Abstract
Preventive interventions are expected to substantially improve the prognosis of patients with primary liver cancer, predominantly hepatocellular carcinoma (HCC) and cholangiocarcinoma. HCC prevention is challenging in the face of the evolving etiological landscape, particularly the sharp increase in obesity-associated metabolic disorders, including metabolic dysfunction-associated steatotic liver disease (MASLD). Next-generation anti-HCV and HBV drugs have substantially reduced, but not eliminated, the risk of HCC and have given way to new challenges in identifying at-risk patients. The recent development of new therapeutic agents and modalities has opened unprecedented opportunities to refine primary, secondary, and tertiary HCC prevention strategies. For primary prevention (before exposure to risk factors), public health policies, such as universal HBV vaccination, have had a substantial prognostic impact. Secondary prevention (after or during active exposure to risk factors) includes regular HCC screening and chemoprevention. Emerging biomarkers and imaging modalities for HCC risk stratification and detection may enable individual risk-based personalized and cost-effective HCC screening. Clinical studies have suggested the potential utility of lipid-lowering, anti-diabetic/obesity, and anti-inflammatory agents for secondary prevention, and some of them are being evaluated in prospective clinical trials. Computational and experimental studies have identified potential chemopreventive strategies directed at diverse molecular, cellular, and systemic targets for etiology-specific and/or agnostic interventions. Tertiary prevention (in conjunction with curative-intent therapies for HCC) is an area of active research with the development of new immune-based neo/adjuvant therapies. Cholangiocarcinoma prevention may advance with recent efforts to elucidate risk factors. These advances will collectively lead to substantial improvements in liver cancer mortality rates.
Keywords: Chemoprevention, generic, hepatocellular carcinoma, cholangiocarcinoma, biomarker, cancer screening
Introduction
Cancer prevention is a cost-effective strategy to reduce cancer mortality, particularly in contrast to the emerging costly anti-cancer drugs with limited survival benefit in patients with advanced-stage cancer.[1] However, the target population for cancer-preventive interventions remains elusive for many cancer types due to limited knowledge about predisposing factors. Primary liver cancer, including hepatocellular carcinoma (HCC) and cholangiocarcinoma (CCA), is an exception with well-defined at-risk conditions in most cases, i.e., patients with chronic hepatic/biliary disease. Liver cancer is the third leading cause of cancer-related deaths worldwide with an estimated 750,000 deaths in 2022, and the most common cause of death from cancer among men in 24 countries and women in 6 countries.[2–4] Globally, liver cancer incidence is expected to increase by 55% between 2020 and 2040.[5, 6] In the U.S., HCC incidence rates have been increasing in more than half of the states with substantial racial/ethnic disparities.[7, 8] The incidence and mortality of CCA have been increasing globally, currently accounting for approximately 15% of primary liver cancers and 2% of cancer-related deaths, respectively.[9] The 5-year overall survival rate is still below 20% due to the limited efficacy of currently available treatment options and highly frequent post-treatment tumor recurrence.[10] Thus, preventive interventions in at-risk patients will be rational strategies to substantially improve the poor prognosis for the patients in the era of next-generation antivirals and obesity epidemic.
Evolving etiological landscape in HCC
Clinically, HCC develops almost exclusively in chronically diseased livers due to various etiological factors, including hepatitis B virus (HBV), hepatitis C virus (HCV), excessive alcohol intake, and metabolic dysfunction-associated steatotic liver disease (MASLD; new nomenclature for non-alcoholic fatty liver disease [NAFLD][11]), and systemic metabolic disorders such as type 2 diabetes (T2D) and dyslipidemia that can accompany MASLD.[12] The prevalence of these etiologies has changed over the past decades with the widespread use of next-generation antivirals and the epidemic of obesity and metabolic disorders. Recent clinical translation of direct-acting antivirals (DAAs) has enabled cure of HCV infection also referred to as sustained virologic response (SVR), in the near totality of treated patients.[13] While HCC incidence rate is reduced by two thirds by achieving an SVR, the risk of HCC development persists, for nearly a decade especially when cirrhosis is present.[13–17] Chronic HBV infection has been the dominant etiology in East/Southeast Asia and sub-Saharan Africa, which is now declining with the use of universal neonatal vaccination and antivirals, but viral cure is still not yet possible.[18, 19] The prevalence of obesity has been rising globally over the past decades, and this trend is closely linked to the rapid increase in MASLD in approximately 30% of the global population.[11, 20, 21] The largest increases are expected in women, smokers, and those without metabolic syndrome, and current smoking and overweight/obesity contribute to increased HCC incidence across etiologies.[22–24] Alcohol-associated liver disease (ALD) is currently the second fastest-growing HCC etiology, and has increased particularly during the COVID-19 pandemic; this surge is expected to lead to increase in incidence and mortality of ALD-related HCC.[25, 26] These changes continue to evolve and pose challenges to the development of effective HCC prevention strategies.
Risk factors for CCA
CCA is the second most common primary liver cancer that originates from biliary tract and is classified into either intrahepatic (iCCA), perihilar (pCCA), or distal (dCCA) CCA according to anatomical location of the tumor.[27] Chronic biliary inflammation associated with Caroli’s disease, choledochal cysts, and hepato/choledocholithiasis are the major CCA risk factors.[9] In Southeast Asia, liver fluke infection is frequently observed in CCA patients. The major HCC risk factors such as HBV, HCV, ALD, and MASLD are also associated with incident iCCA often diagnosed during regular HCC screening. Nearly half of CCA patients lack clinically appreciable risk factors and are often incidentally diagnosed at advanced stages. Thus, early detection of CCA is hampered by highly heterogeneous and still elusive CCA etiologies, which obscure the optimal target population for CCA screening. Enhanced knowledge of CCA risk factors will also inform development of CCA-preventive therapies.
Hcc Prevention Strategies
Framework and levels of HCC prevention
Over the course of cancer initiation and progression, preventive interventions can be classified into three levels, i.e., primary, secondary, and tertiary prevention.[28–30] Primary prevention targets individuals before exposure to risk factors, aiming to avoid at-risk status (Figure 1). Interventions such as vaccination and lifestyle modification belong at this level. Secondary prevention aims to reduce cancer risk and/or mortality in individuals who are exposed to risk factors and develop risk conditions by early cancer detection and chemoprevention. Tertiary prevention encompasses interventions to reduce cancer recurrence, improve survival, and/or maintain quality of life after a cancer diagnosis. In HCC, tertiary prevention is important because of the extremely frequent recurrence (up to 80%) even after curative-intent surgical therapies.[31] Regular monitoring of recurrence after curative-intent therapies aims at early detection of recurrent tumors for medical interventions with survival benefit. Neo/adjuvant therapies with curative-intent therapies are expected to reduce recurrence. This framework serves as a basis to evaluate new strategies of HCC prevention, incorporating specific clinical contexts and new modalities/methodologies.
Figure 1. Conceptual framework for HCC prevention over the natural history of HCC development and progression.
Challenges and unmet needs at each level of HCC prevention
For primary prevention, universal neonatal HBV vaccination has been adopted in 189 countries as of 2019.[32] In Taiwan, the vaccination program has halved HCC incidence and mortality, and nearly eradicated HCC in children and young adults with the four decades of nationwide implementation.[32, 33] However, global coverage with vaccination remains below 50% despite the WHO’s goal of 90% by 2030.[34] Lifestyle modifications may serve as primary prevention of HCC in individuals at risk of cardio-metabolic diseases. It will be critical to properly frame the target population and context of application to achieve clinically meaningful effects of candidate interventions.
Secondary prevention consists of two major components, early detection and chemoprevention. For early detection, practice guidelines currently recommend semi-annual HCC screening with ultrasound and alpha-fetoprotein (AFP) in patients with cirrhosis as an intervention to improve curative treatment receipt and patient survival.[35, 36] However, this recommended regular screening is significantly underutilized (<25% of HCC diagnosis) due to multiple obstacles such as the rapidly growing patient population with metabolic/steatotic liver disease and limited medical resources for HCC screening.[37] The low HCC incidence rates in the growing at-risk population (i.e., patients with MASLD and cured HCV) impair cost-effectiveness of semi-annual HCC screening. To address the problems and improve early detection, new methods are needed for HCC risk stratification to identify at-risk patients to be prioritized for HCC screening. In addition, performance of the current HCC screening modalities (i.e., ultrasound and AFP) is suboptimal, highlighting the need for new methods of HCC detection with improved sensitivity and specificity.[38]
Chemoprevention utilizes drugs or natural compounds to prevent cancer development from precancerous conditions.[29] Next-generation antivirals now enable cure of HCV infection and control of HBV viremia and hepatitis, which have already led to substantial reduction of viral hepatitis-related incident HCC and therefore can serve as chemoprevention.[39–41] Besides antivirals, various classes of compounds have been explored as potential HCC chemoprevention, although none has been adopted in clinical practice to date.[30] Experimental studies in rodent models have suggested multiple molecular pathways and cell types as potential targets for HCC chemoprevention (Figure 2).[42] However, their clinical validation (i.e., “bench-to-bedside” translation) is challenging or practically infeasible in many cases due to the requirements for large and lengthy clinical trials.[28] In addition, chemoprevention clinical trials are rarely successful likely due to the cross-species differences in the molecular mechanisms and timeframe of cancer development and therapeutic response. A “reverse-engineering” approach may help address the issue by starting from “bedside-to-bench” biological hypothesis-free identification of molecular targets surely correlated with clinical HCC incidence.[43] The clinically validated molecular correlates of HCC risk subsequently undergo functional assessments in experimental models, including rodent HCC models, patient-derived precision-cut liver slice (PCLS), and cell-based systems.[43–54] Mechanistically validated targets and chemopreventive agents/strategies can further undergo “back-to-bedside” translation, i.e., prospective clinical trials and eventual clinical implementation (Figure 3). This approach has led to the development of ongoing clinical trials of HCC chemoprevention (e.g., NCT05028829). Recent successes in developing molecular-targeted agents for MASH, biliary diseases, and metabolic disorders suggest their potential utility for HCC chemoprevention.[55–57] For clinical testing and implementation of new chemoprevention therapies, HCC risk biomarkers may play critical roles by guiding their indications and/or monitoring the effect as companion biomarkers.[28] The value of such biomarkers cannot be overemphasized given the low HCC incidence rates (<1% per year) in the rapidly growing major HCC etiologies, including MASLD and cured HCV.[39, 58, 59] Notably, HCC can develop in MASLD patients even before development of cirrhosis,[60] further highlighting the importance of HCC risk biomarkers to select patients from the large pre-cirrhotic MASLD patient population for preventive interventions.
Figure 2. Potential molecular targets and agents for HCC chemoprevention.
(A) Cross-organ and inter-cell type targets for HCC chemoprevention, which can be modulated by candidate agents and interventions. (B) Intra-cellular signaling pathways implicated in hepatocarcinogenesis that are targeted by candidate HCC chemopreventive agents.
Abbreviations: PAMPs, pathogen-associated molecular patterns; LPS, lipopolysaccharides; SCFA, short-chain fatty acid; DAMPs, damage-associated molecular patterns; ECM, extracellular matrix; AGEs, advanced glycation end products; FFA, free fatty acid; TDZ, thiazolidinedione.
Figure 3. “Reverse-engineering” approach for HCC chemoprevention discovery.
A conceptual overview of the ‘reverse-engineering’ approach for HCC chemoprevention discovery is shown, starting with a biological hypothesis-free exploration of clinically relevant chemoprevention targets and agents, followed by experimental validations and clinical testing.
Tertiary prevention aims to control recurrence and improve survival after curative-intent surgical/ablative therapies. HCC recurrence is biologically and clinically more complex compared to other cancer types because of the presence of highly cancerous tissue milieu in the remnant liver. This complexity compromises HCC recurrence risk prediction. Previous attempts to develop neo/adjuvant therapies in HCC have been unsuccessful. However, emerging immune checkpoint inhibitor (ICI)-based regimens may reduce HCC recurrence as neo/adjuvant therapies. The efficacy of these therapies may be affected by the type of HCC recurrence.
Strategies For Early Detection Of HCC Onset and Recurrence
Individual risk-based HCC screening in patients with advanced liver fibrosis or cirrhosis
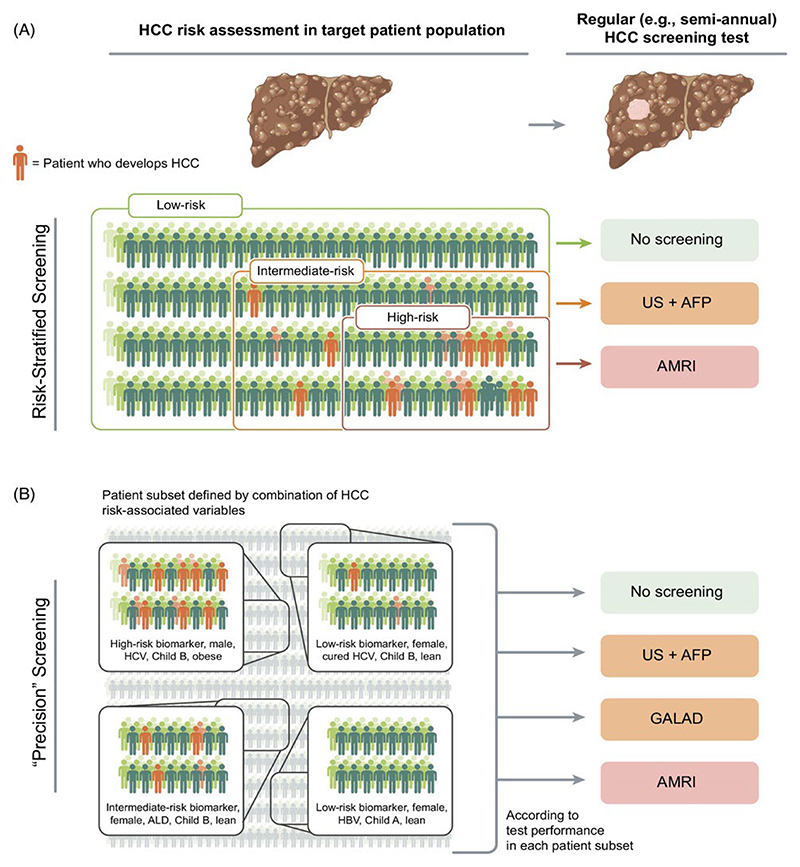
The guideline-recommended semi-annual ultrasound-based HCC screening aims at early tumor detection in patients with advanced liver fibrosis or cirrhosis as a major focus of secondary prevention. However, its clinical implementation is challenging as evidenced by the low utilization rate (<25%) in patients with new HCC diagnosis.[37] Application of this screening protocol is increasingly difficult due to the large and growing population of patients with cirrhosis and metabolic disorders, including pre-cirrhotic MASLD.[61, 62] In addition, the lower HCC incidence rates in the emerging patient populations (<1% per year) diminishes the cost-effectiveness of the current semi-annual screening protocol that were originally tailored to the now diminishing HCC etiology, i.e., active HCV infection associated with higher HCC incidence rates (3~5%).[28] New methods of HCC risk stratification are expected to address this issue by identifying patients with elevated HCC risk who will benefit from HCC screening. New methods for HCC detection are also needed given the suboptimal performance of the current modalities, ultrasound and AFP.[38, 63] HCC risk stratification may be followed by individualized protocol altering intensity of the screening (e.g., frequency, choice of screening tests) according to predicted HCC risk (Figure 4). The rapidly emerging new molecular/imaging-based methods of HCC detection as well as integrative HCC risk scores (e.g., GALAD score, circulating methylated cell-free DNA) may be utilized for personalization of HCC screening.[38] Markov model-based studies showed that risk-stratified HCC screening was more cost-effective compared to the standard-care “all-comer” screening and mitigated screening-related physical, phycological, and socioeconomic harms in low-risk patients who will not benefit from regular screening.[64, 65] Moreover, a ‘precision’ HCC screening strategy tailored based on individual factors (e.g., HCC risk biomarker, sex, etiology, Child-Pugh class, and obesity) was cost-effective in a simulation study.[66] Encouragingly, healthcare providers are receptive to such tailored HCC screening when anticipated HCC risk is provided up front.[67]
Figure 4. Conceptual framework of individual risk-based HCC screening.
(A) Risk-stratified HCC screening. Chronic liver disease patients with advanced fibrosis or cirrhosis are stratified according to biomarker/score-based HCC risk prediction. An HCC screening test modality is then assigned to each risk group to maximize net cost-effectiveness of the screening across the entire target patient population. (B) Individual risk factor-based “precision” HCC screening. Subsets of patients are defined based on combination of the major HCC risk-associated factors such as biomarker-based HCC risk, sex, liver disease etiology, liver disease severity (i.e., Child-Pugh class), and obesity. An HCC screening test modality is then assigned to each patient subset to maximize net cost-effectiveness of the screening across the entire target patient population.
Biological and clinical contexts in HCC recurrence
HCC is a unique cancer type with two biologically distinct types of tumor recurrence due to the highly cancerous cirrhotic liver tissue milieu, presenting the “field effect/cancerization”.[29] Specifically, post-resection/ablation recurrence can be attributable to either (i) growth of disseminated cancer cells from the treated primary tumor (i.e., disseminative recurrence) or (ii) second primary tumor(s) clonally unrelated to the treated first primary tumor (i.e., de novo recurrence). Clinically, these two types of recurrence are often indistinguishable, while each type can be empirically enriched as early or late recurrence (corresponding to disseminative or de novo recurrence, respectively) based on a cut-off of 1 to 2 years after the therapies.[68] Relative prevalence of the recurrence types can vary between patient cohorts depending on various clinical factors such as tumor stages at the treatment, adherence to regular clinical follow-up, and quality of diagnostic and treatment procedures, among other factors. These confounding factors affect performance and generalizability of recurrence-predictive biomarkers/scores. The kinetics of recurrence risk over time can be projected as a recurrence hazard plot,[43, 69] which provides insight about relative prevalence of each recurrence type in a given patient cohort and helps interpretation of efficacy for tertiary prevention (Figure 5).
Figure 5. Hazard of HCC recurrence over time after curative-intent therapy.
The longitudinal hazard kinetics are plotted for two distinct biological types of HCC recurrence, disseminative (red) and de novo (blue) recurrences, after curative-intent therapy. The hazard of disseminative recurrence (arisen from residual HCC cells) usually peaks in one to two years after the therapy, whereas the hazard of de novo recurrence gradually increases over time. Clinically, in the hazard plot, disseminative and de novo recurrences overlap and are not clearly distinguishable. However, they can be enriched as early or late recurrences, respectively, at a cut-off of 1 to 2 years post-treatment. Clonal comparison of somatic DNA aberrations between primary and recurrent tumors may provide insights into their biological distinctions. Although it is not clinically confirmed, experimental studies have suggested that there may be late disseminative recurrence from dormant residual HCC cells.[353]
Predictors/correlates of early (or disseminative) recurrence include clinical, histological, imaging, and molecular features of aggressive tumor such as large tumor size and number, local and/or vascular tumor invasion, poor histological differentiation, increased arterial blood supply, absence of portal blood supply, anatomical proximity to initially treated primary tumor, somatic mutations of oncogenes and/or tumor suppressor genes, and activation of oncogenic and immuno-suppressive molecular pathways.[70] Predictors/correlates of late (or de novo) recurrence include factors associated with severity of underlying liver diseases and/or metabolic disorders, epigenetic imprinting, among others observed in cancer-permissive cirrhotic tissue milieu.[38, 71]
New modalities for HCC risk assessment and detection
Point-of-care (PoC) biomarker assays and imaging devices will improve access to cancer screening tests.[72] AFP can be sensitively measured by using PoC devices with smart phones.[73–75] PoC ultrasound (POCUS) may detect metabolic dysfunction-associated steatohepatitis (MASH) among MASLD patients.[76] Telemedicine tools, widely adopted during the COVID-19 pandemic, may assist use of the PoC-based tests.[77–79] Deep learning model using electronic health records (EHRs) may be utilized to assess HCC risk.[80] Health data collected by smartphones, wearables, and virtual assistance may serve as “digital biomarkers” in HCC risk assessment and monitoring.[81]
Etiology-Directed HCC Prevention Strategies
Medical interventions directed to treat HCC etiologies may serve as secondary or tertiary prevention with clinically meaningful benefits, depending on the clinical context.
HBV
Primary prevention of HBV-related HCC is ahead of other major HCC etiologies. Universal HBV vaccination, implemented in more than 180 countries, has significantly reduced incidence of HBV-related HCC.[32, 82, 83] Taiwan was among the first countries to implement the vaccination and antiviral therapy programs in 1984 and 2003, respectively; the prevalence of HBV infection in HCC patients has decreased from 83.3% (born in 1980–1984) to 55.6% (born in 2000–2004) between the birth cohorts.[32] Notably, HCC incidence has been nearly eliminated in children and young adults after approximately three to four decades of nationwide implementation.[32, 33] In a population-based study in Hong Kong, 30-year cumulative HCC incidence rate was lower in the vaccinated cohort (0.06%) compared to the unvaccinated cohort (0.14%).[84] The accumulating evidence clearly supports and establishes the utility of universal vaccination as an important measure for the primary prevention of HBV-related HCC. A multi-level prevention program, integrating maternal antiviral therapy and neonatal vaccination/testing, controlled the rate of mother-to-child HBV transmission to 0.23%.[85] The low global utilization rate of vaccination (<50%) should be addressed to reach the WHO’s goal of 90% in the near future.[34] Despite the notable reduction in new infections and related mortality, death counts in the aging population with HBV infection are increasing,[86] and secondary prevention in this large patient population is urgently needed.
Secondary prevention needs refined early HCC detection and preventive interventions in patients who are typically on long-term antiviral therapy. Clinical variable-based HCC risk scores have been extensively studied to improve early HCC detection in patients with chronic hepatitis B.[38] Systematic comparisons of the scores in the settings of meta-analysis or in regional patient populations have repeatedly shown superior performance of scores such as REAL-B.[18, 87, 88] Cure rates of HBV infection are low, but significant efforts are ongoing to develop direct-acting anti-HBV drugs and immune-targeted agents toward the goal of functional cure with HBsAg loss and ultimately eliminating HBV infection.[89, 90]
Nevertheless, the current standard care anti-HBV nucleot(s)ide analogs (NAs), tenofovir and entecavir, can achieve potent suppression of viral replication measured by serum/plasma HBV DNA levels, a well-known HCC risk predictor.[38] Suppression of viremia with NAs reduces HCC incidences by 50~70% depending on severity of hepatitis and fibrosis, and the reduction continues beyond 5 years after initiation of the therapies when cirrhosis is present at baseline.[40, 91] Magnitude of HCC risk reduction was comparable between tenofovir and entecavir in a meta-analysis, whereas experimental studies suggest that there may be differences in their mechanisms of action.[92, 93] Even on NAs, patients with persisting low-level viremia have approximately two-fold higher HCC incidence compared to patients without viremia.[91] Non-cirrhotic chronic hepatitis B patients with moderate baseline viral load showed the highest on-treatment HCC risk.[94] HCC still develop even after HBsAg clearance at an annual incidence rate of 0.86% in association with age, presence of cirrhosis, family history of HCC, and more-than-moderate alcohol consumption.[95] Finite, as opposed to continuous, NA therapy in HBeAg-negative cirrhosis patients may reduce HCC incidence possibly due to enhanced antiviral/tumor immunity,[96] although Discontinuation of NA therapy in cirrhotic patients may be controversial. Retrospective clinical studies have suggested that aspirin and statins may serve as chemoprevention of HBV-related HCC.
Tertiary prevention with NAs may control HCC recurrence after curative-intent surgical/ablative therapies. In a meta-analysis of twenty studies including 8,204 patients, NA use was associated with less frequent post-surgical recurrence (relative risk [RR], 0.69).[97] Tenofovir was associated with a lower risk of overall HCC recurrence (adjusted hazard ratio [aHR], 0.73) and late recurrence (aHR, 0.58) compared to entecavir, whereas no difference was observed for early recurrence (aHR, 0.88) in a meta-analysis of nine studies with 5,298 patients.[98] These associations should be evaluated in future, ideally prospective, studies given the high heterogeneities between the studies. A recently completed phase I trial in Taiwan showed that adjuvant P1101 (ropeginterferon alfa-2b) and nivolumab (anti-programmed cell death 1 [PD-1]) post-resection led to reduced HBsAg levels with manageable adverse events,[99] on which a subsequent phase II trial was planned. Several clinical trials are currently ongoing to evaluate the utility of antiviral therapies for secondary or tertiary prevention of HBV-related HCC (Table 1).
Table 1. Ongoing clinical trials for secondary prevention of HCC.
| Etiology of target patient population | Phase | class of agent | Agent | No. enrollment | Liver disease severity | Primary endpoint (Time frame) | Trial acronym | NCT number |
|---|---|---|---|---|---|---|---|---|
| HBV | IV | Anti-viral | NA | 600 | Cirrhosis* | Incident HCC (10 years) | n.a. | NCT03366571 |
| HBV | IV | Anti-viral | NA or peginterferon α | 20,000 | HBV-related Histological changes | Incident HCC (1, 3, 5 years) | OASIS | NCT04896255 |
| HBV | IV | Anti-viral | Entecavir | 245 | Child-Pugh score ≤7 | Incident HCC (5 years) | n.a. | NCT04646928 |
| HCV | IV | Anti-viral | Sofosbuvir-based regimen | 200 | n.a. | Incident HCC (6 years) | LONGHEAD | NCT03042520 |
| Etiology-agnostic | II | Lipid-lowering agent | Atorvastatin | 60 | Child-Pugh class A | Change in HCC risk biomarker (48 weeks) | TORCH | NCT05028829 |
| Etiology-agnostic | II | Lipid-lowering agent | Simvastatin | 80 | Cirrhosis* | Change in serum AFP-L3 (6 months) | n.a. | NCT02968810 |
| Etiology-agnostic | II | Dietary supplement | Epigallocatechin gallate | 60 | Cirrhosis* | Change in HCC risk biomarker (24 weeks) | CATCH-B | NCT06015022 |
| Etiology-agnostic | II | Kinase inhibitor | Erlotinib | 60 | Cirrhosis* | Change in HCC risk biomarker (24 weeks) | n.a. | NCT04172779 |
ClinicalTrials.gov accessed in September 2024.
Clinically and/or histologically diagnosed. n.a., not available. NA, nucleos(t)ide analogue; DAA, direct acting antiviral; AFP, α-fetoprotein.
HCV
While a preventive vaccine is still unavailable, HCV infection is now curable with the use of recently developed next-generation DAAs. However, global HCV elimination remains a distant goal despite the WHO’s ambition to achieve it by 2030 given that >55 million individuals have viremic infections, among which nearly 80% of infections are still undiagnosed.[100, 101] The access to the testing and care is still limited in economically disadvantaged populations affected with various issues such as the opioid crisis.[102, 103] As a pragmatic approach toward this goal, micro-elimination has been proposed to target high-risk populations such as injection drug users, patients on hemodialysis, and inmates with relevant interventions (e.g., HCV screening, reengagement, and antiviral therapies).[104, 105] HCV vaccination may serve as a primary prevention strategy in these high-risk populations but is not yet available.[106, 107] A recent discovery of rodent hepacivirus may accelerate development of HCV vaccines and antivirals.[108, 109]
Antiviral therapies based on DAA- or interferon-based regimens can serve as secondary prevention for HCV-related HCC. HCV cure with antivirals (i.e., SVR) reduces HCC incidence by 50~70%.[13, 110–112] DAA-based regimens were well tolerated in HCV-infected patients with compensated and decompensated cirrhosis and reduced HCC incidence (HR, 0.29).[113] Notably, HCC risk persists for a decade or longer when cirrhosis is present and/or the fibrosis-4 (FIB-4) scores ≥3.25 before antiviral therapies with annual HCC incidences above 2%.[17] In a meta-analysis of 27,711 cirrhotic and 32,123 non-cirrhotic patients, HCC incidence rates were 2.99% and 0.47%, respectively (0.63% in F3 fibrosis).[15] HCC risk gradually decreased over time after achieving SVR, but the HCC incidence rate remained high (1.8% per year) ≥3 years post-SVR in patients with cirrhosis. A therapeutic HCV vaccine with interleukin (IL)-12 DNA analog induced HCV-specific CD4/8 T cell response, but showed little effect on HCV viremia.[114] Epidermal growth factor receptor (EGFR) on the hepatocyte serves as an entry factor for HCV, which can be inhibited by a small molecule inhibitor, erlotinib.[115] In a phase Ib trial, erlotinib (100 mg/day for 14 days) safely reduced HCV RNA levels in non-cirrhotic chronic hepatitis C patients.[116] In rodent models, EGFR activation in hepatic stellate cells and macrophages was reported as a potential HCC chemoprevention target, which can be antagonized by erlotinib.[48, 117–119] More recently, erlotinib was evaluated in patients with compensated cirrhosis in a phase I trial.[120] Seven-day erlotinib treatment suppressed phospho-EGFR in liver tissues at 25 mg/day and favorably modulated an HCC risk transcriptomic signature at 50 mg/day with no dose-limiting adverse event.[49, 120] Epigenetic imprinting by HCV may serve as targets to prevent post-SVR HCC.[52, 53, 121]
Regular HCC screening is another arm of secondary prevention with survival benefit in cirrhosis patients even after HCV cure.[122] The clinical utility of HCC screening is assessed based on cost-effectiveness as a function of multiple influential parameters, particularly annual HCC incidence rate specific to each HCC etiology.[64] According to a recent simulation study, the currently recommended semi-annual HCC screening will meet the “willingness-to-pay” threshold of $100,000 per quality-adjusted life year gained when annual incidence rate exceeds 0.7% in HCV-cured patients.[123] The annual HCC incidence rate is above the threshold in cirrhotic patients (2~3%) within the first year after achieving an SVR, whereas the rate is borderline or below the threshold in non-cirrhotic patients (~0.5%).[15, 124] The low and gradually decreasing HCC incidence rates post SVR highlight the need for refined HCC risk stratification to enable cost-effective HCC screening.[38]
For tertiary prevention, the utility of antiviral therapies has been controversial. In a pooled analysis of 977 patients with HCV-related cirrhosis and successfully-treated HCC, there was a non-significant trend of less HCC recurrence in DAA-exposed patients (RR, 0.64).[125] In a recent randomized controlled trial, post-ablation DAA treatment was associated with less frequent 1-year HCC recurrence (28%) compared to non-DAA group (62%) (p=0.001).[126] Response rate to DAA therapy was lower in patients with HCC (SVR rate, 88%) compared to those without HCC (92%) regardless of cirrhosis status.[127]
There was no difference in both HCC occurrence and recurrence rates between DAA- and interferon-based therapies.[128] The reduction is accompanied by change in HCC risk biomarkers, suggesting their utility as surrogate endpoints in secondary and tertiary prevention.[43, 129, 130]
MASLD/MASH
MASLD/MASH is the fastest rising HCC etiology globally, and HCC prevention is an urgent unmet need in this patient population.[131–133] Any measures mitigating metabolic disorders may serve as secondary prevention. Multiple molecular targeted agents have been actively developed for treatment of MASLD/MASH with specific focus on improving hepatic steatosis and/or fibrosis within time frames of 1 to 2 years.[134] None of these agents have been evaluated for HCC-related endpoints to date, but once approved, their utility for HCC chemoprevention will be clarified with long-term follow up.
Resmetirom, a thyroid hormone receptor β (THR-β) agonist, showed a significant reduction in hepatic fibrosis in non-cirrhotic MASH patients in the phase III MAESTRO-NASH trial and became the first FDA-approved drug for MASH.[55, 135] Epidemiological and experimental studies have shown that hypothyroidism is associated with MASLD and activation of the THR-β pathway reduces hepatic steatosis and gluconeogenesis.[136] A simulation analysis suggested that resmetirom enables cost-effective management of MASH patients.[137] Fibroblast growth factor 21 (FGF21) is a pleiotropic hormone that exerts various beneficial effects on glucose and lipid metabolism through formation of a heterodimeric receptor complex with FGF receptor 1 and β-klotho.[138] In rodent MASH models, FGF21 showed anti-steatotic/fibrotic effects.[139] In a phase II trial, efruxifermin improved markers of liver injury, fibrosis, and glucose and lipid metabolism in patients with compensated MASH cirrhosis.[140] In the phase IIb ENLIVEN trial, pegozafermin improved liver steatosis/fibrosis in pre-cirrhotic MASH patients.[56] A phase IIb trial of FGF19 analog, aldafermin, improved Enhanced Liver Fibrosis (ELF) biomarker in compensated MASH cirrhosis patients.[141] In recent phase II trials, survodutide (agonist of glucagon and glucagon-like peptide-1 [GLP-1] receptors) and tirzepatide (agonist of glucose-dependent insulinotropic polypeptide and GLP-1 receptors) improved MASH with improvement or no worsening of fibrosis.[142, 143] In a phase II trial, semaglutide (GLP-1 receptor agonist [GLP-1 RA]) did not significantly improve fibrosis or MASH in patients with MASH-related compensated cirrhosis.[144] Obeticholic acid, a farnesoid X receptor agonist, improved liver fibrosis and non-invasive scores/tests (NIS/NIT) in phase III trial, but was not recommended for approval due to adverse events.[145–147]
Lifestyle interventions via diet, dietary supplements, and physical activities may act as secondary HCC prevention in MASLD patients.[148–152] The impact of timing, duration, and frequency of fasting has been actively investigated for beneficial and harmful effects.[153] An intermittent fasting 5:2 regimen reduced HCC in rodent MASH models via modulation of PPARγ and PCK1.[154] Retrospective clinical studies have suggested that several lifestyle-related factors are modifiable targets. For example, a high dietary inflammatory potential and low Healthy Eating Index-2015 diet were associated with increased risk of MASLD and HCC.[149, 150] Individuals with high levels of physical activity (≥32 metabolic equivalent hours/week) had a 40% lower risk of MASLD.[150] Despite these clear associations, actual intervention is challenging due to various logistical hurdles, which may be mitigated by IT-based tools.[155, 156] The VITALISE pilot feasibility study is a digital lifestyle intervention that utilizes web-based resources, tele-coaching sessions, and self-reporting system to assist initiation and maintenance of weight loss in MASLD patients.[157] A phase I/II trial showed that energy restriction and exercise improved liver histology in advanced MASH patients.[158] Several clinical variable- and blood test-based NIS/NIT have been assessed for identification of “at-risk” MASH patients with substantial liver fibrosis and will develop progressive diseases, including HCC.[38, 159–161] Some of the biomarkers are therapeutically modifiable and may serve as companion biomarkers for chemoprevention.[50]
ALD
Preventive interventions could be impactful for ALD-related HCC because of its association with a lower screening rate, more advanced stage at diagnosis, lower likelihood of receiving curative therapies, and poorer survival compared to other etiologies.[162] Primary prevention of ALD may be achieved with educational and/or behavioral interventions in high-risk individuals for excess alcohol drinking.[163, 164] Secondary prevention can be considered in patients with established ALD and advanced fibrosis. Alcohol abstinence is an impactful strategy to prevent adverse outcomes from ALD.[165] In a meta-analysis that involved 18,833 ALD cirrhosis patients, abstinence (prevalence, 53.8%) was associated with lower mortality (HR, 0.61) and decompensation (HR, 0.61), whereas available data for HCC was limited and non-significant (HR, 0.86).[166] This highlights the need for further assessment to clarify real utility of abstinence for secondary HCC prevention and the right target population. Monitoring alcohol relapse is critical in assessing the HCC-preventive effect of abstinence and may guide the timing of interventions to control relapse.
ALD-associated molecular aberrations are not limited to hepatic injury directly induced by ethanol byproducts, but also involve various extrahepatic systems, such as the gut-liver-brain axis and systemic immunity.[167] Emerging therapeutic strategies targeting these aberrations may play roles in secondary HCC prevention by directly modulating specific targets such as gut microbiome, neuroendocrine pathways, and incretin hormone system.[167] HCC screening may be improved via refined risk stratification with ALD-specific molecular HCC risk factors, such as single nucleotide polymorphisms (SNP) in WNT3A-WNT9A (rs708113) and TERT (rs2242652) genes[38, 168, 169] Clinical phenotype-based subgroups of ALD-related HCC may also inform definition of at-risk population for regular HCC screening[170] as well as tertiary prevention of post-resection/ablation de novo (or late) HCC recurrence.
Environmental carcinogens
Environmental substances such as microbe-derived products (e.g., aflatoxins B1 [AFB1]), organic compounds (e.g., vinyl chloride), plant-derived compounds (e.g., aristolochic acid [AA]), and heavy metals (e.g., iron, arsenic, cadmium) may increase HCC risk.[171–174]
Food contamination with AFB1, a product of Aspergillus flavus, elevates risk of HBV-related HCC with the characteristic TP53 R249S mutation in specific regions such as Qidong/Guangxi in China.[28, 175, 176] AFB1-related adducts in body fluid specimens can be measured to monitor aflatoxin exposure.[177] Somatic mutations in Mcm8, Bdp1, and Cct6a genes, functional dysregulation of BUB1B and RRM2, and interaction between HBx protein and ALKBH5 demethylase were associated with AFB1-associated hepatocarcinogenesis in experimental models.[178–180] In a phase II trial, ACCS100 (refined calcium montmorillonite clay) reduced serum AFB1-lysine adduct levels in 234 healthy individuals in South Texas.[181, 182] Hepatic AA-DNA adduct and AA-associated mutational signature were observed in 5~10% of liver cancer patients in China in association with somatic mutations in TP53 and JAK1 genes.[183] AA exposure in adulthood may less likely lead to liver tumorigenesis compared to exposure in infancy.[184]
Hemochromatosis occurs due to excessive dietary iron intake and/or germline DNA variants, e.g., HFE C282Y and H63D, and is associated with HCC risk at varying magnitudes.[185–187] Practice guidelines recommend phlebotomy to reduce adverse outcomes, including HCC, as secondary prevention.[188] In 106 Australian patients with the HFE C282Y-positive hemochromatosis and advanced fibrosis, regression of fibrosis by phlebotomy was associated with lower HCC incidence compared to non-responders (0.2% vs. 3.3% per year, respectively; HR, 0.08).[189] An oral iron chelator, deferasirox, was not tolerated in HCC patients.[190] Ferroptosis is an iron-dependent programmed cell death via lipid peroxides and may serve as a target for HCC chemoprevention.[191] A cystine/glutamate antiporter, solute carrier family 7 member 11 (SLC7A11 or xCT), is a potential target to induce ferroptosis as secondary prevention by using generic drugs (e.g., aspirin), cytoprotective agents (e.g., N-acetyl cysteine [NAC]), and natural products (e.g., gallic acid, traditional Chinese herb).[28, 192–196] Glutamine synthase 2 (GLS2) is a tumor suppressor via ferroptosis by regulating glutaminolysis in HCC.[197] Leukemia inhibitory factor receptor (LIFR) in hepatocytes suppresses tumorigenesis by maintaining sensitivity to ferroptosis inducers.[198] F-box and leucine-rich repeat protein 5 (FBXL5), an iron-sensing ubiquitin ligase, is low in patients with hemochromatosis-related HCC, and its ablation induces oxidative stress, tissue damage, inflammation, and compensatory proliferation and malignant transformation of hepatocytes in chemically-induced rodent HCC models.[199] These may serve as targets of iron homeostasis-directed HCC chemoprevention. In a recent case-control study, serum copper levels and copper/zinc ratio were associated with increased HCC risk, and dietary intake and serum levels of zinc were associated with a lower HCC risk in men.[200] Copper overload can be reduced with trientine and D-penicillamine by either facilitating urinary secretion and/or inhibiting intestinal absorption, which may serve as secondary prevention in Wilson’s disease patients.[201–203]
Etiology-Agnostic HCC Prevention Strategies
Molecular mechanisms of hepatocarcinogenesis shared across the liver disease etiologies may serve as targets for etiology-agnostic chemoprevention. Several therapies for metabolic disorders have been associated with lower HCC incidence in non-metabolic etiologies, suggesting their utility as etiology-agnostic HCC-preventive interventions.
Anti-inflammatory/platelet therapies
Chronic hepatic inflammation nearly always accompanies HCC development.[204] Platelets are involved in initiation of systemic cancer-promoting inflammation via various soluble factors, such as thromboxane A2, which contributes to the induction of cyclooxygenase (COX)-1/2 and prostaglandin E2 (PGE2) in the tissue microenvironment.[205] In experimental studies, COX-2 promotes HCC initiation and progression by activating oncogenic pathways, suppressing tumor suppressor pathways, and impairing anti-tumor immunity via various mechanisms that involve YAP oncoprotein, tet methylcytosine dioxygenase 1 (TET1), long non-coding RNA HULC, immunosuppressive myeloid-derived suppressor cells (MDSCs) and regulatory T cells (Tregs), among others.[206–209] Hepatic translocation of gut microbial lipoteichoic and deoxycholic acids enhances COX-2-mediated suppression of anti-tumor immunity in obesity/MASLD-related HCC mouse models.[210] In a phase II trial in 232 patients with curatively treated HCC, a COX-2 inhibitor, meloxicam, did not improve disease-free survival as tertiary prevention, while posthoc subgroup analyses suggested a possible effect in non-viral HCC.[211] A phase III trial of another COX-2 inhibitor, celecoxib, with or without metformin is registered for tertiary prevention of post-surgical recurrence (NCT03184493).
Use of non-steroidal anti-inflammatory drugs (NSAIDs) particularly aspirin, not non-aspirin NSAIDs, has been associated with lower HCC incidence in dose- and duration- (particularly ≥90 days) dependent manners as suggested in meta-analyses of retrospective studies,[30, 212–214] suggesting anti-platelet effect unique to aspirin. Experimental studies have reported various direct and indirect effect of aspirin on cellular proliferation, cell cycle, apoptosis, autophagy, lipid metabolism, and anti-tumor T cell function in HCC.[215] Recent large population-based studies in patients with MASLD and chronic hepatitis B in East Asian counties showed that aspirin use was associated with lower HCC incidence in non-cirrhotic patients (aHRs, 0.6 and 0.7~0.8, respectively).[216–219] A recent meta-analysis involving more than two million patients showed that such an association was obscured when adjusted for concurrent use of statins and metformin.[220] Aspirin use was associated with moderately increased risk of gastrointestinal bleeding.[217, 221] These conflicting findings need to be reconciled and the appropriate target patient population should be clarified. Aspirin use was also associated with less frequent HCC recurrence in a meta-analysis of retrospective studies, suggesting its potential role for tertiary prevention,[221] whereas a recent phase III trial failed to show the benefit of adjuvant aspirin therapy for breast cancer recurrence despite supportive retrospective data.[222] A recent randomized placebo-controlled trial demonstrated that 6-month daily low-dose aspirin reduced hepatic fat content in non-cirrhotic MASLD patients,[223] suggesting its role for secondary prevention in pre-cirrhotic patients. Other anti-platelet agents such as clopidogrel and ticagrelor also suppressed platelet-derived glycoprotein Ib-alpha and reduced HCC development in MASLD-related HCC mouse models.[224]
Immune-based therapies
Recent clinical trials have demonstrated possible utility of ICIs in the context of tertiary prevention.[225] In phase I trials, cemiplimab (anti-PD-1) as neoadjuvant therapy before surgical resection achieved a histological tumor necrosis rate of 70%,[226] and nivolumab (anti-PD-1) with/without ipilimumab as neo/adjuvant therapy achieved radiographic objective response rate of 30%.[227] An interim analysis of a phase III trial of adjuvant atezolizumab (anti-PD-L1) and bevacizumab (anti-VEGF) (IMbrave050) suggested that the combination therapy reduced recurrence-free survival (RFS) after resection/ablation of HCC.[228] However, the RFS benefit was not sustained with extended follow-up, while overall survival remained immature.[229] In a phase III trial, adjuvant immunotherapy with activated autologous cytokine-induced killer cells improved recurrence-free and overall survival.[230] Multiple trials are currently ongoing to test ICI-based combination neo/adjuvant therapies (Table S1). In an experimental study using a chemically induced murine HCC model, anti-PD-1 antibody suppressed tumorigenesis,[231] although immune-related adverse events (irAEs) likely preclude its clinical use as secondary prevention.
Anti-diabetic/obesity therapies
T2D is associated with approximately two-fold higher HCC incidence in meta-analyses of retrospective studies.[232, 233] Obesity is similarly associated with elevated HCC risk; a meta-analysis showed a BMI-dependent increase in the risk of primary liver cancer (HRs, 1.36~3.08).[234] In 14.3 million Korean overweight individuals from a national insurance database, the BMI-dependent increase was confirmed (HR, 1.60 per 5 kg/m2).[235] T2D and obesity can be present not only in patients with MASLD but also in patients with other HCC etiologies and boost HCC risk.[236] Experimental studies have suggested that several anti-diabetic/obesity drugs elicit pleiotropic anti-cancer effects, which may serve as etiology-agnostic HCC prevention strategies.[237, 238]
Metformin, a widely used first-line medication for T2D, elicits anti-cancer effects through suppression of cell proliferation and pathogenic inflammation in various experimental models and cancer types.[239] A meta-analysis showed association of metformin use with reduced HCC incidence (RR, 0.54~0.73) studies.[240] However, the association was obscured when concomitant use of aspirin and statins was adjusted.[220] In a retrospective cohort study of 7,249 HCV-cured patients in Taiwan (T-COACH), the 5-year cumulative HCC incidence was lower in metformin users with diabetes (2.6%) than in non-users (10.9%).[241] In 2,779 Taiwanese patients with diabetes and antiviral-resistant HCV infection, the 5-year HCC incidence was lower in metformin users with diabetes (3.1%) than in non-users (16.5%), mainly in non-cirrhotic patients.[242]
Sodium-glucose cotransporter 2 inhibitors (SGLT2i) have shown anti-tumor effect in experimental studies besides its blood glucose lowering effect by blocking renal reabsorption.[243] SGLT2i showed anti-HCC effect via modulation of the Wnt/β-catenin, AKT/mTOR, hypoxia, and AMPK/ACC pathways.[243] A territory-wide cohort study in Hong Kong showed that SGLT2i was associated with less frequent incident HCC (HR, 0.54) in patients with T2D and chronic hepatitis B.[244] In a case-control study using data from Korea's Health Insurance Review and Assessment Service, SGLT2i use was associated with lower HCC incidence (aHR, 0.45) in MASLD, T2D, and viral hepatitis patients, but not in patients with MASLD and T2D.[245] In a national database that involved 31,215 Taiwanese individuals, SGLT2i use was associated with lower HCC incidence compared to beta-blocker use (aHR, 0.27) among patients with chronic hepatitis B/C and T2D.[246] These studies indicate necessity to identify the right target patient population.
In a National Surveillance, Epidemiology and End Results (SEER) database in the U.S., SGLT2i use was associated with lower HCC-specific mortality (HR, 0.58) and the association was enhanced with longer duration of use (HR, 0.37) in 3,185 T2D patients with HCC, suggesting its potential utility for tertiary prevention.[247] A phase IV trial showed that 52-week treatment with empagliflozin reduced hepatic fat content in MASLD patients,[248] which warrants long-term follow of the participants for incident HCC.
GLP-1 RAs reduce body weight and elicit anti-diabetic effect via “incretin effect”, including stimulation of glucose-dependent insulin release from the pancreatic islets, suppression of glucagon secretion, and stimulation beta cell proliferation.[243] In a MASH mouse model, a GLP-1 RA, liraglutide, ameliorated steatosis, inflammation, and hepatocyte ballooning in the liver and suppressed HCC progression.[249] In a retrospective cohort of 1,890,020 T2D patients from the TriNetX real-world database, the use of GLP-1 RAs was associated with a lower HCC incidence (HRs of 0.20, 0.39, and 0.63 compared with insulin, sulfonylureas, and metformin, respectively).[250] Patients with T2D and cirrhosis treated with GLP-1 RAs plus metformin had less incident HCC (HR, 0.44) compared to metformin alone. [251] An international registry-based Scandinavian cohort study that involves 91,479 T2D patients treated with GLP-1 RAs and 244,004 patients treated with dipeptidyl peptidase-4 inhibitors (DPP-4i), use of GLP-1 RAs was associated with a lower incidence of cirrhosis (aHR, 0.85) but not HCC (HR, 1.05).[252] A recent retrospective cohort study in the U.S. Veterans also reported no significant difference in HCC incidence between GLP-1 RA and dipeptidyl peptidase-4 inhibitor (DPP4i) users (HR, 0.89).[253] These conflicting associations may be attributable to unadjusted confounding factors such as concomitant use of statins, which should be addressed in future prospective studies.[254]
DPP4i elicit anti-diabetic effect by inhibiting degradation of glucose-dependent insulinotropic polypeptide (GIP) and GLP-1. There are somewhat conflicting experimental and clinical evidence regarding anti- or pro-cancer properties of DPP4i.[255] In a mouse MASH HCC model, DDP-4i suppressed the pentose phosphate and p62/Keap1/Nrf2 pathways and prevented HCC progression.[256] In retrospective cohort studies that utilized the Taiwan’s National Health Insurance Research Database, DPP4i use was associated with a lower HCC incidence in 2,166 T2D patients with chronic HCV infection (aHR, 0.59; 0.17 in long-term [>1.49 years] users) and 11,028 T2D patients with chronic HBV infection (aHRs, 0.53) compared to non-users.[257, 258] In a retrospective cohort of 80,178 patients with T2D and MASLD from the Korean National Health Information (NHIS) database, the use of DPP4i and thiazolidinediones was not associated with a lower HCC incidence compared to sulfonylureas, whereas SGLT2i use was associated with a lower HCC incidence rate (adjusted sub-distribution HR [sHR], 0.37).[259] In the T2D and MASLD patients from the NHIS data, incident HCC was less frequent in SGLT2i users compared to DPP4i users (aHR, 0.68).[260]
Lipid-lowering therapies
Stains, beta-hydroxy beta-methylglutaryl-CoA reductase inhibitors, are widely used for hyperlipidemia to reduce adverse cardiovascular outcomes. Experimental studies have shown anti-tumor properties of statins through anti-proliferative, pro-apoptotic, anti-angiogenic, and immunomodulatory effects via mevalonate and other pathways.[261, 262] Statins suppress oncogenic drivers such as Myc, Akt, Rho kinase, NF-κB, tumor necrosis factor (TNF), transforming growth factor β (TGFβ), IL-6, Hippo, Yap, and extracellular signal-regulated kinase 1/2 (ERK1/2) pathways.[263, 264] Retrospective clinical studies and their meta-analyses have suggested a potential role for statins in HCC chemoprevention.[30] In a recent meta-analysis of 10 studies that involves 1,774,476 patients, statin use was associated with lower HCC incidence compared to non-users even after adjusting for concurrent use of aspirin and metformin (HR, 0.52).[220] This association was dose-dependent (aHRs of 0.66~0.34 according to cumulative defined daily dose [cDDD]) in a population-based cohort study in Taiwan.[265] Heterozygosity in the PNPLA3 SNP (rs738409), male sex, diabetes, and high baseline FIB-4 index were associated with HCC risk in subgroup analyses.[266, 267] In a retrospective study of 2,779 chronic hepatitis C patients who failed antiviral therapy in Taiwan, the 5-year cumulative HCC incidence was lower in statin users with hyperlipidemia (3.8%) than in non-users (12.5%).[242]
Use of lipophilic, not hydrophilic, statins was associated with lower HCC incidence compared to non-users (HR, 0.46) in a meta-analysis of 1,083,952 patients from 3 studies.[220] Consistent with this clinical observation, use of lipophilic statins was associated with lower HCC risk levels measured by hepatic transcriptome signature predictive of HCC risk (Prognostic Liver Signature [PLS]) with an aOR of 0.31.[50] In a PLS-inducible cell culture model (cPLS system),[44], a lipophilic statin, atorvastatin, showed the most significant improvement in PLS-based HCC risk level among other statins via favorable modulation of truncated retinoid X receptor-α and metallothionein in addition to suppression of YAP/AKT pathways.[268] In a mouse model of chemically-induced HCC, atorvastatin suppressed angiogenesis and reduced HCC nodules via inhibition of TGF-β and ERK pathways.[269] A phase II trial (TORCH) is underway to test atorvastatin for HCC chemoprevention in patients with advanced fibrosis or cirrhosis, using a serum-based HCC risk biomarker (Prognostic Liver Secretome signature [PLSec]) as the surrogate endpoint (NCT05028829) (Table 1). PLSec modulation will also be evaluated in another ongoing phase II trial (Liver Cirrhosis Network RESCU trial) that tests rosuvastatin (hydrophilic statin) in patients with cirrhosis (NCT05832229), which will provide an opportunity to prospectively compare lipophilic and hydrophilic statins for HCC chemoprevention.
Cellular pathway-targeted therapies
Clinical and experimental studies have suggested that various cellular pathway-targeted drugs approved for unrelated indications may be repurposed for HCC chemoprevention.[30] Many of the agents are available as generic drugs with well-characterized safety profiles and no intellectual property restriction. Therefore, they may be widely accessible as preventive medicines. Several candidate agents have been identified via empirical/serendipitous discoveries and systematic approaches that utilize bioinformatic resources.[270]
Renin-angiotensin system (RAS) is the target for angiotensin-converting enzyme inhibitor (ACEi) and angiotensin II type 1 receptor blocker (ARB) approved for treatment of hypertension. Experimental studies have shown involvement of RAS in pathogenesis of cirrhosis and HCC by promoting fibrogenesis, angiogenesis, and cancer cell proliferation and metastasis through downstream effectors, including TGFβ, AKT/mTOR, ERK1/2, VEGF, and NF-κB pathways.[271] A compound screening in a cell-based model (cPLS system) identified an ACEi, captopril, which favorably modulated a clinical HCC risk biomarker (PLS).[44] In rodent models of cirrhosis- or MASH-related hepatocarcinogenesis, captopril, inhibited the ACE pathway and its crosstalk with EGFR signaling and reduced HCC nodules.[51] Clinical studies support these findings and suggest utility of ACEi/ARB for secondary prevention of HCC. In a territory-wide cohort study of 12,327 MASLD patients with >5-year follow-up in Hong Kong, ACEi/ARB treatment was associated with a lower HCC incidence (sHR, 0.46).[272] In a nationwide cohort of compensated cirrhosis patients from a U.S.-based commercial database, ACEi/ARB users showed a non-significant trend of association with a lower HCC incidence (sHR, 0.83) compared to selective beta-blocker users.[273] These studies warrant further assessment in prospective studies. A systematic review suggested that use of ACEi/ARB alone or with other agents (e.g., vitamin K2, branched chain amino acids [BCAA]) may reduce HCC recurrence as potential tertiary prevention.[274]
Tyrosine kinase inhibitors represent one of the first classes of molecular-targeted drugs for cancer treatment. The expiration of patents for several first-generation kinase inhibitors has opened the path for their repurposing as low-cost chemopreventive agents. EGF +61G allele was associated with elevated tissue and blood EGF levels and HCC incidence (OR, 1.38) in patients with chronic liver diseases.[275–277] Experimental studies have shown that activated EGF signaling in hepatic stellate cells and macrophages promotes HCC development in rodent hepatitis/cirrhosis models.[118, 119] In DEN-induced HCC mouse model, DDX17 stabilized by metadherin promoted HCC initiation through interaction with Y-box binding protein 1 (YB1) and subsequent activation of the EGF/MEK/pERK signaling.[278] A small molecule EGFR inhibitor, erlotinib, reversed a high-risk pattern of the clinical HCC risk signature (PLS) and suppressed HCC development in rodent cirrhosis models.[48] A phase I trial of 7-day erlotinib treatment in cirrhosis patients showed suppression of hepatic phospho-EGFR levels at 25 mg/day and favorably modulated PLS at 50 mg/day (one-sixth and one-third of the approved chemotherapeutic dose, respectively) without any severe adverse events.[120] Based on these findings, a phase II trial of erlotinib to test longer treatment is planned (NCT04172779). The cPLS-based screening also identified nizatidine, a histamine receptor H2 (HRH2) antagonist, which reduced HCC nodules in rodent models via targeting of hepatocytes and HRH2+ CLEC5A+ MARCO- macrophages.[44]
The phosphoinositide 3-kinase (PI3K)/Akt/mammalian target of rapamycin (mTOR) pathway has been studied as a candidate therapeutic and chemoprevention target in HCC.[28, 43, 279] Recent clinical trials have failed to demonstrate a reduction in post-transplant HCC recurrence with mTOR inhibitors (e.g., sirolimus and everolimus), whereas post-hoc subgroup analyses have suggested that subsets of patients (e.g., within the Milan criteria) may benefit from the therapy.[280] The use of mTOR inhibitors as alternative immunosuppressants to calcineurin inhibitors reduces renal injury and extends post-transplant patient survival, but the adverse effects of mTOR inhibitors (e.g., dyslipidemia, hyperglycemia, proteinuria, and impaired wound healing) may limit their use for secondary prevention in cirrhotic patients.[280]
Claudin-1 (CLDN1), a tight junction protein, is expressed on hepatocytes and hepatic stellate cells during liver inflammation, fibrogenesis, and carcinogenesis. CLDN1 is also overexpressed in solid tumors including HCC and CCA. Monoclonal antibodies targeting exposed CLDN1 outside the tight junctions reduced liver fibrosis, prevent/treat HCC, and favorably modulate PLS in mouse models without any sign of toxicity.[281, 282] The antibody is currently under clinical development for treatment of advanced liver fibrosis and solid tumors.
Dietary and nutritional agents
Coffee has been associated with reduced HCC incidence and is recommended in practice guidelines.[35, 283] Meta-analyses consistently showed association of coffee intake with lower HCC risk (RR, 0.53).[284] In a population-based study in the U.K., all coffee types (i.e., decaffeinated, instant, and ground) were associated with lower HCC risk (HR, 0.80).[285] In a U.S.-based multi-center cohort study, coffee consumption of >3 cups/day was associated with lower HCC risk (HR, 0.73); the association was stronger in women (HR, 0.46) compared to men (HR, 0.93) and for caffeinated (HR, 0.71) compared to decaffeinated (HR, 0.92) coffee; there was no association between coffee consumption and iCCA risk.[286] Coffee consumption has been associated with lower levels of hepatic enzymes (AST, ALT, and GGT), inflammatory cytokine (e.g., IL-6), liver stiffness, and histological fibrosis along with favorable modulation of molecular pathways relevant to hepatocarcinogenesis, e.g., PI3K/Akt/mTOR signaling.[28, 287, 288]
Green tea consumption has been associated with lower HCC risk in meta-analyses (RR, 0.80).[284] In rodent models, epigallocatechin gallate (EGCG), a green tea-derived catechin/polyphenol, reduced HCC nodules via induction of hepatic stellate cell senescence, reduction of oxidative damage and DNA mutations, suppression of cell cycle, and induction of apoptosis.[45, 289–293] EGCG also suppressed miR483-3p-induced HCC metastasis in mice.[294] Controlled release of EGCG using near-infrared-responsive gold nanocages enhanced apoptosis of hepatoma cells.[295] EGCG combined with ursolic acid activated innate and acquired anti-cancer immunity.[295] These studies suggest that EGCG may serve for secondary and/or tertiary prevention of HCC. A phase II trial of EGCG in cirrhosis patients was initiated using an HCC risk biomarker, PLSec, as the primary endpoint (NCT06015022).
Dietary patterns have been associated with HCC risk.[296] Meta-analyses have reported that Mediterranean diet, Alternative Healthy Eating Index-2010, Urban Prudent Dietary Pattern, Traditional Cantonese Dietary Pattern, intake of vegetables, whole grains, fiber, fish, poultry, white meat (not processed nor red meat), macronutrients such as monounsaturated fats, and micronutrients such as vitamin E/B9, β-carotene, manganese, and potassium were associated with lower HCC risk.[297–299] In a single-center case-control study in the U.S., mono-unsaturated fatty acid (MUFA) and long-chain omega-3 (not omega-6) polyunsaturated fatty acid (PUFA) intake was associated with lower HCC risk.[300] In prospective cohort studies in the U.S. and Singapore, plant-based low-carbohydrate diet was associated with lower HCC risk.[301, 302] In a single-center cohort of 1,234 MASLD patients, low HDL-cholesterol level was associated with HCC incidence.[303] Sugar-sweetened beverages were associated with increased HCC risk in a prospective cohort of postmenopausal women in the U.S. (aHR, 1.85).[304]
1,25(OH)2D3, the active form of vitamin D3, gradually decreases as liver disease progresses and HCC risk is elevated.[28] A meta-analysis showed that preserved 1,25(OH)2D3 level was associated with lower HCC risk (RR, 0.46) irrespective of geographic regions.[305] In chemical-based rodent models, 1,25(OH)2D3 attenuated liver fibrosis and HCC by restoring hepatic antioxidant Nrf2 and glutathione-S-transferase and suppressing NF-κB and IL-6 pathways.[306–308] In HFD-fed rats, 1,25(OH)2D3 improved MASH histology and suppressed caspase-3 and 3-mercaptopyruvate sulfur transferase.[309] In an RCT in 61 MASLD patients, 1,25(OH)2D3 with fish oil improved gut microbiome, insulin sensitivity, and adiponectin levels.[310] In an RCT enrolling 46 MASLD patients, 12-week 1,25(OH)2D3 reduced ALT, AST, blood sugar, LDL-cholesterol, and fibrogenic proteins (e.g., laminin and hyaluronic acid) and micro-RNAs (e.g., miR-21 and miR-122).[311] A phase IV trial of 1,25(OH)2D3 is planned to test its HCC-preventive effect in patients with chronic hepatitis B on NA treatment (NCT02779465).
BCAA improve nutritional status in cirrhosis patients. In experimental MASH, obesity, HCV, and chemical-based models, BCAA reduces liver fibrosis and HCC nodules by suppressing inflammatory cytokines such as IL-6 and IL-1β, ROS generation, mTOR signaling-mediated cellular senescence, TGFβ1-stimulated pro-fibrogenic gene expression in hepatic stellate cells, and apoptosis of hepatocytes as well as inducing PPAR, p21CIP1 and p27KIP1.[30, 312] In meta-analyses, BCAA increased muscle mass, albumin levels, and BMI, and event-free survival, whereas preventive effects on HCC occurrence/recurrence were unclear.[313–315] Dietary phytochemicals such as curcumin (turmeric extract), resveratrol (polyphenol in grapes, red wine, and berries), silymarin (herbal flavonoid), and carotenoids have been evaluated for chemoprevention that activate cytoprotective mechanisms, such as the Keap1/Nrf2 pathway in carcinogen-induced rodent models, although supporting clinical evidence is lacking.[30, 316]
Gut microbiome-targeted therapies
Gut dysbiosis has been associated with elevated HCC risk in patients with viral and metabolic liver diseases by altering hepatic inflammatory milieu and innate/adoptive anti-cancer immunity.[317–319] Experimental studies have suggested that microbiome-directed treatments may serve as secondary HCC prevention. Dietary cholesterol drives MASLD-related HCC in a mouse model by inducing alterations in gut microbiota and metabolites.[320] In a chemical-induced HCC mouse model, decrease in serum conjugated DCA is associated with HCC, which may be due to reduced bile salt hydrolase-rich intestinal bacteria.[321] Gut microbiota–derived short-chain fatty acids can regulate the function of type 3 innate lymphoid cells (ILC3), essential cells for host defense against infection, through Sox13-dependent signaling, leading HCC development.[322] Probiotic supplementation (Lactobacillus acidophilus) suppressed HCC in experimental MASH models by inhibiting Rho-GTPase pathway in hepatocytes via valeric acid that binds GPR41/43 on the cell surface.[323] Lactoferrin prevents early carcinogenic events, such as necrosis, ROS production, and the surge of facultative liver stem cells in DEN-induced HCC mouse model.[324] An RCT of probiotics is underway in patients with Child A/B cirrhosis for prevention of incident HCC (NCT03853928).
Physical activity-based interventions
Physical activity may reduce development of cancers, including HCC. [325, 326] A meta-analysis showed that higher physical activity was associated with less incident HCC.[327] Improvement of mitochondrial functions by exercise may underlie improvement of insulin sensitivity, histological MASH activity, and dysregulation of cancer-related cellular signaling pathways and cancer stemness.[328–331] A recent RCT showed that serum FGF21 was decreased in response to aerobic exercise in patients with biopsy-proven MASH.[332] A meta-analysis of RCTs showed that an exercise dose of ≥750 metabolic equivalents of task min/week reduced MRI-based liver fat measurements independent of weight loss in MASLD patients.[333]
CCA Prevention
In addition to the known risk factors, several environmental exposures and genetic features have been suggested as predisposing factors and clues to primary prevention.[334] In industrial workers, occupational exposure to several International Agency for Research on Cancer (IARC) class 1 and 2A agents (1,2-dichloropropane, asbestos, and endocrine-disrupting compounds) and rotating shift work were associated with CCA risk.[335] A European registry study suggested that overweight/obesity was associated with CCA risk.[336] A large case-control study that involved 1,292 CCA patients reported CCA risk-associated germline DNA variants in known cancer-predisposing genes, BRCA1, BRCA2, APC, and MSH6 (prevalence, up to 5.5%), linked to homologous recombination deficiency.[337] Large multi-center genome-wide association studies are currently ongoing in the U.S. and Europe.[334]
Early detection biomarkers have been explored by analyzing circulating tumor cells, cell-free DNA, proteins, and exosome-related RNA/DNA, for body fluid-based non-invasive testing,[338] which may complement clinically recommended modalities such as magnetic resonance cholangiopancreatography and CA19-9.[27] Omics profiling studies have revealed heterogeneous molecular abbreviations in CCA tumors and tissue microenvironment that shape molecular subtypes,[339, 340] which may be more sensitively detected by subtype-specific biomarkers. Potential theranostic biomarkers such as CA9, CLDN18, TNC, MMP9, and EGFR were proposed based on the TArget Selection Criteria for Theranosis score.[341] Greater gut microbiome heterogeneity and more abundant Veillonella species may aid in the differential diagnosis of iCCA from HCC.[342]
Medical interventions directed to the risk factors and/or pre-cancerous conditions may serve as secondary prevention. Chronic HBV/HCV infection can promote CCA development via viral DNA integration to host genome, induction of EMT, and generation of immuno-suppressive tissue microenvironment,[343–345] Statin use was associated with lower biliary tract cancer (BTC)/CCA risk (aRR, 0.60) in a meta-analysis.[346] A Swedish population-based cohort study confirmed the finding (aHR, 0.66), whereas no association was observed for low-dose aspirin (aHR, 0.93) and metformin (aHR, 0.98) and higher risk was observed for non-aspirin NSAIDs (aHR, 1.44).[347] A more recent meta-analysis that involved 24,788,738 individuals confirmed no association of metformin with BTC risk (HR, 0.82).[348] No survival benefit of ACEi/ARBs, statins, or, aspirin was observed in 509 patients with established CCA.[349] Coffee consumption was associated with higher GBC incidence (aHR, 1.49), whereas tea consumption was associated with lower GBC incidence (aHR, 0.77) and iCCA (aHR, 0.81) in an international multi-center retrospective study.[350] In thioacetamide-treated rat model of cholangiofibrosis, β-elemene, a Chinese herb Rhizoma Zedoariae extract, reduced intrahepatic cholangial lesions (known to precede CCA initiation) with restored PCDH9 expression.[351] New drugs for primary sclerosing cholangitis and neo/adjuvant therapies may have roles in secondary/tertiary prevention as suggested by recent studies such as adjuvant capecitabine (BILCAP trial).[352] Future studies on molecular-targeted/immunologic agents, molecular characterization of the tumor microenvironment, non-invasive biomarkers, and development of consensus in clinical trial design will facilitate the development of tertiary prevention strategies for CCA.
Future Perspectives
The evolving landscape of HCC etiology continues to hamper the development of effective HCC prevention strategies. Post-SVR HCC risk persists for at least a decade when cirrhosis is present, and there are still many patients with undiagnosed active HCV infection. HBV cure is still an unmet need. MASLD incidence continues to increase with ALD. In parallel, epidemiological studies have revealed considerable heterogeneities and disparities in disease manifestation, clinical care, and outcomes across racial/ethnic and socioeconomic populations, and geographic regions. Emerging technologies such as advanced omics assays and machine learning (ML)/artificial intelligence (AI)-based approaches have enabled molecular and/or clinical characterization of the inter-patient heterogeneities and may provide clues for accurate HCC risk stratification, sensitive early HCC detection, and the rational application of chemopreventive therapies. The ever-growing complexity of new technologies, therapeutic strategies, and clinical contexts underscores the importance of balancing clinical benefits and practical feasibility of testing and implementing new personalized strategies for HCC prevention. Accumulating clinical and experimental evidence has suggested potential chemopreventive agents, such as statins and anti-diabetic, anti-obesity, and anti-inflammatory agents. Innovative strategies for chemoprevention clinical trials are urgently needed to facilitate their clinical testing and translation. CCA prevention is gaining attention as another unmet need. Collectively, these ongoing developments promise to result in transformative refinements of preventive strategies and substantial improvement of poor liver cancer mortality.
Supplementary Material
Key Points.
HCC prevention is increasingly challenging with the evolving etiological landscape, particularly the increase in metabolic etiologies and the widespread use of new anti-HBV/HCV drugs.
Individualized HCC risk assessment and refined screening modalities/tests are expected to improve the limited efficacy of current HCC screening program for early detection.
Combinations of etiology-agnostic and specific preventive interventions will enable effective reduction of the HCC burden and mortality.
Emerging drugs for metabolic disorders and other related conditions may serve for HCC prevention, and surrogate biomarkers will accelerate the assessment of their potential clinical utility.
Prevention of cholangiocarcinoma remains an unmet need owing to the heterogeneous and elusive etiologies.
Acknowledgements
The figures were created using BioRender.com.
Abbreviations
- HCC
hepatocellular carcinoma
- CCA
cholangiocarcinoma
- HBV
hepatitis B virus
- HCV
hepatitis C virus
- MASLD
metabolic dysfunction-associated steatotic liver disease
- NAFLD
non-alcoholic fatty liver disease
- T2D
type 2 diabetes
- DAA
direct-acting antiviral
- SVR
sustained virologic response
- ALD
alcohol-associated liver disease
- AFP
alpha-fetoprotein
- PCLS
precision-cut liver slice
- ICI
immune checkpoint inhibitor
- PoC
point-of-care
- POCUS
point-of-care ultrasound
- MASH
metabolic dysfunction-associated steatohepatitis
- EHR
electronic health record
- NA
nucleot(s)ide analog
- RR
relative risk
- aHR
adjusted hazard risk
- PD-1
programmed cell death-1
- FIB-4
fibrosis-4
- IL
interleukin
- EGFR
epidermal growth factor receptor
- THR
thyroid hormone receptor
- FGF
fibroblast growth factor
- ELF
Enhanced Liver Fibrosis
- GLP-1
glucagon-like peptide 1
- GLP-1RA
GLP-1 receptor agonist
- NIS/NITE
non-invasive scores/tests
- SNP
single nucleotide polymorphisms
- AFB1
aflatoxins B1
- AA
aristolochic acid
- NAC
N-acetyl cysteine
- GLS2
glutamine synthase 2
- LIFR
leukemia inhibitory factor receptor
- FBXL5
F-box and leucine-rich repeat protein 5
- COX
cyclooxygenase
- PGE2
prostaglandin E2
- TET1
tet methylcytosine dioxygenase 1
- MDSC
myeloid-derived suppressor cell
- Tregs
regulatory T cells
- NSAIDs
non-steroidal anti-inflammatory drugs
- irAE
immune-related adverse event
- SGLT2i
sodium-glucose cotransporter 2 inhibitors
- DPP4i
dipeptidyl peptidase-4 inhibitor
- GIP
glucose-dependent insulinotropic polypeptide
- TNF
tumor necrosis factor
- TGF
transforming growth factor
- ERK
extracellularsignal–regulated kinase
- PLS
Prognostic Liver Signature
- FPS
Fibrosis Progression Signature
- PLSec
Prognostic Liver Secretome signature
- RAS
renin-angiotensin system
- ACEi
angiotensin-converting enzyme inhibitor
- ARB
angiotensin II type 1 receptor blocker
- BCAA
branched-chain amino acids
- YB1
Y-box binding protein
- HRH2
histamine receptor H2
- PI3K
phosphoinositide 3-kinase
- mTOR
mammalian target of rapamycin
- CLDN1
claudin-1
- EGCG
epigallocatechin gallate
- MUFA
mono-unsaturated fatty acid
- PUFA
polyunsaturated fatty acid
- ILC3
type 3 innate lymphoid cells
- ML
machine learning
- AI
artificial intelligence.
Footnotes
Financial support and sponsorship
Supported by U.S. National Institutes of Health (CA233794, CA255621, CA282178, CA288375, CA283935, CA256977, DK138474, AI155140); European Commission (ERC-AdG-2020-101021417, HORIZON-HLTH-2021-DISEASE-04-07-101057917); Cancer Prevention and Research Institute of Texas (RR180016, RP200554); Foundation of the University of Strasbourg; Alsace Cancer Foundation; Association pour la Recherche sur le Cancer (ANR-10-IAHU-02, ANR-10-LABX-0028, ANR-21-RHUS-0001); Japan Society for the Promotion of Science (24K11130); Uehara Memorial Foundation.
The funders had no role in the collection of data; the design and conduct of the study; management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript, and decision to submit the manuscript for publication.
Conflict of interest
Yujin Hoshida owns stock in Alentis Therapeutics and Espervita Therapeutics. He advises on Helio Genomics, Espertiva Therapeutics, Roche Diagnostics, Elevar Therapeutics. Takumi Kawaguchi received lecture fee from Janssen Pharmaceutical K.K., Taisho Pharmaceutical Co., Ltd., Kowa Company, Ltd., Otsuka Pharmaceutical Co., Ltd., Eisai Co., Ltd., ASKA Pharmaceutical Co., Ltd., AbbVie GK., EA Pharma. Thomas Baumert is the founder, shareholder, and advisor for Alentis Therapeutics. Raymond T. Chung received research grants from GSK, BMS, Boehringer, and Janssen. Amit G. Singal serves as a consultant or on advisory boards for Genentech, AstraZeneca, Merck, Bayer, Eisai, Exelixis, FujiFilm Medical Sciences, Exact Sciences, Roche, Glycotest, Abbott, and GRAIL.
References
- 1.Williams PA, Zaidi SK, Sengupta R. AACR Cancer Progress Report 2023: Advancing the Frontiers of Cancer Science and Medicine. Clin Cancer Res. 2023;29:3850–3851. doi: 10.1158/1078-0432.CCR-23-2591. [DOI] [PubMed] [Google Scholar]
- 2.Bray F, Laversanne M, Sung H, Ferlay J, Siegel RL, Soerjomataram I, Jemal A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2024;74:229–263. doi: 10.3322/caac.21834. [DOI] [PubMed] [Google Scholar]
- 3.Valle JW, Kelley RK, Nervi B, Oh DY, Zhu AX. Biliary tract cancer. Lancet. 2021;397:428–444. doi: 10.1016/S0140-6736(21)00153-7. [DOI] [PubMed] [Google Scholar]
- 4.Rumgay H, Arnold M, Ferlay J, Lesi O, Cabasag CJ, Vignat J, Laversanne M, et al. Global burden of primary liver cancer in 2020 and predictions to 2040. J Hepatol. 2022;77:1598–1606. doi: 10.1016/j.jhep.2022.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Toh MR, Wong EYT, Wong SH, Ng AWT, Loo LH, Chow PK, Ngeow J. Global Epidemiology and Genetics of Hepatocellular Carcinoma. Gastroenterology. 2023;164:766–782. doi: 10.1053/j.gastro.2023.01.033. [DOI] [PubMed] [Google Scholar]
- 6.Singal AG, Kanwal F, Llovet JM. Global trends in hepatocellular carcinoma epidemiology: implications for screening, prevention and therapy. Nat Rev Clin Oncol. 2023;20:864–884. doi: 10.1038/s41571-023-00825-3. [DOI] [PubMed] [Google Scholar]
- 7.Siegel RL, Giaquinto AN, Jemal A. Erratum to “Cancer statistics, 2024”. Ca-a Cancer Journal for Clinicians. 2024;74:203. doi: 10.3322/caac.21820. (vol 74,pg 12-49,2024) [DOI] [PubMed] [Google Scholar]
- 8.Alvarez CS, Ruhl J, Flynn G, Graubard BI, McGlynn KA. Trends in hepatocellular carcinoma stage by racial/ethnic group in the United States, 1992-2019. JHEP Rep. 2023;5:100868. doi: 10.1016/j.jhepr.2023.100868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Banales JM, Marin JJG, Lamarca A, Rodrigues PM, Khan SA, Roberts LR, Cardinale V, et al. Cholangiocarcinoma 2020: the next horizon in mechanisms and management. Nat Rev Gastroenterol Hepatol. 2020;17:557–588. doi: 10.1038/s41575-020-0310-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee YT, Wang JJ, Luu M, Noureddin M, Kosari K, Agopian VG, Rich NE, et al. The Mortality and Overall Survival Trends of Primary Liver Cancer in the United States. J Natl Cancer Inst. 2021;113:1531–1541. doi: 10.1093/jnci/djab079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee BP, Dodge JL, Terrault NA. National prevalence estimates for steatotic liver disease and subclassifications using consensus nomenclature. Hepatology. 2024;79:666–673. doi: 10.1097/HEP.0000000000000604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huang DQ, Singal AG, Kanwal F, Lampertico P, Buti M, Sirlin CB, Nguyen MH, et al. Hepatocellular carcinoma surveillance - utilization, barriers and the impact of changing aetiology. Nat Rev Gastroenterol Hepatol. 2023;20:797–809. doi: 10.1038/s41575-023-00818-8. [DOI] [PubMed] [Google Scholar]
- 13.Baumert TF, Juhling F, Ono A, Hoshida Y. Hepatitis C-related hepatocellular carcinoma in the era of new generation antivirals. BMC Med. 2017;15:52. doi: 10.1186/s12916-017-0815-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.El-Serag HB, Kanwal F, Richardson P, Kramer J. Risk of hepatocellular carcinoma after sustained virological response in Veterans with hepatitis C virus infection. Hepatology. 2016;64:130–137. doi: 10.1002/hep.28535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim NJ, Vutien P, Cleveland E, Cravero A, Ioannou GN. Fibrosis Stage-specific Incidence of Hepatocellular Cancer After Hepatitis C Cure With Direct-acting Antivirals: A Systematic Review and Meta-analysis. Clin Gastroenterol Hepatol. 2023;21:1723–1738.:e1725. doi: 10.1016/j.cgh.2022.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Singal AG, Lim JK, Kanwal F. AGA Clinical Practice Update on Interaction Between Oral Direct-Acting Antivirals for Chronic Hepatitis C Infection and Hepatocellular Carcinoma: Expert Review. Gastroenterology. 2019;156:2149–2157. doi: 10.1053/j.gastro.2019.02.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ioannou GN, Beste LA, Green PK, Singal AG, Tapper EB, Waljee AK, Sterling RK, et al. Increased Risk for Hepatocellular Carcinoma Persists Up to 10 Years After HCV Eradication in Patients With Baseline Cirrhosis or High FIB-4 Scores. Gastroenterology. 2019;157:1264–1278.:e1264. doi: 10.1053/j.gastro.2019.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wu S, Zeng N, Sun F, Zhou J, Wu X, Sun Y, Wang B, et al. Hepatocellular Carcinoma Prediction Models in Chronic Hepatitis B: A Systematic Review of 14 Models and External Validation. Clin Gastroenterol Hepatol. 2021;19:2499–2513. doi: 10.1016/j.cgh.2021.02.040. [DOI] [PubMed] [Google Scholar]
- 19.Hsu YC, Huang DQ, Nguyen MH. Global burden of hepatitis B virus: current status, missed opportunities and a call for action. Nature Reviews Gastroenterology & Hepatology. 2023;20:524–537. doi: 10.1038/s41575-023-00760-9. [DOI] [PubMed] [Google Scholar]
- 20.Younossi ZM, Golabi P, Paik JM, Henry A, Van Dongen C, Henry L. The global epidemiology of nonalcoholic fatty liver disease (NAFLD) and nonalcoholic steatohepatitis (NASH): a systematic review. Hepatology. 2023;77:1335–1347. doi: 10.1097/HEP.0000000000000004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hartmann P, Zhang X, Loomba R, Schnabl B. Global and national prevalence of nonalcoholic fatty liver disease in adolescents: An analysis of the global burden of disease study 2019. Hepatology. 2023;78:1168–1181. doi: 10.1097/HEP.0000000000000383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Le MH, Yeo YH, Zou B, Barnet S, Henry L, Cheung R, Nguyen MH. Forecasted 2040 global prevalence of nonalcoholic fatty liver disease using hierarchical bayesian approach. Clin Mol Hepatol. 2022;28:841–850. doi: 10.3350/cmh.2022.0239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kanwal F, Khaderi S, Singal AG, Marrero JA, Loo N, Asrani SK, Amos CI, et al. Risk factors for HCC in contemporary cohorts of patients with cirrhosis. Hepatology. 2023;77:997–1005. doi: 10.1002/hep.32434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tan DJH, Ng CH, Muthiah M, Yong JN, Chee D, Teng M, Wong ZY, et al. Rising global burden of cancer attributable to high BMI from 2010 to 2019. Metabolism. 2024;152:155744. doi: 10.1016/j.metabol.2023.155744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Huang DQ, Mathurin P, Cortez-Pinto H, Loomba R. Global epidemiology of alcohol-associated cirrhosis and HCC: trends, projections and risk factors. Nat Rev Gastroenterol Hepatol. 2023;20:37–49. doi: 10.1038/s41575-022-00688-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Julien J, Ayer T, Tapper EB, Barbosa C, Dowd WN, Chhatwal J. Effect of increased alcohol consumption during COVID-19 pandemic on alcohol-associated liver disease: A modeling study. Hepatology. 2022;75:1480–1490. doi: 10.1002/hep.32272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bowlus CL, Arrive L, Bergquist A, Deneau M, Forman L, Ilyas SI, Lunsford KE, et al. AASLD practice guidance on primary sclerosing cholangitis and cholangiocarcinoma. Hepatology. 2023;77:659–702. doi: 10.1002/hep.32771. [DOI] [PubMed] [Google Scholar]
- 28.Fujiwara N, Friedman SL, Goossens N, Hoshida Y. Risk factors and prevention of hepatocellular carcinoma in the era of precision medicine. J Hepatol. 2018;68:526–549. doi: 10.1016/j.jhep.2017.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lippman SM, Abate-Shen C, Colbert Maresso KL, Colditz GA, Dannenberg AJ, Davidson NE, Disis ML, et al. AACR White Paper: Shaping the Future of Cancer Prevention - A Roadmap for Advancing Science and Public Health. Cancer Prev Res (Phila) 2018;11:735–778. doi: 10.1158/1940-6207.CAPR-18-0421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rasha F, Paul S, Simon TG, Hoshida Y. Hepatocellular Carcinoma Chemoprevention with Generic Agents. Semin Liver Dis. 2022;42:501–513. doi: 10.1055/a-1942-6693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vogel A, Meyer T, Sapisochin G, Salem R, Saborowski A. Hepatocellular carcinoma. Lancet. 2022;400:1345–1362. doi: 10.1016/S0140-6736(22)01200-4. [DOI] [PubMed] [Google Scholar]
- 32.Chiang CJ, Jhuang JR, Yang YW, Zhuang BZ, You SL, Lee WC, Chen CJ. Association of Nationwide Hepatitis B Vaccination and Antiviral Therapy Programs With End-Stage Liver Disease Burden in Taiwan. JAMA Netw Open. 2022;5:e2222367. doi: 10.1001/jamanetworkopen.2022.22367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hung GY, Horng JL, Yen HJ, Lee CY, Lin LY. Changing incidence patterns of hepatocellular carcinoma among age groups in Taiwan. J Hepatol. 2015;63:1390–1396. doi: 10.1016/j.jhep.2015.07.032. [DOI] [PubMed] [Google Scholar]
- 34.Howell J, Seaman C, Wallace J, Xiao YZ, Scott N, Davies J, de Santis T, et al. Pathway to global elimination of hepatitis B: HBV cure is just the first step. Hepatology. 2023;78:976–990. doi: 10.1097/HEP.0000000000000430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Singal AG, Llovet JM, Yarchoan M, Mehta N, Heimbach JK, Dawson LA, Jou JH, et al. AASLD Practice Guidance on prevention, diagnosis, and treatment of hepatocellular carcinoma. Hepatology. 2023;78:1922–1965. doi: 10.1097/HEP.0000000000000466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Singal AG, Zhang E, Narasimman M, Rich NE, Waljee AK, Hoshida Y, Yang JD, et al. HCC surveillance improves early detection, curative treatment receipt, and survival in patients with cirrhosis: A meta-analysis. J Hepatol. 2022;77:128–139. doi: 10.1016/j.jhep.2022.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wolf E, Rich NE, Marrero JA, Parikh ND, Singal AG. Use of Hepatocellular Carcinoma Surveillance in Patients With Cirrhosis: A Systematic Review and Meta-Analysis. Hepatology. 2021;73:713–725. doi: 10.1002/hep.31309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lee YT, Fujiwara N, Yang JD, Hoshida Y. Risk stratification and early detection biomarkers for precision HCC screening. Hepatology. 2023;78:319–362. doi: 10.1002/hep.32779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vutien P, Kim NJ, Moon AM, Johnson KM, Berry K, Green PK, Ioannou GN. Hepatocellular carcinoma risk decreases as time accrues following hepatitis C virus eradication. Aliment Pharmacol Ther. 2024;59:361–371. doi: 10.1111/apt.17802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Huang DQ, Tran A, Yeh ML, Yasuda S, Tsai PC, Huang CF, Dai CY, et al. Antiviral therapy substantially reduces HCC risk in patients with chronic hepatitis B infection in the indeterminate phase. Hepatology. 2023;78:1558–1568. doi: 10.1097/HEP.0000000000000459. [DOI] [PubMed] [Google Scholar]
- 41.Huang DQ, Hoang JK, Kamal R, Tsai PC, Toyoda H, Yeh ML, Yasuda S, et al. Antiviral Therapy Utilization and 10-Year Outcomes in Resected Hepatitis B Virus- and Hepatitis C Virus-Related Hepatocellular Carcinoma. J Clin Oncol. 2024;42:790–799. doi: 10.1200/JCO.23.00757. [DOI] [PubMed] [Google Scholar]
- 42.Shankaraiah RC, Gramantieri L, Fornari F, Sabbioni S, Callegari E, Negrini M. Animal Models of Hepatocellular Carcinoma Prevention. Cancers. 2019;11 doi: 10.3390/cancers11111792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nakagawa S, Wei L, Song WM, Higashi T, Ghoshal S, Kim RS, Bian CB, et al. Molecular Liver Cancer Prevention in Cirrhosis by Organ Transcriptome Analysis and Lysophosphatidic Acid Pathway Inhibition. Cancer Cell. 2016;30:879–890. doi: 10.1016/j.ccell.2016.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Crouchet E, Bandiera S, Fujiwara N, Li S, El Saghire H, Fernandez-Vaquero M, Riedl T, et al. A human liver cell-based system modeling a clinical prognostic liver signature for therapeutic discovery. Nat Commun. 2021;12:5525. doi: 10.1038/s41467-021-25468-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sojoodi M, Wei L, Erstad DJ, Yamada S, Fujii T, Hirschfield H, Kim RS, et al. Epigallocatechin Gallate Induces Hepatic Stellate Cell Senescence and Attenuates Development of Hepatocellular Carcinoma. Cancer Prev Res (Phila) 2020;13:497–508. doi: 10.1158/1940-6207.CAPR-19-0383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Li S, Ghoshal S, Sojoodi M, Arora G, Masia R, Erstad DJ, Lanuti M, et al. Pioglitazone Reduces Hepatocellular Carcinoma Development in Two Rodent Models of Cirrhosis. J Gastrointest Surg. 2019;23:101–111. doi: 10.1007/s11605-018-4004-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.DePeralta DK, Wei L, Ghoshal S, Schmidt B, Lauwers GY, Lanuti M, Chung RT, et al. Metformin prevents hepatocellular carcinoma development by suppressing hepatic progenitor cell activation in a rat model of cirrhosis. Cancer. 2016;122:1216–1227. doi: 10.1002/cncr.29912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fuchs BC, Hoshida Y, Fujii T, Wei L, Yamada S, Lauwers GY, McGinn CM, et al. Epidermal growth factor receptor inhibition attenuates liver fibrosis and development of hepatocellular carcinoma. Hepatology. 2014;59:1577–1590. doi: 10.1002/hep.26898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Qian T, Fujiwara N, Koneru B, Ono A, Kubota N, Jajoriya AK, Tung MG, et al. Molecular Signature Predictive of Long-Term Liver Fibrosis Progression to Inform Antifibrotic Drug Development. Gastroenterology. 2022;162:1210–1225. doi: 10.1053/j.gastro.2021.12.250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fujiwara N, Kubota N, Crouchet E, Koneru B, Marquez CA, Jajoriya AK, Panda G, et al. Molecular signatures of long-term hepatocellular carcinoma risk in nonalcoholic fatty liver disease. Sci Transl Med. 2022;14:eabo4474. doi: 10.1126/scitranslmed.abo4474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Crouchet E, Li S, Sojoodi M, Bandiera S, Fujiwara N, El Saghire H, Zhu S, et al. Hepatocellular carcinoma chemoprevention by targeting the angiotensin-converting enzyme and EGFR transactivation. JCI Insight. 2022;7 doi: 10.1172/jci.insight.159254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Juhling F, Hamdane N, Crouchet E, Li S, El Saghire H, Mukherji A, Fujiwara N, et al. Targeting clinical epigenetic reprogramming for chemoprevention of metabolic and viral hepatocellular carcinoma. Gut. 2021;70:157–169. doi: 10.1136/gutjnl-2019-318918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hamdane N, Juhling F, Crouchet E, El Saghire H, Thumann C, Oudot MA, Bandiera S, et al. HCV-Induced Epigenetic Changes Associated With Liver Cancer Risk Persist After Sustained Virologic Response. Gastroenterology. 2019;156:2313–2329.:e2317. doi: 10.1053/j.gastro.2019.02.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jühling F, Bandiera S, Hamdane N, Thumann C, Durand SC, Saghire HE, Davidson I, et al. Hepatitis C virus-induced epigenetic and transcriptional changes persist post cure. J Hepatol. 2017;66:S21 [Google Scholar]
- 55.Harrison SA, Bedossa P, Guy CD, Schattenberg JM, Loomba R, Taub R, Labriola D, et al. A Phase 3, Randomized, Controlled Trial of Resmetirom in NASH with Liver Fibrosis. N Engl J Med. 2024;390:497–509. doi: 10.1056/NEJMoa2309000. [DOI] [PubMed] [Google Scholar]
- 56.Loomba R, Sanyal AJ, Kowdley KV, Bhatt DL, Alkhouri N, Frias JP, Bedossa P, et al. Randomized, Controlled Trial of the FGF21 Analogue Pegozafermin in NASH. N Engl J Med. 2023;389:998–1008. doi: 10.1056/NEJMoa2304286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kowdley KV, Bowlus CL, Levy C, Akarca US, Alvares-da-Silva MR, Andreone P, Arrese M, et al. Efficacy and Safety of Elafibranor in Primary Biliary Cholangitis. N Engl J Med. 2024;390:795–805. doi: 10.1056/NEJMoa2306185. [DOI] [PubMed] [Google Scholar]
- 58.Zou Y, Yue M, Jia L, Wang Y, Chen H, Zhang A, Xia X, et al. Accurate prediction of HCC risk after SVR in patients with hepatitis C cirrhosis based on longitudinal data. BMC Cancer. 2023;23:1147. doi: 10.1186/s12885-023-11628-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hsu CC, Gopalakrishna H, Mironova M, Lee MH, Chen CJ, Yang HI, Wiese M, et al. Risk of Hepatocellular Carcinoma After Spontaneous Clearance of Hepatitis C Virus and in Noncirrhosis Chronic Hepatitis C Patients With Sustained Virological Response: A Systematic Review. Clin Infect Dis. 2023;77:S245–S256. doi: 10.1093/cid/ciad380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mittal S, El-Serag HB, Sada YH, Kanwal F, Duan Z, Temple S, May SB, et al. Hepatocellular Carcinoma in the Absence of Cirrhosis in United States Veterans is Associated With Nonalcoholic Fatty Liver Disease. Clin Gastroenterol Hepatol. 2016;14:124–131.:e121. doi: 10.1016/j.cgh.2015.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Huang DQ, Singal AG, Kono Y, Tan DJH, El-Serag HB, Loomba R. Changing global epidemiology of liver cancer from 2010 to 2019: NASH is the fastest growing cause of liver cancer. Cell Metab. 2022;34:969–977.:e962. doi: 10.1016/j.cmet.2022.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chen YT, Chen TI, Yang TH, Yin SC, Lu SN, Liu XR, Gao YZ, et al. Long-term Risks of Cirrhosis and Hepatocellular Carcinoma Across Steatotic Liver Disease Subtypes. Am J Gastroenterol. 2024 doi: 10.14309/ajg.0000000000002778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tzartzeva K, Obi J, Rich NE, Parikh ND, Marrero JA, Yopp A, Waljee AK, et al. Surveillance Imaging and Alpha Fetoprotein for Early Detection of Hepatocellular Carcinoma in Patients With Cirrhosis: A Meta-analysis. Gastroenterology. 2018;154:1706–1718.:e1701. doi: 10.1053/j.gastro.2018.01.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Goossens N, Singal AG, King LY, Andersson KL, Fuchs BC, Besa C, Taouli B, et al. Cost-Effectiveness of Risk Score-Stratified Hepatocellular Carcinoma Screening in Patients with Cirrhosis. Clin Transl Gastroenterol. 2017;8:e101. doi: 10.1038/ctg.2017.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Parikh ND, Singal AG, Hutton DW, Tapper EB. Cost-Effectiveness of Hepatocellular Carcinoma Surveillance: An Assessment of Benefits and Harms. Am J Gastroenterol. 2020;115:1642–1649. doi: 10.14309/ajg.0000000000000715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kao SZ, Sangha K, Fujiwara N, Hoshida Y, Parikh ND, Singal AG. Cost-effectiveness of a precision hepatocellular carcinoma surveillance strategy in patients with cirrhosis. EClinicalMedicine. 2024;75:102755. doi: 10.1016/j.eclinm.2024.102755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kim NJ, Rozenberg-Ben-Dror K, Jacob DA, Rich NE, Singal AG, Aby ES, Yang JD, et al. Provider Attitudes Toward Risk-Based Hepatocellular Carcinoma Surveillance in Patients With Cirrhosis in the United States. Clin Gastroenterol Hepatol. 2022;20:183–193. doi: 10.1016/j.cgh.2020.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Imamura H, Matsuyama Y, Tanaka E, Ohkubo T, Hasegawa K, Miyagawa S, Sugawara Y, et al. Risk factors contributing to early and late phase intrahepatic recurrence of hepatocellular carcinoma after hepatectomy. J Hepatol. 2003;38:200–207. doi: 10.1016/s0168-8278(02)00360-4. [DOI] [PubMed] [Google Scholar]
- 69.Fujiwara N, Kubota N, Zhu S, Nakagawa S, Baba H, Hoshida Y. Disseminative Recurrence Signature for Hepatocellular Carcinoma From Nonalcoholic Fatty Liver Disease. Gastro Hep Adv. 2023;2:681–683. doi: 10.1016/j.gastha.2023.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Dhanasekaran R, Suzuki H, Lemaitre L, Kubota N, Hoshida Y. Molecular and immune landscape of hepatocellular carcinoma to guide therapeutic decision-making. Hepatology. 2023 doi: 10.1097/HEP.0000000000000513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ding X, He M, Chan AWH, Song QX, Sze SC, Chen H, Man MKH, et al. Genomic and Epigenomic Features of Primary and Recurrent Hepatocellular Carcinomas. Gastroenterology. 2020 doi: 10.1053/j.gastro.2019.09.056. [DOI] [PubMed] [Google Scholar]
- 72.Syedmoradi L, Norton ML, Omidfar K. Point-of-care cancer diagnostic devices: From academic research to clinical translation. Talanta. 2021;225:122002. doi: 10.1016/j.talanta.2020.122002. [DOI] [PubMed] [Google Scholar]
- 73.Zhang J, Li Y, Chai F, Li Q, Wang D, Liu L, Tang BZ, et al. Ultrasensitive point-of-care biochemical sensor based on metal-AIEgen frameworks. Sci Adv. 2022;8:eabo1874. doi: 10.1126/sciadv.abo1874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Li J, Liang X, Zhong R, Liu M, Liu X, Yan HL, Zhou YG. Clinically Applicable Homogeneous Assay for Serological Diagnosis of Alpha-Fetoprotein by Impact Electrochemistry. ACS Sens. 2022;7:3216–3222. doi: 10.1021/acssensors.2c01887. [DOI] [PubMed] [Google Scholar]
- 75.Yao C, Ng E, Wang SX. An automated and mobile magnetoresistive biosensor system for early hepatocellular carcinoma diagnosis. Biosens Bioelectron. 2022;202:113982. doi: 10.1016/j.bios.2022.113982. [DOI] [PubMed] [Google Scholar]
- 76.Sourianarayanane A, McCullough AJ. Accuracy of ultrasonographic fatty liver index using point-of-care ultrasound in stratifying non-alcoholic fatty liver disease patients. Eur J Gastroenterol Hepatol. 2023;35:654–661. doi: 10.1097/MEG.0000000000002544. [DOI] [PubMed] [Google Scholar]
- 77.Rudnick SR, Ugwuegbu J, Soufleris SJ, Bundy R, Dharod A, Russo MW. Telephone-Only Visits Preserved Hepatocellular Cancer Screening Rates in Patients with Cirrhosis Early in the COVID-19 Pandemic. Dig Dis Sci. 2023;68:1791–1796. doi: 10.1007/s10620-022-07786-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hui SM, Sane N, Wang AD, Wan L, Bell S, Dev A. Hepatocellular carcinoma surveillance in the telehealth era: A single-centre review. Journal of Telemedicine and Telecare. 2023:1357633X231166032. doi: 10.1177/1357633X231166032. [DOI] [PubMed] [Google Scholar]
- 79.Choi DT, Sada YH, Sansgiry S, Kaplan DE, Taddei TH, Aguilar JK, Strayhorn M, et al. Using Telemedicine to Facilitate Patient Communication and Treatment Decision-Making Following Multidisciplinary Tumor Board Review for Patients with Hepatocellular Carcinoma. J Gastrointest Cancer. 2023;54:623–631. doi: 10.1007/s12029-022-00844-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Liang CW, Yang HC, Islam MM, Nguyen PAA, Feng YT, Hou ZY, Huang CW, et al. Predicting Hepatocellular Carcinoma With Minimal Features From Electronic Health Records: Development of a Deep Learning Model. JMIR Cancer. 2021;7:e19812. doi: 10.2196/19812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Macias Alonso AK, Hirt J, Woelfle T, Janiaud P, Hemkens LG. Definitions of digital biomarkers: a systematic mapping of the biomedical literature. BMJ Health Care Inform. 2024;31 doi: 10.1136/bmjhci-2023-100914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Bruce MG, Bruden D, Hurlburt D, Morris J, Bressler S, Thompson G, Lecy D, et al. Protection and antibody levels 35 years after primary series with hepatitis B vaccine and response to a booster dose. Hepatology. 2022;76:1180–1189. doi: 10.1002/hep.32474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Chang KC, Chang MH, Chen HL, Wu JF, Chang CH, Hsu HY, Ni YH. Universal Infant Hepatitis B Virus (HBV) Vaccination for 35 Years: Moving Toward the Eradication of HBV. J Infect Dis. 2022;225:431–435. doi: 10.1093/infdis/jiab401. [DOI] [PubMed] [Google Scholar]
- 84.Wong GL, Hui VW, Yip TC, Liang LY, Zhang X, Tse YK, Lai JC, et al. Universal HBV vaccination dramatically reduces the prevalence of HBV infection and incidence of hepatocellular carcinoma. Aliment Pharmacol Ther. 2022;56:869–877. doi: 10.1111/apt.17120. [DOI] [PubMed] [Google Scholar]
- 85.Yin X, Wang W, Chen H, Mao Q, Han G, Yao L, Gao Q, et al. Real-world implementation of a multilevel interventions program to prevent mother-to-child transmission of HBV in China. Nat Med. 2024;30:455–462. doi: 10.1038/s41591-023-02782-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Collaborators GBDHB. Global, regional, and national burden of hepatitis B, 1990-2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet Gastroenterol Hepatol. 2022;7:796–829. doi: 10.1016/S2468-1253(22)00124-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kim HS, Yu X, Kramer J, Thrift AP, Richardson P, Hsu YC, Flores A, et al. Comparative performance of risk prediction models for hepatitis B-related hepatocellular carcinoma in the United States. J Hepatol. 2022;76:294–301. doi: 10.1016/j.jhep.2021.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wu S, Zhou J, Wu X, Sun Y, Wang B, Kong Y, Zhan S, et al. Comparative Performance of 14 HCC Prediction Models in CHB: A Dynamic Validation at Serial On-Treatment Timepoints. Am J Gastroenterol. 2022;117:1444–1453. doi: 10.14309/ajg.0000000000001865. [DOI] [PubMed] [Google Scholar]
- 89.Dusheiko G, Agarwal K, Maini MK. New Approaches to Chronic Hepatitis B. Reply N Engl J Med. 2023;388:1148–1149. doi: 10.1056/NEJMc2301248. [DOI] [PubMed] [Google Scholar]
- 90.Fung S, Choi HSJ, Gehring A, Janssen HLA. Getting to HBV cure: The promising paths forward. Hepatology. 2022;76:233–250. doi: 10.1002/hep.32314. [DOI] [PubMed] [Google Scholar]
- 91.Udompap P, Kim WR. Development of Hepatocellular Carcinoma in Patients With Suppressed Viral Replication: Changes in Risk Over Time. Clin Liver Dis (Hoboken) 2020;15:85–90. doi: 10.1002/cld.904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Tan DJH, Ng CH, Tay PWL, Syn N, Muthiah MD, Lim WH, Tang ASP, et al. Risk of Hepatocellular Carcinoma With Tenofovir vs Entecavir Treatment for Chronic Hepatitis B Virus A Reconstructed Individual Patient Data Meta-analysis. Jama Network Open. 2022;5:e2219407. doi: 10.1001/jamanetworkopen.2022.19407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Murata K, Mizokami M. Possible biological mechanisms of entecavir versus tenofovir disoproxil fumarate on reducing the risk of hepatocellular carcinoma. J Gastroenterol Hepatol. 2023;38:683–691. doi: 10.1111/jgh.16178. [DOI] [PubMed] [Google Scholar]
- 94.Choi WM, Kim GA, Choi J, Choi GH, Lee YB, Sinn DH, Lim YS. Non-linear association of baseline viral load with on-treatment hepatocellular carcinoma risk in chronic hepatitis B. Gut. 2024;73:649–658. doi: 10.1136/gutjnl-2023-330225. [DOI] [PubMed] [Google Scholar]
- 95.Yang H, Bae SH, Nam H, Lee HL, Lee SW, Yoo SH, Song MJ, et al. A risk prediction model for hepatocellular carcinoma after hepatitis B surface antigen seroclearance. J Hepatol. 2022;77:632–641. doi: 10.1016/j.jhep.2022.03.032. [DOI] [PubMed] [Google Scholar]
- 96.Jeng WJ, Chien RN, Chen YC, Lin CL, Wu CY, Liu YC, Peng CW, et al. Hepatocellular carcinoma reduced, HBsAg loss increased, and survival improved after finite therapy in hepatitis B patients with cirrhosis. Hepatology. 2024;79:690–703. doi: 10.1097/HEP.0000000000000575. [DOI] [PubMed] [Google Scholar]
- 97.Zhou YM, Zhang ZS, Zhao YF, Wu LP, Li B. Antiviral Therapy Decreases Recurrence of Hepatitis B Virus-related Hepatocellular Carcinoma after Curative Resection: A Meta-analysis. World Journal of Surgery. 2014;38:2395–2402. doi: 10.1007/s00268-014-2586-z. [DOI] [PubMed] [Google Scholar]
- 98.Giri S, Agrawal D, Afzalpurkar S, Gopan A, Angadi S, Sundaram S. Tenofovir versus entecavir for tertiary prevention of hepatocellular carcinoma in chronic hepatitis B infection after curative therapy: A systematic review and meta-analysis. J Viral Hepat. 2023;30:108–115. doi: 10.1111/jvh.13766. [DOI] [PubMed] [Google Scholar]
- 99.Qin A, Wu CR, Ho MC, Tsai CY, Chen PJ. Sequential Therapy with Ropeginterferon Alfa-2b and Anti-Programmed Cell Death 1 Antibody for Inhibiting the Recurrence of Hepatitis B-Related Hepatocellular Carcinoma: From Animal Modeling to Phase I Clinical Results. Int J Mol Sci. 2023;25 doi: 10.3390/ijms25010433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Cui F, Blach S, Manzengo Mingiedi C, Gonzalez MA, Sabry Alaama A, Mozalevskis A, Seguy N, et al. Global reporting of progress towards elimination of hepatitis B and hepatitis C. Lancet Gastroenterol Hepatol. 2023;8:332–342. doi: 10.1016/S2468-1253(22)00386-7. [DOI] [PubMed] [Google Scholar]
- 101.Polaris Observatory HCVC. Global change in hepatitis C virus prevalence and cascade of care between 2015 and 2020: a modelling study. Lancet Gastroenterol Hepatol. 2022;7:396–415. doi: 10.1016/S2468-1253(21)00472-6. [DOI] [PubMed] [Google Scholar]
- 102.Baumert TF, Berg T, Lim JK, Nelson DR. Status of Direct-Acting Antiviral Therapy for Hepatitis C Virus Infection and Remaining Challenges. Gastroenterology. 2019;156:431–445. doi: 10.1053/j.gastro.2018.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Gezer F, Howard KA, Litwin AH, Martin NK, Rennert L. Identification of factors associated with opioid-related and hepatitis C virus-related hospitalisations at the ZIP code area level in the USA: an ecological and modelling study. Lancet Public Health. 2024;9:e354–e364. doi: 10.1016/S2468-2667(24)00076-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Huang CF, Chen GJ, Hung CC, Yu ML. HCV Microelimination for High-risk Special Populations. J Infect Dis. 2023;228:S168–S179. doi: 10.1093/infdis/jiac446. [DOI] [PubMed] [Google Scholar]
- 105.Morales-Arraez D, Hernandez-Bustabad A, Reygosa Castro C, Benitez-Zafra F, Nicolas-Perez D, Crespo O, Diaz-Flores F, et al. Reengagement strategies for hepatitis C patients lost to follow-up: A randomized clinical trial. Hepatol Commun. 2023;7 doi: 10.1097/HC9.0000000000000080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Barnes E, Cooke GS, Lauer GM, Chung RT. Implementation of a controlled human infection model for evaluation of HCV vaccine candidates. Hepatology. 2023;77:1757–1772. doi: 10.1002/hep.32632. [DOI] [PubMed] [Google Scholar]
- 107.Bandiera S, Billie Bian C, Hoshida Y, Baumert TF, Zeisel MB. Chronic hepatitis C virus infection and pathogenesis of hepatocellular carcinoma. Curr Opin Virol. 2016;20:99–105. doi: 10.1016/j.coviro.2016.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Billerbeck E, Wolfisberg R, Fahnoe U, Xiao JW, Quirk C, Luna JM, Cullen JM, et al. Mouse models of acute and chronic hepacivirus infection. Science. 2017;357:204–208. doi: 10.1126/science.aal1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Brown AJ, Won JJ, Wolfisberg R, Fahnoe U, Catanzaro N, West A, Moreira FR, et al. Host genetic variation guides hepacivirus clearance, chronicity, and liver fibrosis in mice. Hepatology. 2024;79:183–197. doi: 10.1097/HEP.0000000000000547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Baumert TF, Hoshida Y. Addressing the Challenges of Hepatitis C Cure and Persistent Risk of Hepatocellular Carcinoma. Viruses. 2019;11 doi: 10.3390/v11050441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Kanwal F, Kramer J, Asch SM, Chayanupatkul M, Cao Y, El-Serag HB. Risk of Hepatocellular Cancer in HCV Patients Treated With Direct-Acting Antiviral Agents. Gastroenterology. 2017;153:996–1005.:e1001. doi: 10.1053/j.gastro.2017.06.012. [DOI] [PubMed] [Google Scholar]
- 112.Ioannou GN, Green PK, Berry K. HCV eradication induced by direct-acting antiviral agents reduces the risk of hepatocellular carcinoma. J Hepatol. 2017 doi: 10.1016/j.jhep.2017.08.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Calvaruso V, Cabibbo G, Cacciola I, Petta S, Madonia S, Bellia A, Tine F, et al. Incidence of Hepatocellular Carcinoma in Patients With HCV-Associated Cirrhosis Treated With Direct-Acting Antiviral Agents. Gastroenterology. 2018;155:411–421.:e414. doi: 10.1053/j.gastro.2018.04.008. [DOI] [PubMed] [Google Scholar]
- 114.Jacobson JM, Zahrieh D, Strand CA, Cruz-Correa M, Pungpapong S, Roberts LR, Mandrekar SJ, et al. Phase I Trial of a Therapeutic DNA Vaccine for Preventing Hepatocellular Carcinoma from Chronic Hepatitis C Virus (HCV) Infection. Cancer Prev Res (Phila) 2023;16:163–173. doi: 10.1158/1940-6207.CAPR-22-0217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Lupberger J, Zeisel MB, Xiao F, Thumann C, Fofana I, Zona L, Davis C, et al. EGFR and EphA2 are host factors for hepatitis C virus entry and possible targets for antiviral therapy. Nat Med. 2011;17:589–595. doi: 10.1038/nm.2341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Saviano A, Habersetzer F, Lupberger J, Simo-Noumbissie P, Schuster C, Doffoel M, Schmidt-Mutter C, et al. Safety and Antiviral Activity of EGFR Inhibition by Erlotinib in Chronic Hepatitis C Patients: A Phase Ib Randomized Controlled Trial. Clin Transl Gastroenterol. 2022;13:e00492. doi: 10.14309/ctg.0000000000000492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Deshmukh M, Nakagawa S, Higashi T, Vincek A, Venkatesh A, de Galarreta Ruiz, Koh AP, et al. Cell type-specific pharmacological kinase inhibition for cancer chemoprevention. Nanomedicine. 2018;14:317–325. doi: 10.1016/j.nano.2017.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Lanaya H, Natarajan A, Komposch K, Li L, Amberg N, Chen L, Wculek SK, et al. EGFR has a tumour-promoting role in liver macrophages during hepatocellular carcinoma formation. Nat Cell Biol. 2014;16:972–977. doi: 10.1038/ncb3031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Komposch K, Sibilia M. EGFR Signaling in Liver Diseases. Int J Mol Sci. 2015;17 doi: 10.3390/ijms17010030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Tanabe KK, Zahrieh D, Strand CA, Hoshida Y, Flotte TJ, Della’Zanna G, Umar A, et al. Epidermal Growth Factor Receptor Inhibition With Erlotinib in Liver: Dose De-Escalation Pilot Trial as an Initial Step in a Chemoprevention Strategy. Gastro Hep Adv. 2024;3:426–439. doi: 10.1016/j.gastha.2024.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Perez S, Kaspi A, Domovitz T, Davidovich A, Lavi-Itzkovitz A, Meirson T, Alison Holmes J, et al. Hepatitis C virus leaves an epigenetic signature post cure of infection by direct-acting antivirals. PLoS Genet. 2019;15:e1008181. doi: 10.1371/journal.pgen.1008181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Mezzacappa C, Kim NJ, Vutien P, Kaplan DE, Ioannou GN, Taddei TH. Screening for Hepatocellular Carcinoma and Survival in Patients With Cirrhosis After Hepatitis C Virus Cure. JAMA Netw Open. 2024;7:e2420963. doi: 10.1001/jamanetworkopen.2024.20963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Chhatwal J, Hajjar A, Mueller PP, Nemutlu G, Kulkarni N, Peters MLB, Kanwal F. Hepatocellular Carcinoma Incidence Threshold for Surveillance in Virologically Cured Hepatitis C Individuals. Clin Gastroenterol Hepatol. 2024;22:91–101.:e106. doi: 10.1016/j.cgh.2023.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Lockart I, Yeo MGH, Hajarizadeh B, Dore GJ, Danta M. HCC incidence after hepatitis C cure among patients with advanced fibrosis or cirrhosis: A meta-analysis. Hepatology. 2022;76:139–154. doi: 10.1002/hep.32341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Sapena V, Enea M, Torres F, Celsa C, Rios J, Rizzo GEM, Nahon P, et al. Hepatocellular carcinoma recurrence after direct-acting antiviral therapy: an individual patient data meta-analysis. Gut. 2022;71:593–604. doi: 10.1136/gutjnl-2020-323663. [DOI] [PubMed] [Google Scholar]
- 126.Kamal A, Metawea M, Omar H, Ghallab M, Kassem A, Naguib H. Hepatitis C Virus-Related One-Year Hepatocellular Carcinoma Recurrence After Directly Acting Antivirals: A Randomized Controlled Trial. J Gastrointest Cancer. 2024;55:913–923. doi: 10.1007/s12029-024-01035-5. [DOI] [PubMed] [Google Scholar]
- 127.He S, Lockart I, Alavi M, Danta M, Hajarizadeh B, Dore GJ. Systematic review with meta-analysis: effectiveness of direct-acting antiviral treatment for hepatitis C in patients with hepatocellular carcinoma. Aliment Pharmacol Ther. 2020;51:34–52. doi: 10.1111/apt.15598. [DOI] [PubMed] [Google Scholar]
- 128.Waziry R, Hajarizadeh B, Grebely J, Amin J, Law M, Danta M, George J, et al. Hepatocellular carcinoma risk following direct-acting antiviral HCV therapy: A systematic review, meta-analyses, and meta-regression. J Hepatol. 2017;67:1204–1212. doi: 10.1016/j.jhep.2017.07.025. [DOI] [PubMed] [Google Scholar]
- 129.Ono A, Goossens N, Finn RS, Schmidt WN, Thung SN, Im GY, Hoshida Y, et al. Persisting risk of hepatocellular carcinoma after hepatitis C virus cure monitored by a liver transcriptome signature. Hepatology. 2017;66:1344–1346. doi: 10.1002/hep.29203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Fujiwara N, Kobayashi M, Fobar AJ, Hoshida A, Marquez CA, Koneru B, Panda G, et al. A blood-based prognostic liver secretome signature and long-term hepatocellular carcinoma risk in advanced liver fibrosis. Med. 2021;2:836–850.:e810. doi: 10.1016/j.medj.2021.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Wang X, Zhang L, Dong B. Molecular mechanisms in MASLD/MASH-related HCC. Hepatology. 2024 doi: 10.1097/HEP.0000000000000786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Lee HM, Lidofsky SD, Taddei TH, Townshend-Bulson Lisa J. Attacking the public health crisis of hepatocellular carcinoma at its roots. Hepatology. 2022;77 doi: 10.1002/hep.32741. n/a-n/a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Park Y, Jung J, Han S, Kim GA. Metabolic dysfunction-associated steatotic liver disease and MetALD increases the risk of liver cancer and gastrointestinal cancer: A nationwide cohort study. Aliment Pharmacol Ther. 2024 doi: 10.1111/apt.18286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Noureddin M. MASH clinical trials and drugs pipeline: An impending Tsunami. Hepatology. 2024 doi: 10.1097/HEP.0000000000000860. [DOI] [PubMed] [Google Scholar]
- 135.Kingwell K. NASH field celebrates ‘hurrah moment’ with a first FDA drug approval for the liver disease. Nat Rev Drug Discov. 2024;23:235–237. doi: 10.1038/d41573-024-00051-1. [DOI] [PubMed] [Google Scholar]
- 136.Vidal-Cevallos P, Murua-Beltran Gall S, Uribe M, Chavez-Tapia NC. Understanding the Relationship between Nonalcoholic Fatty Liver Disease and Thyroid Disease. Int J Mol Sci. 2023;24 doi: 10.3390/ijms241914605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Javanbakht M, Fishman J, Moloney E, Rydqvist P, Ansaripour A. Early Cost-Effectiveness and Price Threshold Analyses of Resmetirom: An Investigational Treatment for Management of Nonalcoholic Steatohepatitis. Pharmacoecon Open. 2023;7:93–110. doi: 10.1007/s41669-022-00370-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Geng L, Lam KSL, Xu A. The therapeutic potential of FGF21 in metabolic diseases: from bench to clinic. Nat Rev Endocrinol. 2020;16:654–667. doi: 10.1038/s41574-020-0386-0. [DOI] [PubMed] [Google Scholar]
- 139.Nielsen MH, Gillum MP, Vrang N, Jelsing J, Hansen HH, Feigh M, Oro D. Hepatoprotective effects of the long-acting fibroblast growth factor 21 analog PF-05231023 in the GAN diet-induced obese and biopsy-confirmed mouse model of nonalcoholic steatohepatitis. Am J Physiol Gastrointest Liver Physiol. 2023;324:G378–G388. doi: 10.1152/ajpgi.00157.2022. [DOI] [PubMed] [Google Scholar]
- 140.Harrison SA, Ruane PJ, Freilich B, Neff G, Patil R, Behling C, Hu C, et al. A randomized, double-blind, placebo-controlled phase IIa trial of efruxifermin for patients with compensated NASH cirrhosis. JHEP Rep. 2023;5:100563. doi: 10.1016/j.jhepr.2022.100563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Rinella ME, Lieu HD, Kowdley KV, Goodman ZD, Alkhouri N, Lawitz E, Ratziu V, et al. A randomized, double-blind, placebo-controlled trial of aldafermin in patients with NASH and compensated cirrhosis. Hepatology. 2024;79:674–689. doi: 10.1097/HEP.0000000000000607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Sanyal AJ, Bedossa P, Fraessdorf M, Neff GW, Lawitz E, Bugianesi E, Anstee QM, et al. A Phase 2 Randomized Trial of Survodutide in MASH and Fibrosis. N Engl J Med. 2024;391:311–319. doi: 10.1056/NEJMoa2401755. [DOI] [PubMed] [Google Scholar]
- 143.Loomba R, Hartman ML, Lawitz EJ, Vuppalanchi R, Boursier J, Bugianesi E, Yoneda M, et al. Tirzepatide for Metabolic Dysfunction-Associated Steatohepatitis with Liver Fibrosis. N Engl J Med. 2024;391:299–310. doi: 10.1056/NEJMoa2401943. [DOI] [PubMed] [Google Scholar]
- 144.Loomba R, Abdelmalek MF, Armstrong MJ, Jara M, Kjaer MS, Krarup N, Lawitz E, et al. Semaglutide 2.4 mg once weekly in patients with non-alcoholic steatohepatitis-related cirrhosis: a randomised, placebo-controlled phase 2 trial. Lancet Gastroenterol Hepatol. 2023;8:511–522. doi: 10.1016/S2468-1253(23)00068-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Sanyal AJ, Ratziu V, Loomba R, Anstee QM, Kowdley KV, Rinella ME, Sheikh MY, et al. Results from a new efficacy and safety analysis of the REGENERATE trial of obeticholic acid for treatment of pre-cirrhotic fibrosis due to non-alcoholic steatohepatitis. J Hepatol. 2023;79:1110–1120. doi: 10.1016/j.jhep.2023.07.014. [DOI] [PubMed] [Google Scholar]
- 146.Rinella ME, Dufour JF, Anstee QM, Goodman Z, Younossi Z, Harrison SA, Loomba R, et al. Non-invasive evaluation of response to obeticholic acid in patients with NASH: Results from the REGENERATE study. J Hepatol. 2022;76:536–548. doi: 10.1016/j.jhep.2021.10.029. [DOI] [PubMed] [Google Scholar]
- 147.Ng CH, Tang ASP, Xiao J, Wong ZY, Yong JN, Fu CE, Zeng RW, et al. Safety and tolerability of obeticholic acid in chronic liver disease: a pooled analysis of 1878 individuals. Hepatol Commun. 2023;7:e0005. doi: 10.1097/HC9.0000000000000005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Younossi ZM, Zelber-Sagi S, Henry L, Gerber LH. Lifestyle interventions in nonalcoholic fatty liver disease. Nat Rev Gastroenterol Hepatol. 2023;20:708–722. doi: 10.1038/s41575-023-00800-4. [DOI] [PubMed] [Google Scholar]
- 149.Zheng J, Zhao L, Dong J, Chen H, Li D, Zhang X, Hassan MM, et al. The role of dietary factors in nonalcoholic fatty liver disease to hepatocellular carcinoma progression: A systematic review. Clin Nutr. 2022;41:2295–2307. doi: 10.1016/j.clnu.2022.08.018. [DOI] [PubMed] [Google Scholar]
- 150.Heredia NI, Zhang X, Balakrishnan M, Hwang JP, Thrift AP. Association of lifestyle behaviors with non-alcoholic fatty liver disease and advanced fibrosis detected by transient elastography among Hispanic/Latinos adults in the U.S. Ethn Health. 2023;28:299–312. doi: 10.1080/13557858.2022.2027883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Stine JG, Long MT, Corey KE, Sallis RE, Allen AM, Armstrong MJ, Conroy DE, et al. American College of Sports Medicine (ACSM) International Multidisciplinary Roundtable report on physical activity and nonalcoholic fatty liver disease. Hepatol Commun. 2023;7 doi: 10.1097/HC9.0000000000000108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Heinle JW, DiJoseph K, Sabag A, Oh S, Kimball SR, Keating S, Stine JG. Exercise Is Medicine for Nonalcoholic Fatty Liver Disease: Exploration of Putative Mechanisms. Nutrients. 2023;15 doi: 10.3390/nu15112452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Harris E. Study Examines Intermittent Fasting and Cardiovascular Mortality. JAMA. 2024;331:1440. doi: 10.1001/jama.2024.5158. [DOI] [PubMed] [Google Scholar]
- 154.Gallage S, Ali A, Barragan Avila JE, Seymen N, Ramadori P, Joerke V, Zizmare L, et al. A 5:2 intermittent fasting regimen ameliorates NASH and fibrosis and blunts HCC development via hepatic PPARalpha and PCK1. Cell Metab. 2024;36:1371–1393.:e1377. doi: 10.1016/j.cmet.2024.04.015. [DOI] [PubMed] [Google Scholar]
- 155.Zhou R, Gu Y, Zhang B, Kong T, Zhang W, Li J, Shi J. Digital Therapeutics: Emerging New Therapy for Nonalcoholic Fatty Liver Disease. Clin Transl Gastroenterol. 2023;14:e00575. doi: 10.14309/ctg.0000000000000575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Gu Y, Zhou R, Kong T, Zhang W, Chen Y, Wang C, Shi J, et al. Barriers and enabling factors in weight management of patients with nonalcoholic fatty liver disease: A qualitative study using the COM-B model of behaviour. Health Expect. 2023;26:355–365. doi: 10.1111/hex.13665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Avery L, Smith H, McPherson S, Hallsworth K. Feasibility and acceptability of an evidence-informed digital intervention to support self-management in people with non-alcoholic fatty liver disease: protocol for a non-randomised feasibility study (VITALISE) Pilot Feasibility Stud. 2023;9:62. doi: 10.1186/s40814-023-01286-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Mucinski JM, Salvador AF, Moore MP, Fordham TM, Anderson JM, Shryack G, Cunningham RP, et al. Histological improvements following energy restriction and exercise: The role of insulin resistance in resolution of MASH. J Hepatol. 2024 doi: 10.1016/j.jhep.2024.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Castera L, Garteiser P, Laouenan C, Vidal-Trecan T, Vallet-Pichard A, Manchon P, Paradis V, et al. Prospective head-to-head comparison of non-invasive scores for diagnosis of fibrotic MASH in patients with type 2 diabetes. J Hepatol. 2024 doi: 10.1016/j.jhep.2024.03.023. [DOI] [PubMed] [Google Scholar]
- 160.Mozes FE, Lee JA, Vali Y, Alzoubi O, Staufer K, Trauner M, Paternostro R, et al. Performance of non-invasive tests and histology for the prediction of clinical outcomes in patients with non-alcoholic fatty liver disease: an individual participant data meta-analysis. Lancet Gastroenterol Hepatol. 2023;8:704–713. doi: 10.1016/S2468-1253(23)00141-3. [DOI] [PubMed] [Google Scholar]
- 161.Sterling RK, Patel K, Duarte-Rojo A, Asrani SK, Alsawas M, Dranoff JA, Fiel MI, et al. AASLD Practice Guideline on blood-based non-invasive liver disease assessments of hepatic fibrosis and steatosis. Hepatology. 2024 doi: 10.1097/HEP.0000000000000845. [DOI] [PubMed] [Google Scholar]
- 162.Zeng RW, Ong CEY, Ong EYH, Chung CH, Lim WH, Xiao J, Danpanichkul P, et al. Global prevalence, clinical characteristics, surveillance, treatment allocation, and outcomes of alcohol-associated hepatocellular carcinoma. Clin Gastroenterol Hepatol. 2024 doi: 10.1016/j.cgh.2024.06.026. [DOI] [PubMed] [Google Scholar]
- 163.Narro GEC, Diaz LA, Ortega EK, Garin MFB, Reyes EC, Delfin PSM, Arab JP, et al. Alcohol-related liver disease: A global perspective. Ann Hepatol. 2024:101499. doi: 10.1016/j.aohep.2024.101499. [DOI] [PubMed] [Google Scholar]
- 164.Thiele M, Moreno C. Novel interventions against alcohol-related liver disease. Nature Reviews Gastroenterology & Hepatology. 2023;21:78–79. doi: 10.1038/s41575-023-00877-x. [DOI] [PubMed] [Google Scholar]
- 165.Crabb DW, Im GY, Szabo G, Mellinger JL, Lucey MR. Diagnosis and Treatment of Alcohol-Associated Liver Diseases: 2019 Practice Guidance From the American Association for the Study of Liver Diseases. Hepatology. 2020;71:306–333. doi: 10.1002/hep.30866. [DOI] [PubMed] [Google Scholar]
- 166.Lim WH, Tay P, Ng CH, Tan DJH, Ong C, Koh JH, Teng M, et al. Meta-analysis: Prevalence and impact of alcohol abstinence in alcohol-associated cirrhosis. Aliment Pharmacol Ther. 2024;59:730–741. doi: 10.1111/apt.17888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Diaz LA, Winder GS, Leggio L, Bajaj JS, Bataller R, Arab JP. New insights into the molecular basis of alcohol abstinence and relapse in alcohol-associated liver disease. Hepatology. 2023 doi: 10.1097/HEP.0000000000000645. [DOI] [PubMed] [Google Scholar]
- 168.Trépo E, Caruso S, Yang J, Imbeaud S, Couchy G, Bayard Q, Letouzé E, et al. Common genetic variation in alcohol-related hepatocellular carcinoma: a case-control genome-wide association study. Lancet Oncol. 2022;23:161–171. doi: 10.1016/S1470-2045(21)00603-3. [DOI] [PubMed] [Google Scholar]
- 169.Buch S, Innes H, Lutz PL, Nischalke HD, Marquardt JU, Fischer J, Weiss KH, et al. Genetic variation in TERT modifies the risk of hepatocellular carcinoma in alcohol-related cirrhosis: results from a genome-wide case-control study. Gut. 2023;72:381–391. doi: 10.1136/gutjnl-2022-327196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Costentin CE, Minoves M, Kotzki S, Farges O, Goutté N, Decaens T, Bailly S. Alcohol-related hepatocellular carcinoma is a heterogenous condition: Lessons from a latent class analysis. Liver International. 2022;42:1638–1647. doi: 10.1111/liv.15256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Barsouk A, Thandra KC, Saginala K, Rawla P, Barsouk A. Chemical Risk Factors of Primary Liver Cancer: An Update. Hepat Med. 2020;12:179–188. doi: 10.2147/HMER.S278070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Liu S, He L, Bannister OB, Li J, Schnegelberger RD, Vanderpuye CM, Althouse AD, et al. Western diet unmasks transient low-level vinyl chloride-induced tumorigenesis; potential role of the (epi-)transcriptome. Toxicol Appl Pharmacol. 2023;468:116514. doi: 10.1016/j.taap.2023.116514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Nault JC, Letouze E. Mutational Processes in Hepatocellular Carcinoma: The Story of Aristolochic Acid. Semin Liver Dis. 2019;39:334–340. doi: 10.1055/s-0039-1685516. [DOI] [PubMed] [Google Scholar]
- 174.Sadighara P, Abedini AH, Irshad N, Ghazi-Khansari M, Esrafili A, Yousefi M. Association Between Non-alcoholic Fatty Liver Disease and Heavy Metal Exposure: a Systematic Review. Biol Trace Elem Res. 2023;201:5607–5615. doi: 10.1007/s12011-023-03629-9. [DOI] [PubMed] [Google Scholar]
- 175.Szymanska K, Chen JG, Cui Y, Gong YY, Turner PC, Villar S, Wild CP, et al. TP53 R249S mutations, exposure to aflatoxin, and occurrence of hepatocellular carcinoma in a cohort of chronic hepatitis B virus carriers from Qidong, China. Cancer Epidemiol Biomarkers Prev. 2009;18:1638–1643. doi: 10.1158/1055-9965.EPI-08-1102. [DOI] [PubMed] [Google Scholar]
- 176.Qin G, Su J, Ning Y, Duan X, Luo D, Lotlikar PD. p53 protein expression in patients with hepatocellular carcinoma from the high incidence area of Guangxi, Southern China. Cancer Lett. 1997;121:203–210. doi: 10.1016/s0304-3835(97)00352-2. [DOI] [PubMed] [Google Scholar]
- 177.Gramantieri L, Gnudi F, Vasuri F, Mandrioli D, Fornari F, Tovoli F, Suzzi F, et al. Aflatoxin B1 DNA-Adducts in Hepatocellular Carcinoma from a Low Exposure Area. Nutrients. 2022;14 doi: 10.3390/nu14081652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Foley JF, Elgart B, Phadke D, Mav D, Tripodi I, Clausen N, Weick M, et al. Whole exome and transcript profiling of liver following aflatoxin B1 exposure in rats. J Appl Toxicol. 2023;43:1293–1305. doi: 10.1002/jat.4463. [DOI] [PubMed] [Google Scholar]
- 179.Hamdy H, Yang Y, Cheng C, Liu Q. Identification of Potential Hub Genes Related to Aflatoxin B1, Liver Fibrosis and Hepatocellular Carcinoma via Integrated Bioinformatics Analysis. Biology (Basel) 2023;12 doi: 10.3390/biology12020205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Jin J, Kouznetsova VL, Kesari S, Tsigelny IF. Synergism in actions of HBV with aflatoxin in cancer development. Toxicology. 2023;499:153652. doi: 10.1016/j.tox.2023.153652. [DOI] [PubMed] [Google Scholar]
- 181.Pollock BH, Elmore S, Romoser A, Tang L, Kang MS, Xue K, Rodriguez M, et al. Intervention trial with calcium montmorillonite clay in a south Texas population exposed to aflatoxin. Food Addit Contam Part A Chem Anal Control Expo Risk Assess. 2016;33:1346–1354. doi: 10.1080/19440049.2016.1198498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Phillips TD, Wang M, Elmore SE, Hearon S, Wang JS. NovaSil clay for the protection of humans and animals from aflatoxins and other contaminants. Clays Clay Miner. 2019;67:99–110. doi: 10.1007/s42860-019-0008-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Lu ZN, Luo Q, Zhao LN, Shi Y, Wang N, Wang L, Han ZG. The Mutational Features of Aristolochic Acid-Induced Mouse and Human Liver Cancers. Hepatology. 2020;71:929–942. doi: 10.1002/hep.30863. [DOI] [PubMed] [Google Scholar]
- 184.Chen S, Dong Y, Qi X, Cao Q, Luo T, Bai Z, He H, et al. Aristolochic acids exposure was not the main cause of liver tumorigenesis in adulthood. Acta Pharm Sin B. 2022;12:2252–2267. doi: 10.1016/j.apsb.2021.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Atkins JL, Pilling LC, Masoli JAH, Kuo CL, Shearman JD, Adams PC, Melzer D. Association of Hemochromatosis HFE p.C282Y Homozygosity With Hepatic Malignancy. JAMA. 2020;324:2048–2057. doi: 10.1001/jama.2020.21566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Natarajan Y, Patel P, Chu J, Yu X, Hernaez R, El-Serag H, Kanwal F. Risk of Hepatocellular Carcinoma in Patients with Various HFE Genotypes. Dig Dis Sci. 2023;68:312–322. doi: 10.1007/s10620-022-07602-9. [DOI] [PubMed] [Google Scholar]
- 187.Schaefer B, Pammer LM, Pfeifer B, Neururer S, Troppmair MR, Panzer M, Wagner S, et al. Penetrance, cancer incidence and survival in HFE haemochromatosis-A population-based cohort study. Liver Int. 2024;44:838–847. doi: 10.1111/liv.15797. [DOI] [PubMed] [Google Scholar]
- 188.European Association for the Study of the Liver. Electronic address eee, European Association for the Study of the L. EASL Clinical Practice Guidelines on haemochromatosis. J Hepatol. 2022;77:479–502. doi: 10.1016/j.jhep.2022.03.033. [DOI] [PubMed] [Google Scholar]
- 189.Bardou-Jacquet E, Morandeau E, Anderson GJ, Ramm GA, Ramm LE, Morcet J, Bouzille G, et al. Regression of Fibrosis Stage With Treatment Reduces Long-Term Risk of Liver Cancer in Patients With Hemochromatosis Caused by Mutation in HFE. Clin Gastroenterol Hepatol. 2020;18:1851–1857. doi: 10.1016/j.cgh.2019.10.010. [DOI] [PubMed] [Google Scholar]
- 190.Saeki I, Yamamoto N, Yamasaki T, Takami T, Maeda M, Fujisawa K, Iwamoto T, et al. Effects of an oral iron chelator, deferasirox, on advanced hepatocellular carcinoma. World J Gastroenterol. 2016;22:8967–8977. doi: 10.3748/wjg.v22.i40.8967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Tang D, Kroemer G, Kang R. Ferroptosis in hepatocellular carcinoma: from bench to bedside. Hepatology. 2023 doi: 10.1097/HEP.0000000000000390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.He F, Zhang P, Liu J, Wang R, Kaufman RJ, Yaden BC, Karin M. ATF4 suppresses hepatocarcinogenesis by inducing SLC7A11 (xCT) to block stress-related ferroptosis. J Hepatol. 2023;79:362–377. doi: 10.1016/j.jhep.2023.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Liu Y, Ouyang L, Mao C, Chen Y, Li T, Liu N, Wang Z, et al. PCDHB14 promotes ferroptosis and is a novel tumor suppressor in hepatocellular carcinoma. Oncogene. 2022;41:3570–3583. doi: 10.1038/s41388-022-02370-2. [DOI] [PubMed] [Google Scholar]
- 194.Yan X, Liu Y, Li C, Mao X, Xu T, Hu Z, Zhang C, et al. Pien-Tze-Huang prevents hepatocellular carcinoma by inducing ferroptosis via inhibiting SLC7A11-GSH-GPX4 axis. Cancer Cell Int. 2023;23:109. doi: 10.1186/s12935-023-02946-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Wang YF, Feng JY, Zhao LN, Zhao M, Wei XF, Geng Y, Yuan HF, et al. Aspirin triggers ferroptosis in hepatocellular carcinoma cells through restricting NF-kappaB p65-activated SLC7A11 transcription. Acta Pharmacol Sin. 2023;44:1712–1724. doi: 10.1038/s41401-023-01062-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Xie J, Wang H, Xie W, Liu Y, Chen Y. Gallic acid promotes ferroptosis in hepatocellular carcinoma via inactivating Wnt/beta-catenin signaling pathway. Naunyn Schmiedebergs Arch Pharmacol. 2024;397:2437–2445. doi: 10.1007/s00210-023-02770-5. [DOI] [PubMed] [Google Scholar]
- 197.Suzuki S, Venkatesh D, Kanda H, Nakayama A, Hosokawa H, Lee E, Miki T, et al. GLS2 Is a Tumor Suppressor and a Regulator of Ferroptosis in Hepatocellular Carcinoma. Cancer Res. 2022;82:3209–3222. doi: 10.1158/0008-5472.CAN-21-3914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Yao F, Deng Y, Zhao Y, Mei Y, Zhang Y, Liu X, Martinez C, et al. A targetable LIFR-NF-kappaB-LCN2 axis controls liver tumorigenesis and vulnerability to ferroptosis. Nat Commun. 2021;12:7333. doi: 10.1038/s41467-021-27452-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Muto Y, Moroishi T, Ichihara K, Nishiyama M, Shimizu H, Eguchi H, Moriya K, et al. Disruption of FBXL5-mediated cellular iron homeostasis promotes liver carcinogenesis. J Exp Med. 2019;216:950–965. doi: 10.1084/jem.20180900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Liu X, Zhang Y, Yishake D, Luo Y, Liu Z, Chen Y, Zhu H, et al. Dietary intake and serum levels of copper and zinc and risk of hepatocellular carcinoma: A matched case-control study. Chin Med J (Engl) 2024;137:596–603. doi: 10.1097/CM9.0000000000002761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Kirk FT, Munk DE, Swenson ES, Quicquaro AM, Vendelbo MH, Schilsky ML, Ott P, et al. Effects of trientine and penicillamine on intestinal copper uptake: A mechanistic 64 Cu PET/CT study in healthy humans. Hepatology. 2024;79:1065–1074. doi: 10.1097/HEP.0000000000000708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Pfeiffenberger J, Mogler C, Gotthardt DN, Schulze-Bergkamen H, Litwin T, Reuner U, Hefter H, et al. Hepatobiliary malignancies in Wilson disease. Liver Int. 2015;35:1615–1622. doi: 10.1111/liv.12727. [DOI] [PubMed] [Google Scholar]
- 203.van Meer S, de Man RA, van den Berg AP, Houwen RH, Linn FH, van Oijen MG, Siersema PD, et al. No increased risk of hepatocellular carcinoma in cirrhosis due to Wilson disease during long-term follow-up. J Gastroenterol Hepatol. 2015;30:535–539. doi: 10.1111/jgh.12716. [DOI] [PubMed] [Google Scholar]
- 204.Leone V, Ali A, Weber A, Tschaharganeh DF, Heikenwalder M. Liver Inflammation and Hepatobiliary Cancers. Trends Cancer. 2021;7:606–623. doi: 10.1016/j.trecan.2021.01.012. [DOI] [PubMed] [Google Scholar]
- 205.Ballerini P, Contursi A, Bruno A, Mucci M, Tacconelli S, Patrignani P. Inflammation and Cancer: From the Development of Personalized Indicators to Novel Therapeutic Strategies. Front Pharmacol. 2022;13:838079. doi: 10.3389/fphar.2022.838079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Xu Y, Zhao W, Xu J, Li J, Hong Z, Yin Z, Wang X. Activated hepatic stellate cells promote liver cancer by induction of myeloid-derived suppressor cells through cyclooxygenase-2. Oncotarget. 2016;7:8866–8878. doi: 10.18632/oncotarget.6839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Chen H, Cai W, Chu ESH, Tang J, Wong CC, Wong SH, Sun W, et al. Hepatic cyclooxygenase-2 overexpression induced spontaneous hepatocellular carcinoma formation in mice. Oncogene. 2017;36:4415–4426. doi: 10.1038/onc.2017.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Xiong H, Li B, He J, Zeng Y, Zhang Y, He F. lncRNA HULC promotes the growth of hepatocellular carcinoma cells via stabilizing COX-2 protein. Biochem Biophys Res Commun. 2017;490:693–699. doi: 10.1016/j.bbrc.2017.06.103. [DOI] [PubMed] [Google Scholar]
- 209.Xu G, Wang Y, Li W, Cao Y, Xu J, Hu Z, Hao Y, et al. COX-2 Forms Regulatory Loop with YAP to Promote Proliferation and Tumorigenesis of Hepatocellular Carcinoma Cells. Neoplasia. 2018;20:324–334. doi: 10.1016/j.neo.2017.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Loo TM, Kamachi F, Watanabe Y, Yoshimoto S, Kanda H, Arai Y, Nakajima-Takagi Y, et al. Gut Microbiota Promotes Obesity-Associated Liver Cancer through PGE(2)-Mediated Suppression of Antitumor Immunity. Cancer Discov. 2017;7:522–538. doi: 10.1158/2159-8290.CD-16-0932. [DOI] [PubMed] [Google Scholar]
- 211.Takami Y, Eguchi S, Tateishi M, Ryu T, Mikagi K, Wada Y, Saitsu H. A randomised controlled trial of meloxicam, a Cox-2 inhibitor, to prevent hepatocellular carcinoma recurrence after initial curative treatment. Hepatology International. 2016;10:799–806. doi: 10.1007/s12072-016-9704-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Liu Y, Ren T, Xu X, Jin J. Association of aspirin and nonaspirin NSAIDs therapy with the incidence risk of hepatocellular carcinoma: a systematic review and meta-analysis on cohort studies. Eur J Cancer Prev. 2022;31:35–43. doi: 10.1097/CEJ.0000000000000663. [DOI] [PubMed] [Google Scholar]
- 213.Ma S, Qu G, Sun C, Liu H, Jiang Y, Li N, Wu B, et al. Does aspirin reduce the incidence, recurrence, and mortality of hepatocellular carcinoma? A GRADE-assessed systematic review and dose-response meta-analysis. Eur J Clin Pharmacol. 2023;79:39–61. doi: 10.1007/s00228-022-03414-y. [DOI] [PubMed] [Google Scholar]
- 214.Wang Y, Wang M, Liu C, Wang W, Shi J, Dang S. Aspirin Use and the Risk of Hepatocellular Carcinoma: A Meta-analysis. J Clin Gastroenterol. 2022;56:e293–e302. doi: 10.1097/MCG.0000000000001693. [DOI] [PubMed] [Google Scholar]
- 215.Ricciotti E, Wangensteen KJ, FitzGerald GA. Aspirin in Hepatocellular Carcinoma. Cancer Res. 2021;81:3751–3761. doi: 10.1158/0008-5472.CAN-21-0758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Lee TY, Hsu YC, Tseng HC, Yu SH, Lin JT, Wu MS, Wu CY. Association of Daily Aspirin Therapy With Risk of Hepatocellular Carcinoma in Patients With Chronic Hepatitis B. JAMA Intern Med. 2019;179:633–640. doi: 10.1001/jamainternmed.2018.8342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Jang H, Lee YB, Moon H, Chung JW, Nam JY, Cho EJ, Lee JH, et al. Aspirin use and risk of hepatocellular carcinoma in patients with chronic hepatitis B with or without cirrhosis. Hepatology. 2022;76:492–501. doi: 10.1002/hep.32380. [DOI] [PubMed] [Google Scholar]
- 218.Lee CH, Lee YB, Moon H, Chung JW, Cho EJ, Lee JH, Yu SJ, et al. Association between daily aspirin therapy and risk of hepatocellular carcinoma according to metabolic risk factor burden in non-cirrhotic patients with chronic hepatitis B. Aliment Pharmacol Ther. 2023;58:704–714. doi: 10.1111/apt.17643. [DOI] [PubMed] [Google Scholar]
- 219.Lee TY, Hsu YC, Ho HJ, Lin JT, Chen YJ, Wu CY. Daily aspirin associated with a reduced risk of hepatocellular carcinoma in patients with non-alcoholic fatty liver disease: a population-based cohort study. EClinicalMedicine. 2023;61:102065. doi: 10.1016/j.eclinm.2023.102065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Zeng RW, Yong JN, Tan DJH, Fu CE, Lim WH, Xiao J, Chan KE, et al. Meta-analysis: Chemoprevention of hepatocellular carcinoma with statins, aspirin and metformin. Aliment Pharmacol Ther. 2023;57:600–609. doi: 10.1111/apt.17371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Tan RZH, Lockart I, Abdel Shaheed C, Danta M. Systematic review with meta-analysis: The effects of non-steroidal anti-inflammatory drugs and anti-platelet therapy on the incidence and recurrence of hepatocellular carcinoma. Aliment Pharmacol Ther. 2021;54:356–367. doi: 10.1111/apt.16515. [DOI] [PubMed] [Google Scholar]
- 222.Chen WY, Ballman KV, Partridge AH, Hahn OM, Briccetti FM, Irvin WJ, Symington B, et al. Aspirin vs Placebo as Adjuvant Therapy for Breast Cancer: The Alliance A011502 Randomized Trial. JAMA. 2024;331:1714–1721. doi: 10.1001/jama.2024.4840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Simon TG, Wilechansky RM, Stoyanova S, Grossman A, Dichtel LE, Lauer GM, Miller KK, et al. Aspirin for Metabolic Dysfunction-Associated Steatotic Liver Disease Without Cirrhosis: A Randomized Clinical Trial. JAMA. 2024;331:920–929. doi: 10.1001/jama.2024.1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Malehmir M, Pfister D, Gallage S, Szydlowska M, Inverso D, Kotsiliti E, Leone V, et al. Platelet GPIbalpha is a mediator and potential interventional target for NASH and subsequent liver cancer. Nat Med. 2019;25:641–655. doi: 10.1038/s41591-019-0379-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Singal AG, Yarchoan M, Yopp A, Sapisochin G, Pinato DJ, Pillai A. Neoadjuvant and adjuvant systemic therapy in HCC: Current status and the future. Hepatol Commun. 2024;8 doi: 10.1097/HC9.0000000000000430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Marron TU, Fiel MI, Hamon P, Fiaschi N, Kim E, Ward SC, Zhao Z, et al. Neoadjuvant cemiplimab for resectable hepatocellular carcinoma: a single-arm, open-label, phase 2 trial. Lancet Gastroenterol Hepatol. 2022;7:219–229. doi: 10.1016/S2468-1253(21)00385-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Kaseb AO, Hasanov E, Cao HST, Xiao L, Vauthey JN, Lee SS, Yavuz BG, et al. Perioperative nivolumab monotherapy versus nivolumab plus ipilimumab in resectable hepatocellular carcinoma: a randomised, open-label, phase 2 trial. Lancet Gastroenterol Hepatol. 2022;7:208–218. doi: 10.1016/S2468-1253(21)00427-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Qin S, Chen M, Cheng AL, Kaseb AO, Kudo M, Lee HC, Yopp AC, et al. Atezolizumab plus bevacizumab versus active surveillance in patients with resected or ablated high-risk hepatocellular carcinoma (IMbrave050): a randomised, open-label, multicentre, phase 3 trial. Lancet. 2023;402:1835–1847. doi: 10.1016/S0140-6736(23)01796-8. [DOI] [PubMed] [Google Scholar]
- 229.Yopp A, Kudo M, Chen M, Cheng A, Kaseb AO, Lee HC, Qin S, et al. LBA39 - Updated efficacy and safety data from IMbrave050: Phase III study of adjuvant atezolizumab (atezo) + bevacizumab (bev) vs active surveillance in patients (pts) with resected or ablated high-risk hepatocellular carcinoma (HCC) Ann Oncol. 2024;35(suppl_2):1–72. [Google Scholar]
- 230.Lee JH, Lee JH, Lim YS, Yeon JE, Song TJ, Yu SJ, Gwak GY, et al. Adjuvant immunotherapy with autologous cytokine-induced killer cells for hepatocellular carcinoma. Gastroenterology. 2015;148:1383–1391.:e1386. doi: 10.1053/j.gastro.2015.02.055. [DOI] [PubMed] [Google Scholar]
- 231.Chung AS, Mettlen M, Ganguly D, Lu T, Wang T, Brekken RA, Hsiehchen D, et al. Immune Checkpoint Inhibition is Safe and Effective for Liver Cancer Prevention in a Mouse Model of Hepatocellular Carcinoma. Cancer Prev Res (Phila) 2020;13:911–922. doi: 10.1158/1940-6207.CAPR-20-0200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Wang C, Wang X, Gong G, Qiu W, Chen Y, Li G, et al. Increased risk of hepatocellular carcinoma in patients with diabetes mellitus: a systematic review and meta-analysis of cohort studies. Int J Cancer. 2012;130:1639–1648. doi: 10.1002/ijc.26165. [DOI] [PubMed] [Google Scholar]
- 233.Chen J, Han Y, Xu C, Xiao T, Wang B. Effect of type 2 diabetes mellitus on the risk for hepatocellular carcinoma in chronic liver diseases: a meta-analysis of cohort studies. Eur J Cancer Prev. 2015;24:89–99. doi: 10.1097/CEJ.0000000000000038. [DOI] [PubMed] [Google Scholar]
- 234.Sohn W, Lee HW, Lee S, Lim JH, Lee MW, Park CH, Yoon SK. Obesity and the risk of primary liver cancer: A systematic review and meta-analysis. Clin Mol Hepatol. 2021;27:157–174. doi: 10.3350/cmh.2020.0176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Jun BG, Kim M, Shin HS, Yi JJ, Yi SW. Impact of overweight and obesity on the risk of hepatocellular carcinoma: a prospective cohort study in 14.3 million Koreans. Br J Cancer. 2022;127:109–115. doi: 10.1038/s41416-022-01771-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Shin HS, Jun BG, Yi SW. Impact of diabetes, obesity, and dyslipidemia on the risk of hepatocellular carcinoma in patients with chronic liver diseases. Clin Mol Hepatol. 2022;28:773–789. doi: 10.3350/cmh.2021.0383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Kramer JR, Natarajan Y, Dai J, Yu X, Li L, El-Serag HB, Kanwal F. Effect of diabetes medications and glycemic control on risk of hepatocellular cancer in patients with nonalcoholic fatty liver disease. Hepatology. 2022;75:1420–1428. doi: 10.1002/hep.32244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Plaz Torres MC, Jaffe A, Perry R, Marabotto E, Strazzabosco M, Giannini EG. Diabetes medications and risk of HCC. Hepatology. 2022;76:1880–1897. doi: 10.1002/hep.32439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Galal MA, Al-Rimawi M, Hajeer A, Dahman H, Alouch S, Aljada A. Metformin: A Dual-Role Player in Cancer Treatment and Prevention. International Journal of Molecular Sciences. 2024;25 doi: 10.3390/ijms25074083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.O’Connor L, Bailey-Whyte M, Bhattacharya M, Butera G, Hardell KNL, Seidenberg AB, Castle PE, et al. Association of metformin use and cancer incidence: a systematic review and meta-analysis. J Natl Cancer Inst. 2024;116:518–529. doi: 10.1093/jnci/djae021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Tsai PC, Kuo HT, Hung CH, Tseng KC, Lai HC, Peng CY, Wang JH, et al. Metformin reduces hepatocellular carcinoma incidence after successful antiviral therapy in patients with diabetes and chronic hepatitis C in Taiwan. J Hepatol. 2023;78:281–292. doi: 10.1016/j.jhep.2022.09.019. [DOI] [PubMed] [Google Scholar]
- 242.Tsai PC, Huang CF, Yeh ML, Hsieh MH, Kuo HT, Hung CH, Tseng KC, et al. Metformin and statins reduce hepatocellular carcinoma risk in chronic hepatitis C patients with failed antiviral therapy. Clin Mol Hepatol. 2024 doi: 10.3350/cmh.2024.0038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Sun M, Sun J, Sun W, Li X, Wang Z, Sun L, Wang Y. Unveiling the anticancer effects of SGLT-2i: mechanisms and therapeutic potential. Front Pharmacol. 2024;15:1369352. doi: 10.3389/fphar.2024.1369352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Lee CH, Mak LY, Tang EH, Lui DT, Mak JH, Li L, Wu T, et al. SGLT2i reduces risk of developing HCC in patients with co-existing type 2 diabetes and hepatitis B infection: A territory-wide cohort study in Hong Kong. Hepatology. 2023;78:1569–1580. doi: 10.1097/HEP.0000000000000404. [DOI] [PubMed] [Google Scholar]
- 245.Cho HJ, Lee E, Kim SS, Cheong JY. SGLT2i impact on HCC incidence in patients with fatty liver disease and diabetes: a nation-wide cohort study in South Korea. Sci Rep. 2024;14:9761. doi: 10.1038/s41598-024-60133-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Hu WS, Lin CL. Sodium-Glucose Cotransporter-2 Inhibitor versus Beta-Blocker Use for Hepatocellular Carcinoma Risk among People with Hepatitis B or C Virus Infection and Diabetes Mellitus. Cancers (Basel) 2023;15 doi: 10.3390/cancers15072104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Hendryx M, Dong Y, Ndeke JM, Luo J. Sodium-glucose cotransporter 2 (SGLT2) inhibitor initiation and hepatocellular carcinoma prognosis. PLoS One. 2022;17:e0274519. doi: 10.1371/journal.pone.0274519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Cheung KS, Ng HY, Hui RWH, Lam LK, Mak LY, Ho YC, Tan JT, et al. Effects of empagliflozin on liver fat in patients with metabolic dysfunction–associated steatotic liver disease without diabetes mellitus: A randomized, double-blind, placebo-controlled trial. Hepatology. 2024;80:916–927. doi: 10.1097/HEP.0000000000000855. [DOI] [PubMed] [Google Scholar]
- 249.Kojima M, Takahashi H, Kuwashiro T, Tanaka K, Mori H, Ozaki I, Kitajima Y, et al. Glucagon-Like Peptide-1 Receptor Agonist Prevented the Progression of Hepatocellular Carcinoma in a Mouse Model of Nonalcoholic Steatohepatitis. Int J Mol Sci. 2020;21 doi: 10.3390/ijms21165722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Wang L, Berger NA, Kaelber DC, Xu R. Association of GLP-1 Receptor Agonists and Hepatocellular Carcinoma Incidence and Hepatic Decompensation in Patients With Type 2 Diabetes. Gastroenterology. 2024 doi: 10.1053/j.gastro.2024.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Huynh DJ, Renelus BD, Jamorabo DS. Dual metformin and glucagon-like peptide-1 receptor agonist therapy reduces mortality and hepatic complications in cirrhotic patients with diabetes mellitus. Ann Gastroenterol. 2023;36:555–563. doi: 10.20524/aog.2023.0814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Engstrom A, Wintzell V, Melbye M, Svanstrom H, Eliasson B, Gudbjornsdottir S, Hveem K, et al. Association of glucagon-like peptide-1 receptor agonists with serious liver events among patients with type 2 diabetes: A Scandinavian cohort study. Hepatology. 2024;79:1401–1411. doi: 10.1097/HEP.0000000000000712. [DOI] [PubMed] [Google Scholar]
- 253.Kanwal F, Kramer JR, Li L, Yang YX, Cao Y, Yu X, Samuel R, et al. GLP-1 Receptor Agonists and Risk for Cirrhosis and Related Complications in Patients With Metabolic Dysfunction-Associated Steatotic Liver Disease. JAMA Intern Med. 2024 doi: 10.1001/jamainternmed.2024.4661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Li Z, Zhang Y, Li Y, Lan J, Hu Y, Yu J, Meng Q, et al. Letter to the Editor: Statins as potential confounding factors to investigate the association between the use of GLP-1 receptor agonists and risk of HCC. Hepatology. 2024;79:E169–E170. doi: 10.1097/HEP.0000000000000812. [DOI] [PubMed] [Google Scholar]
- 255.Laeeq T, Ahmed M, Sattar H, Zeeshan MH, Ali MB. Role of SGLT2 Inhibitors, DPP-4 Inhibitors, and Metformin in Pancreatic Cancer Prevention. Cancers (Basel) 2024;16 doi: 10.3390/cancers16071325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Kawaguchi T, Nakano D, Koga H, Torimura T. Effects of a DPP4 Inhibitor on Progression of NASH-related HCC and the p62/Keap1/Nrf2-Pentose Phosphate Pathway in a Mouse Model. Liver Cancer. 2019;8:359–372. doi: 10.1159/000491763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Hsu WH, Sue SP, Liang HL, Tseng CW, Lin HC, Wen WL, Lee MY. Dipeptidyl Peptidase 4 Inhibitors Decrease the Risk of Hepatocellular Carcinoma in Patients With Chronic Hepatitis C Infection and Type 2 Diabetes Mellitus: A Nationwide Study in Taiwan. Frontiers in Public Health. 2021;9:711723. doi: 10.3389/fpubh.2021.711723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Chen TI, Lee FJ, Hsu WL, Chen YC, Chen M. Association of Dipeptidyl Peptidase-4 Inhibitors Use with Reduced Risk of Hepatocellular Carcinoma in Type 2 Diabetes Patients with Chronic HBV Infection. Cancers (Basel) 2023;15 doi: 10.3390/cancers15041148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Jang H, Kim Y, Lee DH, Joo SK, Koo BK, Lim S, Lee W, et al. Outcomes of Various Classes of Oral Antidiabetic Drugs on Nonalcoholic Fatty Liver Disease. JAMA Intern Med. 2024;184:375–383. doi: 10.1001/jamainternmed.2023.8029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Chung SW, Moon HS, Shin H, Han H, Park S, Cho H, Park J, et al. Inhibition of sodium-glucose cotransporter-2 and liver-related complications in individuals with diabetes: a Mendelian randomization and population-based cohort study. Hepatology. 2024 doi: 10.1097/HEP.0000000000000837. [DOI] [PubMed] [Google Scholar]
- 261.Longo J, van Leeuwen JE, Elbaz M, Branchard E, Penn LZ. Statins as Anticancer Agents in the Era of Precision Medicine. Clin Cancer Res. 2020;26:5791–5800. doi: 10.1158/1078-0432.CCR-20-1967. [DOI] [PubMed] [Google Scholar]
- 262.Jeon YG, Kim YY, Lee G, Kim JB. Physiological and pathological roles of lipogenesis. Nat Metab. 2023;5:735–759. doi: 10.1038/s42255-023-00786-y. [DOI] [PubMed] [Google Scholar]
- 263.Pham N, Benhammou JN. Statins in Chronic Liver Disease: Review of the Literature and Future Role. Semin Liver Dis. 2024 doi: 10.1055/a-2319-0694. [DOI] [PubMed] [Google Scholar]
- 264.Tripathi S, Gupta E, Galande S. Statins as anti-tumor agents: A paradigm for repurposed drugs. Cancer Rep (Hoboken) 2024;7:e2078. doi: 10.1002/cnr2.2078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Tsan YT, Lee CH, Wang JD, Chen PC. Statins and the risk of hepatocellular carcinoma in patients with hepatitis B virus infection. J Clin Oncol. 2012;30:623–630. doi: 10.1200/JCO.2011.36.0917. [DOI] [PubMed] [Google Scholar]
- 266.Vell MS, Loomba R, Krishnan A, Wangensteen KJ, Trebicka J, Creasy KT, Trautwein C, et al. Association of Statin Use With Risk of Liver Disease, Hepatocellular Carcinoma, and Liver-Related Mortality. JAMA Netw Open. 2023;6:e2320222. doi: 10.1001/jamanetworkopen.2023.20222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Urias E, Tedesco NR, Burkholder DA, Moran IJ, Miller MJ, Jasty VSJ, Patil S, et al. PNPLA3 risk allele is associated with risk of hepatocellular carcinoma but not decompensation in compensated cirrhosis. Hepatol Commun. 2024;8 doi: 10.1097/HC9.0000000000000441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Kim MH, Kim MY, Salloum S, Qian T, Wong LP, Xu M, Lee Y, et al. Atorvastatin favorably modulates a clinical hepatocellular carcinoma risk gene signature. Hepatol Commun. 2022;6:2581–2593. doi: 10.1002/hep4.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Deza Z, Caimi GR, Noelia M, Coli L, Ridruejo E, Alvarez L. Atorvastatin shows antitumor effect in hepatocellular carcinoma development by inhibiting angiogenesis via TGF-beta1/pERK signaling pathway. Mol Carcinog. 2023;62:398–407. doi: 10.1002/mc.23494. [DOI] [PubMed] [Google Scholar]
- 270.Qian T, Zhu S, Hoshida Y. Use of big data in drug development for precision medicine: an update. Expert Rev Precis Med Drug Dev. 2019;4:189–200. doi: 10.1080/23808993.2019.1617632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Zhang HF, Gao X, Wang X, Chen X, Huang Y, Wang L, Xu ZW. The mechanisms of renin-angiotensin system in hepatocellular carcinoma: From the perspective of liver fibrosis, HCC cell proliferation, metastasis and angiogenesis, and corresponding protection measures. Biomed Pharmacother. 2021;141:111868. doi: 10.1016/j.biopha.2021.111868. [DOI] [PubMed] [Google Scholar]
- 272.Zhang X, Wong GL, Yip TC, Tse YK, Liang LY, Hui VW, Lin H, et al. Angiotensin-converting enzyme inhibitors prevent liver-related events in nonalcoholic fatty liver disease. Hepatology. 2022;76:469–482. doi: 10.1002/hep.32294. [DOI] [PubMed] [Google Scholar]
- 273.Elhence H, Dodge JL, Lee BP. Association of Renin-Angiotensin System Inhibition With Liver-Related Events and Mortality in Compensated Cirrhosis. Clin Gastroenterol Hepatol. 2024;22:315–323.:e317. doi: 10.1016/j.cgh.2023.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Barone M, Viggiani MT, Losurdo G, Principi M, Leo AD. Systematic review: Renin-angiotensin system inhibitors in chemoprevention of hepatocellular carcinoma. World J Gastroenterol. 2019;25:2524–2538. doi: 10.3748/wjg.v25.i20.2524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Abu Dayyeh BK, Yang M, Fuchs BC, Karl DL, Yamada S, Sninsky JJ, O’Brien TR, et al. A functional polymorphism in the epidermal growth factor gene is associated with risk for hepatocellular carcinoma. Gastroenterology. 2011;141:141–149. doi: 10.1053/j.gastro.2011.03.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Tanabe KK, Lemoine A, Finkelstein DM, Kawasaki H, Fujii T, Chung RT, Lauwers GY, et al. Epidermal growth factor gene functional polymorphism and the risk of hepatocellular carcinoma in patients with cirrhosis. JAMA. 2008;299:53–60. doi: 10.1001/jama.2007.65. [DOI] [PubMed] [Google Scholar]
- 277.Jiang G, Yu K, Shao L, Yu X, Hu C, Qian P, Xie H, et al. Association between epidermal growth factor gene +61A/G polymorphism and the risk of hepatocellular carcinoma: a meta-analysis based on 16 studies. BMC Cancer. 2015;15:314. doi: 10.1186/s12885-015-1318-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Jin C, Han-Hua D, Qiu-Meng L, Deng N, Peng-Chen D, Jie M, Lei X, et al. MTDH-stabilized DDX17 promotes tumor initiation and progression through interacting with YB1 to induce EGFR transcription in Hepatocellular Carcinoma. Oncogene. 2023;42:169–183. doi: 10.1038/s41388-022-02545-x. [DOI] [PubMed] [Google Scholar]
- 279.Bang J, Jun M, Lee S, Moon H, Ro SW. Targeting EGFR/PI3K/AKT/mTOR Signaling in Hepatocellular Carcinoma. Pharmaceutics. 2023;15 doi: 10.3390/pharmaceutics15082130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Todeschini L, Cristin L, Martinino A, Mattia A, Agnes S, Giovinazzo F. The Role of mTOR Inhibitors after Liver Transplantation for Hepatocellular Carcinoma. Curr Oncol. 2023;30:5574–5592. doi: 10.3390/curroncol30060421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Roehlen N, Saviano A, El Saghire H, Crouchet E, Nehme Z, Del Zompo F, Juhling F, et al. A monoclonal antibody targeting nonjunctional claudin-1 inhibits fibrosis in patient-derived models by modulating cell plasticity. Sci Transl Med. 2022;14:eabj4221. doi: 10.1126/scitranslmed.abj4221. [DOI] [PubMed] [Google Scholar]
- 282.Roehlen N, Muller M, Nehme Z, Crouchet E, Juhling F, Del Zompo F, Cherradi S, et al. Treatment of HCC with claudin-1-specific antibodies suppresses carcinogenic signaling and reprograms the tumor microenvironment. J Hepatol. 2023;78:343–355. doi: 10.1016/j.jhep.2022.10.011. [DOI] [PubMed] [Google Scholar]
- 283.European Association for the Study of the Liver. Electronic address eee, European Association for the Study of the L. EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma. J Hepatol. 2018;69:182–236. doi: 10.1016/j.jhep.2018.03.019. [DOI] [PubMed] [Google Scholar]
- 284.Yu J, Liang D, Li J, Liu Z, Zhou F, Wang T, Ma S, et al. Coffee, Green Tea Intake, and the Risk of Hepatocellular Carcinoma: A Systematic Review and Meta-Analysis of Observational Studies. Nutr Cancer. 2023;75:1295–1308. doi: 10.1080/01635581.2023.2178949. [DOI] [PubMed] [Google Scholar]
- 285.Kennedy OJ, Fallowfield JA, Poole R, Hayes PC, Parkes J, Roderick PJ. All coffee types decrease the risk of adverse clinical outcomes in chronic liver disease: a UK Biobank study. BMC Public Health. 2021;21:970. doi: 10.1186/s12889-021-10991-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Petrick JL, Freedman ND, Graubard BI, Sahasrabuddhe VV, Lai GY, Alavanja MC, Beane-Freeman LE, et al. Coffee Consumption and Risk of Hepatocellular Carcinoma and Intrahepatic Cholangiocarcinoma by Sex: The Liver Cancer Pooling Project. Cancer Epidemiol Biomarkers Prev. 2015;24:1398–1406. doi: 10.1158/1055-9965.EPI-15-0137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Deng Y, Huang J, Wong MCS. Associations between six dietary habits and risk of hepatocellular carcinoma: A Mendelian randomization study. Hepatol Commun. 2022;6:2147–2154. doi: 10.1002/hep4.1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Niezen S, Mehta M, Jiang ZG, Tapper EB. Coffee Consumption Is Associated With Lower Liver Stiffness: A Nationally Representative Study. Clin Gastroenterol Hepatol. 2022;20:2032–2040.:e2036. doi: 10.1016/j.cgh.2021.09.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Aggarwal V, Tuli HS, Tania M, Srivastava S, Ritzer EE, Pandey A, Aggarwal D, et al. Molecular mechanisms of action of epigallocatechin gallate in cancer: Recent trends and advancement. Semin Cancer Biol. 2022;80:256–275. doi: 10.1016/j.semcancer.2020.05.011. [DOI] [PubMed] [Google Scholar]
- 290.Tang YP, Cao J, Cai ZM, An HH, Li YQ, Peng Y, Chen N, et al. Epigallocatechin gallate induces chemopreventive effects on rats with diethylnitrosamine-induced liver cancer via inhibition of cell division cycle 25A. Molecular Medicine Reports. 2020;22:3873–3885. doi: 10.3892/mmr.2020.11463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Liao S, Lin J, Liu J, Chen T, Xu M, Zheng J. Chemoprevention of elite tea variety CFT-1 rich in EGCG against chemically induced liver cancer in rats. Food Sci Nutr. 2019;7:2647–2665. doi: 10.1002/fsn3.1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Coia H, Ma N, Hou Y, Dyba MD, Fu Y, Cruz MI, Benitez C, et al. Prevention of Lipid Peroxidation-derived Cyclic DNA Adduct and Mutation in High-Fat Diet-induced Hepatocarcinogenesis by Theaphenon E. Cancer Prev Res (Phila) 2018;11:665–676. doi: 10.1158/1940-6207.CAPR-18-0160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Li D, Cao D, Cui Y, Sun Y, Jiang J, Cao X. The potential of epigallocatechin gallate in the chemoprevention and therapy of hepatocellular carcinoma. Front Pharmacol. 2023;14:1201085. doi: 10.3389/fphar.2023.1201085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Kang Q, Tong Y, Gowd V, Wang M, Chen F, Cheng KW. Oral administration of EGCG solution equivalent to daily achievable dosages of regular tea drinkers effectively suppresses miR483-3p induced metastasis of hepatocellular carcinoma cells in mice. Food Funct. 2021;12:3381–3392. doi: 10.1039/d1fo00664a. [DOI] [PubMed] [Google Scholar]
- 295.Gao W, Fan X, Bi Y, Zhou Z, Yuan Y. Preparation of NIR-Responsive Gold Nanocages as Efficient Carrier for Controlling Release of EGCG in Anticancer Application. Front Chem. 2022;10:926002. doi: 10.3389/fchem.2022.926002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Luu HN, Neelakantan N, Geng TT, Wang R, Goh GB, Clemente JC, Jin A, et al. Quality diet indexes and risk of hepatocellular carcinoma: Findings from the Singapore Chinese Health Study. Int J Cancer. 2021;148:2102–2114. doi: 10.1002/ijc.33367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.George ES, Sood S, Broughton A, Cogan G, Hickey M, Chan WS, Sudan S, et al. The Association between Diet and Hepatocellular Carcinoma: A Systematic Review. Nutrients. 2021;13 doi: 10.3390/nu13010172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Watling CZ, Wojt A, Florio AA, Butera G, Albanes D, Weinstein SJ, Huang WY, et al. Fiber and whole grain intakes in relation to liver cancer risk: An analysis in 2 prospective cohorts and systematic review and meta-analysis of prospective studies. Hepatology. 2024 doi: 10.1097/HEP.0000000000000819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.Di Y, Ding L, Gao L, Huang H. Association of meat consumption with the risk of gastrointestinal cancers: a systematic review and meta-analysis. BMC Cancer. 2023;23:782. doi: 10.1186/s12885-023-11218-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.Moussa I, Day RS, Li R, Kaseb A, Jalal PK, Daniel-MacDougall C, Hatia RI, et al. Association of dietary fat intake and hepatocellular carcinoma among US adults. Cancer Med. 2021;10:7308–7319. doi: 10.1002/cam4.4256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Liu Y, Yang W, VoPham T, Ma Y, Simon TG, Gao X, Chan AT, et al. Plant-Based and Animal-Based Low-Carbohydrate Diets and Risk of Hepatocellular Carcinoma Among US Men and Women. Hepatology. 2021;73:175–185. doi: 10.1002/hep.31251. [DOI] [PubMed] [Google Scholar]
- 302.Pham YT, Jin A, Wang R, Behari J, Koh WP, Yuan JM, Luu HN. Low-Carbohydrate Diet Score and Risk of Hepatocellular Carcinoma: Findings from a Prospective Cohort Study. Cancer Prev Res (Phila) 2024;17:265–274. doi: 10.1158/1940-6207.CAPR-23-0517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303.Crudele L, De Matteis C, Piccinin E, Gadaleta RM, Cariello M, Di Buduo E, Piazzolla G, et al. Low HDL-cholesterol levels predict hepatocellular carcinoma development in individuals with liver fibrosis. JHEP Rep. 2023;5:100627. doi: 10.1016/j.jhepr.2022.100627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Zhao L, Zhang X, Coday M, Garcia DO, Li X, Mossavar-Rahmani Y, Naughton MJ, et al. Sugar-Sweetened and Artificially Sweetened Beverages and Risk of Liver Cancer and Chronic Liver Disease Mortality. JAMA. 2023;330:537–546. doi: 10.1001/jama.2023.12618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305.Yi Z, Wang L, Tu X. Effect of Vitamin D Deficiency on Liver Cancer Risk: A Systematic Review and Meta-Analysis. Asian Pac J Cancer Prev. 2021;22:991–997. doi: 10.31557/APJCP.2021.22.4.991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306.Goto RL, Tablas MB, Prata GB, Espirito Santo SG, Fernandes AAH, Cogliati B, Barbisan LF, et al. Vitamin D(3) supplementation alleviates chemically-induced cirrhosis-associated hepatocarcinogenesis. J Steroid Biochem Mol Biol. 2022;215:106022. doi: 10.1016/j.jsbmb.2021.106022. [DOI] [PubMed] [Google Scholar]
- 307.Eitah HE, Attia HN, Soliman AAF, Gamal El Din AA, Mahmoud K, Sayed RH, Maklad YA, et al. Vitamin D ameliorates diethylnitrosamine-induced liver preneoplasia: A pivotal role of CYP3A4/CYP2E1 via DPP-4 enzyme inhibition. Toxicol Appl Pharmacol. 2023;458:116324. doi: 10.1016/j.taap.2022.116324. [DOI] [PubMed] [Google Scholar]
- 308.Chen PT, Hsieh CC, Chen MF. Role of vitamin D3 in tumor aggressiveness and radiation response for hepatocellular carcinoma. Mol Carcinog. 2022;61:787–796. doi: 10.1002/mc.23421. [DOI] [PubMed] [Google Scholar]
- 309.Ibrahim MN, Khalifa AA, Hemead DA, Alsemeh AE, Habib MA. 1,25-Dihydroxycholecalciferol down-regulates 3-mercaptopyruvate sulfur transferase and caspase-3 in rat model of non-alcoholic fatty liver disease. J Mol Histol. 2023;54:119–134. doi: 10.1007/s10735-023-10118-9. [DOI] [PubMed] [Google Scholar]
- 310.Li X, Pan C, Ma W, Yang T, Wang C, Han W, Zhang W, et al. Effects of dietary supplementation of fish oil plus vitamin D(3) on gut microbiota and fecal metabolites, and their correlation with nonalcoholic fatty liver disease risk factors: a randomized controlled trial. Food Funct. 2024;15:2616–2627. doi: 10.1039/d3fo02319b. [DOI] [PubMed] [Google Scholar]
- 311.Ebrahimpour-Koujan S, Sohrabpour AA, Giovannucci E, Vatannejad A, Esmaillzadeh A. Effects of vitamin D supplementation on liver fibrogenic factors, vitamin D receptor and liver fibrogenic microRNAs in metabolic dysfunction-associated steatotic liver disease (MASLD) patients: an exploratory randomized clinical trial. Nutr J. 2024;23:24. doi: 10.1186/s12937-024-00911-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 312.Wu T, Wang M, Ning F, Zhou S, Hu X, Xin H, Reilly S, et al. Emerging role for branched-chain amino acids metabolism in fibrosis. Pharmacol Res. 2023;187:106604. doi: 10.1016/j.phrs.2022.106604. [DOI] [PubMed] [Google Scholar]
- 313.Konstantis G, Pourzitaki C, Chourdakis M, Kitsikidou E, Germanidis G. Efficacy of branched chain amino acids supplementation in liver cirrhosis: A systematic review and meta-analysis. Clinical Nutrition. 2022;41:1171–1190. doi: 10.1016/j.clnu.2022.03.027. [DOI] [PubMed] [Google Scholar]
- 314.van Dijk AM, Bruins Slot AS, Portincasa P, Siegerink SN, Chargi N, Verstraete CJR, de Bruijne J, et al. Systematic review with meta-analysis: Branched-chain amino acid supplementation in liver disease. Eur J Clin Invest. 2023;53:e13909. doi: 10.1111/eci.13909. [DOI] [PubMed] [Google Scholar]
- 315.Yap KY, Chi H, Ng S, Ng DH, Shelat VG. Effect of perioperative branched chain amino acids supplementation in liver cancer patients undergoing surgical intervention: A systematic review. World J Gastrointest Surg. 2023;15:2596–2618. doi: 10.4240/wjgs.v15.i11.2596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 316.Li Y, Deng X, Tan X, Li Q, Yu Z, Wu W, Ma X, et al. Protective role of curcumin in disease progression from non-alcoholic fatty liver disease to hepatocellular carcinoma: a meta-analysis. Front Pharmacol. 2024;15:1343193. doi: 10.3389/fphar.2024.1343193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 317.Schneider KM, Mohs A, Gui W, Galvez EJC, Candels LS, Hoenicke L, Muthukumarasamy U, et al. Imbalanced gut microbiota fuels hepatocellular carcinoma development by shaping the hepatic inflammatory microenvironment. Nat Commun. 2022;13:3964. doi: 10.1038/s41467-022-31312-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 318.Huang C, Mei S, Zhang X, Tian X. Inflammatory Milieu Related to Dysbiotic Gut Microbiota Promotes Tumorigenesis of Hepatocellular Carcinoma. J Clin Gastroenterol. 2023;57:782–788. doi: 10.1097/MCG.0000000000001883. [DOI] [PubMed] [Google Scholar]
- 319.Daniel N, Genua F, Jenab M, Mayen AL, Chrysovalantou Chatziioannou A, Keski-Rahkonen P, Hughes DJ. The role of the gut microbiome in the development of hepatobiliary cancers. Hepatology. 2023 doi: 10.1097/HEP.0000000000000406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 320.Zhang X, Coker OO, Chu ES, Fu K, Lau HCH, Wang YX, Chan AWH, et al. Dietary cholesterol drives fatty liver-associated liver cancer by modulating gut microbiota and metabolites. Gut. 2021;70:761–774. doi: 10.1136/gutjnl-2019-319664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 321.Shen R, Ke L, Li Q, Dang X, Shen S, Shen J, Li S, et al. Abnormal bile acid-microbiota crosstalk promotes the development of hepatocellular carcinoma. Hepatol Int. 2022;16:396–411. doi: 10.1007/s12072-022-10299-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 322.Hu C, Xu B, Wang X, Wan WH, Lu J, Kong D, Jin Y, et al. Gut microbiota-derived short-chain fatty acids regulate group 3 innate lymphoid cells in HCC. Hepatology. 2023;77:48–64. doi: 10.1002/hep.32449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 323.Lau HC, Zhang X, Ji F, Lin Y, Liang W, Li Q, Chen D, et al. Lactobacillus acidophilus suppresses non-alcoholic fatty liver disease-associated hepatocellular carcinoma through producing valeric acid. EBioMedicine. 2024;100:104952. doi: 10.1016/j.ebiom.2023.104952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 324.Hernandez-Galdamez HV, Fattel-Fazenda S, Flores-Tellez TNJ, Aguilar-Chaparro MA, Mendoza-Garcia J, Diaz-Fernandez LC, Romo-Medina E, et al. Iron-saturated bovine lactoferrin: a promising chemopreventive agent for hepatocellular carcinoma. Food Funct. 2024;15:4586–4602. doi: 10.1039/d3fo05184f. [DOI] [PubMed] [Google Scholar]
- 325.Hojman P, Gehl J, Christensen JF, Pedersen BK. Molecular Mechanisms Linking Exercise to Cancer Prevention and Treatment. Cell Metab. 2018;27:10–21. doi: 10.1016/j.cmet.2017.09.015. [DOI] [PubMed] [Google Scholar]
- 326.Zhang Y, Cao C, Li C, Witt RG, Huang H, Tsung A, Zhang H. Physical exercise in liver diseases. Hepatology. 2024 doi: 10.1097/HEP.0000000000000941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 327.DiJoseph K, Thorp A, Harrington A, Schmitz KH, Chinchilli VM, Stine JG. Physical Activity and Risk of Hepatocellular Carcinoma: A Systematic Review and Meta-Analysis. Dig Dis Sci. 2023;68:1051–1059. doi: 10.1007/s10620-022-07601-w. [DOI] [PubMed] [Google Scholar]
- 328.Longo M, Meroni M, Paolini E, Macchi C, Dongiovanni P. Mitochondrial dynamics and nonalcoholic fatty liver disease (NAFLD): new perspectives for a fairy-tale ending? Metabolism. 2021;117:154708. doi: 10.1016/j.metabol.2021.154708. [DOI] [PubMed] [Google Scholar]
- 329.Xiao CL, Zhong ZP, Lu C, Guo BJ, Chen JJ, Zhao T, Yin ZF, et al. Physical exercise suppresses hepatocellular carcinoma progression by alleviating hypoxia and attenuating cancer stemness through the Akt/GSK-3beta/beta-catenin pathway. J Integr Med. 2023;21:184–193. doi: 10.1016/j.joim.2023.01.002. [DOI] [PubMed] [Google Scholar]
- 330.Jee H, Park E, Hur K, Kang M, Kim Y. High-Intensity Aerobic Exercise Suppresses Cancer Growth by Regulating Skeletal Muscle-Derived Oncogenes and Tumor Suppressors. Front Mol Biosci. 2022;9:818470. doi: 10.3389/fmolb.2022.818470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 331.Liu Q, Li H, He W, Zhao Q, Huang C, Wang Q, Zheng Z, et al. Role of aerobic exercise in ameliorating NASH: Insights into the hepatic thyroid hormone signaling and circulating thyroid hormones. Front Endocrinol (Lausanne) 2022;13:1075986. doi: 10.3389/fendo.2022.1075986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 332.Stine JG, Welles JE, Keating S, Hussaini Z, Soriano C, Heinle JW, Geyer N, et al. Serum Fibroblast Growth Factor 21 Is Markedly Decreased following Exercise Training in Patients with Biopsy-Proven Nonalcoholic Steatohepatitis. Nutrients. 2023;15 doi: 10.3390/nu15061481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 333.Stine JG, DiJoseph K, Pattison Z, Harrington A, Chinchilli VM, Schmitz KH, Loomba R. Exercise Training Is Associated With Treatment Response in Liver Fat Content by Magnetic Resonance Imaging Independent of Clinically Significant Body Weight Loss in Patients With Nonalcoholic Fatty Liver Disease: A Systematic Review and Meta-Analysis. Am J Gastroenterol. 2023;118:1204–1213. doi: 10.14309/ajg.0000000000002098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 334.Macias RIR, Kanzaki H, Berasain C, Avila MA, Marin JJG, Hoshida Y. The Search for Risk, Diagnostic, and Prognostic Biomarkers of Cholangiocarcinoma and Their Biological and Clinicopathologic Significance. Am J Pathol. 2024 doi: 10.1016/j.ajpath.2024.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 335.Seeherunwong A, Chaiear N, Khuntikeo N, Ekpanyaskul C. Cholangiocarcinoma Attributed to Occupation: A Systematic Reviews. Asian Pac J Cancer Prev. 2022;23:1837–1845. doi: 10.31557/APJCP.2022.23.6.1837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 336.Izquierdo-Sanchez L, Lamarca A, Casta La, Buettner S, Utpatel K, Klümpen HJ, Adeva J, et al. Cholangiocarcinoma landscape in Europe: Diagnostic, prognostic and therapeutic insights from the ENSCCA Registry. J Hepatol. 2022;76:1109–1121. doi: 10.1016/j.jhep.2021.12.010. [DOI] [PubMed] [Google Scholar]
- 337.Okawa Y, Iwasaki Y, Johnson TA, Ebata N, Inai C, Endo M, Maejima K, et al. Hereditary cancer variants and homologous recombination deficiency in biliary tract cancer. J Hepatol. 2023;78:333–342. doi: 10.1016/j.jhep.2022.09.025. [DOI] [PubMed] [Google Scholar]
- 338.Lapitz A, Azkargorta M, Milkiewicz P, Olaizola P, Zhuravleva E, Grimsrud MM, Schramm C, et al. Liquid biopsy-based protein biomarkers for risk prediction, early diagnosis, and prognostication of cholangiocarcinoma. J Hepatol. 2023;79:93–108. doi: 10.1016/j.jhep.2023.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 339.Deng M, Ran P, Chen L, Wang Y, Yu Z, Cai K, Feng J, et al. Proteogenomic characterization of cholangiocarcinoma. Hepatology. 2022;77 doi: 10.1002/hep.32624. n/a-n/a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 340.Carapeto F, Bozorgui B, Shroff RT, Chagani S, Solis Soto L, Foo WC, Wistuba I, et al. The immunogenomic landscape of resected intrahepatic cholangiocarcinoma. Hepatology. 2022;75:297–308. doi: 10.1002/hep.32150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 341.Wijetunga I, McVeigh LE, Charalambous A, Antanaviciute A, Carr IM, Nair A, Prasad KR, et al. Translating Biomarkers of Cholangiocarcinoma for Theranosis: A Systematic Review. Cancers (Basel) 2020;12 doi: 10.3390/cancers12102817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 342.Pomyen Y, Chaisaingmongkol J, Rabibhadana S, Pupacdi B, Sripan D, Chornkrathok C, Budhu A, et al. Gut dysbiosis in Thai intrahepatic cholangiocarcinoma and hepatocellular carcinoma. Sci Rep. 2023;13:11406. doi: 10.1038/s41598-023-38307-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 343.Shen Y, Xu S, Ye C, Li Q, Chen R, Wu W, Jiang Q, et al. Proteomic and single-cell landscape reveals novel pathogenic mechanisms of HBV-infected intrahepatic cholangiocarcinoma. iScience. 2023;26:106003. doi: 10.1016/j.isci.2023.106003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 344.Wang Y, Yuan Y, Gu D. Hepatitis B and C virus infections and the risk of biliary tract cancers: a meta-analysis of observational studies. Infect Agent Cancer. 2022;17:45. doi: 10.1186/s13027-022-00457-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 345.An J, Kim D, Oh B, Oh YJ, Song J, Park N, Kim HI, et al. Comprehensive characterization of viral integrations and genomic aberrations in HBV-infected intrahepatic cholangiocarcinomas. Hepatology. 2022;75:997–1011. doi: 10.1002/hep.32135. [DOI] [PubMed] [Google Scholar]
- 346.Cheung KS, Yeung YWM, Wong WS, Li B, Seto WK, Leung WK. Statins associate with lower risk of biliary tract cancers: A systematic review and meta-analysis. Cancer Med. 2023;12:557–568. doi: 10.1002/cam4.4942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 347.Marcano-Bonilla L, Schleck CD, Harmsen WS, Sadr-Azodi O, Borad MJ, Patel T, Petersen GM, et al. Aspirin, Statins, Non-aspirin NSAIDs, Metformin, and the Risk of Biliary Cancer: A Swedish Population-Based Cohort Study. Cancer Epidemiol Biomarkers Prev. 2022;31:804–810. doi: 10.1158/1055-9965.EPI-20-1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 348.Chen J, Jin H, Zhou H, Liu K. Effects of Metformin on Risk and Prognosis of Biliary Tract Cancer: A Systematic Review and Meta-Analysis. Medicina (Kaunas) 2023;59 doi: 10.3390/medicina59020298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 349.Gunchick V, McDevitt RL, Choi E, Winslow K, Zalupski MM, Sahai V. Survival Analysis of 1140 Patients with Biliary Cancer and Benefit from Concurrent Renin-Angiotensin Antagonists, Statins, or Aspirin with Systemic Therapy. Oncologist. 2023;28:531–541. doi: 10.1093/oncolo/oyad063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 350.Huang YH, Loftfield E, Argirion I, Adami HO, Albanes D, Chan AT, Fedirko V, et al. Association of tea and coffee consumption and biliary tract cancer risk: The Biliary Tract Cancers Pooling Project. Hepatology. 2023;79:1324–1336. doi: 10.1097/HEP.0000000000000748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 351.Wu Q, Shi X, Pan Y, Liao X, Xu J, Gu X, Yu W, et al. The Chemopreventive Role of beta-Elemene in Cholangiocarcinoma by Restoring PCDH9 Expression. Front Oncol. 2022;12:874457. doi: 10.3389/fonc.2022.874457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 352.Wilbur HC, Soares HP, Azad NS. Neoadjuvant and adjuvant therapy for biliary tract cancer: Advances and limitations. Hepatology. 2024 doi: 10.1097/HEP.0000000000000760. [DOI] [PubMed] [Google Scholar]
- 353.Lee TK, Guan XY, Ma S. Cancer stem cells in hepatocellular carcinoma - from origin to clinical implications. Nat Rev Gastroenterol Hepatol. 2022;19:26–44. doi: 10.1038/s41575-021-00508-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.