Abstract
We report a case of a 21-year-old young woman who was initially diagnosed with hyperthyroidism secondary to Graves’ disease and spontaneously switched to hypothyroidism in a year. While most autoimmune hypothyroidism is due to Hashimoto’s disease, in her case, we suspect that her hypothyroidism is due to a switch of antibody dominance from thyroid stimulating hormone (TSH) receptor-stimulating antibody (TS Ab) to TSH receptor-blocking antibody (TB Ab). Switching from dominant TS Ab activity to dominant TB Ab activity is a rare phenomenon. Optimal management of this condition is not known. Loss of follow-up and medication non-adherence has made medical management in this young woman of reproductive age further challenging.
Keywords: endocrine system, thyroid disease, thyrotoxicosis, thyroiditis, endocrinology
Background
Graves’ disease and Hashimoto’s thyroiditis are the common causes of hyperthyroidism and hypothyroidism in adults, respectively, and are a part of autoimmune thyroid disease (AITD).1 AITD is the most frequent autoimmune disease affecting 2%–5% of population with female predominance.2 Thyroid-stimulating hormone (TSH)-receptor antibodies (TR Ab) are involved in the pathogenesis of AITD. All TR Ab block physiological TSH activity on the receptor. Certain antibodies have stimulating effect by mimicking TSH activity leading to hyperthyroidism and other have blocking effect leading to hypothyroidism. Both antibodies can coexist in the same individuals. The clinical presentation in these individuals is determined by the balance between the two types of antibodies.3
Case presentation
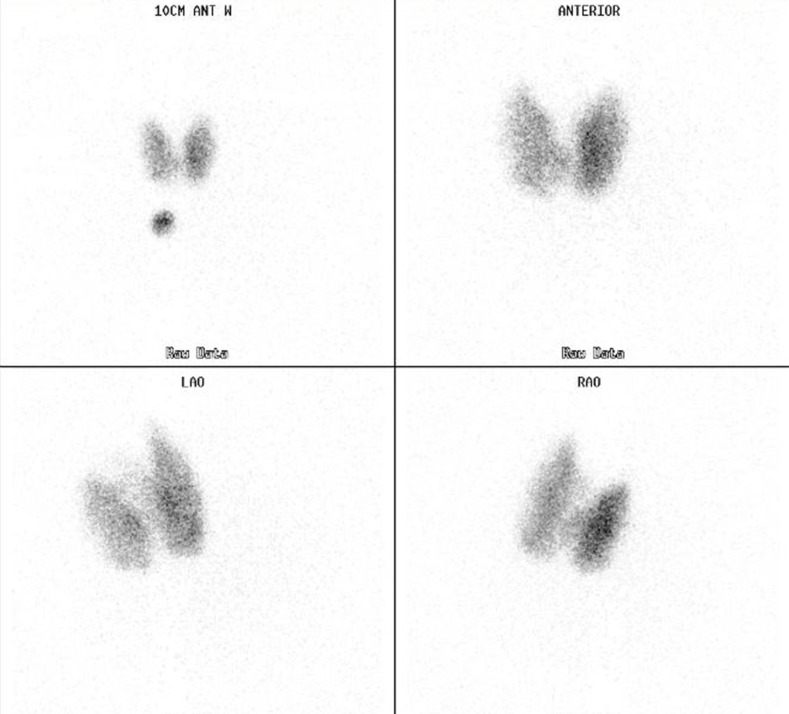
A 21-year-old young woman with no other medical history, non-smoker presented to the endocrine clinic in 2018 to establish care for hyperthyroidism. She reported weight loss of about seven pounds over 6 months, palpitations, enlarged neck, irregular periods, increased sweating and heat intolerance. On physical examination, she was tachycardic and was found to have a painless, enlarged thyroid gland without bruit or orbitopathy. Laboratory data revealed suppressed TSH, elevated thyroid hormones (free T4 and free T3) and elevated thyroid-stimulating immunoglobulin (TSI/TSH receptor-stimulating antibody (TS Ab)) level (see table 1). Ultrasonography showed an enlarged, heterogeneous and hypervascular thyroid gland with no focal nodules. Radioactive uptake scan of thyroid with I-123 revealed homogenous increased uptake of radiotracer in both lobes of thyroid gland (see figure 1). 24- hour radiotracer uptake value was 73% (normal range 10%–30%). Thus, diagnosis of hyperthyroidism due to Graves’ disease was made. At the initial visit, after discussion of various treatment options including surgery and antithyroid medications, patient preferred to undergo radioactive iodine ablation (RAIA) with a plan for a follow-up visit soon thereafter. She however presented 5 months later for her follow-up and did not undergo RAIA as she was working in a day care and could not skip work or stay away from children. She had persistent symptoms of hyperthyroidism including increased appetite, increased bowel movements and tremors. We recommended starting antithyroid medication along with beta blocker therapy while waiting to undergo definitive therapy. She was started on methimazole (MMI) 30 mg daily and asked to get thyroid function tests after 4 weeks of treatment. However, she was lost to follow-up. About a year later, she was referred by her primary care provider again for abnormal thyroid function test results. At that time, she reported a decrease in size of her thyroid gland and a resolution of her hyperthyroid symptoms except for the increased frequency of bowel movements. She disclosed that she had self-discontinued the MMI after 3 months of therapy as she had felt better. While not on any treatment for about 7 months, her thyroid function tests from a month prior showed an elevated TSH, low free T4 and free T3 (see table 1). She denied any weight gain, cold intolerance, fatigue, changes in hair/skin/nails, constipation or other hypothyroid symptoms.
Table 1.
Thyroid function test, thyroid antibodies
| Date (month/ year) |
Free T4 ng/dL (0.82–1.77) |
Free T3 pg/mL (2.0–4.4) |
TSH IU/mL (0.450–4.50) |
TSI/TSAb IU/L (0.00–0.55) |
TR Ab U/L (negative <1.0 U/L) |
| 08/2018 | 4.02 | 19.7 | <0.006 | 28.6 | – |
| 02/2019 | 2.47 | 8.6 | <0.015 | – | – |
| 09/2019 | 0.21 | 0.8 | 47.29 | – | – |
| 10/2019 | 0.21 | 0.8 | 46.02 | 93.60 | >320 |
| 03/2020 | 0.35 | – | 53.5 | – | – |
TR Ab, TSH-receptor antibodies; TSH, thyroid-stimulating hormone; TSI, thyroid-stimulating immunoglobulin.
Figure 1.
Thyroid uptake scan showing diffuse uptake significant for Graves’ disease.
Investigations
Thyroid function tests were done during the recent visit and showed elevated TSH, low free T4, free T3 similar to the values from a month ago. She also had high levels of TS Ab (TSI) and very high levels of TR Ab (TBI) (see table 1).
Differential diagnosis
We considered other causes of alternating thyroid function status including silent thyroiditis, subacute thyroiditis (De Quervain’s) or hypothyroidism from chronic thyroiditis due to Graves’ disease.
Treatment
Given the thyroid function tests were now significant for hypothyroidism, we recommended starting oral levothyroxine 100 μg daily and repeating thyroid labs after 4 weeks to reassess.
Outcome and follow-up
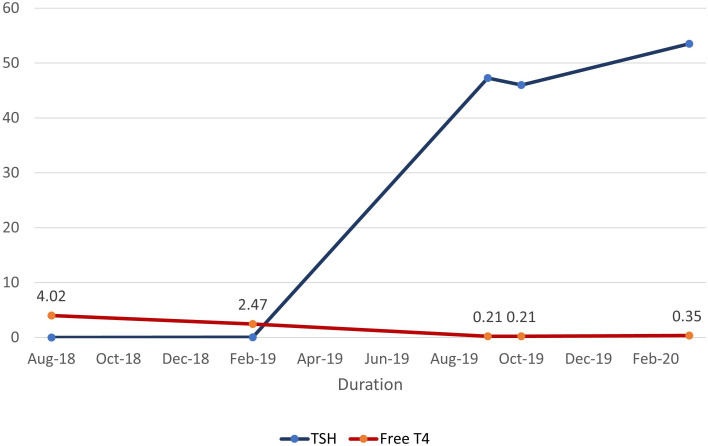
Unfortunately, our biggest challenge in management of this patient has been her lack of compliance with treatment. She did not pick up the medication from pharmacy or repeat laboratory assessment as suggested. After multiple attempts to contact the patient, we were able to convince her to repeat her thyroid function tests. Five months after the initial recommendation to start levothyroxine, the patient continued to be hypothyroid (see figure 2). She disclosed that she did not take any levothyroxine as she was feeling fine. Most recently, following multiple contacts with the endocrine team, she reports she has started taking levothyroxine but often forgets a few doses weekly. We have counselled the patient extensively regarding medication adherence to prevent systemic effects of hypothyroidism. We reviewed the possibility of recurrence of a hyperthyroid phase and reviewed treatment options for her initial Graves’ disease. At this time, she is not a candidate for RAIA as she is hypothyroid, and she is still not interested in pursuing thyroidectomy as definitive treatment. We counselled her about the presence of thyroid antibodies and associated complications in pregnancy. We informed her about the need for close monitoring if she were to conceive. She currently uses oral contraceptives and is not interested in pregnancy.
Figure 2.
Thyroid-stimulating hormone (TSH) versus free T4: thyroid function tests over time.
Discussion
Graves’ disease is a common cause of hyperthyroidism characterised by the presence of antibodies against TR. Though they were first described more than 50 years ago, we do not completely understand the heterogenous nature of TR Ab and their variable clinical presentation.4 Antibodies against TSH receptor (TRAbs) are competitive inhibitors of TSH binding site. TS Ab causes hyperthyroidism, whereas, TSH receptor-blocking antibody (TB Ab) causes hypothyroidism and are involved in pathogenesis of Graves’s disease and autoimmune atrophic thyroiditis, respectively.5 They are essentially two sides of the same coin, which is TR Ab disease.
TR Abs are detected by receptor binding assay and does not differentiate between TS Ab or TB Ab. It is also known as thyrotropin binding inhibitor immunoglobulin (TBII). TSI or TS Ab and TSH receptor-blocking antibody (TB Ab) are measured by bioassay. Apart from TS Ab and TB Ab, neutral TR Ab which neither stimulate nor block TSH have also been demonstrated.6
It was previously considered that patients with Graves’s disease only had stimulating antibodies (TS Ab). However, it is now being increasingly shown that TS Ab and TB Ab can coexist in the same patient. In one study, 18.5% of patients with Graves’s disease were found to have both TS Ab and TB Ab activities and the balance between the two determined whether the patient developed hyperthyroidism or hypothyroidism.7 Similarly, it has been shown that clones of lymphocytes from a patient with autoimmune thyroiditis demonstrated both TS Ab and TB Ab activity.8 Coexisting TS Ab and TB Ab has particularly gained attention in individuals who are being treated with antithyroid medication. There is no dependable correlation in the literature between TS Ab level and TRAb activity in Graves’ disease. This could likely be explained by the presence of TB Ab that are detected in TRAb assay but not while measuring TS Ab activity.3
We are describing a case of spontaneous development of hypothyroidism in a patient who initially presented with hyperthyroidism due to Graves’ disease. Two possible mechanisms have gained consideration over the years as supported by various studies. In patients with Graves’ disease who develop hypothyroidism, it could be the natural course of autoimmune disease over several years leading to hypothyroidism or it could be caused by alternating balance between TS Ab and TB Ab. Hypothyroidism from spent Graves’ disease is reported to develop 10–15 years after initial diagnosis. However, there are other case reports of a similar switch between hyperthyroidism and hypothyroidism mediated by antibodies and it appears to be a rare phenomenon. In one of the largest study of TB Ab over 10 years by Takasu et al,5 it was reported that among 98 patients with TS Ab predominant hyperthyroidism, only 2 developed TB Ab leading to hypothyroidism over 10 years.
Tamai et al9 reported that approximately 20% of patients with Graves’ disease previously treated with antithyroid medication developed hypothyroidism. Of all patients who became hypothyroid after treatment with antithyroidals, 31% were found to have TB Ab and showed marked decrease in thyroid size after treatment with antithyroid drugs. From their findings, it appears that at least one-third of patients with Graves’ disease developed hypothyroidism due to the presence of TB Ab (blocking antibodies) and the cause for hypothyroidism in the remaining two-third could be attributed to chronic autoimmune thyroiditis. There appears to be a positive correlation between antithyroid medication use and TB Ab mediated hypothyroidism. In a 5-year prospective study, Laurberg et al10 showed that treatment with antithyroid medication, especially MMI, was associated with a decrease in TPO autoantibodies and TR Ab (TR Ab and TS Ab). This could tip the balance between TS Ab and TB Ab leading to a switch from hyperthyroidism to hypothyroidism in these patients.
Presence of TRAbs during pregnancy has been associated with fetal/neonatal complications as TRAb is an IgG antibody that crosses the placenta. There have been multiple reports of fetal and neonatal hyperthyroidism as well as hypothyroidism; clinical presentation is likely determined by the balance between TSAb and TBAb activity.11 Measurement of TRAbs in at-risk pregnant patients who have history of Graves’ disease and close monitoring of the fetus is a recommendation shared across various medical societies.
At our institution, TR Ab are measured as TR Ab/TBII and TS Ab. Unfortunately, TB Ab measurement is not available. As mentioned earlier, TBII does not distinguish between TS Ab and TB Ab. However, it can be concluded that when a patient is hypothyroid with elevated TSH and positive TRAb, this may be TB Ab. Similarly, when the patient is hyperthyroid with elevated TRAb, this TRAb may be TSAb. Our patient is hypothyroid with elevated levels of both TR Ab and TS Ab which likely indicates that she has coexisting stimulating, blocking antibodies and that the elevated TRAb level is indicative of dominant TB Ab activity leading to her hypothyroidism. She also has a history of MMI use, although for a short duration which could have influenced the switch. There have been rare case reports of patients switching back to hyperthyroidism from hypothyroid state and vice versa, thereby caught oscillating between the two states multiple times.12 13 There are no definitive predisposing factors that can predict an individual’s risk for switching between hyperthyroidism and hypothyroidism or vice versa, thereby warranting further study in the rare few who do so as well as close monitoring of thyroid function in other patients at risk. A study by Kim et al7 showed that patients with elevated levels of TRAb had higher incidences of Graves’ orbitopathy and implies that patients with eye symptoms would likely benefit from surgery compared with RAIA as radioiodine can cause worsening of orbitopathy. Even though our patient is currently hypothyroid without any eye symptoms, she could still benefit from surgery as she is at an increased risk of switching back to hyperthyroid state given the elevation of both TS Ab and TRAb. In our literature search, we did not come across studies that have looked at the use of block-and-replace method in this population whereby high doses of antithyroid drug (ATD) are given during hyperthyroidism to suppress thyroid hormone production along with levothyroxine replacement to avoid hypothyroidism. It is not associated with higher rates of permanent remission of hyperthyroidism14 but has proven useful in patients with or at risk of Graves’ orbitopathy and individuals with fluctuant thyroid function.15 However, it is contraindicated in pregnancy as it involves use of high doses of ATD.
In patients who refuse definitive therapy (thyroidectomy or RAIA), like our patient, management of euthyroid state becomes challenging requiring frequent thyroid function tests and close follow-up.
Patient’s perspective.
When I was first diagnosed with thyroid disease I was not surprised as I had family history of thyroid disease; my mother has hypothyroidism. I was taking the methimazole daily as advised. I stopped taking the medicine after a while as I was feeling fine. But my primary care physician insisted that I see a thyroid specialist after she did my blood work. She did not tell me that I was hypothyroid. It was during the Endocrine clinic visit that I was informed I was hypothyroid. I just learnt from my doctors that I’m bouncing between hyperthyroidism and hypothyroidism. But I’m not worried and I don’t think it is difficult to manage. I think I will be fine with blood checks and taking medicines. I don’t have any problems taking the thyroid medicine early morning since I have to get up for my job early anyway and don’t eat till I get to work which is an hour later. I do not have any symptoms at this time and that is reassuring.
Learning points.
Thyroid-stimulating hormone (TSH) receptor antibodies (TR Ab) block TSH binding to receptor. TSH receptor stimulating antibody (TS Ab) and TSH receptor blocking antibody (TB Ab) are both TRAbs.
Patients with Graves’ disease can have coexisting TS Ab and TB Ab.
Treatment with antithyroid drugs like methimazole may be associated with reduction in thyroid antibodies and can rarely lead to switch to hypothyroidism in patient with Graves’ disease. Hence, these patients need close monitoring and appropriate evaluation.
Elevated TR Ab in pregnancy can lead to fetal/neonatal complications including hyperthyroidism and hypothyroidism.
Footnotes
Contributors: PP was involved in patient care, acquisition of data, drafting and revision of manuscript. RJ was involved in patient care, revision and final approval of manuscript.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Patient consent for publication: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Stathatos N, Daniels GH. Autoimmune thyroid disease. Curr Opin Rheumatol 2012;24:70–5. 10.1097/BOR.0b013e32834ddb27 [DOI] [PubMed] [Google Scholar]
- 2.Simmonds MJ, Gough SCL. Unravelling the genetic complexity of autoimmune thyroid disease: HLA, CTLA-4 and beyond. Clin Exp Immunol 2004;136:1–10. 10.1111/j.1365-2249.2004.02424.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McLachlan SM, Rapoport B. Thyrotropin-blocking autoantibodies and thyroid-stimulating autoantibodies: potential mechanisms involved in the pendulum swinging from hypothyroidism to hyperthyroidism or vice versa. Thyroid 2013;23:14–24. 10.1089/thy.2012.0374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.ADAMS DD. The presence of an abnormal thyroid-stimulating hormone in the serum of some thyrotoxic patients. J Clin Endocrinol Metab 1958;18:699–712. 10.1210/jcem-18-7-699 [DOI] [PubMed] [Google Scholar]
- 5.Takasu N, Matsushita M. Changes of TSH-Stimulation Blocking Antibody (TSBAb) and Thyroid Stimulating Antibody (TSAb) Over 10 Years in 34 TSBAb-Positive Patients with Hypothyroidism and in 98 TSAb-Positive Graves' Patients with Hyperthyroidism: Reevaluation of TSBAb and TSAb in TSH-Receptor-Antibody (TRAb)-Positive Patients. J Thyroid Res 2012;2012:1–11. 10.1155/2012/182176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cho BY. Clinical applications of TSH receptor antibodies in thyroid diseases. J Korean Med Sci 2002;17:293–301. 10.3346/jkms.2002.17.3.293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kim WB, Chung HK, Park YJ, et al. The prevalence and clinical significance of blocking thyrotropin receptor antibodies in untreated hyperthyroid Graves' disease. Thyroid 2000;10:579–86. 10.1089/thy.2000.10.579 [DOI] [PubMed] [Google Scholar]
- 8.Kohn LD, Suzuki K, Hoffman WH, et al. Characterization of monoclonal thyroid-stimulating and thyrotropin binding-inhibiting autoantibodies from a Hashimoto's patient whose children had intrauterine and neonatal thyroid disease. J Clin Endocrinol Metab 1997;82:3998–4009. 10.1210/jcem.82.12.4433 [DOI] [PubMed] [Google Scholar]
- 9.Tamai H, Hirota Y, Kasagi K, et al. The mechanism of spontaneous hypothyroidism in patients with Graves' disease after antithyroid drug treatment. J Clin Endocrinol Metab 1987;64:718–22. 10.1210/jcem-64-4-718 [DOI] [PubMed] [Google Scholar]
- 10.Laurberg P, Wallin G, Tallstedt L, et al. TSH-receptor autoimmunity in Graves' disease after therapy with anti-thyroid drugs, surgery, or radioiodine: a 5-year prospective randomized study. Eur J Endocrinol 2008;158:69–75. 10.1530/EJE-07-0450 [DOI] [PubMed] [Google Scholar]
- 11.Bucci I, Giuliani C, Napolitano G. Thyroid-Stimulating hormone receptor antibodies in pregnancy: clinical relevance. Front Endocrinol 2017;8:137. 10.3389/fendo.2017.00137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fan W, Tandon P, Krishnamurthy M. Oscillating hypothyroidism and hyperthyroidism - a case-based review. J Community Hosp Intern Med Perspect 2014;4:25734. 10.3402/jchimp.v4.25734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kraiem Z, Baron E, Kahana L, et al. Changes in stimulating and blocking TSH receptor antibodies in a patient undergoing three cycles of transition from hypo to hyper-thyroidism and back to hypothyroidism. Clin Endocrinol 1992;36:211–4. 10.1111/j.1365-2265.1992.tb00960.x [DOI] [PubMed] [Google Scholar]
- 14.Abraham P, Avenell A, Park CM, et al. A systematic review of drug therapy for Graves' hyperthyroidism. Eur J Endocrinol 2005;153:489–98. 10.1530/eje.1.01993 [DOI] [PubMed] [Google Scholar]
- 15.Razvi S, Vaidya B, Perros P, et al. What is the evidence behind the evidence-base? the premature death of block-replace antithyroid drug regimens for Graves' disease. Eur J Endocrinol 2006;154:783–6. 10.1530/eje.1.02169 [DOI] [PubMed] [Google Scholar]