Abstract
Objective
Aortopulmonary window (APW) is a rare congenital heart defect. It occurs as an isolated cardiac lesion or in association with other cardiac anomalies and rarely with abnormal coronary arteries. The spectrum of cardiovascular anomalies associated with APW and overall management and outcome in the current era were reviewed.
Methods
Between 2001 and 2018, all patients diagnosed with APW were included. Based on associated cardiovascular anomalies, those patients were divided into 2 groups: simple APW group and complex APW group (APW with associated other cardiovascular anomalies). All cases were followed longitudinally. The outcomes are described.
Result
Twenty patients underwent APW repair including 2 (10%) in simple APW group and 18 (90%) in complex APW group. Their mean age and weight were 4.8 ± 1.8 months and 4 ± 0.4 kg, respectively. APW Type I was confirmed in 65% followed by Type III in 20% and then Type II in 15% of the patients. In the complex APW group, atrial septal defect was the commonest associated cardiac lesion occurring in 8/20 (40%), followed by ventricular septal defect, interrupted aortic arch, and pulmonary artery anomalies in 25% of each. The presence of patent ductus arteriosus (PDA) was found in 40% of APW cases with 2/3rd of them in association with interrupted aortic arch. Two patients (10%) had unusual coronary anomalies that required repair, both with APW Type I. Associated non-cardiac anomalies were found in 30% of cases. Risk Adjustment for Congenital Heart Surgery (RACHS-1) score frequencies were between 2 and 4. Only one patient had reactive pulmonary hypertension related to chronic lung disease. All patients underwent surgical correction with median age of 2 month at the time of repair (interquartile range, 2 weeks to 4.5 months). Mean duration of mechanical ventilation, pediatric cardiac ICU and hospital length of stay were 2.8 ± 0.5, 9 ± 3 and 26 ± 6 days, respectively. All patients survived with no residual APW with mean follow-up duration of 4.5 years.
Conclusion
Majority of APW are associated with other cardiovascular anomalies (90%) including coronary abnormalities (10%). Early surgical repair of APW and associated lesions showed excellent survival rate, freedom from re-intervention need within an average of 4.5 years of follow up and no evidence of persistent pulmonary hypertension post repair.
Keywords: Aortopulmonary window, Pediatric cardiac surgery, Cardiovascular anomalies
1. Introduction
Aortopulmonary window (APW) was first described in the third decade of last century by Elliotson [1]. It accounts for (0.2%–0.6%) of all congenital heart lesions [2,3]. It is characterized by a communication between the ascending aorta and the pulmonary artery in the presence of two separate semilunar valves arising from separate sub arterial ventricular outflow tracts [4].
In 1978, Mori and associates proposed APW classification into three morphological types. Type I are proximal defects that are located in the proximal aorta above the sinus of Valsalva midway between the semilunar valves and pulmonary bifurcation. Types II are distal defects that are located in the upper portion of the ascending aorta before the aortic branches with aortic origin of right pulmonary artery. Type III are defects that are large and comprise both types I and II, such defect involves the majority of the ascending aorta, pulmonary trunk and the right pulmonary artery [5,6]. In line with this classification, most recent studies reported that type I APW is the commonest type, followed by type II then type III [4,7]. The size of the window, associated cardiac anomalies, and evolution of pulmonary hypertension are important clinical elements in the management of APW. Without repair, congestive heart failure can develop rapidly owing to high pulmonary blood flow [8].
Different types of cardiovascular abnormalities are known to be associated with APW. The reported association ranges between 25 and 35% [9]. These may include intra cardiac lesions, major vessels anomalies, or even coronary abnormalities. The diagnosis of associated cardiovascular lesions is important in order to plan proper surgical management as it may affect early surgical results, late outcome and mortality [10].
Surgical closure of the APW is the standard treatment of choice that to be performed as soon as possible with excellent reported results and outcome [1]. In complex APW, however, the repair can be challenging with small but recognizable reported morbidity and mortality [10].
In this report we aim to review our center's experience with APW in the last two decades, looking for landscape of their different types, spectrum of associated cardiovascular anomalies, results of surgical repair and long-term outcome post repair.
2. Methods
A single-institution retrospective review of patients with APW operated at King Abdulaziz Cardiac Center from 2001 to 2018 was performed after the approval of the hospital's institutional review board. Medical files and electronic medical records were searched. All data were queried and extracted from the electronic health system via chart review. Inclusion criteria consisted of all patients aged (0–14) years those underwent surgical repair for APW. Demographic data, details of cardiac anatomy as reported by echocardiogram, documented surgical findings, hospital course including cardiac ICU stay, and clinic visits after discharge from the hospital were collected.
Based on associated cardiovascular anomalies, we divided our patients into 2 groups: simple APW group and complex APW group. Furthermore, based on original Mori's classification, we classified our APW cases in each group into type I, type II or type III [5]. We have extracted the information about the condition of the patients from the electronic charts. Two patients lost follow up while the rest (18 patients) have completed the follow up.
The statistical analyses were performed with SPSS for Windows, Version 16.0. Chicago, SPSS Inc. The data was expressed as frequencies and percentage for the numerical variables. The normally distributed data was presented as means and standard deviations while the skewed data was presented as medians with ranges.
3. Results
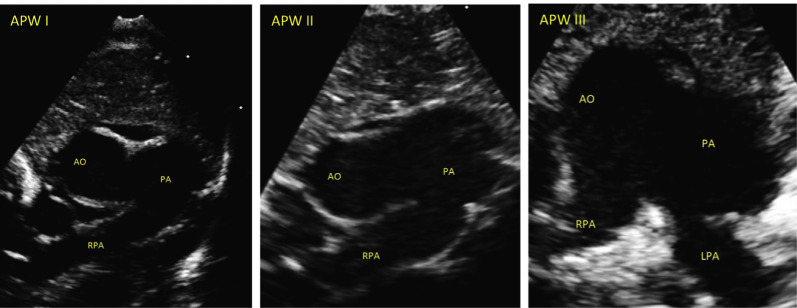
Out of (6861) pediatric cardiac surgeries done in our institute during the study period, twenty patients (0.29%) met the clinical criteria for diagnosis of APW and underwent surgical repair. Their mean age and weight were 4.8 ± 1.8 months and 4 ± 0.4 kg, respectively. Twelve patients (60%) were female and 8 patients were males (40%). There were (2/20, 10%) cases in simple APW group and (18/20, 90%) in complex APW group. APW type I was confirmed in (13/20, 65%), followed by type III in (4/20, 20%), and then type II in (3/20, 15%) of APW cases (Fig. 1).
Fig. 1.
High-parasternal short axis scans demonstrating defect between aorta and main pulmonary artery in each of the 3 types of APW. AO = Aorta; PA= Pulmonary artery; RPA = Right pulmonary artery; LPA = Left pulmonary artery.
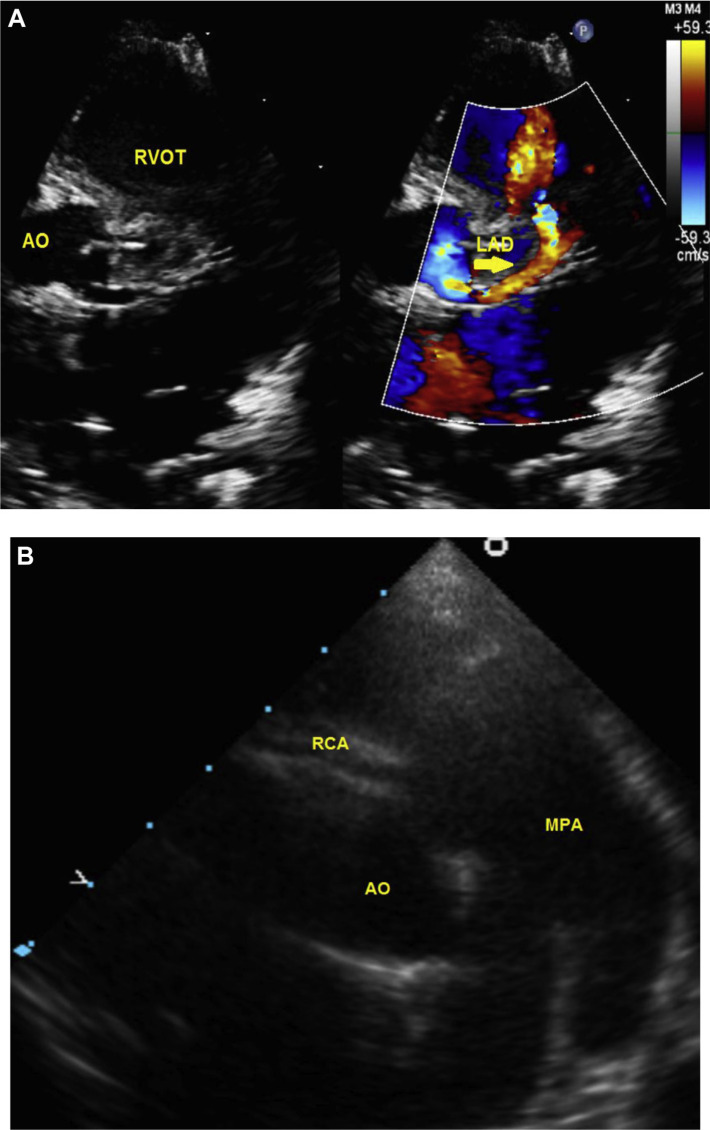
In complex APW group, the most common associated cardiovascular lesion was atrial septal defect in (8/20, 40%). PDA was also seen in (8/20, 40%). One quarter (5/20, 25%) of APW cases had an associated interrupted aortic arch and PDA (Table 1). The commonest form of aortic arch interruption was Type A (4/5 cases). Two female patients (10%) had complex APW Type I with abnormal coronary anatomy that required fistula ligation in the first and re-implantation of anomalous connection of the right coronary artery to pulmonary artery (ARCAPA) in the second case during APW repair (Fig. 2). Right aortic arch was seen in two patients (10%); both had complex APW Type I.
Table 1.
Associated lesions in APW and types of surgical repair.
| Patient | APW type | Associated cardiac lesion | Surgical repair | Age (month) at surgery |
|---|---|---|---|---|
| 1 | I | ASDII, VSD, bilateral SVC, LPA sling | APWR + LPA plasty | 0.75 |
| 2 | I | RCA from pulmonary artery, PDA | APWR + RCA re-implantation | 36 |
| 3 | I | Dextrocardia situs inversus, D-TGA, PDA, BAV, RAA | APWR +arterial switch operation | 1 |
| 4 | I | IAA type A, ASDII, PDA | APW + PDA ligation + IAA repair. | 0.5 |
| 5 | I | VSD, RAA | APWR + VSD closure | 6 |
| 6 | I | Pulmonary atresia, VSD | APWR + RV-PA conduit placement | 9 |
| 7 | I | Supra- PS, LPA stenosis | APWR + LPA plasty | 3 |
| 8 | I | ASDIIa | APWR | 4 |
| 9 | I | ASDII, mitral valve regurgitationa | APWR + ASD repair | 0.5 |
| 10 | I | SAM, VSD, coronary fistula between LAD and RVOT | APWR + resection of SAM, ligation of coronary fistula | 3 |
| 11 | I | IAA type A, BAV, VSD, PDA, and left SVC | APWR + aortic arch repair + ASD closure + VSD closure + PDA ligation | 3 |
| 12 | I | IAA type B, ASDII, PDA, and aberrant RSCA | APWR + aortic arch repair + ASD closure, PDA ligation | 1 |
| 13 | II | IAA type A, ASDIIa, PDA | APWR + IAA repair + PDA ligation | 2 |
| 14 | II | ASDIIa | APWR | 1 |
| 15 | III | ASDII, LPA stenosis | APWR + ASD repair | 0.5 |
| 16 | III | COA, PDA | APWR + aortic arch repair + PDA ligation | 16 |
| 17 | III | PDA, mitral valve regurgitationa | APWR + PDA ligation | 8 |
| 18 | III | IAA type A, PDA | APWR +IAA repair + PDA ligation | 0.25 |
APWR: aortopulmonary window repair; ASDII: secundum atrial septal defect; VSD: ventricular septal defect; LPA: left pulmonary artery; RCA: right coronary artery: PDA: patent ductus arteriosus; d-TGA: transposition of great arteries; BAV: bicuspid aortic valve; RAA: right aortic arch; IAA: interrupted aortic arch; COA: coarctation of aorta; PS: pulmonary stenosis; SAM: sub-aortic membrane; LAD: left anterior descending artery; RVOT: right ventricular outflow tract; SVC: superior vena cava; RV-PA: right ventricle-pulmonary artery.
The associated lesions were judged to cause no significant hemodynamic effects at the time of initial APW repair and their repair were deferred for further forthcoming follow up assessment and evaluation.
Fig. 2.
A: Parasternal short axis scans demonstrating left anterior descending artery (LAD) communicating with right ventricular outflow tract (RVOT); Aorta (AO). B: High-parasternal short axis scans demonstrating anomalous connection of right coronary artery to main pulmonary artery. AO = Aorta; MPA = Main pulmonary artery; RCA = Right coronary artery.
Fourteen out of twenty patients (70%) had no associated extra-cardiac anomalies, while six patients (30%) had different associated non-cardiac anomalies including VACTREL association, schizencephaly, and hypogonadisim, left facial palsy, absent right fibula, bowel malrotation, and aniridia. Three patients underwent genetic screening, none of them had chromosomal or gene abnormalities detected.
All patients were referred for surgical repair, and it was observed that risk adjusted classification for heart surgery (RACHS-1) [11] were between categories 2 to 4 (Table 1). All patients underwent patch repair of APW. Interrupted arch was repaired in the 5 patients using extended end to end anastomosis. None of our patients required patch augmentation of the aortic arch. None of them developed residual arch obstruction. Only one patient had pulmonary hypertension beyond the neonatal period with reactive pulmonary vascular bed. Median age at the time of surgical repair was 2 months (interquartile range, 2 weeks to 4.5 months). Mean duration of mechanical ventilation was 2.8 ± 0.5 days. The pediatric cardiac ICU stay and hospital stay were 9 ± 3 days and 26 ± 6 days respectively. All patients survived with no major complication. The mean follow up duration was 4.5 ± 1 year. Seventeen patients (85%) remained asymptomatic with no residual APW and required no re-intervention. In three patients, there were mild residual lesions on follow-up. The first patient had an associated mild supra-pulmonary valve stenosis which remained with no significant changes post APW repair and during his clinic follow-up, the second patient had small VSD patch leak, and third patient had mild right pulmonary artery stenosis. None of them required re-intervention. No cases were diagnosed to have supra-aortic stenosis in our study.
4. Discussion
APW may occur as an isolated lesion in fifty percent of the cases, and/or in association with minor cardiac anomalies such as ventricular septal defect, PDA, atrial septal defect or patent foramen ovale. It may also be present as part of complex cardiovascular lesions such as tetralogy of Fallot, pulmonary atresia, or in conjunction with various types of aortic arch abnormalities such as arch hypoplasia, coarctation or interruption. Rarely, APW has been reported in association with anomalous origin of coronary arteries [12]. In our cases, we found wide spectrum of associated cardiovascular lesions that were present in 90% of cases, much more than what has been previously reported in (25–35%) of cases [9]. Atrial septal defect and PDA were the most frequently encountered associated lesions among our patients with complex APW (40%). Furthermore, in two thirds of our APW cases with PDA, an interrupted aortic arch was concomitantly present in 5 patients (25%). The commonest form of aortic arch interruption was Type A (4/5 cases). This association between APW and interrupted aortic arch in our patient is much higher that what has been reported by Konstantinov et al. They have reported this association to be found in 4% of the patient with APW [13].
Reviewing case reports about APW revealed various rare cardiovascular anomalies described in associations with APW such as crossed pulmonary arteries [14], aberrant origin of left subclavian artery from the pulmonary artery with right aortic arch [3], and descending aorta to left atrial fistula [15]. We did not encounter any of these rare anomalies in our patients. We have encountered some unusual associated cardiovascular lesions with APW such as bicuspid aortic valve, pulmonary atresia, sub aortic membrane, bilateral superior vena cava, single left superior vena cava and aberrant right subclavian artery. Furthermore, two of our patient (10%) had rare abnormal coronary anatomy that has been seldom reported; the first child had coronary fistula between left anterior descending artery and right ventricular outflow tract. The second child had anomalous right coronary artery from pulmonary artery (ARCAPA) that required surgical re-implantation to the aorta during APW repair. This coronary anomaly association with APW is extremely rare. It was reported in 1999 by Izumoto et al., and recently in Padebettu et al. [16,17].
The association of APW with certain syndromes such as Holt–Oram, Patau's syndrome and VACTREL association has been reported previously [18–21]. Additionally, APW was reported in association with a unique syndrome known as “Berry syndrome” described first in 1982 that embraces distal APW and constellation of other cardiovascular anomalies including aortic origin of the right pulmonary artery, intact ventricular septum and aortic arch obstruction [22]. In our study, genetic testing was done in three patients; none of them had chromosomal abnormality or genetic disorder. None of our patients in complex APW group fit the description of Berry syndrome. Only one of our patients was diagnosed to have VACTREL association.
Late presenting or missed APW cases continue to be a concerning matter in the surgical management. An Indian study reported 50 patients that were identified late at mean age of 1.7 years. The study recommended a high index of suspicion for this lesion in the presence of unexplained severe pulmonary arterial hypertension, and/or unexplained cardiac dilation [20]. Missed diagnosis or late presentation of APW may lead to irreversible pulmonary hypertension and Eisenmenger syndrome [23]. Among our cases, we had one female patient that was referred at 3 years of age and she was diagnosed to have type I APW with anomalous right coronary artery from pulmonary artery (ARCAPA). Despite her late presentation, there was no evidence of pulmonary vascular disease at presentation. Satisfactory result was achieved after APW repair and re-implantation of the coronary artery to the aorta. There was no evidence of pulmonary hypertension or coronary ischemia during her follow-up.
Clinical and echocardiographic evaluations were the principle tool in identifying presence of pulmonary hypertension before subjecting patient to further invasive evaluation. In our series no patient required invasive hemodynamic assessment, except one who was diagnosed at the age of 1 month to have APW. He had chronic lung disease and evidence of pulmonary hypertension which was confirmed by cardiac catheterization and computerized tomography (CT) scan of the chest. He demonstrated favorable response to oxygen and pulmonary vasodilators preoperatively. He underwent corrective surgery with no pulmonary hypertension during follow-up. The presence of reactive pulmonary vascular bed in APW affects surgical outcome and postoperative mortality. In a series done by Tkebuchava et al., late mortality was mainly linked to pulmonary hypertension and pulmonary vascular disease resulting from late correction. In their study, 11/13 patients had late presentation and repair at mean age of 31.2 ± 48.3 months. Pre-operative hemodynamic assessment revealed mean pulmonary artery pressure of 69 ± 7 mmHg and pulmonary vascular resistance equal to 4.95 ± 1.2 U·m2. They reported 9% early death, 15% need for re-intervention and 90% actuarial 10-year-survival rate [24]. In comparison with the aforementioned series, our patient's mean age at operation was 4.8 ± 1.8 months, none of our patients required re-intervention and all survived with a mean follow-up duration of 4.5 years. Our improved results could be related to multiple factors including different historical era, earlier surgical repair and improved peri-operative techniques and management.
5. Limitations
Our limitations in this study include the inherent retrospective nature of the study with a long period of follow up in a single center review. Furthermore, our study did not offer new information related to management of APW. However, looking to the landscape of APW lesions, we described a wider spectrum of cardiovascular anomalies that are associated with this lesion with good mid-term surgical outcome among simple as well as complex APW lesions. In addition, we suggested in our study using the term cardiovascular abnormalities in complex APW to emphasize the importance and incidence of not only great vessel but also coronary abnormalities that deserve more evaluation in further studies as it may affect management plan and outcome.
6. Conclusion
Complex APW is much more frequent than simple APW with wide landscape of concomitant cardiovascular abnormalities. Despite broad spectrum of associated cardiovascular lesions, early surgical repair of APW and associated lesions showed excellent survival rate, almost 5 years freedom from re-intervention and no residual pulmonary hypertension.
Supplementary materials
Abbreviations
- APW
Aortopulmonary window
- PDA
Patent ductus arteriosus
- RACHS-1
Risk Adjustment for Congenital Heart Surgery score
- ARCAPA
Anomalous connection of the right coronary artery to pulmonary artery
- VSD
Ventricular septal defect
Financial support
This research received no specific grant from any funding agency or commercial or not-for-profit sectors.
Conflicts of Interest
The authors have no conflicts of interest relevant to this article.
Ethical standards
The manuscript was completed according to good clinical practice and approved by the Institutional Review Board (IRBC/2054/18) of King Abdullah international medical research center (KAIMRC), Ministry of National Guard Health Affairs.
References
- 1.Ghaderian M. Aortopulmonary window in infants. Heart Views. 2012;13(3):103–6. doi: 10.4103/1995-705X.102153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Richardson JV, Doty DB, Rossi NP, Ehrenhaft JL. The spectrum of anomalies of aortopulmonary septation. J Thorac Cardiovasc Surg. 1979;78:21–7. [PubMed] [Google Scholar]
- 3.Zhu C, Wang T, Zhu Z, Liu K. Aberrant origin of left subclavian artery from the pulmonary artery and right aortic arch in an aortopulmonary window. Interact Cardiovasc Thorac Surg. 2016;23:991–2. doi: 10.1093/icvts/ivw143. [DOI] [PubMed] [Google Scholar]
- 4.Talwar S, Agarwal P, Choudhary SK, et al. Aortopulmonary window: morphology, diagnosis, and long-term results. J Card Surg. 2017;32:138–44. doi: 10.1111/jocs.12936. [DOI] [PubMed] [Google Scholar]
- 5.Mori K, Ando M, Takao A, et al. Distal type of aortopulmonary window: report of 4 cases. Br Heart J. 1978;40:681–9. doi: 10.1136/hrt.40.6.681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jacobs JP, Quintessenza JA, Gaynor JW, Burke RP, Mavroudis C. Congenital heart surgery nomenclature and database project: aortopulmonary window. Ann Thorac Surg. 2000;69:S44–9. doi: 10.1016/s0003-4975(99)01236-9. [DOI] [PubMed] [Google Scholar]
- 7.Alsoufi B, Schlosser B, McCracken C, Kogon B, Kanter K, Border W, et al. Current outcomes of surgical management of aortopulmonary window and associated cardiac lesions. Ann Thorac Surg. 2016;102(2):608–14. doi: 10.1016/j.athoracsur.2016.02.035. [DOI] [PubMed] [Google Scholar]
- 8.Fotaki A, Novaes J, Jicinska H, Carvalho JS. Fetal aorto-pulmonary window: case series and review of the literature. Ultrasound Obstet Gynecol. 2017 Apr;49(4):533–9. doi: 10.1002/uog.15936. [DOI] [PubMed] [Google Scholar]
- 9.Alvarez R, García-Díaz L, Coserria F, Hosseinpour R, Antinolo G. Aortopulmonary window with atrial septal defect: prenatal diagnosis, management and outcome. Fetal Diagn Ther. 2011;30(4):306–8. doi: 10.1159/000324173. [DOI] [PubMed] [Google Scholar]
- 10.Naimo PS, Yong MS, d'Udekem Y, Brizard CP, Kelly A, Weintraub R, et al. Outcomes of aortopulmonary window repair in children: 33 years of experience. Ann Thorac Surg. 2014 Nov;98(5):16749. doi: 10.1016/j.athoracsur.2014.06.043. [DOI] [PubMed] [Google Scholar]
- 11.Jenkins KJ, Gauvreau K, Newburger JW, Spray TL, Moller JH, Iezzoni LI. Consensus based method for risk adjustment for surgery for congenital heart disease. J Thorac Cardiovasc Surg. 2002;123(1):110–8. doi: 10.1067/mtc.2002.119064. [DOI] [PubMed] [Google Scholar]
- 12.Singh J, Loona M, Suryavanshi A, Sahoo M, Mahant TS. Aortopulmonary window with anomalous coronary arteries. J Card Surg. 2015;30(11):846–8. doi: 10.1111/jocs.12636. [DOI] [PubMed] [Google Scholar]
- 13.Konstantinov IE, Karamlou T, Williams WG, Quaegebeur JM, del Nido PJ, Spray TL, et al. Congenital Heart Surgeons Society. Surgical management of aorto-pulmonary window associated with interrupted aortic arch: a Congenital Heart Surgeons Society study. J Thorac Cardiovasc Surg. 2006;131(5):1136–41.e2. doi: 10.1016/j.jtcvs.2005.03.051. [DOI] [PubMed] [Google Scholar]
- 14.Awasthy N, Jawid SA. Aortopulmonary window with crisscross pulmonary arteries: anatomically type 1, physiologically type 2. J Cardiovasc Echography. 2017;27:143–4. doi: 10.4103/jcecho.jcecho_12_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sarkar AK, Sanjeeva NG, Waghmare NS. Association of congenital descending aorto–left atrial fistula with the aortopulmonary window and atrial septal defect. Cardiol Young. 2014;24(1):143–4. doi: 10.1017/S1047951112002156. [DOI] [PubMed] [Google Scholar]
- 16.Izumoto H, Ishihara K, Fujii Y, Oyama K, Kawazoe K. AP window and anomalous origin of right coronary artery from the window. Ann Thorac Surg. 1999;68(2):557–9. doi: 10.1016/s0003-4975(99)00596-2. [DOI] [PubMed] [Google Scholar]
- 17.Padebettu B, Chandana C, Divya M, Girish G. Anomalous origin of the right coronary artery from the pulmonary artery associated with an aortopulmonary window. Ann Pediatr Cardiol. 2018;11(3):325–7. doi: 10.4103/apc.APC_65_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Srinivas SK, Balekundri VI, Manjunath CN. Holt–Oram syndrome with aortopulmonary window- a rare association. Cardiol Young. 2014;24(5):947–9. doi: 10.1017/S1047951113001844. [DOI] [PubMed] [Google Scholar]
- 19.Kiran VS, Singh MK, Shah S, John C, Maheshwari S. Lessons learned from a series of patients with missed aortopulmonary windows. Cardiol Young. 2008;18(5):480–4. doi: 10.1017/S104795110800258. [DOI] [PubMed] [Google Scholar]
- 20.Sharma J, Saleh M, Das BB. Berry syndrome with trisomy 13. Pediatr Cardiol. 2002;23(2):205–9. doi: 10.1007/s00246-001-0048-5. Epub 2002 Feb 19. [DOI] [PubMed] [Google Scholar]
- 21.Trowitzsch E, Schneider M, Urban A, Asfour B. Congenital pulmonary sling, aorto-pulmonary window and pulmonary vein obstruction as a diagnostic and therapeutic challenge in an infant with VACTERL association. Clin Res Cardiol. 2006;95(6):338–43. doi: 10.1007/s00392-006-0383-x. Epub 2006 Apr 3. [DOI] [PubMed] [Google Scholar]
- 22.Berry TE, Bharati S, Muster AJ, Idriss FS, Santucci B, Lev M, et al. Distal aortopulmonary septal defect, aortic origin of the right pulmonary artery, intact ventricular septum, patent ductus arteriosus and hypoplasia of the aortic isthmus: a newly recognized syndrome. Am J Cardiol. 1982;49(1):108–16. doi: 10.1016/0002-9149(82)90284-3. [DOI] [PubMed] [Google Scholar]
- 23.El Dick J, El-Rassi I, Tayeh C, Bitar F, Arabi M. Aorto-pulmonary window in adults: a rare entity leading to Eisenmenger syndrome. Echocardiography. 2019;36(6):1173–8. doi: 10.1111/echo.14368. . Epub2019May22. [DOI] [PubMed] [Google Scholar]
- 24.Tkebuchava T, von Segesser LK, Vogt PR, Bauersfeld U, Jenni R, Künzli A, et al. Congenital aortopulmonary window: diagnosis, surgical technique and longterm results. Eur J Cardio Thorac Surg. 1997;11(2):293–7. doi: 10.1016/s1010-7940(96)01048-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.