Abstract
The pathological assembly of intrinsically disordered proteins/peptides (IDPs) into amyloid fibrils is associated with a range of human pathologies, including neurodegeneration, metabolic diseases and systemic amyloidosis. These debilitating disorders affect hundreds of millions of people worldwide and the number of people affected is increasing sharply. However, the discovery of therapeutic agents has been immensely challenging largely because of i) the diverse number of aggregation pathways and the multi-conformational and transient nature of the related proteins or peptides and ii) the under-development of experimental pipelines for the identification of disease-modifying molecules and their mode-of-action. Here, we describe current approaches used in the search for small-molecule modulators able to control or arrest amyloid formation commencing from IDPs and review recently reported accelerators and inhibitors of amyloid formation for this class of proteins. We compare their targets, mode-of-action and effects on amyloid-associated cytotoxicity. Recent successes in the control of IDP-associated amyloid formation using small molecules highlight exciting possibilities for future intervention in protein-misfolding diseases, despite the challenges of targeting these highly dynamic precursors of amyloid assembly.
Introduction
Amyloidosis is a class of protein-misfolding diseases characterised by the deposition of amyloid fibrils in the tissues or organs of the individuals affected [1, 2]. The most prevalent amyloid disorders, which affect hundreds of millions of individuals worldwide include neurodegenerative diseases (Alzheimer’s disease (AD), Parkinson’s disease (PD), and Amyotrophic Lateral Sclerosis (ALS)). Amyloidosis can also involve systemic or localised amyloid deposits, and includes disorders such as Dialysis-Related Amyloidosis (DRA) immunoglobulin light chain (AL) amyloidosis) and metabolic disease-related (type II diabetes (T2D)). The formation of amyloid involves the aberrant self-association of one or more proteins or peptides into the cross-β structure canonical of amyloid fibrils [3]. While more than 50 human proteins are currently known to be the precursors of human amyloid disease [1, 2], ∼30% of these precursors are intrinsically disordered proteins/peptides (IDPs), which are able to adopt wide variety of conformations in their functional monomeric states. Two types of the most widely studied amyloid precursors are dynamically unstructured peptides: the amyloid β (Aβ) peptides (Aβ40 and Aβ42) [2, 4] and 37-residue human islet amyloid peptide (hIAPP/amylin) [5] which constitute the main amyloid components in plaques detected in people with AD and T2D, respectively. Of particular interest is the increasing recognition of the potential link between T2D and neurodegenerative disorders. Not only have in vitro studies shown the cross-assembly of Aβ and hIAPP [6–8], but mixed amyloid deposits of hIAPP and Aβ have also been detected in AD [9, 10]. These findings raised the proposal of a new neuroendocrine disorder referred to as type 3 diabetes [11, 12].
Recent cryo-electron microscopy (cryo-EM) studies have revealed atomic resolution insights into the structure of amyloid fibrils, with the surprising result that the end products of these self-association processes can be polymorphic, yet specific to each disease kind [3, 13]. Kinetic analysis has shown that the self-assembly of amyloidogenic precursors generally occurs via a nucleation-dependent mechanism, involving the formation of a broad range of conformational ensembles of monomeric and multimeric species [1, 14]. However, the exact mechanism of fibril formation remains unclear, especially regarding the structures of the different species involved and the nature of the different kinetic steps of primary nucleation, secondary nucleation and elongation. As a consequence, routes to intervention in amyloid disease by structure-based design of small molecules is a difficult task [15–18]. An additional complexity in the design of therapies against amyloidosis is the current lack of understanding of the culprit species of the cytotoxicity associated with amyloid formation [16, 19–21], which is partly due to the lack of chemical tools to purposely manipulate amyloidogenic systems, such as stabilising the original transient and dynamic species and limiting the heterogeneity of the aggregation processes. Development of such small-molecule modulators would not only enable us to better understand the mechanisms of aggregation into amyloid and its associated cytotoxicity, but would also provide us a clearer answer to the question of whether it is better to slow down or speed up amyloid formation as a therapeutic strategy.
Here we review current strategies for the discovery of small-molecule modulators against different IDPs involved in amyloid diseases. We then summarise recently reported small-molecule modulators (both inhibitors and accelerators) of the self-assembly of IDPs, mainly focussing on hIAPP and Aβ. We describe current biochemical and biophysical techniques that can be used to define the targets and mode-of-action of these interactions and summarise future challenges and possible solutions to the important question of how to better understand and treat amyloid diseases using small molecules.
The challenges in controlling the aggregation kinetics of IDPs using small molecules
Many protein-based modulators of amyloid formation of various IDPs have been reported, including antibodies, nanobodies and molecular chaperones [22–25], but we still lack effective chemical tools able to purposely manipulate an aggregation energy landscape so as to limit the intermediates formed and/or to control the products of assembly (fibril structures or other aggregation types [26]). Consequently structure-function relationships traditionally used to understand biological mechanisms cannot be carried out. IDPs are extremely challenging to target using small molecules as: 1) they are intrinsically disordered, hence lack structured binding sites; 2) oligomeric intermediates of amyloid assembly are heterogeneous and transient; 3) the toxic species in amyloid assembly remain elusive, such that we currently lack defined species to target. Moreover, protein-misfolding diseases are under kinetic control. Hence, instead of designing chemical tools which target structured protein pockets driven by thermodynamic control, the primary strategy for ligand design lies in the modulation of the aggregation kinetics by entropy-driven binding and the formation of transient and weak interactions between small molecules and IDPs [27–30].
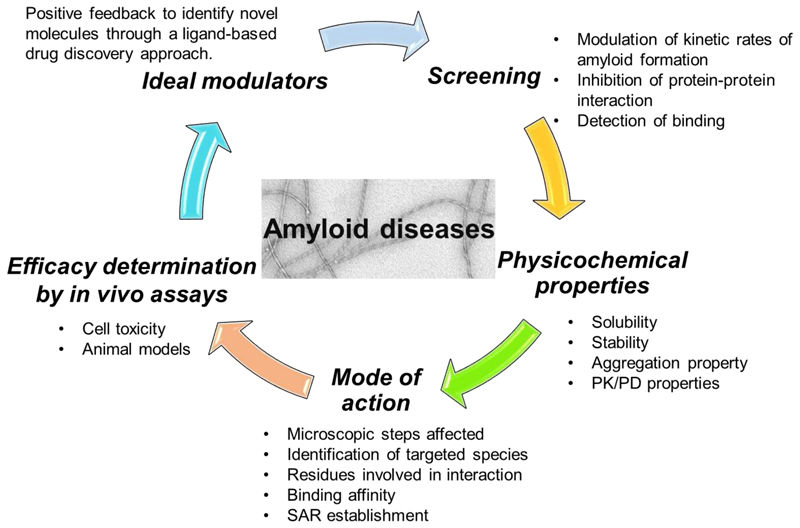
In general, there are five crucial steps for a successful campaign of modulator discovery towards IDPs (Fig. 1). Compared to conventional drug discovery programs, screening for amyloid modulators starts with the lack of defined targets or detailed structural information. Consequently, many studies have relied on in vitro or in vivo biochemical screening of compound libraries, using assays that monitor fibril formation (famously using thioflavin T (ThT) in vitro [31, 32]), directly detecting binding events (e.g. using native electrospray ionisation mass spectrometry (nESI-MS) [33, 34] or using cellular screening [35, 36]). The next crucial stage involves probing the mode-of-action of these “hits”, including identifying the species which bind the small molecules, where the small molecules bind, how strong the interaction is, and the effect(s) of the small molecules on the different steps and the mechanism of amyloid formation. Efficacy in vivo, including evaluation the effect of the small molecules in cellular assays or animal models provides another crucial hurdle. All have to be understood to define the mode-of-action of the small molecule in order to take it forwards for exploration of its effect in an organismal setting.
Fig. 1. Linked steps in a campaign of modulator discovery towards IDPs involved in protein aggregation into amyloid.
The order of the steps for modulator discovery does not matter, as all are needed for successful modulator discovery. PK (Pharmacokinetics), PD (Pharmacodynamics) and SAR (Structure-Activity Relationship).
Methods to search for modulators against the aggregation of IDPs into amyloid
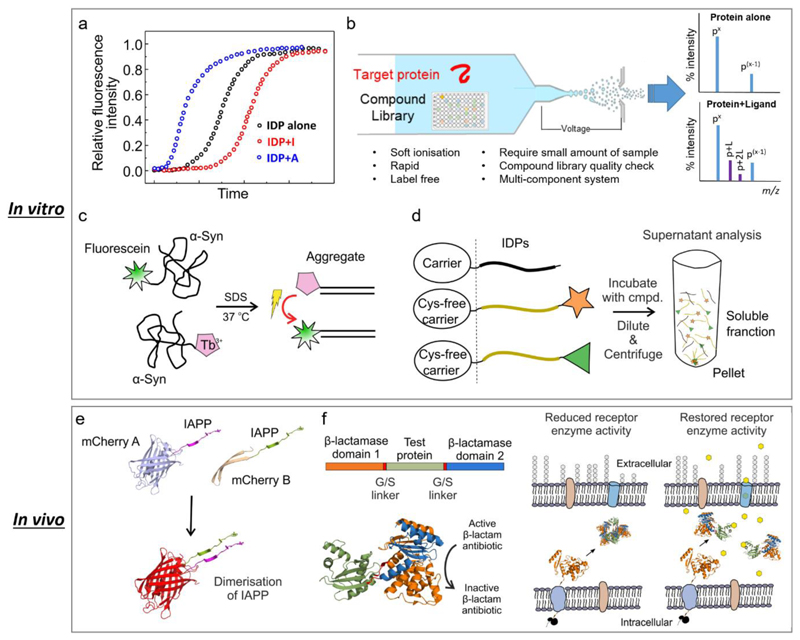
Any of the many steps involved in an amyloid formation are a potential target for small-molecule modifiers of amyloid formation. Some of the approaches developed for conventional drug discovery, as well as newly established approaches specifically targeting IDPs, have been applied in the search for new chemical entities able to modulate amyloidogenesis. These approaches can be broadly grouped into four strategies:1) high-throughput screening (in vitro and in vivo) [31, 33, 36–40]; 2) structure-based drug design (mostly targeting amyloid fibrils) [41, 42]; 3) sequence-based modulator discovery (mostly peptide modulators) [43]; and 4) fragment-based approaches [44]. Despite the activity in this field, only one small molecule therapy against amyloid formation (tafamidis that binds and stabilises the folded tetramer of transthyretin) has reached the market to date [45]. Some of the methods used to screen for small molecules able to modulate amyloid formation in vitro and in cellular assays are shown in Fig. 2. Each is complimentary with advantages and disadvantages, and progress requires application of several of these approaches in parallel as highlighted in Fig. 1.
Fig. 2. Different approaches have been applied for the discovery of small-molecule modulators against IDPs.
(a) Fluorescence-based ThT assays. These assays are usually practiced in miniaturised formats with low cost. However, they require extremely pure proteins/peptides to achieve kinetic data with high reproducibility. Also the assay conditions (such as buffer, temperature, shaking or non-shaking, etc.) need to be carefully controlled. (b) Screening by nESI-MS. Under soft ionisation conditions, the non-covalent interactions between protein and ligand can be maintained. nESI-MS is label-free, can be used in multi-component systems, and confirms the integrity of the compound library at the same time. However caution should be exercised due to the inherent drawbacks of nESI-MS, such as non-specific binding during the electrospray process. (c) FRET-based assays have been developed for HTS of large library to identify small-molecule modulators in vitro. This figure was adapted from ref [46]. (d) Synergistic Aggregation Modulator Assay (SynAggreg) is an in vitro HT platform for the study of protein aggregation and the effect of modulators on protein aggregation [47]. This figure was adapted from ref [47]. (e) A bimolecular fluorescence complementary assay (BiFC) in a constructed E. coli system was developed to monitor the initial transient dimerization stage [35]. Specifically, mCherry protein was split and fused into amyloidogenic peptides/proteins, and a strong fluorescent signal can be detected if the fused biomolecules self-assemble into dimers. This figure was adapted from ref [35]. (f) β-Lactamase tripartite fusion system in E. coli has been introduced to screen inhibitors that prevent protein aggregation [36]. The bioassay can be configured in a 48-well format and has a simple phenotypic antibiotic resistance readout which directly links to the aggregation events of the test proteins/peptides. This figure was adapted from ref [36]. In vivo screening using different organisms have also been reported, such as C. elegans [48, 49]. One of the most important features of these systems is that they are able to investigate the amyloid-forming protein/peptide induced toxicity including those transiently populated low molecular weight oligomers.
Accelerating amyloid formation of IDPs
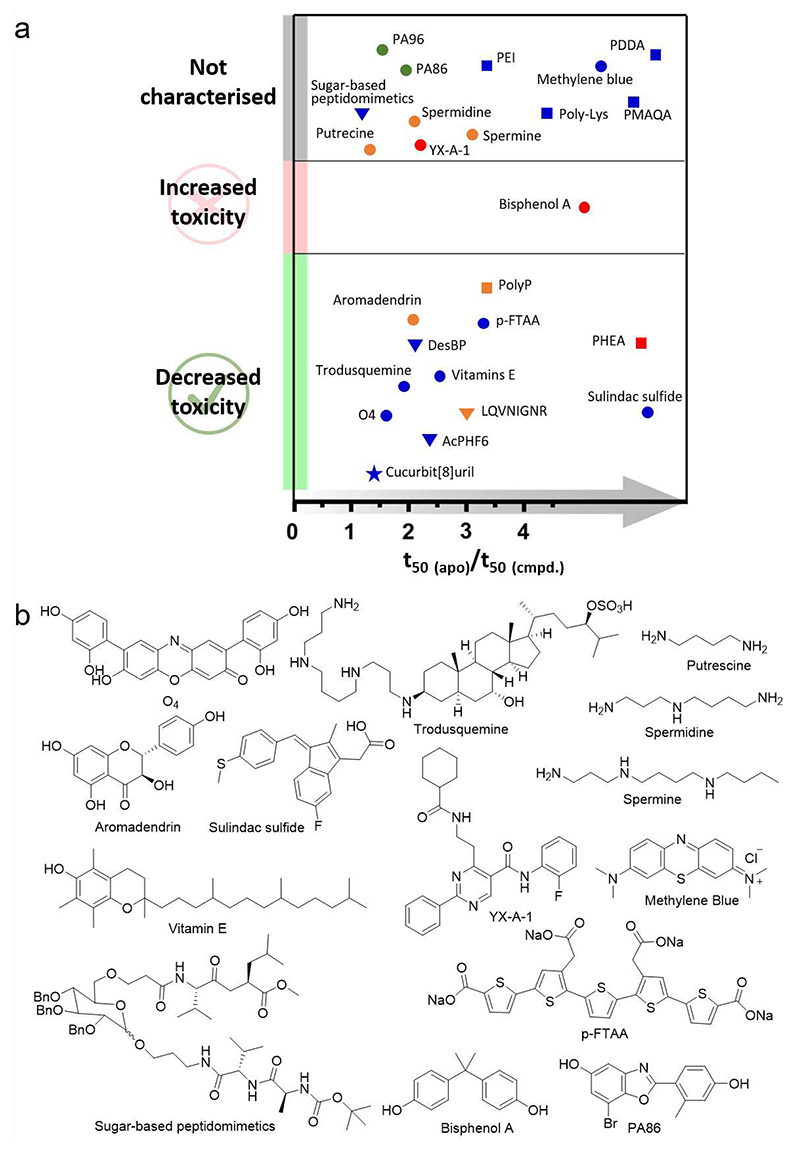
Since the oligomeric species of amyloid assembly are thought to be primarily responsible for amyloid-associated cytotoxicity [21], one approach to treat amyloid would be to decrease their population by shortening their lifetime [16, 32, 50, 51]. One way of achieving this is to accelerate the conversion of oligomers into the stable cross-β amyloid fold. Protein modulators able to accelerate amyloid formation are scarce, with the chaperone SERF being one of very few examples of this kind [52]. Interestingly, SERF is itself an IDP, and has been shown to accelerate the aggregation of Aβ and α-Syn into amyloid (the latter an IDP associated with PD) [52]. Small-molecule accelerators of amyloid formation are also scarce compared with their inhibitory counterparts, but there have been some successes, as shown in Fig. 3 and listed in Table 1.
Fig. 3. Accelerators of IDPs.
(a) Relative t50 of the accelerators and their effect on IDP-induced cytotoxicity. Blue, red, green and orange represent Aβ, hIAPP, α-Syn and multiple targets, respectively. Circle, triangle, square and star represent small molecules, peptides, polymers and macrocycle, respectively. Of note, there are still a large number of accelerators whose effect on IDP-mediated cytotoxicity are not available. It would be interesting to determine the effect of these molecules, which might help to build a greater in-depth view of this possible therapeutic strategy. (b) Chemical structures of small-molecule accelerators discussed in this review.
Table 1. Accelerators of amyloid-formation of different proteins/peptides.
List of small-molecule accelerators that have been development for various amyloid-forming proteins/peptides. They are classified based on their chemical structures, and their mode-of-action and effect on IDP-induced toxicity are summarised.
| Name | Chemical structure |
Amyloid precursor |
Mechanism of action | Toxicity profile | Ref |
|---|---|---|---|---|---|
| O4 | Small molecule | Aβ42 | O4 binds to hydrophobic amino acid residues (12VHHQKLVFFA21 and 24VGSNKGAIIG33) of Aβ42 and stabilises β-sheet rich species. | Reduce cytotoxicity | [62] |
| Bisphenol A (BPA) |
Small molecule | hIAPP | BPA facilitates the secondary structure transition of hIAPP and induces formation of oligomeric species. |
Increase hIAPP- induced membrane disruption and hIAPP-induced INS-1 cell apoptosis. |
[67] |
| PA86 | Small molecule | α-Syn | PA86 increases the elongation growth rate of α-syn. PA86 forms no direct interactions with α-Syn monomer. | N.D. | [46] |
| Aromadendrin | Small molecule | hIAPP and Aβ42 |
Aromadendrin promotes the structural conversion of species in the lag and early growth phases. | Reduce Aβ- and hIAPP-induced toxicity | [66] |
| Trodusquemine | Small molecule | Aβ42 | Trodusquemine increases the rate of surface-catalysed secondary nucleation. |
Inhibit binding of oligomers to neuroblastoma cells. Increase the number of aggregates in C. elegans models, but reduce Aβ42-induced toxicity. |
[50] |
| YX-A-1 | Small molecule | hIAPP | YX-A-1 interacts predominantly with oligomers formed in the lag phase. |
N.D. | [32] |
| Vitamin E | Small molecule | Aβ42 | Vitamin E forms micelles to promote Aβ42 aggregation. | Improve the fitness of AD C. elegans model. | [55] |
| Polyamines (spermine, spermidine, and putrescine) | Small molecule | Aβ40, α- Syn |
All three polyamines bind to the monomeric Aβ40 around residues 4-5, 15-17 and 27-28. | N.D. | [71] [72] |
| Sugar-based peptidomimetics | Small molecule | Aβ42 | The peptides interact with Aβ42 probably via p-stacking. | N.D. | [73] |
| Sulindac sulfide | Small molecule | Aβ40 | Sulindac sulfide forms colloidal particles which recruits monomers and increase local peptide concentration. | Reduced Aβ42-induced toxicity. | [53] |
| p-FTAA | Small molecule | Aβ42 | p-FTAA bound to Aβ oligomers formed at the early stage of aggregation and induced formation of β-sheet structures. | Reduce Aβ42-mediated cytotoxicity | [64] |
| Methylene Blue | Small molecule | Aβ42 | Methylene blue promotes both filament nucleation and elongation. | N.D. | [74] |
| PDDA, PEI and Poly-Lys | Polymer | Aβ42 | PDDA increases the local concentration of Aβ42 and decrease self-repulsion of Aβ42. | N.D. | [58] |
| Polyphosphate (PolyP) | Polymer | Aβ42, α-Syn, Tau, CsgA |
It is speculated that PolyP binds to monomers and increases the local concentration. It also accelerates biofilm formation (a functional amyloid) in bacteria (curli formation of CsgA). |
Reduce α-Syn and Aβ42 induced toxicity in cells and C. elegans models. | [59] |
| Poly(2-hydroxyethyl acrylate) (PHEA) | Star polymers | hIAPP | PHEA interacts with N-terminal residues of hIAPP and increases the local peptide concentration. | Reduce hIAPP-induced toxicity both in vitro and ex vivo. | [75] |
| Polymethacrylat e-copolymer (PMAQA) | Polymer | Aβ40 | PMAQA induces a β-hairpin structure by binding to regions spanning Lys16-Val24 and Ala30-Val40. | N.D. | [60] |
| DesBP | Bicyclic peptide | Aβ42 | DesBP interacts weakly with the monomeric Aβ42 and increases both primary and secondary nucleation are, but not elongation. | Reduce Aβ42-induced toxicity in C. elegansmodels. | [65] |
| LQVNIGNR | Peptide | Ure2, Tau- N244- F378, α- Syn |
This peptide forms vesicular assemblies which promotes conformational transitions of oligomers. | Decrease oligomer-induced membrane leakage. |
[54] |
| AcPHF6 | peptide | Aβ40, Aβ42 | The peptide is likely to interact with Aβ monomer. | Reduce Aβ40 and Aβ42 mediated toxicity in neuronal cells. | [76] |
| Cucurbit[8]uril | Macrocycle | Aβ42 | It preferentially targets Phe residues in Aβ42 and increases the size of the Aβ42 aggregates. | Reduce Aβ42-induced toxicity in neuronal cell line. | [77] |
One mode-of-action by which small molecules can accelerate amyloid formation is by recruiting monomers or oligomers non-specifically and enhancing the nucleation rate of amyloid formation by increasing the local concentration of the protein/peptide precursors. Small molecules which form colloidal particles [53] or micelles [54, 55] have such a mechanism of action, although their general lack of specificity rules them out for development of therapeutics [56, 57]. Highly charged polymers, such as positively charged polyamino acids have also been shown to be effective amyloid accelerators of Aβ and other proteins, by interacting with their target(s) in a non-specific manner [54, 58–60]. In some cases a degree of specificity can still be achieved. For example, colloids formed by sulindac sulfide bind Aβ monomers non-specifically; while sulindac sulfide monomers in solution, or in equilibrium with their colloidal form, bind to hydrophobic cavities (in the vicinity of Gly33) in the Aβ fibril core specifically [53, 61].
Small-molecule accelerators of amyloid formation have also been discovered that bind to oligomeric precursors of amyloid formation [32, 50, 62]. For example, the orcein-related small molecule, O4, binds to hydrophobic residues (12VHHQKLVFFA21 and 24VGSNKGAIIG33) within Aβ42 oligomers [62]. The compound YX-A-1 was recently identified as a potent accelerator of amyloid formation of hIAPP (but not Aβ), enhancing fibril formation substoichiometric concentrations by binding oligomers in the lag phase of assembly [32]. The luminescent oligothiophene, p-FTAA, also binds to oligomers of Aβ (and prion protein [63]), and induces formation of β-sheet structures [64]. These studies highlight the different structural features of oligomers formed from different amyloid precursors or even from different microscopic steps of the sample precursor, which presumably rationalises their specificity.
As shown in Fig. 3, most known accelerators of amyloid formation show a protective effect on IDP-induced cytotoxicity, regardless of their chemical structure, mode-of-action or specificity [50, 65, 66]. Some have a dual benefit. For example, trodusquemine enhances the aggregation of Aβ42 and at the same time reduces the binding affinity of Aβ42 oligomers to cellular membranes, leading to an amelioration of toxicity in neuroblastoma cells [50]. These findings reinforce the notion that driving the formation of inert fibrils from cytotoxic oligomers could be a beneficial strategy for treating amyloid disease. However, this is not always the case. For example, bisphenol A (BPA) was identified as an effective promotor of hIAPP amyloid assembly, however, it failed to alleviate hIAPP-induced cytotoxicity in INS-1 cells [67]. Instead, BPA promotes the formation of hIAPP oligomers with enhanced toxicity and an enhanced ability to disrupt membranes [67]. This is still a valuable outcome in the quest to better understand the structure-function relationship of oligomers. Interestingly, while BPA failed to protect against hIAPP-induced cytotoxicity, derivatives of BPA resulted in inhibitors against hIAPP aggregation [68]. This highlights the fine balance of interactions that control amyloid assembly, with small changes in chemistry able to switch between accelerators and inhibitors by fine-tuning the shape of the aggregation energy landscape.
Some modulators show more complex behaviours on the amyloid formation of IDPs, with their effect depending on the target species, the concentrations of the modulator, and/or the assay conditions employed. For example, trodusquemine enhances the aggregation of Aβ42 [50], but inhibits the aggregation of α-Syn and suppresses α-Syn-induced cytotoxicity in neuronal cells [69]. Some modulators show different effects on the same target, depending on the concentration used in the assay. For example, trehalose inhibits hIAPP aggregation at low concentration, but promotes fibrillation of hIAPP at high concentrations [70]. This different behaviour of the same modulator at various concentrations could result from the ligand altering its own self-association at different concentrations (e.g. colloidal behaviour). Hence, it is crucial to assess the solubility and aggregation propensity of the small molecules themselves before drawing conclusions as to their mode-of-action.
Inhibiting the assembly of IDPs into amyloid
The development of inhibitors of amyloidogenic proteins/peptides as therapeutic agents against protein-misfolding diseases has received much interest over the last decade [16, 18, 51]. The desired outcome of these inhibitors is to retard/block amyloid formation and prevent/reduce the formation of cytotoxic species. Due to the complexity of aggregation pathways, there are potentially many species to target, each of which can change the rate or outcome of assembly: monomeric precursors, oligomers and fibrils. Many different types of small molecules have been designed or identified to target these species, including small molecules, peptides, polymers, and nanoparticles. These inhibitors have been comprehensively reviewed recently by several groups [16, 18, 51, 78–81]. Here we focus on the recent development of small-molecule inhibitors of Aβ and hIAPP, using these systems as exemplars of the challenges and successes in this buoyant field. Several examples are listed in Table 2, and each is discussed below, grouped by the precursor(s) in amyloid formation that they target.
Table 2. Recently-reported small-molecule inhibitors of Aβ and hIAPP.
Small-molecule inhibitors against Aβ and hIAPP are listed, and their mode-of-action and effect on IDP-induced toxicity are summarised.
| Name | Amyloid precursor | Mechanism | Toxicity profile | Ref |
|---|---|---|---|---|
| Bexarotene | Aβ42 | Bexarotene selectively targets the primary nucleation step in Aβ42 aggregation. | Reduce the Aβ42-induced toxicity in neuroblastoma cells and Aβ42 C. elegansmodel. | [92] |
| Rhodanine-based compounds | Aβ42 | These molecules can delay oligomer formation and reduce the overall oligomer production. | N.D. | [93] |
| Polyphenolic biflavonoids | Aβ42 | These molecules inhibit Aβ42 fibrillation and disassemble preformed Aβ42 fibrils. SAR study identified an essential role of the hydroxyl groups on the molecules. | N.D. | [88] |
| 10074-G5 | Aβ42 | 10074-G5 retards primary and secondary nucleation pathways by binding to monomeric Aβ42. | Rescue a C. elegans model of Aβ-associated toxicity. | [82] |
| Anle138b | Aβ42 | Anle138b prevents Aβ42 oligomers induced pore formation in membranes. | Ameliorate hippocampal synaptic, spatial reference memory, and transcriptional homeostasis in AD mouse model. |
[94] |
| Vitamin A (retinoic acid) | Aβ42 | Vitamin A inhibits the primary and secondary processes of Aβ42 fibrillation. | Vitamin A reduces Aβ42 aggregates and increases the total fitness of C. elegans. | [55] |
| Catechol-containing isoflavone | Aβ42 and Tau |
Several derivatives inhibit the aggregation of Aβ42 and Tau. | Reduce the Aβ plaques in the brain and improves the memory deficits in AD mice model. | [95] |
| Genistein | Aβ42 and hIAPP |
Genistein inhibits the aggregation of hIAPP and Aβ42 by preventing the conformational transition of peptide monomers into P-sheet structures. | Prevent hIAPP (RIN-m5F) or Aβ42-induced (SH-SY5Y) cytotoxicity possible thorough reducing peptide-induce membrane leakage. |
[84] |
| AQ-4, THQ-1, DHQ-1, DHQ-2 and BF-3 |
Aβ40 | Compounds were identified through a high-throughput screening in the presence of membrane. | N.D. | [31] |
| BIBA | Aβ40 | BIBA inhibits the aggregation of Aβ40. BIBA interacts weakly with peptide monomers. Docking studies show that BIBA interacts with seven residues which are Glu22, Phe19, Val18, Lys16, Gln15, His6 and Arg5. | Inhibit Aβ-induced paralysis of C. elegans and reduce Aβ plaques in the brain of AD mice model. | [96] |
| Anle145c | hIAPP | Anle145c thermodynamically traps hIAPP in non-cytotoxic oligomers and converts hIAPP amyloid fibril into non-toxic oligomers. | Reduces hIAPP-induced toxicity in INS-1E cells and human MJS cells. | [89] |
| Silybins | hIAPP | Stereospecific inhibition of hIAPP aggregation was observed. MD simulations show that Silybin B interacts with the Ser20-Ser29, His18, the N-terminal domain, and Asn35. | Protect INS-1 cells from hIAPP toxicity more efficiently than silybin A. | [97] |
| Cloridarol | hIAPP | Cloridarol reduces the overall quantity of amyloid fibrils. MD revealed that it binds to C-terminal β-sheet region of hIAPP oligomers. | Protect islet β-cells from hIAPP-induced cytotoxicity. | [87] |
| Yakuchinone B derivatives | hIAPP | Molecular docking shows that molecules interact with hIAPP monomer through hydrogen bonding and hydrophobic interactions. | Reduce IAPP-induced toxicity for BRIN-BD11 cells. |
[98] |
| 2- phenylbenzofurans |
hIAPP | 2-Phenylbenzofurans prevent the fibril formation of hIAPP. | N.D. | [99] |
| YX-I-1 | hIAPP | YX-I-1 inhibits primary nucleation, secondary nucleation and elongation of hIAPP fibrillation by interacting with peptide monomers. | N.D. | [32] |
| Tetracycline derivatives | hIAPP and Aβ | Tetracyclines inhibit the fibril formation of hIAPP and Aβ. They can also effectively disaggregate matured fibrils. MD simulations were carried to study the peptide-small molecule interactions. | Rescue peptide-induced cytotoxicity for INS-1 and SH-SY5Y cell lines. | [90] |
| Tryptophan-galactosylamine conjugates | hIAPP and Aβ42 |
These conjugates inhibit aggregation of hIAPP and Aβ42 and disassemble pre-formed hIAPP and Aβ42 fibrils. |
Reduce the cytotoxicity induced by Aβ42 (SH-SY5Y) and hIAPP (HEK-293). | [91] |
| Lithospermic acid (LA) | hIAPP | LA inhibits hIAPP aggregation by binding to hIAPP monomers. Docking studies show that LA interacts with Arg11, Asn14, Phe15, Asn21, Ala25, Ile26 and Val32. | Reduce hIAPP-induced cytotoxicity of INS-1 cells. | [83] |
| Naphthoquinone-based hybrids | PHF6, hIAPP and Aβ42 |
These molecules inhibit the fibril formation of all the three peptides and disrupt the matured fibrils. Molecular docking shows interaction between small molecules and the monomeric peptides. | Reduce the cytotoxicity induced by PHF6 (SH-SY5Y), Aβ42 (SH-SY5Y) and hIAPP (HEK-293). | [100] |
| DM1 | hIAPP | It interacts with the monomeric peptide by stabilising and/or perturbing the helix conformation at the N-terminus. DM1 is likely to interact strongly with positively charged residues (Lys1 and Arg11) and hydrophobic domain of the peptide (Leu12-Val17). | Reduce hIAPP-induced cytotoxicity in RIN-m cells. | [101] |
| CurDAc | hIAPP | CurDAc inhibits hIAPP aggregation and disassembles hIAPP fibrils. CurDAc promotes the formation of oligomers. NMR studies show that CurDAc interacts residues T4, C7, A8, Q10-N14, L16-S19, I26 and S29. | Increase hIAPP-induced cytotoxicity in RIN-5F cells. | [86] |
| Resveratrol derivatives (4’- DMPR and 4’-O-PR) |
hIAPP | Both molecules abolished hIAPP amyloid growth and protected hIAPP-induced membrane damage. | N.D. | [102] |
| DP-128 | Pan amyloid inhibitor | DP-128 inhibits the aggregation of a number of different amyloidogenic proteins, including hIAPP, Aβ40 and Aβ42. | N.D. | [103] |
| HUP7TH | Pan amyloid inhibitor | HUP7TH inhibits the aggregation of a number of different amyloidogenic proteins, including hIAPP, Aβ40 and Aβ42. | N.D. | [103] |
Discovering small molecules that bind to monomeric IDP precursors of amyloid formation is challenging, because such proteins are dynamically disordered and continually ‘on the move’. Nonetheless, various studies have shown that it is possible to specifically target these species and to inhibit amyloid formation, with successes resulting from combinations of in vitro screens and in-cell assays [32, 82–85]. For example, 10074-G5 which binds to intrinsically disordered c-Myc monomer is able to retard the primary and secondary nucleation pathways of Aβ42 by interacting with the monomeric peptides [82]. This type of interaction leads to a decrease in hydrophobicity and an increase of conformational entropy of Aβ42, which demonstrates the therapeutic possibility for the treatment of protein-misfolding diseases through an ‘entropic expansion’ mechanism [82]. The strategy of targeting monomers of IDPs has also been applied to hIAPP. YX-I-1 was identified as a potent inhibitor against amyloid formation of hIAPP via a combinatorial approach of nESI-MS and ThT bioassays [32]. It can specifically delay primary nucleation, secondary nucleation and elongation by binding to hIAPP monomers and further 2D NMR studies show that the inhibitor mainly interacts with residues in the regions 10-14, 17-20, 23-28 and the C-terminal Tyr37 [32]. Collectively, these studies demonstrate that it is possible to specifically target monomeric IDPs and control their assembly into amyloid.
Successes in targeting oligomers in Aβ/hIAPP amyloid assembly have also been reported. For example, CurDAc, a water-soluble curcumin derivative, induces the formation of hIAPP oligomers and these species reduce RIN-5F cell viability [86]. Via a drug-repurposing strategy, cloridarol (used for the treatment of cardiovascular disease) was identified as an effective inhibitor of hIAPP fibrillation and reduces the hIAPP-induced cytotoxicity in RIN-m5F cells [87]. Molecular Dynamics (MD) simulations revealed that cloridarol preferentially binds to C-terminal β-sheet region of hIAPP oligomers via a combination of hydrophobic interactions, hydrogen bonding and π-π stacking [87]. The different effects of the inhibitors on hIAPP-induced cytotoxicity highlight that inhibition of aggregation does not necessarily correlate with reducing cytotoxicity.
Finally, targeting fibrils themselves with small molecules has been achieved, and could be an effective strategy, assuming that fibrils represent the inert end products of amyloid assembly (which is not necessarily the case, especially given their role in disease transmission, seeding and secondary nucleation that catalyses oligomer and amyloid formation). The near-atomic resolution structures of fibrils generated from pure proteins/peptides in vitro or from patient samples provide a structural basis to specifically target these assembly end products. Most of these type of inhibitors are peptide-based [41, 42]. In some cases, binding to amyloid fibrils causes their disassembly [86, 88–91]. The resulting conversion of fibrils into soluble oligomers can result in the generation of non-toxic [89, 90] or toxic species [86]. These molecules are important chemical tools to stabilise the transient and dynamic oligomeric species for more detailed molecular studies. The different toxicity profiles of the oligomers again highlight targeting aggregation and modulating cytotoxicity should be considered as independent events.
Characterising the interactions between IDPs and small-molecule modulators
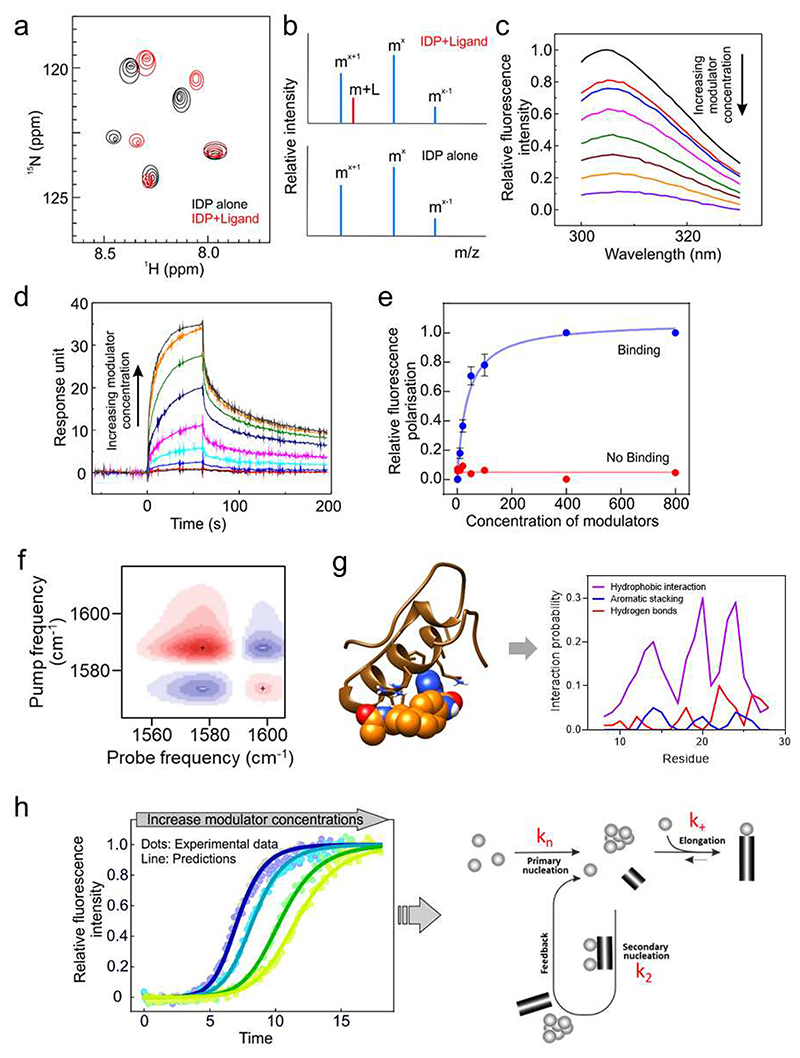
Identifying the targets of small-molecule modulators and probing their mode-of-action have been revolutionised in recent years by the application of kinetic analyses that are able to define the step(s) in amyloid formation that are affected by the addition of a specific ligand [104]. The online programme AmyloFit has been widely applied to study the microscopic steps in amyloid assembly for many proteins, including the Aβ [105, 106] and hIAPP [32, 107], and used to define the targets and mechanism(s) of action of modulators of amyloid assembly [32, 92, 104]. One of the major challenges remaining is to map the binding site of the ligands, so as to understand their mechanism of action in structural/molecular detail. For IDPs it is difficult to answer this critical question because of the dynamics and complexity of the system. As discussed above, most interactions between IDPs and small molecules are weak (∼ μM), involving rapid on/off rates to the protein, which itself is a dynamic moving target [29, 82, 108]. These features make it difficult to detect or quantitatively measure the binding affinity of the modulators and to map their binding site(s). Significant progress has been made recently in both experimental and in silico approaches. These are summarised in Fig. 4. Amongst these, ligand and protein-detected NMR methods, especially at high field strengths (e.g. 950 MHz) are ideal for measuring ligand binding to an IDP and for mapping residues involved in single atom detail [32, 101, 109] (Fig. 4a). nESI-MS coupled with ion mobility-MS (IM-MS) enables discrimination between non-specific and specific binders [33], and can reveal the effect of binding on the conformational distribution of the IDP [110] (Fig. 4b). Methods such as surface plasmon resonance (SPR), fluorescence polarisation (FP) and bio-layer interferometry (BLI) provide methods of determining affinity, with fluorescence titration using Stern-Volmer analysis being particularly fruitful for low affinity measurements [32, 82, 109] (Fig. 4c-e). SPR and BLI can provide information about the association (kon) and dissociation (koff) rate constants of the protein-ligand interactions. By varying the experimental temperature (e.g. using fluorescence titrations), thermodynamic parameters such as the entropy change (ΔS°) and enthalpy change (ΔH°) can be obtained [111]. Two-dimensional infrared spectroscopy (2D FTIR) using isotope-labelled protein can be used to map binding sites of the inhibitor with residue-level resolution [112] (Fig. 4f), while large scale calculations using supercomputers, guided by experimental restraints, can be used to describe the dynamic ensemble of ligand bound states [29]. Linked with parallel kinetic analysis of the mechanism of action of the modulator (Fig. 4g,h), the tools are now in place for a full description of the mode-of-action of the modulators of amyloid formation and how their implementation can tip the energy landscape to favour or disfavour aggregation or even to change its course towards different products of amyloid assembly.
Fig. 4. Biochemical and biophysical approaches used for the characterisation of the interaction between IDPs and small molecules.
(a) Representative 2D NMR spectrum of an IDP with (red) and without (black) a small molecule. (b) nESI mass spectra show the mass addition of a ligand to the target, suggesting the interaction between the target and the ligand.
(c) Fluorescence quenching caused by ligand binding. Stern-Volmer analysis of the fluorescence titration data enables extraction of the Kd. These data were taken from ref [32].
(d) SPR measurement showing ligand binding (Kd) as well as the association (kon) and dissociation (koff) rate constants. These data were taken from ref [32]. (e) Fluorescence polarisation (FP) can provide indications of binding/no binding of ligands. Fitting the FP titration can yield the binding affinity of the ligand. (f) 2D FTIR can provide binding site information of the inhibitor at residue-level resolution. (g) MD simulations can provide detailed descriptions of the conformational ensembles of IDPs and specific information about the IDP-ligand interactions at an atomic-level. MD simulation can predict the favoured interactions formed between IDP and ligand, such as hydrogen bonding, aromatic interaction, charge-charge interaction, etc. (see [29]). (h) Measurement of amyloid formation using ThT fluorescence can be used to determine the mechanism of amyloid formation and the species that bind ligands that affect the rate of assembly into amyloid. These data were taken from ref [32].
Conclusions and outlook
In recent years, substantial progress has also been made in understanding of the kinetic mechanisms of amyloid formation and the structure of the ultimate amyloid fibrils formed [3, 113]. Significant progress has been made in the development of small-molecule modulators towards amyloid-forming proteins/peptides [16, 79, 80]. Here we have reviewed current approaches that are being used in the search for small molecules that are able to modulate the self-association of IDPs into amyloid, and discuss recently reported inhibitors and accelerators of amyloidogenic proteins/polypeptides, focusing on hIAPP and Aβ. We also discuss the advances made in probing small-molecule-IDP interactions by biochemical and biophysical approaches, both experimental and computational. With the development of these methods, it is now possible to detect weak binding of small molecules to IDPs, identify the potential targets of these molecules in IDP-associated amyloid formation, and investigate their mode-of-action in detail. The stage is thus set for an optimistic future that these weak binding molecules may cast new light on how and why IDPs aggregate into amyloid fibrils of different structure specific to different diseases [3, 13]. They also reveal how small changes in the population of different amyloid precursors brought about by weak binding of ligands can fundamentally change the outcomes of amyloid assembly, and can be used to determine the structure-function relationships of the transient and dynamic intermediates of amyloid assembly.
Many questions and challenges in this exciting field remain. Since protein-misfolding diseases are under kinetic control, modulating the aggregation kinetics by altering the rates of different microscopic steps could be an effective way to develop possible therapeutic agents. One fundamental question remaining, however, is whether it is better to slow down or speed up aggregation. The answer to this question will require a better understanding of disease-causing mechanisms, specifically which species in the aggregation energy landscape are cytotoxic and which are benign. It is important to note that cross-seeding of different amyloid protein precursors has been observed and also some proteins are known to aggregate in the same disease (e.g. Dementia with Lewy bodies (DLB) [114], AD, and T2D [10, 11]). A crucial question is whether it is better to develop broad-spectrum modulators able to target multiple protein precursors, e.g. Aβ and α-Syn, Aβ and Tau or Aβ and hIAPP (in LB, AD and T2D, respectively), rather than hunting for small molecules with high specificity. Since the cellular environment is more complex than the experimental conditions of in vitro experiments (such as the presence of metal ions, membranes, molecular chaperones and/or crowding) [115], a better understanding of how modulators behave under physiological conditions is crucial for the transition of small molecules discovered in vitro or in cell lines into effective therapeutic agents. Another question under hot debate is whether it is possible to identify modulators to IDPs with high binding affinity (i.e. sub-μM) and whether tight binding is required for the effectiveness and specificity required for any small molecule in the clinic. Encouragingly, several examples have now been reported of the successful development of modulators which alter the aggregation kinetics of IDPs via weak and dynamical binding to their targets [29, 32, 82]. The physicochemical properties of the modulators needs also to be considered, such as their stability, specificity, solubility, cell permeability, ability to pass through the blood brain barrier (for amyloidosis causing neurodegeneration) and their own aggregation propensity, before conducting further functional studies.
Apart from the reversible modulators of amyloid formation discussed in this review, other promising strategies in term of modulator design have been reported. One of them is the development of covalent modulators which have high potency [116–118]. Another promising strategy is the application of ‘molecular glue’ or proteolysis-targeting chimera (PROTAC), as strategies to target and degrade amyloid precursors. Li and colleagues identified molecules which interact with both huntingtin (mHTT) (the causative agent of Huntington’s disease) and autophagosome protein microtubule-associated protein 1A/1B light chain 3 (LC3) [119]. They showed that the molecules can specifically target mHTT to autophagosomes, reduce the mHTT levels, and rescue disease-relevant phenotypes in cells and in vivo in fly and mouse models of Huntington’s disease [119]. Such approaches could be powerfully applied synergistically with small-molecule modulators of amyloid formation, such that the course of aggregation can be controlled by small switches in the concentration of relevant misfolded precursor states. Since metal ions can play important roles in amyloid formation (such as metal ion-induced Aβ aggregation), bifunctional modulators have been identified that synergistically chelate metal ions and inhibit aggregation into amyloid [120, 121].
In conclusion, despite its long and sometimes tortuous history [1, 2, 113], the amyloid field is currently full of excitement and hope, with the enhancement in our fundamental understanding of aggregation mechanisms and the array of new strategies and small molecules able to control aggregation laying strong foundations for the much-needed breakthroughs in the treatment of amyloid diseases in the years ahead.
Key summary points.
-
–
Protein-misfolding diseases are defined as kinetic diseases which are modulated by different microscopic events with defined rate constants.
-
–
Small-molecule modulators towards IDPs are powerful chemical tools for the investigation of amyloid formation mechanisms and probing molecular mechanisms of amyloid-induced cytotoxicity.
-
–
Targeting amyloid aggregation and modulating IDP-induced cytotoxicity should be considered as two independent events.
-
–
Both inhibitors and accelerators of IDPs are equally important and can inform whether it is better to slow-down or speed-up the self-assembly of IDPs into amyloid.
Acknowledgements
Y.X., R.M.-M. & S.E.R. are funded by Wellcome (204963). SER holds a Royal Society Professorial Fellowship (RSRP\R1\211057). We also thank all of our colleagues in our Amyloid group and in the Astbury Centre at Leeds for their many helpful discussions, especially Richard Foster for all of his insights into small-molecule modulators and Frank Sobott for his help and advice regarding biological mass spectrometry. For the purpose of Open Access, the authors have applied a CC BY public copyright licence to any Author Accepted Manuscript version arising from this submission.
Abbreviations
- Aβ
Amyloid β
- AD
Alzheimer’s disease
- ALS
Amyotrophic Lateral Sclerosis
- BiFC
bimolecular fluorescence complementary assay
- BLI
bio-layer interferometry
- BPA
bisphenol A
- cryo-EM
cryo-electron microscopy
- 2D FTIR
two-dimensional infrared spectroscopy
- FP
fluorescence polarisation
- hIAPP
human islet amyloid peptide
- HTS
high-throughput screening
- IDPs
Intrinsically disordered proteins/peptides
- IM
ion mobility
- LA
lithospermic acid
- MD
molecular dynamics
- nESI-MS
native electrospray ionisation mass spectrometry
- PD
Parkinson’s disease
- PolyP
polyphosphate
- PMAQA
polymethacrylate-copolymer
- PROTAC
proteolysis-targeting chimera
- T2D
type II diabetes
- ThT
Thioflavin T
- αSyn
α-Synuclein
- SynAggreg
Synergistic Aggregation Modulator Assay
- SPR
surface plasmon resonance.
Footnotes
Author Contributions
YX wrote the review and performed the literature search. RMM and SER edited the review.
Conflicts of interest
All authors declare they have no competing interests.
References
- 1.Iadanza MG, Jackson MP, Hewitt EW, Ranson NA, Radford SE. A new era for understanding amyloid structures and disease. Nat Rev Mol Cell Biol. 2018;19:755–73. doi: 10.1038/s41580-018-0060-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Knowles TPJ, Vendruscolo M, Dobson CM. The amyloid state and its association with protein misfolding diseases. Nat Rev Mol Cell Biol. 2014;15:384–96. doi: 10.1038/nrm3810. [DOI] [PubMed] [Google Scholar]
- 3.Gallardo R, Ranson NA, Radford SE. Amyloid structures: much more than just a cross-β fold. Curr Opin Struct Biol. 2020;60:7–16. doi: 10.1016/j.sbi.2019.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tanzi RE, Bertram L. Twenty years of the Alzheimer’s disease amyloid hypothesis: a genetic perspective. Cell. 2005;120:545–55. doi: 10.1016/j.cell.2005.02.008. [DOI] [PubMed] [Google Scholar]
- 5.Milardi D, Gazit E, Radford SE, Xu Y, Gallardo RU, Caflisch A, et al. Proteostasis of islet amyloid polypeptide: A molecular perspective of risk factors and protective strategies for type II diabetes. Chem Rev. 2021;121:1845–93. doi: 10.1021/acs.chemrev.0c00981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Young LM, Mahood RA, Saunders JC, Tu L-H, Raleigh DP, Radford SE, et al. Insights into the consequences of co-polymerisation in the early stages of IAPP and Aβ peptide assembly from mass spectrometry. Analyst. 2015;140:6990–9. doi: 10.1039/c5an00865d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jackson K, Barisone GA, Diaz E, Jin L-w, DeCarli C, Despa F. Amylin deposition in the brain: A second amyloid in Alzheimer disease? Ann Neurol. 2013;74:517–26. doi: 10.1002/ana.23956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Oskarsson ME, Paulsson JF, Schultz SW, Ingelsson M, Westermark P, Westermark GT. In vivo seeding and cross-seeding of localized amyloidosis: a molecular link between type 2 diabetes and Alzheimer disease. Am J Pathol. 2015;185:834–46. doi: 10.1016/j.ajpath.2014.11.016. [DOI] [PubMed] [Google Scholar]
- 9.Moreno-Gonzalez I, Edwards Iii G, Salvadores N, Shahnawaz M, Diaz-Espinoza R, Soto C. Molecular interaction between type 2 diabetes and Alzheimer’s disease through cross-seeding of protein misfolding. Mol Psychiatry. 2017;22:1327–34. doi: 10.1038/mp.2016.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pruzin JJ, Nelson PT, Abner EL, Arvanitakis Z. Review: Relationship of type 2 diabetes to human brain pathology. Neuropathol Appl Neurobiol. 2018;44:347–62. doi: 10.1111/nan.12476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang Y, Song W. Islet amyloid polypeptide: Another key molecule in Alzheimer’s pathogenesis? Prog Neurobiol. 2017;153:100–20. doi: 10.1016/j.pneurobio.2017.03.001. [DOI] [PubMed] [Google Scholar]
- 12.Raimundo AF, Ferreira S, Martins IC, Menezes R. Islet amyloid polypeptide: a partner in crime with Aβ in the pathology of Alzheimer’s Disease. Front Mol Neurosci. 2020;13:35. doi: 10.3389/fnmol.2020.00035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shi Y, Zhang W, Yang Y, Murzin AG, Falcon B, Kotecha A, et al. Structure-based classification of tauopathies. Nature. 2021;598:359–63. doi: 10.1038/s41586-021-03911-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chatani E, Yamamoto N. Recent progress on understanding the mechanisms of amyloid nucleation. Biophys Rev. 2018;10:527–34. doi: 10.1007/s12551-017-0353-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Caflisch A. Kinetic control of amyloidogenesis calls for unconventional drugs to fight Alzheimer’s disease. ACS Chem Neurosci. 2020;11:103–4. doi: 10.1021/acschemneuro.9b00676. [DOI] [PubMed] [Google Scholar]
- 16.Kreiser RP, Wright AK, Block NR, Hollows JE, Nguyen LT, LeForte K, et al. Therapeutic strategies to reduce the toxicity of misfolded protein oligomers. Int J Mol Sci. 2020;21:8651. doi: 10.3390/ijms21228651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Biesaga M, Frigolé-Vivas M, Salvatella X. Intrinsically disordered proteins and biomolecular condensates as drug targets. Curr Opin Chem Biol. 2021;62:90–100. doi: 10.1016/j.cbpa.2021.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Doig AJ, Derreumaux P. Inhibition of protein aggregation and amyloid formation by small molecules. Curr Opin Struct Biol. 2015;30:50–6. doi: 10.1016/j.sbi.2014.12.004. [DOI] [PubMed] [Google Scholar]
- 19.Kulenkampff K, Wolf Perez AM, Sormanni P, Habchi J, Vendruscolo M. Quantifying misfolded protein oligomers as drug targets and biomarkers in Alzheimer and Parkinson diseases. Nat Rev Chem. 2021;5:277–94. doi: 10.1038/s41570-021-00254-9. [DOI] [PubMed] [Google Scholar]
- 20.Cawood EE, Karamanos TK, Wilson AJ, Radford SE. Visualizing and trapping transient oligomers in amyloid assembly pathways. Biophys Chem. 2021;268:106505. doi: 10.1016/j.bpc.2020.106505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nguyen PH, Ramamoorthy A, Sahoo BR, Zheng J, Faller P, Straub JE, et al. Amyloid pligomers: a joint experimental/computational perspective on Alzheimer’s Disease, Parkinson’s Disease, Type II Diabetes, and Amyotrophic Lateral Sclerosis. Chem Rev. 2021;121:2545–647. doi: 10.1021/acs.chemrev.0c01122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Belsare KD, Wu H, Mondal D, Bond A, Castillo E, Jin J, et al. Soluble TREM2 inhibits secondary nucleation of Aβ fibrillization and enhances cellular uptake of fibrillar Aβ. Proc Natl Acad Sci U S A. 2022;119:e2114486119. doi: 10.1073/pnas.2114486119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Linse S, Scheidt T, Bernfur K, Vendruscolo M, Dobson CM, Cohen SIA, et al. Kinetic fingerprints differentiate the mechanisms of action of anti-Aβ antibodies. Nat Struct Mol Biol. 2020;27:1125–33. doi: 10.1038/s41594-020-0505-6. [DOI] [PubMed] [Google Scholar]
- 24.Törner R, Kupreichyk T, Gremer L, Debled EC, Fenel D, Schemmert S, et al. Structural basis for the inhibition of IAPP fibril formation by the co-chaperonin prefoldin. Nat Commun. 2022;13:2363. doi: 10.1038/s41467-022-30042-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wentink AS, Nillegoda NB, Feufel J, Ubartaitė G, Schneider CP, De Los Rios, et al. Molecular dissection of amyloid disaggregation by human HSP70. Nature. 2020;587:483–8. doi: 10.1038/s41586-020-2904-6. [DOI] [PubMed] [Google Scholar]
- 26.Ebo JS, Guthertz N, Radford SE, Brockwell DJ. Using protein engineering to understand and modulate aggregation. Curr Opin Struct Biol. 2020;60:157–66. doi: 10.1016/j.sbi.2020.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Heller GT, Sormanni P, Vendruscolo M. Targeting disordered proteins with small molecules using entropy. Trends Biochem Sci. 2015;40:491–6. doi: 10.1016/j.tibs.2015.07.004. [DOI] [PubMed] [Google Scholar]
- 28.Heller GT, Aprile FA, Bonomi M, Camilloni C, De Simone A, Vendruscolo M. Sequence specificity in the entropy-driven binding of a small molecule and a disordered peptide. J Mol Biol. 2017;429:2772–9. doi: 10.1016/j.jmb.2017.07.016. [DOI] [PubMed] [Google Scholar]
- 29.Robustelli P, Ibanez-de-Opakua A, Campbell-Bezat C, Giordanetto F, Becker S, Zweckstetter M, et al. Molecular basis of small-molecule binding to α-synuclein. J Am Chem Soc. 2022;144:2501–10. doi: 10.1021/jacs.1c07591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen J, Liu X, Chen J. Targeting intrinsically disordered proteins through dynamic interactions. Biomolecules. 2020;10:743. doi: 10.3390/biom10050743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cox SJ, Lam B, Prasad A, Marietta HA, Stander NV, Joel JG, et al. High-throughput screening at the membrane interface reveals inhibitors of amyloid-β. Biochemistry. 2020;59:2249–58. doi: 10.1021/acs.biochem.0c00328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xu Y, Maya-Martinez R, Guthertz N, Heath GR, Manfield IW, Breeze AL, et al. Tuning the rate of aggregation of hIAPP into amyloid using small-molecule modulators of assembly. Nat Commun. 2022;13:1040. doi: 10.1038/s41467-022-28660-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Young LM, Saunders JC, Mahood RA, Revill CH, Foster RJ, Tu L-H, et al. Screening and classifying small-molecule inhibitors of amyloid formation using ion mobility spectrometry–mass spectrometry. Nat Chem. 2015;7:73–81. doi: 10.1038/nchem.2129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Xu M, Loa-Kum-Cheung W, Zhang H, Quinn RJ, Mellick GD. Identification of a new α-synuclein aggregation inhibitor via mass spectrometry based screening. ACS Chem Neurosci. 2019;10:2683–91. doi: 10.1021/acschemneuro.9b00092. [DOI] [PubMed] [Google Scholar]
- 35.Bram Y, Lampel A, Shaltiel-Karyo R, Ezer A, Scherzer-Attali R, Segal D, et al. Monitoring and targeting the initial dimerization stage of amyloid self-assembly. Angew Chem Int Ed. 2015;54:2062–7. doi: 10.1002/anie.201408744. [DOI] [PubMed] [Google Scholar]
- 36.Saunders JC, Young LM, Mahood RA, Jackson MP, Revill CH, Foster RJ, et al. An in vivo platform for identifying inhibitors of protein aggregation. Nat Chem Biol. 2016;12:94–101. doi: 10.1038/nchembio.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pujols J, Peña-Díaz S, Conde-Giménez M, Pinheiro F, Navarro S, Sancho J, et al. High-throughput screening methodology to identify alpha-synuclein aggregation inhibitors. Int J Mol Sci. 2017;18:478. doi: 10.3390/ijms18030478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Staats R, Michaels TCT, Flagmeier P, Chia S, Horne RI, Habchi J, et al. Screening of small molecules using the inhibition of oligomer formation in α-synuclein aggregation as a selection parameter. Commun Chem. 2020;3:191. doi: 10.1038/s42004-020-00412-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lee J, Lee K, Lim CT. Surface plasmon resonance assay for identification of small molecules capable of inhibiting Aβ aggregation. ACS Appl Mater Interfaces. 2021;13:27845–55. doi: 10.1021/acsami.1c04833. [DOI] [PubMed] [Google Scholar]
- 40.Tóth G, Neumann T, Berthet A, Masliah E, Spencer B, Tao J, et al. Novel small molecules targeting the intrinsically disordered structural ensemble of α-synuclein protect against diverse α-synuclein mediated dysfunctions. Sci Rep. 2019;9:16947. doi: 10.1038/s41598-019-52598-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cao Q, Boyer DR, Sawaya MR, Ge P, Eisenberg DS. Cryo-EM structure and inhibitor design of human IAPP (amylin) fibrils. Nat Struct Mol Biol. 2020;27:653–9. doi: 10.1038/s41594-020-0435-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sangwan S, Sahay S, Murray KA, Morgan S, Guenther EL, Jiang L, et al. Inhibition of synucleinopathic seeding by rationally designed inhibitors. eLife. 2020;9:e46775. doi: 10.7554/eLife.46775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Armiento V, Spanopoulou A, Kapurniotu A. Peptide-based molecular strategies to interfere with protein misfolding, aggregation, and cell degeneration. Angew Chem Int Ed. 2020;59:3372–84. doi: 10.1002/anie.201906908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Joshi P, Chia S, Habchi J, Knowles TPJ, Dobson CM, Vendruscolo M. A fragment-based method of creating small-molecule libraries to target the aggregation of intrinsically disordered proteins. ACS Comb Sci. 2016;18:144–53. doi: 10.1021/acscombsci.5b00129. [DOI] [PubMed] [Google Scholar]
- 45.Coelho T, Merlini G, Bulawa CE, Fleming JA, Judge DP, Kelly JW, et al. Mechanism of action and clinical application of tafamidis in hereditary transthyretin amyloidosis. Neurology and Therapy. 2016;5:1–25. doi: 10.1007/s40120-016-0040-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kurnik M, Sahin C, Andersen CB, Lorenzen N, Giehm L, Mohammad-Beigi H, et al. Potent α-synuclein aggregation inhibitors, identified by high-throughput screening, mainly target the monomeric state. Cell Chem Biol. 2018;25:1389–402.:e9. doi: 10.1016/j.chembiol.2018.08.005. [DOI] [PubMed] [Google Scholar]
- 47.Aviolat H, Nominé Y, Gioria S, Bonhoure A, Hoffmann D, Ruhlmann C, et al. SynAggreg: a multifunctional high-throughput technology for precision study of amyloid aggregation and systematic discovery of synergistic inhibitor compounds. J Mol Biol. 2018;430:5257–79. doi: 10.1016/j.jmb.2018.09.009. [DOI] [PubMed] [Google Scholar]
- 48.Lublin AL, Link CD. Alzheimer’s disease drug discovery: in vivo screening using Caenorhabditis elegans as a model for β-amyloid peptide-induced toxicity. Drug Discov Today Technol. 2013;10:e115–e9. doi: 10.1016/j.ddtec.2012.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Faravelli G, Raimondi S, Marchese L, Partridge FA, Soria C, Mangione PP, et al. C. elegans expressing D76N β2-microglobulin: a model for in vivo screening of drug candidates targeting amyloidosis. Sci Rep. 2019;9:19960. doi: 10.1038/s41598-019-56498-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Limbocker R, Chia S, Ruggeri FS, Perni M, Cascella R, Heller GT, et al. Trodusquemine enhances Aβ42 aggregation but suppresses its toxicity by displacing oligomers from cell membranes. Nat Commun. 2019;10:225. doi: 10.1038/s41467-018-07699-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tang Y, Zhang D, Zhang Y, Liu Y, Gong X, Chang Y, et al. Introduction and fundamentals of human islet amyloid polypeptide inhibitors. ACS Appl Bio Mater. 2020;3:8286–308. doi: 10.1021/acsabm.0c01234. [DOI] [PubMed] [Google Scholar]
- 52.Meinen BA, Gadkari VV, Stull F, Ruotolo BT, Bardwell JCA. SERF engages in a fuzzy complex that accelerates primary nucleation of amyloid proteins. Proc Natl Acad Sci U S A. 2019;116:23040–9. doi: 10.1073/pnas.1913316116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Prade E, Barucker C, Sarkar R, Althoff-Ospelt G, Lopez del Amo JM, Hossain S, et al. Sulindac sulfide induces the formation of large oligomeric aggregates of the Alzheimer’s disease amyloid-β peptide which exhibit reduced neurotoxicity. Biochemistry. 2016;55:1839–49. doi: 10.1021/acs.biochem.5b01272. [DOI] [PubMed] [Google Scholar]
- 54.Yang J, Dear AJ, Yao Q-Q, Liu Z, Dobson CM, Knowles TPJ, et al. Amelioration of aggregate cytotoxicity by catalytic conversion of protein oligomers into amyloid fibrils. Nanoscale. 2020;12:18663–72. doi: 10.1039/d0nr01481h. [DOI] [PubMed] [Google Scholar]
- 55.Joshi P, Chia S, Yang X, Perni M, Habchi J, Vendruscolo M. Vitamin A and vitamin E metabolites comodulate amyloid-β aggregation. bioRxiv. 2021:2021.10.30.466561. http://biorxiv.org/content/early/2021/11/02/2021.10.30.466561.abstract . [Google Scholar]
- 56.Feng BY, Toyama BH, Wille H, Colby DW, Collins SR, May BCH, et al. Small-molecule aggregates inhibit amyloid polymerization. Nat Chem Biol. 2008;4:197–9. doi: 10.1038/nchembio.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Torosyan H, Shoichet BK. Protein stability effects in aggregate-based enzyme inhibition. J Med Chem. 2019;62:9593–9. doi: 10.1021/acs.jmedchem.9b01019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Assarsson A, Linse S, Cabaleiro-Lago C. Effects of polyamino acids and polyelectrolytes on amyloid β fibril formation. Langmuir. 2014;30:8812–8. doi: 10.1021/la501414j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cremers CM, Knoefler D, Gates S, Martin N, Dahl J-U, Lempart J, et al. Polyphosphate: a conserved modifier of amyloidogenic processes. Mol Cell. 2016;63:768–80. doi: 10.1016/j.molcel.2016.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sahoo BR, Genjo T, Nakayama TW, Stoddard AK, Ando T, Yasuhara K, et al. A cationic polymethacrylate-copolymer acts as an agonist for β-amyloid and an antagonist for amylin fibrillation. Chem Sci. 2019;10:3976–86. doi: 10.1039/c8sc05771k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Prade E, Bittner HJ, Sarkar R, Lopez del Amo JM, Althoff-Ospelt G, Multhaup G, et al. Structural mchanism of the interaction of Alzheimer Disease Aβ fibrils with the non-steroidal anti-inflammatory drug (NSAID) sulindac sulfide. J Biol Chem. 2015;290:28737–45. doi: 10.1074/jbc.M115.675215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bieschke J, Herbst M, Wiglenda T, Friedrich RP, Boeddrich A, Schiele F, et al. Small-molecule conversion of toxic oligomers to nontoxic β-sheet–rich amyloid fibrils. Nat Chem Biol. 2011;8:93. doi: 10.1038/nchembio.719. [DOI] [PubMed] [Google Scholar]
- 63.Margalith I, Suter C, Ballmer B, Schwarz P, Tiberi C, Sonati T, et al. Polythiophenes inhibit prion propagation by stabilizing prion protein (PrP) aggregates. J Biol Chem. 2012;287:18872–87. doi: 10.1074/jbc.M112.355958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Civitelli L, Sandin L, Nelson E, Khattak SI, Brorsson A-C, Kågedal K. The luminescent oligothiophene p-FTAA converts toxic Aβ1-42 species into nontoxic amyloid fibers with altered properties. J Biol Chem. 2016;291:9233–43. doi: 10.1074/jbc.M115.696229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ikenoue T, Aprile FA, Sormanni P, Ruggeri FS, Perni M, Heller GT, et al. A rationally designed bicyclic peptide remodels Aβ42 aggregation in vitro and reduces its toxicity in a worm model of Alzheimer’s disease. Sci Rep. 2020;10:15280. doi: 10.1038/s41598-020-69626-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zhang Y, Zhang D, Tang Y, Ren B, Liu F, Xu L, et al. Aromadendrin: a dual amyloid promoter to accelerate fibrillization and reduce cytotoxicity of both amyloid-β and hIAPP. Materials Advances. 2020;1:1241–52. doi: 10.1039/D0MA00418A. [DOI] [Google Scholar]
- 67.Gong H, Zhang X, Cheng B, Sun Y, Li C, Li T, et al. Bisphenol A accelerates toxic amyloid rormation of human islet amyloid polypeptide: a possible link between bisphenol A exposure and type 2 diabetes. PLoS One. 2013;8:e54198. doi: 10.1371/journal.pone.0054198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Huang L, Liao M, Yang X, Gong H, Ma L, Zhao Y, et al. Bisphenol analogues differently affect human islet polypeptide amyloid formation. RSC Adv. 2016;6:7239–48. doi: 10.1039/C5RA21792J. [DOI] [Google Scholar]
- 69.Perni M, Flagmeier P, Limbocker R, Cascella R, Aprile FA, Galvagnion C, et al. Multistep inhibition of α-synuclein aggregation and toxicity in vitro and in vivo by trodusquemine. ACS Chem Biol. 2018;13:2308–19. doi: 10.1021/acschembio.8b00466. [DOI] [PubMed] [Google Scholar]
- 70.Chen C-H, Yao T, Zhang Q, He Y-M, Xu L-H, Zheng M, et al. Influence of trehalose on human islet amyloid polypeptide fibrillation and aggregation. RSC Adv. 2016;6:15240–6. doi: 10.1039/C5RA27689F. [DOI] [Google Scholar]
- 71.Antony T, Hoyer W, Cherny D, Heim G, Jovin TM, Subramaniam V. Cellular polyamines promote the aggregation of alpha-synuclein. J Biol Chem. 2003;278:3235–40. doi: 10.1074/jbc.M208249200. [DOI] [PubMed] [Google Scholar]
- 72.Luo J, Yu C-H, Yu H, Borstnar R, Kamerlin SCL, Gräslund A, et al. Cellular polyamines promote amyloid-beta (Aβ) peptide fibrillation and modulate the aggregation pathways. ACS Chem Neurosci. 2013;4:454–62. doi: 10.1021/cn300170x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kaffy J, Brinet D, Soulier J-L, Khemtémourian L, Lequin O, Taverna M, et al. Structure–activity relationships of sugar-based peptidomimetics as modulators of amyloid β-peptide early oligomerization and fibrillization. Eur J Med Chem. 2014;86:752–8. doi: 10.1016/j.ejmech.2014.09.031. https://www.sciencedirect.com/science/article/pii/S0223523414008496 . [DOI] [PubMed] [Google Scholar]
- 74.Necula M, Breydo L, Milton S, Kayed R, van der Veer WE, Tone P, et al. Methylene blue inhibits amyloid Aβ oligomerization by promoting fibrillization. Biochemistry. 2007;46:8850–60. doi: 10.1021/bi700411k. [DOI] [PubMed] [Google Scholar]
- 75.Pilkington EH, Lai M, Ge X, Stanley WJ, Wang B, Wang M, et al. Star polymers reduce islet amyloid polypeptide toxicity via accelerated amyloid aggregation. Biomacromolecules. 2017;18:4249–60. doi: 10.1021/acs.biomac.7b01301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Mohamed T, Gujral SS, Rao PPN. Tau derived hexapeptide AcPHF6 promotes beta-Amyloid (Aβ) fibrillogenesis. ACS Chem Neurosci. 2018;9:773–82. doi: 10.1021/acschemneuro.7b00433. [DOI] [PubMed] [Google Scholar]
- 77.Sonzini S, Stanyon HF, Scherman OA. Decreasing amyloid toxicity through an increased rate of aggregation. Phys Chem Chem Phys. 2017;19:1458–65. doi: 10.1039/c6cp06765d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Boopathi S, Poma AB, Garduño-Juárez R. An overview of several inhibitors for Alzheimer’s disease: characterization and failure. Int J Mol Sci. 2021;22 doi: 10.3390/ijms221910798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Giorgetti S, Greco C, Tortora P, Aprile FA. Targeting amyloid aggregation: an overview of strategies and mechanisms. Int J Mol Sci. 2018;19:2677. doi: 10.3390/ijms19092677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ma L, Yang C, Zheng J, Chen Y, Xiao Y, Huang K. Non-polyphenolic natural inhibitors of amyloid aggregation. Eur J Med Chem. 2020;192:112197. doi: 10.1016/j.ejmech.2020.112197. [DOI] [PubMed] [Google Scholar]
- 81.Pithadia A, Brender JR, Fierke CA, Ramamoorthy A. Inhibition of IAPP aggregation and toxicity by natural products and derivatives. Journal of Diabetes Research. 2016;2016:2046327. doi: 10.1155/2016/2046327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Heller GT, Aprile FA, Michaels TCT, Limbocker R, Perni M, Ruggeri FS, et al. Small-molecule sequestration of amyloid-β as a drug discovery strategy for Alzheimer’s disease. Sci Adv. 2020;6:eabb5924. doi: 10.1126/sciadv.abb5924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Pang B, Bian X, Xing J, Liu S, Liu Z, Song F. Effects of lithospermic acid on hIAPP aggregation and amyloid-induced cytotoxicity by multiple analytical methods. Biochim Biophys Acta Proteins Proteom. 2020;1868:140283. doi: 10.1016/j.bbapap.2019.140283. [DOI] [PubMed] [Google Scholar]
- 84.Ren B, Liu Y, Zhang Y, Cai Y, Gong X, Chang Y, et al. Genistein: a dual inhibitor of both amyloid β and human islet amylin peptides. ACS Chem Neurosci. 2018;9:1215–24. doi: 10.1021/acschemneuro.8b00039. [DOI] [PubMed] [Google Scholar]
- 85.Paul A, Viswanathan GK, Mahapatra S, Balboni G, Pacifico S, Gazit E, et al. Antagonistic activity of naphthoquinone-based hybrids toward amyloids associated with Alzheimer’s disease and type-2 diabetes. ACS Chem Neurosci. 2019;10:3510–20. doi: 10.1021/acschemneuro.9b00123. [DOI] [PubMed] [Google Scholar]
- 86.Cox SJ, Rodriguez Camargo DC, Lee Y-H, Dubini RCA, Rovó P, Ivanova MI, et al. Small molecule induced toxic human-IAPP species characterized by NMR. Chem Commun. 2020;56:13129–32. doi: 10.1039/d0cc04803h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Tang Y, Liu Y, Zhang Y, Zhang D, Gong X, Zheng J. Repurposing a cardiovascular disease drug of cloridarol as hIAPP inhibitor. ACS Chem Neurosci. 2021;12:1419–27. doi: 10.1021/acschemneuro.1c00091. [DOI] [PubMed] [Google Scholar]
- 88.Choi EY, Kang SS, Lee SK, Han BH. Polyphenolic biflavonoids inhibit Amyloid-beta fibrillation and disaggregate preformed Amyloid-beta fibrils. Biomol Ther (Seoul) 2020;28:145–51. doi: 10.4062/biomolther.2019.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Saravanan MS, Ryazanov S, Leonov A, Nicolai J, Praest P, Giese A, et al. The small molecule inhibitor anle145c thermodynamically traps human islet amyloid peptide in the form of non-cytotoxic oligomers. Sci Rep. 2019;9:19023. doi: 10.1038/s41598-019-54919-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Xu J, Zhao C, Huang X, Du W. Tetracycline derivatives resist the assembly behavior of human islet amyloid polypeptide. Biochimie. 2020;174:95–106. doi: 10.1016/j.biochi.2020.04.012. [DOI] [PubMed] [Google Scholar]
- 91.Paul A, Frenkel-Pinter M, Alvarez DE, Milordini G, Gazit E, Zacco E, et al. Tryptophan-galactosylamine conjugates inhibit and disaggregate amyloid fibrils of Aβ42 and hIAPP peptides while reducing their toxicity. Commun Biol. 2020;3:484. doi: 10.1038/s42003-020-01216-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Habchi J, Arosio P, Perni M, Costa Ana R, Yagi-Utsumi M, Joshi P, et al. An anticancer drug suppresses the primary nucleation reaction that initiates the production of the toxic Aβ42 aggregates linked with Alzheimer’s disease. Sci Adv. 2016;2:e1501244. doi: 10.1126/sciadv.1501244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Chia S, Habchi J, Michaels TC, Cohen SI, Linse S, Dobson CM, et al. SAR by kinetics for drug discovery in protein misfolding diseases. Proc Natl Acad Sci U S A. 2018;115:10245–50. doi: 10.1073/pnas.1807884115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Martinez Hernandez A, Urbanke H, Gillman AL, Lee J, Ryazanov S, Agbemenyah HY, et al. The diphenylpyrazole compound anle138b blocks Aβ channels and rescues disease phenotypes in a mouse model for amyloid pathology. EMBO Mol Med. 2018;10:32–47. doi: 10.15252/emmm.201707825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Son SH, Do JM, Yoo J-N, Lee HW, Kim NK, Yoo H-S, et al. Identification of ortho catechol-containing isoflavone as a privileged scaffold that directly prevents the aggregation of both amyloid β plaques and tau-mediated neurofibrillary tangles and its in vivo evaluation. Bioorg Chem. 2021;113:105022. doi: 10.1016/j.bioorg.2021.105022. [DOI] [PubMed] [Google Scholar]
- 96.Yang T, Zhu Z, Yin E, Wang Y, Zhang C, Yuan H, et al. Alleviation of symptoms of Alzheimer’s disease by diminishing Aβ neurotoxicity and neuroinflammation. Chem Sci. 2019;10:10149–58. doi: 10.1039/c9sc03042e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.García-Viñuales S, Ilie IM, Santoro AM, Romanucci V, Zarrelli A, Di Fabio G, et al. Silybins inhibit human IAPP amyloid growth and toxicity through stereospecific interactions. Biochim Biophys Acta Proteins Proteom. 2022;1870:140772. doi: 10.1016/j.bbapap.2022.140772. [DOI] [PubMed] [Google Scholar]
- 98.Hsu J-Y, Rao Sathyan A, Hsu K-C, Chen L-C, Yen C-C, Tseng H-J, et al. Synthesis of Yakuchinone B-inspired inhibitors against islet amyloid polypeptide aggregation. J Nat Prod. 2021;84:1096–103. doi: 10.1021/acs.jnatprod.0c01162. [DOI] [PubMed] [Google Scholar]
- 99.Delogu GL, Era B, Floris S, Medda R, Sogos V, Pintus F, et al. A new biological prospective for the 2-phenylbenzofurans as inhibitors of α-glucosidase and of the islet amyloid polypeptide formation. Int J Biol Macromol. 2021;169:428–35. doi: 10.1016/j.ijbiomac.2020.12.117. [DOI] [PubMed] [Google Scholar]
- 100.Paul A, Viswanathan GK, Mohapatra S, Balboni G, Pacifico S, Gazit E, et al. Antagonistic activity of Naphthoquinone-based hybrids towards amyloids associated with Alzheimer’s disease and Type-2 diabetes. ACS Chem Neurosci. 2019;10:3510–20. doi: 10.1021/acschemneuro.9b00123. [DOI] [PubMed] [Google Scholar]
- 101.Maity D, Kumar S, AlHussein R, Gremer L, Howarth M, Karpauskaite L, et al. Sub-stoichiometric inhibition of IAPP aggregation: a peptidomimetic approach to anti-amyloid agents. RSC Chem Biol. 2020;1:225–32. doi: 10.1039/d0cb00086h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Sciacca MFM, Chillemi R, Sciuto S, Greco V, Messineo C, Kotler SA, et al. A blend of two resveratrol derivatives abolishes hIAPP amyloid growth and membrane damage. Biochim Biophys Acta Biomembr. 2018;1860:1793–802. doi: 10.1016/j.bbamem.2018.03.012. [DOI] [PubMed] [Google Scholar]
- 103.Espargaró A, Pont C, Gamez P, Muñoz-Torrero D, Sabate R. Amyloid pan-inhibitors: one family of compounds to cope with all conformational diseases. ACS Chem Neurosci. 2019;10:1311–7. doi: 10.1021/acschemneuro.8b00398. [DOI] [PubMed] [Google Scholar]
- 104.Meisl G, Kirkegaard JB, Arosio P, Michaels TC, Vendruscolo M, Dobson CM, et al. Molecular mechanisms of protein aggregation from global fitting of kinetic models. Nat Protoc. 2016;11:252. doi: 10.1038/nprot.2016.010. [DOI] [PubMed] [Google Scholar]
- 105.Cohen SI, Linse S, Luheshi LM, Hellstrand E, White DA, Rajah L, et al. Proliferation of amyloid-β42 aggregates occurs through a secondary nucleation mechanism. Proc Natl Acad Sci U S A. 2013;110:9758–63. doi: 10.1073/pnas.1218402110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Meisl G, Yang X, Hellstrand E, Frohm B, Kirkegaard JB, Cohen SI, et al. Differences in nucleation behavior underlie the contrasting aggregation kinetics of the Aβ40 and Aβ42 peptides. Proc Natl Acad Sci U S A. 2014;111:9384–9. doi: 10.1073/pnas.1401564111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Rodriguez Camargo DC, Chia S, Menzies J, Mannini B, Meisl G, Lundqvist M, et al. Surface-catalyzed secondary nucleation dominates the generation of toxic IAPP aggregates. Front Mol Biosci. 2021;8:1037. doi: 10.3389/fmolb.2021.757425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Chen J, Liu X, Chen J. Targeting intrinsically disordered proteins through dynamic interactions. Biomolecules. 2020;10 doi: 10.3390/biom10050743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Ahmed J, Fitch TC, Donnelly CM, Joseph JA, Ball TD, Bassil MM, et al. Foldamers reveal and validate therapeutic targets associated with toxic α-synuclein self-assembly. Nat Commun. 2022;13:2273. doi: 10.1038/s41467-022-29724-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Nahass GR, Sun Y, Xu Y, Batchelor M, Reilly M, Benilova I, et al. Brazilin removes toxic alpha-synuclein and seeding competent assemblies from Parkinson brain by altering conformational equilibrium. J Mol Biol. 2021;433:166878. doi: 10.1016/j.jmb.2021.166878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Suryawanshi VD, Walekar LS, Gore AH, Anbhule PV, Kolekar GB. Spectroscopic analysis on the binding interaction of biologically active pyrimidine derivative with bovine serum albumin. J Pharm Anal. 2016;6:56–63. doi: 10.1016/j.jpha.2015.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Middleton CT, Marek P, Cao P, Chiu C-c, Singh S, Woys AM, et al. Two-dimensional infrared spectroscopy reveals the complex behaviour of an amyloid fibril inhibitor. Nat Chem. 2012;4:355–60. doi: 10.1038/nchem.1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Ke PC, Zhou R, Serpell LC, Riek R, Knowles TPJ, Lashuel HA, et al. Half a century of amyloids: past, present and future. Chem Soc Rev. 2020;49:5473–509. doi: 10.1039/c9cs00199a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Miller RL, Dhavale DD, O’Shea JY, Andruska KM, Liu J, Franklin EE, et al. Quantifying regional α-synuclein, amyloid β, and tau accumulation in lewy body dementia. Annals of Clinical and Translational Neurology. 2022;9:106–21. doi: 10.1002/acn3.51482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Doig AJ, del Castillo-Frias MP, Berthoumieu O, Tarus B, Nasica-Labouze J, Sterpone F, et al. Why is research on amyloid-β failing to give new drugs for Alzheimer’s Disease? ACS Chem Neurosci. 2017;8:1435–7. doi: 10.1021/acschemneuro.7b00188. [DOI] [PubMed] [Google Scholar]
- 116.Cawood EE, Guthertz N, Ebo JS, Karamanos TK, Radford SE, Wilson AJ. Modulation of amyloidogenic protein self-assembly using tethered small molecules. J Am Chem Soc. 2020;142:20845–54. doi: 10.1021/jacs.0c10629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Petri L, Ábrányi-Balogh P, Vagrys D, Imre T, Varró N, Mándity I, et al. A covalent strategy to target intrinsically disordered proteins: discovery of novel tau aggregation inhibitors. Eur J Med Chem. 2022;231:114163. doi: 10.1016/j.ejmech.2022.114163. [DOI] [PubMed] [Google Scholar]
- 118.Palhano FL, Lee J, Grimster NP, Kelly JW. Toward the molecular mechanism(s) by which EGCG treatment temodels mature amyloid fibrils. J Am Chem Soc. 2013;135:7503–10. doi: 10.1021/ja3115696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Li Z, Wang C, Wang Z, Zhu C, Li J, Sha T, et al. Allele-selective lowering of mutant HTT protein by HTT–LC3 linker compounds. Nature. 2019;575:203–9. doi: 10.1038/s41586-019-1722-1. [DOI] [PubMed] [Google Scholar]
- 120.Hindo SS, Mancino AM, Braymer JJ, Liu Y, Vivekanandan S, Ramamoorthy A, et al. Small molecule modulators of copper-induced Aβ aggregation. J Am Chem Soc. 2009;131:16663–5. doi: 10.1021/ja907045h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Beck MW, Derrick JS, Suh J-M, Kim M, Korshavn KJ, Kerr RA, et al. Minor structural variations of small molecules tune regulatory activities toward pathological factors in Alzheimer’s Disease. ChemMedChem. 2017;12:1828–38. doi: 10.1002/cmdc.201700456. [DOI] [PMC free article] [PubMed] [Google Scholar]