Abstract
Introduction
Self-management is an important strategy for cancer survivors. Evaluating self-management is essential for planning nursing interventions that promote self-management and for measuring the contribution of nursing to health outcomes. Many patient-reported outcome measures (PROMs) have been designed and used to assess self-management in cancer survivors. However, it is unclear which PROM has the best reliability and validity. Therefore, the goal is to systematically review the psychometric properties of existing self-management PROMs and determine which PROM is best for cancer survivors.
Methods and analysis
This systematic review will be conducted according to the COnsensus-based Standards for the selection of health Measurement INstruments (COSMIN) guidelines for systematic reviews of PROMs. Ten electronic literature databases (PubMed, EMBASE and so on) and two websites for PROMs will be searched from inception to 1 March 2020. Studies testing the psychometric properties of PROMs assessing self-management for cancer survivors, published in either English or Chinese, will be included. Two independent reviewers determined the eligibility of the studies and will independently extract the data. Risk of bias will be assessed using the COSMIN risk-of-bias checklist, and the quality of the results will be assessed using specific COSMIN quality criteria.
Ethics and dissemination
It is not necessary to obtain ethical approval for this systematic review protocol. The results will be published in a peer-reviewed journal and presented at a relevant conference.
PROSPERO registration number
CRD42020149120.
Keywords: oncology, health & safety, protocols & guidelines
Strengths and limitations of this study.
This is the first systematic review that will identify, evaluate and summarise evidence on patient-reported outcome measures (PROMs) of self-management for cancer survivors and provide comprehensive pictures of their psychometric properties.
Since an accurate, repeatable PROM is a prerequisite for robust results, it is critical to choose an acceptable PROM with strong psychometric properties.
This review will use the most up-to-date COnsensus-based Standards for the selection of health Measurement INstruments methodology to comprehensively report the psychometric properties from multiple validation studies.
This systematic review will include studies published in English and Chinese, which may bias the results since relevant studies in other languages are not included.
A broad definition of self-management may increase the inconsistency of the included studies.
Introduction
An individual is considered a cancer survivor from the time of diagnosis, during and immediately after treatment, and through the balance of his or her life.1 For many patients, living after a diagnosis means living with significant and lasting impact of their cancer and its treatment, including the potential impact on health, physical and mental status, health behaviours, professional and personal identity, sexual behaviour and economic status.1 2 As a result, survivors have a need for ongoing medical and supportive care, yet current models of care largely focused on detecting recurrences and do not adequately address the comprehensive needs of survivors. Self-management programmes may be a strategy to ensure that the long-term physical and psychological health needs of survivors are addressed effectively and are receiving increased attention from medical staff.2–6
In 2019, Van de Velde et al conducted a concept analysis to define self-management in chronic conditions as the intrinsically controlled ability of an active, responsible, informed, and autonomous individual to live with the medical, role and emotional consequences of his chronic condition(s) in partnership with his social network and the healthcare provider(s).7 There are many definitions of self-management in the literature, but most researchers agree that self-management should include two basic elements: medical management and psychosocial management tasks that individuals undertake to deal with their health conditions.6 Based on these two elements, we embrace a broader definition of self-management in this paper. It is worth noting that the term ‘self-management’ is often used interchangeably with ‘self-care’ in research, so as in this paper.8
For patients with chronic conditions, self-management is one of the main goals of nursing practice, and the assessment of self-management is essential for planning nursing interventions that promote self-management and for measuring the contribution of nursing to health outcomes.9 Some patient-reported outcome measures (PROMs) have been developed and applied in research and clinical practice to assess the self-management and related concepts of cancer survivors.10–13 However, PROMS are not always developed and validated according to best practices, and it is not uncommon for them to be developed without reference to theoretical frameworks. These issues can clearly hinder clinicians and researchers from selecting appropriate PROMS. Therefore, there is a need to produce systematic review to provide a comprehensive picture of the psychometric properties of the PROMs in specific domains so that they can select the most appropriate PROMs.
To the best of our knowledge, several systematic reviews have assessed the psychometric properties of PROMs of self-management in other populations, such as diabetes, chronic obstructive pulmonary disease, heart failure and hypertension, and even for healthy children.9 14–17 In populations with cancer, the systematic review of psychometric properties of self-efficacy PROMs has been conducted.18 However, self-management differs from self-efficacy which emphasises the confidence to take action. No psychometric review has summarised and assessed self-management PROMs validated in cancer survivors, the population where it is essential to perform self-management practices after cancer treatment is completed. The COnsensus-based Standards for the selection of health status Measurement INstruments (COSMIN) group provided a recently updated appropriate methodology to conduct a systematic psychometric review.19 As a result, this study aims to systematically review the psychometric properties of existing self-management PROMs for cancer survivors using COSMIN methodology.
Methods
Design
The protocol will be developed according to the Preferred Reporting Items for Systematic reviews and Meta-Analyses Protocols guidelines (PRISMA-P) and was registered in the PROSPERO, an international prospective register of systematic reviews.20 The systematic review will be conducted according to the 10-step procedure for conducting systematic review of PROMs from COSMIN guideline. Figure 1 shows the procedure.19
Figure 1.
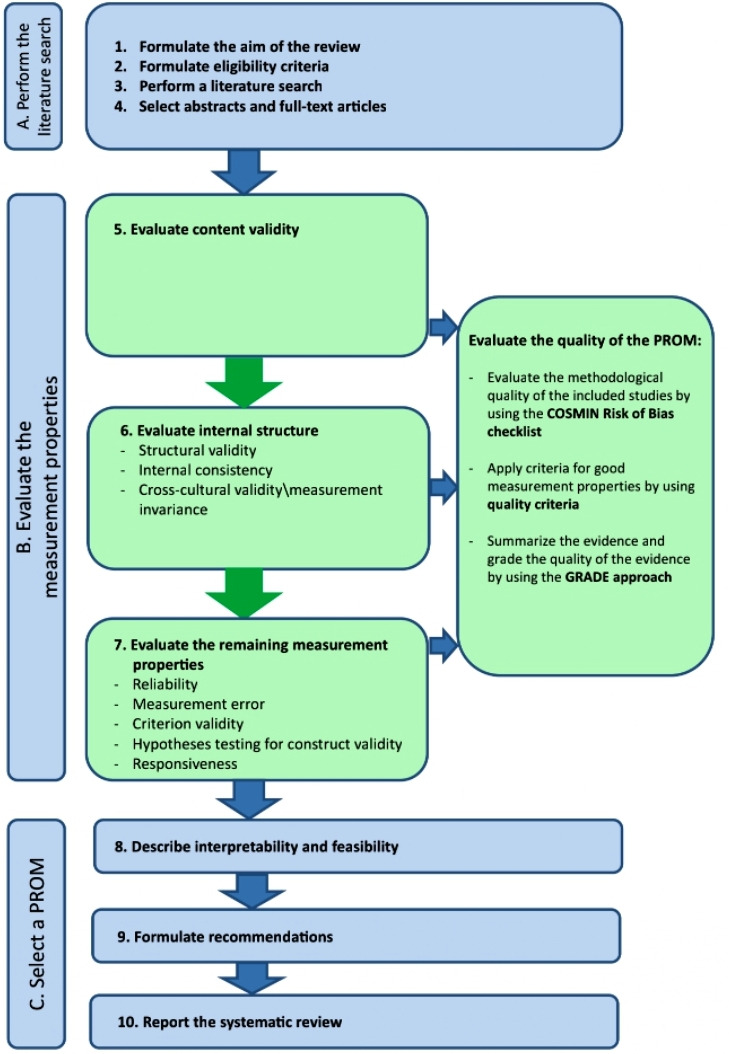
Ten steps for conducting systematic review of PROMs (cited from Prinsen et al19). COSMIN, COnsensus-based Standards for the selection of health Measurement INstruments; GRADE, Grading of Recommendations Assessment, Development and Evaluation; PROMs, patient-reported outcome measures.
Search strategy
The adequate search strategy developed according to the Peer Review of Electronic Search Strategies (PRESS).21 First, the researcher (JP) will develop the ‘primary’ search strategy and fill out the pertinent information in the updated PRESS 2015 Guideline Assessment Form. Then, the other researcher (YC) will check whether there was any need to revise the form against the PRESS 2015 Evidence Based Checklist and decide the final strategy. Besides, a comprehensive PROM filter will be used to find studies on psychometric properties.22 This filter includes terms such as outcome measure, validity, test–retest, reliability and so on, which has been widely used to search psychometric validation papers of PROMs.23 24 Table 1 shows an example of the search strategy in PubMed. Preliminary search was conducted and the self-management PROMs can be identified by the search strategy.12 13
Table 1.
Search strategy for PubMed
| #1 | Cancer*[Title/Abstract] OR carcinoma*[Title/Abstract] OR neoplas*[Title/Abstract] OR tumor*[Title/Abstract] OR tumour*[Title/Abstract] OR malignan*[Title/Abstract] OR leukemi*[Title/Abstract] OR leukaemi*[Title/Abstract] OR metasta*[Title/Abstract] OR oncolog*[Title/Abstract] OR lymphoma*[Title/Abstract] OR myeloma*[Title/Abstract] OR sarcoma*[Title/Abstract] OR Neoplasms[MeSH Terms] |
| #2 | (self-management[Title/Abstract] OR self manag*[Title/Abstract] OR self-care[Title/Abstract] OR self-car*[Title/Abstract] OR Symptom management[Title/Abstract] OR Symptom manag*[Title/Abstract] OR “self-care”[MeSH Terms]) OR “self-management”[MeSH Terms] |
| #3 | Instrument [Title/Abstract] OR instruments [Title/Abstract] OR measure [Title/Abstract] OR measures [Title/Abstract] OR questionnaire[Title/Abstract]] OR questionnaires [Title/Abstract] OR scale [Title/Abstract] OR scales [Title/Abstract] OR tool [Title/Abstract] OR tools [Title/Abstract] OR survey [Title/Abstract] OR test [Title/Abstract] |
| #4 | Sensitive search filter for psychometric properties published by Terwee et al (Terwee et al, 2009) |
| #5 | #1 AND #2 AND #3 AND #4 |
| #6 | (‘Delphi-technique’[title] OR cross-sectional[title] OR “addresses”[Publication Type] OR “biography”[Publication Type] OR “case reports”[Publication Type] OR “comment”[Publication Type] OR “directory”[Publication Type] OR “editorial”[Publication Type] OR “festschrift”[Publication Type] OR “interview”[Publication Type] OR “lectures”[Publication Type] OR “legal cases”[Publication Type] OR “legislation”[Publication Type] OR “letter”[Publication Type] OR “news”[Publication Type] OR “newspaper article”[Publication Type] OR “patient education handout”[Publication Type] OR “popular works”[Publication Type] OR “congresses”[Publication Type] OR “consensus development conference”[Publication Type] OR “consensus development conference, nih”[Publication Type] OR “practice guideline”[Publication Type]) NOT (“animals”[MeSH Terms] NOT “humans”[MeSH Terms]) |
| #7 | #5 NOT #6 |
MeSH, Medical Subject Headings.
From the inception to 1 March 2020, databases or websites will be searched including PubMed, EMBASE, CINAHL, Web of Science, PsycINFO, COSMIN Databases, Sinomed, Wan Fang Database, Chinese National Knowledge Infrastructure and Chongqing VIP Database, as well as the websites for PROMs including HealthMeasures (https://www.healthmeasures.net/) and PROQUALID (http://www.proqolid.org).
Complementary relevant studies will be identified by manually searching the reference list, and for PROMs that have been identified, we will also search for relevant psychometric validation papers from inception to 1 March 2020. We will also update the search prior to the publication of the systematic review. The language will be limited to Chinese and English for both articles presenting the original and translated versions of PROMs.
Inclusion and exclusion criteria
The studies will be included if (1) the participants are cancer survivors1; (2) PROMs for assessing self-management or ‘self-care’ are mentioned by author; or (3) they are articles that develop self-management PROMs for cancer survivors or validate at least one psychometric property of the PROM in line with the COSMIN terminology and definitions of psychometric properties.19
We will exclude a study if it meets the following criteria: (1) it only uses a PROM of self-management to validate another PROM or as an outcome; (2) a self-management PROM was completed by caregivers; (3) self-management is a subscale and psychometric properties results are not reported separately; and (4) an unpublished article or an article for which the full text is not available.
Study screening and selection
References identified by the search strategy will be entered into NoteExpress bibliographic software for de-duplication. Two independent authors in the field of cancer (JP and LS), who have been adequately trained in evidence-based methodologies, will screen the titles and abstracts to assess whether these articles meet the eligibility criteria and independently review the full texts of the articles. Potential discrepancies among the manuscripts selected will be resolved through discussion between the two authors. In case of an inability to reach a consensus agreement, a third author (YH) will be consulted to make a final decision. The process of study selection will be displayed in a PRISMA flow diagram.25
Quality appraisal
The methodological quality of studies on psychometric properties will be assessed independently by the two reviewers (JP and YC) using the COSMIN risk-of-bias checklist, and disagreements will be resolved by the third reviewer (YH).26 The COSMIN risk-of-bias checklist consists of 10 criteria for providing risk-of-bias scores for nine psychometric properties. Each item uses a four-level score: ‘very good’, ‘adequate’, ‘doubtful’ or ‘inadequate’.26 Each study is rated as very good, adequate, doubtful or inadequate quality. To determine the overall rating of the quality of each single study on a measurement property, the lowest rating of any standard in the box is taken. For example, if the lowest rating of all eight items of the reliability box is ‘inadequate’, the overall methodological quality of that specific reliability study is rated as ‘inadequate’.
Data extraction
We will subsequently extract the data on the characteristics of the PROMs (eg, instrument name, construct, theoretical framework, dimension, target population, number of items, response options and so on), on characteristics of the included populations (eg, disease characteristics, instrument administration and so on), on results on the psychometric properties, and on information about interpretability and feasibility of the scores of the PROMs. We will design a data extraction form to record information in Microsoft excel following the COSMIN Guidelines.19 The data will be extracted independently by two reviewers (JP and YC), and any differences in opinion will be resolved by discussion.
Data synthesis
Data synthesis includes three steps. First, the result of each single study on a psychometric property is rated against the updated criteria for good psychometric properties. Each result is rated as either sufficient (+), insufficient (–) or indeterminate (?). Second, we will synthesise the results and come to an overall conclusion of the quality of the PROM as a whole. If the ratings for each study are consistent, the results from different studies on one psychometric property will be qualitatively summarised or statistically pooled in a meta-analysis, and the overall rating will be either sufficient (+), insufficient (–) or indeterminate (?). The use of meta-analysis depends on the availability of quantitative data such as the Cronbach alpha coefficient or correlation coefficient for the psychometric properties, and pooled estimates of psychometric properties can be obtained by calculating weighted means (based on the number of participants included per study) and 95% CIs. If the ratings are inconsistent, we will: (1) find explanations and summarise per subgroup; (2) not summarise the results and do not grade the evidence; or (3) base the conclusion on the majority of consistent results, and downgrade for inconsistency (±). Which strategy is most appropriate is up to the specific situation. Finally, the quality of the evidence will be graded (high, moderate, low, very low evidence), using a modified Grading of Recommendations Assessment, Development and Evaluation (GRADE) approach. The modified GRADE approach uses four factors to determine the quality of the evidence: (1) risk of bias (ie, the methodological quality of the studies), (2) inconsistency (ie, unexplained inconsistency of results across studies), (3) imprecision (ie, total sample size of the available studies) and (4) indirectness (ie, evidence from different populations than the population of interest in the review). Publication bias is not taken into account in this modified GRADE approach because of a lack of registries for these types of studies.
Patient and public involvement
The design of this protocol does not involve the patients or the public.
Ethics and dissemination
It is not necessary to obtain ethical approval for this systematic review protocol. The results will be disseminated to a clinical audience and policymakers though peer-reviewed journals and conferences and will support researchers in choosing the best measure to evaluate the self-management of cancer survivors.
Discussion
To the best of our knowledge, this is the first systematic review of self-management PROMs of cancer survivors. The results of this work will help to identify existing self-management PROMs of cancer survivors and provide a comprehensive picture of their psychometric properties. The results of this systematic review will enable healthcare professionals and policymakers to select the most appropriate PROM based on its psychometric properties, and for guideline developers, the study will also help them gain a more comprehensive understanding of the underlying psychometric properties of existing self-management PROMs for cancer survivor.
Although the systematic review we developed follows the COSMIN guidelines and the PRISMA statement, there are still some potential challenges that may arise. First, due to the diversity of the term and definitions of self-management, a PROM may not fit this study’s description definition of self-management, which would pose a challenge for inclusion in the study. To address this issue, the present study plans to include PROMs as long as they are related to a patient’s personal behaviours in dealing with physical and psychosocial issues and the authors state that they measure self-management or self-care. This study will then report the definition or theoretical framework of self-management used by all included studies in the development of self-management PROMs for the reader’s reference. Second, as with all other systematic evaluations, there is a possibility of publication bias in this study. Therefore, the database and relevant websites will be searched as comprehensively as possible and traced against references to minimise the possibility of missing relevant studies.
To enhance the dissemination of the results, this study will be published in a peer-reviewed journal to attract more attention in the topic of the study. We will also present the results of this study at national and international conferences, and a summary of the results will be presented to healthcare professionals and policymakers by various means, such as briefings, electronic platforms and so on.
Supplementary Material
Footnotes
Contributors: All authors made substantial contributions to conception and design, piloted the inclusion criteria, and provided direction of the data extraction and analysis. JP, YC and LS drafted the protocol. ZZ, WX, GJ and YH critically revised the draft for important intellectual content. All authors agreed on the final version.
Funding: Funding is provided by the FuXing nursing research fund of Fudan University (FNF201929).
Competing interests: None declared.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Patient consent for publication: Not required.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Denlinger CS, Sanft T, Moslehi JJ, et al. NCCN guidelines insights: survivorship, version 2.2020. J Natl Compr Canc Netw 2020;18:1016–23. 10.6004/jnccn.2020.0037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Boland L, Bennett K, Connolly D. Self-Management interventions for cancer survivors: a systematic review. Support Care Cancer 2018;26:1585–95. 10.1007/s00520-017-3999-7 [DOI] [PubMed] [Google Scholar]
- 3.Cuthbert CA, Farragher JF, Hemmelgarn BR, et al. Self-Management interventions for cancer survivors: a systematic review and evaluation of intervention content and theories. Psychooncology 2019;28:2119–40. 10.1002/pon.5215 [DOI] [PubMed] [Google Scholar]
- 4.Cuthbert CA, Samawi HH, Hemmelgarn BR, et al. Effectiveness and components of self-management interventions in adult cancer survivors: a protocol for a systematic review and planned meta-analysis. Syst Rev 2018;7:238. 10.1186/s13643-018-0902-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hammer MJ, Ercolano EA, Wright F, et al. Self-Management for adult patients with cancer: an integrative review. Cancer Nurs 2015;38:E10–26. 10.1097/NCC.0000000000000122 [DOI] [PubMed] [Google Scholar]
- 6.McCorkle R, Ercolano E, Lazenby M, et al. Self-Management: enabling and empowering patients living with cancer as a chronic illness. CA Cancer J Clin 2011;61:50–62. 10.3322/caac.20093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Van de Velde D, De Zutter F, Satink T, et al. Delineating the concept of self-management in chronic conditions: a concept analysis. BMJ Open 2019;9:e027775. 10.1136/bmjopen-2018-027775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Medline Mesh terms for self-management, 2018. Available: https://www.ncbi.nlm.nih.gov/mesh/?term=self-management
- 9.Urpí-Fernández A-M, Zabaleta-Del-Olmo E, Montes-Hidalgo J, et al. Instruments to assess self-care among healthy children: a systematic review of measurement properties. J Adv Nurs 2017;73:2832–44. 10.1111/jan.13360 [DOI] [PubMed] [Google Scholar]
- 10.Paxton RJ, Gao Y, Herrmann SD, et al. Measurement properties of the sedentary behavior strategy self-management instrument in African-American breast cancer survivors. Am J Health Behav 2015;39:175–82. 10.5993/AJHB.39.2.3 [DOI] [PubMed] [Google Scholar]
- 11.Yun YH, Jung JY, Sim JA, et al. Patient-Reported assessment of self-management strategies of health in cancer patients: development and validation of the smart management strategy for health assessment tool (SAT). Psychooncology 2015;24:1723–30. 10.1002/pon.3839 [DOI] [PubMed] [Google Scholar]
- 12.Chan RJ, Yates P, McCarthy AL. The development and preliminary testing of an instrument for assessing fatigue self-management outcomes in patients with advanced cancer. Cancer Nurs 2017;40:48–57. 10.1097/NCC.0000000000000347 [DOI] [PubMed] [Google Scholar]
- 13.Coolbrandt A, Van den Heede K, Clemens K, et al. The Leuven questionnaire for patient self-care during chemotherapy (L-PaSC): instrument development and psychometric evaluation. Eur J Oncol Nurs 2013;17:275–83. 10.1016/j.ejon.2012.07.008 [DOI] [PubMed] [Google Scholar]
- 14.Clari M, Matarese M, Alvaro R, et al. Measurement properties of instruments evaluating self-care and related concepts in people with chronic obstructive pulmonary disease: a systematic review. Heart Lung 2016;45:441–8. 10.1016/j.hrtlng.2016.06.006 [DOI] [PubMed] [Google Scholar]
- 15.Cameron J, Worrall-Carter L, Driscoll A, et al. Measuring self-care in chronic heart failure: a review of the psychometric properties of clinical instruments. J Cardiovasc Nurs 2009;24:E10–22. 10.1097/JCN.0b013e3181b5660f [DOI] [PubMed] [Google Scholar]
- 16.Han H-R, Song H-J, Nguyen T, et al. Measuring self-care in patients with hypertension: a systematic review of literature. J Cardiovasc Nurs 2014;29:55–67. 10.1097/JCN.0b013e3182775fd1 [DOI] [PubMed] [Google Scholar]
- 17.Lu Y, Xu J, Zhao W, et al. Measuring self-care in persons with type 2 diabetes: a systematic review. Eval Health Prof 2016;39:131–84. 10.1177/0163278715588927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Huang F-F, Yang Q, Wang A-N, et al. Psychometric properties and performance of existing self-efficacy instruments in cancer populations: a systematic review. Health Qual Life Outcomes 2018;16:241. 10.1186/s12955-018-1066-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Prinsen CAC, Mokkink LB, Bouter LM, et al. COSMIN guideline for systematic reviews of patient-reported outcome measures. Qual Life Res 2018;27:1147–57. 10.1007/s11136-018-1798-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shamseer L, Moher D, Clarke M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: elaboration and explanation. BMJ 2015;350:g7647. 10.1136/bmj.g7647 [DOI] [PubMed] [Google Scholar]
- 21.McGowan J, Sampson M, Salzwedel DM, et al. PRESS Peer Review of Electronic Search Strategies: 2015 Guideline Statement. J Clin Epidemiol 2016;75:40–6. 10.1016/j.jclinepi.2016.01.021 [DOI] [PubMed] [Google Scholar]
- 22.Terwee CB, Jansma EP, Riphagen II, et al. Development of a methodological PubMed search filter for finding studies on measurement properties of measurement instruments. Qual Life Res 2009;18:1115–23. 10.1007/s11136-009-9528-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Davies CF, Macefield R, Avery K, et al. Patient-Reported outcome measures for post-mastectomy breast reconstruction: a systematic review of development and measurement properties. Ann Surg Oncol 2020:s10434-020-08736-8. 10.1245/s10434-020-08736-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mehdipour A, Beauchamp MK, Wald J, et al. Measurement properties of preference-based measures for economic evaluation in COPD: a systematic review. Qual Life Res 2020:s11136-020-02569-4. 10.1007/s11136-020-02569-4 [DOI] [PubMed] [Google Scholar]
- 25.Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. J Clin Epidemiol 2009;62:1006–12. 10.1016/j.jclinepi.2009.06.005 [DOI] [PubMed] [Google Scholar]
- 26.Mokkink LB, de Vet HCW, Prinsen CAC, et al. COSMIN risk of bias checklist for systematic reviews of patient-reported outcome measures. Qual Life Res 2018;27:1171–9. 10.1007/s11136-017-1765-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


