Abstract
Introduction
This systematic review aimed to compare the effectiveness of nasoalveolar molding (NAM) treatment with no NAM group for babies with nonsyndromic unilateral cleft lip, alveolus, and palate.
Methods
All relevant studies from 1946 to February 2024 were identified using several sources, including The Cochrane Database of Systematic Reviews, Cochrane Central Register of Controlled Trials, LILACS, Scopus, EMBASE, MEDLINE (Ovid), and ePUB ahead of publications and nonindexed citations. The criteria for this review were as follows: (1) studies with a randomized controlled trial (RCT) and controlled clinical trial (CCT) design; (2) the target population included patients with nonsyndromic complete unilateral cleft lip, alveolus, and palate; (3) the intervention group received NAM treatment before lip repair, whereas the comparison group did not; and (4) the primary outcome was nasolabial esthetics measured using Asher McDade index. All articles were screened for the title, abstract, and full text independently and in duplicate by 2 reviewers. The quality assessment of RCT was performed using Cochrane’s risk of bias tool, and the CCT was assessed using the Risk Of Bias In Nonrandomized Studies of Interventions tool.
Results
Out of the 1432 studies retrieved, 2 RCTs and 3 CCTs were included. One RCT was assessed as having a high risk of bias, whereas the other had an unclear risk. All included CCTs were rated as having a high risk of bias. The studies showed a statistically significant reduction in cleft width favoring the NAM group, and 1 study with a high risk of bias reported significantly better long-term intercanine width for NAM. However, none of the included studies examined long-term or short-term nasolabial esthetics or other functional or esthetic outcomes over the long term.
Conclusions
No conclusions can be drawn on the nasolabial esthetics as none of the included studies evaluated this primary outcome in the short or long term. There is low certainty of evidence supporting the short-term effectiveness of NAM for cleft width reduction in patients with complete unilateral cleft lip, alveolus, and palate. Further high-quality studies are needed to evaluate the long-term effectiveness of NAM treatment, enabling clinicians and patients to make informed decisions.
Registration
The protocol for the systematic review was registered with the International Prospective Register of Systematic Reviews from the National Institute for Health Research database (Prospero No. CRD42024503388).
Funding
This work was supported by the DBT/Wellcome Trust India Alliance Grant (No. IA/CPHS/20/1/505255; awarded to Badri Thiruvenkatachari). (Am J Orthod Dentofacial Orthop 2025;■:■-■)
Introduction
Cleft lip and palate (CLP) is one of the most prevalent congenital anomalies, affecting approximately 1 in 700 live births.1 CLP is associated with various dental and skeletal anomalies that can significantly impact facial esthetics, function, and self-esteem over the long term. Affected children often require multiple surgeries throughout their lives, beginning with primary cheiloplasty in infancy.
Presurgical infant orthopedic (PSIO) procedures were introduced to improve the alignment of soft and bony tissues around the cleft before lip repair. Initially proposed by McNeil,2 these techniques have led to the development of several presurgical orthopedic devices over time. These devices include both active models with screws and pins for retention, as well as passive models, some of which use extraoral strapping or nasal stents.3,4
The nasoalveolar molding (NAM) appliance is a type of PSIO device designed to correct alveolar segments while simultaneously shaping the nose and enhancing nasal symmetry. The appliance has 2 main components: alveolar molding and nasal molding. The objectives of the former include aligning and approximating the alveolar segments as closely as possible to reduce the post-surgical relapse and to make it possible for a gingivoperiosteoplasty. The nasal molding is performed to reduce nasal deformity, improve the projection of the nasal tip, increase the columella length, and correct the septal position.4
The NAM appliance is intended for full-time use, with the primary caregiver responsible for its maintenance and the orthodontist conducting weekly adjustments. Despite its potential benefits, the limited long-term evidence supporting this technique has made it a subject of debate, with some practitioners advocating for its use whereas others remain opposed.
Globally, the Eurocleft project reported that 51.7% of cleft teams in Europe do not implement any form of maxillary infant orthopedics.5 In the United Kingdom, a centralized care system for patients with cleft has led to the discontinuation of NAM treatment across all centers.6,7 A 2011 survey of 117 cleft centers in the United States revealed that 37% offered NAM treatment,8 but a more recent survey showed a significant increase, with 86% of centers now providing NAM treatment.9
Proponents of NAM argue that these appliances enhance nasolabial esthetics, reduce cleft severity, minimize scarring, lower the need for secondary surgeries, and promote palatal growth.10–14 Conversely, some studies suggest that NAM may adversely affect future facial growth and increase the caregiver’s burden.4,15 Furthermore, most studies, including systematic reviews, report no significant long-term differences in speech, facial growth, or esthetics among these children.3,4 Not only are the findings inconsistent, but many of the studies are of low quality and retrospective in nature. In addition, there is a notable lack of high-quality, long-term studies to substantiate these claims. A previous study on systematic reviews in the field of CLP reported that 77% of published reviews had a high risk of bias, with 58% being graded as low quality.16 This issue is also evident in earlier systematic reviews on NAM, in which studies of poor quality were included, compromising the overall reliability of the results.17,18
Given the considerable burden of care borne by children with cleft conditions, it is imperative to thoroughly justify every treatment option offered. Therefore, this systematic review aimed to evaluate the existing literature on the short and long-term effectiveness of NAM treatment in patients with nonsyndromic unilateral CLP (UCLP).
Material and Methods
This systematic review was performed in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analysis Guidelines 2020.19
Protocol registration
The protocol for the systematic review was registered with the International Prospective Register of Systematic Reviews from the National Institute for Health Research database (No. CRD42024503388).
Eligibility criteria
Study design
Randomized controlled trials (RCTs) and controlled clinical trials (CCTs) comparing NAM treatment before lip repair with a nontreatment control group were included in this systematic review.
Population
Nonsyndromic patients with complete unilateral cleft lip, alveolus, and palate.
Intervention
NAM treatment before lip repair.
Comparison
No NAM treatment before lip repair.
Primary outcome
Nasolabial esthetics measured using the Asher McDade index.
Secondary outcomes
(1) Cleft width before lip surgery measured in millimeters, (2) arch length measured in millimeters, (3) number of revision surgeries, (4) intercanine width, (5) breakages (yes or no), (6) complications (yes or no), and (7) cost-effectiveness.
Information sources, search strategy, and study selection
Several databases such as The Cochrane Database of Systematic Reviews, Cochrane Central Register of Controlled Trials (CENTRAL), LILACS, Scopus, EMBASE, MEDLINE (Ovid), Web of Science, and ePUB were searched from 1946 to February 29, 2024, for all relevant studies to be included in the systematic review. The studies were included without restrictions on language and date of publication. Google Scholar and PubMed were also searched for completion, including all relevant articles. In addition, the ongoing and unpublished studies were searched using Dissertation data (www.theses.com), Grey literature (www.opengrey.eu), ClinicalTrials.gov (http://www.ClinicalTrials.gov), and ISRCTN registry (http://www.controlled-trials.com). A sample search strategy is shown in Supplementary Table I.
To identify unpublished studies and ongoing trials, the respective first-named authors were contacted. From the identified articles, the reference lists and relevant review articles were also checked for further possible studies to be included.
The potentially relevant articles were screened for the title and abstract independently and in duplicate by 2 reviewers (S.C. and B.T.) after the removal of the duplicates. The reviewers were not blinded to the authors or the results of the research. The full-text articles were examined for eligibility independently by 2 authors (S.C. and B.T.), and disagreements were resolved after discussion.
Data items and collection
Data extraction was performed for the selected articles using a prepiloted data extraction form (Supplementary Table II). The data extraction was completed for all the included studies independently and in duplicate by 2 reviewers (S.C. and B.T.). Discrepancies in the extracted data were resolved through discussions. Details included in the data extraction form were as follows: (1) general information such as extractor details, time, etc.; (2) study information such as title, year, authors, etc.; (3) study eligibility including Population, Intervention, Comparator, and Outcome assessment; (4) risk of bias; (5) characteristics of included studies such as details of the trial, participants, interventions, etc.; and (6) outcomes such as short-term and long-term nasolabial esthetics, cleft width before lip surgery, arch length, breakages, complications, quality of life, and cost-effectiveness.
Risk of bias/quality assessment in individual studies
Cochrane Collaboration’s risk of bias 2, as described in the Cochrane Handbook for Systematic Reviews of Interventions, was used to assess the risk of bias in the included RCTs.20 Risk Of Bias In Nonrandomized Studies of Interventions I tool was used to assess the risk of bias for non-RCTs.21 The risk of bias was assessed separately by 2 independent reviewers (S.C. and B.T.). Any potential disagreements were resolved by discussion between the 2 reviewers. The overall risk of bias for each study was summarized as low, unclear, or high.
Summary measures, approach to synthesis, and analysis
The data collection was completed for the eligible studies, and the authors were contacted if further information was needed. The data were grouped and classified into 2 categories: RCTs and CCTs. The RevMan software (version 5.3; The Nordic Cochrane Centre, Cochrane Collaboration; Copenhagen, Denmark) was used to assess the collected data quantitatively.
Mean differences (MD) with 95% confidence intervals (CIs) were calculated for continuous outcomes. In the instance of dichotomous outcomes, risk ratios with 95% CIs were shown. Random effects model was planned as clinical, and statistical heterogeneity was anticipated.
The study protocol and methodology, treatment interventions, timing of data collection, measurement techniques, and measured outcomes were all reviewed to assess and gauge the clinical heterogeneity of the included studies. The Cochrane’s test for heterogeneity and the I2 test were used to assess statistical heterogeneity.
Publication bias
Standard funnel plots and contoured enhanced funnel plots would be drawn to identify publication bias if >10 studies were included in the meta-analysis.
Additional analyses
Sensitivity analyses were planned to address studies at higher risk of bias, publication bias, and other potential sources of heterogeneity. All analyses were undertaken using the RevMan software version 5.4.
Summarizing findings
A summary of findings table was created to record the results of the main outcomes. The Grading of Recommendation, Assessment, Development, and Evaluation instrument was used to assess the quality of the resultant evidence for each outcome.22
Results
Study selection
The search strategy identified 1432 articles, of which, after removal of duplicates and screening for relevant articles, 20 full-text articles were retrieved for further evaluation. The full-text articles retrieved were assessed for our inclusion criteria, and 15 articles were excluded for specific reasons (Table I). Finally, a total of 5 studies (2 RCTs and 3 CCTs)23–27 were included for the final analysis. The process of selection of articles is shown in the Preferred Reporting Items for Systematic Reviews and Meta-Analysis flowchart (Fig 1).
Table I. Excluded studies and their reasons for exclusion.
| Study No. | Author (y) | Reason for exclusion |
|---|---|---|
| 1 | Alhayyan (2018) | Retrospective |
| 2 | Bhutiani (2020) | DSIC-BCLP included |
| 3 | Broder (2016) | DSIC-questionnaire study |
| 4 | Fuchigami (2017) | DSIC-retrospective |
| 5 | Gomez (2012) | DSIC-no control |
| 6 | Hui Xi (2014) | DSIC-not prospective |
| 7 | Liang (2017) | DSIC-retrospective |
| 8 | Mariqueo (2018) | DSIC-retrospective |
| 9 | Nuryilmaz (2018) | DSIC-retrospective |
| 10 | Quast (2018) | DSIC-grading study |
| 11 | Shen (2015) | DSIC-no control |
| 12 | Singh (2005) | DSIC-no control |
DSIC, does not satisfy inclusion criteria; BCLP, bilateral CLP.
Fig 1.
PRISMA flowchart. PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-Analysis.
Study characteristics
Five studies (2 RCTs23,26 and 3 CCTs24,25,27) that included 262 participants evaluated the effectiveness of the NAM appliance before lip surgery (Table II). The 2 RCT studies23,26 included 68 participants, 42 males and 26 females. Both studies were performed in Egypt. Saad et al23 used broad inclusion criteria and included Egyptian infants with complete UCLP aged <2 weeks. It was a prospective, 2-arm RCT in which 40 patients were randomly allocated to NAM and non-NAM groups through closed envelope concealment and were analyzed at the end of the study. The maxillary casts were blindly analyzed at 3-time points (initial visit, 3 weeks, and after 6 weeks) to check for the amount and rate of maxillary changes, including the cleft gap in horizontal and transverse dimensions and rotation of the alveolar segments.
Table II. Characteristics of included study.
| Study | Participants | Age | Inclusion criteria | Exclusion criteria | Interventions | Outcomes |
|---|---|---|---|---|---|---|
| Saad et al23 Alexandria University, Alexandria, Egypt Prospective, 2-arm RCT |
SG: 20 (11 M, 9F) CG: 20 (12 M, 8 F) |
SG Initial visit: 13.5 ± 1.6 d After 3 wk: 36.5 ± 2.2 d After 6 wk: 60.9 ± 2.7 d CG Initial visit: 13.3 ± 1.6 d After 3 wk: 36.1 ± 2.3 d After 6 wk: 60.3 ± 2.6 d |
Egyptian infants Complete UCLAP Age ≤2 wk Family consented | Non-Egyptian Syndromic CLP Congenital malformations and soft-tissue bands |
SG: NAM CG: non-NAM |
Changes in cleft width between NAM and non-NAM |
| Abd El-Ghafour et al26 Cairo University, Cairo, Egypt Prospective, 2-arm RCT |
SG: 17 CG: 17 Total: 28 (19 M, 9 F) |
SG: 12.58 ± 8.24 CG: 12.74 ± 7.28 |
Infants UCLAP Aged 1-30 d Medically fit |
No information available | SG: D-NAM CG: notreatment |
Changes in maxillary arch dimensions between NAM and non-NAM group |
| Shetty et al25 Nitte University, Mangalore, India Prospective, 3-arm study |
60 participants | SG subgroups
CG subgroups
|
No information available | No information available | SG: NAM CG: non-NAM |
Intersegment distance Intercanine width Posterior arch width |
| Yu et al24 Jiao Tong University, China Prospective CCT Funding: National Natural Science Foundation of China (grant No. 81271 182) |
SG: 15 (7 M, 8 F) CG: 15 (7 M, 8 F) |
SG: 17.53 d CG: 116.64 d |
Nonsyndromic UCLP Complete medical record | No information available | SG: CAD-NAM therapy CG: non-NAM |
Maxillary morphologic changes |
| de Souza et al27 Prospective trial Curitiba, Brazil |
SG: 26 (18 M, 8 F) CG: 12 (7 M, 5 F) | No information available | Nonsyndromic UCL/P | No information available | SG: NAM CG: non-NAM |
Symmetry of face and maxillary arch Changes in arch perimeter, arch length, and labial frenum angle measured in digital models |
SG, study group; M, males; F, females; CG, control group; UCLAP, unilateral cleft lip, alveolus, and palate; D-NAM, digital NAM; CAD, computer-aided design.
Abd El-Ghafour et al26 performed a prospective, balanced, randomized, single-blinded controlled trial with 34 nonsyndromic infants with UCLP (19 males and 9 females) randomly assigned to NAM and non-NAM groups using sequence generation and opaque sealed envelopes. The NAM group received a digitally designed NAM appliance, whereas there was no treatment with the control group. The plaster casts were scanned and analyzed to determine the maxillary arch changes at the start of treatment and after D-NAM but before lip closure to determine the effectiveness of the NAM appliance.
Yu et al24 performed a prospective CCT with 30 patients (14 males and 16 females) grouped into NAM and control groups. Participants in the NAM group received computer-aided design–NAM, whereas the control group did not receive any treatment before lip surgery. The digital models were then analyzed to determine the maxillary alveolar segment changes and compare the results between the computer-aided design–NAM and the nontreatment control group.
Shetty et al25 conducted a CCT in India with 120 patients with UCLP grouped into NAM and nontreatment control groups. Subjects were followed up until 6 years old. The NAM (treatment) group was further subdivided into 3 subgroups (20 patients per group) based on the age at which NAM was initiated: <1 month, 1-6 months, and 6-12 months. Similarly, the non-NAM (control) group was further subdivided into 3 groups based on the age at which undergoing lip surgery was initiated: 6 months, 6-9 months, and 9-15 months. Patients were evaluated at the first visit, before cheiloplasty, and at 6 years old. The intersegment distance, intercanine width, and posterior arch width were analyzed for the groups.
De Souza et al27 evaluated 26 infants with UCLP who were grouped into NAM and nontreatment control groups. The babies were photographed in 2 stages (first month of life and post-NAM but pre-lip surgery) to determine the facial symmetry, and their digital models were analyzed to evaluate the effect of the NAM appliance. The frontal and basilar photographs were taken using a tripod placed from a constant distance using a macro lens digital single-lens reflex camera. The facial symmetry was calculated by measuring the nasal width/intercanthal length ratio, mouth width/interpupillary distance ratio, and columella angle measurement was taken from the standard photographs.
Risk of bias in RCT studies
The methodological quality assessment of 2 RCTs, Abd El-Ghafour et al26 and Saad et al23 are given in Figures 2 and 3. Saad et al23 raised concerns because of the lack of information on sequence generation. Abd El-Ghafour et al26 also raised concerns because of the high risk of bias related to uneven intergroup and intragroup gender distribution. In addition, the authors failed to perform an intention-to-treat analysis.
Fig 2.
Risk of bias 2 summary for RCTs. Green, low risk of bias; yellow, unclear risk of bias; red, high risk of bias.
Fig 3.
Risk of bias graph for RCTs. Specific domains are presented as percentages.
Risk of bias for CCTs
The risk of bias for the included CCTs performed using the Risk Of Bias In Nonrandomized Studies of Interventions tool is shown in Figures 4 and 5. All 3 CCTs24,27 were assessed to be of critical risk. The bias because of the selection of participants was judged as critical in 2 studies as they all used convenience sampling. Shetty et al,25 did not report their sampling technique. Bias in the measurement of outcome was assessed as serious as Yu et al24 and de Souza et al27 did not perform blinding with a high risk that the outcome measure will be influenced by the knowledge of an intervention. In 2 studies, Yu et al24 and Shetty et al25 did not follow an a priori protocol and, thus, introduced selection bias in the reported result.
Fig 4. Risk Of Bias In Nonrandomized Studies of Interventions tool summary for CCTs.
Fig 5.
Risk of bias graph of CCTs. Specific domains are presented as percentages.
Summary of the results
None of the included studies reported on the primary outcome, the short-term or long-term nasolabial esthetics.
Cleft width before lip surgery
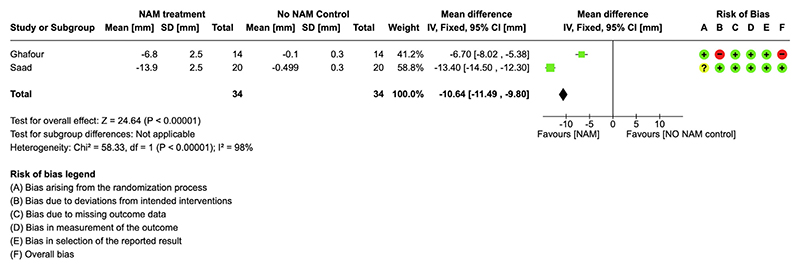
All the included studies, except for de Souza et al,27 investigated cleft width before lip surgery and favored the NAM group. Saad et al23 studied 40 patients and reported a statistically significant change of −13.668 6 0.559 mm in the NAM group and −0.499 6 0.073 mm in the non-NAM group for cleft width at the end of NAM treatment (P <0.001). Abd El-Ghafour et al26 reported a statistically significant change favoring the NAM group with a difference of 6.7 mm (95% CI, 4.28-9.11) between the NAM and non-NAM groups (P <0.001). Shetty et al25 had a sample size of 40 patients and reported a statistically significant difference favoring the NAM group with a difference of −11.45 mm (95% CI, −12.54 to −10.37) between the groups (P <0.001). Yu et al24 had a sample size of 30 subjects and reported a statistically significant difference between the NAM and non-NAM control groups (MD, 4.23; P <0.001).
Arch length before lip surgery
Only 1 of the included studies,27 investigated changes in arch length before lip surgery in NAM and non-NAM groups. The authors defined arch length as the distance from the labial frenum to the center point of the line drawn between the left and right posterior points on the maxillary arch. The results showed a similar arch length in both the NAM and non-NAM groups at the pre-lip surgery time point. However, the pre-NAM baseline arch length was higher in the non-NAM group.
Intercanine width
One study25 evaluated intercanine width at baseline, pre-lip surgery, and 6 years old. The results showed a statistically significantly increased arch length for the NAM group at (3.30 mm; P <0.001) at 6 years old. However, the results were not significant at the post-NAM/pre-lip surgery time point between the NAM and no NAM group (− 0.30 mm; P = 5 0.52).
Other outcomes
Abd El-Ghafour et al26 reported that no significant harms were observed in the treatment group. None of the other outcomes, such as the number of revision surgeries, complications, breakages, quality of life, and cost-effectiveness, were reported in the included studies.
Meta-analysis
Considering the heterogeneity, the CCTs and RCTs were subgrouped for meta-analysis.
Cleft width
The results of the RCTs23,26 comparing cleft width in NAM and non-NAM groups showed that the cleft width in the NAM group was reduced significantly more than in the non-NAM group (MD, 11.00 [95% CI, 11.88-10.11]; I2 98%) (Fig 6).
Fig 6. Meta-analysis of parallel-group RCTs evaluating cleft width change with treatment.
The results from CCTs24,25 on cleft width measurement between NAM and non-NAM control group at precheiloplasty showed a statistically significant difference (MD, 10.57 [95% CI 11.52-9.61]; I2 95%), favoring the NAM group (Fig 7).
Fig 7. Meta-analysis of CCTs evaluating cleft width change with treatment.
Intercanine width
The intercanine width in the long term (6 years) was reported in Shetty et al.25 The results showed a statistically significant difference favoring the NAM treatment group (MD, 3.30 [95% CI, 2.58-4.02]) (Fig 8).
Fig 8. Meta-analysis of CCTs evaluating intercanine arch width at 6 years.
Publication bias was not attempted, as there were not enough studies included in the meta-analysis
Summary of findings
The Grading of Recommendation, Assessment, Development, and Evaluation assessment of the quality of the collected evidence for cleft width immediately post-NAM treatment found low-quality evidence in favor of the NAM group compared with the non-NAM group (Table III). The quality of evidence was down-graded because of the high risk of bias in 1 of the 2 included studies and high heterogeneity.
Table III. Summary of findings for the difference in cleft width between pre-NAM and post-NAM (pre-lip surgery) treatment†.
| Outcomes | Anticipated absolute effects‡ (95% CI) |
Relative effect
(95% CI) |
No. of
Participants (studies) |
Certainty of the evidence (GRADE)$ | Comments | |
|---|---|---|---|---|---|---|
|
Risk with no NAM
control |
Risk with NAM
treatment |
|||||
| Cleft width difference | Tire inean cleft width difference was 0 mm | MD was 10.64 lower (11.49 lower vs 9.8 lower) |
– | 68 (2 RCTs) | ⊕⊕◯◯ Low¶# |
There is some evidence that NAM width reduces cleft width, but the quality of evidence is low |
GRADE, Grading of Recommendation, Assessment, Development, and Evaluation.
†NAM treatment compared with non-NAM control for patients with UCLP. Patient or population: health problem or population. Intervention: NAM treatment. Comparison: non-NAM control; ‡The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI); §GRADE Working Group grades of evidence: (1) high certainty—we are very confident that the true effect lies close to that of the estimate of the effect; (2) moderate certainty—we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different; (3) low certainty—our confidence in the effect estimate is limited: the true effect may be sub-stantially different from the estimate of the effect; and (4) very low certainty—we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect; ¶Downgraded as 1 of the 2 studies that were at high risk of bias; #Downgraded because of high heterogeneity (χ2 = 58.33; degrees of freedom = 1; P <0.00001; I2 + 98%).
Discussion
The NAM treatment performed before lip surgery significantly reduces the width of the cleft. However, none of the studies included in this review specifically addressed the primary objective of NAM treatment, which is to facilitate nasal molding and enhance nasolabial esthetics. The review does not provide any data on long-term outcomes, as none of the included studies assessed meaningful long-term results.
Quality of studies included
This systematic review included 2 RCTs23,26 and 3 CCTs.24,25,27 One RCT26 was considered to have a high risk of bias because of the lack of reporting on sequence generation, significant gender bias, and failure to perform an intention-to-treat analysis. The other RCT23 was assessed as having a low risk of bias. All included CCTs24,25,27 were judged to be at high risk of bias, primarily because of improper participant selection and a lack of assessor blinding.
A previous study on systematic reviews in the field of CLP reported that 77% of published reviews had a high risk of bias, with 58% being graded as low quality.16 This issue is also evident in earlier systematic reviews on NAM, in which studies of poor quality were included, compromising the overall reliability of the results.17,18 In contrast, this review exclusively includes prospective studies (CCTs and RCTs) that evaluate the effectiveness of NAM.
NAM appliance design and technique
There was a huge variation in the NAM appliance technique and protocol used; of the 5 included studies, 324,26,27 used digitally manufactured NAM appliances with nasal stent addition during treatment, whereas the remaining 2 studies23,25 employed conventional acrylic NAM fabrication using Grayson’s technique. Patient follow-up was conducted weekly in 323,24,27 of the 5 studies. Shetty et al25 did not report the frequency of follow-up, whereas Abd El-Ghafour et al26 followed up with patients biweekly. Two studies23,24 initiated nasal stent use around 3 weeks after starting treatment, 1 study began nasal molding from the outset, another study26 started nasal molding when the cleft width was under 4 mm, and 1 study25 reported beginning nasal correction when the cleft width was between 8 and 10 mm.
Outcomes
Cleft width before lip surgery
Numerous studies have examined the effect of NAM on alveolar cleft width reduction before lip surgery, although most of these are short-term and retrospective. A previous systematic review on NAM highlighted the need for high-quality studies to better inform clinicians about the long-term benefits of NAM treatment.28 This systematic review provides moderate evidence of short-term cleft width reduction; however, no long-term data are available to justify the lasting benefits of the NAM appliance.
Short-term improvements in cleft width do not guarantee that these results will be maintained over time to provide superior esthetic and functional outcomes. Long-term, meaningful data on the functional, dentofacial, and esthetic outcomes of NAM are crucial. It is also important to note that the long-term benefits should outweigh the significantly increased burden placed on caregivers by NAM treatment.
Nasolabial esthetics
Several retrospective studies (refer) have demonstrated that NAM treatment improves nasolabial esthetics in the long term.13,15,29,30 However, this review included 5 prospective studies, none of which reported on nasolabial esthetics in either the short or long term. This lack of high-quality studies poses a challenge for clinicians when advising patients about the long-term benefits of NAM. This review highlights the urgent need for well-designed prospective studies to evaluate the long-term impact of NAM on nasolabial esthetics.
Arch width
One study25 reported a significant improvement in arch width among NAM patients in the long term. However, these results should be interpreted with caution, as the study was assessed to have a high risk of bias. In contrast, previous studies have reported no significant improvement in intercanine width in the deciduous dentition or arch length.31,32 The discrepancies among these studies may be attributed to differences in follow-up duration and sample sizes among the studies.
Other secondary outcomes
The other secondary outcomes of this systematic review were the number of revision surgeries, as well as the harms, complications, and breakages associated with the NAM appliance. Although these outcomes are crucial for caregivers in their decision-making process, it is unfortunate that none of the prospective studies reported on them. Although some retrospective studies have claimed a reduction in revision surgeries with NAM,33,34 these findings have not been substantiated by high-quality studies.
Studies on NAM treatment have reported lip and cheek/skin irritation because of taping and gingival ulceration because of excessive pressure.35,36 Although Abd El-Ghafour et al26 reported having no significant harm in their population with no further information, none of the studies investigated these outcomes. It has been shown that these side effects are temporary and do not affect the overall outcome or cause long-term effects on patients.36
NAM treatment has a considerable financial burden on caregivers. Although the total direct medical costs associated with the PSIO group are likely higher than the nontreatment group, some studies have reported that the overall cost is less as there is less likely need for further revision surgeries with NAM, and this out-weighs the cost for NAM treatment.37,38 Unfortunately, none of the studies included in this review reported on this outcome.
Limitations
Because of the difference in study designs and heterogeneity in the outcome measures used, the review was restricted to the number of studies included in the meta-analysis. The other main limitation of this review was the lack of data from the included studies on the primary outcome, nasolabial esthetics. In addition, there was a lack of data on most of the secondary outcomes and a serious lack of data on long-term results from NAM treatment. This shows the urgent need for long-term prospective, high-quality studies on NAM treatment to allow patients to make informed decisions.
Conclusions
This review included 5 studies, 4 of which were judged to have a high risk of bias and 1 a low risk of bias.
No conclusions can be drawn on the nasolabial esthetics as none of the included studies evaluated this primary outcome in the short or long term.
There is low evidence for cleft width reduction with NAM treatment in patients with complete unilateral cleft lip, alveolus, and palate.
Further high-quality long-term studies evaluating the effectiveness of NAM appliances are required to inform clinicians and patients globally.
Supplementary Material
Supplementary Data
Supplementary data associated with this article can be found, in the online version, at https://doi.org/10.1016/j.ajodo.2025.01.012
Acknowledgments
The authors thank Manoj Prathap and Karthika Nambiar, part of the multicentric randomised controlled trial (NAMUC) study team, for their help with the study. The authors also thank our funders, The India Alliance.
This work was supported by the DBT/Wellcome Trust India Alliance Grant (No. IA/CPHS/20/1/505255; awarded to Badri Thiruvenkatachari).
Footnotes
Author Credit Statement
Badri Thiruvenkatachari contributed to conceptualization, funding acquisition, methodology, investigation, formal analysis, and original draft preparation; Subhiksha Chakkaravarthi contributed to investigation, formal analysis, data collection, data analysis, and original draft preparation; and Aarthi Bhuvaraghan contributed methodology and original draft preparation.
All authors have completed and submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest, and none were reported.
References
- 1.Vanderas AP. Incidence of cleft lip, cleft palate, and cleft lip and palate among races: a review. Cleft Palate J. 1987;24:216–25. [PubMed] [Google Scholar]
- 2.McNEIL CK. Orthodontic procedures in the treatment of congenital cleft palate. Dent Rec (London) 1950;70:126–32. [PubMed] [Google Scholar]
- 3.Papadopoulos MA, Koumpridou EN, Vakalis ML, Papageorgiou SN. Effectiveness of pre-surgical infant orthopedic treatment for cleft lip and palate patients: a systematic review and meta-analysis. Orthod Craniofac Res. 2012;15:207–36. doi: 10.1111/j.1601-6343.2012.01552.x. [DOI] [PubMed] [Google Scholar]
- 4.Levy-Bercowski D, Abreu A, DeLeon E, Looney S, Stockstill J, Weiler M, et al. Complications and solutions in presurgical nasoalveolar molding therapy. Cleft Palate Craniofac J. 2009;46:521–8. doi: 10.1597/07-236.1. [DOI] [PubMed] [Google Scholar]
- 5.Shaw B. The Eurocleft Project 1996-2000. Ios Press; Amsterdam: 2000. [Google Scholar]
- 6.Bearn D, Mildinhall S, Murphy T, Murray JJ, Sell D, Shaw WC, et al. Cleft lip and palate care in the United Kingdom–the Clinical Standards Advisory Group (CSAG) Study. Part 4: outcome comparisons, training, and conclusions. Cleft Palate Craniofac J. 2001;38:38–43. doi: 10.1597/1545-1569_2001_038_0038_clapci_2.0.co_2. [DOI] [PubMed] [Google Scholar]
- 7.Sandy J, Williams A, Mildinhall S, Murphy T, Bearn D, Shaw B, et al. The Clinical Standards Advisory Group (CSAG) cleft lip and palate study. Br J Orthod. 1998;25:21–30. doi: 10.1093/ortho/25.1.21. [DOI] [PubMed] [Google Scholar]
- 8.Sischo L, Chan JW, Stein M, Smith C, van Aalst J, Broder HL. Nasoalveolar molding: prevalence of cleft centers offering NAM and who seeks it. Cleft Palate Craniofac J. 2012;49:270–5. doi: 10.1597/11-053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Avinoam SP, Kowalski HR, Chaya BF, Shetye PR. Current presurgical infant orthopedics practices among American cleft palate association-approved cleft teams in North America. J Craniofac Surg. 2022;33:2522–8. doi: 10.1097/SCS.0000000000008790. [DOI] [PubMed] [Google Scholar]
- 10.Ezzat CF, Chavarria C, Teichgraeber JF, Chen JW, Stratmann RG, Gateno J, et al. Presurgical nasoalveolar molding therapy for the treatment of unilateral cleft lip and palate: a preliminary study. Cleft Palate Craniofac J. 2007;44:8–12. doi: 10.1597/06-009. [DOI] [PubMed] [Google Scholar]
- 11.Grayson BH, Cutting C, Wood R. Preoperative columella lengthening in bilateral cleft lip and palate. Plast Reconstr Surg. 1993;92:1422–3. [PubMed] [Google Scholar]
- 12.Lee CTH, Grayson BH, Cutting CB, Brecht LE, Lin WY. Prepubertal midface growth in unilateral cleft lip and palate following alveolar molding and gingivoperiosteoplasty. Cleft Palate Craniofac J. 2004;41:375–80. doi: 10.1597/03-037.1. [DOI] [PubMed] [Google Scholar]
- 13.Maull DJ, Grayson BH, Cutting CB, Brecht LL, Bookstein FL, Khorrambadi D, et al. Long-term effects of nasoalveolar molding on three-dimensional nasal shape in unilateral clefts. Cleft Palate Craniofac J. 1999;36:391–7. doi: 10.1597/1545-1569_1999_036_0391_lteonm_2.3.co_2. [DOI] [PubMed] [Google Scholar]
- 14.Barillas I, Dec W, Warren SM, Cutting CB, Grayson BH. Nasoalveolar molding improves long-term nasal symmetry in complete unilateral cleft lip–cleft palate patients. Plast Reconstr Surg. 2009;123:1002–6. doi: 10.1097/PRS.0b013e318199f46e. [DOI] [PubMed] [Google Scholar]
- 15.Kornbluth M, Campbell RE, Daskalogiannakis J, Ross EJ, Glick PH, Russell KA, et al. Active presurgical infant orthopedics for unilateral cleft lip and palate: intercenter outcome comparison of Latham, modified McNeil, and nasoalveolar molding. Cleft Palate Craniofac J. 2018;55:639–48. doi: 10.1177/1055665618757367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Srivastav S, Tewari N, Antonarakis GS, Duggal R, Saji S, Lokade AK, et al. Evidence mapping and quality analysis of systematic reviews on various aspects related to cleft lip and palate. J Clin Med. 2023;12:6002. doi: 10.3390/jcm12186002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Padovano WM, Skolnick GB, Naidoo SD, Snyder-Warwick AK, Patel KB. Long-term effects of nasoalveolar molding in patients with unilateral cleft lip and palate: a systematic review and meta-analysis. Cleft Palate Craniofac J. 2022;59:462–74. doi: 10.1177/10556656211009702. [DOI] [PubMed] [Google Scholar]
- 18.Jahanbin A, Alizadeh FL, Bardideh E, Sharifi S, Nazari MS. Does presurgical nasoalveolar molding reduce the need for future bone grafting in cleft lip and palate patients? a systematic review and meta-analysis. J Craniofac Surg. 2022;33:2095–9. doi: 10.1097/SCS.0000000000008616. [DOI] [PubMed] [Google Scholar]
- 19.Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. doi: 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al. Cochrane handbook for systematic reviews of interventions. 2nd ed. John Wiley & Sons; Chichester: 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sterne JA, Hernán MA, Reeves BC, Savović J, Berkman ND, Viswanathan M, et al. Robins-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ. 2016;355:i4919. doi: 10.1136/bmj.i4919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brennan SE, Johnston RV. Research note: interpreting findings of a systematic review using GRADE methods. J Physiother. 2023;69:198–202. doi: 10.1016/j.jphys.2023.05.012. [DOI] [PubMed] [Google Scholar]
- 23.Saad MS, Fata M, Farouk A, Habib AMA, Gad M, Tayel MB, et al. Early progressive maxillary changes with nasoalveolar molding: randomized controlled clinical trial. JDR Clin Trans Res. 2020;5:319–31. doi: 10.1177/2380084419887336. [DOI] [PubMed] [Google Scholar]
- 24.Yu Q, Gong X, Shen G. CAD presurgical nasoalveolar molding effects on the maxillary morphology in infants with UCLP. Oral Surg Oral Med Oral Pathol Oral Radiol. 2013;116:418–26. doi: 10.1016/j.oooo.2013.06.032. [DOI] [PubMed] [Google Scholar]
- 25.Shetty V, Agrawal RK, Sailer HF. Long-term effect of presurgical nasoalveolar molding on growth of maxillary arch in unilateral cleft lip and palate: randomized controlled trial. Int J Oral Maxillofac Surg. 2017;46:977–87. doi: 10.1016/j.ijom.2017.03.006. [DOI] [PubMed] [Google Scholar]
- 26.Abd El-Ghafour M, Aboulhassan MA, Fayed MMS, El-Beialy AR, Eid FHK, Hegab SED, et al. Effectiveness of a novel 3D-printed nasoalveolar molding appliance (D-NAM) on improving the maxillary arch dimensions in unilateral cleft lip and palate infants: a randomized controlled trial. Cleft Palate Craniofac J. 2020;57:1370–81. doi: 10.1177/1055665620954321. [DOI] [PubMed] [Google Scholar]
- 27.de Souza TM, Batista ST, de Souza RXS, Rezende SE, Alessi MS, Almeida TFA, et al. The effects of NAM on the symmetry of the face and maxillary arch in babies with unilateral cleft. J Craniofac Surg. 2023;34:1618–24. doi: 10.1097/SCS.0000000000009469. [DOI] [PubMed] [Google Scholar]
- 28.Van Der Heijden P, Dijkstra PU, Stellingsma C, Van Der Laan BF, Korsten-Meijer AGW, Goorhuis-Brouwer SM. Limited evidence for the effect of presurgical nasoalveolar molding in unilateral cleft on nasal symmetry: a call for unified research. Plast Reconstr Surg. 2013;131:62e–71e. doi: 10.1097/PRS.0b013e318267d4a5. [DOI] [PubMed] [Google Scholar]
- 29.Nayak T, Bonanthaya K, Parmar R, Shetty PN. Long-term comparison of the aesthetic outcomes between nasoalveolar molding– and non–nasoalveolar molding–treated patients with unilateral cleft lip and palate. Plast Reconstr Surg. 2021;148:775e–84e. doi: 10.1097/PRS.0000000000008463. [DOI] [PubMed] [Google Scholar]
- 30.Singer E, Daskalogiannakis J, Russell KA, Mercado AM, Hathaway RR, Stoutland A, et al. Burden of care of various infant orthopedic protocols for improvement of nasolabial esthetics in patients with CUCLP. Cleft Palate Craniofac J. 2018;55:1236–43. doi: 10.1177/1055665618766978. [DOI] [PubMed] [Google Scholar]
- 31.Ocak I, Akarsu-Guven B, Karakaya J, Ozgur F, Aksu M. Effects of nasoalveolar molding on maxillary arch dimensions and malocclusion characteristics in primary dentition patients with cleft lip and palate. Int J Paediatr Dent. 2024;34:94–101. doi: 10.1111/ipd.13102. [DOI] [PubMed] [Google Scholar]
- 32.Clark SL, Teichgraeber JF, Fleshman RG, Shaw JD, Chavarria C, Kau CH, et al. Long-term treatment outcome of presurgical nasoalveolar molding in patients with unilateral cleft lip and palate. J Craniofac Surg. 2011;22:333–6. doi: 10.1097/SCS.0b013e318200d874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rubin MS, Clouston S, Ahmed MM, Lowe K, Shetye PR, Broder HL, et al. Assessment of presurgical clefts and predicted surgical outcome in patients treated with and without nasoalveolar molding. J Craniofac Surg. 2015;26:71–5. doi: 10.1097/SCS.0000000000001233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Grayson BH, Maull D. Nasoalveolar molding for infants born with clefts of the lip, alveolus, and palate. Clin Plast Surg. 2004;31:149–58. doi: 10.1016/S0094-1298(03)00140-8. [DOI] [PubMed] [Google Scholar]
- 35.Garcés Alvear GA, Moreno Soza MIB, Ormeño Quintana ADP, Gutiérrez Melis CM. Complications during Grayson presurgical nasoalveolar molding method in nonsyndromic infants with complete unilateral cleft lip and palate. J Craniofac Surg. 2021;32:2159–62. doi: 10.1097/SCS.0000000000007532. [DOI] [PubMed] [Google Scholar]
- 36.Jalil J, Bonanthaya K, Parmar R, Bijapur SU. Nasoalveolar molding: benefits and burdens. Plast Aesthet Res. 2023;10:18. [Google Scholar]
- 37.Pfeifer TM, Grayson BH, Cutting CB. Nasoalveolar molding and gingivoperiosteoplasty versus alveolar bone graft: an outcome analysis of costs in the treatment of unilateral cleft alveolus. Cleft Palate Craniofac J. 2002;39:26–9. doi: 10.1597/1545-1569_2002_039_0026_nmagva_2.0.co_2. [DOI] [PubMed] [Google Scholar]
- 38.Patel PA, Rubin MS, Clouston S, Lalezaradeh F, Brecht LE, Cutting CB, et al. Comparative study of early secondary nasal revisions and costs in patients with clefts treated with and without nasoalveolar molding. J Craniofac Surg. 2015;26:1229–33. doi: 10.1097/SCS.0000000000001729. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.