Abstract
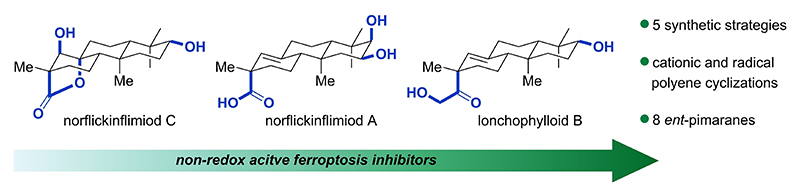
We present a comprehensive account on the evolution of a synthetic platform for a subfamily of ent-pimaranes. For the most complex member, norflickinflimiod C, five distinct strategies relying on either cationic or radical polyene cyclizations to construct the requisite tricyclic carbon scaffold were explored. Insights from early and late stage oxidative and reductive dearomatization studies ultimately led to a mild, rhodium-catalyzed arene hydrogenation for the final synthetic route. A Sharpless asymmetric dihydroxylation was found to be suitable to render the platform enantioselective and diversification of a late-stage key intermediate culminated in the total synthesis of eight ent-pimaranes in 11–16 steps. These compounds were found to inhibit the formation of pro-inflammatory leukotrienes and other 5-lipoxygenase products. Notably, three ent-pimaranes exhibited low micromolar, non-redox active ferroptosis inhibition with remarkable structural specificity.
Keywords: Total synthesis, Natural products, Terpenoids, Polyene cyclization, Ferroptosis
A full account of five distinct synthetic strategies towards a subfamily of ent-pimaranes is given. Highlights include radical and cationic polyene cyclizations for construction of the tricyclic core. Valuable insights from unsuccessful approaches informed the final route, which culminated in the enantioselective synthesis of eight ent-pimaranes. Biological investigations identified ent-pimaranes as novel and unusual non-redox active ferroptosis inhibitors.
Introduction
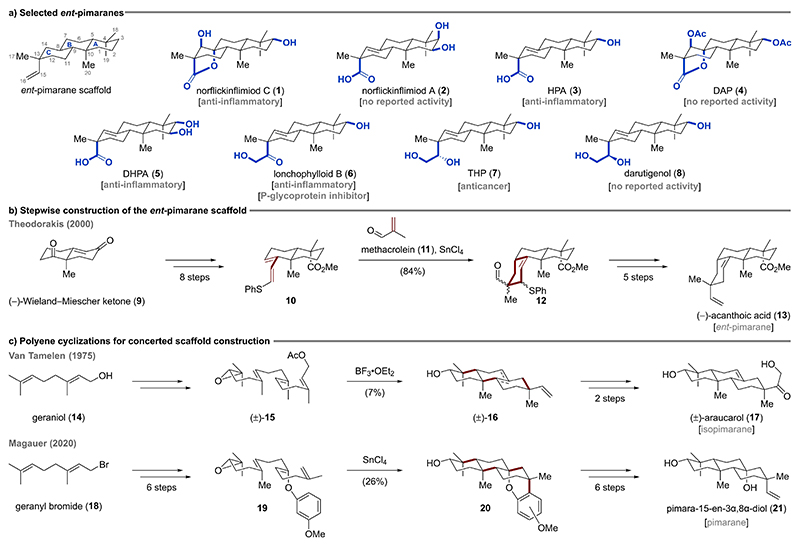
Natural products serve as one of the most important sources for drug discovery and development with nearly 50% of all approved drugs in the last four decades being based on natural products.[1] In the search of novel natural products with potent biological activities, the groups of Yang, Jiang, and Zhao recently isolated more than 20 new ent-pimarane diterpenoids from the orchid Flickingeria fimbriata, which is used in Traditional Chinese Medicine for the treatment of medical conditions associated with inflammation.[2] These studies resulted in the structure elucidation of norflickinflimiod C (1), norflickinflimiod A (2), 2-hydroxy-16-nor-ent-pimar-8(14)-en-15-oic acid (HPA, 3), 3,14-diacetoxy-16-nor-ent-pimar-15α,8-olide (DAP, 4), and 2,3-dihydroxy-16-nor-ent-pimar-8(14)-en-15-oic acid (DHPA, 5) as well as the isolation of known lonchophylloid B (6)[3] (Scheme 1a). Biological assays revealed moderate to potent anti-inflammatory activities for several of the isolated ent-pimaranes by either inhibiting the nuclear factor-kappa B (NF-kB) pathway or inhibiting lipopolysaccharide induced nitric oxide and TNF-α production in the murine RAW264.7 cell system.[2] For some of the compounds moderate antitumor activity against the human breast cancer cell line MCF-7 was identified.[2a] In a different study, lonchophylloid B (6) was shown to be a weak P-glycoprotein inhibitor capable to sensitize cells expressing the multidrug resistant phenotype towards the anticancer drug doxorubicin.[3] In 2017, closely related ent-pimaranes such as the newly described ent-3β,15R,16-trihydroxypimar-8(14)-ene (THP, 7) and the known darutigenol (8)[4] were isolated from aerial parts of Siegesbeckia pubescens (Scheme 1a).[5] THP (7) was found to possess inhibitory effects against the EGF-induced invasion of MB-MDA-231 cancer cells.
Scheme 1.
a) Selected ent-pimaranes with varying oxidation patterns in the A and C ring (highlighted in blue). b) Synthesis of (–)-acanthoic acid (13) through a key Diels–Alder cycloaddition as an example for a stepwise ring construction strategy. c) Selected polyene cyclization approaches accessing pimarane natural products.
From a structural perspective, these ent-pimaranes share a common 6,6,6-tricyclic ring system with five to seven stereo-centers, two of which are quaternary. They mainly differ in their oxidation patterns in the A and C rings. Based on the diverse biological properties of these ent-pimaranes paired with their intriguing, common molecular architecture, we recently reported a divergent synthetic approach to this family of natural products.[6] Here we provide a full account on the evolution of the successful synthetic strategy and the potential of the synthesized ent-pimaranes as novel non-redox active ferroptosis inhibitors is revealed.
To date, only a few total syntheses of pimarane and isopimarane (= inverted C13 stereochemistry) natural products have been reported. Most of them rely on a stepwise construction of the tricyclic core through Diels–Alder cyclo-additions, aldol condensations, or Robinson annulations.[7] As exemplified in Scheme 1b, Theodorakis employed the (–)-Wieland–Miescher ketone (9) to build up the bicyclic diene 10 in eight steps.[7h] A Lewis acid promoted Diels–Alder cycloaddition with methacrolein (11) afforded the tricyclic ent-pimarane scaffold 12, which could be elaborated in five additional steps to (–)-acanthoic acid (13). Alternatively, there have been scarce reports employing polyene cyclizations to forge the requisite tricyclic carbon scaffold in a single transformation from linear precursors.[6,8] In 1975, van Tamelen accessed the 6,6,6-fused core structure 16 of (±)-araucarol (17) via a tandem head-to-tail/tail-to-head polyene cyclization of epoxide 15, which is derived in a convergent manner from two units of geraniol (14) (Scheme 1c).[8c] Even though the key cationic cyclization afforded a mixture of alkene isomers in only 7% yield, three C–C bonds and four stereocenters were generated in a single step. In another example from 2020, geranyl bromide (18) was converted in six steps to enantioenriched dual nucleophilic aryl enol ether 19, which underwent tetracyclization upon treatment with tin(IV) chloride to furnish pentacycle 20. Pimara-15-en-3α,8α-diol (21) was accessed from pentacycle 20 in six additional steps, including an oxidative arene degradation.[8a]
Results and Discussion
With the aim of a general synthetic entry to this family of ent-pimaranes, we selected norflickinflimiod C (1) with the most intricate oxidation pattern as the initial synthetic target. We hypothesized that a successful route to norflickinflimiod C (1) will also enable us to branch out to various closely related congeners. The preparation of an array of ent-pimaranes was expected to allow for more detailed characterization of the bioactivity of these natural products.
Strategy 1 – Tetracyclization of an Allenylic Sulfide
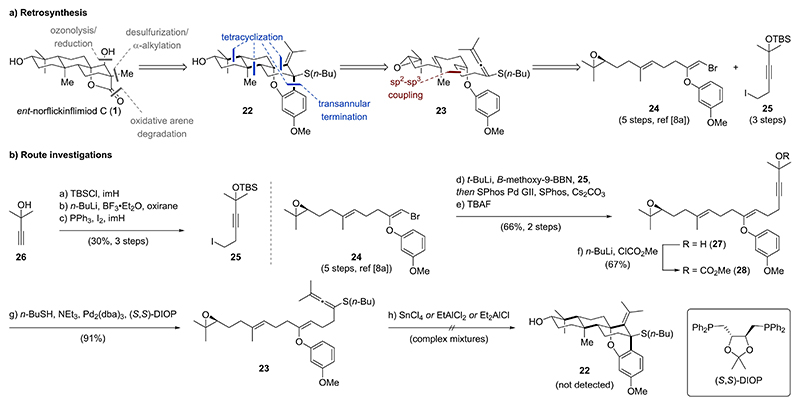
The initial retrosynthetic analysis of ent-norflickinflimiod C (1) revealed a sequence of oxidative arene/alkene degradation and ketone α-alkylation of pentacycle 22 (Scheme 2a). Inspired by previous work[8a,9], we envisioned a tetracyclization of linear epoxide cyclization precursor 23 bearing a dual nucleophilic aryl enol ether and an allenylic sulfide to access pentacycle 22. Installation of an electron-donating group on the allene (e. g., a sulfur moiety) was thought to be critical in controlling the regioselectivity for nucleophilic attack of the arene to the intermediate allylic cation.[9] Allenylic sulfide 23 was planned to arise from a palladium-catalyzed C(sp2)–C(sp3) cross coupling between known enantioenriched vinyl bromide building block 24[8a] and primary iodide 25.
Scheme 2.
a) Retrosynthesis for strategy 1 focusing on a tetracyclization of a linear allenylic sulfide cyclization precursor. b) Precursor synthesis and attempted key cyclization.
Primary iodide 25 was prepared from propargylic alcohol 26 through a three-step sequence involving (1) silyl protection with tert-butyldimethylsilyl chloride (TBSCl) in the presence of imidazole (imH), (2) C2-elongation of an in situ generated lithium acetylide with BF3⋅Et2O and oxirane, and (3) the Garegg–Samuelsson modification[10] of the Appel reaction employing iodine, triphenylphosphine, and imidazole (Scheme 2b). Lithium-halogen exchange of primary iodide 25 with tert-butyllithium (t-BuLi) followed by trapping of the newly formed organolithium species as an ate-complex with B-methoxy-9-borabicyclo[3.3.1]nonane allowed for a Suzuki– Miyaura cross coupling with enantioenriched vinyl bromide 24.[11] Subsequent silyl deprotection employing tetra-n-butylam-monium fluoride (TBAF) afforded propargylic alcohol 27 in 66% yield over two steps. A survey of mild protocols for introduction of the allenylic sulfide, which are required to tolerate both an aryl enol ether and an epoxide, prompted us to investigate a palladium catalyzed coupling between a propargylic mesylate and a thiol.[12] While mesylation of alcohol 27 with mesyl chloride and triethylamine resulted exclusively in elimination to an enyne (see Supporting Information), alcohol 27 could be converted to propargylic carbonate 28 by treating the lithium alkoxide of alcohol 27 with methyl chloroformate. Gratifyingly, coupling of propargylic carbonate 28 with butane-1-thiol in the presence of Pd2(dba)3 and (S,S)-DIOP afforded allenylic sulfide 23 in excellent yield (91 %). Unfortunately, cyclization attempts employing Lewis acids such as tin(IV) tetrachloride, ethyl-aluminum dichloride, or diethylaluminum chloride resulted only in complex mixtures. Partial purification by high performance liquid chromatography (HPLC) and detailed NMR studies indicated no formation of the desired pentacycle 22. Therefore, we abandoned this route.
Strategy 2 – Oxidative Dearomatization/Radical Cyclization Sequence
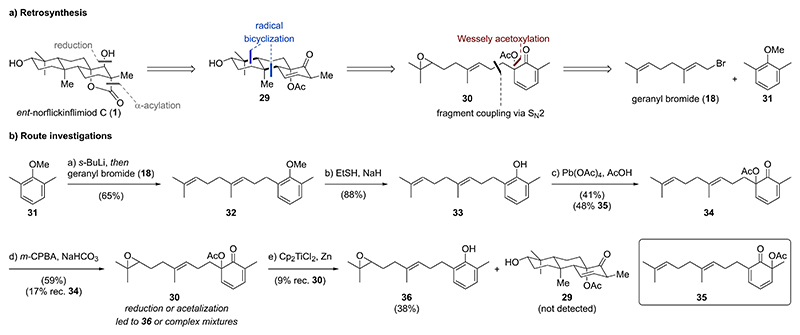
For our next strategy we disconnected ent-norflickinflimiod C (1) to tricycle 29, the substrate of a diastereoselective α-acylation/ketone reduction (Scheme 3a). Tricycle 29 was envisioned to be accessible through a radical cyclization of epoxide 30, which should be terminated by the dienone functionality and generate two quaternary carbons along with five stereocenters. A strategic oxidative dearomatization via Wessely acetoxylation[13] was thought to establish both the dienone and the required, masked tertiary alcohol in ent-norflickinflimiod C (1). Further C–C disconnection of epoxide 30 revealed the commercially available building blocks geranyl bromide (18) and 2,6-dimethylanisole (31).
Scheme 3.
a) Retrosynthesis for strategy 2 focusing on a sequence of oxidative dearomatization/reductive cyclization for construction of the carbon scaffold. b) Synthesis of dienone cyclization precursor 30 and attempted key radical cyclization.
As outlined in Scheme 3b, we commenced with benzylic lithiation of 2,6-dimethylanisole (31) using sec-butyllithium (s-BuLi). Subsequent C–C bond formation through nucleophilic substitution (SN2) with geranyl bromide (18) at –78 °C afforded geranyl arene 32 (65 %). Nucleophilic demethylation of geranyl arene 32 with sodium ethanethiolate at 110 °C gave access to phenol 33 in 88 % yield. Phenol 33 was also obtained in an analogous three-step procedure from 2,6-dimethylphenol (see Supporting Information). Treatment of phenol 33 with lead(IV) acetate and acetic acid (Wessely acetoxylation) in dichloro-methane at 0 °C affected efficient formation of the two regioisomeric dienones 34 and 35 in 41 % and 48 % yield, respectively. Low-temperature epoxidation of dienone 34 (–35 °C→–18 °C) with meta-chloroperoxybenzoic acid (m-CPBA, 1.00 equiv) in the presence of sodium bicarbonate proceeded with high regioselectivity for the terminal trisubstituted alkene over the internal alkene yielding epoxide 30 (59 %) along with recovered dienone 34 (17 %). Increasing the temperature or the m-CPBA equivalents resulted in significantly lower regioselectivity and double epoxidation. Unfortunately, initiation of the key radical cyclization of epoxide 30 with in situ generated RajanBabu–Nugent reagent (Cp2TiCl)[14] failed due to preferential reduction of the dienone over the epoxide. Exclusive formation of phenol 36 in 38 % was observed with no traces of tricycle 29. Attempts to mask the ketone functionality in epoxide 30 either through reduction or acetalization ultimately failed prompting us to revise the synthetic strategy.
Strategy 3 – Cationic Cyclization/Oxidative Dearomatization Sequence
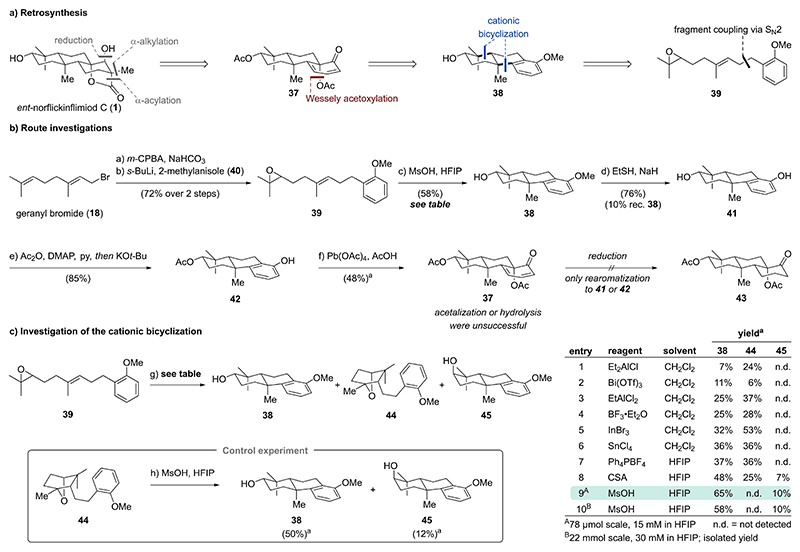
Based on our previous strategy, ent-norflickinflimiod C (1) was envisioned to be accessible from dienone 37 through reduction, α-alkylation, and α-acylation (Scheme 4a). Dienone 37 featuring a protected tertiary alcohol was expected to be formed through a Wessely acetoxylation of a phenol derived from tricycle 38. Tricycle 38 should arise from a cationic bicyclization of epoxide 39. Access to epoxide 39 was intended via an SN2-type fragment coupling analogous to strategy 2. Contrary to the previous strategy, the sequence of Wessely acetoxylation and bicyclization was reversed, thereby avoiding the problem of preferential reduction of the dienone motif over initiation of a reductive radical cyclization cascade. Additionally, epoxide 39 lacks a methyl substituent on the arene (compared to phenol 33). This was thought to control the regioselectivity of the Wessely acetoxylation by directing the acetoxy group to the more electron-rich arene ortho-position despite the inherent steric bias resulting from syn-pentane interactions at this position.
Scheme 4.
a) Retrosynthesis for strategy 3 focusing on a sequence of cationic cyclization/oxidative dearomatization. b) Realization of the key cationic cyclization/oxidative dearomatization sequence to 37 and attempted functionalization. c) Development of a Brønsted acid catalyzed bicyclization in HFIP. aNMR yield.
Commencing from commercially available geranyl bromide (18), regioselective epoxidation of the more electron-rich alkene with m-CPBA proceeded cleanly to a single epoxide product. Chemoselective displacement of the allylic bromide with a nucleophile generated from benzylic lithiation of 2-methylanisol (40) enabled access to racemic epoxide 39 in 72 % over two steps (Scheme 4b). For the investigation of the key cationic bicyclization of epoxide 39, we set out to screen Lewis acids frequently employed for similar polyene cyclizations[15] (e. g., Et2AlCl, Bi(OTf)3, EtAlCl2, BF3⋅Et2O, InBr3, SnCl4; Scheme 4c, entries 1–6). To reduce time, cost, and workload, NMR yields were employed during our screening endeavors. Most of these conditions afforded the desired tricycle 38[16] in 7–36 % NMR yield, albeit as a mixtures with interrupted cyclization products such as oxabicyclo[2.2.1]heptane 44 (6–53 % NMR yield). In addition to the unsatisfying yields, access to large quantities of tricycle 38 was hampered by cumbersome purification involving high performance liquid chromatography (HPLC). Evaluation of alternative protocols to convert epoxide 39 to tricycle 38 led us to investigate conditions reported by Qu in 2016.[17] Subjecting epoxide 39 to tetraphenylphosphonium tetrafluoroborate (Ph4PBF4) in 1,1,1,3,3,3-hexafluoroisopropanol (HFIP) provided comparable results to the previously screened Lewis acids (Scheme 4c, entry 7). Based on the authors’ proposal that the reaction is catalyzed by traces of HF, we reasoned that a sufficiently strong acid might be able to convert oxabicyclo[2.2.1]heptane 44 to the desired tricycle 38. Employing camphorsulfonic acid (CSA) in HFIP (Scheme 4c, entry 8) indeed improved the yield of tricycle 38 (48 % NMR yield) while decreasing the amount of oxabicyclo[2.2.1]heptane 44 (25 % NMR yield). Surprisingly, traces of another tricycle 45 (7 % NMR yield) with an axial hydroxy group were obtained. This product might originate from pseudo-axial alignment of the epoxide in the cyclization step. Alternatively, nucleophilic opening of oxabicyclo[2.2.1]heptane 44 with water at the secondary carbon of the ether bridge (Walden inversion) followed by cyclization would also afford tricycle 45. Notably, even at elevated temperatures (50 °C) and prolonged reaction times (15 h), CSA did not lead to complete conversion of oxabicyclo[2.2.1]heptane 44. After extensive screening of various acids and solvents (see Supporting Information), we identified methanesulfonic acid (MsOH) in HFIP as optimal to cleanly convert epoxide 39 to tricycles 38 (65 % NMR yield) and 45 (10 % NMR yield) (Scheme 4c, entry 9). A control experiment confirmed conversion of oxabicyclo[2.2.1]heptane 44 to tricycles 38 and 45 under the same conditions (MsOH, HFIP). Examination of the choice of solvent revealed that HFIP is crucial, while solvents such as nitromethane, dichloromethane, and acetonitrile performed significantly worse. This can be attributed to HFIP’s unique properties (comparably high acidity, high hydrogen bonding donor strength, high polarity, weak nucleophilicity), which make it exceptionally suitable for the stabilization of cations and thus also for cationic polyene cyclizations.[18] Performing the key cationic bicyclization on a larger scale (22 mmol, 6.1 g) with double the concentration (30 mM vs. 15 mM) led to a slightly lower yield of tricycles 38 (58 %) and 45 (10 %).
Continuing with our route investigations, nucleophilic demethylation of the methyl ether in tricycle 38 with sodium ethanethiolate at 120 °C afforded phenol 41 (76 %). A one-pot procedure consisting of double acetylation with acetic anhydride, 4-(dimethylamino)pyridine (DMAP), and pyridine (py) followed by selective deacetylation of the aryl ester with potassium tert-butoxide (KOt-Bu) enabled access to phenol 42 in 85 % yield. With phenol 42 in hand, the key oxidative dearomatization reaction was investigated by subjecting phenol 42 to hypervalent iodine(III) and lead(IV) oxidants (see Supporting Information). To our delight, acetate addition to the alkyl-substituted ortho-position with the desired stereochemistry was observed for all investigated conditions. While iodine(III) reagents afforded dienone 37 in low yields (< 15 %) along with various side products originating from alternative oxidation at the other ortho- or para-position, lead(IV) acetate and acetic acid (Wessely acetoxylation) turned out to be optimal (48 % NMR yield of dienone 37).[13] Unfortunately, attempts to reduce dienone 37 were met with failure and led exclusively to rearomatization to phenols 41 or 42 (see Supporting Information). Similarly, protection of the ketone in dienone 37 as an acetal or ester hydrolysis resulted only in decomposition or the formation of aromatic side products.
Strategy 4 – Cationic Cyclization/Reductive Dearomatization Sequence
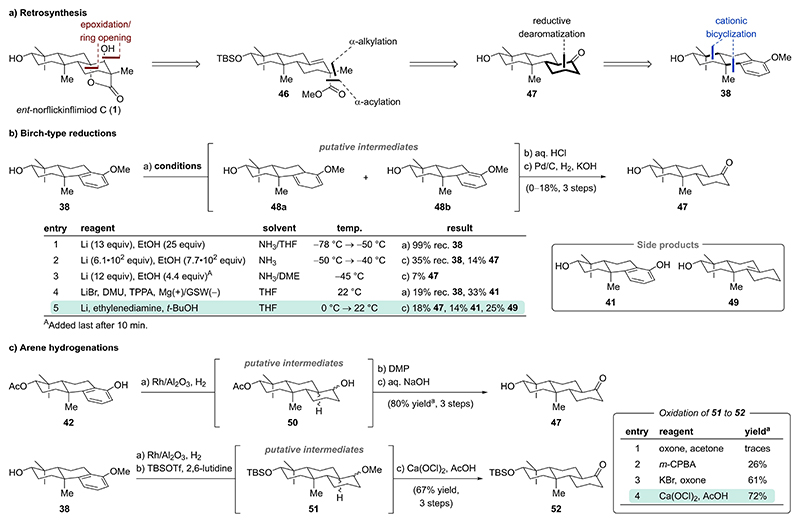
For our fourth strategy, we opted for a diastereoselective epoxidation/epoxide opening sequence to introduce the vital secondary alcohol and γ-lactone motif starting from alkene 46 (Scheme 5a). Alkene 46 was envisioned to arise from ketone 47 through tandem α-alkylation/α-acylation — the flexible interchangeability of these steps was expected to allow for highly diastereoselective introduction of the quaternary stereocenter. Conversion of the ketone functionality to a trisubstituted alkene was expected to proceed through a reduction/elimination or triflation/reduction protocol. Inspired by the advances and informed by the setbacks of our previous strategy, we traced ketone 47 back to already established tricycle 38 via a reductive dearomatization, for which no facile rearomatization process should be possible.
Scheme 5.
a) Retrosynthesis for strategy 4 focusing on a sequence of cationic cyclization/reductive dearomatization for construction of the carbon scaffold. b) Investigation of Birch-type reductions. c) Arene hydrogenation and methyl ether oxidations. aNMR yield.
Having tricycle 38 from the previously established cationic bicyclization, we set out to investigate its reduction (Scheme 5b). Attempted Birch reduction of tricycle 38 in a mixture of liquid ammonia and tetrahydrofuran resulted in no conversion (Scheme 5b, entry 1). Of note, for substrates such as tricycle 38, which require protonation to occur at a site bearing an alkyl substituent, diminished reactivity in Birch-type reductions was reported.[19] This has been addressed in seminal work by Johnson on a similar 2,3-alkylated anisole by addition of a large excess of lithium (433 equiv) leading to about 33 % of regioisomeric enones after acid hydrolysis.[20] Employing these conditions at temperatures slightly below the boiling point of liquid ammonia (–50 °C→–40 °C) followed by hydrolysis and hydrogenation afforded ketone 47 in 14% over three steps (Scheme 5b, entry 2).[16a] Of note, based on NMR monitoring both hydrolysis of the putative intermediate enol ethers (48 a/b) and hydrogenation of the resulting enones under basic conditions (KOH) with simultaneous epimerization to the trans-decalin scaffold proceed with high efficiency. The serious safety concerns of the Birch procedure at temperatures near the boiling point of ammonia paired with the low overall efficiency, warranted further studies. An alternative protocol reported for the reduction of such challenging systems relies on high lithium concentrations and addition of the alcohol last.[21] Unfortunately, subjecting tricycle 38 in a mixture of liquid ammonia and 1,2-dimethoxyethane (DME) to lithium followed by drop-wise addition of ethanol after 10 min yielded only 7 % of desired ketone 47 after hydrolysis and hydrogenation (Scheme 5b, entry 3). An alternative electroreduction developed by Baran, which employs an undivided cell with a magnesium anode, a galvanized steel wire (GSW) cathode, lithium bromide, dimethyl urea (DMU) (a proton source), and tris(pyrrolidino)phosphoramide (TPPA) in tetrahydrofuran under constant current, resulted in exclusive demethylation to phenol 41 in 33 % yield (Scheme 5b, entry 4).[22] Resorting to the ammonia-free Birch conditions by Koide (lithium, ethylenediamine, and tert-butanol in tetrahydrofuran) represented the highest-yielding and safest reduction protocol for tricycle 38. This afforded ketone 47 in 18 % over three steps along with demethylation product 41 (14 %) and over-reduction product 49 (25 %) (Scheme 5b, entry 5).[23] Attempts to improve the yield by adjusting the reagent equivalents, the lithium/alcohol ratio, and the alcohol choice (tert-butanol vs. tert-amyl alcohol) did not result in any further improvement.
Driven by the low overall efficiency, we instead decided to investigate an arene hydrogenation to access ketone 47. This is a well precedented transformation, however, exceedingly harsh conditions have been reported for phenols (e. g., Raney Ni, 37– 158 bar H2, 105–180 °C or RuO2, 103 bar H2, 50 °C).[7a,i,16a,24] Gratifyingly, rhodium-catalyzed conditions reported by Sajiki enabled nearly quantitative reduction of phenol 42 to a mixture of diastereomers 50 under mild conditions (Rh/Al2O3, 12 bar H2, 65 °C) (Scheme 5c).[25] Oxidation of the secondary alcohol in tricycle 50 with Dess–Martin periodinane (DMP) and subsequent acetate hydrolysis/α-epimerization afforded ketone 47 in 80 % NMR yield along with an inseparable impurity. The corresponding cis-decalins were not isolated.
As phenol 42 was accessed from tricycle 38 in two steps, we investigated direct hydrogenation of tricycle 38 under the same conditions to shorten the route and minimize functional group interconversions. Indeed, hydrogenation of the anisole in tricycle 38 proceeded equally well and was followed by treatment of the crude reaction mixture with tert-butyldime-thylsilyl trifluoromethanesulfonate (TBSOTf) in the presence of 2,6-lutidine to afford a mixture of putative diastereomers 51. Investigation of chemoselective methyl ether oxidations with different oxidants such as dimethyldioxirane[26], m-CPBA in trichloroacetonitrile[27], and a mixture of potassium bromide and oxone[28] enabled the desired transformation to ketone 52 in up to 61 % NMR yield (Scheme 5c, entries 1–3). The yield for ketone 52 was further improved to 72 % NMR yield by employing conditions reported by Rutjes (Ca(OCl)2, AcOH; Scheme 5c, entry 4).[29] Overall, tricycle 38 was converted to ketone 52 in 67 % yield over three steps.
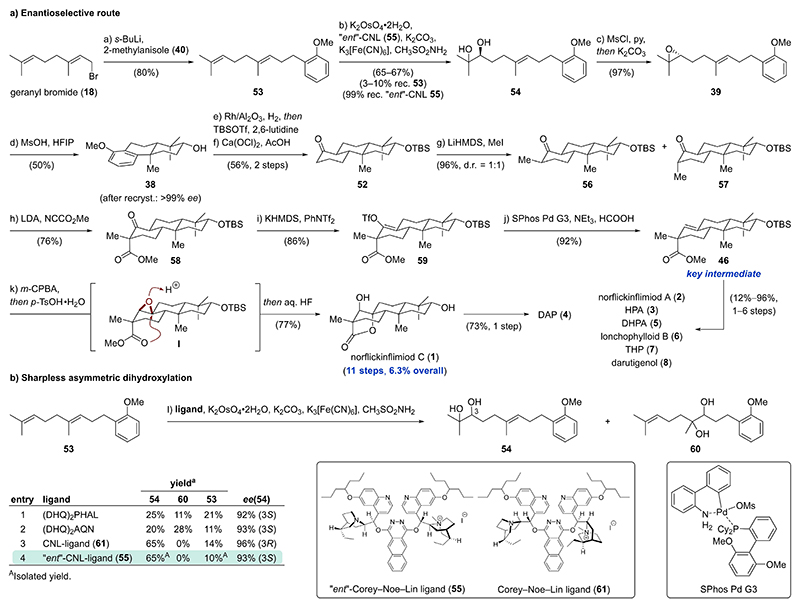
With this promising synthetic entry to ketone 52 in hand, we decided to investigate both an enantioselective access and the final steps leading to norflickinflimiod C (1) (Scheme 6a). First, nucleophilic displacement of geranyl bromide (18) with lithiated 2-methylanisole (40) gave geranyl arene 53. Attempted Shi epoxidation of geranyl arene 53 was plagued by poor regioselectivity, resulting in unselective mono- and diepoxidation.[30] A lack of regio- and enantioselective epoxidation methodologies for trisubstituted alkyl-substituted alkenes, led to investigations of Sharpless asymmetric dihydroxylations (Scheme 6b). While commercial ligands such as (DHQ)2AQN and (DHQ)2PHAL gave excellent enantioselectivities (92–93 % ee), poor ratios of desired diol 54 and undesired, internal diol 60 were observed (Scheme 6b, entries 1–2).[31] Additionally, extensive formation of a mixture of diastereomeric tetraols occurred. Employing the Corey–Noe–Lin ligand (61), which was especially designed to improve the regioselectivity towards terminal alkenes, enabled selective access to diol 54 with excellent enantiomeric excess (96 % ee).[32] Switching to the “ent”-Corey–Noe–Lin ligand (55) provided the desired enantiomer of diol 54 in 65 % yield and 93 % ee.[6,33] The large scale Sharpless asymmetric dihydroxylation of geranyl arene 53 proceeded with similar efficiency (65–67 % of diol 54) and nearly quantitative recovery of the “ent”-Corey–Noe–Lin ligand (55) was possible. To avoid tetraol formation, the reaction was discontinued before complete consumption of geranyl arene 53.
Scheme 6.
a) Enantioselective synthesis of norflickinflimiod C (1) featuring a cationic bicyclization/arene hydrogenation sequence and a late-stage diastereoselective epoxidation/epoxide opening cascade. b) Screening of Sharpless asymmetric dihydroxylation conditions. aNMR yield.
Diol 54 was subjected to mesylation of the more accessible, secondary alcohol with mesyl chloride (MsCl) in the presence of pyridine (py) (Scheme 6a). This was directly followed by an intramolecular nucleophilic substitution of the mesylate with the tertiary alcohol upon addition of K2CO3 and methanol to furnish epoxide 39 (97 %).[34] Subjecting epoxide 39 to the previously optimized cationic bicyclization conditions (MsOH, HFIP) afforded tricycle 38 in 50 % yield, which after recrystallization displayed an ee > 99 %. The slightly decreased yield of tricycle 38 (50 % compared to 58 % previously) was attributed to a higher reaction concentration (48 mM compared to 30 mM) and incomplete conversion of interrupted cyclization products. Following the established conditions involving one-pot arene hydrogenation/silyl protection and subsequent methyl ether oxidation, ketone 52 was accessed in 56 % over two steps.[25,29] Regioselective deprotonation of ketone 52 with lithium bis(trimethylsilyl)amide (LHMDS) at –55 °C→–38 °C and trapping of the in situ generated lithium enolate with methyl iodide afforded a mixture of methylated epimers 56 and 57 (d.r. 1 : 1, 96 %). The inconsequential mixture of methylated epimers 56 and 57 converged trough deprotonation with lithium diisopro-pylamide (LDA) at 0 °C to the same lithium enolate, which was acylated by addition of Mander’s reagent (methyl cyanoformate) at –78 °C to obtain β-ketoester 58 as a single diastereomer (76 %). An alternative sequence of first α-acylation, then α-methylation provided predominantly the opposite stereo-chemistry of the β-ketoester (see Supporting Information). Deprotonation of β-ketoester 58 with potassium bis(trimethylsilyl)amide (KHMDS) at 0 °C and addition of phenyl triflimide (PhNTf2) at –78 °C cleanly furnished triflate 59 (86 %). Reduction of the sterically encumbered triflate 59 using formic acid and triethylamine proceeded under Pd-catalysis (SPhos Pd G3 precatalyst) at 60 °C to afford alkene 46 in excellent yield (92 %).[35] To our delight, subjecting alkene 46 to m-CPBA furnished a putative epoxide intermediate I, which underwent acid-catalyzed epoxide opening upon addition of para-toluene-sulfonic acid (p-TsOH), presumably through nucleophilic attack by the spatially aligned ester. Addition of aqueous hydrofluoric acid led to desilylation affording norflickinflimiod C (1) in 77 % as a single diastereomer. To access further ent-pimaranes, norflickinflimiod C (1) was converted to DAP (4) in 73 % yield. Furthermore, alkene 46 was transformed to six additional ent-pimaranes 2, 3 and 5–8 in 1–6 steps with yields ranging from 12 % to 96 %.[6]
Strategy 5 – Radical Tricyclization
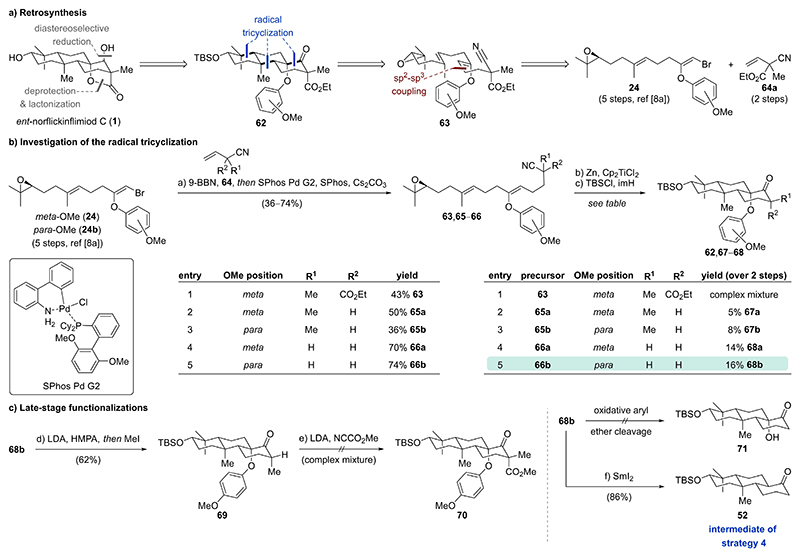
In addition to the previous successful synthetic approach, a different disconnection to norflickinflimiod C (1) was explored. To set the carbocyclic scaffold along with the protected tertiary alcohol, a key radical tricyclization was envisioned (Scheme 7a). Introduction of the γ-lactone motif in ent-norflickinflimiod C (1) was designed through oxidative cleavage of a tertiary alkyl aryl ether in tricyclic ketone 62 followed by lactonization with the spatially aligned ester. The adjacent secondary alcohol would be accessed through diastereoselective ketone reduction. Ketone 62 bearing an arylated acyloin was envisioned to arise from epoxide 63 in a single step through a key nitrile-terminated radical polyene cyclization. Of note, the incorporation of an aryl enol ether in the linear chain is unprecedented for related titanium(III)-mediated radical cyclizations and was expected to strategically introduce oxidation in the carbocyclic scaffold.[36] Epoxide 63 was traced back to two literature known building blocks, vinyl bromide 24[8a] and alkene 64a[37].
Scheme 7.
a) Retrosynthesis for strategy 5 focusing on a radical tricyclization for construction of the carbocyclic scaffold. b) Investigation of the key radical tricyclization. c) Functionalization of tricyclic ketone 68b and interception of strategy 4.
As the steric demand adjacent to the nitril might have a profound influence on the efficiency of the radical tricyclization, we decided to prepare several cyclization precursors (Scheme 7b). Hydroboration of varyingly substituted allyl cyanides 64 with 9-borabicyclo[3.3.1]nonane (9-BBN) at 65 °C enabled in situ generation of trialkylboranes, which were directly subjected to Suzuki–Miyaura cross coupling conditions with meta- and para-methoxy substituted vinyl bromides 24 and 24b.[8a,11b] Coupled products 63, 65, and 66 could be obtained in 36–74 % yield. Next, initiation of the radical tricyclization through reductive epoxide opening with the RajanBabu–Nugent reagent (Cp2TiCl) followed by protection of the newly formed secondary alcohols with TBSCl and imidazole was investigated.[14,38] The silyl protection step was required to aid purification of the cyclization mixture. Unfortunately, fully substituted epoxide 63 only afforded a complex mixture (Scheme 7b, entry 1). Successful radical tricyclization was achieved with α-methyl substituted nitriles 65a and 65b to furnish the desired cyclization products 67a and 67b in 5 % and 8 % over two steps, respectively (Scheme 7b, entries 2–3). Both cyclization products 67a and 67b have an equatorial methyl substituent. Reducing the sterical demand α to the nitril led to increased cyclization efficiency and afforded tricycles 68a (14 % over two steps) and 68b (16 % over two steps) (Scheme 7b, entries 4–5). The para-methoxy aryl ether 68b was investigated for further late-stage functionalizations (Scheme 7c). Deprotonation of aryl ether 68b with LDA in the presence of hexamethylphosphoramide (HMPA) at 0 °C, followed by trapping of the in situ generated lithium enolate with methyl iodide afforded tricycle 69 as a single diastereomer in 62 % yield. Investigation of other methylation conditions resulted in lower yields and diastereose-lectivities (see Supporting Information). Unfortunately, attempts to α-acylate tricycle 69 (analogous to strategy 4) through deprotonation with LDA and addition of Mander’s reagent gave complex mixtures of unidentified products. The same observations were made when attempting to α-acylate tricycle 68b. We ascribe these results to the increased steric demand for axial α-acylation caused by unfavorable interactions with the axial arene. To address this, oxidative degradation of the para-methoxy phenyl group in tricycle 68b was investigated. Despite extensive screening of oxidants and solvent mixtures, the oxidative aryl ether deprotection to tertiary alcohol 71 remained unsuccessful. Finally, tricycle 68b was treated with samarium(II) iodide to allow for efficient reductive deoxygenation to ketone 52 (86 %) and interception of strategy 4 (five steps to norflickinflimiod C (1)).
Biological Investigation – Effects on Lipid Mediator Biosynthesis
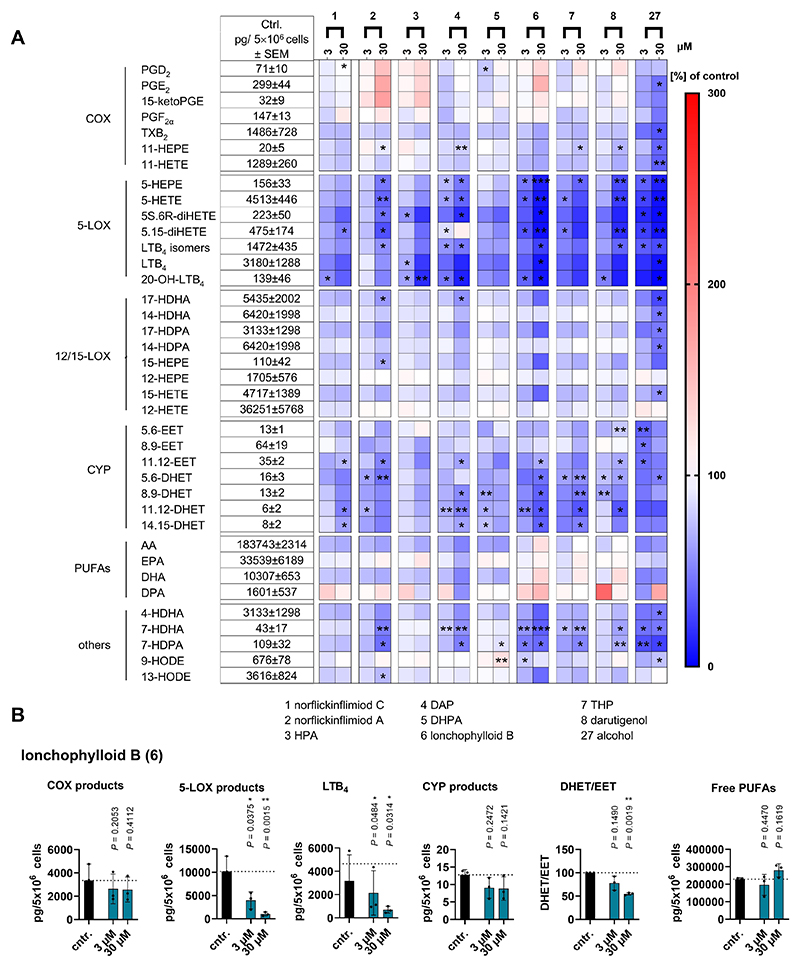
Pimarane natural products possess a broad spectrum of bioactivities including anti-inflammatory, anti-microbial, anti-fungal, anti-viral, anti-malarial, anticancer, cytotoxic, and phytotoxic properties.[2,3,5,39] They also demonstrate anti-diabetic potential[40] and the ability to inhibit vascular contractility[41]. To investigate the effects of ent-pimaranes on the lipid mediator network, we exposed human peripheral blood mononuclear cells (PBMCs) to Staphylococcus aureus conditioned medium (SACM) and performed targeted metabololipidomics to measure key pro- and anti-inflammatory lipid mediators. The effects on lipid mediator biosynthesis are largely consistent among the ent-pimaranes with significant 5-lipoxygenase (5-LOX) inhibition observed and lonchophylloid B (6) being the most active compound (Figure 1A). Most ent-pimaranes reduced 5-LOX activity by approximately 50 % at 3 μM, and lonchophylloid B (6) almost completely suppressed 5-LOX product formation at 30 μM (Figure 1A and B). Other lipid mediator classes, including COX-derived prostanoids, cytochrome P450 enzyme-derived epoxyeicosatrienoic acids (EETs), and 12-/15-LOX products, were less affected, and no redirection of the 5-LOX substrate arachidonic acid to the above mentioned arachidonic acid metabolites was observed (Figure 1A and B). In addition, specific ent-pimaranes seem to inhibit the conversion of EETs to their inactive corresponding diols (DHETs), with lonchophylloid B (6) significantly reducing the DHET/EET ratio (Figure 1B). The ent-pimaranes did not substantially alter the availability of free polyunsaturated fatty acids (Figure 1A and B), ruling out their release from membranes by phospholipases as a major target for the overall decrease in lipid mediator biosynthesis.
Figure 1.
Ent-pimaranes suppress the formation of 5-LOX-derived lipid mediators, suggesting potential anti-inflammatory activity. The effect of ent-pimaranes on the lipid mediator profile was investigated using UPLC-MS/MS in peripheral blood mononuclear cells (PBMCs) stimulated with Staphylococcus aureus conditioned medium (SACM). (A) Heatmap showing the lipid mediator profile upon treatment with vehicle (DMSO, 0.1 %) or ent-pimaranes at concentrations of 3 and 30 μM. Data are shown as % of stimulated control (B). Effects of lonchophylloid B (6) on the formation of COX products, LOX products, LTB4, CYP products (EETs and DHETs), DHET/EET ratio (representing the cellular epoxidase activity) and free PUFAs. Mean (A) or individual values and mean ± SEM (B) of n = 4 independent experiments, * P < 0.05, ** P < 0.01 and *** P < 0.001 vs. stimulated control, paired student’s t-test with log-transformed data. AA, arachidonic acid; COX, cyclooxygenase; CYP, cytochrome P450 monooxygenase; DHA, docosahexaenoic acid; DHET, dihydroxyeicosatrienoic acid; DHET, dihydroxyeicosatrienoic acid; DPA, docosapentaenoic acid; EET, epoxyeicosatrienoic acid; EPA, eicosapentaenoic acid; HDHA, hydroxy docosahexaenoic acid; HDPA, hydroxy docosapentaenoic acid; HEPE, hydroxyeicosapentaenoic acid; HETEs, hydroxydodecanoic acid; HODE, hydroxyoctadecadienoic acid; LOX, lipoxygenase; LTB4, leukotriene B4; PG, prostaglandin; PUFA, polyunsaturated fatty acid; TXB2, Thromboxane B2.
Biological Investigation – Anti-Ferroptotic Properties
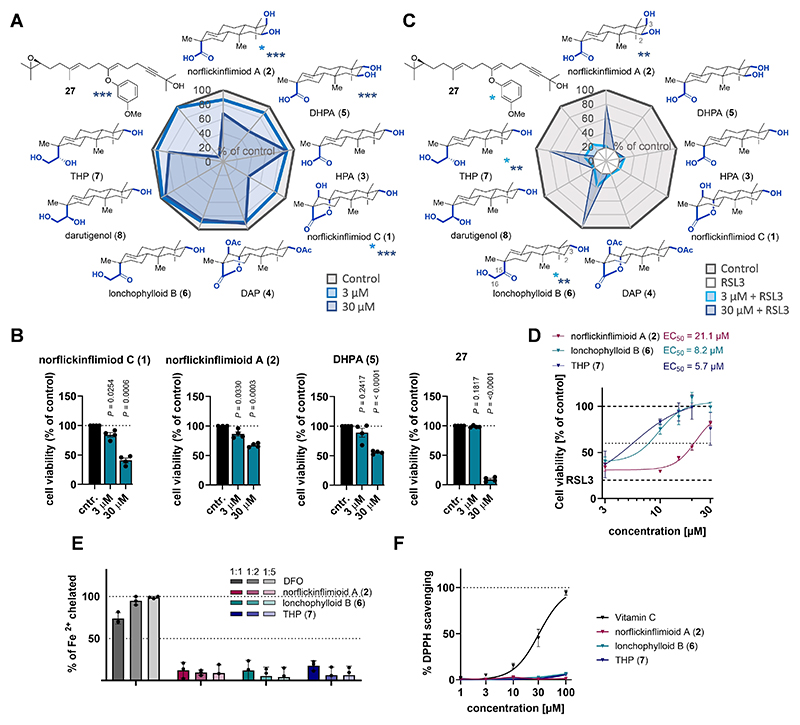
Ferroptosis, a regulated form of cell death, is implicated in various degenerative diseases such as Alzheimer’s, Parkinson’s, multiple sclerosis (MS), degenerative liver disorders, and certain cancers.[42] To explore the anti-ferroptotic potential of ent-pimaranes, we first investigated the cell viability of human HepaRG liver cells upon treatment with these compounds. At 30 μM, norflickinflimiod C (1), norflickinflimiod A (2), DHPA (5), and alcohol 27 exhibited some inherent toxicity (Figure 2A and B).
Figure 2. The configuration of stereocenters critically determines anti-ferroptotic activity of ent-pimaranes.
(A) Radar blot and (B) bar graph showing the viability of HepaRG cells treated with vehicle (DMSO, 0.5 %) or ent-pimaranes for 48 hours as determined by MTT assay. (C) Radar plot and (D) line graph showing the viability of HepaRG cells treated with vehicle (DMSO, 0.5 %), the ferroptosis inducer RSL3 (0.2 μM), or ent-pimaranes alongside RSL3 (0.2 μM) for 48 hours, as determined by MTT assay. (E) Iron-binding capacity measured with the iron chelator ferene with deferoxamine (DFO) as the positive control. (F) DPPH radical scavenging activity with vitamin C as positive control and ethanol (EtOH) as vehicle control, to which all values were normalized. * P < 0.05, ** P < 0.01 vs. vehicle (DMSO, 0.5 %) (A,B) or RSL3 (C), paired Student’s t-test.
To evaluate their ability to inhibit ferroptosis, co-treatments of the ent-pimaranes alongside the ferroptosis inducer RSL3 — a GPX4 inhibitor — were performed (Figure 2C and D). Notably, the ent-pimaranes appeared to require specific structural features for anti-ferroptotic activity. Norflickinflimiod A (2) was highly effective at 30 μM, almost completely preventing ferroptosis, whereas DHPA (5), which differs only in the stereo-chemistry of its C3 OH group, failed to provide any protective effect. HPA (3), which lacks an OH group at C2 and features an equatorial C3 OH compared to the axial C3 OH in norflickin-flimiod A (2), was also ineffective. Similarly, norflickinflimiod C (1) and its doubly acetylated derivative DAP (4), both lacking an axial C3 OH group, were found to be inactive. These findings underscore the critical importance of the axial C3 OH group in maintaining biological activity for these 16-nor-ent-pimaranes. Despite the absence of the crucial axial OH group on C3, lonchophylloid B (6) effectively prevented RSL3-induced ferroptosis at 30 μM. This may be due to additional interactions offered by the C15 and C16 oxidation pattern with a molecular target. It is noteworthy that of the two related epimers THP (7) and darutigenol (8), in which the carbonyl group of lonochophylloid B (6) is replaced by a hydroxy group, only the (S) isomer THP (7) exhibits biological activity, whereas the (R) isomer darutigenol (8) does not. This difference highlights the essential influence of the stereochemistry of a C15 OH on anti-ferroptotic efficacy. EC50 analyses showed that lonochophylloid B (6) and THP (7) were effective in reducing ferroptosis cell death at low micromolar concentrations, whereas norflickin-flimiod A (2) was less potent.
Notably, most ferroptosis inhibitors work by iron chelation or direct radical scavenging.[43] However, none of the ent-pimaranes tested (norflickinflimiod A (2), lonochophylloid B (6), or THP (7)) showed iron-binding capacity (Figure 2E) or radical scavenging activity against DPPH radicals, even at concentrations up to 100 μM (Figure 2F). Thus, it appears that ent-pimaranes inhibit ferroptosis by non-classical mechanisms.
Conclusions
In conclusion, we have explored five different synthetic strategies relying on either radical or cationic polyene cyclizations to provide rapid entry to the family of pimarane natural products. These studies culminated in a synthetic platform that enabled the enantioselective total synthesis of eight ent-pimaranes in 11–16 steps and 1.0–7.8 % overall yield. The initial strategy was abandoned due to unsuccessful key tetracyclization of a dual nucleophilic aryl enol ether paired with an allenylic sulfide. In the second strategy, a Wessely acetoxylation was able to introduce the requisite oxidation and generate a dienone moiety for the radical cyclization. The preferred reduction of the dienone over reductive epoxide opening forced us to abandon this approach. In the third strategy, a cationic bicyclization employing methanesulfonic acid and HFIP was developed. Subsequent oxidative dearomatization allowed installation of the protected tertiary alcohol, but further functionalization failed due to facile rearomatization. In the fourth-generation strategy, a Sharpless asymmetric dihydroxylation enabled enantioselective access to the tricyclic core structure, which was further elaborate via a mild rhodium-catalyzed reductive dearomatization. A sequence of diastereo-selective transformations furnished a key intermediate, which could be converted to eight ent-pimaranes bearing modifications in the A and C ring in 1–6 steps. In the fifth-generation strategy, an unprecedented radical tricyclization featuring an epoxide, an aryl enol ether, and a nitrile successfully generated a tricyclic ketone, which could be used to intercept an intermediate of the fourth strategy. Detailed biological investigations of the lipid mediator profile provided the first evidence that the previously documented anti-inflammatory effects[2] may also be driven by a potent inhibition of 5-LOX-dependent leukotriene formation, as evidenced by a significant decrease in the levels of leukotriene (LT)B4 and other 5-LOX products. In addition, this is the first report of ent-pimaranes inhibiting ferroptosis, a regulated cell death pathway that is critical in numerous disease processes, particularly in degenerative conditions. Notably, unlike for the effect on lipid mediators, the configuration and oxidation pattern of the ent-pimaranes play a critical role in determining the efficacy of these compounds against ferroptosis with their effectiveness ranging from potent agents in the low micromolar range to inactive compounds at concentrations of up to 30 μM, which indicates specific interactions with target biomolecules. This hypothesis is further supported by our findings that, unlike classical ferroptosis inhibitors that act via non-enzymatic pathways, ent-pimaranes do not directly bind ferrous iron or scavenge radicals. Overall, the findings within this study demonstrate the power and the caveats of polycyclization strategies for the total synthesis of terpenoids and are expected to streamline the design of future terpenoid syntheses. Furthermore, ent-pimaranes were identified as a novel scaffold class for non-redox active ferroptosis inhibition.
Supplementary Material
Supporting information for this article is available on the WWW under https://doi.org/10.1002/chem.202403811
Acknowledgements
This research was funded in part by the European Research Council under the European Union’s Horizon 2020 research and innovation program (grant agreement No 101000060 to T.M.), the Tyrolean Science Fund TWF (F.44318/8-2022 to I.P.), and the Austrian Science Fund (FWF) (10.55776/P36299 to A.K.). We thank Nicolas Müller (University of Innsbruck) for helpful discussions during the preparation of this manuscript. We are thankful to the Center for Molecular Biosciences (CMBI). Furthermore, we thank Alilou Mostafa (University of Innsbruck) for assistance with ECD analysis.
Footnotes
Conflict of Interests
The authors declare no conflict of interest.
Data Availability Statement
The data that support the findings of this study are available in the supplementary material of this article.
References
- [1].a) Newman DJ, Cragg GM. J Nat Prod. 2020;83:770–803. doi: 10.1021/acs.jnatprod.9b01285. [DOI] [PubMed] [Google Scholar]; b) Kingston DGI. J Nat Prod. 2011;74:496–511. doi: 10.1021/np100550t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].a) Chen J-L, Zhao Z-M, Xue X, Tang G-H, Zhu L-P, Yang D-P, Jiang L. RSC Adv. 2014;4:14447–14456. [Google Scholar]; b) Chen J-L, Zhong W-J, Tang G-H, Li J, Zhao Z-M, Yang D-P, Jiang L. Molecules. 2014;19:5863–5875. doi: 10.3390/molecules19055863. [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Li H, Zhao J-J, Chen J-L, Zhu L-P, Wang D-M, Jiang L, Yang D-P, Zhao Z-M. Phytochemistry. 2015;117:400–409. doi: 10.1016/j.phytochem.2015.07.005. [DOI] [PubMed] [Google Scholar]
- [3].Ma GX, Wang TS, Yin L, Pan Y, Guo YL, LeBlanc GA, Reinecke MG, Watson WH, Krawiec M. J Nat Prod. 1998;61:112–115. doi: 10.1021/np970065o. [DOI] [PubMed] [Google Scholar]
- [4].Pudles I, Diara A, Lederer E. Bull Soc Chim Fr. 1959:693–700. [Google Scholar]
- [5].Wang J, Duan H, Wang Y, Pan B, Gao C, Gai C, Wu Q, Fu H. J Nat Prod. 2017;80:19–29. doi: 10.1021/acs.jnatprod.6b00150. [DOI] [PubMed] [Google Scholar]
- [6].Plangger I, Wurst K, Magauer T. Org Lett. 2022;24:7151–7156. doi: 10.1021/acs.orglett.2c02843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].For selected examples see:Ireland RE, Schiess PW. J Org Chem. 1963;28:6–16. Chu-Moyer MY, Danishefsky SJ. J Am Chem Soc. 1992;114:8333–8334. Economou C, Tomanik M, Herzon SB. J Am Chem Soc. 2018;140:16058–16061. doi: 10.1021/jacs.8b10891. Germain J, Deslongchamps P. J Org Chem. 2002;67:5269–5278. doi: 10.1021/jo025873l. Jansen BJ, Schepers GC, de Groot A. Tetrahedron. 1989;45:2773–2776. Sicherer-Roetman A, Jansen BJM, de Groot A. Recl Trav Chim Pays-Bas. 1985;104:193–202. Suh YG, Jun RO, Jung JK, Ryu JS. Synth Commun. 1997;27:587–593. Ling T, Kramer BA, Palladino MA, Theodorakis EA. Org Lett. 2000;2:2073–2076. doi: 10.1021/ol0060578. Mori K, Waku M. Tetrahedron. 1985;41:5653–5660.
- [8].a) Feilner JM, Wurst K, Magauer T. Angew Chem Int Ed. 2020;59:12436–12439. doi: 10.1002/anie.202003127. [DOI] [PMC free article] [PubMed] [Google Scholar]; Angew Chem. 2020;132:12536–12539. [Google Scholar]; b) Yajima A, Toda K, Okada K, Yamane H, Yamamoto M, Hasegawa M, Katsuta R, Nukada T. Tetrahedron Lett. 2011;52:3212–3215. [Google Scholar]; c) van Tamelen EE, Marson SA. J Am Chem Soc. 1975;97:5614–5616. doi: 10.1021/ja00838a054. [DOI] [PubMed] [Google Scholar]; d) Li Y, Fu S, Liu B. Org Biomol Chem. 2023;21:4409–4413. doi: 10.1039/d3ob00575e. [DOI] [PubMed] [Google Scholar]
- [9].Feilner JM, Plangger I, Wurst K, Magauer T. Chem - Eur J. 2021;27:12410–12421. doi: 10.1002/chem.202101926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Garegg PJ, Samuelsson B. J Chem Soc, Chem Commun. 1979:978–980. [Google Scholar]
- [11].a) Speck K, Wildermuth R, Magauer T. Angew Chem Int Ed. 2016;55:14131–14135. doi: 10.1002/anie.201608040. [DOI] [PubMed] [Google Scholar]; Angew Chem. 2016;128:14337–14341. [Google Scholar]; b) Miyaura N, Yamada K, Suzuki A. Tetrahedron Lett. 1979;20:3437–3440. [Google Scholar]
- [12].Tsutsumi K, Fujimoto K, Yabukami T, Kawase T, Morimoto T, Kakiuchi K. Eur J Org Chem. 2004;2004:504–510. [Google Scholar]
- [13].a) Wessely F, Lauterbach-Keil G, Sinwel F. Monatsh Chem. 1950;81:811–818. [Google Scholar]; b) Wessely F, Sinwel F. Monatsh Chem. 1950;81:1055–1070. [Google Scholar]
- [14].Nugent WA, RajanBabu TV. J Am Chem Soc. 1988;110:8561–8562. [Google Scholar]
- [15].For selected examples of related cationic bicyclizations, see:Zhao J-F, Zhao Y-J, Loh T-P. Chem Commun. 2008:1353–1355. doi: 10.1039/b718337b. Kim MB, Shaw JT. Org Lett. 2010;12:3324–3327. doi: 10.1021/ol100929z. Castillo A, Del Moral JFQ, Barrero AF. Nat Prod Commun. 2017;12:657–658. Cherney EC, Green JC, Baran PS. Angew Chem Int Ed. 2013;52:9019–9022. doi: 10.1002/anie.201304609. Angew Chem. 2013;125:9189–9192. Mai D, Uchenik D, Vanderwal C. Synlett. 2017;28:1758–1762. Onyango EO, Fu L, Gribble GW. Org Lett. 2014;16:322–324. doi: 10.1021/ol403289y. Rajendar G, Corey EJ. J Am Chem Soc. 2015;137:5837–5844. doi: 10.1021/jacs.5b03229.
- [16].For alternative 7 – 8 step syntheses of tricycle 38, see:Turner RB, Gänshirt KH, Shaw PE, Tauber JD. J Am Chem Soc. 1966;88:1776–1785. Cargill RL, Bushey DF, Dalton JR, Prasad RS, Dyer RD, Bordner J. J Org Chem. 1981;46:3389–3399.
- [17].Tian Y, Xu X, Zhang L, Qu J. Org Lett. 2016;18:268–271. doi: 10.1021/acs.orglett.5b03438. [DOI] [PubMed] [Google Scholar]
- [18].a) Bégué J-P, Bonnet-Delpon D, Crousse B. Synlett. 2004:18–29. [Google Scholar]; b) Eberson L, Hartshorn MP, Persson O, Radner F. Chem Commun. 1996:2105–2112. [Google Scholar]; c) Shuklov I, Dubrovina N, Börner A. Synthesis. 2007:2925–2943. [Google Scholar]; d) Sinha SK, Bhattacharya T, Maiti D. React Chem Eng. 2019;4:244–253. [Google Scholar]; e) Plangger I, Pinkert T, Wurst K, Magauer T. Ang Chem Int Ed. 2023;62:e202307719. doi: 10.1002/anie.202307719. [DOI] [PMC free article] [PubMed] [Google Scholar]; f) Plangger I, Pinkert T, Wurst K, Magauer T. Ang Chem. 2023;135:e202307719. doi: 10.1002/anie.202307719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Rabideau PW, Marcinow Z. Organic Reactions. Vol. 42. Wiley Online Library; 2004. The Birch Reduction of Aromatic Compounds; pp. 1–334. [Google Scholar]
- [20].Johnson WS, Bannister B, Pappo R. J Am Chem Soc. 1956;78:6331–6339. [Google Scholar]
- [21].Wilds AL, Nelson NA. J Am Chem Soc. 1953;75:5360–5365. [Google Scholar]
- [22].Peters BK, Rodriguez KX, Reisberg SH, Beil SB, Hickey DP, Kawamata Y, Collins M, Starr J, Chen L, Udyavara S, Klunder K, et al. Science. 2019;363:838–845. doi: 10.1126/science.aav5606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Burrows J, Kamo S, Koide K. Science. 2021;374:741–746. doi: 10.1126/science.abk3099. [DOI] [PubMed] [Google Scholar]
- [24].a) Lin S-C, Chein R-J. J Org Chem. 2017;82:1575–1583. doi: 10.1021/acs.joc.6b02766. [DOI] [PubMed] [Google Scholar]; b) Johnson WS, Rogier ER, Ackerman J. J Am Chem Soc. 1956;78:6322–6331. [Google Scholar]
- [25].Maegawa T, Akashi A, Yaguchi K, Iwasaki Y, Shigetsura M, Monguchi Y, Sajiki H. Chem - Eur J. 2009;15:6953–6963. doi: 10.1002/chem.200900361. [DOI] [PubMed] [Google Scholar]
- [26].van Heerden FR, Dixon JT, Holzapfel CW. Tetrahedron Lett. 1992;33:7399–7402. [Google Scholar]
- [27].Kamijo S, Matsumura S, Inoue M. Org Lett. 2010;12:4195–4197. doi: 10.1021/ol1018079. [DOI] [PubMed] [Google Scholar]
- [28].Moriyama K, Nakamura Y, Togo H. Org Lett. 2014;16:3812–3815. doi: 10.1021/ol501703y. [DOI] [PubMed] [Google Scholar]
- [29].Gilissen PJ, Blanco-Ania D, Rutjes FPJT. J Org Chem. 2017;82:6671–6679. doi: 10.1021/acs.joc.7b00632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Wang Z-X, Tu Y, Frohn M, Zhang J-R, Shi Y. J Am Chem Soc. 1997;119:11224–11235. [Google Scholar]
- [31].a) Sharpless KB, Amberg W, Bennani YL, Crispino GA, Hartung J, Jeong KS, Kwong HL, Morikawa K, Wang ZM. J Org Chem. 1992;57:2768–2771. [Google Scholar]; b) Becker H, Sharpless KB. Angew Chem Int Ed. 1996;35:448–451. [Google Scholar]; Angew Chem. 1996;108:447–449. [Google Scholar]
- [32].Corey EJ, Noe MC, Lin S. Tetrahedron Lett. 1995;36:8741–8744. [Google Scholar]
- [33].a) Zhang Y, Ji Y, Franzoni I, Guo C, Jia H, Hong B, Li H. Angew Chem. 2021;60:14869–14874. doi: 10.1002/anie.202104014. [DOI] [PubMed] [Google Scholar]; Angew Chem Int Ed. 2021;133:14995–15000. [Google Scholar]; b) Zhuo J, Zhu C, Wu J, Li Z, Li C. J Am Chem Soc. 2022;144:99–105. doi: 10.1021/jacs.1c11623. [DOI] [PubMed] [Google Scholar]
- [34].Crispino GA, Sharpless KB. Synthesis. 1993;1993:777–779. [Google Scholar]
- [35].Bruno NC, Tudge MT, Buchwald SL. Chem Sci. 2013;4:916–920. doi: 10.1039/C2SC20903A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].For a related cyclization, see:Fernández-Mateos A, Teijón P, Clemente R, González R, González F. Synlett. 2007:2718–2722.
- [37].Bunce RA, Burns SE. Org Prep Proced Int. 1999;31:99–106. [Google Scholar]
- [38].Rosales A, Rodríguez-García I, Muñoz-Bascón J, Roldan-Molina E, Padial NM, Morales LP, García-Ocaña M, Oltra JE. Eur J Org Chem. 2015;2015:4567–4591. [Google Scholar]
- [39].Reveglia P, Cimmino A, Masi M, Nocera P, Berova N, Ellestad G, Evidente A. Chirality. 2018;30:1115–1134. doi: 10.1002/chir.23009. [DOI] [PubMed] [Google Scholar]
- [40].Mohammed A, Tajuddeen N, Ibrahim MA, Isah MB, Aliyu AB, Islam MS. Pharmacol Res. 2022;179:106158. doi: 10.1016/j.phrs.2022.106158. [DOI] [PubMed] [Google Scholar]
- [41].Ambrosio SR, Tirapelli CR, Da Costa FB, de Oliveira AM. Life Sci. 2006;79:925–933. doi: 10.1016/j.lfs.2006.05.015. [DOI] [PubMed] [Google Scholar]
- [42].a) Koeberle SC, Kipp AP, Stuppner H, Koeberle A. Med Res Rev. 2023;43:614–682. doi: 10.1002/med.21933. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Stockwell BR. Cell. 2022;185:2401–2421. doi: 10.1016/j.cell.2022.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Berndt C, Alborzinia H, Amen VS, Ayton S, Barayeu U, Bartelt A, Bayir H, Bebber CM, Birsoy K, Böttcher JP, Brabletz S, et al. Redox Biol. 2024;75:103211. doi: 10.1016/j.redox.2024.103211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Vahabi R, Frey W, Pietruszka J. J Org Chem. 2013;78:11549–11559. doi: 10.1021/jo402130u. [DOI] [PubMed] [Google Scholar]
- [45].Grieco PA, Masaki Y. J Org Chem. 1974;39:2135–2136. [Google Scholar]
- [46].Gosselin P, Maignan C, Rouessac F. Synthesis. 1984:876–881. [Google Scholar]
- [47].Kaufman TS, Sindelar RD, Jürgens AR. Magn Reson Chem. 1989;27:1178–1181. [Google Scholar]
- [48].Crabtree SR, Chu WLA, Mander LN. Synlett. 1990;1990:169–170. [Google Scholar]
- [49].Salamone S, Waltl L, Pompignan A, Grassi G, Chianese G, Koeberle A, Pollastro F. Plants. 2022;11:2130. doi: 10.3390/plants11162130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].a) Rao Z, Caprioglio D, Gollowitzer A, Kretzer C, Imperio D, Collado JA, Waltl L, Lackner S, Appendino G, Muñoz E, Temml V, et al. Biochem Pharmacol. 2022;203:115202. doi: 10.1016/j.bcp.2022.115202. [DOI] [PubMed] [Google Scholar]; b) van Anh TT, Mostafa A, Rao Z, Pace S, Schwaiger S, Kretzer C, Temml V, Giesel C, Jordan PM, Bilancia R, Weinigel C, et al. Acta Pharm Sin B. 2021;11:1629–1647. doi: 10.1016/j.apsb.2021.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Neukirch K, Alsabil K, Dinh C-P, Bilancia R, Raasch M, Ville A, Cerqua I, Viault G, Bréard D, Pace S, Temml V, et al. J Med Chem. 2021;64:11496–11526. doi: 10.1021/acs.jmedchem.1c00806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Su F, Descher H, Bui-Hoang M, Stuppner H, Skvortsova I, Rad EB, Ascher C, Weiss A, Rao Z, Hohloch S, Koeberle SC, et al. Redox Biol. 2024;75:103257. doi: 10.1016/j.redox.2024.103257. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available in the supplementary material of this article.