Abstract
Background
Whole blood viscoelastic testing (VET) devices are routinely used in a variety of clinical settings to assess hemostasis. The Quantra QStat System is a cartridge-based point of care VET device that measures changes in clot stiffness during coagulation and fibrinolysis using ultrasound detection of resonance. The objective of this study was to assess the ability of the Quantra QStat System to detect coagulopathies in trauma patients.
Methods
A multicenter observational study was conducted on adult subjects at two level 1 trauma centers. For each subject, whole blood samples were drawn upon arrival to the emergency department and again, in some cases, after administration of blood products and/or antifibrinolytics. Samples were analyzed on the Quantra in parallel to ROTEM delta. The QStat cartridge provides measures of Clot Time (CT), Clot Stiffness (CS), Fibrinogen and Platelet Contributions to clot stiffness (FCS and PCS), and Clot Stability to Lysis (CSL). Data analyses included linear regression of Quantra and ROTEM parameters and an assessment of the concordance of the two devices for the assessment of hyperfibrinolysis.
Results
A total of 56 patients were analyzed. 42% of samples had a low QStat CS value suggestive of an hypocoagulable state. The low stiffness values could be attributed to either low PCS, FCS or combination. Additionally, 13% of samples showed evidence of hyperfibrinolysis based on the QStat CSL parameter. Samples analyzed with ROTEM assays showed a lower prevalence of low CS and hyperfibrinolysis based on EXTEM and FIBTEM results. The correlation of CS, FCS and CT versus equivalent ROTEM parameters was strong with r-values of 0.83, 0.79 and 0.79, respectively.
Discussion
This first clinical experience with the Quantra in trauma patients showed that the QStat Cartridge was strongly correlated with ROTEM parameters and that it could detect coagulopathies associated with critical bleeding.
Level of evidence
Diagnostic test, Level II.
Keywords: blood coagulation, blood coagulation tests, fibrinolysis, hemorrhage
Introduction
Trauma patients often present with an array of coagulation defects during their clinical course, with hemorrhage posing the greatest risk of early mortality following traumatic injury.1–3 Coagulopathy, defined as disturbance of the physiologic balance between bleeding and clotting, is observed in up to one-third of major trauma patients.2 4 5 This condition, in combination with hypothermia and metabolic acidosis, is responsible for significant mortality in the injured patient population. While the pathophysiology of trauma-induced coagulopathy is currently a subject of intense clinical and scientific research, the current understanding indicates a multifactorial condition driven primarily from hypoperfusion, endothelial cell damage, inflammation and tissue trauma.5–11 These factors have been shown to result in variable activation of the protein C pathway resulting in dysfunction of several coagulation factors, autoheparinization from glycocalyx damage, fibrinogen consumption, platelet dysfunction and unregulated fibrinolytic function.5 6 12 13 In particular, hyperfibrinolysis, an increased and unbalanced activation of the fibrinolytic system, has been recognized as one of the main factors contributing to the coagulopathic spectrum observed in trauma and other critical care settings. Recent studies have suggested that measurement of fibrinolysis is a marker for acute traumatic coagulopathy and a predictor for massive transfusion.14 15
Whole blood viscoelastic testing (VET) devices have been used to monitor coagulation and fibrinolytic function in trauma and emergency departments.16–19 The main technologies include thromboelastography, TEG 5000 (Haemonetics Corp, Braintree, Massachusetts, USA) and thromboelastometry, ROTEM delta (Instrumentation Laboratory, Bedford, Massachusetts, USA). To date, many major trauma centers have adopted whole blood VET devices to assess coagulopathies and direct transfusion therapy in bleeding patients with major injury.
The Quantra System (HemoSonics, LLC, Charlottesville, Virginia, USA) was recently introduced as an alternative technology for VET designed for operation at the point of care and in critical care settings.20 The system uses an ultrasound-based technology that does not require moving parts or direct contact with the sample being measured while enabling a comprehensive set of internal quality control measures.21 The performance of the Quantra with the QPlus Cartridge has been previously reported in a number of single-center and multicenter studies involving patients undergoing elective surgical procedures22–26 and, most recently, in patients affected by SARS-CoV-2.27 A new cartridge has been developed for the Quantra, the QStat Cartridge, which provides CT and CS measurements and a test to assess fibrinolytic function. In this study, we sought to compare the Quantra Hemostasis Analyzer with the QStat Cartridge to the ROTEM analyzer in a first multicenter study in the adult trauma population. We hypothesized that there would be a strong correlation in clot parameters between the two instruments.
Methods
A multicenter prospective, observational pilot study evaluating the performance of the Quantra Hemostasis Analyzer with the QStat Cartridge was conducted at two sites in the USA: the Texas Tech University Health Sciences Center, El Paso, Texas, in the University Medical Center of El Paso Emergency Department and at Parkland Memorial Hospital/The University of Texas Southwestern Medical Center, Dallas, Texas. The study protocol was registered under Clinical Trial numbers NCT03912545 and NCT03934983, respectively. Through a deferred consent process, each participant or their legally authorized representative was approached to provide written informed consent to enable release of their data to the study database.
The assessment of the clinical performance of the Quantra QStat System in trauma patients was based on two primary analyses: (1) correlation of QStat results with corresponding ROTEM delta parameters and (2) concordance between the QStat assessment of fibrinolysis (CSL) and the corresponding ROTEM delta lysis parameter (EXTEM ML).
Population and study protocol
The study population included adult patients, 18 years or older, experiencing major trauma requiring care at a level 1 trauma center. Exclusion criteria included the inability to obtain consent (either prior to performing any study related procedure or by deferred consent) or enrollment in another study that might confound results. Both clinical sites currently use site-specific algorithms for ROTEM delta guided trauma resuscitations, including indications for antifibrinolytic therapy. While both centers allow for the empiric, prophylactic administration of antifibrinolytics in bleeding patients, it is rarely done.
From each enrolled subject, whole blood samples were collected in 2.7 mL evacuated tubes containing 3.2% sodium citrate on arrival in the emergency department, prior to the administration of blood products or antifibrinolytics. One sample was used for analysis with the Quantra QStat System and one sample was used in parallel for standard of care analysis on the ROTEM delta running the following assays: INTEM, EXTEM, FIBTEM and APTEM. All ROTEM assays were run for 60 min and were analyzed according to the site’s normal protocols. For some patients, additional samples were taken after the administration of an antifibrinolytic or blood product if significant bleeding persisted and fibrinolysis was suspected.
The Quantra analyzers and the QStat Cartridges used in the study were labeled for Investigational Use Only (IUO). The results from Quantra analysis were blinded to the trauma clinical teams. Clinical decisions were based on each site’s standard of care and were not influenced by the study. Quantra analyzers were located either in a laboratory adjacent to the trauma emergency department/operating rooms or in a clinical research laboratory.
Data collection
For each enrolled subject, in addition to Quantra and ROTEM test results, the following information was documented in the study database: demographics: age, sex, race, ethnicity, height and weight; trauma information: reason for admittance to the hospital, type of injury, injury severity score based on the Revised Trauma Score (RTS); anticoagulant/antifibrinolytic medications taken prior to first test, if known; results of diagnostic tests performed within 30 min of Quantra testing as part of the institution’s standard of care including platelet count, fibrinogen and hematocrit; blood loss recorded within 24 hours of Quantra testing; and blood products and relevant therapies (antifibrinolytics and anticoagulants) administered within 24 hours after Quantra testing.
Quantra QStat System
The Quantra QStat System is a cartridge-based VET device that measures changes in CS resulting from coagulation and fibrinolysis using ultrasound detection of resonance. A detailed description of the system and its operating principle is presented in refs 20 21. The QStat Cartridge is the second single-use disposable developed for the Quantra platform, and it is intended for the management of patients with traumatic injury and in the perioperative setting of liver transplantation. The QStat Cartridge requires a whole blood sample collected in a 2.7 mL citrated tube. The cartridge outputs five parameters: Clot Time (CT), Clot Stiffness (CS), Fibrinogen Contribution to clot stiffness (FCS), Platelet Contribution to clot stiffness (PCS) and Clot Stability to Lysis (CSL). The parameters CT, CS, FCS and PCS and their respective reference range intervals are the same as those previously described for the QPlus Cartridge.22 The CSL parameter is calculated as the normalized difference between the clot stiffness change after maximum clot stiffness in the absence of tranexamic acid and the corresponding clot stiffness change in the presence of tranexamic acid. The CSL reportable range extends from 100% (no fibrinolysis) to 10% (significant fibrinolysis). The reference range for CSL was estimated as between 100% and 93% in a study of 43 healthy adult volunteers. A CSL value below the 93% threshold may indicate a reduction in clot stiffness caused by fibrinolysis. CSL is computed and reported as soon as fibrinolysis is detected.
Statistical analysis
Data analyses were performed using SAS V.9.4 or higher or R V.3.2.1 or higher (https://www.r-project.org/). Summary statistics were determined for each of the Quantra and ROTEM test parameters combined by time point. The Pearson correlation test was used to demonstrate correlation between the Quantra and ROTEM test parameters. A simple linear regression model was used to evaluate the linear relationship between device measurements: CT versus INTEM CT, CS versus EXTEM A20 and FCS versus FIBTEM A20. The strength of the concordance and the correlation (r value) was interpreted according to common definitions: 0.00–0.19: ‘very weak’, 0.20–0.39: ‘weak’, 0.40–0.59: ‘moderate’, 0.60–0.79: ‘strong’ and 0.80–1.0: ‘very strong’.28
A clinical concordance analysis was performed using a 2×2 confusion matrix to determine the agreement between the Quantra and ROTEM clot lysis parameters. As previously stated, for the Quantra QStat Cartridge, clot lysis was defined as positive if CSL was below the normal range threshold of 93%. For ROTEM, positive clot lysis was defined as EXTEM maximum lysis (ML) parameter >15% (determined at 60 min).
Results
A total of 56 adult trauma patients were enrolled in this multicenter study. Patients demographics data are summarized in table 1. Thirty-five subjects had an RTS between 9 and 12; only five subjects had RTS in the lower category of 0–4. Blunt injuries accounted for 64.3% of the cases, penetrating injuries for 39.3% and neurological injuries for 32.1% (note that more than one injury type was recorded for some subjects).
Table 1.
Study demographics
| Characteristic | Value |
| N | 56 |
| Age (mean, SD) | 49.3 (17.1) |
| Male (N, %) | 48 (86) |
| Race (N, %) | |
| Caucasian | 50 (89.3) |
| Hispanic/Latino | 31 (55) |
| Injuries | |
| Revised Trauma Score (RTS) (median) | 12 |
| RTS breakdown (N, %) | N=53 with score |
| 0–4 | 5 (9.4) |
| 5–8 | 13 (24.5) |
| 9–12 | 35 (66.0) |
| Mechanism of injury (N, %) | |
| Motor vehicle accident | 19 (33.9) |
| Gunshot wound | 11 (19.6) |
| Fall | 7 (12.5) |
| Motorcycle accident | 5 (8.9) |
| Other | 14 (25.0) |
| Type of injuries* | |
| Blunt | 36 (64.3) |
| Penetrating | 22 (39.3) |
| Neurologic | 18 (32.1) |
| Outcome | |
| Time in ICU, days (mean, SD) | 7 (8.5) |
| LOS, days (mean, SD) | 10 (10.7) |
| Death (N, %) | 6 (10.7) |
ICU, Intensive Care Unit
LOS, length of stay
*For some subjects more than one type of injury was recorded.
Table 2 provides some basic summary statistics of the data from the Quantra and ROTEM delta devices on samples that were tested in parallel. Forty-two per cent of samples had a low CS value suggestive of an hypocoagulable state. The low stiffness values could be attributed to either low platelet contribution (PCS), low fibrinogen contribution (FCS), or a combination of the two. Fewer samples analyzed on standard ROTEM assays showed evidence of low clot stiffness based on low EXTEM and FIBTEM A20 results and hyperfibrinolysis based on EXTEM ML results.
Table 2.
Summary statistics of QStat and ROTEM data
| Quantra QStat | ROTEM | ||||||||
| CT | CS | PCS | FCS | CSL | INTEM CT | EXTEM A20 | FIBTEM A20 | EXTEM ML* | |
| N obs | 63 | 62 | 59 | 59 | 60 | 54 | 62 | 63 | 61 |
| Mean (SD) | 119.1 (25.3) | 15.7 (7.4) | 14.4 (6.2) | 1.7 (1.3) | 92.4 (20.1) | 157.3 (38.6) | 55.1 (13.6) | 12.9 (6.0) | 9.6 (21.2) |
| Min | 73 | <2.0 | <2.0 | 0.5 | 8.0 | 98 | 0.0 | 0.0 | 0.0 |
| Max | 198 | 46.8 | 37.1 | 9.7 | 100 | 302 | 75 | 34 | 100 |
| Below RR (N,%) | 16 (25.4) | 26 (41.9) | 22 (37.3) | 12 (20.3) | 8 (13.3) | 9 (16.7) | 15 (24.2) | 6 (9.5) | N/A |
| Above RR (N,%) | 3 (4.8) | 1 (1.6) | 1 (1.7) | 2 (3.4) | N/A | 4 (7.4) | 3 (4.8) | 3 (4.8) | 5 (8.2) |
QStat preliminary RR: CT: 104–166 s; CS: 13.0–33.2 hPa; PCS: 11.9–29.8 hPa; FCS: 1.0–3.7 hPa; CSL: 100%–93%.
ROTEM RR: INTEM CT: 122–208 s; EXTEM A20: 50–70 mm; FIBTEM A20: 7–24 mm; EXTEM ML (@60 min): 0%–15%.
*Calculated at 60 min after clot time.
CS, clot stiffness; CSL, clot stability to lysis; CT, clot time; FCS, fibrinogen contribution to clot stiffness; PCS, platelet contribution to clot stiffness; RR, reference ranges.
Fibrinolysis concordance analysis
Eight samples (13.3%) had a QStat CSL value below the reference range threshold of 93%, indicating the presence of hyperfibrinolysis. Conversely, only five samples (8.2%) had an EXTEM ML at 60 min greater than 15%. Table 3 summarizes the results of the concordance analysis between the Quantra CSL and the ROTEM EXTEM ML for the detection of hyperfibrinolysis. Note that for one of the samples determined to be fibrinolysis positive based on CSL <93%, there was no corresponding EXTEM ML reported, thus this sample was excluded from analysis.
Table 3.
Concordance analysis of QStat CSL versus ROTEM EXTEM ML
| ROTEM EXTEM | |||
| ML >15% (fibrinolysis +) | ML ≤15% (fibrinolysis −) | ||
| Quantra QStat | CSL <93% (fibrinolysis +) | 5 | 2 |
| CSL ≥93% (fibrinolysis −) | 0 | 51 | |
| Overall agreement | 96.6% | ||
CSL, clot stability to lysis; ML, maximum lysis.
Table 3 indicates that overall, for 96.6% of samples, QStat and ROTEM results were in agreement with each other with respect identifying fibrinolysis positive and fibrinolysis negative samples. One of the two subjects identified as fibrinolysis positive by the Quantra but not by the ROTEM had a CSL value of 92%, just below the cut-off value of 93%; the second was a patient that received cardiopulmonary resuscitation (CPR) prior to sample collection and analysis.
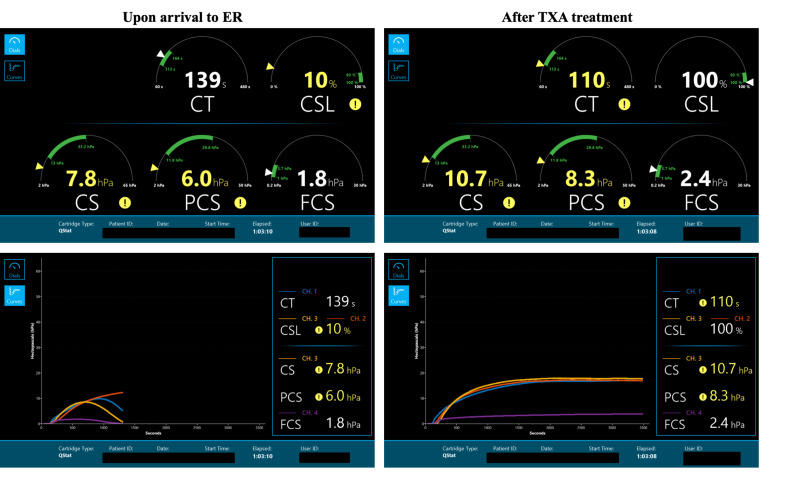
Figure 1 shows the QStat dials and curves generated on arrival to the emergency room and after the administration of antifibrinolytics. Significant hyperfibrinolysis is observed on arrival as indicated by a CSL value of 10%. After administration of TXA, CSL returns within the normal reference range.
Figure 1.
Quantra QStat dials and curves generated on arrival to the emergency room (ER) (left panels) and after the administration of antifibrinolytics (right panels). For each dial, the green bar represents the normal reference range. Values outside the reference range are flagged with a different color and a warning sign. As expected, after the administration of antifibrinolytics, the QStat CSL parameter returns within the normal range from an initial value of 10% indicating significant clot dissolution (as also confirmed from the corresponding curves). CSL, Clot Stability to Lysis; TXA, tranexamic acid.
Correlation analysis
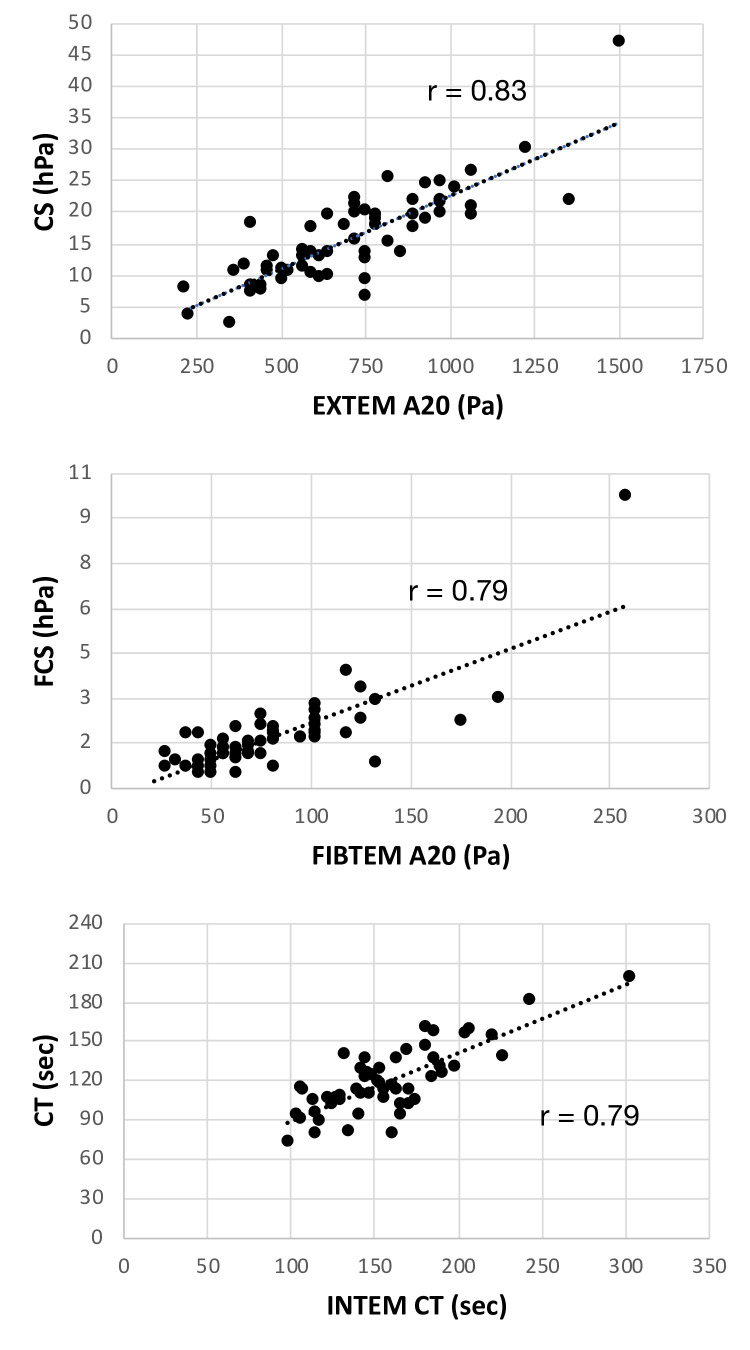
The results of the linear regression analysis are summarized in figure 2, which shows the scatter plots of the three paired QStat and ROTEM parameters: CS versus EXTEM A20, FCS versus FIBTEM A20 and CT versus INTEM CT, respectively. For these analyses, the ROTEM measurements of clot amplitude were converted from millimeters (mm) to units of shear modulus (Pascals) before performing regression analysis.29 30
Figure 2.
Scatter plots of QStat parameters versus corresponding ROTEM delta parameters. (Top) QStat CS and EXTEM A20 in units of Pa, (middle) QStat FCS and FIBTEM A20 in units of Pa and (bottom) QStat CT and INTEM CT. CS, Clot Stiffness; CT, Clot Time; FCS, Fibrinogen Contribution to clot stiffness.
These data demonstrate a strong correlation between QPlus parameters and corresponding ROTEM delta parameters, although in the presence of an inherent bias associated with the differences in measurement principles. As previously discussed with respect to the QPlus parameters, the bias does not introduce concerns regarding the comparison of these devices since measured results are interpreted in relation to the respective reference intervals.22
Although not shown here, the correlation between CSL and EXTEM ML had an r value of 0.95, thus indicating a very strong correlation in addition to high concordance between the two measurements.
Discussion
This manuscript describes the first clinical experience with the Quantra and the QStat Cartridge in adult trauma patients requiring the highest level of trauma activation. It also introduces the CSL, a new parameter generated via viscoelastic analysis of fibrinolysis with the Quantra System. The results of this observational multicenter study showed strong correlation and strong concordance of QStat parameters with equivalent metrics obtained from the ROTEM delta, the standard of care at the level I trauma centers participating in the study. VET with goal-directed treatment algorithm is recommended, when such testing capabilities are available, by several clinical guidelines for the management of massive bleeding and coagulopathies in trauma patients. These include the American College of Surgeons Trauma Quality Improvement Program and the most recent European guideline on management of major bleeding and coagulopathy following trauma.11 31
The comparative data obtained with the QStat parameters CT, CS and FCS versus ROTEM are in agreement with the results previously presented in studies in cardiac and major orthopedic surgeries using the QPlus Cartridge.22–26 Notwithstanding the strong correlation between the two systems, the Quantra-based stiffness results demonstrated a higher number of samples with values below the respective reference ranges as compared with the ROTEM delta. Interestingly, the main contributor to a low Quantra CS value was a low PCS value, highlighting the potential importance of an indicator of platelet contribution to clot stiffness (note that the correlation of PCS with platelet count was moderate with r value of 0.54, data not shown).
The data presented here demonstrated that the CSL parameter had a very strong overall agreement with the EXTEM ML parameter even though only a few patients experienced hyperfibrinolysis. The ROTEM EXTEM ML is a dynamic parameter that measures the per cent clot firmness lost after maximum clot firmness. Although this parameter is routinely used as part of goal-directed treatment algorithms in the trauma and liver transplant populations, for example, there is no reference range interval, nor an approved threshold value provided by the manufacturer. Based on several published studies in civilian and military trauma, clinical practice has converged to the definition of hyperfibrinolysis as a reduction in MCF of greater than 15% (ML >15%) 60 min after the onset of clot formation.18 32–35 Lang et al36 performed a multicenter study on adult normal subjects (n=150) and reported an EXTEM ML reference interval of 0%–18%, thus in line with the clinically used cut-off value of 15%.
Furthermore, the Quantra identified a higher number of samples categorized as ‘fibrinolysis positive’ (ie, with values below the CSL threshold) as compared with the ROTEM delta. The two discordant observations include a sample just below the CSL threshold (CSL of 92% and corresponding EXTEM ML of 9%) and a sample from a patient undergoing CPR that generated a CSL value of 57%, indicating significant hyperfibrinolysis but a corresponding EXTEM ML of 2%. While the reason for this significant difference between the two systems is currently unclear, we hypothesize that out-of-hospital cardiac arrest and longer CPR times may be associated with higher incidence of hyperfibrinolysis.37
The CSL is a new Quantra-based parameter that was designed to quantify the reduction in clot stiffness caused by fibrinolysis. The computation of CSL is based on a differential test strategy with and without tranexamic acid, therefore mitigating the effects of clot relaxation, often observed in viscoelastic testing systems as a reduction in clot stiffness not attributable to fibrinolysis but to the interactions between platelets and fibrinogen with the walls of the measuring chambers. A similar approach can be implemented with the ROTEM delta combining the EXTEM test (no inhibitor of fibrinolysis) and the APTEM test, which inhibits fibrinolysis using aprotinin. However, the majority of ROTEM-based treatment algorithms do not typically rely on the APTEM/EXTEM ML differential. Furthermore, looking at the FIBTEM curve for evidence of fibrinolysis, as plasmin cleaves fibrin, demonstrates further confirmation of true fibrinolysis.38 The QStat FCS curve provided similar evidence of fibrinolysis.
Another difference with respect to other VET platforms is that CSL is not calculated at fixed time points, such as in the case of the TEG LY30 or LY60 parameters or the ROTEM ML at 60 min, but instead it is computed and reported as soon as fibrinolysis is detected. In this study, CSL was computed, on average, within 44.2 min from test initiation (SD of 9.7 min), with a minimum value of 11.6 min recorded in one of the samples with the lowest CSL values. An earlier indication of CSL coupled with the ability to run the test near point-of-care may provide the clinician clinically important information sooner to guide therapy.
Finally, it is important to note that CSL is based on the direct measurement of shear modulus, measured in hectoPascals, which offers a larger available measurement range than the corresponding clot amplitude in units of mm. As previously demonstrated, the relationship between shear modulus and clot amplitude is non-linear, with larger values of shear modulus being compressed more in millimeters.22 30 This means that small relative changes in mm from a high clot stiffness represent large changes in shear modulus, which should be readily measured by CSL.
The study had several limitations that need to be considered. First, the number of enrolled patients was limited and, in particular, the number of hyperfibrinolytic samples/patients was small. Furthermore, even though the ROTEM was used as the comparator in this study, there is no widely accepted gold-standard test/assay for the diagnosis of fibrinolysis. The study was observational; therefore, the actual impact of the Quantra QStat System on patient care remains unknown and should be investigated in the future through interventional studies. Finally, interpretation of the strength of the correlation of the Quantra versus ROTEM was based on the definitions presented by Schober et al28 as previously utilized by Huffmyer et al23 and Naik et al,25 among others, even though we recognize that other definitions with different thresholds exist.
In conclusion, these results support the use of the Quantra QStat System as an aid to monitor coagulation and fibrinolysis status in the trauma population. The ability to perform testing at the point of care, the ability to generate fast results and the closed tube handling of blood samples may provide additional clinical advantages and safety considerations over existing devices. However, additional studies are needed to fully characterize the performance of the system and its ability to affect patient care.
Acknowledgments
We would like to thank Robert Wiard for his support in managing the clinical study sites.
Footnotes
Contributors: EAM, MWC and BR: study design, data collection, data interpretation, writing of the manuscript and critical revision. DAW: literature search, study design, writing of the manuscript and critical revision. FV: literature search, data analysis, data interpretation and initial drafting of the manuscript.
Funding: The study was funded by HemoSonics, LLC (Charlottesville, Virginia, USA).
Competing interests: DAW and FV are employees of HemoSonics, LLC, a medical device company that is commercializing the Quantra QStat System.
Patient consent for publication: Not required.
Ethics approval: The study obtained approval from the Institutional Review Boards (IRB) at the Texas Tech University Health Sciences Center (IRB# E19021) and the UT Southwestern (IRB# STU-2018–0039). Through a deferred consent process, each participant or their legally authorized representative was approached to provide written informed consent to enable release of their data to the study database.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement: All data relevant to the study are included in the article or uploaded as supplementary information. All data relevant to the study are included in the article.
References
- 1.Cannon JW. Hemorrhagic shock. N Engl J Med 2018;378:370–9. 10.1056/NEJMra1705649 [DOI] [PubMed] [Google Scholar]
- 2.Tisherman SA, Schmicker RH, Brasel KJ, Bulger EM, Kerby JD, Minei JP, Powell JL, Reiff DA, Rizoli SB, Schreiber MA. Detailed description of all deaths in both the shock and traumatic brain injury hypertonic saline trials of the resuscitation outcomes Consortium. Ann Surg 2015;261:586–90. 10.1097/SLA.0000000000000837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rhee P, Joseph B, Pandit V, Aziz H, Vercruysse G, Kulvatunyou N, Friese RS. Increasing trauma deaths in the United States. Ann Surg 2014;260:13–21. 10.1097/SLA.0000000000000600 [DOI] [PubMed] [Google Scholar]
- 4.Holcomb JB, Tilley BC, Baraniuk S, Fox EE, Wade CE, Podbielski JM, del Junco DJ, Brasel KJ, Bulger EM, Callcut RA, et al. Transfusion of plasma, platelets, and red blood cells in a 1:1:1 vs a 1:1:2 ratio and mortality in patients with severe trauma: the PROPPR randomized clinical trial. JAMA 2015;313:471–82. 10.1001/jama.2015.12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kornblith LZ, Moore HB, Cohen MJ. Trauma-induced coagulopathy: the past, present, and future. J Thromb Haemost 2019;17:852–62. 10.1111/jth.14450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Moore HB, Gando S, Iba T, Kim PY, Yeh CH, Brohi K, Hunt BJ, Levy JH, Draxler DF, Stanworth S, et al. Defining trauma-induced coagulopathy with respect to future implications for patient management: communication from the SSC of the ISTH. J Thromb Haemost 2020;18:740–7. 10.1111/jth.14690 [DOI] [PubMed] [Google Scholar]
- 7.Schöchl H, Voelckel W, Schlimp CJ. Management of traumatic haemorrhage--the European perspective. Anaesthesia 2015;70(Suppl 1):102–37. 10.1111/anae.12901 [DOI] [PubMed] [Google Scholar]
- 8.Cohen MJ, Christie SA. New understandings of post injury coagulation and resuscitation. Int J Surg 2016;33:242–5. 10.1016/j.ijsu.2016.05.037 [DOI] [PubMed] [Google Scholar]
- 9.Brohi K, Cohen MJ, Davenport RA. Acute coagulopathy of trauma: mechanism, identification and effect. Curr Opin Crit Care 2007;13:680–5. 10.1097/MCC.0b013e3282f1e78f [DOI] [PubMed] [Google Scholar]
- 10.Hess JR, Brohi K, Dutton RP, Hauser CJ, Holcomb JB, Kluger Y, Mackway-Jones K, Parr MJ, Rizoli SB, Yukioka T, et al. The coagulopathy of trauma: a review of mechanisms. J Trauma 2008;65:748–54. 10.1097/TA.0b013e3181877a9c [DOI] [PubMed] [Google Scholar]
- 11.Spahn DR, Bouillon B, Cerny V, Duranteau J, Filipescu D, Hunt BJ, Komadina R, Maegele M, Nardi G, Riddez L, et al. The European guideline on management of major bleeding and coagulopathy following trauma: fifth edition. Crit Care 2019;23:98–172. 10.1186/s13054-019-2347-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moore EE, Moore HB, Chapman MP, Gonzalez E, Sauaia A. Goal-directed hemostatic resuscitation for trauma induced coagulopathy: maintaining homeostasis. J Trauma Acute Care Surg 2018;84:S35. 10.1097/TA.0000000000001797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cohen MJ, Call M, Nelson M, Calfee CS, Esmon CT, Brohi K, Pittet JF. Critical role of activated protein C in early coagulopathy and later organ failure, infection and death in trauma patients. Ann Surg 2012;255:379–85. 10.1097/SLA.0b013e318235d9e6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kashuk JL, Moore EE, Sawyer M, Wohlauer M, Pezold M, Barnett C, Biffl WL, Burlew CC, Johnson JL, Sauaia A. Primary fibrinolysis is integral in the pathogenesis of the acute coagulopathy of trauma. Ann Surg 2010;252:22–33. 10.1097/SLA.0b013e3181f09191 [DOI] [PubMed] [Google Scholar]
- 15.Chapman MP, Moore EE, Ramos CR, Ghasabyan A, Harr JN, Chin TL, Stringham JR, Sauaia A, Silliman CC, Banerjee A. Fibrinolysis greater than 3% is the critical value for initiation of antifibrinolytic therapy. J Trauma Acute Care Surg 2013;75:961–7. 10.1097/TA.0b013e3182aa9c9f [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Da Luz LT, Nascimento B, Shankarakutty AK, Rizoli S, Adhikari NK. Effect of thromboelastography (TEG®) and rotational thromboelastometry (ROTEM®) on diagnosis of coagulopathy, transfusion guidance and mortality in trauma: descriptive systematic review. Crit Care 2014;18:518. 10.1186/s13054-014-0518-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Veigas PV, Callum J, Rizoli S, Nascimento B, da Luz LT. A systematic review on the rotational thromboelastometry (ROTEM) values for the diagnosis of coagulopathy, prediction and guidance of blood transfusion and prediction of mortality in trauma patients. Scand J Trauma 2016;24:114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Abdelfattah K, Cripps MW. Thromboelastography and rotational Thromboelastometry use in trauma. Int J Surg 2016;33:196–201. 10.1016/j.ijsu.2015.09.036 [DOI] [PubMed] [Google Scholar]
- 19.Gonzalez E, Moore EE, Moore HB, Chapman MP, Chin TL, Ghasabyan A, Wohlauer MV, Barnett CC, Bensard DD, Biffl WL, et al. Goal-directed hemostatic resuscitation of trauma-induced coagulopathy: a pragmatic randomized clinical trial comparing a viscoelastic assay to conventional coagulation assays. Ann Surg 2016;263:1051. 10.1097/SLA.0000000000001608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ferrante EA, Blasier KR, Givens TB, Lloyd CA, Fischer TJ, Viola F. A novel device for the evaluation of hemostatic function in critical care settings. Anesth Analg 2016;123:1372–9. 10.1213/ANE.0000000000001413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Leadbetter NH, Givens TB, Viola F. Unique approach to quality assurance in viscoelastic testing. J Appl Lab Med 2020. [Epub ahead of print: 20 May 2020]. 10.1093/jalm/jfaa057 [DOI] [PubMed] [Google Scholar]
- 22.Groves DS, Welsby IJ, Naik BI, Tanaka K, Hauck JN, Greenberg CS, Winegar DA, Viola F. Multicenter evaluation of the Quantra QPlus system in adult patients undergoing major surgical procedures. Anesth Analg 2020;130:899–909. 10.1213/ANE.0000000000004659 [DOI] [PubMed] [Google Scholar]
- 23.Huffmyer JL, Fernandez LG, Haghighian C, Terkawi AS, Groves DS. Comparison of SEER Sonorheometry with rotational Thromboelastometry and laboratory parameters in cardiac surgery. Anesth Analg 2016;123:1390–9. 10.1213/ANE.0000000000001507 [DOI] [PubMed] [Google Scholar]
- 24.Reynolds PS, Middleton P, McCarthy H, Spiess BD. A comparison of a new Ultrasound-Based whole blood viscoelastic test (SEER Sonorheometry) versus thromboelastography in cardiac surgery. Anesth Analg 2016;123:1400–7. 10.1213/ANE.0000000000001362 [DOI] [PubMed] [Google Scholar]
- 25.Naik BI, Durieux ME, Knisely A, Sharma J, Bui-Huynh VC, Yalamuru B, Terkawi AS, Nemergut EC. SEER Sonorheometry versus rotational Thromboelastometry in large volume blood loss spine surgery. Anesth Analg 2016;123:1380–9. 10.1213/ANE.0000000000001509 [DOI] [PubMed] [Google Scholar]
- 26.Baryshnikova E, Di Dedda U, Ranucci M. A comparative study of SEER Sonorheometry versus standard coagulation tests, rotational Thromboelastometry, and multiple electrode aggregometry in cardiac surgery. J Cardiothorac Vasc Anesth 2019;33:1590–8. 10.1053/j.jvca.2019.01.011 [DOI] [PubMed] [Google Scholar]
- 27.Ranucci M, Ballotta A, Di Dedda U, Bayshnikova E, Dei Poli M, Resta M, Falco M, Albano G, Menicanti L. The procoagulant pattern of patients with COVID-19 acute respiratory distress syndrome. J Thromb Haemost 2020;18:1747–51. 10.1111/jth.14854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schober P, Boer C, Schwarte LA. Correlation coefficients: appropriate use and interpretation. Anesth Analg 2018;126:1763–8. 10.1213/ANE.0000000000002864 [DOI] [PubMed] [Google Scholar]
- 29.Lang T, Johanning K, Metzler H, Piepenbrock S, Solomon C, Rahe-Meyer N, Tanaka KA. The effects of fibrinogen levels on thromboelastometric variables in the presence of thrombocytopenia. Anesth Analg 2009;108:751–8. 10.1213/ane.0b013e3181966675 [DOI] [PubMed] [Google Scholar]
- 30.Solomon C, Ranucci M, Hochleitner G, Schöchl H, Schlimp CJ. Assessing the methodology for calculating platelet contribution to clot strength (platelet component) in Thromboelastometry and thrombelastography. Anesth Analg 2015;121:868–78. 10.1213/ANE.0000000000000859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.ACS TQIP Massive transfusion in trauma guidelines. 2014. https://www.https://www.facs.org/-/media/files/quality-programs/trauma/tqip/transfusion_guildelines.ashx (8 May 2020).
- 32.Gall LS, Brohi K, Davenport RA. Diagnosis and treatment of Hyperfibrinolysis in trauma (a European perspective). Semin Thromb Hemost 2017;43:224–34. 10.1055/s-0036-1598001 [DOI] [PubMed] [Google Scholar]
- 33.Schöchl H, Frietsch T, Pavelka M, Jámbor C. Hyperfibrinolysis after major trauma: differential diagnosis of lysis patterns and prognostic value of thrombelastometry. J Trauma 2009;67:125–31. 10.1097/TA.0b013e31818b2483 [DOI] [PubMed] [Google Scholar]
- 34.Theusinger OM, Wanner GA, Emmert MY, Billeter A, Eismon J, Seifert B, Simmen H-P, Spahn DR, Baulig W. Hyperfibrinolysis diagnosed by rotational thromboelastometry (ROTEM) is associated with higher mortality in patients with severe trauma. Anesth Analg 2011;113:1003–12. 10.1213/ANE.0b013e31822e183f [DOI] [PubMed] [Google Scholar]
- 35.Doran CM, Woolley T, Midwinter MJ. Feasibility of using rotational thromboelastometry to assess coagulation status of combat casualties in a deployed setting. J Trauma 2010;69(Suppl 1):S40–8. 10.1097/TA.0b013e3181e4257b [DOI] [PubMed] [Google Scholar]
- 36.Lang T, Bauters A, Braun SL, Pötzsch B, von Pape K-W, Kolde H-J, Lakner M. Multi-centre investigation on reference ranges for ROTEM thromboelastometry. Blood Coagul Fibrinolysis 2005;16:301–10. 10.1097/01.mbc.0000169225.31173.19 [DOI] [PubMed] [Google Scholar]
- 37.Viersen VA, Greuters S, Korfage AR, Van der Rijst C, Van Bochove V, Nanayakkara PW, Vandewalle E, Boer C. Hyperfibrinolysis in out of hospital cardiac arrest is associated with markers of hypoperfusion. Resuscitation 2012;83:1451–5. 10.1016/j.resuscitation.2012.05.008 [DOI] [PubMed] [Google Scholar]
- 38.Wang I-J, Park S-W, Bae B-K, Lee S-H, Choi HJ, Park SJ, Ahn TY, Goh TS, Lee MJ, Yeom SR. FIBTEM improves the sensitivity of Hyperfibrinolysis detection in severe trauma patients: a retrospective study using Thromboelastometry. Sci Rep 2020;10:1–7. 10.1038/s41598-020-63724-y [DOI] [PMC free article] [PubMed] [Google Scholar]