Abstract
Background
Serotonin, originally identified as a neurotransmitter in mammals, functions as an antioxidant to scavenge cellular ROS in plants. In rice, the conversion of tryptamine to serotonin is catalyzed by SL (sekiguchi lesion), a member of cytochrome P450 monooxygenase family. The sl mutant, originated from rice cultivar Sekiguchi-asahi, exhibits spontaneous lesions, whereas its immune responses to pathogens have not been clearly characterized.
Results
Here we identified three allelic mutants of SL in an indica rice restore line Minghui 86 (MH86), named as sl-MH-1, − 2 and − 3, all of which present the typical lesions under normal growth condition. Compared with those in MH86, the serotonin content in sl-MH-1 is dramatically decreased, whereas the levels of tryptamine and L-trytophan are significantly increased. The sl-MH-1 mutant accumulates high H2O2 level at its lesion sites and is more sensitive to exogenous H2O2 treatment than the wild type. When treated with the reductant vitamin C (Vc), the lesion formation on sl-MH-1 leaves could be efficiently suppressed. In addition, sl-MH-1 displayed more resistant to both the blast fungus and blight bacteria, Pyricularia oryzae (P. oryzae, teleomorph: Magnaporthe oryzae) and Xanthomonas oryzae pv. Oryzae (Xoo), respectively. The pathogen-associated molecular patterns (PAMPs)-triggered immunity (PTI) responses, like reactive oxygen species (ROS) burst and callose deposition, were enhanced in sl-MH-1. Moreover, loss function of SL resulted in higher resting levels of the defense hormones, salicylic acid and jasmonic acid. The RNA-seq analysis indicated that after P. oryzae infection, transcription of the genes involved in reduction-oxidation regulation was the most markedly changed in sl-MH-1, compared with MH86.
Conclusions
Our results indicate that SL, involving in the final step of serotonin biosynthesis, negatively regulates rice resistance against (hemi)biotrophic pathogens via compromising the PTI responses and defense hormones accumulation.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12870-020-02724-6.
Keywords: Rice, Serotonin, Reactive oxygen species, PAMP-triggered immunity, Defense hormones, Pyricularia oryzae
Background
In nature, plants are constantly exposed to a wide range of pathogenic microorganisms, thus they have developed a sophisticated innate immune system to protect themselves from infection [1]. PAMP-triggered immunity (PTI), activated upon recognition of conserved pathogen-associated molecular patterns (PAMPs) by plant cell membrane-localized pattern recognition receptors, can rapidly elicit plant defense responses, which includes calcium ions influx, reactive oxygen species (ROS) generation, callose deposition and stomatal closure et al [2]. Another branch of plant immunity is effector-triggered immunity (ETI), caused by direct or indirect perception of pathogen effectors via plant resistance (R) proteins, which is often associated with a hypersensitive response (HR) and renders plants the isolate-specific resistance [3]. As a typical feature of HR, programmed cell death (PCD), accompanied with production of ROS and phytoalexins, is employed to effectively halt the spread of pathogens within the initial penetration site [4]. On the other hand, activation of plant immune responses depends on dramatic changes in the cellular reduction-oxidation (redox) status, resulting in the reprogramming of the transcriptome and the establishment of both local and systemic defense [5]. Upon being infected, the raised oxidative stress in plant cells is essential for HR formation and development [6].
Serotonin (5-hydroxytryptamine) is originally known as a neurotransmitter controlling fundamental physiological processes, such as mood, sleep and anxiety, in mammals [7]. Since its first identification in the Mucuna pruriens, serotonin has been found widely distributed in the plant kingdom and involved in regulation of diverse physiological processes [8, 9]. Serotonin biosynthesis in plants occurs as that tryptophan is converted into tryptamine by tryptophan decarboxylase, following with the catalysis of tryptamine to serotonin by tryptamine 5-hydroxylase [10]. Serotonin is readily oxidized and functions as an antioxidant to scavenge cellular ROS [11]. And the antioxidant activity of serotonin is reported to far exceed that of tryptophan, tryptamine and its derivatives [9]. Thus, it is conceivable that serotonin plays a role in plant innate immunity via regulating cellular redox status. Moreover, serotonin could also be incorporated into the plant cell wall for strengthening the mechanical barrier against pathogens [12].
Rice (Oryza sativa) is the staple food for more than half of the world’s population. Rice blast and bacterial blight, caused by the hemibiotrophic fungal pathogen Pyricularia oryzae (P. oryzae) and the biotrophic bacterial pathogen Xanthomonas oryzae pv. Oryzae (Xoo), respectively, are the most devastating rice diseases [13]. Increasing reports indicate that the serotonin pathway in rice is involved in disease resistance, and most of studies are based on characterizing a lesion mimic mutant, sekiguchi lesion (sl), originated from the rice cultivar Sekiguchi-asahi. The SL gene, cloned by Fujiwara et. al in 2010, encodes CYP71P1, belonging to the cytochrome P450 monooxygenase family [14]. SL possesses tryptamine 5-hydroxylase enzyme activity and can catalyze the conversion of tryptamine to serotonin in rice. As this reason, the accumulation of serotonin after Bipolaris oryzae (B. oryzae, a necrotrophic pathogen) infection was abolished in sl mutant, and sl displayed increased susceptibility to B. oryzae [12]. However, the responses of sl mutant to biotrophic pathogens infection and the mechanisms of SL involved in plant immunity are still ambiguous.
In this study, we identified the sl mutants in MH86 background (sl-MH) and found that the lesions of sl-MH-1 is caused by excessive accumulation of ROS, which could be suppressed by the reductant treatment. The absence of SL results in the enhanced PTI responses and high resting levels of defense hormones, and thus the broad-spectrum resistance against P. oryzae and Xoo.
Results
Identification of the sl mutants in MH86 background
We have identified a rice runaway-cell death mutant, rcd1–1, in an indica rice restore line Minghui 86 (MH86). This mutant spontaneously exhibits orange-colored lesions on its leaves when grown in field or greenhouse (Fig. S1). Through map-based cloning, a G to T mutation was found at 1205 nucleotide of SL ORF, which leads to the 370 Arg mutated to Leu [15]. Two allelic mutants, rcd1–2 and rcd1–3, were obtained by 60Co ~ γ-ray radiation, which carries C85 and A1420 deletion in SL coding region, respectively (Fig. S2). Both rcd1–2 and rcd1–3 spontaneously present sekiguchi lesions, similar with rcd1–1 (Fig. S1). Therefore, hereafter we named rcd1–1, rcd1–2 and rcd1–3 mutants as sl-MH-1, sl-MH-2 and sl-MH-3, respectively.
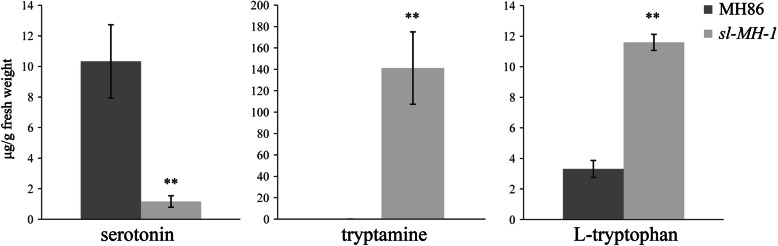
To investigate difference of the metabolites levels in serotonin biosynthesis pathway in sl-MH-1 mutant and MH86, the contents of serotonin, tryptamine and L-trytophan from the leaves of 8-week-old plants grown in a greenhouse with natural light were measured. The data indicated that serotonin level was significantly decreased in sl-MH-1 compared with MH86, whereas tryptamine and L-trytophan, the upstream metabolites in serotonin biosynthesis, were accumulated to significantly higher levels in sl-MH-1 (Fig. 1), proving the function of SL in catalyzing the conversion of tryptamine to serotonin. Moreover, we found that the content of L-glutamine in sl-MH-1 was significantly higher than that in MH86 (Fig. S3). Taken together, our results indicate that mutation of SL in MH86 background also results in the typical sekiguchi lesion and compromised serotonin biosynthesis.
Fig. 1.
Levels of serotonin, tryptamine and L-tryptophan in MH86 and sl-MH-1. The contents of serotonin, tryptamine and L-tryptophan in the leaves of 8-week-old MH86 and sl-MH-1 plants were measured by Ultra High Performance Liquid Chromatography (UHPLC). Bars represent mean values ± standard error (SE) from five biological replicates. Statistically significant difference was indicated by ** (p < 0.01, Student’s t-test)
Endogenous oxidative stress results in lesion formation on sl-MH-1 leaves
To determine the relationship between cell death and ROS accumulation in absence of SL, we stained the leaves of 8-week-old sl-MH-1 and MH86 with trypan blue and 3,3-diaminobenzidine tetrahydrochloride (DAB) to detect cell death and H2O2 accumulation, respectively. As shown in Fig. 2a, compared with MH86, the lesion sites on sl-MH-1 leaves (shown by the dark trypan blue staining) displayed more H2O2 accumulation as indicated by DAB staining (dark brown color). To assess the defects of sl-MH-1 responding to exogenous ROS stress, sl-MH-1 and MH86 seeds were germinated on Murashige and Skoog (MS) medium with or without H2O2. After kept in a 28 °C growth chamber with 12-h light for 1 week, we found that there was no significant difference between sl-MH-1 and MH86 seedlings on MS medium without H2O2 (Fig. 2b). The H2O2 application could retard the growth of both sl-MH-1 and MH86 seedlings, whereas sl-MH-1 seedlings displayed more stressed (with leaves turning yellow) than the wild type to H2O2 treatment (Fig. 2b). Therefore, SL mutation leads to high endogenous ROS accumulation and increased sensitivity to exogenous ROS stress.
Fig. 2.

Responses of sl-MH-1 to exogenous H2O2 and VC treatments. a Trypan blue (left) and DAB (right) staining of the 8-week-old sl-MH-1 and MH86 leaves to detect cell death and H2O2 accumulation, respectively. b sl-MH-1 and MH86 seeds were germinated on MS medium with or without 0.02% H2O2. The seedlings were imaged after kept in a growth chamber for 7 days. Bars = 1 cm. c. The leaves of sl-MH-1 plants were imaged after treated with 0.1% VC for 10 days. CK means water treatment as control
In order to further determine whether lesion of sl-MH-1 is resulted from its high internal ROS accumulation, we treated the mutant with the antioxidant Vitamin C (VC, ascorbic acid), which is one of the major redox buffers in plant cell and functions as an antioxidant to conduct ROS detoxification [16]. And it has been reported that exogenous application of VC could alleviate oxidative stress in rice induced by abiotic stress [17]. 3-week-old sl-MH-1 seedlings grown in green house, whose leaves did not exhibit visible lesions, were treated with 0.1% VC or water (as control) by spraying for 10 days. Then the lesion numbers on the top second and third leaves were counted. As shown in Fig. 2c and Table 1, the lesion numbers on sl-MH-1 leaves was significantly decreased by VC treatment, indicating that exogenous antioxidant application could suppress sl-MH-1 lesion formation. Taken together, our data suggest that the cell death in absence of SL is caused by high endogenous ROS accumulation.
Table 1.
Lesion numbers on the sl-MH-1 leaves after VC and CK treatments
| treatment | average number of lesions per leaf | |
|---|---|---|
| top second leaf | top third leaf | |
| CK (86 plants) | 1.33 + 1.38 | 1.24 + 1.41 |
| 0.1%VC (42 plants) | 0.05 + 0.22** | 0.70 + 0.26** |
** means statistically significant with p < 0.01 with One-way analysis of variance (ANOVA)
sl-MH-1 mutant displays enhanced resistance to P. oryzae and Xoo
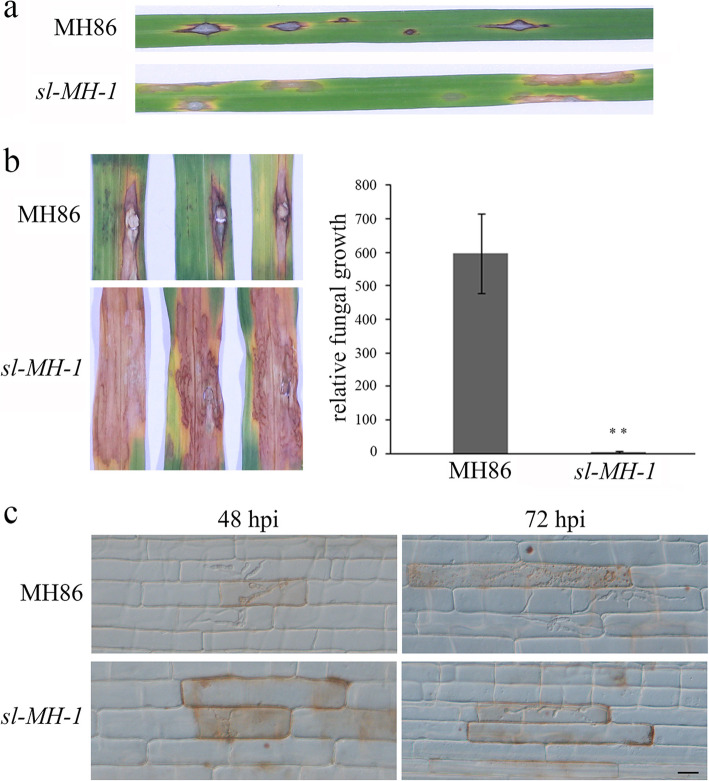
As ROS production is an important defense response of plants to pathogen invasion, we wondered whether sl-MH-1 mutant was more resistant to rice pathogens. Firstly, we challenged 3-week-old sl-MH-1 and MH86 seedlings with conidial spores of the compatible blast fungal isolate, FJ-1, by spraying. As shown in Fig. 3a, inoculation with FJ-1 caused typical disease symptom on MH86 leaf, whereas sl-MH-1 mutant exhibited sekiguchi lesions at the FJ-1 infection sites. To quantify the resistance of sl-MH-1 to the blast fungus, we carried out punch inoculation on sl-MH-1 and MH86 leaves and investigated the relative fungal biomass within the infected region, which showed that the sl-MH-1 mutant supported significantly less blast fungus growth than MH86 (Fig. 3b). In order to further monitor the process of M. oryzae invasion in sl-MH-1 and MH86, a rice leaf sheath inoculation assay was performed. It was found that the invasive hyphae (IH) in MH86 could extend to the neighboring cells of the first infected cell at 48 h post inoculation (hpi) and further extended to the adjacent cells at 72 hpi (Fig. 3c). However, at both 48 and 72 hpi, the IH was still constricted in the first infected cell of sl-MH-1 (Fig. 3c). Although the IH could elongate from 48 to 72 hpi in sl-MH-1 cells, the infection rate of blast fungus was markedly slower than that in MH86. Taken together, these results indicate that knockout of SL enhances rice resistance to blast fungus.
Fig. 3.
sl-MH-1 mutant displays increased resistance to P. oryzae. a 3-week-old MH86 and sl-MH-1 seedlings were inoculated with P. oryzae conidia by spraying. At 7 days post inoculation (dpi), the diseased leaves were imaged. b Punch inoculation with P. oryzae conidia was carried out on the leaves of 4-week-old MH86 and sl-MH-1 plants. The infected leaves were photographed at 9 dpi (left); meanwhile fungal biomass was measured to quantify the relative P. oryzae growth in MH86 and sl-MH-1 leaves (right). Bars represent mean values ± SD from three biological replicates. Statistically significant difference was indicated by ** (p < 0.01, Student’s t-test). c. Leaf sheathes of MH86 and sl-MH-1 were inoculated with P. oryzae conidia to monitor the infection process. The infected cells were imaged at 48 and 72 hpi with a microscope under bright field. Bar = 20 μm
To determine whether sl-MH-1 mutant possesses the increased resistance to bacterial pathogens, we further inoculated sl-MH-1 and MH86 plants with Xoo. As shown in Fig. 4, via comparing blight lesion length and bacterial population at 14 days after inoculation, it was found that sl-MH-1 indeed displayed enhanced resistance to blight bacteria as well. Furthermore, to support the resistant phenotypes of sl-MH-1 is resulted from loss function of SL, another allelic mutant, sl-MH-3, was subjected to the above inoculation assays. The results showed that similar with sl-MH-1, sl-MH-3 displayed more resistance to P. oryzae and Xoo (Fig. S4a-d). Therefore, SL negatively regulates rice broad-spectrum resistance to the (hemi)biotrophic pathogens.
Fig. 4.
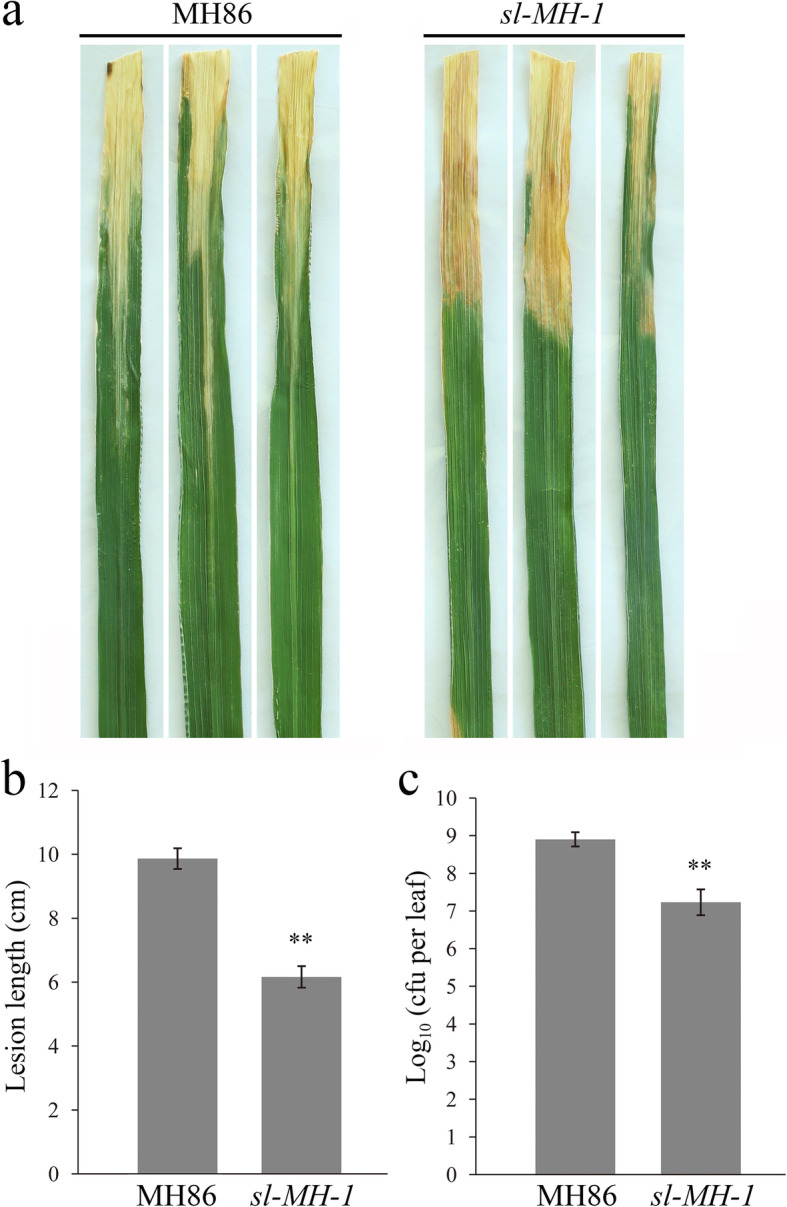
sl-MH-1 mutant showed enhanced resistance to Xoo. a MH86 and sl-MH-1 plants were inoculated with Xoo at the tilling stage. The infected leaves from three independent MH86 or sl-MH-1 plants were imaged at 14 dpi. b Blight lesion length on sl-MH-1 and MH86 leaves were measured. Bars represent mean values ± SD (n = 6, from three independent plants). c Blight bacterial populations were counted 14 dpi with bars representing mean values ± SD from three independent MH86 or sl-MH-1 plants. Cfu means colony-forming units. Statistically significant difference in b and c was indicated by ** (p < 0.01, Student’s t-test)
SL mutation leads to enhanced PTI responses
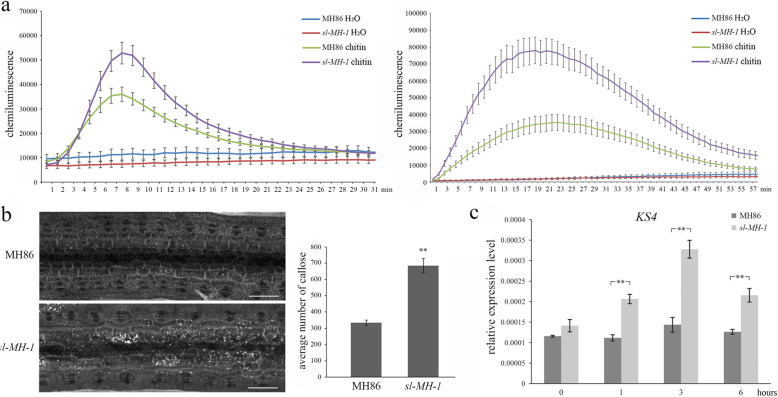
In order to decipher the mechanism of enhanced resistance by SL deletion, the PTI responses of sl-MH-1 and MH86 were analyzed. We firstly measured the levels of ROS burst in sl-MH-1 and MH86 upon flg22 and chitin treatments. It was found that ROS burst could be stimulated in both sl-MH-1 and MH86 leaves by the PAMPs treatments, whereas the levels of ROS production were significantly higher in sl-MH-1 than MH86 at the indicated time points (Fig. 5a). Additionally, the callose deposition on sl-MH-1 and MH86 leaves after chitin treatment was investigated. As shown in Fig. 5b, compared with MH86, there was significantly more callose deposited on sl-MH-1 leaves. And the enhanced callose deposition was similarly observed on sl-MH-3 leaves (Fig. S4e). Furthermore, transcriptional profile of the PTI-related defense gene KS4 was investigated in sl-MH-1 and MH86 before and after chitin treatment. KS4 is a diterpene cyclase enzyme involving in momilactone biosynthesis [18, 19]. We found that at all the indicated time points after chitin treatment, the transcriptional levels of KS4 in sl-MH-1 were significantly higher than those in MH86 (Fig. 5c). Taken together, our data demonstrate that loss function of SL enhances rice PTI responses.
Fig. 5.
PTI responses are enhanced in sl-MH-1 mutant. a ROS burst from MH86 and sl-MH-1 leaf discs treated with 400 nM chitin or 500 nM flg22 was detected at the indicated time points. Error bars represent the SE (n = 8). The data are from one of three independent experiments performed with similar results. B Callose deposition on MH86 and sl-MH-1 leaves after chitin treatment was imaged with a microscope under UV light (left). Number of the callose deposition per view was analysed by Image J (right). Bars represent mean values ± SE (n = 5). Statistically significant difference was indicated by ** (p < 0.01, Student’s t-test). This assay was performed in three independent replicates with similar results. c The relative transcriptional levels of KS4 in 3-week-old MH86 and sl-MH-1 leaves at the indicated time points after treated with 400 nM chitin. Rice ubiquitin-encoding gene (UBQ) was used as the internal control. Bars represent mean values ± SD (n = 3). Statistically significant difference was indicated by ** (p < 0.01, Student’s t-test). This assay was performed in two independent replicates with similar results
sl-MH mutants accumulate higher resting levels of defense hormones
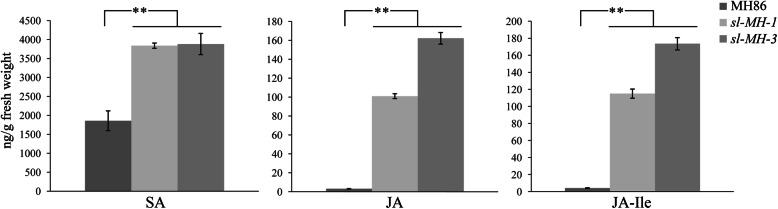
Salicylic acid (SA) and jasmonic acid (JA) are the major phytohormones involved in defense against pathogens. To assess whether SL mutation alters contents of these defense hormones, we detected the levels of free SA, JA and JA-isoleucine (JA-Ile, the active form of JA) in 6 weeks-old sl-MH-1 and MH86 plants, when the lesions were fully appeared on sl-MH-1 leaves. As shown in Fig. 6, the free SA level in sl-MH-1 was about twice as high as that in MH86; both JA and JA-Ile contents in sl-MH-1 were burst to approximately 30 times higher than those in MH86. Meanwhile, levels of SA, JA and JA-Ile were also measured in sl-MH-3 to confirm that the high accumulation of the defense hormones was resulted from SL mutation. It was found that sl-MH-3 accumulated similar level of free SA with sl-MH-1 and even higher levels of JA and JA-Ile than those in sl-MH-1 (Fig. 6). Moreover, the levels of defense hormones were also measured in 3 weeks-old sl-MH-1 and MH86 plants, when the lesions were not observed on sl-MH-1 leaves. As shown in Fig. S5, the free SA level in 3-week-old sl-MH-1 seedlings was similarly about two-fold higher than that in MH86; JA-Ile content in sl-MH-1 was about 3 times higher than that in MH86, whereas no significant difference of JA level was detected between 3-week-old sl-MH-1 and MH86. These results suggest that SL mutation leads to constantly more SA production and dramatically increased JA and JA-Ile contents along with the lesion appearance.
Fig. 6.
Contents of SA, JA and JA-Ile in MH86 and sl-MH mutants. The resting levels of SA, JA and JA-Ile in 6-week-old MH86, sl-MH-1 and sl-MH-3 plants were measured by UPLC. Bars represent mean values ± SD from three biological replicates. Statistically significant difference was indicated by ** (p < 0.01, Student’s t-test)
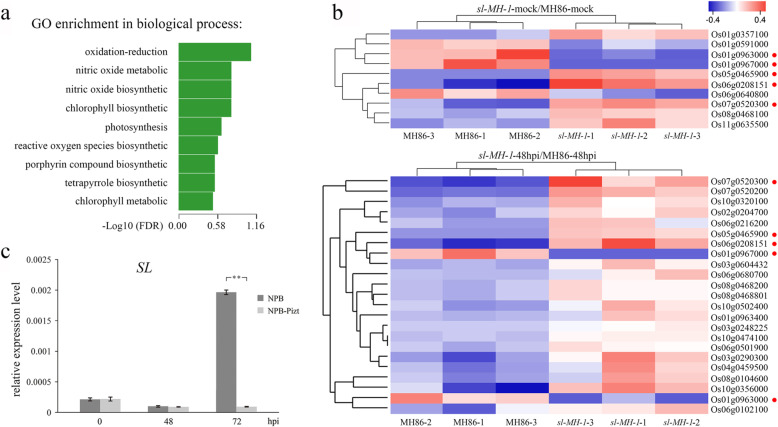
SL mutation mainly alters transcription of the genes in reduction-oxidation pathway upon blast fungus infection
To determine the difference of gene transcription in sl mutant and wild type in response to pathogen infection, we carried out transcritome analysis in sl-MH-1 and MH86 plants with and without P. oryzae inoculation. The leaf tissues of sl-MH-1 and MH86 were harvested at 48 hpi with FJ-1 to conduct RNA-seq analysis, water treatment was used as control (mock). The number of significant differentially expressed loci (SDEL) in sl-MH-1-mock compared to MH86-mock (sl-MH-1-mock/MH86-mock) and sl-MH-1-48hpi compared to MH86-48hpi (sl-MH-1-48hpi/MH86-48hpi) with fold change ≥2 or ≤ 0.5 were 95 and 149, respectively (supplementary Table S1 and Table S2). We used Gene Ontology enrichment analysis to investigate the transcript profiles in various biological processes of sl-MH-1 and MH86 following blast fungus infection. The results indicated that compared with MH86, the most markedly affected biological process in sl-MH-1 is the redox pathway (Fig. 7a). The significant differentially expressed genes (DEGs) related to redox regualtion were 10 and 23 in sl-MH-1-mock/MH86-mock and sl-MH-1-48hpi/MH86-48hpi, respectively, whose transcript levels were displayed as heatmaps shown in Fig. 7b. There are five redox-related genes had similar trend of transcriptional change in both sl-MH-1-mock/MH86-mock and sl-MH-1-48hpi/MH86-48hpi (Fig. 7b, indicated by red dots), suggesting their transcriptional changes caused by defective SL was not affected by blast fungus infection. Among them, Os07g0520300 encodes phytohormone-related protein of cytochrome P450 family, whose transcriptional level was significantly increased in sl-MH-1; Os01g0963000 and Os01g0967000 were the only two down-regulated redox genes in sl-MH-1 after inoculation, which encodes cationic peroxidase SPC4 and LSM domain containing protein, respectively. SPC4 potentially involved in ROS generation in rice [20].
Fig. 7.
Transcritome analysis in MH86 and sl-MH-1 plants with and without blast fungus inoculation. a Gene Ontology enrichment analysis showed the changed transcript profiles in various biological processes of sl-MH-1, compared with MH86, after P. oryzae inoculation. b Heat maps of the significant differentially expressed genes (DEGs) in the redox pathway from sl-MH-1-mock compared with MH86-mock and sl-MH-1-48hpi compared with MH86-48hpi. The red dots indicate the genes with similar trend of transcriptional change in sl-MH-1-mock/MH86-mock and sl-MH-1-48hpi/MH86-48hpi. c The relative transcriptional levels of SL in NPB and NPB-Pizt plants before and after inoculation with P. oryzae isolate KJ201. UBQ was used as the internal control. Bars represent mean values ± SD (n = 3). Statistically significant difference was indicated by ** (p < 0.01, Student’s t-test). The data are from one of three independent experiments performed with similar results
Among the other eighteen redox-related DEGs in sl-48hpi/MH86-48hpi, all of which were up-regulated in sl-MH-1 mutant, five genes, Os06g0102100, Os02g0204700, Os06g0680700, Os10g0320100 and Os06g0501900, encode cytochrome P450 family proteins; Os10g0356000 and Os10g0502400 mainly expressed in photosynthetic system, the later was regulated by phytochromes and cryptochromes under different light conditions [21, 22]; Os08g0104600 encodes an chloroplast-located ferredoxin, involved in transferring electron from photosystem I to NADP+ and the various acceptor systems of metabolic processes [23]; Os03g0290300 and Os06g0216200 (OsOPR2) encodes a ω-3 fatty acid desaturase and a OPR isozyme, respectively, which are involved in JA biosynthesis [24–26]; Os04g0459500 encodes a glyceraldehyde-3-phosphate dehydrogenase (GAPDH), which translocates to the nucleus during apoptosis and influences cytotoxicity [27]. To confirm the above RNA-seq results, we performed quantitative real-time PCR (qRT-PCR) analysis of the key genes in redox pathway, including Os01g0963000, Os08g0104600 and Os03g0290300, in MH86 and sl-MH-3 before and at 48 h after P. oryzae inoculation. As shown in Fig. S6, the transcriptional changes of these genes in sl-MH-3 are similar with those in sl-MH-1 transcritome data.
In addition, we investigated SL transcription in the japonica rice cultivar Nipponbare (NPB) and Piz-t-transgenic NPB (NPB-Pizt) plants before and after inoculation with the blast fungal isolate KJ201, which is avirulent to Piz-t [19, 28]. As shown in Fig. 7c, no significant difference of SL transcriptional levels in NPB and NPB-Pizt was detected before or at 48 h after inoculation. With disease development, SL transcription in NPB was significantly up-regulated at 72 hpi, when it was not obviously altered in NPB-Pizt (Fig. 7c). These results suggest that successful P. oryzae infection could up-regulate SL expression in rice.
Discussion
Sekiguchi lesion is resulted from high accumulation of endogenous ROS
The biological functions of serotonin in rice are mainly explored via characterizing phenotypes of the sekiguchi lesion mutant, as SL is required for converting tryptamine to serotonin. In this study, we identified the mutants of SL gene in MH86 background, which also exhibit sekiguchi lesion under normal growth condition and markedly decreased serotonin content. sl-MH-1 mutant accumulates high level of H2O2 at its lesion sites and is more sensitive to exogenous H2O2 treatment. Furthermore, VC treatment could suppress the lesion formation on sl-MH-1 leaves, and the similar result has been described in the sl mutant [29]. These results suggest that when SL is defective, high endogenous oxidation level leads to cell death occurrence, which is consistent with the role of serotonin as an effective internal ROS scavenger [30]. Meanwhile, when SL is mutated, the H2O2 generated from oxidation of the excessive tryptamine by monoamine oxidase could also contribute to the lesion formation [29]. Excessive ROS production could contribute to or execute PCD by rapidly oxidizing and damaging cellular components, including proteins, nucleic acids and lipids. ROS-induced PCD has been described as a mechanism of photooxidative damage in plants during photosynthesis [31]. It has been reported that the most effective wavelength for inducing typical sekiguchi lesion was 400–700 nm; whereas in the dark, only brown spots and necrotic spot lesions formed on the sl leaves [32]. In addition, sekiguchi lesion could be significantly inhibited by the photosynthetic inhibitor [33]. Therefore, we speculate that under normal growth condition, serotonin is essential for scavenging endogenous oxygen radicals produced during photosynthesis, and thus protecting rice from oxidative damage.
SL negatively regulates rice resistance to biotrophic pathogens
In this study, we found that sl-MH mutants displayed enhanced resistance to both blast fungus and blight bacteria. sl-MH-1 formed the typical lesion surrounding the infection sites, which could efficiently inhibit the pathogen extension. Moreover, the transcriptional level of SL was up-regulated in NPB but not NPB-Pizt at 72 hpi with the blast fungal isolate avirulent to Piz-t, suggesting that a compatible interaction between rice and P. oryzae could induce SL expression. It has been supposed that 72 hpi is the stage when P. oryzae transits from the biotrophic to the necrotrophic phase during its disease cycle [34]. Therefore, the up-regulation of SL expression at this time point may be required for alleviating high oxidative stress caused by host cell collapse upon entering necrotrophic phase. Similarly, serotonin was indicated to function in protecting uninfected tissues from oxidative damage caused by the HR [35].
Our data also showed that sl-MH-1 mutant displayed more robust PTI responses, including PAMPs-induced ROS burst and callose deposition. High oxidative level in plant cells plays multifaceted signaling roles in mediating the establishment of immune responses [36]. For example, ROS could stimulate a rapid Ca2+ influx upon elicitation [37]. All the plant plasma membrane-localized respiratory burst oxydase homologs (Rbohs), which are essential for apoplastic ROS production [38], contain the Ca2+ − binding EF hand motifs at their N terminus, implying that ROS production and Ca2+ influx co-regulate each other [39]. Except that, ROS directly mediates the cross-linking of plant cell wall components to strength the structural barriers against pathogens [40]. Moreover, ROS could also contribute to the activation of plant immune responses by inducing changes in gene expression [41]. Thus, we suppose that the enhanced PTI responses of the sl-MH mutant may also be resulted from its high endogenous ROS accumulation.
In contrast to its enhanced resistance to P. oryzae and Xoo, the sl mutant was reported to be more susceptible to B. oryzae [12]. The divergent pathogenic phenotypes on sl mutants could be resulted from the different trophic types of these pathogens. B. oryzae is a necrotrophic pathogen, which takes nutrition from dead host cells. In this case, the spontaneous cell death of sl mutants could be a favorable term for B. oryzae growth. However, P. oryzae and Xoo are hemibiotrophic and biotrophic pathogens, respectively, to whose colonization, lesion of sl mutants could set a limitation.
SL functions in suppressing accumulation of the defense hormones
SA and JA are the important phytohormones that trigger and mediate a series of defense responses. In Arabidopsis, JA is involved in resistance against necrotrophic pathogens and herbivorous insects [42], whereas SA contributes to defense responses against biotrophic pathogens [43]. And the SA and JA-mediated immune pathways in Arabidopsis are always antagonistic to each other. Although the role of SA in defense is conserved in rice, the SA content is less essential for inducing rice resistance, as rice plants usually accumulate high level of SA during normal growth [44, 45]. Interestingly, SA could also contribute to rice defense against brown planthopper [46]. Furthermore, in contrast to the finding in Arabidopsis, JA in rice positively regulates immune responses against (hemi)biotrophic pathogens [47, 48].
Our results indicated that SA constantly accumulates in sl-MH-1 mutant to a level about twice as high as that in the wild type, and the contents of JA and JA-Ile are dramatically increased in sl-MH-1 when the lesions appear. Considering that SA in rice is essential for modulating redox balance and scavenging the endogenous ROS [49], we speculate that the high SA accumulation in sl-MH-1 may be employed to alleviate its internal oxidative stress. JA is a lipid-derived hormone, whose chloroplastic intermediate, cis-(1)-12-oxophytodienoic acid (OPDA), is derived from oxidatively modified polyunsaturated fatty acids [50]. Thus the increased JA and JA-Ile contents in sl-MH-1 may also be caused by the raised oxidative level, as high levels of JA and its derivant were detected in Arabidopsis when more ROS was accumulated by deletion of Fd2, the major ferredoxin in chloroplasts [23]. On the other hand, Os03g0290300, encoding a ω-3 fatty acid desaturase that is involved in the synthesis of unsaturated fatty acids serving as JA precursor, was significantly up-regulated in sl-MH mutants, which could contribute to JA and JA-Ile production as well.
Conclusions
In this study, we identified sl mutants in MH86 background (sl-MH) and revealed the roles of SL in rice innate immunity. Our results suggest the following conclusions: (i) the sl-MH mutant is more sensitive to exogenous ROS stress, its lesions formation is mainly resulted from excessive accumulation of internal ROS; (ii) SL negatively regulates rice defense against blast fungus and blight bacteria via compromising the PTI responses and suppressing the defense hormones accumulation; (iii) loss function of SL dramatically alters transcription of the genes involved in redox pathway after inoculation with P. oryzae; (iv) success P. oryzae infection up-regulates the transcriptional level of SL in rice. Taken together, our study decipher the negative roles of SL in rice defense against (hemi)biotrophic pathogens.
Methods
Plant materials and blast isolates
The indica rice restore line Minghui 86 was originally bred at Institute of Rice, Fujian Academy of Agricultural Sciences, Fuzhou, China. The sl-MH-1 mutant was generated from the tissue culture-induced mutation of MH86 as described previously [15]. The sl-MH-2 and sl-MH-3 mutants in this study were generated from 60Co ~ γ-ray irradiation of MH86 by us. The rice cultivar Nipponbare, its transgenic line carrying Piz-t (NPB-Pizt) and the P. oryzae isolate KJ201 were originally obtained from Dr. Guo-Liang Wang’s laboratory (Department of Plant Pathology, Ohio State University, Columbus, Ohio). The P. oryzae isolate FJ-1 (virulent to Minghui86) was provided by Dewei Yang (Institute of Rice, Fujian Academy of Agricultural Sciences, Fuzhou, China). The Xoo strain race 6 was provided by Dr. Dingzhong Tang (Plant Immunity Center, Fujian Agriculture and Forestry University, Fuzhou, China). For the H2O2 treatment, P. oryzae inoculation, PTI responses detection and defense hormones measurement assays, the plants were germinated and grown in a growth chamber at 28 °C under a 12-h light (600–800 μmol/m2.s) /12-h dark cycle. For the metabolites measurement, lesion staining, VC treatment and Xoo inoculation assays, the plants were grown in a humidity-controlled greenhouse under natural conditions during summer season.
Blast fungus and blight bacteria inoculation
P. oryzae and Xoo inoculations were carried out in growth chamber and greenhouse, respectively. P. oryzae isolate FJ-1 was cultured on CMII medium for 2 weeks under light for sporulation. Then a conidial suspension with 3 × 105 spores/ml was sprayed on 3-week-old rice leaves. After inoculation, the seedlings were maintained in the dark for 24 h at 28 °C with high humidity, then transferred into the growth chamber for 5 ~ 7 days to evaluate their disease symptom. Four-week-old rice plants were used to perform punch inoculation as previously described [19], and a 10-μl volume of a spore suspension (2 × 105 spores/ml) was applied. Investigation of the fungal biomass in infected rice leaf tissue was carried out as the method described previously [51]. Two punched leaves from one single plant were collected as one biological replicate for the statistic analysis. For leaf sheath inoculation, conidial suspension was injected to the detached sheath cavum of 21-day-old rice plants. Then, the inoculated sheath was kept in an incubator with 80% humidity for the indicated time. Prior to microscopy observing, the surface cell of inner sheath was peeled and made as a slide sample. Inoculation with Xoo was conducted during the tilling stage by the leaf clipping method [52]. The bacterial suspension with optical density at 600 nm (OD600) = 0.5 was used to inoculate rice leaves as follows: scissor was dipped in the bacterial suspension and then used to cut the tip of a rice leaf. The inoculated plants were kept in the greenhouse for 14 days before disease symptom analysis. The blight lesion length and bacterial population accumulated in the infected leaves were evaluated as reported previously [53].
VC treatment
VC (0.1%, diluted with distilled water) was sprayed onto the 3-week-old rice seedlings three times per day (at 9:00, 12:00 and 15:00) for 10 days. Water spraying was used as control.
Analysis of metabolites content
1000 μl of precooled extract solution (acetonitrile:methanol:water, 2:2:1) was added to each 50 mg ground leave samples of MH86 and sl-MH-1. Metabolites extraction was performed as described previously [54] with minor modification as below. After homogenate-sonicate circles and centrifugation, 100 μl of the supernatant was transferred and dried under a nitrogen flow, then 100 μl of 10% methanol was used to reconstitute the residual. Following centrifugation, 80 μl of the supernatant was transferred into an auto-sampler vial for UHPLC-MS/MS analysis. Preparing the standard solution and the UHPLC separation was carried out as described before [54]. L-2-Chloro-phenylalanine was used as the internal standard (IS) at a concentration of 200 nmol/l. Mobile phase A was 0.1% acetic acid in water, and mobile phase B was methanol. The elution time and gradient for UHPLC separation and quantification analysis of the metabolites content were shown in Table S3. Reproducibility was assessed using five biological replicates in each experiment.
SA and JA contents measurement
For measuring the defense hormones contents, the leaves from three 6-week-old MH86, sl-MH-1 or sl-MH-3 plants were harvested as one biological replicate; the leaves from eight 3-week-old MH86 or sl-MH-1 plants were harvested as one biological replicate. The method of measurement is as previously described [55].
DAB and Trypan blue staining
The leaf samples of 8-week-old MH86 and sl-MH-1 plants were soaked in 2 mg/mL 3,3′-diaminobenzidine (DAB) (2 mg/mL DAB,0.05% Tween 20,10 mM Na2HPO4) for 4 ~ 5 h then transferred to the destaining solution (ethanol:lactic acid: Glycerol = 3:1:1). After heating to 90–95 °C for 15 min, the leaves were transferred to a fresh destaining solution and shaked gently overnight. Trypan blue staining was carried out as previously described [56].
Callose deposition
For observing callose deposition, the rice leaves from 7-day-old seedling were treat with chitin (hexa-N-acetylchitohexaose). The assay was carried out as previously described [57]. Finally the leaves were observed under UV light (340 to 380 nm; Zeiss LSM880).
Seedling treated with H2O2
The dehusking rice seeds were sterilized with 75% ethanol for 2 min and 3% sodium hypochlorite for 30 min, then germinated on 1/2 MS medium with or without 0.02% H2O2. The plates were put in a 28 °C growth chamber with a 12-h light/12-h dark cycle for 7 days. Then the seedlings were imaged for analyzing sensitivity to H2O2 treatment.
ROS burst detection
Leaf discs from 7-day-old rice seedlings were floated on sterilized water overnight, then put into 100 ul reaction solution (20 uM luminal and 2.5 μg/mL peroxidase) containing 500 nM flg22 or 400 nM chitin to immediately test the ROS burst. The luminescence was measured by Berthold Mithras luminometer every 1–2 min for 1 h. Each data point represented eight replicates.
RNA-seq sequencing and qRT-PCR analysis
The total RNA from MH86 and sl-MH-1 leaves before and after blast fungus spraying inoculation was extracted using the RNAeasy kit (Qiagen, Germany) and treated with an RNase-Free DNAse Set (Qiagen, Germany), according to the manufacturer’s instructions. The RNA quality, library construction and size were assayed using a 2100 Bioanalyzer system (Agilent, USA). The libraries were synthesized using the TruSeq RNA Sample Preparation v2 kit (Illumina, USA). Total RNA from each treatment was pooled and then two libraries were constructed and used for sequencing. The samples were run in the NovaSeq system and raw sequences of paired 150-bp were obtained.
The analysis below was run on Majorbio (https://www.i-sanger.com). Raw reads were evaluated using fastx_toolkit_0.0.14. SeqPrep and Sickle software were used for clean data. TopHat2 was used to map reads individually for each biological replicate in MH86 or sl-MH-1 against O. sativa japonica cultivar Nipponbare sequence according to MSU 7.0. Cufflinks was used for assembly into transcripts with reference annotation to guide assembly [58]. All the produres were completed according to the settled parameter. The significant differentially expressed loci (SDEL) in sl-MH-1 mutant compared to MH86 were identified after applying multiple corrections (FDR adjusted p ≤ 0.05). The SDEL with fold change ≥2 or ≤ 0.5 were used for further analysis. Functional enrichment analysis (FEA) was performed on SDEL in sl-MH-1 compared to MH86. Functional annotation and expression correlation analysis were conducted on the gene set related to oxidation-reduction biological process. Heat map was generated using cluster analysis.
qRT-PCR analysis was carried out as previously described [23]. The primers used for qRT-PCR in this study are listed in Table S4.
Supplementary Information
Additional file 1: Fig. S1. Lesion phenotype of the allelic sl-MH mutants The leaves of MH86, sl-MH-1, sl-MH-2 and sl-MH-3 were photographed after grown in greenhouse for 6 weeks.
Additional file 2: Fig. S2. Schematic representation of SL gene structure and the mutation sites. Black boxes and lines indicate exons and introns, respectively, and untranslated regions are shown in grey boxes. The arrows indicate the mutation sites of the allelic sl-MH mutants.
Additional file 3: Fig. S3. The content of L-glutamine in MH86 and sl-MH-1. The levels of L-glutamine in 8-week-old MH86 and sl-MH-1 plants were measured by UPLC. Bars represent mean values ± SD from five biological replicates. Statistically significant difference was indicated by ** (p < 0.01, Student’s t-test).
Additional file 4: Fig. S4. Resistance of sl-MH-3 to P. oryzae and Xoo. a. 3-week-old MH86 and sl-MH-3 seedlings were inoculated with P. oryzae conidia by spraying and the diseased leaves were imaged at 7 dpi. b. Punch inoculation with P. oryzae conidia was performed on the leaves of 4-week-old MH86 and sl-MH-3 plants. The diseased leaves were photographed at 9 dpi (left), and the fungal biomass was measured (right). Bars represent mean values ± SD from three biological replicates. Statistically significant difference was indicated by ** (p < 0.01, Student’s t-test). c. MH86 and sl-MH-3 plants were inoculated with Xoo. The infected leaves from three independent MH86 or sl-MH-3 plants were imaged at 14 dpi. d. Blight lesion length on sl-MH-3 and MH86 leaves were measured (left), bars represent mean values ± SD (n = 6, from three independent plants; * means p < 0.05 by Student’s t-test). Blight bacterial populations were counted 14 dpi with bars representing mean values ± SD from three independent MH86 or sl-MH-3 plants (right), ** means p < 0.01 by Student’s t-test. e. Callose deposition on MH86 and sl-MH-3 leaves after chitin treatment was imaged with a microscope under UV light (left). Number of the callose deposition per view was counted (right). Bars represent mean values and SD (n = 5). Statistically significant difference was indicated by ** (p < 0.01, Student’s t-test).
Additional file 5: Fig. S5. Contents of defense hormones in 3-week-old MH86 and sl-MH-1 plants. The resting levels of SA, JA and JA-Ile in 3-week-old MH86 and sl-MH-1 leaves were measured by UPLC. Bars represent mean values ± SD from three biological replicates. Statistically significant difference was indicated by ** (p < 0.01, Student’s t-test).
Additional file 6: Fig. S6. qRT-PCR analysis of the representative genes of redox pathway in MH86 and sl-MH-3. The transcriptional levels of Os01g0963000, Os08g0104600 and Os03g0290300 were determined in MH86 and sl-MH-3 at 48 hpi with P. oryzae (water spraying was employed as mock treatment). UBQ was used as the internal control. Bars represent mean values ± SD (n = 3). Statistically significant difference was indicated by ** (p < 0.01, Student’s t-test).
Additional file 7: Table S1. Significant differentially expressed genes in sl-MH-1-mock/MH86-mock.
Additional file 8: Table S2. Significant differentially expressed genes in sl-MH-1-48hpi/MH86-48hpi.
Additional file 9: Table S3. Quantification of serotonin, tryptamine, L-trytophan and L-glutamine by UHPLC-MS.
Additional file 10: Table S4. Sequences of the qRT-PCR primers in this study.
Acknowledgements
We thank Dr. Songbiao Chen at Institute of Oceanography, Minjiang University, Fuzhou, China for sharing sl-MH-1 seeds. Dr. Dingzhong Tang at Plant Immunity Center, Fujian Agriculture and Forestry University, Fuzhou, China for providing the Xoo strain race 6, Dewei Yang at Institute of Rice, Fujian Academy of Agricultural Sciences, Fuzhou, China for providing the P. oryzae isolate FJ-1, and Jinfang Chu at Institute of Genetics and Developmental Biology, Chinese Academy of Sciences, Beijing, China for helping us to measure and analyze contents of the defense hormones.
Abbreviations
- PCD
Programmed cell death
- Vc
Vitamin C
- PAMPs
Pathogen-associated molecular patterns
- PTI
PAMPs-triggered immunity
- ROS
Reactive oxygen species
- P. oryzae
Pyricularia oryzae
- Xoo
Xanthomonas oryzae pv. Oryzae
- B
oryzae: Bipolaris oryzae
- redox
Reduction–oxidation
- ETI
Effector-triggered immunity
- HR
Hypersensitive response
- sl
sekiguchi lesion
- DAB
3,3-diaminobenzidine tetrahydrochloride
- MS
Murashige and Skoog
- SA
Salicylic acid
- JA
Jasmonic acid
- JA-Ile
JA-isoleucine
- hpi
Hours post inoculation
- SDEL
Significant differentially expressed loci
- DEGs
Differentially expressed genes
- RNA-Seq
RNA-sequencing
- qRT-PCR
quantitative real-time polymerase chain reaction
Authors’ contributions
MW and DT conceived and designed the project. FY, YN and DT performed the experiments. DT, FY, YN, YL, ZC and GL analyzed the data. MW, DT, QL and FW wrote and edited the manuscript. All the authors have read and approved the final manuscript.
Funding
This work was supported by the Natural Science Foundation of Fujian Province (Number: 2014 J01102), Youth Program of National Natural Science Foundation of China (Number: 31301654), China Postdoctoral Science Foundation (Number: 2019 M662919) and National R&D Project of Transgenic Crops of Ministry of Science and Technology of China (Number: 2017ZX08001–001).
Availability of data and materials
All data sustaining the results in this study are included in this manuscript or its supplementary information files. The datasets analyzed during the current study are available from the corresponding author on reasonable request. The raw sequencing data reported in this article have been deposited in NCBI SRA database, under accession number PRJNA634690, which are publicly accessible at http://www.ncbi.nlm.nih.gov/bioproject/634690.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no conflicts of interest.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Dagang Tian, Fang Yang and Yuqing Niu contributed equally to this work.
Contributor Information
Feng Wang, Email: wf@fjage.org.
Mo Wang, Email: wangmo108@163.com.
References
- 1.Jones JDG, Dangl JL. The plant immune system. Nature. 2006;444(7117):323–329. doi: 10.1038/nature05286. [DOI] [PubMed] [Google Scholar]
- 2.Tang D, Wang G, Zhoud J-M. Receptor kinases in plant-pathogen interactions: more than pattern recognition. Plant Cell. 2017;29:618–637. doi: 10.1105/tpc.16.00891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Peng Y, Wersch RV, Zhang Y. Convergent and divergent signaling in PAMP-triggered immunity and effector-triggered immunity. Mol Plant-Microbe Interact. 2017;31(4):403–409. doi: 10.1094/MPMI-06-17-0145-CR. [DOI] [PubMed] [Google Scholar]
- 4.Hofius D, Tsitsigiannis DI, Jones JDG, Mundy J. Inducible cell death in plant immunity. Semin Cancer Biol. 2007;17(2):166–187. doi: 10.1016/j.semcancer.2006.12.001. [DOI] [PubMed] [Google Scholar]
- 5.Spoel SH, Loake GJ. Redox-based protein modifications: the missing link in plant immune signalling. Curr Opin Plant Biol. 2011;14(4):358–364. doi: 10.1016/j.pbi.2011.03.007. [DOI] [PubMed] [Google Scholar]
- 6.Coll NS, Epple P, Dangl JL. Programmed cell death in the plant immune system. Cell Death Differ. 2011;18(8):1247–1256. doi: 10.1038/cdd.2011.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Veenstra-Vanderweele J, Anderson GM, Cook EH. Pharmacogenetics and the serotonin system: initial studies and future directions. Eur J Pharmacol. 2000;410(2):165–181. doi: 10.1016/S0014-2999(00)00814-1. [DOI] [PubMed] [Google Scholar]
- 8.Bowden K, Brown BG, Batty JE. 5-Hydroxytryptamine: its occurrence in cowhage (Mucuna pruriens) Nature. 1954;174:925–926. doi: 10.1038/174925a0. [DOI] [PubMed] [Google Scholar]
- 9.Ramakrishna A, Giridhar P, Ravishankar GA. Phytoserotonin: a review. Plant Signal Behav. 2011;6(6):800–809. doi: 10.4161/psb.6.6.15242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schröder P, Abele C, Gohr P, Stuhlfauth-Roisch U, Grosse W. Latest on enzymology of serotonin biosynthesis in walnut seeds. Adv Exp Med Biol. 1999;467:637–644. doi: 10.1007/978-1-4615-4709-9_81. [DOI] [PubMed] [Google Scholar]
- 11.Huether G, Fettkötter I, Keilhoff G, Wolf G. Serotonin acts as a radical scavenger and is oxidized to a dimer during the respiratory burst of activated microglia. J Neurochem. 2010;69(5):2096–2101. doi: 10.1046/j.1471-4159.1997.69052096.x. [DOI] [PubMed] [Google Scholar]
- 12.Ishihara A, Hashimoto Y, Tanaka C, Dubouzet JG, Nakao T, Matsuda F, Nishioka T, Miyagawa H, Wakasa K. The tryptophan pathway is involved in the defense responses of rice against pathogenic infection via serotonin production. Plant J. 2008;54(3):481–495. doi: 10.1111/j.1365-313X.2008.03441.x. [DOI] [PubMed] [Google Scholar]
- 13.Liu W, Liu J, Triplett L, Leach JE, Wang GL. Novel insights into rice innate immunity against bacterial and fungal pathogens. Annu Rev Phytopathol. 2014;52(1):213–241. doi: 10.1146/annurev-phyto-102313-045926. [DOI] [PubMed] [Google Scholar]
- 14.Fujiwara T, Maisonneuve S, Isshiki M, Mizutani M, Chen L, Wong HL, Kawasaki T, Shimamoto K. Sekiguchi lesion gene encodes a cytochrome P450 monooxygenase that catalyzes conversion of tryptamine to serotonin in rice. J Biol Chem. 2010;285(15):11308–11313. doi: 10.1074/jbc.M109.091371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gao X, Chen Z, Song Y, Chen Z, Tian D, Lin Y, Yang S, Chen S, Wang F. Identification and gene mapping of a runaway cell death mutant rcd1 in Rice. Mol Plant Breed. 2015;13:1433–1440. [Google Scholar]
- 16.Pavet V, Olmos E, Kiddle G, Mowla S, Kumar S, Antoniw J. Alvarez MaE, foyer CH: ascorbic acid deficiency activates cell death and disease resistance responses in Arabidopsis. Plant Physiol. 2005;139(3):1291–1303. doi: 10.1104/pp.105.067686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang R, Liu S, Zhou F, Ding C. Exogenous ascorbic acid and glutathione alleviate oxidative stress induced by salt stress in the chloroplasts of Oryza sativa L. Z Naturforsch C. 2014;69:226–236. doi: 10.5560/znc.2013-0117. [DOI] [PubMed] [Google Scholar]
- 18.Hasegawa M, Mitsuhara I, Seo S, Imai T, Koga J, Okada K, Yamane H, Ohashi Y. Phytoalexin accumulation in the interaction between Rice and the blast fungus. Mol Plant-Microbe Interact. 2010;23(8):1000–1011. doi: 10.1094/MPMI-23-8-1000. [DOI] [PubMed] [Google Scholar]
- 19.Park CH, Shirsekar G, Bellizzi M, Chen S, Songkumarn P, Xie X, Shi X, Ning Y, Zhou B, Suttiviriya P, et al. The E3 ligase APIP10 connects the effector AvrPiz-t to the NLR receptor Piz-t in Rice. PLoS Pathog. 2016;12(3):e1005529. doi: 10.1371/journal.ppat.1005529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu X, Williams CE, Nemacheck JA, Wang H, Subramanyam S, Zheng C, Chen M-S. Reactive oxygen species are involved in plant defense against a gall midge. Plant Physiol. 2010;152(2):985–999. doi: 10.1104/pp.109.150656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mccormac AC, Fischer A, Kumar AM, Söll D, Terry MJ. Regulation of HEMA1 expression by phytochrome and a plastid signal during de-etiolation in Arabidopsis Thaliana. Plant J. 2001;25(5):549–561. doi: 10.1046/j.1365-313x.2001.00986.x. [DOI] [PubMed] [Google Scholar]
- 22.Tanaka R, Tanaka A. Chlorophyll cycle regulates the construction and destruction of the light-harvesting complexes. Biochimica et Biophysica Acta (BBA)/Bioenergetics. 2011;1807(8):968–976. doi: 10.1016/j.bbabio.2011.01.002. [DOI] [PubMed] [Google Scholar]
- 23.Wang M, Rui L, Yan H, Shi H, Zhao W, Lin JE, Zhang K, Blakeslee JJ, Mackey D, Tang D, et al. The major leaf ferredoxin Fd2 regulates plant innate immunity in Arabidopsis. Mol Plant Pathol. 2018;19(6):1377–1390. doi: 10.1111/mpp.12621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sobajima H, Takeda M, Sugimori M, Kobashi N, Kiribuchi K, Cho E-M, Akimoto C, Yamaguchi T, Minami E, Shibuya N, et al. Cloning and characterization of a jasmonic acid-responsive gene encoding 12-oxophytodienoic acid reductase in suspension-cultured rice cells. Planta. 2003;216(4):692–698. doi: 10.1007/s00425-002-0909-z. [DOI] [PubMed] [Google Scholar]
- 25.Taki N, Sasaki-Sekimoto Y, Obayashi T, Kikuta A, Kobayashi K, Ainai T, Yagi K, Sakurai N, Suzuki H, Masuda T, et al. 12-oxo-phytodienoic acid triggers expression of a distinct set of genes and plays a role in wound-induced gene expression in Arabidopsis. Plant Physiol. 2005;139(3):1268–1283. doi: 10.1104/pp.105.067058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Goepfert S, Poirier Y. β-Oxidation in fatty acid degradation and beyond. Curr Opin Plant Biol. 2007;10(3):245–251. doi: 10.1016/j.pbi.2007.04.007. [DOI] [PubMed] [Google Scholar]
- 27.Hara MR, Agrawal N, Kim SF, Cascio MB, Fujimuro M, Ozeki Y, Takahashi M, Cheah JH, Tankou SK, Hester LD, et al. S-nitrosylated GAPDH initiates apoptotic cell death by nuclear translocation following Siah1 binding. Nat Cell Biol. 2005;7(7):665–674. doi: 10.1038/ncb1268. [DOI] [PubMed] [Google Scholar]
- 28.Zhou B, Qu S, Liu G, Dolan M, Sakai H, Lu G, Bellizzi M, Wang GL. The eight amino-acid differences within three leucine-rich repeats between Pi2 and Piz-t resistance proteins determine the resistance specificity to Magnaporthe grisea. Mol Plant-Microbe Interact. 2006;19(11):1216–1228. doi: 10.1094/MPMI-19-1216. [DOI] [PubMed] [Google Scholar]
- 29.Ueno M, Shibata H, Kihara J, Honda Y, Arase S. Increased tryptophan decarboxylase and monoamine oxidase activities induce Sekiguchi lesion formation in rice infected with Magnaporthe grisea. Plant J. 2003;36(2):215–228. doi: 10.1046/j.1365-313X.2003.01875.x. [DOI] [PubMed] [Google Scholar]
- 30.Huether G, Fettkötter I, Keilhoff G, Wolf G. Serotonin acts as a radical scavenger and is oxidized to a dimer during the respiratory burst of activated microglia . J Neurochem. 2002;69(5):2096–2101. doi: 10.1046/j.1471-4159.1997.69052096.x. [DOI] [PubMed] [Google Scholar]
- 31.Triantaphylides C, Krischke M, Hoeberichts FA, Ksas B, Gresser G, Havaux M, Van Breusegem F, Mueller MJ. Singlet oxygen is the major reactive oxygen species involved in Photooxidative damage to plants. Plant Physiol. 2008;148(2):960–968. doi: 10.1104/pp.108.125690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Arase S, Fukuyama R, Honda Y. Light-dependent induction of Sekiguchi lesion formation by Bipolaris oryzae in rice cv. Sekiguchi-asahi. J Phytopathol. 2000;148(4):193–196. doi: 10.1046/j.1439-0434.2000.00497.x. [DOI] [Google Scholar]
- 33.Iedome M, Arase S, Honda Y, Nozu M. Light-dependent necrosis formation by Magnaporthe grisea toxin(s) in Rice cv. Sekiguchi-asahi. J Phytopathol. 2008;143:325–328. doi: 10.1111/j.1439-0434.1995.tb00269.x. [DOI] [Google Scholar]
- 34.Mathioni SM, Beló A, Rizzo CJ, Dean RA, Donofrio NM. Transcriptome profiling of the rice blast fungus during invasive plant infection and in vitro stresses. BMC Genomics. 2011;12:49. doi: 10.1186/1471-2164-12-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hayashi K, Fujita Y, Ashizawa T, Suzuki F, Nagamura Y, Hayano-Saito Y. Serotonin attenuates biotic stress and leads to lesion browning caused by a hypersensitive response to Magnaporthe oryzae penetration in rice. Plant J Cell Mol Biol. 2016;85(1):46–56. doi: 10.1111/tpj.13083. [DOI] [PubMed] [Google Scholar]
- 36.Lehmann S, Serrano M, L’Haridon F, Tjamos SE, Metraux J-P. Reactive oxygen species and plant resistance to fungal pathogens. Phytochemistry. 2014;112:54–62. doi: 10.1016/j.phytochem.2014.08.027. [DOI] [PubMed] [Google Scholar]
- 37.Levine A, Pennell RI, Alvarez ME, Palmer R, Lamb C. Calcium-mediated apoptosis in a plant hypersensitive disease resistance response. Curr Biol. 1996;6(4):427–437. doi: 10.1016/S0960-9822(02)00510-9. [DOI] [PubMed] [Google Scholar]
- 38.Torres MA, Dangl JL, Jones JDG. Arabidopsis gp91phox homologues AtrbohD and AtrbohF are required for accumulation of reactive oxygen intermediates in the plant defense response. Proc Natl Acad Sci. 2002;99(1):517–522. doi: 10.1073/pnas.012452499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Torres MA, Dangl JL. Functions of the respiratory burst oxidase in biotic interactions, abiotic stress and development. Curr Opin Plant Biol. 2005;8(4):397–403. doi: 10.1016/j.pbi.2005.05.014. [DOI] [PubMed] [Google Scholar]
- 40.Almagro L, Gomez Ros LV, Belchi-Navarro S, Bru R, Ros Barcelo A, Pedreno MA. Class III peroxidases in plant defence reactions. J Exp Bot. 2009;60(2):377–390. doi: 10.1093/jxb/ern277. [DOI] [PubMed] [Google Scholar]
- 41.Kotchoni SO, Gachomo EW. The reactive oxygen species network pathways:an essential prerequisite for perception of pathogen attack and the acquired disease resistance in plants. J Biosci. 2006;31(3):389–404. doi: 10.1007/BF02704112. [DOI] [PubMed] [Google Scholar]
- 42.Glazebrook J. Contrasting mechanisms of defense against biotrophic and necrotrophic pathogens. Annu Rev Phytopathol. 2005;43:205–227. doi: 10.1146/annurev.phyto.43.040204.135923. [DOI] [PubMed] [Google Scholar]
- 43.Vlot AC, Dempsey DMA, Klessig DF. Salicylic acid, a multifaceted hormone to combat disease. Annu Rev Phytopathol. 2009;47:177–206. doi: 10.1146/annurev.phyto.050908.135202. [DOI] [PubMed] [Google Scholar]
- 44.Yang D-L, Yang Y, He Z. Roles of plant hormones and their interplay in Rice immunity. Mol Plant. 2013;6:675–685. doi: 10.1093/mp/sst056. [DOI] [PubMed] [Google Scholar]
- 45.Silverman P, Seskar M, Kanter D, Schweizer P, Métraux J-P, Raskin I. Salicylic acid in Rice: biosynthesis, conjugation and possible role. Plant Physiol. 1995;108:633–639. doi: 10.1104/pp.108.2.633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lu HP, Luo T, Fu HW, Wang L, Tan Y-y, Huang J-z, Wang Q, Ye G-y, Gatehouse A, Lou Y-g, et al. Resistance of rice to insect pests mediated by suppression of serotonin biosynthesis. Nature Plants. 2018;4(6):338–344. doi: 10.1038/s41477-018-0152-7. [DOI] [PubMed] [Google Scholar]
- 47.Yamada S, Kano A, Tamaoki D, Miyamoto A, Shishido H, Miyoshi S, Taniguchi S, Akimitsu K, Gomi K. Involvement of OsJAZ8 in Jasmonate-induced resistance to bacterial blight in Rice. Plant Cell Physiol. 2012;53(12):2060–2072. doi: 10.1093/pcp/pcs145. [DOI] [PubMed] [Google Scholar]
- 48.Mei C, Qi M, Sheng G, Yang Y. Inducible overexpression of a rice allene oxide synthase gene increases the endogenous jasmonic acid level, PR gene expression, and host resistance to fungal infection. Mol Plant-Microbe Interact. 2006;19:1127–1137. doi: 10.1094/MPMI-19-1127. [DOI] [PubMed] [Google Scholar]
- 49.Yang Y, Qi M, Mei C. Endogenous salicylic acid protects rice plants from oxidative damage caused by aging as well as biotic and abiotic stress. Plant J. 2004;40(6):909–919. doi: 10.1111/j.1365-313X.2004.02267.x. [DOI] [PubMed] [Google Scholar]
- 50.Wasternack C, Hause B. Jasmonates: biosynthesis, perception, signal transduction and action in plant stress response, growth and development. Ann Bot. 2013;111:1021–1058. doi: 10.1093/aob/mct067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Park C-H, Chen S, Shirsekar G, Zhou B, Khang CH, Songkumarn P, Afzal AJ, Ning Y, Wang R, Bellizzi M, et al. The Magnaporthe oryzae effector AvrPiz-t targets the RING E3 ubiquitin ligase APIP6 to suppress pathogen-associated molecular pattern-triggered immunity in rice. Plant Cell. 2012;24(11):4748–4762. doi: 10.1105/tpc.112.105429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kauffman HE, Reddy APK, Hsieh SPY, Merca SD. An improved technique for evaluating resistance to rice varieties of Xanthomonas oryzae pv. Oryzae. Plant Dis Rep. 1973;57:537–541. [Google Scholar]
- 53.Chern M, Canlas PE, Fitzgerald HA, Ronald PC. Rice NRR, a negative regulator of disease resistance, interacts with Arabidopsis NPR1 and rice NH1. Plant J. 2005;43:623–635. doi: 10.1111/j.1365-313X.2005.02485.x. [DOI] [PubMed] [Google Scholar]
- 54.Ou J, Peng Y, Yang W, Zhang Y, Hao J, Li F, Chen Y, Zhao Y, Xie X, Wu S, et al. ABHD5 blunts the sensitivity of colorectal cancer to fluorouracil via promoting autophagic uracil yield. Nat Commun. 2019;10(1):1078. doi: 10.1038/s41467-019-08902-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Fu J, Chu J, Sun X, Wang J, Yan C. Simple, rapid, and simultaneous assay of multiple carboxyl containing Phytohormones in wounded tomatoes by UPLC-MS/MS using single SPE purification and isotope dilution. Anal Sci. 2012;28(11):1081–1087. doi: 10.2116/analsci.28.1081. [DOI] [PubMed] [Google Scholar]
- 56.Akhter D, Qin R, Nath UK, Alamin M, Jin X, Shi C. The Brown Midrib Leaf (bml) Mutation in Rice (Oryza sativa L.) Causes Premature Leaf Senescence and the Induction of Defense Responses. Genes. 2018;9(4):203. doi: 10.3390/genes9040203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yang C, Yu Y, Huang J, Meng F, Pang J, Zhao Q, Islam MA, Xu N, Tian Y, Liu J. Binding of the Magnaporthe oryzae chitinase MoChia1 by a rice tetratricopeptide repeat protein allows free chitin to trigger immune responses. Plant Cell. 2019;31(1):172–188. doi: 10.1105/tpc.18.00382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Trapnell C, Williams BA, Pertea G, Mortazavi A, Kwan G. Baren MJv, Salzberg SL, Wold BJ, Pachter L: transcript assembly and quantification by RNA-Seq reveals unannotated transcripts and isoform switching during cell differentiation. Nat Biotechnol. 2010;28(5):511–515. doi: 10.1038/nbt.1621. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Fig. S1. Lesion phenotype of the allelic sl-MH mutants The leaves of MH86, sl-MH-1, sl-MH-2 and sl-MH-3 were photographed after grown in greenhouse for 6 weeks.
Additional file 2: Fig. S2. Schematic representation of SL gene structure and the mutation sites. Black boxes and lines indicate exons and introns, respectively, and untranslated regions are shown in grey boxes. The arrows indicate the mutation sites of the allelic sl-MH mutants.
Additional file 3: Fig. S3. The content of L-glutamine in MH86 and sl-MH-1. The levels of L-glutamine in 8-week-old MH86 and sl-MH-1 plants were measured by UPLC. Bars represent mean values ± SD from five biological replicates. Statistically significant difference was indicated by ** (p < 0.01, Student’s t-test).
Additional file 4: Fig. S4. Resistance of sl-MH-3 to P. oryzae and Xoo. a. 3-week-old MH86 and sl-MH-3 seedlings were inoculated with P. oryzae conidia by spraying and the diseased leaves were imaged at 7 dpi. b. Punch inoculation with P. oryzae conidia was performed on the leaves of 4-week-old MH86 and sl-MH-3 plants. The diseased leaves were photographed at 9 dpi (left), and the fungal biomass was measured (right). Bars represent mean values ± SD from three biological replicates. Statistically significant difference was indicated by ** (p < 0.01, Student’s t-test). c. MH86 and sl-MH-3 plants were inoculated with Xoo. The infected leaves from three independent MH86 or sl-MH-3 plants were imaged at 14 dpi. d. Blight lesion length on sl-MH-3 and MH86 leaves were measured (left), bars represent mean values ± SD (n = 6, from three independent plants; * means p < 0.05 by Student’s t-test). Blight bacterial populations were counted 14 dpi with bars representing mean values ± SD from three independent MH86 or sl-MH-3 plants (right), ** means p < 0.01 by Student’s t-test. e. Callose deposition on MH86 and sl-MH-3 leaves after chitin treatment was imaged with a microscope under UV light (left). Number of the callose deposition per view was counted (right). Bars represent mean values and SD (n = 5). Statistically significant difference was indicated by ** (p < 0.01, Student’s t-test).
Additional file 5: Fig. S5. Contents of defense hormones in 3-week-old MH86 and sl-MH-1 plants. The resting levels of SA, JA and JA-Ile in 3-week-old MH86 and sl-MH-1 leaves were measured by UPLC. Bars represent mean values ± SD from three biological replicates. Statistically significant difference was indicated by ** (p < 0.01, Student’s t-test).
Additional file 6: Fig. S6. qRT-PCR analysis of the representative genes of redox pathway in MH86 and sl-MH-3. The transcriptional levels of Os01g0963000, Os08g0104600 and Os03g0290300 were determined in MH86 and sl-MH-3 at 48 hpi with P. oryzae (water spraying was employed as mock treatment). UBQ was used as the internal control. Bars represent mean values ± SD (n = 3). Statistically significant difference was indicated by ** (p < 0.01, Student’s t-test).
Additional file 7: Table S1. Significant differentially expressed genes in sl-MH-1-mock/MH86-mock.
Additional file 8: Table S2. Significant differentially expressed genes in sl-MH-1-48hpi/MH86-48hpi.
Additional file 9: Table S3. Quantification of serotonin, tryptamine, L-trytophan and L-glutamine by UHPLC-MS.
Additional file 10: Table S4. Sequences of the qRT-PCR primers in this study.
Data Availability Statement
All data sustaining the results in this study are included in this manuscript or its supplementary information files. The datasets analyzed during the current study are available from the corresponding author on reasonable request. The raw sequencing data reported in this article have been deposited in NCBI SRA database, under accession number PRJNA634690, which are publicly accessible at http://www.ncbi.nlm.nih.gov/bioproject/634690.