Abstract
Background
Transcatheter Mitral Valve-in-Valve Implantation (TMViVI) has recently emerged as a novel therapy for degenerated mitral valve bioprosthesis. Re-operative mitral valve surgery is associated with a substantial risk of mortality and morbidity. The objective of this study was to describe the outcomes of transcatheter mitral valve-in-valve implantations in our cardiac center.
Methods
Twenty-two patients underwent the valve-in-valve procedure because of bioprosthesis degeneration from March 2017 to October 2018. Clinical, echocardiographic, procedural details and survival at follow up were assessed.
Results
Eight patients refused re-operative cardiac surgery while others were deemed a high risk for conventional re-operative sternotomy. All patients had TMViVI performed via a trans-septal approach, and the prosthesis was implanted successfully with immediate hemodynamic improvement in 20 patients. One patient had tamponade (4.55%), two had permanent pacemaker insertion (9.09%), two patients had a renal impairment (9.09%), and three patients had vascular complications (13.64%). There was one aborted procedure for the failure to cross the tissue valve with a transcatheter valve, and one patient was converted to an emergency mitral valve surgery. All patients were discharged in NYHA class I/II and NYHA class was markedly improved at one-year follow-up (p = 0.002).
Conclusions
Trans-septal mitral valve-in-valve implantation can be performed safely for degenerative mitral valve bioprosthesis and with favorable early clinical and hemodynamic outcomes.
Keywords: Degenerated mitral bioprosthesis, Transcatheter mitral valve implantation, Trans-septal mitral valve in valve
Introduction
Transcatheter Mitral Valve-in-Valve Implantation (TMViVI) has recently emerged as a novel therapy for degenerated mitral valve bio-prosthesis. [1] Reoperative sternotomy for mitral valve surgery is associated with a substantial risk of mortality and morbidity. Additionally, the patients who undergo re-operative mitral valve replacement are older and more fragile. [2] Therefore the popularity of TMViVI is increasing for treating degenerated bioprosthesis in patients with high surgical risk.
Transcatheter valve-in-valve replacement was added to the armamentarium of mitral valve intervention; since then, the discussion and the trade-offs between mechanical and bioprosthetic valves are changing. [3] The recent AHA/ACC focused update of the 2014 AHA/ACC guideline for the management of patients with valvular disease defined the role of valve-in-valve as a class IIb recommendation. [3] valve-in-valve, valve-in-ring, and valve-in-mitral annular calcification were previously evaluated with inconsistent results, and there is fast-growing literature on this subject. [4]
Most of the available publications discussed the short-term outcomes, while the intermediate and long-term outcomes remain to be evaluated. Since TMViVI is increasingly used, we aimed to present our experience in transcatheter mitral valve-in-valve for the management of degenerated mitral bioprosthesis, describe the patients who had this procedure, and their outcomes.
Patients and methods
Patient population
From March 2017 to September 2018, thirty–thirty patients were admitted to our tertiary referral cardiac center with a degenerated mitral bioprosthesis. Eleven patients had re-operative isolated mitral valve surgery, and 22 patients were assigned to catheter-based intervention based on the decision of the interdisciplinary heart team, and all patients were deemed to be high-risk candidates. Patients who refused surgery were considered suitable candidates for the transcatheter mitral valve-in-valve procedure (n = 8). Patient who underwent trans-catheter mitral valve in valve had symptomatic severe mitral regurgitation (n = 10; 45%), severe mitral stenosis (n = 6; 27%) or mixed pathology (n = 6; 27%).
A pre-procedural secure database of patients’ demographics, preoperative risk assessment, and procedural data were created and included procedure details and operative outcomes. Follow-up data were collected at 30, 180, and 365 days. Trans-thoracic echocardiography was performed in all patients pre- and post-procedure. Patients were routinely scheduled for clinical follow-up 30 days after the procedure.
We do not perform transcatheter mitral valve in valve in patients with infective endocarditis, mitral valve vegetations and patients with a thick inter-atrial septum. Patients with valve size less than 25 were considered unfit for transcatheter intervention for the possibility of patient prosthesis mismatch. Patients with a pre-procedure paravalvular leak can be managed concomitantly; however, none of our patients had a concomitant paravalvular leak. All valve types were suitable for the valve in valve implantation.
Ethical considerations
The nature of valve-in-valve implantation in the non-aortic position was discussed with each patient as part of the informed consent process by the procedure's assigned consultant interventionist. The study was approved by the Institutional Review Board (IRB), and the patient's consent to participate in the study was waived because of the retrospective nature of the research.
Echocardiography
All valve-in-valve procedures were concluded with a thorough assessment of valvular hemodynamics and the flow characteristics before, during, and immediately following valve implantation with echocardiography. Transthoracic echocardiography (TTE) was done before the procedure to evaluate the prosthesis morphology and function as well as for the routine measurements. Transesophageal echocardiogram (TEE) was the guidance tool throughout the procedure. Pre-procedural TEE was conducted to confirm the absence of left atrial appendage (LAA) thrombus and affirm the findings of the TTE as regard to the morphological and functional status of the bioprosthesis.
Intraprocedural TEE and fluoroscopy were used in septal puncture guidance. It was crucial to assess the appropriate positioning of the valve, the mitral valve gradients, and the absence of a periprosthetic leak. TEE was pivotal to rule out any encroachment effect of the prosthesis, particularly on the left ventricular outflow tract (LVOT).
The trans-septal approach in valve-in-valve implantation
The valve-in-valve implantation procedures were performed in our hybrid operative room. Twenty-one patients had the procedure performed under general anesthesia, and one patient had moderate sedation. All patients had trans-esophageal echocardiography (TEE) continuous guidance. The right femoral vein access was used to perform the trans-septal puncture. After securing the Guideright™ J tip (St. Jude Medical, Inc., St. Paul, MN, USA) long wire into the left innominate vein, the trans-septal sheath was introduced over the wire. The trans-septal needle BRK™ 1 (St. Jude Medical, Inc., St. Paul, MN, USA) was then introduced into the sheath and kept few millimeters short of the tip of the sheath. Under both fluoroscopy and TEE guidance, the sheath-needle assembly was pulled back slowly in a clockwise rotation to achieve a posterior direction. At the superior-posterior position, the septum was punctured, and the sheath was secured into the left atrium (LA). A five French diagnostic Judkins right catheter (JR4) was introduced into the LA via the trans-septal sheath. A Terumo soft exchange wire inside the JR4 (Terumo, Somerset, NJ, USA) was introduced into the left ventricle (LV) via the mitral prosthesis, and JR4 was then further introduced into the LV. The extra stiff Confida™ Brecker guidewire (Medtronic Inc, Minneapolis, Minnesota, USA) was secured into the LV through the JR4. After securing the trans-septal sheath into the LA, heparin was given intravenously to keep activated clotting time (ACT) more than 250 s throughout the procedure.
A fourteen French (14F) Edward sheath was then secured in the right femoral vein over the stiff wire. The septum is dilated using Z-MED-X™ (NuMED Canada Inc., Cornwall, ON, Canada) 14F balloon to facilitate the passage of the transcatheter Heart Valve across the septum. Edwards Sapien 3 Trans-catheter Heart Valve (Edwards life sciences, Irvin California, USA) was used in all patients (Fig. 1). The transcatheter heart valve was crimped in a reverse fashion onto the balloon compared with the same valve used in the aortic position; so that the outer skirt was directed towards the LA. The valve-in-valve mitral app version 2.2 developed by the technology company UBQO and Dr. Vinayak Bapat (Retrieved from http://www.ubqo.com/vivmitral), was used to choose the valve size to be used according to the size of the degenerated mitral bio-prosthesis to be treated in every patient. Similar to Cheung and associates,[5] the transcatheter valve was placed to slightly overlapping the stent of the degenerated bioprosthesis into the LA for sufficient anchoring. No ventricular pacing was used at any stage of the procedure.
Fig. 1.
Trans-septal Approach in mitral valve-in-valve implantation. After right femoral vein access, septal puncture, and dilatation by ballooning (A), the transcatheter heart valve in a reverse fashion was positioned and deployed in the degenerated bioprosthesis (B; C). Edwards Sapien 3 transcatheter valve successfully implanted on a degenerated mitral bioprosthesis (D).
Pre-implantation balloon dilatation was performed in 10 patients (45%), and one patient had post-implantation balloon dilatation. Subsequently, valve performance was assessed with TEE and fluoroscopy (Fig. 2).
Fig. 2.
Intraprocedural transesophageal echocardiographic guidance. A. intra-atrial septum tenting B. Trans-septal sheath into the left atrium C. ballooning the septum D. the degenerative mitral bioprosthesis with immobile leaflet E. Competent mitral valve-in-valve F and G. Pre- and post-procedure mitral valve gradients.
In the case of concomitant transcatheter aortic valve implantation, the aortic valve was deployed retrogradely via the transfemoral arterial approach into the aortic valve before the mitral valve intervention. Concomitant intervention to the tricuspid valve was done after transcatheter mitral valve implantation.
After the procedure, all patients with no other indications for anti-coagulation were started on warfarin combined with Aspirin for 3 months with target INR between 2 and 3. This is followed by lifelong Aspirin only.
Statistical analysis
Continuous data were described as median and (25th- 75th percentiles) and categorical data as number and percent. A comparison between the pre and post-procedural echocardiographic data was performed using Wilcoxon matched-pairs signed-rank test, and pre and post-procedural categorical variables were compared using the McNemar test. Kaplan–Meier curve was used to present survival distribution. Stata 14.2 was used to analyze the data (Stata Corp- College Town- Texas–USA).
Results
Baseline data
Patients characteristics and co-morbidities are presented in Table 1. Most patients presented in NYHA class III/IV (n = 17; 77.27%) One patient had a history of stroke (4.55%), three patients had a permanent pacemaker (13.64%), two patients had cardiomyopathy (9.09%), and one patient (4.55%) had a previous percutaneous coronary intervention (PCI). Six patients had a history of coronary artery bypass grafting (CABG) (27.27%), and seven patients had aortic valve replacement (AVR) with a tissue valve (31.82%). Ten patients had prior tricuspid valve (TV) intervention (45.45%): ring repair in 5 patients (22.73%), De Vega repair in 4 patients (18.18%), and TV replacement with bioprosthetic valves in 3 patients (13.64%). Median creatinine clearance was 67.5 mg/dl (25th- 75th percentiles; 40–78) and hemoglobin was 11.9 mg/dl (25th- 75th percentiles; 10.1–13.4). STS score for mitral valve replacement was 5.59 (25th- 75th percentiles; 4.4–7.6), and Euro-Score II (ES II) was 6.63 (25th- 75th percentiles; 5.5–9.5). All patients had prior mitral valve replacement with a bioprosthetic valve (median size 25; 25th- 75th percentiles: 25–27). Median time from mitral valve replacement to transcatheter mitral valve implantation was 6.33 years (25th- 75th percentiles: 4.35–10.65). Pre-procedural echocardiographic data are shown in Table 1.
Table 1.
Baseline patients' data. Continuous variables are presented as median (25th- 75th percentiles) and categorical variables as number (percent). BMI: body mass index; BSA: body surface area; COPD: chronic obstructive pulmonary disease; LV: left ventricle; LVEDD; left ventricular end-diastolic diameter; LVESD: left ventricular end-systolic diameter; PASP: pulmonary artery systolic pressure.
| Variable | Total number of patients (22) |
|---|---|
| Age (Years) | 73 (67–78) |
| Female | 16 (72.73%) |
| BMI (Kg/m2) | 29.45 (25.22–33.73) |
| BSA (m2) | 1.7 (1.6–1.9) |
| Diabetes mellitus | 11 (50%) |
| Hypertension | 15 (68.18%) |
| COPD | 3 (13.64%) |
| Heart Failure in the previous 2 weeks | 6 (28.57%) |
| Chronic kidney disease | 2 (9.09%) |
| Atrial fibrillation | 11 (50%) |
| Mitral valve bioprosthesis model | |
| Mosaic | 6 (27.27%) |
| Hancock | 1 (4.55%) |
| Perimount | 9 (40.9%) |
| Epic | 3 (13.64%) |
| Carpentier- Edward (Magna) | 1 (4.55%) |
| LVEDD (mm) | 45 (41–53) |
| LVESD (mm) | 30 (27–36) |
| PASP (mmHg) | 60 (55–75) |
| Indexed LV mass (g/m2) | 96.4 (80.6–121.3) |
| Ejection fraction (%) | 55 (50–60) |
Procedural and post-procedural details
All patients had a trans-septal approach to the mitral valve via the right femoral vein. Twenty-one patients (95.45%) had general anesthesia, and one patient had moderate sedation. There was one aborted procedure for the failure to cross the tissue valve with a transcatheter valve, and the patient was enlisted for re-operative mitral valve replacement (MVR). This patient had Carpentier-Edward (Magna) valve size 27, and the leaflets were fused and stuck. On the other hand, one patient was converted to an emergency MVR because the deployment was to LVOT. Pre-implantation balloon insufflation was used in 10 (45.45%). Edward Sapien (Edwards life sciences, Irvin California, USA) was used in all patients (median size 26 mm; 25th- 75th percentiles: 23–29). One patient (4.5%) required inotropic support during the procedure, and four patients had mild MR post-implantation (20%).
Concomitant procedures were performed in 4 patients, 2 had a tricuspid valve in valve implantation, one patient had an aortic valve in valve implantation, and one had transcatheter aortic valve implantation.
Post-procedure complications and discharge echocardiographic measurements are shown in Table 2. No patient had a paravalvular leak, device migration, or thrombosis post-implantation. Discharge echocardiography showed a significant decrease in mean and peak mitral valve pressure gradient and pulmonary artery systolic pressure (Table 3).
Table 2.
Post-procedural complications and discharge echocardiographic measurements. Continuous variables are presented as median (25th- 75th percentiles) and categorical variables as number (percent). AF: atrial fibrillation; CCU; coronary care unit; LVOT: left ventricular outflow tract; LV: left ventricle; LVEDD; left ventricular end-diastolic diameter; LVESD: left ventricular end-systolic diameter; PASP: pulmonary artery systolic pressure; PMM: permanent pacemaker.
| Variable | |
|---|---|
| New-onset AF | 3 (13.64%) |
| PMM insertion | 2 (9.09%) |
| LVOT obstruction | 1 (4.55%) |
| Perforation and tamponade | 1 (4.55%) |
| Renal impairment and dialysis | 2 (9.09%) |
| Hemorrhagic stroke | 2 (9.09%) |
| Vascular complications | 3 (13.64%) |
| In-hospital death | 2 (9.09%) |
| CCU stay (hours) | 27.5 (23–92) |
| Hospital stay (days) | 14 (6–20) |
| Ejection fraction (%) | 55 (50–60) |
| LVEDD (mm) | 41.5 (31–47) |
| LVESD (mm) | 26 (21.5–31) |
| PASP (mmHg) | 50 (45–55) |
| Indexed LV mass (g/m2) | 92.15 (80.25–124.45) |
Table 3.
Echocardiographic changes pre and post-procedure. Continuous variables are presented as median (25th- 75th percentiles). MV: mitral valve; PASP: pulmonary artery systolic pressure.
| Pre-procedure | Post-procedure | p | |
|---|---|---|---|
| Ejection fraction (%) | 55 (50–60) | 55 (50–60) | 0.74 |
| MV peak gradient (mmHg) | 21.1 (18.8–25.2) | 15.7 (13.2–19.6) | 0.0002 |
| MV mean gradient (mmHg) | 8.3 (6–10.9) | 6.9 (6–8.2) | 0.0030 |
| PASP (mmHg) | 60 (55–75) | 50 (45–55) | 0.0037 |
The median follow-up duration was 12.5 months (25th and 75th percentiles: 4–17). During the follow-up, mitral valve replacement was performed after three months for LV pseudoaneurysm in one patient, and one patient had MVR after eight months for mitral valve infective endocarditis. At one year, there was a marked improvement in the NYHA class, and two patients were in class III (p = 0.002). Seven patients required re-hospitalization after the procedure (4 of them for cardiac causes) with a median time to re-hospitalization five months (25th-75th percentiles: 3.5–8.5). Survival at 12 months was 86% (Fig. 3).
Fig. 3.
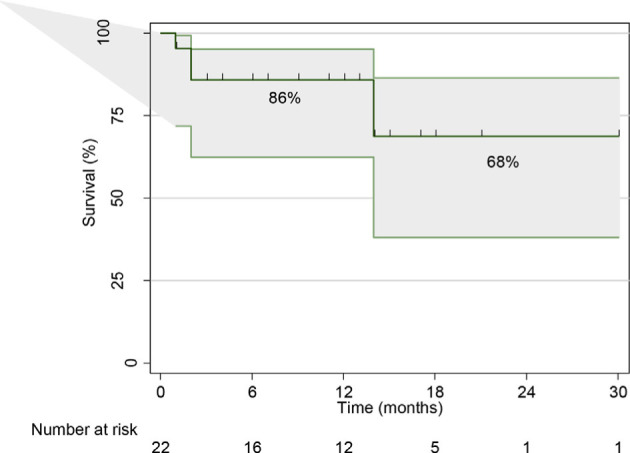
Kaplan–Meier survival distribution.
Discussion
Re-operative mitral valve replacement surgery is associated with increased morbidity and mortality, particularly in elderly and fragile patients. The reported operative mortality can reach 15%. [6,7] Transcatheter valve-in-valve implantation was endorsed in the new European Society of Cardiology (ESC)/European Association for Cardio-Thoracic Surgery (EACTS) Guidelines (2017) as an option for the management of degenerated bio-prosthesis in high surgical risk patients and after multidisciplinary heart team discussion to individualize the approach to each patient. [8] In the annual transcatheter valve therapy registry report of The Society of Thoracic Surgeons/American College of Cardiology, the patients who had mitral valve-in-valve implantation were high risk, with an STS predicted risk of mortality for mitral valve replacement of 11%. They found 7.2% hospital mortality and 8.5% 30-day mortality. [9]
All patients included in this study were discussed by our heart team, which consists of cardiologists and cardiac surgeons. The patients were assigned to transcatheter mitral valve-in-valve implantation if they were either considered high risk for conventional mitral surgery or if they refused it.
We used the trans-septal approach with successful implantation in 90% of the patients. Trans-septal access is simple and less invasive compared to the trans-apical approach. It causes no LV damage, which can happen with the latter approach. [10] Significant bleeding is usually experienced from the apical cannulation, making the trans-septal approach a better option. [11]
Yoon and coworkers, [12] in their study about transcatheter mitral valve implantation for degenerated bioprosthesis enrolled 248 patients who were deemed high surgical risk based on STS score, and they concluded that transcatheter mitral valve replacement provided an acceptable outcome in this cohort of patients.
The correct sizing of the transcatheter heart valve is extremely important for a successful procedure, and multimodal information should be used. These include TEE, cardiac computerized tomography scan, and the valve-in-valve app. [13] We used the valve-in-valve app version 2.2 as our sizing reference after confirming the surgical valve label in the cardiac surgery document for every specific patient. Balloon valvuloplasty was used in 10 patients before valve-in-valve implantation, and one patient had difficulty in crossing the degenerated valve and was scheduled for mitral valve surgery.
Immediately after the procedure, the mitral valve function improved with a significant reduction to the complete disappearance of mitral regurgitation (MR), and three patients had mild MR. Two patients who had predominant MS had a significant reduction of the mean gradient across the mitral valve (8.8 mmHg–2 mmHg; 12.3 mmHg–5 mmHg) at the same heart rate indicating the successful treatment of the MS. No dye was needed in any patients because we used the radiopaque frame of the surgical prosthesis to deploy the valve. Our results are consistent with other published series, which showed that mitral valve-in-valve for degenerated bioprosthesis was associated with low periprocedural complications and good long-term outcomes. [14]
In-hospital mortality was reported in 2 patients (9.09%), and survival at one year was 86%. In a meta-analysis on mitral valve-in-valve for degenerated bioprostheses, the trans-apical approach was used in 55% of the patients and hospital mortality 5.7%, and 23.4% at six months. [15]
During the one-year follow-up, there was no reported valve-related complications or structural degeneration. Clinical status improved, and NYHA functional class improved immediately and was significantly better after a one-year follow-up. Ye and associates reported eight years single-center experience involving 42 cases of Aortic valve-in-valve and 31 mitral valve-in-valve and stated that the procedure could be performed safely with a high success rate and encouraging midterm clinical outcome in high surgical risk patients. [16] Additionally, reporting and analyzing the long term data of this approach in a large cohort of patients is recommended. [16]
Study strengths and limitations
The major limitation of the study is the retrospective nature with its inherited referral and selection biases. Another limitation is the small patients' number, which is considered acceptable for this newly introduced technique. Finally, there is a lack of comparison group with surgical mitral valve replacement; however, most of our patients were included because they were deemed unfit for surgical interventions. Of particular interest, this retrospective study reported unique techniques and work-up. The procedures were done in a hybrid operative room. No CT scan was needed for peri-procedural work-up. The approach was trans-septal in all patients, and neither pacing nor contrast was used.
Conclusion
Trans-septal mitral valve-in-valve implantation can be performed safely for degenerative mitral valve bioprosthesis and with favorable clinical and hemodynamic early outcomes. It can offer a viable option in patients with high surgical risk and or in patients who reject conventional surgery.
Abbreviations
- ACT
activated clotting time
- AVR
Aortic valve replacement
- ES II
EuroScore II
- IRB
Institutional Review Board
- LA
Left atrium
- LAA
Left atrial appendage
- LV
Left ventricle
- LVOT
Left ventricular outflow tract
- NYHA
New York Heart Association
- TEE
Trans-esophageal echocardiography
- TMViVI
Transcatheter Mitral Valve-in-Valve Implantation
- TTE
Trans-thoracic echocardiography
Funding
None.
Declaration of Competing Interest
The authors have nothing to disclose.
References
- 1.Testa L, Popolo Rubbio A, Casenghi M, Pero G, Latib A, Bedogni F. Transcatheter mitral valve replacement in the transcatheter aortic valve replacement Era. J Am Heart Assoc. 2019 Nov;8(22):e013352. doi: 10.1161/JAHA.119.013352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Maganti M, Rao V, Armstrong S, Feindel CM, Scully HE, David TE. Redo valvular surgery in elderly patients. Ann Thorac Surg. 2009 Feb;87(2):521–5. doi: 10.1016/j.athoracsur.2008.09.030. [DOI] [PubMed] [Google Scholar]
- 3.Nishimura RA, Otto CM, Bonow RO, Carabello BA, Erwin 3rd JP, Fleisher LA, et al. 2017 AHA/ACC focused update of the 2014 AHA/ACC guideline for the management of patients with valvular heart disease: a report of the American College of Cardiology/American heart association task force on clinical practice guidelines. Circulation. 2017 Jun;135(25):e1159–95. doi: 10.1161/CIR.0000000000000503. [DOI] [PubMed] [Google Scholar]
- 4.Ribeiro RVP, Yanagawa B, Légaré JF, Hassan A, Ouzounian M, Verma S, et al. Clinical outcomes of mitral valve intervention in patients with mitral annular calcification: a systematic review and meta-analysis. J Card Surg. 2019 Jan;35(1):66–74. doi: 10.1111/jocs.14325. [DOI] [PubMed] [Google Scholar]
- 5.Cheung AW, Gurvitch R, Ye J, Wood D, Lichtenstein SV, Thompson C, et al. Transcatheter transapical mitral valve-in-valve implantations for a failed bioprosthesis: a case series. J Thorac Cardiovasc Surg. 2011 Mar;141(3):711–5. doi: 10.1016/j.jtcvs.2010.11.026. [DOI] [PubMed] [Google Scholar]
- 6.Balsam LB, Grossi EA, Greenhouse DG, Ursomanno P, Deanda A, Ribakove GH, et al. Reoperative valve surgery in the elderly: predictors of risk and long-term survival. Ann Thorac Surg. 2010 Oct;90(4):1195–200. doi: 10.1016/j.athoracsur.2010.04.057. discussion 1201. [DOI] [PubMed] [Google Scholar]
- 7.Kilic A, Helmers MR, Han JJ, Kanade R, Acker MA, Hargrove WC, et al. Redo mitral valve surgery following prior mitral valve repair. J Card Surg. 2018 Dec;33(12):772–7. doi: 10.1111/jocs.13944. [DOI] [PubMed] [Google Scholar]
- 8.Falk V, Baumgartner H, Bax JJ, De Bonis M, Hamm C, Holm PJ, et al. 2017 ESC/EACTS Guidelines for the management of valvular heart disease. Eur J Cardio Thorac Surg. 2017 Oct;52(4):616–64. doi: 10.1093/ejcts/ezx324. [DOI] [PubMed] [Google Scholar]
- 9.Grover FL, Vemulapalli S, Carroll JD, Edwards FH, Mack MJ, Thourani VH, et al. 2016 annual report of the society of thoracic Surgeons/American College of Cardiology trans-catheter valve therapy registry. J Am Coll Cardiol. 2017 Mar;69(10):1215–30. doi: 10.1016/j.jacc.2016.11.033. [DOI] [PubMed] [Google Scholar]
- 10.Eleid MF, Whisenant BK, Cabalka AK, Williams MR, Nejjari M, Attias D, et al. Early outcomes of percutaneous transvenous transseptal transcatheter valve implantation in failed bioprosthetic mitral valves, ring annuloplasty, and severe mitral annular calcification. JACC Cardiovasc Interv. 2017 Oct;10(19):1932–42. doi: 10.1016/j.jcin.2017.08.014. [DOI] [PubMed] [Google Scholar]
- 11.Seiffert M, Conradi L, Baldus S, Schirmer J, Knap M, Blankenberg S, et al. Transcatheter mitral valve-in-valve implantation in patients with degenerated bioprostheses. JACC Cardiovasc Interv. 2012 Mar;5(3):341–9. doi: 10.1016/j.jcin.2011.12.008. [DOI] [PubMed] [Google Scholar]
- 12.Yoon S-H, Whisenant BK, Bleiziffer S, Delgado V, Schofer N, Eschenbach L, et al. Transcatheter mitral valve replacement for degenerated bioprosthetic valves and failed annuloplasty rings. J Am Coll Cardiol. 2017 Aug;70(9):1121–31. doi: 10.1016/j.jacc.2017.07.714. [DOI] [PubMed] [Google Scholar]
- 13.Keenan NM, Bennetts JS, McGavigan AD, Rice GD, Joseph MX, Baker RA, et al. Transcatheter transseptal mitral valve-in-valve replacement: an early Australian case series and literature Review. Heart Lung Circ. 2019 Aug; doi: 10.1016/j.hlc.2019.07.010. [DOI] [PubMed] [Google Scholar]
- 14.Urena M, Brochet E, Lecomte M, Kerneis C, Carrasco JL, Ghodbane W, et al. Clinical and haemodynamic outcomes of balloon-expandable transcatheter mitral valve implantation: a 7-year experience. Eur Heart J. 2018 Jul;39(28):2679–89. doi: 10.1093/eurheartj/ehy271. [DOI] [PubMed] [Google Scholar]
- 15.Hu J, Chen Y, Cheng S, Zhang S, Wu K, Wang W, et al. Transcatheter mitral valve implantation for degenerated mitral bioprostheses or failed surgical annuloplasty rings: a systematic review and meta-analysis. J Card Surg. 2018 Sep;33(9):508–19. doi: 10.1111/jocs.13767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ye J, Cheung A, Yamashita M, Wood D, Peng D, Gao M, et al. Transcatheter aortic and mitral valve-in-valve implantation for failed surgical bioprosthetic valves: an 8-year single-center experience. JACC Cardiovasc Interv. 2015 Nov;8(13):1735–44. doi: 10.1016/j.jcin.2015.08.012. [DOI] [PubMed] [Google Scholar]




