Abstract
Ageing is a biological process that is linked to a functional decline, ultimately resulting in death. Large interindividual differences exist in terms of life- and healthspan, representing life expectancy and the number of years spent in the absence of major diseases, respectively. The genetic and molecular mechanisms that are involved in the regulation of the ageing process, and those that render age the main risk factor for many diseases are still poorly understood.
Nevertheless, a growing number of compounds have been put forward to affect this process. However, for scientists and laypeople alike, it is difficult to separate fact from fiction, and hype from hope. In this review, we discuss the currently pursued pharmacological anti-ageing approaches. These are compared to non-pharmacological interventions, some of which confer powerful effects on health and well-being, in particular an active lifestyle and exercise. Moreover, functional parameters and biological clocks as well as other molecular marks are compared in terms of predictive power of morbidity and mortality. Then, conceptual aspects and roadblocks in the development of anti-ageing drugs are outlined. Finally, an overview on current and future strategies to mitigate age-related pathologies and the extension of life- and healthspan is provided.
Keywords: ageing, exercise, healthspan, lifespan, longevity, rapamycin, reprogramming, rejuvenation, senolytics
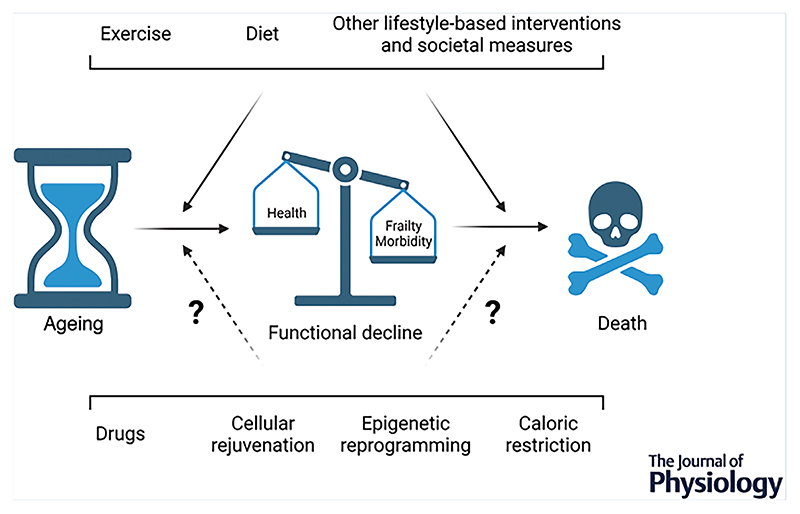
Abstract figure legend.
Modulation of the human ageing process. Ageing leads to a functional decline with increased frailty and morbidity, ultimately resulting in death. This process is affected by the genetic set-up, epigenetic pre-disposition and random events, leading to large interindividual differences in regard to health, well-being and life expectancy. Exercise, diet and other lifestyle-based interventions as well as societal measures can help to maximize the chance for healthy ageing. Proposed drugs, cellular, epigenetic and other non-pharmacological approaches such as caloric restriction await validation for safe and efficacious application in human geroprotection.
Introduction
In all higher organisms, ageing is an inescapable process ultimately resulting in death. In evolutionary terms, removing individuals from the population after the reproductive phase might present an advantage in light of limited food and other resources. Nevertheless, it is unclear whether human ageing represents an active, controlled pathway or is a byproduct of lack of maintenance pressure after reproduction (Kyriazis, 2020). The uncertainty about and the fear of death, coupled witha steady functional deterioration and related health consequences in ageing thus led to endeavours to restore youth or even vanquish mortality throughout human history. Such feats as the search for the Holy Grail, the fountain of youth or the philosopher’s stone are amply documented in myths and fables in almost all human cultures. In modern times, the biology of ageing is the object of intense scientific scrutiny. However, the molecular mechanisms that are involved in ageing remain poorly defined and understood, hinting at a pleiotropic, multifactorial and highly complex sequence of events. Several pathways and processes have been linked to ageing, and proposed as ‘hallmarks’ (Lopez-Otin et al., 2013) or ‘pillars’ (Kennedy et al., 2014), but caveats have been raised and general applicability remains debated (Gems & de Magalhaes, 2021; Keshavarz et al., 2022). Reproducible and robust interventions not only would have the potential to slow down ageing progression, but also reduce the risk for many age-associated chronic diseases (Partridge et al., 2020). A number of these pathologies could be ameliorated by one instead of multiple segregated approaches, thereby overcoming the slow progression in finding directed therapies for some of these, for example neurodegenerative pathologies including dementia, Alzheimer’s or Parkinson’s disease.
Human and animal models to study ageing
Studies of naturally occurring ‘mutants’, that is centenarians with an extraordinarily long lifespan and, in most cases, an above average number of years in good health (‘healthspan’), are hampered by the small numbers of such individuals (approx. 0.01% of the US population) (Melzer et al., 2020). The identification of more than 50 genetic loci associated with longevity with small effect size underlines the putative multifactorial and polygenic aspects of ageing. Moreover, many of these genetic variants have been linked to the risk for developing a number of chronic diseases, the pre-valence of which increases with age. It thus is unclear whether the genetic polymorphisms that have been described so far directly pertain to the ageing process, or help to stave off age-associated pathologies. Other human studies have been performed in patients suffering from progerias, in which symptoms are observed that resemble an accelerated ageing phenotype (Melzer et al., 2020). Three prototypical forms of progeria, Werner, Hutchinson–Gilford and Cockayne syndrome, are caused by mutations in genes encoding proteins involved in DNA repair, fidelity of DNA replication and/or nuclear architecture (Coppede, 2021). Similarly, mutations in the Werner gene involved in genomic maintenance and DNA repair lead to adult-onset Werner syndrome. However, whether these pathologies represent bona fide accelerated ageing remains debatable, in particular in light of an average lifespan of around 15 years in childhood progeria. Thus, to circumvent the limitations imposed by small populations, diseases with questionable relevance for normal human ageing, and the necessity to study human ageing over years to decades, geroscience to a large extent relies on model organisms, including Saccharomyces cerevisiae (yeast), Drosophila melanogaster (fruit fly), Caenorhabditis elegans (a nematode), rodents and, albeit much rarer, non-human primates.
Numerous genes, pathways and processes have been identified that affect lifespan of various species. Notably, most monogenic mutations that evoke an extension in lifespan in these model organisms affect proteins involved in anabolic metabolism (e.g. insulin, insulin-like growth factor 1 (IGF-1) and mammalian target of rapamycin (mTOR) signalling) and growth (e.g. pituitary and growth hormone signalling), or reproduction (e.g. related to fertility and fecundity) (Gems & Partridge, 2013). To a large extent, these insights have fuelled a growing pipeline of compounds to potentially affect the ageing process in humans (Fig. 1) (Raffaele & Vinciguerra, 2022). However, since many of these compounds failed to consistently evoke effects in different species, mouse lines, dietary and other contexts, or even both sexes of the same strain, systematic and standardized attempts to study life-extending effects of dietary and pharmacological interventions were initiated by the National Institute on Aging in the Interventions Testing Program (ITP) (Nadon et al., 2017). However, care has to be taken to extrapolate findings from model organisms to humans. First, many of these organisms have unique traits affecting longevity that are absent or at least disputable in humans (e.g. formation of spores in lower organisms, the Dauer stage in nematodes, torpor in mice, or reproduction–lifespan trade-offs in fruit flies). Second, many of the age-expanding mutations discovered in such organisms also confer physiological and functional disadvantages (such as decreased neuromuscular function in the methuselah fly), or fail to be associated with a longevity phenotype in humans (for example in dwarf syndromes with mutations in the same growth-related genes as in mice) (Bartke, 2021). Third, laboratory organisms are studied under tightly controlled genetic and environmental conditions and constraints, in many cases failing to reproduce the complex environment of human daily life. Examples include pathogen-limited or -free rodent breeding and housing stations, in which infections, cancers or other pathological events often result in removal of the respective animals from experimental cohorts, specific ad libitum diets, exposure to temperatures below thermoneutrality, or a severely sedentary activity pattern. Fourth, the lifespan of yeast (approx. 14 days, representing chronological and non-dividing, not replicative ageing), C. elegans (approx. 20 days), D. melanogaster (approx. 2−3 months), Mus musculus (approx. 2−3 years), Rattus norvegicus (approx. 3−4 years) or Macaca mulatta (rhesus monkey, approx. 27−40 years) are far from that achieved in humans (world-wide average life expectancy of approx. 73 years, but over 80 years in a number of countries for both sexes). In fact, based on the number of cortical neurons, and compared to body size, humans might already be long-lived (Herculano-Houzel, 2019; Austad, 2010). Besides the biological considerations of these differences, the much longer lifespan of humans also poses a markedly higher hurdle on tolerability, safety, adverse effects, development of tolerance, evasion or feedback mechanisms of chronic treatments and interventions, which might not be of importance or even observed in short-lived model organisms.
Figure 1. Putative anti-ageing drugs and some of their proposed targets.
Many natural and synthetic compounds have been proposed to extend lifespan in model organisms, and thus act as potential anti-ageing drugs in humans. Notably, however, most of these compounds failed to do this reproducibly, exhibiting profoundly varying effects in different species, contexts and sexes, and all of them still await human validation in clinical trials. Moreover, for most of these interventions, tolerability, safety and adverse effects are unknown, in particular in long-term administration as would be required for anti-ageing therapy. Finally, it is unclear whether the proposed molecular targets, pathways and mechanisms are indeed dysregulated in human ageing, and if sex-, tissue- and cell type-specific differences exist. Abbreviations: mTOR: mammalian target of rapamycin; RAA, renin–angiotensin–aldosterone; ROS, reactive oxygen species. Image: from Freepik.com (created by Racool_studio). Created with BioRender.com.
Pharmacological and non-pharmacological approaches proposed, but not proven to slow down human ageing
Based on these caveats, it is not surprising that many pharmacological and interventional approaches have failed to live up to initial reports and promises (Keshavarz et al., 2022) (Fig. 1). For example, for the polyphenolic compound resveratrol, there is strong evidence that it does not directly bind to the proposed target, sirtuin 1 (SIRT1); many of the effects are only observed in specific mouse strains at very high doses and are dependent on co-administration of a high fat diet; and effects in human clinical trials were small or non-existent (Furrer & Handschin, 2020; Handschin, 2016). Accordingly, clinical development of resveratrol derivatives was stopped 5 years after the acquisition of Sirtris Pharmaceuticals by GlaxoSmithKline in 2008, despite the 720 million US$ price tag. Currently, metformin and rapamycin belong to the most discussed drugs to be repurposed for anti-ageing treatments (Blagosklonny, 2021; Campisi et al., 2019). Clinical trials are planned for these drugs to assess geroprotective and lifespan-enhancing properties. However, in addition to mixed outcomes on mortality in humans or animal models (metformin) (Blagosklonny, 2021; Mohammed et al., 2021), and the immuno-suppressing properties at least at some concentrations (rapamycin), other potential adverse effects have been reported. Importantly, potential beneficial anti-ageing effects would have to outweigh the restrictions imposed on the many benefits of physical activity and other life style-based interventions. Metformin seems to consistently reduce the effects of endurance and resistance training in humans, similar to what has been reported previously for resveratrol (e.g. see Gliemann et al., 2013; Handschin, 2016; Konopka et al., 2019; Kulkarni et al., 2020; Walton et al., 2019). Likewise, rapamycin-induced inhibition of mTOR blunts muscle hypertrophy in different contexts of muscle unloading and reloading in animal models (Morley, 2016). Unfortunately, the metformin and rapamycin studies in model organisms did not include an exercise arm. Such effects thus could not be assessed, and might be of limited relevance in sedentary animals. However, this outcome is of high significance in the human population and is inadequately modelled by caged mice (Booth & Laye, 2009). Other adverse effects of these and other drugs will also have to be identified and evaluated in long-term treatment of humans, as indicated by the potential of mTOR inhibition to affect β-amyloid plaques and the development of Alzheimer’s disease (Shi et al., 2022). Although often neglected as an anti-ageing drug, aspirin is another example of a reproducible compound in longevity studies (Campisi et al., 2019), with controversial outcomes on all-cause mortality in healthy elderly (McNeil et al., 2018). Of note, the exact identity, pharmacokinetics, tissue distribution, target mechanism and other crucial pharmacological parameters of many of the proposed anti-ageing compounds are not clear. Similarly, dosing, timing, age of initiation of treatment and other considerations will have to be elucidated (Nelson et al., 2017).
Failures, controversies and question marks are, however, not limited to proposed pharmacological anti-ageing interventions. Caloric restriction robustly extends lifespan in various laboratory models, albeit with decreasing relative payoff when moving from simple to more complex, higher organisms (Dakic et al., 2022; Sohal & Forster, 2014). Notably, in rodents, sex- and strain-specific effects of caloric restriction were reported, which, in some strains, even had a negative effect on lifespan. Thus, the genetic background, time-of-day of feeding, nutrient composition, age at initiation of caloric restriction, extent of caloric restriction and other parameters seem to affect the outcome in rodent, non-human primate and human studies (Campisi et al., 2019; Dakic et al., 2022; Sohal & Forster, 2014). Moreover, questions about adequate controls, and thus the baseline of caloric intake, have been raised. Ad libitum feeding might be detrimental to health in control groups of rodents and primates, and the health benefits of caloric restriction in part caused by the amelioration of overeating and obesity-associated pathologies, distinct from any bona fide pro-lifespan effects. In humans, the epidemiological data clearly show an unhealthy baseline of ad libitum food intake in many societies, linked to inadequate physical activity. Furthermore, basal, non-exercise activated thermogenic (NEAT) and activity-linked energy expenditure exhibits high interindividual differences, and for any caloric restriction intervention, baseline values and the targeted restriction would have to be strictly personalized. Finally, implementation might be hampered by psychological factors, and side effects, for example on frailty or cognitive performance, might occur (Hofer et al., 2022).
Cellular reprogramming and epigenetic rejuvenation refer to a collection of approaches attempting to leverage recent insights into tissue differentiation and de-differentiation (de Lima Camillo & Quinlan, 2021). Even though promising results in model organisms have been reported, many limitations and potential adverse effects will have to be overcome before safe application in humans can be envisioned. For example, the choice of reprogramming factors, tissue delivery and control of expression, potential tissue de-differentiation or development of cancers have to be considered, in particular in the time scale of human ageing and treatment (de Lima Camillo & Quinlan, 2021). Moreover, many of the proposed anti-ageing drugs, including metformin, resveratrol and rapamycin, decrease reprogramming efficiency at the concentrations for which life-extending benefits have been reported, described as the age reversal–age extension (Arae) paradox in which one anti-ageing treatment adversely affects another (de Lima Camillo & Quinlan, 2021). As a final example, the usage of senolytics, aiming at the selective destruction of senescent cells, will have to address the biological function of cell senescence in differentiation, regeneration, tissue function maintenance and remodelling, wound healing, prevention of tumourigenesis and other processes (Rhinn et al., 2019; Ring et al., 2022).
Lifestyle interventions that improve healthspan
In contrast to the yet to be proven potential of these pharmacological and non-pharmacological approaches, exercise provides true, effective robust and reproducible geroprotective effects in model organisms as well as in humans. This is not only achieved by reducing the risk for and incidence of many age-associated chronic diseases, but also by improving quality of life, mean and maximal lifespan (Campisi et al., 2019; Furrer & Handschin, 2020; Garatachea et al., 2015; Goh et al., 2021; Gubert & Hannan, 2021; Handschin, 2016). Importantly, such effects are already reported after moderate activities, and, if performed correctly, training has no adverse effects and outcomes (Booth & Laye, 2009). Physical activity is complemented by other behaviours to affect human health, morbidity and mortality. Collectively, such lifestyle-based interventions currently are the best modalities to promote healthy ageing in humans (Fig. 2), including stress reduction and adequate sleep patterns (Friedman, 2020). Furthermore, a calorically controlled diet balanced in macro- and micronutrients seems to surpass the effects of extreme diet regimens, many of which have been proposed based on experiments in rodents, but with questionable translatability to humans (Lee et al., 2021), for example for low protein-containing diets (Kitada et al., 2019). Then, intellectual and cognitive challenges as well as social interactions help to reduce neuronal declines. Disease prevention, for example in the form of non-smoking and controlled UV exposure, and evidence-based health monitoring and prevention, with proven effectiveness, benefits and minimized risks (Raffle et al., 2019), likewise contribute to health. Societal factors, including access to high quality health care and socio-economic status, are often neglected, albeit highly significant, contributors to life expectancy and health (McMaughan et al., 2020). All of these factors are modifiable by individuals and the society, and have a proven effect on healthy ageing. To a much smaller extent, epigenetic remodelling can be influenced in a targeted manner by exercise or nutrition. Thus, the epigenetic landscape (influenced by hereditability and behaviour), genetic endowment obtained from parents, and the outcome of random events (such as accidents), of which frequency is related to risk behaviour, represent events that affect human lifespan, but on which we have minimal or no influence. The discovery of anti-ageing drugs could have an impact in diverging ways. In the most benevolent manner, such compounds help to promote health up to old age. As outlined above, the use of such drugs might, however, also entail adverse effects, some of which could directly counteract other anti-ageing interventions, for example those elicited by exercise or rejuvenation strategies. Psychologically, the broad availability of anti-ageing drugs could also result in an even further reduction of adherence to and compliance with proven lifestyle-based interventions, now erroneously perceived to be ‘substituted’ by the administration of a pill, with an ultimate net negative outcome on healthy ageing. Overall, the idea of a monotherapy to improve health- and lifespan might still be fiction, and combination polytherapies still years in the making.
Figure 2. Determinants of healthy ageing.
Modifiable lifestyle-based interventions have a proven effect on morbidity and mortality in humans. While some of these might affect epigenetic remodelling, the epigenetic drift in ageing, genetic endowment and random events represent age modifiers on which we have minimal or no influence. Novel pharmacological or non-pharmacological interventions could positively affect healthy ageing, but also harbour the potential for adverse effects, and might decrease adherence to and compliance with lifestyle-based approaches. Created with BioRender.com.
Assessing and monitoring biological age using clocks
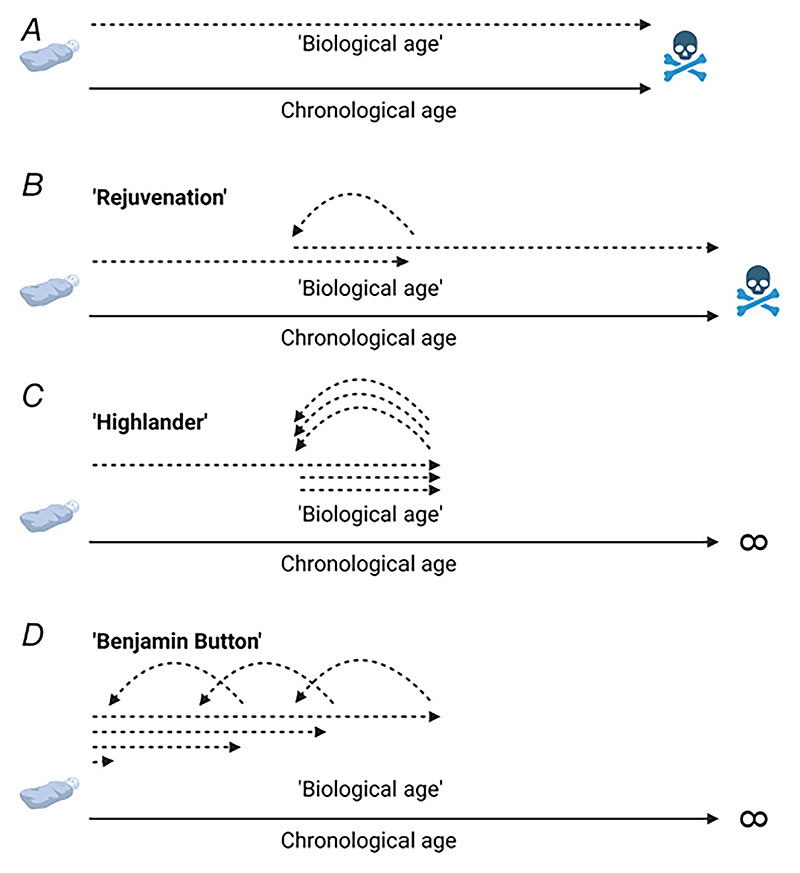
The development of potential anti-ageing therapies is hampered by the difficulty for human translation. First, even though hotly debated (Gems, 2015), ageing currently is not classified as a disease by the FDA, EMA and other regulatory agencies, in contrast to many age-associated diseases, for example sarcopenia (ICD-10-CM code M62.84). Therefore, the design and approval of geroprotective clinical trials face high regulatory and ethical hurdles of treating by definition ‘healthy’ individuals, in addition to the logistical and financial problems of following large cohorts over years to decades. Indeed, many of the data on interventions with a proven effect on health- and lifespan, that is exercise, originate from cross-sectional studies, epidemiological assessments, or cohort observations such as meta-analyses of morbidity and mortality of former athletes (Runacres et al., 2021). The necessity for elaborate and costly prospective clinical anti-ageing studies could be mitigated by the use of biomarkers that accurately reflect age and react to treatments in shorter timeframes (Jylhava et al., 2017; Rutledge et al., 2022). In recent years, various molecular biomarkers to benchmark the ‘biological age’, as opposed to the chronological age (Fig. 3A), have been suggested and tested, based on the observation of large interindividual differences in ageing progression, health- and lifespan. Proposed markers include telomere length, transcriptomic profiles, metabolomics age scores, and protein-/glycan-derived age (Jylhava et al., 2017; Rutledge et al., 2022). Most prominently, the changes in the epigenome that occur during development, differentiation and ageing are leveraged to define ‘epigenetic clocks’ (Seale et al., 2022). However, so far, different epigenetic clocks exhibit poor correlation between each other, as well as with functional phenotypes (Fohr et al., 2021; Jylhava et al., 2017). Second, the naked mole rat is an exceptionally long-lived rodent, with very rare occurrences of cancer, cardio-vascular or other age-associated diseases, and thus has been defined as a demographically non-ageing animal, despite clear signs of ‘epigenetic ageing’ (Kerepesi et al., 2022). Furthermore, ageing clocks seem to differ between organs (Nie et al., 2022), in some of which epigenetic clocks provide poor prediction of tissue-specific ageing, for example in skeletal muscle (Sillanpaa et al., 2021). Finally, epigenetic clocks might represent multifactorial composites, in which individual modules are affected differently by perturbations, including epigenetic reprogramming (Levine et al., 2022). Future research will thus not only have to provide further evidence that the molecular basis of different ageing clocks indeed correlates with age, but also that a change in such biomarkers provides meaningful predictive power for phenotypic and functional adaptations. Conceptually, it is finally unclear how the biological compared to a chronological age should be interpreted and applied. Do reported reductions in the biological age, for example after rejuvenation, predict a corresponding increase in chronological age/lifespan (Fig. 3B)? If true, would repetitive rejuvenation eliminate chronological ageing, leading to immortality at a chosen chronological age (as depicted in the ‘Highlander’ movie) (Fig. 3C)? Are there biological constraints to push rejuvenation and hence the biological clock to ever younger ages, leading to a ‘Benjamin Button’-like outcome (Fig. 3D)? Until such questions are resolved on the conceptual and biological levels, reported alterations in a loosely defined biological age, and the differences to chronological age are of limited value.
Figure 3. The concept of biological and chronological age.
A, the theoretical concept of a biological age is based on the observation of the vast differences in life-, health- and morbidityspan in individuals. B, rejuvenation strategies claim to reduce the biological age, which therefore should result in an increase in chronological age and lifespan, and potentially healthspan. C, if rejuvenation strategies indeed would work, and a biological clock accurately represents the relative age of an individual, repeated interventions should result in a ‘Highlander’ effect, effectively pausing chronological ageing. D, furthermore, if such rejuvenation interventions were staggered, chronological ageing would be reversed analogous to ‘Benjamin Button’. In both cases, immortality would be achieved. Conceptual and biological arguments for and against such scenarios remain to be formulated and described, respectively. Created with BioRender.com.
Intriguingly, ageing biomarkers often prioritize disease risk and health outcomes over bona fide ageing progression, with high potential clinical relevance (Li et al., 2020; Macdonald-Dunlop et al., 2022). In the current state, most molecular clocks are inferior to functional parameters to predict morbidity and mortality in humans (Fig. 4). Endurance and cardiovascular function (for example (Strasser & Burtscher, 2018), maximal endurance (Kodama et al., 2009) or daily steps (Paluch et al., 2022)), skeletal muscle function (for example muscle and grip strength (Sayer & Kirkwood, 2015), muscle power (Losa-Reyna et al., 2022) or muscle mass (Liu et al., 2022)) and neuromuscular function (for example gait speed (White et al., 2013)) all show strong prospective correlation with health. Importantly, these parameters represent at least part of the main decline in functional capacity with age that strongly contributes to loss of independence, hospitalization and admission to nursing homes (Nwagwu et al., 2020). In contrast to the molecular clocks, these biomarkers are inexpensive and easy to determine, provide direct functional readouts, but might lack the mechanistic insights included in the former (Jylhava et al., 2017; Libert et al., 2022; Rutledge et al., 2022; Tzemah-Shahar et al., 2022). Thus, in the future, composite indices that include functional and molecular biomarkers besides diagnostic parameters such as blood pressure, lipids, glucose and others, will improve the determination of health, and the prediction of morbidity and mortality (Libert et al., 2022; Tzemah-Shahar et al., 2022).
Figure 4. Predictors of ageing, morbidity and mortality.
Functional parameters, mostly related to neuromuscular capacity, have a high predictive power for human morbidity and mortality. These are easy and inexpensive to measure and implement. Molecular biomarkers are still being refined, and require more invasive collection and specialized analysis. In the future, compound indices that also include diagnostic markers might help to further improve and refine the prediction of healthy ageing. Images: from Freepik.com (created by Rawpixel.com and freepik). Created with BioRender.com.
Conclusions and perspectives
Ageing research has undergone a tremendous change in recent decades, and is accelerating at a breathtaking speed due to technological advances, conceptual insights and an increasing availability of financial resources. Nevertheless, due to its multifactorial and complex nature, the mechanistic, cellular, evolutionary and organismal underpinnings of the ageing process are still only rudimentarily understood, in particular in regards to humans. It is not even established whether human lifespan has a limit, or whether future scientific and technological advances will help to break the current ageing records (Colchero et al., 2021; Eisenstein, 2022). Therefore, it is unclear whether the concept of increased lifespan, as reported in model organisms, can be translated to humans, in particular if this would result in an extended morbidity-, but no change in healthspan (Fig. 5A and B). Alternatively, anti-ageing strategies might help to optimize the longevity potential towards a theoretical personal maximum. Optimally, this should be modelled after the long healthspan observed in centenarians (Fig. 5C). However, on the individual as well as the societal level, significant advances would already be achieved if healthspan could be maximized, and morbidityspan minimized without increases in absolute lifespan (Fig. 5D). As long as breakthroughs in novel pharmacological and non-pharmacological approaches are elusive, the current focus should be on proven interventions (Fig. 2). Moreover, these interventions could also help to guide mechanistic studies in geroscience. For example, exercise improves many of the known molecular aspects that have been linked to ageing, including epigenetic marks, mitochondrial function, inflammation, DNA damage or telomere shortening (Garatachea et al., 2015; Goh et al., 2021; Guan & Yan, 2022; Rebelo-Marques et al., 2018; Sellami et al., 2021). Thus, while the influx of funding from individual billionaires facing their own mortality certainly helps to push ageing research, individual and societal measures, for example aimed at promoting physical activity, affordable access to healthy food or balancing socio-economic differences, will have immediate, direct and measurable impacts on our health in old (and young) age.
Figure 5. Life-, health- and morbidityspan.
A, lifespan can be divided into healthspan, the number of years spent in good health, and mobidityspan, the number of years in which quality of life is impaired due to age-related functional declines, frailty and the occurrence of an increasing number of age-associated diseases. B, anti-ageing strategies aimed at elongation of lifespan are of limited desirability if primarily reached by longer mobidityspans. C, centenarians, exceptionally long-lived humans, often have an extended health- and a compressed morbidityspan. D, if an upper limit of human lifespan exists, an extension of healthspan, analogous to that in centenarians, should be aimed at to improve quality of life for the individual and relieve health care costs for society. Created with BioRender.com.
Supplementary Material
Additional supporting information can be found online in the Supporting Information section at the end of the HTML view of the article.
Acknowledgments
Figures were created with BioRender.com. Images were obtained from Freepik.com on a free licence.
Open-access access funding provided by Universitat Basel.
Funding
Research in our laboratory is supported by grants from the Swiss National Science Foundation (SNSF, grant 310030_184832), Innosuisse (grant 44112.1 IP-LS), the Swiss Society for Research on Muscle Diseases (SSEM), the Jain Foundation, the Novartis Stiftung für Medizinisch-Biologische Forschung and the University of Basel.
Biography
 Regula Furrer completed her MSc and PhD in Human Movement Sciences at the Vrije Universiteit (VU) Amsterdam, The Netherlands. She subsequently joined Prof. Handschin’s group at the Biozentrum of the University of Basel in Switzerland as a postdoc, investigating various aspects of exercise and muscle plasticity in health and disease.
Regula Furrer completed her MSc and PhD in Human Movement Sciences at the Vrije Universiteit (VU) Amsterdam, The Netherlands. She subsequently joined Prof. Handschin’s group at the Biozentrum of the University of Basel in Switzerland as a postdoc, investigating various aspects of exercise and muscle plasticity in health and disease.  Christoph Handschin is a group leader at the Biozentrum of the University of Basel in Switzerland. He acquired his PhD in Biochemistry at the Biozentrum before moving to Prof. Bruce Spiegelman’s laboratory at the Dana-Farber Cancer Institute, Harvard Medical School in Boston (USA) as a postdoc. He returned to Switzerland to the Institute of Physiology of the University of Zurich as Assistant Professor, and subsequently joined the faculty of the Biozentrum first as Associate, and then as Full Professor. His research group aims at linking molecular mechanisms to physiology and pathophysiology in skeletal muscle.
Christoph Handschin is a group leader at the Biozentrum of the University of Basel in Switzerland. He acquired his PhD in Biochemistry at the Biozentrum before moving to Prof. Bruce Spiegelman’s laboratory at the Dana-Farber Cancer Institute, Harvard Medical School in Boston (USA) as a postdoc. He returned to Switzerland to the Institute of Physiology of the University of Zurich as Assistant Professor, and subsequently joined the faculty of the Biozentrum first as Associate, and then as Full Professor. His research group aims at linking molecular mechanisms to physiology and pathophysiology in skeletal muscle.
Additional information
Competing interests
None.
Author contributions
R.F. and C.H. designed and created the figures, wrote, edited and approved the final version of the manuscript. All authors agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All persons designated as authors qualify for authorship, and all those who qualify for authorship are listed.
References
- Austad SN. Methusaleh’s Zoo: How nature provides us with clues for extending human health span. Journal of Comparative Pathology. 2010;142(Suppl 1):S10–S21. doi: 10.1016/j.jcpa.2009.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartke A. Growth hormone and aging. Reviews in Endocrine & Metabolic Disorders. 2021;22:71–80. doi: 10.1007/s11154-020-09593-2. [DOI] [PubMed] [Google Scholar]
- Blagosklonny MV. The goal of geroscience is life extension. Oncotarget. 2021;12(3):131–144. doi: 10.18632/oncotarget.27882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booth FW, Laye MJ. Lack of adequate appreciation of physical exercise’s complexities can pre-empt appropriate design and interpretation in scientific discovery. Journal of Physiology. 2009;587(23):5527–5539. doi: 10.1113/jphysiol.2009.179507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campisi J, Kapahi P, Lithgow GJ, Melov S, Newman JC, Verdin E. From discoveries in ageing research to therapeutics for healthy ageing. Nature. 2019;571(7764):183–192. doi: 10.1038/s41586-019-1365-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colchero F, Aburto JM, Archie EA, Boesch C, Breuer T, Campos FA, Collins A, Conde DA, Cords M, Crockford C, Thompson ME, et al. The long lives of primates and the ‘invariant rate of ageing’ hypothesis. Nature Communications. 2021;12(1):3666. doi: 10.1038/s41467-021-23894-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coppede F. Mutations involved in premature-ageing syndromes. The Application of Clinical Genetics. 2021;14:279–295. doi: 10.2147/TACG.S273525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dakic T, Jevdjovic T, Vujovic P, Mladenovic A. The less we eat, the longer we live: Can caloric restriction help us become centenarians? International Journal of Molecular Sciences. 2022;23(12):6546. doi: 10.3390/ijms23126546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Lima Camillo LP, Quinlan RBA. A ride through the epigenetic landscape: Aging reversal by reprogramming. Geroscience. 2021;43(2):463–485. doi: 10.1007/s11357-021-00358-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenstein M. Does the human lifespan have a limit? Nature. 2022;601(7893):S2–S4. doi: 10.1038/d41586-022-00070-1. [DOI] [PubMed] [Google Scholar]
- Fohr T, Tormakangas T, Lankila H, Viljanen A, Rantanen T, Ollikainen M, Kaprio J, Sillanpaa E. The association between epigenetic clocks and physical functioning in older women: A three-year follow-up. Journals of Gerontology Series A, Biological Sciences and Medical Sciences. 2021;77(8):1569–1576. doi: 10.1093/gerona/glab270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman SM. Lifestyle (Medicine) and healthy aging. Clinics in Geriatric Medicine. 2020;36(4):645–653. doi: 10.1016/j.cger.2020.06.007. [DOI] [PubMed] [Google Scholar]
- Furrer R, Handschin C. Lifestyle vs. pharmacological interventions for healthy aging. Aging. 2020;12(1):5–7. doi: 10.18632/aging.102741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garatachea N, Pareja-Galeano H, Sanchis-Gomar F, Santos-Lozano A, Fiuza-Luces C, Moran M, Emanuele E, Joyner MJ, Lucia A. Exercise attenuates the major hallmarks of aging. Rejuvenation Research. 2015;18(1):57–89. doi: 10.1089/rej.2014.1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gems D. The aging-disease false dichotomy: Understanding senescence as pathology. Frontiers in Genetics. 2015;6:212. doi: 10.3389/fgene.2015.00212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gems D, de Magalhaes JP. The hoverfly and the wasp: A critique of the hallmarks of aging as a paradigm. Ageing Research Reviews. 2021;70:101407. doi: 10.1016/j.arr.2021.101407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gems D, Partridge L. Genetics of longevity in model organisms: Debates and paradigm shifts. Annual Review of Physiology. 2013;75(1):621–644. doi: 10.1146/annurev-physiol-030212-183712. [DOI] [PubMed] [Google Scholar]
- Gliemann L, Schmidt JF, Olesen J, Bienso RS, Peronard SL, Grandjean SU, Mortensen SP, Nyberg M, Bangsbo J, Pilegaard H, Hellsten Y. Resveratrol blunts the positive effects of exercise training on cardio-vascular health in aged men. Journal of Physiology. 2013;591(20):5047–5059. doi: 10.1113/jphysiol.2013.258061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goh J, Wong E, Soh J, Maier AB, Kennedy BK. FEBS Journal. Advance online publication; 2021. Targeting the molecular & cellular pillars of human aging with exercise. [DOI] [PubMed] [Google Scholar]
- Guan Y, Yan Z. Molecular mechanisms of exercise and healthspan. Cells. 2022;11(5):872. doi: 10.3390/cells11050872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gubert C, Hannan AJ. Exercise mimetics: Harnessing the therapeutic effects of physical activity. Nature Reviews Drug Discovery. 2021;20(11):862–879. doi: 10.1038/s41573-021-00217-1. [DOI] [PubMed] [Google Scholar]
- Handschin C. Caloric restriction and exercise “mimetics”: Ready for prime time? Pharmacological Research. 2016;103:158–166. doi: 10.1016/j.phrs.2015.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herculano-Houzel S. Longevity and sexual maturity vary across species with number of cortical neurons, and humans are no exception. Journal of Comparative Neurology. 2019;527(10):1689–1705. doi: 10.1002/cne.24564. [DOI] [PubMed] [Google Scholar]
- Hofer SJ, Carmona-Gutierrez D, Mueller MI, Madeo F. The ups and downs of caloric restriction and fasting: From molecular effects to clinical application. EMBO Molecular Medicine. 2022;14(1):e14418. doi: 10.15252/emmm.202114418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jylhava J, Pedersen NL, Hagg S. Biological age predictors. EBioMedicine. 2017;21:29–36. doi: 10.1016/j.ebiom.2017.03.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy BK, Berger SL, Brunet A, Campisi J, Cuervo AM, Epel ES, Franceschi C, Lithgow GJ, Morimoto RI, Pessin JE, Rando TA, et al. Geroscience: Linking aging to chronic disease. Cell. 2014;159(4):709–713. doi: 10.1016/j.cell.2014.10.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerepesi C, Meer MV, Ablaeva J, Amoroso VG, Lee SG, Zhang B, Gerashchenko MV, Trapp A, Yim SH, Lu AT, Levine ME, et al. Epigenetic aging of the demographically non-aging naked mole-rat. Nature Communications. 2022;13(1):355. doi: 10.1038/s41467-022-27959-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keshavarz M, Xie K, Schaaf K, Bano D, Ehninger D. Targeting the “hallmarks of aging” to slow aging and treat age-related disease: Fact or fiction? Molecular Psychiatry. 2022 doi: 10.1038/s41380-022-01680-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitada M, Ogura Y, Monno I, Koya D. The impact of dietary protein intake on longevity and metabolic health. EBioMedicine. 2019;43:632–640. doi: 10.1016/j.ebiom.2019.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kodama S, Saito K, Tanaka S, Maki M, Yachi Y, Asumi M, Sugawara A, Totsuka K, Shimano H, Ohashi Y, Yamada N, et al. Cardiorespiratory fitness as a quantitative predictor of all-cause mortality and cardiovascular events in healthy men and women: A meta-analysis. JAMA. 2009;301(19):2024–2035. doi: 10.1001/jama.2009.681. [DOI] [PubMed] [Google Scholar]
- Konopka AR, Laurin JL, Schoenberg HM, Reid JJ, Castor WM, Wolff CA, Musci RV, Safairad OD, Linden MA, Biela LM, Bailey SM, et al. Metformin inhibits mitochondrial adaptations to aerobic exercise training in older adults. Aging Cell. 2019;18(1):e12880. doi: 10.1111/acel.12880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulkarni AS, Peck BD, Walton RG, Kern PA, Mar JC, Windham ST, Bamman MM, Barzilai N, Peterson CA. Metformin alters skeletal muscle transcriptome adaptations to resistance training in older adults. Aging. 2020;12(20):19852–19866. doi: 10.18632/aging.104096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyriazis M. Ageing throughout history: The evolution of human lifespan. Journal of Molecular Evolution. 2020;88(1):57–65. doi: 10.1007/s00239-019-09896-2. [DOI] [PubMed] [Google Scholar]
- Lee MB, Hill CM, Bitto A, Kaeberlein M. Antiaging diets: Separating fact from fiction. Science. 2021;374(6570):eabe7365. doi: 10.1126/science.abe7365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine ME, Higgins-Chen A, Thrush K, Minteer C, Niimi P. Clock work: Deconstructing the epigenetic clock signals in aging, disease, and reprogramming. bioRxiv. 2022 doi: 10.1101/2022.02.13.480245. [DOI] [Google Scholar]
- Li X, Ploner A, Wang Y, Magnusson PK, Reynolds C, Finkel D, Pedersen NL, Jylhava J, Hagg S. Longitudinal trajectories, correlations and mortality associations of nine biological ages across 20-years follow-up. eLife. 2020;9:e51507. doi: 10.7554/eLife.51507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Libert S, Chekholko A, Kenyon C. A physiology clock for human aging. bioRxiv. 2022 doi: 10.1101/2022.04.14.488358. [DOI] [Google Scholar]
- Liu M, Zhang Z, Zhou C, Ye Z, He P, Zhang Y, Li H, Liu C, Qin X. Predicted fat mass and lean mass in relation to all-cause and cause-specific mortality. Journal of Cachexia, Sarcopenia and Muscle. 2022;13(2):1064–1075. doi: 10.1002/jcsm.12921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Otin C, Blasco MA, Partridge L, Serrano M, Kroemer G. The hallmarks of aging. Cell. 2013;153(6):1194–1217. doi: 10.1016/j.cell.2013.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Losa-Reyna J, Alcazar J, Carnicero J, Alfaro-Acha A, Castillo-Gallego C, Rosado-Artalejo C, Rodriguez-Manas L, Ara I, Garcia-Garcia FJ. Impact of relative muscle power on hospitalization and all-cause mortality in older adults. Journals of Gerontology Series A, Biological Sciences and Medical Sciences. 2022;77(4):781–789. doi: 10.1093/gerona/glab230. [DOI] [PubMed] [Google Scholar]
- Macdonald-Dunlop E, Taba N, Klaric L, Frkatovic A, Walker R, Hayward C, Esko T, Haley C, Fischer K, Wilson JF, Joshi PK. A catalogue of omics biological ageing clocks reveals substantial commonality and associations with disease risk. Aging. 2022;14(2):623–659. doi: 10.18632/aging.203847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMaughan DJ, Oloruntoba O, Smith ML. Socioeconomic status and access to healthcare: Interrelated drivers for healthy aging. Frontiers in Public Health. 2020;8:231. doi: 10.3389/fpubh.2020.00231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNeil JJ, Nelson MR, Woods RL, Lockery JE, Wolfe R, Reid CM, Kirpach B, Shah RC, Ives DG, Storey E, Ryan J, et al. Effect of aspirin on all-cause mortality in the healthy elderly. New England Journal of Medicine. 2018;379(16):1519–1528. doi: 10.1056/NEJMoa1803955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melzer D, Pilling LC, Ferrucci L. The genetics of human ageing. Nature Reviews Genetics. 2020;21(2):88–101. doi: 10.1038/s41576-019-0183-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohammed I, Hollenberg MD, Ding H, Triggle CR. A critical review of the evidence that metformin is a putative anti-aging drug that enhances healthspan and extends lifespan. Frontiers in Endocrinology. 2021;12:718942. doi: 10.3389/fendo.2021.718942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morley JE. The mTOR conundrum: Essential for muscle function, but dangerous for survival. Journal of the American Medical Directors Association. 2016;17(11):963–966. doi: 10.1016/j.jamda.2016.09.001. [DOI] [PubMed] [Google Scholar]
- Nadon NL, Strong R, Miller RA, Harrison DE. NIA Interventions testing program: Investigating putative aging intervention agents in a genetically heterogeneous mouse model. EBioMedicine. 2017;21:3–4. doi: 10.1016/j.ebiom.2016.11.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson KM, Dahlin JL, Bisson J, Graham J, Pauli GF, Walters MA. Curcumin may (Not) defy science. ACS Medicinal Chemistry Letters. 2017;8(5):467–470. doi: 10.1021/acsmedchemlett.7b00139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nie C, Li Y, Li R, Yan Y, Zhang D, Li T, Li Z, Sun Y, Zhen H, Ding J, Wan Z, et al. Distinct biological ages of organs and systems identified from a multi-omics study. Cell Reports. 2022;38(10):110459. doi: 10.1016/j.celrep.2022.110459. [DOI] [PubMed] [Google Scholar]
- Nwagwu VC, Cigolle C, Suh T. Reducing frailty to promote healthy aging. Clinics in Geriatric Medicine. 2020;36(4):613–630. doi: 10.1016/j.cger.2020.06.005. [DOI] [PubMed] [Google Scholar]
- Paluch AE, Bajpai S, Bassett DR, Carnethon MR, Ekelund U, Evenson KR, Galuska DA, Jefferis BJ, Kraus WE, Lee IM, Matthews CE, Steps for Health C et al. Daily steps and all-cause mortality: a meta-analysis of 15 international cohorts. Lancet Public Health. 2022;7(3):e219–e228. doi: 10.1016/S2468-2667(21)00302-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Partridge L, Fuentealba M, Kennedy BK. The quest to slow ageing through drug discovery. Nature Reviews Drug Discovery. 2020;19(8):513–532. doi: 10.1038/s41573-020-0067-7. [DOI] [PubMed] [Google Scholar]
- Raffaele M, Vinciguerra M. The costs and benefits of senotherapeutics for human health. The Lancet Global Health. 2022;3:E67–E77. doi: 10.1016/S2666-7568(21)00300-7. [DOI] [PubMed] [Google Scholar]
- Raffle AE, Mackie A, Gray JAM. Screening: evidence and practice. Oxford University Press; 2019. [Google Scholar]
- Rebelo-Marques A, De Sousa Lages A, Andrade R, Ribeiro CF, Mota-Pinto A, Carrilho F, Espregueira-Mendes J. Aging hallmarks: The benefits of physical exercise. Frontiers in Endocrinology. 2018;9:258. doi: 10.3389/fendo.2018.00258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhinn M, Ritschka B, Keyes WM. Cellular senescence in development, regeneration and disease. Development. 2019;146(20):dev151837. doi: 10.1242/dev.151837. [DOI] [PubMed] [Google Scholar]
- Ring NAR, Valdivieso K, Grillari J, Redl H, Ogrodnik M. The role of senescence in cellular plasticity: Lessons from regeneration and development and implications for age-related diseases. Developmental Cell. 2022;57(9):1083–1101. doi: 10.1016/j.devcel.2022.04.005. [DOI] [PubMed] [Google Scholar]
- Runacres A, Mackintosh KA, McNarry MA. Health consequences of an elite sporting career: Long-term detriment or long-term gain? A meta-analysis of 165,000 former athletes. Sports Medicine. 2021;51(2):289–301. doi: 10.1007/s40279-020-01379-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutledge J, Oh H, Wyss-Coray T. Nature Reviews Genetics. Advance online publication; 2022. Measuring biological age using omics data. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sayer AA, Kirkwood TB. Grip strength and mortality: A biomarker of ageing? Lancet. 2015;386(9990):226–227. doi: 10.1016/S0140-6736(14)62349-7. [DOI] [PubMed] [Google Scholar]
- Seale K, Horvath S, Teschendorff A, Eynon N, Voisin S. Making sense of the ageing methylome. Nature Reviews Genetics. 2022;23(10):585–605. doi: 10.1038/s41576-022-00477-6. [DOI] [PubMed] [Google Scholar]
- Sellami M, Bragazzi N, Prince MS, Denham J, Elrayess M. Regular, intense exercise training as a healthy aging lifestyle strategy: Preventing DNA damage, telomere shortening and adverse DNA methylation changes over a lifetime. Frontiers in Genetics. 2021;12:652497. doi: 10.3389/fgene.2021.652497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Q, Chang C, Saliba A, Bhat MA. Microglial mTOR activation upregulates Trem2 and enhances beta-Amyloid plaque clearance in the 5XFAD alzheimer’s disease model. Journal of Neuroscience. 2022;42(27):5294–5313. doi: 10.1523/JNEUROSCI.2427-21.2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sillanpaa E, Heikkinen A, Kankaanpaa A, Paavilainen A, Kujala UM, Tammelin TH, Kovanen V, Sipila S, Pietilainen KH, Kaprio J, Ollikainen M, et al. Blood and skeletal muscle ageing determined by epigenetic clocks and their associations with physical activity and functioning. Clinical Epigenetics. 2021;13(1):110. doi: 10.1186/s13148-021-01094-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohal RS, Forster MJ. Caloric restriction and the aging process: A critique. Free Radical Biology & Medicine. 2014;73:366–382. doi: 10.1016/j.freeradbiomed.2014.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strasser B, Burtscher M. Survival of the fittest: VO2max, a key predictor of longevity? Frontiers in Bioscience. 2018;23(8):1505–1516. doi: 10.2741/4657. [DOI] [PubMed] [Google Scholar]
- Tzemah-Shahar R, Hochner H, Iktilat K, Agmon M. What can we learn from physical capacity about biological age? A systematic review. Ageing Research Reviews. 2022;77:101609. doi: 10.1016/j.arr.2022.101609. [DOI] [PubMed] [Google Scholar]
- Walton RG, Dungan CM, Long DE, Tuggle SC, Kosmac K, Peck BD, Bush HM, Villasante Tezanos AG, McGwin G, Windham ST, Ovalle F, et al. Metformin blunts muscle hypertrophy in response to progressive resistance exercise training in older adults: A randomized, double-blind, placebo-controlled, multicenter trial: The MASTERS trial. Aging Cell. 2019;18(6):e13039. doi: 10.1111/acel.13039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White DK, Neogi T, Nevitt MC, Peloquin CE, Zhu Y, Boudreau RM, Cauley JA, Ferrucci L, Harris TB, Satterfield SM, Simonsick EM, et al. Trajectories of gait speed predict mortality in well-functioning older adults: The Health, Aging and Body Composition study. Journals of Gerontology Series A, Biological Sciences and Medical Sciences. 2013;68(4):456–464. doi: 10.1093/gerona/gls197. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.