Abstract
The exposome was proposed following the realization that most human diseases have an environmental rather than a genetic (hereditary) origin. Non-communicable diseases are, in fact, the consequence of multiple exposures that activate a sequence of stages in a multistage process that already starts in early life. This attracted attention to both the multiplicity (in fact, potentially the totality) of exposures humans are exposed to since conception and to the life-long perspective of disease causation. In this paper, we examine an extension of the exposome concept that incorporates a Darwinian approach based on the concept of phenotypic plasticity. One of the theses is that interpreting exposome science as “precision environmental research” is only a partial interpretation, largely focused on chemical exposures, while a broadening of the perspective is needed, also in light of the planetary crisis. Such broadening involves the incorporation of basic concepts from evolutionary biology and medicine, including the ability of organisms to adapt to rapidly changing environments. We refer in particular to cancer and “Darwinian carcinogenesis.”
Keywords: evolutionary theory, phenotypic plasticity, agnostic research, theory-driven research, environment, human health
Synopsis
Exposome research is more than agnostic, technology-driven research or “precision” environmental research but needs to be connected to the theory of evolution, ie, the basic laws of biology.
The concept of exposome has gained traction in the last decade, partly because of large investments, particularly by the European Commission. The concept was introduced to allow a leap forward in the science of environmental health and, more generally, in the investigation of the causes of diseases, following the acknowledgment that these are mainly environmental rather than genetic (inherited). Non-communicable diseases are the consequence of multiple exposures that activate a sequence of stages in a multistage process that already starts in early life. This attracted attention to both a multiplicity (in fact, potentially the totality) of exposures humans are exposed to since conception and to a life-long perspective of disease causation; these are two of the principal components of the exposome concept (for completeness, we report the main definitions of exposome in the Box 1).
Box 1. Definitions of exposome.
The first definition is due to Christopher Wild: the exposome “encompasses life-course environmental exposures (including life-style factors), from the prenatal period onwards.”3 Porta’s Dictionary of Epidemiology defines the exposome as “a potential measure of the effects of life-course exposures on health. It comprises the totality of exposures to which an individual is subjected from conception to death, including those resulting from environmental agents, socioeconomic conditions, lifestyle, diet, and endogenous processes. Characterization of the exposome could permit addressing possible associations with health outcomes and their significance, if any, alone or in combination with genomic factors.”34
According to Miller and Jones, the exposome is “the cumulative measure of environmental influences and associated biological responses throughout the lifespan, including exposures from the environment, diet, behavior, and endogenous processes.”35
According to a slightly different perspective, Rappaport wrote that “The exposome concept promotes environmental analogues of genome-wide association studies, which employ untargeted omic methods to compare biospecimens from diseased and healthy subjects. The goal of such investigations is to discover key biomarkers of exposure that enable follow-up hypotheses to be explored regarding sources of exposure, dose–response relationships, mechanisms of action, disease causality and public health interventions.”3
Again Rappaport: “The concept of the exposome, representing the totality of exposures received by a person during life, encompasses all sources of toxicants and, therefore, offers scientists an agnostic approach for investigating the environmental causes of chronic diseases. In this context, it is appropriate to regard the ‘environment’ as the body’s internal chemical environment and to define ‘exposures’ as levels of biologically active chemicals in this internal environment. To explore the exposome, it makes sense to employ a top-down approach based upon biomonitoring (eg, blood sampling) rather than a bottom-up approach that samples air, water, food, and so on.”36
The exposome concept has been coined recently, and there is still uncertainty about its interpretation. For example, one can think that it is too broad since it encompasses all potential exposures, that is, it is unfeasible. Another concern is the fact that traditional environmental research has been redefined by some as exposome research, thus distorting the initial aims. One way to clarify the boundaries of the concept is to stress (1) that “single exposure—single disease” approaches do not fall within the exposome remit; and (2) that a time-related (possibly life-course) approach is integral to the concept. In addition, it is useful to reaffirm that the term exposome covers three domains: (1) the exposures that occur in the real world, and also the molecular changes they induce in the human body; (2) the tools that are available to investigate such exposures and their biological effects; (3) the “community of practice,” that is the scientific community that shares common goals and tools inspired by the exposome concept.
It is important to keep these three components separate to avoid misinterpretations: exposures and their molecular and biochemical impacts cannot be known in their entirety, and we can only proceed by selecting subsets according to priorities; tools are under continuous development (typically, omics), possibly on the basis of the mentioned priorities; and the concept of community of practice is important because it defines the internal rules, definitions, ethos, etc., of those involved in such research.
One of the main consequences of launching exposome research has been a proliferation of “agnostic” or untargeted investigations. This originated from the understanding that many, if not most, of the relevant environmental exposures at the roots of human diseases are unknown, so that we need to provide powerful untargeted tools for discovery (see in particular reference Rappaport and Smith1). However, the exposome should not be interpreted only as the application of new technologies (sensors, omics) to epidemiology for hypothesis generation but requires a stronger theoretical basis, rooted in biology. Also, we believe that mimicking precision medicine by referring to precision prevention (or “precision environmental research”: see Baccarelli et al2) is too limited an interpretation of the exposome. The attempt here is to use evolutionary concepts (like phenotypic plasticity and Darwinian carcinogenesis) to add new dimensions to the exposome concept and practice.
What we knew already: migrants, geographic distribution and trends of diseases
The usual narrative says that the exposome was introduced because of the acknowledgment that most human diseases have an environmental origin, so that as many resources should be put in the study of environmental exposures as in genomics and inheritance.1,3 This is the first pillar of the exposome. In fact, much of pre-exposome epidemiological understanding of non-communicable diseases already supported the view that human diseases are mainly of environmental origin. We do not need particularly sophisticated tools to reach the conclusion of a predominance of the environment over inheritance. Nevertheless, the exposome approach can strongly reinforce this conclusion.
Clear evidence in the past came in particular from the study of migrant populations. As many examples show (French to Québec, Japanese to the United States, Italians to Australia, etc.), those who migrate acquire the risk of non-communicable diseases (NCD) that is typical of the host population. The Japanese in the United States (Hawaii) at the second generation after migration showed risks of colorectal cancer much higher than the Japanese who stayed home, and much lower risks of stomach cancer. After migration, their risks tended to align with the risks of the American whites, an observation that strongly argues against a genetic, inherited origin of these cancers and other NCD.4
Apart from migrants, the simple observation of the distribution of NCD over space and time speaks in favor of environmental factors: there is little matching between gene variants and the incidence of the majority of NCD at a geographic level, and such incidence changes too quickly to be explained by modifications in the gene pool. Typical is the case of the crossing of curves for breast and cervical cancers in low-income countries that occurs in a few years after countries develop economically.5 Despite these simple observations, in the past, there have been great expectations towards genetics and genomics to address NCD.
Agnostic or theory-driven?
Epidemiology had already suggested that most NCD have an environmental origin, but it failed to identify at least 50% of those causes.1 This is the reason why the need for an agnostic/untargeted component in the exposome has been put forward, aiming to generate new hypotheses on the causes of diseases. An agnostic approach (ie, hypothesis-free) made sense and has been successful in the case of genomics (through genome-wide association studies, GWAS) because genes are fixed, that is, they do not vary in the course of time in a given individual, with the exception of rare somatic mutations. Conversely, exposures and molecular markers that are relevant to human diseases (microbiota, chemical exposures, diet, smoking, stress, etc.) constantly vary in time, interact in complex ways with molecules in the body, induce alterations in the stages that form the multistage process (and these alterations can be repaired, eg, DNA repair), and effects can be cumulative, etc. Therefore, an agnostic (untargeted) type of investigation of the exposome at any point in time has a high probability of being limited, capturing only a small proportion of all the events that occur in a life-long perspective.
The interplay of genetic susceptibility, pathogenic hits from outside and inside the body, repair, etc., should also be examined under the concept of “phenotypic plasticity,” that is the ability of organisms to adapt to environmental challenges, mediated by epigenetic modifications. Molecular changes, rather than being looked at in isolation like genetic variants, should be analyzed in this context, that is, in a continuum that goes from external exposures to diseases, with a historical-biographical approach. This is also the reason why the concept of meet-in-the-middle,6 that is, intercalating evidence of mechanistic events between exposures and health outcomes, has been put forward as a strategic approach to epidemiological study designs.
Bottom-up and top-down
How should we apply the concept of agnostic investigations? Starting from exposures or from diseases? The bottom-up versus top-down debate was started in particular by a paper by Steven Rappaport7 that suggested that the exposome tools can be used to look at the multiple consequences for health stemming from exposures in a certain environmental compartment (bottom-up); or vice-versa, to look at the multiple exposures associated with a given disease (top-down approach).
These two views are complementary, but not in an obvious way. They correspond by and large to another old controversy in science, reductionism versus holism. “Ontological reductionism” is the belief that everything existing in nature is “made up of a small set of primitive and indivisible material elements,” and “Epistemological reductionism supposes that knowledge about one field of scientific enquiry, which pertains to phenomena at a given level, can be reduced, at least in principle, to another body of knowledge, which concerns a lower and more basic level.”8 Complementary to reductionism, holism is the idea that systems possess properties as wholes apart from the properties of their component parts.
Both the bottom-up and the top-down applications of exposomic tools can be reductionist in that they can isolate single exposures or single diseases to consider for an agnostic search. In fact, the top-down and bottom-up views are methodological approaches, whereas the reductionism versus holism controversy mainly refers to the ontological interpretation of scientific phenomena. Holism and reductionism tend to be dichotomous, but in fact, they can be complementary, exactly like the bottom-up and top-down approaches. Getting rid of the “mystical” element in the “holistic” terminology, the underlying idea is that health and disease, and their interactions with the environment, are too complex to be reduced to single components like gene variants or single exposures (the “anti-reductionist” view).
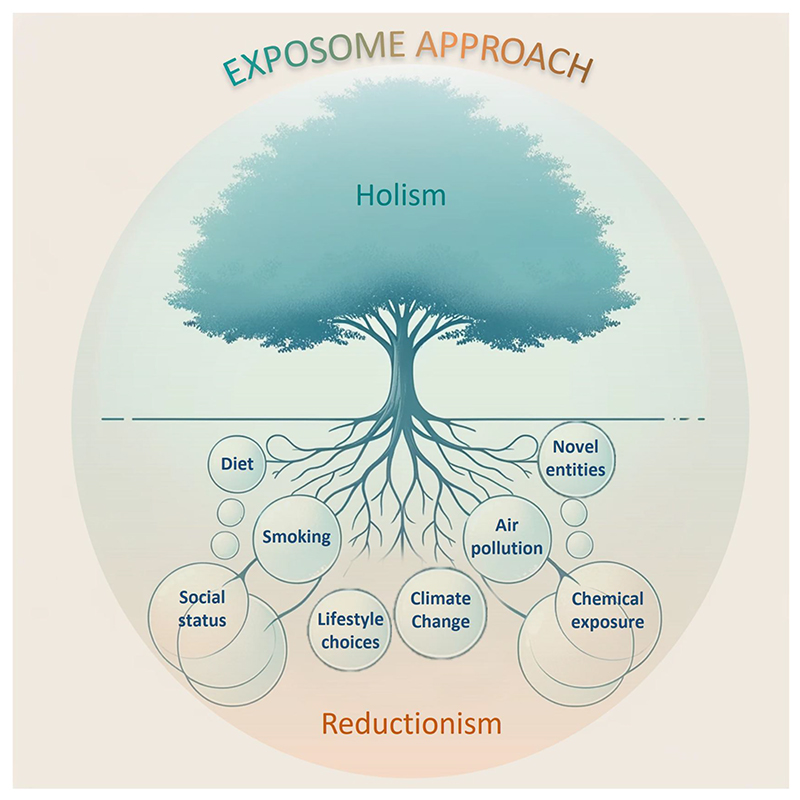
For example, social epidemiology provides good evidence of the overarching role of social circumstances—starting at birth or even before—in prompting a chain of events that lead to poor aging and health.9 This is true also of “planetary health,” in which human health depends on the broad transformations occurring at multiple levels (climate, biodiversity, chemical pollution, diet, etc.) and their interactions. However, this reasoning is clearly insufficient since it does not acknowledge that before reconstructing the “holistic” picture, we need to isolate single causes or risk factors according to a “reductionist” methodological approach (Figure 1).
Figure 1.
The complementarity of the reductionist and holistic approaches in the understanding of the exposome. The roots of the tree represent the reductionist approach. This approach focuses on breaking down the exposome into individual elements or factors. For instance, examining specific chemical exposures, lifestyle choices, or social conditions separately. The trunk of the tree interconnects all these influences (in this sense it is holistic) and then it leads to the multiple health effects of the combination of exposures. The tree, as a whole, illustrates how both approaches are essential in understanding the human exposome (This image was generated with the assistance of AI tool DALL-E-2)
The interplay between complexity and reductionism has been termed “the arch of knowledge” in philosophy of science,10 that is, researchers switch between broad, holistic perspectives and detailed, reductionist analyses to gain a more comprehensive understanding of complex phenomena. All major theories in science have emerged from an interplay between observations (ie, DNA’s crystallographic images), formulation of broader hypotheses (the double strand and DNA’s replication), further observations confirming or falsifying the hypotheses, and finally consolidated theories (DNA as a template for inheritance and cell’s metabolism).
One way to proceed in epidemiology is, for example, first to conduct top-down omics investigations that use biospecimens from nested case-control studies to generate hypotheses. Then subsequent bottom-up studies would test hypotheses that eventually result in potentially causal exposures and pathways.
This line of research that incorporates agnostic hypothesis generation is particularly relevant to the study of “new entities,” a concept put forward by Persson and others at the Stockholm Environment Institute to indicate chemicals that are novel in a geological sense, that is, are not present in nature or occur today at much higher concentrations than in nature.11 It is estimated that there are at least 350 000 chemicals on the global market: clearly, the current speed of research and regulation (with a one-by-one approach) is totally inefficient and does not take into consideration potential interactions. The current pace of identification of toxic properties of chemicals, and their regulation as performed by EPA in the United States, or REACH in Europe is too slow. In this case, the bottom-up exposome has much to offer in terms of agnostic investigations followed by selection of high-priority compounds and further toxicological investigations.
An evolutionary perspective
“Risk factor” epidemiology has been powerful in identifying important causes of NCD like smoking, dietary factors, air pollution, occupational exposures, and others. Its limitations have been already pointed out several times and we will not repeat them here.12 In addition to overlooking the importance of life-course experiences, risk factor epidemiology also ignored the foundations of biology, that is, the evolutionary perspective.
For example, if we look at the ways the obesity epidemic is dealt with, research driving public health is focused on dietary components, excess calories, and physical exercise. Of course, this makes a lot of sense and is a prima facie very reasonable approach (though not very successful in curbing the epidemic). However, a closer evolutionary look suggests that fat deposition in the body follows more complex temporal pathways that are determined by evolutionary pressures, so that fat deposition is different at different ages and in the two genders and has multiple determinants and phenotypes (in fact, it would be better to refer to “obesities”).13
The “crafty phenotype” hypothesis put forward by Wells14 consists in “a genetic basis for accommodating variability in the ‘fitness value’ of fat through phenotypic plasticity, depending on the endogenous and exogenous characteristics of each individual” (for phenotypic plasticity see below). In practice, fat is differentially distributed between peripheral and abdominal depots (at different ages and in the two genders), reflecting variable prioritization of survival versus reproduction in the course of life.14
The temporalized perspective suggested by Wells is the consequence of the evolutionary history of the species and of the need to respond to different challenges in different periods of life. This is true for many other defense and repair mechanisms that animals have developed in response to external threats. Fat deposition and its modulation in the life-course is a response to particular groups of challenges (nutrition, temperature, and reproduction). But a similar scheme is also valid for another group of threats that are at the center of the exposome concept, chemical exposures.
One of the pillars of current knowledge on defense mechanisms is the Xenobiotic Metabolic Pathway (XMP), which can handle apparently an infinite number of molecules (the so-called promiscuity).15 However, given its evolutionary origin, it is not clear how this system manages totally new synthetic entities and the interaction of multiple xenobiotics in mixtures, and whether it is possible to predict (for public health purposes) which chemicals will not be dealt with. One surprising feature of reactive pathways is that they are based on a very limited number of xenobiotic receptors.16 Needless to say, defense mechanisms, including receptors, are the product of a long evolutionary history, that cannot be ignored when dealing with exposome research.
They go back in time to the exposure of animals to natural poisons, including the “plant-animal warfare,” but now we are dealing with a large number of new artificial toxins. Reference to the handling of xenobiotics and the role of receptors is relevant to a better science of the exposome and to public health measures, for example because of saturation of defense pathways: this might be a serious issue because of the disproportion between the large number of xenobiotics now in the environment (hundreds of thousands?) and the few receptors dealing with them.
In practice, our suggestion is to consider the evolutionary perspective when planning exposome research and refining its tools. One concept that is central in branches of biological research, such as plant biology, is “phenotypic plasticity.”
Phenotypic plasticity: why did it not attract attention in epidemiology (yet)?
All species have been exposed to rapid environmental transformations in history (eg, in temperature, food availability), and they developed the ability to adapt. Both fat deposition and the management of toxicants are examples of plasticity. “Phenotypic plasticity” can be defined as the multiple ways in which organisms cope with environmental variation, including morphological, physiological, and behavioral changes. Organisms that have a greater capacity of adaptation to rapidly changing environments have been selected, and this is based on the selection of a multiplicity of gene variants that increase the range of potential responses (“norms of reaction” to environmental challenges). It is also likely that phenotypic plasticity is mediated by epigenetic modifications that occur more rapidly than genetic mutations fixed in cells and then selected.17,18
Humankind has gone through periods of intense environmental change and transformation in the remote past, particularly temperature and food. However, in the last decades, such transformation has occurred in a number of different fields, if not their totality (eg, the nine planetary boundaries by Rockstrom et al19), creating a severe challenge to all species. We know little about the concept of resilience, which has been studied only from limited perspectives.
Phenotypic plasticity is at the basis of resilience and is represented, for example, by the “allostatic load,” that is, the ensemble of physiological reactions—like the cortisol response to stress—that enable animals to cope with stressful environments. It is a reserve of adaptive capacity that, however, can be overcome by excessive stimuli and lead to disease.
Phenotypic plasticity does not apply only to organisms and their populations, but also to cells; for example, it is plausible that changing and harmful environments select cells that have a greater ability to cope via adaptation, including a “mutator phenotype” and cell transformation and proliferation. This could be a way to incorporate phenotypic plasticity into the Darwinian interpretation of carcinogenesis, where the “hallmarks of cancer” express different strategies to overcome stressful circumstances. This is explained in more detail below.
In summary, human evolution occurred in extremely unstable environments, where a polygenic inheritance stabilized phenotypes. This stability underpins “reaction norms” through which different biological systems (including, eg, adiposity, XMP) can respond sensitively to ecological factors.
Non-communicable diseases as multistage processes: a Darwinian interpretation of cancer
The theory of Darwinian carcinogenesis has a long history and stemmed in part from mathematical models. Mathematical multistage models originated from contributions from several epidemiologists and statisticians (Armitage, Doll-Peto, Moolgavkar, Day-Brown, Knudson, and others).20–24 In particular, the impact of starting and quitting different exposures such as smoking, arsenic, asbestos, or heavy metals led to thinking that certain exposures may act early in the sequence of stages and others late. This was reminiscent of the very early biological models (mainly based on animal experiments) that led to coining the terms of initiators and promoters of cancer. In fact, historically, cancer has been interpreted as the product of genetic mutations or epimutations (“initiation”) plus events not involving DNA (“promotion”). Biological theories of carcinogenesis have evolved separately within epidemiology on one side (identification of risk factors) and molecular biology on the other side (eg, characterization of mutations such as in retinoblastoma, Vogelstein’s model of colorectal cancer, identification of oncogenes and tumor-suppressor genes, etc.). The two fields (epidemiology and molecular biology) have only recently intersected (“molecular epidemiology”).
The interpretation of carcinogenesis has also been enriched recently with the concept of the “hallmarks of cancer.” Hallmarks of cancer are components of the cancer phenotype including apoptosis, cell proliferation, inflammation, immune response, etc.25 However, (1) their temporal sequence, (2) the relationship with causes/risk factors, and (3) their integration in leading to the cancer phenotype need yet to be clarified.
Cancer is nowadays interpreted as a Darwinian process at the cellular level, that is, based on mutations or epimutations plus selection.17,26 Exposures related to the individual’s external and internal exposures/risk factors can provide a selective advantage to cells, either directly (mutagenesis) or indirectly (selection of cells bearing advantageous mutations). Exposures that are not mutagenic may be selectogenic, such as those inducing inflammatory or altered immune responses, or hormonal disequilibrium. In research, there has been a disconnection between mechanistic investigations—culminating in the paradigm of the “hallmarks of cancer”—and research on the causes of cancer, including, for example, viral and chemical carcinogenesis or the metabolic syndrome. The hallmarks of cancer describe the cancer phenotype cross-sectionally, but do not address the carcinogenic process in its unfolding, including the role of risk factors. Notably, the hall-marks of cancer fit very well with the Darwinian theory of carcinogenesis because they express the ability of the cells to adapt to a stressful environment by increasing their fitness (eg, abolishing apoptosis, producing new vascular networks, opposing the immune response, etc.). In doing so, they alter the surrounding tissues, creating an evolutionary niche.
The Darwinian interpretation of cancer reconciles the external exposome as the sum of mutagens and selectogens, with the internal exposome as a series of cellular modifications that express a strategy of adaptation to the surrounding environment.
Conclusions: broadening the concept of exposome
The social component
We believe that tendencies to reconduct the exposome solely to the agnostic identification of new causes of diseases (and particularly chemical causes) is a limitation. A conceptual refinement is needed that considers the new challenges created by the crossing of the planetary boundaries and exposure to “novel entities.” We propose that the theory of evolution in its different aspects is useful for such a refinement. This also involves a better integration of molecular and social components of the exposome. There has been a tendency—also in the exposome community—to believe that “molecular” is more credible (and measurable) than “social.” In fact, this problem was perceived by the founders of the exposome, and the social exposome is one of the components of the “general external” exposome according to Wild’s definition.3
In spite of the fact that social inequalities are the single most important risk factor for disease and mortality,27 people are reluctant to use the word “cause” referring to social aspects of disease, a term which is used instead, for example, for p53 mutations. In practice, it is easier to be reductionist—from a biological-molecular perspective—than “holistic”—from a social perspective. This is just an example of the fact that the concept of exposome needs to be broadened, incorporating also a theory of society and social relationships.28
Even more, the concept of exposome needs to be adapted to the current tendency to address the planetary boundaries and in general planetary health, where the social component is crucial given the human origin of the environmental crisis. Molecular epidemiology is essentially driven by technology, thanks to the developments of high-resolution mass spectrometry, genomic and epigenomic platforms, and other technologies. Obviously, there has not been such a spectacular and tangible evolution in social techniques.
Does this alone justify the greater credibility that molecular pathways tend to have over social pathways? We do not believe so, and in fact, we have tried to claim elsewhere that molecular and sociological reconstructions are complementary and should converge and reinforce each other, and there is no reason to consider one more persuasive than the other: the exposome can be reinterpreted—at least at the individual level—as the science of how biography translates into biology (the embodiment).28
From this point of view, we need to specify a theory of society that allows us to set up more effective causal constructs. There is a rhetoric of causality, or “construction of causality,” that characterizes different sciences: in biomedical sciences this is based on randomized trials and on molecular pathways, but the construct is definitely limited if we want to understand more complex societal impacts on health. A broader construction of causality is needed and this requires a more systemic view on the side of biomedical researchers and perhaps a more analytical and granular approach in social sciences. The social exposome can be the integration of the two, not only because it examines the upstream determinants of exposures, but also for the understanding that people affect the environment of other people.
The evolutionary component
Other scholars have already considered the intersection of natural and social sciences, for example in the eco-social theory of disease,29–33 but we are not aware of an incorporation of the evolutionary dimension. For example, co-evolution of humans and other species needs to be addressed as a research paradigm within the exposome community, as a way of understanding what are the limits of phenotypic plasticity as an additional planetary boundary.
The integration of the social component and phenotypic plasticity can lead to a more robust “community exposome,” that is the ability to integrate the multiple layers that characterize a specific population: social stratification, productive activities, cultural components, mobility, behaviors, etc., and their embodiment into molecules and life trajectories of individuals. Diet and obesity are examples of such a need for integration at the community level: dietary habits are rooted in local traditions but are also intersected by commercial trends favored by globalization, and by the co-evolution with edible and competing species.
The erosion of the microbiome is the product of social changes and an example of co-evolution. In addition, if we want to develop the concept of “community exposome,” we should also integrate the community voices, once again a problem relevant to social sciences: there are early and still limited examples of such work.
Acknowledgments
We are grateful to Stephen Rappaport for thoughtful suggestions and to two anonymous reviewers for very accurate comments that led to a substantial rewriting of the paper.
Funding
P.V. was funded by the MRC Centre for Environment and Health, Imperial College, London. S.D. acknowledges funding from PNR-ANSES-EXPOL - 22-EST-210.
Footnotes
Author contributions
Paolo Vineis (Conceptualization [equal], Methodology [equal], Resources [equal], Writing—original draft [equal], Writing—review & editing [equal]), and Sonia Dagnino (Conceptualization [equal], Methodology [equal], Writing—review & editing [equal])
Conflict of interest statement
None declared.
Data availability
Not relevant.
References
- 1.Rappaport SM, Smith MT. Epidemiology. Environment and disease risks. Science. 2010;330(6003):460–461. doi: 10.1126/science.1192603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baccarelli A, Dolinoy DC, Walker CL. A precision environmental health approach to prevention of human disease. Nat Commun. 2023;14(1):2449. doi: 10.1038/s41467-023-37626-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wild CP. The exposome: from concept to utility. Int J Epidemiol. 2012;41(1):24–32. doi: 10.1093/ije/dyr236. [DOI] [PubMed] [Google Scholar]
- 4.Haenszel W, Kurihara M, Segi M, Lee RK. Stomach cancer among Japanese in Hawaii. J Natl Cancer Inst. 1972;49(4):969–988. [PubMed] [Google Scholar]
- 5.Ahmedin J, Vineis P, Bray F, Torre L, Forman D. Cancer Atlas, American Cancer Society. 2014 [Google Scholar]
- 6.Chadeau-Hyam M, Athersuch TJ, Keun HC, et al. Meeting-in-the-middle using metabolic profiling—a strategy for the identification of intermediate biomarkers in cohort studies. Biomarkers. 2011;16(1):83–88. doi: 10.3109/1354750X.2010.533285. [DOI] [PubMed] [Google Scholar]
- 7.Rappaport SR. Discovering environmental causes of disease. J Epidemiol Commun Health. 2012;66(2):99–102. doi: 10.1136/jech-2011-200726. [DOI] [PubMed] [Google Scholar]
- 8.Mazzocchi F. Complexity and the reductionism-holism debate in systems biology. Wiley Interdiscip Rev Syst Biol Med. 2012;4(5):413–427. doi: 10.1002/wsbm.1181. [DOI] [PubMed] [Google Scholar]
- 9.Krieger N. Public health, embodied history, and social justice: looking forward. Int J Health Serv. 2015;45(4):587–600. doi: 10.1177/0020731415595549. [DOI] [PubMed] [Google Scholar]
- 10.Oldroyd DR. The Arch of Knowledge: An Introductory Study of the History of the Philosophy and Methodology of Science Paperback. Methuen: 1986. [Google Scholar]
- 11.Persson L, Carney Almroth BM, Collins CD, et al. Outside the safe operating space of the planetary boundary for novel entities. Environ Sci Technol. 2022;56(3):1510–1521. doi: 10.1021/acs.est.1c04158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vineis P, Robinson O, Chadeau-Hyam M, Dehghan A, Mudway I, Dagnino S. What’s new in the exposome? Environ Int. 2020;143:105887. doi: 10.1016/j.envint.2020.105887. [DOI] [PubMed] [Google Scholar]
- 13.Yang C-H, Fagnocchi L, Apostle S, et al. PERMUTE Independent phenotypic plasticity axes define distinct obesity sub-types. Nat Metab. 2022;4(9):1150–1165. doi: 10.1038/s42255-022-00629-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wells JCK. Natural selection and human adiposity: crafty genotype, thrifty phenotype. Philos Trans R Soc Lond B Biol Sci. 2023;378(1885):20220224. doi: 10.1098/rstb.2022.0224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kliewer SA, Goodwin B, Willson TM. The nuclear pregnane X receptor: a key regulator of xenobiotic metabolism. Endocr Rev. 2002;23(5):687–702. doi: 10.1210/er.2001-0038. [DOI] [PubMed] [Google Scholar]
- 16.Mackowiak B, Hodge J, Stern S, Wang H. The roles of xenobiotic receptors: beyond chemical disposition. Drug Metab Dispos. 2018;46(9):1361–1371. doi: 10.1124/dmd.118.081042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vineis P, Schatzkin A, Potter JD. Models of carcinogenesis: an overview. Carcinogenesis. 2010;31(10):1703–1709. doi: 10.1093/carcin/bgq087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pigliucci M. Phenotypic Plasticity: Beyond Nature and Nurture. Johns Hopkins University Press; 2001. [Database] [Google Scholar]
- 19.Richardson K, Steffen W, Lucht W, et al. Earth beyond six of nine planetary boundaries. Sci Adv. 2023;9(37):eadh2458. doi: 10.1126/sciadv.adh2458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wilkins A, Corbett R, Eeles R. Age distribution and a multi-stage theory of carcinogenesis: 70 years on. Br J Cancer. 2023;128(3):404–406. doi: 10.1038/s41416-022-02009-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Peto R, Darby S, Deo H, Silcocks P, Whitley E, Doll R. Smoking, smoking cessation, and lung cancer in the UK since 1950: combination of national statistics with two case-control studies. BMJ. 2000;321(7257):323–329. doi: 10.1136/bmj.321.7257.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Day NE, Brown CC. Multistage models and primary prevention of cancer. J Natl Cancer Inst. 1980;64(4):977–989. [PubMed] [Google Scholar]
- 23.Moolgavkar SH, Dewanji A, Venzon DJ. A stochastic two-stage model for cancer risk assessment. I. The hazard function and the probability of tumor. Risk Anal. 1988;8(3):383–392. doi: 10.1111/j.1539-6924.1988.tb00502.x. [DOI] [PubMed] [Google Scholar]
- 24.Chernoff J. The two-hit theory hits 50. Mol Biol Cell. 2021;32(22):rt1. doi: 10.1091/mbc.E21-08-0407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hanahan D. Hallmarks of cancer: new dimensions. Cancer Discov. 2022;12(1):31–46. doi: 10.1158/2159-8290.CD-21-1059. [DOI] [PubMed] [Google Scholar]
- 26.Vineis P, Berwick M. The population dynamics of cancer: a Darwinian perspective. Int J Epidemiol. 2006;35(5):1151–1159. doi: 10.1093/ije/dyl185. [DOI] [PubMed] [Google Scholar]
- 27.Vaccarella S, Georges D, Bray F, et al. Socioeconomic inequalities in cancer mortality between and within countries in Europe: a population-based study. Environ Int. 28(25):100551. doi: 10.1016/j.lanepe.2022.100551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vineis P, Barouki R. The exposome as the science of social-to-biological transitions. Environ Int. 2022;165:107312. doi: 10.1016/j.envint.2022.107312. [DOI] [PubMed] [Google Scholar]
- 29.Juarez PD, Matthews-Juarez P, Hood DB, et al. The public health exposome: a population-based, exposure science approach to health disparities research. Int J Environ Res Public Health. 2014;11(12):12866–12895. doi: 10.3390/ijerph111212866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Senier L, Brown P, Shostak S, Hanna B. The socio-exposome: advancing exposure science and environmental justice in a postgenomic era. Environ Sociol. 2017;3(2):107–121. doi: 10.1080/23251042.2016.1220848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zota AR, VanNoy BN. Integrating intersectionality into the exposome paradigm: a novel approach to racial inequities in uterine fibroids. Am J Public Health. 2021;111(1):104–109. doi: 10.2105/AJPH.2020.305979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Krieger N. Methods for the scientific study of discrimination and health: an ecosocial approach. Am J Public Health. 2012;102(5):936–944. doi: 10.2105/AJPH.2011.300544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Phelan JC, Link BG, Tehranifar P. Social conditions as fundamental causes of health inequalities: theory, evidence, and policy implications. J Health Soc Behav. 2010;51(Suppl):S28–40. doi: 10.1177/0022146510383498. https://emoryhercules.com/ [DOI] [PubMed] [Google Scholar]
- 34.Porta M. A Dictionary of Epidemiology. 6th. Oxford University Press; 2014. [Google Scholar]
- 35.Miller GW, Jones DP. The nature of nurture: refining the definition of the exposome. Toxicol Sci. 2014;137(1):1–2. doi: 10.1093/toxsci/kft251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rappaport SR. Implications of the exposome for exposure science. J Expo Sci Environ Epidemiol. 2011;21(1):5–9. doi: 10.1038/jes.2010.50. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not relevant.