Abstract
Background
Dog-assisted interventions (DAIs) to improve health-related outcomes for people with mental health or neurodevelopmental conditions are becoming increasingly popular. However, DAIs are not based on robust scientific evidence.
Aims
To determine the effectiveness of DAIs for children and adults with mental health or neurodevelopmental conditions, assess how well randomised controlled trials (RCTs) are reported, and examine the use of terminology to classify DAIs.
Methods
A systematic search was conducted in EMBASE, PsycINFO, PubMed, CINAHL, Web of Science and The Cochrane Library. RCTs were grouped by commonly reported outcomes and described narratively with forest plots reporting standardised mean differences plus 95% confidence intervals without a pooled estimate. The quality of reporting of RCTs and DAIs were evaluated by assessing adherence to CONSORT and the template for intervention description and replication (TIDieR) guidelines. Suitability of use of terminology was assessed by mapping terms to the intervention content described.
Results
Thirty-three papers were included, reporting 29 RCTs (with five assessed as overall high quality); 57% (8/14) found a positive impact of DAIs on social skills/behaviour; 50% (5/10) for symptom frequency/severity; 43% (6/14) for depression, and 33% (2/6) for agitation. The mean proportion of adherence to the CONSORT statement was 48.6%. The TIDieR checklist also indicated considerable variability in intervention reporting. Most DAIs were assessed as having clear alignment for terminology, but improvement in reporting information is still required.
Conclusions
DAIs may be promising for improving mental health and behavioural outcomes for those with mental health or neurodevelopmental conditions, particularly for conditions requiring social skill support. However, the quality of reporting requires improvement.
Keywords: dog-assisted interventions, canine-assisted interventions, mental health conditions, neurodevelopmental conditions, systematic review
Introduction
Mental health conditions constitute a leading cause of disability worldwide (1). The World Health Organisation (2) defines the term “mental disorders” as a range of mental and behavioural conditions that fall within the International Statistical Classification of Diseases and Related Health Problems (ICD-11) (3). These include disorders that cause a high burden of disease such as depression, bipolar affective disorder, schizophrenia, anxiety disorders, dementia, intellectual disabilities, and developmental and behavioural disorders with onset usually occurring in childhood and adolescence (e.g., autism spectrum condition (ASC)) (2, 3). The need to develop and test new interventions to improve outcomes and quality of life related to these conditions is widely acknowledged (4–7).
Animal-assisted interventions (AAIs) have been receiving increasing interest as (complementary) interventions to improve health-related outcomes, especially those focused on mental health, across various age groups (8–11). In a health-focused context, AAIs intentionally include animals in health, education and social services contexts for therapeutic or otherwise ameliorative purposes. Health-focused AAIs include animal-assisted therapy, which is goal-orientated, structured, documented, and delivered by trained professionals; and animal-assisted activities, which are also goal-orientated, but typically based on spontaneous interaction, delivered usually by volunteers and non-specialist trained animals. Although a variety of species (e.g., dogs, horses, small mammals, farm animals) can be involved in AAIs in research and practice, dog-assisted interventions (DAIs) are the most commonly provided and researched type of AAI (12).
Research suggests that DAIs might improve a range of mental health and behavioural outcomes such as anxiety, agitation, feelings of depression and loneliness, while enhancing positive social interaction (13–16). Although overall poorly understood, mechanisms underlying these effects have been hypothesised to be related to, for example, the calming and motivating effects of the dog’s presence, which in turn might catalyse participants’ engagement with therapy (17). Recently, there has been much enthusiasm for and a rapid increase in the provision of DAIs for a wide range of mental health and neurodevelopmental conditions in practice (14, 15, 18–21), with DAIs being increasingly offered by third sector organisations or by teams affiliated with health and social care or educational settings.
However, DAIs are currently not based on robust evidence. Although findings from generally small randomised controlled trials (RCTs) have been reported (15, 22–24), evidence synthesis has unanimously highlighted common methodological problems and a lack of rigour in study design (8, 25–27). Key issues include small sample sizes and consequently a lack of statistical power, and an absence of manualised intervention protocols and well-designed control conditions (8, 28, 29). Design issues are further compounded by limited intervention reporting, restricting the opportunity for reproducibility and comparability (28, 30). The complex nature of DAIs in health-related contexts, which involve inter-species interactions between several actors including a dog and vulnerable patient(s), also requires consideration of welfare and safety for the participant, dog and handler that exceeds current design and reporting practice in the field (29, 31). Notably, common terminological and conceptual confusion with regard to the definition of DAIs and their application in practice and research contexts has been identified, further compounding transparency (32).
Several evidence syntheses have been conducted to explore the impact of DAIs in populations with mental health and neurodevelopmental conditions (25, 29, 33, 34), with a wide variation in review focus (e.g. on specific diagnostic groups, settings, or age groups), methodological quality and terminology used. No existing systematic reviews have formally evaluated the reporting quality of RCTs delivering DAIs by assessing adherence to gold standard reporting guidelines, such as CONSORT (35); or evaluated the quality and completeness of reporting DAIs, for example, by assessing intervention reporting in accordance with the template for intervention description and replication (TIDieR) guide (36). Likewise, no existing systematic reviews have examined the use of how these interventions are described, practiced and reported. Thus, the research aims for this review were:
To examine the use of terminology and definitions chosen to classify DAIs in the included RCTs;
To determine the effectiveness of mental health-focused DAIs for populations with mental health and neurodevelopmental conditions, in clinical and community (including educational) settings;
To assess how well RCTs delivering DAIs to people with mental health and neurodevelopmental conditions are reported based on internationally recognised gold standard reporting guidelines (CONSORT and TIDieR).
Methods
We report methodology in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) guidelines (37), following a pre-registered International Prospective Register of Systematic Reviews (PROSPERO) protocol [CRD42024526375]. An amendment to the protocol was made to add the first review question. We believed this was an important addition due to the ambiguity and inconsistent terminology for DAIs used across this research area.
Inclusion criteria
Studies were assessed for inclusion based on the population, intervention, comparator, outcome and study design (PICOS; Table 1) (38).
Table 1. Inclusion criteria based on PICOS.
| Population | Studies that included children (aged up to 18 years) and/or adults (aged 18 years and above) with a diagnosis of a mental health or neurodevelopmental condition (as defined by the ICD-11 (3)), in clinical and community (including educational) settings. Studies evaluating DAIs delivered to participants with dementia were included as a diagnosis of dementia is categorised in ‘mental, behavioural or neurodevelopmental disorders’ in the ICD-11 (3). |
| Intervention | DAIs (including dog-assisted therapy and dog-assisted activity) delivered to participants with a diagnosis of a mental health and/or neurodevelopmental condition. |
| Comparator | Studies with the following controls were considered: normal practice (‘usual care’), waiting-list control, or any other intervention described by the authors as a comparator. |
| Outcomes | Studies that reported: mental health and behavioural outcomes (e.g., agitation, anxiety, social behaviour, verbalisation). |
| Study designs | RCTs (including randomised feasibility and pilot trials). |
Exclusion criteria
Studies were excluded if: (1) they described or evaluated the impact of living with pet dogs or assistance dogs; (2) the DAIs were primarily education interventions with educational outcomes (e.g. reading), as DAIs were only included if they were delivered for health-related/therapeutic purposes; (3) interventions involved species other than dogs; (4) interventions involved robotic dogs; (5) they did not assess the impact on outcomes for people with a mental health or neurodevelopmental condition, or (6) they were systematic reviews, theses, dissertations or not original research.
Search strategy
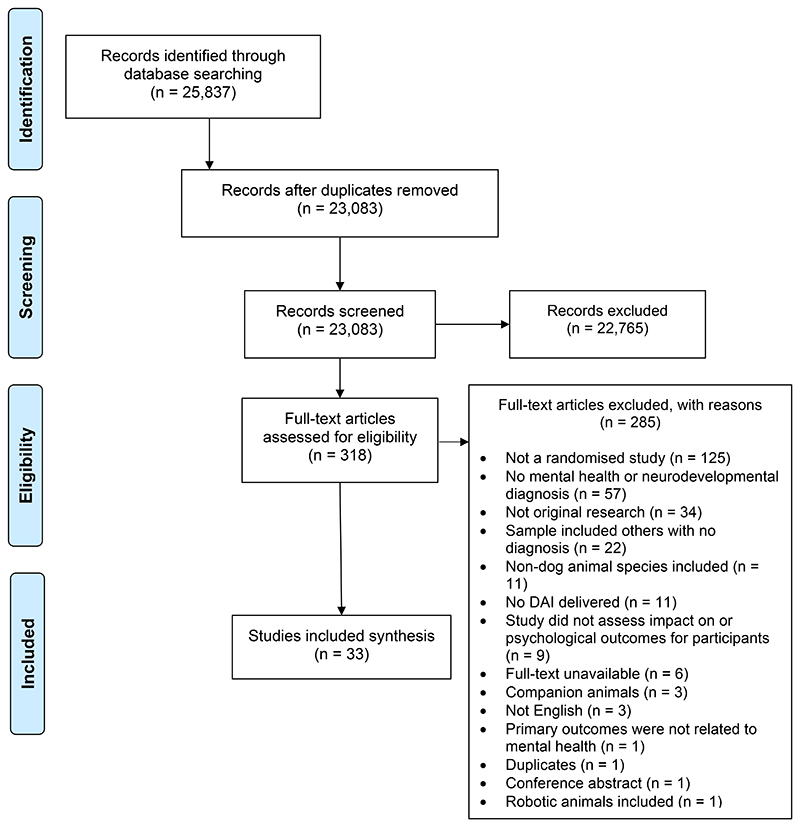
EMBASE, PsycINFO, PubMed, CINAHL, Web of Science and The Cochrane Library were searched up to 30th April 2024. A comprehensive search strategy was developed using subject headings and words that related to DAIs (e.g., dog-assisted therapy, dog-assisted activities, dog-assisted interventions, animal-assisted interventions, therapy dogs, therapy animals) and mental health or neurodevelopmental conditions in children and adult populations. Searches were limited to studies published in English. The search strategy for EMBASE is provided in Supplementary Material 1 and was adapted for the other included databases. Reference lists of included papers and systematic reviews of DAIs for mental health and neurodevelopmental conditions were manually screened to identify potential further studies. Covidence was used to record publications at all stages of the selection process (Figure 1). Titles and abstracts were screened independently by two authors (ES and JP). If there was a disagreement, studies were included in the full-text review. Full-text screening was undertaken independently by two authors (ES and JP), and any disagreements were resolved with a third author (ER).
Figure 1. PRISMA diagram of paper selection process.
Data extraction
Using a pre-defined data extraction worksheet in Microsoft Excel, relevant data were extracted by one author (ES). Information included research methodology, sample size, follow-up periods, type and content of the intervention and control groups, mode of delivery, frequency and duration, participant details including diagnosis, diagnostic criteria, role of animal handlers, aspects related to selection, training, and safety of the animals involved, and outcomes of the intervention. The complete list of data extracted is provided in Supplementary Material 2. Data extraction commenced on 12th May 2024.
Risk of bias assessment
Two authors (ES and JP) independently assessed the risk of bias of each RCT using the Cochrane risk of bias tool (39). Consensus was reached through discussion between the two authors. Data from the risk of bias assessment were entered into Review Manager 5.3 (40) to generate a summary figure. Risk of bias assessment was used as a tool for critique of the research evidence and not as an exclusion criterion. The risk of bias for all domains was summarised to produce an overall risk of bias for each RCT. RCTs were classified as overall high risk of bias if they scored ‘unclear’ or ‘high’ in any bias domain other than performance bias, due to the nature of DAIs making the blinding of participants and personnel difficult to mask.
Data synthesis
As per the protocol, a meta-analysis was planned, provided low heterogeneity, assessed using the I2 statistic. However, due to clinical and methodological heterogeneity, we determined that a statistical meta-analysis was inappropriate, and a narrative synthesis is provided to summarise the effectiveness of DAIs. Trials were grouped by commonly reported mental health and behavioural outcomes and findings were described narratively with forest plots reporting standardised mean difference (SMD) plus 95% confidence interval (CI) without a pooled estimate. SMD is the mean difference in outcome scores between the intervention and control group divided by the pooled SD at follow-up, resulting in a unit-free effect size. By convention, SMD effect sizes of 0.2, 0.5 and 0.8 are considered small, medium and large intervention effects, respectively (41). The direction of effect was assessed based on the effects reported by authors of the included studies and the forest plots produced. The direction of effect (or if there was no difference between intervention and control), was used to determine the effectiveness of DAIs. The findings are presented in three categories: mental health conditions, neurodevelopmental conditions, and dementia. While a diagnosis of dementia is categorised in ‘mental, behavioural or neurodevelopmental disorders’ in the ICD-11 (3), studies involving adults with dementia are presented separately to those with other mental health conditions due to the distinct aetiology of the condition.
The CONSORT statement (42) was used to assess the quality of reporting of RCTs. Two authors (ES and HS) individually assessed each paper, and each item was scored ‘yes’ when adequately reported or ‘no’ when inadequately, inconsistently, or not at all reported. Reporting of an item in Supplementary Material was considered acceptable only if clearly cited in the main text. Additionally, the Template for Intervention Description and Replication (TIDieR) checklist (36) was used for the appraisal of quality and completeness of reporting intervention details. Data in each of the papers and any Supplementary Material cited within the papers were used. Two authors (ES and JP) individually assessed each study, and each item was scored ‘yes’, when adequately reported or ‘no’ when inadequately, inconsistently reported, or not applicable. Cohen’s kappa (k) was calculated to assess the agreement between reviewers for both appraisals using the CONSORT statement and the TIDieR checklist. Interpretation of the coefficient is described as: ‘none’ = 0 – 0.20; ‘minimal’ = 0.21 – 0.39; ‘weak’ = 0.40 – 0.59; ‘moderate’ = 0.60 – 0.79’; ‘strong’ = 0.80 – 0.90, and ‘almost perfect’ = > 0.90 (43). Data were analysed in IBM SPSS Version 28 (44).
Terminology used by authors to classify DAIs as ‘dog-assisted therapy’ or ‘dog-assisted activity’ was extracted for each study and assessed for suitability of use by two authors (ES and HS) based on the intervention content described, using internationally recognised definitions by the International Association of Human-Animal Interaction Organisations (45) and Animal-Assisted Intervention International (46). We did not use the new terminology proposed in early 2024 (32), as this would not have corresponded to terminology and classification used in the studies, all of which were conducted before the 2024 recommendations were published. We assessed alignment between study terminology and conceptual definitions using three categories: 1) ‘clear alignment of content and terminology’, 2) ‘unclear alignment of content and terminology’ (e.g., due to limited information in the manuscript), and 3) ‘misalignment of content and terminology’ (e.g., a DAI was described as therapy, but the content description clearly depicted activity).
Results
Description of studies
Database searches yielded a total of 25,837 records. After the removal of duplicates and screening of titles, abstracts, and full-text papers, 33 papers were included in the review (Figure 1), reporting a total of 29 studies. The independent screening of titles and abstracts and full-texts both yielded a Cohen’s kappa of 0.77. Two papers evaluating a DAI delivered to adults with ASC refer to the same RCT (21, 23); two papers delivered to adults with schizophrenia refer to the same RCT (14, 47), and three papers delivered to children with ADHD refer to the same RCT (15, 48, 49). All papers were included as they assessed different relevant outcomes. A list of all included papers are presented in Supplementary Material 3.
Thirty-three papers described 29 small-scale RCTs (intervention sample size range = 5-186; control sample size range = 4-185). Study follow-ups ranged from immediately post-intervention (14, 16, 18, 19, 24, 47, 50–63) to 3 months (20, 64–67).
DAIs were delivered to a variety of study populations, including: dementia (n=11), schizophrenia (n=5), ASC (n=3), attention deficit hyperactivity disorder (ADHD) (n=2), any acute psychiatric diagnosis (n=2), Foetal Alcohol Spectrum Disorder (FASC) (n=2), intellectual disabilities (n=1), anxiety or depression (n=1), post-traumatic stress disorder (PTSD) (n=1), or mixed diagnoses (e.g., ASC, ADHD, intellectual disabilities) (n=1).
DAIs were delivered to a variety of age groups, including children (4-12 years; n=5), children and adolescents (6-17 years; n=5), adults (18-65 years; n=8), and older adults (65+ years; n=11). For those including children, all participants were diagnosed with a neurodevelopmental condition (ASC or ADHD), and for those including older adults, all participants were diagnosed with dementia.
Of 28 studies (96.6%), just over half of all participants were female (n=784, 54.9%). One study did not report participant gender (68). Only five studies (17.2%) reported ethnicity (13, 15, 24, 48, 49, 59), and two thirds were White Caucasians (n=141, 66.5%). Of 25 studies (86.2%) reporting participant age, the mean age was 42.7 years (standard deviation; SD = 32.8). Four studies (13.8%) did not provide information on participant age (52, 61, 62, 68). For those reporting diagnosis severity at baseline data collection (n=13, 44.8%), participants with dementia (16, 57–65, 69) were most commonly diagnosed with mild, moderate or mild-moderate dementia (n=678, 96.9%), and participants with schizophrenia (47, 50) were most commonly considered ‘mildly ill’ according to the Positive and Negative Syndrome Scale (PANSS) scores (n=64, 100%). Six studies (20.7%) reported participant characteristics related to animal ownership (19, 57, 63–65, 70). Of these, 196 participants (68.7%) reported they were current or previous animal owners or enjoyed interaction with animals. Table 2 presents demographics reported by the studies.
Table 2. Participant demographics available in included studies, separated by age group.
| Gender (n, %) |
Ethnicity (n, %) |
Mean age (years) |
Age standard deviation (SD) |
Experience with animals (n, %) |
|
|---|---|---|---|---|---|
| Children and adolescents | |||||
| Mental health conditions | Female (49, 56.3%) |
White (21, 63.6%) |
14.8 | 1.8 | Not reported |
| Neurodevelopmental conditions | Female (85, 26.5%) |
White (83, 59.3%) |
8.7 | 2.4 | Yes (18, 81.8%) |
| Adults | |||||
| Mental health conditions | Female (120, 52.4%) |
Not reported | 50.1 | 2.8 | Not reported |
| Neurodevelopmental conditions | Female (46, 50.0%) |
Not reported | 38.2 | 1.2 | Yes (18, 33.9%) |
| Older adults | |||||
| Dementia | Female (484, 69.1%) |
White (37, 94.9%) |
83.7 | 2.1 | Yes (160, 76.2%) |
The majority of studies were conducted in Europe (n=17; 58.6%), followed by Asia (n=5; 17.2%), USA (n=4; 13.8%), Australia (n=2; 6.9%), and the UK (n=1; 3.5%). Study settings varied substantially and included hospitals (n=11) and care facilities (e.g., nursing homes, care homes) (n=10). Supplementary Material 4 provides an overview of study characteristics.
Intervention characteristics
Interventions varied by type (therapy or activity), content, role of intervention providers, group size, and frequency and duration (Supplementary Material 5). Of the 29 studies, DAIs included were described by authors as therapy (n=23; 79.3%) and activities (n=6; 20.7%).
Studies used various controls, including the same therapy or activity without the presence of a dog (13, 15, 24, 48, 49, 51–53, 55, 61, 63), usual care activities or treatment (14, 16, 18–20, 22, 47, 50, 60, 62, 64, 65, 68, 71), waitlist control groups (21, 23), relaxation or reminiscing interventions (54, 56, 59), and a discussion group about animals (67). Two studies did not provide detailed information regarding the control group content (57, 58).
Risk of bias assessment
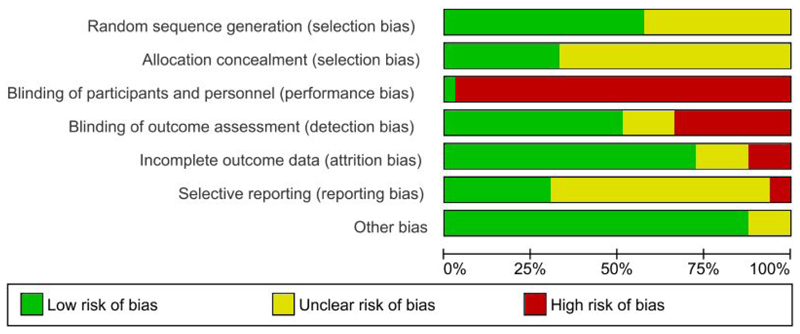
Risk of bias results are presented in Figure 2.
Figure 2. Risk of bias graph: review authors’ judgements about each risk of bias item presented as percentages across all included studies.
Thirteen studies (44.8%) were judged to be of unclear risk of bias for random sequence generation due to insufficient information regarding method of randomisation (dementia, n=5; neurodevelopmental, n=4; mental health, n=4). The remaining studies reported participants were allocated using various methods (e.g., computer randomisation, coin flip method) and were judged as low risk of bias. Twenty studies (69.0%) did not provide a statement regarding allocation concealment so were judged as having an unclear risk of bias (dementia, n=10; mental health, n=6; neurodevelopmental, n=4). Twenty-eight studies (96.6%) reported in 33 papers were judged to be of high risk of bias for blinding of participants and personnel due to the inability to blind individuals to the presence of a dog. Only one study (3.5%) was judged to be of low risk of bias as both participants and personnel were blinded. Psychiatric rehabilitation institutions were randomised, and in those randomised to the control group, participants watched animal documentaries (67). No information was provided regarding whether the participants were debriefed about the blinding and their group allocation once participation had concluded (67).
Just over half of the studies (n=15; 51.7%) reported blinding outcome assessments, so were judged as low risk of bias. However, nine studies (31.0%; reported in 12 papers focusing on different outcomes) did not blind outcome assessors and were judged as high risk of bias (mental health, n=4; neurodevelopmental, n=3; dementia, n=2). Six of these reported that blinding was impossible due to the nature of the intervention or limited resources (14, 19, 47, 50, 64, 65), and four involved self-report or the child’s parents completing the outcome measures so could not be blinded (13, 15, 48, 52). Five studies (17.2%) were judged as unclear risk of bias due to insufficient information related to blinding of outcome assessments (dementia, n=4; neurodevelopmental, n=1).
Most studies (n=21; 72.4%) were judged as low risk of bias for incomplete outcome data as above 85% of participants completed the study. Four studies (13.8%) were judged as a high risk of bias due to withdrawals and exclusions that may have imbalanced groups, and lack of intention-to-treat analysis and/or use of a per protocol analysis (mental health, n=2; neurodevelopmental, n=1; dementia, n=1) (18, 50, 54, 68). The remaining four studies (13.8%) were judged as unclear risk of bias due to insufficient information (dementia, n=2; mental health, n=2).
Eighteen studies (62.1%; reported in 20 papers) were judged to be of unclear bias for selective outcome reporting due to the absence of a pre-published registration/protocol explicitly stating the primary outcomes and assessment timepoints (dementia, n=9; neurodevelopmental, n=6, mental health, n=3). Two studies (6.9%) were judged to be of high risk of bias for selective outcome reporting. One study reported their aims were to investigate physiological and psychological aspects of schizophrenia, but no physiological measures were reported (68). One study reported they measured depression pre- and post-intervention for participants with anxiety or mixed anxiety-depressive disorders, but depression scores were not available (52). The remaining nine studies (31.0%; reported in 12 papers) were judged as low risk of bias as they cited a pre-published registration/protocol clearly stating their primary outcomes and assessment timepoints (neurodevelopmental, n=6; mental health, n=4; dementia, n=2). Risk of bias across individual studies is presented in Supplementary Material 6.
What terminology and definitions are used to classify DAIs in the included RCTs? (RQ1)
For the 23 studies (79.3%) evaluating dog-assisted therapy, 20 (69.0%) were assessed as having clear alignment of content and terminology (mental health, n=5; neurodevelopmental, n=9; dementia, n=6) based on internationally accepted definitions (45, 46). One study (3.4%) delivered to adults with schizophrenia was assessed as misaligned, as a member of the research team delivered the sessions, no goals were reported, and the sessions were described as ‘activities’ (50). Lastly, two studies (6.9%) delivered to participants with dementia were assessed as unclear alignment, as limited information was reported on the training/experience of the dog-handler team, and how content was developed to meet goals (58, 61). For those reporting dog-assisted activities, all six (100%) were classed as clear alignment (mental health, n=2; neurodevelopmental, n=1; dementia, n=3). Supplementary Material 7 reports content presented from studies that describe details related to goals/content and the dog-handler team, and whether the study has been assessed as having clear alignment, unclear alignment, or misalignment.
What is the effectiveness of DAIs for populations with mental health and neurodevelopmental conditions? (RQ2)
Studies included a wide range of mental health and behavioural outcome measures (Supplementary Material 4), most commonly evaluating depression (n=14; 48.3%); social skills (n=14; 48.3%); symptom frequency/severity (n=10; 34.5%), and agitation (n=6; 20.7%).
Depression
Fourteen studies (48.3%) reported depression as an outcome (dementia, n=10; mental health, n=3; neurodevelopmental, n=1). The measures used to assess depression varied (Supplementary Material 4), but for participants with dementia, depression was most commonly evaluated using the Cornell Scale for Depression in Dementia (n=5) or the Geriatric Depression Scale (n=3).
Six studies showed a positive impact on depression compared to the control group (dementia, n=5; mental health, n=1) (16, 20, 58, 61, 62, 65). Of these, five evaluated individual or group dog-assisted therapy delivered by a professionally trained animal handler, but only one was accompanied by an experienced therapist (16). The remaining study evaluated group dog-assisted activities delivered by a professionally trained animal handler (65).
Seven studies showed no benefits of DAIs on depression scores compared to the control group (dementia, n=4; mental health, n=2; neurodevelopmental, n=1) (24, 47, 57, 59, 60, 63, 71). Of these, six evaluated group or individual dog-assisted therapy delivered by an experienced therapist and professional animal handler (47, 63, 71), a professional animal handler alone (57, 60), or an experienced clinician trained by an animal handler (24). One evaluated group dog-assisted activities delivered by a staff nurse (59). Lastly, one study aimed to evaluate depression, but post-intervention depression scores were not reported (52).
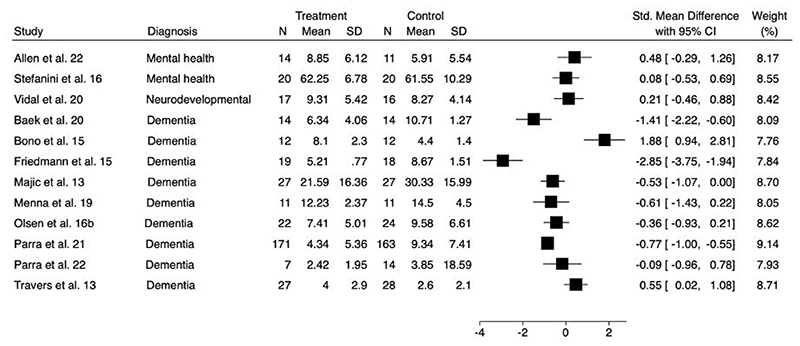
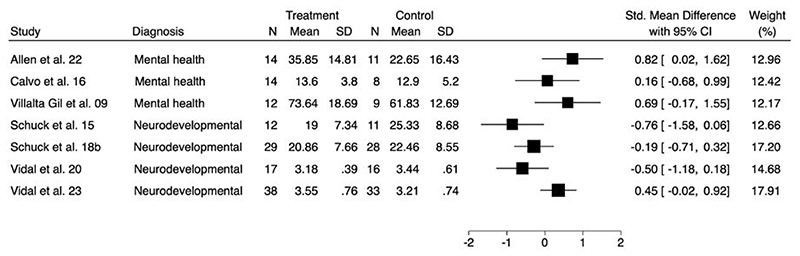
A forest plot to show the comparison of depression at longest follow-up is presented in Figure 3a. As improvement in depressive symptoms was associated with lower scores on all outcome measures, SMDs less than zero indicate improvements for the intervention arm. Of the fourteen studies evaluating this outcome, two trials were excluded as one did not report mean values or standard deviations (47), and one did not provide post-intervention depression scores (52).
Figure 3a. Forest plot for comparison of depression at longest follow-up.
Social skills/behaviour
Fourteen studies (48.3%) evaluated the impact of DAIs on social skills/behaviour using various measures (Supplementary Material 4). Of these, eight studies showed a positive impact of group dog-assisted therapy on social skills/behaviour compared to the control group (neurodevelopmental, n=6; mental health, n=2) (13–15, 49, 55, 56, 67, 71). Interventions in all eight studies were delivered by a professional animal handler, and three of those were accompanied by an experienced therapist or psychologist (14, 56, 71).
Six studies showed no benefits of group or individual dog-assisted therapy on social skills/behaviour compared to the control group (mental health, n=3; neurodevelopmental, n=2; dementia, n=1) (20, 21, 51, 53, 63, 66). Of these, four were delivered by a professional animal handler (20, 51, 53, 66), and one was accompanied by an experienced psychologist (51). Two were delivered by a therapist who had been trained in dog behaviour and welfare, working with either their own accredited dogs (63) or dogs provided by a service dog foundation (21).
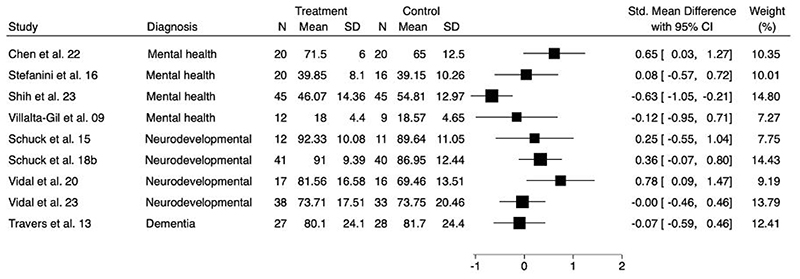
A forest plot to show the comparison of social skills/behaviours at longest follow-up is presented in Figure 3b. As improvement in social skills/behaviour was associated with higher scores on all outcome measures, SMDs higher than zero indicate improvements for the intervention arm. Of the fourteen studies evaluating this outcome, five trials were excluded as post-intervention means and standard deviations were not reported (21, 49, 53, 55, 66).
Figure 3b. Forest plot for comparison of social skills/behaviour at longest follow-up.
Symptom frequency/severity
Ten studies (34.5%) measured changes in symptom frequency/severity. Measures varied by diagnosis (Supplementary Material 4), however, all those delivered to participants with schizophrenia evaluated symptomology using the PANSS, and those delivered to participants with ADHD used the ADHD-Rating-Scale. Five studies showed a positive impact of DAIs on symptom frequency/severity compared to the control group (neurodevelopmental, n=3; mental health, n=2) (13, 15, 47, 68, 71). Of these, four studies evaluated group dog-assisted therapy delivered by a professional animal handler and experienced therapist/psychologist (47, 71) or a professional animal handler alone (13, 15). The remaining study evaluated group dog-assisted activities delivered by a member of the research team (68).
Five studies showed no benefits of dog-assisted therapy on symptom frequency/severity compared to the control group (mental health, n=3; neurodevelopmental, n=2) (24, 50, 51, 56, 70). Of these, one intervention was delivered on an individual basis by experienced therapists who had completed advanced courses in dog behaviour and welfare (21), one was group-based and delivered by a professional animal handler (50), one was delivered to groups and on an individual basis by a professional animal handler and psychologist (56), and two were delivered by experienced clinicians trained by an animal handler to a group or on an individual basis, respectively (24, 51).
A forest plot to show the comparison of symptom frequency/severity at longest follow-up is presented in Figure 3c. As improvement in symptom frequency/severity was associated with lower scores on all outcome measures, SMDs lower than zero indicate improvements for the intervention arm. Of the ten studies evaluating this outcome, three trials were excluded from the forest plot as means and standard deviations were not reported (21, 47, 68).
Figure 3c. Forest plot for comparison of symptom frequency/severity at longest follow-up.
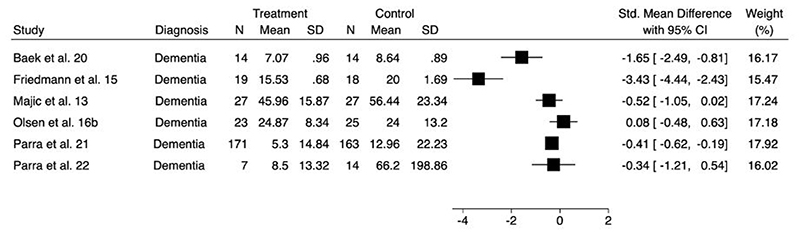
Agitation
Six studies (20.7%) of participants with dementia evaluated the impact of DAIs on agitation using various measures (Supplementary Material 4). Two studies showed a positive impact of dog-assisted therapy compared to the control group (16, 62), and both were delivered by a professional animal handler and experienced therapist.
However, four studies showed no benefits of DAIs on agitation compared to the control group (57, 59, 60, 65). Of these, two evaluated group dog-assisted activities delivered by a professional animal handler (65) or a nurse practitioner (59). Two evaluated dog-assisted therapy delivered by a professional animal handler only, one of which was delivered on a group basis (57), and one did not specify whether the sessions were group-based or on a one-to-one basis (60).
A forest plot to show the comparison of agitation at longest follow-up is presented in Figure 3d. As improvement in agitation was associated with lower scores on all outcome measures, SMDs lower than zero indicate improvements for the intervention arm.
Figure 3d. Forest plot for comparison of agitation at longest follow-up.
In summary, for all of the commonly reported outcomes, findings were mixed. While this is likely due to the small sample sizes, this may also be attributable to the diverse range of DAIs delivered, as they varied considerably by type (therapy or activity), characteristics of provision, such as group size, frequency and duration, and intervention content (Supplementary Material 5). There was also substantial variation in intervention intensity (Table 3), with total intervention intensity (hours) ranging from 0.3 to 48 for participants with mental health conditions; 3 to 54 for participants with neurodevelopmental conditions, and 8 to 70 for participants with dementia.
Table 3. Intervention frequency, duration and intensity for each study, and average intervention intensity for dementia, neurodevelopmental conditions and mental health conditions.
| Author/Year | Duration (weeks) |
Frequency | Session length (minutes) |
Intervention intensity (hours) |
Average intervention intensity (hours) |
|---|---|---|---|---|---|
| Mental health conditions | |||||
| Allen et al. (2021) | 12 | 1 x weekly | 90 | 18 | 14.8 |
| Calvo et al. (2016) | 24 | 2 x weekly | 60 | 48 | |
| Chen et al. (2021; 2022) | 12 | 1 x weekly | 60 | 12 | |
| Chu et al. (2009) | 8 | 1 x weekly | 50 | 7 | |
| Shih et al. (2023) | 12 | 1 x weekly | 60 | 12 | |
| Stefanini et al. (2015) | 12 | 1 x weekly | 45 | 9 | |
| Stefanini et al. (2016) | 12 | 1 x weekly | 45 | 9 | |
| Villalta-Gil et al. (2009) | 12 | 2 x weekly | 45 | 18 | |
| Wolynczyk-Gmaj et al. (2021) | 1 | Once | 20 | 0.3 | |
| Neurodevelopmental disorders | |||||
| Fung et al. (2014) | 7 | 3 x weekly | 20 | 7 | 18.3 |
| Hill et al. (2020) | 9 | 1 x weekly | 60 | 9 | |
| Meints et al. (2022) | 4 | 2 x weekly | 20 | 3 | |
| Scorzato et al. (2017) | 20 | 1 x weekly | 30 | 10 | |
| Schuck et al. (2015) | 12 | 2 x weekly | 120/150 | 54 | |
| Schuck et al. (2018a; 2018b) & Nieforth et al. (2024) |
12 | 2 x weekly | 120/150 | 54 | |
| Vidal et al. (2020) | 12 | 1 x weekly | 45 | 9 | |
| Vidal et al. (2023) | 12 | 1 x weekly | 45 | 9 | |
| Wijker et al. (2020; 2021) | 10 | 1 x weekly | 60 | 10 | |
| Dementia | |||||
| Baek et al. (2020) | 8 | 2 x weekly | 60 | 16 | 25 |
| Bono et al. (2015) | 35 | 2 x weekly | 60 | 70 | |
| Briones et al. (2021) | 39 | 1 x weekly | 50 | 33 | |
| Friedmann et al. (2015) | 12 | 2 x weekly | 90 | 36 | |
| Majic et al. (2013) | 10 | 1 x weekly | 45 | 8 | |
| Menna et al. (2019) | 12 | 1 x weekly | NS | N/A | |
| Olsen et al. (2016a) | 12 | 2 x weekly | 30 | 12 | |
| Olsen et al. (2016b) | 12 | 2 x weekly | 30 | 12 | |
| Parra et al. (2021) | 35 | 1 x weekly | 45 | 26 | |
| Parra et al. (2022) | 26 | 1 x weekly | 45 | 20 | |
| Travers et al. (2015) | 11 | 2 x weekly | 45 | 17 | |
How well reported are RCTs delivering DAIs to people with mental health and neurodevelopmental conditions? (RQ3)
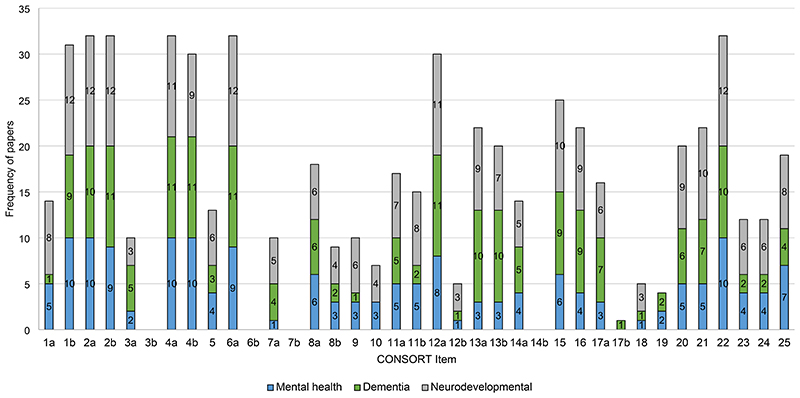
The mean proportion of adherence to the CONSORT statement was calculated at 48.6% with standard deviation = 13.4% (minimum and maximum adherence were 13.51% and 75.7%, respectively). Only nine items were reported in over 75% of the included RCTs. Notably, seventeen papers (51.5%) were published across 13 journals that did not explicitly require authors to follow the CONSORT statement.
Compliance per CONSORT item is presented in Table 4 and Figure 4. Overall, Cohen’s kappa (k) indicated a statistically significant ‘strong’ level of agreement [k = 0.88 (95% CI = 0.53 – 1.23, p < .001]. Cohen’s kappa (k) was also calculated to assess the agreement by CONSORT item (Table 4). Four items were assessed as ‘no’, as they were not applicable to the included RCTs. These included changes to methods after trial commencement, changes to trial outcomes after the trial commenced, explanation of any interim analyses and stopping guidelines, and why the trial ended or was stopped. Additionally, many of the RCTs did not report binary outcomes, so item 17b (presentation of effect sizes for binary outcomes) was not applicable to the majority of the studies (n=23). The lowest scoring item was ‘important harms or unintended effects’ (item 19; n=4, 12.1%).
Table 4. Assessment of the quality of reporting of RCTs using the CONSORT statement.
| Item/Sub-item | Item No |
Checklist | N of RCTs (%) |
Kappa (k) |
Significance (p) |
|---|---|---|---|---|---|
| Title/abstract | |||||
| 1a | Identification as an RCT in the title | 14 (42.4) | 1.00 | 0.001 | |
| 1b | Structured summary of trial design, methods, results, and conclusions | 31 (93.9) | 1.00 | 0.001 | |
| Introduction | |||||
| Background | 2a | Scientific background and explanation of rationale | 32 (96.9) | 1.00 | 0.001 |
| Objectives | 2b | Specific objectives or hypotheses | 32 (96.9) | 1.00 | 0.001 |
| Methods | |||||
| Trial design | 3a | Description of trial design (such as parallel, factorial) including allocation ratio | 10 (30.3) | 0.82 | 0.001 |
| 3b | Important changes to methods after trial commencement (such as eligibility criteria), with reasons | 0 (0) | 1.00 | 0.001 | |
| Participants | 4a | Eligibility criteria for participants | 32 (96.9) | 1.00 | 0.001 |
| 4b | Settings and locations where the data were collected | 30 (90.9) | 1.00 | 0.001 | |
| Interventions | 5 | The interventions for each group with sufficient details to allow replication, including how and when they were actually administered | 13 (39.4) | 0.64 | 0.071 |
| Outcomes | 6a | Completely defined pre-specified primary and secondary outcome measures, including how and when they were assessed | 32 (96.9) | 1.00 | 0.001 |
| 6b | Any changes to trial outcomes after the trial commenced, with reasons | 0 (0) | 1.00 | 0.001 | |
| Sample size | 7a | How sample size was determined | 10 (30.3) | 1.00 | 0.001 |
| 7b | When applicable, explanation of any interim analyses and stopping guidelines | 0 (0) | 1.00 | 0.001 | |
| Randomisation: | 8a | Method used to generate the random allocation sequence | 18 (54.6) | 0.82 | 0.001 |
| Sequence generation | 8b | Type of randomisation; details of any restriction (such as blocking and block size) | 9 (27.3) | 1.00 | 0.001 |
| Randomisation: Allocation concealment mechanism |
9 | Mechanism used to implement the random allocation sequence (such as sequentially numbered containers), describing any steps taken to conceal the sequence until interventions were assigned | 10 (30.3) | 1.00 | 0.001 |
| Randomisation: Implementation |
10 | Who generated the random allocation sequence, who enrolled participants, and who assigned participants to interventions | 7 (21.2) | 1.00 | 0.001 |
| Blinding | 11a | If done, who was blinded after assignment to interventions (for example, participants, care providers, those assessing outcomes) and how | 17 (51.5) | 1.00 | 0.001 |
| 11b | If relevant, description of the similarity of interventions | 15 (45.5) | 0.64 | 0.071 | |
| Statistical methods | 12a | Statistical methods used to compare groups for primary and secondary outcomes | 30 (90.9) | 1.00 | 0.001 |
| 12b | Methods for additional analyses, such as subgroup analyses and adjusted analyses | 5 (15.2) | 1.00 | 0.001 | |
| Results | |||||
| Participant flow | 13a | For each group, the numbers of participants who were randomly assigned, received intended treatment, and were analysed for the primary outcome | 22 (66.7) | 0.93 | 0.001 |
| 13b | For each group, losses and exclusions after randomisation, together with reasons | 20 (60.6) | 1.00 | 0.001 | |
| Recruitment | 14a | Dates defining the periods of recruitment and follow-up | 14 (42.4) | 1.00 | 0.001 |
| 14b | Why the trial ended or was stopped | 0 (0) | 1.00 | 0.001 | |
| Baseline data | 15 | A table showing baseline demographic and clinical characteristics for each group | 25 (75.8) | 0.93 | 0.001 |
| Numbers analysed | 16 | For each group, number of participants (denominator) included in each analysis and whether the analysis was by original assigned groups | 22 (66.7) | 0.93 | 0.001 |
| Outcomes and estimation | 17a | For each primary and secondary outcome, results for each group, and the estimated effect size and its precision (such as 95% confidence interval) | 16 (48.5) | 0.93 | 0.001 |
| 17b | For binary outcomes, presentation of both absolute and relative effect sizes is recommended | 1 (3.0) | 1.00 | 0.001 | |
| Ancillary analyses | 18 | Results of any other analyses performed, including subgroup analyses and adjusted analyses, distinguishing pre-specified from exploratory | 5 (15.2) | 0.82 | 0.001 |
| Harms | 19 | All important harms or unintended effects in each group | 4 (12.1) | 0.93 | 0.001 |
| Discussion | |||||
| Limitations | 20 | Trial limitations, addressing sources of potential bias, imprecision, and, if relevant, multiplicity of analyses | 20 (60.6) | 0.76 | 0.001 |
| Generalisability | 21 | Generalisability (external validity, applicability) of the trial findings | 22 (66.7) | 0.82 | 0.001 |
| Interpretation | 22 | Interpretation consistent with results, balancing benefits and harms, and considering other relevant evidence | 32 (96.9) | 1.00 | 0.001 |
| Other information | |||||
| Registration | 23 | Registration number and name of trial registry | 12 (36.4) | 1.00 | 0.001 |
| Protocol | 24 | Where the full trial protocol can be accessed, if available | 12 (36.4) | 1.00 | 0.001 |
| Funding | 25 | Sources of funding and other support (such as supply of drugs), role of funders | 19 (57.6) | 1.00 | 0.001 |
Figure 4. Graphical presentation of CONSORT compliance per item and by diagnosis category.
For studies that reported important harms or unintended effects, three (10.3%) reported adverse events related to the DAI. These included treatment disrupting events due to the dog (24), participants exhibiting behaviours that threatened to compromise the welfare of the dogs (50), and participants presenting fearful reactions to the dog (60). One study reported an adverse event unrelated to the DAI, indicating an infectious outbreak may have negatively influenced outcomes (63). Although adverse events related to DAIs were only reported in three studies (10.3%), selection criteria for the dogs were reported in 20 studies (69.0%; e.g., free of veterinary infectious diseases; certified in accordance with a national standard; completion of vaccinations; previous participation in DAIs, and appropriate ratings on aptitude and temperament tests). Ten studies (34.5%) specifically reported information about training in varying detail. Eight only reported dogs were trained to work with people (24, 50, 58, 60, 64, 68, 70, 71), whereas two reported information about the dog being trained on specific exercises of the intervention (19, 62). Information on dog safety and welfare was most commonly reported in studies delivering interventions to participants with neurodevelopmental conditions. These studies reported the dog’s working time was limited per day (23, 53, 54, 70), and/or dog welfare and stress behaviours were documented or monitored (23, 54, 55, 70).
Less than half of the RCTs (n=13, 39.4%) adequately reported details relating to the intervention according to the CONSORT statement, and further assessment using the TIDieR checklist indicated considerable variability in intervention reporting (Table 5). Only one of the 33 papers reported all of the information expected (19). Items most likely to achieve a ‘yes’ agreement included intervention name (100%), rationale (100%), procedures and processes (100%), and frequency (100%). Those least likely to achieve a ‘yes’ agreement included items relating to the description of the intervention provider (51.5%), location (51.5%) and materials (18.2%).
Table 5. Frequency of papers achieving ‘yes’, ‘no’ or ‘N/A’ agreement for each TIDieR Checklist Item.
| TIDieR Checklist Item | n | Yes, % (n) |
No, % (n) |
N/A, % (n) |
Kappa (k) |
Significance (p) |
|---|---|---|---|---|---|---|
| Name or phrase describing the intervention | 33 | 100 (33) |
0 (0) |
0 (0) |
1.00 | 0.001 |
| Intervention rationale | 33 | 100 (33) |
0 (0) |
0 (0) |
1.00 | 0.001 |
| Description of intervention materials | 33 | 18.2 (6) |
81.8 (27) |
0 (0) |
1.00 | 0.001 |
| Intervention procedures and processes | 33 | 100 (33) |
0 (0) |
0 (0) |
1.00 | 0.001 |
| Intervention provider | 33 | 51.5 (17) |
48.5 (16) |
0 (0) |
0.93 | 0.001 |
| Mode(s) of delivery | 33 | 87.9 (29) |
12.1 (4) |
0 (0) |
1.00 | 0.001 |
| Intervention location | 33 | 51.5 (17) |
48.5 (16) |
0 (0) |
1.00 | 0.001 |
| Intervention frequency | 33 | 100 (33) |
0 (0) |
0 (0) |
1.00 | 0.001 |
| If undertaken, tailoring of the intervention | 33 | 6.1 (2) |
30.3 (10) |
63.6 (21) |
0.74 | 0.005 |
| If undertaken, modification of the intervention | 0 | N/A | N/A | N/A | N/A | N/A |
| How intervention adherence or fidelity assessed where appropriate | 33 | 12.2 (4) |
3.0 (1) |
84.8 (28) |
1.00 | 0.001 |
| If undertaken, actual intervention adherence or fidelity | 33 | 15.2 (5) |
0 (0) |
84.8 (28) |
1.00 | 0.001 |
Overall, Cohen’s kappa (k) indicated a statistically significant ‘almost perfect’ level of agreement [k = 0.99 (95% CI = 0.97 – 1.01, p < .001]. Cohen’s kappa (k) was also calculated to assess the agreement by TIDieR checklist item (Table 5).
Discussion
The aims of this review were to synthesise findings of published research to determine whether DAIs are effective for people with mental health or neurodevelopmental conditions and to formally assess the quality of reporting and use of terminology in RCTs for the first time. Findings for the effectiveness of DAIs across outcome categories were mixed, as determined by using direction of effect and forest plots. However, they clearly signal promise and point towards opportunities to improve future research in this area (e.g., the development of guidelines for clear terminology and reporting standards, and the conduct of rigorous RCTs with larger sample sizes to ensure studies are adequately powered). Due to small sample sizes, heterogeneity of study quality, outcome measures and variation in the types of DAIs provided (in terms of content and delivery), it is challenging or impossible to interpret results in terms of DAIs’ promise for a specific population. However, taking into consideration the three core outcome groups (depression, social skills and agitation, recognising that symptom frequency/severity is not symptom specific), it is evident that 57% (8/14) of the studies reported a positive outcome of DAI for social skills, 43% (6/14) for depression, and 33% (2/6) for agitation. Of 14 studies evaluating social skills, only two were rated as overall high quality (56, 71), both of which reported positive outcomes. Of the 14 studies evaluating depression, only one study was rated as overall high quality (24), and no benefits of the DAI were reported. No studies evaluating agitation were rated as overall high quality.
Without delving more deeply into potential mechanistic pathways, which is beyond the scope of this review, it could therefore be tentatively proposed that DAIs show particular promise for conditions that might benefit from social skill support. It will be crucial for future research to investigate potential mechanisms of action for DAIs (and AAIs in general) in greater detail, so these can be closely linked to specific outcome measures and populations, including hypotheses involving longer-term impact beyond intervention completion. It would be important to justify any hypotheses more rigorously according to which symptoms of mood disorders (e.g., depression/anxiety), would still be improved six months post-intervention, considering any mediating or ‘catalysing’ factors, such as improved engagement and rapport building facilitated by the DAI compared to standard care.
Methodological considerations
While improvements were found for depression, social skills/behaviour, symptom frequency/severity and agitation in some RCTs, other trials did not find benefits of DAIs on these outcomes. Although the quality of the evidence base is improving, there is largely an absence of rigorous methodology to enable demonstration of the potential effectiveness of DAIs. For example, the studies frequently included small or very small sample sizes, rendering studies inadequately powered to detect potential differences in effect sizes between study groups and likely undermining the internal and external validity of the studies (72). Other examples of limited rigour include generally short or no follow-up periods for assessing outcomes, and an overall high risk of bias assessed for the vast majority of included studies (n=25).
Additionally, there are several limitations to be addressed in relation to generalisability to our study population groups. For children and adolescents with neurodevelopmental conditions, females were notably underrepresented (n=85, 26.5%). This could be attributed to the fact males are more likely to be diagnosed with ASC or ADHD than females (73, 74), and future research should aim to include more female participants to adequately reflect the population of children and young people with neurodevelopmental conditions (75). Secondly, only five studies reported information regarding participant ethnicity. The collection and reporting of ethnicity data is essential for understanding the generalisability of findings and the likely impact of an intervention for particular ethnic groups (76). Likewise, studies providing information regarding the severity of a participant’s condition was only reported for those with dementia or schizophrenia. As severity is not consistently reported, it cannot be determined if the effects of DAIs are attributed to the intervention or the severity of the condition (77), and this limitation has been highlighted in previous systematic reviews exploring AAIs for ASC (75) and schizophrenia (78).
Although our findings cannot offer definitive conclusions about the effectiveness of DAIs for our study population groups, they clearly signal promise of DAIs to improve a variety of psychosocial outcomes, aligning with findings from previous observational studies (79–82). Recent evidence syntheses also highlight promise for DAIs in improving outcomes for a range of mental health and neurodevelopmental conditions, such as schizophrenia (78), mental health conditions (8, 83, 84), PTSD and trauma (9, 85), ASC (11, 34), and ADHD (86). Despite this, evidence syntheses unanimously emphasise the need for more rigorous and sufficiently powered RCTs (25, 78, 87, 88) to determine the true impact of DAIs for these populations. However, the focus on determining effectiveness raises an important issue: reporting of RCTs of DAIs is often insufficiently accurate, comprehensive and transparent. For example, authors often did not report data on intervention implementation (e.g., adaptation/tailoring of the intervention to specific groups or materials, materials used to support intervention implementation). Inadequate reporting can make it challenging for researchers to replicate trials, for intervention developers to design effective interventions, and for providers to implement interventions in practice (89). A lack of sharing protocols, outcome data, and intervention materials has been identified as a possible reason for limitations in the ability of human-animal interaction researchers to reproduce trial procedures, replicate trial results, and effectively synthesise evidence on these interventions (90).
This review also revealed that many CONSORT items were poorly reported in the DAI literature. Such items included descriptions of trial design; information about how the sample size was determined; randomisation information, and important harms or unintended effects in each group. Only 14 of 27 journals included referenced reporting guidelines in ‘Instructions to Authors’. This inefficient use of resources for research likely contributes to the suboptimal dissemination of potentially effective interventions and overestimations of intervention efficacy. As in other areas of research, transparent, detailed and adequately subject-specific reporting of DAI RCTs is needed to minimise reporting biases and maximise the credibility and utility of this research evidence (91).
Beyond effectiveness
It is important to extend this focus beyond ‘what works’ and consider ‘under what circumstances and how these interventions work’ (92). The effect of complex DAIs (or AAIs generally), which involve poorly understood interspecies interactions between several actors including a dog, may depend on elements of difficult-to-control, dynamic systems in which they occur (93). For example, aspects related to the physical environment in which interventions take place, which may vary greatly between or even within study settings but may have substantial effects on the dogs involved; considerations relating to ‘matching’ dogs and participants, and the role of all actors involved (participant, handler and/or therapist) would be important to investigate. To unlock the true potential of DAIs (and AAIs generally) in the future, it will be crucial to complement evidence from applied intervention research with findings from well-designed and well-conducted observational studies focused on exploring layers of AAIs/DAIs (such as mechanistic impact-outcome pathways; environmental aspects; the role of all actors, and interspecies reciprocity) (94) that have so far received little attention but will be fundamental in advancing this promising area. Future research needs to explore how and why these interventions work, for whom, and under what conditions (95). Interdisciplinary mixed-method research and process evaluations conducted alongside outcome evaluations could facilitate our understanding of how DAIs may work and highlight issues that may impact effectiveness in real-world settings.
Intervention terminology, practice and reporting
Despite expansion of practice, inconsistencies remain in how DAIs are described, practiced, and reported upon within the evidence base (32). While most DAIs described in studies in this review were assessed as having clear alignment for content and terminology, improvement in reporting certain information was still required (e.g., training of the dog-handler team, measures used to assess dog aptitude, temperament and behaviour, access to intervention materials to identify how content was developed to align with goals, in the case of therapy). The absence of this detailed information makes it challenging to ascertain the preparation, training, and expectations of the handler and the dogs that work in different roles. Recent research has argued these difficulties may have hindered the development of the field in terms of establishing agreed standards of practice, qualifications and competencies, and adopting good animal welfare practices (32). As a result, new uniform terminology has been suggested to improve clarify for those involved in the delivery and receipt of DAIs (32). This review uses original terminology to be consistent with the taxonomy and definitions reported in the included RCTs. Seeing the extensive variety of intervention content, engagement and delivery modalities reported for DAIs (Supplementary Material 5), future work could usefully focus on efforts to classify further subtypes of DAIs, building on the classification by Binder et al. (32), specifying the role of the dog and type of intervention content. This would allow future evidence syntheses to summarise study findings more specifically in relation to the effectiveness of ‘DAI types’ for specific populations and would further facilitate our understanding of what works for whom under what circumstances.
Limitations
Firstly, the clinical and methodological heterogeneity did not allow for meta-analyses to definitively determine the benefits of DAIs for participants with mental health or neurodevelopmental conditions. Analyses to separate studies by those evaluating dog-assisted therapy and those evaluating dog-assisted activities was considered. However, due to the number imbalance, this was not possible. For example, only two of the 14 studies evaluating depression were dog-assisted activities (compared to 12 of which were therapy). For studies evaluating symptom frequency/severity, only one of 10 studies evaluated dog-assisted activity, and all studies evaluating social skills delivered dog-assisted therapy only. Therefore, the effectiveness results should be interpreted with some caution. Secondly, while this review aimed to determine the effectiveness of DAIs for individuals with neurodevelopmental and mental health conditions across all age groups, a significant proportion of the studies included focused on older participants with dementia. Subsequently, the findings related to depression and agitation are not generalisable to younger populations with mental health or neurodevelopmental conditions. Future research targeting these subgroups is required to clarify the impact of DAIs across diverse age ranges and conditions. Lastly, included papers were those published in English, non-English language studies may have contributed to further understanding.
Conclusion
The implementation of DAIs for a wide range of mental health and neurodevelopmental conditions has been rapidly increasing in practice. The existing body of evidence indicates that DAIs may be promising for improving mental health and behavioural outcomes for these population groups, possibly specifically for conditions that benefit from improving social skills, however, the current literature has considerable methodological concerns. There remains significant room for improvement in relation to the design and reporting of DAI RCTs, with potential to develop DAI (or AAI)-specific extensions to existing guidelines. Further rigorous interdisciplinary research is required to help advance research in this field.
Supplementary Material
Funding Statement
This review is funded by the National Institute for Health Research (NIHR) Programme Development Grants (Reference: NIHR205656). ES, JP, HS, JR, DMc, QW & ER are supported by the NIHR Yorkshire and Humber Applied Research Collaboration: https://www.arc-yh.nihr.ac.uk. The views expressed are those of the authors, and not necessarily those of the NIHR.
Footnotes
Author Contributions: Conceptualisation: E.S., S.H and E.R.; methodology: E.S., S.H., A.M., H.S., J.P. and E.R.; software: E.S., J.P and H.S.; formal analysis: E.S., J.P. and H.S.; investigation: E.S., J.P., H.S., and E.R.; resources: E.S., S.H., A.S., H.S., J.P., J.R., D.Mc, D.M., C.C., Q.W., S.G., and E.R.; data curation: E.S., J.P., H.S., S.H., and E.R., writing—original draft preparation: E.S., writing—review and editing: E.S., S.H., A.S., H.S., J.P., J.R., D.Mc, D.M., C.C., Q.W., S.G., and E.R.; visualisation: E.S., S.H., A.S., H.S., J.P., J.R., D.Mc, D.M., C.C., Q.W., S.G., and E.R.; supervision: S.H., A.M., D.Mc., D.M., E.R.; project administration: E.S., S.H., H.S., J.P., and E.R. All authors have read and agreed to the published version of the manuscript.
Declaration of Interest: The authors declare no conflict of interest.
Data Availability
Data availability is not applicable to this article as no new data were created or analysed in this study.
References
- 1.Patel V, Saxena S, Lund C, Thornicroft G, Baingana F, Bolton P, et al. The Lancet Commission on global mental health and sustainable development. The lancet. 2018;392(10157):1553–98. doi: 10.1016/S0140-6736(18)31612-X. [DOI] [PubMed] [Google Scholar]
- 2.Organisation WH. Comprehensive Mental Health Action Plan 2013-2030. World Health Organisation; Geneva: 2021. [Google Scholar]
- 3.Stein DJ, Szatmari P, Gaebel W, Berk M, Vieta E, Maj M, et al. Mental, behavioral and neurodevelopmental disorders in the ICD-11: an international perspective on key changes and controversies. BMC Medicine. 2020;18(1):21. doi: 10.1186/s12916-020-1495-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.World Health Organization. Survive, thrive, transform. World Health Organization; Geneva: 2016. Global strategy for women’s, children’s and adolescents’ health (2016–2030) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.World Health Organization. Nurturing care for early childhood development: A framework for helping children survive and thrive to transform health and human potential. World Health Organization; Geneva: 2018. [Google Scholar]
- 6.World Health Organization. Comprehensive Mental Health Action Plan 2013-2030. 2021. Available from: https://www.who.int/publications/i/item/9789240031029.
- 7.World Health Organization. Autism. 2023. Available from: https://www.who.int/news-room/fact-sheets/detail/autism-spectrum-disorders.
- 8.Jones MG, Rice SM, Cotton SM. Incorporating animal-assisted therapy in mental health treatments for adolescents: A systematic review of canine assisted psychotherapy. PLoS One. 2019;14(1):e0210761. doi: 10.1371/journal.pone.0210761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hediger K, Wagner J, Künzi P, Haefeli A, Theis F, Grob C, et al. Effectiveness of animal-assisted interventions for children and adults with post-traumatic stress disorder symptoms: a systematic review and meta-analysis. Eur J Psychotraumatol. 2021;12(1):1879713. doi: 10.1080/20008198.2021.1879713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hüsgen CJ, Peters-Scheffer NC, Didden R. A systematic review of dog-assisted therapy in children with behavioural and developmental disorders. Advances in Neurodevelopmental Disorders. 2022:1–10. [Google Scholar]
- 11.Nieforth LO, Schwichtenberg A, O’Haire ME. Animal-Assisted Interventions for Autism Spectrum Disorder: A Systematic Review of the Literature from 2016 to 2020. Review Journal of Autism and Developmental Disorders. 2021:1–26. doi: 10.1007/s40489-021-00291-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nimer J, Lundahl B. Animal-assisted therapy: A meta-analysis. Anthrozoos. 2007;20(3):225–38. [Google Scholar]
- 13.Schuck SEB, Emmerson NA, Fine AH, Lakes KD. Canine-assisted therapy for children with ADHD: Preliminary findings from the Positive Assertive Cooperative Kids study. Journal of Attention Disorders. 2015;19(2):125–37. doi: 10.1177/1087054713502080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen CR, Hung CF, Lee YW, Tseng WT, Chen ML, Chen TT. Functional Outcomes in a Randomized Controlled Trial of Animal-Assisted Therapy on Middle-Aged and Older Adults with Schizophrenia. International Journal of Environmental Research and Public Health. 2022;19(10):6270. doi: 10.3390/ijerph19106270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schuck SE, Emmerson NA, Abdullah MM, Fine AH, Stehli A, Lakes KD. A Randomized Controlled Trial of Traditional Psychosocial and Canine-Assisted Interventions for Children with ADHD. CABI International; 2018. p. 16. [Google Scholar]
- 16.Parra E, Manuel Hernández Garre J, Echevarría Pérez P. Impact of Dog-Assisted Therapy for Institutionalized Patients With Dementia: A Controlled Clinical Trial. Alternative Therapies in Health & Medicine. 2022;28(1):26–31. [PubMed] [Google Scholar]
- 17.Fine AH. Handbook on animal-assisted therapy: Theoretical foundations and guidelines for practice. academic press; 2010. [Google Scholar]
- 18.Briones MA, Pardo-Garcia I, Escribano-Sotos F. Effectiveness of a dog-assisted therapy program to enhance quality of life in institutionalized dementia patients. Clinical Nursing Research. 2021;30(1):89–97. doi: 10.1177/1054773819867250. [DOI] [PubMed] [Google Scholar]
- 19.Hill J, Ziviani J, Driscoll C, Teoh AL, Chua JM, Cawdell-Smith J. Canine Assisted Occupational Therapy for Children on the Autism Spectrum: A Pilot Randomised Control Trial. Journal of Autism and Developmental Disorders. 2020;50(11):4106–20. doi: 10.1007/s10803-020-04483-7. [DOI] [PubMed] [Google Scholar]
- 20.Stefanini MC, Martino A, Bacci B, Tani F. The effect of animal-assisted therapy on emotional and behavioral symptoms in children and adolescents hospitalized for acute mental disorders. European Journal of Integrative Medicine. 2016;8(2):81–8. [Google Scholar]
- 21.Wijker C, Leontjevas R, Spek A, Enders-Slegers M-J. Effects of dog assisted therapy for adults with autism spectrum disorder: An exploratory randomized controlled trial. Journal of autism and developmental disorders. 2020;50(6):2153–63. doi: 10.1007/s10803-019-03971-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stefanini MC, Martino A, Allori P, Galeotti F, Tani F. The use of Animal-Assisted Therapy in adolescents with acute mental disorders: A randomized controlled study. Complementary therapies in clinical practice. 2015;21(1):42–6. doi: 10.1016/j.ctcp.2015.01.001. [DOI] [PubMed] [Google Scholar]
- 23.Wijker C, Kupper N, Leontjevas R, Spek A, Enders-Slegers MJ. The effects of Animal Assisted Therapy on autonomic and endocrine activity in adults with autism spectrum disorder: A randomized controlled trial. General Hospital Psychiatry. 2021;72:36–44. doi: 10.1016/j.genhosppsych.2021.05.003. [DOI] [PubMed] [Google Scholar]
- 24.Allen B, Shenk CE, Dreschel NE, Wang M, Bucher AM, Desir MP, et al. Integrating Animal-Assisted Therapy Into TF-CBT for Abused Youth With PTSD: A Randomized Controlled Feasibility Trial. Child maltreatment. 2022;27(3):466–77. doi: 10.1177/1077559520988790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zafra-Tanaka JH, Pacheco-Barrios K, Tellez WA, Taype-Rondan A. Effects of dog-assisted therapy in adults with dementia: a systematic review and meta-analysis. BMC Psychiatry. 2019;19(1):41. doi: 10.1186/s12888-018-2009-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hill J, Ziviani J, Driscoll C, Cawdell-Smith J. Can canine-assisted interventions affect the social behaviours of children on the autism spectrum? A systematic review. Review Journal of Autism and Developmental Disorders. 2019;6(1):13–25. [Google Scholar]
- 27.Shoesmith E, Surr C, Ratschen E. Animal-assisted and robotic animal-assisted interventions within dementia care: A systematic review. Dementia. 2023:14713012231155985. doi: 10.1177/14713012231155985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Santaniello A, Dicé F, Claudia Carratú R, Amato A, Fioretti A, Menna LF. Methodological and terminological issues in animal-assisted interventions: an umbrella review of systematic reviews. Animals. 2020;10(5):759. doi: 10.3390/ani10050759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Santaniello A, Garzillo S, Cristiano S, Fioretti A, Menna LF. The research of standardized protocols for dog involvement in animal-assisted therapy: a systematic review. Animals. 2021;11(9):2576. doi: 10.3390/ani11092576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fine AH, Beck AM, Ng Z. The state of animal-assisted interventions: addressing the contemporary issues that will shape the future. International journal of environmental research and public health. 2019;16(20):3997. doi: 10.3390/ijerph16203997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Serpell J, McCune S, Gee N, Griffin JA. Current challenges to research on animal-assisted interventions. Applied Developmental Science. 2017;21(3):223–33. doi: 10.1080/10888691.2016.1243988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Binder AJ, Parish-Plass N, Kirby M, Winkle M, Skwerer DP, Ackerman L, et al. Recommendations for uniform terminology in animal-assisted services (AAS. Human-Animal Interactions. 2024 [Google Scholar]
- 33.Jain B, Syed S, Hafford-Letchfield T, O’Farrell-Pearce S. Dog-assisted interventions and outcomes for older adults in residential long-term care facilities: A systematic review and meta-analysis. Int J Older People Nurs. 2020;15(3):e12320. doi: 10.1111/opn.12320. [DOI] [PubMed] [Google Scholar]
- 34.Dimolareva M, Dunn TJ. Animal-Assisted Interventions for School-Aged Children with Autism Spectrum Disorder: A Meta-Analysis. J Autism Dev Disord. 2021;51(7):2436–49. doi: 10.1007/s10803-020-04715-w. [DOI] [PubMed] [Google Scholar]
- 35.Altman DG, Schulz KF, Moher D, Egger M, Davidoff F, Elbourne D, et al. The revised CONSORT statement for reporting randomized trials: explanation and elaboration. Annals of internal medicine. 2001;134(8):663–94. doi: 10.7326/0003-4819-134-8-200104170-00012. [DOI] [PubMed] [Google Scholar]
- 36.Hoffmann TC, Glasziou PP, Boutron I, Milne R, Perera R, Moher D, et al. Better reporting of interventions: template for intervention description and replication (TIDieR) checklist and guide. Bmj. 2014;348 doi: 10.1136/bmj.g1687. [DOI] [PubMed] [Google Scholar]
- 37.Moher DL, Tetzlaff AJ, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(6):e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Saaiq M, Ashraf B. Modifying “Pico” Question into “Picos” Model for More Robust and Reproducible Presentation of the Methodology Employed in A Scientific Study. World J Plast Surg. 2017;6(3):390–2. [PMC free article] [PubMed] [Google Scholar]
- 39.Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. Bmj. 2011;343 doi: 10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Manager R. 5.3 ed The Nordic Cochrane Centre, The Cochrane Collaboration; Copenhagen: 2014. [Google Scholar]
- 41.Higgins J, Green S. Cochrane Handbook for Systematic Reviews of Interventions. 2011. Available from: https://handbook-5-1.cochrane.org/
- 42.Schulz KF, Altman DG, Moher D. CONSORT 2010 statement: updated guidelines for reporting parallel group randomised trials. Trials. 2010;11(1):1–8. doi: 10.1186/1745-6215-11-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.McHugh ML. Interrater reliability: the kappa statistic. Biochem Med (Zagreb) 2012;22(3):276–82. [PMC free article] [PubMed] [Google Scholar]
- 44.Corp I. IBM SPSS Statistics (Version 280) 2020.
- 45.Fine AH, editor. International Association of Human Animal Interaction Organisations. Handbook of animal assisted therapy: foundations and guidelines for animal-assisted interventions. 4th ed. Elsevier; 2015. The IAHAIO definitions for animal-assisted intervention and guidelines for wellness of animals involved; p. 416. [Google Scholar]
- 46.Animal-Assisted Intervention International. Animal-Assisted Intervention. 2020. Available from: https://aai-int.org/aai/animal-assisted-intervention/
- 47.Chen TT, Hsieh TL, Chen ML, Tseng WT, Hung CF, Chen CR. Animal-Assisted Therapy in Middle-Aged and Older Patients With Schizophrenia: A Randomized Controlled Trial. Frontiers in Psychiatry. 2021;12:713623. doi: 10.3389/fpsyt.2021.713623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schuck SEB, Johnson HL, Abdullah MM, Stehli A, Fine AH, Lakes KD. The Role of Animal Assisted Intervention on Improving Self-Esteem in Children With Attention Deficit/Hyperactivity Disorder. FRONTIERS IN PEDIATRICS. 2018;6 doi: 10.3389/fped.2018.00300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nieforth LO, Guerin NA, Stehli A, Schuck S, Yi K, O’Haire ME. Observation of human-animal interaction for research (OHAIRE) behavior coding in a randomized control trial of children with attention-deficit hyperactivity disorder (ADHD) and a canine-assisted intervention. Frontiers in Psychiatry. 2024;15 doi: 10.3389/fpsyt.2024.1327380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Calvo P, Fortuny JR, Guzmán S, Macías C, Bowen J, García ML, et al. Animal assisted therapy (AAT) program as a useful adjunct to conventional psychosocial rehabilitation for patients with schizophrenia: results of a small-scale randomized controlled trial. Frontiers in psychology. 2016;7:631. doi: 10.3389/fpsyg.2016.00631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Villalta-Gil V, Roca M, Gonzalez N, Domenec E, Escanilla A, et al. Dog-assisted therapy in the treatment of chronic schizophrenia inpatients. Anthrozoos. 2009;22(2):149–59. [Google Scholar]
- 52.Wolynczyk-Gmaj D, Ziolkowska A, Rogala P, Scigala D, Bryla L, Gmaj B, et al. Can dog-assisted intervention decrease anxiety level and autonomic agitation in patients with anxiety disorders? Journal of Clinical Medicine. 2021;10(21):5171. doi: 10.3390/jcm10215171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fung S-c, Leung AS-m. Pilot study investigating the role of therapy dogs in facilitating social interaction among children with autism. Journal of Contemporary Psychotherapy: On the Cutting Edge of Modern Developments in Psychotherapy. 2014;44(4):253–62. [Google Scholar]
- 54.Meints K, Brelsford VL, Dimolareva M, Marechal L, Pennington K, Rowan E, et al. Can dogs reduce stress levels in school children? effects of dog-assisted interventions on salivary cortisol in children with and without special educational needs using randomized controlled trials. PLoS ONE. 2022 June;17(6):e0269333. doi: 10.1371/journal.pone.0269333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Scorzato I, Zaninotto L, Romano M, Menardi C, Cavedon L, Pegoraro A, et al. Effects of Dog-Assisted Therapy on Communication and Basic Social Skills of Adults With Intellectual Disabilities: A Pilot Study. Intellectual and developmental disabilities. 2017;55(3):125–39. doi: 10.1352/1934-9556-55.3.125. [DOI] [PubMed] [Google Scholar]
- 56.Vidal R, Vidal L, Lugo J, Ristol F, Domenec E, Casas T, et al. Dog-Assisted Therapy vs Relaxation for Children and Adolescents with Fetal Alcohol Spectrum Disorder: A Randomized Controlled Study. Journal of Autism and Developmental Disorders. 2023 doi: 10.1007/s10803-023-06023-5. [DOI] [PubMed] [Google Scholar]
- 57.Baek SM, Lee Y, Sohng KY. The psychological and behavioural effects of an animal-assisted therapy programme in Korean older adults with dementia. Psychogeriatrics. 2020;20(5):645–53. doi: 10.1111/psyg.12554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bono AV, Benvenuti C, Buzzi M, Ciatti R, Chiarelli V, Chiambretto P, et al. Effects of animal assisted therapy (AAT) carried out with dogs on the evolution of mild cognitive impairment. Giornale di Gerontologia. 2015;63(1):32–6. [Google Scholar]
- 59.Friedmann E, Galik E, Thomas SA, Hall PS, Chung SY, McCune S. Evaluation of a pet-assisted living intervention for improving functional status in assisted living residents with mild to moderate cognitive impairment: A pilot study. American Journal of Alzheimer’s Disease and other Dementias. 2015;30(3):276–89. doi: 10.1177/1533317514545477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Majic T, Gutzmann H, Heinz A, Lang UE, Rapp MA. Animal-assisted therapy and agitation and depression in nursing home residents with dementia: A matched caseecontrol trial. American Journal of Geriatric Psychiatry. 2013;21(11):1052–9. doi: 10.1016/j.jagp.2013.03.004. [DOI] [PubMed] [Google Scholar]
- 61.Menna LF, Santaniello A, Gerardi F, Sansone M, Di Maggio A, Di Palma A, et al. Efficacy of animal-assisted therapy adapted to reality orientation therapy: measurement of salivary cortisol. Psychogeriatrics. 2019;19(5):510–2. doi: 10.1111/psyg.12418. [DOI] [PubMed] [Google Scholar]
- 62.Parra EV, Garre JMH, Perez PE. Benefits of dog-assisted therapy in patients with dementia residing in aged care centers in Spain. International Journal of Environmental Research and Public Health. 2021;18(4):1–12. doi: 10.3390/ijerph18041471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Travers C, Perkins J, Rand J, Bartlett H, Morton J. An evaluation of dog-assisted therapy for residents of aged care facilities with dementia. Anthrozoos. 2013;26(2):213–25. [Google Scholar]
- 64.Olsen C, Pedersen I, Bergland A, Enders-Slegers MJ, Ihlebak C. Effect of animal-assisted activity on balance and quality of life in home-dwelling persons with dementia. Geriatric nursing (New York, NY) 2016;37(4):284–91. doi: 10.1016/j.gerinurse.2016.04.002. [DOI] [PubMed] [Google Scholar]
- 65.Olsen C, Pedersen I, Bergland A, Enders-Slegers MJ, Patil G, Ihlebaek C. Effect of animal-assisted interventions on depression, agitation and quality of life in nursing home residents suffering from cognitive impairment or dementia: a cluster randomized controlled trial. International Journal of Geriatric Psychiatry. 2016;31(12):1312–21. doi: 10.1002/gps.4436. [DOI] [PubMed] [Google Scholar]
- 66.Stefanini MC, Martino A, Allori P, Galeotti F, Tani F. The use of Animal-Assisted Therapy in adolescents with acute mental disorders: A randomized controlled study. Complement Ther Clin Pract. 2015;21(1):42–6. doi: 10.1016/j.ctcp.2015.01.001. [DOI] [PubMed] [Google Scholar]
- 67.Shih CA, Yang MH. Effect of Animal-Assisted Therapy (AAT) on Social Interaction and Quality of Life in Patients with Schizophrenia during the COVID-19 Pandemic: An Experimental Study. Asian Nurs Res (Korean Soc Nurs Sci) 2023;17(1):37–43. doi: 10.1016/j.anr.2023.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Chu C, Liu C, Sun C, Lin J. The effect of animal-assisted activity on inpatients with schizophrenia. Journal of Psychosocial Nursing & Mental Health Services. 2009;47(12):42–8. doi: 10.3928/02793695-20091103-96. [DOI] [PubMed] [Google Scholar]
- 69.Briones MA, Pardo-Garcia I, Escribano-Sotos F. Effectiveness of a Dog-Assisted Therapy Program to Enhance Quality of Life in Institutionalized Dementia Patients. Clinical nursing research. 2019:1054773819867250. doi: 10.1177/1054773819867250. [DOI] [PubMed] [Google Scholar]
- 70.Wijker C, Leontjevas R, Spek A, Enders-Slegers MJ. Effects of Dog Assisted Therapy for Adults with Autism Spectrum Disorder: An Exploratory Randomized Controlled Trial. Journal of Autism and Developmental Disorders. 2020;50(6):2153–63. doi: 10.1007/s10803-019-03971-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Vidal R, Vidal L, Ristol F, Domenec E, Segu M, Vico C, et al. Dog-assisted therapy for children and adolescents with fetal alcohol spectrum disorders a randomized controlled pilot study. Frontiers in Psychology. 2020;11 doi: 10.3389/fpsyg.2020.01080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Faber J, Fonseca LM. How sample size influences research outcomes. Dental press journal of orthodontics. 2014;19:27–9. doi: 10.1590/2176-9451.19.4.027-029.ebo. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Martin J. Why are females less likely to be diagnosed with ADHD in childhood than males? The Lancet Psychiatry. 2024;11(4):303–10. doi: 10.1016/S2215-0366(24)00010-5. [DOI] [PubMed] [Google Scholar]
- 74.Gould J. Towards understanding the under-recognition of girls and women on the autism spectrum. Autism. 2017;21(6):703–5. doi: 10.1177/1362361317706174. [DOI] [PubMed] [Google Scholar]
- 75.Rehn AK, Caruso VR, Kumar S. The effectiveness of animal-assisted therapy for children and adolescents with autism spectrum disorder: A systematic review. Complementary Therapies in Clinical Practice. 2023;50:101719. doi: 10.1016/j.ctcp.2022.101719. [DOI] [PubMed] [Google Scholar]
- 76.Gaias LM, Duong MT, Pullmann MD, Brewer SK, Smilansky M, Halbert M, et al. Race and ethnicity in educational intervention research: A systematic review and recommendations for sampling, reporting, and analysis. Educational Research Review. 2020;31:100356 [Google Scholar]
- 77.Becker JL, Rogers EC, Burrows B. Animal-assisted social skills training for children with autism spectrum disorders. Anthrozoös. 2017;30(2):307–26. [Google Scholar]
- 78.Hawkins EL, Hawkins RD, Dennis M, Williams JM, Lawrie SM. Animal-assisted therapy for schizophrenia and related disorders: A systematic review. Journal of Psychiatric Research. 2019;115:51–60. doi: 10.1016/j.jpsychires.2019.05.013. [DOI] [PubMed] [Google Scholar]
- 79.Lutwack-Bloom P, Wijewickrama R, Smith B. Effects of pets versus people visits with nursing home residents. Journal of Gerontological Social Work. 2005;44(3/4):137–59. [Google Scholar]
- 80.Ávila-Álvarez A, Alonso-Bidegain M, De-Rosende-Celeiro I, Vizcaíno-Cela M, Larrañeta-Alcalde L, Torres-Tobío G. Improving social participation of children with autism spectrum disorder: Pilot testing of an early animal-assisted intervention in Spain. Health & Social Care in the Community. 2020;28(4):1220–9. doi: 10.1111/hsc.12955. [DOI] [PubMed] [Google Scholar]
- 81.Morales-Moreno I, Cerezo-Chuecos F, Balanza-Galindo S, Gómez-Díaz M, Echevarría-Pérez P. Implementation of Assisted Therapy With Dogs in the Therapeutic Approach to People With Autistic Spectrum Disorder. Holistic Nursing Practice. 2020;34(5) doi: 10.1097/HNP.0000000000000403. [DOI] [PubMed] [Google Scholar]
- 82.Signal T, Taylor N, Prentice K, McDade M, Burke KJ. Going to the dogs: A quasi-experimental assessment of animal assisted therapy for children who have experienced abuse. Applied developmental science. 2017;21(2):81–93. [Google Scholar]
- 83.Hoagwood KE, Acri M, Morrissey M, Peth-Pierce R. Animal-Assisted Therapies for Youth with or at risk for Mental Health Problems: A Systematic Review. Appl Dev Sci. 2017;21(1):1–13. doi: 10.1080/10888691.2015.1134267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Yakimicki ML, Edwards NE, Richards E, Beck AM. Animal-assisted intervention and dementia: A systematic review. Clinical nursing research. 2019;28(1):9–29. doi: 10.1177/1054773818756987. [DOI] [PubMed] [Google Scholar]
- 85.Germain SM, Wilkie KD, Milbourne VM, Theule J. Animal-assisted psychotherapy and trauma: A meta-analysis. Anthrozoös. 2018;31(2):141–64. [Google Scholar]
- 86.Pérez-Gómez J, Amigo-Gamero H, Collado-Mateo D, Barrios-Fernandez S, Muñoz-Bermejo L, Garcia-Gordillo M, et al. Equine-assisted activities and therapies in children with attention-deficit/hyperactivity disorder: A systematic review. J Psychiatr Ment Health Nurs. 2021;28(6):1079–91. doi: 10.1111/jpm.12710. [DOI] [PubMed] [Google Scholar]
- 87.Batubara SO, Tonapa SI, Saragih ID, Mulyadi M, Lee B-O. Effects of animal-assisted interventions for people with dementia: A systematic review and meta-analysis. Geriatric Nursing. 2022;43:26–37. doi: 10.1016/j.gerinurse.2021.10.016. [DOI] [PubMed] [Google Scholar]
- 88.Narvekar H, Narvekar H. Canine-Assisted Therapy in Neurodevelopmental Disorders: A Scoping Review. European Journal of Integrative Medicine. 2022;50 [Google Scholar]
- 89.Nosek BA, Alter G, Banks GC, Borsboom D, Bowman SD, Breckler SJ, et al. Promoting an open research culture. Science. 2015;348(6242):1422–5. doi: 10.1126/science.aab2374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Rodriguez KE, Green FLL, Binfet J-T, Townsend L, Gee NR. Complexities and considerations in conducting animal-assisted intervention research: A discussion of randomized controlled trials. Human-Animal Interactions. 2023 [Google Scholar]
- 91.Cybulski L, Mayo-Wilson E, Grant S. Improving transparency and reproducibility through registration: The status of intervention trials published in clinical psychology journals. J Consult Clin Psychol. 2016;84(9):753–67. doi: 10.1037/ccp0000115. [DOI] [PubMed] [Google Scholar]
- 92.Booth A, Noyes J, Flemming K, Moore G, Tunçalp Ö, Shakibazadeh E. Formulating questions to explore complex interventions within qualitative evidence synthesis. BMJ Global Health. 2019;4(Suppl 1):e001107. doi: 10.1136/bmjgh-2018-001107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Sniehotta FF, Araújo-Soares V, Brown J, Kelly MP, Michie S, West R. Complex systems and individual-level approaches to population health: a false dichotomy? The Lancet Public Health. 2017;2(9):e396–e7. doi: 10.1016/S2468-2667(17)30167-6. [DOI] [PubMed] [Google Scholar]
- 94.Leconstant C, Spitz E. Integrative model of human-animal interactions: A one health–one welfare systemic approach to studying HAI. Frontiers in Veterinary Science. 2022;9:656833. doi: 10.3389/fvets.2022.656833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Weiss MJ, Bloom HS, Brock T. A conceptual framework for studying the sources of variation in program effects. Journal of Policy Analysis and Management. 2014;33(3):778–808. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data availability is not applicable to this article as no new data were created or analysed in this study.