Abstract
Introduction
Atrial tachyarrhythmias especially atrial fibrillation are the most commonly encountered arrhythmias in clinical practice. Most atrial tachyarrhythmia episodes are subclinical. Cardiac implantable electronic devices with atrial sensing function enable detection of atrial tachyarrhythmias through means of atrial high rate event algorithms. Prolonged atrial high rate episodes(AHRE) above a defined rate and duration threshold represent episodes of atrial fibrillation, atrial flutter, and longer atrial tachycardias that correlate strongly with risk for thromboembolic events.
Objective
1. To examine the occurrence of prolonged AHRE in dual-chamber pacemaker recipients over the study period. 2. To examine the factors which influence the occurrence of prolonged AHRE in these patients.
Methods
In this study, we analyzed data of 398 patients without valvular heart disease or history of atrial fibrillation who underwent dual chamber permanent pacemaker implantation at our center from January 2013 to June 2018. Patient demographics, cardiovascular comorbidities, medications, echocardiographic parameters such as ejection fraction and left atrial(LA) dimension were obtained. Also, we collected pacing characteristics such as paced QRS duration(QRSd), ventricular pacing site and cumulative percentage ventricular paced beats.
Results
Prolonged AHRE occurred in 59 patients(14.8%). Baseline LA dimension was greater in patients with prolonged AHRE(median 35 mm, IQR 33–37 vs median 35 mm, IQR 34–38, P = 0.004) compared to those without. Paced QRSd was significantly longer in patients with prolonged AHRE (median of 147 ms, IQR 139–160 ms vs 140 ms, IQR 132–150 ms; P < 0.001). On multivariable logistic regression, paced QRSd(OR 1.04, 95%CI 1.02–1.06; P = 0.001) and baseline LA dimension(OR 1.14, 95%CI 1.03–1.27; P = 0.01) significantly co-predicted AHRE. On Kaplan Meier analysis, patients with paced QRSd≥142 ms had more likelihood of developing prolonged AHRE during follow up (HR 2.46, CI 1.40–4.3, P = 0.001). After adjusting for baseline values, patients with paced QRSd≥142 ms had significant decline in left ventricular ejection fraction (adjusted mean difference −1.27%; P = 0.02) and significant LA dilation (adjusted mean difference 0.62 mm; P = 0.05)
Conclusion
In our study, paced QRSd and LA dimension were the strongest predictors for prolonged AHRE. The incidence of AHRE may be reduced by achieving the narrowest possible paced QRSd during device implantation.
Keywords: Device detected atrial high rate episodes, Device detected atrial arrhythmia, Sub-clinical atrial fibrillation
1. Introduction
A trial tachyarrhythmias (atrial fibrillation, atrial flutter, and atrial tachycardia) are commonly encountered arrhythmias in clinical practice. Among these, atrial fibrillation(AF) is the most common [1,2] and clinically relevant arrhythmia with a global prevalence of 1–2%. AF is strongly associated with thromboembolic events(especially ischemic stroke), worsening of left ventricular(LV) function and worsening cardiac status [3]. Additionally, AF is associated with worsening quality of life, increased hospital admissions and significant health-care costs [4]. Most AF episodes are subclinical and asymptomatic. Nevertheless, the clinical importance of these difficult to detect asymptomatic AF episodes cannot be underestimated as subclinical AF (SCAF) episodes are associated with an increased risk of stroke [5].
Cardiac Implantable Electronic Devices (CIED) with atrial sensing function enable us to detect and analyze these atrial tachyarrhythmias. Modern Atrial High Rate Episode (AHRE) detection algorithms integrated into pacemakers & other CIEDs allow detection of atrial tachyarrhythmias(AT) with high sensitivity and specificity [6,7]. In addition to ATs, AHRE algorithms may also pick up other events such as myopotentials, lead noise & pacemaker mediated arrhythmic events such as repetitive non-re-entrant ventriculoatrial synchrony (RNRVAS), endless loop tachycardia (ELT), far-field R wave (FFRW) [8] which are easily identifiable by evaluating device stored tracings. Various studies have defined thresholds for the rate and duration for AHRE episodes that help to differentiate prolonged AHREs from sinus tachycardia, slower & shorter atrial tachyarrhythmias which are of unknown clinical significance. Employing these rate and duration thresholds, the studies have also been able to correlate prolonged AHRE with thromboembolic risk [5,9–12]. More recently, there is evidence that prolonged AHRE increases the risk of all major cardiovascular outcomes(MACE) [13]. Thus, CIED detected AHRE is emerging as a powerful tool in the arsenal of the clinician to predict the risk of such events. Prolonged AHREs above the defined threshold represent clinically relevant episodes of atrial fibrillation [14], atrial flutter, and longer atrial tachycardias which lead to cardioembolic and cardiovascular events. The thresholds used for prolonged AHREs in previous studies and the corresponding risk of thromboembolism have been summarized in Table 1.
Table 1.
Summary of trials on AHREs detected by CIED and risk of thromboembolism(adapted from EHRA consensus document on device detected atrial tachyarrhythmias)[15].
| Year | Name of trial | Number of participants(N) | Follow up (Months) | AHRE rate cut off (beats per minute) | AHRE duration cut off (minutes) | HR for TE event | P-value |
|---|---|---|---|---|---|---|---|
| 2003 | Ancillary MOST[9] | 312 | 27(median) | >220 | 5 | 6.7 | 0.02 |
| 2005 | AT500 registry[10] | 725 | 22(median) | >174 | 1440 | 3.1 | 0.04 |
| 2009 | TRENDS[16] | 2486 | 17(mean) | >175 | 330 | 2.2 | 0.06 |
| 2012 | HomeMonitor CRT[11] | 560 | 12(median) | >180 | 228 | 9.4 | 0.006 |
| 2012 | ASSERT[5] | 2580 | 30(mean) | >190 | 6 | 2.5 | 0.007 |
| 2014 | SOS AF[12] | 10016 | 24(median) | >175 | 60 | 2.11 | 0.008 |
| 2019 | Miyazawa et al.[17] | 856 | 48(mean) | >175 | 5 | 3.40 | 0.01 |
AHRE- Atrial high rate episode, HR – Hazard ratio, TE – Thromboembolic, MOST- Mode selection trial, TRENDS – The relationship between daily atrial tachyarrhythmia burden from implantable device diagnostics and stroke, CRT – cardiac resynchronization therapy, ASSERT – Asymptomatic atrial fibrillation and stroke evaluation in pacemaker patients and atrial fibrillation reduction atrial pacing trial
Age, previous history of AF, left ventricular function, left atrial size [18], the cumulative percentage of ventricular paced beats [19,20] and more recently inflammatory markers [21] have been independently associated with AHREs in previous studies.
1.1. Aim of the study
In this retrospective study, we sought to examine the occurrence of prolonged AHRE in patients undergoing dual chamber permanent pacemaker implantation at a high-volume center in south India. Also, we sought to examine the factors which influence the occurrence of prolonged AHREs.
2. Materials and methods
2.1. Definitions
Atrial tachyarrhythmia – is a collective term which includes atrial fibrillation, atrial flutter, and atrial tachycardia
- Prolonged AHRE - From a review of literature, these indicate AHRE episodes above a certain rate and duration threshold. These represent episodes of atrial fibrillation, atrial flutter or longer atrial tachycardias. These episodes have been strongly linked with the risk of thromboembolism (see Table 1). For this study, we considered AHREs with any of the following characteristics as significant
- AHRE occurring at a rate of ≥220bpm and lasting ≥5 min
- AHRE occurring at a rate of ≥190bpm and lasting ≥6 min
- AHRE occurring at a rate of ≥175bpm and lasting ≥60 min
Virtual CHA2DS2VASc score – The determined CHA2DS2-VASc score in the absence of atrial fibrillation
2.2. Study design
We conducted a retrospective study of patients who underwent dual chamber permanent pacemaker implantation at our center over a period of 5 ½ years (January 2013 to June 2018). Data was collected from admission & discharge records, inpatient & outpatient medical records and pacemaker interrogation records.
Baseline and demographic data such as age at implantation, gender, pacemaker indication, cardiac comorbidities, previously diagnosed AF, presence of moderate to severe valvular heart disease, drug history, virtual CHA2DS2VASC score, ejection fraction, left atrial (LA) dimension, right ventricular (RV) pacemaker lead position based on chest x-ray in posteroanterior and lateral view, pacemaker mode and paced QRS duration were collected.
Medical records and pacemaker interrogation reports were reviewed and information such as AHRE episodes, cumulative percentage of paced ventricular beats, ejection fraction (biplane Simpson's method) & LA dimension was collected.
At our center, we routinely implant RV leads in non-apical positions (such as the lower, upper septum or the outflow tract septum) in preference to the RV apical position if permitted by pacing thresholds. Right atrial leads are usually placed in RA appendage or high right atrial free wall. AV hysteresis, managed ventricular pacing, Search AV+ or other dedicated RV pace reduction algorithms are not generally used at our institution. Paced AV delay is usually set between 170 and 220 ms. Sensed AV delay is usually set between 150 and 200 ms. A post-procedure ECG is obtained on the first postoperative day while applying a magnet over the device to document the paced QRS duration.
2.3. Inclusion criteria
Consecutive patients aged more than 17 years who underwent dual chamber permanent pacemaker implantation(DDD or DDDR mode) at our center over the study period.
Patients who had follow-up visits at our center during the study period with the most recent visit at least 1 year after pacemaker implantation
2.4. Exclusion criteria
Previously diagnosed atrial fibrillation
History of anticoagulation use
Moderate or severe valvular heart disease
Incomplete medical records or pacemaker interrogation reports
Episodes detected within 48 h of device implantation were ignored as they could be related to atrial lead implantation.
2.5. Ethics
This is a retrospective study. Institutional ethical clearance was obtained prior to commencement of study and data collection. All electronic patient data was stored in a password-protected computer. Only the principal and co-investigator had access to any of the patient data collected for the purpose of this study.
2.6. Statistical analysis
Categorical variables were described using frequencies and percentages. Continuous variables were described using mean & standard deviation (normally distributed data) and median & inter-quartile range (non-normally distributed data). Shapiro Wilk test of normality was used to differentiate between normal and non-normal distribution. Categorical data were compared using the chi-square test. Independent samples T-test was used to compare parametric data. Non-parametric data were compared using Mann Whitney U test. Univariable logistic regression was used to determine the association of various variables with the detection of AHRE. The variables with significant association were used in a multivariable binary logistic regression to obtain their adjusted association with the detection of AHRE. Analysis of covariance (ANCOVA) was used to compare the effect of QRS duration on ejection fraction (EF) and left atrial (LA) to control for the baseline values. Kaplan Meier plot was used to assess the hazard of development of prolonged AHRE over the study period. A 5% level of significance was considered to be significant in all the analyses. All statistics were done using SPSS version 23(IBM corporation, USA).
3. Results
3.1. Patient demographics
A total of 564 patients underwent dual chamber permanent pacemaker implantation at our center over the study period. 92 patients were excluded owing to insufficient records. 42 were excluded for moderate to severe valvular heart disease on echocardiogram and 32 were excluded for prior documentation of AF or anticoagulation use (Fig. 1).
Fig. 1.
Study design. VHD – Valvular heart disease, AF – Atrial fibrillation, OAC – Oral anticoagulant.
The most common indication for pacing was symptomatic bifascicular block (27.1%) followed by complete heart block (26.4%). Sinus node dysfunction accounted for 20.4% of pacemaker implantation at our center. Other indications for pacing included Mobitz type II AV block(10.8%), symptomatic trifascicular block(8.3%) and high-grade AV block(5.5%).
The mean age of patients in the cohort was 59.89 years (SD –11.93). The majority of our patient population were males (72.6%). The median duration of follow up was 18.58 months (IQR, 13.22–30.75).
3.2. Baseline characteristics (Table 2)
Table 2.
Comparison of baseline & pacing characteristics between patients with and without prolonged AHRE.
| Variables | Total (N = 398) | AHRE (N = 59) | No AHRE (N = 339) | P-value |
|---|---|---|---|---|
| Age (mean, SD) | 59.89(11.93) | 61.46(12.82) | 59.62(11.77) | 0.28 |
| Males (n, %) | 289 (72.6) | 40 (73.5) | 249(67.8) | 0.43 |
| Diabetes Mellitus (n, %) | 142(35.7) | 23(39) | 119(35.1) | 0.56 |
| Hypertension (n, %) | 245(61.6) | 37(62.7) | 208(61.4) | 0.89 |
| Dyslipidemia (n, %) | 192(48.2) | 23(39) | 169(49.9) | 0.16 |
| CHA2DS2VASc (median, IQR) | 2(1–3) | 2(1–3) | 2(1–3) | 0.11 |
| Aspirin use (n, %) | 103(25.9) | 19(32.2) | 84(24.8) | 0.26 |
| Statin use (n, %) | 158(39.7) | 24(40.7) | 134(39.5) | 0.89 |
| Beta blocker use (n, %) | 92(23.1) | 16(27.1) | 76(22.4) | 0.41 |
| Ejection fraction (median, IQR) | 57(56–59) | 57(56–59) | 57(56–59) | 0.78 |
| LA dimension (median, IQR, mm) | 35(33–37) | 35(34–38) | 35(32–36) | 0.004 |
| Paced QRS duration (median, IQR, ms) | 142(134–152) | 147 (139–160) | 140(132.75–150) | <0.001 |
| Apical RV lead position (n, %) | 99(24.9) | 20(33.9) | 79(23.3) | 0.10 |
| Cum. %V paced (median, IQR) | 67(5–99) | 92(17–99) | 62(3–99) | 0.17 |
We compared the baseline & pacing characteristics between patients who were detected to have prolonged AHRE against those patients who did not. The baseline variables were comparable across both groups except for the median baseline LA dimension which was significantly greater in patients with AHRE (35, IQR 34–38 vs 35, IQR 32–36; P = 0.004).
3.3. Post pacing characteristics and cumulative pacing percentage on follow-up (Table 2)
We also compared the post pacing characteristics among both the groups. The median paced QRS duration was significantly prolonged in patients with significant AHRE (147, IQR 139–160 vs 140(132.75–150; P < 0.001). Other variables did not vary significantly between both groups.
3.4. Predictors of prolonged AHRE during follow-up (Table 3)
Table 3.
Univariable logistic regression analysis looking at the unadjusted association of various variables to the detection of prolonged AHRE.
| Variables | OR | 95% CI | P-value | |
|---|---|---|---|---|
| Age | 1.01 | 0.99 | 1.04 | 0.27 |
| Gender(male) | 1.31 | 0.72 | 2.39 | 0.37 |
| Diabetes Mellitus | 1.18 | 0.67 | 2.09 | 0.57 |
| Hypertension | 1.06 | 0.60 | 1.88 | 0.84 |
| Dyslipidemia | 0.64 | 0.37 | 1.13 | 0.13 |
| CHA2DS2VASc | 1.15 | 0.94 | 1.40 | 0.18 |
| Aspirin use | 1.44 | 0.79 | 2.63 | 0.23 |
| Statin use | 1.05 | 0.60 | 1.84 | 0.87 |
| Beta blocker use | 1.29 | 0.69 | 2.41 | 0.43 |
| Ejection fraction | 1.02 | 0.95 | 1.10 | 0.55 |
| Baseline LA dimension | 1.17 | 1.06 | 1.29 | 0.002 |
| Paced QRS duration | 1.04 | 1.02 | 1.07 | <0.001 |
| Lead position(apical) | 1.69 | 0.93 | 3.06 | 0.09 |
| Cum. %V paced | 1.07 | 1.00 | 1.14 | 0.05 |
On follow up, device detected prolonged AHRE occurred in 59 patients (14.8%). Univariable logistic regression analysis (Table 3) revealed that among the various factors, baseline LA dimension (OR 1.17, CI 1.06–1.29; P = 0.002), paced QRS duration (OR 1.04, CI 1.02–1.07; P < 0.001) & cumulative percentage of ventricular pacing (OR 1.07, CI 1.0–1.14, P = 0.05) significantly predicted prolonged AHRE episodes.
3.5. Adjusted predictors of prolonged AHRE during follow-up (Table 4)
Table 4.
Multivariable logistic regression looking at the adjusted association of significant variables to the detection of prolonged AHRE.
| Variables | OR | 95% CI | P-value | |
|---|---|---|---|---|
| Paced QRS duration | 1.04 | 1.02 | 1.06 | 0.001 |
| Baseline LA dimension | 1.14 | 1.03 | 1.27 | 0.01 |
| Cum. %V paced | 1.04 | 0.97 | 1.12 | 0.30 |
After adjusting for other factors, baseline LA dimension and paced QRS duration (QRSd) were significant co-predictors for prolonged AHRE episodes. Every 1 ms prolongation of paced QRS duration increased the risk of prolonged AHRE by 4%. Every 1 mm increase in LA dimension increased the risk of prolonged AHRE by 14%.
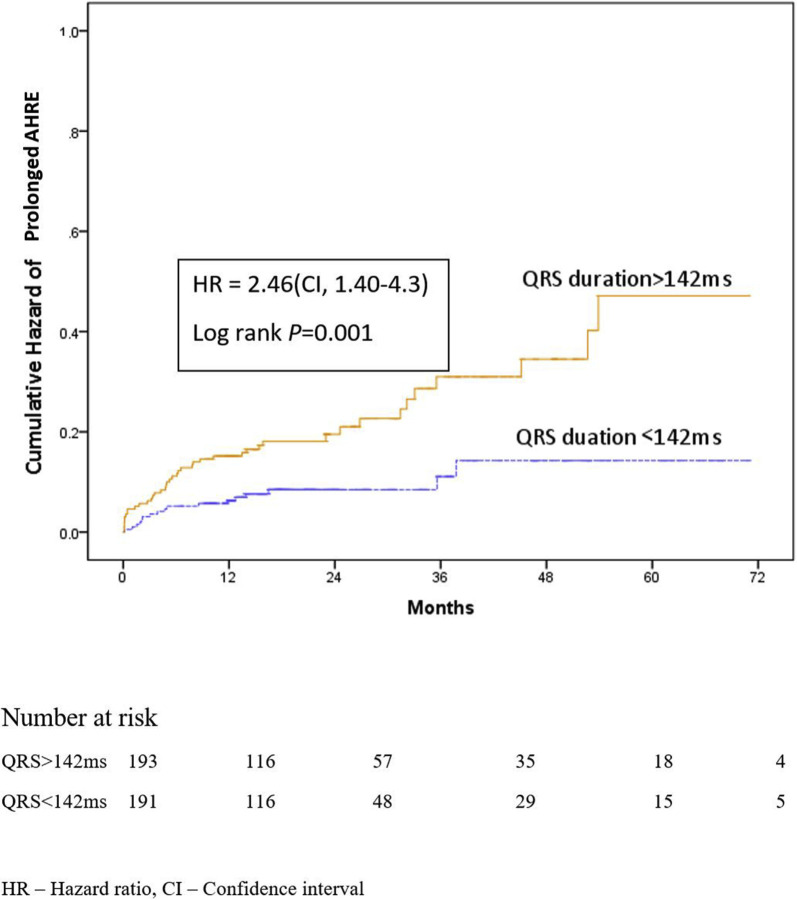
3.6. Paced QRS duration and hazard of AHRE (Fig. 2)
Fig. 2.
Kaplan Meier hazard plot showing the likelihood of AHRE in pacemaker patients with paced QRS duration >142 ms as compared to those with less than 142 ms. HR – Hazard ratio, CI – Confidence interval.
As QRS duration was found to significantly predict the risk of prolonged AHRE, we decided to examine the association of a longer paced QRS duration (≥ 50th percentile for the cohort, ≥142 ms) on the hazard of developing significant AHRE during follow-up. Patients who had a paced QRSd ≥142 ms were more likely to have a prolonged AHRE episode (P = 0.001) over the period of follow up.
3.7. Paced QRSd and its effect on EF & LA dimension(Table 5)
Table 5.
Analysis of covariance for assessing the relation between paced QRS and changes in EF & LA dimension after adjusting for baseline values.
| Variable | Observed mean
|
Adjusted mean
|
Adjusted mean difference | Confidence interval | P-value | |||
|---|---|---|---|---|---|---|---|---|
| QRSd ≥142 ms | QRSd ≤142 ms | QRSd ≥142 ms QRS>142 |
QRSd ≤142msaced QRS<142 |
|||||
| EF (%) at follow up | 54.80 | 56.59 | 55.06 | 56.33 | −1.27 | −2.34 | −0.20 | 0.02 |
| LA dimension(mm) at follow up | 36.83 | 35.63 | 36.54 | 35.92 | 0.62 | 0.01 | 1.24 | 0.05 |
Even after correcting for baseline values, there was significant decrease in EF in patients with paced QRS duration ≥142 ms (adjusted mean difference −1.27, P = 0.01). Also, after correcting for baseline values, there was significant increase in LA dimension in patients with paced QRS duration ≥142 ms (adjusted mean difference 0.62, P = 0.05)
4. Discussion
This study was designed to look at the incidence of prolonged AHRE in pacemaker patients and further analyze its relationship to other factors. Prolonged AHRE episodes were detected in 59 patients (14.8%). This was lower than in most previous studies. This may be due to several reasons.
Our study completely excluded patients who had moderate to severe valvular heart disease involving any valve. Most of the previous studies have not explicitly excluded moderate/ severe valvular heart disease in their study protocol. Unlike the other populations in whom comorbidities such as hypertension & diabetes constitute the dominant risk factors for AF, in India up to 40% of AF burden is attributable to valvular heart disease [23].
Our study also excluded patients with previously diagnosed AF. In unselected studies, the detection of AHRE has been reported to be as high as 50%. Past history of AF has been found in as much as 63% of the study population in prior studies [9,24,25]. In fact, this may have influenced the results to such an extent that AF history was the only significant predictor of device detected AF in one such study [24]. In studies where past AF was excluded the incidence of device detected AHRE has been much more conservative in the range of 24–35% [5,19,26].
The mean age at pacemaker implantation in our study population was 59.89(SD –11.93). In comparison, the mean age of device implantation in previous AHRE studies (derived from the meta-analysis by Mahajan et al.) [27] was 72(IQR, 65–77). AF being a disease of the elderly is likely to be less common in a younger study population such as ours. A decade difference in the onset of degenerative conduction system disease in our population is an interesting topic for future research.
Next, we looked at the association of prolonged AHRE with various variables after adjustment for various cofactors using multivariable logistic regression. Here paced QRS duration (OR 1.04, CI 1.02–1.06, P = 0.001), and baseline LA dimension (OR 1.14, CI 1.03–1.27, P = 0.01) stood out as significant. LA enlargement has been debated as both a cause and effect of atrial fibrillation [28]. Nevertheless, there is a strong association between LA enlargement and risk of AF [29]. In the Framingham heart study, for every 1 mm increase in the LA dimension, there was an increase in the risk of AF by 8% [30]. Our study also arrived at the same conclusion.
The QRS duration is a measure of the degree of ventricular dyssynchrony. In several previous studies, prolonged paced QRSd has been shown to be associated with worsening LV function. [31–33] In our study, QRSd has also been shown to be associated with prolonged AHRE in pacemaker recipients. A recent study by Xing et al. [34] arrived at the same conclusion. This is of immense clinical importance. Paced ECG is easily available and achieving a QRS as narrow as possible has been traditionally used as a goal to guide optimal lead placement. In most cases, non-apical lead placement such as septal, RV outflow tract (RVOT) and His Bundle area (HBA) leads to shorter QRS durations. This has been done in hopes of restoring ventricular synchrony and preserving LV function. However, our study shows that this strategy also reduces the rates of AHRE; thus, accentuating the potential benefit of non-apical pacing. In fact, a study by Pastore et al. [35] revealed that HBA pacing led to a lower occurrence of AF on follow up over a period of 5 years.
Finally, we also looked at the changes in EF & LA dimension and their relation to paced QRS duration. As seen in previous studies, there was a significant decline in LV function (adjusted mean difference −1.27, P = 0.01) with broadening of QRS implying the importance of ventricular dyssynchrony in promoting LV dysfunction. Also, there was significant LA enlargement (adjusted mean difference 0.62, P = 0.05) among patients with QRSd ≥142 ms. LA remodeling and LA dilation are critical pathogenetic factors promoting & maintaining AF. This finding highlights the role of ventricular dyssynchrony in promoting LA remodeling.
Implanted devices give us a unique opportunity to diagnose atrial tachyarrhythmias early. A recent meta-analysis that combines evidence from 11 major trials show that the relationship between device detected atrial tachyarrhythmias and stroke risk is unequivocal (OR 2.4, CI 1.8–3.3, P < 0.001) [27]. Prolonged AHRE has also been identified as an independent predictor of subclinical silent ischemic brain lesions [36]. However, the adoption of anti-coagulation in this patient population is low [18]. A recent small scale study looked into the efficacy and safety of oral anticoagulation in patients with prolonged AHRE [37]. The study concluded that OAC therapy was not associated with an increase in major bleeding. Two large scale studies [38,39] are underway examining the safety and efficacy of OAC therapy in patients with prolonged AHRE. The EHRA consensus document on device detected subclinical atrial tachyarrhythmias recommends consideration of anticoagulation for all patients with prolonged AHRE and CHA2DS2VASc score ≥2[15].
4.1. Limitations of the study
Our study has a few limitations. First of all, it is a retrospective study. Also, there is a heterogeneity in the indications for device implantation in the study population. Further, we did not assess the cumulative burden of AHRE in each individual which has been previously shown to correlate with stroke risk [16]. It is possible that patients with subclinical AF prior to device implantation may have been included in the analysis – however, there is no reliable method to exclude such patients. We have measured LA dimension instead of the more reliable indexed LA volume (the former underestimates LA enlargement as it measures it along one axis only). There is new evidence that AHRE detected up to 3 months post-implantation may be related to the atrial lead fixation used and the procedure itself without a significant effect on clinical outcomes [22]. However, we have not excluded such episodes from our study as our study protocol was finalized before this paper was published. Also, dedicated algorithms to reduce RV pacing such as managed ventricular pacing, Search AV+ are not generally used in our institution. Therefore, the potential benefit of these algorithms in reducing AHRE will not reflect in this study. Lastly, no outcome measures such as stroke were included in this study.
5. Conclusion
In pacemaker implanted patients without valvular heart disease or history of atrial fibrillation, prolonged AHREs are mainly predicted by paced QRS duration and left atrial dilation. Achieving the narrowest possible QRS duration during implantation by optimal non-apical lead positioning and increasing adoption of His-Bundle area pacing strategy can potentially decrease pacemaker detected atrial tachyarrhythmias in addition to preserving LV function.
Acknowledgments
We acknowledge the help provided by Mr. Bijesh Yadav, who helped us with the statistical analysis of this study.
Abbreviations
- AF
Atrial Fibrillation
- AHRE
Atrial high rate episode
- ANCOVA
Analysis of co-variance
- CI
Confidence Interval
- CIED
Cardiac implantable electronic devices
- EF
Ejection fraction
- EHRA
European Heart Rhythm Association
- ELT
Endless loop tachycardia
- FFRW
Far field R wave
- HBA
Bis Bundle Area
- HR
Hazard ratio
- IQR
Interquartile range
- LA
Left atrium
- LV
Left ventricle
- MACE
Major cardiovascular outcomes
- MVP
Managed ventricular pacing
- OAC
Oral anticoagulant
- OR
Odds ratio
- QRSd
QRS duration
- RNRVAS
Repeated Non-reentrant ventriculo atrial synchrony
- RV
Right ventricle
- RVOT
Right ventricular outflow tract
- SAV
Search AV
- SCAF
Subclinical atrial fibrillation
- VHD
Valvular heart disease
Future directions
The adoption of more physiological pacing strategies is likely to decrease the risk of AHRE post pacemaker implantation. Further studies examining the occurrence of AHRE with physiological pacing protocols are required. Although the correlation between device detected AHRE and stroke risk is robust, there are still major gaps in our understanding with regard to the pathophysiologic basis of AT leading to stroke, the temporal relationship of stroke with AHRE as well as appropriate use and safety of oral anticoagulation for device detected AHRE. The ongoing trials such as ARTESiA [39] (Apixaban for the Reduction of Thrombo-Embolism in patients with Device-Detected Sub-Clinical Atrial fibrillation, ClinicalTrials.gov Identifier: NCT01938248) and NOAH-AFNET 6 [38] (Non-vitamin K Antagonist Oral Anticoagulants in Patients With Atrial High Rate Episodesd—Atrial Fibrillation NETwork6,ClinicalTrials.gov Identifier: NCT02618577) are likely to shed more light on appropriate management of device detected AHRE.
Disclosure of any funding to the study
No funding was used for this study.
Disclosure of any conflict of interest
We declare no conflict of interest.
References
- 1.Go AS, Hylek EM, Phillips KA, Chang Y, Henault LE, Selby JV, et al. Prevalence of diagnosed atrial fibrillation in adults: national implications for rhythm management and stroke prevention: the AnTicoagulation and Risk Factors in Atrial Fibrillation (ATRIA) study. JAMA. 2001;285:2370–5. doi: 10.1001/jama.285.18.2370. [DOI] [PubMed] [Google Scholar]
- 2.European Heart Rhythm Association. European Association for Cardio-Thoracic Surgery. Camm AJ, Kirchhof P, Lip GYH, Schotten U, et al. Guidelines for the management of atrial fibrillation: the task force for the management of atrial fibrillation of the European society of cardiology (ESC) Eur Heart J. 2010;31:2369–429. doi: 10.1093/eurheartj/ehq278. [DOI] [PubMed] [Google Scholar]
- 3.Chugh SS, Blackshear JL, Shen W-K, Hammill SC, Gersh BJ. Epidemiology and natural history of atrial fibrillation: clinical implications. J Am Coll Cardiol. 2001;37:371–8. doi: 10.1016/S0735-1097(00)01107-4. [DOI] [PubMed] [Google Scholar]
- 4.Thrall G, Lane D, Carroll D, Lip GYH. Quality of life in patients with atrial fibrillation: a systematic review. Am J Med. 2006;119:448.e1–448.e19. doi: 10.1016/j.amjmed.2005.10.057. [DOI] [PubMed] [Google Scholar]
- 5.Healey JS, Connolly SJ, Gold MR, Israel CW, Van Gelder IC, Capucci A, et al. Subclinical atrial fibrillation and the risk of stroke. N Engl J Med. 2012;366:120–9. doi: 10.1056/NEJMoa1105575. [DOI] [PubMed] [Google Scholar]
- 6.Purerfellner H, Gillis AM, Holbrook R, Hettrick DA. Accuracy of atrial tachyarrhythmia detection in implantable devices with arrhythmia therapies. Pacing Clin Electrophysiol PACE. 2004;27:983–92. doi: 10.1111/j.1540-8159.2004.00569.x. [DOI] [PubMed] [Google Scholar]
- 7.Defaye P, Dournaux F, Mouton E. Prevalence of supraventricular arrhythmias from the automated analysis of data stored in the DDD pacemakers of 617 patients: the AIDA study. The AIDA Multicenter Study Group. Automatic Interpretation for Diagnosis assistance. Pacing Clin Electrophysiol PACE. 1998;21:250–5. doi: 10.1111/j.1540-8159.1998.tb01098.x. [DOI] [PubMed] [Google Scholar]
- 8.Kohno R, Oginosawa Y, Abe H. Identifying atrial arrhythmias versus pacing-induced rhythm disorders with state-of-the-art cardiac implanted devices. J Arrhythmia. 2014;30:82–7. doi: 10.1016/j.joa.2014.03.001. [DOI] [Google Scholar]
- 9.Glotzer TV, Hellkamp AS, Zimmerman J, Sweeney MO, Yee R, Marinchak R, et al. Atrial high rate episodes detected by pacemaker diagnostics predict death and stroke: report of the Atrial Diagnostics Ancillary Study of the MOde Selection Trial (MOST) Circulation. 2003;107:1614–9. doi: 10.1161/01.CIR.0000057981.70380.45. [DOI] [PubMed] [Google Scholar]
- 10.Capucci A, Santini M, Padeletti L, Gulizia M, Botto G, Boriani G, et al. Monitored atrial fibrillation duration predicts arterial embolic events in patients suffering from bradycardia and atrial fibrillation implanted with anti-tachycardia pacemakers. J Am Coll Cardiol. 2005;46:1913–20. doi: 10.1016/j.jacc.2005.07.044. [DOI] [PubMed] [Google Scholar]
- 11.Shanmugam N, Boerdlein A, Proff J, Ong P, Valencia O, Maier SKG, et al. Detection of atrial high-rate events by continuous home monitoring: clinical significance in the heart failure-cardiac resynchronization therapy population. Eur Eur Pacing Arrhythm Card Electrophysiol J Work Groups Card Pacing Arrhythm Card Cell Electrophysiol Eur Soc Cardiol. 2012;14:230–7. doi: 10.1093/europace/eur293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Boriani G, Glotzer TV, Santini M, West TM, De Melis M, Sepsi M, et al. Device-detected atrial fibrillation and risk for stroke: an analysis of >10,000 patients from the SOS AF project (Stroke preventiOn Strategies based on Atrial Fibrillation information from implanted devices) Eur Heart J. 2014;35:508–16. doi: 10.1093/eurheartj/eht491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pastori D, Miyazawa K, Li Y, Szekely O, Shahid F, Farcomeni A, et al. Atrial high-rate episodes and risk of major adverse cardiovascular events in patients with cardiac implantable electronic devices. Clin Res Cardiol Off J Ger Card Soc. 2019 doi: 10.1007/s00392-019-01493-z. [DOI] [PubMed]
- 14.Benezet-Mazuecos J, Rubio JM, Farre J. Atrial high rate episodes in patients with dual-chamber cardiac implantable electronic devices: unmasking silent atrial fibrillation. Pacing Clin Electrophysiol PACE. 2014;37:1080–6. doi: 10.1111/pace.12428. [DOI] [PubMed] [Google Scholar]
- 15.Gorenek B, Bax J, Boriani G, Chen S-A, Dagres N, Glotzer TV, et al. Device-detected subclinical atrial tachyarrhythmias: definition, implications and managementdan European heart rhythm association (EHRA) consensus document, endorsed by heart rhythm society (HRS), asia pacific heart rhythm society (APHRS) and sociedad latinoamericana de Estimulacion cardíaca y electrofisiología (SOLEACE) EP Eur. 2017;19:1556–78. doi: 10.1093/europace/eux163. [DOI] [PubMed] [Google Scholar]
- 16.Glotzer TV, Daoud EG, Wyse DG, Singer DE, Ezekowitz MD, Hilker C, et al. The relationship between daily atrial tachyarrhythmia burden from implantable device diagnostics and stroke risk: the TRENDS study. Circ Arrhythm Electrophysiol. 2009;2:474–80. doi: 10.1161/CIRCEP.109.849638. [DOI] [PubMed] [Google Scholar]
- 17.Miyazawa K, Pastori D, Li Y-G, Szekely O, Shahid F, Boriani G, et al. Atrial high rate episodes in patients with cardiac implantable electronic devices: implications for clinical outcomes. Clin Res Cardiol. 2019;108:1034–41. doi: 10.1007/s00392-019-01432-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Healey JS, Martin JL, Duncan A, Connolly SJ, Ha AH, Morillo CA, et al. Pacemaker-detected atrial fibrillation in patients with pacemakers: prevalence, predictors, and current use of oral anticoagulation. Can J Cardiol. 2013;29:224–8. doi: 10.1016/j.cjca.2012.08.019. [DOI] [PubMed] [Google Scholar]
- 19.Cheung JW, Keating RJ, Stein KM, Markowitz SM, Iwai S, Shah BK, et al. Newly detected atrial fibrillation following dual chamber pacemaker implantation. J Cardiovasc Electrophysiol. 2006;17:1323–8. doi: 10.1111/j.1540-8167.2006.00648.x. [DOI] [PubMed] [Google Scholar]
- 20.Sweeney Michael O, Hellkamp Anne S, Ellenbogen Kenneth A, Greenspon Arnold J, Freedman Roger A, Lee Kerry L, et al. Adverse effect of ventricular pacing on heart failure and atrial fibrillation among patients with normal baseline QRS duration in a clinical trial of pacemaker therapy for sinus node dysfunction. Circulation. 2003;107:2932–7. doi: 10.1161/01.CIR.0000072769.17295.B1. [DOI] [PubMed] [Google Scholar]
- 21.Pastori D, Miyazawa K, Li Y, Shahid F, Hado H, Lip GYH. Inflammation and the risk of atrial high-rate episodes (AHREs) in patients with cardiac implantable electronic devices. Clin Res Cardiol Off J Ger Card Soc. 2018;107:772–7. doi: 10.1007/s00392-018-1244-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Benezet-Mazuecos J, Iglesias JA, Cortes M, Rubio JM, de la Vieja JJ, Del Río A, et al. Silent atrial fibrillation in pacemaker early post-implantation period: an unintentionally provoked situation? Eur Eur Pacing Arrhythm Card Electrophysiol J Work Groups Card Pacing Arrhythm Card Cell Electrophysiol Eur Soc Cardiol. 2018;20:758–63. doi: 10.1093/europace/eux053. [DOI] [PubMed] [Google Scholar]
- 23.Narasimhan C, Verma JS, Ravi Kishore AG, Singh B, Dani S, Chawala K, et al. Cardiovascular risk profile and management of atrial fibrillation in India: real world data from RealiseAF survey. Indian Heart J. 2016;68:663–70. doi: 10.1016/j.ihj.2015.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gillis AM, Morck M. Atrial fibrillation after DDDR pacemaker implantation. J Cardiovasc Electrophysiol. 2002;13:542–7. doi: 10.1046/j.1540-8167.2002.00542.x. [DOI] [PubMed] [Google Scholar]
- 25.Israel CW, Neubauer H, Olbrich H-G, Hartung W, Treusch S, Hohnloser SH, et al. Incidence of atrial tachyar-rhythmias in pacemaker patients: results from the Balanced Evaluation of Atrial Tachyarrhythmias in Stimulated patients (BEATS) study. Pacing Clin Electrophysiol PACE. 2006;29:582–8. doi: 10.1111/j.1540-8159.2006.00405.x. [DOI] [PubMed] [Google Scholar]
- 26.Ziegler PD, Glotzer TV, Daoud EG, Singer DE, Ezekowitz MD, Hoyt RH, et al. Detection of previously undiagnosed atrial fibrillation in patients with stroke risk factors and usefulness of continuous monitoring in primary stroke prevention. Am J Cardiol. 2012;110:1309–14. doi: 10.1016/j.amjcard.2012.06.034. [DOI] [PubMed] [Google Scholar]
- 27.Mahajan R, Perera T, Elliott AD, Twomey DJ, Kumar S, Munwar DA, et al. Subclinical device-detected atrial fibrillation and stroke risk: a systematic review and meta-analysis. Eur Heart J. 2018;39:1407–15. doi: 10.1093/eurheartj/ehx731. [DOI] [PubMed] [Google Scholar]
- 28.Andersen JS, Egeblad H, Abildgaard U, Aldershvile J, Godtfredsen J. Atrial fibrillation and left atrial enlargement: cause or effect? J Intern Med. 1991;229:253–6. doi: 10.1111/j.1365-2796.1991.tb00340.x. [DOI] [PubMed] [Google Scholar]
- 29.Patel DA, Lavie CJ, Milani RV, Shah S, Gilliland Y. Clinical implications of left atrial enlargement: a review. Ochsner J. 2009;9:191–6. [PMC free article] [PubMed] [Google Scholar]
- 30.Vaziri SM, Larson MG, Benjamin EJ, Levy D. Echocardiographic predictors of nonrheumatic atrial fibrillation. The Framingham Heart study. Circulation. 1994;89:724–30. doi: 10.1161/01.cir.89.2.724. [DOI] [PubMed] [Google Scholar]
- 31.Sweeney MO, Hellkamp AS. Heart failure during cardiac pacing. Circulation. 2006;113:2082–8. doi: 10.1161/CIRCULATIONAHA.105.608356. [DOI] [PubMed] [Google Scholar]
- 32.Sharma G, Shetkar SS, Patel CD, Singh H, Naik N, Roy A, et al. Paced QRS duration predicts left ventricular function in patients with permanent pacemakers – one-year follow-up study using equilibrium radionuclide angiography (ERNA) Indian Pacing Electrophysiol J. 2015;15:90–5. doi: 10.1016/j.ipej.2015.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sumiyoshi M, Nakata Y, Tokano T, Yasuda M, Ohno Y, Hisaoka T, et al. Clinical significance of QRS duration during ventricular pacing. Pacing Clin Electrophysiol PACE. 1992;15:1053–64. doi: 10.1111/j.1540-8159.1992.tb03099.x. [DOI] [PubMed] [Google Scholar]
- 34.Xing F, Jiang J, Hu X, Feng C, He J, Dong Y, et al. Association between paced QRS duration and atrial fibrillation after permanent pacemaker implantation: a retrospective observational cohort study. Medicine (Baltim) 2018;97:e9839. doi: 10.1097/MD.0000000000009839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pastore G, Zanon F, Baracca E, Aggio S, Corbucci G, Boaretto G, et al. The risk of atrial fibrillation during right ventricular pacing. EP Eur. 2016;18:353–8. doi: 10.1093/europace/euv268. [DOI] [PubMed] [Google Scholar]
- 36.Benezet-Mazuecos J, Rubio JM, Cort es M, Iglesias JA, Calle S, de la Vieja JJ, et al. Silent ischaemic brain lesions related to atrial high rate episodes in patients with cardiac implantable electronic devices. Eur Eur Pacing Arrhythm Card Electrophysiol J Work Groups Card Pacing Arrhythm Card Cell Electrophysiol Eur Soc Cardiol. 2015;17:364–9. doi: 10.1093/europace/euu267. [DOI] [PubMed] [Google Scholar]
- 37.Marinheiro R, Parreira L, Amador P, Lopes C, Fernandes A, Mesquita D, et al. Clinical impact of oral anticoagulation in patients with atrial high-rate episodes. J Stroke Cerebrovasc Dis Off J Natl Stroke Assoc. 2019;28:971–9. doi: 10.1016/j.jstrokecerebrovasdis.2018.12.019. [DOI] [PubMed] [Google Scholar]
- 38.Kirchhof P, Blank BF, Calvert M, Camm AJ, Chlouverakis G, Diener H-C, et al. Probing oral anticoagulation in patients with atrial high rate episodes: rationale and design of the Non-vitamin K antagonist Oral anticoagulants in patients with Atrial High rate episodes (NOAH-AFNET 6) trial. Am Heart J. 2017;190:12–8. doi: 10.1016/j.ahj.2017.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lopes RD, Alings M, Connolly SJ, Beresh H, Granger CB, Mazuecos JB, et al. Rationale and design of the apixaban for the reduction of thrombo-embolism in patients with device-detected sub-clinical atrial fibrillation (ARTESiA) trial. Am Heart J. 2017;189:137–45. doi: 10.1016/j.ahj.2017.04.008. [DOI] [PubMed] [Google Scholar]