y, Huddleston S, Li P, Xiao B, Farha OK, Ameer GA. ACS Nano. 2018;12:1023. doi: 10.1021/acsnano.7b01850. [DOI] [PubMed] [Google Scholar]
- [242].Yin M, Wu J, Deng M, Wang P, Ji G, Wang M, Zhou C, Blum NT, Zhang W, Shi H, Jia N, et al. ACS Nano. 2021;15:17842. doi: 10.1021/acsnano.1c06036. [DOI] [PubMed] [Google Scholar]
- [243].Mitragotri S, Burke PA, Langer R. Nat Rev Drug Discov. 2014;13:655. doi: 10.1038/nrd4363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [244].Frid AH, Kreugel G, Grassi G, Halimi S, Hicks D, Hirsch LJ, Smith MJ, Wellhoener R, Bode BW, Hirsch IB, Kalra S, et al. Mayo Clinic Proceedings. 2016;91:1231. doi: 10.1016/j.mayocp.2016.06.010. [DOI] [PubMed] [Google Scholar]
- [245].Doonan C, Riccò R, Liang K, Bradshaw D, Falcaro P. Acc Chem Res. 2017;50:1423. doi: 10.1021/acs.accounts.7b00090. [DOI] [PubMed] [Google Scholar]
- [246].Chen Y, Li P, Modica JA, Drout RJ, Farha OK. J Am Chem Soc. 2018;140:5678. doi: 10.1021/jacs.8b02089. [DOI] [PubMed] [Google Scholar]
- [247].Zhou Y, Liu L, Cao Y, Yu S, He C, Chen X. ACS Appl Mater Interfaces. 2020;12:22581. doi: 10.1021/acsami.0c04303. [DOI] [PubMed] [Google Scholar]
- [248].Zhang C, Hong S, Liu M-D, Yu W-Y, Zhang M-K, Zhang L, Zeng X, Zhang X-Z. J Control Release. 2020;320:159. doi: 10.1016/j.jconrel.2020.01.038. [DOI] [PubMed] [Google Scholar]
- [249].Yang X-X, Feng P, Cao J, Liu W, Tang Y. ACS Appl Mater Interfaces. 2020;12:13613. doi: 10.1021/acsami.9b20774. [DOI] [PubMed] [Google Scholar]
- [250].Jarai BM, Stillman Z, Attia L, Decker GE, Bloch ED, Fromen CA. ACS Appl Mater Interfaces. 2020;12:38989. doi: 10.1021/acsami.0c10900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [251].Xiao B, Wheatley PS, Zhao X, Fletcher AJ, Fox S, Rossi AG, Megson IL, Bordiga S, Regli L, Thomas KM, Morris RE. J Am Chem Soc. 2007;129:1203. doi: 10.1021/ja066098k. [DOI] [PubMed] [Google Scholar]
- [252].Luzuriaga MA, Herbert FC, Brohlin OR, Gadhvi J, Howlett T, Shahrivarkevishahi A, Wijesundara YH, Venkitapathi S, Veera K, Ehrman R, Benjamin CE, et al. ACS Nano. 2021;15:17426. doi: 10.1021/acsnano.1c03092. [DOI] [PubMed] [Google Scholar]
- [253].Cheng H, Zhu J-Y, Li S-Y, Zeng J-Y, Lei Q, Chen K-W, Zhang C, Zhang X-Z. Advanced Functional Materials. 2016;26:7847. [Google Scholar]
- [254].Ni K, Lan G, Guo N, Culbert A, Luo T, Wu T, Weichselbaum RR, Lin W. Science Advances. 2020;6:eabb5223. doi: 10.1126/sciadv.abb5223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [255].Luzuriaga MA, Welch RP, Dharmarwardana M, Benjamin CE, Li S, Shahrivarkevishahi A, Popal S, Tuong LH, Creswell CT, Gassensmith JJ. ACS Appl Mater Interfaces. 2019;11:9740. doi: 10.1021/acsami.8b20504. [DOI] [PubMed] [Google Scholar]
- [256].Anselmo AC, Mitragotri S. Bioeng Transl Med. 2019;4:e10143. doi: 10.1002/btm2.10143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [257].Matoori S, Veves A, Mooney DJ. Science Translational Medicine. 2021;13:eabe4839. doi: 10.1126/scitranslmed.abe4839. [DOI] [PubMed] [Google Scholar]
- [258].Liang Y, He J, Guo B. ACS Nano. 2021;15:12687. doi: 10.1021/acsnano.1c04206. [DOI] [PubMed] [Google Scholar]
- [259].Loh QL, Choong C. Tissue Engineering Part B: Reviews. 2013;19:485. doi: 10.1089/ten.teb.2012.0437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [260].Baryakova TH, Pogostin BH, Langer R, McHugh KJ. Nat Rev Drug Discov. 2023;22:387. doi: 10.1038/s41573-023-00670-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [261].Sullivan R, Pramesh CS, Booth CM. Nature. 2017;549:325. doi: 10.1038/549325a. [DOI] [PubMed] [Google Scholar]
- [262].Hirschle P, Preiß T, Auras F, Pick A, Völkner J, Valdepérez D, Witte G, Parak WJ, Rädler JO, Wuttke S. CrystEngComm. 2016;18:4359. [Google Scholar]
- [263].Modena MM, Rühle B, Burg TP, Wuttke S. Advanced Materials. 2019;31:1901556. doi: 10.1002/adma.201901556. [DOI] [PubMed] [Google Scholar]
- [264].Yaghi OM. ACS Cent Sci. 2019;5:1295. doi: 10.1021/acscentsci.9b00750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [265].Jiang H, Alezi D, Eddaoudi M. Nat Rev Mater. 2021;6:466. [Google Scholar]
- [266].Mason JA, Oktawiec J, Taylor MK, Hudson MR, Rodriguez J, Bachman JE, Gonzalez MI, Cervellino A, Guagliardi A, Brown CM, Llewellyn PL, et al. Nature. 2015;527:357. doi: 10.1038/nature15732. [DOI] [PubMed] [Google Scholar]
- [267].Kim H, Yang S, Rao SR, Narayanan S, Kapustin EA, Furukawa H, Umans AS, Yaghi OM, Wang EN. Science. 2017;356:430. doi: 10.1126/science.aam8743. [DOI] [PubMed] [Google Scholar]
- [268].NuMat Brings First MOF-Enabled Gas Storage Product to Market. Institute for Sustainability and Energy at Northwestern (ISEN); [Google Scholar]
- [269].Silva P, Vilela SMF, Tomé JPC, Almeida Paz FA. Chem Soc Rev. 2015;44:6774. doi: 10.1039/c5cs00307e. [DOI] [PubMed] [Google Scholar]
- [270].Martin L, Hutchens M, Hawkins C, Radnov A. Nature Reviews Drug Discovery. 2017;16:381. doi: 10.1038/nrd.2017.70. [DOI] [PubMed] [Google Scholar]
- [271].Mullard A. Nature Reviews Drug Discovery. 2014;13:877. doi: 10.1038/nrd4218. [DOI] [PubMed] [Google Scholar]
- [272].Wouters OJ, McKee M, Luyten J. JAMA. 2020;323:844. doi: 10.1001/jama.2020.1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [273].Anselmo AC, Mitragotri S. Journal of Controlled Release. 2014;190:15. doi: 10.1016/j.jconrel.2014.03.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [274].Hare JI, Lammers T, Ashford MB, Puri S, Storm G, Barry ST. Advanced Drug Delivery Reviews. 2017;108:25. doi: 10.1016/j.addr.2016.04.025. [DOI] [PubMed] [Google Scholar]
- [275].Anselmo AC, Mitragotri S. Bioengineering & Translational Medicine. 2021;6:e10246. doi: 10.1002/btm2.10246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [276].Shan X, Gong X, Li J, Wen J, Li Y, Zhang Z. Acta Pharmaceutica Sinica B. 2022;12:3028. doi: 10.1016/j.apsb.2022.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [277].Mak IW, Evaniew N, Ghert M. Am J Transl Res. 2014;6:114. [PMC free article] [PubMed] [Google Scholar]
- [278].Sun D, Zhou S, Gao W. ACS Nano. 2020;14:12281. doi: 10.1021/acsnano.9b09713. [DOI] [PubMed] [Google Scholar]
- [279].Ioannidis JPA, Kim BYS, Trounson A. Nat Biomed Eng. 2018;2:797. doi: 10.1038/s41551-018-0314-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [280].Atkins JT, George GC, Hess K, Marcelo-Lewis KL, Yuan Y, Borthakur G, Khozin S, LoRusso P, Hong DS. Br J Cancer. 2020;123:1496. doi: 10.1038/s41416-020-01033-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [281].Kumari S, Howlett TS, Ehrman RN, Koirala S, Trashi O, Trashi I, Wijesundara YH, Gassensmith JJ. Chem Sci. 2023;14:5774. doi: 10.1039/d3sc00500c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [282].Bigini P, Gobbi M, Bonati M, Clavenna A, Zucchetti M, Garattini S, Pasut G. Nat Nanotechnol. 2021;16:1169. doi: 10.1038/s41565-021-01001-3. [DOI] [PubMed] [Google Scholar]
- [283].Wang TC, Vermeulen NA, Kim IS, Martinson ABF, Stoddart JF, Hupp JT, Farha OK. Nat Protoc. 2016;11:149. doi: 10.1038/nprot.2016.001. [DOI] [PubMed] [Google Scholar]
- [284].Geng S, Lin E, Li X, Liu W, Wang T, Wang Z, Sensharma D, Darwish S, Andaloussi YH, Pham T, Cheng P, et al. J Am Chem Soc. 2021;143:8654. doi: 10.1021/jacs.1c02108. [DOI] [PubMed] [Google Scholar]
- [285].Lin J-B, Nguyen TTT, Vaidhyanathan R, Burner J, Taylor JM, Durekova H, Akhtar F, Mah RK, Ghaffari-Nik O, Marx S, Fylstra N, et al. Science. 2021;374:1464. doi: 10.1126/science.abi7281. [DOI] [PubMed] [Google Scholar]
- [286].Ruiz-Zambrana CL, Malankowska M, Coronas J. Dalton Trans. 2020;49:15139. doi: 10.1039/d0dt02207a. [DOI] [PubMed] [Google Scholar]
- [287].Hirai K, Sada K. Chem Commun. 2017;53:5275. doi: 10.1039/c7cc00702g. [DOI] [PubMed] [Google Scholar]
- [288].Rohra N, Gaikwad G, Dandekar P, Jain R. ACS Appl Mater Interfaces. 2022;14:8251. doi: 10.1021/acsami.1c22153. [DOI] [PubMed] [Google Scholar]
- [289].Mao C, Wang S, Li J, Feng Z, Zhang T, Wang R, Fan C, Jiang X. ACS Nano. 2023;17:2840. doi: 10.1021/acsnano.2c11241. [DOI] [PubMed] [Google Scholar]
- [290].Koryakina IG, Bachinin SV, Gerasimova EN, Timofeeva MV, Shipilovskikh SA, Bukatin AS, Sakhatskii A, Timin AS, Milichko VA, Zyuzin MV. Chemical Engineering Journal. 2023;452:139450 [Google Scholar]
- [291].Severino MI, Gkaniatsou E, Nouar F, Pinto ML, Serre C. Faraday Discuss. 2021;231:326. doi: 10.1039/d1fd00018g. [DOI] [PubMed] [Google Scholar]
- [292].Pobłocki K, Drzeżdżon J, Gawdzik B, Jacewicz D. Green Chem. 2022;24:9402. [Google Scholar]
- [293].Escobar-Hernandez HU, Shen R, Papadaki MI, Powell JA, Zhou HC, Wang Q. ACS Chem Health Saf. 2021;28:358. [Google Scholar]
- [294].Howarth AJ, Liu Y, Li P, Li Z, Wang TC, Hupp JT, Farha OK. Nat Rev Mater. 2016;1:1. [Google Scholar]
- [295].Kuznicki A, Lorzing GR, Bloch ED. Chem Commun. 2021;57:8312. doi: 10.1039/d1cc02104d. [DOI] [PubMed] [Google Scholar]
- [296].Luo W-C, O’Reilly Beringhs A, Kim R, Zhang W, Patel SM, Bogner RH, Lu X. European Journal of Pharmaceutics and Biopharmaceutics. 2021;169:256. doi: 10.1016/j.ejpb.2021.10.014. [DOI] [PubMed] [Google Scholar]
- [297].Ding M, Cai X, Jiang H-L. Chem Sci. 2019;10:10209. doi: 10.1039/c9sc03916c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [298].Freund R, Lächelt U, Gruber T, Rühle B, Wuttke S. ACS Nano. 2018;12:2094. doi: 10.1021/acsnano.8b00932. [DOI] [PubMed] [Google Scholar]
- [299].Faria M, Björnmalm M, Thurecht KJ, Kent SJ, Parton RG, Kavallaris M, Johnston APR, Gooding JJ, Corrie SR, Boyd BJ, Thordarson P, et al. Nature Nanotech. 2018;13:777. doi: 10.1038/s41565-018-0246-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [300].Lammers T, Storm G. Nat Nanotechnol. 2019;14:626. doi: 10.1038/s41565-019-0497-8. [DOI] [PubMed] [Google Scholar]
- [301].Kang Y, Park H, Smit B, Kim J. Nat Mach Intell. 2023;5:309. [Google Scholar]
- [302].Luo Y, Bag S, Zaremba O, Cierpka A, Andreo J, Wuttke S, Friederich P, Tsotsalas M. Angewandte Chemie International Edition. 2022;61:e202200242. doi: 10.1002/anie.202200242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [303].Glasby LT, Gubsch K, Bence R, Oktavian R, Isoko K, Moosavi SM, Cordiner JL, Cole JC, Moghadam PZ. Chem Mater. 2023;35:4510. doi: 10.1021/acs.chemmater.3c00788. [DOI] [PMC free article] [PubMed] [Google Scholar]