Abstract
Itch is a unique sensory experience that is responded to by scratching. How pruritogens, which are mechanical and chemical stimuli with the potential to cause itch, engage specific pathways in the peripheral and central nervous system has been a topic of intense investigation over the last few years. Studies employing recently developed molecular, physiological, and behavioral techniques have delineated the dedicated mechanisms that transmit itch information to the brain. This review outlines the genetically defined and evolutionary conserved circuits for itch ranging from the skin-innervating peripheral neurons to the cortical neurons that drive scratching. Moreover, scratch suppression of itch is attributed to the concurrent activation of pain and itch pathways. Hence, we discuss the similarities between circuits driving pain and itch.
Keywords: Ciruits, itch, modulation, pain
Abbreviations
- AITC
allyl isothiocynate
- DRG
dorsal root gangalion
- GRP
gastrin releasing peptide
- GRPR
gastrin releasing peptide receptor
- PAG
periaqueductal gray
- PBN
parabrachial Nucleus
- TG
trigeminal gangalion
1. Introduction
Frequent mosquito bites in the evening, especially after the monsoon, are an integral part of the living experience of people residing in a tropical country such as India. These mosquito bites typically cause pain, followed by an intense urge to itch. The itch due to a mosquito bite is caused by components of the mosquito’s saliva which is released into the superficial layer of skin (Ohtsuka et al. 2001). The exact mechanism of mosquito bite-induced pruritus is not completely understood; however, bite-induced itching may be caused by activation of neural pathways that can sense histamine and other itch-causing agents (Fostini et al. 2019; Vander Does et al. 2022). Histamine in the mosquito’s saliva activates histamine receptors on itch-sensing sensory neurons and causes the sensation of itch. Receptors on itch-sensitive sensory neurons in the skin transmit information regarding the presence of histamine to the brain through dedicated neural pathways in the spinal cord. As a result, we sense itch, orient our arms towards the site of the mosquito bite, and scratch with the nails on our fingers. Mosquito bite-induced itch is short-lasting, and this sort of itching is protective. However, chronic itch conditions such as atopic dermatitis and psoriasis cause uncontrollable scratching, leading to physical injuries. The loss of quality of life due to chronic itch is a significant burden on society and has no good clinical resolution. Moreover, chronic itch caused by persistent release of histamines can be treated by anti-histamines, but non-histaminergic itch is more challenging to treat. For example, one prominent side-effect of chloroquine treatment in malarial patients is persistent itch (Buszman et al. 1984; Adebayo et al. 1997; Liu et al. 2009; Aghahowa et al. 2010).
The entire process, from sensing the itch to scratching, is facilitated by neural circuits that begin with multipolar sensory neurons feeding itch information derived from the skin into the dorsal horn of the spinal cord. In the spinal cord, two sets of neurons are recruited to process and transmit itch information to the brain. The first are the projection neurons with axon terminals in the brainstem and thalamus that transmit itch (Chen and Sun 2020; Wercberger et al. 2021; Ren et al. 2023). Second, interneurons gate the interactions between the sensory and projection neurons in the spinal cord to adjust the latency and frequency of scratching (Sun and Chen 2007; Sun et al. 2009; Braz et al. 2014; Bourane et al. 2015; Acton et al. 2019; Pan et al. 2019; Chen and Sun 2020). The brain areas that receive the spinal inputs, such as the parabrachial nucleus (PBN) and periaqueductal gray (PAG), transmit the information to limbic areas such as the amygdala, regions involved in sensory discrimination such as the thalamus, and sensorimotor nuclei in the brainstem such as reticular formation (Ikoma et al. 2006; Mu and Sun 2022). Here, we have detailed the neuronal population in the peripheral, spinal, and supraspinal levels that transmit information from itch at the skin to various nuclei across the brain. We have stressed how cell populations defined by differential gene expression at each of the nodes of itch information and processing allow us to interrogate dedicated neural pathways.
2. Peripheral pruriceptors
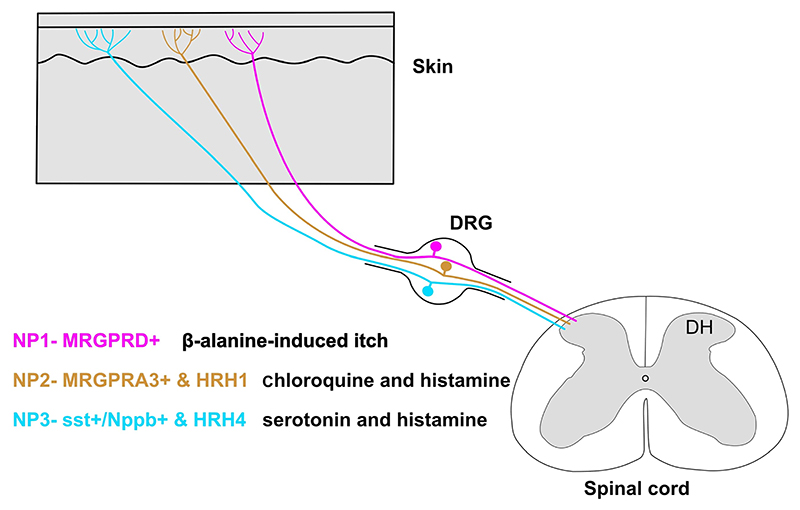
Itch-sensing primary sensory neurons in the dorsal root ganglion (DRG) and the trigeminal ganglion (TG), also known as pruriceptors, innervate the skin covering the body and head, respectively. The pruriceptors form free nerve endings in the superficial skin layers and are slow-conducting C-fibers (Schmelz et al. 1997; Namer et al. 2008). Pruritogens that activate these neurons are of two main types: histaminergic and non-histaminergic. In the last few decades, many studies have focused on isolating the dedicated sensory neuron population for various somatosensory stimuli including itch. These efforts were driven by the labeled line theory for sensory modalities, which proposed that distinct somatosensory stimuli such as gentle touch and chemical itch are transmitted by non-overlapping sensory neuron populations (Schmelz et al. 1997; Liu et al. 2009; Han et al. 2013). Initial painstaking electrophysiological experiments helped identify sensory neurons dedicated to sensing itch stimuli. Microneurographic recordings from human skin showed that there is a population of slow-conducting sensory neurons that are mechanically insensitive but are excited by iontophoretic injections of histamine (Schmelz et al. 1997). Later studies by the same group showed that a population of histamine-sensitive neurons respond to noxious chemical stimuli such as capsaicin (active component of chilli pepper) and allyl isothio-cyanate (AITC) (active component of mustard oil) (Schmelz et al. 2003), thus indicating that the itch-responsive primary sensory neurons are polymodal, i.e., respond to multiple classes of stimuli. Recently, gene expression in modality-specific somatosensory neuron populations has provided access to itch-responsive neurons. For example, neurons expressing the chloroquine receptor MrgprA3 (Mas-related G-proteins-coupled receptor member A3) respond to histamine and other pruritogens such as serotonin (Xing et al. 2020). The genetic classification of somatosensory neurons is important for two aspects. First, it allows us to study the molecular characteristics and physiology of the neurons of our interest in a targeted manner; for example, studies performed on transgenic strains driven by the MrgprA3 promoter revealed their morphology in the skin and relationship with thermal or mechanical somatosensory stimuli (Han et al. 2013). Second, this approach can reveal molecular drug targets that are specifically expressed in itch neurons, so that the other somatosensory modalities remain unaffected. Another independent itch-sensitive sensory neuron population expresses the neuropeptide gene, natriuretic polypeptide b (Nppb). The Nppb molecule is critical for itch transmission since pruritic stimuli induce Nppb release from the peripheral sensory neurons into the spinal cord; Nppb binds to the spinal cord neurons expressing natriuretic peptide receptor 1 (Npr1) and facilitates itch (Mishra and Hoon 2013). With the advent of single-cell transcriptome profiling techniques, we can now comprehensively assign molecular identities to modality-specific sensory neuronal populations. For example, a recent single-cell sequencing technique study categorized itch-sensing DRG neurons into three independent groups: NP1, NP2, and NP3 (Usoskin et al. 2015) (figure 1). NP1 neurons are marked by the expression of MrgprD receptors which mediate β-alanine-induced itch (Liu et al. 2012). NP2 neurons are characterized by the expression of MrgprA3 receptors (Liu et al. 2009; Han et al. 2013). In contrast, the NP3 population of neurons expresses somatostatin and brain natriuretic peptide (Nppb). The NP3 population also expresses noxious heat- and chemical-sensing channels TrpV1 and TrpA1, respectively (Usoskin et al. 2015). Thus, itch fibers are mostly multimodal and thus can respond to both noxious thermal and chemical stimuli.
Figure 1. The three major groups of pruriceptors in mice.
Unbiased single-cell RNA-sequencing classifies itch-sensing DRG neurons into three categories: NP1, NP2, and NP3. These neurons innervate the epidermis of the skin, and hence are positioned to sense external as well as internal pruritogens. The NP1 population of sensory neurons expresses MrgprD receptors and is involved in β-alasnine-induced itch. NP2 and NP3 both expresses the histamine receptors HRH1 and HRH4, respectively, and hence are involved in histaminergic itch. The distinction between NP2 and NP3 being, NP2 can evoke itch through chloroquine as it also expresses the MrgprA3 receptor, whereas NP3 expresses both Sst (somatostatin) and Nppb (natriuretic polypeptide b), which play an important role in the transmission of itch signals at the periphery and spinal cord levels.
Sensory neurons use a variety of neurotransmitters and neuropeptides to transmit itch signals to spinal dorsal horn neurons. Glutamate is one of the prominent neurotransmitters in the primary sensory neurons. However, knocking out glutamate from TrpV1+ neurons, which include the itch neurons, abrogated pain-induced behaviors but did not affect itch-induced scratching (Lagerström et al. 2010; Liu et al. 2010), indicating that itch transmission from the periphery to the spinal cord is primarily peptidergic. In contrast, a recent study has shown that glutamate release from itch-selective primary sensory MrgprA3+ neurons is important in the transmission of itch signals from the sensory neurons to the spinal cord, while the itch-selective neuropeptide NMB is involved in enhancing the activity of spinal itch-responsive neurons (Cui et al. 2022). Interestingly, this study shows that the effect of glutamate deletions on itch is persistent for 6 months, suggesting that previous reports of no effect of glutamate on itch sensation may be an underestimation of the effect of glutamate on pruriception. Serotonin, an essential brain neurotransmitter, can also drive itch. Serotonin receptors are expressed in mice and human DRG neurons (Flegel et al. 2015; Usoskin et al. 2015). The serotonin receptor HTR7 mediates acute itch triggered by serotonin application or selective serotonin reuptake inhibitors. Genetic deletion of HTR7 in mice abolishes serotonin-induced acute itch and reduces skin lesions in chronic itch (Morita et al. 2015).
3. Spinal GRP–GRPR circuitry
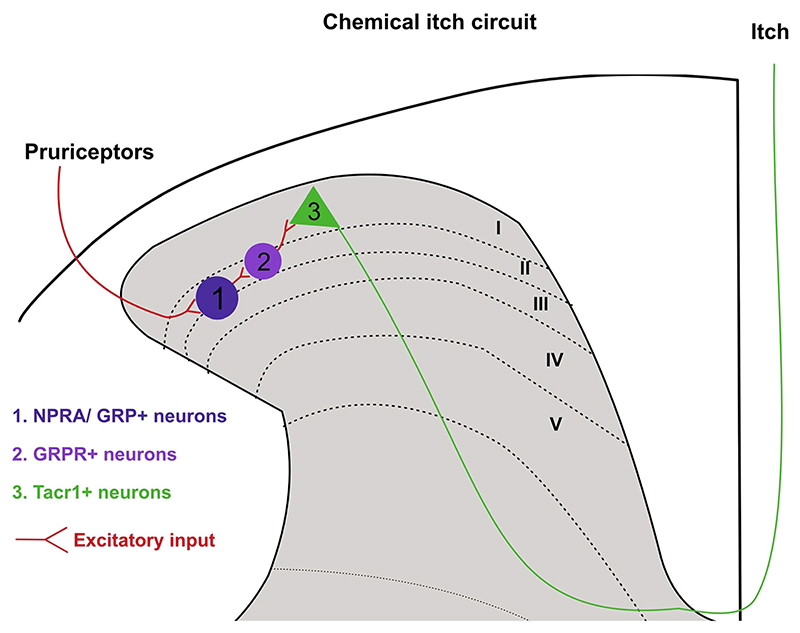
The neuropeptide Grp and its receptor Grpr-expressing neurons in the spinal dorsal horn are important for transmission of chemical itch in mice (Sun and Chen 2007; Sun et al. 2009). Grp belongs to the bombesin family of neuropeptides, and these peptides were originally discovered in frog skin (Erspamer et al. 1972; Jensen et al. 2008). Their homologs in mammals are gastrin-related peptide (Grp) and neuromedin b (Nmb). Grp and Nmb neuropeptides signal through their dedicated high-affinity receptors, gastrin-releasing peptide receptor (Grpr) and neuromedin B receptor (Nmbr), respectively (Battey and Wada 1991; Kroog et al. 1995). These neuropeptides and their receptors are expressed in various organs in the body, including the nervous system, and have been found to have important roles in stress response and metabolic regulation (Gonzalez et al. 2008). Surprisingly, more recently it was found that the peptides Grp and Nmb are sufficient to induce itch (Sun and Chen 2007; Sun et al. 2009; Su and Ko 2011). Of the two bombesin-like neuropeptides, the Grp neuropeptide and its receptor Grpr have been well studied in itch and itch-induced scratching behavior (Barry et al. 2018; figure 2). Grp was initially found to be ina small subset of primary sensory neurons and Grpr in the dorsal horn of the spinal cord (Panula et al. 1983; Sun and Chen 2007). Intrathecal Grp induced spontaneous scratching, whereas simultaneous administration of Grp and Grpr-antagonist abrogated the pruritic effects of Grp. Furthermore, when spinal cord neurons expressing Grpr were ablated using bombesin-conjugated cytotoxins or when Grpr was knocked out in mutant mice, scratching behavior decreased in response to pruritogens (Sun et al. 2009; Sun and Chen 2007). However, careful gene expression studies revealed that Grp was present at very low levels in DRG neurons (Solorzano et al. 2015). In contrast, Grp was highly expressed in a small subset of interneurons in the dorsal horn (Fleming et al. 2012). Mishra and Hoon (2013) found that Npbb neuropeptide-expressing DRG neurons activate Npr1 (Npbb receptor1)-expressing neurons in the spinal cord and are involved in itch induction in mice. Npr1 neurons also express Grp; thus, activation of these neurons results in Grp release. Grp, in turn, activates Grpr-expressing neurons and thus facilitates itch transmission. More recent studies using optogenetic neuromodulatory techniques showed that the activation of Grpr neurons is sufficient to induce scratching (Chen et al. 2021; figure 2). Interestingly, Grpr neurons synapse onto the tachykinin receptor 1 (Tacr1) (Bardoni et al. 2019), expressing projection neurons (Todd et al. 2000; Spike et al. 2003; Al-Khater and Todd 2009; Cameron et al. 2015; figure 2). Tacr1 neurons form the core of the spinoparabrachial pathway that is intrumental in transmitting pain from the cord to the parabrachial area in the brainstem (Mantyh et al. 1997). Neurotoxic ablation of Tacr1-expressing neurons in the spinal dorsal horn reduces acute and chronic itch-induced scratching (Carstens et al. 2010; Akiyama et al. 2015), thus indicating that itch and pain can be co-transmitted through spinal Tacr1 neurons. However, how itch and pain signals are transmitted by spinal Tacr1 neurons remains to be explored. Further, Grpr neurons are under supraspinal modulation, where descending inputs from the periaqueductal gray can suppress their activity and thus mediate itch-induced scratching (Gao et al. 2019; Liu et al. 2019). Descending serotonergic projections to the spinal cord were shown to modulate the Grp–Grpr circuitry directly and affect scratching behavior (Zhao et al. 2014). Importantly, the Grp–Grpr pathway in the spinal cord primarily transmits non-histaminergic itch, with a minor role in histaminergic itch (Akiyama et al. 2014; Zhao et al. 2014). Thus, the Grp–Grpr pathway is well suited for the targeted development of drugs to treat histamine-resistant chronic itch.
Figure 2. The role of spinal Grp–Grpr circuitry in the itch pathway.
The Npra (natriuretic peptide receptor A), which is a receptor for neuropeptide Nppb, also expresses Grp. Hence, when Nppb-expressing pruriceptors activate dorsal horn Npra-expressing neurons during itch, Grp is released, which in turn activates Grpr interneurons. The Grpr neurons synapse onto the Tacr1 projection neurons which have been implicated in both the itch and pain pathways. This makes the Grp–Grpr circuitry important in evoking itch responses.
4. Spinal neurons for mechanical itch
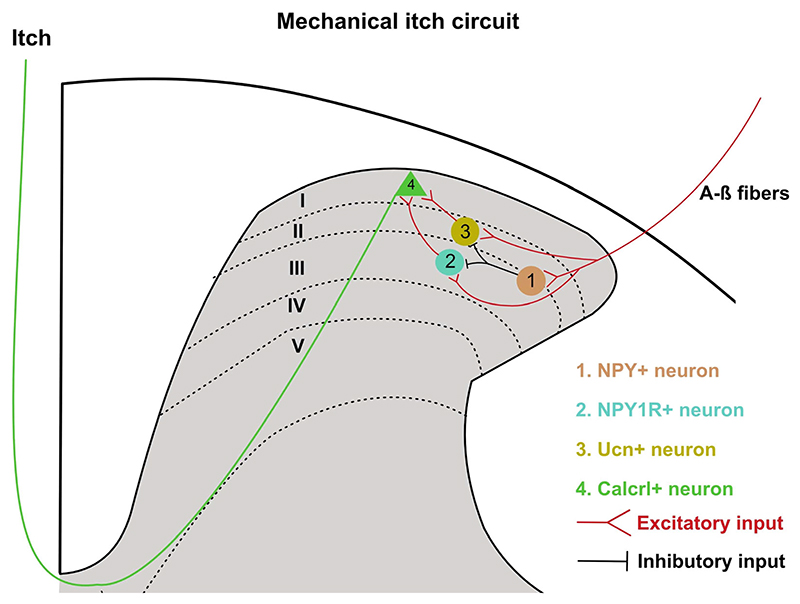
Recent studies have pointed out a segregation between the chemical and mechanical itch pathways in the spinal cord. Both types of itch are gated by inhibitory and excitatory interneurons located in the dorsal horn. The neuropeptide Y (Npy)-expressing inhibitory neurons are distributed in laminas III and IVof the dorsal horn and are distinct from the ones expressing dynorphin, galanin, or parvalbumin. When the spinal cord Npy neurons were ablated or chemogenetically silenced, mice developed mechanical itch. At the same time, pain or chemical itch remained unchanged (Bourane et al. 2015). The excitatory neurons expressing the gene encoding for the receptor of Npy, Npy1r, receive inhibitory inputs from the Npy-expressing neurons (Acton et al. 2019; Pan et al. 2019; figure 3). Ablation or inhibition of the Npy1r neurons suppressed mechanical itch, whereas activation resulted in spontaneous itch and enhanced mechanical itch (Acton et al. 2019). Moreover, a recent study showed that the ablation of excitatory interneurons expressing neuropeptide urocortin 3 (Ucn3+) in mice abrogated mechanical itch without affecting pain or chemical itch, while activation of these neurons increased mechanical itch (Pan et al. 2019). Interestingly, although manipulating Npy1r and Ucn3+ spinal neurons in mice had similar effects on mechanical itch, these populations did not overlap (figure 3), implying that the Npy1r and Ucn3+ spinal neurons can independently mediate mechanical itch and probably play a key role in the transition from acute to chronic itch.
Figure 3. The pathway for mechanical itch sensation.
Both Npy1r- and Ucn-expressing interneurons promote mechanical itch but can be inhibited through Npy neurons. This indicates that transmission of mechanical itch sensation relies on the type of inputs received from the pruriceptors as they can directly synapse onto Npy1r- and Ucn-expressing interneurons. They synapse onto Calcrl-expressing neurons which project to the PBN.
Recently, a Tacr1-independent projection neuron population was discovered that expresses the gene calcitonin receptor-like receptor (Calcrl) (Ren et al. 2023; figure 3). The Calcrl+ neurons, such as seen with the Npy1r- and Ucn3+-expressing neurons, specifically modulate mechanical itch. These neurons are distributed in laminas I and II of the dorsal horn of the spinal cord, and a few are distributed in the lateral spinal nucleus. Inhibition of the axonal terminals of spinal Calcrl+ neurons in the PBN suppressed transmission of mechanical itch from the cord to the brain. Calcr+ projection neurons overlap with Gpr83-expressing projection neurons. Gpr83 neurons are another recently identified population in the spinoparabrachial pathway that specifically transmits innocuous and noxious mechanical stimuli (Choi et al. 2020). Thus, chemical and mechanical itches can be processed and transmitted through independent networks of interneurons and spinal projection neurons.
5. PBN is a gateway for transmitting pain and itch information
Somatosensory information from the spinal cord is relayed to the brain via the spinoparabrachial and spinothalamic pathways. The spinoparabrachial pathway is critical for the aversive, motivational, and motor components of pain and itch response, while the spinothalamic pathway is responsible for sensory discrimination. The PBN is central to the spinopara-brachial pathway and directs pruriceptive information to the thalamus, hypothalamus, basal ganglia, amygdala, insula, and reticular formation (figure 4). Thus, the PBN is positioned to modulate the behavioral and physiological responses to itch; indeed, when neurons in the PBN are silenced, itch is suppressed (Mu et al. 2017). Importantly, the activity of PBN neurons coincides with scratching bouts.
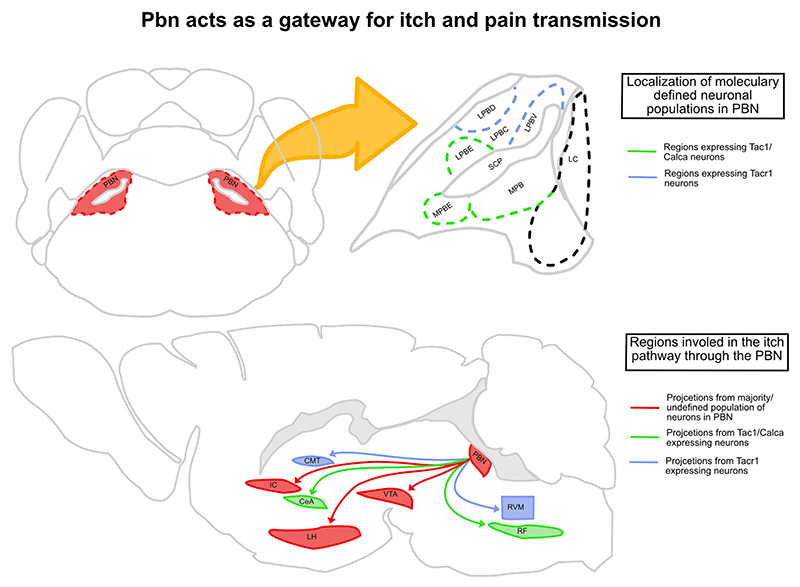
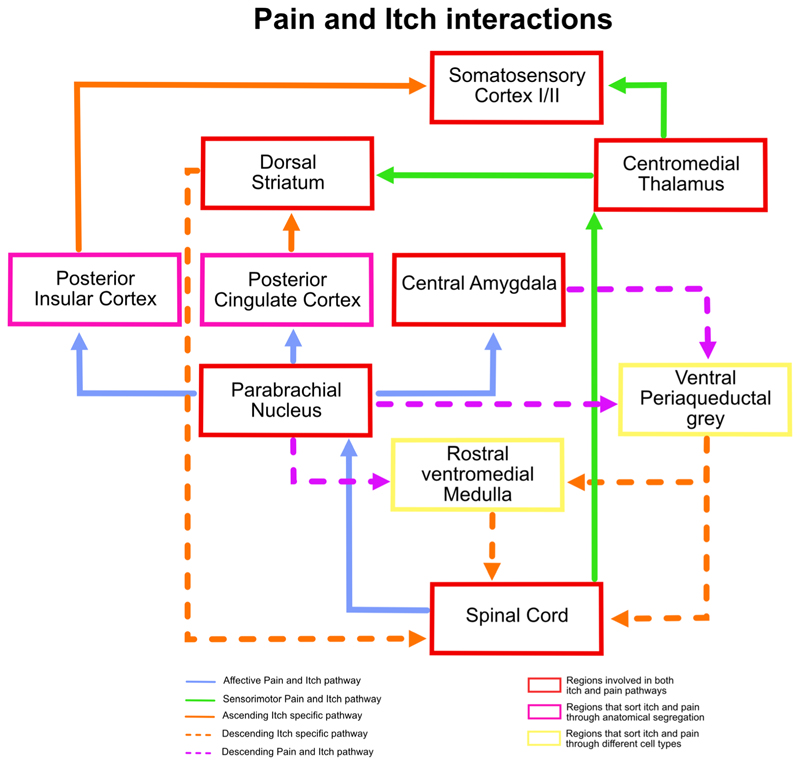
Figure 4. The PBN acts as a gateway for itch and pain transmission.
The PBN lies laterally to the locus coeruleus (LC) and is divided into the lateral PBN and medial PBN. The majority of Tacr1-expressing cells are in the ventro-lateral (LPBV) and dorso-lateral (LPBD) part of the PBN, and majority of the Tac1- and Calca-expressing cells are in the external-lateral (LPBE), external-medial (MPBE), and medial (MPB) PBN. The Tac1/Calca cells projecting to CeA and RF (reticular formation) can mediate/affect both up- and downstream itch pathways, respectively. The Tacr1 cells projecting to CMT (centro-medial thalamus) and RVM can mediate inhibition of itch through pain. The VTA, IC (insular cortex), and LH (lateral hypothalamus) also receive strong inputs from the PBN and have been implicated in affecting various aspects of both itch and pain pathways.
Since the PBN is synaptically connected to sensorimotor nuclei such as the medullary reticular formation, PBN neurons, through reticular connections, can initiate or modulate scratching bouts (figure 4). The inhibitory neurons in the central amygdala (CeA) are instrumental in learning emotional memories and using this information for adaptive sensorimotor behaviors (Fadok et al. 2018). Notably, pruritic stimuli engage the CeA neurons (Samineni et al. 2021), and stimulation of pruritogen-sensitive CeA neurons exacerbate chloroquine-induced itch. In addition, activation of the same CeA neurons is sufficient to drive itch-induced aversion (Samineni et al. 2021). The lPBN neurons expressing the neuropeptide genes Calca and Tac1 synapse onto the CeA neurons and can thus regulate various aspects of itch through the CeA (Barik et al. 2018; Palmiter et al. 2018). Recently, the ventral tegmental area (VTA), a midbrain region rich in dopaminergic neurons and important in motivated behaviors, was implicated in itch-induced scratching behavior (Yuan et al. 2018). The firing of VTA neurons was tuned to the bouts of scratching, and suppression of VTA activity shortened the bouts. Intriguingly, PBN neurons project to the VTA and thus can affect itch-scratching bouts by affecting dopamine release in the VTA outputs, such as the ventral striatum and nucleus accumbens (Yang et al. 2021). Notably, itch is suppressed when spinal Tacr1 or post-synaptic neurons in the LPBN are chemogenetically stimulated. This observation implies that even if pain and itch are transmitted along the same ascending pathway from the spinal cord, pain and itch are processed differently in downstream brain circuits. Recently it was shown that spinal cord Tacr1 neurons synapse onto a PBN population that expresses Tacr1. The synaptic targets of the PBN Tacr1 neurons may elucidate circuit mechanisms for pain-induced suppression of itch in the brain (figure 4). Grp (Karthik et al. 2022) and Grpr are also expressed in the lateral PBN, and the role of these neurons in pain or itch is unknown. The role of Grp–Grpr signaling in the PBN in itch remains to be tested.
6. Pain–itch interactions in the brain
Itch and pain share an antagonistic relationship (Davidson and Giesler 2010; Piyush Shah and Barik 2022). Scratching, a mild form of noxious mechanical stimulus, is the de facto behavioral response to itch and is sufficient to resolve itch. Opioids, which are potent analgesics, can exacerbate itch (Neal and Rathmell 2012). Under inflammatory conditions, mechanical pruritic stimuli are often sensed as pain since high-threshold mechanoreceptors are sensitized and activated by pruritic stimuli (Woolf and Ma 2007; Gold and Gebhart 2010). Similarly, in patients with chronic itch, painful noxious stimuli can evoke intense scratching (Ikoma et al. 2004). Several lines of evidence indicate that the interaction between pain and itch occur in the brain. PBN neurons are involved in driving both pain- and itch-induced behaviors and, as discussed in the previous section, can exaggerate pain responses while suppressing itch. The periaqueductal gray (PAG), an integral part of the descending pain modulatory pathway, receives inputs from the central amygdala and PBN, and thus can integrate descending and ascending pruritic information (Li et al. 2021; Samineni et al. 2021) (figure 5). The PAG output neurons can be excitatory or inhibitory. The stimulation of inhibitory PAG neurons results in pain hypersensitivity and itch-suppression (Samineni et al. 2019), whereas activation of PAG excitatory neurons has a contrasting effect on pain and itch (figure 5). In an independent study, rostral ventromedial medulla (RVM) projecting Tac1+ PAG neurons were shown to gate itch through GRPR-expressing interneurons in the spinal cord (Gao et al. 2019; Liu et al. 2019). In vivo extracellular recordings from anesthetized rats showed that RVM-On and RVM-Off cells are activated and inhibited by pruritogens such as histamine and chloroquine (Follansbee et al. 2018), respectively. Interestingly, scratching stimuli at the site of pruritogen administration elicited similar responses as pruritogens did in RVM-On and RVM-Off cells, indicating that a high-threshold mechanical stimulus (scratching) can inhibit itch by activating RVM-On cells or inhibiting RVM-Off cells (figure 5). Internal states such as stress can modulate pain thresholds. Recent evidence suggests that RVM neurons act as the final output for stress-mediated pain-modulatory brain pathways. Stress-responsive nuclei such as the posterior and lateral hypothalamus synapse onto RVM neurons and can affect nociception through RVM–spinal cord connections. Similarly, stress can affect pruriception, and hypothalamic–RVM–spinal cord circuits may play an important role.
Figure 5. Interactions of pain and itch transmission in both ascending and descending circuits.
Both pain and itch information are transmitted from the spinal cord to the CMT and PBN through the spinothalamic and spinoparabrachial pathways, respectively. The CMT directly projects to the somatosensory cortex (SSC), while the PBN projects to the IC and cingulate cortex (CC), where the anterior and posterior side of the regions code for itch and pain distinctively. They then project to the SSC and striatum. The dorsal striatum (DS) is part of an itch-specific descending pathway. The ventral periaqueductal grey (VPAG) is part of the descending pathway where the excitatory and inhibitory neurons cause an increase in itch and pain responses, respectively. Similarly, the rostral ventromedial medulla (RVM) is part of the descending pathway where the On and Off cells have a contrasting effect on pain and itch responses.
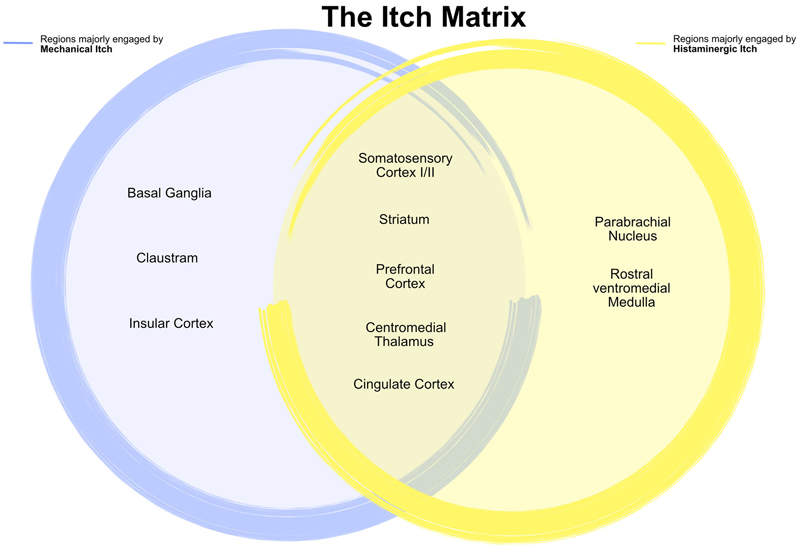
7. Itch matrix
One well-held notion is that to solve the mystery of the brain mechanisms underlying pain, one needs to understand how distributed pain-responsive nodes across the brain interact to give rise to pain perception. Similarly, there has been a reckoning for an itch matrix. Human functional imaging studies with PET-H215O, ASL-fMRI, or BOLD-fMRI indicate the involvement of several areas, including the primary or secondary somatosensory cortex (SI or SII), prefrontal cortex (PFC), cingulate cortex, insular cortex (IC), claustrum, basal ganglia, thalamus, precuneus, parietal cortex, and motor cortex in itch (figure 6). Here, we discuss a few brain areas implicated in itch processing in human studies and investigated in animal models. Both chemical and mechanical itch stimuli evoke S1 activity in rodents and humans (Gross et al. 2007; Kim et al. 2017). Importantly, in neural recordings done in the rat S1 at the cellular level, it was found that no neurons are exclusively activated by pruritic stimuli, and the same neurons responded to both mechanical and chemical itch (Khasabov et al. 2020). Thus, it is improbable that there are itch-specific neurons in the S1. It is more likely that mildly noxious stimuli that can cause itch are encoded by ensembles of S1 neurons. A recent study performed two-photon imaging to capture neural activity and found multiplexed representation in large areas of the S1. It was found that mechanical, thermal, and pruritic stimuli activate overlapping neuronal populations (Chen et al. 2021). Regions such as the thalamus, S1/2, and parietal cortex are engaged by both histaminergic and non-histaminergic itch (Papoiu et al. 2012; figure 6). However, the basal ganglia, claustrum, and insular cortex are preferentially activated by mechanical non-histaminergic itch (figure 6).
Figure 6. Regions involved in either mechanical or histaminergic itch that make up the itch matrix.
Mechanical itch majorly evokes activity from the claustrum, insular cortex, and regions in the basal ganglia, whereas histaminergic itch majorly evokes activity from the parabrachial nucleus and the rostral ventromedial medulla. The somatosensory cortex I/II, striatum, prefrontal cortex, centromedial thalamus, and cingulate cortex are involved in all types of itch.
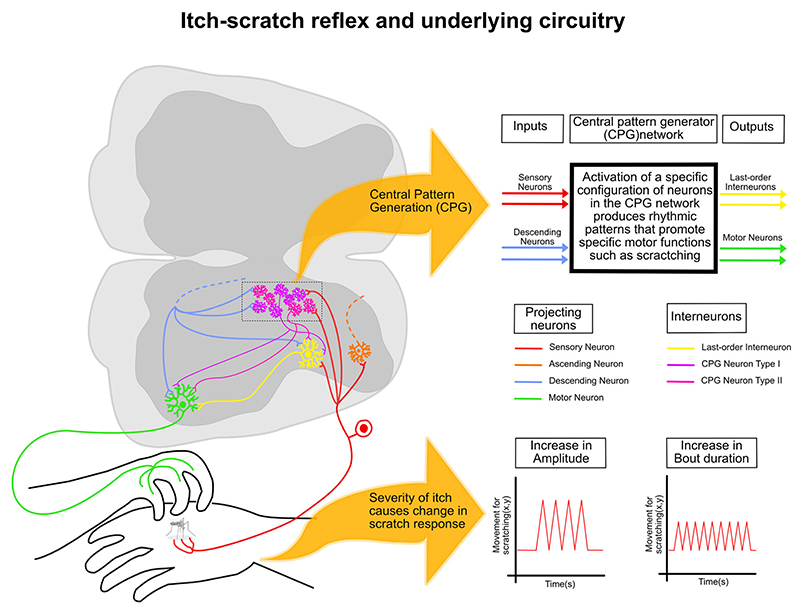
8. Itch–scratch reflex and underlying circuitry
In his seminal studies, Sherrington (1906) observed that mechanical or electrical stimuli elicited spontaneous scratches in animals with cervical transections. He concluded that the scratching frequency was independent of the stimulus intensity or nature (Sherrington 1906). Rather, the intensity of the stimulus altered the scratching amplitude and the duration of the scratching bouts. Sherrington’s observations of scratch reflexes induced in spinalized mammals and later studies done in turtles (Currie and Stein 1988; Field and Stein 1997; Berkowitz and Hao 2011) indicate that there are central pattern generators (CPGs) within the spinal cord that are sufficient to generate scratching (figure 7). However, the threshold for the induction of spontaneous scratching generated in these spinalized preparations is much lower in intact animals, indicating that scratch CPGs are under descending supra-spinal modulation. Spinal CPGs are constituted of interneurons and have been widely studied in locomotion (Marder and Bucher 2001) (figure 7). Studies have suggested that locomotory and scratching CPGs share neuronal loci (Berkowitz and Hao 2011). The molecularly circumscribed spinal cord interneurons mediating itch described before may play an integral part in spinal CPGs. Identifying scratch CPGs and upstream brain neurons will show how the brain drives scratch reflex in acute and chronic itch. Apart from the spinal CPGs designed to generate scratching in animals and humans, there might be intra-spinal mechanisms that help the coordinate scratching events. For example, propriospinal neurons in cervical spinal segments can synapse onto the lumbar pre-motor neurons to direct the paws in animals to the site of itching to scratch.
Figure 7. The underlying circuitry for the itch–scratch reflex.
A greater sensation of itch leads to greater scratch responses where there is an increase in the force applied during scratching (increase in amplitude) and/or an increase in duration of the scratches (increase in bout duration). Scratch response is generated at the spinal cord level through a network of interneurons that receive inputs from sensory neurons and descending neurons which can code for a multitude of motor responses known as the CPG network. Activation of a specific configuration of neurons in this network leads to a scratch response as this network synapses onto the last-order interneurons and motor neurons.
Acute itch serves a protective purpose; however, uncontrolled scratching is a hallmark of several chronic dermatological and neurological diseases, and they are notoriously difficult to manage clinically. In clinical settings, the severity of itch in patients is measured through questionnaires that largely focus on qualitative aspects of pain (Darsow et al. 1997; Dawn et al. 2009; O’Neill et al. 2011). The severity of the itch can be alternatively and accurately measured by the frequency and vigor of scratching bouts (Ikoma et al. 2019; Wimalasena et al. 2021) (figure 7). Indeed, the amplitude and frequency of scratching differ in patients with scabies compared with other chronic itch conditions (Lam Hoai et al. 2021; figure 7). The itch–scratch reflex can also be understood as a coping behavior. Somatosensory coping behaviors are recruited when noxious stimuli induce a strong enough sensation to be relieved by attending the exposure site (Ma et al. 2022). Interpreting itch-induced behaviors in pre-clinical models from a coping perspective can provide novel insights into the pathophysiology of chronic itch.
9. Conclusion and future directions
This review outlined the ascending neural pathways through which itch is transmitted from the periphery to the cortex and described how the descending pathways coordinate a behavioral scratching response. We detailed the recent advances in mapping the neural circuits and molecular signaling pathways specific to itch. As discussed, pain and itch interact at multiple levels in the nervous system, resulting in pain inhibition of itch. However, why and how the behavioral output of pain stimuli takes precedence over itch is not understood. One possibility is that an itch is a mild form of pain; hence, intense pain can override itch sensation. However, the discovery of itch-specific cellular and molecular pathways indicates that itch and pain might be distinct sensory modalities (Sun et al.2009; Bardoni et al. 2019; Jiang et al. 2022). Alternatively, top–down mechanisms can prioritize pain behavior by simultaneously activating motor programs for pain-induced withdrawal or escape and inhibiting itch–scratch reflexes, for example, in the ‘leaky gate model’ in the spinal cord where Grp-expressing interneurons are responsive to both itch and weak noxious stimuli (Sun et al. 2017); with the exposure of strong noxious stimuli, the itch pathway is inhibited from disabling the possibilities of noxious stimuli being transmitted by two parallel pathways. Similarly, gating may exist in the somatosensory cortex or downstream structures, where itch-preferring pathways are suppressed, and pain-related circuits are promoted when an individual is simultaneously exposed to pain and itch-causing stimuli.
The neural circuits for pain and itch in the brain and the spinal cord are neuropeptide rich. How these neuropeptides function in these circuits and how they contribute chronification of pain and itch will be important for developing circuit-based clinical solutions for itch. Recent developments in genetically encoded probes for neuropeptide activity monitoring can shed light on itch-related peptide release in the circuits of interest (Al-Hasani et al. 2018). Since brain circuits for histaminergic and non-histaminergic or chemical and mechanical itch coincide, circuit-based interventions can be effective for chronic itch disorders irrespective of their causal origins. Moreover, reflexive protective behaviors such as coughing (Pecova et al. 2020; Chung et al. 2022), induced by irritants in the airway, may have similar underlying neural mechanisms as itch. It will be interesting to test the role of the molecularly defined itch circuits in airway irritation and persistent cough.
Funding statement
AB is supported by the IndiaAlliance Intermediate Fellowship. JNP is supported by the Indian Institute of Science.
Declarations
Conflict of interest The authors declare no conflict of interests.
Corresponding editor: Renee M Borges
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
Author contributions
JNP, PR, and AB conceived, researched, illustrated, and wrote the review.
References
- Acton D, Ren X, Di Costanzo S, et al. Spinal neuropeptide Y1 receptor-expressing neurons from an essential excitatory pathway for mechanical itch. Cell Rep. 2019;28:625–39.:e6. doi: 10.1016/j.celrep.2019.06.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adebayo RA, Sofowora GG, Onayemi O, et al. Chloroquine-induced pruritus in malaria fever: contribution of malaria parasitaemia and the effects of prednisolone, niacin, and their combination, compared with antihistamine. Br J Clin Pharmacol. 1997;44:157–161. doi: 10.1046/j.1365-2125.1997.00612.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aghahowa SE, Obianwu HO, Isah AO, et al. Chloroquine-induced pruritus. Indian J Pharm Sci. 2010;72:283–289. doi: 10.4103/0250-474X.70471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akiyama T, Nguyen T, Curtis E, et al. A central role for spinal dorsal horn neurons that express neurokinin-1 receptors in chronic itch. Pain. 2015;156:1240–1246. doi: 10.1097/j.pain.0000000000000172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akiyama T, Tominaga M, Takamori K, et al. Roles of glutamate, substance P, and gastrin-releasing peptide as spinal neurotransmitters of histaminergic and nonhistaminergic itch. Pain. 2014;155:80–92. doi: 10.1016/j.pain.2013.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Hasani R, Wong JMT, Mabrouk OS, et al. In vivo detection of optically-evoked opioid peptide release. eLife. 2018;7:e36520. doi: 10.7554/eLife.36520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Khater KM, Todd AJ. Collateral projections of neurons in laminae I, III, and IV of rat spinal cord to thalamus, periaqueductal gray matter, and lateral parabrachial area. J Comp Neurol. 2009;515:629–646. doi: 10.1002/cne.22081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bardoni R, Shen K-F, Li H, et al. Pain inhibits GRPR neurons via GABAergic signaling in the spinal cord. Sci Rep. 2019;9:15804. doi: 10.1038/s41598-019-52316-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barry DM, Munanairi A, Chen Z-F. Spinal mechanisms of itch transmission. Neurosci Bull. 2018;34:156–164. doi: 10.1007/s12264-017-0125-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barik A, Thompson JH, Seltzer M, et al. A brainstem-spinal circuit controlling nocifensive behavior. Neuron. 2018;100:1491–503.:e3. doi: 10.1016/j.neuron.2018.10.037. [DOI] [PubMed] [Google Scholar]
- Battey J, Wada E. Two distinct receptor subtypes for mammalian bombesin-like peptides. Trends Neurosci. 1991;14:524–528. doi: 10.1016/0166-2236(91)90005-f. [DOI] [PubMed] [Google Scholar]
- Berkowitz A, Hao Z-Z. Partly shared spinal cord networks for locomotion and scratching. Integr Comp Biol. 2011;51:890–902. doi: 10.1093/icb/icr041. [DOI] [PubMed] [Google Scholar]
- Bourane S, Duan B, Koch SC, et al. Gate control of mechanical itch by a subpopulation of spinal cord interneurons. Science. 2015;350:550–554. doi: 10.1126/science.aac8653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braz J, Solorzano C, Wang X, et al. Transmitting pain and itch messages: a contemporary view of the spinal cord circuits that generate gate control. Neuron. 2014;82:522–536. doi: 10.1016/j.neuron.2014.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buszman E, Kopera M, Wilczok T. Electron spin resonance studies of chloroquine-melanin complexes. Biochem Pharmacol. 1984;33:7–11. doi: 10.1016/0006-2952(84)90363-0. [DOI] [PubMed] [Google Scholar]
- Cameron D, Polgár E, Gutierrez-Mecinas M, et al. The organisation of spinoparabrachial neurons in the mouse. Pain. 2015;156:2061–2071. doi: 10.1097/j.pain.0000000000000270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carstens EE, Carstens MI, Simons CT, et al. Dorsal horn neurons expressing NK-1 receptors mediate scratching in rats. Neuroreport. 2010;21:303–308. doi: 10.1097/WNR.0b013e328337310a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X-J, Sun Y-G. Central circuit mechanisms of itch. Nat Commun. 2020;11:3052. doi: 10.1038/s41467-020-16859-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X-J, Liu Y-H, Xu N-L, et al. Multiplexed representation of itch and mechanical and thermal sensation in the primary somatosensory cortex. J Neurosci. 2021;41:10330–10340. doi: 10.1523/JNEUROSCI.1445-21.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi S, Hachisuka J, Brett MA, et al. Parallel ascending spinal pathways for affective touch and pain. Nature. 2020;587:258–263. doi: 10.1038/s41586-020-2860-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung KF, McGarvey L, Song WJ, et al. Cough hypersensitivty and chronic cough. Nat Rev Dis Primers. 2022;8:45. doi: 10.1038/s41572-022-00370-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui L, Guo J, Cranfill SL, et al. Glutamate in primary afferents is required for itch transmission. Neuron. 2022;110:809–823. doi: 10.1016/j.neuron.2021.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Currie SN, Stein PS. Electrical activation of the pocket scratch central pattern generator in the turtle. J Neurophysiol. 1988;60:2122–2137. doi: 10.1152/jn.1988.60.6.2122. [DOI] [PubMed] [Google Scholar]
- Darsow U, Mautner VF, Bromm B, et al. The Eppendorf pruritus questionnaire. Hautarzt. 1997;48:730–733. doi: 10.1007/s001050050651. [DOI] [PubMed] [Google Scholar]
- Davidson S, Giesler GJ. The multiple pathways for itch and their interactions with pain. Trends Neurosci. 2010;33:550–558. doi: 10.1016/j.tins.2010.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawn A, Papoiu ADP, Chan YH, et al. Itch characteristics in atopic dermatitis: results of a web-based questionnaire. Br J Dermatol. 2009;160:642–644. doi: 10.1111/j.1365-2133.2008.08941.x. [DOI] [PubMed] [Google Scholar]
- Erspamer V, Erspamer GF, Inselvini M, et al. Occurrence of bombesin and alytesin in extracts of the skin of three European discoglossid frogs and pharmacological actions of bombesin on extravascular smooth muscle. Br J Pharmacol. 1972;45:333–348. doi: 10.1111/j.1476-5381.1972.tb08087.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fadok JP, Markovic M, Tovote P, et al. New perspectives on central amygdala function. Curr Opin Neurobiol. 2018;49:141–147. doi: 10.1016/j.conb.2018.02.009. [DOI] [PubMed] [Google Scholar]
- Field EC, Stein PS. Spinal cord coordination of hindlimb movements in the turtle. J Neurophysiol. 1997;78:1394–1403. doi: 10.1152/jn.1997.78.3.1394. [DOI] [PubMed] [Google Scholar]
- Flegel C, Schöbel N, Altmüller J, et al. RNA-Seq Analysis of human trigeminal and dorsal root ganglia with a focus on chemoreceptors. PLoS One. 2015;10:e0128951. doi: 10.1371/journal.pone.0128951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleming MS, Ramos D, Han SB, et al. The majority of dorsal spinal cord gastrin releasing peptide is synthesized locally whereas neuromedin B is highly expressed in pain- and itch-sensing somatosensory neurons. Mol Pain. 2012;8:52. doi: 10.1186/1744-8069-8-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Follansbee T, Akiyama T, Fujii M, et al. Effects of pruritogens and algogens on rostral ventromedial medullary ON and OFF cells. J Neurophysiol. 2018;120:2156–2163. doi: 10.1152/jn.00208.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fostini AC, Golpanian RS, Rosen JD, et al. Beat the bite: pathophysiology and management of itch in mosquito bites. Itch. 2019;4:e19. [Google Scholar]
- Gao ZR, Chen WZ, Liu MZ, et al. Tac1-expressing neurons in the periaqueductal gray facilitate the itch-scratching cycle via descending regulation. Neuron. 2019;101:45–59.:e9. doi: 10.1016/j.neuron.2018.11.010. [DOI] [PubMed] [Google Scholar]
- Gold M, Gerald Nociceptor Sensitization in Pain Pathogenesis. Nat Med. 2010;16:1248–1257. doi: 10.1038/nm.2235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez N, Moody TW, Igarashi H, et al. Bombesin-related peptides and their receptors. Curr Opin Endocrinol Diabetes Obes. 2008;15:58–64. doi: 10.1097/MED.0b013e3282f3709b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross J, Schnitzler A, Timmermann L, et al. Gamma oscillations in human primary somatosensory cortex reflect pain perception. PLoS Biol. 2007;5:e133. doi: 10.1371/journal.pbio.0050133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han L, Ma C, Liu Q, et al. A subpopulation of nociceptors specifically linked to itch. Nat Neurosci. 2013;16:174–182. doi: 10.1038/nn.3289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikoma A, Ebata T, Chantalat L, et al. Measurement of nocturnal scratching in patients with pruritus using a smartwatch: initial clinical studies with the itch tracker app. Acta Derm Venereol. 2019;99:268–273. doi: 10.2340/00015555-3105. [DOI] [PubMed] [Google Scholar]
- Ikoma A, Fartasch M, Heyer G, et al. Painful stimuli evoke itch in patients with chronic pruritus. Neurology. 2004;62:212–217. doi: 10.1212/wnl.62.2.212. [DOI] [PubMed] [Google Scholar]
- Ikoma A, Steinhoff M, Ständer S, et al. The neurobiology of itch. Nat Rev Neurosci. 2006;7:535–547. doi: 10.1038/nrn1950. [DOI] [PubMed] [Google Scholar]
- Jensen RT, Battey JF, Spindel ER, et al. International Union of Pharmacology. LXVIII. Mammalian bombesin receptors: nomenclature, distribution, pharmacology, signaling, and functions in normal and disease states. Pharmacol Rev. 2008;60:1–42. doi: 10.1124/pr.107.07108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang S, Wang YS, Zheng XX, et al. Itch-specific neurons in the ventrolateral orbital cortex selectively modulate the itch processing. Sci Adv. 2022;8:eabn4408. doi: 10.1126/sciadv.abn4408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karthik S, Huang D, Delgado Y, et al. Molecular ontology of the parabrachial nucleus. J Comp Neurol. 2022;530:1658–1699. doi: 10.1002/cne.25307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khasabov SG, Truong H, Rogness VM, et al. Responses of neurons in the primary somatosensory cortex to itch- and pain-producing stimuli in rats. J Neurophysiol. 2020;123:1944–1954. doi: 10.1152/jn.00038.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim W, Kim SK, Nabekura J. Functional and structural plasticity in the primary somatosensory cortex associated with chronic pain. J Neurochem. 2017;141:499–506. doi: 10.1111/jnc.14012. [DOI] [PubMed] [Google Scholar]
- Kroog GS, Jensen RT, Battey JF. Mammalian bombesin receptors. Med Res Rev. 1995;15:389–417. doi: 10.1002/med.2610150502. [DOI] [PubMed] [Google Scholar]
- Lagerström MC, Rogoz K, Abrahamsen B, et al. VGLUT2-dependent sensory neurons in the TRPV1 population regulate pain and itch. Neuron. 2010;68:529–542. doi: 10.1016/j.neuron.2010.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam Hoai X-L, De Maertelaer V, Simonart T. Real-world adherence to topical therapies in patients with moderate acne. Int J Dermatol. 2021;60:70–72. doi: 10.1016/j.jdin.2020.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li JN, Ren JH, He CB, et al. Projections from the lateral parabrachial nucleus to the lateral and ventral lateral periaqueductal gray subregions mediate the itching sensation. Pain. 2021;162:1848–1863. doi: 10.1097/j.pain.0000000000002193. [DOI] [PubMed] [Google Scholar]
- Liu Q, Sikand P, Ma C, et al. Mechanisms of itch evoked by β-alanine. J Neurosci. 2012;32:14532–14537. doi: 10.1523/JNEUROSCI.3509-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Q, Tang Z, Surdenikova L, et al. Sensory neuron-specific GPCR Mrgprs are itch receptors mediating chloroquine-induced pruritus. Cell. 2009;139:1353–1365. doi: 10.1016/j.cell.2009.11.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Abdel Samad O, Zhang L, et al. VGLUT2-dependent glutamate release from nociceptors is required to sense pain and suppress itch. Neuron. 2010;68:543–556. doi: 10.1016/j.neuron.2010.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu MZ, Chen XJ, Liang TY, et al. Synaptic control of spinal GRPR+ neurons by local and long-range inhibitory inputs. Proc Natl Acad Sci USA. 2019;116:27011–27017. doi: 10.1073/pnas.1905658116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Q, et al. A functional subdivision within the somatosensory system and its implications for pain research. Neuron. 2022;110:749–769. doi: 10.1016/j.neuron.2021.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantyh PW, Rogers SD, Honore P, et al. Inhibition of hyperalgesia by ablation of lamina I spinal neurons expressing the substance P receptor. Science. 1997;278:275–279. doi: 10.1126/science.278.5336.275. [DOI] [PubMed] [Google Scholar]
- Marder E, Bucher D. Central pattern generators and the control of rhythmic movements. Curr Biol. 2001;11:986–996. doi: 10.1016/s0960-9822(01)00581-4. [DOI] [PubMed] [Google Scholar]
- Mishra SK, Hoon MA. The cells and circuitry for itch responses in mice. Science. 2013;340:968–971. doi: 10.1126/science.1233765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morita T, McClain SP, Batia LM, et al. HTR7 mediates serotonergic acute and chronic itch. Neuron. 2015;87:124–138. doi: 10.1016/j.neuron.2015.05.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mu D, Deng J, Liu KF, et al. A central neural circuit for itch sensation. Science. 2017;357:695–699. doi: 10.1126/science.aaf4918. [DOI] [PubMed] [Google Scholar]
- Mu D, Sun Y-G. Circuit mechanisms of itch in the brain. J Invest Dermatol. 2022;142:23–30. doi: 10.1016/j.jid.2021.09.022. [DOI] [PubMed] [Google Scholar]
- Namer B, Carr R, Johanek LM, et al. Separate peripheral pathways for pruritus in man. J Neurophysiol. 2008;100:2062–2069. doi: 10.1152/jn.90482.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neal J, Rathmell JP. Complications in regional anesthesia and pain medicine. Lippincott Williams & Wilkins; 2012. [Google Scholar]
- O’Neill JL, Chan YH, Rapp SR, et al. Differences in itch characteristics between psoriasis and atopic dermatitis patients: results of a web-based questionnaire. Acta Derm Venereol. 2011;91:537–540. doi: 10.2340/00015555-1126. [DOI] [PubMed] [Google Scholar]
- Ohtsuka E, Kawai S, Ichikawa T, et al. Roles of mast cells and histamine in mosquito bite-induced allergic itch-associated responses in mice. Jap J Pharmacol. 2001;86:97–105. doi: 10.1254/jjp.86.97. [DOI] [PubMed] [Google Scholar]
- Palmiter RD. The parabrachial nucleus: CGRP neurons function as a general alarm. Trends Neurosci. 2018;41:280–293. doi: 10.1016/j.tins.2018.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan H, Fatima M, Li A, et al. Identification of a spinal circuit for mechanical and persistent spontaneous itch. Neuron. 2019;103:1135–1149.:e6. doi: 10.1016/j.neuron.2019.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panula P, Hadjiconstantinou M, Yang HY, et al. Immunohistochemical localization of bombesin/gastrin-releasing peptide and substance P in primary sensory neurons. J Neurosci. 1983;3:2021–2029. doi: 10.1523/JNEUROSCI.03-10-02021.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papoiu ADP, Coghill RC, Kraft RA, et al. A tale of two itches. Common features and notable differences in brain activation evoked by cowhage and histamine induced itch. Neuroimage. 2012;59:3611–3623. doi: 10.1016/j.neuroimage.2011.10.099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pecova T, Kocan I, Vysehradsky R, et al. Itch and cough - similar role of sensory nerves in their pathogenesis. Physiol Res. 2020;69:S43–S54. doi: 10.33549/physiolres.934403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren X, Liu S, Virlogeux A, et al. Identification of an essential spinoparabrachial pathway for mechanical itch. Neuron. 2023;111:1812–1829.:e6. doi: 10.1016/j.neuron.2023.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samineni VK, Grajales-Reyes JG, Grajales-Reyes GE, et al. Cellular, circuit and transcriptional framework for modulation of itch in the central amygdala. eLife. 2021;10:e68130. doi: 10.7554/eLife.68130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samineni VK, Grajales-Reyes JG, Sundaram SS, et al. Cell type-specific modulation of sensory and affective components of itch in the periaqueductal gray. Nat Commun. 2019;10:1–15. doi: 10.1038/s41467-019-12316-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmelz M, Schmidt R, Bickel A, et al. Specific C-receptors for itch in human skin. J Neurosci. 1997;17:8003–8008. doi: 10.1523/JNEUROSCI.17-20-08003.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmelz M, Schmidt R, Weidner C, et al. Chemical response pattern of different classes of C-nociceptors to pruritogens and algogens. J Neurophysiol. 2003;89:2441–2448. doi: 10.1152/jn.01139.2002. [DOI] [PubMed] [Google Scholar]
- Shah PD, Barik A. The spino-parabrachial pathway for itch. Front Neural Circuits. 2022;16:805831. doi: 10.3389/fncir.2022.805831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherrington CS. Observations on the scratch-reflex in the spinal dog. J Physiol. 1906;34:1–50. doi: 10.1113/jphysiol.1906.sp001139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solorzano C, Villafuerte D, Meda K, et al. Primary afferent and spinal cord expression of gastrin-releasing peptide: message, protein, and antibody concerns. J Neurosci. 2015;35:648–657. doi: 10.1523/JNEUROSCI.2955-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spike RC, Puskár Z, Andrew D, et al. A quantitative and morphological study of projection neurons in lamina I of the rat lumbar spinal cord. Eur J Neurosci. 2003;18:2433–2448. doi: 10.1046/j.1460-9568.2003.02981.x. [DOI] [PubMed] [Google Scholar]
- Su P-Y, Ko M-C. The role of central gastrin-releasing peptide and neuromedin B receptors in the modulation of scratching behavior in rats. J Pharmacol Exp Ther. 2011;337:822–829. doi: 10.1124/jpet.111.178970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y-G, Chen Z-F. A gastrin-releasing peptide receptor mediates the itch sensation in the spinal cord. Nature. 2007;448:700–703. doi: 10.1038/nature06029. [DOI] [PubMed] [Google Scholar]
- Sun S, Xu Q, Guo C, et al. Intensity-dependent coding of pain and itch in the spinal cord. Neuron. 2017;93:840–53.:e5. doi: 10.1016/j.neuron.2017.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y-G, Zhao Z-Q, Meng X-L, et al. Cellular basis of itch sensation. Science. 2009;325:1531–1534. doi: 10.1126/science.1174868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todd AJ, McGill MM, Shehab SA. Neurokinin 1 receptor expression by neurons in laminae I, III and IV of the rat spinal dorsal horn that project to the brainstem. Eur J Neurosci. 2000;12:689–700. doi: 10.1046/j.1460-9568.2000.00950.x. [DOI] [PubMed] [Google Scholar]
- Usoskin D, Furlan A, Islam S, et al. Unbiased classification of sensory neuron types by large-scale single-cell RNA sequencing. Nat Neurosci. 2015;18:145–153. doi: 10.1038/nn.3881. [DOI] [PubMed] [Google Scholar]
- Vander Does A, Labib A, Yosipovitch G. Update on mosquito bite reaction: Itch and hypersensitivity, pathophysiology, prevention, and treatment. Front Immunol. 2022;13:1024559. doi: 10.3389/fimmu.2022.1024559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wercberger R, Braz JM, Weinrich JA, et al. Pain and itch processing by subpopulations of molecularly diverse spinal and trigeminal projection neurons. Proc Natl Acad Sci USA. 2021;118:e2105732118. doi: 10.1073/pnas.2105732118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wimalasena NK, Milner G, Silva R, et al. Dissecting the precise nature of itch-evoked scratching. Neuron. 2021;109:3075–87.:e2. doi: 10.1016/j.neuron.2021.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolf CJ, Ma Q. Nociceptors–Noxious stimulus detectors. Neuron. 2007;55:353–364. doi: 10.1016/j.neuron.2007.07.016. [DOI] [PubMed] [Google Scholar]
- Xing Y, Chen J, Hilley H, et al. Molecular signature of pruriceptive MrgprA3+ neurons. Invest Dermatol. 2020;140:2041–2050. doi: 10.1016/j.jid.2020.03.935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H, de Jong JW, Cerniauskas I, et al. Pain modulates dopamine neurons via a spinal-parabrachial-mesencephalic circuit. Nat Neurosci. 2021;24:1402–1413. doi: 10.1038/s41593-021-00903-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan L, Liang T-Y, Deng J, et al. Dynamics and functional role of dopaminergic neurons in the ventral tegmental area during itch processing. J Neurosci. 2018;38:9856–9869. doi: 10.1523/JNEUROSCI.1483-18.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao ZQ, Liu XY, Jeffry J, et al. Descending control of itch transmission by the serotonergic system via 5-HT1A-facilitated GRP-GRPR signaling. Neuron. 2014;84:821–834. doi: 10.1016/j.neuron.2014.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]