Abstract
Regulatory T (Treg) cells are immunosuppressive CD4 T cells defined by expression of the transcription factor Foxp3. Genetic loss-of-function mutations in Foxp3 cause lethal multi-organ autoimmune inflammation resulting from defects in Treg cell development and suppressive activity. Whether Treg cells are continuously dependent on Foxp3 is still unclear. Here, we leveraged chemically-induced protein degradation to show that functionally suppressive Treg cells in healthy organs can persist in the near-complete absence of Foxp3 protein for at least 10 days. Conversely, Treg cells responding to type 1 inflammation in settings of autoimmunity, viral infection, or cancer were selectively lost upon Foxp3 protein depletion. Acute degradation experiments revealed that Foxp3 acts mostly as a direct transcriptional repressor and modulates responsiveness to cytokine stimulation. This inflammation-dependent requirement for continuous Foxp3 activity enabled induction of a selective anti-tumor immune response upon systemic Foxp3 depletion, without causing deleterious T cell expansion in healthy organs.
Introduction
Regulatory T (TREG) cells are an essential immunosuppressive CD4 T cell lineage defined by expression of the X-linked, forkhead box transcription factor Foxp3 (1). TREG cells are present in most tissues and continuously required to suppress the activation and expansion of self-reactive conventional T (TCON) cells (1). TREG cells can also infiltrate tumors where they inhibit anti-tumor T cell responses and promote disease progression (2–7). Systemic TREG cell ablation effectively inhibits tumor growth in mice but also causes severe autoimmunity. Clinical translation of TREG cell-based immunotherapies therefore hinges on the development of strategies to selectively deplete TREG cells from tumors but not other organs (8).
Genetic loss-of-function mutations in Foxp3 cause lethal multi-organ autoimmunity resulting from defects in TREG cell-suppressive activity, fitness, and lineage stability (9–15). However, whether TREG cells are continuously dependent on Foxp3 is less clear. Deletion of a loxP-flanked Foxp3 allele (Foxp3flox) using tamoxifen-inducible Cre recombinase caused an approximately 2-fold expansion of splenic CD4 T cells after 4 weeks, a much more modest phenotype than that of mice constitutively lacking Foxp3 (16). However, because the Foxp3flox allele lacks a fluorescent reporter that would enable direct identification of Foxp3-transcribing cells after the deletion of essential exons, it is unclear if lineage-committed TREG cells are maintained in this setting. Another study used retrovirally-expressed Cre recombinase to induce Foxp3 deletion before transferring TREG cells together with wildtype CD4 T cells into lymphopenic mice (11). In this setting, TREG cells downregulated non-deleted portions of the Foxp3 transcript as well as other TREG cell signature genes, suggesting that the transferred TREG cells quickly de-differentiated upon loss of Foxp3. Whether this strict and continuous requirement for Foxp3 also applies to TREG cells residing in their native tissue environments or within tumors is still unclear.
Foxp3 binds to approximately 10,000 regions in the genome and interacts with hundreds of other nuclear proteins (17–20). How these events impact gene expression has been difficult to define. Genetic deficiency in Foxp3 affects the transcription of a subset of Foxp3-bound genes; however, many genes that are not bound by Foxp3 also change their expression. Foxp3 can thus be assumed to drive both direct and indirect transcriptional changes. Indirect changes may arise as a secondary consequence of direct changes in the expression of genes encoding signaling molecules, transcription factors, co-factors, and other factors that impact the TREG cell transcriptome (19). Importantly, indirect regulation may also affect genes that are bound by Foxp3, which can therefore not be assumed to represent direct transcriptional targets. It is therefore still unclear if Foxp3 acts as a direct transcriptional activator, a repressor or both, and exactly which genes it regulates (17–23). Similarly, while Foxp3-containing protein complexes can encompass both transcriptional co-activators and co-repressors, it is unknown which of these are important for direct target gene regulation (20, 21, 24, 25).
TREG cell functionality is extensively modulated by environmental stimuli, including antigen, co-stimulatory molecules, cytokines, chemokines, alarmins, hormones, and xenobiotics (26, 27). Transcription factors and co-factors whose activity or expression is induced by these stimuli can alter the TREG cell transcriptome and epigenome and direct cell migration, proliferation, and suppressive functions (28). Some of these factors can also physically interact with Foxp3 or bind to the same sites in the genome (17, 19–21, 24). This raises the possibility that the direct target genes regulated by Foxp3 are context-dependent, and that a differential requirement for Foxp3 may exist in TREG cells exposed to inflammatory environments, such as a tumor, compared to TREG cells residing in healthy organs.
Here, we developed a genetically modified mouse model that enables rapid chemically-induced degradation of Foxp3 protein in fluorescently-labeled TREG cells. Using this system, we found that Foxp3-expressing cells in lymphoid and non-lymphoid tissues of healthy mice were largely maintained and continued to suppress T cell expansion following the near-complete depletion of Foxp3 protein for 10 days. In contrast, a specialized subset of T-bet+CXCR3+ TREG cells that arises in settings of autoimmunity, viral infection, or cancer was continuously dependent on Foxp3 for its maintenance. Acute Foxp3 degradation revealed its function as an activation-dependent transcriptional repressor, capable of modulating responses to cytokine stimulation. This inflammation-dependent requirement for continuous Foxp3 activity enabled induction of a selective anti-tumor immune response upon systemic Foxp3 protein depletion, without causing deleterious T cell expansion in healthy organs.
Results
Chemically-induced Foxp3 protein degradation in mice
To study the continuous requirement for Foxp3 in mature TREG cells, we employed the auxin-inducible degron (AID) system, which leverages the Oryza sativa Tir1 adaptor protein (29). Tir1 mediates chemically-induced protein degradation by recruiting a Skp1-Cul1-F-box E3 ligase complex to an AID peptide-tagged target protein in response to the plant hormone auxin (indole-3-acetic-acid, IAA) (Fig. 1A). The application of the AID system in vivo has been limited by high levels of background degradation (30). A recently developed “AID2” system overcomes this limitation by introducing a phenylalanine-to-glycine point mutation in Tir1-F74G, which diminishes binding to IAA and instead enables binding to a synthetic high-affinity ligand, 5-phenol-indole-3-acetic-acid (5-Ph-IAA) (29), which we employ here.
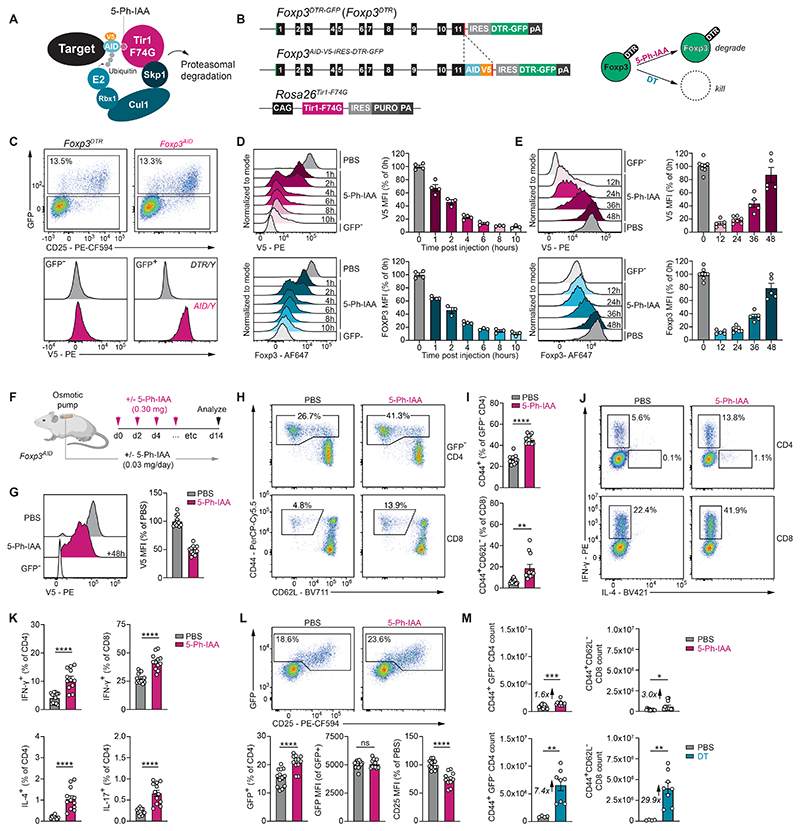
Fig. 1. Chemically-induced degradation of Foxp3.
(A) Schematic illustration of the AID2 system. (B) Schematic illustration of the Foxp3AID-V5-IRES-DTR-GFP and Rosa26Tir1-F74G alleles and their function. (C) Representative flow cytometry plots showing GFP fluorescence and V5 staining in the spleen. (D) Flow cytometry measurements of Foxp3 degradation kinetics following i.p. injection of 5-Ph-IAA. Geometric mean fluorescence intensity (MFI) of V5 or Foxp3 intracellular antibody staining in GFP+ TREG cells was normalized to the average of PBS-treated control samples after subtracting background fluorescence from the GFP- CD4 T cell population. Pooled data from two independent experiments a total of 3 to 4 mice per timepoint. (E) Foxp3 protein recovery after 5-Ph-IAA injection. Pooled data from three independent experiments with 5-8 mice per timepoint (F) Experimental setup. Osmotic pumps delivering ∼0.03 mg/day 5-Ph-IAA or PBS were implanted into male and female Foxp3AID mice. 0.3 mg 5-Ph-IAA or PBS was injected every 48 hours for 14 days. (G) Foxp3-V5 levels 48 hours after the final 5-Ph-IAA injection. (H-I) Flow cytometry analysis of CD44 and CD62L on splenic CD4 and CD8 T cells after 14 days of continuous 5-Ph-IAA treatment. (J-K) Cytokine production measured by intracellular staining and flow cytometry. (L) GFP and CD25 levels on splenic CD4 T cells following 14-day continuous Foxp3 protein depletion. (M) Total activated GFP- CD4 and CD8 T cell counts after 14 days of Foxp3 protein degradation (top) or 11 days after two consecutive daily injections of DT (bottom). Panels G-M show pooled data from three independent experiments with 12-13 mice per group (Foxp3 degradation) or pooled data from two independent experiments with 4-8 mice per group (TREG cell depletion). Error bars show mean with SEM. P values were calculated using unpaired two-tailed Student’s t-test. ns: P > 0.05, *: P ≤ 0.05, **: P ≤ 0.01, ***: P ≤ 0.001, ****: P ≤ 0.0001.
To enable auxin-inducible Foxp3 protein degradation, we generated a Foxp3AID-V5-IRES-DTR-GFP allele, expressing a C-terminal Foxp3-AID fusion protein, detectable via the V5 epitope tag (Fig. 1B). The inclusion of an IRES-GFP moiety in the allele enables continued visualization of TREG cells after Foxp3 protein degradation is complete, while the diphtheria toxin receptor (DTR) cassette enables inducible TREG cell ablation (3). These mice were bred to Rosa26Tir1-F74G mice, which constitutively express Tir1-F74G in all cells (30, 31). Hemizygous male Foxp3AID-V5-IRES-DTR-GFP/YRosa26Tir1-F74G/Tir1-F74G and homozygous female Foxp3AID-V5-IRES-DTR-GFP/AID-V5-IRES-DTR-GFPRosa26Tir1-F74G/Tir1-F74G animals, henceforth referred to as Foxp3AID mice, were healthy and showed no signs of spontaneous T cell activation, which could be effectively induced by diphtheria toxin (DT)-mediated TREG cell depletion (Fig. S1A-C). GFP+ TREG cells from Foxp3AID mice were uniformly V5+ (Fig. 1C). To define the kinetics of Foxp3 degradation, we injected Foxp3AID mice with 5-Ph-IAA at different timepoints prior to the isolation and analysis of splenic GFP+ TREG cells (Fig. S1D-E). Using intracellular antibody staining for either the V5 tag or an endogenous Foxp3 epitope, we found that approximately 90-95% of Foxp3 protein was degraded 10 hours after 5-Ph-IAA injection (Fig. 1D). Foxp3 protein gradually recovered to pre-treatment levels after approximately 48 hours (Fig 1E). Thus, the Foxp3-AID fusion protein is correctly expressed, functional, and rapidly degraded upon in vivo administration of 5-Ph-IAA.
Foxp3 protein is dispensable for short-term maintenance of steady-state TREG cells
High levels of Foxp3 protein are essential to establish immunological tolerance. Previous work has shown that a constitutive reduction in Foxp3 protein levels of approximately 80% caused by an mRNA-destabilizing reporter gene insertion in the 3’ UTR of the mouse Foxp3 gene was sufficient to induce aggressive early onset autoimmunity (32). Whether Foxp3 protein is continuously required to maintain TREG cell identity and suppress T cell activation in steady-state adult animals is less clear. To address this question, we induced continuous Foxp3 protein depletion by injecting Foxp3AID mice with 5-Ph-IAA every 48 hours for two weeks. To ensure protein degradation between injection timepoints, we also implanted a small osmotic pump continuously delivering a low dose of 5-Ph-IAA (Fig. 1F). GFP+ TREG cells isolated 48 hours after the final 5-Ph-IAA injection showed a 55% reduction in Foxp3 protein levels compared to PBS-treated control animals (Fig. 1G). Thus, we achieved a continuous reduction in Foxp3 protein levels fluctuating between 95% depletion and 55% depletion, measured 10 hours and 48 hours post-injection, respectively (Fig. 1D, F, G).
This sustained reduction was associated with an increase in the frequencies of activated CD44+ GFP- CD4 and CD8 T cells in the spleen and increased production of the pro-inflammatory cytokines IFN-γ, IL-4, and IL-17 (Fig. 1H-K). However, GFP+ CD4 T cell frequencies were not reduced and even slightly increased compared to PBS-treated controls (Fig. 1L). These cells showed reduced expression of the high-affinity interleukin-2 receptor (IL-2R) α-chain CD25 but maintained normal Foxp3 gene transcription, as measured by GFP intensity. These results therefore suggested that continuously high levels of Foxp3 protein were dispensable for sustained Foxp3 expression and TREG cell maintenance under steady-state conditions, but required for suppressive function. However, when we directly compared the effects of Foxp3 protein depletion to DT-induced TREG cell ablation, it became apparent that the latter caused much more dramatic effector CD4 and CD8 T cell expansion than the former (Fig. 1I, M, S1A-C). Thus, despite losing some of their activity, TREG cells depleted of Foxp3 protein were still capable of suppressing T cell expansion to a considerable degree. Consistently, these cells also maintained their expression of various TREG cell surface markers and suppressive molecules, including CD39, CD73, CTLA-4, GARP (LRRC32), and GITR (Fig. S1F-G). We therefore conclude that splenic TREG cells were not only maintained but also continued to exert some of their suppressive functions following Foxp3 protein depletion under steady-state conditions.
We hypothesized that it was the quiescent, non-proliferative state of splenic Treg cells that enabled them to tolerate the short-term depletion of their lineage-defining transcription factor. To examine this hypothesis, we analyzed the effects of Foxp3 protein degradation on non-lymphoid tissue TREG cells, which depend on T cell receptor (TCR) stimulation for their differentiation and migration into organs and are much more proliferative than their splenic counterparts (33–35). To ensure more complete depletion of Foxp3 protein in these experiments, we administered 5-Ph-IAA every 24 hours for 10 consecutive days before analyzing the vascular (i.v. anti-CD45+) and extravascular (i.v. anti-CD45-) compartments of the liver, lungs, and large intestine (Fig 2A-B, S2A-B). Consistent with an established role for Foxp3 in maintaining anergy (9), TREG cells across multiple different organs more highly expressed the proliferation marker Ki-67 in the absence of Foxp3 protein (Fig. 2C-D). However, although up to 70% of GFP+ non-lymphoid tissue TREG cells were Ki-67+, these cells were largely maintained in the absence of Foxp3, with a slight decrease observed only in the extravascular compartment of the liver (Fig. 2E). GFP levels were also largely unaffected by Foxp3 protein depletion, with minor reductions observed in the extravascular compartment of the liver and the large intestine lamina propria (Fig. 2F). In contrast, CD25 levels on TREG cells were uniformly reduced upon Foxp3 protein depletion (Fig. 2G, S2C). Thus, even highly proliferative non-lymphoid tissue TREG cells could persist for at least 10 days in the absence of Foxp3.
Fig. 2. Proliferative non-lymphoid tissue TREG cells persist following short-term Foxp3 depletion.
(A-B) Male and female Foxp3AID mice were injected with 5-Ph-IAA or PBS for 10 consecutive days. An eFluor450-labeled antibody against CD45 was injected intravenously (i.v.) 3 minutes before euthanizing the animals to distinguish vascular-associated (i.v. CD45+) and tissue-associated (i.v. CD45-) immune cells. Foxp3 (V5) protein levels were measured in GFP+ CD4 T cells from spleen, liver, lung, and large intestine lamina propria (LI). GFP- CD4 T cells are shown as a negative control. (C-D) Flow cytometry data showing Ki-67 staining on GFP+ CD4 T cells. (E) Frequency of GFP+ cells among CD4 T cells in indicated organs. (F) GFP geometric mean fluorescence intensity (MFI) among GFP+ CD4 T cells. (G) CD25 levels on GFP+ TREG cells, normalized to PBS-treated controls. (H) GFP- CD4 and CD8 T cell counts across different organs after 10 days of continuous Foxp3 protein depletion. Panels A-H show pooled data from 2 independent experiments with a total of 6-8 mice per group. (I) Foxp3AID mice were injected with diphtheria toxin (DT) on day 0, 1, and 6. GFP+ CD4 T cell frequencies and GFP- CD4 and CD8 T cell counts analyzed on days 10-12. Pooled data from 2 independent experiments with a total of 6 mice per group. Error bars show mean with SEM. P values were calculated using unpaired two-tailed Student’s t-test. *: P ≤ 0.05, **: P ≤ 0.01, ***: P ≤ 0.001, ****: P ≤ 0.0001.
To determine if these cells also maintained their suppressive activity, we compared the effects of 5-Ph-IAA-induced Foxp3 protein depletion and DT-induced TREG cell ablation on GFP- CD4 and CD8 T cell numbers. While the former had no significant effect (Fig. 2H), the latter induced dramatic T cell expansion in all organs (Fig. 2I). Thus, TREG cells across different tissues of steady-state animals continued to suppress rampant T cell expansion for at least 10 days in the near-complete absence of their lineage-defining transcription factor, Foxp3.
Foxp3 is essential for TREG cells responding to type 1 inflammation
The results of our protein degradation experiments were in apparent contrast with a previous study that reported a continuous requirement for Foxp3 in maintaining the identity and function of mature TREG cells (11). This study used retrovirally-expressed Cre recombinase to induce the deletion of a loxP-flanked Foxp3 allele in mature TREG cells before transferring these cells, together with conventional CD4 T cells, into lymphopenic recipients. TREG cells in this experiment downregulated the expression of Foxp3 and other TREG cell signature genes and lost their suppressive activity, leading to the conclusion that TREG cell maintenance is continuously dependent on Foxp3. We considered the possibility that this apparent discrepancy with our own results was attributable to the small amount of Foxp3 protein left after 5-Ph-IAA-induced protein degradation, compared to the complete absence of functional Foxp3 protein following the genetic deletion of Foxp3. Alternatively, because adoptively transferred TREG cells become highly activated in response to the lymphopenic environment and pro-inflammatory cytokines produced by the co-transferred conventional CD4 T cells, they may be more strongly dependent on Foxp3 than their resting counterparts residing in healthy organs of unperturbed mice.
To distinguish between these possibilities, we transferred 3x105 double-sorted (>99% pure) GFP+ TREG cells isolated from Foxp3AID mice together with equal numbers of congenically-marked naïve CD4 T cells into Rag2-/- mice before inducing Foxp3 protein degradation (Fig. 3A). In PBS-treated animals, we found that approximately 75% of Foxp3-sufficient TREG cells maintained GFP expression 19 days post-transfer (Fig. 3B-D). In contrast, and consistent with prior observations (11), depletion of Foxp3 protein from day 9 onwards led to a significant loss of GFP+ cells, with the remaining TREG cells showing reduced Foxp3 gene transcription and CD25 levels (Fig. 3B-D). Thus, Foxp3 protein degradation using the AID2 system could recapitulate a previously reported phenotype associated with inducible genetic Foxp3 deficiency. Our results therefore suggested that not all TREG cells are equally dependent on a continuously high level of Foxp3 protein and that a particularly strong requirement for Foxp3 may exist in TREG cells exposed to inflammatory environments.
Fig. 3. Acutely activated TREG cells require Foxp3.
(A) Experimental setup. (B) Representative CD45 staining on splenic CD4 T cells and summary data showing percentage of CD45.2+ CD4 T cells. (C-D) V5, GFP, and CD25 levels on splenic CD45.2+ CD4 T cell populations. Panels A-D show pooled data from two independent experiments with 6-7 mice per group. (E) Experimental setup. (F) Splenic effector CD4 and CD8 T cell frequencies on day 12. Pooled data from 4 independent experiments with 14-20 mice per group (G-H) GFP+ and V5+ cell frequencies among splenic CD4 T cells and GFP MFI on day 12. Panel H shows pooled data from three independent experiments with 13-16 mice per group. (I-J) GFP and CXCR3 staining on CD4 T cells on day 12. Pooled data from two independent experiments with a total of 8-9 mice per group. (K) Experimental setup. Foxp3AID mice were infected with 2x105 pfu LCMV Armstrong (LCMV Arm.). Foxp3 protein was depleted from day 5 onwards through daily injections of 5-Ph-IAA. (L-M) GFP and CXCR3 staining on splenic CD4 T cells on day 12 post-infection. Pooled data from two independent experiments with 7-10 mice per group. Error bars show mean with SEM. P values were calculated using unpaired two-tailed Student’s t-tests (D, H) or one-way ANOVA with Tukey’s multiple comparisons test (F, J, M). ns: P > 0.05, *: P ≤ 0.05, **: P ≤ 0.01, ***: P ≤ 0.001, ****: P ≤ 0.0001.
To determine if TREG cells in other inflammatory settings were similarly sensitive to Foxp3 protein depletion, we first analyzed TREG cells exposed to systemic autoimmune inflammation. The DT-induced depletion of DTR-expressing TREG cells causes the rapid expansion and activation of self-reactive TCON cells producing a variety of cytokines (5). Upon cessation of DT treatment, newly generated TREG cells expanding in this inflammatory environment acquire transiently enhanced suppressive functions and dampen autoimmunity until homeostasis is restored (17, 36). To induce systemic autoimmunity, we injected Foxp3AID mice with two consecutive daily doses of DT. Starting from day 7, when TREG cell frequencies have already recovered to a considerable degree (3), we depleted Foxp3 protein by daily injection of 5-Ph-IAA (Fig. 3E). Analysis of splenic T cell populations on day 12 revealed dramatic expansion of CD44+ effector CD4 and CD8 T cells relative to healthy control animals (Fig. 3F). Foxp3 degradation under these conditions caused a reduction in the frequency of GFP+ TREG cells, with the remaining population showing lower Foxp3 gene transcription as measured by GFP levels (Fig. 3G-H). Thus, TREG cells responding to DT-induced autoimmune inflammation were continuously dependent on Foxp3. The observation that most, but not all GFP+ cells were lost in this setting also raised the possibility that certain TREG cell subpopulations were more strongly affected by Foxp3 protein depletion than others. A specialized TREG cell subset expressing the transcription factor T-bet and the chemokine receptor CXCR3 has been shown to arise under conditions of type 1 inflammation in response to the cytokine IFN-#x03B3; (37–39). We found that these TH1-like TREG cells, identified by either T-bet or CXCR3 staining, accounted for approximately 70% of TREG cells responding to DT-induced autoimmune inflammation and that this subset was selectively lost following Foxp3 depletion (Fig. 3I-J, S3A-C).
We next asked if TH1-like TREG cells generated during an acute viral infection were similarly dependent on Foxp3. To this end, we infected mice with the Armstrong strain of lymphocytic choriomeningitis virus (LCMV), which induces a potent TH1 and CD8 T cell response and the production of IFN-γ. As previously reported, approximately 50% of TREG cells in the spleens of LCMV-infected mice expressed CXCR3 on day 12 post-infection (Fig. 3K-M) (40, 41). Depletion of Foxp3 protein from days 5 to 12 caused a selective loss of GFP+ CXCR3+ TREG cells, consistent with the notion that Foxp3 is continuously required to sustain this subset of inflammation-induced TREG cells. Thus, in contrast to resting TREG cells residing in healthy organs of unperturbed mice, TREG cells actively responding to autoimmune inflammation and viral infection are continuously dependent on high levels of Foxp3 protein.
Immediate gene-regulatory functions of Foxp3
We next sought to define the immediate transcriptional targets of Foxp3 under steady-state and inflammatory conditions. To this end, we performed total RNA-seq analysis on GFP+ CD4 T cells isolated from pooled secondary lymphoid tissues 6 hours after injection of 5-Ph-IAA (Fig. 4A). To induce acute inflammation, animals were transiently treated with DT 11 days prior to Foxp3 protein depletion (Fig. 4A). Intronic reads from the resulting datasets were used as a proxy for nascent transcripts as previously described (42); however, because many genes lack introns or may be co-transcriptionally spliced, we also separately analyzed exonic reads. Acute Foxp3 protein depletion only minimally affected the transcriptome of steady-state TREG cells (Fig. 4B). In contrast, we identified many differentially expressed genes in acutely activated TREG cells, including transcription factors (e.g. Lef1, Rora, Nr4a1, Nr4a3, and Id2), signaling molecules (e.g. Rasa3, Dock2, and Rictor), and cell surface proteins (e.g. Il18r1, Il21r, and Cd40lg) (Fig. 4C-D, Data File S1). Transcriptional features characteristic of activated TREG cells such as the downregulation of Bach2 and upregulation of Prdm1, Gzmb, and the proliferation marker Mki67 were unaffected by acute Foxp3 protein depletion (Fig. 4E).
Fig. 4. Immediate transcriptional functions of Foxp3 in activated TREG cells.
(A) Experimental setup. (B-C) Differential gene expression analysis in steady-state (left) or activated (right) GFP+ CD4 T cells from pooled secondary lymphoid tissues of animals treated with 5-Ph-IAA or PBS for 6 hours. Analysis of intronic (top) and exonic (bottom) reads. Genes with adjusted P <0.01 are highlighted. RNA-sequencing samples were pooled from 7 independent sorts, with a total of 6-10 biological replicates per condition. (D-E) Expression of selected genes in transcripts per million (TPM). (F) Each cell shows the number of upregulated (top) and downregulated (bottom) genes at a given P value and fold change (FC) cutoff. Cells are colored according to the ratio of up- and downregulated genes. (G) Gene Ontology (GO) term enrichment analysis for differentially expressed (DE) genes (top). Each dot represents a GO term. Dot size reflects statistical significance of the enrichment. Upregulated genes associated with GO term lymphocyte activation (bottom). (H) DE genes identified in activated TREG cells were linked to Foxp3 binding sites identified by ChIP-seq, CUT&RUN, the intersection of peaks identified by both techniques or the intersection of the top 2000 most highly bound sites identified by both techniques using public data from GSE154680. (I) Model for activation-dependent gene regulation by Foxp3. (J) Differential gene expression between activated TREG (aTreg) cells and resting TREG (rTreg) cells, measured in the presence (PBS) or absence (5-Ph-IAA) of Foxp3 protein. Analysis was performed for three gene sets: all expressed genes (all genes), genes upregulated after Foxp3 degradation in activated TREG cells (Up genes) and genes downregulated after Foxp3 degradation in activated TREG cells (Down genes). P values from hypergeometric test (H) or one-sided Kolmogorov-Smirnov test (J).
Although the immediate transcriptional targets of Foxp3 included both repressed and activated genes, we found that repression was much more pronounced at various fold-change and P value cutoffs (Fig. 4F). Moreover, while genes upregulated after Foxp3 degradation were strongly enriched for genes involved in T cell activation and differentiation, genes downregulated upon Foxp3 depletion were not (Fig. 4G). Finally, we found that upregulated genes were more strongly enriched for Foxp3 binding, as measured by ChIP-seq and CUT&RUN analysis (19) (Fig. 4H). Together, these observations suggest that Foxp3 acts predominantly as a direct transcriptional repressor in activated TREG cells.
While very few individual genes were significantly affected by Foxp3 degradation in steady-state TREG cells, we found that these cells to a lesser degree also showed significant differential expression of the collective Foxp3-dependent gene set identified in activated TREG cells (Fig. S4A). Moreover, when steady-state TREG cells were re-analyzed independently of their activated counterparts, more statistically significant differential gene expression could be detected; however, the magnitude of these changes was minimal (Fig. S4B-D). Notable genes included Tcf7, Lef1, and Foxp3 itself, which was slightly de-repressed upon Foxp3 protein depletion (Fig. S4D). We first considered the possibility that protein degradation was simply more efficient in activated versus steady-state TREG cells. However, a time course analysis revealed only minor differences in Foxp3 degradation kinetics. The largest difference was observed 2-hours after 5-Ph-IAA injection, at which time activated TREG cells contained approximately 25% less Foxp3 protein than resting TREG cells (Fig. S4E). At the timepoint of the analysis, 6-hours after 5-Ph-IAA injection, no statistically significant difference in Foxp3 protein levels could be detected between steady-state and activated TREG cells.
To further account for the potentially confounding effects of these small differences in degradation kinetics, we analyzed how the genes differentially expressed after acute Foxp3 protein degradation were affected by constitutive genetic Foxp3 deficiency at different stages of TREG cell development. Here, we took advantage of a previously generated dataset comparing wildtype TREG to “reporter-null” cells isolated from female Foxp3GFPKO/WT mice (19). These animals remain healthy due to random X-chromosome inactivation and expression of the wildtype Foxp3 allele in half of their TREG cells. Wildtype TREG and GFPKO cells were compared at the CD62L+ “naïve” stage and the CD62L- “effector” stage, induced by T cell receptor (TCR) stimulation (43) (Fig. S5A-C). Genes that were differentially expressed following protein degradation in activated TREG cells were also differentially expressed in “effector” TREG versus GFPKO cells, but not in their “naïve” counterparts (Fig. S5D-F). Thus, complete genetic Foxp3 deficiency in “naïve” TREG cells was insufficient to recapitulate the transcriptional changes resulting from acute Foxp3 protein degradation in activated TREG cells. We therefore conclude that Foxp3 has bona fide activation-dependent gene-regulatory functions that are acutely required to maintain TREG cell transcriptional identity under inflammatory conditions.
We considered several mechanisms through which an inflammation-dependent requirement for Foxp3 might arise. First, Foxp3 might bind to different genes under inflammatory conditions and alter the expression of these new targets (20). Second, Foxp3 may recruit activation-dependent co-factors whose expression or activity is induced under inflammatory conditions (17). Finally, Foxp3-dependent repression of its constitutively bound targets may counteract inflammation-induced transcriptional activators that are absent in steady-state TREG cells (Fig. 4I). Arguing against the first model, we previously found that genome-wide Foxp3 binding patterns are largely unchanged under conditions of DT-induced autoimmune inflammation (17). Moreover, both the first and second models would predict that directly repressed Foxp3 target genes are downregulated in Foxp3-sufficient activated versus resting TREG cells; however, we did not find this to be the case (Fig. 4J). Rather, expression of these genes was increased in activated Foxp3-depleted TREG cells compared to all other conditions, which is most consistent with the third model (Fig. 4I, J). Thus, while other modes of regulation cannot be ruled out, our results suggest that the activation-dependent function of Foxp3 in maintaining TREG cell transcriptional identity arises mostly from its ability to counteract inflammation-induced transcriptional activators.
Cytokines dictate the effect of Foxp3 depletion
Because TREG cells responding to autoimmune inflammation or infection in vivo are exposed to a complex mix of signals, we next sought to define the requirements for Foxp3 in TREG cells responding to defined cytokine stimuli. The selective effects of Foxp3 protein depletion on the maintenance of T-bet+CXCR3+ TREG cells suggested a role in modulating the response to TH1 polarizing cytokines. This was further supported by an enrichment for genes involved in the regulation of type II IFN production and response to interleukin-12 (IL-12) among the directly repressed transcriptional targets of Foxp3, including Ifngr1, Jak2, Stat4, and Tbx21 (Fig. 4G, S6A-B). Moreover, using a previously published single-cell RNA-seq compendium of immune cell responses to 86 different cytokines (44), we found that the expression of genes directly repressed by Foxp3 was induced in TCON cells responding to IL-12 administration (Fig. S6C). Thus, Foxp3 directly represses genes involved in TH1 responses.
The development of naïve CD4 T cells into TH1 cells is initiated by TCR stimulation and IFN-γ-dependent activation of STAT1, which induces expression of the transcription factor T-bet and upregulation of the IL-12 receptor component IL-12Rβ2 (45, 46). IL-12-dependent activation of STAT4 then synergizes with T-bet to induce expression of the TH1 cell signature cytokine IFN-γ (47). TREG cells are known to be less responsive to TH1 polarizing cytokines than TCON cells (38). This is thought to prevent TREG cells from producing IFN-γ, while still enabling the expression of T-bet and the downstream chemokine receptor CXCR3, necessary for migration to sites of type I inflammation. The role of Foxp3 in this “abortive” TH1 cell differentiation process is still unknown. We therefore investigated if Foxp3 was acutely required to limit IFN-γ production by TREG cells stimulated under TH1 polarizing conditions in vitro (Fig. 5A). Foxp3-depleted TREG cells activated in the presence of TH1 polarizing cytokines expressed higher levels of T-bet and more frequently produced IFN-γ than either Foxp3-sufficient TREG cells activated under the same conditions or Foxp3-depleted TREG cells activated in the absence of polarizing cytokines (Fig. 5B-D). Thus, Foxp3 directly represses multiple genes in the TH1 cell differentiation pathway and prevents IFN-γ production by TREG cells upon exposure to type 1 inflammation.
Fig. 5. Cytokines dictate the effects of Foxp3 depletion.
(A) Experimental setup. FACS-sorted GFP+ TREG or GFP- naïve TCON CD4 T cells were isolated from pooled secondary lymphoid tissues of Foxp3AID mice and activated in vitro on anti-CD3-coated plates with anti-CD28 and IL-2. 5-Ph-IAA and TH1-polarizing cytokines (IFN-γ and IL-12) were added to the cultures 48 hours post-activation, as indicated. (B-D) Flow cytometry analysis of GFP, Foxp3, T-bet, and IFN-γ levels. TCON cells activated in the presence or absence of TH1-polarizing cytokines are gated on GFP- CD4 T cells. TREG cells are gated on GFP+ CD4 T cells. Cytokine production was measured after 3-hours of restimulation with PMA/Ionomycin/Brefeldin A/Monensin. T-bet levels are normalized to the positive control population (TCON + IFN-γ/IL-12) after subtracting background fluorescence. Pooled data from 4 independent experiments with a total of 3-6 biological replicates per group. (E) Experimental design. (F) GFP and CD25 levels on splenic CD4 T cells of the indicated treatment groups. (G-J) Summary data showing Foxp3 (V5) and CD25 levels on splenic GFP+ CD4 T cells and the percentage of GFP+ cells among CD4 T cells. V5 levels are normalized to the average of their respective negative control groups that did not receive 5-Ph-IAA. Pooled data from three independent experiments with a total of 6-9 mice per group. Error bars show mean with SEM. P values were calculated using one-way ANOVA with Tukey’s multiple comparisons test. ns: P > 0.05, *: P ≤ 0.05, **: P ≤ 0.01, ***: P ≤ 0.001, ****: P ≤ 0.0001.
Because of its central role in TREG cell maintenance, we next analyzed how responsiveness to IL-2 was impacted by Foxp3 depletion. Both TREG cells and activated TCON cells rely on IL-2 as a critical growth factor to support their proliferation and survival (48). Naïve T cell activation via the TCR and co-stimulatory receptors triggers IL-2 production and expression of CD25, which pairs with the IL-2Rβ-chain and common γ-chain to form the high affinity IL-2 receptor (IL-2R). IL-2 then acts in an autocrine or paracrine manner to activate the IL-2R and downstream STAT5 signaling, which induces expression of genes associated with T cell proliferation and survival as well as further upregulation of CD25 (49). Because TREG cells are incapable of producing IL-2, they rely solely on IL-2 produced by TCON cells. Moreover, IL-2-induced STAT5 acts directly on regulatory elements in the Foxp3 locus to maintain its transcriptional activity (50, 51). Finally, because TREG cells express constitutively high levels of CD25, they can effectively outcompete TCON cells for IL-2 and suppress their expansion (52, 53).
To define the direct gene-regulatory functions of Foxp3 in IL-2-stimulated TREG cells, we injected mice with IL-2/anti-IL-2 (JES6-1) monoclonal antibody complexes that selectively engage and expand TREG, but not TCON cells (54, 55), before acutely degrading Foxp3 protein for 6 hours (Fig. S7A). IL-2 stimulation induced transcriptional changes in approximately 2,500 genes, including those encoding the IL-2Rα- and β-chains and the anti-apoptotic protein Bcl-2 (Fig. S7B). Compared to TREG cells responding to autoimmune inflammation, Foxp3 degradation in IL-2-stimulated TREG cells affected a relatively small number of genes (Fig. S7C-D). Again, Foxp3 acted primarily as a transcriptional repressor. Among its directly regulated targets were Pde3b, whose downregulation contributes to the metabolic fitness of TREG cells (9); Tcf7, encoding a chromatin remodeling transcription factor with widespread effects on the epigenetic landscape of TREG and TCON cells (19); Themis, a modulator of TCR signaling involved in thymocyte selection (56); and Il7r, another STAT5-activating cytokine receptor whose downregulation underlies the selective dependence of TREG cells on IL-2. Finally, Foxp3 repressed Cdk8, encoding a negative regulator of STAT5 activity, inhibition of which is sufficient to induce TREG cell differentiation (57) (Fig. S7E).
To test how Foxp3 depletion affected TREG cell responsiveness to IL-2, we injected 5-Ph-IAA for 5 consecutive days in mice treated with either activating IL-2/anti-IL-2 (JES6-1) antibody complexes, or a distinct anti-IL-2 antibody (S4B6) that blocks the binding of IL-2 to CD25 (Fig. 5E). IL-2/anti-IL-2 complexes induced the expansion of GFP+ TREG cells, while IL-2 neutralization induced their contraction, neither of which were significantly impacted by Foxp3 protein depletion (Fig. 5F-I). However, we found that the IL-2-induced upregulation of CD25 on TREG cells was entirely Foxp3 dependent (Fig. 5J). Conversely, when IL-2 was neutralized, Foxp3 protein degradation no longer had any effect on CD25 levels. Importantly, TREG cells continued to express higher levels of CD25 thanGFP- TCON cells even under conditions of IL-2 neutralization and Foxp3 protein depletion (Fig. 5F). This observation is consistent with previous work showing that Foxp3GFPKO “reporter-null” cells express CD25 levels lower than wildtype TREG cells, but higher than resting TCON cells (9). Thus, while higher baseline expression of CD25 by TREG cells is a Foxp3-independent feature, the upregulation of CD25 in response to IL-2 is Foxp3-dependent. We therefore conclude that Foxp3 regulates CD25 levels in an IL-2-dependent manner.
Based on our observations, we hypothesized that the Foxp3-dependent regulation of CD25 may be selectively important during an ongoing immune response to ensure that TREG cells appropriately scale their CD25 levels and can continue to outcompete activated TCON cells for IL-2. To test this, we analyzed the effects of Foxp3 protein depletion on CD25 expression by TREG and TCON cells in the context of autoimmune inflammation (Fig. S7I). The GFP- CD4 and CD8 T cells that expanded after transient TREG cell depletion more frequently expressed CD25 than their steady-state counterparts, which was further enhanced when Foxp3 protein was depleted for the final 5 days of the experiment (Fig. S7F-H). A comparison of CD25 levels between TREG cells and CD25+ TCON cells revealed that the former expressed higher levels of CD25 in steady-state or DT-treated animals (Fig. S7I-J). In contrast, in animals treated with DT and 5-Ph-IAA, TCON cells expressed higher levels of CD25 than TREG cells. These results are consistent with the notion that Foxp3-dependent feedback regulation of CD25 is critical to enable effective competition for IL-2 with activated TCON cells (Fig. S7K).
Together, our data show that the effects of Foxp3 protein depletion are dictated by cytokine exposure. Foxp3-dependent suppression of IFN-γ production occurs only in the presence of TH1 polarizing cytokines, while Foxp3-dependent regulation of CD25 occurs when IL-2 is present. These two examples thus illustrate how an inflammation-dependent requirement for continuous Foxp3 activity might arise.
Systemic Foxp3 protein depletion induces selective anti-tumor immune response
Similar to TREG cells exposed to other inflammatory settings, tumor-associated TREG cells become highly activated in response to stimulatory cues in their microenvironment (17, 58). TH1-like TREG cells accumulate in tumors and tumor-draining lymph nodes and interact with CD8 T cells and CXCL9-producing type 1 dendritic cells (DC1s) to suppress anti-tumor immunity through a variety of different mechanisms, including inhibition of antigen cross-presentation, downregulation of costimulatory molecules, and suppression of pro-inflammatory cytokine production (59–61). We found that nearly all TREG cells infiltrating subcutaneously implanted MC38 colorectal tumors were T-bet+ (Fig. S8A-B). Based on our observations in the autoimmunity and viral infection models, we hypothesized that systemic depletion of Foxp3 protein would selectively interfere with these tumor-associated TREG cells, while leaving TREG cells elsewhere in the body relatively unaffected. A single intraperitoneal injection of 5-Ph-IAA was sufficient to degrade approximately 75% of Foxp3 protein in GFP+ tumor-infiltrating TREG cells,15 hours post-injection (Fig. S9A). Daily treatments with 5-Ph-IAA initiated on day 3 after tumor implantation caused the expansion of tumor-infiltrating effector T cells (Fig. S9B-E) and effectively inhibited tumor growth to a similar degree as complete DT-induced TREG cell ablation (Fig. 6A-C). Initiation of 5-Ph-IAA treatment on day 7 also inhibited the growth of established tumors and prolonged survival (Fig. S9F). Conversely, withdrawal from 5-Ph-IAA led to tumor re-growth within approximately one to two weeks (Fig. S9G). Thus, Foxp3 protein depletion was as effective as TREG cell ablation at inhibiting tumor growth. However, while TREG cell ablation induced splenomegaly and systemic T cell expansion, Foxp3 protein depletion did not (Fig. 6D, 2H-I). This was consistent with a selective reduction in GFP+ TREG cell frequencies in tumors, but not secondary lymphoid tissues of 5-Ph-IAA-treated mice (Fig. 6E-F, S9H). CD25 levels were reduced on TREG cells across all tissues, but most dramatically within the tumor. Thus, Foxp3 protein depletion selectively affects highly activated tumor-infiltrating TREG cells, while leaving TREG cells in healthy organs relatively unperturbed.
Fig. 6. Short-term Foxp3 depletion drives localized anti-tumor immunity.
A) Experimental setup. 1x106 MC38 tumor cells were subcutaneously (s.c.) injected into the right flank of Foxp3AID mice, which were subsequently treated with PBS, DT, or 5-Ph-IAA as indicated. Animals were analyzed on day 12 (experiment 2) or day 13 (experiment 1), when tumors from PBS-treated animals reached a critical size. B) Tumor volume measurements at indicated timepoints. C) Tumor weight at the end of the experiment. D) Spleen weight and effector T cell counts in the spleen at the end of the experiment. E-F) GFP and CD25 levels on CD4 T cells from the spleen, non-draining brachial and axillary lymph nodes (ndLN), draining brachial and axillary lymph nodes (dLN), or the tumor. G) Effector CD4 and CD8 T cell counts in the indicated organs. H-I) CD25 and Ki-67 staining on effector CD4 and CD8 T cells from the indicated organs. Pooled data from two independent experiments with 10-12 mice per experimental group. P values from one-way ANOVA with Tukey’s multiple comparisons test (C-D), or two-tailed unpaired (F) or paired (G, I) Student’s t-tests. ns: P > 0.05, *: P ≤ 0.05, **: P ≤ 0.01, ***: P ≤ 0.001, ****: P ≤ 0.0001.
To confirm the localized effect of Foxp3 protein depletion, we next compared the tumor-draining and non-draining lymph nodes of mice within the same treatment groups (Fig. 6G). Tumor-bearing animals treated with PBS showed low CD4 and CD8 effector T cell counts in both the draining and non-draining lymph nodes, while TREG cell-depleted mice showed dramatic, but non-specific T cell expansion at both sites. In contrast, Foxp3 protein depletion caused a preferential expansion of effector CD4 and CD8 T cells in the tumor-draining, but not the non-draining lymph node. Further analysis of these cells revealed
an expanded population of proliferative Ki-67+CD25+ CD8 T cells in the tumor-draining lymph node of 5-Ph-IAA-treated mice that was largely absent from non-draining lymph nodes, or from PBS-treated controls (Fig. 6H-I). Thus, short-term systemic depletion of Foxp3 protein induces a localized anti-tumor immune response without causing significant T cell expansion or loss of GFP+ TREG cells elsewhere in the body. Together, our results identify an inflammation-dependent requirement for continuous Foxp3 activity that can be exploited to selectively target tumor-infiltrating TREG cells.
Discussion
Despite extensive characterization of Foxp3-deficient mice under various conditions, the functional requirements for Foxp3 in mature TREG cells have remained poorly defined. Using the AID2 system in combination with a bicistronic Foxp3 reporter, we found that high levels of Foxp3 protein were largely dispensable for the short-term maintenance of Foxp3-expressing cells under steady-state conditions. Foxp3-depleted TREG cells also continued to restrain TCON cell expansion to a considerable degree. Conversely, TREG cells that were activated in response to autoimmune-inflammation, viral infection, cancer, or adoptive transfer into lymphopenic hosts were acutely dependent on Foxp3 for their maintenance. Specifically, a subset of T-bet+CXCR3+ TREG cells that arises in settings of type 1 inflammation was lost following Foxp3 protein depletion. Because TREG cells that died or shut down Foxp3 transcription can no longer be identified using our model, the exact fate of these cells is still unclear.
Our observations also shed new light on the direct gene regulatory functions of Foxp3, which in the past have been difficult to distinguish from indirect secondary consequences of constitutive Foxp3 deficiency. We found that Foxp3 controls gene expression in an activation-dependent manner and acts predominantly as a transcriptional repressor by counteracting inflammation-induced transcriptional activators. Although not directly supported by our data, other activation-dependent modes of gene regulation, such as re-localization of Foxp3 to different target genes or recruitment of inflammation-induced co-factors may also contribute (17, 20). Importantly, because some of the proteins encoded by Foxp3-regulated genes can still undergo a degree of turnover within the 6-hour 5-Ph-IAA treatment period, we cannot fully exclude the possibility that minor secondary transcriptional changes occur even within this short time frame (62). While our data suggest that Foxp3 acts predominantly as a transcriptional repressor, a direct role for Foxp3 in activating gene expression cannot be ruled out. Moreover, certain modes of gene repression, such as DNA methylation or polycomb-mediated silencing may take more than 6 hours to reverse and could therefore be missed in our analysis. Among the genes acutely repressed by Foxp3 were Tcf7, a chromatin-remodeling transcription factor whose downregulation contributes indirectly to shaping the TREG cell accessible chromatin landscape (19); Cdk8, a negative regulator of STAT5 activity, inhibition of which is sufficient to induce TREG cell differentiation (57); the mammalian target of rapamycin (mTOR) complex 2 (mTORC2) adapter protein Rictor, genetic deletion of which can partially rescue the fitness and suppressive function of Foxp3 deficient “reporter-null” cells (63); and the transcriptional regulator Id2, overexpression of which is sufficient to cause loss of Foxp3 expression under inflammatory conditions (64). Thus, inhibition or genetic deletion of genes directly repressed by Foxp3 can induce TREG cell differentiation or partially rescue Foxp3 deficiency, while their overexpression can drive TREG cell instability.
Our work suggests that Foxp3 controls distinct facets of TREG cell biology depending on exposure to specific external stimuli. Foxp3 directly repressed genes involved in the regulation of IFN-γ production and the response to IL-12. Accordingly, Foxp3-depleted TREG cells exposed to TH1-polarizing cytokines in vitro expressed increased levels of T-bet and began to produce IFN-γ. Another cytokine that modulated the activity of Foxp3 was IL-2. We found that the Foxp3-dependent regulation of CD25 was induced by exposure to IL-2 and abrogated upon IL-2 neutralization. Thus, the effects of Foxp3 protein depletion are determined by the cytokine environment. It is likely that exposure to additional signals such as TCR stimulation, alarmins, glucocorticoids, prostaglandins, and various cytokines and chemokines influence the activity and cellular requirements for Foxp3 in distinct ways. We speculate that the loss of Foxp3-depleted Treg cells in settings of autoimmune inflammation, viral infection, or cancer may result from their exposure to a complex combination of these stimuli.
While systemic Foxp3 degradation might have been expected to cause catastrophic autoimmunity, we find that it rather specifically augments anti-tumor T cell responses. This effect was associated with a selective loss of GFP+ TREG cells from tumors, but not from healthy organs. It is possible that prolonged depletion of Foxp3 protein for multiple weeks or months will eventually cause more severe autoimmunity; however, this is likely to be reversible when Foxp3-transcribing cells are maintained. In support of this notion, a previous report demonstrated that the restoration of Foxp3 protein levels in mice expressing a Foxp3 reporter-null allele could effectively rescue TREG cell suppressive activity and cure a pre-existing autoimmune phenotype (65). Thus, transient Foxp3 protein depletion in adult animals is unlikely to have long-term deleterious consequences.
Consistent with the relatively modest effects of Foxp3 protein depletion in steady-state adult mice, human TREG cells subject to acute genetic FOXP3 deletion were shown to retain a degree of in vitro suppressive activity for at least two weeks (66). Together, these observations raise the question as to why constitutive genetic Foxp3 deficiency causes such severe and early-onset disease. Based on the results of our adoptive transfer experiments, we speculate that one factor may be that a strict requirement for Foxp3 exists in neonates, when the first cohorts of newly generated T cells undergo a phase of lymphopenia-driven activation (67). Together, our findings shed new light on the cellular and molecular functions of Foxp3 and provide a rationale for direct therapeutic targeting of Foxp3 in cancer.
Materials and Methods
Study design
The goal of the study was to understand the continuous requirement for high levels of Foxp3 protein in TREG cells exposed to type 1 inflammation, in settings of autoimmunity, viral infection, and cancer. To this end, we developed a Foxp3AID-V5-IRES-DTR-GFP allele that enables acute Foxp3 protein depletion, visualization and isolation of Foxp3-depleted TREG cells, and inducible ablation of Foxp3-expressing cells. The effects of Foxp3 protein depletion under various conditions were analyzed using flow cytometry and RNA-sequencing.
Mice
Animals were housed at the IMP/IMBA shared animal facility under standard specific pathogen-free (SPF) conditions at a room temperature of 22 °C and 55% humidity on a 14-hour light/10-hour dark cycle and unrestricted access to food and water. Mice were euthanized by carbon dioxide inhalation or cervical dislocation. All experimental and control animals were age-matched and included both male and female mice between 6-14 weeks of age. All animal experiments were carried out under valid project licenses approved by the Austrian Veterinary Authorities.
The Foxp3AID-V5-IRES-DTR-GFP (Foxp3AID) and Rosa26Tir1-F74G alleles were generated by CRISPR/Cas9-mediated genome editing in zygotes using the EASI-CRISPR approach (68). To generate the Foxp3AID allele, a single-stranded DNA (ssDNA) repair template encoding a glycine-serine linker, the minimal AID polypeptide (69), and a V5 epitope tag, flanked by ∼100 bp sequences homologous to the genomic regions immediately upstream and downstream of the Foxp3 stop codon was obtained from integrated DNA technologies (IDT). The repair template was injected together with recombinant Cas9 protein (IDT) and an sgRNA overlapping the Foxp3 stop codon (IDT) into Foxp3IRES-DTR-GFP mouse zygotes (3). Correct targeting was confirmed by Sanger sequencing PCR products amplified using primers outside of the 3’ and 5’ homology arms. The Rosa26Tir1-F74G allele was generated by injecting a 200 bp repair template together with Cas9 protein and a sgRNA targeting codon 73 of Tir1 into Rosa26Tir1/Tir1 zygotes (30, 31). Rag2tm1Fwa (Rag2-/-) mice and Ptprca (CD45.1) mice were bred in-house.
Diphtheria toxin, 5-Ph-IAA, and antibody administration
DT was obtained from List Biological Laboratories (cat #150) and dissolved in PBS. Animals were injected intraperitoneally with 40μg/kg per dose. 5-Phenol-indole-3-acetic acid (5-Ph-IAA) was purchased from BioAcademia (cat #30-003-10), dissolved in PBS and injected at 0.3mg per dose via intraperitoneal injection. For continuous 5-Ph-IAA administration, 200μl Alzet osmotic pumps (cat #2002) were filled with 5-Ph-IAA or PBS solution according to manufacturer’s instructions and implanted subcutaneously. IL-2/anti-IL-2 treatments were performed as previously described (55). Briefly, 1μg recombinant IL-2 (Pepro Tech, 212-12-250UG) was pre-incubated with 5μg JES6-1 antibody (BioXcell, BE0043) for 30 min at 37°C and intraperitoneally injected into mice on three consecutive days before analysis on day five. For RNA-seq analysis of IL-2-expanded TREG cells, mice were injected with IL-2/anti-IL-2 complexes on days 0 to 3. Mice were injected again with IL-2/anti-IL-2 complexes, with or without addition of 5-Ph-IAA, 6-hours before analysis on day 5. To discriminate vascular from tissue lymphocytes, we intravenously injected 2 μg of anti-CD45.2 (104, Invitrogen, 48-0454-82) in 150 μL of PBS three minutes before tissue harvesting, as previously described (70).
Antibodies
The following antibodies were used for flow cytometry, with clones, venders, catalog numbers and dilutions as indicated: anti-CD25 (PC61, BD, 562694, 1:400), anti-CD39 (Duha59, BioLegend, 143812, 1:400), anti-CD4 (GK1.5, BioLegend, 100449, 1:400), anti-CD44 (IM7, Thermo Fisher Scientific, 45-0441-82, 1:400), anti-CD45.1 (A20, Cytek Biosciences, 60-0453-U100, 1:400), anti-CD45.2 (104, Invitrogen, 48-0454-82, 1:400), anti-CD5 (53-7.3, BD Biosciences, 612809, 1:400), anti-CD62L (MEL-14, BioLegend, 104445, 1:400), anti-CD73 (TY/11.8, BioLegend, 127206, 1:400), anti-CD8α (53-6.7, BioLegend, 100744, 1:400), anti-CD90.2 (30-H12, Cytek Biosciences, 60-0903-U100, 1:1000), Anti-CXCR3/CD183 (CXCR3-173, Biolegend, 126521, 1:200), anti-CTLA-4 (UC10-4B9, BioLegend, 106316, 1:200), anti-FOXP3 (150D/E4, BioLegend, B320014, 1:200), anti-GARP (F011-5, BioLegend, 142904, 1:400), anti-GITR (DTA-1, BioLegend, 126312, 1:400), anti-IFN-γ (50-7311, Cytek Biosciences, 50-7311-U100, 1:400), anti-IL-4 BV421 (11B11, BioLegend, 504127, 1:400), anti-T-bet (4B10, Biolegend, 644824, 1:400), anti-TCRβ (H57-597, BioLegend, 109249), Anti-TNF-α (MP6-XT22, Biolegend, 506314, 1:800), anti-V5 (TCM5, Thermo Fisher Scientific, 12-6796-42, 1:400).
Lymphocyte isolation
For cell isolation from spleens, lymph nodes and livers, organs were collected in ice-cold FACS buffer (PBS with 2mM EDTA and 1% fetal calf serum) and meshed through a 100μm strainer (Corning, cat# 07-201-432) using the back of a syringe plunger. Erythrocytes were lysed using ACK lysis buffer (Thermo Fisher Scientific, cat# A1049201).
For isolation of immune cells from intestinal tissues, organs were incubated with DTT/EDTA buffer (PBS with 2mM L-glutamine, 10mM HEPES, 1x penicillin/streptomycin, 5% FCS, 1mM DTT and 2mM EDTA) for 20 minutes at 37°C shaking at 250 RPM. The intraepithelial lymphocytes released in this step were discarded before enzymatically digesting the remaining tissue with Collagenase/DNase buffer (RPMI-1640 with 2mM L-glutamine, 10mM HEPES, 1x penicillin/streptomycin, 5% FCS, 1 mg/mL collagenase A from Clostridium histolyticum (Sigma-Aldrich, cat #11088793001), and 1 μg/mL DNaseI (Sigma-Aldrich: Cat #10104159001)) for 25 minutes at 37°C shaking at 250 RPM in the presence of five 0.25-inch ceramic beads (MP Biomedicals, cat #116540412). Lamina propria lymphocytes released in this step were filtered through a 100μm strainer and washed in FACS buffer. Immune cells from lungs and tumors were isolated by digesting finely minced tissues with Collagenase/DNase buffer for 25 minutes at 37°C shaking at 250 RPM in the presence of five ceramic beads. Tumors were digested in a similar manner using digestion buffer containing 0.125U/mL collagenase D (Roche cat #11088858001) instead of collagenase A. Digested samples were filtered through a 100μm strainer and washed in FACS buffer. Liver and tumor samples were enriched for lymphocytes by centrifugation in 40% (vol/vol) PBS-adjusted Percoll solution (Thermo Fisher, cat #45–001-747).
Cell Purification and flow cytometry
Single cell suspensions were prepared in ice-cold FACS buffer, washed once in PBS, and stained with Ghost Dye™ Red 780 (Cytek Biosciences, cat #13-0865-T500) for 10 minutes at 4°C to label dead cells. Cell surface antigens were stained for 20 minutes at 4°C using a mix of fluorophore-conjugated antibodies diluted in FACS buffer. Unfixed cells were analyzed immediately or fixed and permeabilized using the eBioscience Foxp3/Transcription Factor staining buffer set (ThermoFisher, cat #00-5523-00), before intracellular staining according to the manufacturer’s instructions. For intracellular cytokine measurements, cells were re-stimulated in vitro with 50ng/mL PMA (Sigma-Aldrich, P8139-1MG), 1μg/mL ionomycin (Sigma-Aldrich, I0634), 1μg/mL brefeldin A (Sigma-Aldrich, B6542-5MG) and 2μM monensin (Sigma-Aldrich, M5273-1G) in complete RPMI for 3 hours at 37°C before fixation and permeabilization (BD, Cytofix/Cytoperm kit, 554714) and antibody staining. Cells were washed and resuspended in FACS buffer and filtered through a 100-μm nylon mesh before analysis on a Cytek Aurora spectral flow cytometer. For TREG cell sorting, pooled spleen and lymph node cells were enriched for CD4 T cells using the Mojosort™ Mouse CD4 T Cell Isolation Kit (BioLegend cat # 480033), stained with antibody cocktails as described, and sorted on BD Aria II.
T cell transfer
3x105 FACS-purified (>99% pure) GFP+ TREG cells from pooled secondary lymphoid tissues of Foxp3AID mice were mixed with an equal number of FACS-purified naïve CD44-CD62L+CD25-CD4+ T cells isolated from Ptprca (CD45.1) mice and retroorbitally injected into Rag2-/- mice. Starting on day 9 post-transfer, animals received daily injections of 5-Ph-IAA before analysis on day 19.
LCMV infection
Mice were infected intraperitoneally with 2x105 pfu LCMV Armstrong. Starting on day 5 post-infection, the animals were injected daily with 0.3 mg of 5-Ph-IAA for a period of 7 consecutive days before analysis on day 12.
Subcutaneous tumor implantation
106 MC38 cells in PBS were mixed with an equal volume of Matrigel and injected subcutaneously into the right flank. Mice were treated with 5-Ph-IAA, DT, or PBS as described. Tumor size was measured every 2-3 days using calipers. Tumor volume (V) was calculated as V = 0.5 x length x width2.
In vitro Treg cell polarization and TH1 cell differentiation
TREG cells were expanded in vivo using IL-2/anti-IL-2 complexes, as previously described. Pooled spleen and lymph node cells were enriched for CD4 T cells using the Mojosort™ Mouse CD4 T Cell Isolation Kit (BioLegend cat # 480033). GFP+ TREG cells and GFP- CD44loCD62Lhi naïve CD4 T cells were isolated by flow cytometry. 3x105 GFP+ Treg cells were cultured in 24-well plates coated with 10 µg/mL anti-CD3 (clone 145-2C11, Bio X Cell cat # BE0001-1) and 10 µg/mL anti-CD28 (clone 37.51, Bio X Cell cat # BE0015-1) in complete RPMI supplemented with 2000U/mL IL-2 (Peprotech cat # 212-12). Cells were cultured for 2 days before adding fresh media supplemented with either 10 µg/mL anti-IFNγ (clone XMG1.2, BD Biosciences cat # 554409) and IL-2, or 67 ng/mL IFN-γ (Biolegend cat # 575306), 67 ng/mL IL-12 (R&D systems cat # 419-ML/CF) and IL-2. Foxp3 protein was degraded by adding 0.5 µg/mL 5-Ph-IAA to the culture media. For TH1 cell differentiation, 500k naïve CD4 T cells were activated under the same conditions as the TREG cells but immediately supplemented with 100 ng/mL IFN-γ, 100 ng/mL IL-12 and 2000 U/mL IL-2. Cells were analyzed on day 6.
RNA-sequencing
GFP+ TREG cells were FACS purified from pooled spleen and lymph nodes of Foxp3AID mice. RNA was extracted using an in-house kit. Briefly, sorted cells were lysed with a buffer containing guanidine thiocyanate, Triton X-100 and β-mercapthoethanol. RNA was captured using magnetic beads. Following a series of wash steps, the beads were incubated with a DNAse I-containing buffer (NEB, cat #M0303) for 15 minutes at 37°C to degrade genomic DNA. After washing, the RNA was eluted in 20uL nuclease-free water for 5 mins at 60°C from pre-dried beads. RNA quality and concentration were assessed using the Agilent DNF-471 RNA kit and Agilent Fragment Analyzer. Sequencing libraries were prepared using the SMARTer Stranded Total Pico Input RNA Kit v3 (Takara cat. #634487) according to the manufacturer protocol, with 10ng of input RNA. Sequencing was performed using NovaSeqX 10B XP flowcell, using paired-end reads with a length of 150bp.
Bioinformatic analysis of RNA-sequencing data
After DNA sequencing, reads were trimmed using Cutadapt and filtered using standard illumina quality metrics. Reads from ribosomal DNA were removed using Bowtie 2. Remaining reads were aligned to the mm10 genome version of December 2011 (GRCm38) using STAR version 2.4.2a. To quantify reads aligning to exons and introns, we used a custom-curated and extended version of mm10 RefSeq (downloaded on December 6, 2018) in which intron annotations were created by extrapolating from the annotated exons of each transcript. Read quantification was performed separately for exons and introns over the whole gene body using subread featureCounts version 2.0.2. Reads were separated into two groups: those entirely overlapping with exons (exonic reads) and those overlapping entirely with introns or with intron-exon junctions (intronic reads). Raw counts were converted to normalized values calculated as transcripts per million (TPM) using concatenated exon and intron lengths. For differential gene expression analysis, read counts from all samples were normalized together and pairwise differential expression between 5-Ph-IAA-treated and control groups was analyzed using DESeq2 version 1.34.0 in R-4.1.2 (https://www.r-project.org). Two versions of this analysis were performed: 1) steady-state and activated TREG cells analyzed together, or 2) steady-state and activated TREG cells analyzed separately. False Discovery Rate (FDR) correction for multiple testing took into account the total number of gene-level tests carried out across all the pairwise comparisons. Numbers of differentially expressed genes were calculated at various adjusted P value and fold-change cutoffs among genes with TPM>20 in at least one condition. Enrichment for Foxp3 binding at differentially expressed genes was analyzed using publicly available ChIP-seq and CUT&RUN data (GSE154680). Foxp3 binding sites identified by ChIP-seq, CUT&RUN, the intersection of peaks identified by both techniques, or the intersection of the top 2000 most highly bound sites identified by both techniques were linked to the nearest gene. Enrichment for Foxp3 binding to different gene sets was determined using a hypergeometric test.
Statistical analysis
Statistical analysis was performed using Prism (GraphPad, v10.4.1). Statistical tests for individual experiments are reported in the figure legends. Briefly, two-tailed paired or unpaired t tests were used to compare two groups of samples. Comparisons between multiple groups were performed using one-way or two-way analysis of variance (ANOVA), followed by post hoc Tukey or Šidák multiple comparisons tests. A log-rank test was used to compare survival curves.
Supplementary Material
One-sentence summary.
Acute Foxp3 protein depletion selectively affects Treg cells responding to type 1 inflammation.
Acknowledgments
We thank Clarissa Campbell, Chrysothemis Brown, Ariella Glasner, Johannes Zuber and all members of the van der Veeken and Busslinger laboratories for helpful discussions; Christian Theussl and Jacek R. Wojciechowski at the IMP transgenic facility for the generation of genetically modified mouse models, staff of the IMP/IMBA shared animal facility for maintaining our mouse colony, the VBCF BioOptics facility for help with flow cytometry and cell sorting, the preclinical phenotyping facility for help with osmotic pump implantation, and the NGS facility for high throughput sequencing and data pre-processing.
Funding
E.M. is supported by the Boehringer Ingelheim Fonds. Research in the van der Veeken laboratory is supported by Boehringer Ingelheim, the Austrian Science Fund (FWF, 10.55776/PAT4163824) and the European Research Council (ERC) under the Horizon 2020 research and innovation program (ERC-2023-STG 101116251). Research in the laboratory of A.C.O. is supported by Boehringer Ingelheim, the Austrian Research Promotion Agency (Headquarter grant FFG-852936), the ERC (‘UnlockIT’ grant no. 101125797) and the European Union’s Horizon 2020 research and innovation program under the Marie Skłodowska–Curie grant agreement No 955951.
Footnotes
Author contributions: C.J. and J.v.d.V. designed the study, analyzed data, prepared figures, and wrote the manuscript. C.J. performed most of the experiments. Q.S. generated the Foxp3AID mice together with the IMP transgenic facility. P.D. contributed to the IL-2/anti-IL-2 treatments, non-lymphoid tissue TREG cell isolation, and tumor experiments. J.T. contributed to in vitro TREG cell polarization assays. G.E. and A.O. assisted with tumor experiments. M.J. performed bioinformatics analysis. E.M. and R.R. helped optimize conditions for in vivo protein degradation experiments. M.B. provided the Rosa26Tir1-F74G mice and technical advice on the project.
Competing interests: The institution that A.O. is affiliated with has received fees for her participation on an advisory board or for presentation at a company-sponsored symposium from Genentech, Roche, InvIOs, AstraZeneca, and Boehringer Ingelheim. All other authors declare that they have no competing interests.
Data and materials availability
The RNA sequencing data generated in this study are available through the Gene Expression Omnibus (GEO) database under accession number GSE289371. Tabulated data underlying the figures is provided in data file S2. All other data needed to support the conclusions of the paper are present in the paper or the Supplementary Materials. Custom code for intronic RNA-seq data analysis is available from https://github.com/Jaritz/jaeger_2025. The Foxp3AID-V5-IRES-DTR-GFP mice will be shared upon request after completion of a material transfer agreement (MTA) with the Research Institute of Molecular Pathology.
References
- 1.Josefowicz SZ, Lu LF, Rudensky AY. Regulatory T cells: mechanisms of differentiation and function. Annu Rev Immunol. 2012;30:531–564. doi: 10.1146/annurev.immunol.25.022106.141623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bos PD, Plitas G, Rudra D, Lee SY, Rudensky AY. Transient regulatory T cell ablation deters oncogene-driven breast cancer and enhances radiotherapy. J Exp Med. 2013;210:2435–2466. doi: 10.1084/jem.20130762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kim JM, Rasmussen JP, Rudensky AY. Regulatory T cells prevent catastrophic autoimmunity throughout the lifespan of mice. Nat Immunol. 2007;8:191–197. doi: 10.1038/ni1428. [DOI] [PubMed] [Google Scholar]
- 4.Klages K, et al. Selective depletion of Foxp3+ regulatory T cells improves effective therapeutic vaccination against established melanoma. Cancer Res. 2010;70:7788–7799. doi: 10.1158/0008-5472.CAN-10-1736. [DOI] [PubMed] [Google Scholar]
- 5.Lahl K, et al. Selective depletion of Foxp3+ regulatory T cells induces a scurfy-like disease. J Exp Med. 2007;204:57–63. doi: 10.1084/jem.20061852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li X, Kostareli E, Suffner J, Garbi N, Hammerling GJ. Efficient Treg depletion induces T-cell infiltration and rejection of large tumors. Eur J Immunol. 2010;40:3325–3335. doi: 10.1002/eji.201041093. [DOI] [PubMed] [Google Scholar]
- 7.Teng MW, et al. Conditional regulatory T-cell depletion releases adaptive immunity preventing carcinogenesis and suppressing established tumor growth. Cancer Res. 2010;70:7800–7809. doi: 10.1158/0008-5472.CAN-10-1681. [DOI] [PubMed] [Google Scholar]
- 8.Tay C, Tanaka A, Sakaguchi S. Tumor-infiltrating regulatory T cells as targets of cancer immunotherapy. Cancer Cell. 2023;41:450–465. doi: 10.1016/j.ccell.2023.02.014. [DOI] [PubMed] [Google Scholar]
- 9.Gavin MA, et al. Foxp3-dependent programme of regulatory T-cell differentiation. Nature. 2007;445:771–775. doi: 10.1038/nature05543. [DOI] [PubMed] [Google Scholar]
- 10.Lin W, et al. Regulatory T cell development in the absence of functional Foxp3. Nat Immunol. 2007;8:359–368. doi: 10.1038/ni1445. [DOI] [PubMed] [Google Scholar]
- 11.Williams LM, Rudensky AY. Maintenance of the Foxp3-dependent developmental program in mature regulatory T cells requires continued expression of Foxp3. Nat Immunol. 2007;8:277–284. doi: 10.1038/ni1437. [DOI] [PubMed] [Google Scholar]
- 12.Wildin RS, et al. X-linked neonatal diabetes mellitus, enteropathy and endocrinopathy syndrome is the human equivalent of mouse scurfy. Nat Genet. 2001;27:18–20. doi: 10.1038/83707. [DOI] [PubMed] [Google Scholar]
- 13.Chatila TA, et al. JM2, encoding a fork head-related protein, is mutated in X-linked autoimmunity-allergic disregulation syndrome. J Clin Invest. 2000;106:R75–81. doi: 10.1172/JCI11679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brunkow ME, et al. Disruption of a new forkhead/winged-helix protein, scurfin, results in the fatal lymphoproliferative disorder of the scurfy mouse. Nat Genet. 2001;27:68–73. doi: 10.1038/83784. [DOI] [PubMed] [Google Scholar]
- 15.Bennett CL, et al. The immune dysregulation, polyendocrinopathy, enteropathy, X-linked syndrome (IPEX) is caused by mutations of FOXP3. Nat Genet. 2001;27:20–21. doi: 10.1038/83713. [DOI] [PubMed] [Google Scholar]
- 16.Tai Y, Sakamoto K, Takano A, Haga K, Harada Y. Dysregulation of humoral immunity in Foxp3 conditional-knockout mice. Biochem Biophys Res Commun. 2019;513:787–793. doi: 10.1016/j.bbrc.2019.04.090. [DOI] [PubMed] [Google Scholar]
- 17.Arvey A, et al. Inflammation-induced repression of chromatin bound by the transcription factor Foxp3 in regulatory T cells. Nat Immunol. 2014;15:580–587. doi: 10.1038/ni.2868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Samstein RM, et al. Foxp3 exploits a pre-existent enhancer landscape for regulatory T cell lineage specification. Cell. 2012;151:153–166. doi: 10.1016/j.cell.2012.06.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.van der Veeken J, et al. The Transcription Factor Foxp3 Shapes Regulatory T Cell Identity by Tuning the Activity of trans-Acting Intermediaries. Immunity. 2020;53:971–984.:e975. doi: 10.1016/j.immuni.2020.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.He M, et al. Dynamic Foxp3-chromatin interaction controls tunable Treg cell function. J Exp Med. 2024;221 doi: 10.1084/jem.20232068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kwon HK, Chen HM, Mathis D, Benoist C. Different molecular complexes that mediate transcriptional induction and repression by FoxP3. Nat Immunol. 2017;18:1238–1248. doi: 10.1038/ni.3835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ramirez RN, Chowdhary K, Leon J, Mathis D, Benoist C. FoxP3 associates with enhancer-promoter loops to regulate T(reg)-specific gene expression. Sci Immunol. 2022;7:eabj9836. doi: 10.1126/sciimmunol.abj9836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.DuPage M, et al. The chromatin-modifying enzyme Ezh2 is critical for the maintenance of regulatory T cell identity after activation. Immunity. 2015;42:227–238. doi: 10.1016/j.immuni.2015.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rudra D, et al. Transcription factor Foxp3 and its protein partners form a complex regulatory network. Nat Immunol. 2012;13:1010–1019. doi: 10.1038/ni.2402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ichiyama K, et al. Transcription factor Ikzf1 associates with Foxp3 to repress gene expression in Treg cells and limit autoimmunity and anti-tumor immunity. Immunity. 2024;57:2043–2060.:e2010. doi: 10.1016/j.immuni.2024.07.010. [DOI] [PubMed] [Google Scholar]
- 26.Chaudhry A, Rudensky AY. Control of inflammation by integration of environmental cues by regulatory T cells. J Clin Invest. 2013;123:939–944. doi: 10.1172/JCI57175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Huang X, Rudensky AY. Regulatory T cells in the context: deciphering the dynamic interplay with the tissue environment. Curr Opin Immunol. 2024;89:102453. doi: 10.1016/j.coi.2024.102453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Trujillo-Ochoa JL, Kazemian M, Afzali B. The role of transcription factors in shaping regulatory T cell identity. Nat Rev Immunol. 2023;23:842–856. doi: 10.1038/s41577-023-00893-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nishimura K, Fukagawa T, Takisawa H, Kakimoto T, Kanemaki M. An auxin-based degron system for the rapid depletion of proteins in nonplant cells. Nat Methods. 2009;6:917–922. doi: 10.1038/nmeth.1401. [DOI] [PubMed] [Google Scholar]
- 30.Fedl AS, et al. Transcriptional function of E2A, Ebf1, Pax5, Ikaros and Aiolos analyzed by in vivo acute protein degradation in early B cell development. Nat Immunol. 2024;25:1663–1677. doi: 10.1038/s41590-024-01933-7. [DOI] [PubMed] [Google Scholar]
- 31.Baker O, et al. RAC-tagging: Recombineering And Cas9-assisted targeting for protein tagging and conditional analyses. Sci Rep. 2016;6:25529. doi: 10.1038/srep25529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wan YY, Flavell RA. Regulatory T-cell functions are subverted and converted owing to attenuated Foxp3 expression. Nature. 2007;445:766–770. doi: 10.1038/nature05479. [DOI] [PubMed] [Google Scholar]
- 33.Delacher M, et al. Precursors for Nonlymphoid-Tissue Treg Cells Reside in Secondary Lymphoid Organs and Are Programmed by the Transcription Factor BATF. Immunity. 2020;52:295–312.:e211. doi: 10.1016/j.immuni.2019.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fan X, et al. CD49b defines functionally mature Treg cells that survey skin and vascular tissues. J Exp Med. 2018;215:2796–2814. doi: 10.1084/jem.20181442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Munoz-Rojas AR, Mathis D. Tissue regulatory T cells: regulatory chameleons. Nat Rev Immunol. 2021;21:597–611. doi: 10.1038/s41577-021-00519-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.van der Veeken J, et al. Memory of Inflammation in Regulatory T Cells. Cell. 2016;166:977–990. doi: 10.1016/j.cell.2016.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Koch MA, et al. The transcription factor T-bet controls regulatory T cell homeostasis and function during type 1 inflammation. Nat Immunol. 2009;10:595–602. doi: 10.1038/ni.1731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Koch MA, et al. T-bet(+) Treg cells undergo abortive Th1 cell differentiation due to impaired expression of IL-12 receptor beta2. Immunity. 2012;37:501–510. doi: 10.1016/j.immuni.2012.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hall AO, et al. The cytokines interleukin 27 and interferon-gamma promote distinct Treg cell populations required to limit infection-induced pathology. Immunity. 2012;37:511–523. doi: 10.1016/j.immuni.2012.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Littringer K, et al. Common Features of Regulatory T Cell Specialization During Th1 Responses. Front Immunol. 2018;9:1344. doi: 10.3389/fimmu.2018.01344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gocher-Demske AM, et al. IFNgamma-induction of T(H)1-like regulatory T cells controls antiviral responses. Nat Immunol. 2023;24:841–854. doi: 10.1038/s41590-023-01453-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gaidatzis D, Burger L, Florescu M, Stadler MB. Analysis of intronic and exonic reads in RNA-seq data characterizes transcriptional and post-transcriptional regulation. Nat Biotechnol. 2015;33:722–729. doi: 10.1038/nbt.3269. [DOI] [PubMed] [Google Scholar]
- 43.Levine AG, Arvey A, Jin W, Rudensky AY. Continuous requirement for the TCR in regulatory T cell function. Nat Immunol. 2014;15:1070–1078. doi: 10.1038/ni.3004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cui A, et al. Dictionary of immune responses to cytokines at single-cell resolution. Nature. 2024;625:377–384. doi: 10.1038/s41586-023-06816-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Afkarian M, et al. T-bet is a STAT1-induced regulator of IL-12R expression in naive CD4+ T cells. Nat Immunol. 2002;3:549–557. doi: 10.1038/ni794. [DOI] [PubMed] [Google Scholar]
- 46.Mullen AC, et al. Role of T-bet in commitment of TH1 cells before IL-12-dependent selection. Science. 2001;292:1907–1910. doi: 10.1126/science.1059835. [DOI] [PubMed] [Google Scholar]
- 47.Thieu VT, et al. Signal transducer and activator of transcription 4 is required for the transcription factor T-bet to promote T helper 1 cell-fate determination. Immunity. 2008;29:679–690. doi: 10.1016/j.immuni.2008.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shouse AN, LaPorte KM, Malek TR. Interleukin-2 signaling in the regulation of T cell biology in autoimmunity and cancer. Immunity. 2024;57:414–428. doi: 10.1016/j.immuni.2024.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kim HP, Kelly J, Leonard WJ. The basis for IL-2-induced IL-2 receptor alpha chain gene regulation: importance of two widely separated IL-2 response elements. Immunity. 2001;15:159–172. doi: 10.1016/s1074-7613(01)00167-4. [DOI] [PubMed] [Google Scholar]
- 50.Rubtsov YP, et al. Stability of the regulatory T cell lineage in vivo. Science. 2010;329:1667–1671. doi: 10.1126/science.1191996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Feng Y, et al. Control of the inheritance of regulatory T cell identity by a cis element in the Foxp3 locus. Cell. 2014;158:749–763. doi: 10.1016/j.cell.2014.07.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Busse D, et al. Competing feedback loops shape IL-2 signaling between helper and regulatory T lymphocytes in cellular microenvironments. Proc Natl Acad Sci U S A. 2010;107:3058–3063. doi: 10.1073/pnas.0812851107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chinen T, et al. An essential role for the IL-2 receptor in T(reg) cell function. Nat Immunol. 2016;17:1322–1333. doi: 10.1038/ni.3540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Boyman O, Kovar M, Rubinstein MP, Surh CD, Sprent J. Selective stimulation of T cell subsets with antibody-cytokine immune complexes. Science. 2006;311:1924–1927. doi: 10.1126/science.1122927. [DOI] [PubMed] [Google Scholar]
- 55.Webster KE, et al. In vivo expansion of T reg cells with IL-2-mAb complexes: induction of resistance to EAE and long-term acceptance of islet allografts without immunosuppression. J Exp Med. 2009;206:751–760. doi: 10.1084/jem.20082824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Choi S, Cornall R, Lesourne R, Love PE. THEMIS: Two Models, Different Thresholds. Trends Immunol. 2017;38:622–632. doi: 10.1016/j.it.2017.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Akamatsu M, et al. Conversion of antigen-specific effector/memory T cells into Foxp3-expressing T(reg) cells by inhibition of CDK8/19. Sci Immunol. 2019;4 doi: 10.1126/sciimmunol.aaw2707. [DOI] [PubMed] [Google Scholar]
- 58.Magnuson AM, et al. Identification and validation of a tumor-infiltrating Treg transcriptional signature conserved across species and tumor types. Proc Natl Acad Sci U S A. 2018;115:E10672–E10681. doi: 10.1073/pnas.1810580115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Moreno Ayala MA, et al. CXCR3 expression in regulatory T cells drives interactions with type I dendritic cells in tumors to restrict CD8(+) T cell antitumor immunity. Immunity. 2023;56:1613–1630.:e1615. doi: 10.1016/j.immuni.2023.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zagorulya M, et al. Tissue-specific abundance of interferon-gamma drives regulatory T cells to restrain DC1-mediated priming of cytotoxic T cells against lung cancer. Immunity. 2023;56:386–405.:e310. doi: 10.1016/j.immuni.2023.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Okamoto M, et al. A genetic method specifically delineates Th1-type Treg cells and their roles in tumor immunity. Cell Rep. 2023;42:112813. doi: 10.1016/j.celrep.2023.112813. [DOI] [PubMed] [Google Scholar]
- 62.Wolf T, et al. Dynamics in protein translation sustaining T cell preparedness. Nat Immunol. 2020;21:927–937. doi: 10.1038/s41590-020-0714-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Charbonnier LM, et al. Functional reprogramming of regulatory T cells in the absence of Foxp3. Nat Immunol. 2019;20:1208–1219. doi: 10.1038/s41590-019-0442-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hwang SM, et al. Inflammation-induced Id2 promotes plasticity in regulatory T cells. Nat Commun. 2018;9:4736. doi: 10.1038/s41467-018-07254-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hu W, et al. Regulatory T cells function in established systemic inflammation and reverse fatal autoimmunity. Nat Immunol. 2021;22:1163–1174. doi: 10.1038/s41590-021-01001-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lam AJ, et al. Optimized CRISPR-mediated gene knockin reveals FOXP3-independent maintenance of human Treg identity. Cell Rep. 2021;36:109494. doi: 10.1016/j.celrep.2021.109494. [DOI] [PubMed] [Google Scholar]
- 67.Min B, et al. Neonates support lymphopenia-induced proliferation. Immunity. 2003;18:131–140. doi: 10.1016/s1074-7613(02)00508-3. [DOI] [PubMed] [Google Scholar]
- 68.Miura H, Quadros RM, Gurumurthy CB, Ohtsuka M. Easi-CRISPR for creating knock-in and conditional knockout mouse models using long ssDNA donors. Nat Protoc. 2018;13:195–215. doi: 10.1038/nprot.2017.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Morawska M, Ulrich HD. An expanded tool kit for the auxin-inducible degron system in budding yeast. Yeast. 2013;30:341–351. doi: 10.1002/yea.2967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Anderson KG, et al. Intravascular staining for discrimination of vascular and tissue leukocytes. Nat Protoc. 2014;9:209–222. doi: 10.1038/nprot.2014.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The RNA sequencing data generated in this study are available through the Gene Expression Omnibus (GEO) database under accession number GSE289371. Tabulated data underlying the figures is provided in data file S2. All other data needed to support the conclusions of the paper are present in the paper or the Supplementary Materials. Custom code for intronic RNA-seq data analysis is available from https://github.com/Jaritz/jaeger_2025. The Foxp3AID-V5-IRES-DTR-GFP mice will be shared upon request after completion of a material transfer agreement (MTA) with the Research Institute of Molecular Pathology.