Abstract
Aggregation of the peptide hormone amylin into amyloid deposits is a pathological hallmark of type-2 diabetes (T2D). While no causal link between T2D and amyloid has been established, the S20G mutation in amylin is associated with early-onset T2D. Here we report cryo-EM structures of amyloid fibrils of wild-type human amylin and its S20G variant. The wild-type fibril structure, solved to 3.6-Å resolution, contains two protofilaments, each built from S-shaped subunits. S20G fibrils, by contrast, contain two major polymorphs. Their structures, solved at 3.9-Å and 4.0-Å resolution, respectively, share a common two-protofilament core that is distinct from the wild-type structure. Remarkably, one polymorph contains a third subunit with another, distinct, cross-β conformation. The presence of two different backbone conformations within the same fibril may explain the increased aggregation propensity of S20G, and illustrates a potential structural basis for surface-templated fibril assembly.
A cross-β structure was identified as the generic building block of amyloid fibrils over 50 years ago (reviewed in ref. 1). Famously associated with pathology in Alzheimer’s, Parkinson’s and Huntington’s diseases, amyloid fibrils formed by 36 human proteins have been associated with more than 50 diseases1. This stable amyloid fold also plays a functional role in bacteria, fungi, plants and man2. Driven by developments in cryo-EM, electron diffraction and solid-state NMR, our understanding of amyloid structures has been revolutionized in recent years with near-atomic-resolution structures of amyloid fibrils formed in vitro and extracted from tissues of patients with Alzheimer’s disease3,4, Pick’s disease5, chronic traumatic encephalopathy6, corticobasal degeneration7 and multiple system atrophy8, and with systemic amyloidosis diseases caused by the misfolding of serum amyloid A9, immunoglobulin light chains10,11 and transthyretin12. These structures reveal a remarkable diversity of the common cross-β structure, with the same (or similar) protein sequences forming an array of structures very different to the one sequence–one structure relationship common to globular proteins. This body of work also shows that different fibril structures formed by the same, or similar, protein sequence can result in different diseases, as illustrated by fibrils from disease-associated variants of α-synuclein in Parkinson’s disease and dementia with Lewy bodies13,14, or the eight different fibril structures currently determined for different isotypes of tau associated with Alzheimer’s disease, Pick’s disease, chronic traumatic encephalopathy and corticobasal degeneration3,5–7.
The aggregation of the 37-residue peptide hormone amylin (human islet amyloid polypeptide) into amyloid fibrils has been linked to T2D15, in which amyloid deposition in pancreatic islets is a recognized histopathological hallmark. Amylin is cosecreted with insulin by pancreatic β-cells and is essential for glucose homeostasis. While no direct causal link between amylin and T2D has been established, numerous reports implicate amylin and its amyloid formation in β-cell death16,17. Importantly, a single genetic mutation in amylin causing the S20G variant is associated with a familial, early-onset form of T2D18; this substitution renders amylin more aggregation prone and increases the risk of disease19–21.
Biochemical experiments have shown that peptides spanning the amylin sequence from residues 8–37 can form amyloid aggregates22, with the sequence 22NFGAIL27 playing a central role in amyloid formation23–25. Using X-ray crystallography, it has been shown that several fragments of amylin can self-interact, forming amyloid-like assemblies with different backbone conformations26,27. While it is improbable that all of these very different homotypic interactions could occur simultaneously in amyloid fibrils formed from full-length amylin, these structures show that a single amylin fragment can adopt different conformations to stabilize an amyloid fold, consistent with polymorphism seen in other protein fibrils3,5–7,9,11.
Here we describe a 3.6-Å resolution structure of amylin amyloid fibrils formed in vitro from the wild-type protein, as well as fibrils with two different structures formed by the S20G variant, at 3.9-Å and 4.0-Å resolution. The results show that wild-type amylin fibrils are formed from two protofilaments, each containing a single amylin subunit per molecular layer. The subunits adopt an S-shaped conformation in each protofilament, reminiscent of the structures of fibrils formed by the Alzheimer’s disease-associated Aβ42 peptide28–31, which has a similar length and has 56% sequence similarity in its central-aggregation-prone region. The structures of the S20G variant show that the serine-to-glycine substitution results in a different protofilament fold at the core of the fibril. Strikingly, this new fold generates a surface onto which a third subunit binds while adopting a completely different backbone conformation. This arrangement generates a unique three-protofilament fibril that contains three asymmetric amylin subunits per molecular layer. This lateral association may represent the molecular basis of surface-catalyzed amyloid growth via secondary nucleation, and a potential mechanism for increased aggregation of the S20G variant.
Results
Polymorphism in amylin amyloid fibrils
We synthesized peptides corresponding to wild-type human amylin and the S20G variant using solid-phase peptide synthesis. Peptides were C-terminally amidated and contained the native disulfide bridge between residues 2 and 7. Purity and completeness of disulfide formation were assessed by high-performance liquid chromatography (HPLC) and ESI–MS, respectively (Supplementary Fig. 1). Both wild-type amylin and the S20G variant formed amyloid fibrils within 2 days of incubation under identical conditions at pH 6.8 (see Methods), as judged by negative-stain EM (Extended Data Fig. 1). Both samples are polymorphic. For wild-type amylin the predominant polymorph, accounting for ~80% of all fibrils imaged using cryo-EM, is long (>1 μm) and thin (<20 nm in diameter) and has an overt crossover length of ~25 nm (Fig. 1a and Extended Data Fig. 1a,b). Fibrils of the S20G variant were more heterogeneous, but the majority (~79% of all fibrils imaged) present an overt crossover length of ~50 nm (Fig. 1b and Extended Data Fig. 1c,d). We did not identify any fibrils with a 25-nm crossover (that is, the ‘wild-type morphology’) in S20G fibril samples, but fibrils with a 50-nm cross-over (that is, the ‘S20G morphology’) were found in the wild-type sample (~10%) as determined by atomic force microscopy (AFM), although these were seldom observed with cryo-EM. All fibrils of all polymorphs, both wild type and S20G, have an unambiguous, left-handed helical twist as determined by AFM (Fig. 1a,b insets, and Extended Data Fig. 1e,f). The fibril height profiles, determined using AFM, confirm the predominant crossover lengths observed by EM, at 22 ± 2 nm for wild-type and 43 ± 5 nm for S20G fibrils.
Fig. 1. Morphology of amylin fibrils.
a,b, raw cryo-eM images of wild-type (a) and S20G (b) amylin fibrils. Overt crossover lengths of 25 nm (wild type) and 50 nm (S20G) are indicated by arrows. All fibrils have a left-handed twist, as indicated by AFM (insets).
Cryo-EM structure of wild-type amylin fibrils
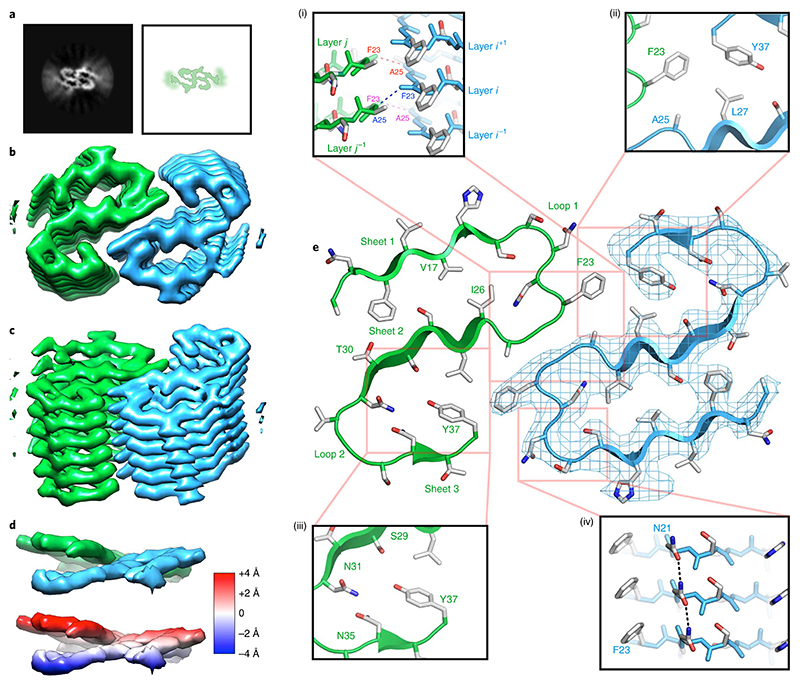
The wild-type fibril sample described above was used for cryo-EM analyses (see Methods). After manual selection from the raw micrographs and two-dimensional (2D) and three-dimensional (3D) classification, we obtained a homogeneous dataset of segments from the fibrils with a 25-nm crossover length (Extended Data Fig. 2a–e), from which a 3D structure was determined at 3.6-Å resolution (Table 1). The reconstruction has two symmetric protofilaments consisting of stacked layers of density (Fig. 2). Each subunit is characterized by a continuous density that corresponds to the peptide backbone, preceded at one end by a localized disordered region (LDR)32 (Fig. 2a), and has a helical twist of 178.23° and rise of 2.43 Å, characteristic of a left-handed, 21-screw axis symmetry. This led to a left-handed helical fibril in which the layers of density in each protofilament are separated by β-strand spacing of ~4.9 Å (Fig. 2b,c and Supplementary Fig. 2a). However, the layers are not perpendicular to the fibril long axis but are tilted by ~10° in opposite directions in each protofilament (Fig. 2d and Extended Data Fig. 3a–c). This tilt means that each monomer (i) contacts four different monomers in the opposing protofilaments (j – 1, j, j + 1 and j + 2) at its C-terminal (residue Y37) and central regions (residues 21–27). Each layer in each protofilament can accommodate a single amylin subunit (Fig. 2e). The quality of the density was sufficient to build an unambiguous model for the C-terminal 23 residues of the wild-type amylin sequence (residues 14–37), consistent with a wealth of biochemical, biophysical and structural information that this region is most likely to be structured in the fibril core22,23,33–36. The density of the bulky side chains of F15, H18, F23 and Y37 is consistent with this interpretation (Fig. 2e). Each monomer folds into a planar, S-shaped back-bone conformation forming three β-strands (β1, residues 14–19; β2, 26–31; and β3, 35–36) separated by two loops (L1, residues 20–25; and L2, 32–34) (Fig. 2e and Extended Data Fig. 3d).
Table 1. Cryo-EM data collection, refinement and validation statistics.
| Wild type (EMD-11380, PDB 6ZRF) |
S20G 2PF (EMD-11382, PDB 6ZRQ) |
S20G 3PF (EMD-11383, PDB 6ZRR) |
|
|---|---|---|---|
| Data collection and processing | |||
| Magnification | 130,000 | 130,000 | |
| Voltage (kV) | 300 | 300 | |
| Electron exposure (e-/Å2) | 1.01 | 1.05 | |
| Defocus range (μm) | −1.3 to −2.9, step 0.2 | −0.6 to −2.8, step 0.2 | |
| Pixel size (Å) | 1.065 | 1.065 | |
| Symmetry imposed | C1 | C1 | |
| Initial particle images (no.) | 117,316 | 64,274 | |
| Final particle images (no.) | 32,846 | 11,901 | 6,447 |
| Helical twist (°) | 178.23 | 179.05 | 358.1 |
| Helical rise (Å) | 2.43 | 2.41 | 4.81 |
| Map resolution (Å) | 3.6 | 4.0 | 3.9 |
| FSC threshold | 0.143 | 0.143 | 0.143 |
| Refinement | |||
| Initial model used | de novo | de novo | de novo |
| Model resolution (Å) | 3.9 | 4.4 | 4.5 |
| FSC threshold | 0.5 | 0.5 | 0.5 |
| Map sharpening B factor (Å2) | −150 | −93 | −98 |
| Model composition | |||
| Nonhydrogen atoms | 2,200 | 2,040 | 3,060 |
| Protein residues | 288 | 276 | 396 |
| R.m.s. deviations | |||
| Bond lengths (Å) | 0.010 | 0.006 | 0.010 |
| Bond angles (°) | 0.9 | 1.172 | 1.175 |
| Validation | |||
| MolProbity score | 2.41 | 2.20 | 2.29 |
| Clashscore | 38 | 23.19 | 29.63 |
| Favored rotamers (%) | 95 | 94 | 96 |
| Poor rotamers (%) | 0 | 0 | 0 |
| Ramachandran plot | |||
| Favored (%) | 95 | 95 | 95 |
| Allowed (%) | 5 | 5 | 5 |
Fig. 2. Structure of protofilaments and near-atomic resolution model for the wild-type amylin fibril.
a, Cross-sections through unsharpened 3D reconstructions for the wild type and its schematic representation, showing an ordered core composed of two protofilaments surrounded by diffuse density for more poorly resolved regions of the map. b, Fibril cross-section with the two protofilaments of wild-type fibrils colored blue (protofilament A) and green (protofilament B). c, As in b but with 45° tilt. d, Two molecular layers, the upper colored as in b and c and the lower, in identical view, with density colored according to height (in Å) along the fibril long axis, as indicated in the key (with zero corresponding to the center of mass of the molecular layer). e, The two symmetry-related subunits, each comprising a protofilament (A in blue and B in green). (i) Main-chain interactions consistent with H-bonds across the interprotofilament interface between F23 of one subunit and A25 of the other, zig-zagging up and down the interface; (ii) a hydrophobic core stabilizes the interprotofilament interface and the subunit fold; (iii) polar residues stabilize the subunit fold; (iv) stacking of aromatic side chains and asparagine ladders stabilizes the fibril.
The loop L1 contains a substantial portion of the interprotofilament interface, where residues 23FGA25 pack against the same region in the opposite protofilament forming a homotypic, face-to-face, dry steric zipper stabilized by two main chain-main chain interactions consistent with H-bonds between the zipper residues (Fig. 2e(i)). The tilt of each monomer is especially important in this region as the main-chain H-bonds of a monomer i in one protofilament bond to different opposing monomers in its same plane (j) and above its plane (j + 1) in the opposite protofilament (Extended Data Fig. 3a–c). This arrangement generates a network of interactions consistent with H-bonds that expand across the interprotofilament interface (Fig. 2e(i)). The remainder of the interprotofilament interface is generated by the formation of a hydrophobic core consisting of residue F23 with A25 and L27, and with Y37 of the opposing monomer (Fig. 2e(ii)).
Intramonomer interactions that stabilize the S-shaped fold involve both hydrophobic (V17 and I26 (Fig. 2e)) and polar interactions (S29, N31, N35, Y37 (Fig. 2e(iii))). There are also extensive interlayer interactions consistent with H-bonds via the formation of asparagine ladders (N21 (Fig. 2e(iv)), N22 and N35). Collectively, these interactions define two cores in each protofilament. A first core forms a tube of polar residues along the fibril and is predominantly formed by residues S29, N31 and N35, together with T30 and the phenolic -OH of Y37. The second core is apolar and is formed by residues F15, V17 and I26, together with the residues at the interprotofilament interface (Fig. 2e). In combination, these fibrils employ all of the stabilizing features observed in amyloid fibrils formed from other proteins and peptides to date, contained within a single structure: homotypic dry zippers, polar and apolar cores, aromatic stacking, asparagine ladders and the formation of parallel β-sheets.
S20G fibrils contain two distinct polymorphs
A cryo-EM dataset of S20G fibrils was also collected. Fibrils were manually selected from the raw cryo-EM images and, after 2D classification, an apparently homogeneous dataset of fibril segments consistent with a ~50-nm crossover length was obtained and in which β-strands appear to be out of register as judged from 2D class averages (Extended Data Fig. 4a–g and Supplementary Fig. 2b,c).
Extensive subsequent 3D classifications suggested that the dataset contains two similar structures with an identical overt crossover length. Once this had been established, we were able to solve one of these structures to 3.9 Å and discovered that it contained three protofilaments. We then used this structure as a model for further rounds of 3D classification to separate the dataset into its component parts. These separated datasets were then each solvable, generating two fibrils, one with two protofilaments (2PF; 4.0-Å resolution; ~76% of fibril segments with a 50-nm repeat) and one with three protofilaments (3PF; 3.9-Å resolution; ~24% of fibril segments with a 50-nm repeat), which we describe below.
Cryo-EM structure of the 2PF fibril
The 2PF fibril contains two identical monomers per molecular layer, one in each protofilament (Fig. 3a,b). Each of these monomers adopts a planar (Fig. 3c,d), 2D structure that is tilted away from the plane perpendicular to the fibril long axis by approximately 1.7° (Extended Data Fig. 5). The layers in each protofilament are out of register with each other by precisely half of the interstrand spacing (2.4 Å) (Fig. 3d), which corresponds to a 21 pseudo screw symmetry with a twist of 179.05° per subunit (Table 1). Each monomer contains four β-strands formed by residues 15–18 (β1), 21–23 (β2), 28–31 (β3) and 35–36 (β4) (Fig. 3e and Extended Data Fig. 3d). Strands β1, β3 and β4 partially over-lap in sequence with strands β1, β2 and β3 observed in the wild-type structure, respectively, while strand β2 from S20G is not observed in the wild-type structure (Extended Data Fig. 3d).
Fig. 3. Conformation of 2PF fibrils and details of its molecular structure.
a, Cross-sections through unsharpened 3D reconstructions for S20G 2PF fibrils and their schematic representation, showing two protofilaments in an ordered core surrounded by diffuse density for more poorly resolved regions of the map. b, Fibril cross-section (view and coloring as in Fig. 2b). c, Side view of out-of-register stacking of layers in the two protofilaments. d, The two protofilaments colored by height along the fibril axis. e, The atomic model with and without the density from which it is derived. (i) The interface between protofilaments, dominated by interactions between A25 in one protofilament and L27 in the other; (ii) the hydrophobic core of the subunit fold; (iii) polar interactions stabilizing the subunit fold, especially involving H18, which is solvent exposed in the wild-type structure.
The spatial arrangement of secondary structure elements in the S20G 2PF structure is completely different to that in the wild-type fibrils. In fact, the backbone of these structures cannot be super-posed (Extended Data Fig. 6a)—only the segment containing residues 31NVGSNT36 can be superposed between these structures (root mean squared deviation (r.m.s.d.) = 0.95 Å; Extended Data Fig. 6b). The interface between the protofilaments is also less interdigitated in the S20G 2PF structure, and is formed by residues 25AIL27 arranged in a face-to-face, homotypic, dry steric zipper (Fig. 3e(i)). These amino acids are consecutive to those that form the interface in the wild-type structure (23FGA25), but neither the interfacial nor the adjacent residues can be superposed between wild-type and 2PF structures. Both polar and apolar interactions stabilize the 2PF monomer fold. The apolar interactions are between residues F23, I26 and V32 (Fig. 3e(ii)), while the polar interactions involve side chain–side chain contacts between residues H18 and N21, and between N31 and N35 (Fig. 3e(iii)). Some of the interactions that stabilize the stacking of monomers are conserved between wild-type and S20G structures; these include common features of amyloid fibrils such as stacking of the aromatic side chains of residues F15, F23 and Y37.
The structure of the S20G fibrils shows a single apolar core within each S20G monomer formed by residues F23, I26 and V32, and the 25AIL27 sequence at the interface between protofilaments (Fig. 3e). This core extends across the interface between monomers and constitutes a large hydrophobic region that gives stability to the protofilaments that form the 2PF fibril, and is shielded from the solvent by patches of polar residues formed by residues S19–N22 and S28–N31 on the surface of the fibril (Fig. 3e). There is also a polar core that contributes to the stability of the fibril formed by side chains of H18 from β1, N21 from β2 and S34 from β4 (Fig. 3e(iii)).
The 3PF and 2PF fibrils share a common core architecture
The second major polymorph found in the S20G sample is an unprecedented structure that we term 3PF and is composed of three monomers per molecular layer, each of which stacks to generate three protofilaments (Fig. 4). One of these monomers (protofilament C, shown in red in Fig. 4b–f) has a completely different fold to the other two monomers (protofilaments A and B: blue and green, respectively, in Fig. 4b,c), the latter of which are symmetrical and have a backbone conformation that is indistinguishable (at 3.9-Å resolution) from the two monomers in a layer of the 2PF structure. Indeed, the backbone superposition of the symmetrical monomers A and B observed in 3PF fibrils with those of 2PF fibrils has r.m.s.d. = ~1.7 Å over 23 residues (Extended Data Fig. 6c). Moreover, the symmetric monomers in 3PF are also out of register (Fig. 4c,d) and related by a 21 pseudo screw axis symmetry as observed in the 2PF fibrils, with a very similar interface (residues 25AIL27) engaged in a homotypic, apolar steric zipper (Fig. 4e,f). Despite their similarities, the relative tilt angles and stacking arrangement of subunits in protofilaments A and B in 3PF fibrils are subtly different to those observed in 2PF fibrils, presumably because of the binding of the third monomer on one side of the ‘2PF-like’ core, which inserts its C-terminal end into the groove between protofilaments A and B (Fig. 4e,f) and distorts the even spacing between monomeric planes observed in the 2PF fibrils (Extended Data Fig. 5e–g). Instead, this generates an alternating short distance (~1.33 Å) and long distance (~3.44 Å) between monomeric planes (Extended Data Fig. 5h).
Fig. 4. Conformation of S20G 3PF fibrils and details of their molecular structure.
a, Cross-sections through unsharpened 3D reconstructions for S20G 3PF fibrils and their schematic representation, showing three protofilaments in an ordered core surrounded by diffuse density for more poorly resolved regions of the map. b, Cross-section view with the two protofilaments resembling 2PF fibrils colored blue (protofilament A) and green (protofilament B), and an additional protofilament associated on one face of the fibril, colored red (protofilament C). c, Side view. d, Single layer colored by height along the fibril axis. e, Atomic model with (protofilaments A and C) and without (protofilament B) the density from which it is derived. f, The C terminus of protofilament C interdigitates into the gap between protofilaments A and B.
The outcome of the docking of protofilament C onto the fibril side is that the molecular layers of 3PF are rendered asymmetric by the presence of the monomers in protofilament C. This asymmetry and distortion of the spacing between monomer planes means that the 3PF polymorph cannot be reconstructed by applying the same symmetry operators of 2PF. Instead, this polymorph was reconstructed by applying C1 symmetry with a helical rise of 4.81 Å and twist per subunit of 358.1° (Table 1).
The subunits within the symmetric 2PF-like core of the 3PF fibril have the same β-strands and interconnecting loops observed in the S20G 2PF fibril (Extended Data Fig. 3d). In contrast, the monomers in protofilament C have only two β-strands (β1, residues 15–18; and β2, residues 25–29), which partially overlap with β1 and β2 observed in the wild-type structure (Extended Data Fig. 3d). Indeed, subunits in protofilament C are more similar to those in the wild-type structure than those in protofilaments A and B to which they are opposed. The superposition of the backbone of monomer C (red in Fig. 4b,c,e) and the wild-type monomer has r.m.s.d. = 3.1 Å over the 14 N-terminal residues observed in the structure (residues 15–29), indicating that they have, in part, a similar fold (Extended Data Fig. 6d). Unlike protofilaments A and B in the 3PF structure, the monomers in protofilament C are neither planar nor perpendicular to the fibril axis. The insertion of the C terminus of monomer C into the groove between protofilaments A and B is above the plane of the neighbor monomer B. The remainder of the chain of monomer C descends along the fibril axis and enters in register with monomer B by its N-terminal region (Fig. 4d and Extended Data Fig. 5e). Through the insertion of their C-terminal ends, monomers in protofilament C interact with those in protofilaments A and B. The side chain of the C-terminal Y37 in monomer C contacts the side chains of L27 and S28 in an A-type monomer, while its backbone makes an interaction consistent with an H-bond with the side chain of N22 in a B-type monomer (Fig. 4f). The interface between monomers B and C is larger than that between A and B, and is formed by both polar and apolar residues that cluster at two different points on the interface (Fig. 4e). The apolar interactions are between residues A25 and L27 of the asymmetric monomer C, with F15 and V17 on neighbor monomer B. The polar interactions are between S29 of monomer C and S19 of monomer B. The polar cluster also includes the side chains N31 and T36 of monomer C, and N22 of monomer B. The backbone of monomer C describes a broad turn in this position, which is 12 Å away from the backbone of monomer B at its most distant point. As a consequence there is a loose interdigitation of residues in the polar patch (Fig. 4e). The 3PF fibril therefore strongly resembles a 2PF fibril, with the addition of a third monomer having a unique fold, and constitute an unprecedented three-protofilament asymmetric amyloid fibril structure.
Symmetric and asymmetric interfaces in wild-type, 2PF and 3PF fibrils
The structures described highlight how the single-substitution S20G results in large differences in the conformation of amylin subunits forming the protofilaments of the amyloid fibrils (Fig. 5a–c) and in profoundly different protofilament interfaces (Fig. 5d–f), even though the side chain of residue 20 does not participate in fibril-stabilizing interactions and is solvent exposed in both wild-type and S20G polymorphs (Fig. 5d–f). To understand how the difference in protofilament interfaces might influence fibril stability, we analyzed the different interfaces using the software PDBePISA37. We generated fibril segments of six molecular layers organized in two protofilaments for wild-type and S20G 2PF fibrils (Figs. 2 and 3), or three protofilaments for 3PF fibrils (Fig. 4), and compared their buried surface area, solvation free energy gain following formation of the interface (ΔiG) and the free energy of assembly dissociation (ΔGdiss) (Table 2).
Fig. 5. Schematic views of backbone fold and interprotofilament interactions in the structures of wild-type, S20G 2PF and S20G 3PF amylin fibrils.
a–c, The amylin backbone is represented as a tube and colored like a rainbow from the N-terminal (N) to the C-terminal (C) residues observed in the structure (residues 14–37) for wild-type (a), S20G 2PF (b) and S20G 3PF (c) fibrils. d–f, The backbone trace of each monomer for wild-type (d), S20G 2PF (e) and S20G 3PF (f) fibrils. The backbones are colored by protofilament, as in Figs. 2–4, and the amino acid side chains are schematically represented by spheres. The sequence number for selected amino acids is indicated by numbers colored according to the protofilament to which they refer. The one-letter code is used to identify each amino acid. Polar and apolar cores that extend across protofilament interfaces are indicated as green and yellow regions, respectively.
Table 2. Protofilament interface analysis of wild-type, S20G 2PF and S20G 3PF fibrils.
| Wild type | S20G 2PF | S20G 3PF | |
|---|---|---|---|
| ΔiG (kcal mol-1) | −28.3 | −8.8 | −41.5 A–B interface, −12.1 B–C interface, −20.0 |
| Surface area (Å2) | 10,662 | 11,068 | 14,320 |
| Protofilament interface area (Å2) | 1,960 | 360 | 2,571 A–B interface, 482 B–C interface, 1,644 |
| ΔGdiss (kcal mol−1) | 22 | −1.7 | 8.6 A–B interface, 1.1 B–C interface, 5.2 |
| No. and type of monomer based on structure | 12A | 12A | 12A + 6C |
| Protofilament species | AB | AB | ABC |
| Dissociation | A + B | A + B | A + BC |
In the wild-type fibril, the buried surface area between protofilaments is 1,960 Å2; ΔiG for the interface is ~−28 kcal mol−1 and ΔGdiss of the fibril into two protofilaments is ~22 kcal mol–1. If the number of molecular layers is decreased and then ΔGdiss re-evaluated, a minimum length for the fibril to be considered stable can be obtained (ΔGdiss > 0) which, for the wild-type structure, is two layers (giving ΔGdiss = ~0.4 kcal mol–1) (Extended Data Fig. 6e). In the S20G 2PF fibril the interprotofilament interface is 360 Å2, confirming the visual impression of a smaller interface. The ΔiG of the interface between protofilaments is ~−9 kcal mol–1 and ΔGdiss of the two protofilaments is ~−2 kcal mol–1, which suggests that the minimum length for a stable S20G 2PF fibril would be seven or more molecular layers. Finally, the combined interface areas between the monomers in each molecular layer of the 3PF fibril was determined to be 2,571 Å2, the combined ΔiG of these interfaces is ~−42 kcal mol–1 and ΔGdiss = ~9 kcal mol–1. Decreasing the number of molecular layers for this polymorph suggests that the minimum length for stability of a 3PF fibril would be four molecular layers, in which case ΔGdiss = ~3 kcal mol–1. Hence, as expected by their larger buried surface area, the 3PF fibrils are predicted to be considerably more stable than their 2PF counterparts.
The interface between all protofilaments in 3PF fibrils can be broken down into two subinterfaces (Table 2). The interface between symmetric monomers (A and B in Fig. 4) is similar to that in the S20G 2PF fibril (Fig. 3), but the presence of the asymmetric monomer expands the hydrophobic core of the fibril (Fig. 5f) and distorts the spacing between planes of symmetric monomers (Extended Data Fig. 5h). We estimate that the total buried area of this 2PF-like interface is ~482 Å2. This is greater than that of the S20G 2PF, and results in ΔiG = ~−12 kcal mol–1 and ΔGdiss = ~1 kcal mol–1. The interface between monomers B and C in 3PF fibrils is larger than that between monomers A and B. The estimated buried area in this interface is ~1,644 Å2, ΔiG = −20 kcal mol–1 and ΔGdiss = ~5 kcal mol–1. Therefore, the predicted dissociation pattern for 3PF fibrils would be for the interface between symmetric monomers (that is, A and B) to break first, with that between asymmetric partners (that is, B and C) being more resilient.
Discussion
Here we present the structure of amyloid fibrils of wild-type amylin at 3.6-Å resolution and two major polymorphs of the S20G variant associated with early-onset T2D (at 4.0-Å and 3.9-Å resolution)18,38. Two recent papers have reported cryo-EM structures of wild-type amylin fibrils. Röder et al.39 describe three polymorphs, the most abundant of which had its structure determined at 4.2-Å resolution; this polymorph had a 25-nm crossover length and a monomer fold that is very similar to the wild-type structure described here (within the resolution limit of the reconstructions; Extended Data Fig. 7a). However, based on AFM analysis of dried fibrils, the structure described by Röder et al. is a right-handed fibril whereas AFM of the hydrated material created here shows that the fibrils are unambiguously left-handed, consistent with previous reports for wild-type amylin fibrils with a 25-nm crossover23,40,41. Cao et al.42 report a completely different architecture for fibrils formed by an N-terminally SUMOylated amylin variant, again with two protofilaments. However, that monomer fold is unrelated to the one reported here (Extended Data Fig. 7b), perhaps due to the addition of a SUMO tag (~13 kDa) that is substantially larger than amylin itself (~4 kDa).
The wild-type amylin fibril structure obtained here is different from that determined previously using solid-state NMR43, which was derived from a sample containing two major polymorphs. One polymorph (described as a ‘striated ribbon’) also contained two symmetric amylin molecules per layer, but each formed two parallel β-sheets connected by a loop (Extended Data Fig. 8a,b); the other polymorph (described as a ‘twisted fibril’), with a period of 25–50 nm and height 4–10 nm, was not structurally elucidated but it may correspond to the wild-type amylin fibrils described here (25-nm repeat, height 8 nm). Importantly, the experimental conditions in our study are different from those employed by others who also observed twisted amylin fibrils23,35,40, suggesting that this twisted morphology is an inherent feature of amylin amyloids.
The wild-type amylin structure presented here is consistent with previous biochemical22,23, bioinformatic35 and structural studies33,34,36 that collectively suggest that the N-terminal region does not form part of the fibril core, that the C-terminal region is folded and that Y37 is buried in a quasi-crystalline state44. The overall S-shape of the monomers within the wild-type amylin protofilaments is reminiscent of a previous ‘β-serpentine’ model for amylin amyloid (Extended Data Fig. 8c,d)35. Previous X-ray crystal structures of short segments of amylin covered most of the residues visible in our structure and showed that several of those segments self-interact, and do so in multiple conformations26,27,45,46. Six of those fragment structures superpose on the cryo-EM structure of wild-type amylin presented here27,46 (Extended Data Fig. 8e), including one that covers the interprotofilament interface27 (21NNFGAIL27). This fragment structure overlays almost perfectly with our structure (r.m.s.d. = ~0.4 Å; Extended Data Fig. 8f), but the fibril interface itself cannot be reproduced based on the fragment structure as it does not capture the tilted relationship between monomers in the fibril.
Many of the features previously observed to contribute to the stability of other amyloid fibrils are also present in the wild-type amylin fibril structure. These include asparagine ladders30, where residue N21 (Fig. 2d(iv)) is particularly interesting because the substitution N21P increases the rate of amylin aggregation, possibly by inducing a turn in the backbone47, consistent with its position in our structure. Other stabilizing features include interactions between each monomer and multiple monomers in the opposing protofilament. Such interactions have been observed in fibrils of Aβ42 prepared in vitro29, and in ex vivo fibrils extracted from patients with systemic AA amyloidosis9. This feature is particularly important in the wild-type amylin fibril (Extended Data Fig. 3a–c), but also in the interaction of monomer C with the core of 3PF fibrils (Extended Data Fig. 5e,g). Finally, both wild-type and S20G amylin fibrils are stabilized by (different) polar and apolar interactions (Fig. 5d–f). In particular, H18 was reported to play a role in Zn2+ binding48 and it is surface exposed in wild-type fibrils but buried in 2PF and 3PF, suggesting these fibrils could have different zinc-binding properties (Extended Data Fig. 9a,b). Interprotofilament interfaces of pathological amyloids seem to contain mostly apolar cores, as observed in fibrils of human prion protein49, Aβ40/42 (refs. 29,30,50) and α-synuclein51. By contrast, the structure of Orb2, a functional amyloid implicated in memory persistence in Drosophila52, featured exclusively polar interfaces. In the present study, the interface between monomers B and C in S20G 3PF fibrils contains both apolar and polar cores (Fig. 5f). Whether this particular interface composition gives 3PF fibrils distinctive properties regarding its thermodynamic stability, or characteristic kinetics of assembly or disassembly, remain to be studied.
The S-shaped monomer in wild-type amylin fibrils is reminiscent of structures of the Alzheimer’s disease-related Aβ42 fibrils determined using solid-state NMR28,30,31 and cryo-EM29. The clinical relationship between Alzheimer’s and T2D has been known for some time53, and the risk of dementia (including Alzheimer’s disease and vascular dementia, both linked to Aβ deposition) is increased in patients with T2D54. Indeed, a recent study linked T2D to a subset of dementias termed diabetes-related dementia55. The molecular explanation for this link remains unclear, but it may involve cross-seeding of aggregation56 possibly via direct interaction of aggregation-prone regions57. A superposition of the wild-type amylin fibrils here with those of Aβ42 (Extended Data Fig. 10a–e) supports this hypothesis, as noted previously39. Indeed, the most similar segments in amylin and Aβ42 (residues 10–27 in amylin, 15–32 in Aβ42; Extended Data Fig. 10a) include the interprotofilament interfaces in both wild-type and S20G amyloid structures. Whether coaggregation results from cross-seeding, coaggregation, secondary nucleation or association between preformed protofilaments remains unresolved58,59. Strikingly, superposition of the amylin and Aβ42 fibril structures shows that the disease-related mutations (S20G in amylin and E22G in Aβ42 (also known as the Arctic mutation)) occupy structurally equivalent positions39 (Extended Data Fig. 10b). However, it is not possible to predict whether the effects of the Arctic mutation in Alzheimer’s disease60 arise from structural manifestations in the architecture of Aβ42 fibrils akin to those described here for amylin.
The effects of S20G substitution in amylin on fibril architecture are unanticipated and remarkable. The wild-type and 2PF structures have distinct folds, interprotofilament interfaces, steric zippers and stabilizing cores, but S20 is solvent exposed in each. The 3PF structure is unprecedented, revealing a single fibril composed of two different protofilament structures: a 2PF-like core with the lateral association of a third protofilament. There are currently four structures of amyloid fibrils with three protofilaments: unseeded in vitro fibrils of Aβ40 (ref. 61) and of the D23N ‘Iowa’ mutant of Aβ40 (ref. 50), in vitro seeded fibrils from an Alzheimer’s disease brain sample62 and ex vivo extracted functional Orb2 amyloid fibrils from Drosophila brains52; in each case the fibrils are symmetric. By contrast, the backbone fold of subunit C of S20G 3PF fibrils is completely different to that of subunits A and B in the same fibril. Thus, the same amylin sequence is able to adopt multiple conformations, even within the same molecular assembly, a phenomenon strikingly reminiscent of how quasi-equivalence is utilized by viruses to build their capsids63.
Although the precision of the stability of different fibrils and their interfaces is limited by the resolution of the structural data presented here, PDBePISA establishes a clear trend: wild-type fibrils are most stable, followed by the S20G 3PF fibril, with the S20G 2PF fibril being the least thermodynamically favorable structure. However, 2PF fibrils are more common than 3PF fibrils. This is consistent with the aggregation of S20G being under kinetic control, whereby aggregation into the 3PF polymorph is thermodynamically favorable but occurs from a nucleation event that is rarer. The observation that S20G aggregates more rapidly than wild-type amylin is consistent with such a hypothesis19–21. An alternative explanation is that 3PF arises directly from a 2PF core, via seeding of a new protofilament on the preformed fibril surface. If this were the case, the 3PF structure shown here would describe the structural details of the kind of interactions that support secondary nucleation. Detailed study of the aggregation kinetics of S20G, together with analysis of large cryo-EM datasets acquired at different time points within the aggregation reaction, will probably shed light on this in the future.
Online content
Any methods, additional references, Nature Research reporting summaries, source data, extended data, supplementary information, acknowledgements, peer review information; details of author contributions and competing interests; and statements of data and code availability are available at https://doi.org/10.1038/s41594-020-0496-3.
Methods
Preparation of wild-type and S20G amylin peptide
Wild-type and S20G amylin were synthesized using a Liberty Blue automated microwave peptide synthesizer (CEM Microwave Technology) on a 0.1-mmol scale, as reported previously64,65. We used 9-fluorenylmethyloxycarbonyl (Fmoc)-protected amino acids and PAL-NovaSyn TG resin (Merck), allowing the generation of amylin with an amidated C terminus. Three pseudo proline dipeptides (Fmoc-Ala-Thr(psiMe,MePro)-OH, Fmoc-Ser(tBu)-Ser(psiMe,MePro)-OH and Fmoc-Leu-Ser(psiMe,Mepro)-OH, Merck) were used for the insertions of Ala-8 and Thr-9, Ser-19 and Ser-20, and Leu-27 and Ser-28. All residues and the three pseudo proline dipeptides were double coupled. The peptides were cleaved from the resin in a cleavage cocktail of trifluoroacetic acid (TFA, 9.4 ml), 3,6-dioxa-1,8-octanedithiol (250 μl), H2O (250 μl) and triisopropylsilane (100 μl). The mixture was stirred at room temperature for 3.5 h and then concentrated under a nitrogen stream. Subsequently the crude peptide was precipitated in cold diethyl ether, followed by three washes with the same solvent. The peptide was then dissolved in a 50% acetonitrile aqueous solution containing 0.1% TFA, and lyophilized. The peptide was then dissolved in 50% DMSO aqueous solution to promote formation of the internal disulfide bond between Cys-2 and Cys-7. The oxidized peptides were then purified by reverse-phase HPLC using a Kinetex EVO C18 column (Phenomenex). The buffers used in the HPLC purification were acetonitrile with 0.1% formic acid and H2O with 0.1% formic acid. The masses of the purified peptides were confirmed by ESI–MS as 3,902.9 for wild-type amylin (expected, 3,903.3) and 3,872.9 for amylin-S20G (expected, 3,873.3). The purity of the two peptides was assessed by analytical HPLC and was >95%. After purification, peptides were again lyophilized and stored at −20 °C until use.
Fibril growth
Lyophilized peptides were monomerized by dissolution into hexafluoroisopropanol (Sigma) at a final concentration of 1 mg ml–1. Samples were incubated for 15 min at room temperature with occasional agitation to allow complete monomerization. Monomerized samples where then aliquoted in 1.5-ml glass vials containing 50 μg of peptide per vial. The solvent was evaporated to dryness by gently blowing a stream of nitrogen gas while swirling the vial around to generate a film of peptide around the walls of the vial. Vials containing dried peptide films were stored at −20 °C until use. At the point of use, peptide vials were allowed to reach room temperature before opening and then an aliquot of ice-cold aggregation buffer (freshly prepared 20 mM ammonium acetate, pH 6.8 filtrated through a 0.2-μm polyvinylidene difluoride filter immediately before use) was added to obtain stock concentrations no higher than 100 μM of peptide. The concentration of the stock was estimated by absorbance at 280 nm using the calculated molar extinction coefficient of 1,615 M−1 cm−1. The concentration of the stocks was adjusted to 30 μM by addition of aggregation buffer, and samples were incubated quiescently at room temperature to allow the formation of amyloid fibrils as confirmed by negative staining transmission electron microscopy and thioflavin T fluorescence. Typically, amyloid fibrils were observed within 24–48 h. After the observation of fibrils, samples where maintained at 4 °C until their use for cryo-EM sample preparation and were used within 2 weeks of that.
AFM sample preparation and imaging
The handedness of fibrils was unambiguously determined using AFM. A sample volume of 40 μl of either wild-type (at 30 μM) or S20G (at 15 μM) amylin fibrils was deposited onto freshly cleaved mica and allowed to incubate for 4 min. The mica surface was then rinsed with buffer (50 mM NaPO4, 300 mM KCl, pH 7.5) via fluid exchange, maintaining the fibrils in a liquid environment. AFM observations were performed in tapping mode using a Dimension FastScan Bio with FastScan-D-SS probes (Bruker) in the same buffer. The force applied by the tip on the sample was minimized by maximizing the set point whilst maintaining tracking of the surface.
Cryo-EM imaging
Amylin fibrils (either wild type or S20G) at a concentration of 30 μM monomer equivalent were diluted 1:1 with 300 mM NaCl, to achieve a final concentration of 15 µM monomer equivalent of the peptides. A 300-mesh copper EM grid with lacey carbon film (Agar Scientific) was cleaned in a Tergeo-EM plasma cleaner (Pie Scientific) for 1 min at power 40 mA. Four microlitres of the sample was applied to the grid, which was then blotted with Whatman no. 40 filter paper and plunge-frozen in liquid ethane using a Vitrobot mark IV (Thermo Fisher).
Electron microscopy images were collected using a Titan Krios (Thermo Fisher) electron microscope operating at 300 keV, and recorded on an energy-filtered K2 direct detector (Gatan), with a pixel size of 1.06 Å per pixel. For the wild-type sample, a set of 800 micrographs was recorded with a defocus range between −1.3 and −2.9 μm every 0.2 μm, and total electron doses of 50.7 e−/Å2. The dose was fractionated into 50 frames for a per-frame dose of 1.01 e−/Å2. For the S20G sample a set of 684 micrographs was recorded with a defocus range between −0.6 and −2.8 μm every 0.2 μm, and total electron doses of 54.7 e−/Å2. The dose was fractionated into 52 frames for a per-frame dose of 1.05 e−/Å2.
Frames 3–50 of wild-type micrograph movies, and 3–52 of S20G micrograph movies, were motion corrected, dose weighted and merged using motioncor2 (ref. 66). The contrast transfer function (CTF) for each micrograph was determined using gCTF67 on motion-corrected, but non-dose-weighted, micrographs.
Helical reconstruction
Helical reconstruction68 was performed using Relion 3.0 (ref. 69) except for initial model generation, which was performed in Relion 3.1 (ref. 70). The data were assessed visually, and start and end points for fibrils that appeared to be composed of a single set of protofilaments were interactively selected by hand. For the S20G dataset, the fibrils were segmented into 300 × 300 pixel boxes (corresponding to 319.5 × 319.5 Å), with an overlap of 90% (an offset of 30 pixels, 31.95 Å). For the wild-type dataset the fibrils were segmented into 200 × 200 pixel boxes (corresponding to 213.0 × 213.0 Å), with an overlap of 90% (an offset of 20 pixels, 21.3 Å). For the wild-type dataset the initial 117,316 extracted segments were subjected to multiple rounds of 2D classification; classes showing an obvious ~4.8-Å repeating feature were taken forward into the next classification. The 84,597 segments ultimately taken forward from this iterative 2D classification process were then subjected to iterative rounds of 3D classification using a de novo starting model generated with Relion 3.1. The same procedure was followed for the S20G dataset, where 64,274 segments were extracted initially and, after multiple rounds of 2D classification where only those classes showing an obvious ~4.8-Å repeating feature were taken forward into the next classification iteration, this yielded a final set of 25,137 segments that were taken forward to iterative rounds of 3D classification using a de novo starting model generated with Relion 3.1.
The initial 3D classification rounds were performed with searching of helical rise and twist around values estimated from crossover lengths measured from 2D class averages. In these classification steps the wild-type and S20G datasets were separated into three classes each. After the first round of classification, the initial model was updated to that showing the best separation of density stacks along the fibril axis. For the wild type, after several rounds of update of the initial model a final 3D classification was performed with fixed values of 178.23° helical twist and 2.43-Å helical rise.
For S20G the initial 3D classification with a search of helical rise and twist yielded two distinct polymorphs that contained either two or three protofilaments. We followed the 3D classification strategy employed for the wild-type dataset using the three-protofilament model obtained as the initial model for the next 3D classification round. This allowed us to separate particles contributing to the three-protofilament structure from those contributing to the two-protofilament structure. The class of particles belonging to the three-protofilament structure was further 3D classified with searches of helical symmetry. These symmetry searches converged to values for a rise and twist of 4.81 Å and 358.1°, respectively. The class showing the best separation of stacks of density along the helical axis was used as the initial model for a new 3D classification round, with fixed helical parameters found in the precedent step. The resulting model was employed as the initial model for 3D classification against the original mixed dataset, into two- and three-protofilament and ambiguous classes. This step generated a class containing 6,447 for the 3PF reconstruction. Particles contributing to the 2PF structure were 3D classified using the 2PF map as starting model. The symmetry searches converged to values for rise and twist of 2.41 Å and 179.05°, respectively. A final 3D classification with the resulting model and fixed helical parameters against the 2PF particles resulted in a class containing 11,901 particles.
Each of the resulting 3D classes was subjected to 3D refinement using their respective 3D maps as reference maps, a t-value of 50 and fixed helical parameters. After refinement, all models were masked (wild type, 15% of Z length; S20G, 10% of Z length) and postprocessed in Relion. Fourier shell correlation plots for wild type, S20G 2PF and S20G 3PF are shown in Supplementary Fig. 3.
Model building and refinement
Atomic models of single layers for each of the fibril reconstruction maps were built de novo in COOT71. The initial model of S20G 2PF was used to guide the model building of 3PF. For each of the models, six copies of the single-layer models were fit into the respective cryo-EM maps using Chimera72 to preserve nearest neighbor interactions during subsequent refinement steps. The stack of six layers was then subjected to multiple rounds of real-space refinement in Phenix73, alternated with real-space refinement in COOT. The refinements in Phenix were restrained by defining noncrystallographic symmetry restraint groups for each of the protofilaments. Side chain clashes were detected using MOLPROBITY74 and corrected by iterative cycles of real-space refinement in both COOT and Phenix. All refinements were performed using information relating to 3.6-Å resolution for wild type, 3.9-Å resolution for S20G 2PF and 4.0-Å resolution for S20G 3PF.
Reporting Summary
Further information on experimental design is available in the Nature Research Reporting Summary linked to this article.
Extended Data
Extended Data Fig. 1. Negative-stain TEM and AFM characterization of wild-type and S20G amylin fibrils.
a, Wild-type (WT) amylin fibrils as observed by negative-stain TeM. The proportion of all fibrils in the dominant polymorph determined here (~80%) are indicated by the pie chart in row (a). b, As in (a), but digitally magnified. c, S20G mutant amylin fibrils as observed by negative-stain TeM. Again, the proportion of all fibrils in the dominant two polymorphs determined here (2PF (60%) and 3PF (19%)) are indicated by the pie chart in row (c). d, Same as (c) but digitally magnified. e, In solution AFM analysis of wild type amylin fibrils. f, In solution AFM analysis of S20G mutant amylin fibrils. Scale bars and their accompanying dimensions are indicated on each panel.
Extended Data Fig. 2. 2D-classification and class average details of wild-type amylin data-set.
a, 2D classification of the initial cryoeM data-set. Shown are selected classes ranked on class distribution from higher to lower (left to right and then top to bottom). b, representative 2D class showing a cross-over point along the long fibril axis. c, Detail of the representative 2D class shown in (b) marked in red. d, 2D-projection of the final electron potential map. e, Detail of the 2D-projection of the final electron potential map marked in red shown in D.
Extended Data Fig. 3. Inter-protofilament interactions that stabilised the wild-type amylin fibril and secondary structure assignments.
a, Monomer “i” in red, and apposing monomer “j” in brown have 10º tilt angle respect to the main fibril axis, which generates an effective 20º tilt between monomers in opposing protofilaments. b, Top view of the interaction depicted in (a), showing monomer “i” in red, and the stack of opposing monomers “j-1”, “j”, “j+1” and “j+2” in brown shades, with which monomer “i” interacts. The N-terminal and C-terminal ends of monomers in both protofilaments are indicated by letters (N and C) in the respective colours. c, Side-view of the interaction shown in (b), showing the opposing monomers as molecular surface in shades of brown, where the red surface indicates the contacts with monomer “i”, which is shown as red ribbon. d, Secondary structure assignments for wild-type and S20G amylin subunits in their amyloid fibril state as determined in this study and compared to previously published structures33,35,36,43. Dashed lines indicate sequence segments for which structure is not determined in the experiment. Continuous lines indicate loops. Arrows indicate β-strands.
Extended Data Fig. 4. 2D-classification and class average details of the S20G amylin data-set.
a, 2D classification of the initial cryo-EM data-set. Shown are selected classes ranked on class distribution (from left to right and then top to bottom). b, Representative 2D class showing a cross-over point along the long fibril axis. c, Detail of representative 2D class shown in (b) highlighted in red. d, 2D-projection of the final 3D-reconstructed map of 2PF fibrils. e, Detail of the 2D-projection of the final 3D-reconstructed map highlighted in red shown in (d). f, 2D-projection of the final 3D-reconstructed map of 3PF fibrils. g, Detail of the 2D-projection of the final 3D-reconstructed map highlighted in red shown in f.
Extended Data Fig. 5. Inter-protofilament interactions in the 2PF and 3PF polymorphs of S20G amylin.
a, Monomer “i” in red, and apposing monomer “j-1” in brown have 1º tilt angle respect to the main fibril axis, which generates an effective 2º tilt between monomers in opposing protofilaments. b, Top view of the interaction depicted in (a), showing monomer “i” in red, and the stack of opposing monomers “j-1” and “j” in brown shades, with which monomer “i” interacts. The N-terminal (N) and C-terminal (C) ends of monomer “i” are indicated by red letters. c, Side-view of the interaction shown in (b), showing the opposing monomers “j” and “j-1” as molecular surface in shades of brown, where the red surface indicates the contacts with monomer “i”, which is shown as a red ribbon. d, Side view as in (b), where the backbone of each protofilament is shown as ribbons. The even inter-strand distance of 2.4-Å is indicated by alternating black and yellow lines. e, Monomers “jB” and “jA” are out of register and form the “2PF-like” core of 3PF polymorph. Monomer “i”, in red, binds on the side of monomer “jB” and inserts its C-terminal end in the groove between “jA” and jB”. The N-terminal end of monomer “i” is in register with monomer “jB”, while the C-terminal end of monomer “i” is in register with monomer “jA”. f, Top view of the interaction depicted in (e), showing monomer “i” in red, and the stack of opposing monomers “jA-1”, “jA” and “jA+1” in brown shades that form protofilament A. Protofilament B also contains 3 monomers, but their labels have not been included. The N-terminal (N) and C-terminal (C) ends of monomer “i” are indicated by red letters. g, Side-view of the interaction shown in (f), where monomers of protofilaments jA and jB are indicated. The red surface in protofilaments A and B represents the surfaces of interaction with monomer “i” of protofilament C. h, Side view as in (f), where monomer C has been removed for clarity, and where the backbone of each protofilament is shown as ribbons. The uneven inter-strand distance of 1.3-Å and 3.4-Å is indicated by alternating black and yellow lines.
Extended Data Fig. 6. Monomer superposition from amyloid fibrils form by wild-type and S20G amylin, and dependency of fibril stability on number of molecular layers for amylin fibrils.
a, Global superposition of the Cα trace of the wild-type fibril monomer (blue) on the Cα trace of S20G 2PF fibril monomer (red). b, The same structures superposed in the segment 31NVGSNT36 (residues numbered in red on figure), where the side chains of this segment (plus the terminal Tyrosine residue) are shown as sticks. c, Global superposition of the Cα trace of the S20G 2PF monomer (red) on the Cα trace of S20G 3PF symmetric monomers A or B (green). d, Superposition of the Cα trace of the S20G 3PF monomer C (green) on the Cα trace of wild-type monomers A or B (blue) in the region of residues 15–29 (indicated by numbers of the respective colours for each structure). In all the above panels the N-terminal and C-terminal ends of each structure are indicated with letters (N and C) in the respective colour of each monomer. e, The ΔGdiss for wild-type (blue circles) or S20G mutant polymorph 2PF (orange circles) or 3PF (red circles) as estimated by PDBePISA is plotted in function of the number of molecular layers on each model. The data is well represented by linear fit (shown as segmented lines).
Extended Data Fig. 7. Comparison of recently published structures of wild-type and sumoylated amylin fibrils.
Structural superposition of a molecular layer of a, S-shaped monomers of wild-type amylin from a right-handed fibril (blue, PDB code 6Y1A39) onto S-shaped monomers of wild-type amylin determined in our study (left-handed fibril, red), b, N-terminally sumoylated amylin monomers (green, PDB code 6VW242) superposed on the S-shaped monomers determined in our study (red).
Extended Data Fig. 8. Previously proposed structure and model for wild-type amylin, and superposition of X-ray structures from fragments onto cryo-EM structure of wild-type amylin.
a, Structural model for the striated ribbon polymorph studied by Luca and co-workers43 where two amylin monomers are represented as ribbons coloured from N-terminal yellow to C-terminal blue. b, Stick representation of one of the atomic models proposed by Luca and co-workers43. c, Diagram of the “β-serpentine” fold proposed by Kajava and co-workers35 for wildtype amylin. d, Ball-and-stick model of the “β-serpentine” shown in (c). e, Cα trace of fragments of amylin determined by X-ray crystallography (aa 14-20, PDB: 3FTH46, green; aa 14-19, PDB: 3FR146, yellow; aa 21-27, PDB: 3DGJ27, red; aa 28-33, PDB: 3DG127, orange; aa 31-37, PDB: 3FTL46, cyan; aa 31-37, PDB: 3FTK46, purple) superposed to the Cα trace of the S-shaped monomer of wild-type amylin fibrils (blue). The N-terminal and C-terminal ends of the S-shaped monomer of wild-type amylin are indicated by letters N and C in blue colour, respectively. f, As in (e) but also showing the side chains of those amino acids that superpose.
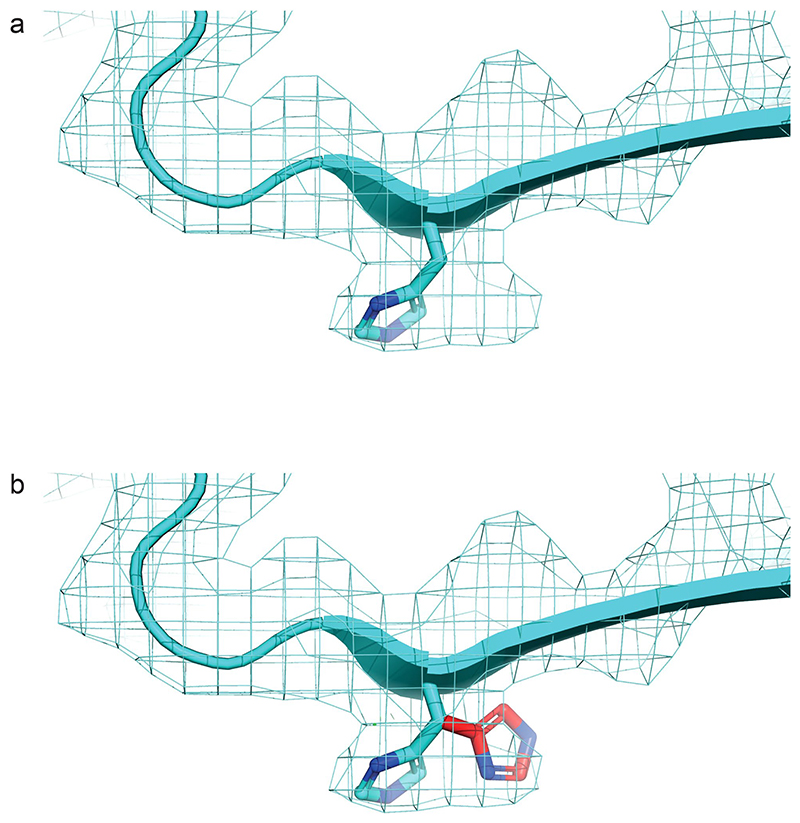
Extended Data Fig. 9. The electron potential map around His18 in the wild-type amylin fibril structure is difficult to interpret.
The density could accommodate two distinct His rotamers (shown in panels a and b). It is possible that both rotomers are present in the structure, or that the dataset contains two polymorphs that differ only in this position. His18 has been proposed to be involved in binding metal ions such as zinc and calcium48. Interestingly, it has been proposed that Lys1 is also part of the coordination system of Zn2+, together with His18, which would require the N-terminal sequence of amylin, including Lys1, to fold back towards His1848. This hypothesis would be consistent with the position of the N-terminal LDR in the wild-type fibril structure presented here (Fig. 1c).
Extended Data Fig. 10. The S-shaped fold of wild-type amylin is similar to the fold of Aβ42 fibril fold.
a, Sequence alignment of human amylin and Aβ42. The highest sequence similarity region (56%) is highlighted by a black box. The aggregation prone region with highest similarity between amylin (residues 22-27) and Aβ42 (residues 27-32) is highlighted by a red box. Conservation symbols and sequence colour are according to Clustal. b, c, d and e, Cα trace of wild-type amylin (blue) superposed onto the Cα trace of Aβ42 fibrils solved by cryo-EM (panel b, cyan, PDB: 5OQV29), and solved by ssNMR (panel c, green, PDB: 2NAO30; panel d, pink, PDB: 5KK328; panel e, orange, PDB: 2MXU31). The side chains of S20 in amylin, and E22 in Aβ, are shown as sticks in panel (b). The N-terminal and C-terminal ends of each structure are indicated by N and C letters, respectively.
Supplementary Material
Acknowledgements
R.G., Y.X., R.F., N.A.R. and S.E.R thank Wellcome for generous support (no. 204963). M.G.I. is supported by the MRC (no. MR/P018491/1). All EM was performed at the Astbury Biostructure Laboratory, which was funded by the University of Leeds and the Wellcome Trust (no. 108466/Z/15/Z), and we thank R. Thompson, E. Hesketh, D. Maskell and C. Scarff for assistance with cryo-EM data collection. We thank V. Zorzini for assistance in model building. Peptide synthesis was performed using instrumentation funded by EPSRC (no. EP/N013573/1). The AFM experiments were performed with instrumentation funded by Wellcome (no. 101497/Z/13/Z). All data processing was performed using ARC4 at the University of Leeds. Finally, we thank colleagues in the Radford and Ranson laboratories for many helpful discussions while preparing this manuscript.
Footnotes
Author contributions
R.G., M.G.I., Y.X., R.F., S.E.R. and N.A.R. designed the experiments. Y.X. synthesized and characterized peptides. R.G. and Y.X. optimized fibril growth conditions and prepared fibrils. R.G. and G.R.H. performed AFM experiments. R.G. prepared EM samples and collected data. R.G. and M.G.I. performed image processing and reconstruction. R.G. performed model building and refinement. All authors analyzed some or all of the data, and wrote or edited the manuscript.
Competing interests
The authors declare no competing interests.
Peer review information Inês Chen was the primary editor on this article and managed its editorial process and peer review in collaboration with the rest of the editorial team.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Data availability
The cryo-EM maps and atomic models have been deposited in the EMDB and wwPDB, respectively, with the following accession codes: wild-type amylin (EMD-11380, PDB 6ZRF), S20G 2PF (EMD-11382, PDB 6ZRQ) and S20G 3PF (EMD-11383, PDB 6ZRR).
References
- 1.Iadanza MG, Jackson MP, Hewitt EW, Ranson NA, Radford SE. A new era for understanding amyloid structures and disease. Nat Rev Mol Cell Biol. 2018;19:755–773. doi: 10.1038/s41580-018-0060-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Otzen D, Riek R. Functional amyloids. Cold Spring Harb Perspect Biol. 2019 doi: 10.1101/cshperspect.a033860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fitzpatrick AWP, et al. Cryo-EM structures of tau filaments from Alzheimer’s disease. Nature. 2017;547:185–190. doi: 10.1038/nature23002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kollmer M, et al. Cryo-EM structure and polymorphism of Aβ amyloid fibrils purified from Alzheimer’s brain tissue. Nat Commun. 2019;10:4760. doi: 10.1038/s41467-019-12683-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Falcon B, et al. Structures of filaments from Pick’s disease reveal a novel tau protein fold. Nature. 2018;561:137–140. doi: 10.1038/s41586-018-0454-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Falcon B, et al. Novel tau filament fold in chronic traumatic encephalopathy encloses hydrophobic molecules. Nature. 2019;568:420–423. doi: 10.1038/s41586-019-1026-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang W, et al. Novel tau filament fold in corticobasal degeneration. Nature. 2020;580:283–287. doi: 10.1038/s41586-020-2043-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schweighauser M, et al. Structures of α-synuclein filaments from multiple system atrophy. Nature. 2020 doi: 10.1038/s41586-020-2317-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liberta F, et al. Cryo-EM fibril structures from systemic AA amyloidosis reveal the species complementarity of pathological amyloids. Nat Commun. 2019;10:1104. doi: 10.1038/s41467-019-09033-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Radamaker L, et al. Cryo-EM structure of a light chain-derived amyloid fibril from a patient with systemic AL amyloidosis. Nat Commun. 2019;10:1103. doi: 10.1038/s41467-019-09032-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Swuec P, et al. Cryo-EM structure of cardiac amyloid fibrils from an immunoglobulin light chain AL amyloidosis patient. Nat Commun. 2019;10:1269. doi: 10.1038/s41467-019-09133-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schmidt M, et al. Cryo-EM structure of a transthyretin-derived amyloid fibril from a patient with hereditary ATTR amyloidosis. Nat Commun. 2019;10:5008. doi: 10.1038/s41467-019-13038-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Boyer DR, et al. Structures of fibrils formed by α-synuclein hereditary disease mutant H50Q reveal new polymorphs. Nat Struct Mol Biol. 2019;26:1044–1052. doi: 10.1038/s41594-019-0322-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Boyer DR, et al. The α-synuclein hereditary mutation E46K unlocks a more stable, pathogenic fibril structure. Proc Natl Acad Sci USA. 2020;117:3592–3602. doi: 10.1073/pnas.1917914117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Westermark P, Wernstedt C, Wilander E, Sletten K. A novel peptide in the calcitonin gene related peptide family as an amyloid fibril protein in the endocrine pancreas. Biochem Biophys Res Commun. 1986;140:827–831. doi: 10.1016/0006-291x(86)90708-4. [DOI] [PubMed] [Google Scholar]
- 16.Birol M, Kumar S, Rhoades E, Miranker AD. Conformational switching within dynamic oligomers underpins toxic gain-of-function by diabetes-associated amyloid. Nat Commun. 2018;9:1312. doi: 10.1038/s41467-018-03651-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Raleigh D, Zhang X, Hastoy B, Clark A. The β-cell assassin: IAPP cytotoxicity. J Mol Endocrinol. 2017;59:R121–R140. doi: 10.1530/JME-17-0105. [DOI] [PubMed] [Google Scholar]
- 18.Sakagashira S, et al. Missense mutation of amylin gene (S20G) in Japanese NIDDM patients. Diabetes. 1996;45:1279–1281. doi: 10.2337/diab.45.9.1279. [DOI] [PubMed] [Google Scholar]
- 19.Meier DT, et al. The S20G substitution in hIAPP is more amyloidogenic and cytotoxic than wild-type hIAPP in mouse islets. Diabetologia. 2016;59:2166–2171. doi: 10.1007/s00125-016-4045-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sakagashira S, et al. S20G mutant amylin exhibits increased in vitro amyloidogenicity and increased intracellular cytotoxicity compared to wild-type amylin. Am J Pathol. 2000;157:2101–2109. doi: 10.1016/S0002-9440(10)64848-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Young LM, Tu LH, Raleigh DP, Ashcroft AE, Radford SE. Understanding co-polymerization in amyloid formation by direct observation of mixed oligomers. Chem Sci. 2017;8:5030–5040. doi: 10.1039/c7sc00620a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jaikaran ET, et al. Identification of a novel human islet amyloid polypeptide β-sheet domain and factors influencing fibrillogenesis. J Mol Biol. 2001;308:515–525. doi: 10.1006/jmbi.2001.4593. [DOI] [PubMed] [Google Scholar]
- 23.Goldsbury C, et al. Amyloid fibril formation from full-length and fragments of amylin. J Struct Biol. 2000;130:352–362. doi: 10.1006/jsbi.2000.4268. [DOI] [PubMed] [Google Scholar]
- 24.Westermark P, Engstrom U, Johnson KH, Westermark GT, Betsholtz C. Islet amyloid polypeptide: pinpointing amino acid residues linked to amyloid fibril formation. Proc Natl Acad Sci USA. 1990;87:5036–5040. doi: 10.1073/pnas.87.13.5036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ashburn TT, Auger M, Lansbury PT., Jr The structural basis of pancreatic amyloid formation: isotope-edited spectroscopy in the solid state. J Am Chem Soc. 1992;114:790–791. [Google Scholar]
- 26.Soriaga AB, Sangwan S, Macdonald R, Sawaya MR, Eisenberg D. Crystal structures of IAPP amyloidogenic segments reveal a novel packing motif of out-of-register beta sheets. J Phys Chem B. 2016;120:5810–5816. doi: 10.1021/acs.jpcb.5b09981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wiltzius JJ, et al. Atomic structure of the cross-β spine of islet amyloid polypeptide (amylin) Protein Sci. 2008;17:1467–1474. doi: 10.1110/ps.036509.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Colvin MT, et al. Atomic resolution structure of monomorphic Aβ42 amyloid fibrils. J Am Chem Soc. 2016;138:9663–9674. doi: 10.1021/jacs.6b05129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gremer L, et al. Fibril structure of amyloid-β(1–42) by cryo-electron microscopy. Science. 2017;358:116–119. doi: 10.1126/science.aao2825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Walti MA, et al. Atomic-resolution structure of a disease-relevant Aβ(1–42) amyloid fibril. Proc Natl Acad Sci USA. 2016;113:E4976–E4984. doi: 10.1073/pnas.1600749113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xiao Y, et al. Aβ(1–42) fibril structure illuminates self-recognition and replication of amyloid in Alzheimer’s disease. Nat Struct Mol Biol. 2015;22:499–505. doi: 10.1038/nsmb.2991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gallardo R, Ranson NA, Radford SE. Amyloid structures: much more than just a cross-β fold. Curr Opin Struct Biol. 2020;60:7–16. doi: 10.1016/j.sbi.2019.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Alexandrescu AT. Amide proton solvent protection in amylin fibrils probed by quenched hydrogen exchange NMR. PLoS ONE. 2013;8:e56467. doi: 10.1371/journal.pone.0056467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jayasinghe SA, Langen R. Identifying structural features of fibrillar islet amyloid polypeptide using site-directed spin labeling. J Biol Chem. 2004;279:48420–48425. doi: 10.1074/jbc.M406853200. [DOI] [PubMed] [Google Scholar]
- 35.Kajava AV, Aebi U, Steven AC. The parallel superpleated beta-structure as a model for amyloid fibrils of human amylin. J Mol Biol. 2005;348:247–252. doi: 10.1016/j.jmb.2005.02.029. [DOI] [PubMed] [Google Scholar]
- 36.Weirich F, et al. Structural characterization of fibrils from recombinant human islet amyloid polypeptide by solid-state NMR: the central FGAILS segment is part of the β-sheet core. PLoS ONE. 2016;11:e0161243. doi: 10.1371/journal.pone.0161243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Krissinel E, Henrick K. Inference of macromolecular assemblies from crystalline state. J Mol Biol. 2007;372:774–797. doi: 10.1016/j.jmb.2007.05.022. [DOI] [PubMed] [Google Scholar]
- 38.Seino S Study Group of Comprehensive Analysis of Genetic Factors in Diabetes Mellitus. S20G mutation of the amylin gene is associated with Type II diabetes in Japanese. Diabetologia. 2001;44:906–909. doi: 10.1007/s001250100531. [DOI] [PubMed] [Google Scholar]
- 39.Röder C, et al. Cryo-EM structure of islet amyloid polypeptide fibrils reveals similarities with amyloid-β fibrils. Nat Struct Mol Biol. 2020;27:660–667. doi: 10.1038/s41594-020-0442-4. [DOI] [PubMed] [Google Scholar]
- 40.Goldsbury CS, et al. Polymorphic fibrillar assembly of human amylin. J Struct Biol. 1997;119:17–27. doi: 10.1006/jsbi.1997.3858. [DOI] [PubMed] [Google Scholar]
- 41.Bedrood S, et al. Fibril structure of human islet amyloid polypeptide. J Biol Chem. 2012;287:5235–5241. doi: 10.1074/jbc.M111.327817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cao Q, Boyer DR, Sawaya MR, Ge P, Eisenberg DS. Cryo-EM structure and inhibitor design of human IAPP (amylin) fibrils. Nat Struct Mol Biol. 2020;27:653–659. doi: 10.1038/s41594-020-0435-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Luca S, Yau WM, Leapman R, Tycko R. Peptide conformation and supramolecular organization in amylin fibrils: constraints from solid-state NMR. Biochemistry. 2007;46:13505–13522. doi: 10.1021/bi701427q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Padrick SB, Miranker AD. Islet amyloid polypeptide: identification of long-range contacts and local order on the fibrillogenesis pathway. J Mol Biol. 2001;308:783–794. doi: 10.1006/jmbi.2001.4608. [DOI] [PubMed] [Google Scholar]
- 45.Krotee P, et al. Common fibrillar spines of amyloid-β and human islet amyloid polypeptide revealed by microelectron diffraction and structure-based inhibitors. J Biol Chem. 2018;293:2888–2902. doi: 10.1074/jbc.M117.806109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wiltzius JJ, et al. Molecular mechanisms for protein-encoded inheritance. Nat Struct Mol Biol. 2009;16:973–978. doi: 10.1038/nsmb.1643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Godin E, Nguyen PT, Zottig X, Bourgault S. Identification of a hinge residue controlling islet amyloid polypeptide self-assembly and cytotoxicity. J Biol Chem. 2019;294:8452–8463. doi: 10.1074/jbc.RA118.006454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rowinska-Zyrek M. Coordination of Zn2+ and Cu2+ to the membrane disrupting fragment of amylin. Dalton Trans. 2016;45:8099–8106. doi: 10.1039/c6dt00628k. [DOI] [PubMed] [Google Scholar]
- 49.Glynn C, et al. Cryo-EM structure of a human prion fibril with a hydrophobic, protease-resistant core. Nat Struct Mol Biol. 2020;27:417–423. doi: 10.1038/s41594-020-0403-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sgourakis NG, Yau WM, Qiang W. Modeling an in-register, parallel “Iowa” Aβ fibril structure using solid-state NMR data from labeled samples with Rosetta. Structure. 2015;23:216–227. doi: 10.1016/j.str.2014.10.022. [DOI] [PubMed] [Google Scholar]
- 51.Li B, et al. Cryo-EM of full-length α-synuclein reveals fibril polymorphs with a common structural kernel. Nat Commun. 2018;9:3609. doi: 10.1038/s41467-018-05971-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hervas R, et al. Cryo-EM structure of a neuronal functional amyloid implicated in memory persistence in Drosophila. Science. 2020;367:1230–1234. doi: 10.1126/science.aba3526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Han W, Li C. Linking type 2 diabetes and Alzheimer’s disease. Proc Natl Acad Sci USA. 2010;107:6557–6558. doi: 10.1073/pnas.1002555107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Biessels GJ, Staekenborg S, Brunner E, Brayne C, Scheltens P. Risk of dementia in diabetes mellitus: a systematic review. Lancet Neurol. 2006;5:64–74. doi: 10.1016/S1474-4422(05)70284-2. [DOI] [PubMed] [Google Scholar]
- 55.Hanyu H. Diabetes-related dementia. Adv Exp Med Biol. 2019;1128:147–160. doi: 10.1007/978-981-13-3540-2_8. [DOI] [PubMed] [Google Scholar]
- 56.Hu R, Zhang M, Chen H, Jiang B, Zheng J. Cross-seeding interaction between β-amyloid and human islet amyloid polypeptide. ACS Chem Neurosci. 2015;6:1759–1768. doi: 10.1021/acschemneuro.5b00192. [DOI] [PubMed] [Google Scholar]
- 57.Andreetto E, et al. Identification of hot regions of the Aβ–IAPP interaction interface as high-affinity binding sites in both cross- and self-association. Angew Chem Int Ed Engl. 2010;49:3081–3085. doi: 10.1002/anie.200904902. [DOI] [PubMed] [Google Scholar]
- 58.O’Nuallain B, Williams AD, Westermark P, Wetzel R. Seeding specificity in amyloid growth induced by heterologous fibrils. J Biol Chem. 2004;279:17490–17499. doi: 10.1074/jbc.M311300200. [DOI] [PubMed] [Google Scholar]
- 59.Young LM, et al. Insights into the consequences of co-polymerisation in the early stages of IAPP and Aβ peptide assembly from mass spectrometry. Analyst. 2015;140:6990–6999. doi: 10.1039/c5an00865d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Nilsberth C, et al. The ‘Arctic’ APP mutation (E693G) causes Alzheimer’s disease by enhanced Aβ protofibril formation. Nat Neurosci. 2001;4:887–893. doi: 10.1038/nn0901-887. [DOI] [PubMed] [Google Scholar]
- 61.Paravastu AK, Leapman RD, Yau WM, Tycko R. Molecular structural basis for polymorphism in Alzheimer’s β-amyloid fibrils. Proc Natl Acad Sci USA. 2008;105:18349–18354. doi: 10.1073/pnas.0806270105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lu JX, et al. Molecular structure of β-amyloid fibrils in Alzheimer’s disease brain tissue. Cell. 2013;154:1257–1268. doi: 10.1016/j.cell.2013.08.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Caspar DL, Klug A. Physical principles in the construction of regular viruses. Cold Spring Harb Symp Quant Biol. 1962;27:1–24. doi: 10.1101/sqb.1962.027.001.005. [DOI] [PubMed] [Google Scholar]
- 64.Abedini A, Raleigh DP. The role of His-18 in amyloid formation by human islet amyloid polypeptide. Biochemistry. 2005;44:16284–16291. doi: 10.1021/bi051432v. [DOI] [PubMed] [Google Scholar]
- 65.Khemtemourian L, Guillemain G, Foufelle F, Killian JA. Residue specific effects of human islet polypeptide amyloid on self-assembly and on cell toxicity. Biochimie. 2017;142:22–30. doi: 10.1016/j.biochi.2017.07.015. [DOI] [PubMed] [Google Scholar]
- 66.Zheng SQ, et al. MotionCor2: anisotropic correction of beam-induced motion for improved cryo-electron microscopy. Nat Methods. 2017;14:331–332. doi: 10.1038/nmeth.4193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zhang K. Gctf: real-time CTF determination and correction. J Struct Biol. 2016;193:1–12. doi: 10.1016/j.jsb.2015.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.He S, Scheres SHW. Helical reconstruction in RELION. J Struct Biol. 2017;198:163–176. doi: 10.1016/j.jsb.2017.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zivanov J, et al. New tools for automated high-resolution cryo-EM structure determination in RELION-3. Elife. 2018;7:e42166. doi: 10.7554/eLife.42166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Scheres SHW. Amyloid structure determination in RELION-3.1. Acta Crystallogr D Struct Biol. 2020;76:94–101. doi: 10.1107/S2059798319016577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Emsley P, Lohkamp B, Scott WG, Cowtan K. Features and development of Coot. Acta Crystallogr D Struct Biol. 2010;66:486–501. doi: 10.1107/S0907444910007493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Pettersen EF, et al. UCSF Chimera—a visualization system for exploratory research and analysis. J Comput Chem. 2004;25:1605–1612. doi: 10.1002/jcc.20084. [DOI] [PubMed] [Google Scholar]
- 73.Liebschner D, et al. Macromolecular structure determination using X-rays, neutrons and electrons: recent developments in Phenix. Acta Crystallogr D Struct Biol. 2019;75:861–877. doi: 10.1107/S2059798319011471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Chen VB, et al. MolProbity: all-atom structure validation for macromolecular crystallography. Acta Crystallogr D Struct Biol. 2010;66:12–21. doi: 10.1107/S0907444909042073. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The cryo-EM maps and atomic models have been deposited in the EMDB and wwPDB, respectively, with the following accession codes: wild-type amylin (EMD-11380, PDB 6ZRF), S20G 2PF (EMD-11382, PDB 6ZRQ) and S20G 3PF (EMD-11383, PDB 6ZRR).