Abstract
Deriving a complete understanding of protein self-association into amyloid fibrils across multiple distance and time scales is an enormous challenge. At small length scales, a detailed description of the partially folded protein ensemble that participates in self-assembly remains obscure. At larger length scales, amyloid fibrils are often heterogeneous, can form along multiple pathways, and are further complicated by phenomena such as phase-separation. Over the last 5 years, we have used an array of biophysical approaches in order to elucidate the structural and molecular mechanism of amyloid fibril formation, focusing on the all β-sheet protein, β2-microglubulin (β2m). This protein forms amyloid deposits in the human disease ‘dialysis-related amyloidosis’ (DRA). We have shown that under acidic conditions β2m rapidly associates in vitro to form amyloid-like fibrils that have different morphological properties, but which contain an underpinning cross-β structure. In this review, we discuss our current knowledge of the structure of these fibrils, as well as the structural, kinetic and thermodynamic relationship between fibrils with different morphologies. The results provide some of the first insights into the shape of the self-assembly free-energy landscape for this protein and highlight the parallel nature of the assembly process. We include a detailed description of the structure and dynamics of partially folded and acid unfolded species of β2m using NMR, and highlight regions thought to be important in early self-association events. Finally, we discuss briefly how knowledge of assembly mechanisms in vitro can be used to inform the design of therapeutic strategies for this, and other amyloid disorders, and we speculate on how the increasing power of biophysical approaches may lead to a fuller description of protein self-assembly into amyloid in the future.
Keywords: Beta-2-microglobulin, Amyloid, NMR, Intermediate, AFM
1. Overview
Since first being recognised as a human amyloid protein 20 years ago [1,2], an increasing number of groups world-wide has applied an array of medical, cellular and, more recently, biochemical and biophysical approaches, to try to elucidate how and why β2-microglobulin (β2m) aggregates to form amyloid fibrils that specifically and characteristically deposit in the joints of all patients undergoing long term renal dialysis [3]. Many of the important discoveries involving medical and cellular aspects of dialysis-related amyloidosis (DRA), as well as developments and improvements in the dialysis procedure itself, are reviewed elsewhere in this issue. Here, we review the current understanding of the structural molecular mechanism of β2m amyloid formation in vitro obtained using a wide range of biophysical methods. From a biophysical viewpoint, β2m provides a highly tractable system with which to elucidate the structural molecular mechanism of amyloid formation, since it is small (99 residue, ∼12 kDa), monomeric, and has a well-known β-sandwich structure that involves seven β-strands stabilised by a single disulphide bond (Fig. 1). Most importantly, DRA involves the self-association of full length wild-type β2m [4] (although small amounts of truncated products, commonly lacking the N-terminal 6 amino acids, are also found in fibrils ex vivo [5,6], most likely from post-deposition proteolysis). Compared with many of the more complex proteins that are involved in human amyloid disease, or peptides which lack a well-defined starting structure [7], therefore, β2m represents a powerful system with which to define the principles of molecular recognition in self-assembly, as well as being involved in an important human disease.
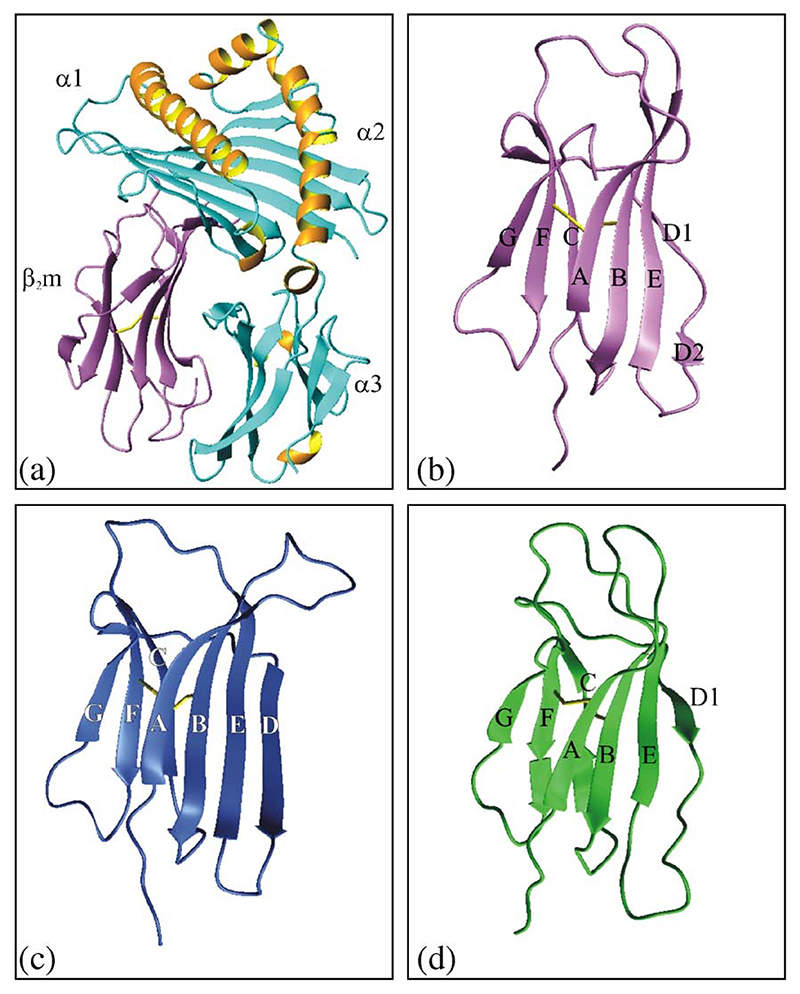
Fig. 1. Ribbon diagram of the crystal structure of the MHC-I complex.
(a); the structure of β2m in the MHC complex (b); the crystal structure of monomeric human β2m (c); the solution structure of monomeric human β2m (d). The structures were taken from 1DUZ (for (a) and (b) [75]), 1LDS (for (c) [17]) and 1JNJ (for (d) [16]). Individual subunits in the MHC heavy chain (α1 – 3), as well as individual β-strands in native β2m, (A–G) are shown. The figures were drawn using the program MOLMOL [76].
2. Key stages in the development of β2m aggregation and the onset of DRA
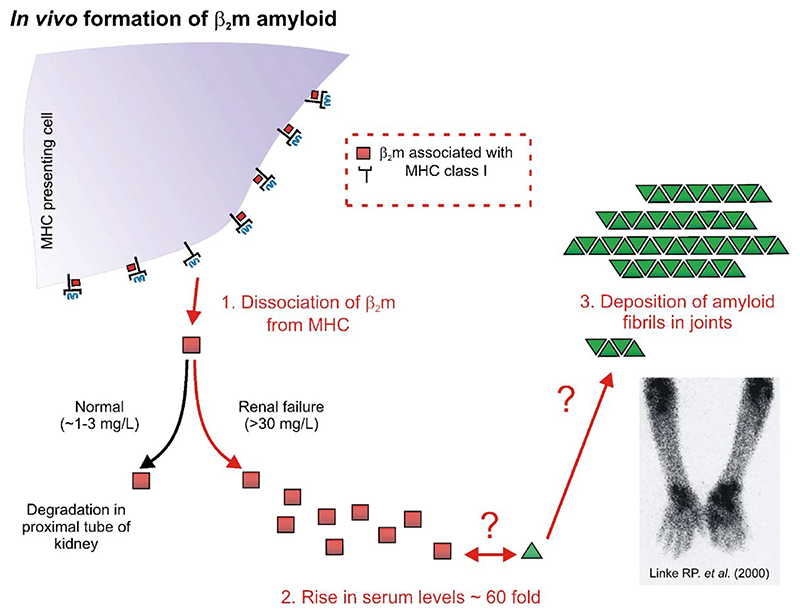
A general schematic outlining important key stages in the development of DRA is shown in Fig. 2. In its normal biological function β2m plays a central role in cellular immunology in that the protein is required for the successful folding and assembly of the MHC class I complex required for antigen display [8,9]. As part of its normal catabolic cycle β2m dissociates from the heavy chain of the MHC-I complex, whereupon it is carried in the blood to the kidneys where more than 95% of the protein is removed by degradation in the proximal tubule [10,11]. In healthy individuals, the plasma concentration of β2m is kept constant at 1 – 3 mg/L by renal catabolism [12]. Since β2m cannot be removed from the serum by the kidney or the dialysis membrane in patients with renal dysfunction, β2m concentrations are increased by up to ∼60-fold in patients with end-stage renal disease [13,14]. As a consequence of its elevated concentration, and by a mechanism that is currently poorly understood, full-length, wild-type β2m then self-associates to form amyloid plaques that deposit in the joints of all patients undergoing long term renal therapy [3]. However, despite the identification of full-length, disulphide-bridged, wild-type β2m as the major culprit protein in DRA some 20 years ago [1,2], until very recently, virtually nothing was known about how this normally soluble protein assembles into fibrils with the cross-β structure of amyloid. To address this important question, various groups world-wide have used a variety of approaches to determine how this might occur, by driving β2m fibril formation in vitro. The purpose of these experiments is to find biophysically-compatible conditions that allow this complex process to be dissected and key stages identified. Here, we review recent insights into the mechanism of self-assembly initiated under acidic conditions in vitro, focusing on (i) the structure of monomeric β2m; (ii) the morphology of the amyloid fibrils formed in vitro; (iii) the pathway(s) of assembly; and (iv) the structural properties of possible amyloid precursor states.
Fig. 2.
Schematic diagram outlining events involved in the development of DRA. The molecular mechanism of self-association in vivo is unknown. Several biological factors may be involved in the development of the disease. Lower right image: an anterior view of a DRA patient imaged using 111In-β2m scintigraphy showing the accumulation of β2m amyloid deposits in the elbows and wrists (reproduced with permission from reference [77]).
3. The structure of monomeric β2m
The deposition of β2m into amyloid fibrils commences with the dissociation of the protein from the MHC heavy chain. Key to developing a molecular understanding of β2m fibril formation, therefore, is to determine the structural properties of monomeric human β2m. Surprisingly, despite the determination of the structures of more than 80 MHC complexes in the protein data bank [15], until recently, the structure of monomeric human β2m was unknown. In 2002, however, Verdone et al. solved the solution structure of human β2m using NMR [16], whilst in the same year, Trinh et al. elucidated the first crystal structure of the same protein [17]. Although differing in detail in dynamic regions of the protein structure (including the N- and C-termini, the loops (specifically residues 12–20, 49–60 and 71–77) and the D-strand (residues 51–56)), the two crystal structures (Figs. 1b and c) demonstrate that free monomeric β2m and its MHC-bound counterpart are very similar, established by the fact that superposition of all Cα atoms of the two structures gives an RMSD of 3.1 Å. Indeed, if the largest deviations involving residues 12–20, 49–60, and 71–77 are omitted from the alignment an RMSD of only 0.5 Å is obtained [17]. Most of the regions showing greatest deviation from the MHC-bound structure involve residues in direct interaction with the MHC heavy chain. Of these, the most intriguing are residues in the D-strand, a region that is divided into two short β-strands in the MHC complex (Fig. 1a, b), only the first of which is retained as a highly ordered entity in the monomeric protein in solution (Fig. 1d). However, in the crystal form of the monomeric protein, a structural rearrangement of residues 51–56 occurs, involving removal of the β-bulge and a slippage of one residue in hydrogen bond partners in the D1 strand, resulting in residues 51–56 forming one long continuous β-strand (Fig. 1c). Examination of the protein structure in solution under conditions very similar to those used to crystallise β2m, however, failed to detect any significant population of molecules with the continuous D-strand, suggesting that this conformation is rarely formed in solution and was trapped by crystallisation. The presence of short edge strands has been proposed to be an effective method of preventing edge-edge strand association of β-sheet proteins [18], and hence conformational changes such as those seen in the D-strand, even if only rarely populated in solution, will increase the propensity of β2m to self-associate. Since this time, the crystal structure of a variant of monomeric human β2m has been solved and interestingly does not show the conformational changes observed in the D-strand of the wild-type protein [19,20]. Whether the D-strand is indeed involved in molecular recognition in the self-assembly mechanism of β2m, therefore, remains an open question that will require further experimentation, and ultimately a high-resolution structure of an amyloid fibril, to resolve.
4. DRA—an unfolding disease
Perhaps, one of the most intriguing discoveries in β2m fibrillogenesis is that the full-length wild-type protein is remarkably intransigent to self-assembly at neutral pH. β2m incubated at mM concentrations in H2O at pH 7.0 remains monomeric in solution [21], despite being ca. 1000-times higher in concentration than that found in the serum of dialysis patients. In order to form β2m fibrils in vitro, therefore, a number of different approaches have been used, which are summarised in Table 1. Studies in our laboratory have focused on the mechanism of spontaneous (unseeded) fibril growth, initiated by acidification of the protein solution [21,22], a method commonly used to generate amyloid-like fibrils in vitro [23]. Whilst the relationship of the fibrils formed under these conditions with those generated in vivo remains to be resolved (and will require detailed structural analysis of both fibril types (see below)), the reproducible rates of fibril formation under acidic conditions provide an ideal framework for the elucidation of the structural mechanism of amyloid formation. Although differing in their approach, an important common theme to emerge from these studies is that conformational rearrangements of the normally highly soluble native β2m are required in order for amyloid fibrils to form in vitro. The native protein, therefore, is not amyloidogenic, but instead requires partial or substantial unfolding to populate non-native protein conformations that initiate the formation of amyloid-like fibrils. Studies using mutagenesis methods to create β2m variants differing significantly in their global stability [24,25], as well as generating species in which the protein is considerably destabilised by reduction of the single disulphide bond, also failed to produce species capable of rapid and spontaneous assembly at neutral pH into the long straight fibrils characteristic of amyloid in vitro [26–28]. These data suggest, therefore, that DRA is a disorder of protein unfolding, whereupon transient, local fluctuations of one or more regions of the polypeptide chain generate the precursors to amyloid formation capable of spontaneous self-assembly.
Table 1. Some conditions employed in the literature to generate fibrils from full-length β2m in vitro. SDS is sodium dodecyl sulphate and SAP is serum amyloid P component.
| Conditions for extension | Conditions for seed generation | Reference |
|---|---|---|
| Acidic pH with varying ionic strength | – | [21,29,30,34,54,74] |
| Dialysis with pH 7.4 buffer containing 200 μg mL −1 SAP | – | [78] |
| Dialysis against deionised water and subsequent evaporation of solvent | – | [79] |
| Incubation in the supernatants of cultured peripheral blood mononuclear cells | – | [80] |
| pH 7.4, 150 mM KCl, 1M urea with 200 μM Cu2+ | – | [81] |
| pH 7.4 | Ex vivo amyloid fibrils | [61,82] |
| Extension of seeds during refolding experiments, pH 7.4 | Ex vivo amyloid fibrils | [82] |
| Extension of seeds in the presence of ∼0.5 mM SDS pH 7.5 | Ex vivo amyloid fibrils extended at low pH in vitro | [83,84] |
| Extension of seeds in the presence of ∼20% v/v TFE and ∼10−200 μg mL−1 heparin, pH 7.5 | Ex vivo amyloid fibrils extended at low pH in vitro | [85] |
5. Amyloid-like fibrils of β2m formed in vitro can be classified into a range of different morphological types
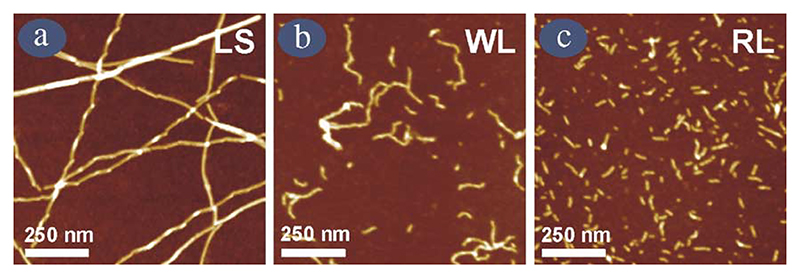
With analogy to chemical reactions, a full understanding of the mechanism of amyloid formation requires structural elucidation of every species populated on a given reaction coordinate, together with determination of the stability and the kinetics of interconversion of all species on the reaction path. In the case of amyloid formation, this is especially important, but experimentally extremely challenging, since the fibrils formed can be highly heterogeneous, suggesting that the energy landscape for fibril formation is more complex than that describing a simple chemical reaction. For β2m fibrils generated in acidic conditions in vitro, this is certainly the case. Detailed studies using electron microscopy (EM) and atomic force microscopy (AFM) have now defined and characterised multiple, distinct classes of β2m fibrils, that differ in their height, twist, persistence length, and morphological appearance (Fig. 3 and Table 2) [22,29,30]. By careful choice of the assembly environment, such as the pH, buffer, salt concentration and agitation conditions, β2m fibril types can be generated in a homogeneous fashion [30]. For example at ~pH 3.5 (and below) in low-ionic strength buffer, long-straight fibrils (LS) form that are comprised of several protofilaments twisted in a left-handed helical array (Fig. 3a). At pH 3.5 with 200 mM NaCl, fibrils that are seemingly flexible and nodular in appearance are generated (we name these worm-like (WL) fibrils, Fig. 3b), whilst in the absence of NaCl, but moderate ionic-strength buffer at this pH, much shorter rod-like (RL) fibrils are formed (Fig. 3c). Importantly, LS and WL fibrils formed under these conditions appear to have the cross-β structure of amyloid, inferred by their ability to bind the dye thioflavin T, as well as their reactivities to antibodies known to recognise a generic epitope of amyloid fibrils [30,31]. These fibrils also give rise to an X-ray fibre diffraction pattern consistent with a cross-β structure [24]. Collectively, these data demonstrate that the fibrils formed from β2m under acidic conditions in vitro are amyloid-like.
Fig. 3. AFM images of the different fibril morphologies formed from β2m at pH 3.5 under different solution conditions.
(a) long-straight (LS); (b) worm-like (WL) and (c) rod-like (RL). RL fibrils were formed in buffer containing 25 mM sodium phosphate and 25 mM sodium acetate, pH 3.5 (no agitation), whilst WL fibrils were formed under identical conditions but containing 200 mM NaCl (no agitation), and LS fibrils were formed in 5 mM ammonium acetate and 5 mM ammonium formate buffer at pH 3.5 with agitation (200 rpm). All images were taken after an incubation time of at least 2 weeks at 37 °C.
Table 2. The properties of different amyloid-like fibrils formed from wild-type β2m under acidic conditions in vitro, and brief details of conditions under which each fibril type forms under non-agitating conditions [29,30].
| Fibril type | Conditions | Height (nm) | Helical period (nm) | Persistence length (μm) | Length (nm) |
|---|---|---|---|---|---|
| (1) ‘Long straight’ | pH 1.5–4.0 | ≥1.7 | ≥1000 | ||
| Protofilament | Ionic strength ≤ 50 mM | ∼4–5 | – | ||
| Type I | ∼5 | 50–80 | |||
| Type II | ∼8 | 30–60 | |||
| Type III | ∼5 | 40–100 | |||
| (2) ‘Worm-like’ | pH 2.5–4.0 Ionic strength ≥ 100 mM |
∼3.5 | – | ∼0.1 | ∼150–600 |
| (3)‘Rod-like’ | pH 3.0–4.0 Ionic strength ≥ 50 mM |
∼3.5 | – | ∼0.1 | ∼20–150 |
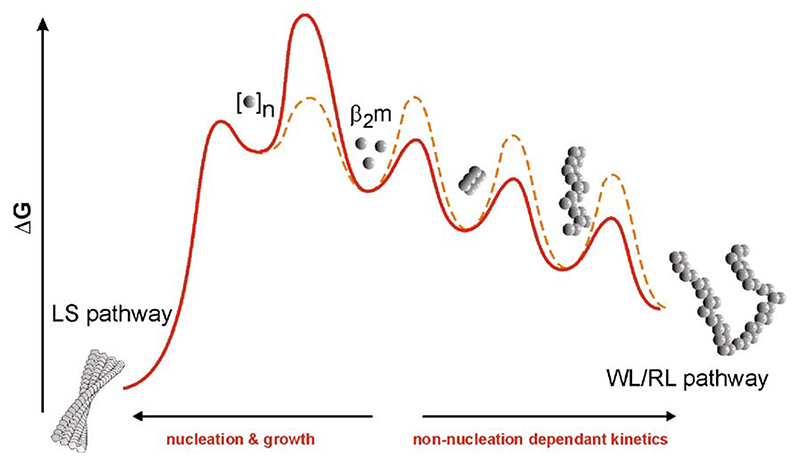
The discovery that amyloid fibril formation for almost all proteins results in a highly heterogeneous fibrillar product is often used to support a linear hierarchical mechanism of assembly, involving the consecutive assembly of increasingly well-ordered fibrillar structures until the long, straight fibrils characteristic of amyloid develop [32,33]. Such a mechanism suggests, therefore, that fibril structures that lack the rigidity of fully assembled amyloid (such as the WL fibrils seen for β2m) represent on-pathway assembly intermediates. Excitingly, in recent experiments using AFM single particle image analysis and a detailed survey of the dependence of fibril formation on the solution conditions, we have shown that such a simple mechanism does not hold for β2m, but instead, self-assembly of this protein occurs by a more complex mechanism involving at least two parallel assembly routes (Fig. 4) [30]. The first route occurs with characteristic non-nucleation dependent growth kinetics, and results in the formation of WL fibrils, via RL particles that are on-pathway in this assembly route. By contrast, the formation of LS fibrils occurs with nucleation-dependent kinetics involving a lag phase (as has been demonstrated previously [34]), without the production of substantial intermediates [30]. Importantly, these data demonstrate that the assembly of β2m amyloid fibrils, at least under acidic conditions in vitro, occurs on a complex energy landscape, involving multiple and competing paths that lead to fibrils of different morphological types. This realisation has several important implications for our understanding of amyloid-based biological phenomena. Firstly, it reinforces the conjecture that proteins can assemble into more than one cross-β structure, which is thought to be the molecular origin of ‘strains’ in prion biology [35,36] and human disease [37]. Secondly, it suggests that not all amyloid fibrils form by nucleation and growth (similar conclusions have been obtained with transthyretin [38] and phosphoglycerate kinase [39] self-assembly in vitro, which both incidentally, result in worm-like fibrils similar to those of β2m presented here). Since nucleation and growth and the infectivity of some amyloid diseases (i.e., prion-based diseases) has been argued to be synonymous [40], this may explain why some amyloid fibrils are infectious [41], and some are not [42]. Finally, a vast array of data implicates small oligomeric and so-called protofibrils as the species responsible for cellular toxicity [43,44]. Since these species are more likely to be generated along a non-nucleated pathway than during nucleation and growth, modulating the flux of protein through a particular pathway may open up a potential therapeutic avenue for those amyloid diseases that involve cellular toxicity.
Fig. 4.
At low pH, β2m assembly can be depicted on an energy diagram involving at least two competing pathways [30], the shape of which can be manipulated by solution conditions (different coloured lines). At the molecular level, how these pathways are ‘gated’ is currently unknown.
6. Towards higher resolution structures of amyloid fibrils
Whilst the AFM images presented above provide a powerful method of identifying and characterising different fibril types, this technique cannot provide the information needed to generate detailed structural models of amyloid. Amyloid fibrils are remarkably difficult materials to work with in this regard, since their size, heterogeneity and insolubility rule out high-resolution structural analysis using crystallography or solution NMR methods [45] (although if the peptide length is small, and the intermolecular interactions regular, three dimensional crystals have been shown to result [46], which are amenable to conventional X-ray crystallography). Solid state NMR can be used to determine the structure of relatively small proteins (for example, SH3 domain [47]), and distance constraints required for building high-resolution models of amyloid protofilaments [48] have been applied to fibrils formed from several peptides (the largest examples so far being Aβ1–40 [49] and a 72-residue fragment of the fungal prion protein, HET-s [50,51]. Solving the structures of amyloid fibrils derived from larger proteins by solid-state NMR remains a challenge. How then can the structure of an amyloid fibril of a protein 99 residues in length be determined? Like many biophysical questions, this one is also best addressed by combining a number of different methods. Thus, assuming fibrils of suitable quality can be obtained, single particle reconstruction using cryo-electron microscopy can provide information about the number and arrangement of protofilaments in the fibril array, provided that the fibrils have a clear helical period [52]. Being a single molecule method this technique also has the advantage that fibril segments belonging to different structural classes can be separated for averaging and three-dimensional reconstruction (see [53] for a review of cryo-EM methods). Combined with information from hydrogen exchange, limited proteolysis, EPR and/or cross-linking experiments, individual subunits (of unknown structure) can then be built into the electron density map. Towards this goal, in collaboration with Helen Saibil, Sara Cohen-Krausz and Julie Hodgkinson, we have recently generated images of the long-straight fibrils formed from β2m in vitro at pH 1.5 and 2.5, the results showing a remarkable, extended organisation of protofibrils unlike any seen previously. In combination with the results of elegant hydrogen exchange studies that have suggested that residues 21–82 form stable hydrogen bonds in these fibrils [54,55], and the results from our own laboratory which suggest that residues 1–9 are highly exposed to solvent in these fibrils (as assessed by limited proteolysis with pepsin, Myers, S.L., Ashcroft, A. E., and SER unpublished results), the next step will be to increase the resolution of the electron density maps and to generate as many constraints as possible, so that models for β2m amyloid can be generated and used to test, refine, and improve models in the future. Only then will the question of how so many different morphological types of fibrils, each based on a cross-β structure, can be generated from the same 99 residue amino acid sequence.
7. Structure and dynamics of the amyloid precursor conformation(s)
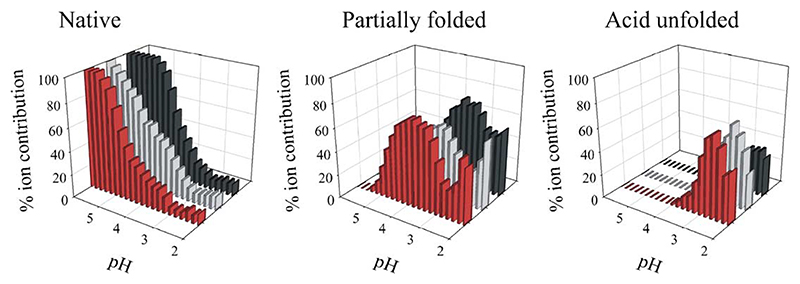
A number of different approaches have now been used to elucidate the conformational properties of different proteins at the onset of fibril formation (i.e., whilst they remain monomeric). This includes using circular dichroism in the near and far UV (providing information about tertiary and secondary structure); the binding of hydrophobic dyes such as 1-anilino naphthalene sulphonate (ANS) (acting as a probe of solvent exposed hydrophobic surface area); hydrogen exchange (permitting analysis of the presence or absence of stable hydrogen bonds) and temperature or urea denaturation (providing information about the stability and cooperativity of different ensembles) [21,22,24,26]. Whilst these techniques probe only the average properties of different ensembles, they have provided the important result that the amyloidogenic precursors for many, if not all proteins, are hydrophobically collapsed species that contain different degrees of secondary and tertiary structure formation and lack the cooperativity of structure that is unique to the native state. The techniques listed above have been used to determine the structural properties of monomeric β2m under the acidic conditions used to generate fibrils in vitro [21,22,24,26]. However, as described above, assembly is often heterogeneous, as is the unfolded ensemble, which makes a direct correlation between the initial monomer conformation and the morphology of the assembly product difficult to make. Nevertheless, we have extensively characterised the non-native ensembles of monomeric β2m at pH 3.5, where a partially folded ensemble is populated, and at pH 2.5, where more highly unfolded species are generated [56,57]. More recently, using the ability of electrospray ionisation mass spectrometry (ESI-MS) to resolve different species co-populated in solution, we have demonstrated that these ensembles are highly heterogeneous in that at least three distinct species are co-populated in solution at both pH values, including a compact, native-like form, as well as partially folded and more highly expanded states (Fig. 5) [58,59]. The concentration of these species varies with pH and can be measured and quantified using this technique. By correlating the concentration of each species with the rate of amyloid formation, given that suitable kinetic models for assembly are found in the future, MS could provide a powerful opportunity to identify which of these species is key to the initiation of amyloid formation under different conditions.
Fig. 5.
Populations of native, partially folded and acid unfolded molecules of β2m as determined by fitting the charge state distribution in ESI mass spectra to a series of Gaussian distributions at several pH values [58]. Data are shown for wild-type β2m (black) and two variants, one with an amino acid substitution in native strand-A (V9A, grey) and one with an amino acid substitution in the core of the protein (F30A, red).
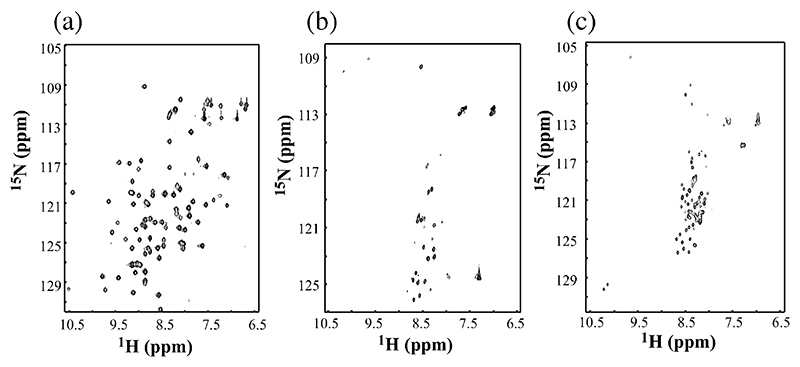
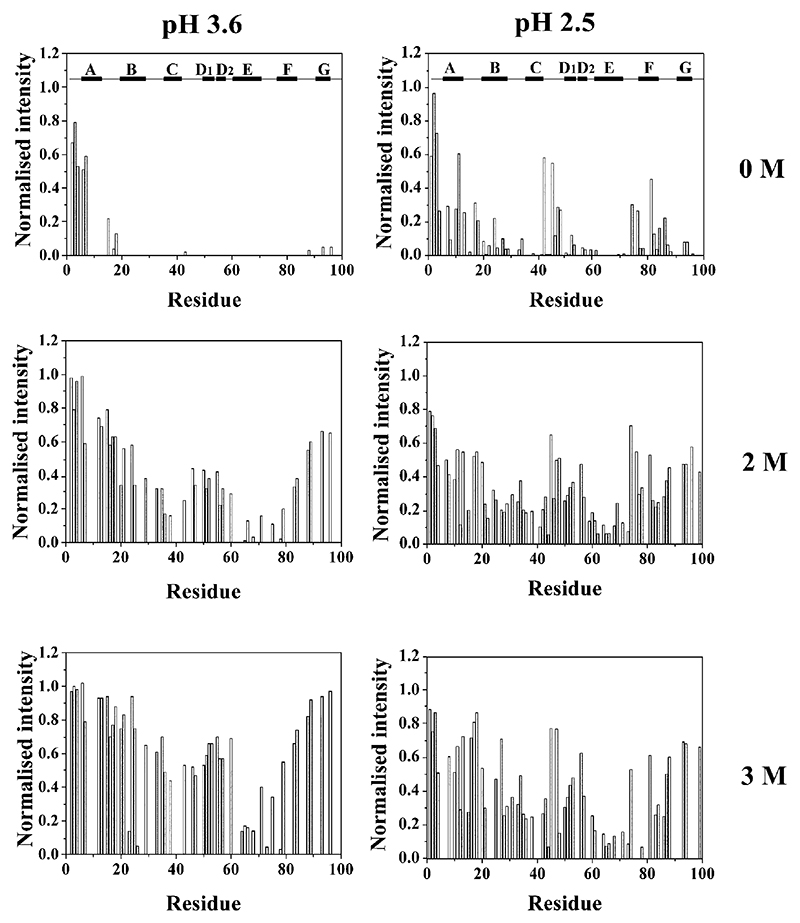
Although the partially unfolded states of some proteins give rise to well-dispersed NMR spectra, in general the conformational heterogeneity and dynamic fluctuations of these species result in broad spectra that preclude direct determination of their structural properties by NMR. The acid unfolded species of β2m at pH 3.6 and 2.5 are no exception. Thus, at 37 °C and pH 7.0, all 86 main chain amide resonances of β2m are well resolved with a wide chemical shift dispersion in the 1H–15N HSQC spectrum characteristic of a natively folded state (Fig. 6). At lower pH, significant broadening of resonances and limited chemical shift dispersion of NMR spectra indicate that the protein has unfolded, forming one or more species in dynamic equilibrium. For example, at pH 3.6, only 28 resonances are resolved in the spectrum of partially unfolded β2m, whilst 63 resonances are observed at pH 2.5, indicating substantial unfolding may have occurred in the latter conditions (Fig. 6). The broadening of resonances and limited chemical shift dispersion in the spectra obtained at pH 3.6 and 2.5 are consistent with interconversion, on an intermediate timescale measured by NMR, between the different species known to be co-populated in solution at these pH values. How then can structural information be elucidated at the residue-specific level? We have attacked this problem using two approaches. First, using an NMR method pioneered by Redfield and Dobson [60], urea titration experiments were used to identify individual residues that are resistant to denaturation to different extents. Using this method, residues involved in the structural core of the partially folded or acid unfolded proteins, and hence resistant to urea denaturation, can be identified at the residue-specific level. By categorising individual residues into different classes based on their stability to denaturation, therefore, residues can be classified as integral, or peripheral, to structure in these non-native states. The data from such an experiment for β2m at pH 2.5 and 3.6 are shown in Fig. 7. The results are dramatic, showing that at pH 2.5 β2m is more highly unfolded than its counterpart at pH 3.6, consistent with the number of resonances resolved in the NMR spectra of these states (Fig. 6), and the MS results shown in Fig. 5. More importantly, however, the results show that the N-terminal twenty residues in both species are either highly denatured in the absence of urea, or denature at low (<2 M) concentrations of this chaotrope. The C-terminal 15 amino acids are also seen to be unfolded, but only at pH 2.5 in 0 M urea. By contrast, the most stable region in both structures involves residues lying between the disulphide bond that links residues 25 and 80, with residues in the region corresponding to the native E strand (residues 60–71) being particularly resistant to denaturation in both cases [56,57]. The disulphide bond is also thought to preserve tertiary contacts in a variant of β2m lacking the N-terminal six residues at pH 6.5. This species has been shown to form amyloid fibrils at pH 6.5, by contrast with the behaviour of the wild-type protein [61], whilst reduction of the disulphide bond reduces residual structure in acid unfolded wild-type β2m [28,57]. Together with the observation that an intact disulphide bond is necessary for the formation of LS fibrils [26,62], these data suggest that residual structure in residues 25 – 80 could play an important role in the amyloidogenicity of this protein.
Fig. 6.
1H– 15N HSQC spectra of β2m obtained in 90% (v/v) H2O, 10% (v/v) D2O at pH 7.0 (a); pH 3.6 (b) and pH 2.5 (c) at 37 °C recorded at 500 MHz.
Fig. 7.
Titration of partially unfolded β2m (pH 3.6) and acid unfolded β2m (pH 2.5) with urea at 25 °C. In each case, the intensity of individual residues is shown as a function of the concentration of urea (0 – 3 M).
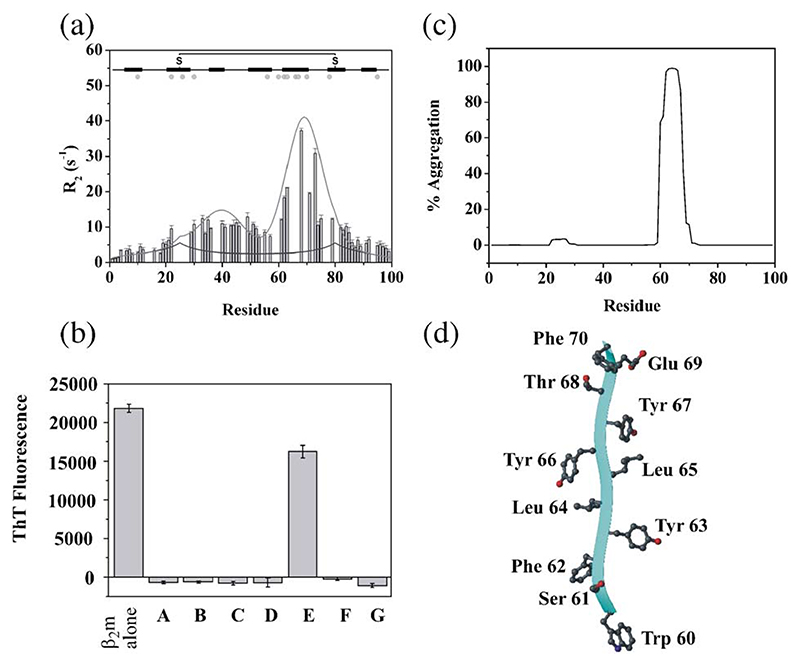
The second NMR approach taken to determine the conformational properties of acid unfolded β2m makes useof the sharper linewidths observed in the spectrum of β2m denatured at pH 2.5 (Fig. 6), which permit a more direct analysis of the dynamic and structural properties of this ensemble. Using resonance assignments of acid unfolded β2m [28], 15N NMR transverse relaxation experiments revealed that the acid denatured ensemble, although predominantly unfolded in the N- and C-terminal regions, contains substantial non-native structure in the central region of the polypeptide chain, especially in residues corresponding to the native β-strand E, consistent with the results presented above [57] (Fig. 8a). Interestingly, this aromatic-rich region has been implicated in amyloidogenesis by the observation that of seven synthetic peptides equivalent to the seven β-strands of native β2m, only the peptide corresponding to the native strand E (DWSFYL-LYYTEFT) is able to form amyloid-like fibrils at acidic pH in vitro (Fig. 8b), whilst all other peptides in this study were unable to form amyloid, despite surveying a wide range of solution conditions [63]. Furthermore, residues in strand E are also predicted to be uniquely and highly amyloidogenic using the algorithm TANGO [64] (Fig. 8c), possibly because of the large number of aromatic residues in this region (Fig. 8d) [65–67].
Fig. 8. Residue specific NMR transverse relaxation rates for wild-type β2m in water at pH 2.5, 25 °C, obtained at 500 MHz.
(a). The black line is a fit to a model of a random coil containing a single disulphide bond, and the grey line is a fit to the observed rates, showing significant residual structure in the core of the protein, specifically in the residues that comprise the native E-strand (residues 60–71). The position of β-strands in native β2m is shown above the plot and residues that are aromatic are marked as a grey dot. Thioflavin-T fluorescence of fibrils formed from β2m and different peptides corresponding to individual strands of β2m incubated at pH 3.6 in 400 mM NaCl [63] (b). Prediction of the aggregation propensity of the amino acid sequence of β2m using the TANGO algorithm [64] (c). The structure of residues 60 – 70 in native β2m (equivalent to the native E strand) showing the high content of aromatic residues in this region is shown in (d). The image was drawn using MOLMOL [76].
A more detailed analysis of the origins of the line width in the NMR spectra of β2m at pH 2.5 using relaxation dispersion experiments [57,68], revealed that the acid unfolded ensemble involves two or more distinct species in conformational equilibrium on the μs–ms timescale, again consistent with the results from ESI-MS (Fig. 5) [58]. Further studies of variant proteins with replacement of aromatic residues in the E-strand (Y66E and F62AY63AY67A) revealed that these species are hydrophobically collapsed, as removal of these aromatic moieties disrupts the residual structure across the entire polypeptide chain and causes a consequent decrease in the population of this conformer in the acid unfolded ensemble [57]. Whether strand E plays a role in the self-assembly of the full-length protein, for example in providing a surface for initiating amyloid-formation, remains to be seen.
8. Summary and implications
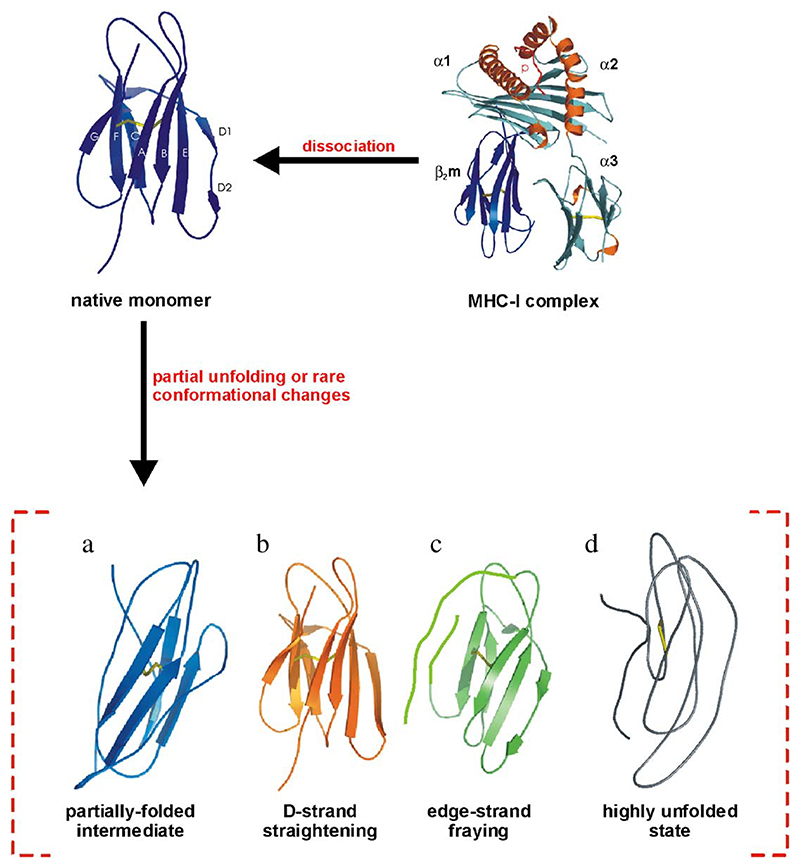
Large volumes of experimental data have now identified the key conformational changes that promote the formation of amyloid-like fibrils from β2m in vitro (Fig. 9). Primarily, local or more global unfolding events are thought to initiate self-assembly, suggesting that the key amyloidogenic precursors are destabilised states that have increased conformational dynamics and decreased cooperativity. Although exact structural details of these precursor states remain to be elucidated, a number of general features are now known. For instance, the central β-strands form the protected core in the ultimate protein fibril (Myers, S. L., Ashcroft, A. E., and SER unpublished data [55,69]), and are the most structurally stable region in the acid denatured and partially folded states [56,57,69]. Moreover, an intact disulphide bond is required for generation of long, straight fibrils (but not WL fibrils) in vitro at very low pH [26,28,70]. Together, these data demonstrate that an increased population of non-native species that exposes new assembly-competent surfaces, perhaps involving the aromatic-rich E-strand or straightening of the D-strand, may promote fibril formation [17,56,57,63]. The regions of β2m that could be involved in promoting fibril formation are diverse, other laboratories suggesting that the B/C strands [70–72] and the F/G region [73,74] are involved, as well as the conformational differences arising either from dissociation of β2m from MHC-I [16,17], or from deletion of the N-terminal six amino acids [61]. These various proposals highlight the complexity of the assembly mechanism (i.e., different regions may play different roles in assembly under different conditions), and may explain the variety of fibril morphologies produced. Intriguingly, the most vulnerable strands A, D and G, are all involved in contacts with the heavy chain in the intact MHC-I complex, emphasising the pivotal role of the heavy chain in stabilising β2m and preventing its self-assembly. The next exciting stages of research in this area will be to elucidate the assembly mechanism at higher resolution, so that the role of individual residues in assembly under different conditions can be deduced and used to determine the structure of amyloid fibrils themselves. Additionally, structural elucidation of early aggregation states in these pathways will be needed for a fuller description of the assembly mechanism. The discovery that fibril formation occurs via multiple, competing pathways adds to the complexity of any emerging model of amyloidosis. In addition, the conformational properties, as well as the population of different precursor states, and the structure and kinetics of assembly of the fibril itself, may also be highly dependent on the growth conditions employed. Finally, the mechanism of action of biological factors in the facilitation of β2m assembly in vivo must be determined in detail using the biophysical and biochemical analyses described above.
Fig. 9.
Schematic diagram of possible models for β2m amyloid fibril formation in vitro. As indicated, β2m dissociates from the MHC heavy chain and under amyloidogenic conditions, is destabilised. Possible conformational changes which increase the propensity to self-assemble are depicted in the figure as (a) population of one or more partially folded conformations; (b) the loss of the β-bulge in strand D; (c) the displacement of the edge-strands; (d) formation of extensively unfolded structures.
The insights into the mechanism of β2m fibril formation from biophysical studies offer possible avenues for therapeutic investigations. Stabilisation of the native fold of free monomers will reduce the amyloidogenic potential of this protein, whilst identification of regions of the protein that are involved in amyloid assembly may offer other targets for ligands. The concept of competing pathways could lend itself to a therapeutic rationale whereby fibril formation is biased in favour of a relatively harmless pathway over a competing route that contains a toxic product. Deriving new perspectives on the events occurring during aggregation of β2m in vitro, combined with identification of the factors involved in sequestration of β2m in the joints over the forthcoming years, will form an important platform upon which to derive new insights into the structural molecular mechanism of amyloid formation, and may guide future therapeutic strategies for this and other amyloid diseases.
Acknowledgements
We would like to thank past and current members of the Radford research group and our collaborators and colleagues for many helpful discussions over the years, all of whom have contributed enormously to the work reviewed in this article. We particularly acknowledge David Smith, Toni Borysik, Victoria McParland, Clemens Stilling, Sue Jones and Thomas Jahn for providing some of the figures. SER is a BBSRC Professorial Fellow, WSG is funded by the BBSRC and GWP by the Wellcome Trust.
References
- [1].Gejyo F, Yamada T, Odani S, Nakagawa Y, Arakawa M, Kunitomo T, Kataoka H, Suzuki M, Hirasawa Y, Shirahama T, Cohen AS, et al. A new form of amyloid protein associated with chronic hemodialysis was identified as β2-microglobulin. Biochem Biophys Res Commun. 1985;129:701–706. doi: 10.1016/0006-291x(85)91948-5. [DOI] [PubMed] [Google Scholar]
- [2].Gorevic PD, Casey TT, Stone WJ, Diraimondo CR, Prelli FC, Frangione B. β2-microglobulin is an amyloidogenic protein in man. J Clin Invest. 1985;76:2425–2429. doi: 10.1172/JCI112257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Floege J, Ketteler M. β2-microglobulin-derived amyloidosis: an update. Kidney Int. 2001;59:S164–S171. doi: 10.1046/j.1523-1755.2001.59780164.x. [DOI] [PubMed] [Google Scholar]
- [4].Campistol JM, Bernard D, Papastoitsis G, Sole M, Kasirsky J, Skinner M. Polymerization of normal and intact β2-microglobulin as the amyloidogenic protein in dialysis-amyloidosis. Kidney Int. 1996;50:1262–1267. doi: 10.1038/ki.1996.436. [DOI] [PubMed] [Google Scholar]
- [5].Linke RP, Hampl H, Lobeck H, Ritz E, Bommer J, Waldherr R, Eulitz M. Lysine-specific cleavage of β2-microglobulin in amyloid deposits associated with hemodialysis. Kidney Int. 1989;36:675–681. doi: 10.1038/ki.1989.245. [DOI] [PubMed] [Google Scholar]
- [6].Bellotti V, Stoppini M, Mangione P, Sunde M, Robinson CV, Asti L, Brancaccio D, Ferri G. β2-microglobulin can be refolded into a native state from ex vivo amyloid fibrils. Eur J Biochem. 1998;258:61–67. doi: 10.1046/j.1432-1327.1998.2580061.x. [DOI] [PubMed] [Google Scholar]
- [7].Selkoe DJ. Folding proteins in fatal ways. Nature. 2003;426:900–904. doi: 10.1038/nature02264. [DOI] [PubMed] [Google Scholar]
- [8].Bjorkman PJ, Saper MA, Samraoui B, Bennett WS, Strominger JL, Wiley DC. Structure of the human class-I histocompatibility antigen, HLA-A2. Nature. 1987;329:506–512. doi: 10.1038/329506a0. [DOI] [PubMed] [Google Scholar]
- [9].Hill DM, Kasliwal T, Schwarz E, Hebert AM, Chen T, Gubina E, Zhang L, Kozlowski S. A dominant negative mutant β2-microglobulin blocks the extracellular folding of a major histocompatibility complex class I heavy chain. J Biol Chem. 2003;278:5630–5638. doi: 10.1074/jbc.M208381200. [DOI] [PubMed] [Google Scholar]
- [10].Sundin DP, Cohen M, Dahl R, Falk S, Molitoris BA. Characterization of the β2-microglobulin endocytic pathway in rat proximal tubule cells. Am J Physiol. 1994;267:F380–F389. doi: 10.1152/ajprenal.1994.267.3.F380. [DOI] [PubMed] [Google Scholar]
- [11].Bellotti V, Mangione P, Stoppini M. Biological activity and pathological implications of misfolded proteins. Cell Mol Life Sci. 1999;55:977–991. doi: 10.1007/s000180050348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Floege J, Bartsch A, Schulze M, Shaldon S, Koch KM, Smeby LC. Clearance and synthesis rates of β2-microglobulin in patients undergoing hemodialysis and in normal subjects. J Lab Clin Med. 1991;118:153–165. [PubMed] [Google Scholar]
- [13].Floege J, Ehlerding G. β2-microglobulin-associated amyloidosis. Nephron. 1996;72:9–26. doi: 10.1159/000188801. [DOI] [PubMed] [Google Scholar]
- [14].Koch KM, Gennari FJ, Vanypersele C, Rees A, Goldman M, Mihatsch MJ, Mion CM, Borsatti A, Winearls CG, Davison AM, Cohen JJ, et al. Dialysis-related amyloidosis. Kidney Int. 1992;41:1416–1429. doi: 10.1038/ki.1992.207. [DOI] [PubMed] [Google Scholar]
- [15].Berman HM, Westbrook J, Feng Z, Gilliland G, Bhat TN, Weissig H, Shindyalov IN, Bourne PE. The Protein Data Bank. Nucleic Acids Res. 2000;28:235–242. doi: 10.1093/nar/28.1.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Verdone G, Corazza A, Viglino P, Pettirossi F, Giogetti S, Mangione P, Andreola A, Stoppini M, Bellotti V, Esposito G. The solution structure of human β2-microglobulin reveals the prodromes of its amyloid transition. Protein Sci. 2002;11:487–499. doi: 10.1110/ps.29002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Trinh CH, Smith DP, Kalverda AP, Phillips SEV, Radford SE. Crystal structure of monomeric human β2-microglobulin reveals clues to its amyloidogenic properties. Proc Natl Acad Sci U S A. 2002;99:9771–9776. doi: 10.1073/pnas.152337399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Richardson JS, Richardson DC. Natural β-sheet proteins use negative design to avoid edge-to-edge aggregation. Proc Natl Acad Sci U S A. 2002;99:2754–2759. doi: 10.1073/pnas.052706099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Rosano C, Zuccotti S, Mangione P, Giorgetti S, Bellotti V, Pettirossi F, Corazza A, Viglino P, Esposito G, Bolognesi M. β2-microglobulin H31Y variant 3D structure highlights the protein natural propensity towards intermolecular aggregation. J Mol Biol. 2004;335:1051–1064. doi: 10.1016/j.jmb.2003.11.040. [DOI] [PubMed] [Google Scholar]
- [20].Zuccotti S, Rosano C, Mangione P, Bellotti V, Bolognesi M. Preliminary crystallographic characterization of the human β2-microglobulin His31Tyr mutant in a tetrameric assembly. Acta Crystallogr, Sect D Biol Crystallogr. 2003;59:1270–1272. doi: 10.1107/s0907444903009193. [DOI] [PubMed] [Google Scholar]
- [21].McParland VJ, Kad NM, Kalverda AP, Brown A, Kirwin-Jones P, Hunter MG, Sunde M, Radford SE. Partially unfolded states of β2-microglobulin and amyloid formation in vitro. Biochemistry. 2000;39:8735–8746. doi: 10.1021/bi000276j. [DOI] [PubMed] [Google Scholar]
- [22].Kad NM, Thomson NH, Smith DP, Smith DA, Radford SE. β2-microglobulin and its deamidated variant, N17D form amyloid fibrils with a range of morphologies in vitro. J Mol Biol. 2001;313:559–571. doi: 10.1006/jmbi.2001.5071. [DOI] [PubMed] [Google Scholar]
- [23].Uversky VN, Fink AL. Conformational constraints for amyloid fibrillation: the importance of being unfolded. Biochim Biophys Acta. 2004;1698:131–153. doi: 10.1016/j.bbapap.2003.12.008. [DOI] [PubMed] [Google Scholar]
- [24].Smith DP, Jones S, Serpell LC, Sunde M, Radford SE. A systematic investigation into the effect of protein destabilisation on β2-microglobulin amyloid formation. J Mol Biol. 2003;330:943–954. doi: 10.1016/s0022-2836(03)00687-9. [DOI] [PubMed] [Google Scholar]
- [25].Corazza A, Pettirossi F, Viglino P, Verdone G, Garcia J, Dumy P, Giorgetti S, Mangione P, Raimondi S, Stoppini M, Bellotti V, et al. Properties of some variants of human β2-microglobulin and amyloidogenesis. J Biol Chem. 2004;279:9176–9189. doi: 10.1074/jbc.M310779200. [DOI] [PubMed] [Google Scholar]
- [26].Smith DP, Radford SE. Role of the single disulphide bond of β2-microglobulin in amyloidosis in vitro. Protein Sci. 2001;10:1775–1784. doi: 10.1110/ps.4901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Chiba T, Hagihara Y, Higurashi T, Hasegawa K, Naiki H, Goto Y. Amyloid fibril formation in the context of full-length protein—Effects of proline mutations on the amyloid fibril formation of β2-micro-globulin. J Biol Chem. 2003;278:47016–47024. doi: 10.1074/jbc.M304473200. [DOI] [PubMed] [Google Scholar]
- [28].Katou H, Kanno T, Hoshino M, Hagihara Y, Tanaka H, Kawai T, Hasegawa K, Naiki H, Goto Y. The role of disulfide bond in the amyloidogenic state of β2-microglobulin studied by heteronuclear NMR. Protein Sci. 2002;11:2218–2229. doi: 10.1110/ps.0213202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Kad NM, Myers SL, Smith DP, Smith DA, Radford SE, Thomson NH. Hierarchical assembly of β2-microglobulin amyloid in vitro revealed by atomic force microscopy. J Mol Biol. 2003;330:785–797. doi: 10.1016/s0022-2836(03)00583-7. [DOI] [PubMed] [Google Scholar]
- [30].Gosal WS, Morten IJ, Hewitt EW, Smith DA, Thomson NH, Radford SE. Competing pathways determine fibril morphology in the self-assembly of β2-microglobulin into amyloid. J Mol Biol. doi: 10.1016/j.jmb.2005.06.040. (in press) [DOI] [PubMed] [Google Scholar]
- [31].O’Nuallain B, Wetzel R. Conformational Abs recognizing a generic amyloid fibril epitope. Proc Natl Acad Sci U S A. 2002;99:1485–1490. doi: 10.1073/pnas.022662599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Zerovnik E. Amyloid-fibril formation—Proposed mechanisms and relevance to conformational disease. Eur J Biochem. 2002;269:3362–3371. doi: 10.1046/j.1432-1033.2002.03024.x. [DOI] [PubMed] [Google Scholar]
- [33].Ross CA, Poirier MA. Protein aggregation and neurodegenerative disease. Nat Med. 2004:S10–S17. doi: 10.1038/nm1066. [DOI] [PubMed] [Google Scholar]
- [34].Naiki H, Hashimoto N, Suzuki S, Kimura H, Nakakuki K, Gejyo F. Establishment of a kinetic model of dialysis-related amyloid fibril extension in vitro, Amyloid-Int. J Exp Clin Investig. 1997;4:223–232. [Google Scholar]
- [35].Tanaka M, Chien P, Naber N, Cooke R, Weissman JS. Conformational variations in an infectious protein determine prion strain differences. Nature. 2004;428:323–328. doi: 10.1038/nature02392. [DOI] [PubMed] [Google Scholar]
- [36].King CY, Diaz-Avalos R. Protein-only transmission of three yeast prion strains. Nature. 2004;428:319–323. doi: 10.1038/nature02391. [DOI] [PubMed] [Google Scholar]
- [37].Safar J, Wille H, Itrri V, Groth D, Serban H, Torchia M, Cohen FE, Prusiner SB. Eight prion strains have PrPSc molecules with different conformations. Nat Med. 1998;4:1157–1165. doi: 10.1038/2654. [DOI] [PubMed] [Google Scholar]
- [38].Hurshman AR, White JT, Powers ET, Kelly JW. Transthyretin aggregation under partially denaturing conditions is a downhill polymerization. Biochemistry. 2004;43:7365–7381. doi: 10.1021/bi049621l. [DOI] [PubMed] [Google Scholar]
- [39].Modler AJ, Gast K, Lutsch G, Damaschun G. Assembly of amyloid protofibrils via critical oligomers—A novel pathway of amyloid formation. J Mol Biol. 2003;325:135–148. doi: 10.1016/s0022-2836(02)01175-0. [DOI] [PubMed] [Google Scholar]
- [40].Eigen M. Prionics or the kinetic basis of prion diseases. Biophys Chem. 1996;63:A1–A18. doi: 10.1016/s0301-4622(96)02250-8. [DOI] [PubMed] [Google Scholar]
- [41].Lundmark K, Westermark GT, Nystrom S, Murphy CL, Solomon A, Westermark P. Transmissibility of systemic amyloidosis by a prion-like mechanism. Proc Natl Acad Sci U S A. 2002;99:6979–6984. doi: 10.1073/pnas.092205999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Tagoe CE, French D, Gallo G, Buxbaum JN. Amyloidogenesis is neither accelerated nor enhanced by injections of preformed fibrils in mice transgenic for wild-type human transthyretin: the question of infectivity. Amyloid-J Protein Fold Disord. 2004;11:21–26. doi: 10.1080/13506120410001674982. [DOI] [PubMed] [Google Scholar]
- [43].Caughey B, Lansbury PT. Protofibrils, pores, fibrils, and neuro-degeneration: separating the responsible protein aggregates from the innocent bystanders. Annu Rev Neurosci. 2003;26:267–298. doi: 10.1146/annurev.neuro.26.010302.081142. [DOI] [PubMed] [Google Scholar]
- [44].Klein WL, Stine WB, Jr, Teplow DB. Small assemblies of unmodified amyloid β protein are the proximate neurotoxin in Alzheimer’s disease. Neurobiol Aging. 2004;25:569–580. doi: 10.1016/j.neurobiolaging.2004.02.010. [DOI] [PubMed] [Google Scholar]
- [45].Tycko R. Progress towards a molecular-level structural understanding of amyloid fibrils. Curr Opin Struct Biol. 2004;14:96–103. doi: 10.1016/j.sbi.2003.12.002. [DOI] [PubMed] [Google Scholar]
- [46].Nelson R, Sawaya MR, Balbirnie M, Madsen AO, Riekel C, Grothe R, Eisenberg D. Structure of the cross-β spine of amyloid-fibrils. Nature. 2005;435:773–778. doi: 10.1038/nature03680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Castellani F, van Rossum B, Diehl A, Schubert M, Rehbein K, Oschkinat H. Structure of a protein determined by solid-state magic-angle-spinning NMR spectroscopy. Nature. 2002;420:98–102. doi: 10.1038/nature01070. [DOI] [PubMed] [Google Scholar]
- [48].Tycko R. Insights into the amyloid folding problem from solid-state NMR. Biochemistry. 2003;42:3151–3159. doi: 10.1021/bi027378p. [DOI] [PubMed] [Google Scholar]
- [49].Petkova AT, Leapman RD, Guo Z, Yau WM, Mattson MP, Tycko R. Self-propagating, molecular-level polymorphism in Alzheimer’s β-amyloid fibrils. Science. 2005;307:262–265. doi: 10.1126/science.1105850. [DOI] [PubMed] [Google Scholar]
- [50].Siemer AB, Ritter C, Ernst M, Riek R, Meier BH. High resolution solid-state NMR spectroscopy of the prion protein HET-s in its amyloid conformation. Angew Chem, Int Ed Engl. 2005;44:2441–2444. doi: 10.1002/anie.200462952. [DOI] [PubMed] [Google Scholar]
- [51].Ritter C, Maddelein ML, Siemer AB, Luhrs T, Ernst M, Meier BH, Saupe SJ, Riek R. Correlation of structural elements and infectivity of the HET-s prion. Nature. 2005;435:844–848. doi: 10.1038/nature03793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Jimenez JL, Guijarro JL, Orlova E, Zurdo J, Dobson CM, Sunde M, Saibil HR. Cryo-electron microscopy structure of an SH3 amyloid fibril and model of the molecular packing. EMBO J. 1999;18:815–821. doi: 10.1093/emboj/18.4.815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Saibil HR. Macromolecular structure determination by cryo-electron microscopy. Acta Crystallogr, Sect D Biol Crystallogr. 2000;56:1215–1222. doi: 10.1107/s0907444900010787. [DOI] [PubMed] [Google Scholar]
- [54].Hoshino M, Katou H, Hagihara Y, Hasegawa K, Naiki H, Goto Y. Mapping the core of the β2-microglobulin amyloid fibril by H/D exchange. Nature Struct Biol. 2002;9:332–336. doi: 10.1038/nsb792. [DOI] [PubMed] [Google Scholar]
- [55].Yamaguchi KI, Katou H, Hoshino M, Hasegawa K, Naiki H, Goto Y. Core and heterogeneity of β2-microglobulin amyloid fibrils as revealed by H/D exchange. J Mol Biol. 2004;338:559–571. doi: 10.1016/j.jmb.2004.02.067. [DOI] [PubMed] [Google Scholar]
- [56].McParland VJ, Kalverda AP, Homans SW, Radford SE. Structural properties of an amyloid precursor of β2-microglobulin. Nat Struct Biol. 2002;9:326–331. doi: 10.1038/nsb791. [DOI] [PubMed] [Google Scholar]
- [57].Platt GW, McParland VJ, Kalverda AP, Homans SW, Radford SE. Dynamics in the unfolded state of β2-microglobulin studied by NMR. J Mol Biol. 2005;346:279–294. doi: 10.1016/j.jmb.2004.11.035. [DOI] [PubMed] [Google Scholar]
- [58].Borysik AJ, Radford SE, Ashcroft AE. Co-populated conformational ensembles of β2-microglobulin uncovered quantitatively by electrospray ionisation mass spectroscopy. J Biol Chem. 2004;279:27069–27077. doi: 10.1074/jbc.M401472200. [DOI] [PubMed] [Google Scholar]
- [59].Borysik AJ, Read P, Little DR, Bateman RH, Radford SE, Ashcroft AE. Separation of β2-microglobulin conformers by high-field asymmetric waveform ion mobility spectrometry (FAIMS) coupled to electrospray ionisation mass spectrometry. Rapid Commun Mass Spectrom. 2004;18:2229–2234. doi: 10.1002/rcm.1613. [DOI] [PubMed] [Google Scholar]
- [60].Schulman BA, Kim PS, Dobson CM, Redfield C. A residue-specific NMR view of the non-cooperative unfolding of a molten globule. Nat Struct Biol. 1997;4:630–634. doi: 10.1038/nsb0897-630. [DOI] [PubMed] [Google Scholar]
- [61].Esposito G, Michelutti R, Verdone G, Viglino P, Hernandez H, Robinson CV, Amoresano A, DalPiaz F, Monti M, Pucci P, Mangione P, et al. Removal of the N-terminal hexapeptide from human β2-microglobulin facilitates protein aggregation and fibril formation. Protein Sci. 2000;9:831–845. doi: 10.1110/ps.9.5.831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Ohhashi Y, Hagihara Y, Kozhukh GV, Hoshino M, Hasegawa K, Yamaguchi I, Naiki H, Goto Y. The intrachain disulfide bond of β2-microglobulin is not essential for the immunoglobulin fold at neutral pH, but is essential for amyloid fibril formation at acidic pH. J Biochem. 2002;131:45–52. doi: 10.1093/oxfordjournals.jbchem.a003076. [DOI] [PubMed] [Google Scholar]
- [63].Jones S, Manning J, Kad NM, Radford SE. Amyloid-forming peptides from β2-microglobulin—Insights into the mechanism of fibril formation in vitro. J Mol Biol. 2003;325:249–257. doi: 10.1016/s0022-2836(02)01227-5. [DOI] [PubMed] [Google Scholar]
- [64].Fernandez-Escamilla AM, Rousseau F, Schymkowitz J, Serrano L. Prediction of sequence-dependent and mutational effects on the aggregation of peptides and proteins. Nat Biotechnol. 2004;22:1302–1306. doi: 10.1038/nbt1012. [DOI] [PubMed] [Google Scholar]
- [65].de la Paz M, Serrano L. Sequence determinants of amyloid fibril formation. Proc Natl Acad Sci U S A. 2004;101:87–92. doi: 10.1073/pnas.2634884100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Gazit E. A possible role for pi-stacking in the self-assembly of amyloid fibrils. FASEB J. 2002;16:77–83. doi: 10.1096/fj.01-0442hyp. [DOI] [PubMed] [Google Scholar]
- [67].Tartaglia GG, Cavalli A, Pellarin R, Caflisch A. The role of aromaticity, exposed surface, and dipole moment in determining protein aggregation rates. Protein Sci. 2004;13:1939–1941. doi: 10.1110/ps.04663504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Mulder FAA, Skrynnikov NR, Hon B, Dahlquist FW, Kay LE. Measurement of slow (μs–ms) time scale dynamics in protein side chains by 15N relaxation dispersion NMR spectroscopy: application to Asn and Gln residues in a cavity mutant of T4 lysozyme. J Am Chem Soc. 2001;123:967–975. doi: 10.1021/ja003447g. [DOI] [PubMed] [Google Scholar]
- [69].Monti M, Principe S, Giorgetti S, Mangione P, Merlini G, Clark A, Bellotti V, Amoresano A, Pucci P. Topological investigation of amyloid fibrils obtained from β2-microglobulin. Protein Sci. 2002;11:2362–2369. doi: 10.1110/ps.0206902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Hasegawa K, Ohhashi Y, Yamaguchi I, Takahashi N, Tsutsumi S, Goto Y, Gejyo F, Naiki H. Amyloidogenic synthetic peptides of β2-microglobulin—A role of the disulfide bond. Biochem Biophys Res Commun. 2003;304:101–106. doi: 10.1016/s0006-291x(03)00543-6. [DOI] [PubMed] [Google Scholar]
- [71].Wadai H, Yamaguchi K, Takahashi S, Kanno T, Kawai T, Naiki H, Goto Y. Stereospecific amyloid-like fibril formation by a peptide fragment of β2-microglobulin. Biochemistry. 2005;44:157–164. doi: 10.1021/bi0485880. [DOI] [PubMed] [Google Scholar]
- [72].Kozhukh GV, Hagihara Y, Kawakami T, Hasegawa K, Naiki H, Goto Y. Investigation of a peptide responsible for amyloid fibril formation of β2-microglobulin by Achromobacter protease I. J Biol Chem. 2002;277:1310–1315. doi: 10.1074/jbc.M108753200. [DOI] [PubMed] [Google Scholar]
- [73].Ivanova MI, Gingery M, Whitson LJ, Eisenberg D. Role of the C-terminal 28 residues of β2-microglobulin in amyloid fibril formation. Biochemistry. 2003;42:13536–13540. doi: 10.1021/bi0301486. [DOI] [PubMed] [Google Scholar]
- [74].Ivanova MI, Sawaya MR, Gingery M, Attinger A, Eisenberg D. An amyloid-forming segment of β2-microglobulin suggests a molecular model for the fibril. Proc Natl Acad Sci U S A. 2004;101:10584–10589. doi: 10.1073/pnas.0403756101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Khan AR, Baker BM, Ghosh P, Biddison WE, Wiley DC. The structure and stability of an HLA-A0201/octameric tax peptide complex with an empty conserved peptide-N-terminal binding site. J Immunol. 2000;164:6398–6405. doi: 10.4049/jimmunol.164.12.6398. [DOI] [PubMed] [Google Scholar]
- [76].Koradi R, Billeter M, Wuthrich K. MOLMOL: a program for display and analysis of macromolecular structures. J Mol Graph. 1996;14:51–55. doi: 10.1016/0263-7855(96)00009-4. [DOI] [PubMed] [Google Scholar]
- [77].Linke RP, Schaeffer J, Gielow P, Lindner P, Lottspeich F, Pluckthun A, Weiss EH. Production of recombinant human β2-micro-globulin for scintigraphic diagnosis of amyloidosis in uremia and hemodialysis. Eur J Biochem. 2000;267:627–633. doi: 10.1046/j.1432-1327.2000.01071.x. [DOI] [PubMed] [Google Scholar]
- [78].Ono K, Uchino F. Formation of amyloid-like substance from β2-microglobulin in vitro-role of serum amyloid-P component—A preliminary study. Nephron. 1994;66:404–407. doi: 10.1159/000187854. [DOI] [PubMed] [Google Scholar]
- [79].Connors LH, Shirahama T, Skinner M, Fenves A, Cohen AS. In vitro formation of amyloid fibrils from intact β2-microglobulin. Biochem Biophys Res Commun. 1985;131:1063–1068. doi: 10.1016/0006-291x(85)90198-6. [DOI] [PubMed] [Google Scholar]
- [80].Campistol JM, Sole M, Bombi JA, Rodriguez R, Mirapeix E, Munoz-Gomez J, Revert OW. In vitro spontaneous synthesis of β2-microglobulin amyloid fibrils in peripheral blood mononuclear cell culture. Am J Pathol. 1992;141:241–247. [PMC free article] [PubMed] [Google Scholar]
- [81].Morgan CJ, Gelfand M, Atreya C, Miranker AD. Kidney dialysis-associated amyloidosis: a molecular role for copper in fiber formation. J Mol Biol. 2001;309:339–345. doi: 10.1006/jmbi.2001.4661. [DOI] [PubMed] [Google Scholar]
- [82].Chiti F, De Lorenzi E, Grossi S, Mangione P, Giorgetti S, Caccialanza G, Dobson CM, Merlini G, Ramponi G, Bellotti V. A partially structured species of β2-microglobulin is significantly populated under physiological conditions and involved in fibrillo-genesis. J Biol Chem. 2001;276:46714–46721. doi: 10.1074/jbc.M107040200. [DOI] [PubMed] [Google Scholar]
- [83].Yamamoto S, Hasegawa K, Yamaguchi I, Tsutsumi S, Kardos J, Goto Y, Gejyo F, Naiki H. Low concentrations of sodium dodecyl sulfate induce the extension of β2-microglobulin-related amyloid fibrils at a neutral pH. Biochemistry. 2004;43:11075–11082. doi: 10.1021/bi049262u. [DOI] [PubMed] [Google Scholar]
- [84].Kihara M, Catani E, Sakai M, Hasegawa K, Naiki H, Goto Y. Seeding-dependent maturation of β2-microglobulin amyloid fibrils at neutral pH. J Biol Chem. 2005;280:12012–12018. doi: 10.1074/jbc.M411949200. [DOI] [PubMed] [Google Scholar]
- [85].Yamamoto S, Yamaguchi I, Hasegawa K, Tsutsumi S, Goto Y, Gejyo F, Naiki H. Glycosaminoglycans enhance the trifluoroethanol-induced extension of β2-microglobulin-related amyloid fibrils at a neutral pH. J Am Soc Nephrol. 2004;15:126–133. doi: 10.1097/01.asn.0000103228.81623.c7. [DOI] [PubMed] [Google Scholar]