Abstract
We determined the relationships between DNA sequence variation and DNA methylation using blood samples from 3,799 Europeans and 3,195 South Asians. We identify 11,165,559 SNP-CpG associations (meQTLs, P<10-14), including 467,915 meQTLs that operate in trans. The meQTLs are enriched for functionally relevant characteristics, including shared chromatin state, HiC interaction, and association with gene expression, metabolic and clinical traits. We use molecular interaction and colocalisation analyses to identify multiple nuclear regulatory pathways linking meQTL loci to phenotypic variation, including UBASH3B (body mass index), NFKBIE (rheumatoid arthritis), MGA (blood pressure), and COMMD7 (white cell counts). For rs6511961, ChIP-seq validates ZNF333 as the likely trans-acting effector protein. Finally, we used interaction analyses to identify population and lineage specific meQTLs, including rs174548 in FADS1, with strongest effect in CD8T cells, thus linking fatty acid metabolism with immune dysregulation and asthma. Our study advances understanding of the potential pathways linking genetic variation to human phenotype.
Introduction
Methylation of DNA plays a key role in determination of genomic structure and function, including regulation of cellular differentiation, and coordination of gene expression.1–4 Disturbances in DNA methylation have been implicated in the development of atherosclerosis, cancer, obesity, type 2 diabetes and neuropsychiatric illness, and other complex multifactorial diseases, and predict all-cause mortality.5–12 Improved understanding of the mechanisms influencing DNA methylation is therefore anticipated to provide new insights into the biological pathways that determine genome regulation, molecular phenotypes, and development of disease.
DNA methylation is strongly influenced by underlying genetic variation, both in cis (same chromosome) and in trans (across chromosomes).9,13–23 Genetic variants which influence DNA methylation in trans are of particular interest, and identify nuclear regulatory pathways which play a critical role in the coordination of genomic function, and impact multiple biological processes.14,18–20,23 We aimed to build on this previous work, and advance understanding of the molecular mechanisms linking regulatory genetic variation to gene expression, molecular interactions, phenotypic variation and disease susceptibility.
Results
Genome-wide association and replication testing
Our study design is summarised in Extended Data Figure 1. We first carried out a genome-wide association study of DNA methylation in peripheral blood, with replication testing, amongst 3,799 Europeans (N=1,731 discovery; N=2,068 replication) and 3,195 South Asians (N=1,841 discovery; N=1,354 replication). DNA methylation was quantified using the Illumina Infinium HumanMethylation450 BeadChip. Genome-wide association was done in Europeans and South Asians separately.24 Methylation Quantitative Trait Loci (meQTLs) reaching genome-wide significance (P<10-14) were selected for replication testing. This stringent statistical threshold for genome-wide significance provides complete Bonferroni correction for the ~4.3 trillion statistical tests carried out, and reduces the risk of false positive results (see Methods). Replication testing was first done using an ancestry specific approach; this was followed by a final trans-ancestry analysis (Extended Data Figure 1). At each stage of replication, we required meQTLs reach i. P<0.05 with consistent direction of effect, and ii. P<10-14 in combined analysis of discovery and replication results. The meQTLs identified by genome-wide association showed a high rate of replication (>90%) in both ancestry specific, and cross-ancestry replication testing (Supplementary Table 1, Extended Data Figure 2). Replication rates are comparable or higher than for meQTLs reported in published studies (Supplementary Tables 2 and 3). Our meQTLs replicate in data generated by Illumina methylation EPIC array (>96% at P<0.05 with same direction of effect, N=1,848 samples, see Methods) and by MeDIP-seq peripheral-blood DNA methylomes (47% of testable meQTLs at P<0.05, Supplementary Table 4, Extended Data Figure 2),25 demonstrating that our findings are generalisable across platforms.
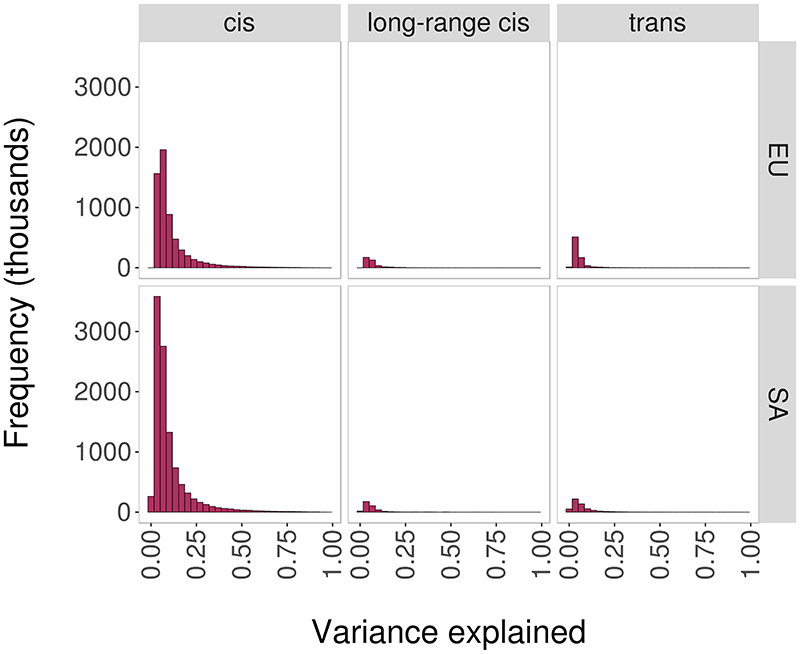
The output is a high-confidence cosmopolitan set of 11,165,559 meQTLs (comprising 2,709,428 SNPs and 70,709 CpGs) that are experimentally stringent, highly reproducible, and which operate across human populations (Figure 1 and Supplementary Table 5). The median effect size for the 11.2M meQTLs is 2.0% (IQR: 1.2 to 3.5%) absolute change in methylation per allele copy. On average the SNPs explain 10.3% (IQR: 4.4 to 11.5%) of variation in methylation at the respective CpGs (Supplementary Table 6 and Extended Data Figure 3).
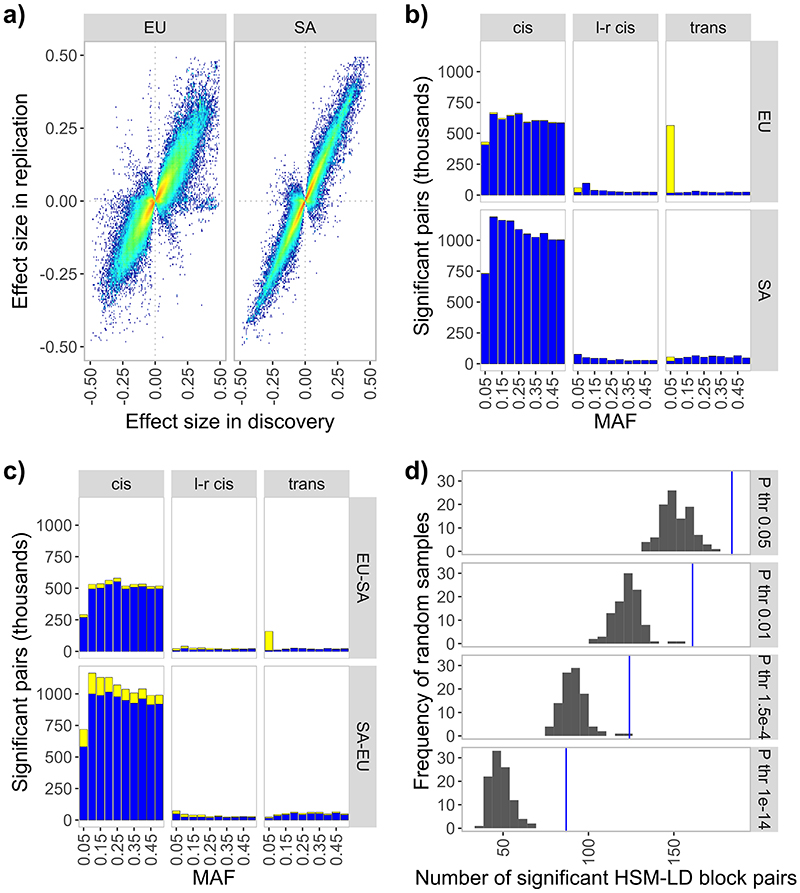
Figure 1. Summary of results for genome-wide association and replication testing.
1a. Chessboard plot. Each dot represents a unique SNP-CpG pair reaching genome-wide significance in discovery (P<10-14) and showing both ancestry specific and cross-ancestry replication. CpG position and background CpG density (450K array) are annotated on the x-axis, and SNP position and background SNP density are annotated on the y-axis. SNP-CpG pairs are colour coded according to proximity of SNP and CpG: cis – within 1Mb (N=10,346,172, green markers appearing as a diagonal line); long-range cis – distance >1Mb but on the same chromosome (N=351,472, purple markers); trans – SNP and CpG are on different chromosomes (N=467,915, black markers). 1b. Manhattan plot of trans-acting SNP-CpG associations. Each marker represents the number of CpG sites associated in trans with the identified trans-acting SNPs. Results are for the cosmopolitan set of SNP-CpG pairs showing both ancestry specific and cross-ancestry replication. SNPs with the highest number of CpGs in trans (top 1%) are highlighted in black and the gene nearest the sentinel SNP is displayed.
The identified meQTLs operate across diverse cell types
We show that 80%-87% of the 11.2M meQTLs have consistent direction of effect and 26%-37% replicate at P<0.05, in isolated white cell subsets (N=57 samples, Figure 2, Supplementary Table 7). We also show that 72%-86% of our meQTLs have consistent direction of effect in isolated adipocytes (subcutaneous and visceral, N=47 samples) and in adipose tissue (N=603 samples; P<1×10-324 for each comparison, binomial test). Further 19.2% replicate in isolated visceral adipocytes, 19.4% in subcutaneous adipocytes and 44.2% in subcutaneous adipose tissue (P<0.05 and same direction of effect, Figure 2, Supplementary Table 7). These proportions are consistent with expectations based on sample size. Our results demonstrate that many of the meQTLs operate across diverse cell lineages, and are thus likely to be relevant to tissues and biological systems other than blood.
Figure 2. Replication in isolated white cells, isolated adipocytes, and adipose tissue.
Density plot summarising replication of the SNP-CpG pairs identified by genome-wide association. Rows i-iv. four isolated white cell subsets (CD4+ lymphocytes, CD8+ lymphocytes, neutrophils and monocytes), rows v-vi. isolated visceral and subcutaneous adipocytes and row vii. whole adipose tissue. Results are presented as the effect size (change in methylation, on 0-1 scale where 1 represents 100% methylation) per allele copy of the identified SNP in whole blood (x-axis) and in the respective isolated cell type (y-axis), stratified by SNP-CpG proximity (cis, long-range cis, and trans associations). Plotting area is limited to effect sizes between -0.5 and 0.5. Results show highly concordant effect sizes between whole blood and each cell type. Inset in each panel are replication rates in the respective cell type (‘Rep’: P<0.05 and same direction of effect), as well as percent of directional consistency between effect sizes (‘Dir’).
Annotation of the meQTLs identified
SNPs are enriched for association with DNA methylation on their cis-chromosome, even beyond the conventional 1Mb interval (Extended Data Figure 4, see Methods). Since underlying genomic mechanisms may differ according to proximity, we separate our findings into: i. cis-meQTLs (SNP-CpG distance <1Mb, N=10,346,172 pairs; 2,650,691 SNPs and 67,694 CpGs); ii. long-range cis-meQTLs (>1Mb apart but on the same chromosome, N=351,472 pairs; 120,593 SNPs and 1,846 CpGs) and iii. trans-meQTLs (associations between SNPs and CpGs on different chromosomes, N=467,915 pairs; 200,761 SNPs and 3,592 CpGs). We used conditional analyses, correlation structure and genomic distance to estimate the total number of independent loci in our cosmopolitan SNP-CpG associations (Supplementary Figure 1, see Methods). This identified 34,001 independent genetic loci associated with 46,664 independent methylation loci in cis; 467 independent genetic loci associated with 499 independent methylation loci in long-range cis; and 1,847 independent genetic loci associated with 3,020 independent methylation loci in trans. For each of these we selected a single sentinel SNP and a single CpG site (lowest P-value in any pairwise association, Supplementary Table 8 and 9, and Methods) to represent the individual loci in downstream analyses.
Functional genomic evaluation of the meQTL SNPs and CpGs
Sentinel meQTL SNPs are enriched for location in multiple active chromatin regions, supporting a role in genome regulation (Extended Data Figure 5).14 Expression array data for our cohort participants (Europeans: N=853, South Asians: N=693; see Methods) identifies 2,696 sentinel SNPs to be expression Quantitative Trait Loci (total eQTL pairs 3,131: cis 3,018; long-range cis 50; trans 63) at P<7.98×10-11 (P<0.05 after Bonferroni correction for all possible SNP-transcript tests, Supplementary Table 10), and shows that sentinel SNPs are enriched for eQTLs both in cis and in trans (range 4.1 to 22.1 fold compared to expectations under the null hypothesis, P=8.10×10-18 to P=2.45×10-66; Extended Data Figure 6). We separately show that sentinel meQTL SNPs are strongly enriched for protein-QTLs (1.6 to 2.1 fold; P<0.001), metabolite-QTLs in cis (1.4 fold, P<0.001), and for association with phenotypic traits and diseases (1.9 to 3.4, P<0.001). Results are summarised in Extended Data Figure 7 and Supplementary Figure 2.
Sentinel CpGs influenced by genetic variants in cis are enriched in flanking regions of active TSS and enhancers, and depleted in heterochromatin regions, while SNP-CpG pairs in trans are additionally enriched at active TSS (Extended Data Figure 5).14 Using the extensive baseline phenotypic data for our participants, we show that meQTL CpGs are enriched for association with metabolic, physiologic and clinical traits (252 of 277 available traits at P<1.8x10-4 (Bonferroni correction for 277 tests) compared to expectations under the null hypothesis (median enrichment 1.10, interquartile range 1.06 to 1.15; Extended Data Figure 7, Supplementary Table 11). These findings support a potential role for the identified CpGs (or their correlated markers) in determining phenotypic traits.
Next, we defined both the cis- and trans- relationships between DNA methylation and gene expression (expression quantitative trait methylation loci, eQTMs) in our participants. Using similar analytic approaches to published studies initially suggested 90,666 putative cis-eQTMs in our dataset at P=8.7x10-12 (P<0.05 after Bonferroni correction for the number of possible CpG-expression pairs).14 However, this result appears strongly confounded by variation in white cell composition, and adjustment for estimated cell type proportions reduced the number of cis-eQTMs identified to 769, of which 155 overlap our sentinel CpGs. We use Summary-data-based Mendelian Randomisation (SMR)26 to further confirm this interpretation; putative cis-eQTMs identified with correction for white cell subsets were strongly replicated by SMR, while uncorrected eQTMs were not (SMR P<0.05/N tests: 73% vs 17% respectively, P=2.0x10-29; Supplementary Table 12). In parallel, we identify 97,281 trans-eQTMs, of which 11,562 overlap one of our sentinel CpGs; 627 of these trans-eQTMs are supported by SMR (Supplementary Table 12), a proportion consistent with the statistical power of our analysis (Supplementary Table 13). Finally, we show that sentinel CpGs which are part of cis-meQTL pairs, are strongly enriched for being cis-eQTMs (ie associated with gene expression in cis, Extended Data Figure 6). Our results confirm the potential for white cell subset composition to confound analyses of gene expression in whole blood, and provide experimental approaches for resolving the potential biases.
Physical and regulatory interactions between meQTL SNPs and CpGs
We tested whether cis-meQTLs might represent a direct effect of the sequence variant on the interaction between chromatin associated factors and cis-regulatory elements harbouring the CpG site.27,28 Using data from the Roadmap Epigenomics Consortium, we show that 88% of CpGs with cis-acting meQTLs are associated with SNPs localising to the same chromatin state (empirical P=9.9x10-3; Extended Data Figure 5). We similarly hypothesised that long-range cis-meQTLs might reflect physical interactions between distal enhancers and promoters.14,29,30 In support of this, we show that long-range cis associations occur more frequently within topologically associated domains (15.5 fold, empirical P<0.01, Extended Data Figure 5) and more frequently have a HiC contact between SNP and CpG sites at promoter regions in 17 primary blood cell types characterised in the BLUEPRINT project31 (2.5 fold, empirical P<0.01, Extended Data Figure 5). Annotating these associated pairs with chromHMM epigenetic states reveals 145 promoter-promoter, 178 enhancer-promoter and 49 enhancer-enhancer interactions. We demonstrate that the trans-acting SNP-CpG pairs are also enriched for location in regions of chromosomal interaction in primary blood cells (3.7 fold, empirical P=6.6×10-3, Extended Data Figure 5), and in lymphoblastoid cell lines (1.8 fold, empirical P<0.01; Supplementary Table 14).31,32 Taken together, these results indicate that genetic variants associate with methylation levels of CpG sites localised in the same or in physically interacting regulatory elements, consistent with a co-ordinated role in genomic regulation.
Intersection of DNA methylation and gene expression at meQTLs
Few studies have explored trans-acting relationships between DNA methylation and gene expression. Trans-meQTLs in particular, provide new opportunities to understand the co-ordination of genomic function, including identification of the proximal candidate gene(s) underlying the trans-acting effect of meQTL SNPs.23 To address this systematically, we first use data from eQTLGen (N=31,684 samples) to identify 4,811 cis-eQTLs associated with the 1,847 trans-acting sentinel meQTL SNPs (P<1x10-6, Bonferroni correction for 48,237 eQTL tests). We then test the 4,811 eQTL genes for association with DNA methylation in our participants, and find 1,607 trans-eQTMs at P<0.05. SMR supports 929 of these trans-eQTMs (SMR P<3.1x10-5; Bonferroni correction for 1607 tests), while 34 trans-eQTMs are likely to be regulated by a common genetic mechanism (coloc PP4 >0.6)33 The 34 cis-eQTLs identified as likely to be mediating trans-methylation signatures identified include ZFP57 (associated with trans-meQTL SNP rs2747429), which encodes a DNA binding protein critical for maintenance of epigenetic memory,33,34 as well as other ZNF/ZFP genes anticipated to be involved in genome regulation (Supplementary Table 15).
Intersection of DNA methylation with clinical phenotypes at meQTLs
We used our meQTLs as genetic instruments to examine the potential causal relationships between DNA methylation and body mass index (BMI), as a model phenotype of global public health significance. Our sentinel meQTL SNPs and CpGs are both strongly enriched for association with BMI (Extended Data Figure 7 and Supplementary Table 11 respectively), consistent with a role in the aetiology of adiposity. Using the 941 SNPs independently associated with BMI at P<10-8 in GWAS as genetic instruments,35 SMR suggests a potential causal relationship between DNA methylation and BMI at 374 loci (P<0.05 after Bonferroni correction, Supplementary Table 16, of which 239 show evidence for a shared underlying causal variant (coloc PP4>0.6). At the UBASH3B locus we identify SNP rs7115089 as influencing both DNA methylation and BMI (SMR P=2.5x10-10, coloc PP4=1.0). UBASH3B encodes a protein with tyrosine phosphatase activity, that has been previously linked to advanced neoplasia.36 SNP rs7115089 is strongly associated with BMI,35 and is in LD (R2>0.8) with genetic variants linked to other cardiovascular and metabolic traits in GWAS studies.37–40 SNP rs7115089 is associated with differential methylation at our sentinel CpG (cg26684673), which we have previously shown to be associated with BMI in adults.8 SNP rs7115089 is associated with expression of UBASH3B (P=1.7x10-17). Animal models show that expression of Ubash3b is an early transcriptomic-based biomarker of gestational calorie restriction which may drive programmed susceptibility to obesity and other chronic diseases in later life,41 and expression of UBASH3B in peripheral blood is also strongly associated with BMI and other measures of adiposity in humans (Supplementary Table 17).42 Our results thus identify UBASH3B as a potential mediator of both genetic and environmental exposures underlying adiposity and cardiometabolic disease.
Integrating molecular information at trans-acting loci
We identify 467,915 trans-acting SNP-CpG pairs, comprising pairwise relationships between 200,761 unique SNPs and 3,592 unique CpGs. Based on conditional analysis, these represent 1,847 distinct loci with genetic variants that influence DNA methylation in trans (range 1-298 trans-CpG sites per genetic locus, Figure 1). The genes in cis to the sentinel trans-acting SNPs are enriched for genes with known regulatory function (1.64 mean fold enrichment, empirical P=5.99×10-3; gene list and pathway analysis in Supplementary Tables 18 and 19 respectively), including documented transcription factors such as CTCF, NFKB1, REST and TBX6. Our results support the view that the trans meQTLs identify genetic loci with key roles as master regulators of genome structure and function, and that the effects of these trans-acting loci may be mediated through their remote effects on DNA methylation.
To generate new knowledge of the nuclear proteins involved in mediating the trans SNP-CpG relationships, we next identified known transcription factors with binding sites that overlap the trans-CpG signatures of the trans-acting genetic loci. Based on power calculations, we limited the analysis to the 115 sentinel trans-meQTLs with N≥5 CpG sites associated (see Methods). At 45 genetic loci (39%), the trans-CpGs of the respective sentinel SNPs overlap binding sites of one or more known transcription factors (Figure 3; Extended Data Figure 8; Supplementary Table 20; FDR<0.05). This represents a 1.8 fold enrichment compared to expectation under the null hypothesis (P=7.4x10-6, binomial test, see Methods). As a sensitivity analysis, we repeated the experiment using data generated on a methylationEPIC array, to test the impact of increased coverage of methylation markers on identification of overlapping transcription factors (Methods and Supplementary Table 21). There was no evidence for false positive findings, but the higher density marker set of the EPIC array did increase the number of overlapping transcription factors identified by 14% (Supplementary Table 21).
Figure 3. Candidate genes for sentinel SNPs that are associated with trans-CpG sites which overlap transcription factor binding sites.
Panel 3a shows the evidence for each candidate: i. genes that are transcription factors in cis, and which overlap the trans-CpG signatures (‘enriched cis-TF’); ii. genes selected by the random walk analysis including protein-protein interactions (‘PPI’), and iii. genes that are cis-eQTL for the sentinel SNPs. The heatmap in panel 3b shows the percentage of associated CpG sites with trans-eQTM at each locus (x-axis). The heatmap in panel 3c shows the enrichment or depletion of binding of transcription factors (y-axis) at the associated CpG sites of each locus (x-axis). Odds ratios comparing the frequency of state annotations at associated CpGs with background CpGs are colour coded. Odds ratios greater than 10 or less than 0.1 have been set to 10 or 0.1 for improved readability of the colour scale. Odds ratios greater than 1 indicate enrichment, while odds ratios less than 1 indicate depletion.
At 4 of the 45 genetic loci with trans-CpG signature overlapping a transaction factor, the genes in cis to the sentinel SNP encode the respective nuclear transcription factor (REST, NFE2, CTCF and NFKB1; FET P=1.7x10-5 to 3.4x10-89, Extended Data Figure 9, Supplementary Table 22). For this subset of loci, the identified cis-encoded transcription factor is likely to be directly responsible for the respective trans-methylation signature. In contrast, at the remaining 41 loci, the genes in cis to the sentinel SNP do not encode the transcription factor overlapping the trans-CpG sites (Supplementary Table 23). We hypothesised that the causal gene in cis at these trans-acting genetic loci may either encode a previously unreported transcription factor, co-factor, or interacting protein influencing nuclear regulatory pathways. To identify the most likely candidate gene and accompanying molecular pathway for these loci, we integrate the comprehensive SNP-methylation (meQTL), SNP-expression (eQTL) and methylation-expression (eQTM) data generated in our study, with publicly available protein-protein interaction networks and transcription factor binding maps, using an approach based on random walks (see Methods). Our approach identifies strong candidate genes and their corresponding molecular networks at 19 loci (Figure 3; Supplementary Tables 24 and 25; Extended Data Figure 9; Supplementary Figure 3). In addition, we prioritise 6 candidate genes for the remaining loci, which were unambiguous cis-eQTLs for only a single gene (see Methods). To corroborate the candidate genes identified in cis at these 25 genetic loci, we quantified the number of trans-eQTMs associated with expression for each of the candidate genes. We observed significantly more trans-eQTMs compared to the remaining genes encoded at the trans-acting loci (P=4.5x10-6, Wilcoxon test, Supplementary Figure 4).
The NFKBIE locus
To illustrate the results of our approach, we highlight SNP rs730775, which is associated with 49 CpG sites in trans (Figure 4). NFKBIE (NFKB inhibitor epsilon, empirical P<0.01; Supplementary Table 24) is the most likely trans-acting gene for this locus. The SNP is located in the first intron of NFKBIE and is a cis-eQTL for NFKBIE in whole blood (eQTLGen P=1.2x10-23). NFKBIE directly inhibits NFKB1 activity and is significantly co-expressed (P=2.2x10-4) with NFKB1, which directly binds at 31 of the 49 trans-associated CpG sites (OR=7.8, P=9.1x10-7, Supplementary Table 23). The trans-CpG sites localise to genes of the NFKB pathway such as IKBE and TRAF6, and are enriched for the GO term ‘regulation of interleukin-6 (IL-6) biosynthetic process’ (GO:0045408; P=3.75x10-5; hypergeometric test). The NFKBIE locus is associated with rheumatoid arthritis (RA),43 which is characterised by IL-6 mediated autoimmunity and can be treated with IL-6 targeting drugs.44,45 We performed a colocalisation analysis of molecular QTL and GWAS using enloc.46 On average the posterior colocalisation probability was 70% at the sentinel SNP rs730775 (Figure 4a), supporting a shared causal variant for the majority of the CpG sites. Our results suggest genetic variation at the NFKBIE locus is linked to rheumatoid arthritis through trans-acting regulation of DNA methylation by NFKB.
Figure 4. Regulatory networks and locus colocalisation analyses.
Panels 4A through 4D show the identified random walk networks and results for the individual colocalisation analyses for the NFKBIE, MGA, COMMD7 and SENP7 loci, respectively. The networks illustrate the connections between the genotype at SNPs (yellow rectangle), the identified candidate genes (yellow ellipse), which are connected through a network of protein-protein and protein-DNA interactions to methylation at the trans-associated CpG sites (beige rectangles), and the expression of genes encoded at the CpG sites. Ellipses represent genes: i. encoded at the genetic locus identified by the sentinel and prioritised by the random walk (yellow fill), ii. encoded at the CpG loci (beige border) or iii. part of the protein-protein interaction network (black border). For genes in the protein-protein interaction network, the fill colour of ellipses represents the random walk score as indicated in the colour bar legend. Edges connecting genes, SNPs and CpG sites represent: i. protein-protein interactions, ii. protein-DNA interactions identified by TFBS overlap and iii. genomic proximity (<1Mb). Bold edges indicate significant correlation with gene expression. Other plots show the i. GWAS signal (-log10(P)) and ii. colocalisation signal (mean per-SNP colocalisation probability (mean SCP) over all trans CpGs) on the y-axis for available SNPs in the genomic region around the respective genetic loci (x-axis). Colouring of individual SNPs indicates LD (R^2) to the lead SNP in the locus.
The MGA, COMMD7 and SENP7 loci
The trans-CpG sites linked to rs17677199 overlap the binding sites of three transcription factors encoded at other loci: MAX (MYC associated factor X), E2F6 (E2F Transcription Factor 6) and NFYB (Nuclear transcription factor Y subunit beta) (Figure 4b). SNP rs17677199 lies in cis to MGA, a known interacting protein for MAX, and MGA, MAX and E2F6 expression shows strong co-variation. MGA is thus a strong candidate linking rs17677199 with disturbances in MAX and E2F6 binding. SNP rs17677199 is associated with raised blood pressure, aortic aneurysms and subarachnoid haemorrhage. Both MAX and E2F6 are compelling candidates for mediating the effects of rs17677199 on DNA methylation, and vascular disease. Mutations in MAX are associated with abnormalities of blood pressure regulation, including development of phaeochomocytoma, a catecholamine secreting tumour.47 In addition, the E2F family of transcription factors is implicated in vascular function and blood pressure regulation.48 E2F transcription factors regulate synthesis of DHFR (Dihydrofolate Reductase), the rate-limiting salvage enzyme for tetrahydrobiopterin, an essential cofactor for endothelial nitric oxide synthase. Colocalisation analysis with fastenloc supports a shared causal variant underlying DNA methylation of trans-meQTL CpG sites and diastolic blood pressure (Figure 4b).
SNP rs6141779 is associated with 10 trans-CpG sites. The only gene at this locus is COMMD7 (COMM Domain Containing 7), which is also an eQTL for the sentinel SNP, and thus a highly plausible cis-candidate gene. Our pathway analysis links COMMD7 to NKFB1 through covariation in expression (Figure 4c). COMMD7 interacts with the NF-kappa-B complex and suppresses its transcriptional activity.49 Sentinel SNP rs6141779 is strongly associated with white cell subset composition.50 Colocalisation analysis supports multiple shared causal variants for basophil counts, and DNA methylation with average posterior probabilities over CpG sites ranging from 7%-66% (Figure 4c).
We also replicate and extend results for the known trans-acting locus SENP7,18,23 identified by SNP rs9859077 (Figure 4d). Our pathway and colocalisation analyses provide new insights into the molecular mechanism linking SENP7 with trans-regulation of both DNA methylation and gene expression on chromosome 19, and to the body composition, leucocyte traits and inflammatory diseases linked to this locus.51
Experimental validation at the ZNF333 locus
At the genetic locus identified by rs6511961, the putative candidate gene is ZNF333 (Supplementary Table 24).52 Expression of ZNF333 in our participants is associated with rs6511961, and covaries with expression of TAL1 and CDK9, genes known to encode for nuclear transcription factors (Extended Data Figure 10). SMR supports a causal relationship between cis-expression of ZNF333, and trans-methylation, with colocalisation analyses providing some evidence for rs6511961 as a common underlying genetic driver (coloc PP4: 0.27).
To further test the hypothesis that ZNF333 contributes to the relationship of rs6511961 with its trans-CpG signature, we carried out ChIP-seq using FLAG/Myc-tagged ZNF333 constructs. ChIP-seq confirmed site-specific DNA binding (Figure 5, Extended Data Figure 10 and Source Data Figure 1). The putative binding motif for ZNF333 is TG[AG]*TCA. The binding sites for ZNF333 are enriched for motifs of known transcription factors (P<10-700), supporting the view that ZNF333 binds sites involved in genome regulation. Furthermore, we find that 35% of the CpGs associated with rs6511961 in trans are in or near (<500bp) ZNF333 DNA binding sites (FET P<0.05, Figure 5). Immunoprecipitation mass-spectrometry (IP-MS, Supplementary Note; Supplementary Tables 26 to 28) experiments provided further experimental evidence to support the hypothesis that ZNF333 encodes a DNA binding protein that determines, at least in part, the trans-CpG signature of rs6511961.
Figure 5. Experimental evaluation of ZNF333 by ChIP-seq.
5a. Regional plot illustrating the overlap of the trans-CpG signature for SNP rs6511961, with the ChIP-seq signature for ZNF333. Upper panel shows the -log10(P-value) (y-axis) of the association of each CpG site in the region (genomic position on the x-axis) to the trans-acting SNP rs6511961. The lead CpG associated with rs6511961 is identified by a diamond; colour coding of other CpGs at locus (circles) describes their correlation (r) with the lead CpG. The middle panel shows genomic coordinates of binding sites of ZNF333 identified by ChIP-seq as purple boxes. The lower panel shows the gene annotation (exons: blue boxes, introns: blue lines). 5b. Venn diagram showing the overlap between binding sites from biological replicates of ZNF333 ChIP-seq using either FLAG or Myc antibodies. 5c. Circos plot summarising i. the genomic distribution of CpGs associated in trans [inner connections] with rs6511961 at the ZNF333 locus, and ii. the DNA binding sites of ZNF333 identified by ChIP-seq studies (green bars). 5d. The observed and expected proportions of CpG sites that overlap ZNF333 DNA binding sites (interval size around peak of 500bp), compared to the background frequency of all tested CpG sites. Significant enrichment is shown by permutation testing with matched background (see Methods). Enrichment is robust to selection of interval size around the peak: from 100bp (2.7 fold) to 1000bp (4.5 fold).
Population specific effects at meQTLs
Amongst our 11.2M meQTLs, 1,354,623 (12%) showed evidence for an interaction with ancestry at P<4.5x10-9 (i.e. P<0.05 after Bonferroni correction for 11.2M tests). Identified SNPs are enriched for blood composition, immune and cardiometabolic traits compared to background expectations (Supplementary Table 29 and 30, and Extended Data Figure 7). Our results are in line with findings that genetic loci associated with blood cell counts display substantial heterogeneity between populations, and that gene regulatory programs in immune cells are subject to recent population specific adaptation.53,54
Interaction analysis of meQTLs with environmental context
As a final experiment, we re-examined the relationship of SNP with CpG in the cosmopolitan set of meQTLs, seeking evidence for an interaction with white blood cell composition, body mass index or cigarette smoking (see Methods), as examples of biological traits that are anticipated or previously reported to have a strong relationship with DNA methylation.8,55–59 We found that, 130,016 (~1.1%) of our 11.2M meQTLs showed evidence for an interaction with one or more of the phenotypes tested (at a Bonferroni-corrected threshold of P<4.5x10-9, Supplementary Table 31). White cell subsets generated the highest number of interaction-meQTLs (‘iQTLs’), and these showed evidence for replication between Europeans and South Asians (Figure 6a). In contrast, there was little evidence for an effect of body mass index or smoking on the genetic regulation of methylation in blood cells.
Figure 6. White-cell iQTLs.
6a. Plot shows replication of effect sizes of significant iQTL (CD8T) between KORA and LOLIPOP cohorts. Axes indicate genotype:celltype interaction effect sizes, points show individual associations. 6b. Barplots indicate replication of iQTL in isolated cells. Y-axis shows the total number of associations and x-axis the respective cell-types. Dark blue areas indicate the proportion of replicating associations, light blue areas the proportion of non-replicating associations. 6c. ‘Volcano’ plots highlighting the enrichment of iQTL SNPs with GWAS information in diverse traits. Y-axis shows -log10 of the QTLenrich P-value, x-axis shows the log2 fold enrichment of observed GWAS SNP among iQTL compared to expected. Plots are split by analysed cell types. Points reflect individual GWAS studies, their colours the respective phenotype category. 6d. An example association plot for the rs174548-cg21709803 iQTL in KORA data, separated into individuals with ‘high’ and ‘low’ abundance (above and below median, respectively) of CD8T cells. Y-axis indicates methylation residuals, x-axis genotypes. Boxplots indicate medians (center lines), first and third quartiles (lower and upper box limits, respectively; whisker extents: 1.5-fold interquartile ranges). Points indicate outliers. 6e. Same association plot as in 6D, but using data from isolated cells (indicated by different shades of grey). 6f. Manhattan plot of meQTL, asthma GWAS and iQTL results for the selected iQTL example show colocalisation of association signals. X-axis indicates the genomic region around the rs174548 SNP, y-axis the -log10 of association P-values. Individual points represent SNPs in the locus.
Significant interactions with blood cell proportions can be indicative of meQTLs with stronger or weaker effects in specific cell types.60 Cell type specificity of iQTLs is supported by the high replication rates of iQTLs in isolated CD4 and CD8T-cells (Figure 6b). We expand our iQTL analysis from cosmopolitan meQTL to a genome-wide cis-iQTL analysis and discover a total of 16,135 iQTLs (P<8.8x10-11; Supplementary Table 31), of which 64% are independent of cosmopolitan meQTLs (LD R2<0.2). The presence of an iQTL indicates that the relationship between methylation levels and genotype varies depending on the abundance of a specific cell type. SNPs which are part of white cell iQTLs are enriched for association with phenotypic variation in GWA studies (number of phenotypes enriched at FDR<0.05 in QTLEnrich analysis: CD4T, N=18; CD4T, N=11; monocytes, N=23; Supplementary Table 32), including blood cell traits, immune traits and allergies (Figure 6c). We show that rs174548 in the FADS1 gene shows increased correlation with DNA methylation in subjects with high abundance of CD8T cells (Figure 6d and Figure 6e). FADS1 is a key enzyme in the metabolism of fatty acids. SNP rs174548 is strongly associated with concentrations of arachidonic acid and other metabolites fatty acid metabolism,61,62 blood eosinophil counts,50 and inflammatory diseases such as asthma (GWAS P = 2.5x10-10).63 Colocalisation analysis indicates a shared causal variant for rs174548 and asthma (coloc PP4=0.63, Figure 6f), providing a pathway linking fatty acid metabolism in CD8T cells with immune phenotypes. This SNP is not detected as a cosmopolitan meQTL, highlighting the potential for iQTL analysis to improve annotation of functional genetic variants, and to generate hypotheses about the cellular specificity of traits.
Discussion
We identify 11.2 million unique SNP-CpG associations in peripheral blood, including 467,915 meQTL associations that operate in trans and that comprise pairwise relationships between 1,847 genetic loci and 3,020 methylation loci. Key strengths of our study design, include use of stringent statistical thresholds, and replication testing across population groups and tissues, to enable identification of high-confidence generalisable meQTLs. Both the SNPs and CpGs that form meQTL pairs are enriched for multiple functionally relevant characteristics, including shared chromatin state, HiC interaction, association with cis and trans gene expression, and links to multiple metabolic and clinical traits. Candidate genes at trans-acting genetic loci are enriched for nuclear transcription factors and their interacting proteins. Molecular interaction data, supported by colocalisation analyses, identify multiple nuclear regulatory pathways, linking sequence variation to disturbances in DNA methylation, molecular and phenotypic variation. This includes the UBASH3B (body mass index), NFKBIE (rheumatoid arthritis), MGA (blood pressure), and COMMD7 (white cell counts). As proof of principle, we use ChIP-seq to provide experimental support for ZNF333 as a novel trans-acting genomic regulator. Finally, we use interaction analyses to identify both population and cell-lineage specific meQTL effects that are biologically relevant. This includes meQTL SNP rs174548 in FADS1, with strongest effect in CD8T cells, linking fatty acid metabolism with immune dysregulation and asthma. Our study thus advances understanding of the relationships between DNA sequence variation and DNA methylation, thereby providing new insights into the molecular networks involved in nuclear regulation, and the potential pathways linking genetic variation to human phenotype.
To move beyond investigation of cosmopolitan regulatory effects in mixed-cellular populations, we extended our analyses to identify cell-lineage and population specific processes. White-cell subset interaction analyses revealed meQTLs with stronger or weaker effects in specific cell types. We identified many thousands of white cell specific iQTLs, which were strongly supported by high replication rates in isolated CD4 and CD8T cells. SNPs that are part of white cell iQTLs are enriched for association with phenotypic variation in GWA studies, notably blood cell traits, immune traits and allergies. We highlight the iQTL SNP rs174548 in the FADS1 gene, which shows increased correlation with methylation in CD8+ T cells. FADS1 plays a key role in fatty acid metabolism, and genetic variation at this locus is well known to be a determinant of concentrations for arachidonic acid, eicosanoids and blood lipid levels.61,62 Our iQTL analysis suggests that genetic variation at FADS1 has a specific impact on regulation of FADS1 in CD8+ T cells, and may help explain the relationship of this locus with inflammatory diseases such as asthma.63 CD8+ T cells contribute to the development of asthma, including recruitment to pulmonary sites, and secretion of the pro-inflammatory cytokines IL-13 and Il-4.64 People with asthma have increased cytokine release by CD8+ T cells, and cytokine activity is related to asthma severity.65 Our interaction analyses of meQTL data thus shed new light on the mechanisms impacting DNA methylation in white blood cells, an approach that may enable identification of cell-specific patterns of DNA regulation in other studies of tissues samples with mixed cellular composition.60
Our study provides new insights into the genetic regulation of DNA methylation, and reveals multiple novel nuclear regulatory networks. Our findings advance understanding of the biological pathways underpinning phenotypic variation, and will inform hypothesis driven experimental studies to define the specific molecular mechanisms involved.
Methods
Further details of experimental Methods and data analyses are provided in the Supplementary Note.
Discovery and replication of genetic variants influencing DNA methylation
A summary of the participating population cohorts is provided in Supplementary Tables 33 and 34. Genome wide association was carried out in Europeans and South Asians separately.24 First, methylation residuals were derived from a linear regression of the percentage methylation (outcome) with technical and clinical predictors: age, gender, estimates of white-blood cell subpopulations and principal components of control-probe intensities (Supplementary Table 34). Association testing of methylation residuals with genotypes was carried out using Quicktest. Genome-wide significance was set to P<10-14, which corresponds to P<0.05 after Bonferroni correction for the ~4.3 trillion statistical tests performed, a choice consistent with other recent publications.19,20 Replication testing was done using linear regression in R, and combined analysis of discovery and replication data by inverse-variance meta-analysis (R package meta). Associations were considered replicated when the association showed consistent direction of effect between discovery and replication, a replication P<0.05 and a combined P<10-14. We assessed our meQTLs for enrichment with SNPs known to influence white blood cell count, to test for confounding by variation in white cell subsets (Supplementary Table 35).
Replication across-platforms and cell types
We used DNA methylome data to carry out cross platform replication of meQTLs, with permutation testing to establish whether the overlaps observed were more than expected by chance.25 Replication across tissues was initially tested using genomic DNA from i. isolated white cell subsets (N=60 individuals), ii. isolated visceral adipocytes (N=48 individuals), and iii. isolated subcutaneous adipocytes (N=48 individuals). Genome-wide genotyping (Illumina OmniExpress) and quantification of DNA methylation (Illumina EPIC array) was done according to manufacturer’s recommended protocols. Imputation of unmeasured genotypes was done using the reference panel from the 1000 Genomes project Phase 3. We tested the associations between SNPs and CpGs using linear regression. We additionally carried out replication testing in 603 subcutaneous adipose tissue samples collected in the MuTHER study. Methylation profiling was performed using the Illumina Infinium HumanMethylation450 BeadChip. Genotyping was done with a combination of Illumina arrays (HumanHap300, Human- Hap610Q, 1M-Duo, and 1.2MDuo 1M). Associations between SNPs and DNA methylation levels were tested in samples of related individuals using GEMMA software.66
Conditional analysis and linkage disequilibrium pruning
Local correlations between SNPs (LD) and between neighbouring CpG sites lead to redundant pairs of SNPs and CpG sites representing the same meQTL. We used a two-stage approach to identify independent associations among all identified SNP-CpG pairs (Supplementary Figure 1). We first performed iterative conditional analysis using individual level data from the European and South Asian discovery datasets. For each CpG the most strongly associated SNP (lowest P) was selected. Association testing was then repeated for all SNPs that had previously been associated at P<10-14 with that CpG, but including the most strongly associated SNP as a predictor in the regression model. Analysis was carried out in Europeans and South Asians separately, followed by meta-analysis. From the SNPs that remained significantly associated (P<10-14), the most strongly associated SNP was selected and the process repeated until no SNPs remained. Independently associated SNPs for the respective CpG were then carried forward. This yielded a parsimonious set of 84,456 SNPs independently associated with one or more CpG sites (Supplementary Table 8).
Whilst this step reduces redundancy introduced by LD between SNPs, it creates a scenario where the same genetic locus can be represented by different SNPs. This is caused by the fact that the most strongly associated SNP for each genetic locus (i.e. the SNP conditioned on) will vary from one CpG to another. To further reduce the impact of local correlation (Supplementary Figure 1) we combined highly correlated SNPs into SNP loci, and highly correlated CpGs into methylation loci. To achieve this, the most strongly associated marker (lowest P) was selected and all markers with R2>0.2 and distance<1Mb were then assigned to a corresponding locus. Of the remaining markers, the most strongly associated marker was again chosen and the process was repeated until no markers remained. This approach was applied to SNPs and CpGs within each category (cis, long-range cis, trans) separately. Supplementary Figure 5 shows a sensitivity analysis on the number of independent loci for varying R2 thresholds.
Enrichment of meQTLs within chromatin states
We obtained chromatin state annotations (15 state model) defined by chromHMM segmentation of histone modification ChIP-seq data,67 from the Roadmap Epigenomics Project for primary blood cells.68 Since we were working with whole blood, we combined these primary epigenomes into a weighted epigenome annotation based on estimated cell fractions in whole blood (Supplementary Note and Supplementary Table 36). We used permutation testing to assess for enrichment compared to expectations under the null hypothesis.
Genetic variants influencing gene expression in Europeans and South Asians
Transcriptome-wide measurements of gene expression in blood along with measurements of DNA methylation from the same blood sample are available for European (N=853) and South Asian (N=693) participants of the KORA and LOLIPOP studies (Illumina HumanHT-12 v3 and 450K methylation arrays respectively). These data enable evaluation of relationships between SNP, methylation and gene expression using individual level data, in relevant populations, and with a range of statistical models to allow for sensitivity analyses and investigation of potential confounding effects. Expression values were summarised to gene level estimates by averaging the log2 transformed expression levels of probes mapping to the same gene. To quantify the relationship between genetic variation and gene expression we first derived residuals for gene expression using linear regression of gene expression levels against sex, age, RNA integrity number, RNA amplification plate (KORA) / RNA conversion batch (LOLIPOP) and sample storage time (KORA) / RNA extraction batch (LOLIPOP). Expression residuals were then used as outcome variables in a linear regression model with SNP dosage as the independent variable, corresponding to the following linear model formulae: 1) Gene ~ SNP + sex + age + RIN + RNA_Ampli_Plate + Storage_Time (KORA) and 2) Gene ~ SNP + sex + age + RIN + RNA_Conv_Batch + RNA_Extract_Batch (LOLIPOP). Data analysis was performed using MatrixEQTL.69 and results analysed separately for Europeans and South Asians. We then combined results between Europeans and South Asians using inverse-variance meta-analysis. Statistical significance was inferred at P= 7.98×10-11 (P<0.05 after Bonferroni correction for the number of SNP-expression pairs tested). We supplemented results from our participants (‘KORA-LOLIPOP eQTL dataset’) with eQTL results from publicly available resources (GTEx and eQTLGen).70,71 The specific datasets used for each experiment are documented.
SNPs influencing DNA methylation are enriched for association with gene expression
To confirm that SNPs influencing methylation are more likely to affect gene expression, we randomly selected 100 sets comprising 1,000 SNPs ‘observed’ to be associated with DNA methylation from the list of SNP-CpG associations after pruning. For each ‘observed’ set, we generated a ‘background’ set of SNPs to quantify expectations under the null hypothesis. Each set of ‘background’ SNPs comprised 1000 SNPs that are i. not part of a significantly associated SNP-CpG pair, and ii. matched with the ‘observed’ SNPs on minor allele frequency (±2%) and distance to the nearest gene (±10kb), but selected otherwise at random. We then determined the proportion of SNPs associated with gene expression in 100 ‘observed’ sets and 100 ‘background’ sets. Association of observed and background SNPs with gene expression was tested in our KORA-LOLIPOP eQTL dataset (statistical significance was inferred at P= 5.06×10-11, as above). The probability of enrichment was calculated by comparison of ‘observed’ sets with ‘background’ sets using a t-test.
Association of DNA methylation with gene expression
We quantified the associations of DNA methylation with gene expression using our KORA-LOLIPOP gene expression dataset (Europeans: N=853; South Asians: N=693). To test for and exclude CpG-gene pairs that arise due to confounding by underlying genetic background, we derived methylation residuals by correcting methylation (beta) values for the sentinel SNP(s) associated with the corresponding CpG (formula: CpG ~ Σ SNPsassociated). Gene expression residuals were used as outcome variables in a regression model with methylation residuals as the independent variable (formula: Generesiduals ~ CpGresiduals). Data analysis was performed using MatrixeQTL69 and results analysed in Europeans and South Asians separately. At Bonferroni corrected P-value thresholds, there was a high degree of reproducibility for eQTM results between the populations (Supplementary Table 37). We therefore combined results between Europeans and South Asians using inverse-variance meta-analysis (R-package meta). Statistical significance was inferred at P=8.7x10-12 (P<0.05 after Bonferroni correction for all possible CpG-expression pairs). We carried out association tests with/without adjustment of the methylation residuals for white cell subsets (i.e. with/without Houseman white cell subset estimates, formula: CpGresiduals ~ CD8T + CD4T + NK + Bcell + Mono), to test for confounding by cell subset composition (Supplementary Table 38).
In addition, we compared the proportion of putative cis-eQTMs from analyses with and without correction for white cell subsets that were supported by Summary-data-based Mendelian Randomisation (SMR). SMR tests for association of an exposure with an outcome using summary-level data from GWAS and other QTL studies, and using a genetic variant as the instrumental variable to avoid non-genetic confounding.26 Coloc analysis was subsequently performed for loci with a potentially causal relationship between DNA methylation levels and gene expression in cis (PP4>0.6).46
Enrichment of meQTL SNPs and CpGs for association with phenotypes
We performed enrichment analyses of meQTL and iQTL SNPs for association with clinical traits using QTLEnrich,72 which includes uniformly processed summary statistics of 114 GWAS studies. We tested meQTL SNPs for enrichment as protein-QTLs (pQTLs) and metabolite-QTLs (mQTLs) using the Phenoscanner v2 database.63,73 To evaluate the biological relevance of our sentinel CpGs, we first quantified the association of DNA methylation with 49 clinical traits (physical measures, health status, lifestyle behaviours and biochemical traits), and with concentration of 228 metabolites measured by NMR metabolomics in the LOLIPOP cohort (N=2,866 participants with DNA methylation data available). We used permutation testing to determine expectations under the null hypothesis (see Supplementary Note and Supplementary Table 39).
Identification of cis-eQTLs influencing CpGs in trans
We used SMR analysis to assess whether the proximal candidate gene at a trans-acting genetic locus shows covariation with the trans-methylation signature (triangulation of cis-eQTL, trans-meQTL and trans-eQTM data). Results for cis SNP-expression (cis-eQTL) associations were obtained from eQTLgen,71 while trans SNP-methylation (trans-meQTL) and SNP-expression (trans-eQTM) associations were as reported in the current study. We started with trans sentinel meQTL SNPs reported in our current study, and identified significant cis eQTL associations at a Bonferroni corrected threshold. For loci whereby SMR estimates suggest a potential causal relationship between cis gene expression and trans methylation levels (P<0.05 after Bonferroni correction), this was followed up with coloc analysis (PP4>0.6). In addition, we also evaluated the complementary model whereby the causal inference analysis started with observed trans-eQTMs and assessed the proportion that was correctly inferred by SMR.
Enrichment of trans-CpGs in Transcription Factor Binding Sites
We obtained transcription factor binding sites (TFBS) for 145 distinct DNA binding proteins from 246 ChIP-seq experiments performed on blood related cell lines (Supplementary Table 20). Data were uniformly processed by the remap resource.74 We defined a CpG site to be bound if a binding site was located within a window of 100 bp (50 bp in each direction; see Supplementary Figure 6). To examine the relationship between the trans-CpG signatures of the sentinel SNPs and the TFBS of DNA binding proteins, we first determined the minimum number of trans-CpGs associated with a sentinel SNP needed for detection of enrichment in TFBS. This number depends on whether the smallest achievable P-value in the Fisher test is less than an adjusted significance level padj. (see Supplementary Note). Based on this analysis, we tested each of the 115 sentinel SNPs with ≥5 associated trans-CpGs, for over- or underrepresentation in the TFBS for each of the 246 ChIP-seq datasets for DNA binding proteins. For each sentinel SNP, we resampled 10,000 sets of CpG sites of equal size, to compute empirical P-values for the overlap of the observed trans-CpG sites with TFBS. We carried out similar analyses using the MethylEpic array to validate our findings.
Random walk analysis
We set out to identify the most likely trans-acting gene for each locus with at least five trans-acting SNP-CpG pairs overlapping a transcription factor binding site, by linking the genes in the locus to the associated CpG sites through a sequence of protein-protein interactions (PPI) and protein-DNA interaction. We used PPIs that had experimental evidence or database information available in STRING.75 The initial network comprised 12,769 proteins and 186,674 interactions. In addition, we restricted the network to 8,880 proteins that were expressed (median reads per kilobase per million sequenced (RPKM) > 0.1) in whole blood in the GTEx dataset,70 and further to the largest connected component of the network comprising 8,668 proteins and 99,143 interactions used for the analysis. Formally, we defined the PPI network P = (VP, EP), where VP is the set of nodes (or vertices) corresponding to proteins and EP is the set of undirected edges corresponding to interactions between proteins. Similarly, we represent protein-DNA interactions as graph D = (VD, ED), where VD is the union of 145 proteins for which ChIP-seq data was available (see above) and the CpG sites that were within 50 bp of sites bound by these proteins.
For each locus, we identified the set of candidate genes C as all genes encoded at the SNP locus that are part of the PPI network. Locus regions were defined based on the results of the pruning analysis that identified sentinel SNPs. Specifically, we identified all trans-associated CpG sites that were assigned to the same sentinel SNP. For these trans-CpG sites we obtained all SNPs that are i. associated with the CpG in the complete, cosmopolitan pairwise analysis of SNP-CpG associations, and ii. located in cis (within 1 Mb) of the sentinel SNP. In this way, the trans-acting loci are refined by patterns of LD and observed associations with methylation levels, but are not larger than 1 Mb.
Next, we identified the set of CpG sites S that were associated with the respective sentinel SNP at the trans-acting genetic locus. We added the CpG sites S and their protein-DNA interactions ED(S) to the PPI graph P to form the locus graph G = (VP+S, EP+ ED(S)). Finally, we used the topology of the locus graph G to rank candidate genes C. The ranking is based on random walks and is conceptually similar to published studies.111,112 We represent graphs (V, E) by their adjacency matrix A = (aij) with entries aij = 1 if (i, j) in E and 0 otherwise. We defined the symmetric transition matrix T = (tij) with tij = aij / sqrt(d(i) d(j)), where d(i) is the degree of node i, specifying the probability to move from node i to j in one step of the random walk.76 Consequently, transition probability matrices for paths with t steps can be computed as Tt. We initiated random walks at the CpG sites S and computed the transition probability Ttsc to start at CpG site s in S and reach candidate gene c in C in t steps. Since the lengths of the paths t are not known a priori, we sum the transition probabilities over all possible path lengths t = (0, …, ∞). The random walk has a stationary state with a distribution that is defined by the degree distribution of the nodes, which corresponds to the first eigenvector ψ0 of the transition matrix T with eigenvalue λ0 = 1.76 We are not interested in this stationary state, so we remove the contribution of the first eigenvector from the transition matrix and compute the aggregated transition probability matrix . This infinite sum has a closed form solution,77 however, the resulting matrix M is not sparse and therefore the computation is very memory intensive. Alternatively, the solution can be approximated using spectral decomposition of the transition matrix:77
To compute the ranking of candidate genes, while saving memory, we approximated the aggregated transition matrix M using the first n=500 eigenvectors and stored only the submatrix of M that holds the transitions from CpG sites s in S and candidate genes c in C. The final ranking of candidate gene c was computed as the average aggregated transition probability over all CpG sites pc = 1 / |S| ΣsMsc. To assess whether the score pc of a candidate gene was significantly higher than expected by chance, we performed the same analysis on B > 100 randomised graphs and computed scores pbc for all genes in C to determine the empirical P-values for the maximum score at each locus P(pc) = 1 / B Σb δ(pc > maxC pbc). Randomised graphs were constructed by randomly sampling the same number of |S| CpG sites Sb with matched mean and standard deviation of methylation levels (see TFBS analysis). The random CpG sites Sb were then added to the PPI graph P to form the background locus graph Gb = (VP+Sb, EP+ ED(Sb)). This way we empirically assess the probability of ranking scores as extreme as the one observed, by transitioning from a random set of CpGs through the original PPI and ChIP-seq graph to each of the candidate genes. For each locus the set of significant candidate genes was defined as C* =[c | P(pc) < 0.05].
To visualise the results of the random walk analysis we first defined weights wi for each node i of the locus graph G by the sum of the random walk score to transition from the CpG sites in S to node i and of the random walk score for transitioning from i to the selected candidate genes in C* in the trans locus. These weights were normalised and inverted to w*i = maxi (wi) - wi, such that highest scoring nodes receive the lowest weights. These weights w* were then used to determine the minimal weight paths from each of the CpG sites in S to the candidate genes in C* in the trans locus, thus representing paths through nodes with high random walk scores. Nodes on these minimal weight paths were recorded in the set Q. For each locus we defined the candidate pathway Gc as the subgraph of the locus graph G with the nodes defined by the union of C*, Q, S and all edges of G between this subset of nodes.
Identification of candidate genes for sentinel SNPs at trans-acting genetic loci
We combined all available information from transcription factor signatures, PPI random walks and eQTL results (nominal P<0.01 in our data or in GTEx whole blood data) to select candidate gene(s) responsible for the effect of the sentinel SNPs on DNA methylation in trans. We evaluated random walk based candidate predictions using gene ontology enrichment analysis and overlap with eQTL results (Supplementary Figure 7). We observed that a definition of SNP locus based on the association results (LD regions) yielded a higher proportion of candidates annotated to GO terms for regulators such as “regulation of biological process”, “DNA binding” and “regulation of transcription, DNA−templated”. We separately noted that the number of candidates with cis-eQTL was higher for the analysis in which only PPIs between genes expressed in whole blood were considered. Therefore, we used PPIs of genes expressed in whole blood, and the LD based definition of trans loci, to identify candidates by random walk analysis. We established the following order of evidence for prioritisation: i. Transcription factors encoded at the trans-acting genetic locus that are enriched for binding at the associated CpG sites and that are a cis-eQTL for the sentinel SNP; ii. Transcription factors encoded at the trans-acting locus that are enriched for binding at the associated CpG sites, but that do not have an eQTL with the sentinel SNP; iii. Candidates that were identified through the random walk analysis (empirical P<0.05) and have a cis-eQTL; iv. Random walk candidates without a cis-eQTL; v. Singular cis-eQTLs at the trans-acting genetic locus, without other evidence.
Integrated network analysis
To set the results of the random walk analysis into context, we integrated the candidate pathways defined above for each trans-acting genetic locus with genotype, gene expression and methylation data for Europeans (KORA) and South Asians (LOLIPOP). Hence, we collected for both cohorts the 1) genotype data for the sentinel SNP 2) methylation beta residuals (see Methylation Data above) for all CpG sites associated in trans and 3) gene transcript expression residuals (see Gene Expression Data above) for all genes within a 1Mb window of the respective SNP and CpG sites as well as the genes utilised in the random walk analysis. Genetic variation in cis could also influence expression and methylation measurements. To avoid confounding by cis effects, we therefore adjusted expression and methylation data for previously reported cis-eQTLs,78 and for cis-acting SNPs identified in our study, using a linear regression model (i.e. getting residuals 1) for genes using: GeneA ~ GeneA + eQTL_SNP1 + eQTL_SNP2 + … + eQTL_SNPi) and 2) for CpGs using: CpGA ~ CpGA + meQTL_SNP1 + meQTL_SNP2 + … + meQTL_SNPi). The residuals were used to test for association individually in each cohort and subsequently combined using fixed effects meta-analysis. Resulting P-values were adjusted for multiple testing using the Benjamini-Hochberg method.79 In the resulting network, vertices represent variables (genotype, gene expression and methylation) and edges represent significant correlation between these variables (FDR < 0.05). Correlation edges found between a CpG and a CpG-gene (i. e. a gene found within the 1Mb window around the CpG) were added to the candidate pathway graph (see Random walk analysis) for each locus.
Colocalisation analysis of trans meQTL
Colocalisation analysis of trans meQTL and GWAS was performed using fastenloc,46 a Bayesian method to determine the probability of a shared causal variant for a pair of molecular (meQTL) and physiological (GWAS) traits. First, we used Phenoscanner v263,73 and the GWAS catalog,80 to select GWAS traits and studies of interest for each locus. We obtained GWAS summary statistics for each trait of interest for the region (+/- 500 kb) around the sentinel SNP (Supplementary Table 40). Fastenloc was used to determine SNP level posterior colocalisation probabilities for molecular and physiological traits for all CpG site associated with the same locus in trans. We summarised the colocalisation probabilities across all trans CpG sites using the average SNP level posterior colocalisation probabilities.
ChIP-seq validation of ZNF333 binding at the identified DNA methylation sites
Plasmid overexpressing dual-tagged (Myc and FLAG) human ZNF333 transcript (RC216457) was purchased from OriGene Technologies. ZNF333 and control GFP plasmid (pmax-GFP, Lonza) were transfected into HCT116 cells with JetPrime transfection reagent (Polyplus) according to manufacturer’s instructions in 15-cm tissue culture dishes. Culture media was refreshed after 24h and cells maintained for another 24h. At 48h cell lysates were used for ChIP-seq. Western blot using Myc and FLAG antibodies was also performed to confirm high ZNF333 expression abundance. Raw sequencing from ChIP-seq experiments were mapped using BWA. The overlap between ZNF333 ChIP-seq peaks (union of Myc and FLAG) and rs6511961 target CpGs (in trans) was calculated using a window size of 500 bp. Statistical significance was calculated based on permutation testing.
Interaction analysis of meQTLs with their environmental context
We ran interaction analyses for the cosmopolitan SNP-CpG pairs using linear regression models with the methylation beta value as the dependent variable, and an interaction between the SNP and phenotype of interest as the independent variable of interest. The phenotypes of interest examined were: smoking (yes/no), BMI (kg/m2) and estimated proportions of CD8T, CD4T and monocytes. The analyses were run in KORA F4 and LOLIPOP separately. Significant results in one cohort were examined for replication (P<0.05, same direction of effect) in the other cohort. In a second step we repeated the interaction analysis with the covariates age, sex, BMI and white blood cell count for all CpG-SNP pairs in cis using tensorQTL (v1.0.3).81 Statistical significance was inferred at Bonferroni-corrected p-value of 0.05/number of tested pairs. We used GOstats for pathway analysis of the iQTLs (Supplementary Table 41)
Extended Data
Extended Data Figure 1.
Extended Data Figure 2.
Extended Data Figure 3.
Extended Data Figure 4.
Extended Data Figure 5.
Extended Data Figure 6.
Extended Data Figure 7.
Extended Data Figure 8.
Extended Data Figure 9.
Extended Data Figure 10.
Supplementary Material
Acknowledgments
KORA study
The KORA study was initiated and financed by the Helmholtz Zentrum München –German Research Center for Environmental Health, which is funded by the German Federal Ministry of Education and Research (BMBF) and by the State of Bavaria. KORA research was supported within the Munich Center of Health Sciences (MC-Health), Ludwig-Maximilians-Universität, as part of LMUinnovativ. The work was supported by the German Federal Ministry of Education and Research (BMBF) within the framework of the EU Joint Programming Initiative ‘A Healthy Diet for a Healthy Life’ (DIMENSION grant number 01EA1902A). The work was further supported by the Bavarian State Ministry of Health and Care through the research project DigiMed Bayern (www.digimed-bayern.de). The German Diabetes Center (DDZ) is supported by the Ministry of Culture and Science of the State of North Rhine-Westphalia and the German Federal Ministry of Health. This study was supported in part by a grant from the German Federal Ministry of Education and Research to the German Center for Diabetes Research (DZD).
The LOLIPOP study
The LOLIPOP study is supported by the National Institute for Health Research (NIHR) Comprehensive Biomedical Research Centre Imperial College Healthcare NHS Trust, the British Heart Foundation (SP/04/002), the Medical Research Council (G0601966, G0700931), the Wellcome Trust (084723/Z/08/Z), the NIHR (RP-PG-0407-10371), European Union FP7 (EpiMigrant, 279143), European Union Horizon 2020 (iHealth-T2D, 643774). BL is supported by the Imperial College Junior Research Fellowship scheme as well as the Academy of Medical Sciences Springboard award. JCC is also supported by the Singapore NMRC (NMRC/STaR/0028/2017). We thank the participants and research staff who made the study possible.
The NFBC studies
MW was supported by the European Union’s Horizon 2020 research and innovation programme (grant 633212). NFBC1966 received financial support from the Academy of Finland (grants 104781, 120315, 129269, 1114194, 24300796, Center of Excellence in Complex Disease Genetics and SALVE), University Hospital Oulu, Biocenter, University of Oulu, Finland (75617), NHLBI grant 5R01HL087679-02 through the STAMPEED program (1RL1MH083268-01), NIH/NIMH (5R01MH63706:02), ENGAGE project and grant agreement HEALTH-F4-2007-201413, EU FP7 EurHEALTHAgeing -277849, the Medical Research Council, UK (G0500539, G0600705, G1002319, PrevMetSyn/SALVE) and the MRC, Centenary Early Career Award. NFBC1986 received financial support from EU QLG1-CT-2000-01643 (EUROBLCS) Grant E51560, NorFA Grant no. 731, 20056, 30167, USA / NIHH 2000 G DF682 Grant 50945. The NFBC programmes are also funded by the H2020-633595 DynaHEALTH action, academy of Finland EGEA-project (285547) and EU H2020 ALEC project (Grant Agreement 633212), and Exposomic, Genomic and Epigenomic Approach to Prediction of Metabolic and Cardiorespiratory function and Ill-Health (EGEA), Grant no. 285547.
The MuTHER Study
MuTHER was funded by the WT (081917/Z/07/Z). TwinsUK was funded by the WT and European Community’s Seventh Framework Programme (FP7/2007-2013). The study also received support from the National Institute for Health Research (NIHR) Clinical Research Facility at Guy’s & St. Thomas’ and King’s College London. Analysis was funded by British Heart Foundation (BHF) grant RG/14/5/30893 to P.D. and form part of the research themes contributing to the translational research portfolio of Barts Cardiovascular Biomedical Research Unit which is funded by the National Institute for Health Research (NIHR).
The Saguenay Youth Study
The Saguenay Youth Study has been funded by the Canadian Institutes of Health Research (T.P., Z.P.), Heart and Stroke Foundation of Canada (Z.P.) and the Canadian Foundation for Innovation (Z.P.).
We acknowledge Dr. Gabriele Möller and Prof. Dr. Jerzy Adamski (Helmholtz Center Munich), for their support in the IP-MS transfection experiment. We used data generated by the PCHI-C Consortium (PubmedI ID: 27863249), funded by the UK NIHR, Medical Research Council (MR/L007150/1) and Biotechnology and Biological Research Council (BB/J004480/1).
MuTHER consortium
Kourosh R. Ahmadi38, Chrysanthi Ainali39, Amy Barrett40, Veronique Bataille38, Jordana T. Bell38, Alfonso Buil41, Panos Deloukas42, Emmanouil T. Dermitzakis41, Antigone S. Dimas41, Richard Durbin43, Daniel Glass38, Elin Grundberg44, Neelam Hassanali40, Åsa K. Hedman45, Catherine Ingle43, David Knowles46, Maria Krestyaninova47, Cecilia M. Lindgren45, Christopher E. Lowe48,49, Mark I. McCarthy40,45, Eshwar Meduri43, Paola di Meglio50, Josine L. Min42, Stephen B. Montgomery41, Frank O. Nestle50, Alexandra C. Nica41, James Nisbet43, Stephen O’Rahilly48,49, Leopold Parts43, Simon Potter43, Johanna Sandling43, Magdalena Sekowska43, So-Youn Shin43, Kerrin S. Small38, Nicole Soranzo43, Tim D. Spector38, Gabriela Surdulescu38, Mary E. Travers40, Loukia Tsaprouni43, Sophia Tsoka39, Alicja Wilk43, Tsun-Po Yang43, Krina T. Zondervan45.
38. Department of Twin Research and Genetic Epidemiology, King’s College London, London, UK
39. Department of Informatics, School of Natural and Mathematical Sciences, King’s College London, Strand, London, UK
40. Oxford Centre for Diabetes, Endocrinology & Metabolism, University of Oxford, Churchill Hospital, Headington, Oxford, UK
41. Department of Genetic Medicine and Development, University of Geneva Medical School, Geneva, Switzerland
42. William Harvey Research Institute, Queen Mary University of London, London, UK
43. Wellcome Trust Sanger Institute, Hinxton, Cambridge, UK
44. Children's Mercy Hospitals and Clinics, Kansas City, MO, 64108, USA
45. Wellcome Trust Centre for Human Genetics, University of Oxford, Oxford, UK
46. University of Cambridge, Cambridge, UK
47. European Bioinformatics Institute, Hinxton, UK
48. University of Cambridge Metabolic Research Labs, Institute of Metabolic Science Addenbrooke’s Hospital Cambridge, UK
49. Cambridge NIHR Biomedical Research Centre, Addenbrooke’s Hospital, Cambridge, UK
50. St. John's Institute of Dermatology, King's College London, London, UK.
Footnotes
Author contributions
Data collection and analysis in the contributing population studies
KORA: Annette Peters, Brigitte Kühnel, Christian Gieger, Christian Herder, Clemens Baumbach, Eva Reischl, Holger Prokisch, Konstantin Strauch, Liliane Pfeiffer, Melanie Waldenberger, Michael Roden, Rory Wilson, Thomas Illig, Thomas Meitinger, Wolfgang Rathmann
Lolipop: Benjamin C Lehne, James Scott, Jaspal S Kooner, John C Chambers, Weihua Zhang, William R Scott
MuTHER: Eirini Marouli, MuTHER Consortium, Panos Deloukas, Stephane Bourgeois
NFBC: Marjo-Riitta Jarvelin, Matthias Wielscher, Silvain Sebert, Ville Karhunen
SYS: Jean Shin, Manon Bernard, Tomas Paus, Zdenka Pausova
Data collection and molecular follow up analyses
ChIP-seq: Dominic P Lee, Matias I Autio, Roger SY Foo, Wilson LW Tan
ChIP-MS: Stefanie M. Hauck, Juliane Merl-Pham, Pamela Matías-Garcia
Data analysis and writing group (alphabetical order)
John C Chambers, Johann S Hawe, Matthias Heinig, Christian Gieger, Benjamin C Lehne, Marie Loh, Katharina Schmid, Melanie Waldenberger, Rory Wilson
Competing Interests
The authors declare no competing interests.
Data Availability
Summary statistics for the 11.2M SNP-CpG pairs reaching genome-wide significance are available at https://zenodo.org/record/5196216#.YRZ3TfJxeUk. ChIP-seq data for ZNF333 are available through the NCBI SRA (accession code: SRP284104). Raw genotype, methylation and expression data can be made available upon reasonable request by the authors. Controlled data access to data of the KORA cohort can be obtained through https://epi.helmholtz-muenchen.de. Source data are provided with this paper.
The web-links for the publicly available datasets used in the study are as follows:
Phenoscanner v2:
http://www.phenoscanner.medschl.cam.ac.uk
GWAS catalog:
https://www.ebi.ac.uk/gwas/docs/file-downloads
meQTL and eQTM data from Bonder et al 2015:
https://molgenis26.gcc.rug.nl/downloads/biosqtlbrowser/2015_09_02_trans_meQTLsFDR0.05-CpGLevel.txt
https://molgenis26.gcc.rug.nl/downloads/biosqtlbrowser/2015_09_02_cis_eQTMsFDR0.05-CpGLevel.txt
GTEx v6 eQTL results:
eQTLgen cis eQTL results
https://molgenis26.gcc.rug.nl/downloads/eqtlgen/cis-eqtl/cis-eQTLs_full_20180905.txt.gz
TWAShub
http://twas-hub.org/genes/UBASH3B/
GWAS summary statistics of 114 traits for colocalization analysis https://zenodo.org/record/3629742
ChIP-seq binding sites http://hgdownload.cse.ucsc.edu/goldenPath/hg19/encodeDCC/wgEncodeRegTfbsClustered/wgEncodeRegTfbsClusteredWithCellsV3.bed.gz
http://tagc.univ-mrs.fr/remap/download/All/filPeaks_public.bed.gz
ChromHMM states: http://egg2.wustl.edu/roadmap/data/byFileType/chromhmmSegmentations/ChmmModels/coreMarks/jointModel/final/all.mnemonics.bedFiles.tgz
Hi-C data (EGAD00001003106):
https://ega-archive.org/datasets/EGAD00001003106/
Protein - protein interactions:
http://string90.embl.de/newstring_download/protein.links.detailed.v9.0.txt.gz
Code Availability
Code for the analysis is available through GitHub: https://github.com/heiniglab/hawe2021_meQTL_analyses, and also through zenodo DOI: 10.5281/zenodo.5529828.82
References
- 1.Bird A. Perceptions of epigenetics. Nature. 2007;447:396–8. doi: 10.1038/nature05913. [DOI] [PubMed] [Google Scholar]
- 2.Schubeler D. Function and information content of DNA methylation. Nature. 2015;517:321–6. doi: 10.1038/nature14192. [DOI] [PubMed] [Google Scholar]
- 3.Parry A, Rulands S, Reik W. Active turnover of DNA methylation during cell fate decisions. Nat Rev Genet. 2021;22:59–66. doi: 10.1038/s41576-020-00287-8. [DOI] [PubMed] [Google Scholar]
- 4.Jaenisch R, Bird A. Epigenetic regulation of gene expression: how the genome integrates intrinsic and environmental signals. Nat Genet. 2003;33(Suppl):245–54. doi: 10.1038/ng1089. [DOI] [PubMed] [Google Scholar]
- 5.Chambers JC, et al. Epigenome-wide association of DNA methylation markers in peripheral blood from Indian Asians and Europeans with incident type 2 diabetes: a nested case-control study. Lancet Diabetes Endocrinol. 2015;3:526–534. doi: 10.1016/S2213-8587(15)00127-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Marioni RE, et al. DNA methylation age of blood predicts all-cause mortality in later life. Genome Biol. 2015;16:25. doi: 10.1186/s13059-015-0584-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.van der Harst P, de Windt LJ, Chambers JC. Translational Perspective on Epigenetics in Cardiovascular Disease. J Am Coll Cardiol. 2017;70:590–606. doi: 10.1016/j.jacc.2017.05.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wahl S, et al. Epigenome-wide association study of body mass index, and the adverse outcomes of adiposity. Nature. 2017;541:81–86. doi: 10.1038/nature20784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang Y, et al. DNA methylation signatures in peripheral blood strongly predict all-cause mortality. Nat Commun. 2017;8:14617. doi: 10.1038/ncomms14617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sugiura M, et al. Epigenetic modifications in prostate cancer. Int J Urol. 2020 doi: 10.1111/iju.14406. [DOI] [PubMed] [Google Scholar]
- 11.Blokhin IO, Khorkova O, Saveanu RV, Wahlestedt C. Molecular mechanisms of psychiatric diseases. Neurobiol Dis. 2020;146:105–136. doi: 10.1016/j.nbd.2020.105136. [DOI] [PubMed] [Google Scholar]
- 12.Darwiche N. Epigenetic mechanisms and the hallmarks of cancer: an intimate affair. Am J Cancer Res. 2020;10:1954–1978. [PMC free article] [PubMed] [Google Scholar]
- 13.Bonder MJ, et al. Genetic and epigenetic regulation of gene expression in fetal and adult human livers. BMC Genomics. 2014;15:860. doi: 10.1186/1471-2164-15-860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bonder MJ, et al. Disease variants alter transcription factor levels and methylation of their binding sites. Nat Genet. 2017;49:131–138. doi: 10.1038/ng.3721. [DOI] [PubMed] [Google Scholar]
- 15.Gibbs JR, et al. Abundant quantitative trait loci exist for DNA methylation and gene expression in human brain. PLoS Genet. 2010;6:e1000952. doi: 10.1371/journal.pgen.1000952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Grundberg E, et al. Global analysis of DNA methylation variation in adipose tissue from twins reveals links to disease-associated variants in distal regulatory elements. Am J Hum Genet. 2013;93:876–90. doi: 10.1016/j.ajhg.2013.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gutierrez-Arcelus M, et al. Passive and active DNA methylation and the interplay with genetic variation in gene regulation. Elife. 2013;2:e00523. doi: 10.7554/eLife.00523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lemire M, et al. Long-range epigenetic regulation is conferred by genetic variation located at thousands of independent loci. Nat Commun. 2015;6:6326. doi: 10.1038/ncomms7326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huan T, et al. Genome-wide identification of DNA methylation QTLs in whole blood highlights pathways for cardiovascular disease. Nat Commun. 2019;10:4267. doi: 10.1038/s41467-019-12228-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hannon E, et al. Leveraging DNA-Methylation Quantitative-Trait Loci to Characterize the Relationship between Methylomic Variation, Gene Expression, and Complex Traits. Am J Hum Genet. 2018;103:654–665. doi: 10.1016/j.ajhg.2018.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gaunt TR, et al. Systematic identification of genetic influences on methylation across the human life course. Genome Biol. 2016;17:61. doi: 10.1186/s13059-016-0926-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McRae AF, et al. Identification of 55,000 Replicated DNA Methylation QTL. Sci Rep. 2018;8:17605. doi: 10.1038/s41598-018-35871-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hop PJ, et al. Genome-wide identification of genes regulating DNA methylation using genetic anchors for causal inference. Genome Biol. 2020;21:220. doi: 10.1186/s13059-020-02114-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Peterson RE, et al. Genome-wide Association Studies in Ancestrally Diverse Populations: Opportunities, Methods, Pitfalls, and Recommendations. Cell. 2019;179:589–603. doi: 10.1016/j.cell.2019.08.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bell CG, et al. Obligatory and facilitative allelic variation in the DNA methylome within common disease-associated loci. Nat Commun. 2018;9:8. doi: 10.1038/s41467-017-01586-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhu Z, et al. Integration of summary data from GWAS and eQTL studies predicts complex trait gene targets. Nat Genet. 2016;48:481–7. doi: 10.1038/ng.3538. [DOI] [PubMed] [Google Scholar]
- 27.Brenner C, et al. Myc represses transcription through recruitment of DNA methyltransferase corepressor. EMBO J. 2005;24:336–46. doi: 10.1038/sj.emboj.7600509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Esteve PO, Chin HG, Pradhan S. Human maintenance DNA (cytosine-5)-methyltransferase and p53 modulate expression of p53-repressed promoters. Proc Natl Acad Sci U S A. 2005;102:1000–5. doi: 10.1073/pnas.0407729102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shlyueva D, Stampfel G, Stark A. Transcriptional enhancers: from properties to genome-wide predictions. Nat Rev Genet. 2014;15:272–86. doi: 10.1038/nrg3682. [DOI] [PubMed] [Google Scholar]
- 30.Visel A, Rubin EM, Pennacchio LA. Genomic views of distant-acting enhancers. Nature. 2009;461:199–205. doi: 10.1038/nature08451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Javierre BM, et al. Lineage-Specific Genome Architecture Links Enhancers and Non-coding Disease Variants to Target Gene Promoters. Cell. 2016;167:1369–1384.:e19. doi: 10.1016/j.cell.2016.09.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rao SS, et al. A 3D map of the human genome at kilobase resolution reveals principles of chromatin looping. Cell. 2014;159:1665–80. doi: 10.1016/j.cell.2014.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu Y, Toh H, Sasaki H, Zhang X, Cheng X. An atomic model of Zfp57 recognition of CpG methylation within a specific DNA sequence. Genes Dev. 2012;26:2374–9. doi: 10.1101/gad.202200.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shi H, et al. ZFP57 regulation of transposable elements and gene expression within and beyond imprinted domains. Epigenetics Chromatin. 2019;12:49. doi: 10.1186/s13072-019-0295-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yengo L, et al. Meta-analysis of genome-wide association studies for height and body mass index in approximately 700000 individuals of European ancestry. Hum Mol Genet. 2018;27:3641–3649. doi: 10.1093/hmg/ddy271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lee ST, et al. Protein tyrosine phosphatase UBASH3B is overexpressed in triple-negative breast cancer and promotes invasion and metastasis. Proc Natl Acad Sci U S A. 2013;110:11121–6. doi: 10.1073/pnas.1300873110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pulit SL, et al. Meta-analysis of genome-wide association studies for body fat distribution in 694 649 individuals of European ancestry. Hum Mol Genet. 2019;28:166–174. doi: 10.1093/hmg/ddy327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kichaev G, et al. Leveraging Polygenic Functional Enrichment to Improve GWAS Power. Am J Hum Genet. 2019;104:65–75. doi: 10.1016/j.ajhg.2018.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhu Z, et al. Shared genetic and experimental links between obesity-related traits and asthma subtypes in UK Biobank. J Allergy Clin Immunol. 2020;145:537–549. doi: 10.1016/j.jaci.2019.09.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Richardson TG, et al. Evaluating the relationship between circulating lipoprotein lipids and apolipoproteins with risk of coronary heart disease: A multivariable Mendelian randomisation analysis. PLoS Med. 2020;17:e1003062. doi: 10.1371/journal.pmed.1003062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Konieczna J, Sanchez J, Palou M, Pico C, Palou A. Blood cell transcriptomic-based early biomarkers of adverse programming effects of gestational calorie restriction and their reversibility by leptin supplementation. Sci Rep. 2015;5:9088. doi: 10.1038/srep09088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mancuso N, et al. Integrating Gene Expression with Summary Association Statistics to Identify Genes Associated with 30 Complex Traits. Am J Hum Genet. 2017;100:473–487. doi: 10.1016/j.ajhg.2017.01.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Okada Y, et al. Genetics of rheumatoid arthritis contributes to biology and drug discovery. Nature. 2014;506:376–81. doi: 10.1038/nature12873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Emery P, et al. IL-6 receptor inhibition with tocilizumab improves treatment outcomes in patients with rheumatoid arthritis refractory to anti-tumour necrosis factor biologicals: results from a 24-week multicentre randomised placebo-controlled trial. Ann Rheum Dis. 2008;67:1516–23. doi: 10.1136/ard.2008.092932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Navarro-Millan I, Singh JA, Curtis JR. Systematic review of tocilizumab for rheumatoid arthritis: a new biologic agent targeting the interleukin-6 receptor. Clin Ther. 2012;34:788–802.:e3. doi: 10.1016/j.clinthera.2012.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wen X, Pique-Regi R, Luca F. Integrating molecular QTL data into genome-wide genetic association analysis: Probabilistic assessment of enrichment and colocalization. PLoS Genet. 2017;13:e1006646. doi: 10.1371/journal.pgen.1006646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Burnichon N, et al. MAX mutations cause hereditary and sporadic pheochromocytoma and paraganglioma. Clin Cancer Res. 2012;18:2828–37. doi: 10.1158/1078-0432.CCR-12-0160. [DOI] [PubMed] [Google Scholar]
- 48.Li H, et al. Novel Treatment of Hypertension by Specifically Targeting E2F for Restoration of Endothelial Dihydrofolate Reductase and eNOS Function Under Oxidative Stress. Hypertension. 2019;73:179–189. doi: 10.1161/HYPERTENSIONAHA.118.11643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Burstein E, et al. COMMD proteins, a novel family of structural and functional homologs of MURR1. J Biol Chem. 2005;280:22222–32. doi: 10.1074/jbc.M501928200. [DOI] [PubMed] [Google Scholar]
- 50.Astle WJ, et al. The Allelic Landscape of Human Blood Cell Trait Variation and Links to Common Complex Disease. Cell. 2016;167:1415–1429.:e19. doi: 10.1016/j.cell.2016.10.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Suhail A, et al. DeSUMOylase SENP7-Mediated Epithelial Signaling Triggers Intestinal Inflammation via Expansion of Gamma-Delta T Cells. Cell Rep. 2019;29:3522–3538.:e7. doi: 10.1016/j.celrep.2019.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jing Z, Liu Y, Dong M, Hu S, Huang S. Identification of the DNA binding element of the human ZNF333 protein. J Biochem Mol Biol. 2004;37:663–70. doi: 10.5483/bmbrep.2004.37.6.663. [DOI] [PubMed] [Google Scholar]
- 53.Chen MH, et al. Trans-ethnic and Ancestry-Specific Blood-Cell Genetics in 746,667 Individuals from 5 Global Populations. Cell. 2020;182:1198–1213.:e14. doi: 10.1016/j.cell.2020.06.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nedelec Y, et al. Genetic Ancestry and Natural Selection Drive Population Differences in Immune Responses to Pathogens. Cell. 2016;167:657–669.:e21. doi: 10.1016/j.cell.2016.09.025. [DOI] [PubMed] [Google Scholar]
- 55.Joehanes R, et al. Epigenetic Signatures of Cigarette Smoking. Circ Cardiovasc Genet. 2016;9:436–447. doi: 10.1161/CIRCGENETICS.116.001506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Singmann P, et al. Characterization of whole-genome autosomal differences of DNA methylation between men and women. Epigenetics Chromatin. 2015;8:43. doi: 10.1186/s13072-015-0035-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zeilinger S, et al. Tobacco smoking leads to extensive genome-wide changes in DNA methylation. PLoS One. 2013;8:e63812. doi: 10.1371/journal.pone.0063812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Giri AK, et al. DNA methylation profiling reveals the presence of population-specific signatures correlating with phenotypic characteristics. Mol Genet Genomics. 2017;292:655–662. doi: 10.1007/s00438-017-1298-0. [DOI] [PubMed] [Google Scholar]
- 59.Breeze CE, et al. eFORGE: A Tool for Identifying Cell Type-Specific Signal in Epigenomic Data. Cell Rep. 2016;17:2137–2150. doi: 10.1016/j.celrep.2016.10.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Westra HJ, et al. Cell Specific eQTL Analysis without Sorting Cells. PLoS Genet. 2015;11:e1005223. doi: 10.1371/journal.pgen.1005223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Guan W, et al. Genome-wide association study of plasma N6 polyunsaturated fatty acids within the cohorts for heart and aging research in genomic epidemiology consortium. Circ Cardiovasc Genet. 2014;7:321–331. doi: 10.1161/CIRCGENETICS.113.000208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Shin SY, et al. An atlas of genetic influences on human blood metabolites. Nat Genet. 2014;46:543–550. doi: 10.1038/ng.2982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kamat MA, et al. PhenoScanner V2: an expanded tool for searching human genotype-phenotype associations. Bioinformatics. 2019;35:4851–4853. doi: 10.1093/bioinformatics/btz469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gelfand EW, Dakhama A. CD8+ T lymphocytes and leukotriene B4: novel interactions in the persistence and progression of asthma. J Allergy Clin Immunol. 2006;117:577–82. doi: 10.1016/j.jaci.2005.12.1340. [DOI] [PubMed] [Google Scholar]
- 65.Cho SH, Stanciu LA, Holgate ST, Johnston SL. Increased interleukin-4, interleukin-5, and interferon-gamma in airway CD4+ and CD8+ T cells in atopic asthma. Am J Respir Crit Care Med. 2005;171:224–30. doi: 10.1164/rccm.200310-1416OC. [DOI] [PubMed] [Google Scholar]
- 66.Zhou X, Stephens M. Genome-wide efficient mixed-model analysis for association studies. Nat Genet. 2012;44:821–4. doi: 10.1038/ng.2310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ernst J, Kellis M. Discovery and characterization of chromatin states for systematic annotation of the human genome. Nat Biotechnol. 2010;28:817–25. doi: 10.1038/nbt.1662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Roadmap Epigenomics C, et al. Integrative analysis of 111 reference human epigenomes. Nature. 2015;518:317–30. doi: 10.1038/nature14248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Shabalin AA. Matrix eQTL: ultra fast eQTL analysis via large matrix operations. Bioinformatics. 2012;28:1353–8. doi: 10.1093/bioinformatics/bts163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Consortium GT, et al. Genetic effects on gene expression across human tissues. Nature. 2017;550:204–213. doi: 10.1038/nature24277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kim KA, et al. Environmental risk factors and comorbidities of primary biliary cholangitis in Korea: a case-control study. Korean J Intern Med. 2020 doi: 10.3904/kjim.2019.234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Consortium GT. The GTEx Consortium atlas of genetic regulatory effects across human tissues. Science. 2020;369:1318–1330. doi: 10.1126/science.aaz1776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Staley JR, et al. PhenoScanner: a database of human genotype-phenotype associations. Bioinformatics. 2016;32:3207–3209. doi: 10.1093/bioinformatics/btw373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Griffon A, et al. Integrative analysis of public ChIP-seq experiments reveals a complex multi-cell regulatory landscape. Nucleic Acids Res. 2015;43:e27. doi: 10.1093/nar/gku1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Franceschini A, et al. STRING v9.1: protein-protein interaction networks, with increased coverage and integration. Nucleic Acids Res. 2013;41:D808-15. doi: 10.1093/nar/gks1094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Haghverdi L, Buettner F, Theis FJ. Diffusion maps for high-dimensional single-cell analysis of differentiation data. Bioinformatics. 2015;31:2989–98. doi: 10.1093/bioinformatics/btv325. [DOI] [PubMed] [Google Scholar]
- 77.Haghverdi L, Buttner M, Wolf FA, Buettner F, Theis FJ. Diffusion pseudotime robustly reconstructs lineage branching. Nat Methods. 2016;13:845–8. doi: 10.1038/nmeth.3971. [DOI] [PubMed] [Google Scholar]
- 78.Schramm K, et al. Mapping the genetic architecture of gene regulation in whole blood. PLoS One. 2014;9:e93844. doi: 10.1371/journal.pone.0093844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Benjamini Y, Drai D, Elmer G, Kafkafi N, Golani I. Controlling the false discovery rate in behavior genetics research. Behav Brain Res. 2001;125:279–84. doi: 10.1016/s0166-4328(01)00297-2. [DOI] [PubMed] [Google Scholar]
- 80.Buniello A, et al. The NHGRI-EBI GWAS Catalog of published genome-wide association studies, targeted arrays and summary statistics 2019. Nucleic Acids Res. 2019;47:D1005–D1012. doi: 10.1093/nar/gky1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Taylor-Weiner A, et al. Scaling computational genomics to millions of individuals with GPUs. Genome Biol. 2019;20:228. doi: 10.1186/s13059-019-1836-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Analysis code available at. [DOI]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Summary statistics for the 11.2M SNP-CpG pairs reaching genome-wide significance are available at https://zenodo.org/record/5196216#.YRZ3TfJxeUk. ChIP-seq data for ZNF333 are available through the NCBI SRA (accession code: SRP284104). Raw genotype, methylation and expression data can be made available upon reasonable request by the authors. Controlled data access to data of the KORA cohort can be obtained through https://epi.helmholtz-muenchen.de. Source data are provided with this paper.
The web-links for the publicly available datasets used in the study are as follows:
Phenoscanner v2:
http://www.phenoscanner.medschl.cam.ac.uk
GWAS catalog:
https://www.ebi.ac.uk/gwas/docs/file-downloads
meQTL and eQTM data from Bonder et al 2015:
https://molgenis26.gcc.rug.nl/downloads/biosqtlbrowser/2015_09_02_trans_meQTLsFDR0.05-CpGLevel.txt
https://molgenis26.gcc.rug.nl/downloads/biosqtlbrowser/2015_09_02_cis_eQTMsFDR0.05-CpGLevel.txt
GTEx v6 eQTL results:
eQTLgen cis eQTL results
https://molgenis26.gcc.rug.nl/downloads/eqtlgen/cis-eqtl/cis-eQTLs_full_20180905.txt.gz
TWAShub
http://twas-hub.org/genes/UBASH3B/
GWAS summary statistics of 114 traits for colocalization analysis https://zenodo.org/record/3629742
ChIP-seq binding sites http://hgdownload.cse.ucsc.edu/goldenPath/hg19/encodeDCC/wgEncodeRegTfbsClustered/wgEncodeRegTfbsClusteredWithCellsV3.bed.gz
http://tagc.univ-mrs.fr/remap/download/All/filPeaks_public.bed.gz
ChromHMM states: http://egg2.wustl.edu/roadmap/data/byFileType/chromhmmSegmentations/ChmmModels/coreMarks/jointModel/final/all.mnemonics.bedFiles.tgz
Hi-C data (EGAD00001003106):
https://ega-archive.org/datasets/EGAD00001003106/
Protein - protein interactions:
http://string90.embl.de/newstring_download/protein.links.detailed.v9.0.txt.gz
Code for the analysis is available through GitHub: https://github.com/heiniglab/hawe2021_meQTL_analyses, and also through zenodo DOI: 10.5281/zenodo.5529828.82