Abstract
Pneumopericardium is a rare complication of pericardiocentesis (PC), occurring as a result of either a direct pleuropericardial communication or a leaky drainage system. Pneumopericardium is often self-limiting; however, physicians should be aware of this complication as it may progress to tension pneumopericardium, which requires immediate recognition and management. PC has been associated with pneumothorax, pneumomediastinum or subcutaneous emphysema, but the association with pleural effusion has been less reported. The authors present the case of a 14-year-old healthy boy who developed post-PC pneumopericardium and pleural effusion, a rare association reported in the literature. The diagnosis of this potential life-threatening event was made using readily available complementary diagnostic methods, such as transthoracic echocardiography and chest X-ray.
Keywords: air leaks, pericardial disease, paediatric intensive care
Background
Pneumopericardium is defined as the presence of an air-fluid level in the pericardial sac. It is a rare entity that has been reported most commonly in the context of invasive mechanical ventilation or spontaneously without any underlying cause.1 Pneumopericardium resulting after pericardiocentesis (PC) is even rarer and has been attributed either to a direct pleuropericardial communication or to an air leakage in the pericardial drainage system.2 3 Usually, it is a stable condition, but it may generate a tension effect on the heart, becoming a life-threatening event.4–8
In this case, post-PC pneumopericardium was associated with pleural effusion, a unique combination with few cases reported in the literature so far. This case also highlights the potential risks associated with this entity and the need for preventive measures and diagnostic means during emergency procedures.
Case presentation
A 14-year-old boy, without relevant medical history, besides attention deficit hyperactivity disorder medicated with methylphenidate and risperidone, was referred to our hospital due to a 3-week-old history of intermittent fever (axillar maximum temperature of 38.9°C) and mild upper respiratory tract symptoms. Four days before admission, he started becoming tired and had precordial pain, suggestive of pericarditis.
On examination, he was dyspnoeic and tachycardic with a normal blood pressure. Heart sounds were muffled, and an intermittent friction rub was audible. Pulsus paradoxus was present (11 mm Hg). Transthoracic echocardiography (TTE) showed a large circumferential pericardial effusion with expiratory diastolic collapse of the right atrium and ventricle (figure 1, video 1).
Figure 1.
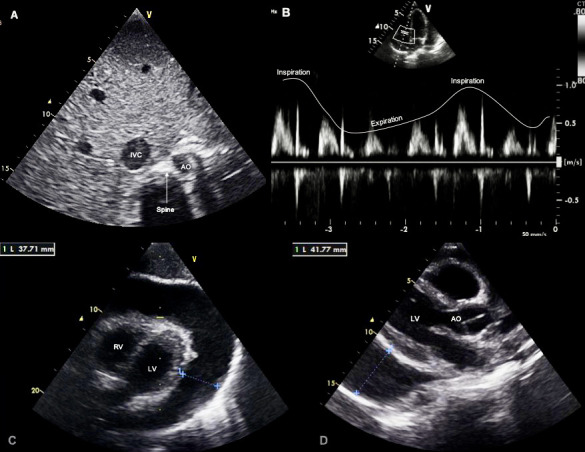
Transthoracic echocardiogram confirmed the tamponade physiology. (A) The abdominal short-axis view showed a dilated IVC, an indirect sign of increased pressure in the right cavities. (B) Doppler study revealed a significant respiratory variation of tricuspid valve inflow with an increase in velocity during inspiration. (C) Subcostal view and (D) parasternal long-axis view showed extensive (37–41 mm) circumferential pericardial effusion (**) in diastole. AO, aorta; IVC, inferior vena cava; LV, left ventricle; RV, right ventricle.
Video 1.
An echo-guided subxiphoid percutaneous PC was performed with a 10 Fr dilator, with an 8.5 Fr pigtail pericardial drain left in situ and attached to a Pleur-evac chest drainage system. The procedure was incident-free, and 700 mL of serosanguineous fluid was immediately drained under tension. Overall, 850 mL of pericardial fluid was drained. A postprocedural TTE showed a significant reduction in pericardial effusion with relief of the right ventricular wall diastolic collapse (video 2).
Video 2.
After PC, the patient’s chest pain and haemodynamic parameters immediately improved. Twelve hours later, a pneumopericardium with pleural effusion but without pneumothorax was diagnosed (figure 2A, A″). About 250 mL of air was aspirated. Due to the patient’s haemodynamic stability, he was managed conservatively. Although the pneumopericardium steadily resolved, pleural effusion worsened, requiring a thoracocentesis draining of 140 mL of serosanguineous fluid similar to the pericardial fluid (figure 2B).
Figure 2.
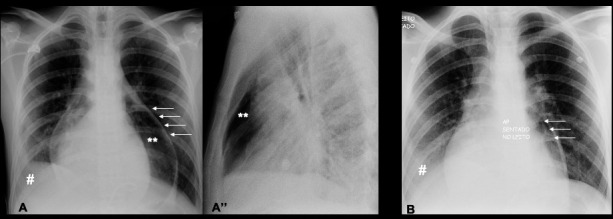
(A, A″) Chest X-ray, day 1 after pericardiocentesis (PC), confirmed the pneumopericardium. (A) The posteroanterior view showed a sharply defined linear structure representing the pericardial sac (←) encompassing an air density (**) that surrounds the cardiac silhouette; pleural effusion on the right side was also visible (#). (A″) The lateral view showed the air confined to the level of great arteries being different from a pneumomediastinum. (B) Day 3 after PC, the posteroanterior view showed a reduction in pneumopericardium (←) after syringe aspiration and the worsening of right pleural effusion (#).
Three days after PC, the pericardial fluid ceased to drain, and the pericardial drain was removed. Pleural effusion also improved, as did the pneumopericardium (figure 3A).
Figure 3.
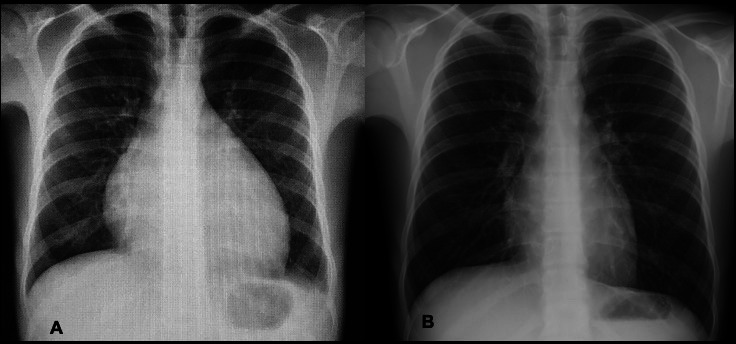
(A) Posteroanterior chest X-ray on admission showed cardiomegaly (cardiothoracic index: 62%) with clear lung fields. (B) On day 12 after pericardiocentesis, there was regression of pericardial air after conservative treatment.
Despite its rarity, pneumopericardium can result in life-threatening cardiac tamponade, and clinicians need to be aware of this entity.
Investigations
The admission 12-lead ECG revealed classic signs of pericarditis, with ST-segment elevation more evident in precordial leads V2 and V3 and diffuse PR-segment depression. The cardiothoracic index was 62% on chest X-ray (CXR), and the lung fields were clear (figure 3A).
The first TTE showed signs of impending cardiac tamponade (figure 1, video 1)—inferior vena cava ectasia without respiratory modulation, a large (40 mm) circumferential pericardial effusion with right atrial and ventricular diastolic collapse and left-sided interventricular shift; the pulsed-wave Doppler showed an exaggerated respiratory variation in the tricuspid valve inflow, with a significant increase in velocity during inspiration; however, there were no significant variations in mitral inflow or aortic flow velocities. Left ventricular systolic function was preserved with adequate free wall motion. Pulmonary artery pressure was within the normal range. Immediately after PC, TTE showed a significant reduction in pericardial effusion and the absence of right ventricular wall diastolic collapse (video 2).
The pericardial fluid analysis revealed an exudate containing 85 mg/dL of glucose (serum glucose—99 mg/dL), 5.7 g/dL of protein (serum proteins—6 g/dL), 842 U/L of lactate dehydrogenase and 3.3×109/L of white blood cells (45% neutrophils, 40% lymphocytes). Pericardial histopathology and culture were negative for specific pericardial diseases and microorganisms.
A TTE performed 12 hours after PC failed to clearly show the heart. Air being a poor conductor of ultrasound, the diagnosis of a pneumopericardium was hypothesised. A CXR showed a lucent outline separating the pericardium from the cardiac silhouette (figure 2A-A″). A bilateral pleural effusion, more pronounced on the right side, was noted in this first control CXR and worsened progressively on serial evaluations (figure 2B), causing atelectasis of the ipsilateral lung segment as shown by a thoracic ultrasound, leading to a thoracocentesis.
Pleural and pericardial fluid shared the same analytical and histopathological exudate characteristics.
The possibility of a polyserositis was raised, so an abdominal ultrasound was performed, showing no evidence of peritoneal effusion.
The blood analysis showed normal white blood cell count, procalcitonin of 0.38 ng/mL, C reactive protein of 15 mg/dL, sedimentation velocity of 78 mm/1st h and normal cardiac enzymes. Autoimmunity studies and thyroid hormones levels were normal. Protein chain reaction (PCR) for cytomegalovirus, mycoplasma, parvovirus, Epstein-Barr virus, enterovirus and adenovirus were negative. PCR in respiratory secretions was positive for rhinovirus.
Differential diagnosis
Our patient’s presentation was compatible with a viral pericarditis as it is the most common cause of this entity in children: he had a recent history of flu-like symptoms, and the etiological agent identified was a rhinovirus. Pericardial effusion was a complication of his pericarditis.
Pneumopericardium is defined as the presence of air in the pericardial space being superiorly confined to the level of great arteries, as found in our patient (figure 2), and it differs from a pneumomediastinum in that the air moves up to the superior mediastinum and neck.5 9 However, it is a rare complication of pericarditis per se, and in our case, the most likely cause was iatrogenic; it usually occurs within minutes to hours after PC, as it happened in this case.10
Post-PC pneumopericardium could be caused by suction of air secondary to a defect in the drainage system. This did not appear to be the case as the drainage system connections were airtight, and the actual pericardial drain was in an adequate position (no sideholes extended outside the pericardial space).
Another possible explanation was that the skin orifice created by the dilator (10 Fr) left a potential space around the pericardial drain (8.5 Fr), causing a Venturi effect during the respiratory cycle. Under these circumstances, air was suctioned into the pericardial sac. For this mechanism to be responsible for the pneumopericardium, considerable negative intrathoracic pressures have to be generated. It is worth noting that our patient was in room air, without significant respiratory distress.11
We also hypothesise that, as a consequence of the procedure, a pericardial-pleural fistula was created during the insertion of the needle or catheter advancement. This newly created communication, coupled with high-pressure fluid within the pericardial space, promoted a flow gradient for the effusion to move from the pericardial space to the pleural cavity, explaining the occurrence of a pleural effusion. However, it does not explain the accumulation of air in the pericardial cavity as there was no concomitant pneumothorax. Moreover, in pericardial-pleural fistulae, as the fluid moves the pericardial sac to the pleural space, the latter’s effusion worsens as the former improves. None of these situations occurred.12
For our patient, the likely cause was iatrogenic introduction of air during PC or a leakage secondary to a defect in the drainage system.
Treatment
Given that pleural effusion was probably secondary to pericarditis, he was managed with ibuprofen 600 mg three times per day for 2 weeks after the end of symptoms and colchicine 0.5 mg two times per day for 3 months to avoid recurrence. Cephazolin was given as prophylaxis at the time of the PC.
Outcome and follow-up
The patient was discharged 6 days after PC and was completely asymptomatic. By the 12th day after PC, the pneumomediastinum had been reabsorbed (figure 3B). Three months after discharge, 12-lead ECG and TTE were normal.
As in most cases of viral pericarditis, he will continue to be observed for a period of 12 months as the risk of recurrence is approximately 10%.13
Discussion
PC is both a diagnostic and a potentially life-saving therapeutic procedure. Currently, echocardiography-guided PC is considered the standard clinical practice in the treatment of large pericardial effusions and cardiac tamponade, as seen in our patient, with high success rates of more than 95%. Although considered relatively safe, this invasive procedure may be associated with certain risks. Mortality associated with echocardiography-guided PC is low (<1%), and the overall complication rate may vary between 4% and 20%.3
An infrequent, but important complication of PC is pneumopericardium.2 4–10 14–17 This develops when there is a direct communication between the pleura and the pericardium, when air is introduced during the aspiration or when there is a leak in the drainage system.3
Pneumopericardium can have other causes, but none related to our case. In fact, the aetiology of pneumopericardium can be classified into three other major and more frequent categories besides iatrogenic, which is the rarest: (1) dull or penetrating chest injury and barotrauma, often caused by positive pressure ventilation (most often encountered in neonates); (2) fistulae between the pericardium and air-containing organs and structures; and (3) secondary production of gas by bacteria in the fluid in the pericardial sac, such as Clostridium perfringens or Klebsiella.1
Clinical presentation is variable, ranging from asymptomatic patients or those with nonspecific symptoms (dyspnoea, chest pain, palpitations) to a more serious condition, such as cardiac tamponade.2 A ‘mill-wheel’ murmur, a churning or splashing auscultatory sound due to blood mixing with air in the pericardial cavity, is a pathognomonic finding on auscultation.5 6
The CXR is usually the initial examination of choice. It typically reveals air-fluid level and radiolucency surrounding the cardiac boarder outlined by a fine line representing the pericardial sac, as observed in our case. However, the anteroposterior projection may fail to show mediastinal air in up to 50% of cases, and a lateral projection is very useful for differentiating a pneumopericardium from a pneumomediastinum. As ultrasound beams cannot easily pass through air, echocardiography is not routinely indicated for the diagnosis of pneumopericardium. Notwithstanding this limitation, the loss of the cardiac image during systole and its reappearance during diastole could offer a diagnostic clue. During systole, as the heart contracts, air fills the anterior mediastinum and the acoustic signal is lost. During diastole, air is displaced by the heart and the image reappears. This effect is known as the ‘air gap sign’. Hydropneumopericardium also produces a characteristic two-dimensional image similar to microbubbles swirling within the pericardial fluid.18–20 Other studies include axial CT, which can confirm the air and fluid in the pericardial sac and may be performed to rule out other plausible causes, such as fistulae or associated injuries in case of trauma.7
Iatrogenic pneumopericardium is often self-limiting and requires no specific therapy. However, in some patients, life-threatening complications may occur, especially pericardial tamponade, requiring prompt decompression.4–8 If the pericardial drain is still in place, treatment can be limited to removal of air by syringe aspiration, as we did in our case, maintaining observation. As shown in this case, postural changes to improve air aspiration can be effective because the catheter is often positioned posteriorly and intrapericardial air moves upward consistently. If no drain is present and there is evidence of haemodynamic compromise, a repeat PC is necessary.7 17
Pneumopericardium after PC has been associated with pneumothorax, pneumomediastinum and subcutaneous emphysema, but the association with pleural effusion is unique. To the best of our knowledge, this association has not been described in children. In our literature review, we found a case of an elderly woman with similar features to our case.17 There is also another report of pneumopericardium and pleural effusion after PC in an adult man with tuberculous pericarditis and pleural involvement.9
Few cases in the literature report pericardial-pleural fistulae as a complication of PC, and some associate pleural and pericardial effusions. Nevertheless, they describe a rapid resolution of pericardial effusion, with simultaneous formation of a pleural one, unlike our case.12
As far as we are aware, there is no evidence in the literature relating pneumomediastinum to pleural effusions. Regarding the pneumomediastinum formation in our patient, the likely cause was the iatrogenic introduction of air during PC or a leakage secondary to a defect in the drainage system.
The current case demonstrates how a rare, but potentially life-threatening PC complication can be easily diagnosed using readily available and inexpensive complementary diagnostic methods, such as TTE and CXR. This case also highlights the need for strong clinical suspicion, since manifestations are variable and non-specific. Moreover, patients are usually asymptomatic, making the timely diagnosis of pneumopericardium a challenge prior to haemodynamic compromise. Although a rare complication, clinicians need to be aware of this possibility.
Another important aspect to remember is the fact that the pneumopericardium can resolve spontaneously in a few days, as was the case with our patient. However, close monitoring for signs of cardiac tamponade is essential, as this complication could have a sudden onset. Finally, all efforts should be done during the procedure to avoid this complication.
Patient’s perspective.
The worst part of all this process were not the drains or the stay at the hospital; instead, it was when I felt the chest pain for the first time. Not because of the pain per se, because it was bearable, but because I feared for my life. As soon as the doctors explained to me what was happening with my heart and that they needed to take some liquid out, I started to feel more secure because, at least, I knew what was happening and that they were taking care of me.
The doctors explained to me that my situation was quite rare, and I am happy to help other physicians learn from my case.
Learning points.
A few clinical manifestations are non-specific; thus, a strong clinical suspicion is important for early diagnosis of a pneumopericardium to prevent fatal outcome.
Pneumopericardium can be easily diagnosed by readily available and inexpensive complementary diagnostic methods, such as transthoracic echocardiogram and chest radiography.
Pneumopericardium is a rare clinical entity, often self-limiting but can be life-threatening.
A patient with a pneumopericardium should be monitored closely, and a prompt decompression is warranted in a selected group of patients with clinical deterioration.
Physicians should take precautions in handling drainage devices to avoid this iatrogenic complication.
Even without complications, viral pericarditis should maintain follow-up at least in the first year after the event because the risk of recurrence is about 10%.
Footnotes
Contributors: AP is the main author since she designed the work and has done the acquisition, analysis and interpretation of data. CH is a co-author who became responsible for the acquisition of complementary diagnostic methods. PS and AP are co-authors who revised the work and ensured its accuracy.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Patient consent for publication: Parental/guardian consent obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Gołota JJ, Orłowski T, Iwanowicz K, et al. Air tamponade of the heart. Kardiochir Torakochirurgia Pol 2016;13:150–3. 10.5114/kitp.2016.61052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Iskander S, Amar H, Audrey B, et al. Pneumopericardium: a rare complication of pericardiocentesis. J Cardiovasc Ultrasound 2016;24:55–9. 10.4250/jcu.2016.24.1.55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kumar R, Sinha A, Lin MJ, et al. Complications of pericardiocentesis: a clinical synopsis. Int J Crit Illn Inj Sci 2015;5:206–12. 10.4103/2229-5151.165007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wakabayashi Y, Hayashi T, Mitsuhashi T, et al. Tension pneumopericardium after pericardiocentesis: useful echocardiographic obscured heart sign and effective postural change during air aspiration. Heart Rhythm 2018;15:1116 10.1016/j.hrthm.2018.02.021 [DOI] [PubMed] [Google Scholar]
- 5.Lee SH, Kim WH, Lee SR, et al. Cardiac tamponade by iatrogenic pneumopericardium. J Cardiovasc Ultrasound 2008;16:26–8. 10.4250/jcu.2008.16.1.26 [DOI] [Google Scholar]
- 6.Lee J, Kang BS, Kim C, et al. Tension pneumopericardium after pericardiocentesis. J Korean Med Sci 2016;31:470–2. 10.3346/jkms.2016.31.3.470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pandey AK, Singh SK, Devenraj V, et al. Pneumopericardium: a rare complication following pericardiocentesis. Indian J Thorac Cardiovasc Surg 2019;35:493–5. 10.1007/s12055-018-00785-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Methachittiphan N, Boonyaratavej S, Kittayarak C, et al. Pneumohydropericardium with cardiac tamponade after pericardiocentesis. Heart 2012;98:93. 10.1136/heartjnl-2011-300614 [DOI] [PubMed] [Google Scholar]
- 9.Abrahan Iv LL, Obillos SMO, Aherrera JAM, et al. A rare case of pneumopericardium in the setting of tuberculous constrictive pericarditis. Case Rep Cardiol 2017;2017:1–6. 10.1155/2017/4257452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yuce M, Sari I, Davutoglu V, et al. Bubbles around the heart: pneumopericardium 10 days after pericardiocentesis. Echocardiography 2010;27:E115–6. 10.1111/j.1540-8175.2010.01245.x [DOI] [PubMed] [Google Scholar]
- 11.Visser F, Heine M, Levin AI. Pneumopericardium: two case reports and a review. SAJAA 2008;14:41–5. [Google Scholar]
- 12.Winter M, Lim I, Joseph M. The disappearing pericardial effusion: a pericardial-pleural fistula. J Am Soc Echocardiogr 2009;22:973.e5–973.e7. 10.1016/j.echo.2009.03.029 [DOI] [PubMed] [Google Scholar]
- 13.Tunuguntla H, Jeewa A, Denfield SW. Acute myocarditis and pericarditis in children. Pediatr Rev 2019;40:14–25. 10.1542/pir.2018-0044 [DOI] [PubMed] [Google Scholar]
- 14.Özkartal T, Schlossbauer SA, Faletra F, et al. Pericardiocenteses complicated by pneumopericardium. JACC: Case Reports 2019;1:249–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Choi WH, Hwang YM, Park MY, et al. Pneumopericardium as a complication of pericardiocentesis. Korean Circ J 2011;41:280–2. 10.4070/kcj.2011.41.5.280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mullens W, Dupont M, De Raedt H. Pneumopericardium after pericardiocentesis. Int J Cardiol 2007;118:e57. 10.1016/j.ijcard.2006.12.082 [DOI] [PubMed] [Google Scholar]
- 17.Deng J, Wang C, Liu Y, et al. Pneumopericardium-Complication of pericardiocentesis: a rare case. J Clin Radiol Case Rep 2019;3:1–5. [Google Scholar]
- 18.Bedotto JB, McBride W, Abraham M, et al. Echocardiographic diagnosis of pneumopericardium and hydropneumopericardium. J Am Soc Echocardiogr 1988;1:359–61. 10.1016/S0894-7317(88)80012-9 [DOI] [PubMed] [Google Scholar]
- 19.Reid CL, Chandraratna AN, Kawanishi D, et al. Echocardiographic detection of pneumomediastinum and pneumopericardium: the air gap sign. J Am Coll Cardiol 1983;1:916–21. 10.1016/S0735-1097(83)80209-5 [DOI] [PubMed] [Google Scholar]
- 20.Antonini-Canterin F, Nicolosi GL, Mascitelli L, et al. Direct demonstration of an air-fluid interface by two-dimensional echocardiography: a new diagnostic sign of hydropneumopericardium. J Am Soc Echocardiogr 1996;9:187–9. 10.1016/S0894-7317(96)90027-9 [DOI] [PubMed] [Google Scholar]


